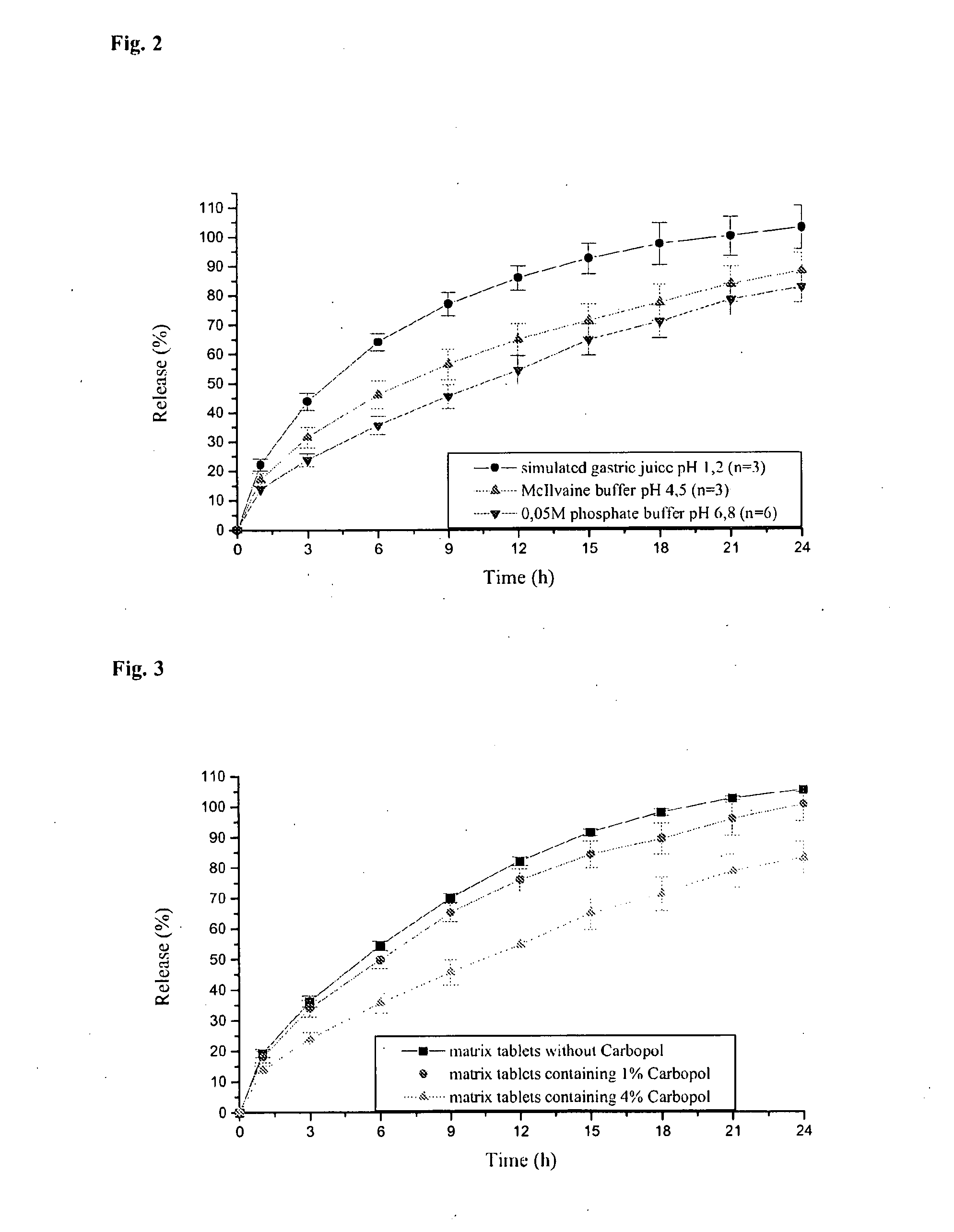
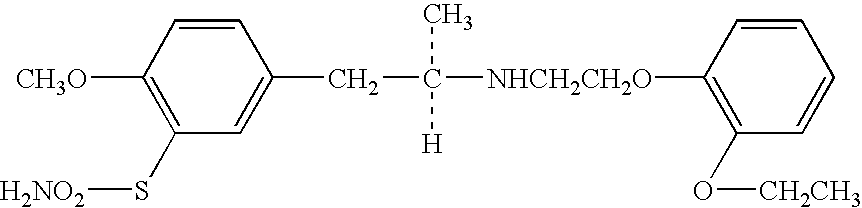
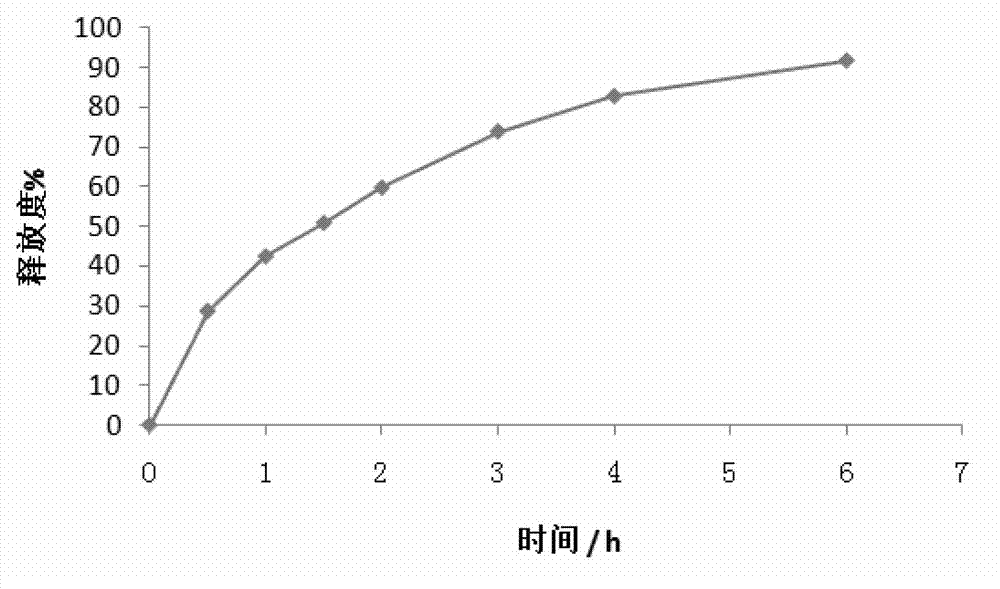
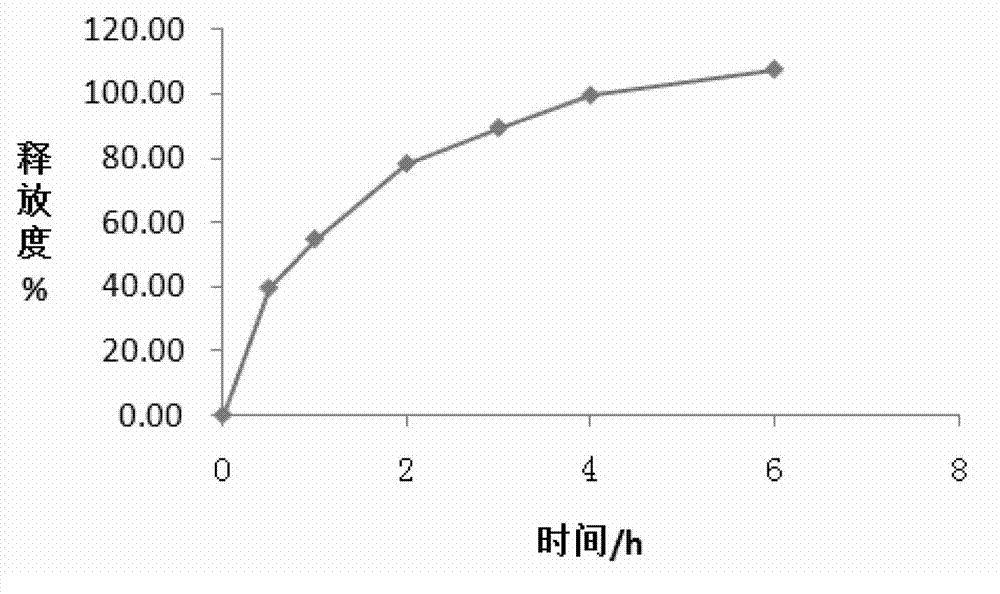
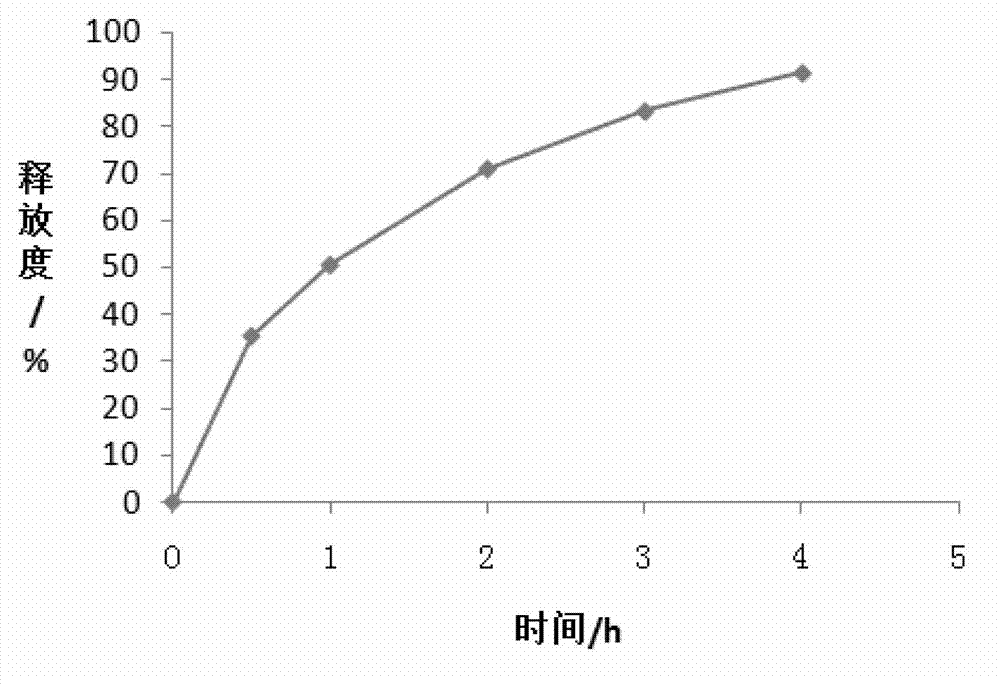
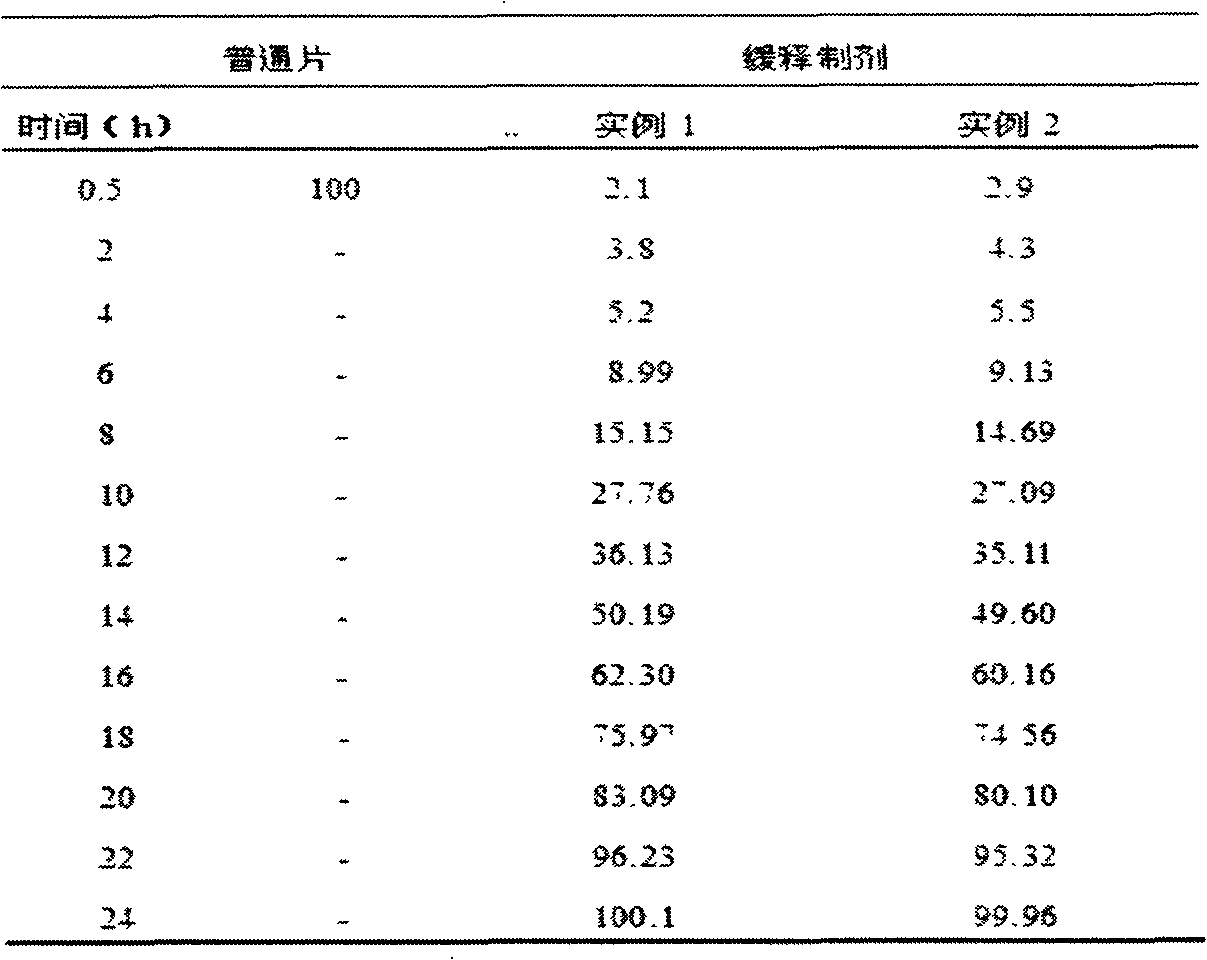
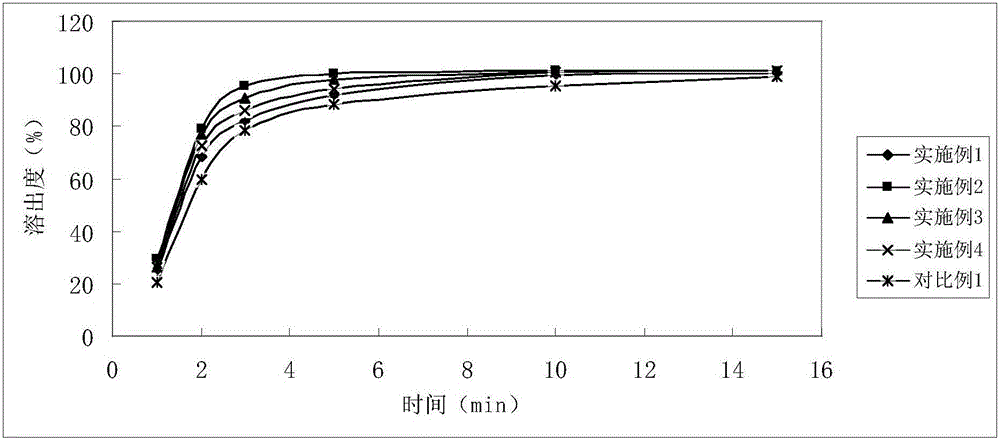
Patents
Literature
432results about How to "Good content uniformity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
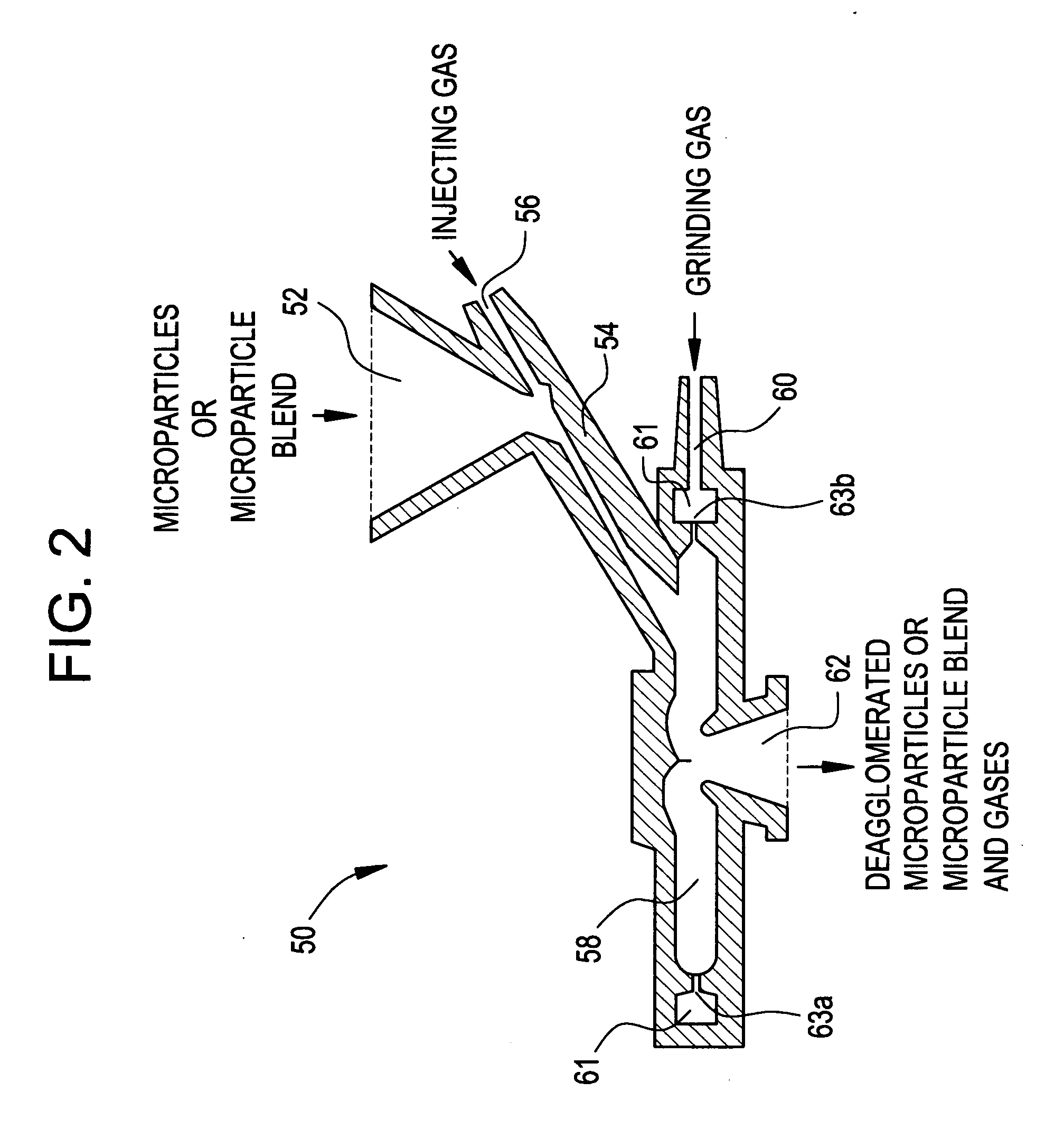
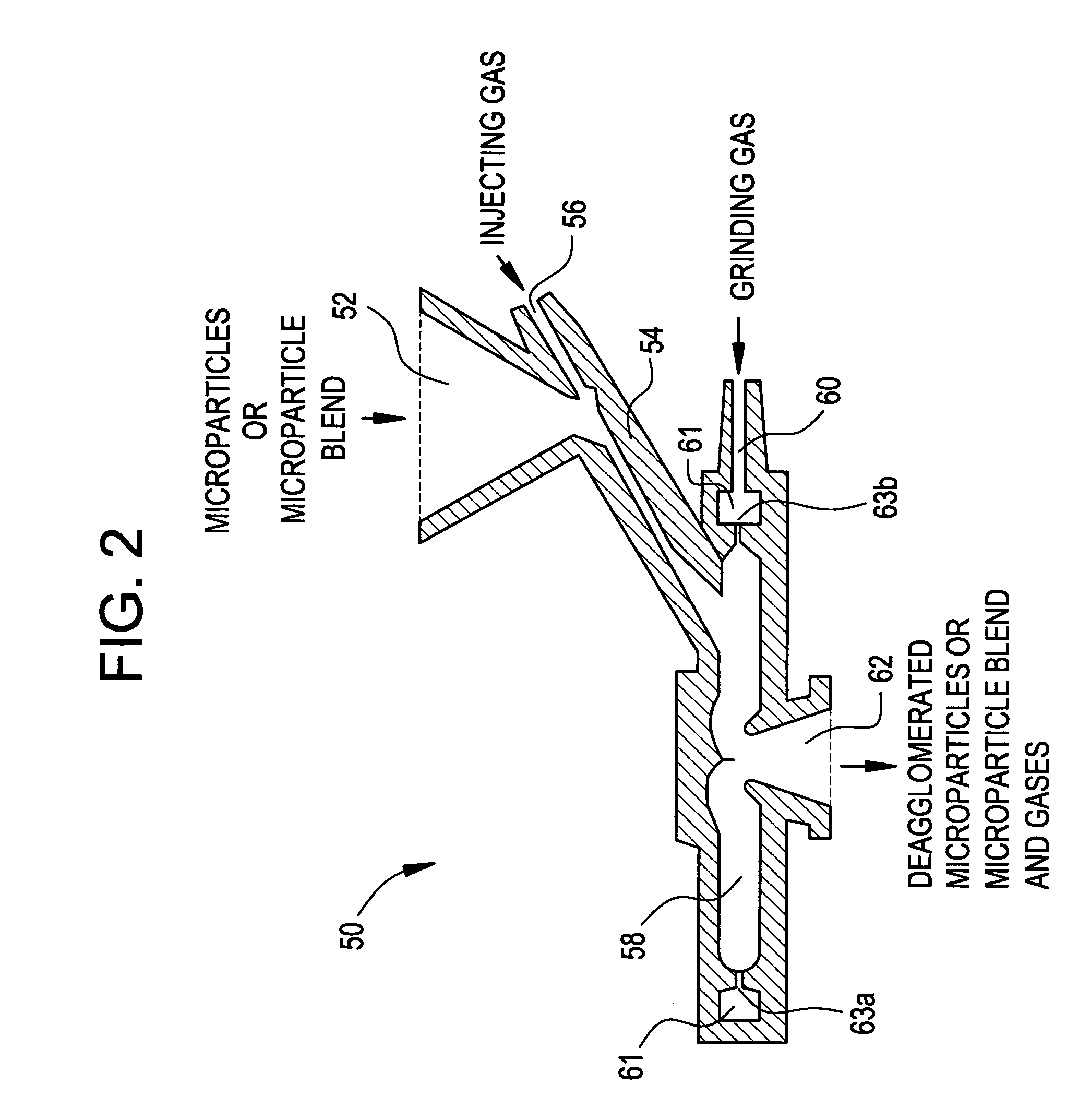
Methods for making pharmaceutical formulations comprising deagglomerated microparticles
InactiveUS20060093678A1High crystallinityReduce contentPowder deliveryGranulation by liquid drop formationMicroparticleVolume average
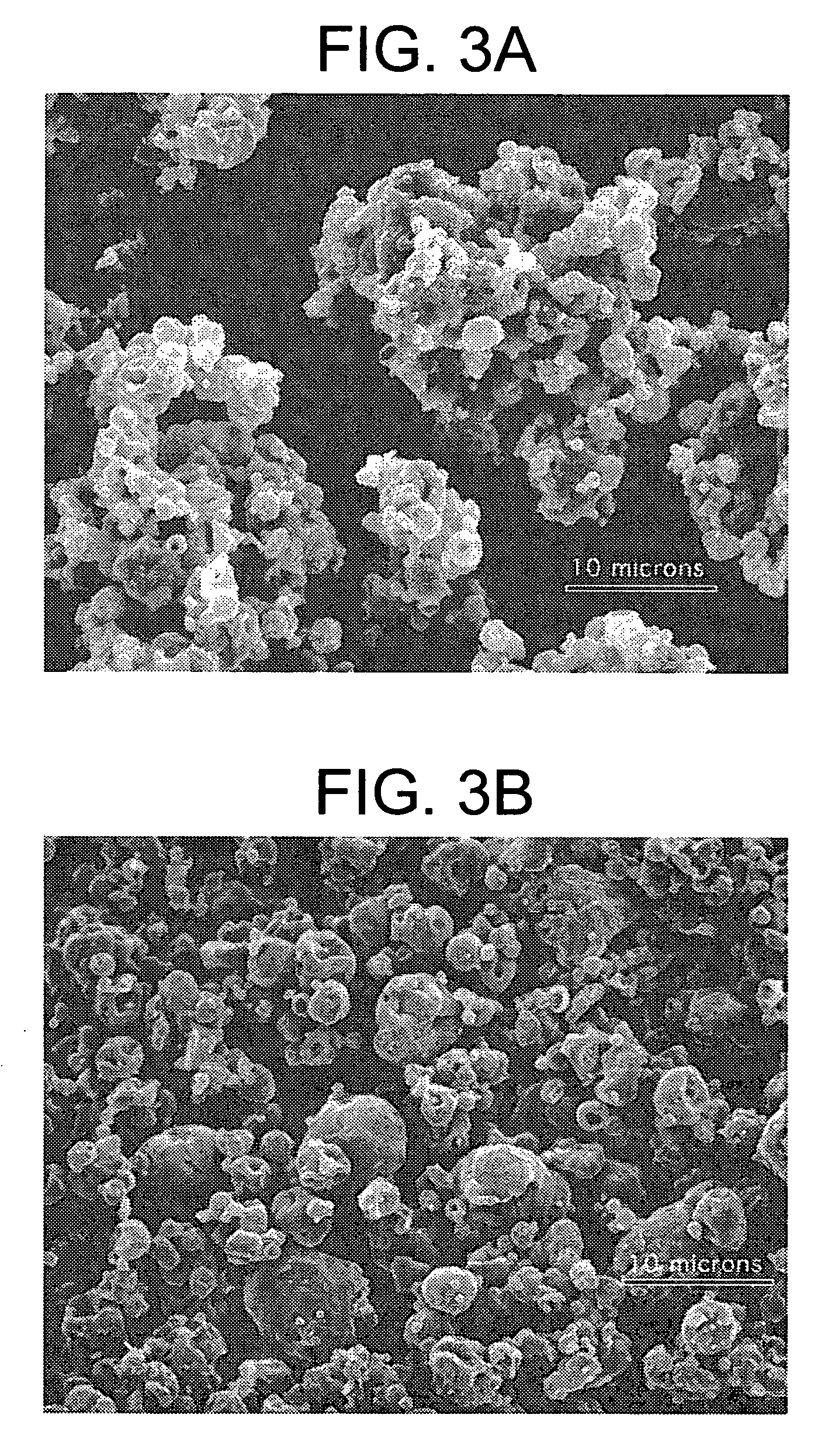
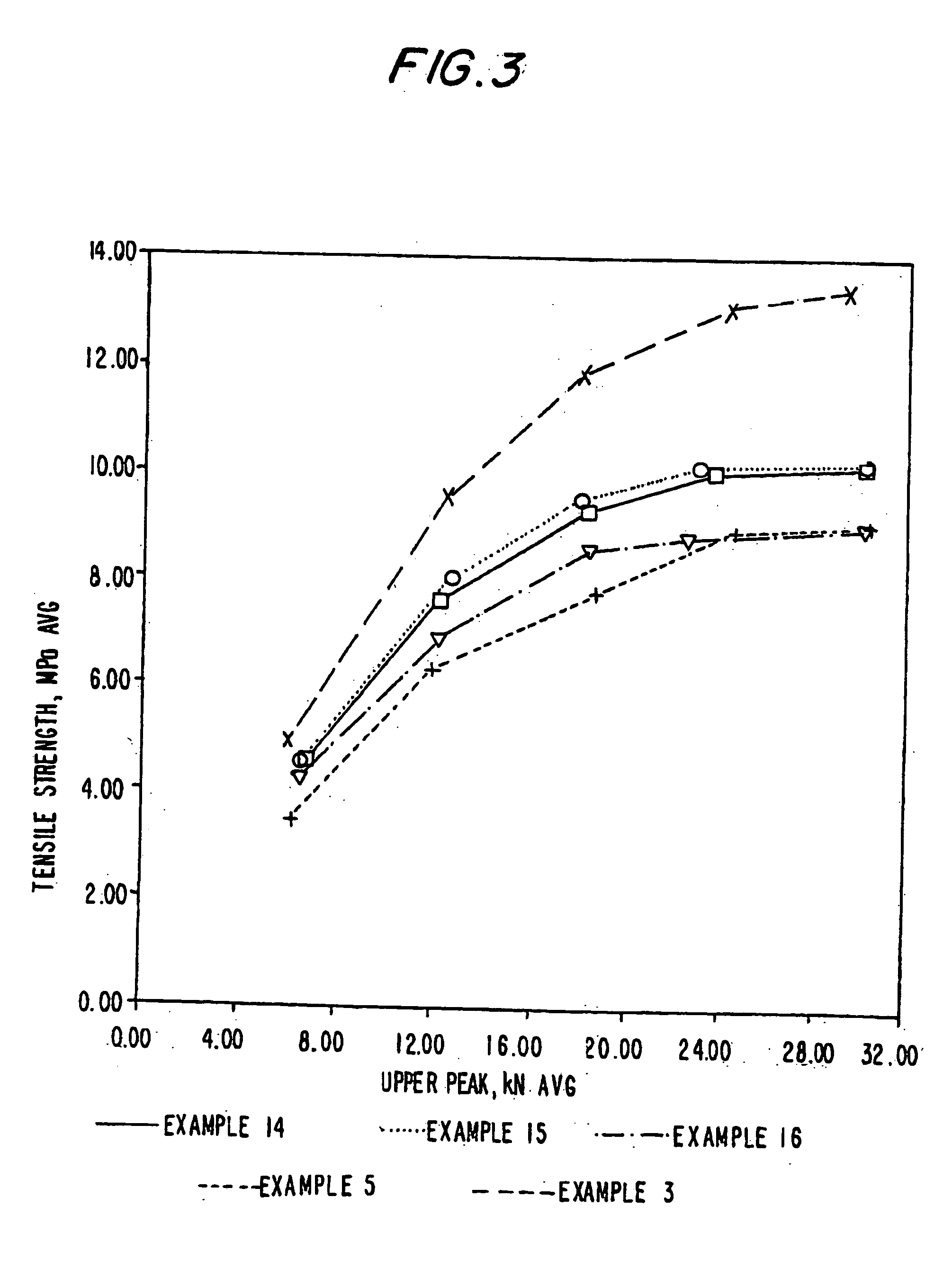
Methods are provided for making a dry powder blend pharmaceutical formulation comprising (i) forming microparticles which comprise a pharmaceutical agent; (ii) providing at least one excipient in the form of particles having a volume average diameter that is greater than the volume average diameter of the microparticles; (iii) blending the microparticles with the excipient to form a powder blend; and (iv) jet milling the powder blend to deagglomerate at least a portion of any of the microparticles which have agglomerated, while substantially maintaining the size and morphology of the individual microparticles. Jet milling advantageously can eliminate the need for more complicated wet deagglomeration processes, can lower residual moisture and solvent levels in the microparticles (which leads to better stability and handling properties for dry powder formulations), and can improve wettability, suspendability, and content uniformity of dry powder blend formulations.
