Pantoprazole sodium freeze dried injection and preparation method thereof
A technology of pantoprazole sodium and freeze-dried powder injection, which is applied in the field of pantoprazole sodium freeze-dried powder injection and its preparation, can solve problems such as bone calcium loss, and achieve the effects of alleviating pain, avoiding side effects, and reducing impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, preparation and detection of pantoprazole sodium freeze-dried powder injection
[0044] One, prepare pantoprazole sodium freeze-dried powder injection
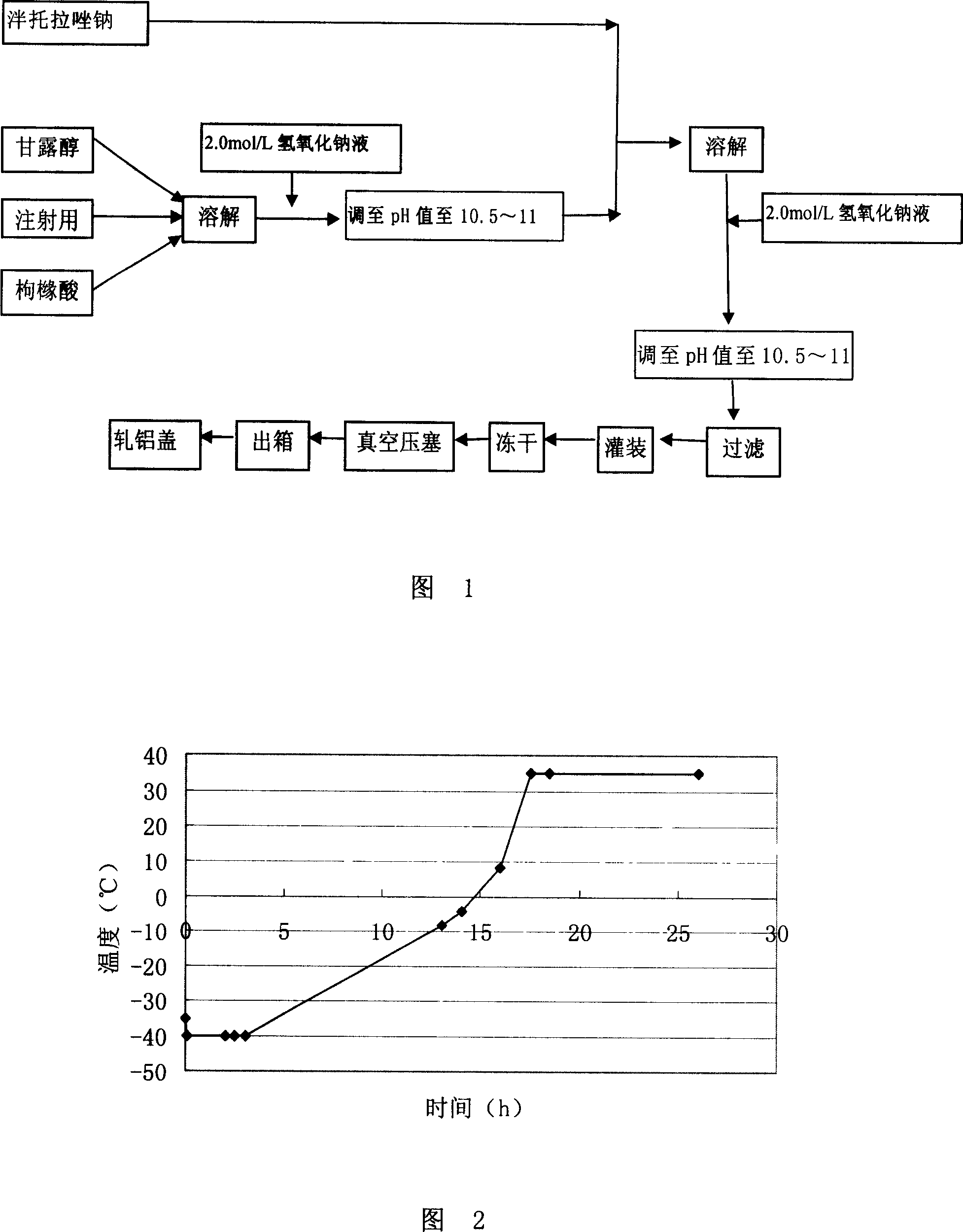
[0045] With reference to the process flow diagram shown in Fig. 1, prepare 1000 bottles (marked amount: containing 40g pantoprazole sodium) pantoprazole sodium freeze-dried powder injection with method of the present invention, concrete process comprises the following steps:
[0046] 1) Take materials by the following weight: mannitol 40.00g, sodium citrate 2.00g, pantoprazole sodium 44.72g;
[0047] 2) Dissolve mannitol and sodium citrate with 1800g water for injection, adjust the pH value to 10.5 with 2mol / L sodium hydroxide solution, add pantoprazole sodium, fully dissolve and then adjust the pH value with 2mol / L sodium hydroxide solution value to 10.5, and finally add water for injection at 4°C to 2000g;
[0048] 3) Sterilize by filtering twice with a filter membrane with a pore size of 0.22 μm;
[...
Embodiment 2
[0065] Embodiment 2, preparation and detection of pantoprazole sodium freeze-dried powder injection
[0066] Prepare 1000 bottles of pantoprazole sodium freeze-dried powder injection with the method of the present invention, concrete process comprises the following steps:
[0067] 1) Take materials by the following weight: dextran 24.00g, pantoprazole sodium 44.72g;
[0068] 2) Dissolve dextran with 1800g water for injection, adjust the pH value to 9.5 with 2mol / L disodium hydrogen phosphate solution, add pantoprazole sodium, fully dissolve and then adjust the pH value to 9.5 with 2mol / L disodium hydrogen phosphate solution , and finally add water for injection at 10°C to 2000g;
[0069] 3) Sterilize by filtering 3 times with a filter membrane with a pore size of 0.3 μm;
[0070] 4) Use an automatic filling machine (Nanjing Bojian Technology Co., Ltd. KFG-300 linear semi-dropper aseptic filling machine) to dispense the filtrate into 1000 bottles (specification: 10mL) of Xili...
Embodiment 3
[0073] Embodiment 3, preparation and detection of pantoprazole sodium freeze-dried powder injection
[0074] Prepare 1000 bottles of pantoprazole sodium freeze-dried powder injection with the method of the present invention, concrete process comprises the following steps:
[0075] 1) Take materials by the following weight: glucose 44.72g, potassium citrate 2.68g, pantoprazole sodium 44.72g;
[0076] 2) Dissolve glucose and potassium citrate with 1800g water for injection, adjust the pH value to 11.5 with 1mol / L potassium hydroxide solution, add pantoprazole sodium, fully dissolve and then adjust the pH value with 1mol / L potassium hydroxide solution value to 11.5, and finally add water for injection at 6°C to 2000g;
[0077] 3) Sterilize by filtering twice with a filter membrane with a pore size of 0.1 μm;
[0078] 4) Use an automatic filling machine (Nanjing Bojian Technology Co., Ltd. KFG-300 linear semi-dropper aseptic filling machine) to dispense the filtrate into 1000 bo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com