Preparation method for esomeprazole magnesium trihydrate crystalline form
A technology of esomeprazole magnesium trihydrate and esomeprazole magnesium, applied in the field of preparation of esomeprazole magnesium trihydrate crystal form, can solve the problem of not providing XRPD spectrum and data, etc. Achieve high crystallinity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Preparation of esomeprazole magnesium trihydrate crystal form
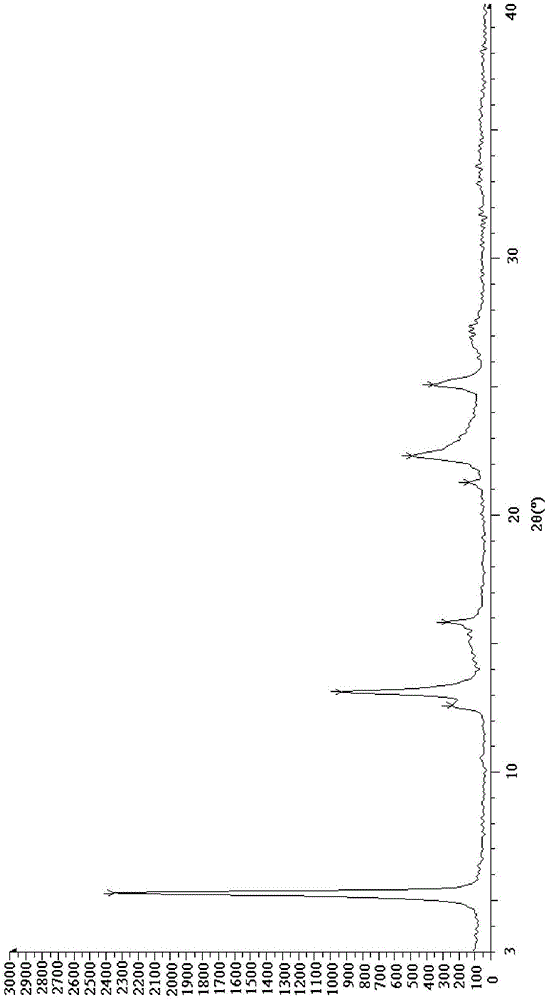
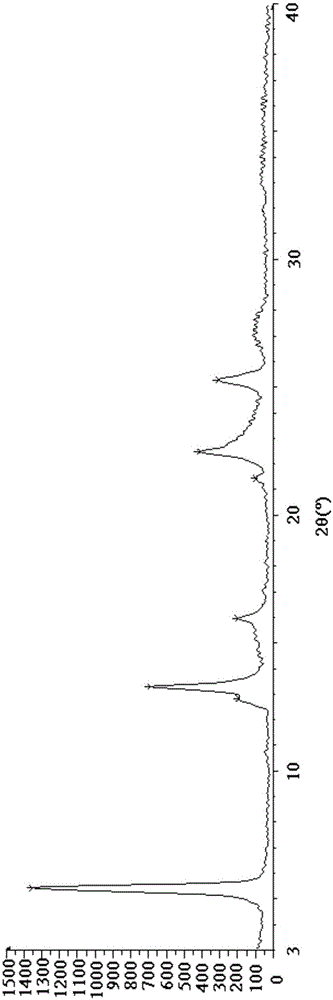
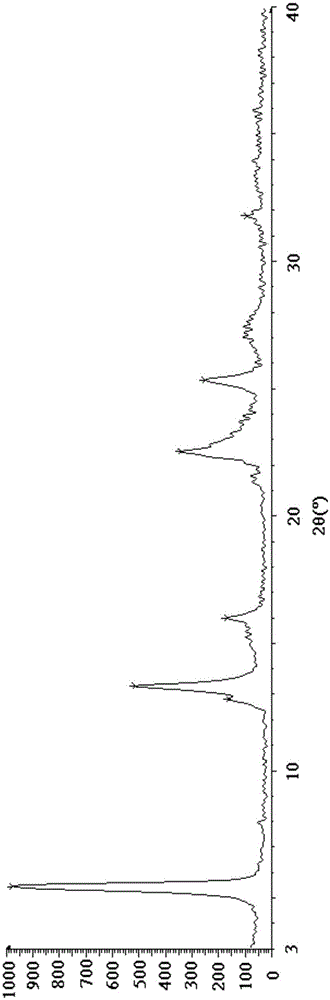
[0048] Put 100g of crude esomeprazole magnesium in 500ml of methanol and stir at 10°C until dissolved. Use 2g of activated carbon as a filter aid, filter, and concentrate the filtrate to 1 / 2 of the original volume at 40°C under reduced pressure. Add 500 ml of acetone, and stir at 10° C. for 2 hours. After filtering, the filter cake was stirred and washed with 200 ml of methanol / acetone (v / v=1 / 3) mixed solvent for 10 minutes. Filter, dissolve the filter cake in 150ml of methanol, stir to dissolve, add 600ml of water, stir and crystallize at 30°C for 3 hours. Filter and dry the filter cake under vacuum at 40°C to obtain 85g of off-white solid, with a molar yield of 85%, a moisture content of 7.1%, a chromatographic purity of 99.92%, and an optical purity of 99.89%. figure 1 .
Embodiment 2
[0049] Embodiment 2 Preparation of esomeprazole magnesium trihydrate crystal form
[0050] Put 100g of crude esomeprazole magnesium in 1000ml of methanol and stir at 25°C until dissolved. Use 5g of activated carbon as a filter aid, filter, and concentrate the filtrate to 1 / 3 of the original volume at 45°C under reduced pressure. Add 1000 ml of acetone, and stir at 20° C. for 4 hours. After filtering, the filter cake was stirred and washed twice with 350 ml of methanol / acetone (v / v=1 / 4) mixed solvent, 20 minutes each time. Filtrate, dissolve the filter cake in 200ml of methanol, stir and dissolve, add 1200ml of water, stir and crystallize at 35°C for 4 hours. Filtrate, and dry the filter cake under vacuum at 45°C to obtain 90g of off-white solid, with a molar yield of 90%, a moisture content of 7.4%, a chromatographic purity of 99.95%, and an optical purity of 99.90%. figure 1 unanimous.
Embodiment 3
[0051] Embodiment 3 Preparation of esomeprazole magnesium trihydrate crystal form
[0052] 100g of crude esomeprazole magnesium was dissolved in 1500ml of methanol at 30°C until dissolved. Use 10 g of activated carbon as a filter aid, filter, and concentrate the filtrate under reduced pressure at 50°C to 1 / 5 of the original volume. Add 1500 ml of acetone, and stir at 30° C. for 6 hours. After filtering, the filter cake was stirred and washed with 600 ml of methanol / acetone (v / v=1 / 5) mixed solvent for 30 minutes. Filtrate, dissolve the filter cake in 300ml of methanol, stir and dissolve, add 3000ml of water, stir and crystallize at 45°C for 5 hours. Filtrate, and dry the filter cake under vacuum at 50°C to obtain 92g of off-white solid, with a molar yield of 92%, a moisture content of 7.0%, a chromatographic purity of 99.96%, and an optical purity of 99.91%. figure 1 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com