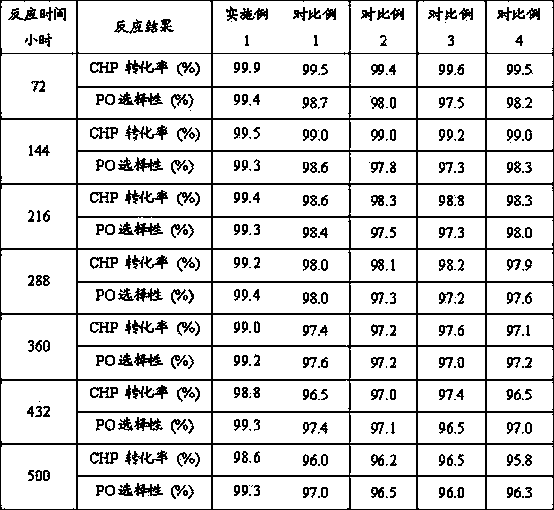
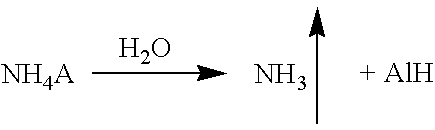Patents
Literature
283 results about "Cumene hydroperoxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cumene hydroperoxide is an organic hydroperoxide intermediate in the cumene process for synthesizing phenol and acetone from benzene and propene. It is typically used as an oxidizing agent. Products of decomposition of cumene hydroperoxide are methylstyrene, acetophenone, and cumyl alcohol. Its formula is C₆H₅C(CH₃)₂OOH.
Self-curing system for endodontic sealant applications
InactiveUS7275932B2Fasten curingImprove shelf life stabilityImpression capsTeeth fillingThioureaSelf curing
A two-part self-curing endodontic sealing system comprises a thiourea derivative, such as acetyl thiourea, and a hydroperoxide, such as cumene hydroperoxide. The thiourea derivative is used as a reducing agent and the hydroperoxide is used as an oxidizing agent.
Owner:PENTRON CLINICAL TECH
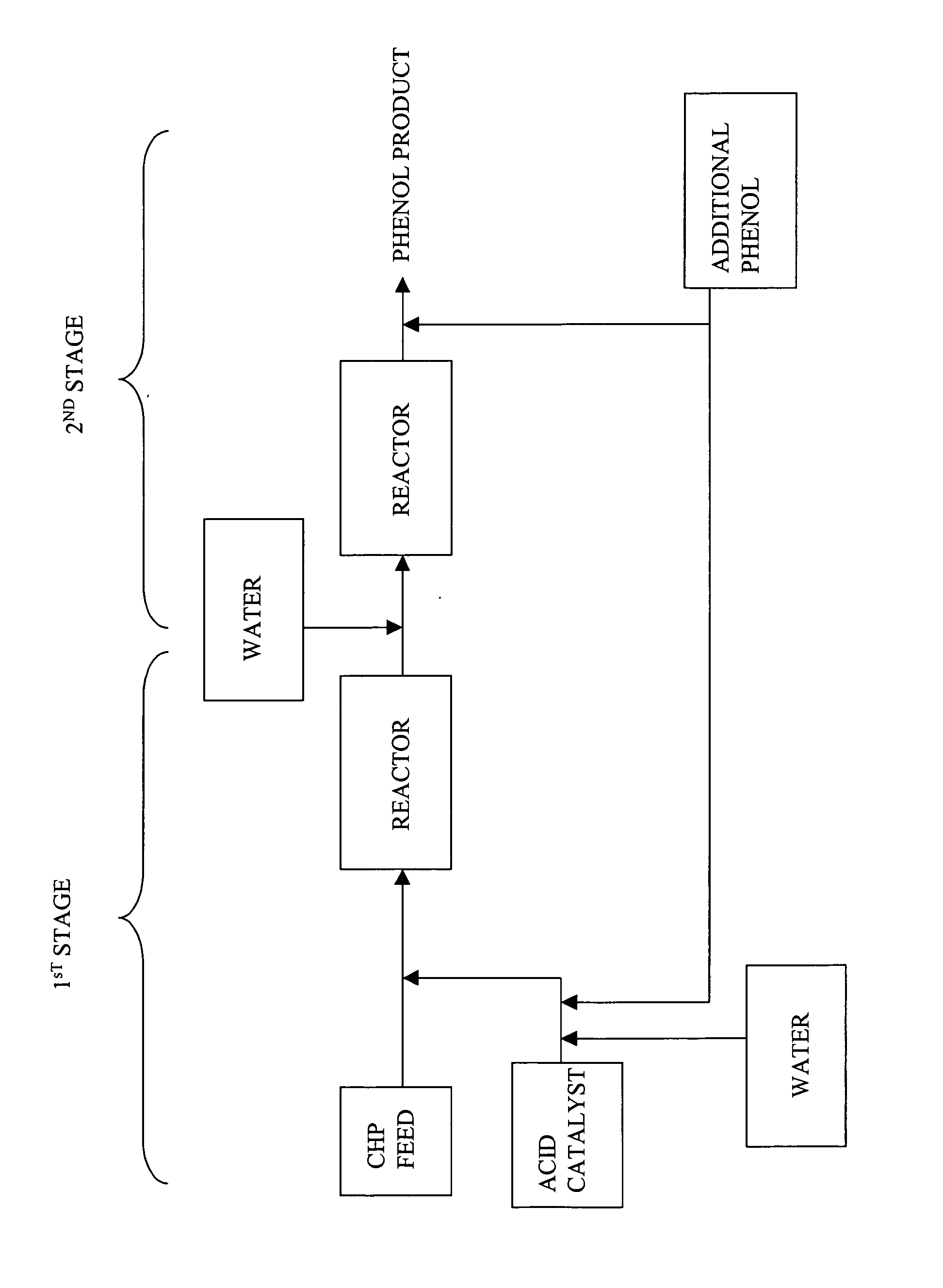
Process for producing phenol
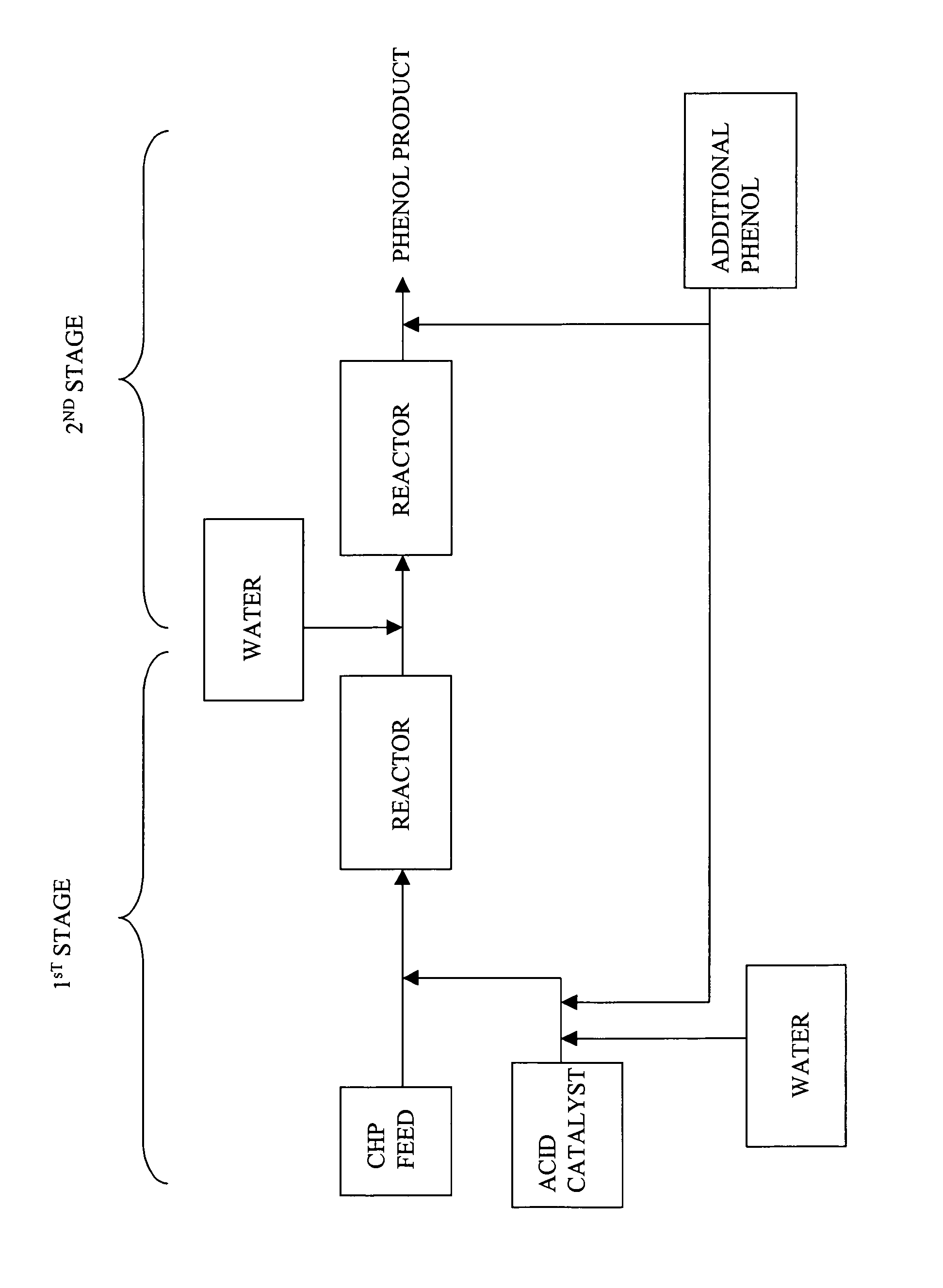
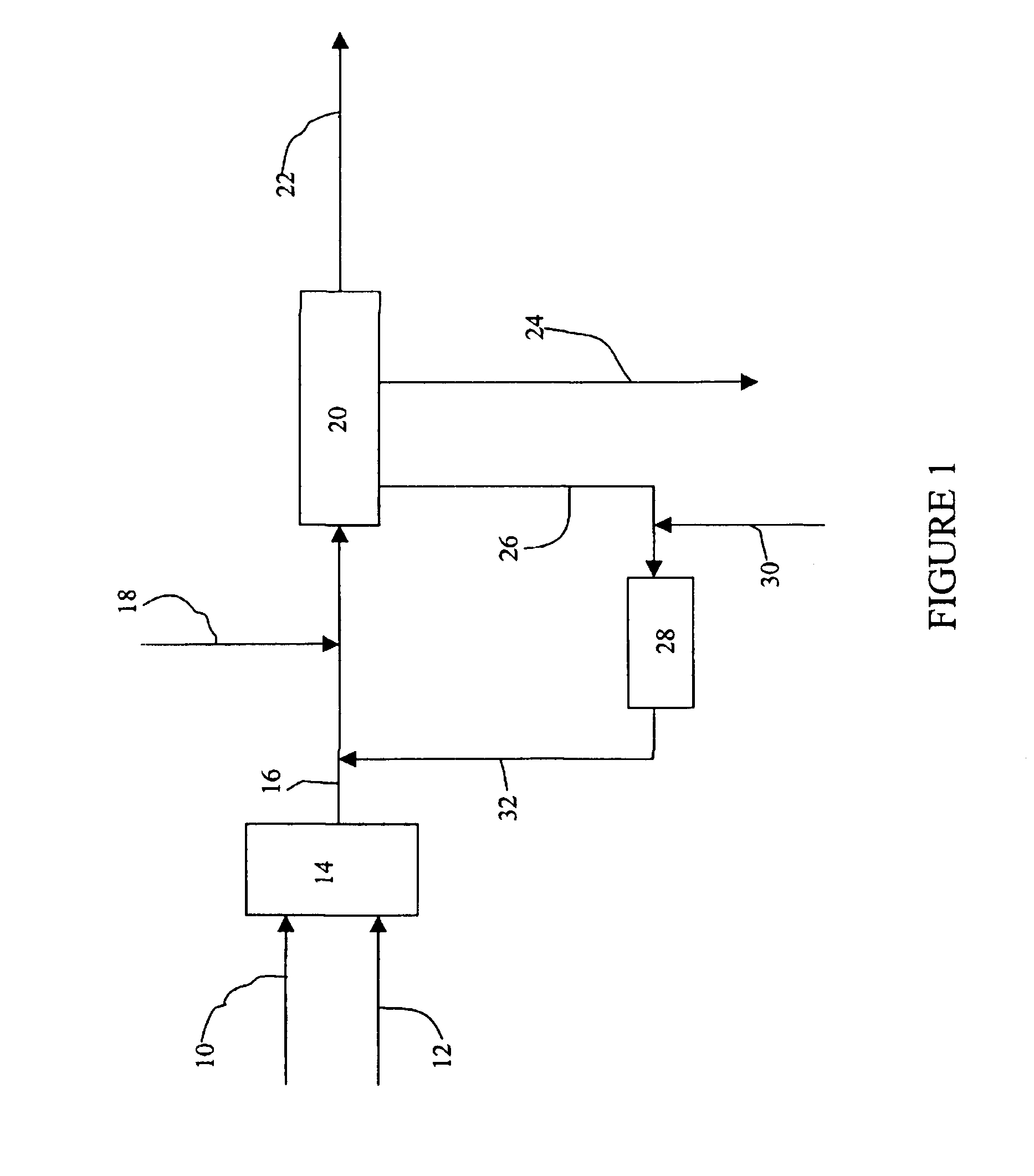
A process for producing a phenol product generally comprises a first step comprising reacting in a first reactor a feed stream comprising cumene hydroperoxide and water with an acid catalyst to produce an effluent comprising the phenol product, acetone, and at least 1% by weight residual cumene hydroperoxide, and a second step comprising passing the effluent into a second reactor and decomposing the residual cumene hydroperoxide, wherein during said process the ratio of phenol to acetone is maintained at a molar ratio of greater than 1:1, and wherein the water in each of the first and second steps is present in an amount more than 0 and less than or equal to 5 weight percent based on the total weight of the feed stream or effluent, and wherein the process is continuous.
