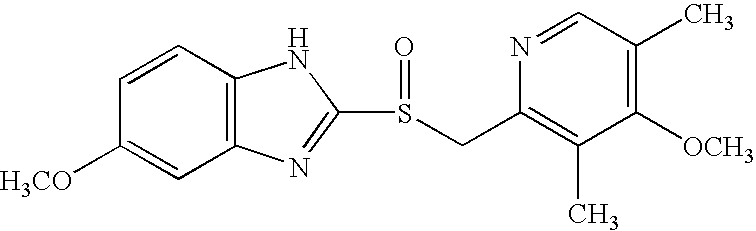
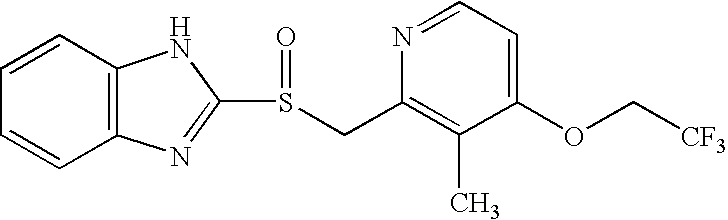
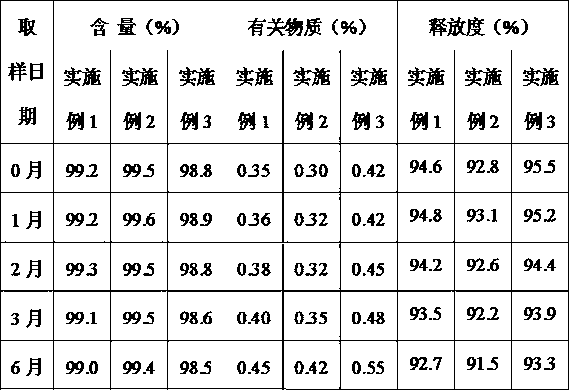
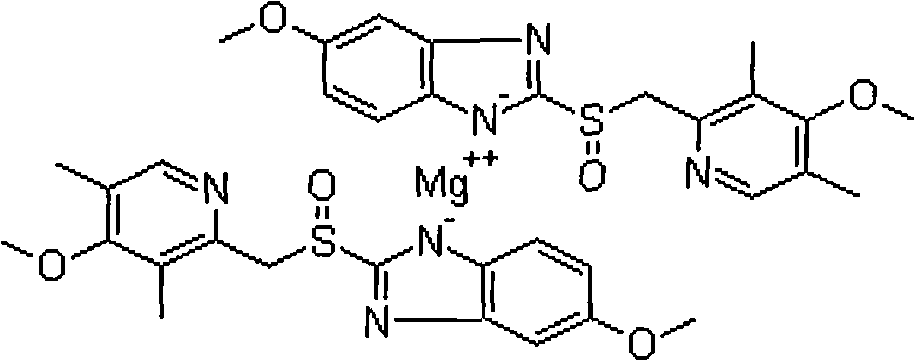
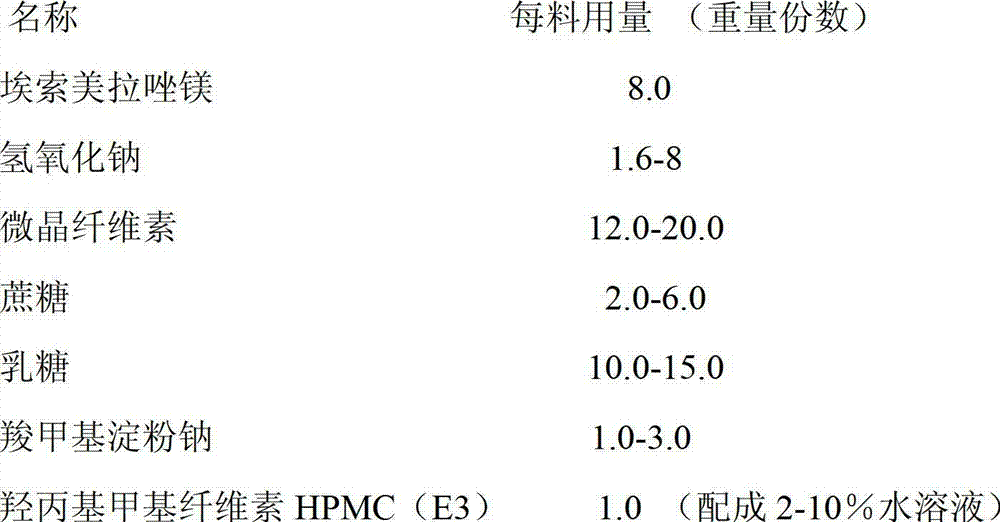
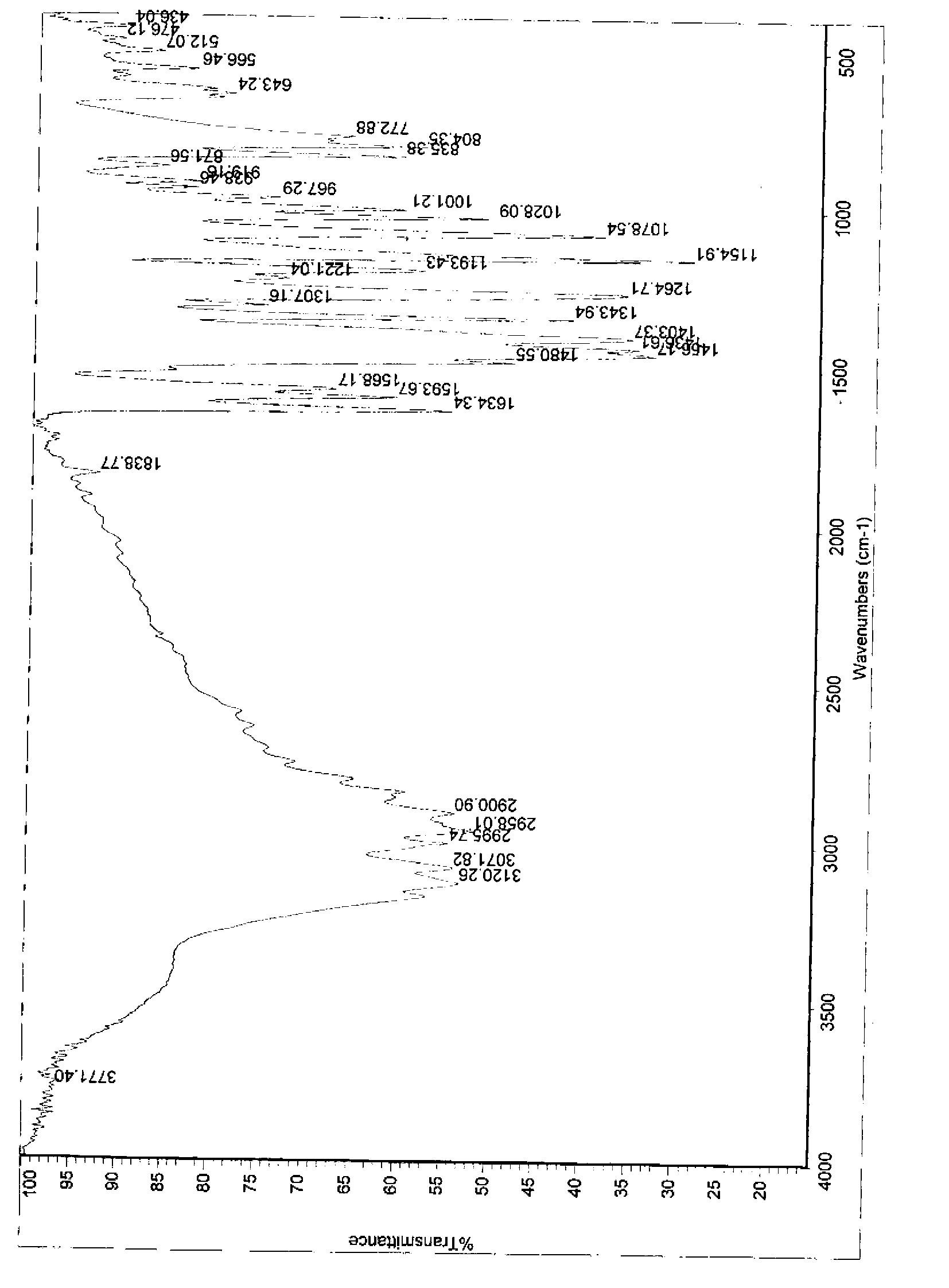
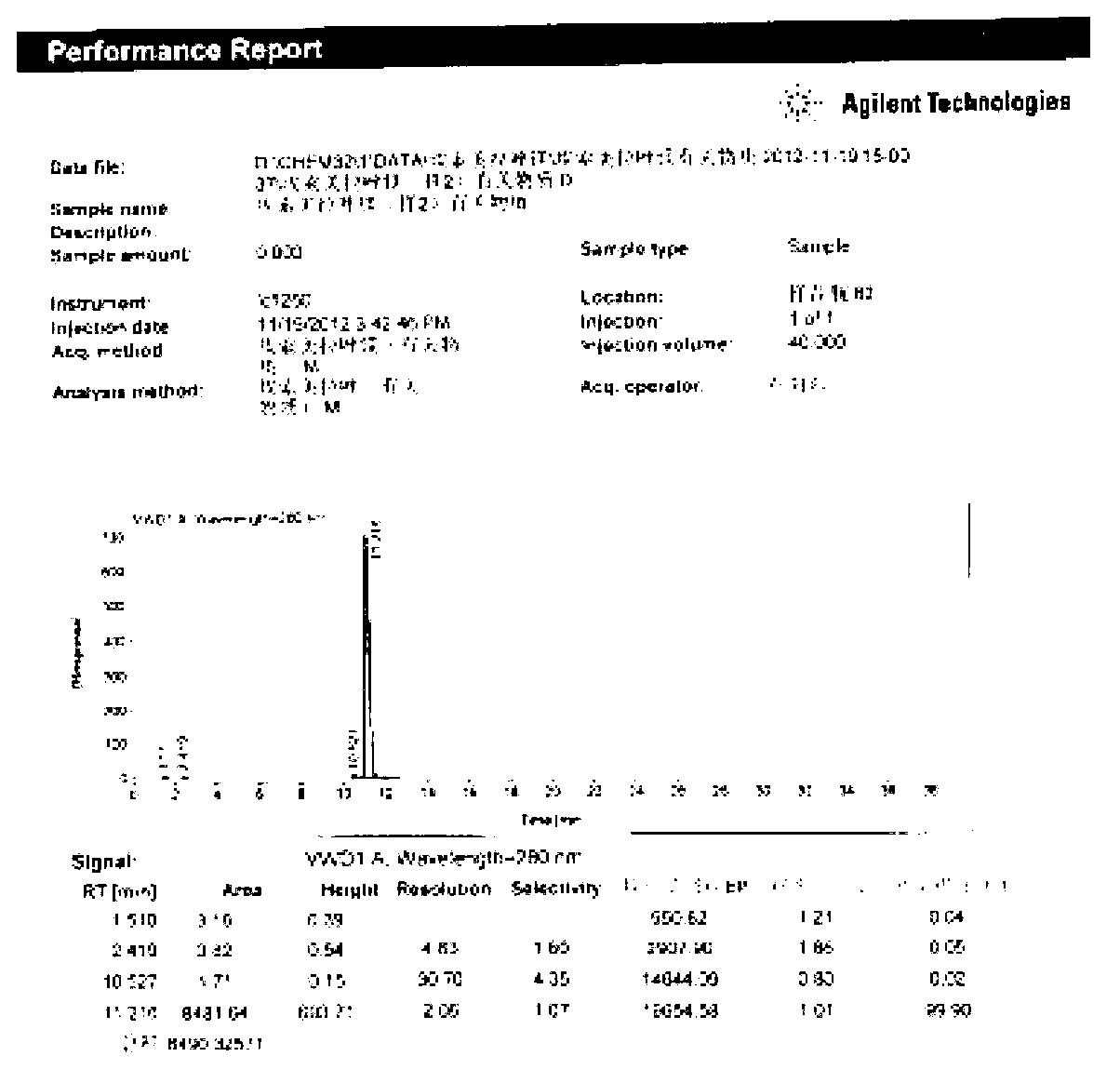
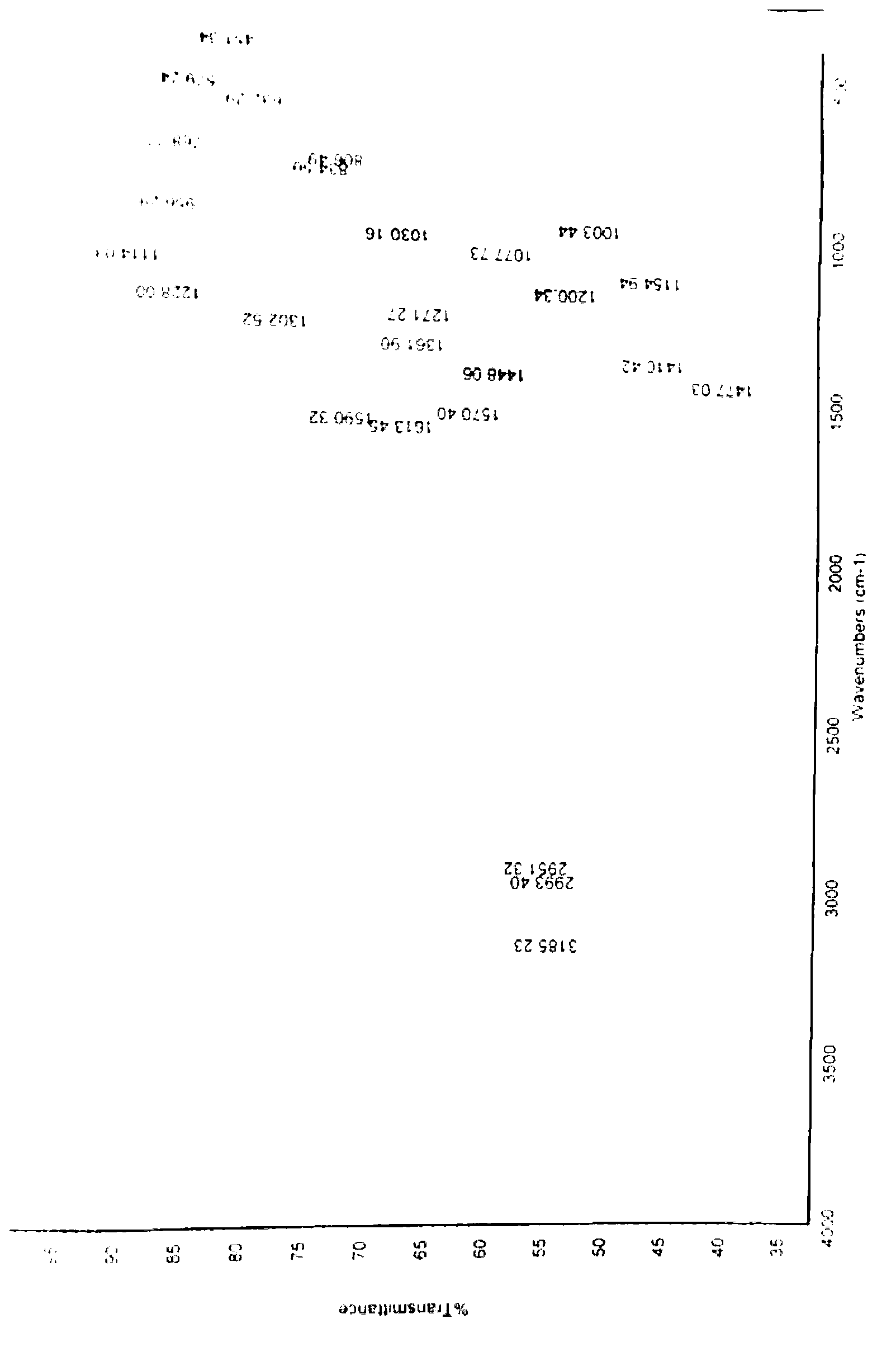
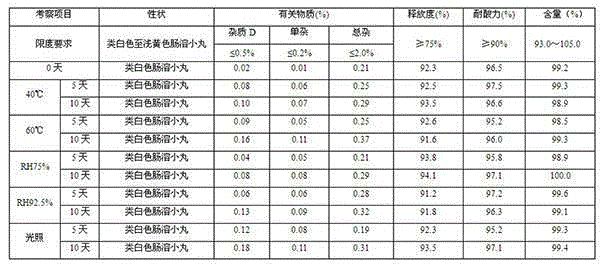
Patents
Literature
105 results about "Esomeprazole Magnesium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
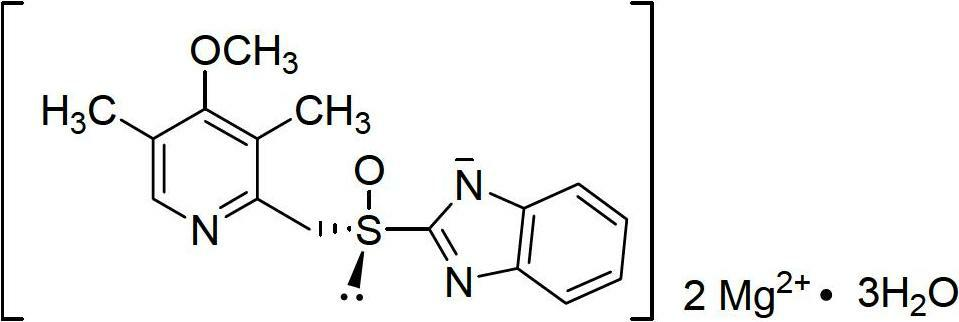
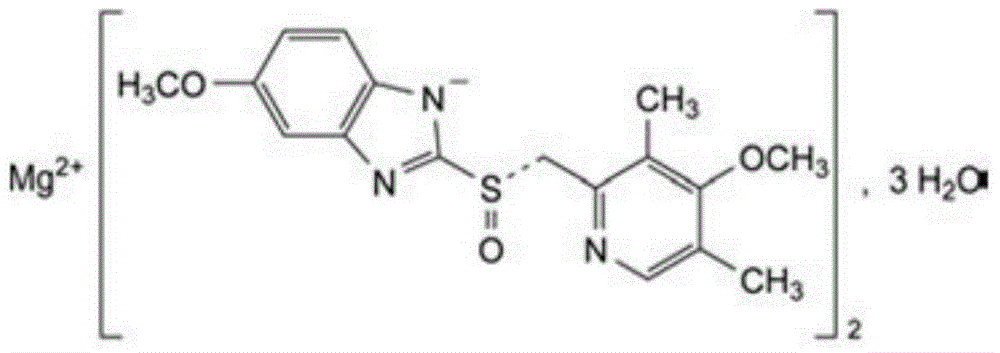
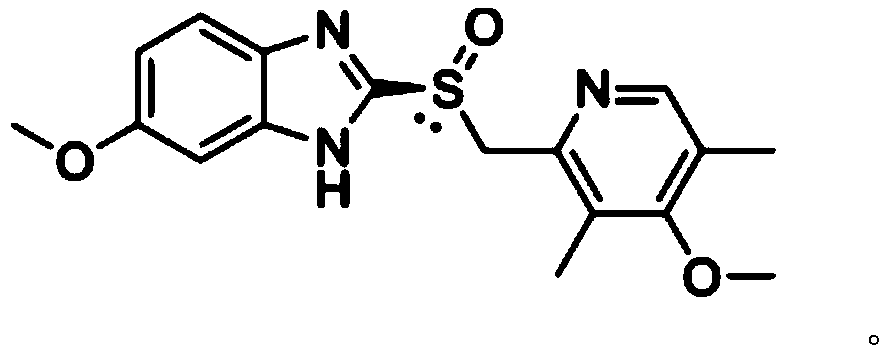
