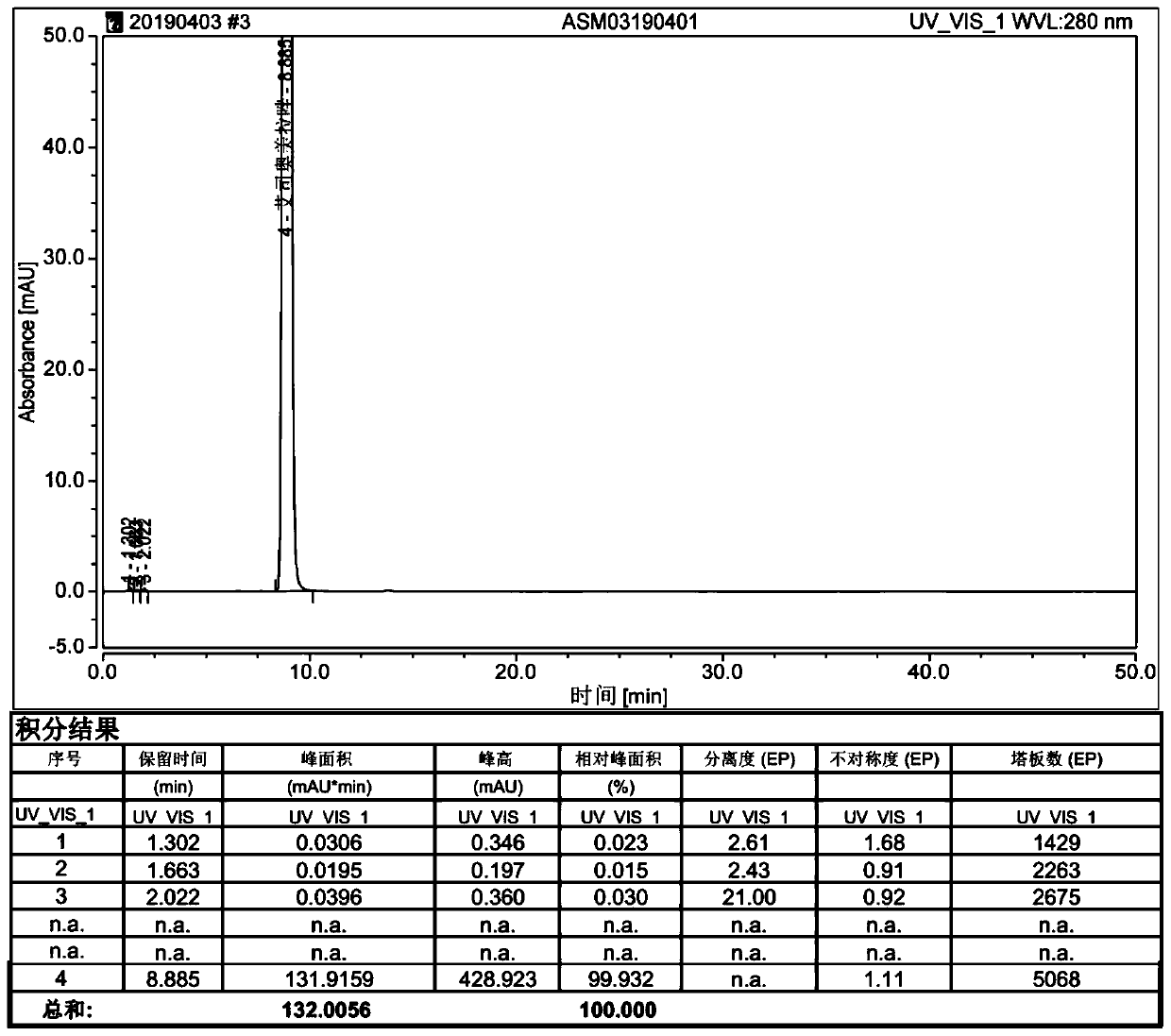
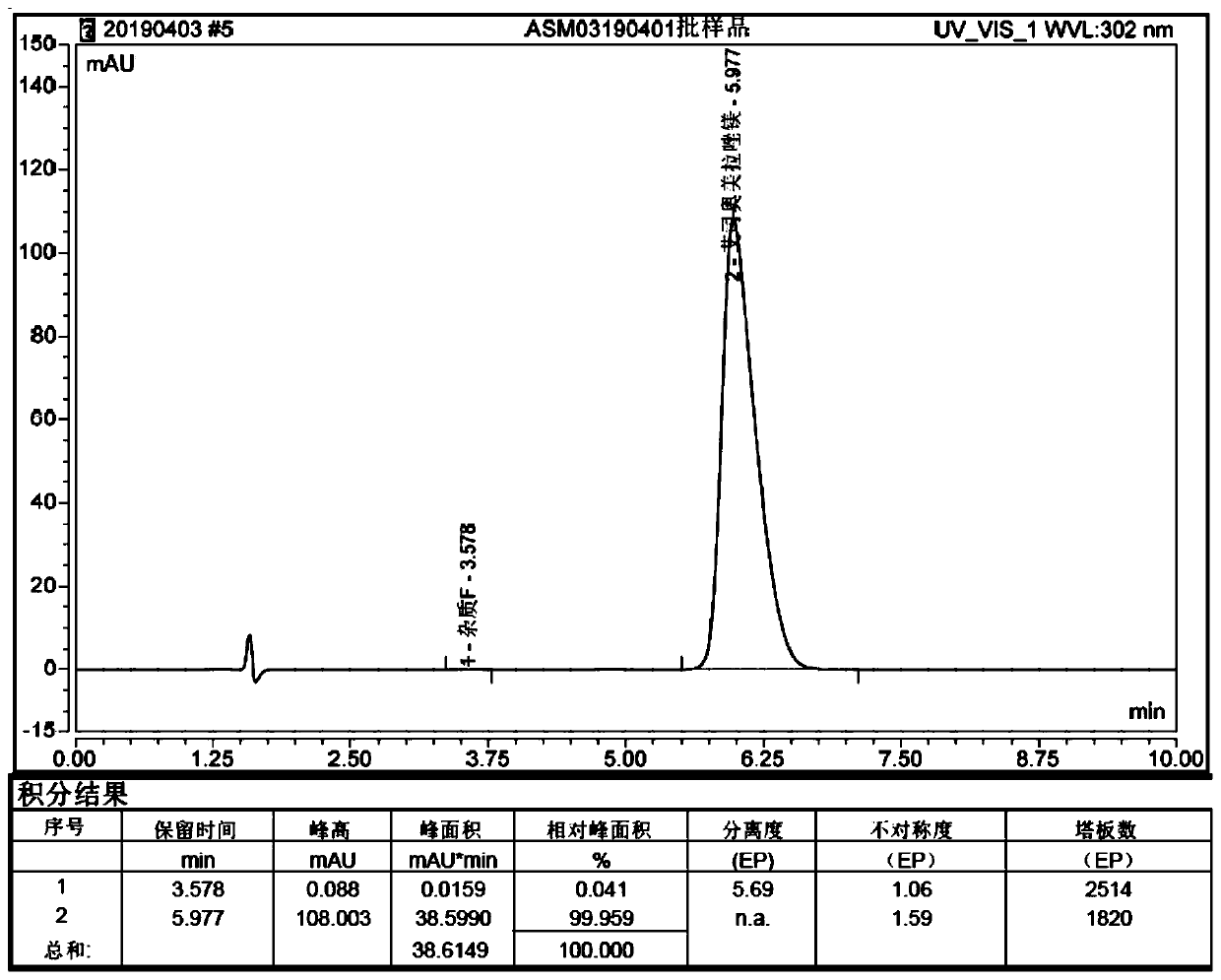
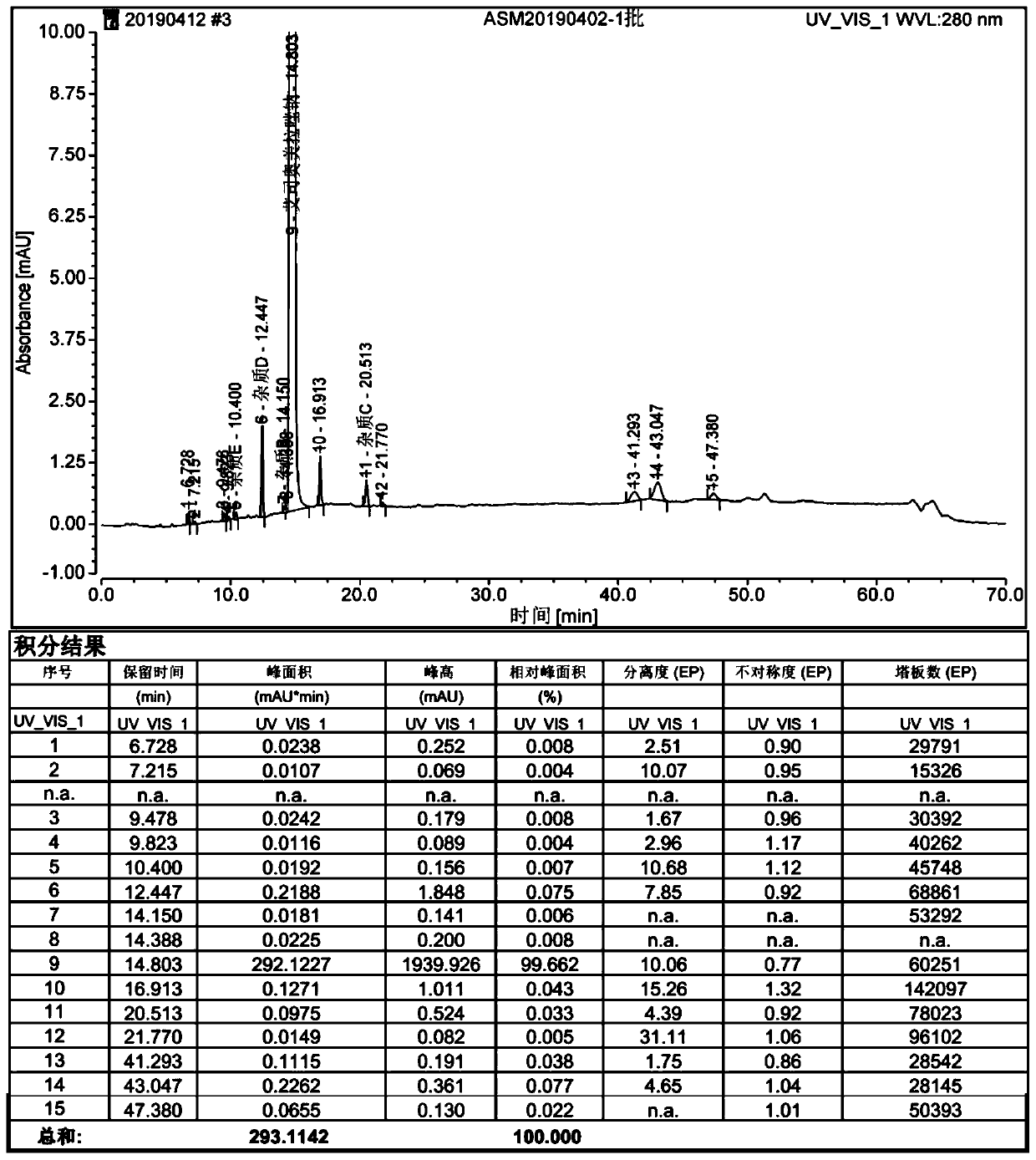
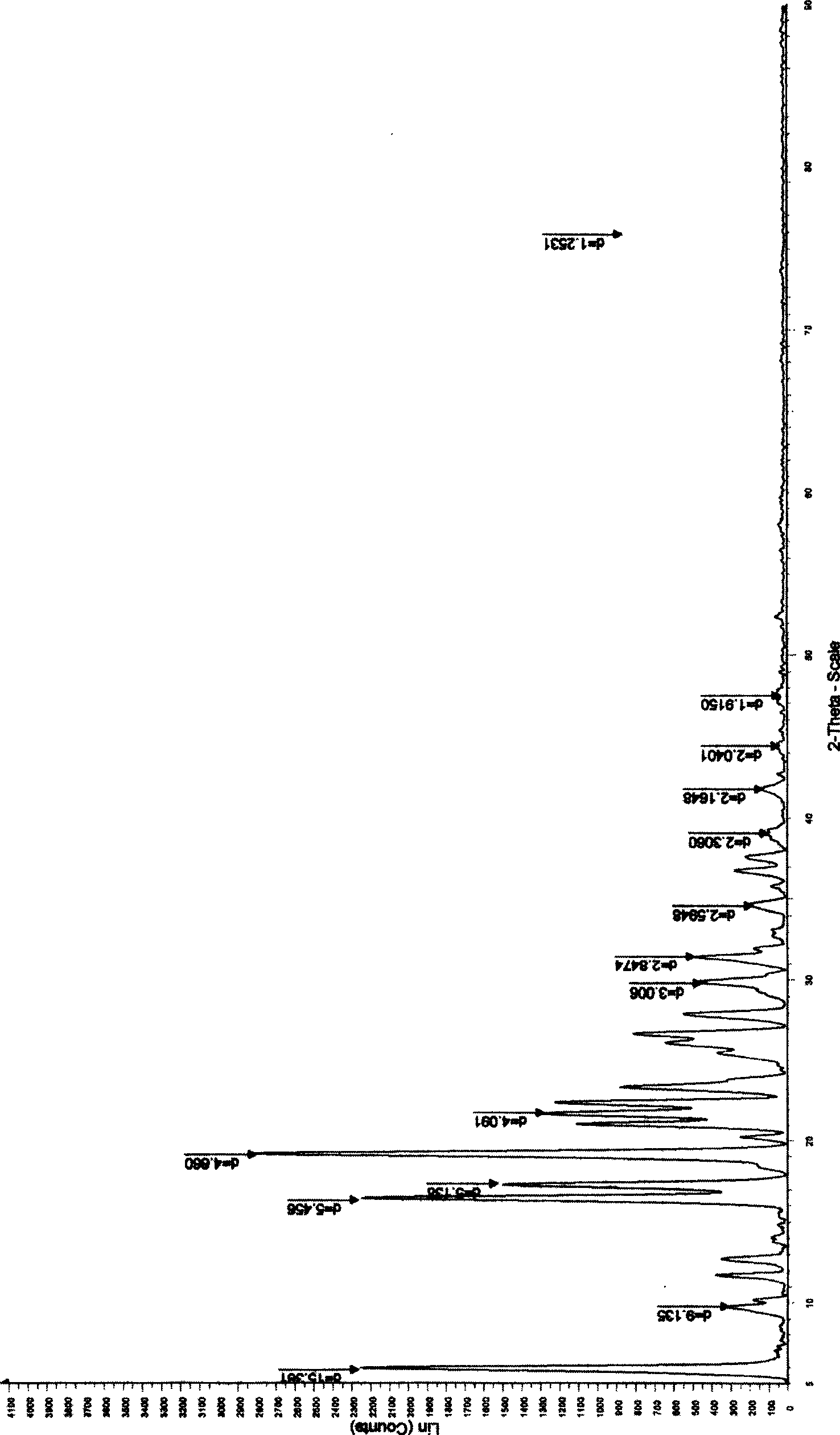
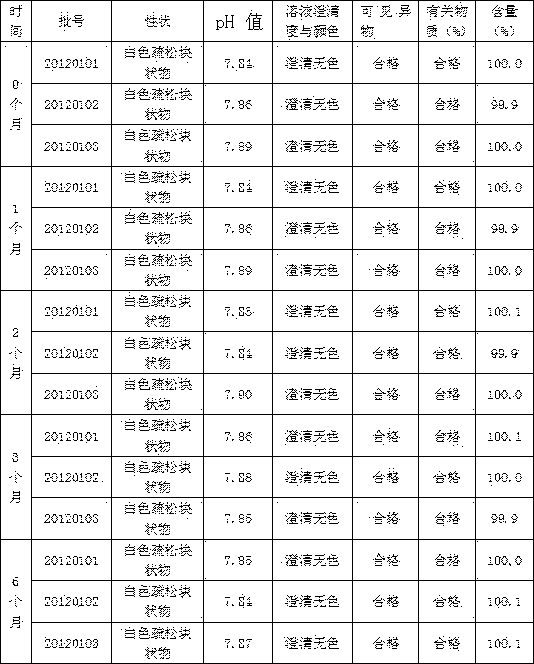
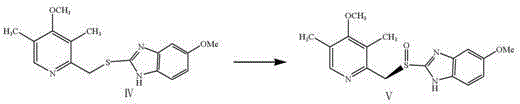Patents
Literature
50 results about "Diethyl tartrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diethyl tartrate is an organic compound, the ethyl ester of tartaric acid. It exists in both as a chiral isomer, showing both left- and right-handed forms, as well as a meso stereoisomer, which is not chiral. The chiral isomer is far more common.
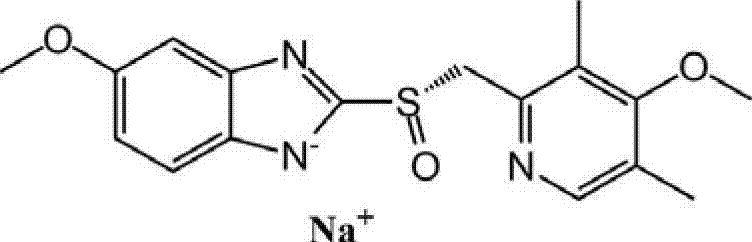
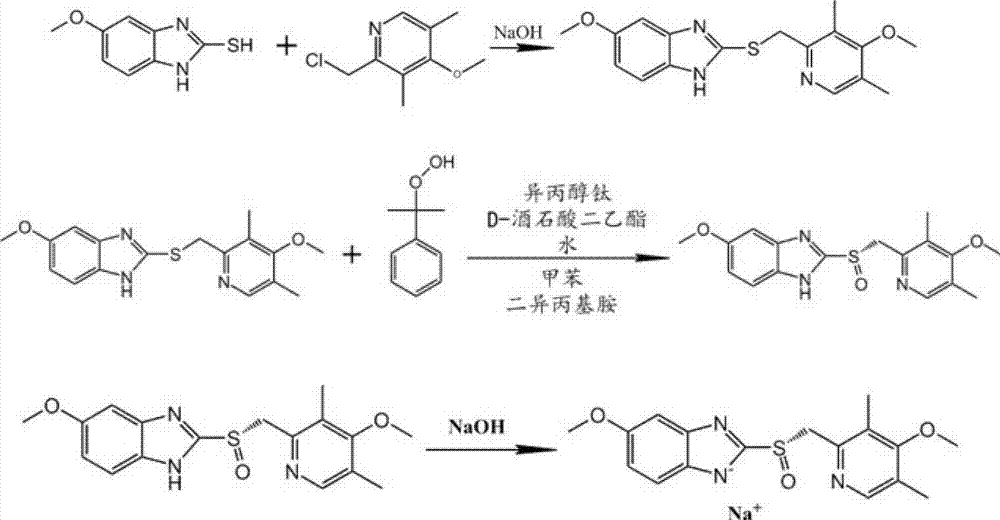
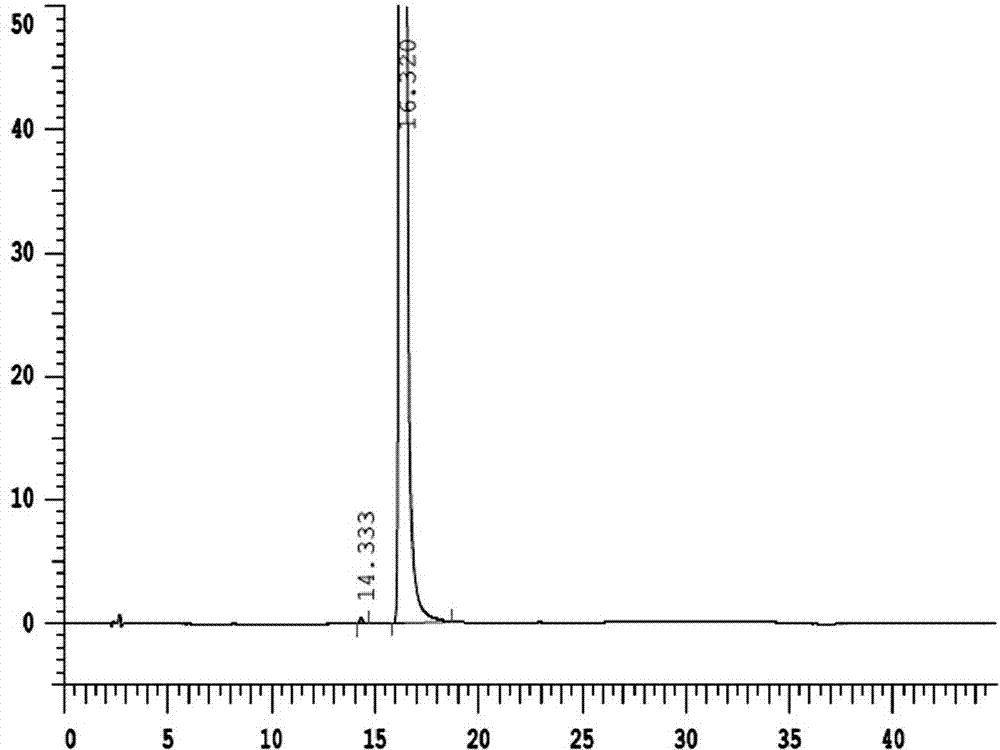
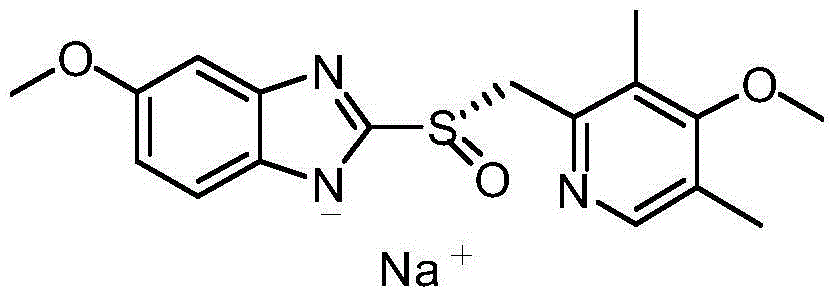
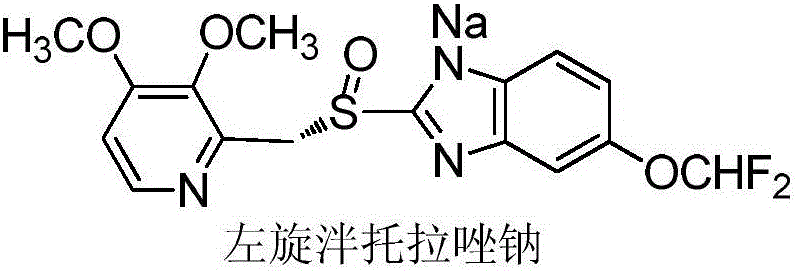
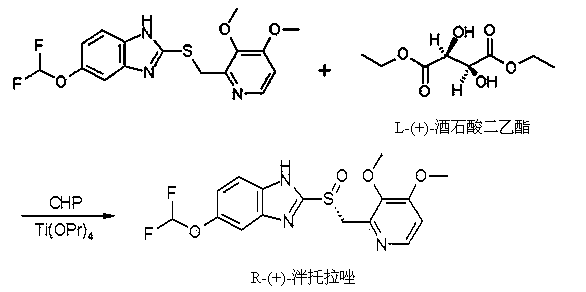
Method for preparing and purifying (L)-pantoprazole sodium
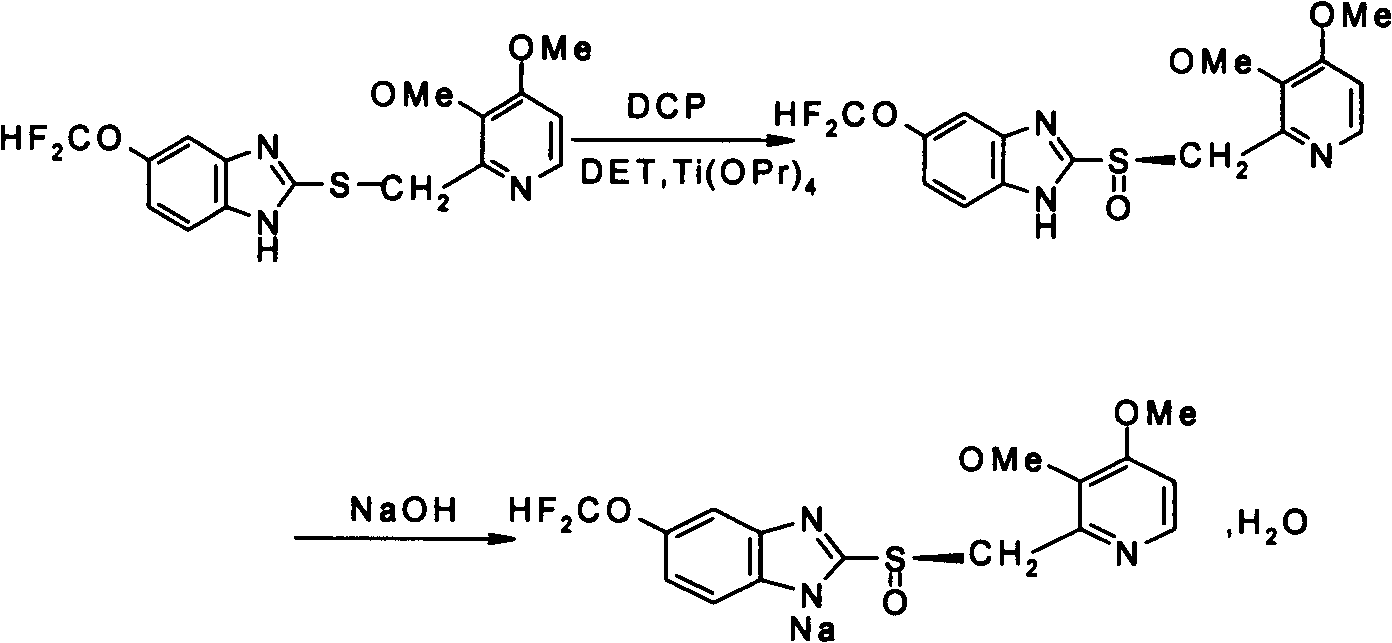
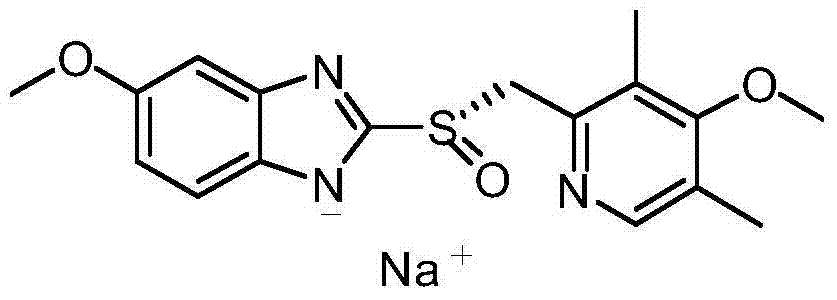
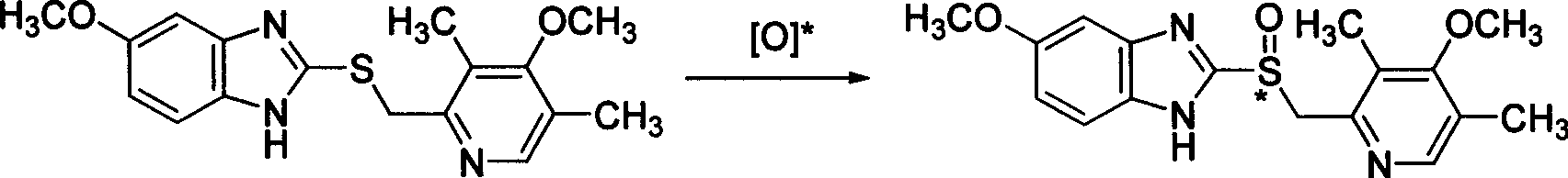
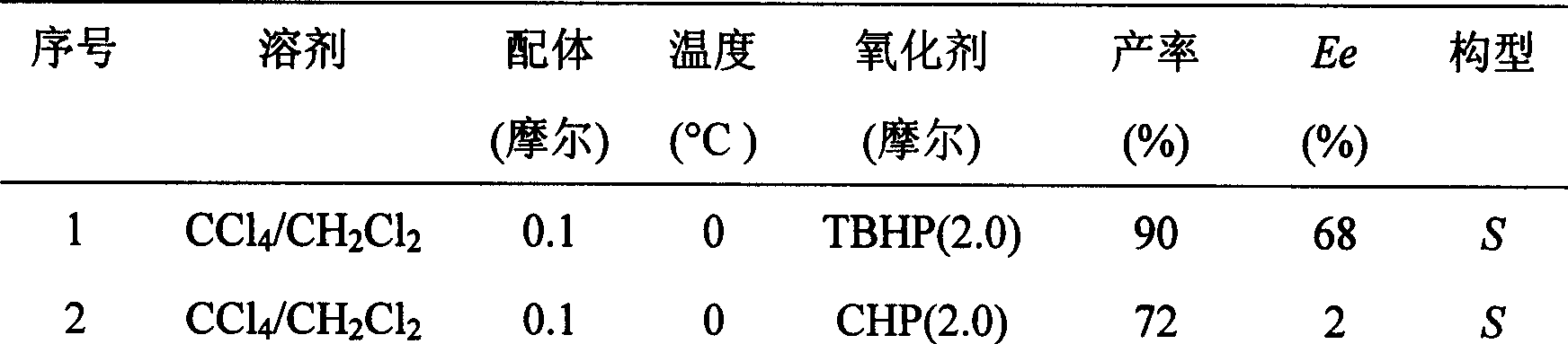
The invention provides a method for preparing (L)-pantoprazole sodium, which comprises the following steps of: oxidizing 5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]thio}-1H-benzimidazole by using 3,5-diisopropylbenzene hydroperoxide under the catalysis of a tetraisopropyl titanate, D-(-)-diethyl tartrate and N,N-diisopropylethylamine system to obtain S-(-)-5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl}-1H-benzimidazole, namely (L)-pantoprazole, refining the (L)-pantoprazole, and preparing a salt to obtain the (L)-pantoprazole sodium.
Owner:HC SYNTHETIC PHARMA CO LTD
Industrial production method of high-purity esomeprazole sodium
ActiveCN102321071AReduce the impactOxidation reaction time is shortOrganic chemistryOrganic basePotassium hydroxide
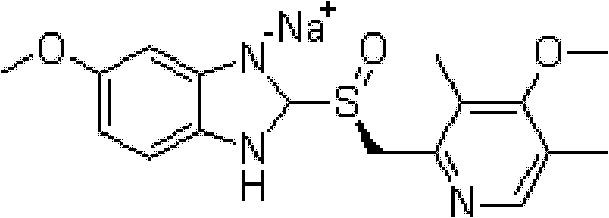
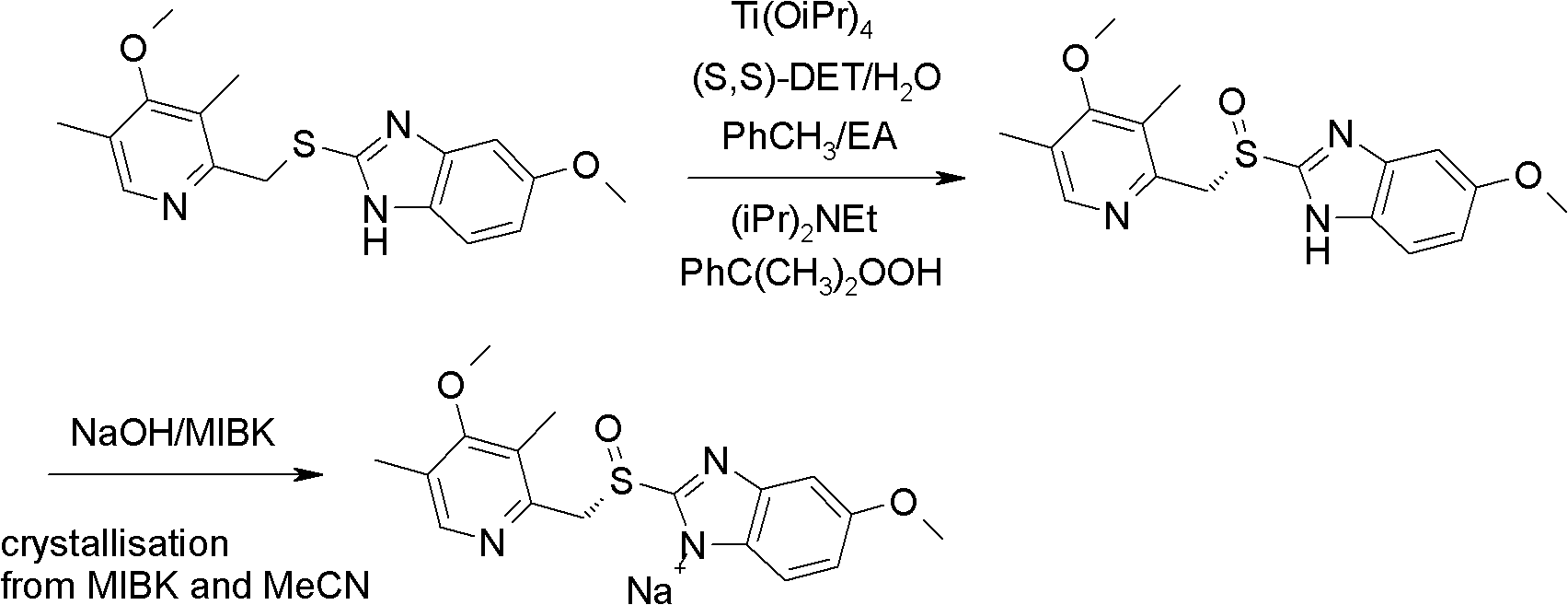
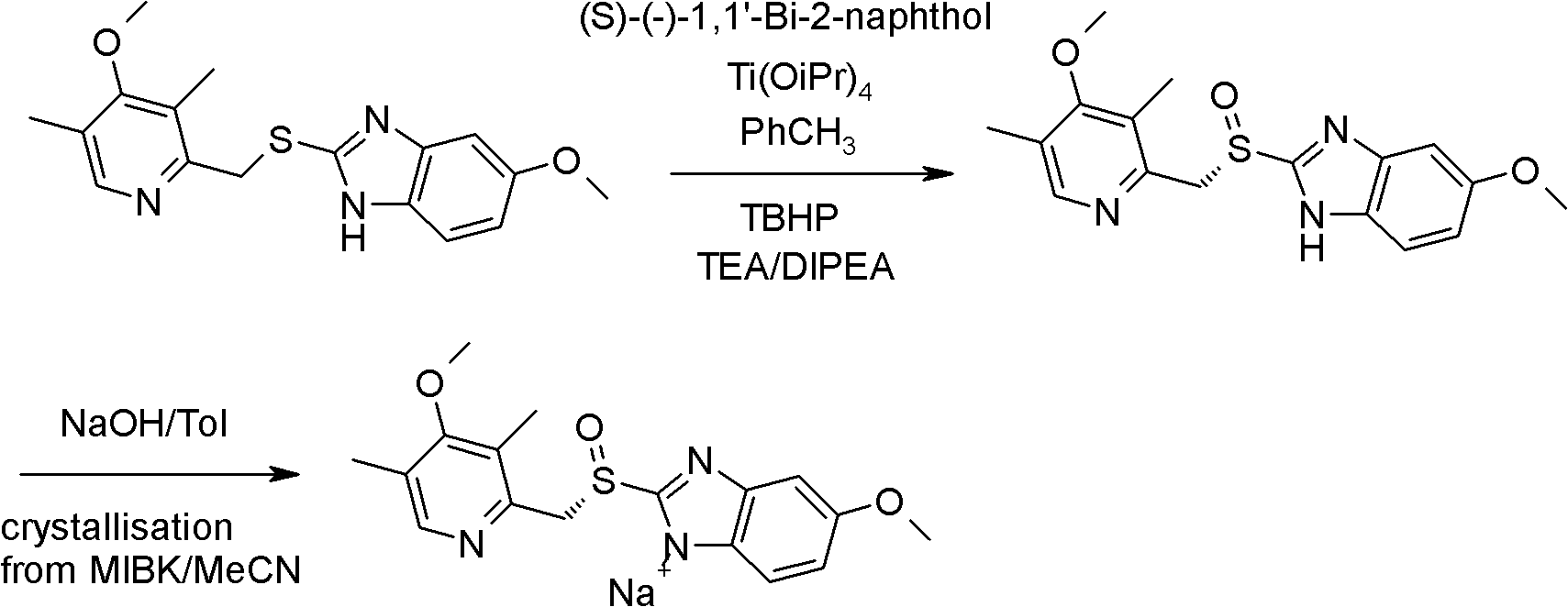
The invention relates to an industrial production method of high-purity esomeprazole sodium. The industrial production method is characterized by comprising the following steps: mixing a raw material 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole with a solvent for dissolving 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole; and