Owner:SABIC GLOBAL TECH BV
Process for preparing isopropyl benzene by catalytically hydrogenolysis alpha, alpha dimethyl benzyl alcohol
InactiveCN1616383ACreate pollutionMild reaction conditionsHydrocarbon from oxygen organic compoundsReaction temperatureSolvent
The present invention hydrogenolyzes alpha, alpha-dimethyl benzyl alcohol in solvent into isopropyl benzene with hydrogen or organic compound as hydrogen source at reaction temperature of 30-100 deg.c under multiphase Pd base catalyst. The present invention has alpha, alpha-dimethyl benzyl alcohol converting rate greater than 96 % and isopropyl benzene selectivity greater than 99 %. The said process may be used in the hydrogenolysis of alpha, alpha-dimethyl benzyl alcohol as the co-product of propylene epoxidation process to prepare propylene oxide with cumene hydroperoxide as oxidant to realize circular utilization of isopropyl benzene. The said technology may be used in recovering propylene from tail gas generated in the process of producing propylene chloride or polypropylene, and may be also used in the propylene oxide producing line.
Owner:EAST CHINA UNIV OF SCI & TECH
Process for preparing isopropyl benzene by catalytically hydrogenolysis alpha, alpha dimethyl benzyl alcohol
InactiveCN1308273CCreate pollutionMild reaction conditionsHydrocarbon from oxygen organic compoundsPtru catalystBENZYL ALCOHOL/WATER
The present invention hydrogenolyzes alpha, alpha-dimethyl benzyl alcohol in solvent into isopropyl benzene with hydrogen or organic compound as hydrogen source at reaction temperature of 30-100 deg.c under multiphase Pd base catalyst. The present invention has alpha, alpha-dimethyl benzyl alcohol converting rate greater than 96 % and isopropyl benzene selectivity greater than 99 %. The said process may be used in the hydrogenolysis of alpha, alpha-dimethyl benzyl alcohol as the co-product of propylene epoxidation process to prepare propylene oxide with cumene hydroperoxide as oxidant to realize circular utilization of isopropyl benzene. The said technology may be used in recovering propylene from tail gas generated in the process of producing propylene chloride or polypropylene, and may be also used in the propylene oxide producing line.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for production of cumene hydroperoxide
ActiveUS7393984B1Improve productivityRemoving and reducing formation of undesirableOrganic compound preparationPeroxy compound preparationBenzoic acidGas phase
A continuous method of cumene oxidation in a gas-liquid system is provided, where the liquid phase is represented by cumene and its oxidation products and the gas phase is represented by air. The oxidation process can be carried out either in a reactor series or in a single reactor at least one of which is preferably equipped with at least two airlift-type trays. When specific CHP concentration is achieved, the oxidation products are discharged from the to reaction zone and treated in a mixing device with aqueous ammonia or water to remove organic acids such as formic acid, benzoic acid, etc. and to remove phenol, which is an inhibitor of oxidation reaction. The cumene oxidation product stream, free of organic acids and phenol is recycled to the same reactor in the case of single reactor, or is passed to the next reactor of the series in the case of reactor series. In all cases, the oxidation products treated with water or aqueous ammonia is first directed to a unit for separation of aqueous phase from organic products and then anhydrous organic product stream is forwarded to the next reactor of the series, or recycled to the single reactor for the continued cumene oxidation until the required CHP concentration is achieved. The airlift-type trays in at least one process reactor accelerate the cumene oxidation reaction therein while increasing the process selectivity and enabling the process to be conducted at lower temperature, improving safety thereof. Advantageously, lower quality cumene having impurities such as sulfur-containing trace elements, can be used in the inventive process while maintaining a high process selectivity.