Owner:CHICKERING DONALD E III +6
Pharmaceutical excipient having improved compressibility
InactiveUS6936277B2Improve compression performanceHigh compression compactibilityPowder deliveryPill deliveryActive agentMedicine
A method of preparing an excipient composition includes forming an aqueous slurry containing a mixture of microcrystalline cellulose in the form of a wet cake and a surfactant, said surfactant being present in an amount from about 0.1% to about 0.5% by weight of the wet-cake microcrystalline cellulose; and drying said slurry to obtain an excipient comprising a plurality of agglomerated particles of microcrystalline cellulose in intimate association with said surfactant. The excipient may be mixed with a therapeutically active agent to form a dosage form. The surfactant provides a hydrophobic boundary at cellulose surfaces, and improves absorptivity of the therapeutically active agent.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Stable low digestive enzyme content formulation
ActiveUS20120201875A1Good content uniformityMinimal loss of enzymatic activityPeptide/protein ingredientsMetabolism disorderMedicineDigestive enzyme
The present invention is directed to a pharmaceutical composition or dosage form having a stable, low (diluted) digestive enzyme content comprising at least one digestive enzyme and at least one carrier, or a dosage form thereof. The invention is also directed to a process of preparation of the composition or the dosage form. In addition the invention is directed to the treatment and prevention of disorders or conditions associated with a digestive enzyme deficiency in a patient in need thereof, comprising administering to said patient a pharmaceutically acceptable amount of the composition having a stable low digestive enzyme content or dosage form thereof.
Owner:SOC DES PROD NESTLE SA
Manufacture of multiple minicapsules
ActiveUS20110052645A1Pharmaceutical effectivenessReduce colonic bacterial flora damageBiocideGlass/slag layered productsEngineeringMechanical engineering
Owner:SUBLIMITY THERAPEUTICS LTD
Pharmaceutical excipient having improved compressibility
InactiveUS20060008522A1Improve compression performanceHigh compression compactibilityWood working apparatusPill deliveryActive agentSlurry
A method of preparing an excipient composition includes forming an aqueous slurry containing a mixture of microcrystalline cellulose in the form of a wet cake and a surfactant, said surfactant being present in an amount from about 0.1% to about 0.5% by weight of the wet-cake microcrystalline cellulose; and drying said slurry to obtain an excipient comprising a plurality of agglomerated particles of microcrystalline cellulose in intimiate association with said surfactant. The excipient may be mixed with a therapeutically active agent to form a dosage form. The surfactant provides a hydrophobic boundary at cellulose surfaces, and improves absorptivity of the therapeutically active agent.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Methods for making pharmaceutical formulations comprising deagglomerated microparticles
InactiveUS20060093677A1High crystallinityReduce contentPowder deliveryGranulation by liquid drop formationMicroparticleVolume average
Methods are provided for making a dry powder blend pharmaceutical formulation comprising (i) forming microparticles which comprise a pharmaceutical agent; (ii) providing at least one excipient in the form of particles having a volume average diameter that is greater than the volume average diameter of the microparticles; (iii) blending the microparticles with the excipient to form a powder blend; and (iv) jet milling the powder blend to deagglomerate at least a portion of any of the microparticles which have agglomerated, while substantially maintaining the size and morphology of the individual microparticles. Jet milling advantageously can eliminate the need for more complicated wet deagglomeration processes, can lower residual moisture and solvent levels in the microparticles (which leads to better stability and handling properties for dry powder formulations), and can improve wettability, suspendability, and content uniformity of dry powder blend formulations.
Owner:CHICKERING DONALD E III +6
Process for preparing stabilized vitamin D
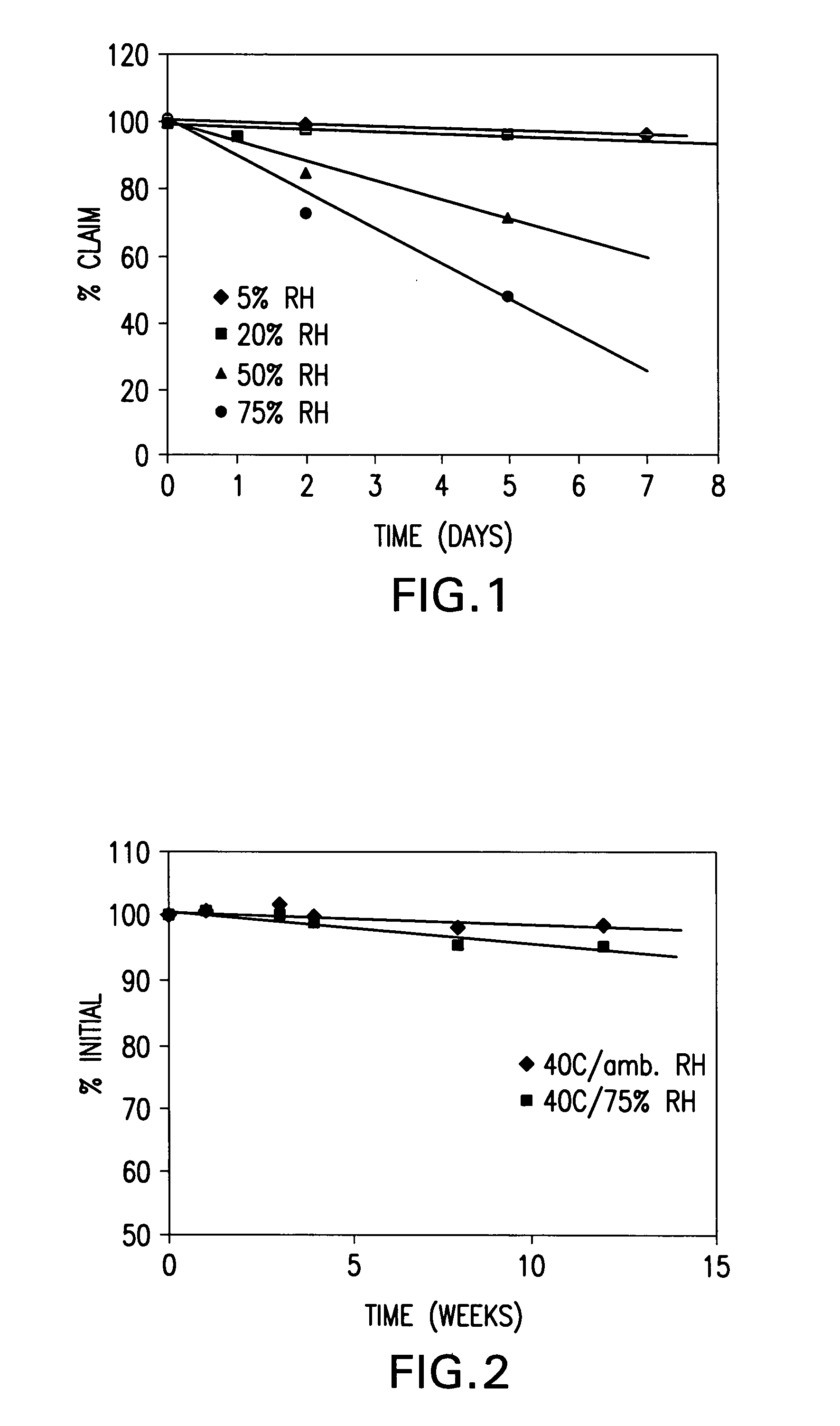
InactiveUS20060019933A1Improve stabilityGood content uniformityBiocideOrganic active ingredientsSURFACTANT BLENDVitamin D+Metabolites
This invention is related to a process for preparing a stabilized form of vitamin D which comprises the steps of: a) dissolving vitamin D in a solution that contains water and at least one surfactant to produce a mixture; b) spraying the mixture onto an inert carrier to produce a wet mass; and c) drying the wet mass obtain the stabilized form of vitamin D.
Owner:MERCK & CO INC
Cellulose powder having excellent segregation preventive effect, and compositions thereof
ActiveUS20110062630A1Easy to solveGood content uniformityOrganic active ingredientsCosmetic preparationsChemistryCellulose Powders
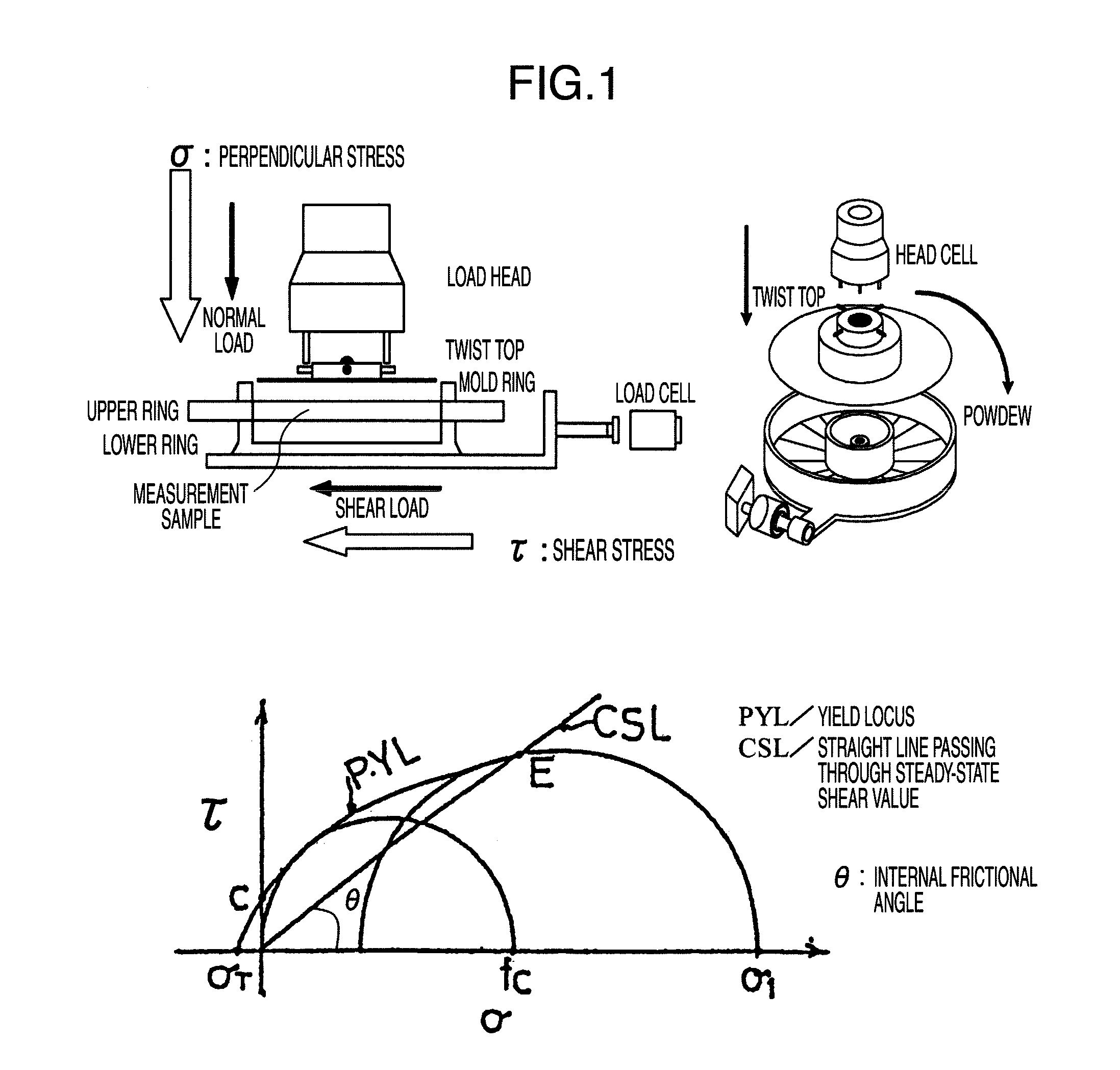
With regard to compositions derived from active ingredients and other additives in medical, food and industrial fields, there have been problems that the active ingredients cannot be uniformly mixed, and that the active ingredients become segregated and lose uniformity as the active ingredients undergo transport, input, and filling processes. There is provided a cellulose powder which improves the uniformity of compositions containing active ingredients or other additives to prevent segregation of the active ingredients, wherein the cellulose powder contains cellulose I type crystals, has an average particle diameter of less than 30 μm, a powder density of 0.1 to 0.45 g / cm3, a tapping density of 0.1 to 0.5 g / cm3, a repose angle of 35 to 50°, a specific surface of more than or equal to 0.1 m2 / g and less than 20 m2 / g, an internal friction angle of 36 to 42°, and is a cellulose powder including a secondary flocculation structure in which primary particles are flocculated.
