Pantoprazole sodium freeze-dried powder injection and preparation method thereof
A technology of pantoprazole sodium and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of excipients affecting product pre-freezing and drying, and unsatisfactory medicinal effects of powder injections, and achieve good clarity, stable quality and reliable control and avoid side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
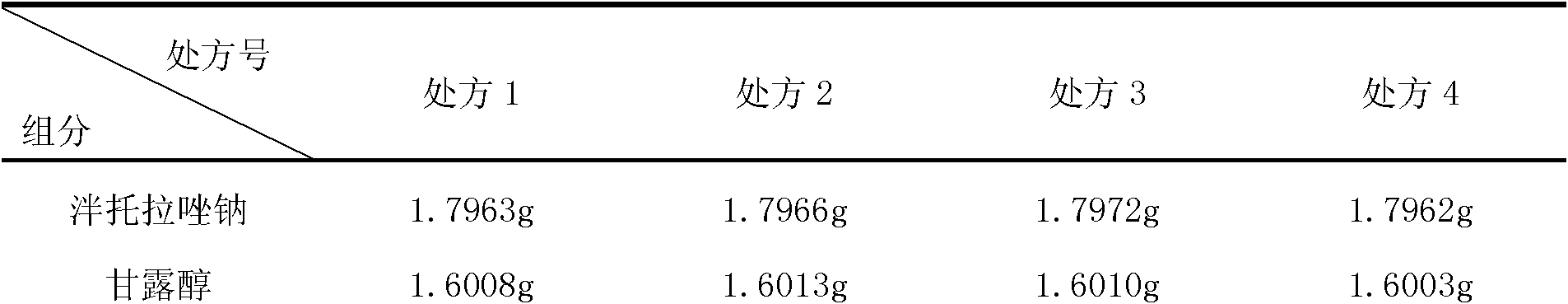
[0099] (1) Prescription: Specification 40mg:1.2ml
[0100] Pantoprazole Sodium (as Pantoprazole) 40g
[0101] Mannitol 40g
[0102] Add water for injection to 1200ml
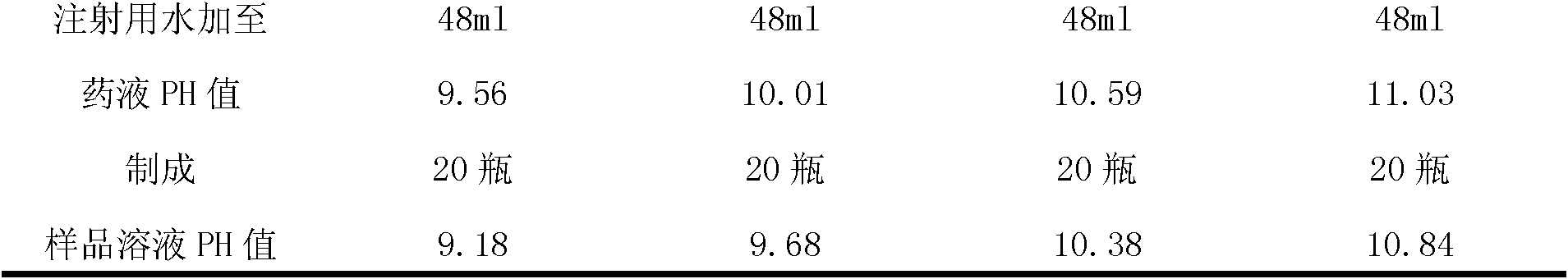
[0103]
[0104] 1000 bottles
[0105] (2) Preparation process:
[0106] Weigh the prescription amount of pantoprazole sodium and mannitol, add 80% of the total amount of water for injection (15℃) in the preparation tank, add the prescription amount of mannitol and pantoprazole sodium in turn, stir to make Dissolve into a clear solution; add water for injection to the full amount, mix well, adjust the pH value of the solution to 10.8 with 5mol / L sodium hydroxide solution, add 0.10% (g / ml) of the total amount of the prepared medicinal activated carbon, stir and absorb 30 Minutes, after decarbonization and the first 0.22μm sterilization filtration, after the intermediates pass the test, the medicine is hydraulically filtered to the medicine storage tank in the medicine receiving room, and then ...
Embodiment 2
[0112] (1) Prescription: Specification 80mg:2.4ml
[0113] Pantoprazole Sodium (as Pantoprazole) 80.0g
[0114] Mannitol 80.0g
[0115] Add water for injection to 2400ml
[0116]
[0117] Made into 1000 bottles
[0118] (2) Preparation process:
[0119] Weigh the prescription amount of pantoprazole sodium and mannitol, add 80% of the total amount of water for injection (15℃) in the preparation tank, add the prescription amount of mannitol and pantoprazole sodium in turn, stir to make Dissolve into a clear solution; add water for injection to the full amount, mix well, adjust the pH value of the solution to 10.8 with 5mol / L sodium hydroxide solution, add 0.10% (g / ml) medicinal activated carbon of the total solution, stir and absorb 30 Minutes, after decarbonization and the first 0.22μm sterilization filtration, after the intermediates pass the test, the medicine is hydraulically filtered to the medicine storage tank in the medicine receiving room, and th...
Embodiment 3
[0125] (1) Prescription: Specification 80mg:2.4ml
[0126] Pantoprazole Sodium (as Pantoprazole) 80.0g
[0127] Mannitol 64.0g
[0128] Add water for injection to 2400ml
[0129]
[0130] 1000 bottles
[0131] (2) Preparation process:
[0132] Weigh the prescription amount of pantoprazole sodium and mannitol, first add 80% of the total amount of water for injection (18°C) in the preparation tank, add the prescription amount of mannitol and pantoprazole sodium in turn, stir to make Dissolve into a clear solution; replenish the water for injection to the full amount, mix well, adjust the pH of the solution to 10.5 with 4mol / L sodium hydroxide solution, add 0.10% (g / ml) medicinal activated carbon of the total solution, stir and absorb 30 Minutes, after decarbonization and the first 0.22μm sterilization filtration, after the intermediates pass the test, the medicine is hydraulically filtered to the medicine storage tank in the medicine receiving room, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com