Owner:ILLA INT
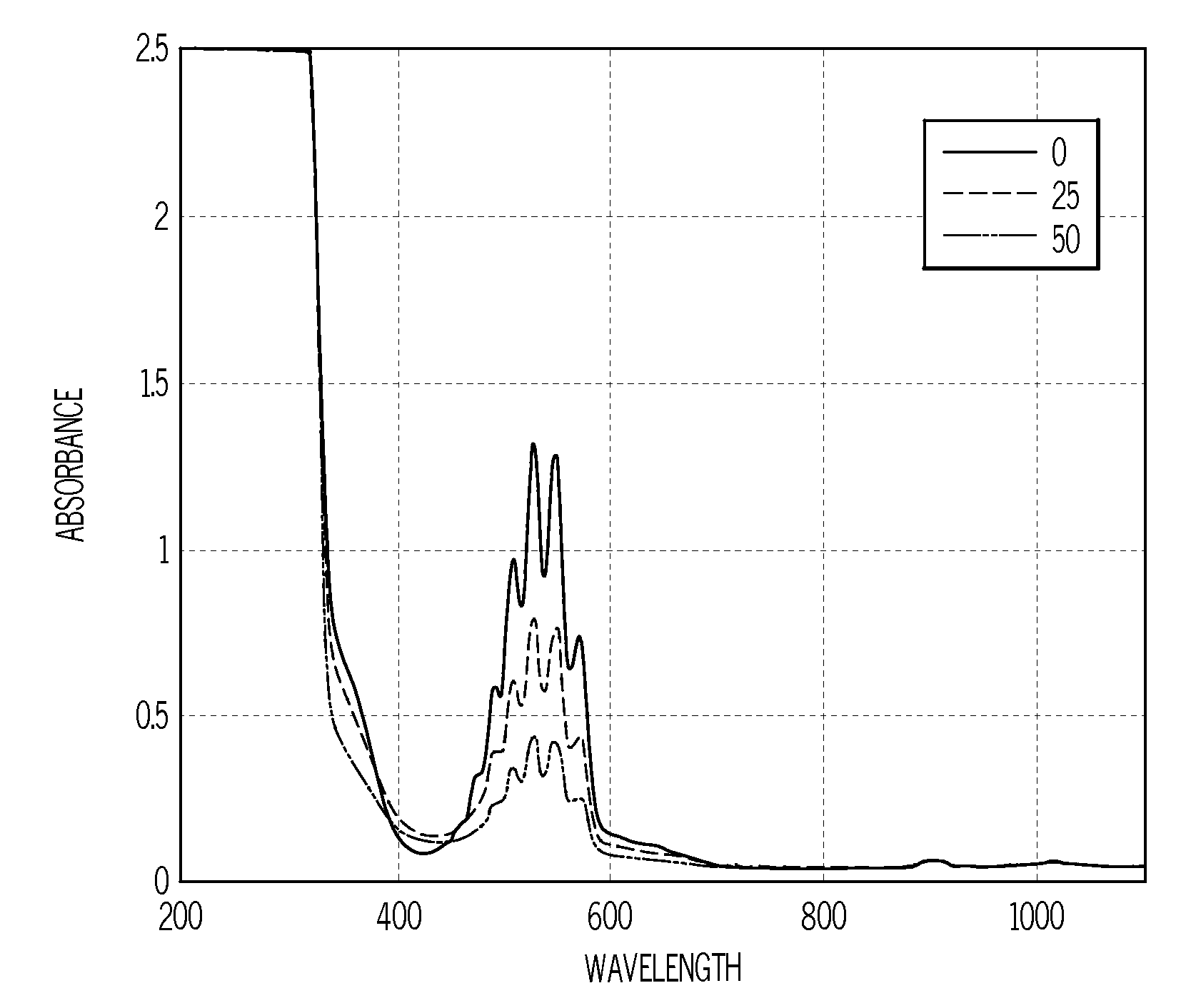
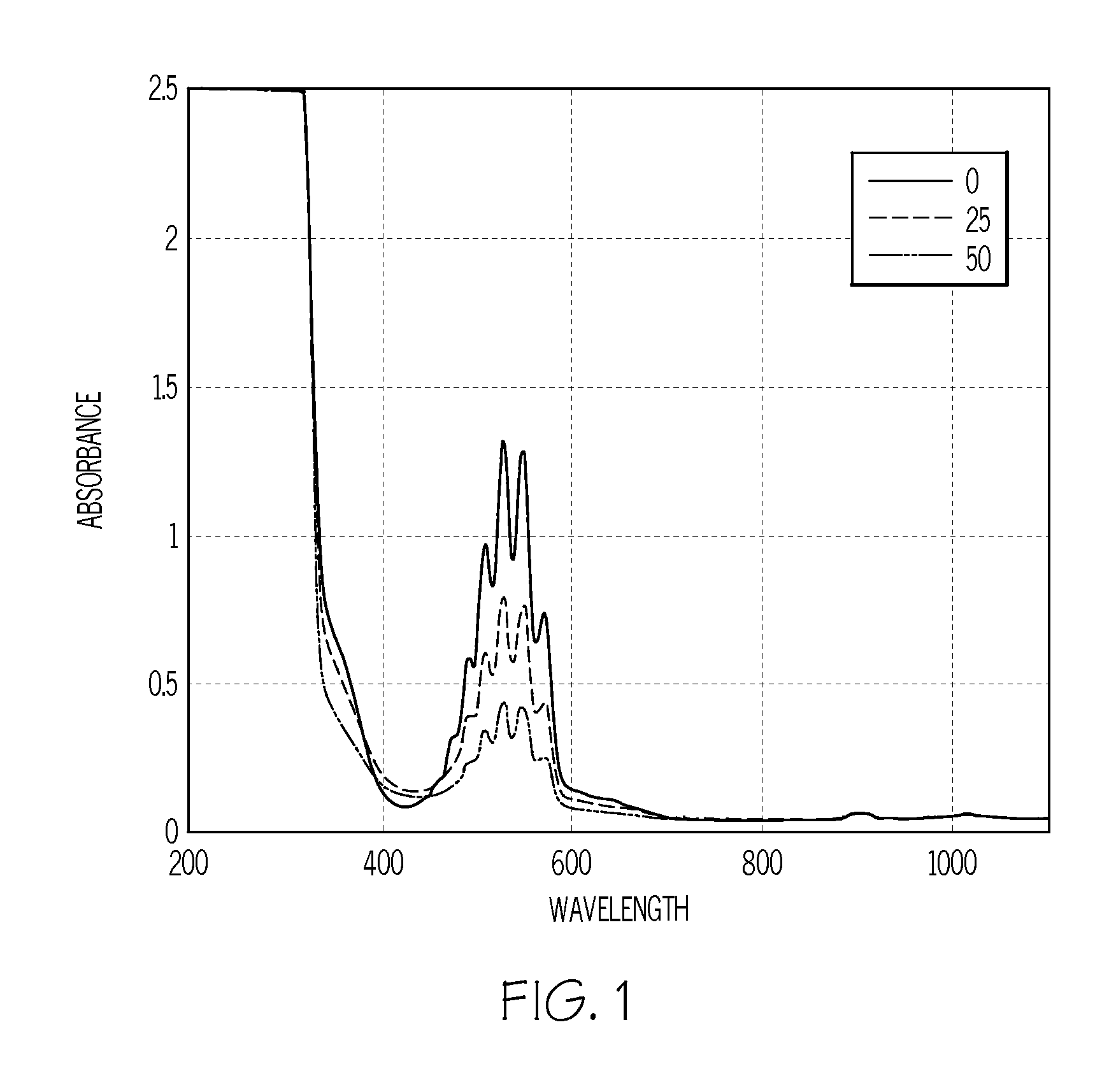
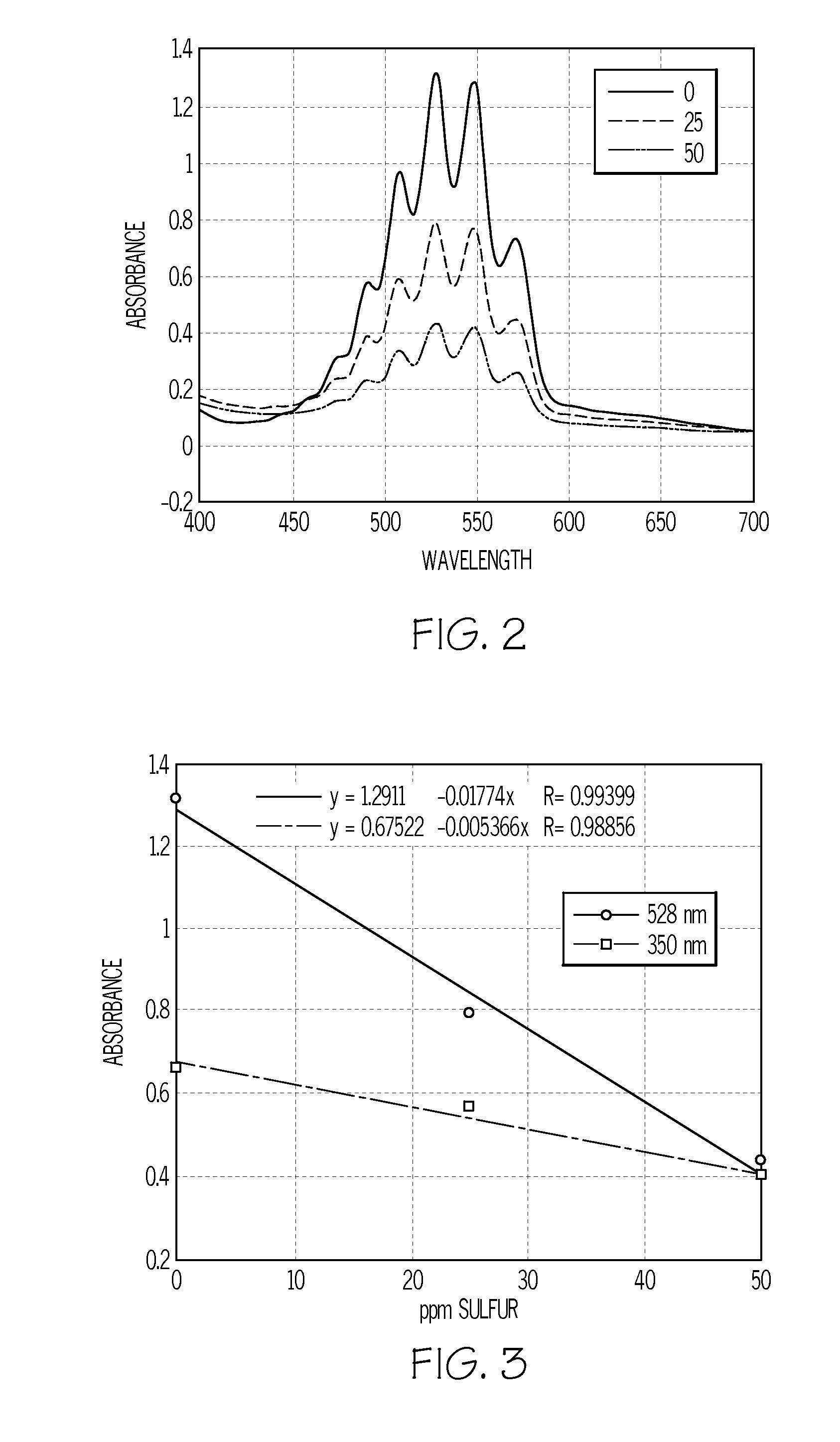
Method of analyzing sulfur content in fuels
InactiveUS20080165361A1Radiation pyrometryAnalysis using chemical indicatorsSolventColorimetric analysis
A method of detecting the amount of sulfur compounds in fuels such as ultra low sulfur diesel (ULSD) fuels is provided in which a fuel sample is reacted with a solvent and an oxidizing agent to produce a reaction product which may be analyzed by visual observation and / or in combination with spectrophotometric or colorimetric analysis. The oxidizing agent may be selected from potassium permanganate, sodium dichromate, nitric acid, hydrogen peroxide, cumene hydroperoxide, and sodium hypochlorite, and may be used in combination with an acid.
Owner:UNIV OF DAYTON
Method for the production of phenol and acetone
ActiveUS7482493B2Organic compound preparationCarbonyl compound preparationPhenolCumene hydroperoxide
A method for the production of phenol and acetone from a cumene hydroperoxide mixture comprises: decomposing the cumene hydroperoxide mixture in the presence of a catalyst mixture to form a mixture comprising phenol and acetone, wherein the method further comprises: a) forming the catalyst mixture in a catalyst formation reactor by combining sulfuric acid and phenol in a weight ratio of from 2:1 to 1:1000; b) holding the catalyst mixture in the catalyst formation reactor at a temperature of about 20 to 80° C. for about 1 to 600 minutes; and c) adding the catalyst mixture to the cumene hydroperoxide mixture to form the phenol and acetone mixture. Running the process in this manner reduces the yield of hydroxyacetone and, consequently, improves the quality of the commercial phenol. Moreover, this method reduces consumption of sulfuric acid in comparison with the process in which sulfuric acid is used as catalyst.
Owner:SABIC GLOBAL TECH BV
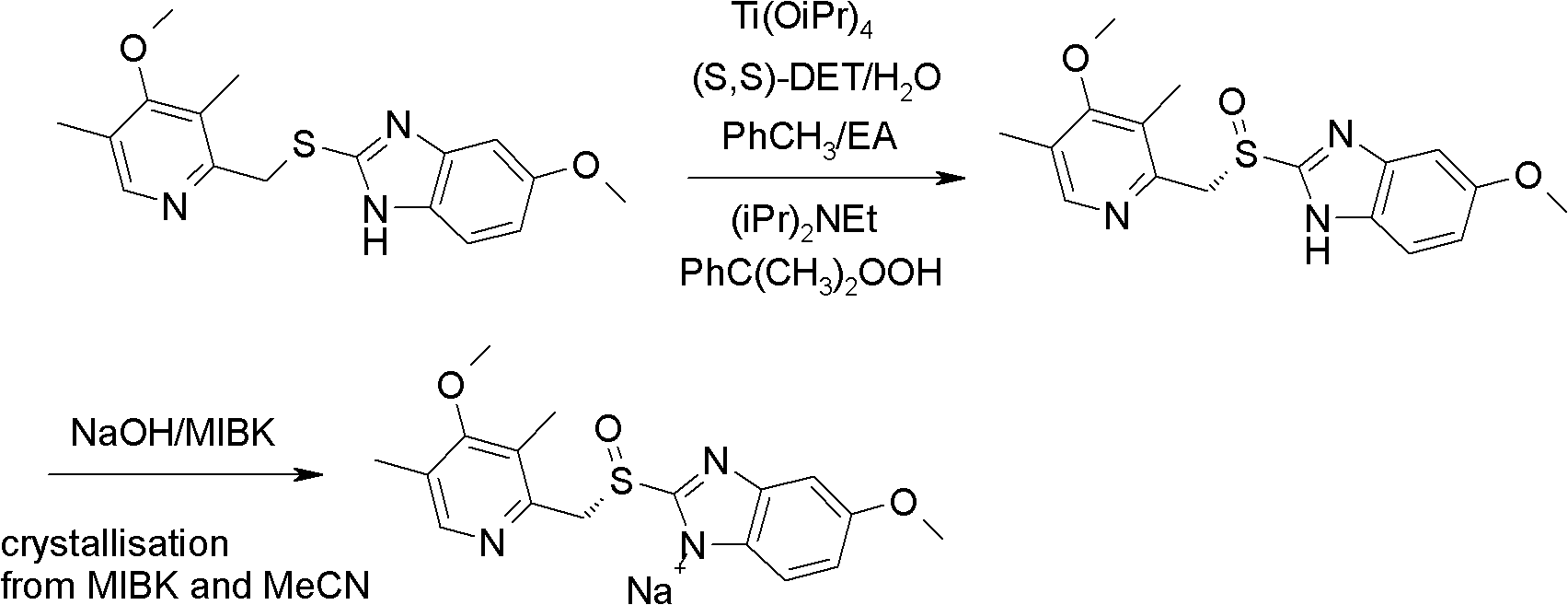
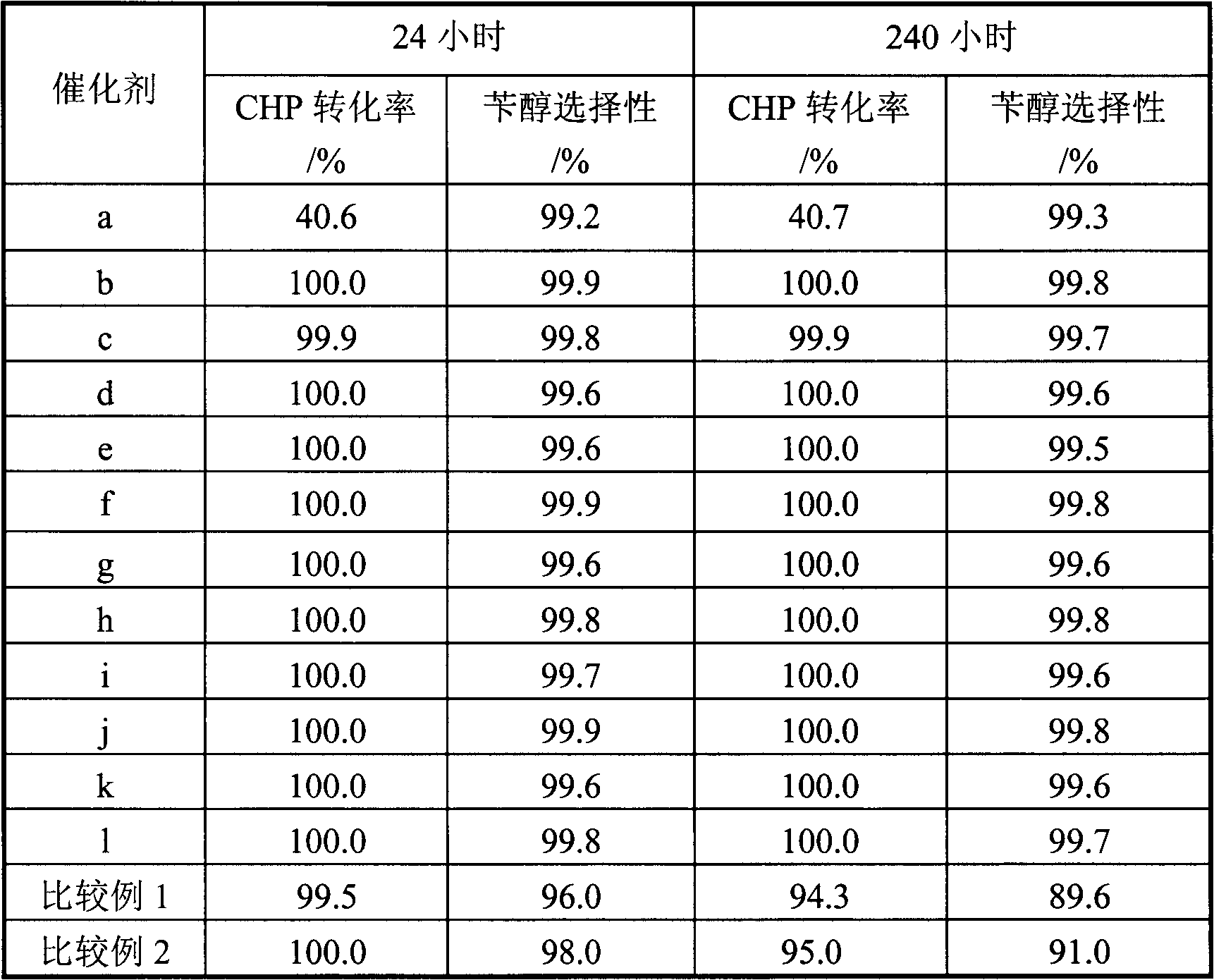
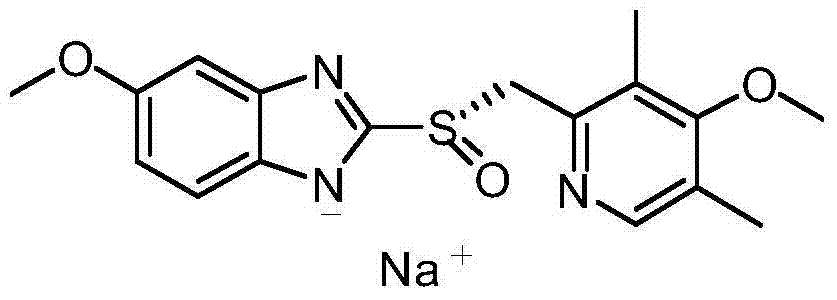
Industrial production method of high-purity esomeprazole sodium
ActiveCN102321071AReduce the impactOxidation reaction time is shortOrganic chemistryOrganic basePotassium hydroxide
The invention relates to an industrial production method of high-purity esomeprazole sodium. The industrial production method is characterized by comprising the following steps: mixing a raw material 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole with a solvent for dissolving 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole; and successively adding water, D-diethyl tartrate and titanium iso-propoxide as well as an inorganic base, then adding cumene hydroperoxide, adding methanol or ethanol after reaction, filtering, carrying out posttreatment and salifying to prepare high-purity esomeprazole sodium, wherein the inorganic base is one of potassium carbonate, sodium carbonate, sodium hydroxide and potassium hydroxide. By using the method in the invention, the defects of high cost and serious environment pollution which are caused by using the organic base in the prior art are solved, and the defects of difficult posttreatment, poor repeatability and difficult industrialization in the prior art are solved simultaneously. According to the invention, the inorganic base is used as the raw material, thus the industrial production method has the advantages of low cost, little environment pollution, short reaction time and high product purity, is easy to operate and industrially produce.