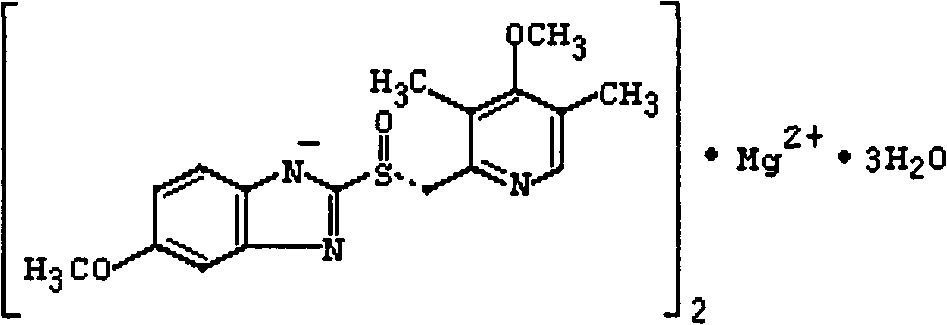
The magnesium salt of esomeprazole, the S-isomer of omeprazole, with gastric proton pump inhibitor activity. In the acidic compartment of parietal cells, esomeprazole is protonated and converted into the active achiral sulphenamide; the active sulphenamide forms one or more covalent disulfide bonds with the proton pump hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase), thereby inhibiting its activity and the parietal cell secretion of H+ ions into the gastric lumen, the final step in gastric acid production. H+/K+ ATPase is an integral membrane protein of the gastric parietal cell.