successively adding water, D-diethyl tartrate and titanium iso-propoxide as well as an inorganic base, then adding cumene hydroperoxide, adding methanol or ethanol after reaction, filtering, carrying out posttreatment and salifying to prepare high-purity esomeprazole sodium, wherein the inorganic base is one of potassium carbonate, sodium carbonate, sodium hydroxide and potassium hydroxide. By using the method in the invention, the defects of high cost and serious environment pollution which are caused by using the organic base in the prior art are solved, and the defects of difficult posttreatment, poor repeatability and difficult industrialization in the prior art are solved simultaneously. According to the invention, the inorganic base is used as the raw material, thus the industrial production method has the advantages of low cost, little environment pollution, short reaction time and high product purity, is easy to operate and industrially produce.
Owner:NANJING HAIRUN PHARM CO LTD
Method for synthesizing esomeprazole sodium
The invention discloses a method for synthesizing esomeprazole sodium. The method comprises the steps as follows: preparing 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole, namely prochirality thioether; preparing crude esomeprazole sodium; refining the crude esomeprazole sodium; adding the prepared prochirality thioether and dried methylbenzene into D-(-) diethyl tartrate and water by stirring, adding titanium isopropylate, and stirring; and adding diisopropylamine at constant temperature, stirring, dropwise adding cumyl hydroperoxide with the mass concentration of 80%, ending the reaction, extracting, salifying, concentrating, washing and carrying out vacuum drying to obtain a crude product, and refining the crude product to obtain the esomeprazole sodium. The method is low in cost, toxicity and pollution, easy to operate, short in reaction time, high in product purity and easy for industrial production.
Owner:KAMP PHARMA
Preparation method for high-purity esomeprazole sodium
ActiveCN103288801ASolve the prone to titanium complex suspensionSolve the difficulty of splittingOrganic chemistrySodium bicarbonateOmeprazole Sodium