Owner:NANJING HAIRUN PHARM CO LTD
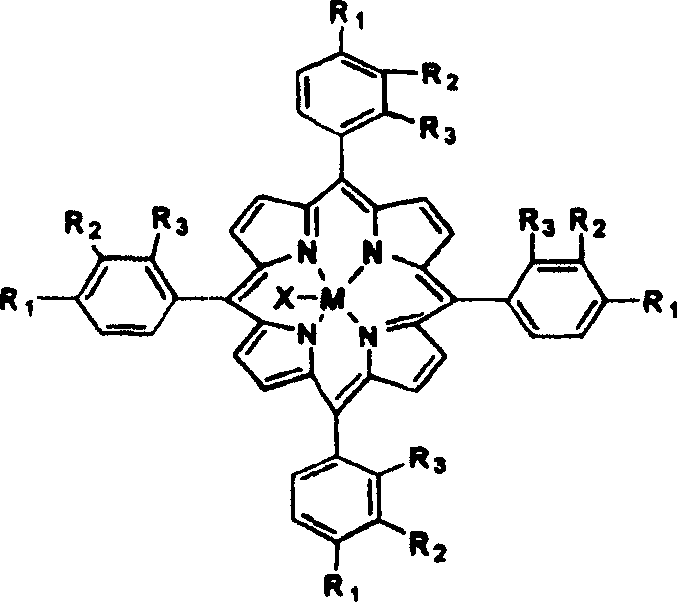
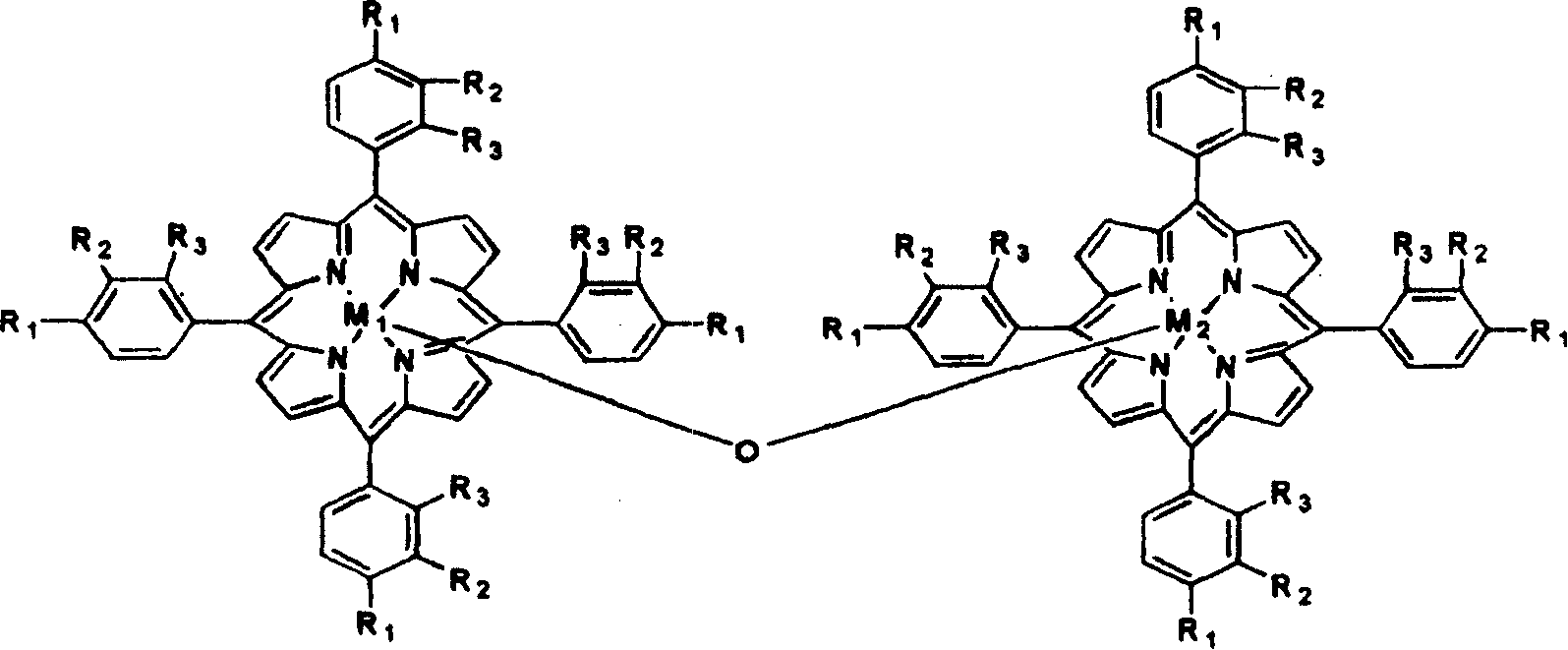
Method for preparing isopropyl benzene hydrogen peroxide by catalytically oxidizing isopropyl benzene
InactiveCN101235007AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperaturePorphyrin
The invention discloses a method for catalyzing and selectively oxidizing cumene into cumene hydroperoxide, which comprises in a mixture of oxidant air, oxygen-enriched air, or of oxygen gas and inertia gas, at an absolute reaction pressure of 0.1 to 1.0 MPa and a reaction temperature of 70 to130 DEG C, using the single metalloporphyrin of a formula (1), mu-oxgen double metalloporphyrin or relative carrier as catalyst at the density of 1 to 80 ppm without additional materials. The metalloporphyrin of low density can effectively and high selectively catalyze air to oxidize cumene into cumene hydroperoxide. The catalyst of the invention has low consumption, low synthesis cost, low catalysis reaction temperature, quick initiation, less byproduct, high selectivity and simple process, while cumene hydroperoxide yield is higher than industrial non-catalysis reaction yield and the reaction time is shorter than industrial non-catalysis reaction.
Owner:HUNAN UNIV
Epoxypropane production method
InactiveCN104109140AOrganic chemistryBulk chemical productionCumene hydroperoxideEnvironmental chemistry
The invention relates to an epoxypropane production method. The problems of low epoxypropane selectivity and low catalyst stability existing in the prior art are mainly solved. The method comprises the following steps: 1, oxidizing isopropyl benzene by molecular oxygen to obtain a cumene hydroperoxide oxidation solution; 2, controlling the weight percentage concentration of acidic substances in the cumene hydroperoxide oxidation solution not greater than 0.3%, the weight percentage concentration of alkali metal or alkali earth metal ions in the cumene hydroperoxide oxidation solution not greater than 0.2% and the weight percentage concentration of water in the cumene hydroperoxide oxidation solution not greater than 0.5%; 3, reacting the cumene hydroperoxide oxidation solution with propylene under liquid phase conditions under the action of a catalyst to generate epoxypropane and alpha, alpha-dimethylbenzylalcohol; and 4, carrying out a hydrogenolysis reaction on alpha, alpha-dimethylbenzylalcohol by hydrogen in the presence of a catalyst to generate isopropyl benzene, wherein the generated isopropyl benzene is circulated to step a and is used as a raw material for preparing cumene hydroperoxide. The epoxypropane production method well solves the problems, and can be used in the industrial production of epoxypropane preparation.
Owner:CHINA PETROLEUM & CHEM CORP +1
Reactor and method for preparing epoxypropane by reactor
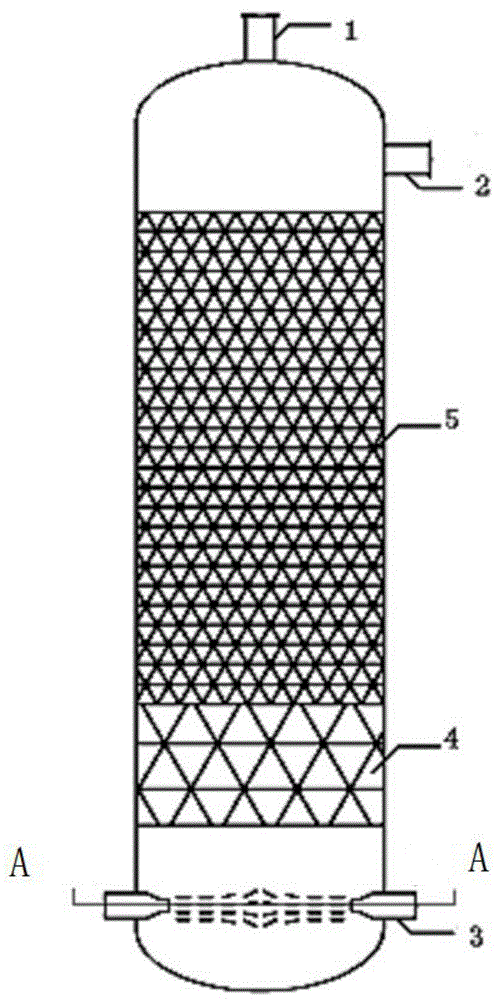
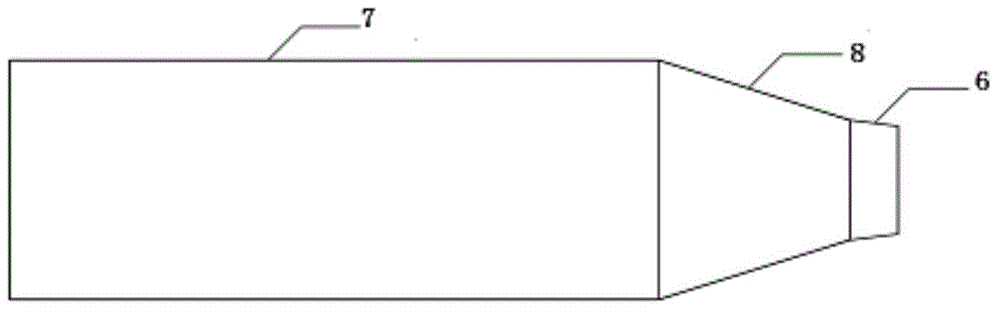
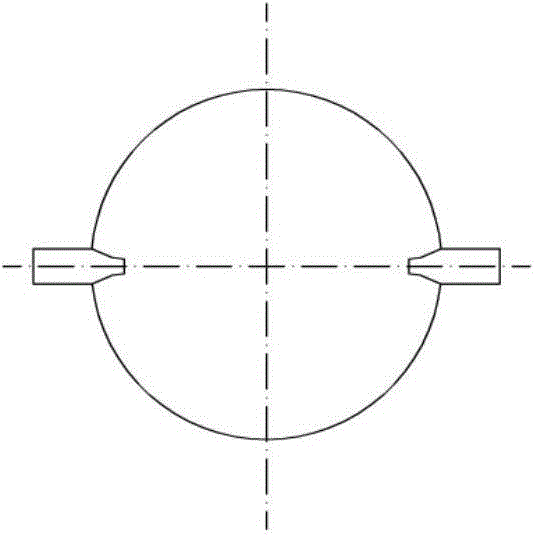
ActiveCN104907009AIntensified interphase mixingHigh selectivityOrganic chemistryChemical/physical processesSpray nozzleFixed bed
The invention discloses a reactor. The middle of the reactor is filled with an epoxidation catalyst, the top wall of the reactor is provided with a gas outlet, the upper side wall of the reactor is provided with a liquid outlet, the lower side wall of the reactor is provided with one or two groups of injectors, each group of the injectors comprises two injectors symmetrically distributed on the same plane, the injector comprises a jet pipe and a nozzle connected to the jet pipe, the nozzle is frustoconical, the diameter of one end of the nozzle connected to the jet pipe is greater than that of the other end, the nozzle stretches into a fixed bed reactor, the jet pipe comprises two sections, the section of the jet pipe far away from the nozzle is cylindrical, the other section is frustoconical, and the diameter of one end of the jet pipe far away from the nozzle is greater than the diameter of one end of the jet pipe connected to the nozzle. The invention discloses a method for preparing epoxypropane by the reactor and a reaction system composed of the reactors connected in series. The reactor realizes reactant high-speed clashing mixing, improves reactant mixing effects, improves heat and mass transfer effects and improves a cumene hydroperoxide conversion rate and epoxypropane selectivity.
