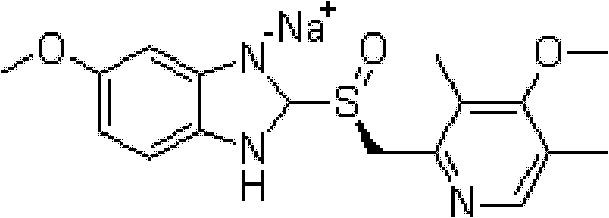
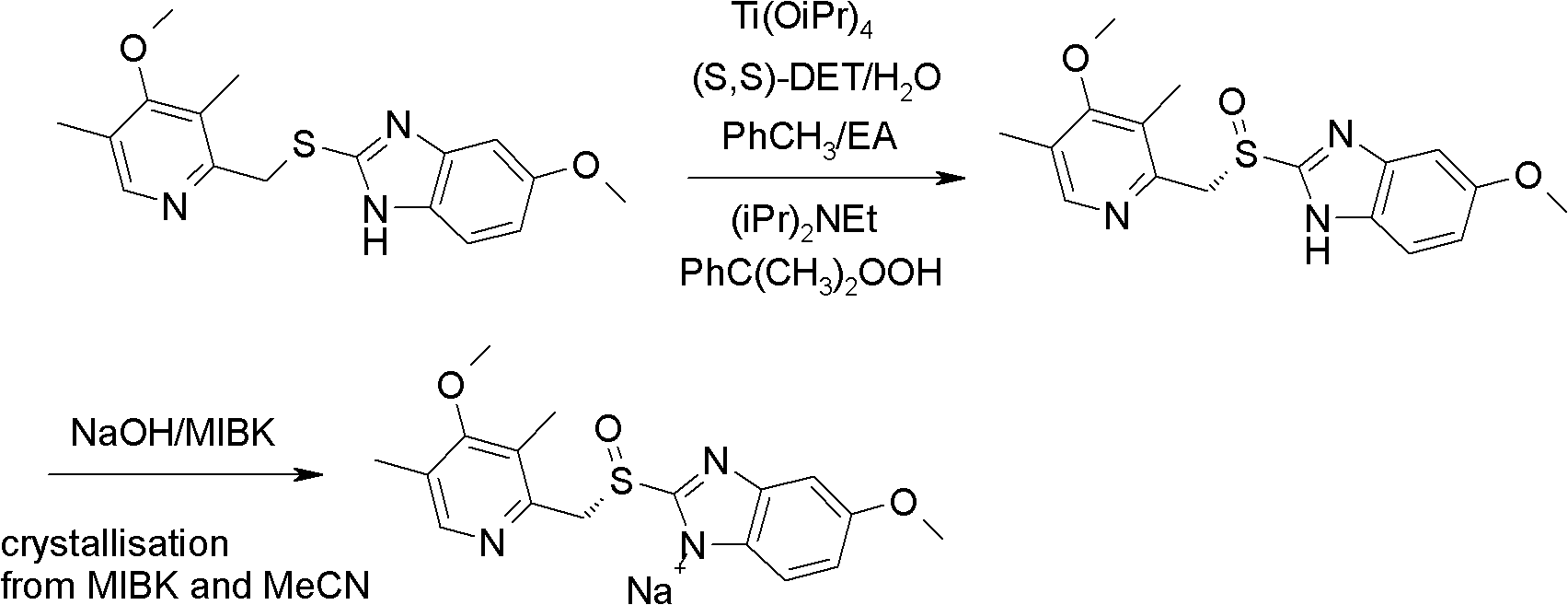
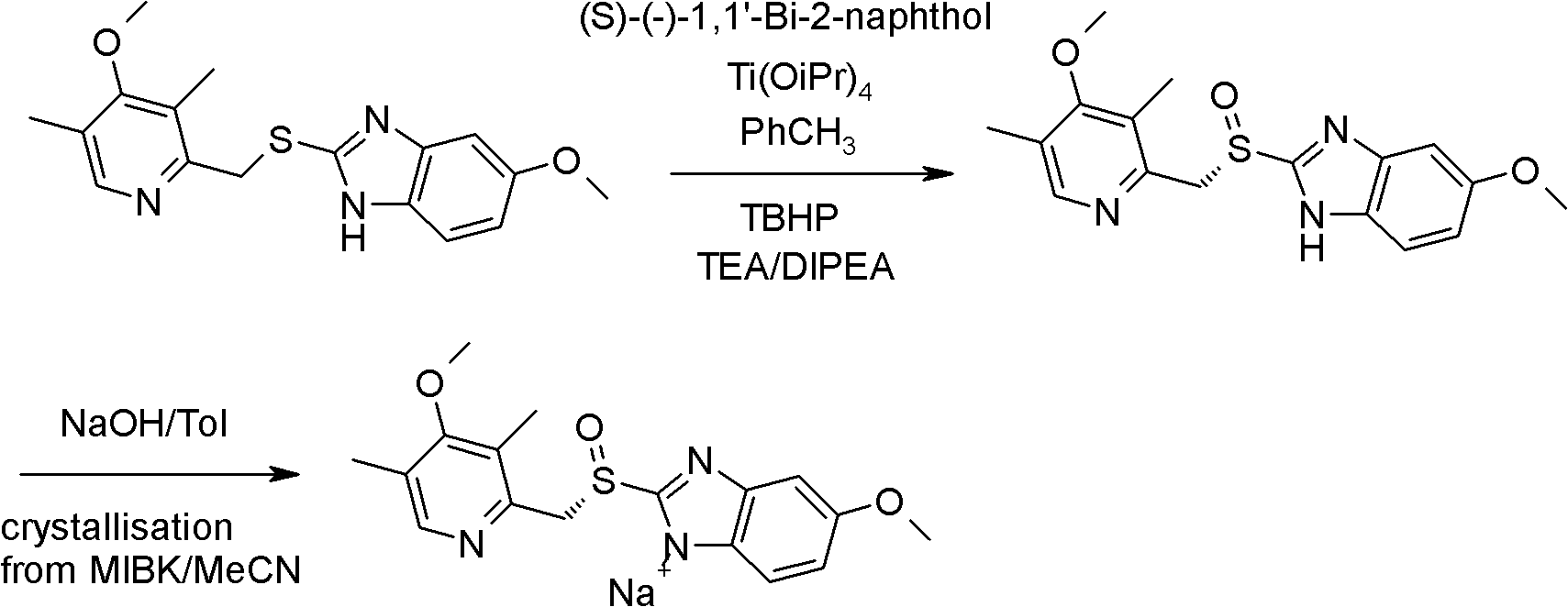
Industrial production method of high-purity esomeprazole sodium
A technology for esomeprazole sodium and a production method is applied in the field of high-purity esomeprazole sodium, can solve the problems of taking a long time, cannot be separated, and is difficult to industrially scale up production, and achieves high economic value and social benefit. , The oxidation reaction time is short, and the effect of reducing gastric acid secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of esomeprazole sodium
[0049] Add 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio-1H-benzimidazole 16g (48.6mmol) and 100mL (86.6g) toluene, stir to dissolve, add 0.15mL (8.1mmol) of water, 10g (48.6mmol) of D-diethyl tartrate and 6.9g (24.3mmol) of titanium isopropoxide, and keep the reaction at 50°C for about 1h. Add potassium carbonate 3.35g (24.3mmol), cool to 20°C, add cumene hydroperoxide 8.7g (content 85%, 48.6mmol), react at 20°C and monitor the reaction liquid by HPLC, react for about 2 hours, raw material 5 -Methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio-1H-benzimidazole basic reaction is completed (less than 2% raw material);
[0050] Add 20ml of methanol (15.8g) to the above reaction solution, stir for 10 minutes, filter the insoluble matter, add ammonia water to the filtrate to extract the organic layer (60ml*4), adjust the pH of the obtained ammonia solution to about 8 with acetic acid, and use ethyl acetate Extracted, then...
Embodiment 2
[0055] Preparation of esomeprazole sodium