The invention discloses a preparation method for high-purity esomeprazole sodium. The preparation method comprises the steps of: including and splitting esomeprazole sodium and D-(-)-diethyl tartrate, titanium iso-propylate, triethylamine and L-(+)-mandelic acid in the presence of a proper amount of water, and separating to obtain an inclusion complex; dissolving the inclusion complex with ethyl acetate, washing inclusion complex with sodium carbonate water solution, carrying out ammonia hydroxide eluting on an ethyl acetate layer, slowly regulating the pH value to 6-7 with glacial acetic acid, then extracting with dichloromethane, and concentrating to obtain crude esomeprazole free alkali product; carrying out silica gel adsorption and elution on the crude product to obtain a pure esomeprazole free alkali product; and enabling the pure product and the methanol-ethanol-acetonitrile solution of sodium hydroxide to form salt, and then crystallizing with isopropyl ether to obtain the high-purity esomeprazole sodium. According to the preparation method, the difficulties that when inclusion and splitting are carried out, the titanium complex suspension body are difficult to split and the ammonia complex of titanium is difficult to remove can be solved, the industrialization production can be realized, the industrialized production cost is low, the product purity is high, the yield is high, and no harmful gas is generated.
Owner:SICHUAN BAILI PHARM CO LTD
Preparation method of esomeprazole and magnesium salt thereof
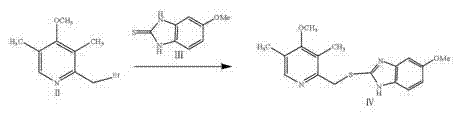
The invention discloses a preparation method of esomeprazole and a magnesium salt thereof. The preparation method comprises the steps: with 4-methoxy-2-hydroxymethyl-3,5-dimethyl pyridine as raw material, carrying out a reaction with hydrobromic acid to obtain 4-methoxy-2-bromomethyl-3,5-dimethyl pyridine, namely a compound II; dripping a methanol solution of the compound II into a methanol solution of a compound III, and carrying out a reaction to obtain 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl thio-1H-benzimidazole, namely a compound IV; dissolving the compound IV in dichloromethane, adding D-diethyl tartrate, tetrabutyl titanate and water for reaction, and thus obtaining esomeprazole; and carrying out a reaction of esomeprazole with magnesium methoxide to obtain magnesium esomeprazole. The method is utilized to prepare esomeprazole and the magnesium salt thereof, and can effectively reduce the production cost.