Owner:HONGBAOLI GRP CO LTD +1
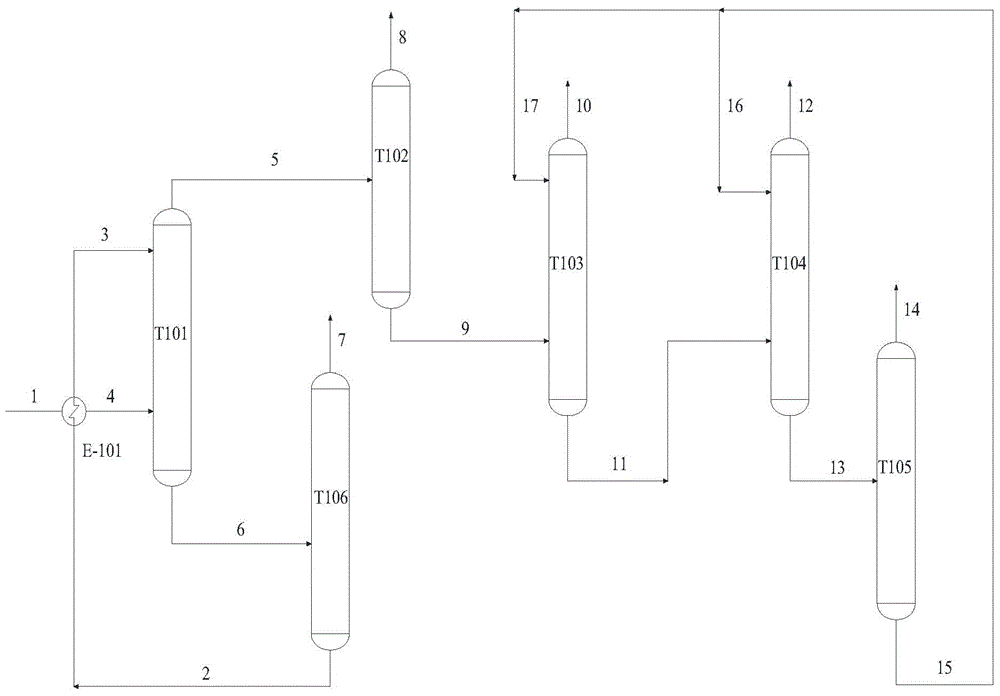
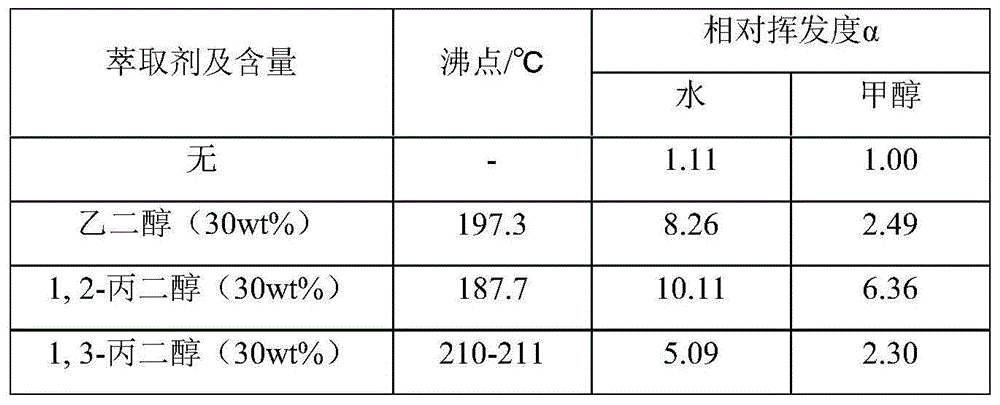
Propylene oxide refining method
The invention provides a refining method of propylene oxide (PO). The method is a method for refining a crude propylene oxide product prepared through a process for preparing propylene oxide through a CHP (cumene hydroperoxide) method. According to the invention, in a de-heavy tower, C2-C6 alkyldiol is adopted as an extraction agent for removing impurities such as water, propionaldehyde and acetone; bottom liquid enters a solvent recovery tower wherein the extraction agent is recovered, and the extraction agent is circulated and fed from the upper part of the de-heavy tower; in a de-light tower, impurities such as C3-C4 light hydrocarbon and acetaldehyde in de-heavy tower top distillate are removed; de-light tower bottom liquid enters an extraction tower wherein C7-C20 hydrocarbon is adopted as an extraction agent for removing impurities such as residual acetaldehyde and water; extraction tower bottom liquid enters a product tower wherein C7-C20 hydrocarbon is adopted as an extraction agent for removing C5-C6 light hydrocarbon, and a propylene oxide product is obtained at the tower top; and product tower bottom liquid enters a solvent stripping tower wherein the extraction agent C7-C20 hydrocarbon is recovered.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing epoxy butane
The invention relates to a method for preparing epoxy butane, which comprises the following step: in an isopropyl benzene solution containing 25 wt% of cumene hydroperoxide solute, preparing epoxy butane from butylene oxide by using the cumene hydroperoxide solute as an oxidizer and a titanium-silicon molecular sieve with three-dimensional pore canal structure as a catalyst, wherein the fixed bed reaction conditions are as follows: the mole ratio of butylene to the cumene hydroperoxide solute is (5.0-12.0):1, the weight hourly space velocity of the cumene hydroperoxide is 1.0-5.0 h<-1>, the reaction pressure is 1.0-6.0 MPa, and the temperature is 60.0-120.0 DEG C. The catalyst is the titanium-silicon molecular sieve with three-dimensional pore canal structure; the molecular sieve has hysteresis loop on the low-temperature nitrogen adsorption and desorption isotherm; the average pore size is 2.0-8.0nm, and the specific area is 650.0-1100.0 m<2> / g; and the catalyst has the advantages of favorable activity and high epoxy butane selectivity, and can be widely popularized and applied to industrial production of epoxy butane by butylene epoxidation.
Owner:CHINA PETROLEUM & CHEM CORP
Process for producing phenol
InactiveUS20050222466A1Easy to understandOrganic compound preparationCarbonyl compound preparationPHENOL LIQUIDPhenol product
A process for producing a phenol product generally comprises a first step comprising reacting in a first reactor a feed stream comprising cumene hydroperoxide and water with an acid catalyst to produce an effluent comprising the phenol product, acetone, and at least 1% by weight residual cumene hydroperoxide, and a second step comprising passing the effluent into a second reactor and decomposing the residual cumene hydroperoxide, wherein during said process the ratio of phenol to acetone is maintained at a molar ratio of greater than 1:1, and wherein the water in each of the first and second steps is present in an amount more than 0 and less than or equal to 5 weight percent based on the total weight of the feed stream or effluent, and wherein the process is continuous.
Owner:SABIC GLOBAL TECH BV
Method and system for purifying cumene hydroperoxide cleavage products
InactiveUS6576798B1High materialEliminate needOrganic compound preparationPH-change processesWater solubleAqueous solution
A system for purifying a cumene hydroperoxide cleavage product mixture comprises a cumene hydroperoxide cleavage product mixture feed containing impurities in fluid communication with an aqueous alkaline solution feed; the cumene hydroperoxide cleavage product mixture and aqueous alkaline solution feeds in fluid communication with a neutralization drum having a aqueous salt phase outlet; a aqueous salt phase feed containing impurities in fluid communication with a decomposer reactor having an oxidized aqueous salt phase outlet; an oxidizing agent feed in fluid communication with the aqueous salt phase feed containing the impurities prior to the decomposer reactor; and an oxidized aqueous salt phase feed containing water-soluble oxidized derivatives of the impurities in fluid communication with the cumene hydroperoxide cleavage product mixture prior to the neutralization drum.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Self-Curing System For Endodontic Sealant Applications
InactiveUS20080003542A1Fasten curingImprove shelf life stabilityImpression capsTeeth fillingThioureaSelf curing
A two-part self-curing endodontic sealing system comprises a thiourea derivative, such as acetyl thiourea, and a hydroperoxide, such a cumene hydroperoxide. The thiourea derivative is used as a reducing agent and the hydroperoxide is used as an oxidizing agent.
Owner:PENTRON CLINICAL TECH
Polyester resin composition, switch made from the same, and method of producing the switch
InactiveUS6046258APromote productionImprove insulation performanceCouplings bases/casesTumbler/rocker switch detailsInorganic compoundNeopentyl glycol
PCT No. PCT / JP97 / 00404 Sec. 371 Date May 8, 1998 Sec. 102(e) Date May 8, 1998 PCT Filed Feb. 14, 1997 PCT Pub. No. WO98 / 36028 PCT Pub. Date Aug. 20, 1998A polyester resin composition comprising 21-29% by weight of a resin component consisting of fumaric acid neopentyl glycol propylene glycol type polyester and cumene hydroperoxide as a hardening agent; 52-60% by weight of one or more kinds of inorganic compounds which undergo dehydration reaction at a temperature not later than 150 DEG C. as an inorganic filler; and 15-23% by weight of a reinforcing material. The composition of the present invention when used for an insulation component member of a switch, improves the insulation property after the cut-off of a large electric current. More specifically, the composition of the present invention can prevent the formation of the resin skin layer on the surface of the molded product, so that the inorganic compound which can be decomposed by the heat from the electric arc to produce a gas which changes the free carbon and metal vapor to nonconductive oxides can be present in the surface layer of the molded product and the insulation property can be secured even after the cut-off of the electric current.
Owner:MITSUBISHI ELECTRIC CORP
Maleic anhydride acidation chitosan salt/nature rubber graft copolymer and preparation method thereof
InactiveCN101864051AImprove antibacterial propertiesImprove mechanical propertiesBenzoyl peroxidePeroxydisulfate
The invention provides maleic anhydride acidation chitosan salt / nature rubber graft copolymer and a preparation method thereof. The method comprises the following steps: adding stabilizing agent non-ionic surface active agents Peregal-O into concentrated natural rubber for maintaining the stability and the temperature rise of the nature rubber; regulating the pH value of the maleic anhydride acidation chitosan salt solution between 8 and 12; then, adding obtained materials into the nature rubber; adding triggering agents of one kind or two kinds of materials in benzoyl peroxide, tert-butyl hydroperoxide, azodiisobutyronitrile, sulphur, cumene hydroperoxide, potassium peroxydisulfate and N, N-methylene-bisacrylamide; carrying out stilling standing for 24 hours; adding activating agents of tetraethylenepentamine; continuously raising the temperature; carrying out reaction for 2 to 24 hours at the temperature between 60 and 125 DEG C; and obtaining the milky maleic anhydride acidation chitosan salt / nature rubber graft copolymer. The copolymer has good antibacterial property, good physiological activity, good hydrophilcity and good heat resistance performance, and further widens the application range of the nature rubber in the fields of medical sanitation and the like.
