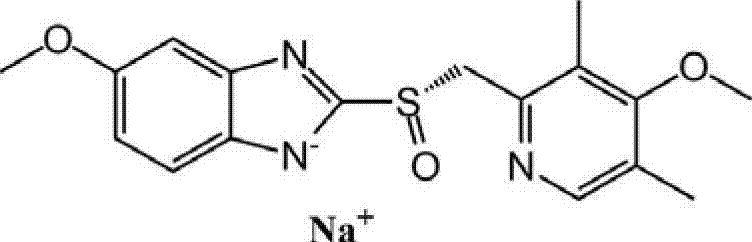
Method for synthesizing esomeprazole sodium
A technology for esomeprazole sodium and a production method is applied in the field of drug synthesis, can solve the problems of high raw material cost, harsh operating conditions, low purity and the like, and achieves the effects of reducing production cost, easily controlling temperature, and simplifying operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
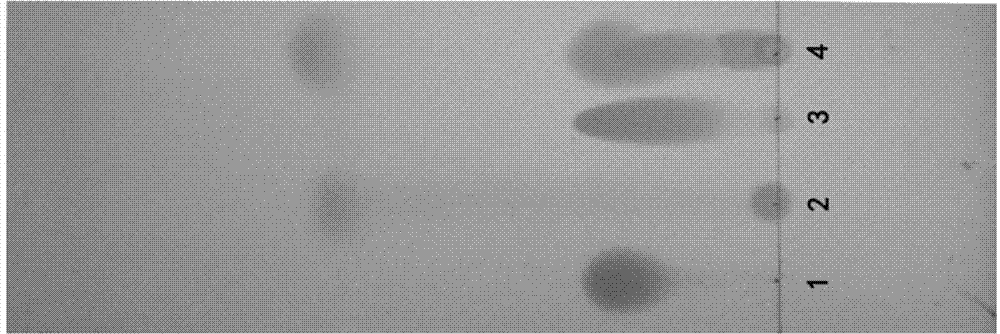
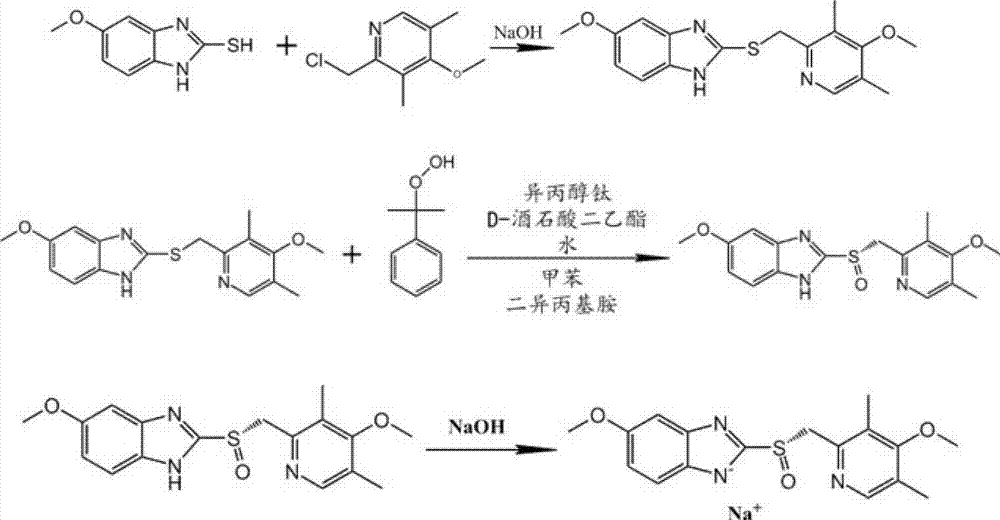
[0054] Reduce to 25°C for suction filtration, and the filtrate (red-brown) was evaporated under reduced pressure at 45°C to remove about 1 / 2 of the methanol (with solid precipitation), and then extracted with 12L of water and 12L of dichloromethane (boiling point 39.8). The aqueous layer was extracted once more with 8 L of dichloromethane. The combined dichloromethane layers were washed with 5 L of water and 5 L of saturated brine, dried with 2000 g of anhydrous sodium sulfate for 2 hours, filtered with suction, and the filter cake was washed with dichloromethane. Dichloromethane was distilled off under reduced pressure to obtain an oil. Heat the oily product with 10L of acetonitrile (boiling point 81.1°C) at 85°C for 30 minutes, then cool and stir to crystallize naturally (overnight, at least 3.5 hours), then suction filter (centrifugal filtration), wash the filter cake (filter residue) with 2L of acetonitrile, vacuum (drum) at 30°C wind) drying (over 4 hours) to obtain 38...
Embodiment 2
[0063] Add 1568g esomeprazole intermediate (5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H- in 20L reactor benzimidazole), then slowly stirred and dissolved with 6.4L toluene, and then added 600g D-(-) diethyl tartrate after heating up to 50°C-55°C, then added 40ml purified water and stirred for 0.5 hours, then added 410g titanium isopropoxide , continue stirring for 50min. Cool down to 25±2°C, add 190g of diisopropylamine and stir for 5min, add dropwise 820g of cumene hydroperoxide with a mass concentration of 80% (the temperature is controlled at 25±2°C during the dropwise addition). After the dropping, stir and react at 25±2°C for 3 hours, and check by TLC every hour. After the reaction was completed, 3 L of concentrated ammonia water and 3 L of purified water were added to the reaction liquid, stirred for 30 min, and then the ammonia water layer was extracted. The reaction solution was then extracted once with 3L of concentrated ammonia water and 3L of puri...
Embodiment 3
[0065] Add 1568g esomeprazole intermediate (5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H- in 20L reactor benzimidazole), then slowly stirred and dissolved with 6.4L toluene, and then added 600g D-(-) diethyl tartrate after heating up to 50°C-55°C, then added 40ml purified water and stirred for 0.5 hours, then added 410g titanium isopropoxide , continue stirring for 50min. Cool down to 20°C, add 190g of diisopropylamine and stir for 5 minutes, then add 820g of cumene hydroperoxide with a mass concentration of 80% dropwise (the temperature is controlled at 20±2°C during the dropwise addition). After dropping, stir and react at 20±2°C for 5 hours. After the reaction was completed, 1362.3 g of the crude product was obtained after extraction, salt formation, concentration, washing, and vacuum drying, with a yield of 77.2% and an enantiomeric purity of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com