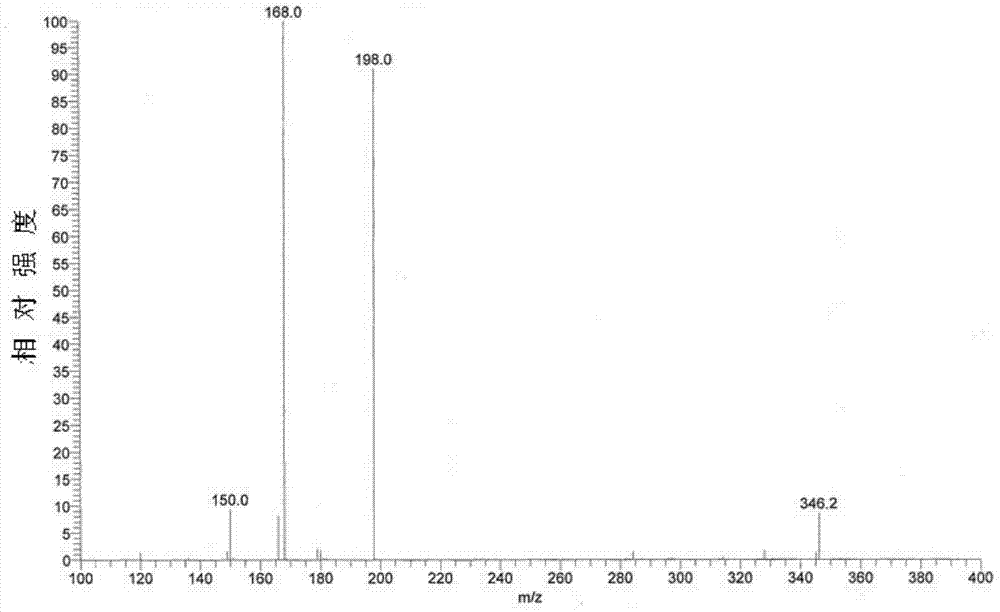
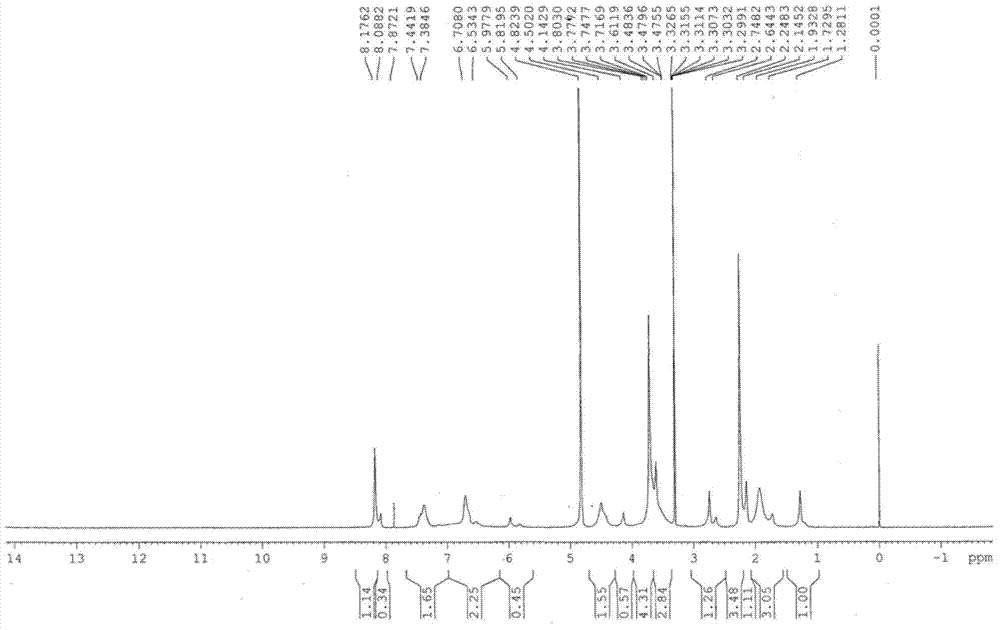
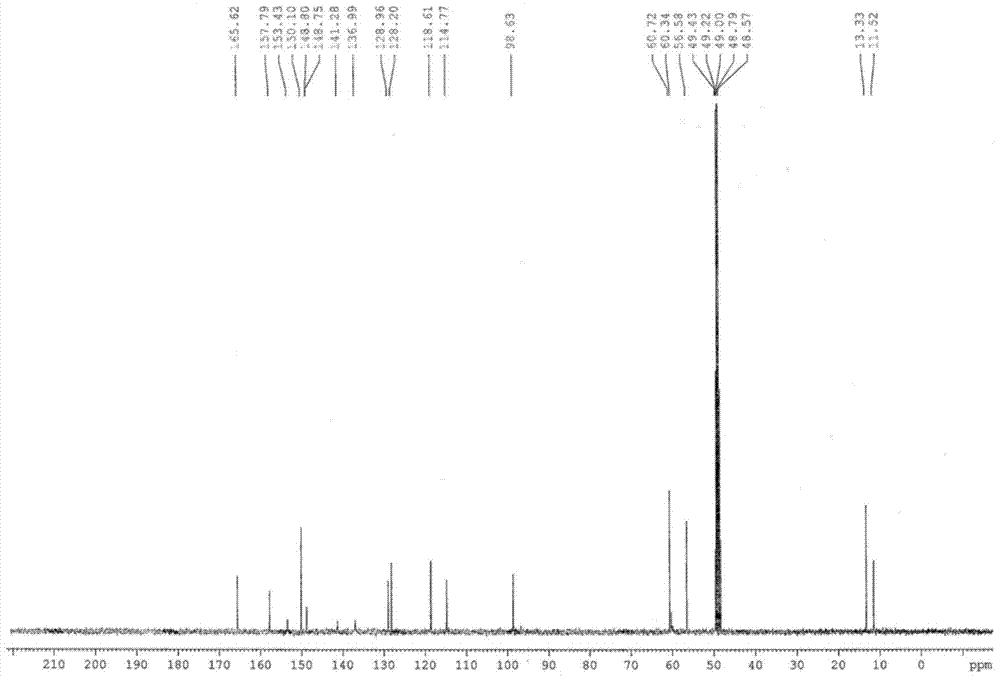
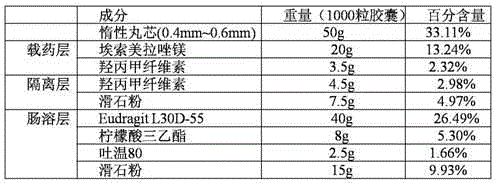
Esomeprazole magnesium enteric-coated pellet and preparation method thereof
ActiveCN102670521AProtect active ingredientsFix stability issuesOrganic active ingredientsDigestive systemMedicineIsolation layer
The invention belongs to the field of pharmacy and relates to a medicinal preparation taking esomeprazole magnesium as an active ingredient, in particular to an esomeprazole magnesium enteric-coated pellet and a preparation method thereof. According to the esomeprazole magnesium enteric-coated pellet, medicines can be rapidly released in intestinal tracts. The esomeprazole magnesium enteric-coated pellet comprises the following structural layers in sequence from inside to outside: a medicine-contained layer, an isolation layer and an enteric-coated layer.
Owner:珠海润都制药股份有限公司
Stable pharmaceutical formulations of benzimidazole compounds
Provided are stable pharmaceutical formulations of benzimidazole compounds, particularly esomeprazole magnesium, and processes for their preparation.
Owner:TEVA PHARM USA INC
Esomeprazole magnesium contained enteric-coated tablet and preparation method thereof
ActiveCN102940611AHigh safety complianceImprove stabilityOrganic active ingredientsDigestive systemCoated tabletsInsulation layer
The invention provides an esomeprazole magnesium contained enteric-coated tablet and a preparation method thereof. The enteric coated tablet is composed of an inner tablet core layer which uses esomeprazole as an active ingredient, an intermediate insulation layer and an enteric coating protection layer. According to the esomeprazole magnesium contained enteric-coated tablet, the conditions that basic remedies are instable and prone to be oxidized and decomposed are overcome, the prepared enteric-coated tablet is even in coating, compact in coating layer, stable and reliable in quality and capable of meeting large-scale production requirements of enterprises at the present stage and has good market development prospects, the releasing rate can reach over 90%, the bioavailability is obviously improved, and safe and effective drug using is guaranteed.
Owner:KAMP PHARMA
Esomeprazole magnesium sustained-release dropping pill and preparation method thereof
InactiveCN101269037ASubstance content changeSuitable for useOrganic active ingredientsDigestive systemDiseaseReflux esophagitis
The invention discloses a pharmaceutical preparation used for treating erosive reflux oesophagitis, a gastroesophageal reflux disease, maintaining a long-term therapy to prevent the recurrence of oesophagitis for a cured patient, controlling the symptoms of gastroesophageal reflux diseases, eradicating helicobacter pylori jointly medicated with an appropriate antimicrobial therapy, healing duodenal ulcer related to helicobacter pylori infections and preventing the recurrence of peptic ulcer related to helicobacter pylori, and particularly relates to a prepared sustained-release oral formulation adopting esomeprazole magnesium as the ingredient. The pharmaceutical preparation aims to supplement the deficiency of the prior art and provide a sustained-release esomeprazole magnesium dropping pill. The sustained-release esomeprazole magnesium dropping pill overcomes the defects in the prior art effectively, guarantees no occurrence of an obvious quality change for the drug during the effective storage period and has the advantages of controllable release time, full release and high bioavailability simultaneously.
Owner:北京博智绿洲医药科技有限公司
Granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablet
ActiveCN103040774AGuaranteed insolublePromote dissolutionOrganic active ingredientsPill deliveryEnteric-coated granulesEsomeprazole Sodium
The invention provides a granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablets. The granulating and coating process comprises the following steps of: firstly preparing esomeprazole magnesium granules; then preparing esomeprazole magnesium enteric-coated granules sequentially through isolating layer coating and enteric-coated layer coating; and finally blending adjuvants with the esomeprazole magnesium enteric-coated granules and tabletting to prepare the esomeprazole magnesium enteric-coated tablets. An appropriate granulating method comprises the following steps of: crushing and grinding the esomeprazole magnesium and the adjuvants into powder, and then uniformly mixing; mixing with an adhesive to obtain a water solution, stirring for 8-10 minutes in a wet type granulator to prepare appropriate granules; drying at 40-45 DEG C, and screening to obtain the granules with grain size being between 40 meshes and 80 meshes. According to the process including granulating and coating of raw materials, the enteric-coated granules are prepared firstly and then blended with the adjuvants and finally tabletting is carried out, pellets are not used, the content uniformity of the prepared esomeprazole magnesium enteric-coated tablet product is greatly enhanced, and the problem of unqualified uniformity of the product content caused by excessive material flowability in an original process is solved.
Owner:SHANGHAI SINE WANXIANG PHARMA
Esomeprazole magnesium injection liquid
InactiveCN101513387AConvenient for clinical operationImprove bioavailabilityOrganic active ingredientsDigestive systemPatient needReflux
The invention discloses an esomeprazole magnesium injection liquid capable of treating gastroesophageal reflux disease. At present, esomeprazole magnesium in the market is only tablets, and the patient needs to take the esomeprazole magnesium for 1 to 3 times per day; and because the dosage taken by the patient per day is large and the frequency is high, side effects generated by the medicine are large, too. In order to solve the problems, the invention aims to provides the esomeprazole magnesium injection liquid with convenient clinical use, high bioavailability and low price, which can make the medicine quickly reach effective treatment concentration in vivo through direct intravenous injection or intramuscular injection, reduce first-pass effect of the medicament in liver and improve the bioavailability of the medicament in vivo. The esomeprazole magnesium injection liquid provided by the invention mainly comprises the esomeprazole magnesium or pharmaceutically acceptable salts thereof and solvent for injection; and 1 ml of the injection liquid comprises 10 to 200 mg of the esomeprazole magnesium or the pharmaceutically acceptable salts thereof, and the pH of the injection liquid is between 3.0 and 8.0.
Owner:李铁军
Preparation method of esomeprazole magnesium
InactiveCN103936714AHigh yieldMild reaction conditionsOrganic chemistryEsomeprazole Sodium4-methoxypyridine
The invention discloses a preparation and refinement method of esomeprazole magnesium. The preparation and refinement method comprises the following steps: condensing 2-chloromethyl-3, 5-dimethyl-4-methoxyl pyridine hydrochloride and 2-sulfydryl-5-methoxy-benzimidazole serving as starting materials, carrying out improved sharpless asymmetric oxidation so as to prepare esomeprazole sodium, then carrying out salt displacement so as to prepare esomeprazole magnesium, and finally refining to obtain high-purity esomeprazole magnesium. The preparation method is mild in reaction conditions, simple to operate, good in repeatability and high in yield and facilitates industrial production. The chromatographic purity of esomeprazole magnesium prepared by the method is above 99.8%; the optical purity of esomeprazole magnesium reaches above 99.6%; esomeprazole magnesium is stable in morphology and can meet medicinal requirements.
Owner:北京华禧联合科技发展有限公司
Preparation method for esomeprazole magnesium trihydrate
The invention discloses a preparation method for esomeprazole magnesium trihydrate, and the preparation method comprises the following steps: 1) taking omeprazole sulfide, then adding a chiral ligand, a catalyst and an organic solvent, heating and mixing for reaction, so as to form a chiral omeprazole sulfide compound; 2) adding an inorganic oxidant for oxidation reaction, and oxidizing omeprazole sulfide into esomeprazole; 3) adding inorganic base aqueous solution, extracting so as to enable the esomeprazole, obtained in the step 2), to form an esomeprazole inorganic salt, and dissolving the esomeprazole inorganic salt into the inorganic base aqueous solution layer; 4) adding inorganic magnesium salt into the inorganic base aqueous solution layer, stirring for reaction, and then carrying out centrifugation and drying, thus obtaining the esomeprazole magnesium trihydrate. The preparation method has the advantages of being high in product purity, high in yield, simple in technique, high-efficiency, environment-friendly, low in cost and the like.
Owner:珠海润都制药股份有限公司
Esomeprazole enteric-coated tablets and preparation method thereof
The invention discloses esomeprazole enteric-coated tablets and a preparation method thereof. The formula of the tables comprises esomeprazole magnesium, and an inert pellet core, a binder, a dispersant and a disintegrant which are used for tablet preparation. The preparation method comprises spraying and coating esomeprazole magnesium on the blank pellet core, coating an isolating layer and an enteric coated layer, making into enteric-coated pellets, and making into the required enteric-coated preparation by using the specific tablet adjuvants and tabletting method. The esomeprazole enteric-coated tablets and the preparation method thereof disclosed by the invention have the characteristics that by adopting specific tablet preparation method, the shortcoming that the enteric coated layers of the enteric-coated pellets are easy to be broken during tabletting process to affect the acid resistance of esomeprazole enteric-coated tablets is effectively overcome.