Owner:AGRI PRODS PROCESSING RES INST CHINESE ACAD OF TROPICAL AGRI SCI +1
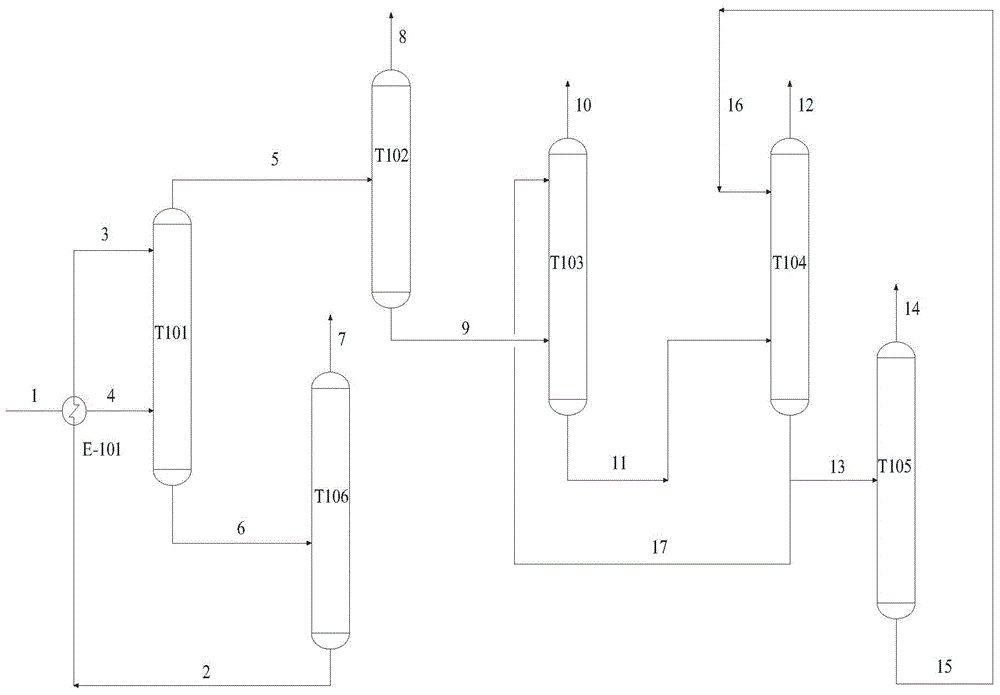
Epoxypropane purifying method
InactiveCN104109138AChanging the relative volatilityReduce adverse effectsOrganic chemistryExtractive distillationBiochemical engineering
The invention relates to an epoxypropane purifying method. The problems of high extractant cost and bad product quality due to the introduction of a system outside medium in the prior art are mainly solved. The epoxypropane purifying method is characterized in that a crude epoxypropane solution containing epoxypropane, water, acetaldehyde, propionaldehyde, methanol, acetone and C5C7 hydrocarbon impurities and obtained after a reaction of cumene hydroperoxide and propylene is extracted and distilled by an extractant, wherein the extractant is isopropyl benzene. The epoxypropane purifying method well solves the problems, and can be used in the industrial production of epoxypropane purification.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for Production of Cumene Hydroperoxide
ActiveUS20070260093A1Enhances CHP productionIncreasing CHP productionOrganic compound preparationChemical/physical/physico-chemical nozzle-type rreactorsOxygen contentLiquid phase
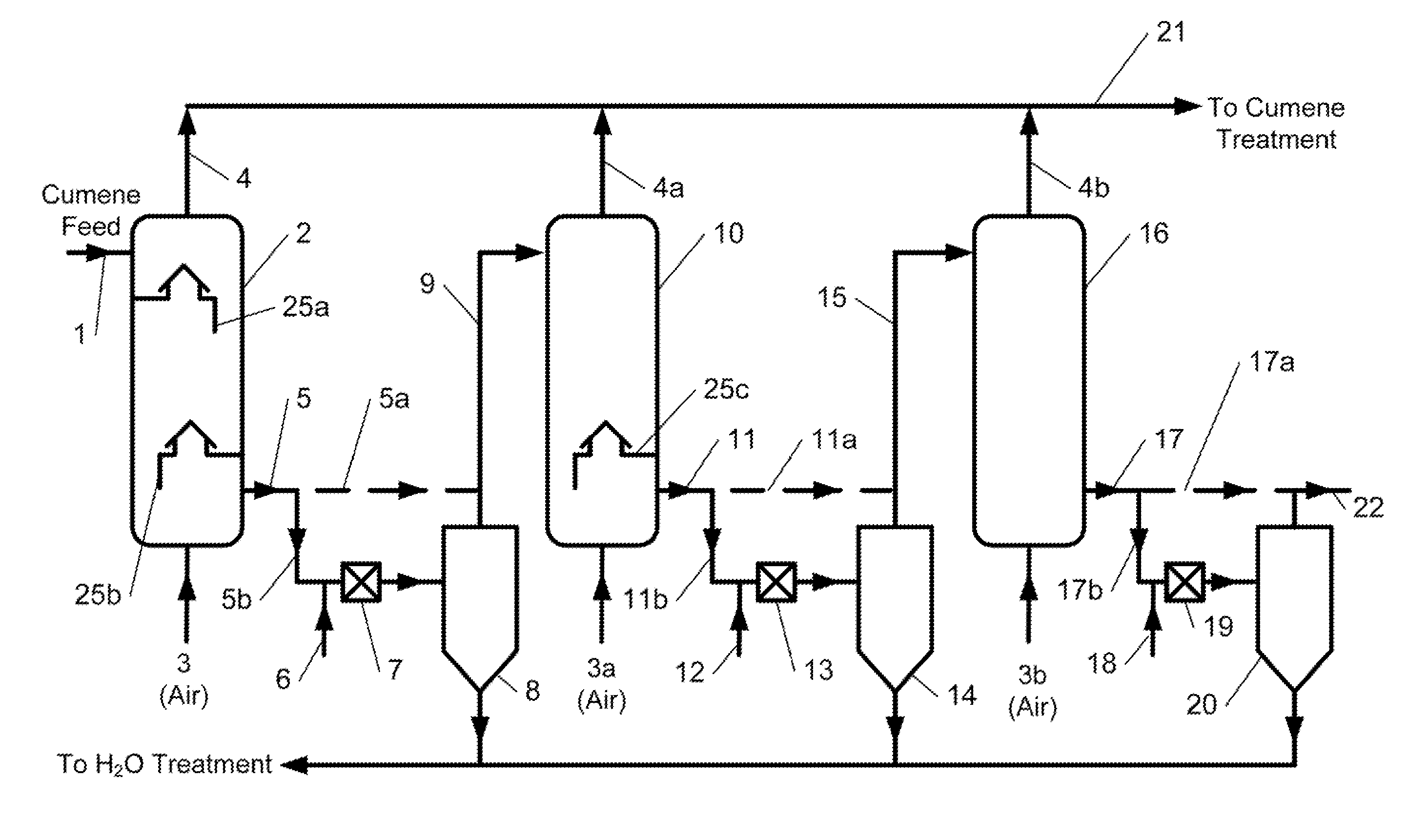
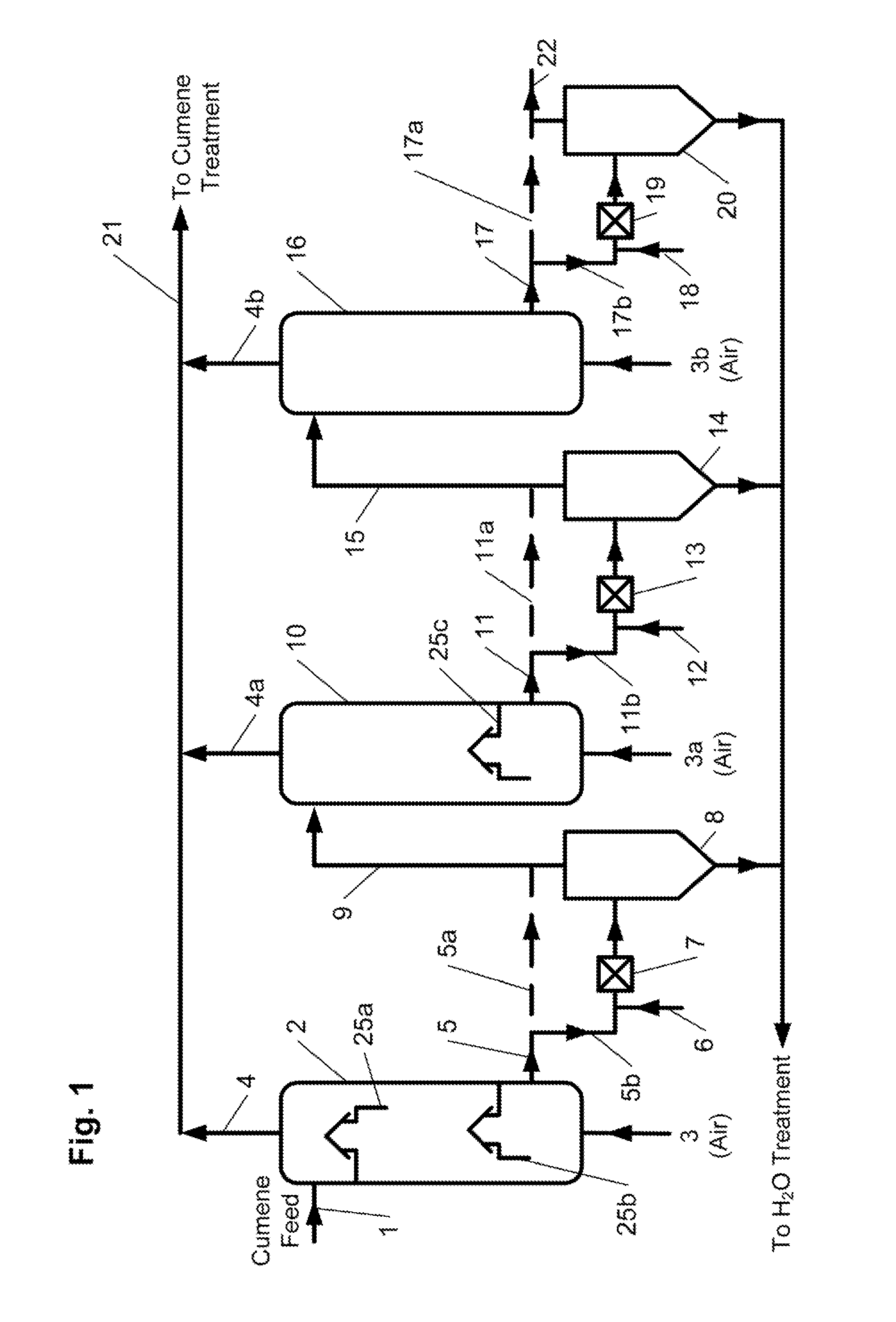
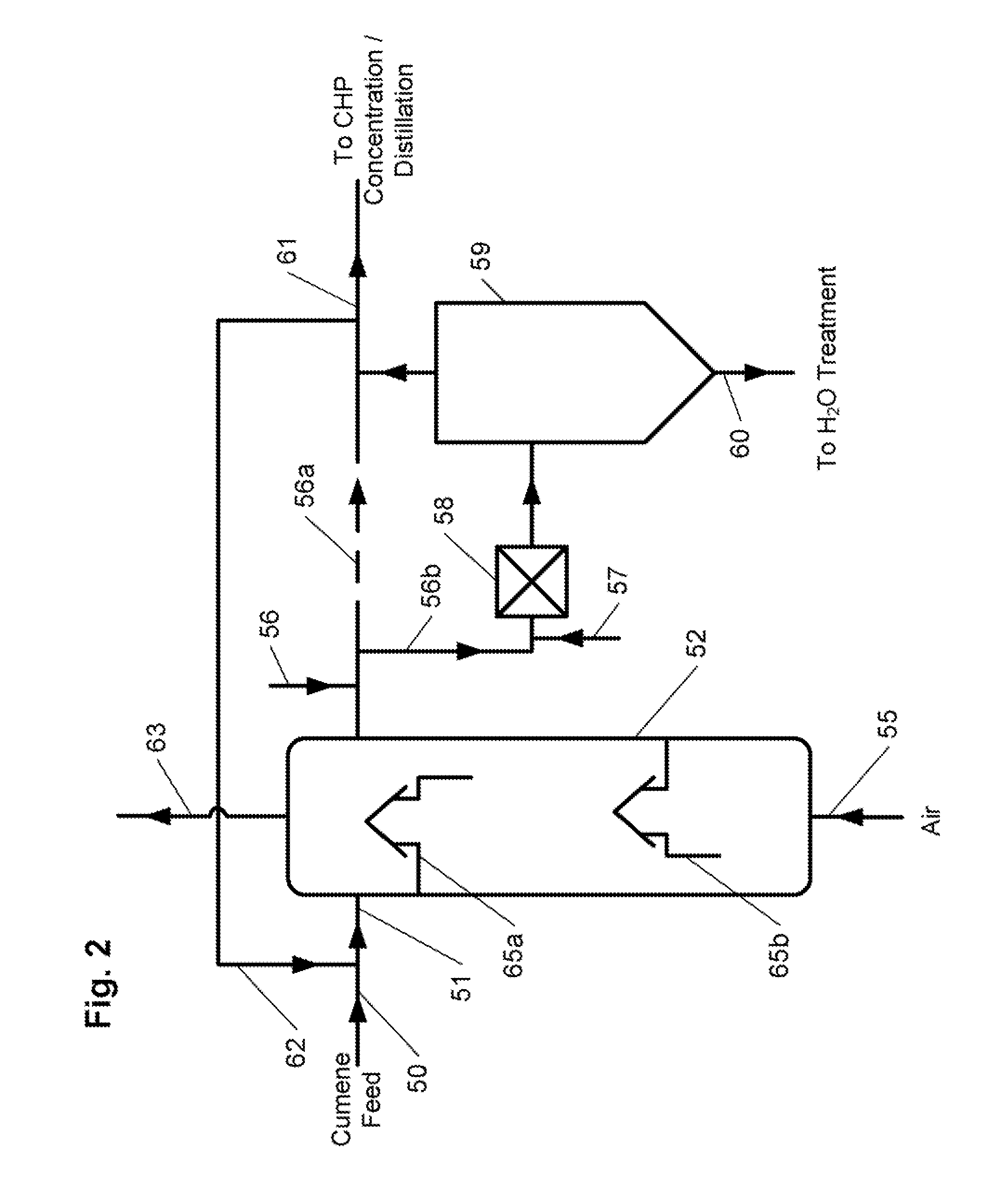
A process for continuous production of cumene hydroperoxide comprising liquid phase oxidation of cumene in a reactor in the presence of an oxygen-containing gas under such conditions that an oxygen content of the total oxygen-containing gas volume fed into the liquid phase in the reactor is adjusted to not less than 22 mol % and not more than 50 mol %, and (1) the cumene hydroperoxide production per unit volume of the reaction fluid in the reactor is not less than 22 kg / m3 / hr, (2) an oxygen content of an exhaust gas of the reactor is not less than 2 mol % and not more than 10 mol % or (3) said oxygen-containing gas is fed into the reactor using a sparger whose aperture pitch is at least twice the aperture diameter. By use of the process, CHP production per unit volume of reaction fluid in the reactor can be enhanced, thus the process is capable of miniaturizing the reactor allowing required CHP production or enhancing CHP production in an existing reactor.
