Method for the decomposition of cumene hydroperoxide
a technology of cumene hydroperoxide and cumene hydroperoxide, which is applied in the field of cumene hydroperoxide decomposition, can solve the problems of increasing complexity in both equipment count and control, reducing the yield of cumene hydroperoxide, so as to maximize the yield of dicumyl peroxide and minimize residual dicumyl peroxide , the effect of maximizing the overall di
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
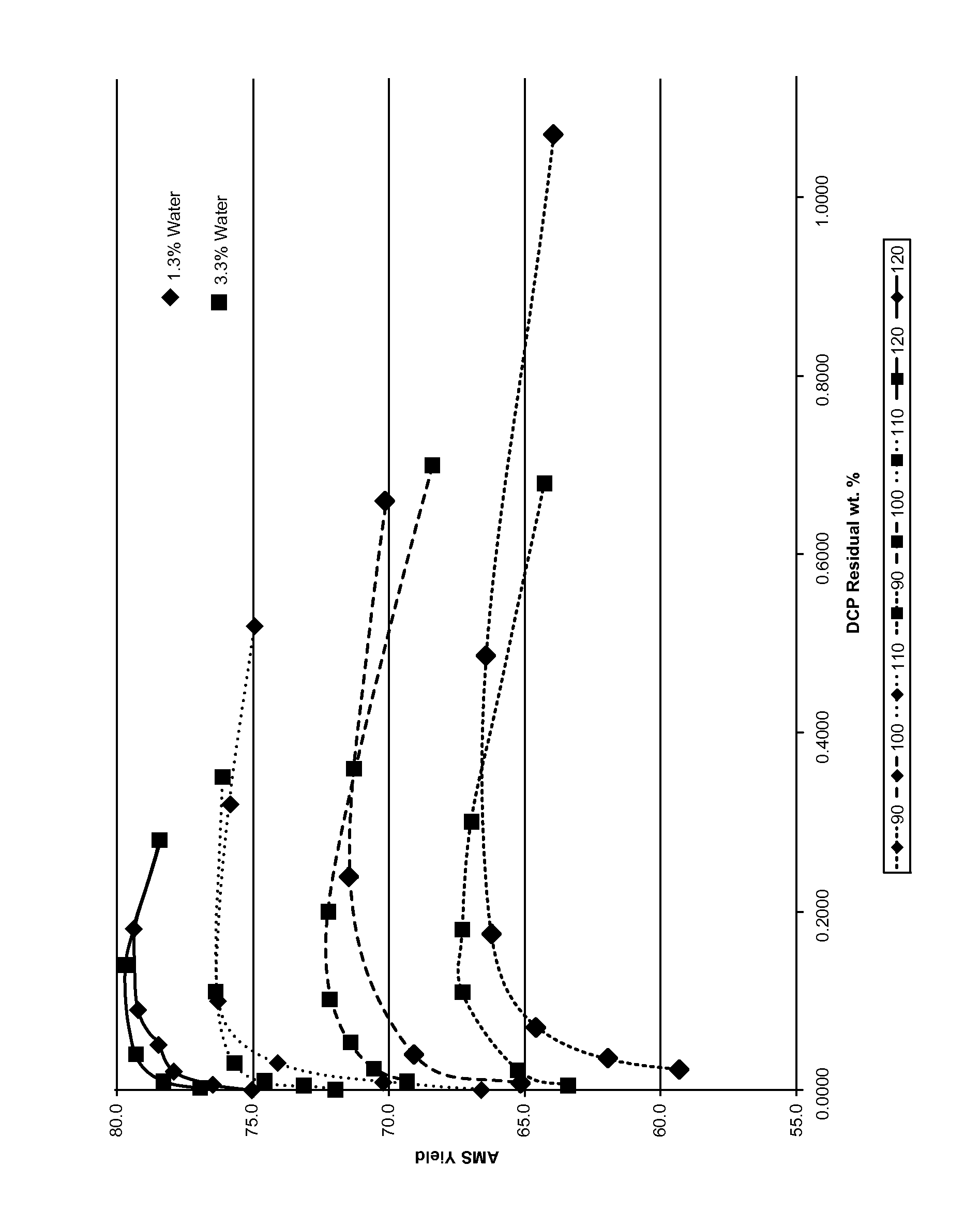
[0012]The results in FIG. 1 were generated using a well stirred glass reactor that was charged with 15 ml of a solution of 1 / 1 molar phenol / acetone spiked with approximately 8.2% DCP, 1.3% DMBA, 1.4% AMS, 12% cumene, and sufficient water added to give either 1.3% or 3.3% water content. The solution was brought to target temperature, and 8 μL of 0.5 molar sulfuric acid added (approximately 25 ppm in the bulk reaction) to start the reaction. Samples were taken at various times, neutralized with a small amount of base, and analyzed for a complete component profile.
example 2
[0013]A CHP-containing stream with 80% CHP, 3.6% DMBA, 0.4% acetophenone (AP), and the residual cumene, was fed to the back mixed first stage of a commercial CHP decomposer operating under conditions of vigorous boiling at 550-600 mm Hg pressure, 78-80° C., a 1.25-1.35 mole ratio of acetone to CHP, 5-6 minute residence time, 300-350 ppm of sulfuric acid, and 1.0-1.3 wt. % water under optimum conditions. With no additional water added ahead of a plug flow second stage with 0.8-1.0 minutes of residence time, an average AMS yield of 80.8% was obtained at 108 C, with 0.09 to 0.12 wt. % DCP, and 0.16 to 0.18% DMBA residuals exiting the second stage.
example 3
[0014]Conditions were as in example 2 with 1.5 wt. % additional water added ahead of the second stage. An optimal average AMS yield of 82.1% was obtained at 122° C. with 0.02 to 0.04% DCP, and 0.16 to 0.18 wt. % DMBA exiting the second stage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com