Owner:ASAHI KASEI CHEM CORP
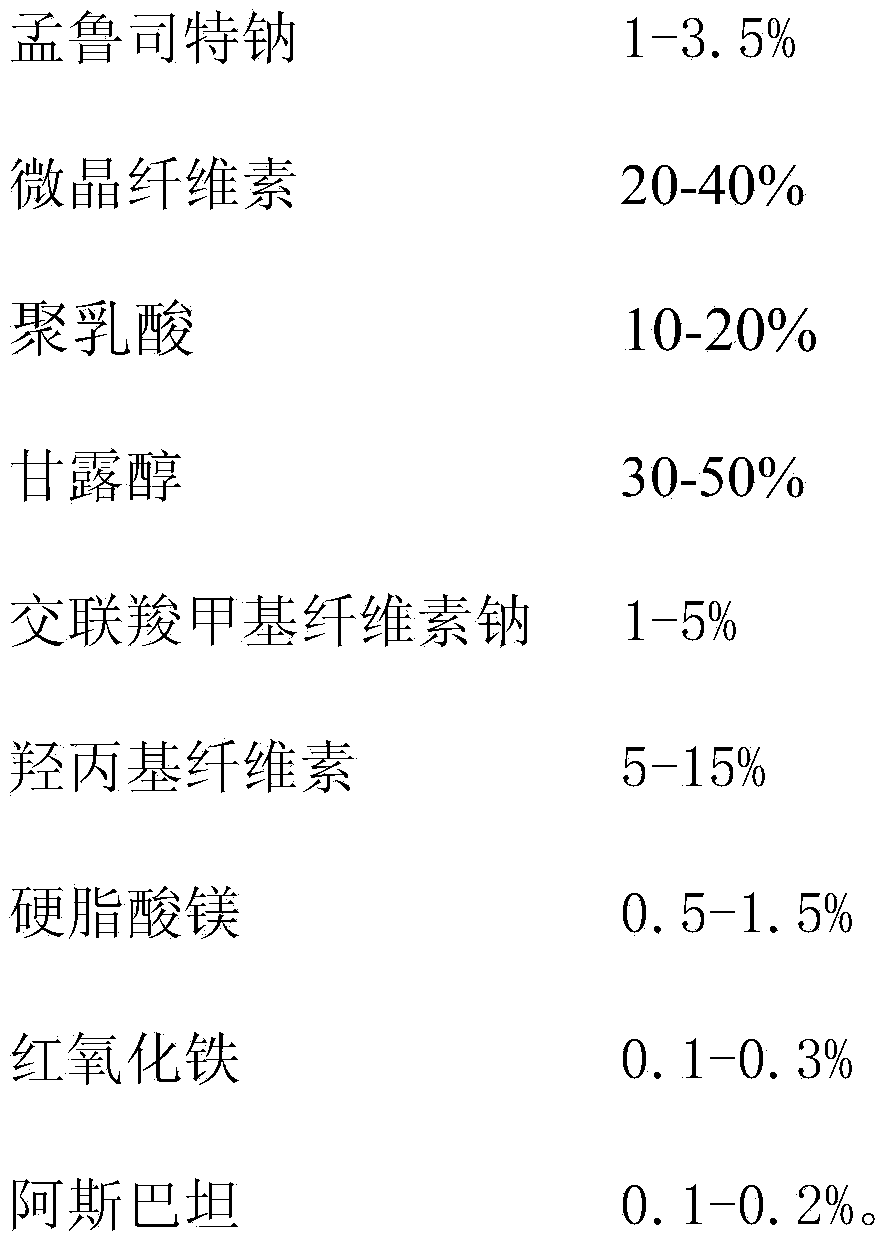
Montelukast sodium chewing tablet prescription and preparation process thereof
ActiveCN103494781AImprove complianceQuality improvementPill deliveryPharmaceutical non-active ingredientsMontelukast SodiumMedical prescription
The invention relates to montelukast sodium chewing tablets and a preparation process thereof. The tablets comprise montelukast sodium and a filling agent, a diluting agent, a bonding agent, a disintegrating agent, and a flavoring. The tablets are prepared with a powder direct tabletting method. The fluidity and compressibility are good. The chewing tablets have long-term storage stability. The tablets are used in prevention and long-term treatment of asthma of children aged 2 to 14, and are used in relieving symptoms caused by allergic rhinitis.