Owner:MITSUBISHI CHEM CORP
Process for production of propylene oxide
InactiveCN1612870ASolution to short lifeHigh activityOrganic compound preparationOrganic chemistry methodsOrganic acidAlcohol
A process for producing propylene oxide, comprising the following steps: oxidation step: a step of obtaining cumene hydroperoxide by oxidizing cumene; epoxidation step: a step of obtaining propylene oxide and cumyl alcohol by reacting a cumene solution containing cumene hydroperoxide with an excess of propylene in a liquid phase in the presence of a solid catalyst; and hydrogenolysis step: a step of obtaining cumene through hydrogenolysis of cumyl alcohol obtained in the epoxidation step, and recycling the cumene to the oxidation step as the raw material of the oxidation step, wherein the concentration of organic acids in cumyl alcohol supplied to the hydrogenolysis step is adjusted to 200 ppm by weight or less.
Owner:SUMITOMO CHEM CO LTD
Method of producing cumene hydroperoxide
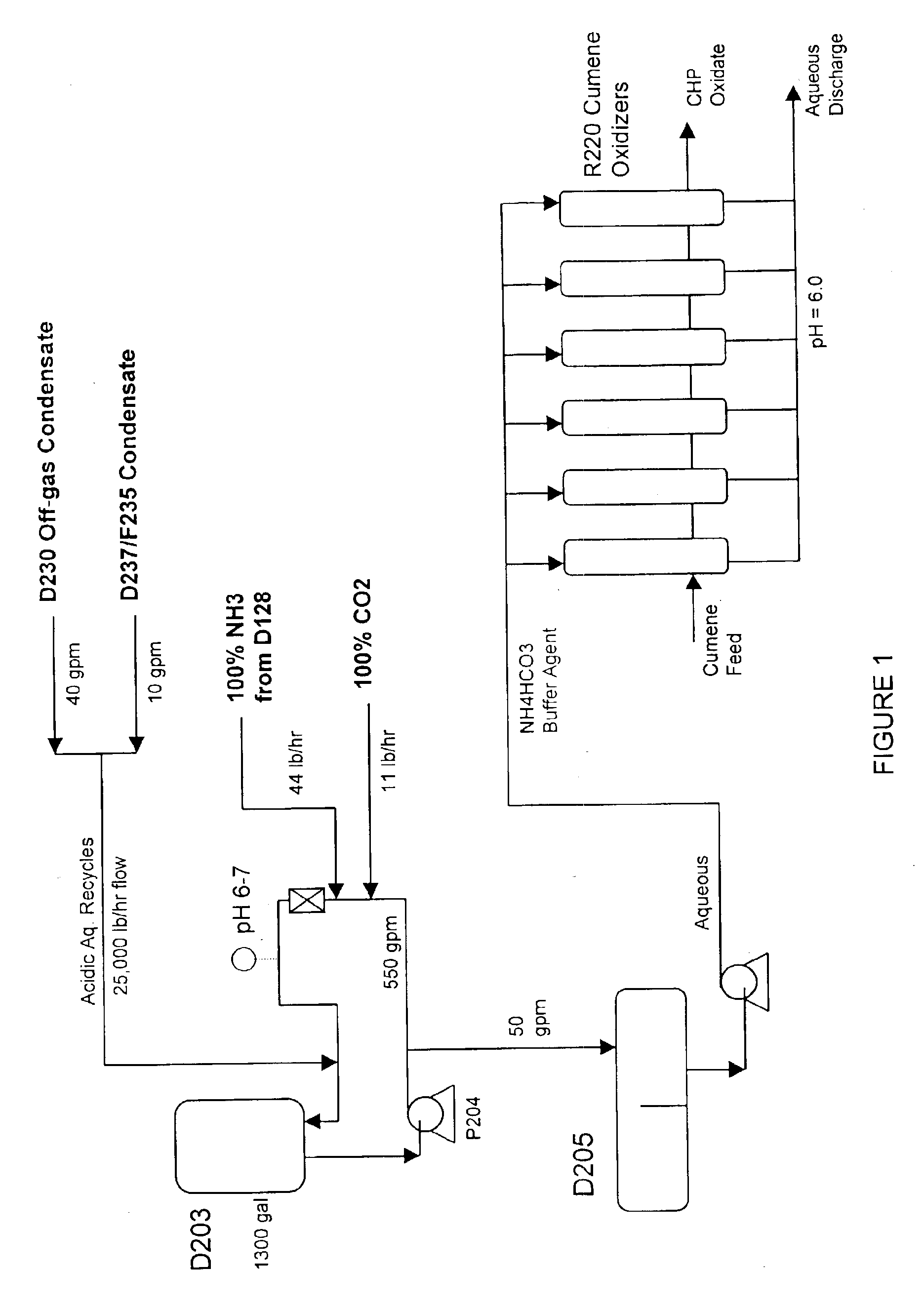
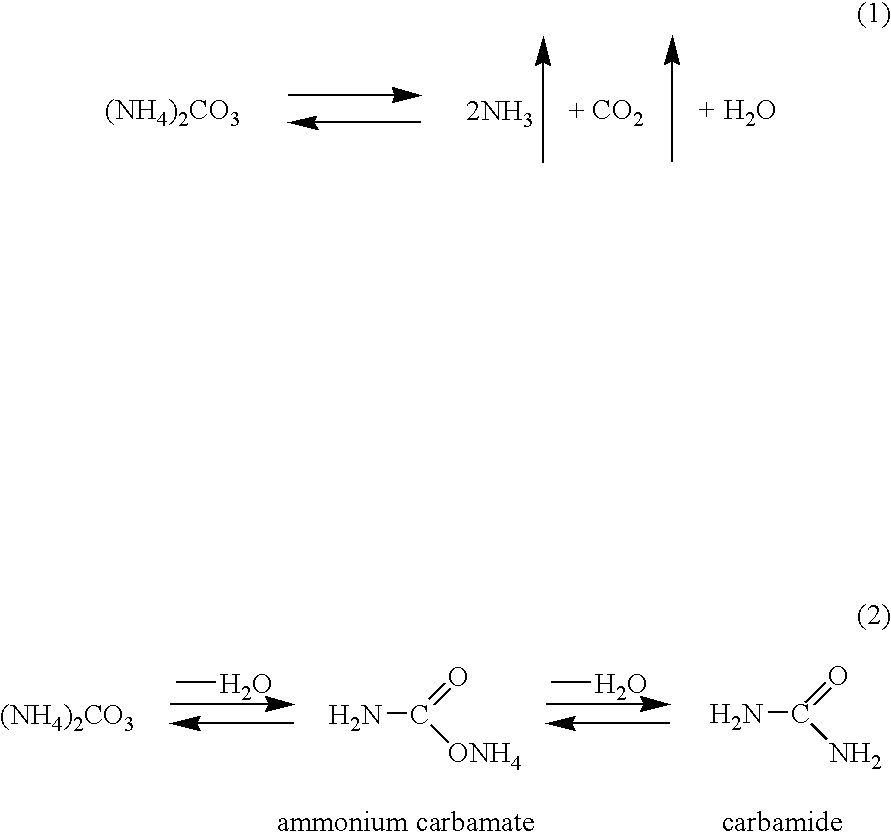
The specification provides a method of producing cumene hydroperoxide by continuous aqueous-emulsion oxidation at a high temperature and pressure in a cascade of reactors, wherein the process is conducted in the presence of a mixture of an aqueous solution of an ammonium salt with a concentration of 0.001-0.5 mass % and an aqueous solution of ammonia with a concentration of 0.001-0.5 mass %, which mixture is fed into each oxidation reactor in an ammonia:ammonium salt mass ratio of 1:100 to 100:1.
Owner:SABIC GLOBAL TECH BV
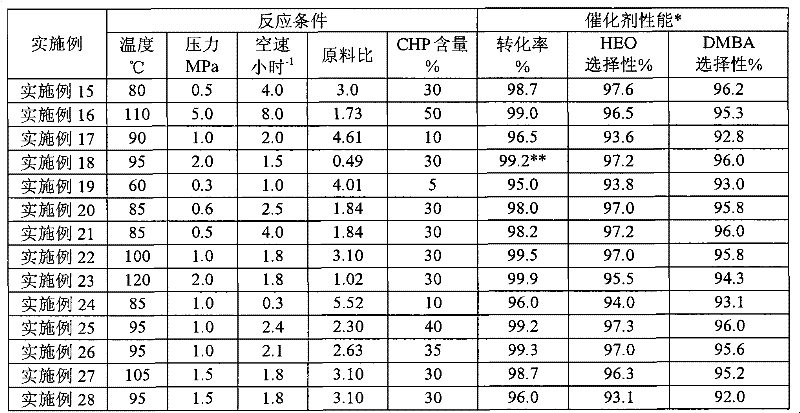
Method for the decomposition of cumene hydroperoxide
InactiveUS20110306800A1Yield maximizationMinimize residual dicumyl peroxideOxygen-containing compound preparationOrganic compound preparationDecompositionPhenol
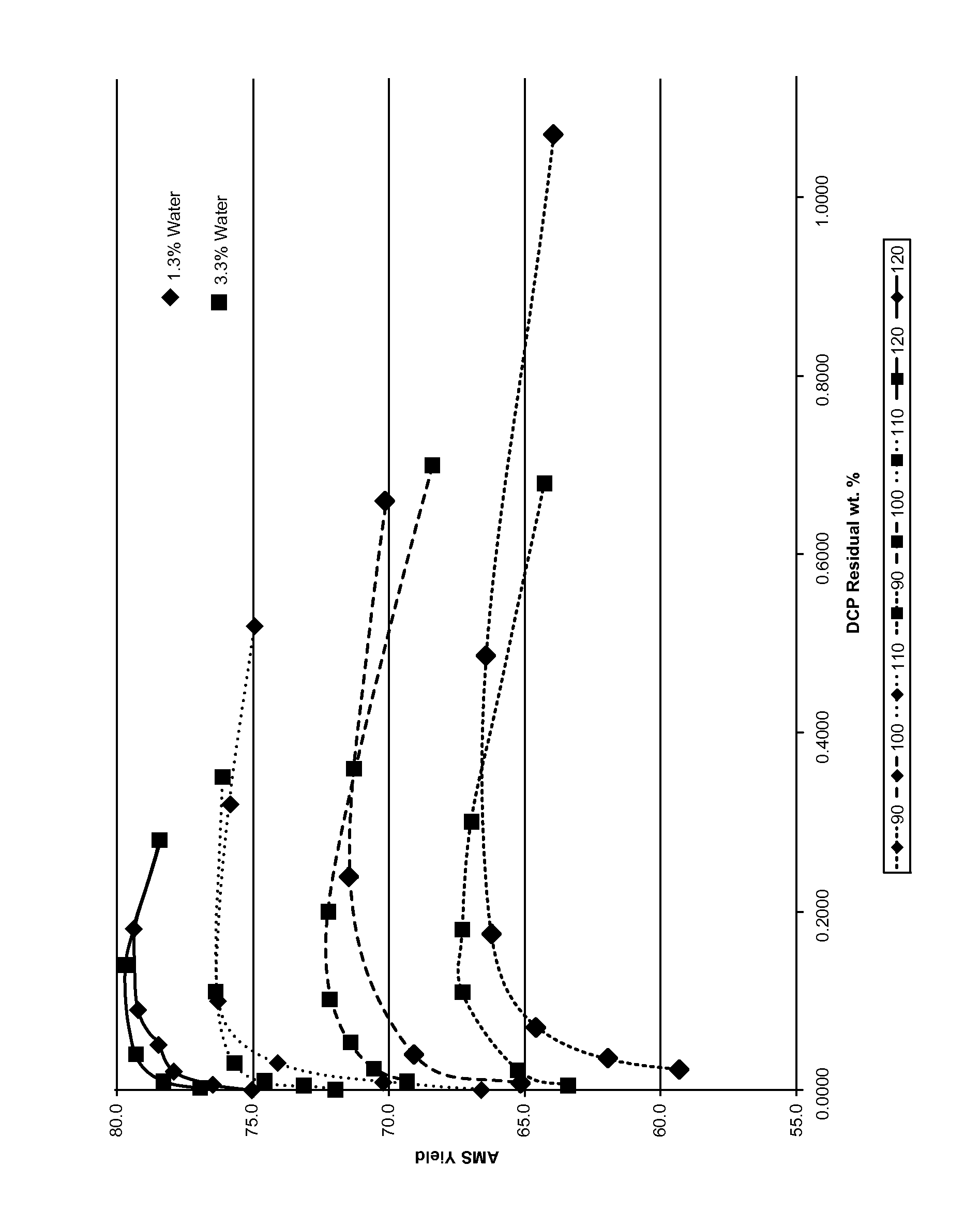
An improved method for the production of phenol, acetone and alpha-methyl styrene (AMS) from a cumene hydroperoxide and dimethylbenzyl alcohol (DMBA) mixture is described, wherein 0.5-5% additional water by weight is added prior to the final DMBA dehydration step, carried out in the presence of about 20-400 ppm mineral acid catalyst at 110-150° C. for 0.5 to 40 minutes residence time. The use of additional water allows greater flexibility in maintaining optimum temperature in the second stage over a much broader turndown range with fixed equipment, decreases the residual dicumyl peroxide (DCP) at the yield optimum for a given temperature, and increases the overall yield of AMS at optimum conditions at a given temperature.