Owner:KAIFENG MINGREN PHARMA
Method for synthesizing and refining esomeprazole sodium
ActiveCN103570686AConvenient sourceSimple and fast operationOrganic chemistryEsomeprazole SodiumTetraisopropyl titanate
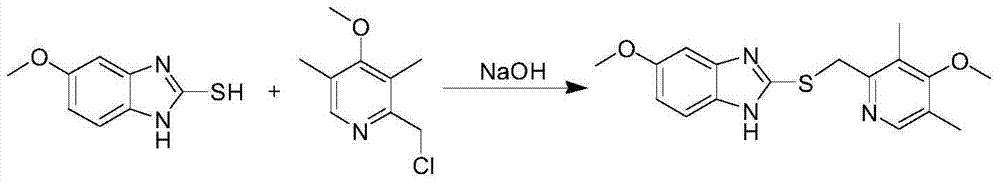
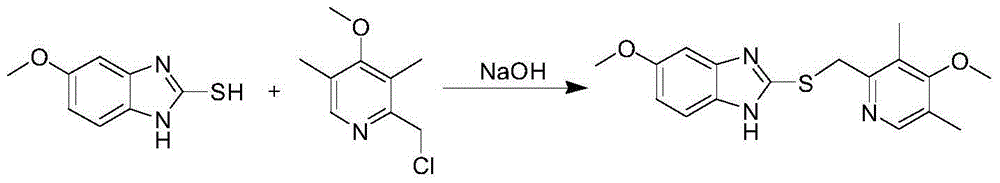
The invention relates to a method for synthesizing and refining esomeprazole sodium. The method comprises the following several steps: (1) carrying out condensation by taking 2-sulfydryl-5-methoxybenzimidazole and 2-chloromethyl-3.5-dimethyl-4-methoxypyridine hydrochloride as raw materials to generate prochiral thioether; (2) synthesizing the prochiral thioether into an esomeprazole crude product under the action of D-(-) diethyl tartrate, water, tetraisopropyl titanate, diisopropylethylamine and cumene hydroperoxide, and refining to generate esomeprazole potassium salt; (3) dissolving the esomeprazole potassium salt, then transforming into esomeprazole sodium salt, and refining to obtain a final product. The process of the esomeprazole sodium has the advantages of easiness for operation, good reaction repeatability and higher yield; the obtained product is high in purity and suitable for industrialized production.
Owner:哈药集团股份有限公司 +1
Esomeprazole magnesium preparation method
The invention discloses an esomeprazole preparation method and an esomeprazole magnesium preparation method. The esomeprazole preparation method is characterized in that a catalytic oxidation of omeprazole sulfide is carried out under the action of an added oxidant in the presence of bidentate chiral aminoalcohol and titanium alkoxide at room temperature to obtain a chiral proton pump inhibitor esomeprazole in a single enantiomer or enriched enantiomer form. The above preparation methods have the advantages of no need of the addition of an alkaline reagent, easy obtaining and reuse of the bidentate chiral aminoalcohol participating in the above reaction, increase of the utilization rate of the chiral aminoalcohol, substitution of expensive D-(-)-diethyl tartrate, and production cost reduction; and the chemical purity and the reaction overall yield of the prepared esomeprazole magnesium reach 99.7% and 66% respectively.