[0056] Add 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio-1H-benzimidazole 500g (1.52mol) and 4.5 kg toluene, stir to dissolve, add water 5.5mL (0.31mol), D-diethyl tartrate 313g (1.52mol) and titanium isopropoxide 217.5g (0.76mol), keep the reaction at 50°C for about 1h, add potassium carbonate 4.2g (0.03mol), cooled to 5°C, added cumene hydroperoxide 272g (content 85%, 1.52mol), reacted at 5°C and monitored the reaction liquid by HPLC, reacted for about 2 hours, raw material 5-methoxy -2-(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio-1H-benzimidazole basic reaction is completed (less than 2% raw material);
[0057] Add 475ml (375g) of methanol to the above reaction solution, stir for 15 minutes, filter the insoluble matter, add ammonia water to the filtrate to extract the organic layer (2000ml*4), adjust the pH of the obtained ammonia solution to about 7.5 with acetic acid, and extract with ethyl acetate , and then dried,...
Embodiment 3
[0062] Preparation of esomeprazole sodium (with reference to Chinese patent CN1070489)
[0063] Add 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio-1H-benzimidazole 500g (1.52mol) and 4L to the reaction flask Heat toluene to 54°C, stir to dissolve, add 3.55mL (0.2mol) of water, 190g (0.92mol) of D-diethyl tartrate and 129g (0.45mol) of titanium isopropoxide, and keep the reaction at 54°C for about 50 minutes , cooled to 10°C, and then 58g (0.45mol) of diisopropylethylamine was added to the solution, 272g (content 85%, 1.52mol) of cumene hydroperoxide was added, and the reaction was monitored by HPLC at 10°C. React about 3 hours, raw material 5-methoxyl group-2-(4-methoxyl group-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole basic reaction finishes (raw material 2 %the following);
[0064] Add ammonia water to the above reaction solution to extract the organic layer (1600ml*3), add 2000ml methyl isobutyl ketone to the obtained ammonia solution, adjust the pH to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com