Owner:哈药集团股份有限公司 +1
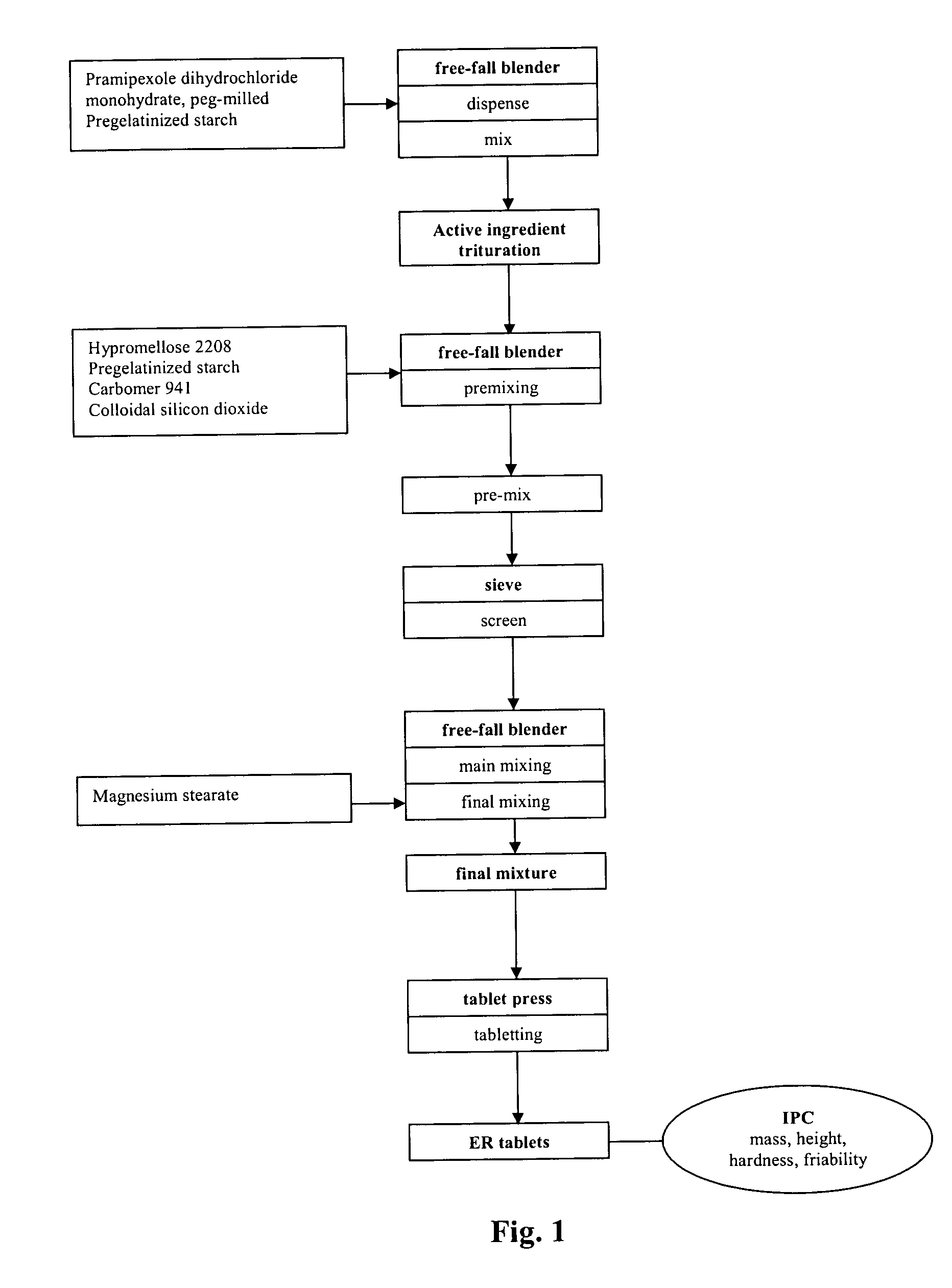
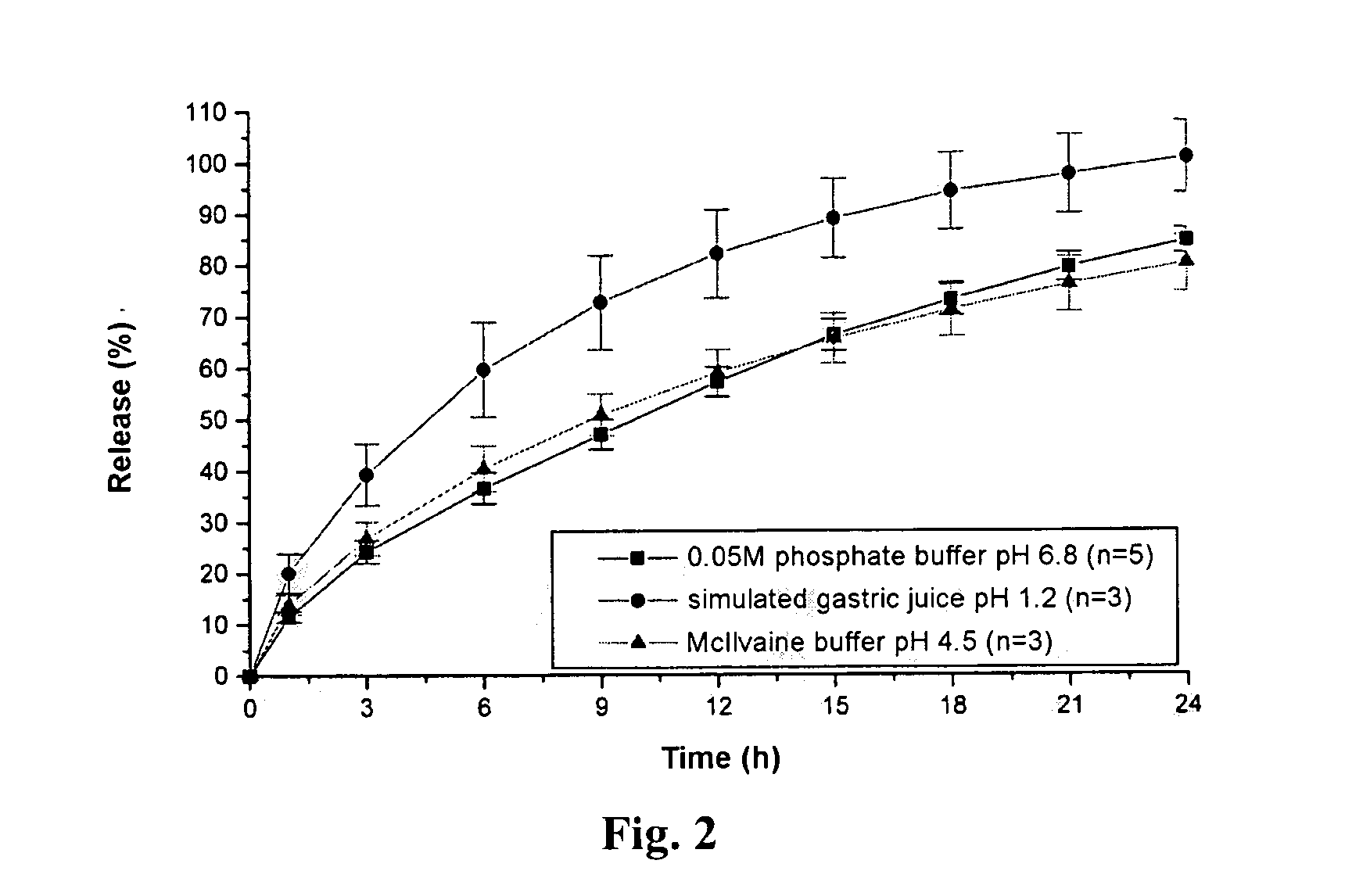
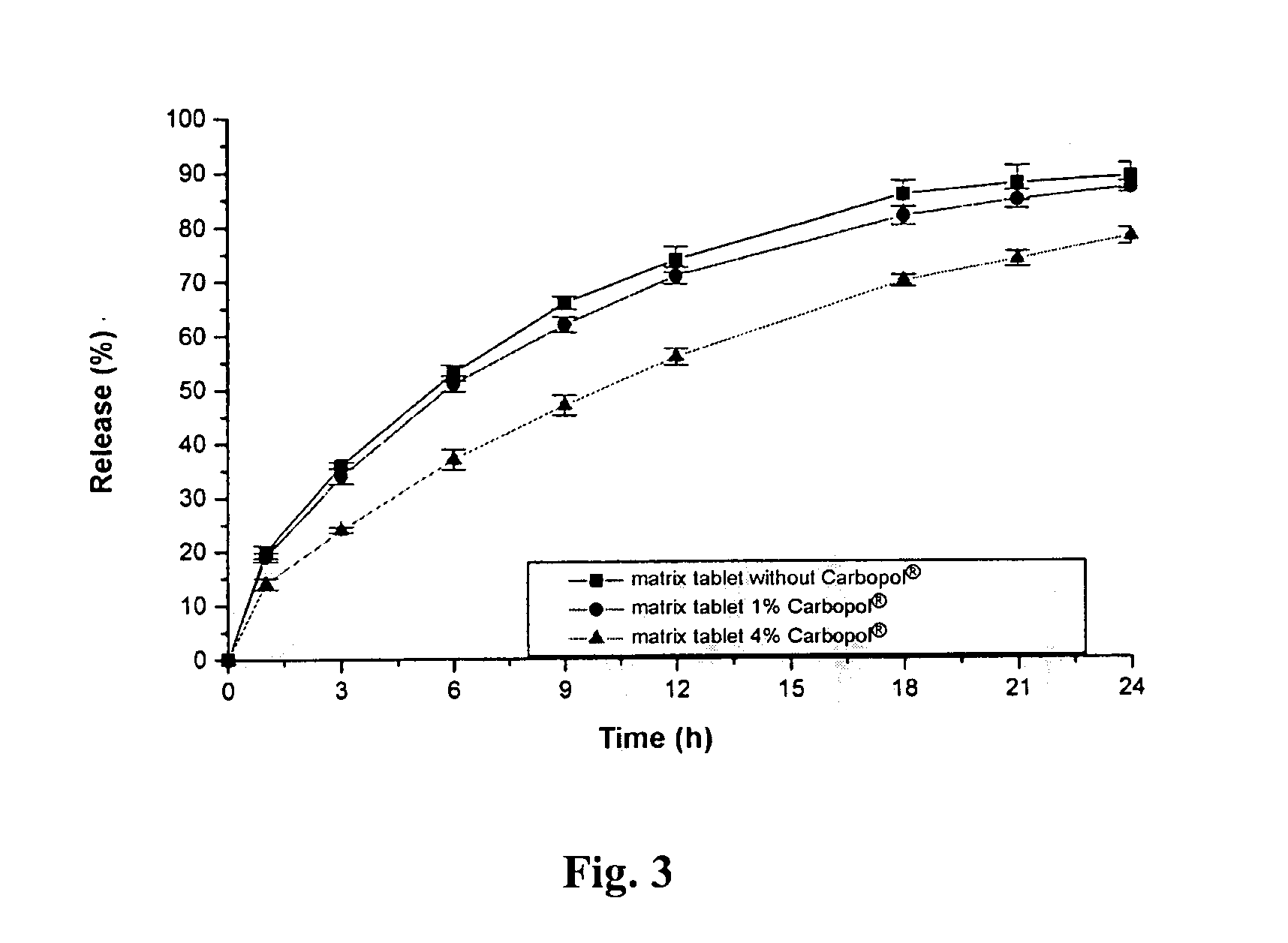
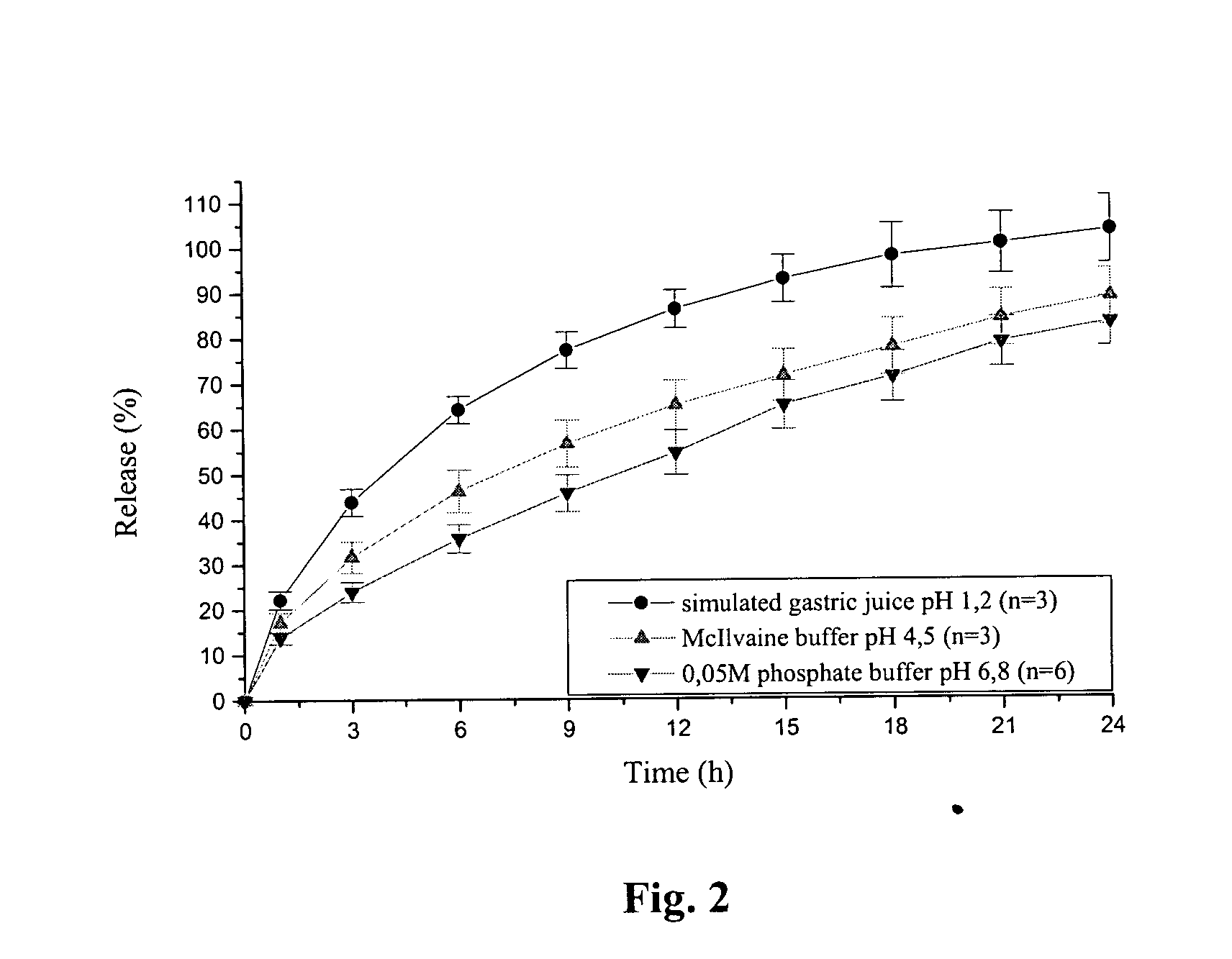
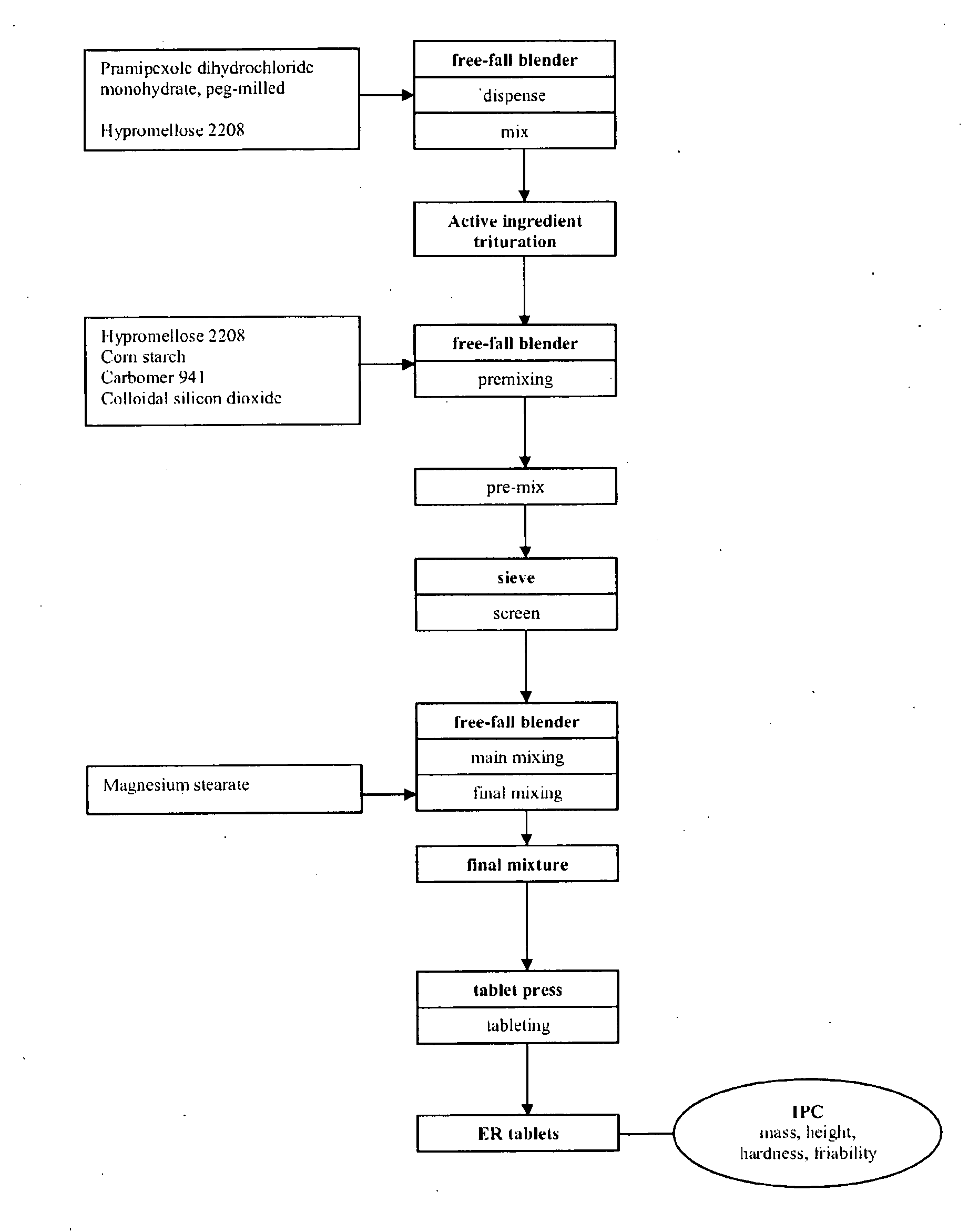
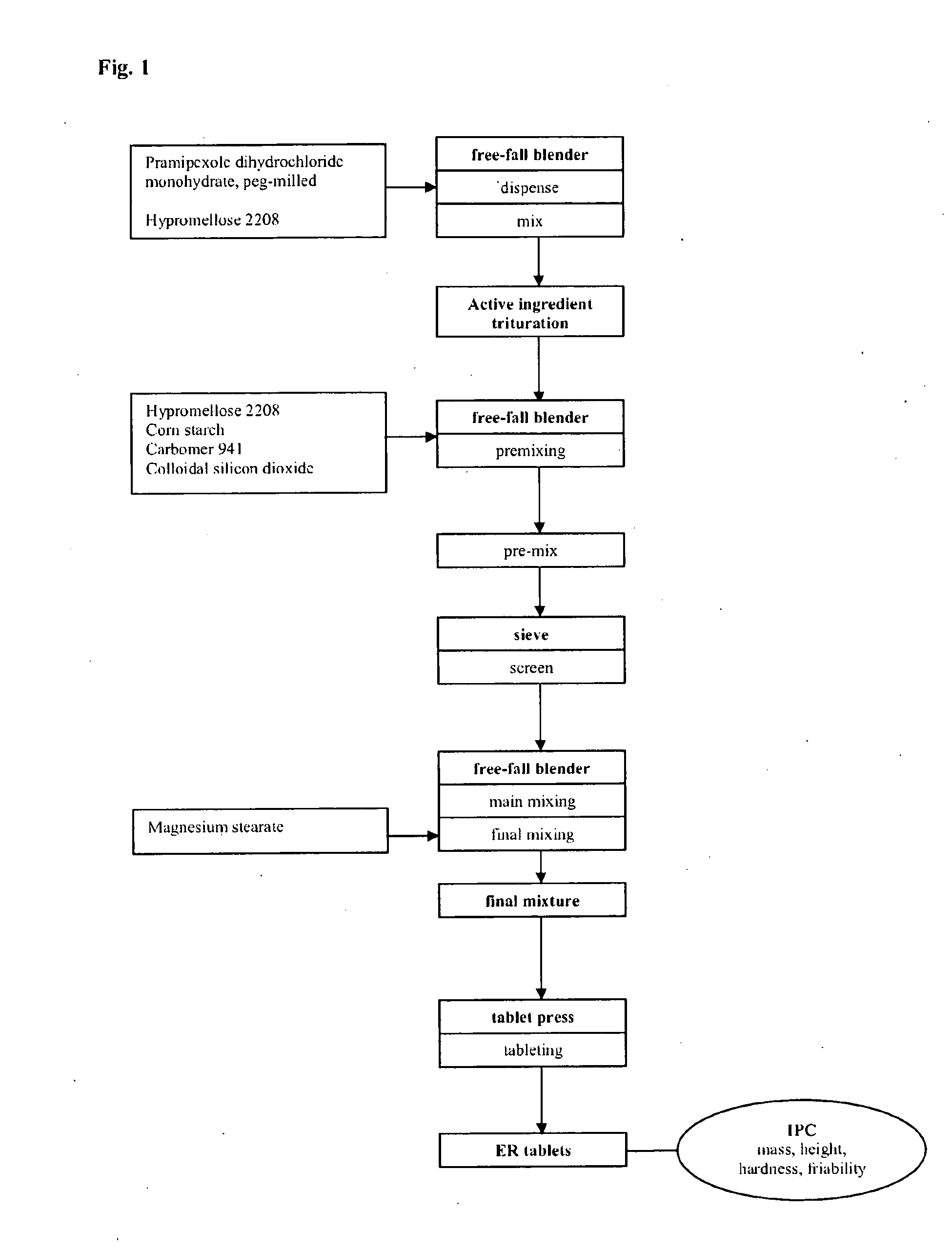
Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
InactiveUS20060051417A1Improve complianceImprove conveniencePowder deliveryOrganic active ingredientsExtended release tabletsPramipexole
An extended release tablet formulation comprising pramipexole or a pharmaceutically acceptable salt thereof in a matrix, the matrix comprising at least two water swelling polymers, wherein one of the polymers is pregelatinized starch, and wherein another one of the polymers is an anionic polymer.
