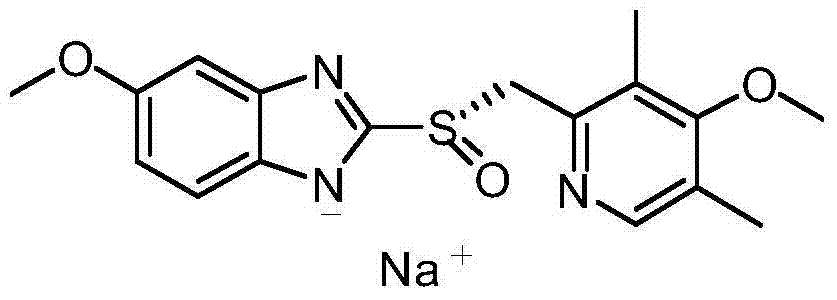
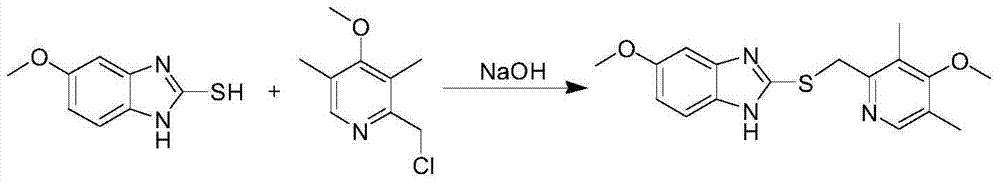
Method for synthesizing and refining esomeprazole sodium
A technology for esomeprazole sodium and meprazole potassium, applied in the field of medicine, can solve the problems of low product purity, difficult to control, influence on yield of qualified products, etc., and achieves high reaction selectivity, easy operation and high production efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 prepares prochiral sulfide thing
[0048] Take 80.0g (2.0mol) of sodium hydroxide and add 300ml of water to dissolve it, cool down to below 40°C, and then add it to 900ml of methanol under stirring. Add 150.0g (0.83mol) of 2-mercapto-5-methoxybenzimidazole into the above solution and stir until dissolved, which takes about 30 minutes. After dissolving, 185.0 g (0.83 mol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride was added to the reaction liquid. Heated to 65°C and reacted for 3 hours. When the temperature of the reaction solution drops below 30°C, add 600ml of dichloromethane and 300ml of water, stir for about ten minutes to dissolve the turbidity, separate the dichloromethane layer, extract the water layer with 300ml of dichloromethane three times, and combine the dichloromethane The methane layer was washed once with 300 ml of saturated brine. Dry over anhydrous sodium sulfate and concentrate in vacuo to obtain the crude product of...
Embodiment 2
[0049] Embodiment 2 prepares the crude product of esomeprazole potassium
[0050] Add 240.0g (0.73mol) of sulfide to 1500ml of toluene, heat to 70°C under stirring to dissolve, then cool down to 47°C to 50°C, add 150.4g (0.73mol) of D-(-)-diethyl tartrate ), 103.8g (0.37mol) of tetraisopropyl titanate, 4g (0.06mol) of water, and kept at 47℃~50℃ for 2.5h. Then lower the temperature to 0℃~5℃, add 94.4g (0.73mol) of diisopropylethylamine, stir for 10min, keep 0℃~5℃ and dropwise add 196.6g (1.09mol) of cumene hydroperoxide toluene solution (mass Concentration 85%). After the dropwise addition was completed, the reaction was continued for 4.5 hours at 0°C to 5°C. Dissolve 81.8g (0.73mol) of potassium hydroxide in 800ml of methanol. After the reaction is complete, add potassium hydroxide methanol solution dropwise into the reaction solution at 0°C to 5°C, and stir for 2 hours to crystallize. Filtration, washing with a small amount of methanol, and vacuum drying yielded 243.4 g of...
Embodiment 3
[0051] Embodiment 3 prepares the crude product of esomeprazole potassium
[0052] Add 240.0g (0.73mol) of sulfide to 1500ml of toluene, heat to 70°C under stirring to dissolve, then cool down to 47°C to 50°C, add 150.4g (0.73mol) of D-(-)-diethyl tartrate ), 103.8g (0.37mol) of tetraisopropyl titanate, 4g (0.06mol) of water, and kept at 47℃~50℃ for 2.5h. Then cool down to 5°C-10°C, add 94.4g (0.73mol) of diisopropylethylamine, stir for 10min, keep 7°C-10°C and dropwise add 157.3g (0.87mol) of cumene hydroperoxide toluene solution (mass Concentration 85%). After the dropwise addition was completed, the reaction was continued at 7°C to 10°C for 5 hours. Dissolve 81.8g (0.73mol) of potassium hydroxide in 800ml of methanol. After the reaction is complete, cool down to 0°C to 5°C, add potassium hydroxide methanol solution dropwise into the reaction solution, and stir for 2 hours to crystallize. Filtration, washing with a small amount of methanol, and vacuum drying yielded 245.7 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com