Owner:北京华禧联合科技发展有限公司
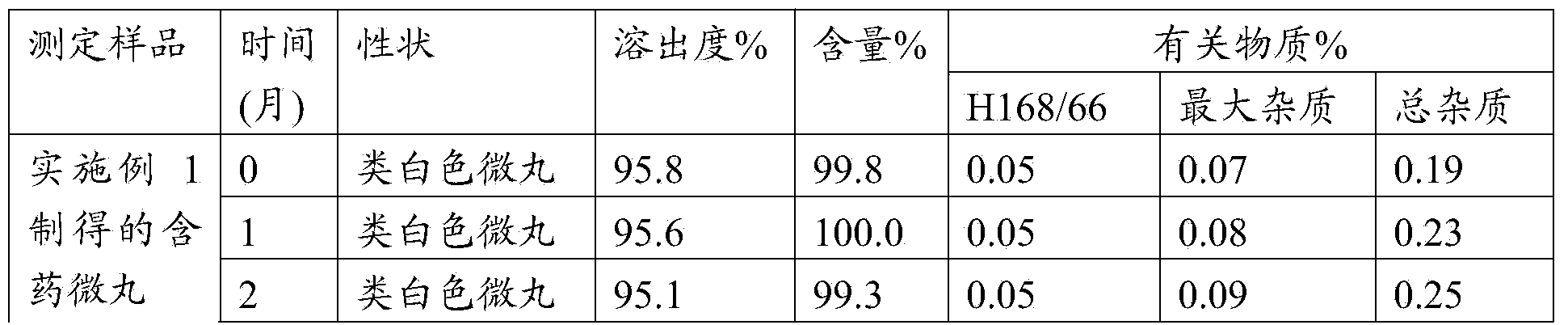
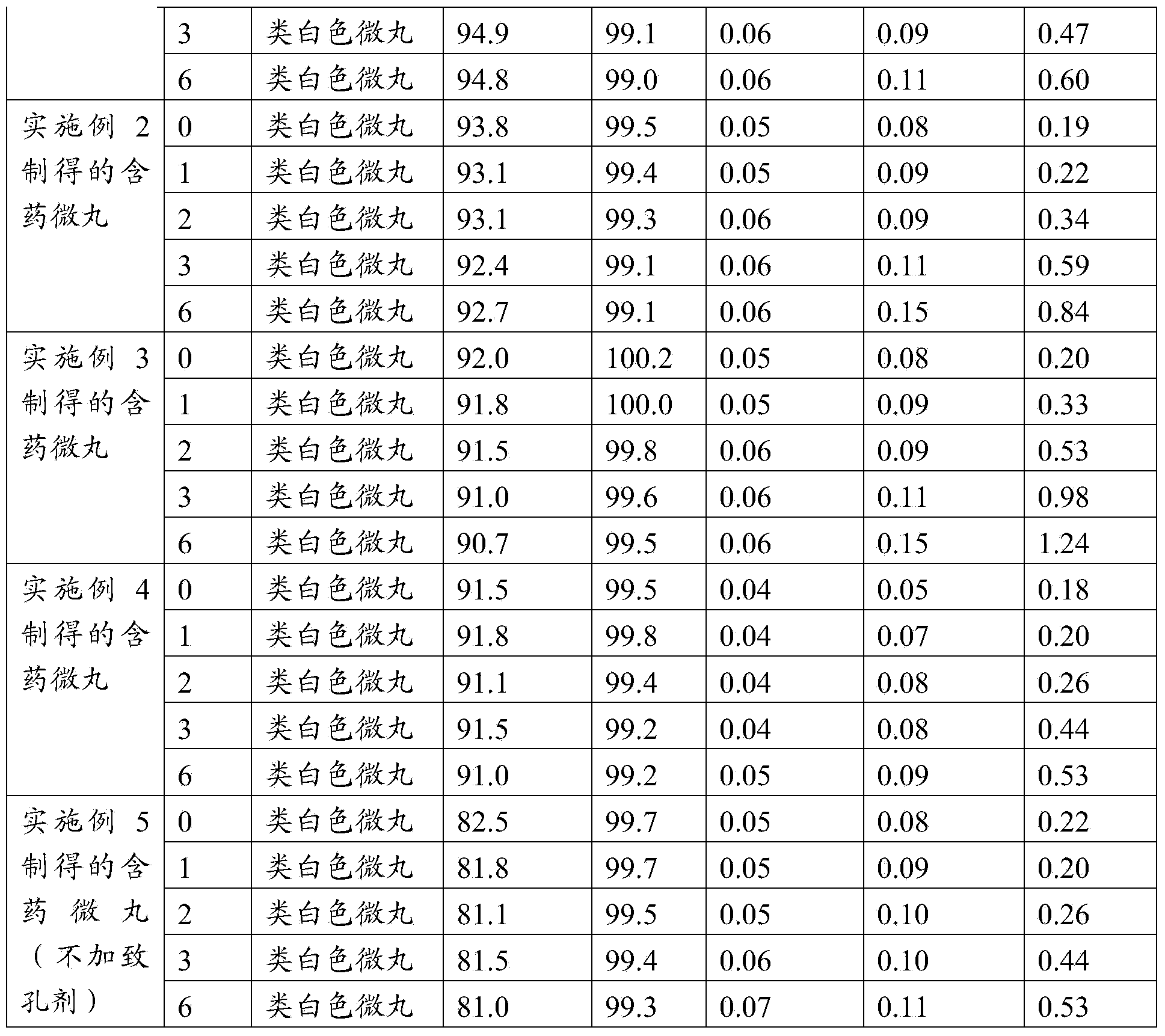
Enteric-coated micropellet containing esomeprazole magnesium
ActiveCN104414978APrevent Mutual AggregationAvoid being sucked by the wall of the fluidized cylinderOrganic active ingredientsDigestive systemMethacrylic acid-ethyl acrylate copolymerStearic acid
The invention relates to an enteric-coated micropellet containing esomeprazole magnesium. The enteric-coated micropellet comprises an inert pellet core, a medicament-loaded layer, an isolation layer and an enteric-coated layer, wherein the enteric-coated layer contains a methacrylic acid / ethyl acrylate 1: 1 copolymer, glyceryl monostearate, Tween 80 and talcum powder, wherein the methacrylic acid / ethyl acrylate 1: 1 copolymer is preferably Eudragit L30D-55. The enteric-coated micropellet containing esomeprazole magnesium, provided by the invention, can give consideration to acid resistance, release rate and stability of a product for a long time in the placement process, can meet or exceed existing preparation quality standards; and furthermore, the enteric-coated micropellet is prepared by simple process steps, is suitable for a relatively large pellet core (0.3mm-1mm), has the characteristic of low production cost, and is more suitable for large-scale industrialized production.
Owner:SICHUAN GOWELL PHARMA
Esomeprazole-containing medicinal composition
InactiveCN102397277AQuickly neutralizes stomach acidProlong the action timeOrganic active ingredientsDigestive systemBlood concentrationBlood drug concentration
The invention discloses an esomeprazole-containing medicinal composition, which contains esomeprazole and a buffering agent. The problem that an esomeprazole magnesium enteric preparation is slowly absorbed is solved through the medicinal composition; and the medicinal composition is convenient to takeeat, is takeneaten for only one time everyday when a patient is in an empty belly state (at least 1 hour before meal), and can quickly reach the maximal blood concentration (within about 30 minutes) and control gastric acid very effectively.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Preparation method of esomeprazole and magnesium salt thereof
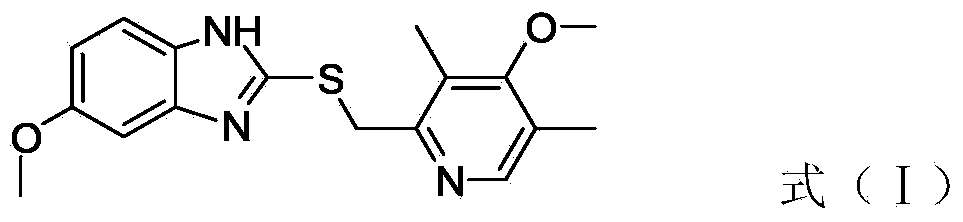
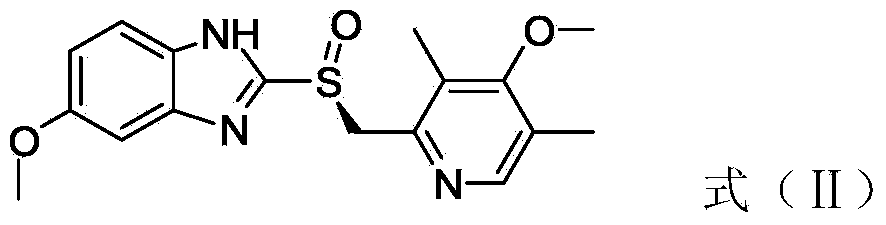
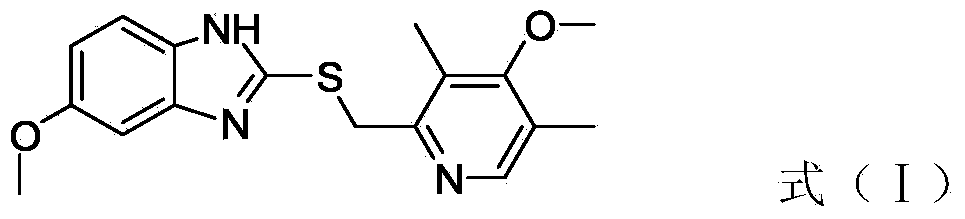
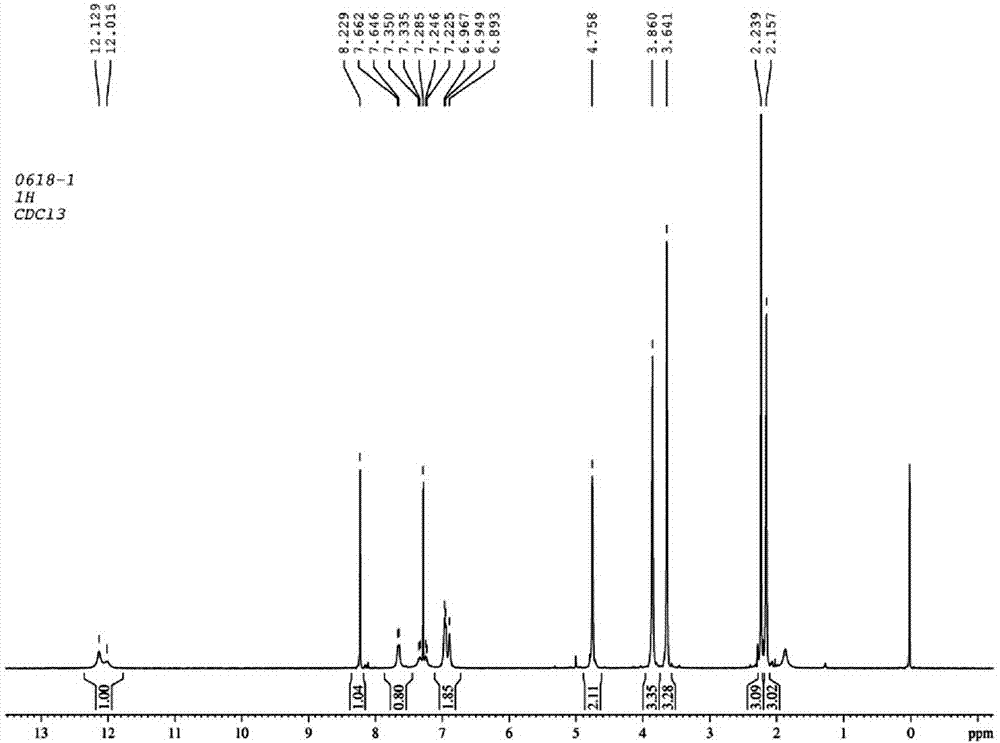
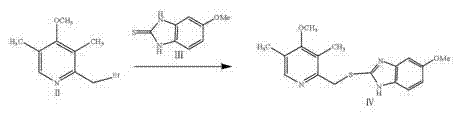
The invention discloses a preparation method of esomeprazole and a magnesium salt thereof. The preparation method comprises the steps: with 4-methoxy-2-hydroxymethyl-3,5-dimethyl pyridine as raw material, carrying out a reaction with hydrobromic acid to obtain 4-methoxy-2-bromomethyl-3,5-dimethyl pyridine, namely a compound II; dripping a methanol solution of the compound II into a methanol solution of a compound III, and carrying out a reaction to obtain 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl thio-1H-benzimidazole, namely a compound IV; dissolving the compound IV in dichloromethane, adding D-diethyl tartrate, tetrabutyl titanate and water for reaction, and thus obtaining esomeprazole; and carrying out a reaction of esomeprazole with magnesium methoxide to obtain magnesium esomeprazole. The method is utilized to prepare esomeprazole and the magnesium salt thereof, and can effectively reduce the production cost.