Owner:湖南千金湘江药业股份有限公司
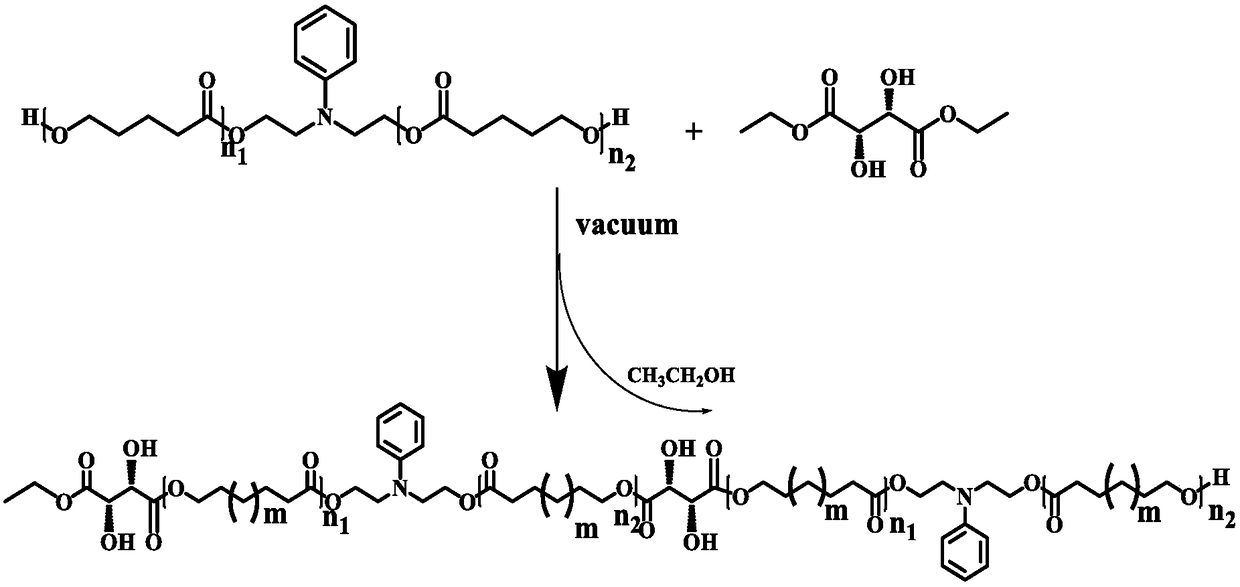
Method for preparing polycaprolactone polyol by using enzymatic catalysis method
ActiveCN108424512AThe polycondensation reaction has low priorityControl ratioFermentationSide chainDiethyl tartrate
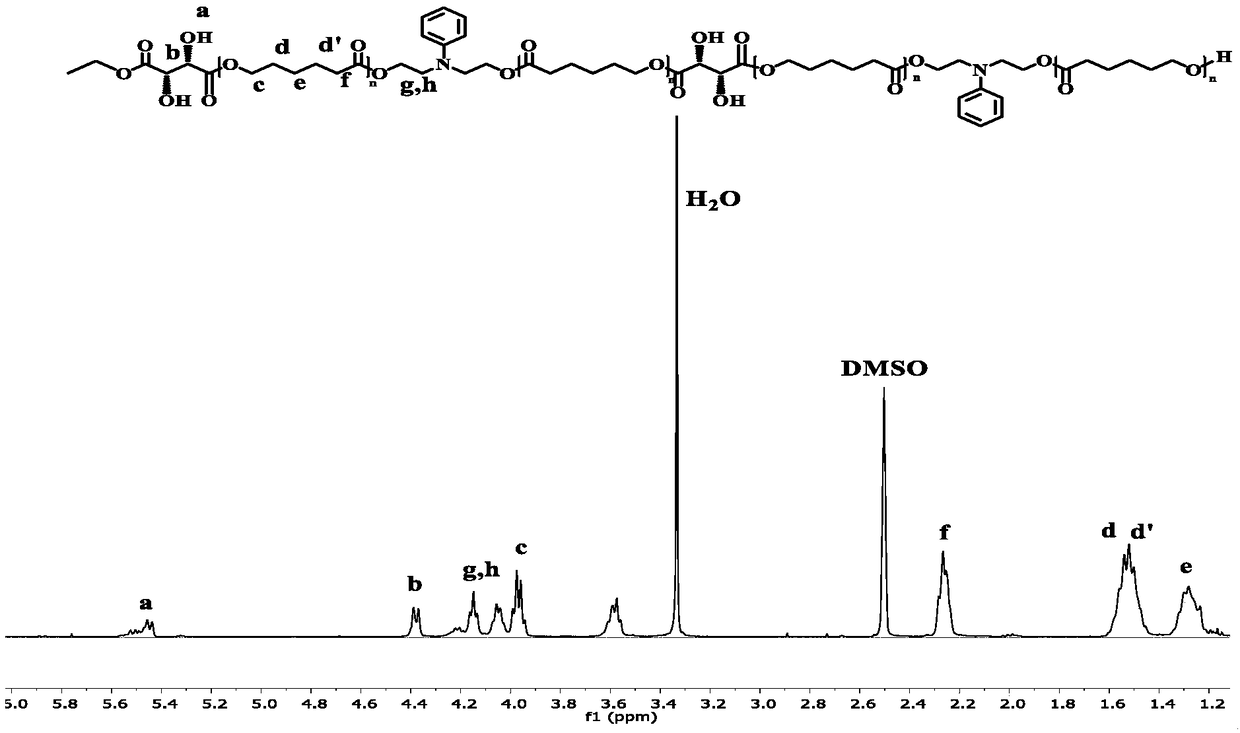
The invention discloses a method for preparing polycaprolactone polyol by using an enzymatic catalysis method. The method comprises: S1, under a solvent condition, carrying out ring opening polymerization by using N-phenyldiethanolamine or binary primary alcohol as an initiator, using caprolactone as a monomer and using immobilized enzyme as catalyst to obtain a prepolymer with a structure represented by a formula V defined in the specification, wherein the sum of n1 and n2 is 1-50; and S2, carrying out a reaction on the prepolymer prepared in the step S1 and D-diethyl tartrate to obtain a compound represented by a formula IV defined in the specification, wherein the sum of n1 and n2 is 1-50. According to the present invention, the product can improve the thermodynamic property and the hydrophilic property of the polycaprolactone material to a certain extent, can introduce a certain amount of hydroxyl groups into the side chain of the polymer, and can be used for post-modification of the polymer or other applications.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for enhancing production yield and rate of esomeprazole
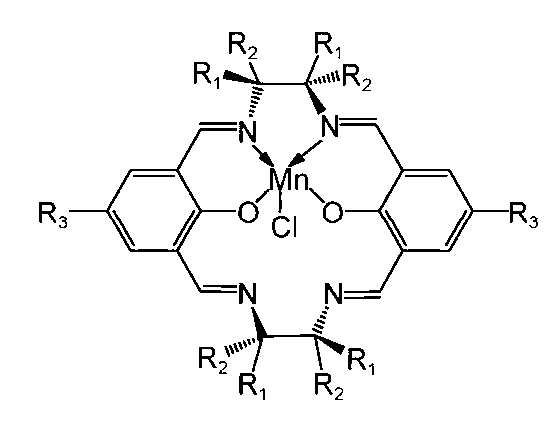
The invention aims to enhance the conversion and generation yield and rate of esomeprazole from omeprazole precursor-thioether through a chiral catalyst Mn(salen). The method comprises the following steps: weighing a right amount of esomeprazole precursor-thioether, evenly mixing with dichloromethane, adding the chiral catalyst Mn(salen) and D-(-)diethyl tartrate to carry out chiral conversion, adding cumene hydroperoxide for oxidation to generate esomeprazole, filtering to remove the chiral catalyst, distilling out dichloromethane to obtain an esomeprazole crude product, and finally, crystallizing with acetone to obtain the esomeprazole pure product. High-performance liquid chromatography is utilized to detect the content. The chiral catalyst Mn(salen) can enhance the generation yield of esomeprazole sodium by 50%, and save the reaction time by 2 hours or so. The method is simple to operate, and has the advantages of low reagent toxicity, low required initial raw material cost and high product yield.
Owner:CP PHARMA QINGDAO CO LTD
Water-soluble S, S-heptaplatin derivative
ActiveCN102079761AGood water solubilitySmall weight effectOrganic active ingredientsPowder deliverySolubilityPlatinum
The invention relates to a new water-soluble S, S-heptaplatin derivative-cis-[(4S, 5S)-4, 5-bi (aminomethyl)-2-isopropyl-1, 3-dioxolane 3-hydroxy-1, 1-cyclobutanedicarboxylate platinum (II)]. The derivative is prepared by the following steps of: (1) preparing a required amine ligand by using L-diethyl tartrate as an initial raw material through condensation, ammonolysis and LiAlH4 reduction reaction; and (2) adding LI to K2[PtCl4] used as an initial raw material, transforming the raw material into K2PtI4, making the K2PtI4 react with the amine ligand (4S, 5S)-4, 5-bi (aminomethyl)-2-isopropyl-1, 3-dioxolane to prepare a corresponding diiodo intermediate, and making the diiodo intermediate react with 3-hydroxy-1, 1-cyclobutanedicarboxylate in equimolar amounts to obtain a target compound. The compound has the characteristics that the antitumaous effect of the compound is higher than that of carboplatin, heptaplatin and R, R-derivatives thereof, and the toxicity is lower than that of carboplatin, heptaplatin and R, R-derivatives thereof. The compound can be used for treating cancers clinically. Moreover, the compound also has the advantages of good water solubility and stability, and can be prepared into lyophiled powder or aqueous solution.
Owner:KUNMING INST OF PRECIOUS METALS +1
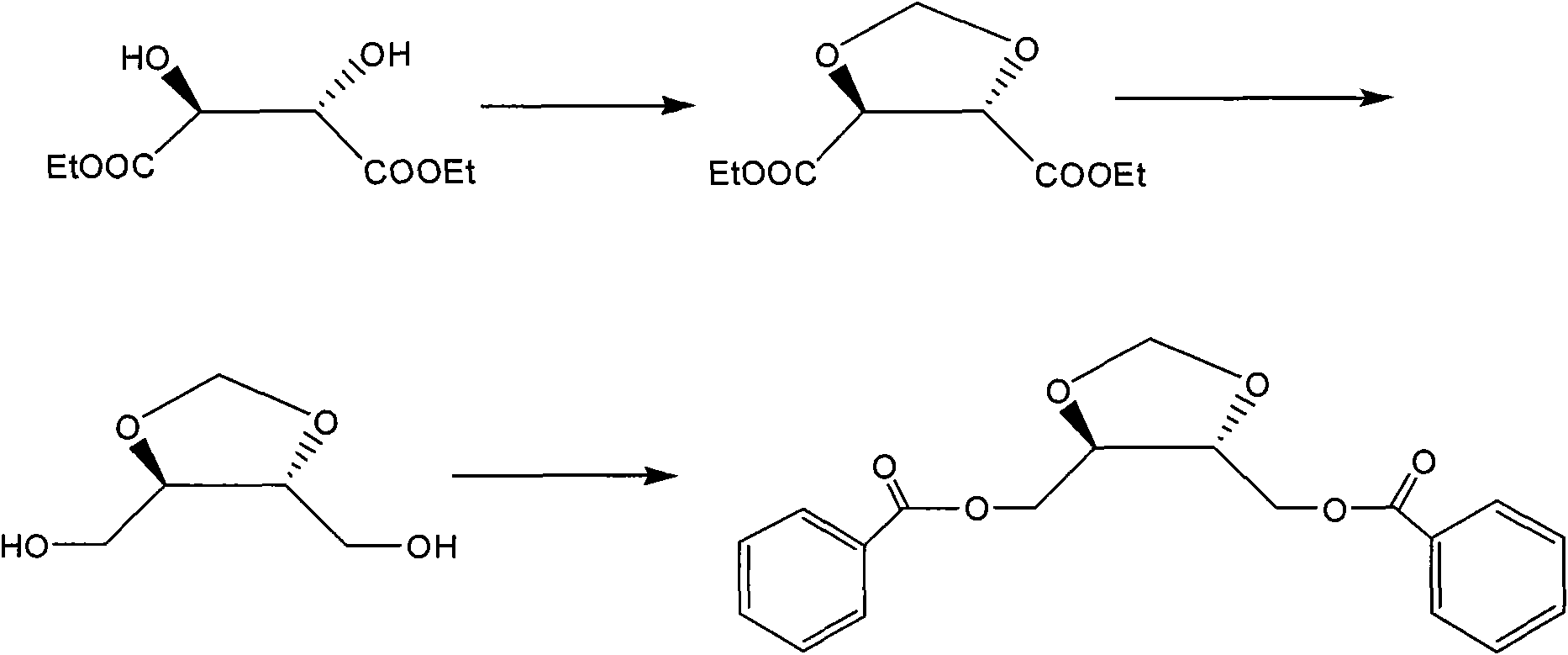
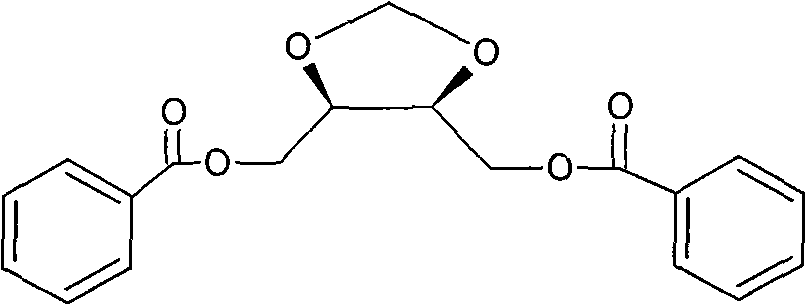
Method for synthesizing diastereoisomer of Doranidazole intermediate
The invention discloses a method for synthesizing diastereoisomer of Doranidazole intermediate [(4RS,5SR)-4,5-di(benzoyl oxido-methyl)-1,3-dioxolane]. The method is realized in the following steps: using L-diethyl tartrate or D-diethyl tartrate as the initial raw material, cyclizing, reducing and acidylating to obtain the diastereoisomer [(4R,5R)-4,5-di(benzoyl oxido-methyl)-1,3-dioxolane or (4S,5S)-4,5-di(benzoyl oxido-methyl)-1,3-dioxolane]. The invention has the advantages that the initial raw material with optical activity is easy to purchase, the raw material and the reagents are safe and stable, and the method is convenient for operation and amplified production.
