Method for preparing isopropyl benzene hydrogen peroxide by catalytically oxidizing isopropyl benzene
A technology of cumene hydroperoxide and cumene, which is applied to the preparation of peroxygen compounds, chemical instruments and methods, and the preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
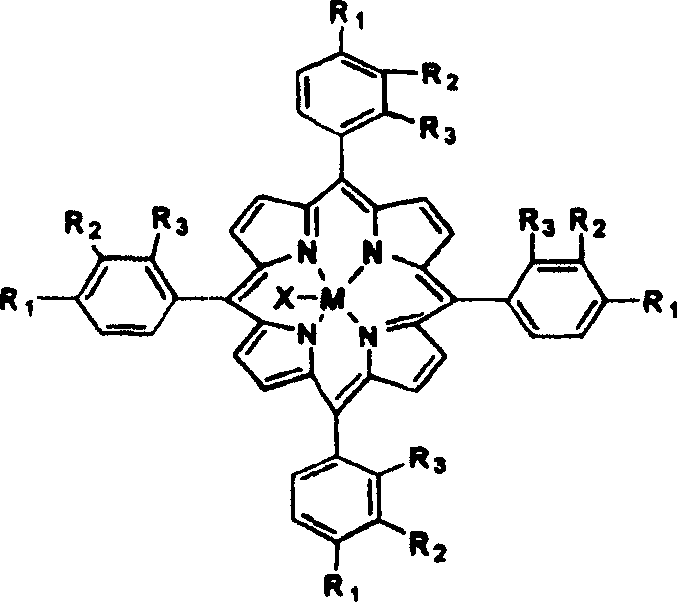
[0012] 1 mg of metalloporphyrins with general formula (1) structure, R 1 = Cl, R 2 = R 3 =H, M=Mn, X=Cl, add into 150ml of cumene, and feed air with an absolute pressure of 0.1MPa at a flow rate of 600ml / min. The reaction was stirred at 110°C for 4 hours, the yield of cumene hydroperoxide was 24.9%, and the selectivity was 94.5%. However, in the non-catalyzed oxidation process without metal porphyrin under this condition, the yield of cumene hydroperoxide is only 2.4%, and the selectivity is 96.1%.
Embodiment 2
[0014] With 2mg of metalloporphyrins with general formula (1) structure, R 1 = Cl, R 2 = R 3 =H, M=Fe, X=Cl, add into 300ml of cumene, and feed air with an absolute pressure of 0.1MPa at a flow rate of 500ml / min. The reaction was stirred at 110° C. for 4 hours, the yield of cumene hydroperoxide was 20.6%, and the selectivity was 96.0%. In the non-catalyzed oxidation process without adding metal porphyrins under this condition, the yield of cumene hydroperoxide is only 2.2%, and the selectivity is 96.5%.
Embodiment 3
[0016] The metalloporphyrin that 5mg has general formula (1) structure is dissolved in the 150ml cumene, R 1 = Cl, R 2 = R 3 =H, M=Mn, feed the air with absolute pressure of 0.1MPa with 600ml / min gas velocity, reactant 3 hours under 120 ℃, cumene hydroperoxide productive rate is 38.85%, and selectivity is 90.5%. However, in the non-catalyzed oxidation process without adding metal porphyrins under this condition, the yield of cumene hydroperoxide is only 2.9%, and the selectivity is 95.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com