Owner:KAIFENG MINGREN PHARMA
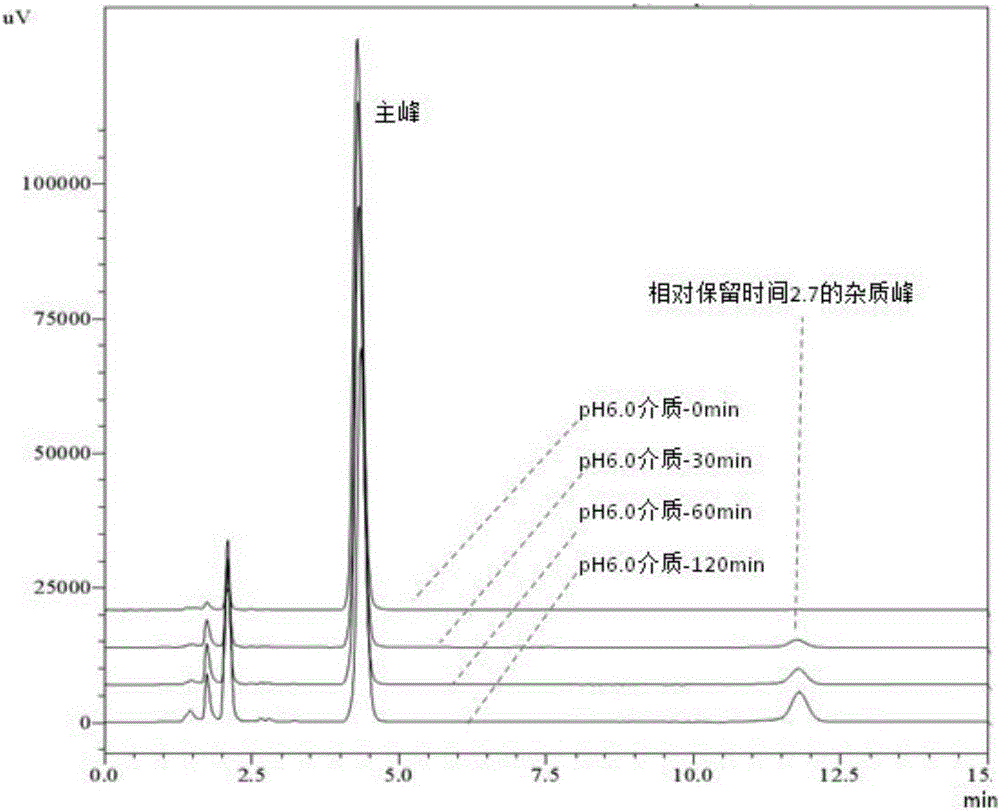
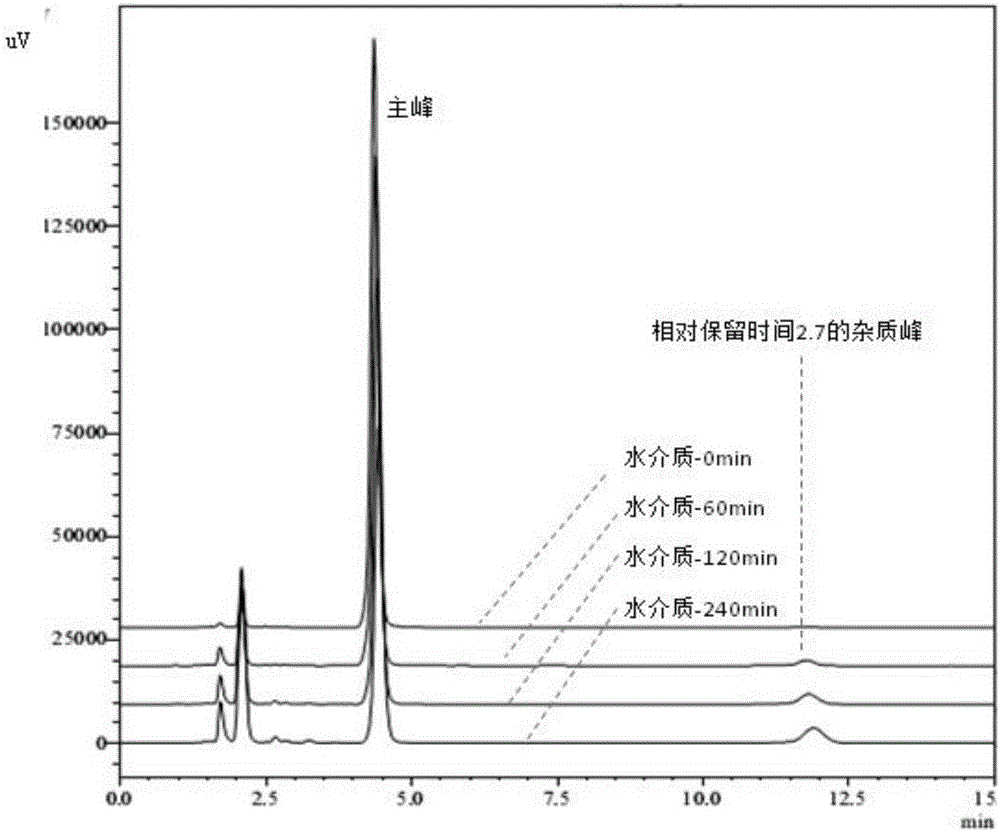
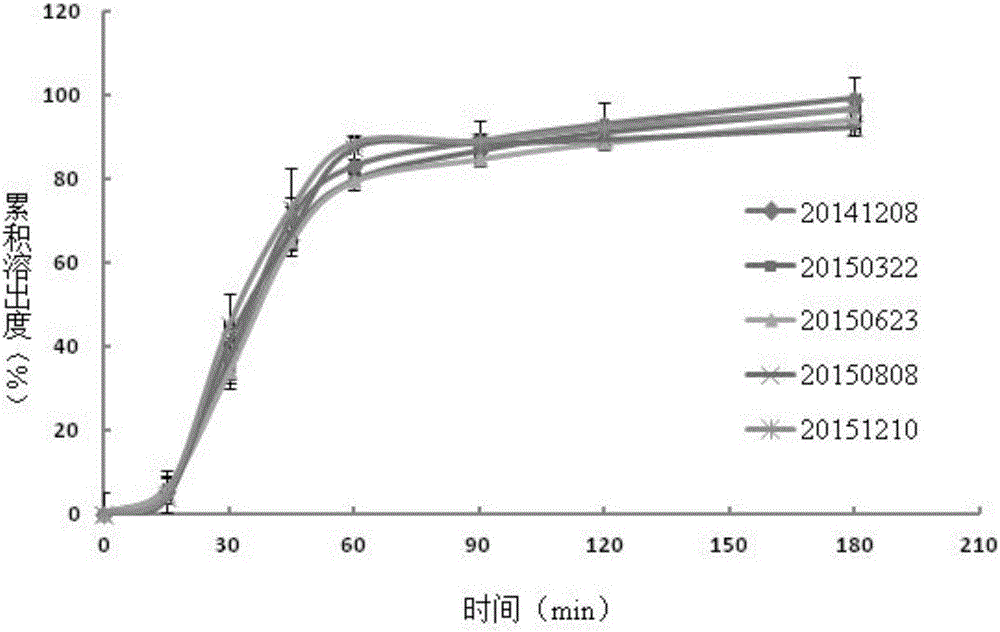
Method for measuring dissolution rates of esomeprazole magnesium enteric-coated preparation in different media
ActiveCN106018604ASolving the problem of inability to accurately determine the dissolution rate of esomeprazole magnesium enteric-coated preparations in acidic mediaAccurate methodComponent separationWater bathsFiltration
The invention discloses a method for measuring the dissolution rates of an esomeprazole magnesium enteric-coated preparation in different media. The method is characterized in that a step of establishing a linear regression equation is added, that is, a sodium hydroxide solution and ethyl alcohol are added into the content of an esomeprazole magnesium enteric-coated tablet or capsule to perform ultrasonic dissolution, then filtration is conducted, the filtrate is taken out, subjected to constant-volume treatment and then placed in a water bath of 37+ / -0.5 DEG C, a sample is taken at each dissolution time and is injected into a high performance liquid chromatograph, chromatograms are recorded, and the linear relationship between a main peak and a relative retention time 2.7 impurity peak is found out from the area change relationship between the main peak and the relative retention time 2.7 impurity peak to obtain the linear regression equation which is applied to calculation of the dissolution quantity of esomeprazole magnesium. The method solves the problem that the dissolution rate of the esomeprazole magnesium enteric-coated preparation cannot be accurately measured in the prior art, and has the advantages of being convenient, quick, accurate, excellent in repeatability, high in sensitivity and strong in practicability during operation.
Owner:SUN YAT SEN UNIV +1
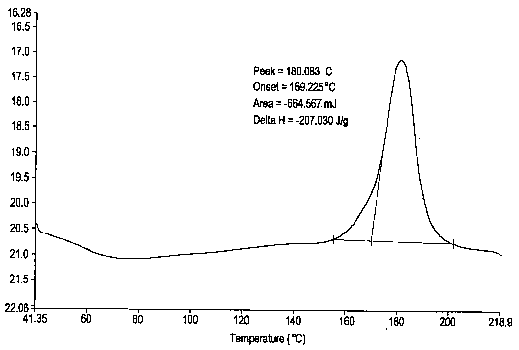
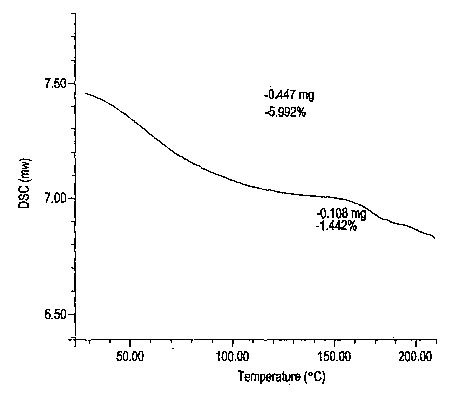
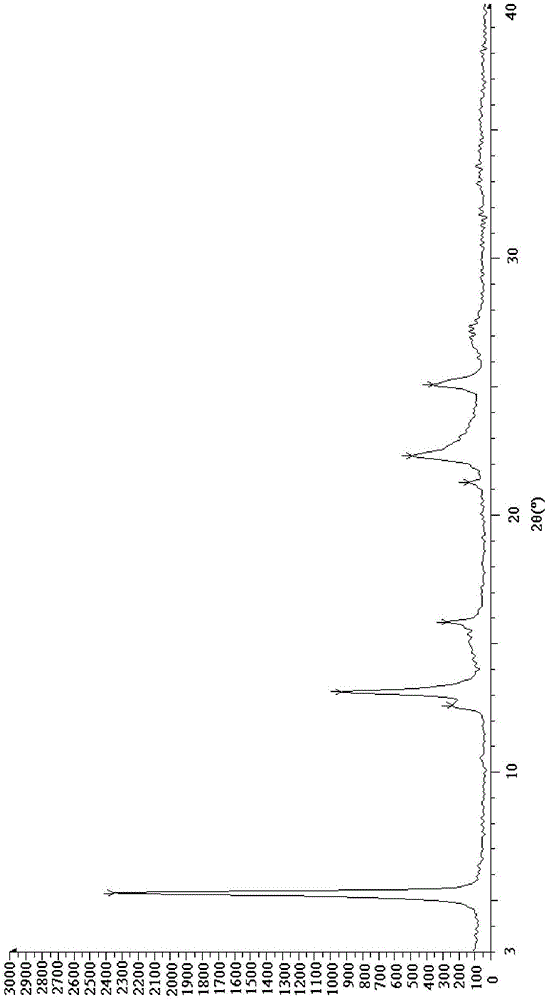
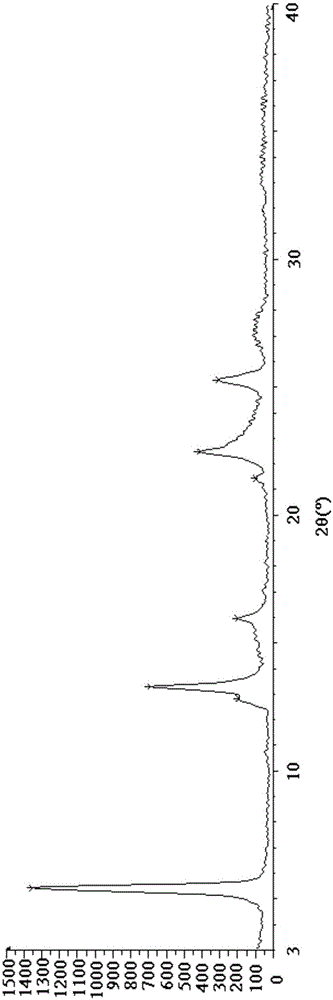
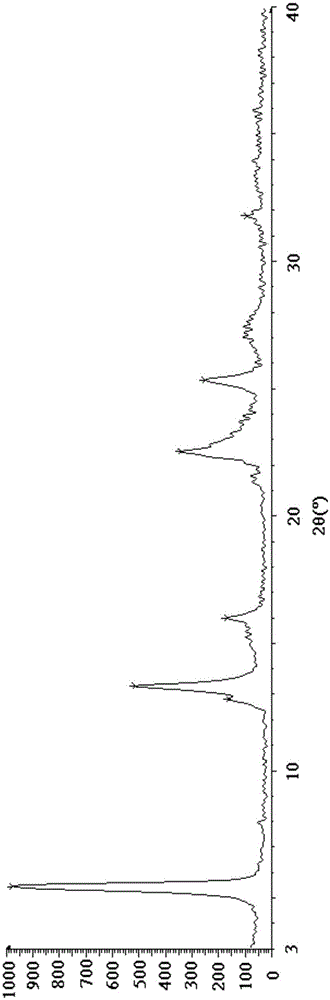
Esomeprazole magnesium trihydrate and preparation method thereof
ActiveCN103509001AHigh chemical purityHigh optical purityOrganic active ingredientsOrganic chemistryPhysical chemistryPharmaceutical Substances
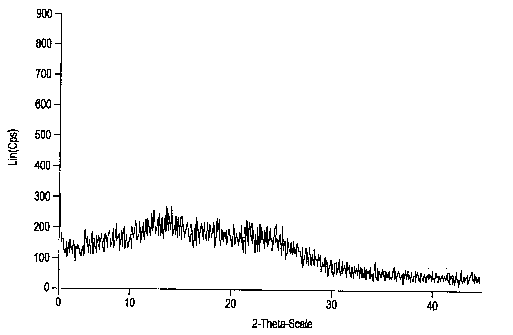
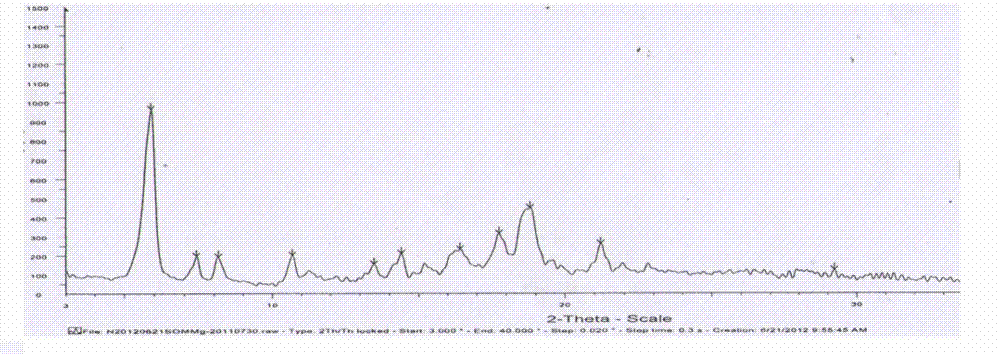
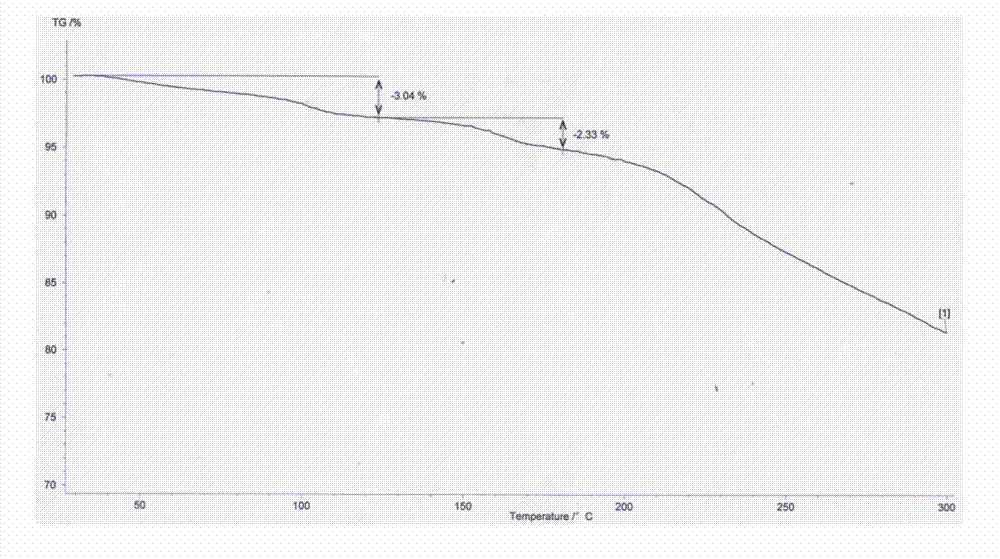
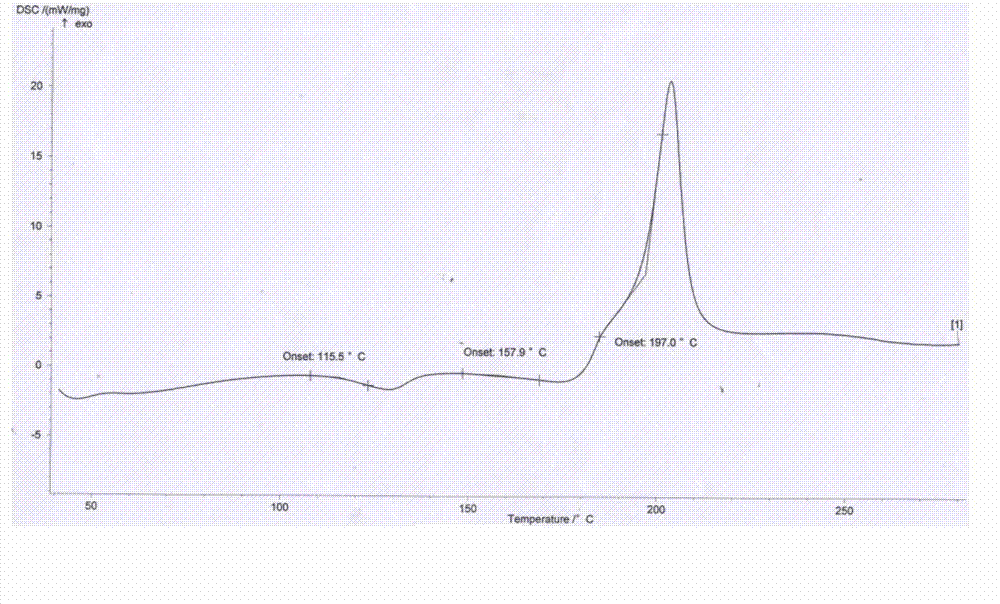
The invention relates to amorphous esomeprazole magnesium trihydrate, pharmaceutical compositions containing the amorphous esomeprazole magnesium trihydrate, and a preparation method of the amorphous esomeprazole magnesium trihydrate. The amorphous esomeprazole magnesium trihydrate is alpha-radiated by CuK, wherein X-ray powder diffraction pattern has broad peaks at parts that 2theta is 7+-1 degree and 18+-1 degree; according to peak intensity of broad peaks at parts that 2theta is 18+-1 degree being 100%, peak intensity ratios of the broad peaks at parts that 2theta is 7+-1 degree is larger than 50% and smaller than 100%. The esomeprazole magnesium trihydrate has advantages of high chemical and optical purity, stable properties, simple and controllable preparation method, and high yield, and is suitable for large scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Esomeprazole magnesium preparation method
The invention discloses an esomeprazole preparation method and an esomeprazole magnesium preparation method. The esomeprazole preparation method is characterized in that a catalytic oxidation of omeprazole sulfide is carried out under the action of an added oxidant in the presence of bidentate chiral aminoalcohol and titanium alkoxide at room temperature to obtain a chiral proton pump inhibitor esomeprazole in a single enantiomer or enriched enantiomer form. The above preparation methods have the advantages of no need of the addition of an alkaline reagent, easy obtaining and reuse of the bidentate chiral aminoalcohol participating in the above reaction, increase of the utilization rate of the chiral aminoalcohol, substitution of expensive D-(-)-diethyl tartrate, and production cost reduction; and the chemical purity and the reaction overall yield of the prepared esomeprazole magnesium reach 99.7% and 66% respectively.
Owner:湖南千金湘江药业股份有限公司
Esomeprazole magnesium enteric capsules and preparation method thereof
ActiveCN105106168AAvoid damageIntegrity guaranteedOrganic active ingredientsDigestive systemActive agentPharmaceutical drug