Owner:深圳万乐药业有限公司
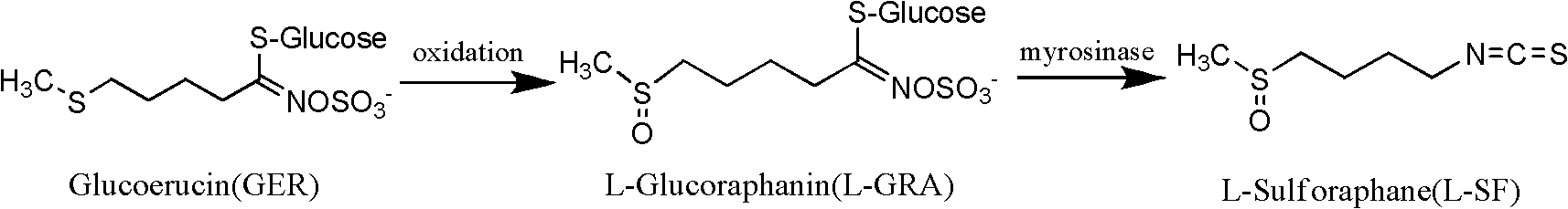
Method for preparing sulforaphane by using glucoerucin
The invention relates to a method for preparing sulforaphane by using glucoerucin, comprising the following steps: (1) preparing glucoerucin; (2) preparing L-glucose sulforaphane: adding sulforaphane obtained by the step (1), (+)-L-diethyl tartrate, alkoxy titanium or alkoxy vanadium compound in a mixed solution of methanol, ethanol and ethyl acetate to obtain a product of L-glucose sulforaphane;(3) taking broccoli seeds and crushing, filtering through gauze to obtain a filtrate, adding ammonium sulfate in the filtrate at a temperature of 4 DEG C with a saturation of 55 %, mixing uniformly, centrifuging the precipitate to dissolve the precipitate in deionized water, and dialyzing overnight to obtain a crude enzyme of hydrolase; and (4) purifying the L-glucose sulforaphane. The method aims at overcoming the disadvantages of high cost of raw materials, low yield of sulforaphane and the like. According to the invention, sesame seeds which are cheaply obtained are used as raw materials, thus the cost of the raw materials is one fifth that of broccoli seeds, and the sulforaphane yield obtained by using sesame seeds is 3 times the yield obtained by using broccoli seeds.
Owner:BEIJING UNIV OF CHEM TECH
Method for synthesizing and refining esomeprazole sodium
ActiveCN103570686BConvenient sourceSimple and fast operationOrganic chemistryEsomeprazole SodiumTetraisopropyl titanate
The invention relates to a method for synthesizing and refining esomeprazole sodium. The method comprises the following several steps: (1) carrying out condensation by taking 2-sulfydryl-5-methoxybenzimidazole and 2-chloromethyl-3.5-dimethyl-4-methoxypyridine hydrochloride as raw materials to generate prochiral thioether; (2) synthesizing the prochiral thioether into an esomeprazole crude product under the action of D-(-) diethyl tartrate, water, tetraisopropyl titanate, diisopropylethylamine and cumene hydroperoxide, and refining to generate esomeprazole potassium salt; (3) dissolving the esomeprazole potassium salt, then transforming into esomeprazole sodium salt, and refining to obtain a final product. The process of the esomeprazole sodium has the advantages of easiness for operation, good reaction repeatability and higher yield; the obtained product is high in purity and suitable for industrialized production.
Owner:哈药集团股份有限公司 +1
Method for preparing high-purity esomeprazole magnesium
ActiveCN110305108ATo achieve the purpose of decolorizationOrganic chemistry methodsAqueous acetoneFiltration
The invention relates to a method for preparing high-purity esomeprazole magnesium. The method comprises the following steps: mixing omeprazole sulfide, diethyl tartrate and titanium isopropoxide, adding diisopropylethylamine, performing stirring, dropping cumene hydroperoxide, and performing separation so as to obtain an oil substance; adding a strong base, performing stirring, adding the strongbase time by time, performing extraction and washing, performing TLC (thin-layer chromatography) monitoring, and stopping adding the strong base when trace points are generally vanished; performing cooling, crystal separation, filtration and leaching so as to obtain an esomeprazole salt; dissolving the esomeprazole salt with water, reducing the temperature to 20 DEG C or less, adjusting the pH value, dropping a magnesium sulfate heptahydrate solution, and after dropping, performing stirring, filtration, leaching and drying so as to obtain a crude product of esomeprazole; dissolving the crude product of the esomeprazole with methanol, performing decoloring with activated carbon, performing filtration, concentration and secondary dissolution, adding an acetone solution, and performing stirring, suction filtration and drying, so as to obtain the esomeprazole magnesium. The product prepared by using the method is high in purity, high in yield and small in impurity.
Owner:湖南协创药品开发有限公司
Method for high enantiomer selection preparation of (S)-Omeprazole
The present invention relates to antimer selective catalytic oxidation process for preparing optical active antimer or optically pure antimer (S)-omeprazole. In the presence of the titanium containing catalyst in-situ created with metal titanium reagent and chiral glycol ligand and the oxidant, omeprazole sulfide is catalytically oxidized with antimer. Compared with the process utilizing tetraisopropanol titanium-diethyl tartrate system, the process of the present invention has the advantages of cheap material, high antimer selectivity and high yield. The present invention also relates to thepreparation of (S)-omeprazole in neutralized state, partial crystallized state or completely crystallized state.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing L-pantoprazole sodium
The invention discloses a method for preparing L-pantoprazole sodium in an asymmetric oxidation manner. Under the condition that toluene is used as solvent, a chiral coordination compound is generated through pantoprazole thioether under the effect of D-(-)-diethyl tartrate, titanium tetra-isopropoxide and purified water in a brand-new feeding manner; then, the chiral coordination compound is oxidized through an oxidizing agent, namely, p-Dipropylbenzene hydroperoxide, and then L-pantoprazole sodium is prepared; and the high-purity L-pantoprazole sodium can be obtained by conducting one time of refining on the L-pantoprazole sodium and conducting salt formation on the L-pantoprazole sodium and sodium hydroxide. By means of the preparation method, influences of moisture on reaction can be effectively avoided, and therefore the preparation method is suitable for large-batch industrialized production; and by only conducting one time of recrystallization on the L-pantoprazole sodium before and after salt formation, the chromatographic purity and the optical purity of the product can reach 99.5% or above, and the total yield can reach 60% or above. In addition, the preparation method is gentle in reaction condition, small in environment pollution, high in yield and product purity and suitable for industrialized production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Heat-aging-resistant polylactic acid composite material and preparation method thereof
The invention discloses a heat-aging-resistant polylactic acid composite material and a preparation method thereof, and relates to the technical field of biodegradable plastics, and the heat-aging-resistant polylactic acid composite material is prepared from the following components in percentage by weight: 20-80% of polylactic acid, 10-50% of poly (butylene adipate-co-terephthalate), 5-10% of a compatilizer, 1-5% of an antioxidant and 1-5% of a lubricant. The adhesive comprises the following components in percentage by weight: 5-20% of diethyl tartrate modified terpene resin grafted modified monomethoxy polyethylene glycol-polylactic acid double-block copolymer, 0.2-5% of plasticizer and 0.2-5% of lubricant. The polylactic acid material can be used in food fields of meal boxes, water cups and the like, has certain light aging resistance, can be applied to the fields of mulching films, food packaging films and the like, and expands the application field of the polylactic acid material.
Owner:WANHUA CHEMICAL (NINGBO) CO LTD
Polyamide-alginate fiber dialysis membrane and preparation method thereof
InactiveCN108816062AGood biocompatibilityNo adverse effectsSemi-permeable membranesMembranesDialysis membranesFiber
The invention provides a polyamide-alginate fiber dialysis membrane and a preparation method thereof. The dialysis membrane is a hollow fiber membrane and is prepared from the following components inparts by weight: 25-30 parts of polyamide, 12-18 parts of alginate fibers, 5-10 parts of a copolymer of L-diethyl tartrate and polyethyleneimine, 5-10 parts of poly-2-methyl-2,3-epoxypropiorote ethylene glycol methacrylate, 2-8 parts of polylactic acid, 1-2 parts of diatomite, 1-5 parts of polyethylene glycol, 1-2 parts of hexanol or heptanol, 0.5-1 part of zinc oxide and 40-60 parts of dimethylsulfoxide, wherein for hollow fibers, the average inside diameter is 120-280 microns, and the wall thickness is 25-45 microns. The preparation method of the polyamide-alginate fiber dialysis membrane adopts a dry-wet method preparation process. The polyamide-alginate fiber dialysis membrane can effectively solve the technical problems that the biocompatibility is not ideal enough, and medium molecular substances such as beta 2-microglobulins cannot be eliminated and has an excellent application prospect.
