Method for preparing stable esomeprazole enteric-coated pills
A technology for esomeprazole enteric and esomeprazole magnesium is applied in the field of preparation of esomeprazole enteric-coated pellets, and can solve the problems of enteric-coated drugs disintegration and poor drug dissolution, influence and complicated preparation process and other problems to achieve the effect of saving time, overcoming the complexity of the preparation process and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Compatibility experiment between embodiment 1 bulk drug and auxiliary material
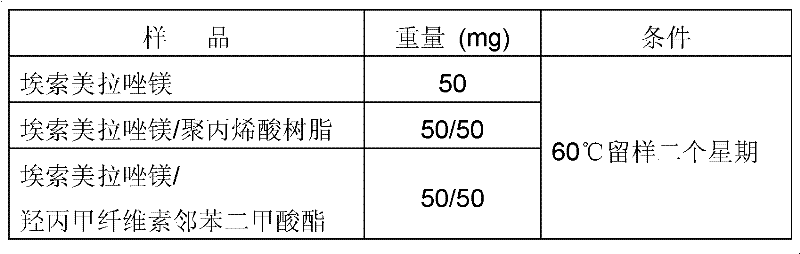
[0030] 1. Mix 50mg of esomeprazole magnesium and 50mg of enteric-coated materials together, put them in a sample box at 60°C under the condition of sealing and avoiding light, and take samples at one week and two weeks for Esso Meprazole magnesium content detection. The proportioning ratio between raw materials and auxiliary materials is shown in Table 1.
[0031] Under table 1 anhydrous condition, the proportioning between bulk drug and auxiliary material
[0032]
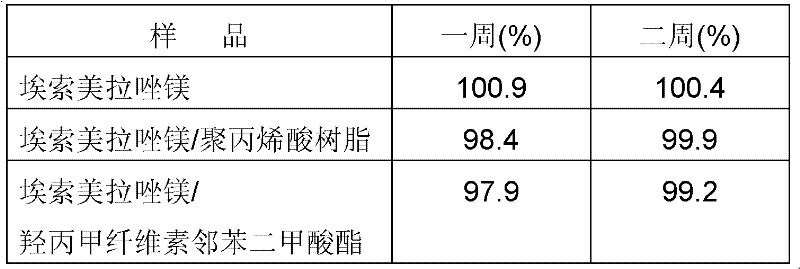
[0033] Result: The content of esomeprazole magnesium in samples determined at one week and two weeks is shown in Table 2.
[0034] Under table 2 anhydrous condition, the compatibility test result between bulk drug and auxiliary material
[0035]
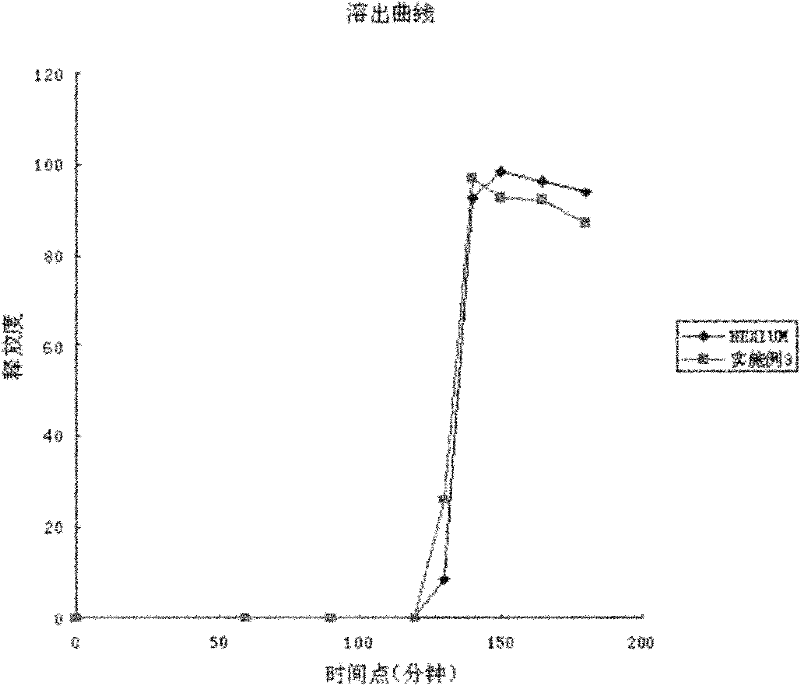
[0036]According to the content detection results in Table 2, the content of the active ingredient did not change significantly in the two-week reserved sample experiment, and...
Embodiment 2
[0045] 1. Preparation of pill core:
[0046] The ratio of each substance is as follows:
[0047]
[0048] The preparation steps are as follows:
[0049] 1) Dissolve hypromellose in 1000g of water, add esomeprazole magnesium into the above solution, stir and disperse evenly;
[0050] 2) Calcium carbonate and talcum powder are evenly dispersed in the water of 500g;
[0051] 3) Add the dispersion in step 2) to the dispersion in step 1), stir evenly, and set aside;
[0052] 4) Wrap the mixed solution in step 3) on the surface of the blank sugar core with a fluidized bed to prepare a pill-containing core. During the whole process, the temperature of the product is controlled at 25-30°C, and the ventilation volume is 1.0-1.4m 3 / min / kg, the spraying speed is 10-15g / min / kg, and the free moisture (detection value: 1.54%) of control containing pill core.
[0053] 2. Coating of enteric coating:
[0054] The ratio of each substance is as follows:
[0055]
[0056] The prepar...
Embodiment 3
[0065] 1. Preparation of drug-containing pill core:
[0066] The ratio of each substance is as follows:
[0067]
[0068] The preparation steps are as follows:
[0069] 1) Dissolve hypromellose in 1000g of water, add esomeprazole magnesium into the above solution, stir and disperse evenly;
[0070] 2) Calcium carbonate and talcum powder are evenly dispersed in 500g of water;
[0071] 3) Add the dispersion in step 2) to the dispersion in step 1), stir evenly, and set aside;
[0072] 4) Wrap the mixed solution in step 3) on the surface of the blank sugar core with a fluidized bed to prepare a pill-containing core. During the whole process, the temperature of the product is controlled at 30-40°C, and the ventilation volume is 0.7-1.0m 3 / min / kg, the spraying speed is 5-8g / min / kg, and the free moisture (detection value: 1.81%) of control containing pill core.
[0073] 2. Coating of enteric coating:
[0074] The ratio of each substance is as follows:
[0075]
[0076] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com