The invention discloses esomeprazole magnesium enteric capsules and a preparation method thereof. Esomeprazole magnesium is prepared into enteric coated pellets, and the enteric coated pellets are filled into gastric coated capsules. Each enteric coated pellet consists of an empty pellet core, a medicine carrying layer, two isolating layers, an enteric coated layer and a film coating layer. In order to guarantee stability of medicines, alkaline materials are added in the empty pellet cores; alkali stabilizers and antioxidant are added in the medicine carrying layer; and a high-alkalinity modifier and a low-alkalinity modifier are respectively added in the two isolating layers of each enteric coated pellet. In order to guarantee a dissolution effect, surfactant is added in the enteric coated layers; and stability of products is improved owing to the film coating layers wrapping the outer layers of the enteric coated pellets. Owing to optimized formulation and technology of the pellets, smoothness of a technology is improved, work efficiency is also improved, coating time is shortened, consumption of labor and materials is reduced, and production cost is reduced.
Owner:DEZHOU DEYAO PHARMA
Naproxen esomeprazole enteric preparation and preparation method thereof
ActiveCN104208039AUniform coatingImprove distributionOrganic active ingredientsAntipyreticCoated tabletsBiochemistry
The invention discloses a naproxen esomeprazole enteric preparation and a preparation method thereof, wherein the preparation is obtained by filling a naproxen enteric pill and an esomeprazole thin membrane coated tablet into the same capsule, wherein each esomeprazole thin membrane coated tablet contains 20 mg esomeprazole, and the naproxen enteric pill in each capsule contains 375mg or 500mg naproxen. The preparation method for the naproxen esomeprazole enteric preparation is that the naproxen and the esomeprazole are prepared into the enteric pill and tablets respectively, and thus, influences of the acid enteric material on the stability of the esomeprazole are avoided; moreover, the method is reasonable in design, simplified in a coating step and simple in process; and the prepared naproxen esomeprazole enteric preparation has stable quality, high bioavailability, a high economic value and important social meaning.
Owner:杭州新诺华医药有限公司
Esomeprazole pharmaceutical composition and preparation thereof
InactiveCN103845734AAvoid degradation damageRaise the pHOrganic active ingredientsDigestive systemAluminium hydroxideStrong acids
The invention provides an esomeprazole pharmaceutical composition with stronger acid resistance and a preparation thereof. The esomeprazole pharmaceutical composition comprises esomeprazole, one or more antacids and common pharmaceutical adjuvants, wherein the esomeprazole comprises esomeprazole salt, particularly esomeprazole magnesium salt and esomeprazole lithium salt; and the antacids comprise sodium bicarbonate, sodium carbonate, potassium carbonate, potassium bicarbonate, calcium carbonate, aluminum carbonate, magnesium carbonate, magnesium hydroxide, magnesium oxide, aluminium hydroxide, magnesium aluminum carbonate and the like. The preparation of the esomeprazole pharmaceutical composition is prepared by adding the antacids via interior addition and exterior addition; and in an optimized preparation method, the weight ratio of the antacids in the interior addition and the exterior addition is 2: 3, so that the antacids play double efficacies, the dosage of acid neutralization agent is reduced and the acid resisting effect is better.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH +1
Esomeprazole magnesium enteric pill coated tablet and preparation method thereof
InactiveCN104586809AGood drug content uniformityGood content uniformityOrganic active ingredientsDigestive systemCoated tabletsAdjuvant
The invention discloses an esomeprazole magnesium enteric pill coated tablet. The esomeprazole magnesium enteric pill coated tablet is prepared from the following raw materials in percentage by mass: 4.7-19.7% of a quick-release enteric coated pill, 18.6-30.9% of a slow-release enteric coated pill, 60.7-66.4% of a buffer adjuvant, 1.0% of a lubricant and 2-3% of a thin film coating. The esomeprazole magnesium enteric pill coated tablet is less frequently taken by patients, is reduced in stimulation to the enteric canal, is stable in plasma concentration and is free from peak-valley phenomenon.