Owner:SUZHOU BEC BIOLOGICAL TECH
Preparation method of (2R,3R)-2,3-dimethoxy-1,1,4,4-tetraphenyl-1,4-butanediol
InactiveCN108276256AOrganic chemistry methodsEther preparation by ester reactions1,4-ButanediolRegioselectivity
The invention relates to a preparation method of C2 symmetric chiral diol, and belongs to the field of chiral compound preparation chemistry. The invention particularly discloses a preparation methodof (2R3R)-2,3-dimethoxy-1,1,4,4-tetraphenyl-1,4-butanediol, which comprises the steps of performing phenylating on natural diethyl tartrate to prepare (2R,3R)-1,1,4,4-tetraphenyl butantetraol; at a certain temperature and in an appropriate reaction medium, carrying out high-regioselectivity 2,3-dimethylation reaction on the (2R,3R)-1,1,4,4-tetraphenyl butantetraol, with NaH and a methylating agentto obtain the (2R,3R)-2,3-dimethoxy-1,1,4,4-tetraphenyl-1,4-butanediol. The raw materials of the method are easy to get, the method is simple and convenient to operate, and low in cost.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Preparation method of (2R,3R)-or (2S,3S)-1,4-dimethoxy-1,1,4,4-tetraphenyl-2,3-butanediol
InactiveCN103804152ARaw materials are easy to getShort synthetic routeOrganic chemistryOrganic compound preparation2,3-ButanediolDiethyl tartrate
The invention discloses a preparation method of (2R,3R)-or (2S,3S)-1,4-dimethoxy-1,1,4,4-tetraphenyl-2,3-butanediol. The preparation method comprises the steps of phenylating optically pure diethyl tartrate to obtain (2R,3R)-or (2S,3S)-1,1,4,4-tetraphenyl butantetraol; reacting with sulfoxide chloride to generate (4R,5R)-or (4S,5S)-4,5-bis diphenyl chloromethyl cyclic sulfite; with presence of alkali, implementing substitution reaction with methanol in a suitable medium to introduce a methoxy group, thus preparing (4R,5R)-or (4S,5S)-4,5-bismethoxy diphenylmethyl cyclic sulfite; and with presence of alkali, removing a sulfinyl protecting group in a suitable medium to obtain (2R,3R)-or (2S,3S)-1,4-dimethoxy-1,1,4,4-tetraphenyl-2,3-butanediol. The method disclosed by the invention is easily available in raw material, simple and convenient to operate, and low in cost.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Ophthalmic Pharmaceutical Composition, Ophthalmic Kit, and Pharmaceutical Application Thereof
PendingUS20200215079A1Improve stabilityExtended shelf lifeOrganic active ingredientsSenses disorderGlutaric acidPolythylene glycol
The present invention relates to an ophthalmic pharmaceutical composition, comprising 0.06 to 40 parts by weight of a solid matrix, 0.4 to 55 parts by weight of a liquid matrix and 1 part by weight of an active pharmaceutical ingredient; the solid matrix is selected from the group consisting of polylactic acid, polyglycolic acid, polylactic acid-co-glycolic acid copolymer, and polyethylene glycol with a number average molecular weight of 800 to 10,000; the liquid matrix is a first liquid matrix and / or a second liquid matrix; the first liquid matrix is selected from the group consisting of diethyl succinate, diethyl tartrate, dimethyl glutarate and glycerol triacetate; when the liquid matrix is the first and second liquid matrixes, the second liquid matrix is selected from the group consisting of glycerol, propylene glycol, and polyethylene glycol with a number average molecular weight 70-750; when the liquid matrix is the second liquid matrix, the second liquid matrix is glycerol and / or propylene glycol, or a combination thereof with polyethylene glycol having a number average molecular weight of 70 to 750.
Owner:SHENYANG XINGQI PHARM CO LTD
Organic-inorganic hybrid chiral sorbent and process for the preparation thereof
InactiveUS20130317244A1High Enantiomeric Excess (eeGroup 4/14 element organic compoundsOther chemical processesPropanoic acidSorbent
The present invention provides an organic-inorganic hybrid chiral sorbent for chiral resolution of various racemic compounds viz. racemic mandelic acid, 2-phenyl propionic acid, diethyl tartrate, 2,2′-dihydroxy-1,1′-binaphthalene (BINOL) and cyano chromene oxide with excellent chiral separation (enantiomeric excess, 99%) in case of mandelic acid under medium pressure column chromatography. These optically pure enantiomers find applications as intermediates in pharmaceutical industries.
Owner:COUNCIL OF SCI & IND RES
A kind of preparation method of high-purity esomeprazole sodium
ActiveCN103288801BRealize industrial productionLow cost of industrializationOrganic chemistrySodium bicarbonateSodium hydroxide
The invention discloses a preparation method for high-purity esomeprazole sodium. The preparation method comprises the steps of: including and splitting esomeprazole sodium and D-(-)-diethyl tartrate, titanium iso-propylate, triethylamine and L-(+)-mandelic acid in the presence of a proper amount of water, and separating to obtain an inclusion complex; dissolving the inclusion complex with ethyl acetate, washing inclusion complex with sodium carbonate water solution, carrying out ammonia hydroxide eluting on an ethyl acetate layer, slowly regulating the pH value to 6-7 with glacial acetic acid, then extracting with dichloromethane, and concentrating to obtain crude esomeprazole free alkali product; carrying out silica gel adsorption and elution on the crude product to obtain a pure esomeprazole free alkali product; and enabling the pure product and the methanol-ethanol-acetonitrile solution of sodium hydroxide to form salt, and then crystallizing with isopropyl ether to obtain the high-purity esomeprazole sodium. According to the preparation method, the difficulties that when inclusion and splitting are carried out, the titanium complex suspension body are difficult to split and the ammonia complex of titanium is difficult to remove can be solved, the industrialization production can be realized, the industrialized production cost is low, the product purity is high, the yield is high, and no harmful gas is generated.
Owner:SICHUAN BAILI PHARM CO LTD
Preparation method of esomeprazole potassium
The invention discloses a preparation method of esomeprazole potassium. The preparation method comprises the following steps: (1) putting methylbenzene into a dry reaction kettle, and introducing nitrogen into the methylbenzene until the end of centrifugation; (2) putting omeprazole sulfide, rising the temperature to 30 to 32 DG C, and putting 26 KG of D-(-)diethyl tartrate; (3) rising the temperature to 44 to 46 DEG C, and putting isopropyl titanate (IV) after stirring for 30 minutes; (4) cooling to 31 to 35 DEG C, and putting diisopropylethylamine; (5) keeping the temperature be 31 to 33 DEG C, and dropwise adding cumyl hydroperoxide within 30 minutes to 2 hours; (6) keeping the temperature be 31 to 33 DEG C for 15 minutes for reacting; checking the content of sulfide through QC (Quality Control), and monitoring whether the reaction ends or not; (7) after the end of the reaction, stirring a methanol solution of potassium hydroxide; (8) cooling to 25 to 30 DEG C and keeping for 1 hour; (9) centrifuging, thus obtaining a coarse product, and refining further.
Owner:上海华源医药科技发展有限公司
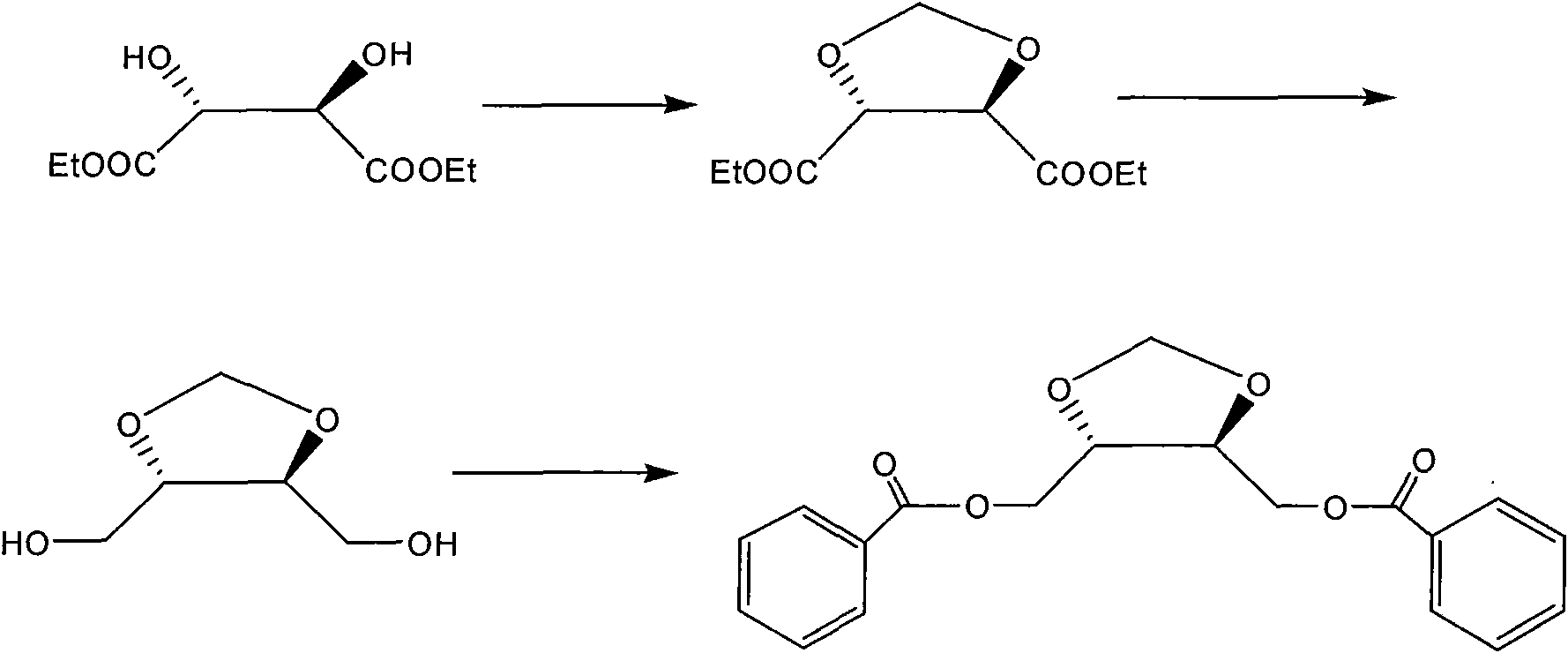
Method for preparing and purifying 2,3-O-isopropylidene threitol
The invention discloses a method for preparing and purifying 2,3-O-isopropylidene threitol. The preparation method comprises the following steps of uniformly mixing 2,3-O-isopropylidene diethyl tartrate, a solvent and a reducer at 0-5 DEG C, preserving heat at 0-70 DEG C, performing reduction reaction for 16-36 hours, preserving heat, and adding a quenching agent for quenching reaction to obtain 2,3-O-isopropylidene threitol. The purification method comprises the steps of performing reduced pressure distillation on a crude product of 2,3-O-isopropylidene threitol, collecting fractions at 140-170 DEG C, uniformly dispersing the fractions with ethanol, adding dry ice, uniformly mixing, and separating out white solids, namely purified 2,3-O-isopropylidene threitol. The method is simple in process, low in production cost and high in product yield, and has an important application value.