Owner:BOEHRINGER INGELHEIM INT GMBH
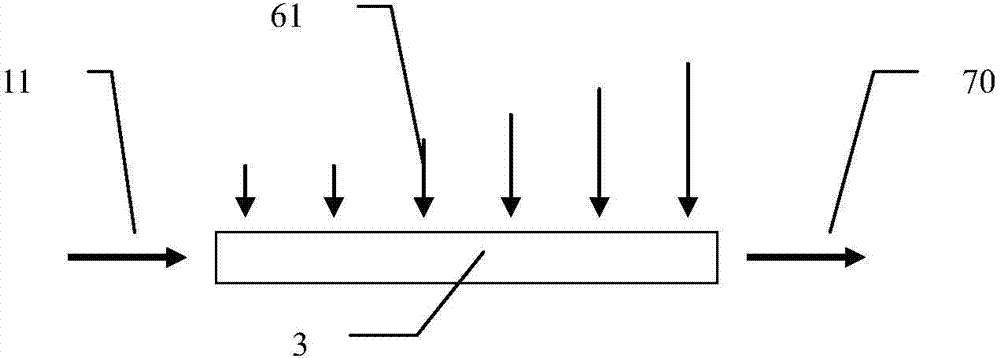
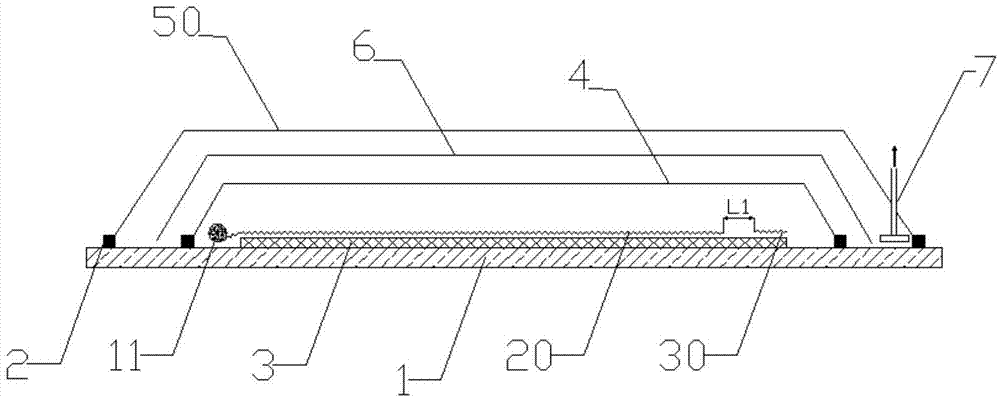
Method for molding resin matrix composite material by zero-adhesive-discharge vacuum assisted resin infusion (VARI)
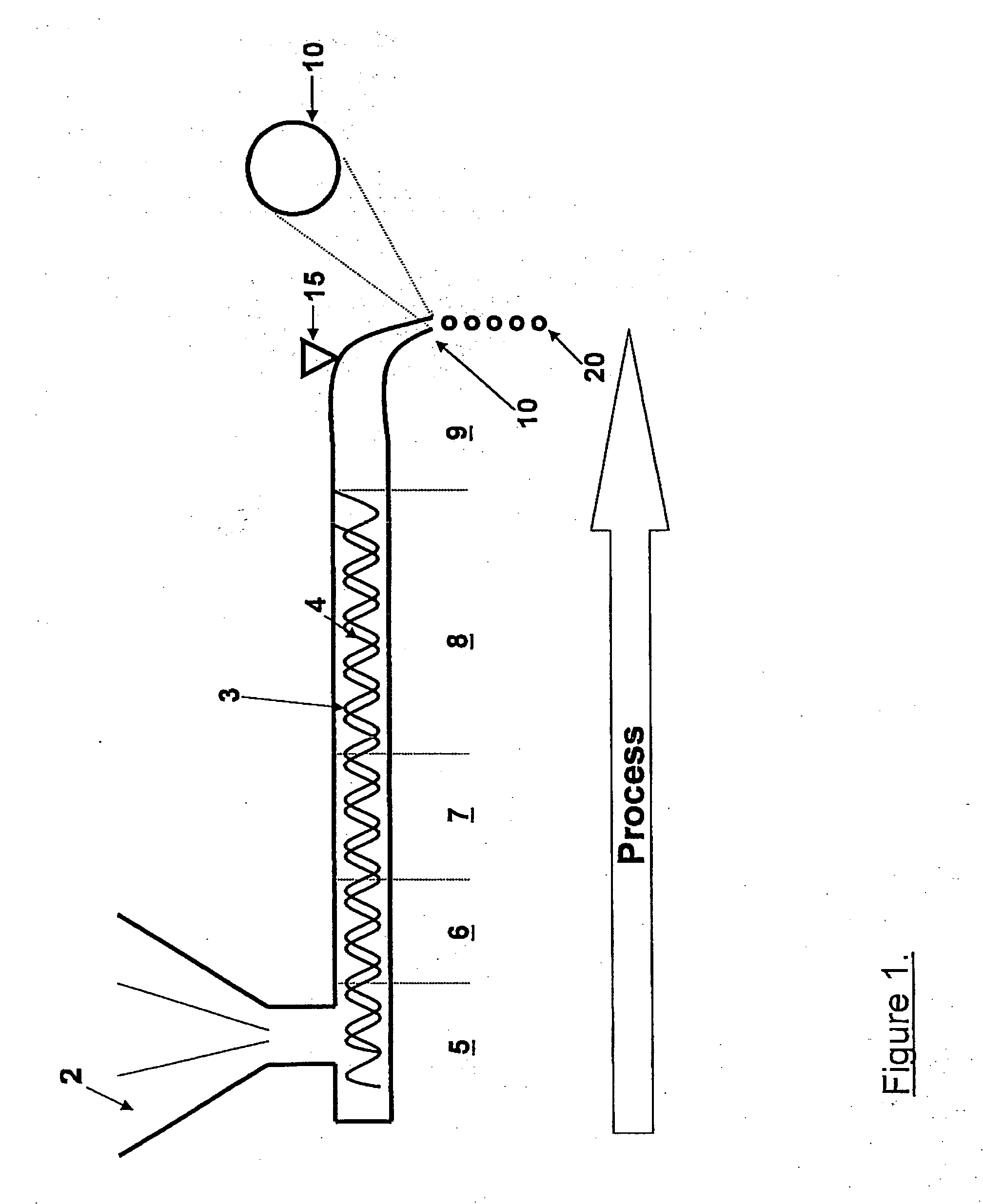
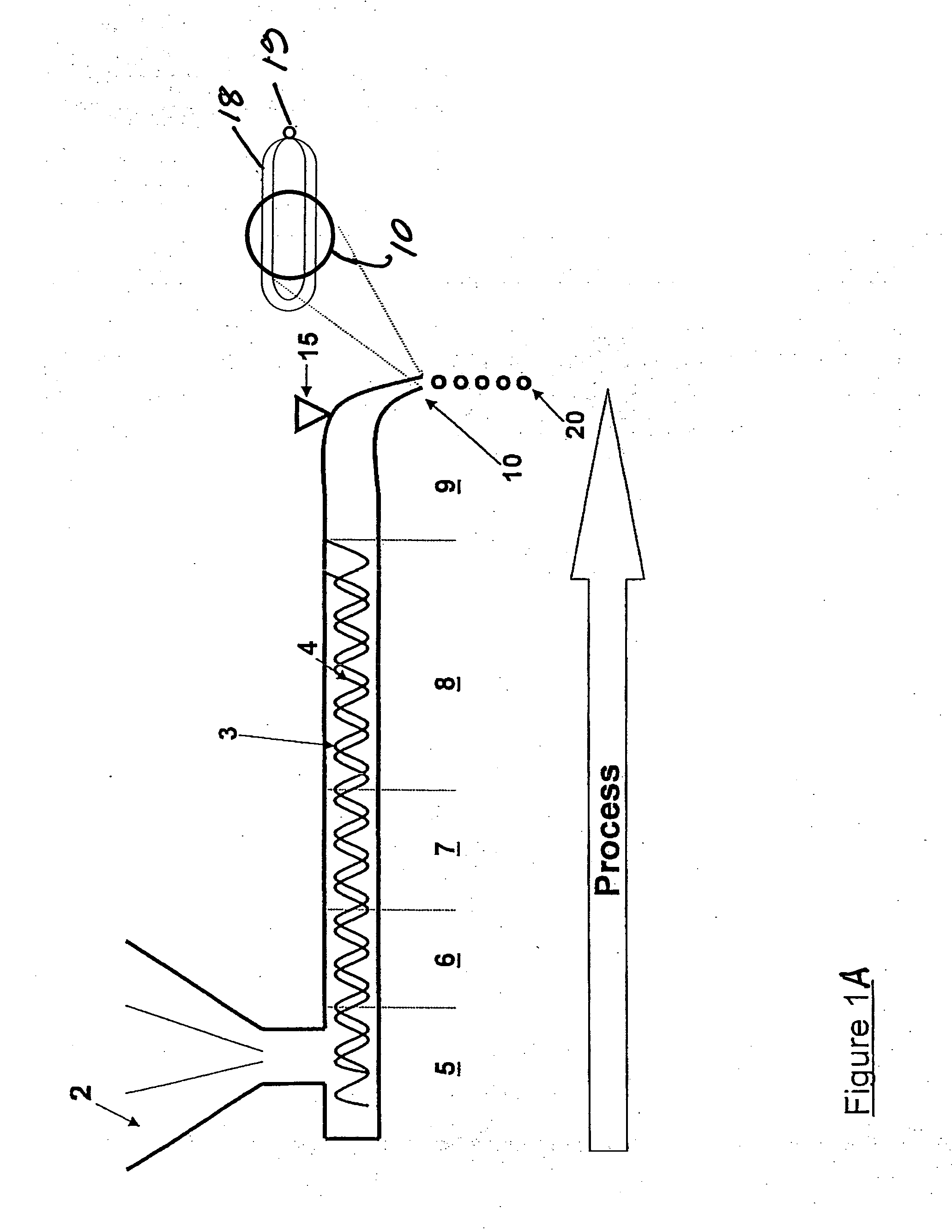
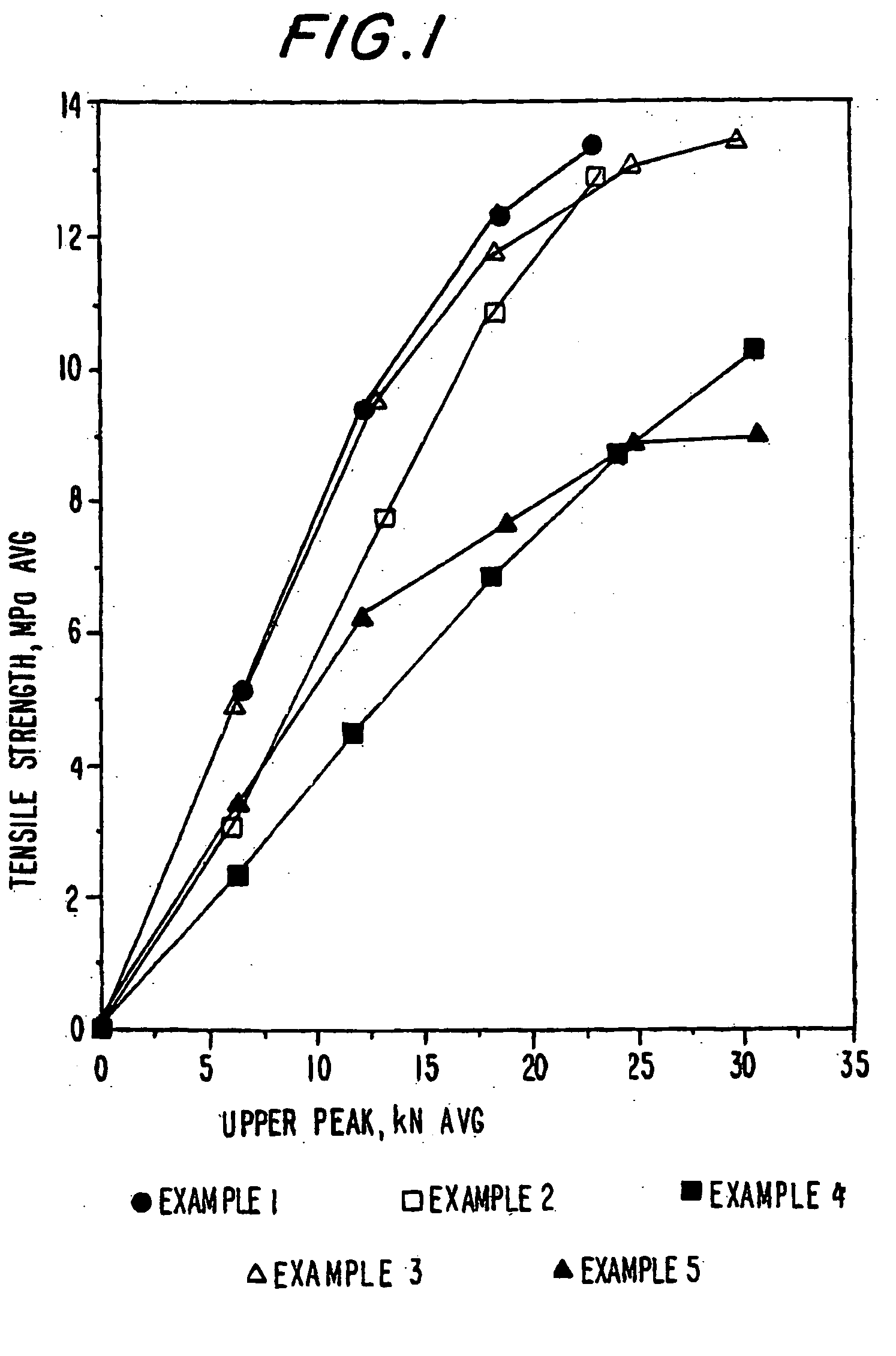
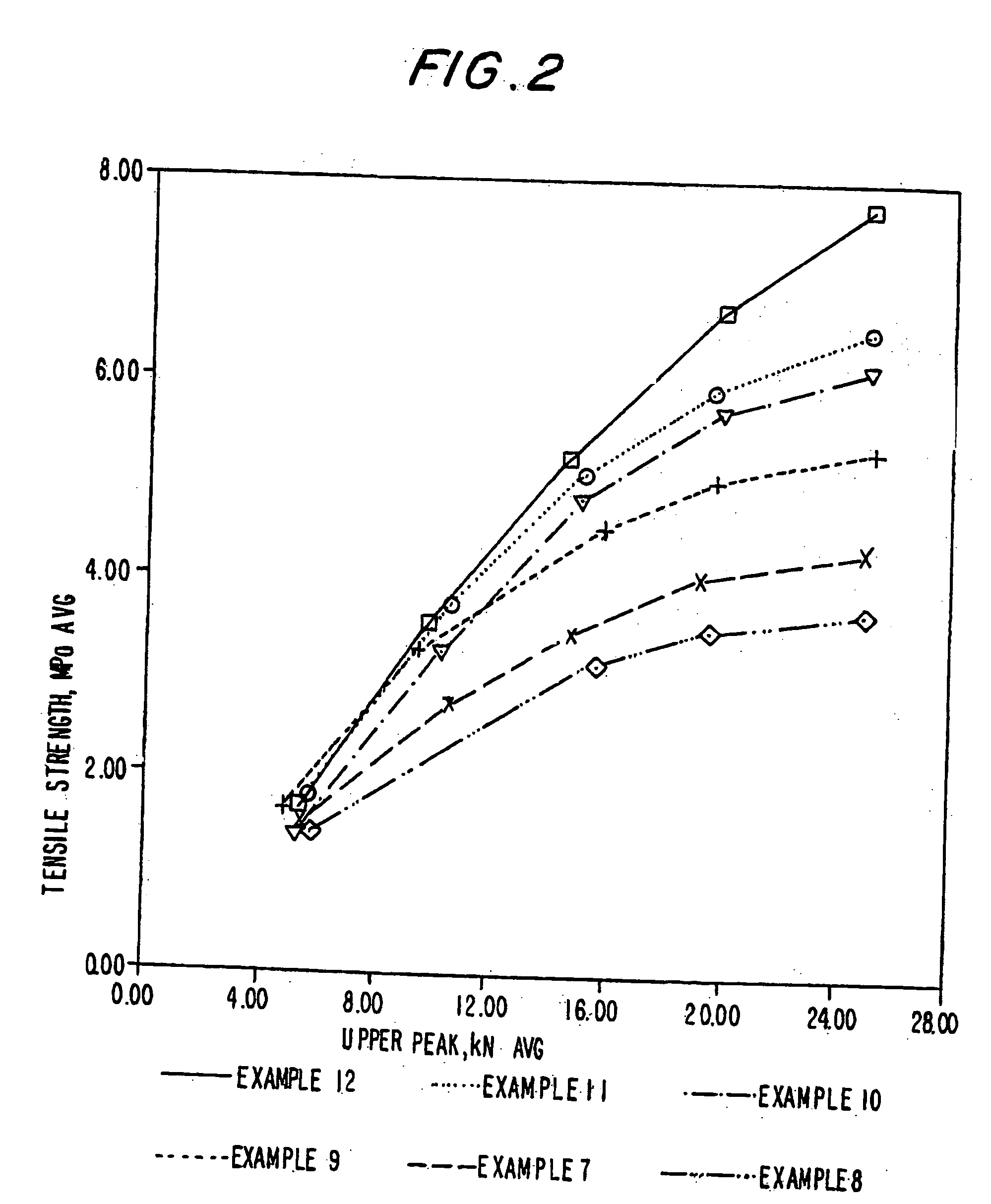
The invention belongs to a resin matrix composite material liquid molding technology, and relates to a method for molding a resin matrix composite material by zero-adhesive-discharge vacuum assisted resin infusion (VARI). A glue inlet diversion net (20) and a resin drawing layer (30) are arranged on a fiber-reinforced premolded body (3); the outer surface of the fiber-reinforced premolded body (3) is covered with a micro-ventilation layer (4) and a glue blocking layer (5). According to the method, a resin glue discharge channel is omitted, so that zero adhesive discharge of the VARI molding technology is realized, and the using amount of resin is accurately controlled; the micro-ventilation layer (4) and the glue blocking layer (5) are additionally arranged, so that pressure is evenly transmitted into the fiber-reinforced premolded body (3); furthermore, the excessive gas in the resin and the fiber-reinforced premolded body (3) can be fully discharged, so that the porosity of parts can be reduced, the molding quality and the thickness uniformity of the parts can be improved, and the production cost is remarkably lowered.