Owner:ZHEJIANG YATAI PHARMA
Preparation method for esomeprazole magnesium trihydrate crystalline form
ActiveCN105111188AHigh crystallinityImprove stabilityOrganic chemistry methodsActivated carbonSolvent
The present invention discloses a preparation method for an esomeprazole magnesium trihydrate crystalline form. The preparation method comprises: (a)dissolving esomeprazole magnesium crude into C1-C3 alkanol; (b) taking activated carbon as a filter aid to filter, and then concentrating filtrate under reduced pressure; (c) adding acetone; (d) filtering a mixture which is subjected to temperature-preserving and stirring to obtain a filter cake 1, and stirring and washing the filter cake 1 with a mixed solvent of C1-C3 alkanol and acetone; (e) filtering the mixture to obtain a filter cake 2, dissolving the filter cake 2 with C1-C3 alkanol, adding water into the dissolved filter cake, and separating out a crystal while stirring; and (f) filtering the mixture to obtain a filter cake 3, carrying out vacuum drying on the filter cake 3 at the temperature of 40-50 DEG C to obtain the esomeprazole magnesium trihydrate . The method provided by the present invention is good in repeatability, simple in operation and high in product yield, purity and crystallinity and is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation method of esomeprazole magnesium
The invention relates to a preparation method of esomeprazole magnesium. The method comprises the steps that: esomeprazole is dissolved in an organic solvent; a potassium-containing alkali or salt is subjected to a reaction with esomeprazole, such that esomeprazole potassium is produced; and esomeprazole potassium is subjected to a displacement reaction with a magnesium salt, such that esomeprazole magnesium is prepared. The invention also relates to a refining method of esomeprazole magnesium. With the method provided by the invention, the purity of the prepared esomeprazole magnesium is higher than 99.9%, the yield is higher than 82%, and esomeprazole magnesium has good morphological stability. With the method, requirements by factory pharmacy for purity and yield can be satisfied.
Owner:NANJING YOKO PHARMA
Esomeprazole magnesium enteric-coated tablet containing esomeprazole magnesium pellets and preparation method of esomeprazole magnesium enteric-coated tablet
InactiveCN104546787AImprove acid resistanceQuick releaseOrganic active ingredientsDigestive systemWaxActive component
The invention belongs to the field of medicines and discloses an esomeprazole magnesium enteric-coated tablet containing an esomeprazole magnesium pellet and a preparation method of the esomeprazole magnesium enteric-coated tablet. The enteric-coated tablet is prepared by pressing pellets taking esomeprazole magnesium as active components, a thinner, a disintegrating agent, a lubricating agent and the like. According to the method provided by the invention, an enteric-coated pellet is firstly made into a medicine contained core pellet and then is sequentially wrapped with an insulating layer, an enteric coated layer and a wax protecting layer. According to the tablet provided by the invention, the enteric-coated pellet is wrapped with the wax protecting layer, so that the problems of acid tolerance descending caused by the breakage of the enteric coated layer during the tabletting process of the enteric-coated pellet, and disqualification in content uniformity caused by excess liquidity can be solved.
Owner:深圳市国源医药科技有限公司 +1
Esomeprazole drug-containing pellet composition and preparation method thereof
ActiveCN103816124AImprove stabilityHigh dissolution rateOrganic active ingredientsDigestive systemRefluxEsomeprazole Sodium
The invention belongs to the field of medicines, and discloses an esomeprazole drug-containing pellet composition and a preparation method thereof. The esomeprazole drug-containing pellet composition provided by the invention comprises esomeprazole magnesium and hydrate thereof, a filling agent and a pore-forming agent. The filling agent and the pore-forming agent are mixed with esomeprazole according to a certain proportion, so that the stability of an esomeprazole medicament can be effectively improved, and adverse reactions such as allergy and the like caused by the use of a stabilizing agent are avoided. Moreover, the pore-forming agent can be quickly dissolved in water and can form pore passages in pellets to accelerate dissolution of the medicament. Experiments show that the esomeprazole drug-containing pellet composition disclosed by the invention is good in stability, high in dissolution degree and significant in gastric acid inhibition, so that the composition can be widely applied to treatment of gastroesophageal reflux diseases and peptic ulcers with positive helicobacter pylori. The preparation method disclosed by the invention is simple in operation, low in production cost and high in efficiency, and is suitable for industrial large-scale production of the esomeprazole drug-containing pellet composition.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
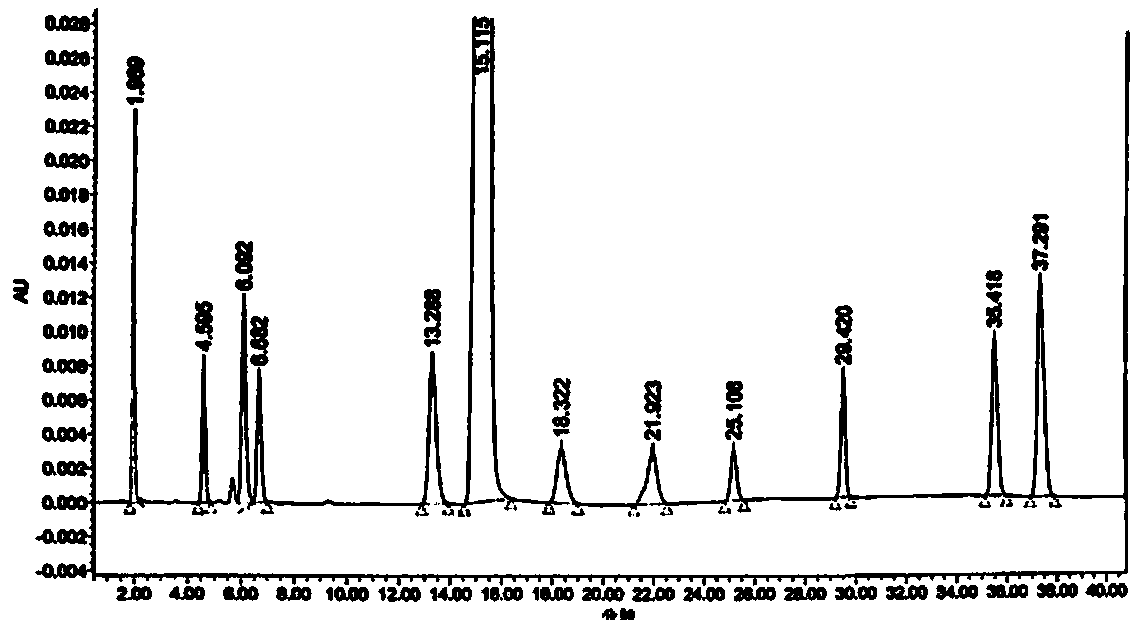
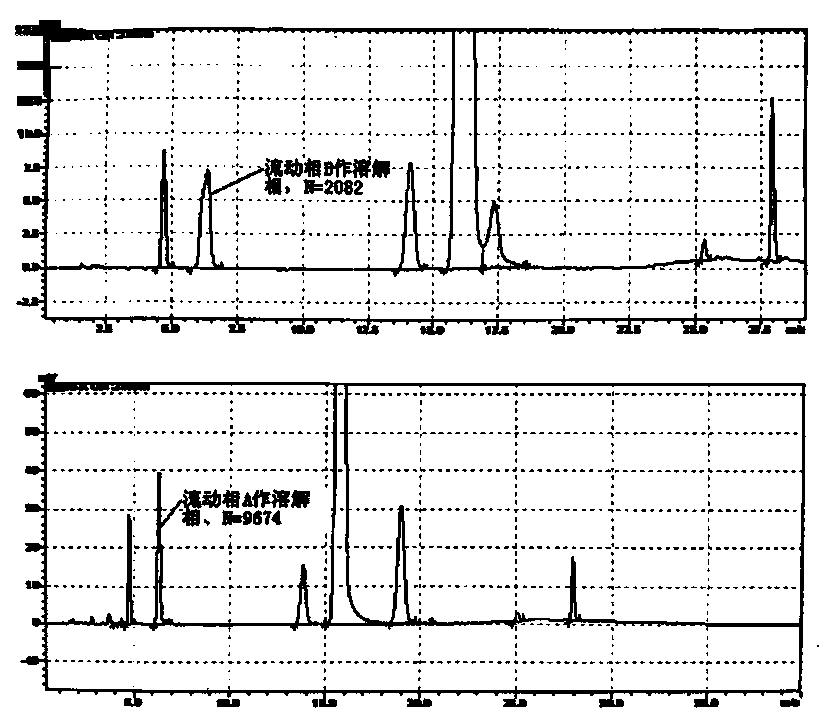
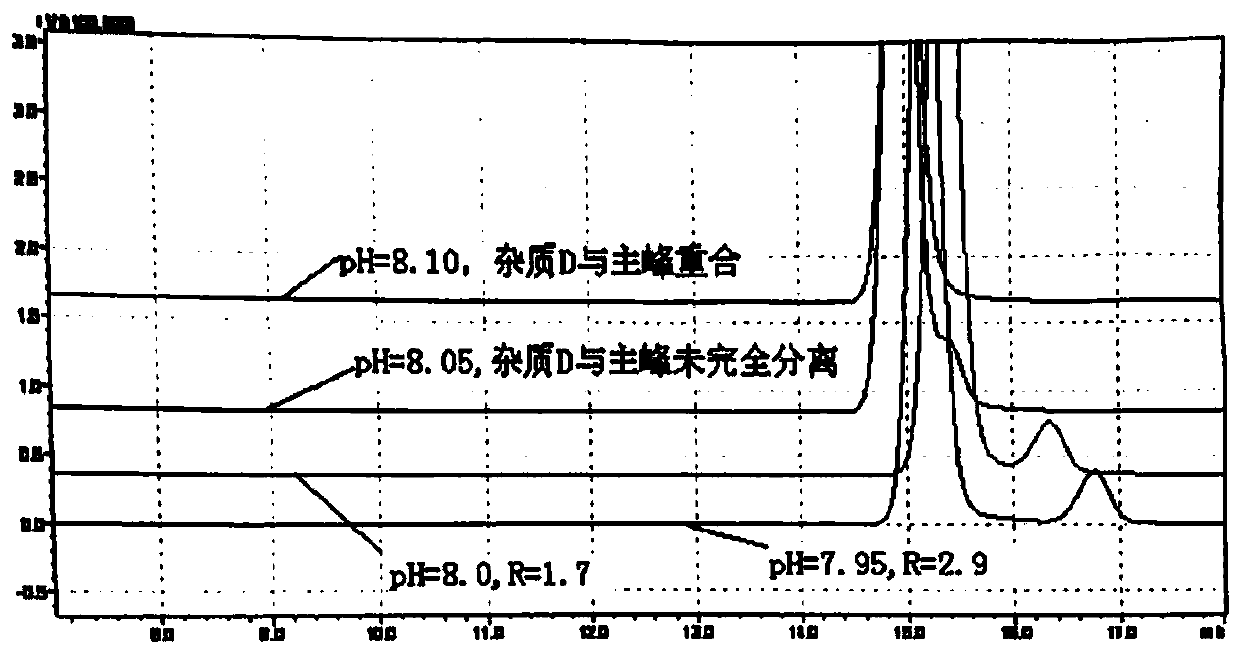
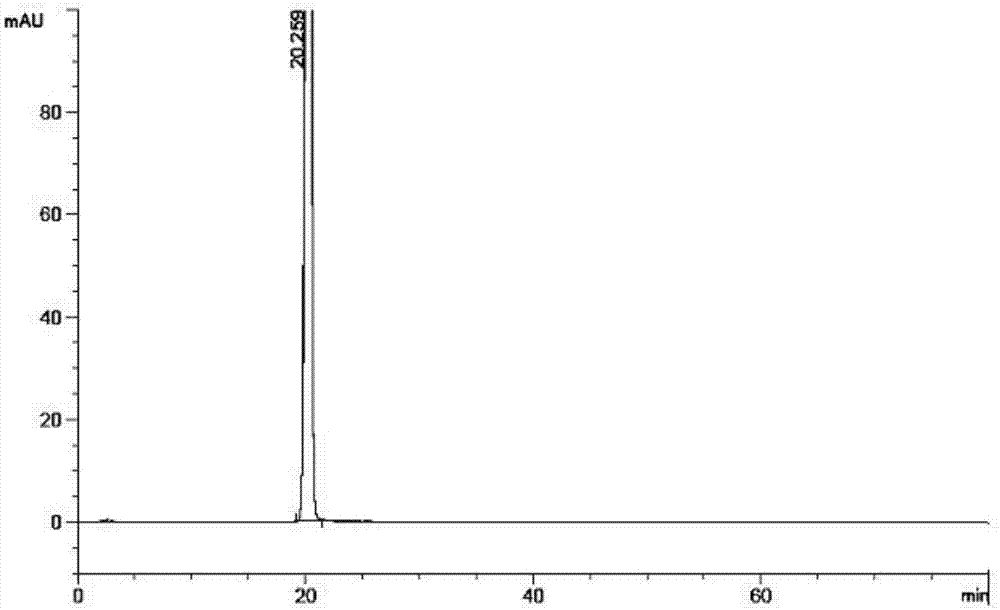
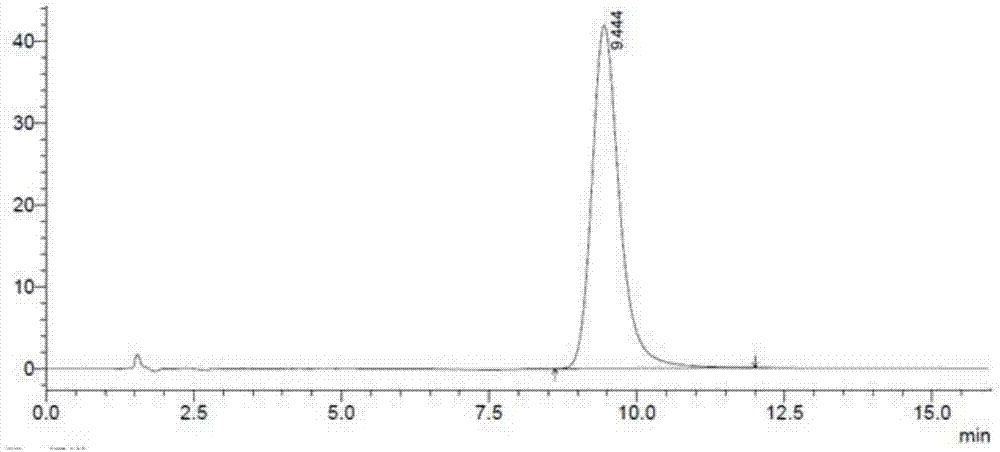
Esomeprazole magnesium related substance analysis method based on impurity spectrum
InactiveCN110988180AAvoid underdetectionReaction Impurity LevelComponent separationTheoretical platePhysical chemistry
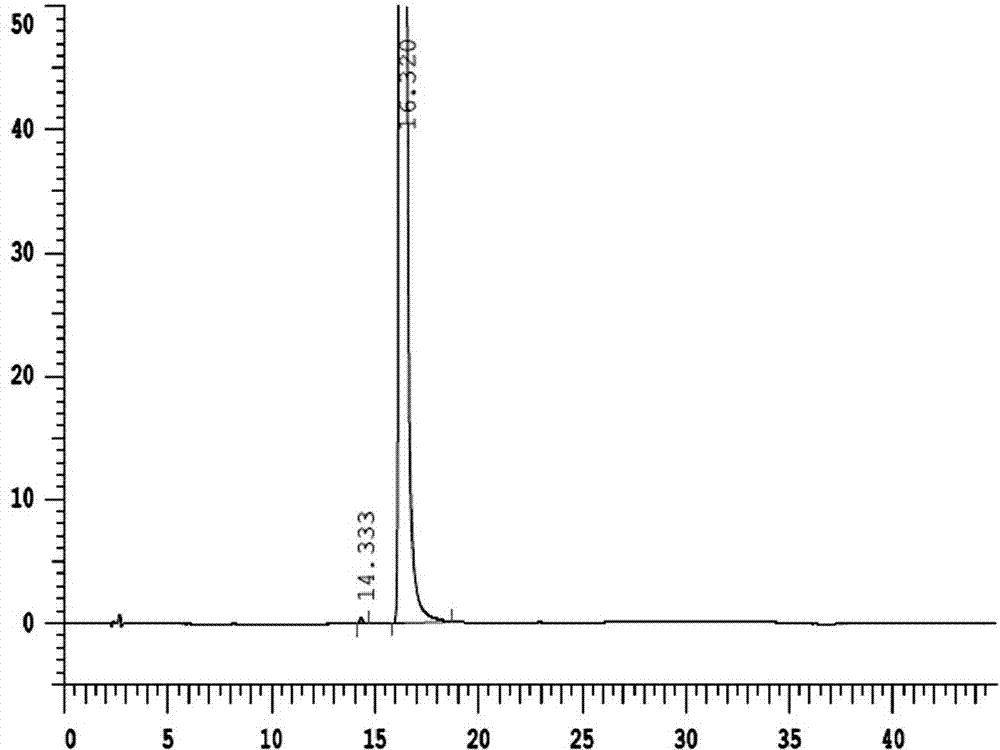
The invention provides a method for effectively separating known and potential impurities in esomeprazole magnesium based on an esomeprazole magnesium impurity spectrum. The retention time of the esomeprazole main peak is about 15 minutes, the resolution between impurity peaks is greater than 1.5, the number N of theoretical plates of the main peak is greater than or equal to 3000, the main peak tailing factor T is greater than or equal to 0.8 and less than or equal to 1.2, and the peak pattern is good. According to the method, a C8 chromatographic column (250 mm * 4.6 nm, 5 microns) and a UVdetector are mainly adopted, the detection wavelength is 280 nm, the column temperature is 25 DEG C, the flow velocity of a mobile phase is 1.5 mL / min, and the sample introduction volume is 20 microliters; a mobile phase A: the ratio of acetonitrile to ammonium acetate is 1: 3, and the pH value is adjusted to 7.5 by ammonia water; and a mobile phase B: the ratio of acetonitrile: ammonium acetate is 6: 4, and the pH value is adjusted to 7.5 by ammonia water. Experiments prove that the method can effectively and successfully separate 12 impurities and main peaks in an impurity spectrum, realizeseffective detection, and can be used for quality monitoring in the production process of the esomeprazole magnesium bulk drug.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD +1
