Preparation method for esomeprazole magnesium trihydrate
A technology for esomeprazole magnesium trihydrate and esomeprazole, which is applied in the field of pharmaceutical preparation, can solve the problems of long production cycle, difficulty in reaching quality standards for final products, and difficulty in drying, etc. Stereoselective and specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
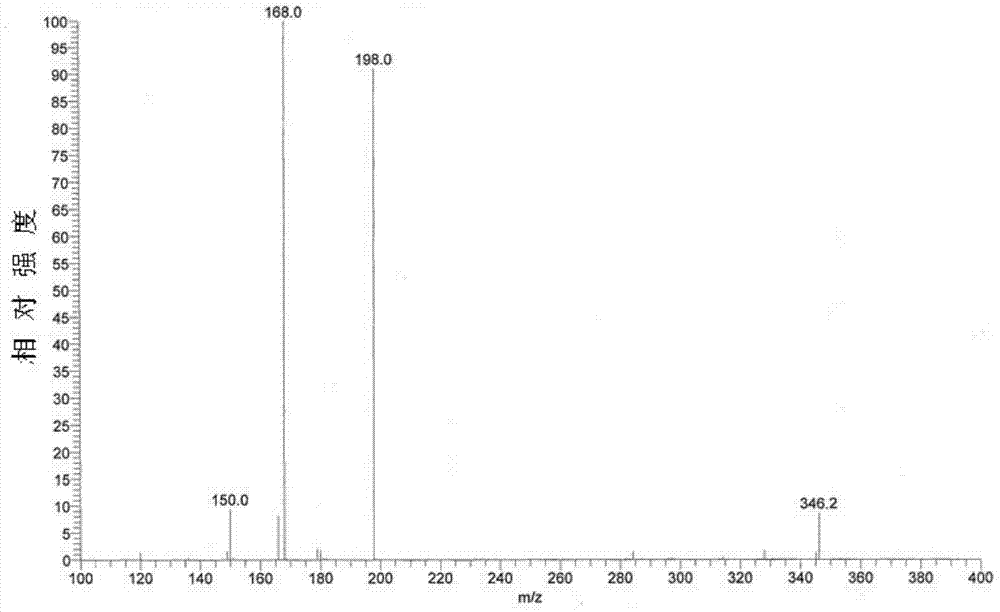
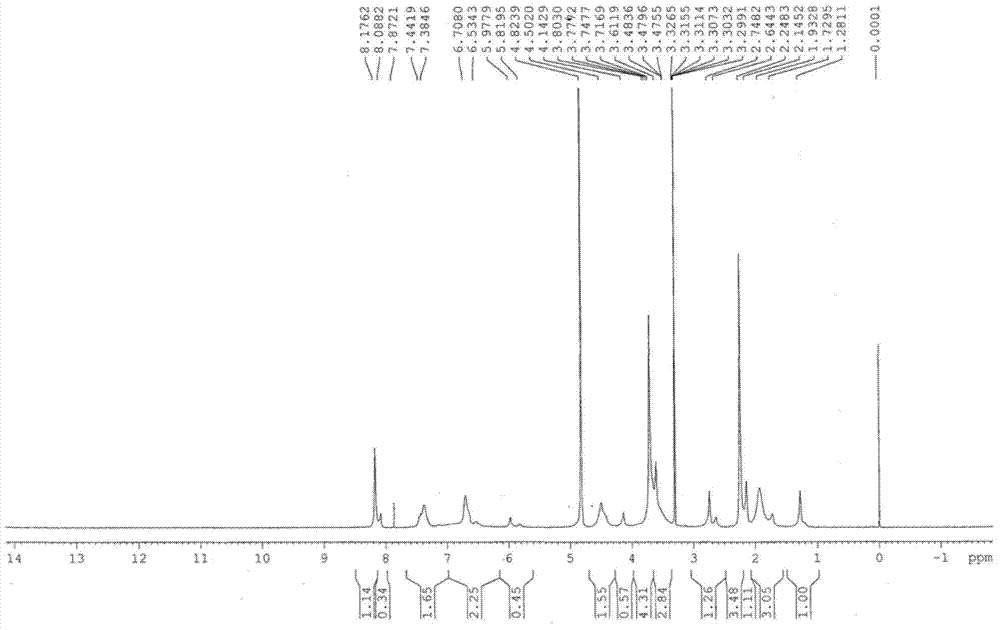
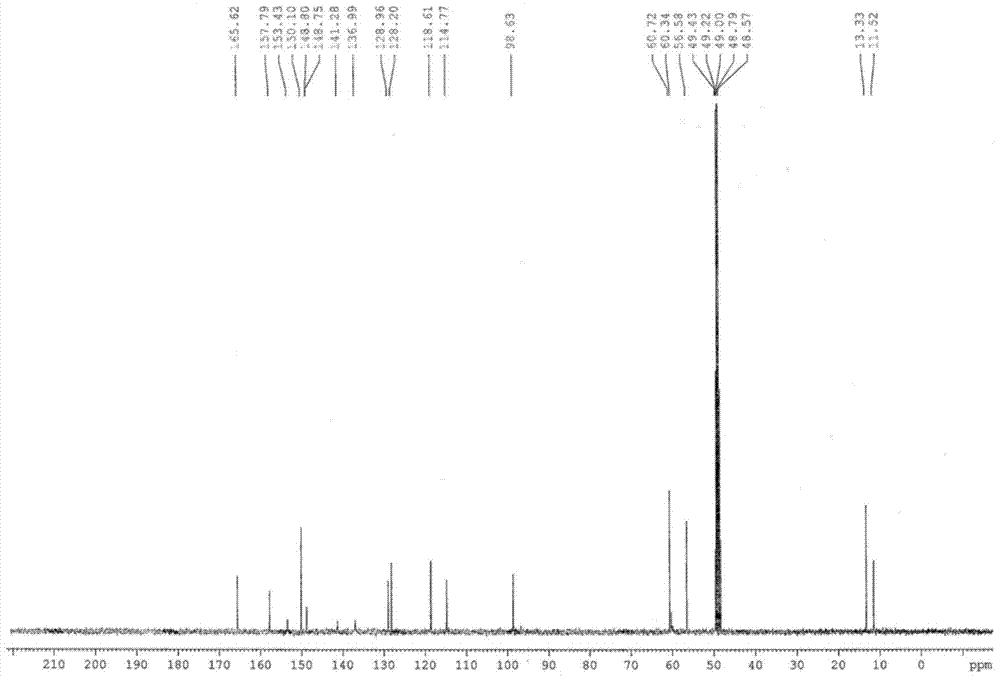
[0038] Embodiment one: the preparation of esomeprazole magnesium trihydrate
[0039] Add 62g (0.188mol) omeprazole sulfide, 20g (0.097mol) D-diethyl tartrate, 21g (0.074mol) tetraisopropyl titanate, 220g n-butyl acetate, 0.4g water into 500mL three-neck In the bottle, heat up to 50°C, stir and react for 1.5 hours; cool down to 10°C, add 2.0g benzyltriethylammonium chloride (TEBA), 240g sodium hypochlorite solution (available chlorine content is 4% to 5%), and control the temperature The reaction was carried out at 10°C for 3 hours. The reaction is monitored by high-performance liquid chromatography, and when the omeprazole sulfide content is below 1%, the reaction is stopped to obtain esomeprazole. Subsequently, add 700g mass concentration to reaction system and be that the sodium hydroxide aqueous solution of 12.5% extracts, and esomeprazole forms esomeprazole sodium and is dissolved in alkali water layer; Discard n-butyl acetate layer, collects alkali water layer, add 22...
Embodiment 2
[0041] Embodiment two: the preparation of esomeprazole magnesium trihydrate