Owner:AVIC COMPOSITES
Method for preparing solid preparation and solid preparation
ActiveCN102106806AEasy to operateImprove securityOrganic active ingredientsNervous disorderWater insolubleWater soluble
The invention discloses a method for preparing a solid preparation, which comprises the following steps of: dissolving active ingredients of water-insoluble and / or lowly water-soluble alkaline medicaments in acidulant-containing acid solution to prepare medicament-containing acid solution; and mixing the medicament-containing acid solution and auxiliary materials uniformly to perform wet-method pelletizing. The invention also discloses the solid preparation prepared by the method. By the method, the defects of serious pollution, large loss and serious potential safety hazard which are caused by mechanical pulverization are overcome, and the method is easy and convenient to operate, has high safety factors and is applied to industrial production easily. The solid preparation prepared by the method has the excellent dissolution characteristic, stability and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Hydrochloric acid cefetamet pivoxil dispersible tablet and method for preparing the same
ActiveCN101219124AThe content of the main drug is uniformGood content uniformityAntibacterial agentsOrganic active ingredientsDissolutionBULK ACTIVE INGREDIENT
The invention discloses a cefetamet pivoxil hydrochloride tablet and a preparation method thereof. On the premise of specific active ingredients of the cefetamet pivoxil hydrochloride, the invention takes into account the varieties and dosages of disintegrating agents and joint usage thereof, suitable components and ratios of bonding agents and other filling agents, and the micronization treatment of the raw and auxiliary materials and selection of corresponding optimized process conditions. Experiment results indicate that compared with the prior art, the product of the invention has quick disintegration and dissolution and stable quality.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
ActiveUS20060198887A1Improve complianceImprove conveniencePowder deliveryOrganic active ingredientsExtended release tabletsPramipexole
Owner:BOEHRINGER INGELHEIM INT GMBH
Modified Release Formulation
InactiveUS20090041844A1Improve complianceImprove convenienceBiocideOrganic active ingredientsExtended release tabletsPramipexole
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical composition for controlled release of active substances and manufacturing method thereof
InactiveUS7442387B2Reducing pressure appliedImprovement of dysuriaHeavy metal active ingredientsBiocideControlled releasePolyethylene oxide
The present invention pertains to a sized product, which contains a drug, polyethylene oxide with a molecular weight of 2,000,000 or higher, and a specific size controlling agent for polyethylene oxide (substance with the appropriate plasticity and binding force) and wherein at least the above-mentioned specific size controlling agent is uniformly dispersed in the above-mentioned polyethylene oxide, a controlled-release pharmaceutical composition containing this sized product, and a method of manufacturing a controlled-release pharmaceutical composition containing this sized product.A controlled-release pharmaceutical composition with good uniformity of content can be presented by using powder particles of polyethtylene oxide with powder properties suitable for tableting, which is obtained by uniform dispersion of the specific size controlling agent for polyethylene oxide of the present invention.
Owner:ASTELLAS PHARMA INC
Entecavir dispersible tablet and preparation thereof
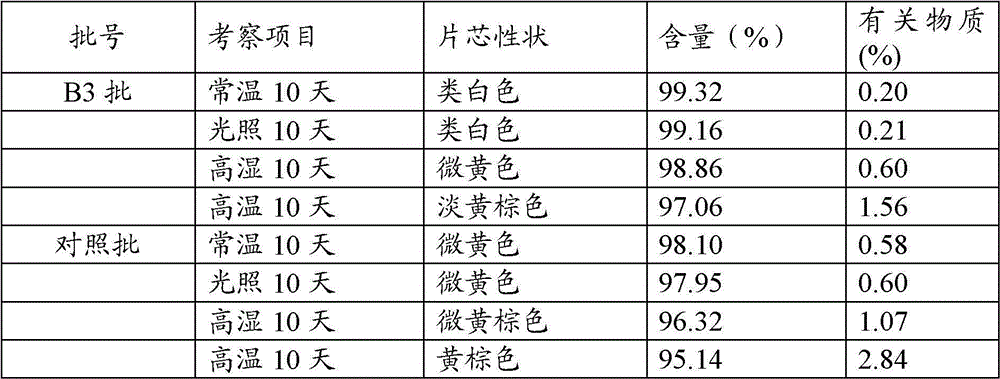
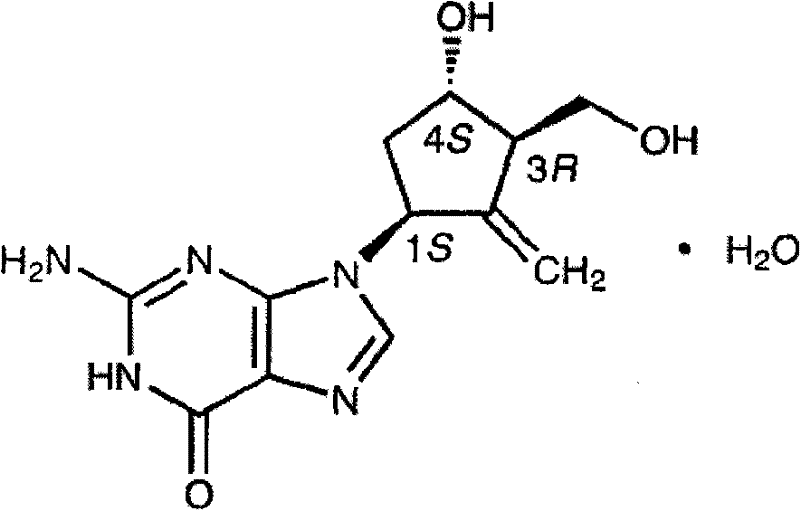
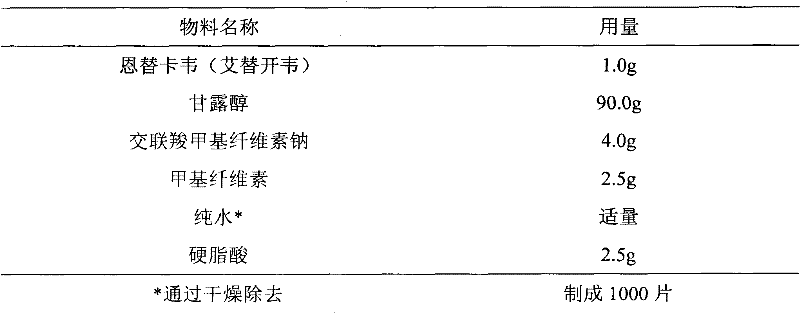
InactiveCN101244044AGood content uniformitySimple manufacturing methodDigestive systemAntiviralsEntecavirPharmacology
The invention relates to an Entecavir dispersible tablet and the preparation method; wherein, the Entecavir dispersible tablet comprises Entecavir, binder, filler, disintegrating agent, and lubricant; the uniformity of dosage units (A+1.8S) is not larger than 10.0. The preparation method for the dispersible tablet in the invention has the advantages of simple process, and suitability for large-scale industrial production.
Owner:上海国创医药股份有限公司
Trimetazidine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN102885795AThe solution is not easy to cleanSimple preparation stepsOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazidine dihydrochloride sustained-release tablet and a preparation method thereof. The trimetazidine dihydrochloride sustained-release tablet comprises the following constituents in percentage by mass: 5-60% of trimetazidine dihydrochloride, 10-25% of sustained-release framework material, 1-8% of adhesive, 20-80% of filler, 0.1-5% of glidant and 0.2-3% of lubricant. According to the trimetazidine dihydrochloride sustained-release tablet, medicine can be slowly and uniformly released by adding the sustained-release framework material, so as to achieve regulation and control for a release speed, reduce the peak-valley ratio of the medicine, improve the efficacy, reduce the toxic and side effects of the medicine, reduce daily medicine-taking times and enhance the compliance of the patient on the medicine. The preparation method of the trimetazidine dihydrochloride sustained-release tablet disclosed by the invention is simple in process, does not need specially process production equipment, and is low in cost and good for batch amplification and industrialized production for products.
Owner:AC PHARMA CO LTD
Memantine hydrochloride capsule sustained-release preparation and preparation method for same
InactiveCN102552218ALarge distribution areaImprove bioavailabilityNervous disorderPharmaceutical delivery mechanismControl layerSide effect
The invention discloses a memantine hydrochloride capsule sustained-release preparation, wherein the preparation is composed of two parts, namely, immediate-release grains and sustained-release grains; the sustained-release part is composed of a blank pellet core, a medicine layer and a release control layer; and the immediate-release part is composed of a blank pellet core and a medicine layer. The capsule is uniform in content, good in release effect, stable in blood concentration, good for reducing the toxic and side effects of medicines, and is capable of being used for treating moderate-to-severe Alzheimer-type dementia, and the medicine-taking times of the patient are reduced,.
Owner:无锡万全医药技术有限公司
Tofacitinib citrate tablets and preparation method thereof
InactiveCN105878202AGood content uniformityDissolution rate is fastOrganic active ingredientsAntipyreticFiller ExcipientLactose
The invention discloses Tofacitinib citrate tablets composed of Tofacitinib citrate and pharmaceutically acceptable auxiliary materials. The auxiliary materials comprise filler, disintegrating agent, flow aid and coating materials. The filler is microcrystalline cellulose lactose premix with the model of Celactose80, and the weight ratio of the Tofacitinib citrate to the microcrystalline cellulose lactose premix with the model of Celactose80 is 1:5-25. The invention further discloses a preparation method. The Tofacitinib citrate tablets has high content uniformity and have the advantages of being high in dissolving-out speed, stable in chemical property and the like.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
Immunomodulator slow-release preparation and preparation method thereof
ActiveCN103610658AProlong the action timeUniform and constant action timeOrganic active ingredientsPill deliveryBlood concentrationProlonged-release tablet
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
Solid dispersoid containing progestational hormone, preparation method thereof and composite comprising same
InactiveCN102018657AHigh dissolution rateImprove bioavailabilityPowder deliveryOrganic active ingredientsDissolutionHormones regulation
The invention relates to a solid dispersoid comprising progestational hormone and at least one water-soluble carrier material, wherein the progestational hormone is dispersed into the water-soluble carrier material, and the weight ratio of the progestational hormone to the water-soluble carrier material is 1:0.3-300. The solid dispersoid has the advantages of higher dissolution rate and bioavailability, good content uniformity degree, high physical stability and high chemical stability. The invention also relates to a preparation method of the solid dispersoid and a composite comprising the solid dispersoid.
Owner:上海市生物医药技术研究院
Diclofenac sodium sustained-release tablet and its preparation process
ActiveCN102274200AAvoid condensationReduce usageOrganic active ingredientsAntipyreticDiclofenacAdhesive
The invention relates to the technical field of medicine, in particular to diclofenac sodium sustained release tablets and a preparation process thereof. The diclofenac sodium sustained release tablets provided by the invention comprise the following components in percentage by mass: 16.5 to 39.0 percent of diclofenac sodium, 10.0 to 35.5 percent of sustained release agent, 33.5 to 65.0 percent of filling agent, 2.01 to 8.0 percent of glidant, 0 to 2.5 percent of lubricating agent, and 0 to 8.0 percent of adhesive. According to the diclofenac sodium sustained release tablets and a whole-powder direct tabletting method thereof provided by the invention, by changing the components and the preparation method, the production process is simplified, the production cost is reduced, the production efficiency is improved, the yield is improved to 98.0 to 100 percent, granule condensation is avoided, the surface of the tablets is smooth, meanwhile, consumption of high-concentration alcohol is avoided, and the potential safety hazard in the production process is reduced.