Preparation method of esomeprazole trihydrate
The invention relates to the field of medicinal chemistry, in particular to a preparation method of esomeprazole trihydrate. The preparation method of esomeprazole trihydrate comprises the steps as follows: omeprazole is taken as an original raw material and subjected to complexation, separation, hydrolysis and salifying thato obtain the esomeprazole trihydrate. With the adoption of the preparation method, the yield and the purity of esomeprazole trihydrate can be remarkably improved, further, the reaction steps are simple, the environment protection is realized, the cost is controllable, and accordingly, the preparation method is suitable for industrial mass production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Preparation method of esomeprazole magnesium
The invention relates to the field of chemical synthesis, in particular to a novel synthesis process for active pharmaceutical ingredients of a PPI (proton pump inhibitor), and especially relates to a preparation method of esomeprazole magnesium. According to the method, 5-methoxy-2-mercaptobenzimidazole and 2-chloromethyl-3,5-dimethyl-4-methoxy pyridine hydrochloride are taken as the starting raw materials and subjected to condensation, asymmetric oxidation and salifying to prepare esomeprazole magnesium, a wet product is refined with water and methanol, an intermediate does not require crystallization, filtering and drying, the operation steps are simplified, the production cycle is shortened, material loss and energy consumption in a processing process are reduced, the yield is improved, and good economic benefits and social benefits are realized. The preparation method is good in repeatability and simple to operate, improves the yield and purity of esomeprazole magnesium and facilitates industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Esomeprazole magnesium micro-tablet
InactiveCN102846571AOrganic active ingredientsDigestive systemBULK ACTIVE INGREDIENTMaterials science
The invention discloses an esomeprazole magnesium micro-tablet, which is provided with a cylindrical space shape. The height of the esomeprazole magnesium micro-tablet is 1-3mm, and the diameter of the esomeprazole magnesium micro-tablet is 1-3mm. The content of the esomeprazole magnesium which is the active ingredient in the micro-tablet is 1-5mg, and the adopted enteric coating material is methacrylic acid copolymer (Eudragit L100). The enteric coating material of the esomeprazole magnesium micro-tablet also comprises triethyl citrate which is used as plasticizer, the content of the triethyl citrate is 0.05-0.08mg, glidant is glycerin monostearate, the content of the glycerin monostearate is 0.1-0.15mg, surfactant is polysorbate, and the content is 0.1-0.2mg. The micro-tablet and acceptable pharmaceutic adjuvants are prepared into the esomeprazole magnesium tablet, and the acceptable pharmaceutic adjuvants consist of lactose, croscarmellose sodium, polyvinylpyrrolidone and talcum powder. The esomeprazole magnesium micro-tablet disclosed by the invention can be used for treating the digestive tract ulcer disease.
Owner:NANJING ZENKOM PHARMA
Method for preparing stable esomeprazole enteric-coated pills
InactiveCN102406628AMeet stability requirementsSimple processOrganic active ingredientsDigestive systemMedicineFree water
The invention provides a method for preparing stable esomeprazole enteric-coated pills, which comprises: (1) preparing pill cores containing a medicine and controlling the free water in the pill cores containing the medicine to be less than 2.5 weight percent; and (2) performing enteric coating under a condition that the free water content in the pill cores containing the medicine is controlled to be less than 2.5 weight percent. The invention provides a new feasible process method for replacing an isolating layer, thereby overcoming the complexity of a preparation process and adverse effect on enteric-coated medicine disintegration, exsolution or the like in the prior art.
Owner:SHANGHAI SUNTECH PHARMA +1
Method for preparing high-purity esomeprazole magnesium trihydrate
InactiveCN105418588AHigh yieldImprove reaction efficiencyOrganic chemistry methodsManganesePotassium hydroxide
The invention discloses a method for preparing a high-purity esomeprazole magnesium trihydrate. The method comprises the following steps: (1), mixing omeprazole sulfide, (R)-1,1 minute-binaphthyl-2,2 minute-diamine and inorganic metal salt in acetone, thereby obtaining a mixture M; (2), at a condition of 15-45 DEG C, dipping 30% H2O2 into the mixture M of the step (1) to perform an oxidization reaction, and after the oxidization reaction is completed, adding potassium hydroxide and methanol solution, thereby obtaining esomeprazole potassium; (3), mixing the esomeprazole potassium obtained in step (2) with anhydrous magnesium chloride in methyl alcohol, stirring to react, and centrifuging and separating, thereby obtaining the esomeprazole magnesium trihydrate, wherein in step (1), the inorganic metal salt is cobalt (II), iron (II) or magnesium (II) metal salt. The method for preparing the high-purity esomeprazole magnesium trihydrate has high yield, good selectivity and high reaction efficiency, and is suitable for industrial mass production.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Esomeprazole magnesium suspension tablet and preparation method thereof
InactiveCN104027320APrecise split doseSuitable for childrenOrganic active ingredientsDigestive systemEsomeprazole PillSuspending Agents
The invention provides an esomeprazole magnesium suspension tablet which is prepared by mixing and tabletting an esomeprazole pill, a filler, corrigent, a disintegrating agent, a suspending agent, a lubricant and the like, wherein the esomeprazole pill is prepared by carrying the medicine in a pill core, wrapping an isolating layer and wrapping an enteric coating. Because of nicking, the esomeprazole magnesium suspension tablet can be torn apart for taking; a small pill tabletting process is adopted, the divided dose is accurate, the enteric effect of the pill is not affected after the tablet is torn apart, the tablet can be reasonably taken according to the age and the weight of children, and the clinical compliance is improved. The esomeprazole magnesium suspension tablet provided by the invention can be rapidly dispersed into suspension, is beneficial for distribution of the medicine in gastrointestinal tracts, can improve the bioavailability of the medicine, and is also applicable to patients with dysphagia.
Owner:杭州新诺华医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com