Owner:HONEYWELL INT INC
Catalyst for producing alpha, alpha-dimethyl benzyl alcohol by hydrogenation of cumene hydroperoxide and preparation method thereof
ActiveCN101992086ACatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsSilicon oxideBENZYL ALCOHOL/WATER
The invention relates to a catalyst for producing alpha, alpha-dimethyl benzyl alcohol by hydrogenation of cumene hydroperoxide and a preparation method thereof, mainly solving the problem of poor Pd catalyst stability in the process of producing the alpha, alpha-dimethyl benzyl alcohol in the prior art. In the invention, the adopted technical scheme is as follows: the catalyst comprises the following components in percentage by weight: (a) 0.1-2.0% of mixture selected from metals or oxides of Pd, (b) 85.0-99.7% of at least one of aluminum oxide and silicon oxide, (c) 0.1-5.0% of at least oneof metals or oxides of Sn, Pb or In, and (d) 0.1-10.0% of at least one of metals or oxides of Na, K, Cs, Mg, Ca or Ba. The invention provides the preparation method of the catalyst, thereby better solving the problem in the prior art. The invention can be used for industrially producing the alpha, alpha-dimethyl benzyl alcohol by the hydrogenation of cumene hydroperoxide.
Owner:CHINA PETROLEUM & CHEM CORP +1
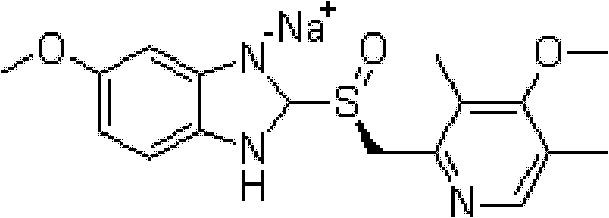
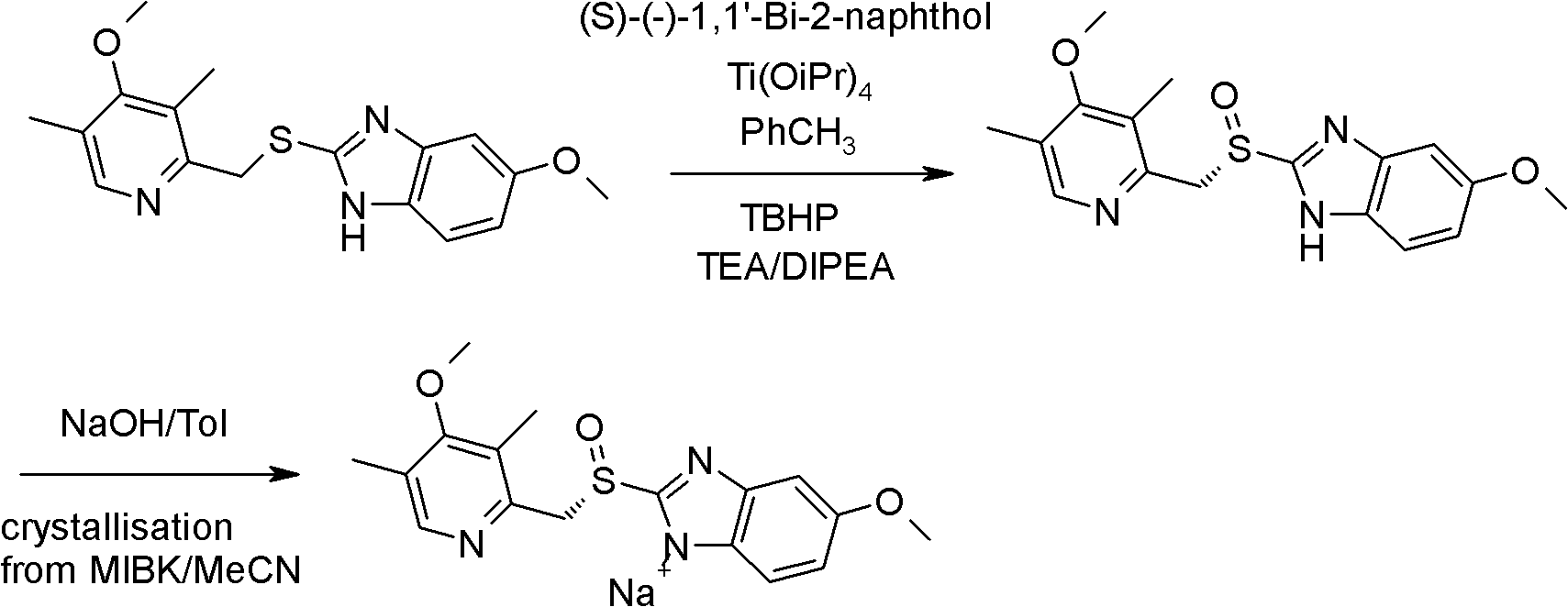
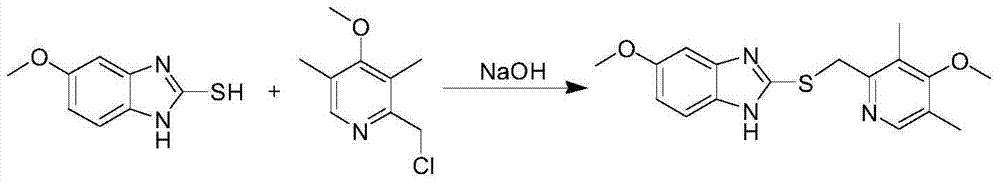
Method for synthesizing and refining esomeprazole sodium
ActiveCN103570686AConvenient sourceSimple and fast operationOrganic chemistryEsomeprazole SodiumTetraisopropyl titanate
The invention relates to a method for synthesizing and refining esomeprazole sodium. The method comprises the following several steps: (1) carrying out condensation by taking 2-sulfydryl-5-methoxybenzimidazole and 2-chloromethyl-3.5-dimethyl-4-methoxypyridine hydrochloride as raw materials to generate prochiral thioether; (2) synthesizing the prochiral thioether into an esomeprazole crude product under the action of D-(-) diethyl tartrate, water, tetraisopropyl titanate, diisopropylethylamine and cumene hydroperoxide, and refining to generate esomeprazole potassium salt; (3) dissolving the esomeprazole potassium salt, then transforming into esomeprazole sodium salt, and refining to obtain a final product. The process of the esomeprazole sodium has the advantages of easiness for operation, good reaction repeatability and higher yield; the obtained product is high in purity and suitable for industrialized production.
Owner:哈药集团股份有限公司 +1
Composite catalytic viscosity reducer for steam injection to thickened oil and preparation method and use thereof
ActiveCN101423754APlay the role of hydrogenation modificationViscosity is easy to recoverDrilling compositionReducerSURFACTANT BLEND
The invention relates to a composite catalytic viscosity reducer for thick oil steam injection production, as well as a preparation method and application thereof. The viscosity reducer is formed by combining three slugs, wherein a slug I comprises the following components in weight percentage content: 1 to 2 percent of cumene hydroperoxide, 1 to 3 percent of manganese oleate, 0.2 to 0.5 percent of sodium dihydrogen phosphate, 0.1 to 0.5 percent of alkaline matter and the balance being water; a slug II comprises the following components in weight percentage content: 0.05 to 0.1 percent of hydrothermal cracking catalysts, 0.05 to 0.2 percent of alkaline matter and the balance being water; and a slug III comprises the following components in weight percentage content: 0.1 to 0.5 percent of surfactant, 0.1 to 0.3 percent of alkaline matter and the balance being water. The composite viscosity reducer formed by combining three viscosity reducers has the advantages of good viscosity reducing effect, as well as wider adaptive range and better effects during field application through combination. Experiments prove that the composite viscosity reducer can reduce the viscosity of crude oil from 309,028 mPa.s to 55 mPa.s within 24 hours at a temperature between 100 and 200 DEG C, and reaches the viscosity reducing rate of over 99.9 percent.
Owner:中国石油化工股份有限公司河南油田分公司石油工程技术研究院 +1
Simultaneous preparation of 1,2-epoxycyclohexane and α,α-dimethylbenzyl alcohol
InactiveCN102295626AHigh activityGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by oxygen reductionEpoxyCyclohexene
The invention relates to a method for simultaneously preparing 1,2-epoxycyclohexane and α,α-dimethylbenzyl alcohol, which mainly solves the problem of separately producing 1,2-epoxycyclohexane and α,α in the prior art -When dimethyl benzyl alcohol, there are the problems of serious pollution of the production process, poor product quality and high production cost. In the present invention, cyclohexene and cumene hydroperoxide are used as raw materials, and a non-polar organic compound that is inert to the reaction system is used as a solvent, under mild reaction conditions, on a titanium-containing porous silica catalyst A redox reaction between cumene hydroperoxide and cyclohexene, in which cumene hydroperoxide is reduced to α,α-dimethylbenzyl alcohol, while cyclohexene is oxidized to 1,2-epoxy The technical scheme of cyclohexane solves this problem well, and can be used in the industrial production of simultaneously preparing 1,2-epoxycyclohexane and α,α-dimethylbenzyl alcohol.
Owner:CHINA PETROLEUM & CHEM CORP +1
Homogeneous molybdenum base epoxidation catalyst
ActiveCN103418434AImprove conversion rateHigh selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsState of artRoom temperature
The present invention relates to a homogeneous molybdenum base epoxidation catalyst, wherein problems of low cumene hydroperoxide conversion rate and low propylene oxide selectivity in the prior art are mainly solved with the present invention. The technical scheme comprises that: the homogeneous molybdenum base epoxidation catalyst preparation method comprises: a) dissolving a molybdenum source and an acid substance in a solvent to obtain a mixture I; b) adding an organic amine to the mixture I, heating to a temperature of 120-200 DEG C, and stirring for 1-24 h to obtain a mixture II; and c) adding a basic substance to the mixture II, and stirring for 0.5-12 h at a room temperature to obtain the homogeneous molybdenum base epoxidation catalyst. With the technical scheme, the problems in the prior art are mainly solved with the present invention, and the homogeneous molybdenum base epoxidation catalyst can be used for industrial production of propylene oxide production through cumene hydroperoxide and propylene epoxidation.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for the production of phenol and acetone
A method for the production of phenol and acetone from a cumene hydroperoxide mixture comprises a first stage and a second stage and at least two serially connected reactors, wherein the first stage comprises decomposition of a cumene hydroperoxide mixture in the presence of a catalyst mixture to form a dicumyl peroxide mixture, and the second stage comprises formation of a phenol and acetone mixture from decomposition of the dicumyl peroxide mixture formed in the first stage, wherein, the first stage further comprises: a) forming a catalyst mixture by combining sulfuric acid and phenol in a weight ratio of from 2:1 to 1:1000 in a catalyst formation reactor, b) holding the catalyst mixture in the catalyst formation reactor at a temperature of about 20 to 80° C. for about 1 to 600 minutes; and, c) adding the catalyst mixture to the cumene hydroperoxide mixture to form the phenol and acetone mixture. The proposed method permits a significant reduction in the yield of hydroxyacetone that causes deterioration in the quality of commercial phenol.
Owner:SABIC GLOBAL TECH BV
Strongly basic carbon nanotube composite resin and its preparation method
The invention relates to strongly basic carbon nanotube composite resin and its preparation method, and mainly aims to solve the problems of poor heat resistance and swelling resistance of ion exchange resin involved in previous technologies. The composite resin of the invention comprises: (1) 75-90% of a monomer; (2) 5-15% of a comonomer; (3) 0.1-5% of a nano-material; (4) 0.1-10% of an initiator. Specifically, the monomer is at least one of p-chloromethyl styrene, 4-(3-chloropropyl) styrene, 4-(3-bromopropyl) styrene, 4-(4-chlorobutyl) styrene, 4-(4-brombutyl) styrene, 4-(5-chloropentyl) styrene or 4-(5-bromopentyl) styrene; the comonomer is at least one of glycol dimethacrylate, 2-propenylbenzene, divinyl phenylmethane, and divinylbenzene;the nano-material is at least one of a multi-walled carbon nanotube, a single-walled carbon nanotube, C60 or C70 etc. fullerene; and the initiator is at least one of benzoyl peroxide, azodiisobutyronitrile, lauroyl peroxide, and cumene hydroperoxide. The composite resin and its preparation method of the invention well solves the above problems, and can be used in the industrial production of oxirane catalytic hydration.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com