Owner:SINOPHARM GRP ZHIJUN SHENZHEN PINGSHAN PHARMA CO LTD
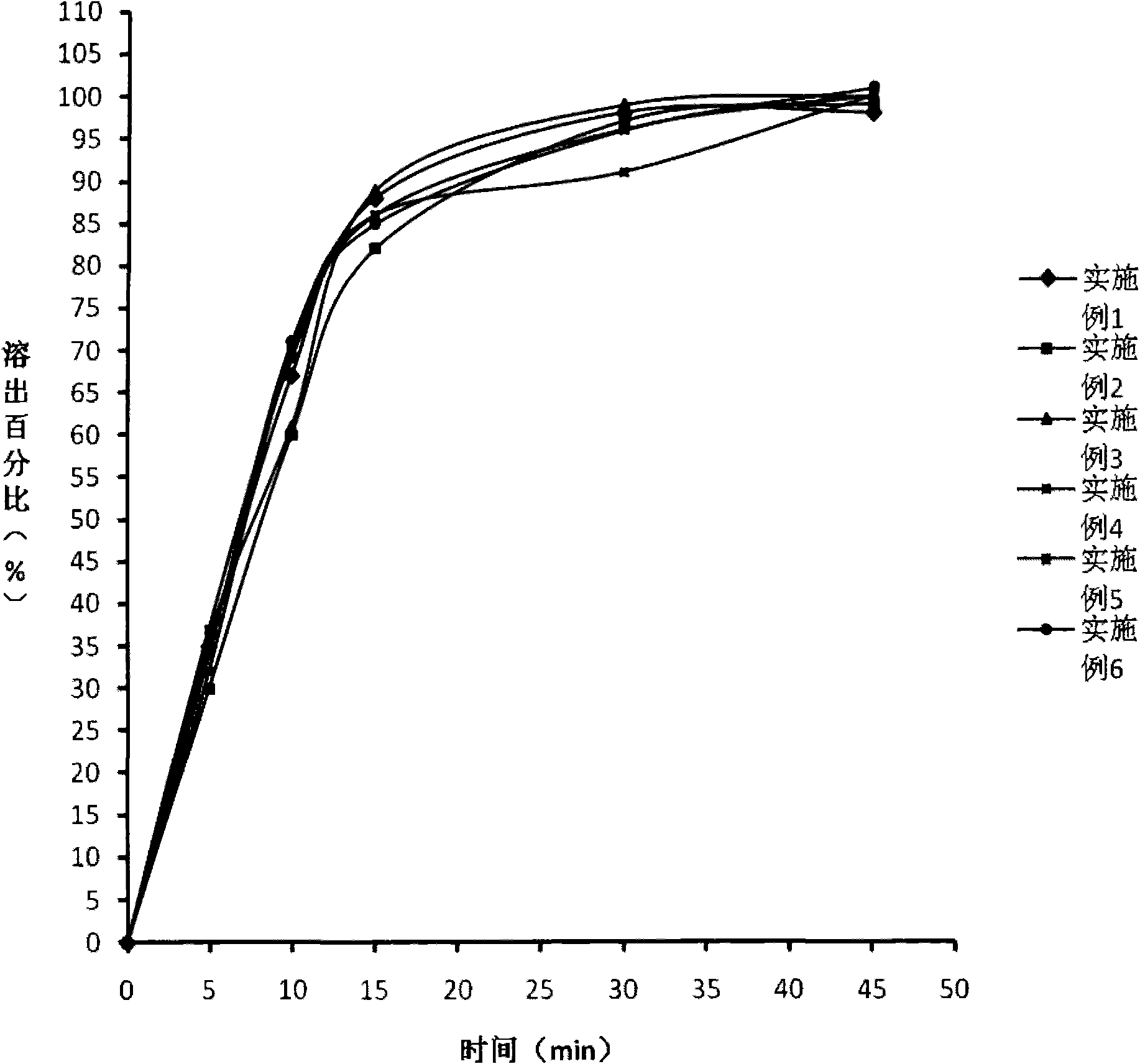
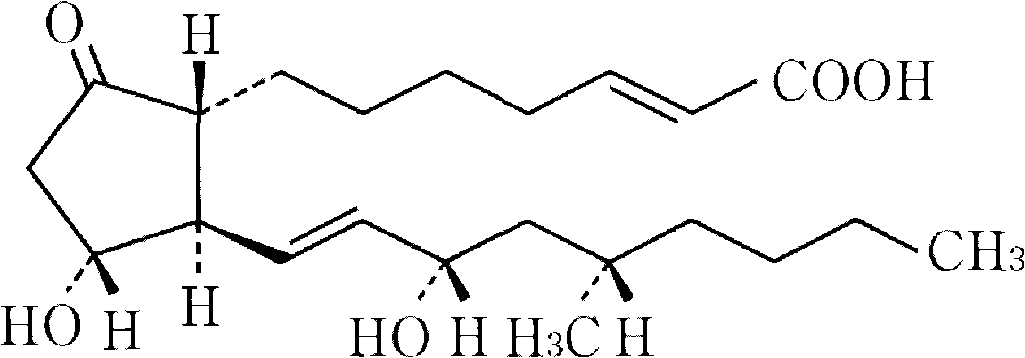
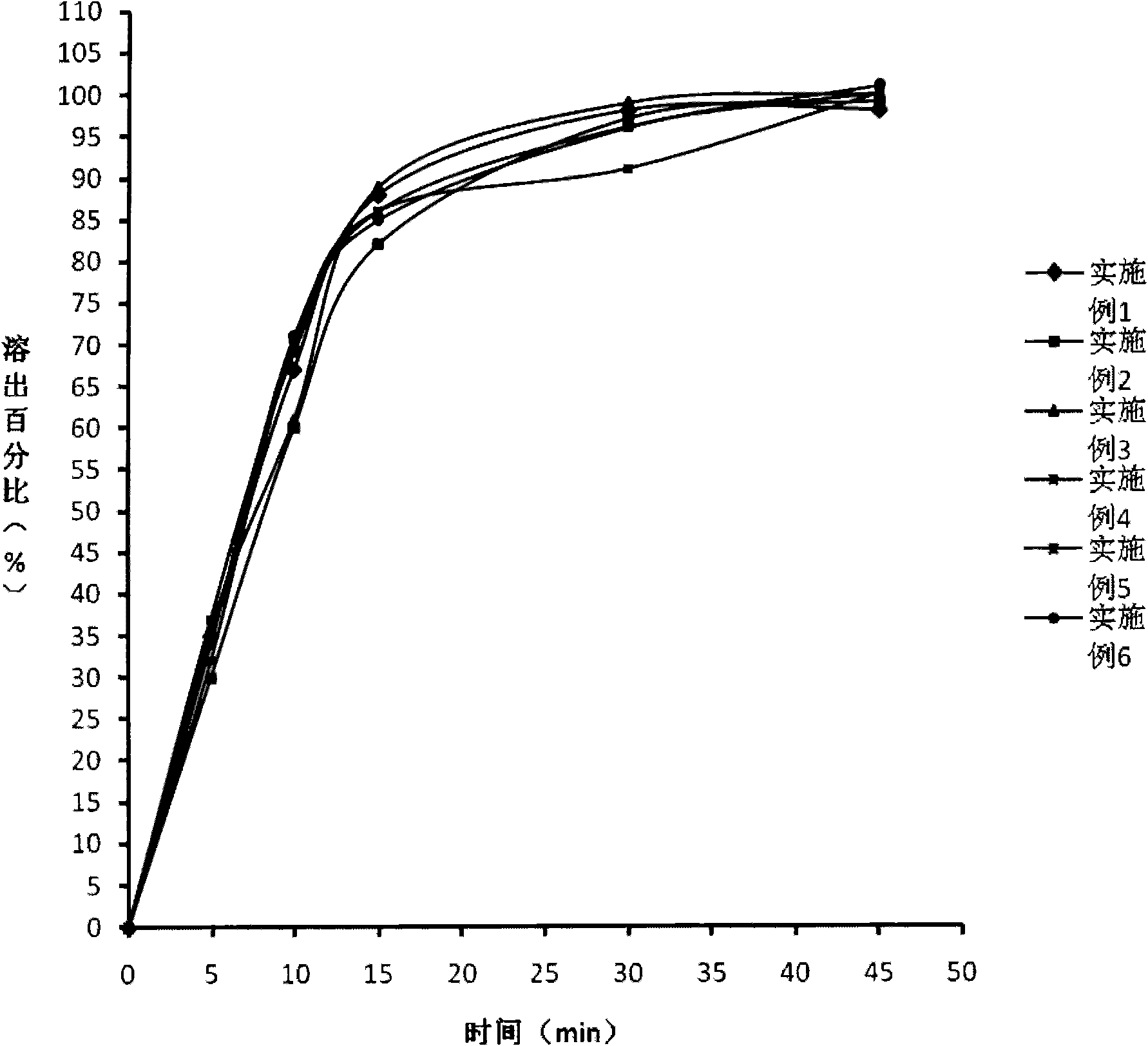
Drug composite containing limaprost and preparation method thereof
ActiveCN101862337ADoes not affect dissolutionDoes not affect disintegrationOrganic active ingredientsPharmaceutical product form changeCoated tabletsMANNITOL/SORBITOL
The invention provides a drug composite containing limaprost and a preparation method thereof, wherein the drug composite of the invention contains 0.01-1% (weight) of the cyclodextrin inclusion compound of limaprost, 0.5-10% (weight) of freeze-drying stabilizer and other pharmaceutically acceptable excipients, wherein the free-drying stabilizer contains mannitol. The drug composite adopts a freeze drying and dry granulating combined preparation technology, greatly overcomes the defects of easy moisture adsorption and extremely bad stability of limaprost which is the main drug, and simultaneously cannot influence the slaking characteristic and the dissolution of the main drug, in addition, the product is not a coated tablet, and thereby the invention prevents the defect of slower dissolution of the conventional coated tablets.
Owner:BEIJING TIDE PHARMA
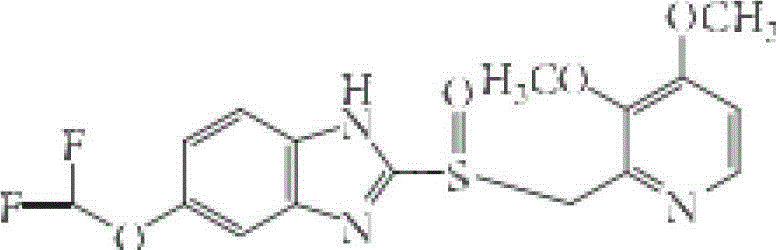
Enteric-coated tablet of S-pantoprazole or salt of S-pantoprazole, and preparation method thereof
ActiveCN102871980AAccurate doseEasy to carryOrganic active ingredientsDigestive systemChemistryPharmaceutic Adjuvant
The invention relates to an enteric-coated tablet of S-pantoprazole or salt of the S-pantoprazole, and a preparation method thereof, wherein the enteric-coated tablet comprises the components: a) a tablet core formed by the S-pantoprazole or the salt of the S-pantoprazole and pharmaceutic adjuvant; b) an isolating layer; and c) an enteric layer. The tablet core of the S-pantoprazole or the salt of the S-pantoprazole is formed by directly tabletting full powder, so that the damage of high temperature to the S-pantoprazole or the salt of the S-pantoprazole in the process of drying water and granules during wet granulation can be effectively avoided, and the stability of the preparation is improved. Furthermore, the isolating layer and the enteric layer have less composition, so that the preparation is simple and convenient. According to the invention, the preparation technology is simplified and the production cost is reduced; and the prepared S-pantoprazole enteric-coated tablet is better in dissolution rate and good in stability.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation containing vitamin D3 and calcium carbonate and preparation method for preparation
ActiveCN106420808AImprove stabilityGood content uniformityOrganic active ingredientsMetabolism disorderVitaminCalcium EDTA
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a preparation containing vitamin D3 and calcium carbonate and a preparation method for the preparation. The preparation containing vitamin D3 and calcium carbonate comprises calcium carbonate, vitamin D3 and pharmaceutically acceptable auxiliary materials, wherein the vitamin D3 is vitamin D3 coating powder obtained by coating vitamin D3 with opadry. The vitamin D3 coating powder in the preparation containing vitamin D3 and calcium carbonate has relatively good stability, and is relatively high in uniformity of content of vitamin D3. Meanwhile, the preparation containing vitamin D3 and calcium carbonate provided by the invention can effectively promote absorption, on calcium, of a human body.
Owner:北京远方通达医药技术有限公司
Entecavir tablet and preparation method thereof
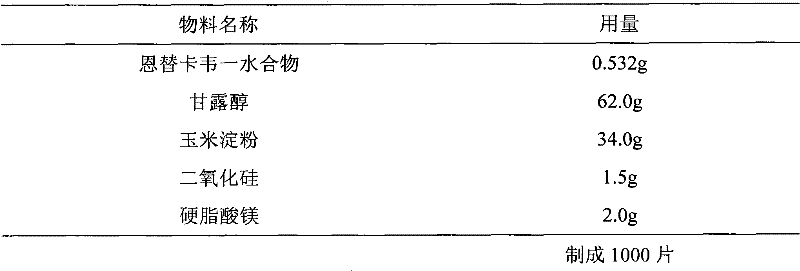
InactiveCN102652737AStable in natureGood content uniformityDigestive systemAntiviralsMedicinePharmacology
The invention relates to an Entecavir tablet. The Entecavir tablet contains 0.2-1% of Entecavir monohydrate, 85-98% of filler, 0-5% of binder, 0-10% of disintegrating agent, 0-2% of flow aid and 0.5-2% of lubricating agent by weight, wherein the Entecavir monohydrate is added in the form of powder. The invention also relates to a preparation method of the Entecavir tablet.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Flupentixol-melitracen pharmaceutical composition and preparation method thereof
InactiveCN108498470AGood process reproducibility and stabilityGood uniformityOrganic active ingredientsNervous disorderDrugChemistry
The invention designs an anti-depression drug flupentixol-melitracen pharmaceutical composition and a preparation method thereof, wherein the composition comprises flupentixol hydrochloride, melitracen hydrochloride, a filler, a binder, a lubricant and a stabilizer, and is prepared by using wet granulation tableting, and the method comprises: granulation, drying, granulation and total mixing, tableting, film coating, and other steps. According to the present invention, the process effectively solves the problems of dissolution and content uniformity due to the high difference in the contents of the two main drugs, and further protects the stability of the active ingredient; and the prepared flupentixol-melitracen tablet has characteristics of stable quality, safety and good effectiveness.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Solid medicinal preparation containing mannitol or lactose
InactiveUS20100004279A1Good content uniformityTreatment and/or prophylaxis of thrombosisPowder deliveryBiocideMANNITOL/SORBITOLLactose
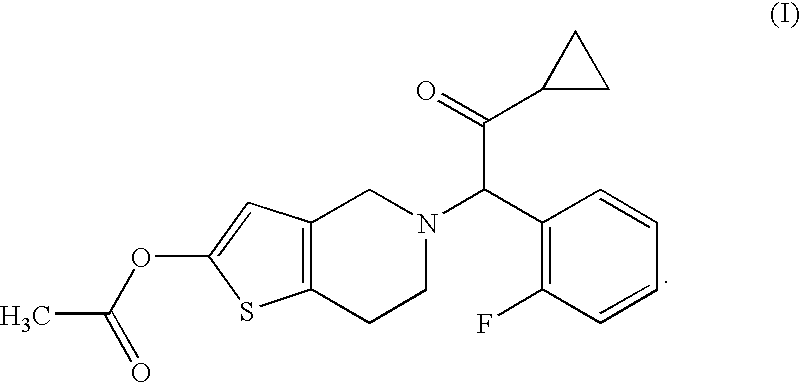
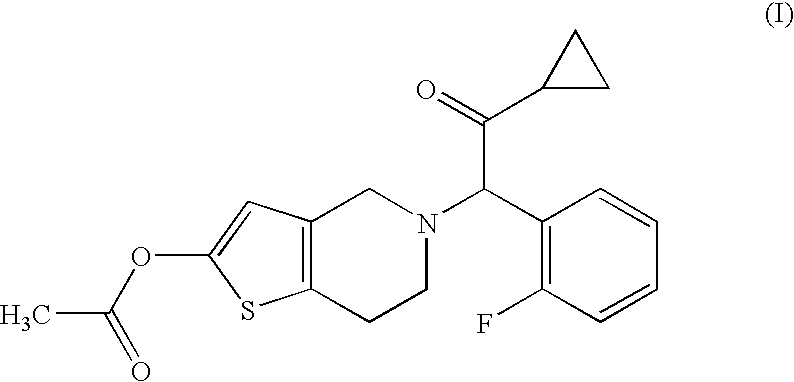
A solid medicinal preparation which contains: (A) a compound of the following formula (I) or a pharmacologically acceptable salt thereof; and (B) mannitol or lactose which, under specific conditions, has a particle size distribution in which the 90% cumulative diameter is 80 to 300 μm. The compound of the formula (I) has the following formula:The solid medicinal preparation has improved content uniformity.
Owner:DAIICHI SANKYO CO LTD +1
Tetrodotoxin quick-release pellet preparation and its preparation method and use
ActiveCN106063780AHigh rate of drug useGood content uniformityOrganic active ingredientsNervous disorderDrug release rateOral medication
The invention discloses a tetrodotoxin quick-release pellet preparation and its preparation method and use and relates to the field of a medicinal preparation technology and use. The tetrodotoxin quick-release pellet preparation is suitable for oral administration and utilizes a blank pellet core as a carrier and a tetrodotoxin aqueous solution containing a cosolvent and a binder as a drug feeding solution. A weight ratio of the tetrodotoxin to the pellet core is 0.0025%-0.3% and an oral dosage of the tetrodotoxin is less than or equal to 300 micrograms. The tetrodotoxin quick-release pellet preparation has the advantages of high superimpose rate, good content uniformity, fast drug release rate and fast pain easing. The tetrodotoxin quick-release pellet preparation has the characteristics of good clinical use compliance and high safety. The blank pellet core fluidized bed-based drug superimpose method is suitable for preparation of a very low specification tetrodotoxin oral preparation.
Owner:XIAMEN ZHAOYANG BIOLOGICAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com