Owner:BEIJING ODYSSEY CHEM
Specific L-pantoprazole sodium superfine powder lyophilized preparation and preparation method thereof
InactiveCN104138357AHigh clarityImprove stabilityOrganic active ingredientsPowder deliveryMeth-Pantoprazole
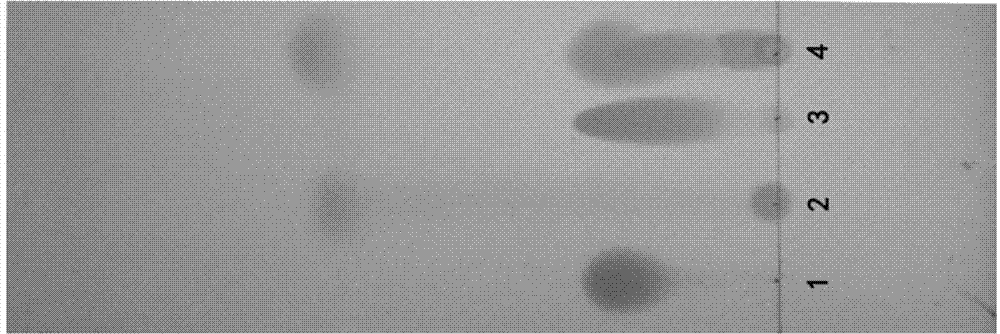
The invention discloses a specific L-pantoprazole sodium superfine powder lyophilized preparation and a preparation method thereof. The preparation method uses 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)methyl] sulfur]-1H-benzimidazole and cumene hydroperoxide as raw materials, and comprises the following steps: preparing a L-pantoprazole crude product by catalysis of tetraisopropyl orthotitanate, D-(-)diethyl tartrate and triethylamine system; recrystallizing with acetone to obtain a L-pantoprazole refined product; reacting with NaOH to obtain L-pantoprazole sodium; performing air-jet superfine crushing, and lyophilizing. The specific L-pantoprazole sodium superfine powder lyophilized preparation has the advantages of good clarity, high stability, high purity, few impurities, small particles, large specific surface area, good solubility, small toxic and side effect, allergy prevention and the like.
Owner:浙江磐谷药源有限公司
Preparation and purification method of pantoprazole optical antimer
InactiveCN103804355AShort reaction timeHigh synthesis efficiencyOrganic chemistryPurification methodsSulfur
The invention provides a preparation and purification method of a pantoprazole optical antimer. The method comprises the following steps: carrying out a reaction on 5-difloromethoxyl-2-{[(3, 4-dimethoxyl-2-pyridyl)methyl]sulfur}-1H-benzimidazole and optically active diethyl tartrate (D-(-)-diethyl tartrate or L-(+)-diethyl tartrate) according to different feeding sequences; reacting completely within 2 hours and filtering to obtain optically active pantoprazole; then, purifying to obtain a pure product S-(-)-pantoprazole or R-(+)-pantoprazole. The method provided by the invention is simple and convenient to operate, higher in product purity and high in yield, and the reaction time is shortened and the production cost is saved, so that the method is more suitable for industrialized large-scale production.
Owner:HC SYNTHETIC PHARMA CO LTD
Preparation method of esomeprazole and magnesium salt thereof
ActiveCN103709143BEasy to getReduce manufacturing costOrganic chemistryMagnesium saltMethyl palmoxirate
The invention discloses a preparation method of esomeprazole and its magnesium salt. Using 4-methoxy-2-hydroxymethyl-3,5-lutidine as raw material, react with hydrobromic acid to obtain 4-methoxy-2-bromomethyl-3,5-lutidine Compound II; compound II methanol solution was added dropwise to compound III methanol solution to react to obtain 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio- 1H-benzimidazole is compound IV; compound IV is dissolved in dichloromethane, adding D-diethyl tartrate, tetrabutyl titanate and water to react to obtain esomeprazole; reacting esomeprazole with magnesium methanol Get esomeprazole magnesium. Utilizing the technical scheme of the invention to prepare esomeprazole and its magnesium salt can effectively reduce the production cost.
Owner:KAIFENG MINGREN PHARMA
Preparation method and application of drug-loading and permeation-promoting integrated transdermal drug delivery system matrix
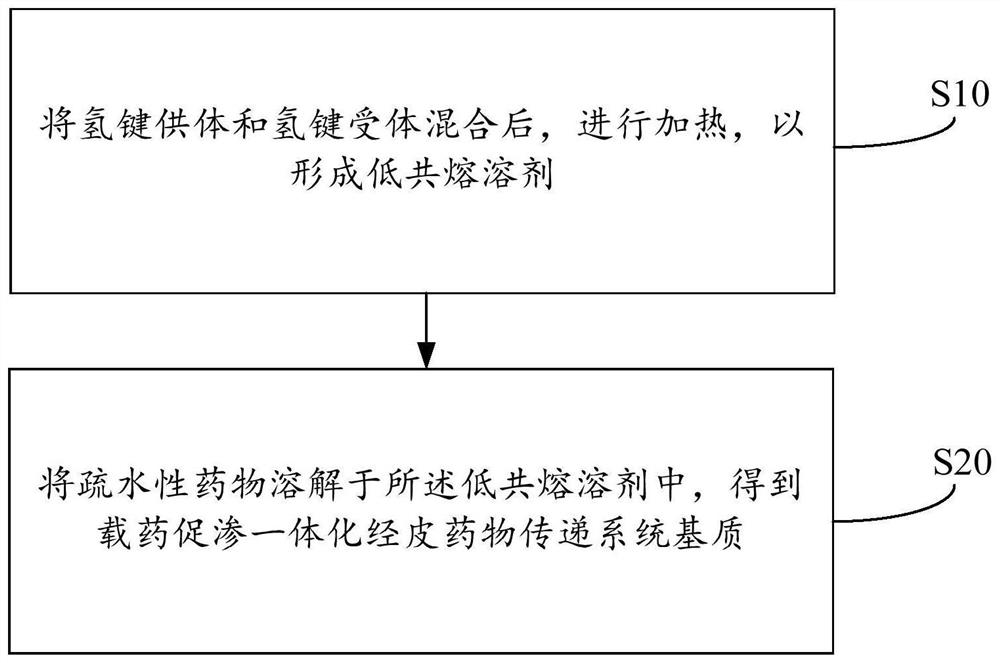
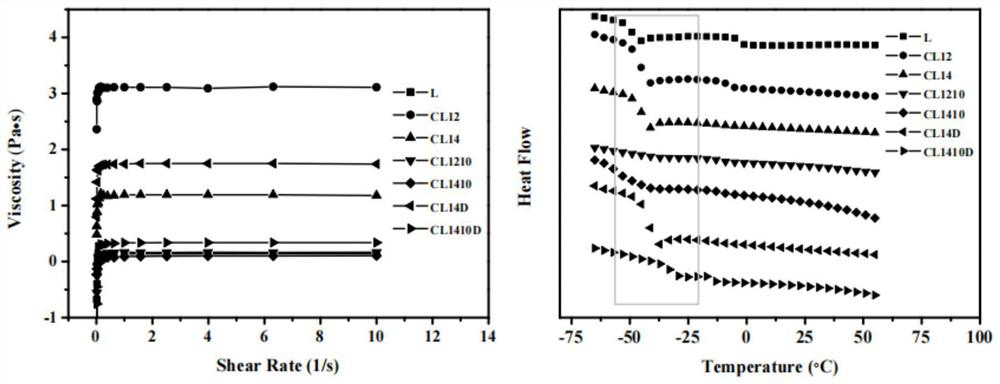
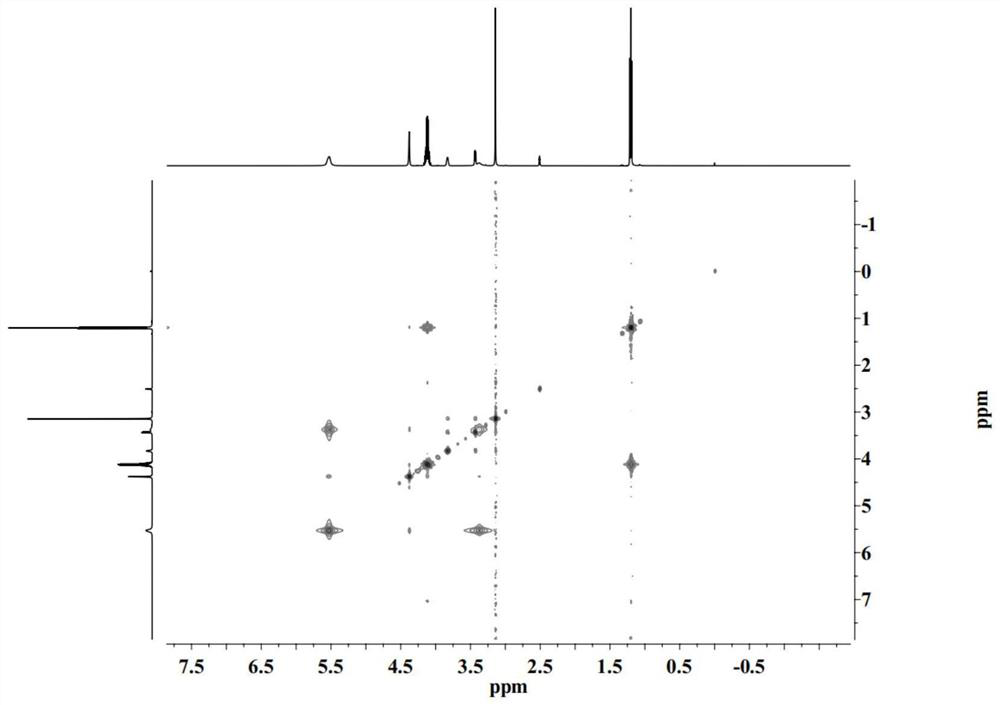
PendingCN114432274AAvoid stimulationOrganic active ingredientsPharmaceutical non-active ingredientsDiethyl tartrateCombinatorial chemistry
The invention discloses a preparation method and application of a drug-loading and permeation-enhancing integrated transdermal drug delivery system matrix, and the preparation method of the drug-loading and permeation-enhancing integrated transdermal drug delivery system matrix comprises the following steps: mixing a hydrogen bond donor and a hydrogen bond receptor, and heating to form a eutectic solvent; and dissolving a hydrophobic drug in the deep-eutectic solvent to obtain the drug-loading and permeation-promoting integrated transdermal drug delivery system matrix. The deep-eutectic solvent is constructed through hydrogen bond interaction of the L-diethyl tartrate and the choline chloride, then the hydrophobic drug is dissolved in the deep-eutectic solvent, and the hydrophobic drug integrally shows certain viscosity and can be used as a matrix material of a transdermal drug delivery system. Meanwhile, the system has a certain solubilizing effect and permeation enhancing performance on hydrophobic drugs, and irritation to the skin is avoided.
Owner:JIHUA LAB +1
A kind of preparation method of esomeprazole magnesium
ActiveCN110305108BStandards compliantTo achieve the purpose of decolorizationOrganic chemistry methodsAqueous acetoneOmeprazole
The invention relates to a preparation method of esomeprazole magnesium, comprising the following steps: mixing omeprazole sulfide, diethyl tartrate and isopropyl titanate, adding diisopropylethylamine, stirring, Add cumene hydroperoxide dropwise, separate to obtain an oily substance; add strong base, stir, then add strong base one by one, extract, wash, do TLC monitoring, stop adding strong base when there is basically no spot; cool down, crystallize, Filter and rinse to obtain esomeprazole salt; dissolve esomeprazole salt in water, cool down to below 20°C, adjust pH, add magnesium sulfate heptahydrate aqueous solution dropwise, after the dropwise addition, stir and filter , rinse, and dry to obtain crude esomeprazole magnesium; dissolve the crude esomeprazole magnesium with methanol, decolorize activated carbon, filter, concentrate, dissolve again, add acetone aqueous solution, stir, suction filter, dry, The esomeprazole magnesium is obtained, and the product prepared by the invention has high purity, good yield and less impurities.
Owner:湖南协创药品开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com