[0042] Add 80g (0.243mol) omeprazole sulfide, 28.4g (0.139mol) D-diethyl tartrate, 41.4g (0.146mol) tetraisopropyl titanate, 320g n-pentyl acetate, 0.7g water into 500mL In the three-necked flask, heat up to 50°C, stir and react for 1.5 hours; cool down to 10°C, add 1.0g benzyltriethylammonium chloride (TEBA), 320g sodium hypochlorite solution (available chlorine content is 4% to 5%), The temperature was controlled and reacted at 9°C for 4 hours. The reaction is monitored by high-performance liquid chromatography, and when the omeprazole sulfide content is below 1%, the reaction is stopped to obtain esomeprazole. Subsequently, add 850g mass concentration to reaction system and be that the aqueous sodium hydroxide solution of 12.5% extracts, and esomeprazole forms esomeprazole sodium and is dissolved in alkaline water layer; Discards n-pentyl acetate layer, collects alkaline water layer, add 320g ...
Embodiment 3
[0044] Embodiment three: the preparation of esomeprazole magnesium trihydrate
[0045] Get the n-butyl acetate layer discarded after the completion of the reaction of Example 1, wash 3 times with 200g water, wash the n-butyl acetate layer to neutrality, then add anhydrous sodium sulfate and molecular sieves to dry, and n-butyl acetate The moisture content of ester layer is controlled below 0.08%, obtains the n-butyl acetate that reclaims.
[0046] Add 100g (0.304mol) omeprazole sulfide, 50g (0.176mol) tetraisopropyl titanate, 0.5g water and recovered n-butyl acetate (containing catalyst D-diethyl tartrate) into a 500mL three-necked flask In the medium, heat up to 50°C, stir and react for 1.5 hours; cool down to 5°C, add 1.0g benzyltriethylammonium chloride (TEBA), 380g sodium hypochlorite solution (available chlorine content is 4% to 5%), and control the temperature at React at 8°C for 4 hours. The reaction is monitored by high-performance liquid chromatography, and when the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com