Trimetazidine dihydrochloride sustained-release tablet and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release tablets, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. The production process is complex, the drug release stability is greatly affected, and the PEO gel strength is not high. It can solve the problems of adjuvant adhesion equipment, excellent quality stability, and suitable for process production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
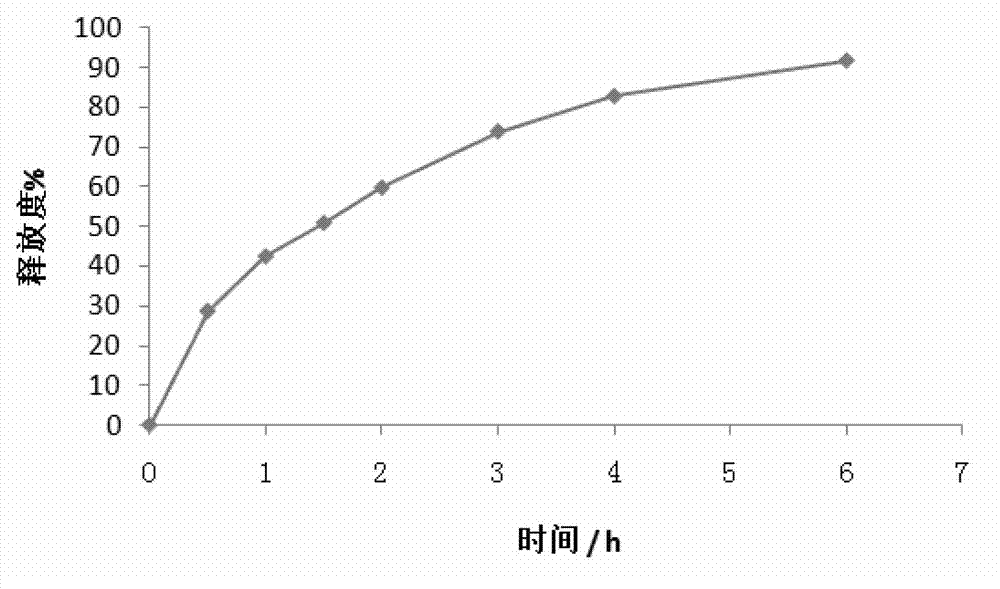
Embodiment 1
[0053] A trimetazidine hydrochloride sustained-release tablet, comprising the following components (calculated in 1000 tablets):
[0054]
[0055] The method for preparing the above-mentioned trimetazidine hydrochloride sustained-release tablet comprises the following steps:
[0056] (1), grinding and sieving: grind trimetazidine hydrochloride into fine powder, get trimetazidine hydrochloride fine powder, hydroxypropyl methylcellulose (12000mPa s-21000mPa s), povidone, phosphoric acid Calcium hydrogen is passed through a 40-mesh sieve, and micronized silica gel and stearic acid are passed through an 80-mesh sieve;
[0057] (2), Weighing: Accurately weigh 35.00g of trimetazidine hydrochloride, 48.00g of hydroxypropyl methylcellulose (12000mPa s-21000mPa s), 12.00g of povidone, 103.60g of calcium hydrogen phosphate, and micronized silica gel 0.40g, stearic acid 2.00g, Opadry (film coating material is hypromellose) 10.00g for subsequent use;
[0058] (3), mixing: mix the wei...
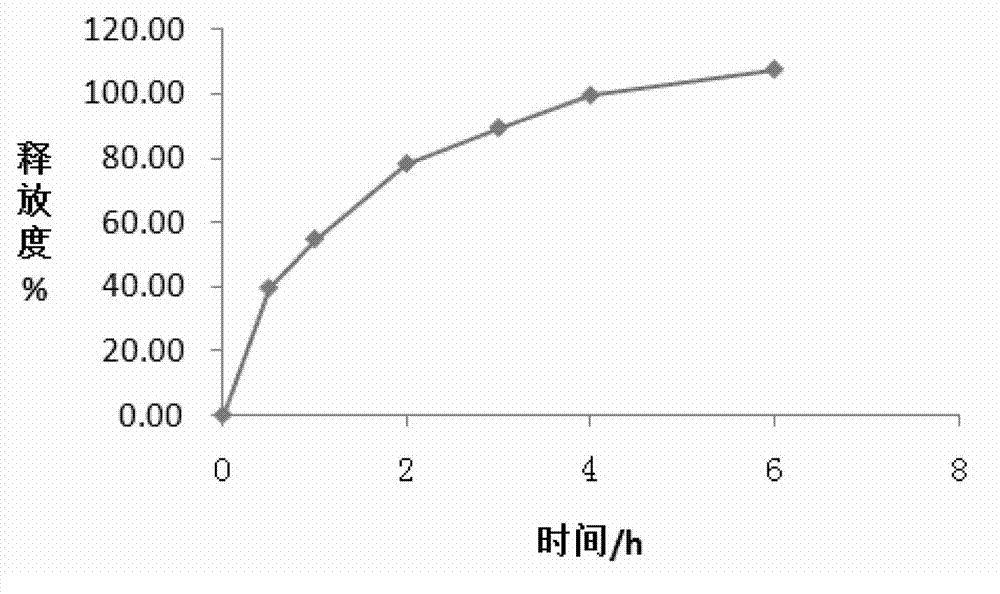
Embodiment 2
[0064] A trimetazidine hydrochloride sustained-release tablet, comprising the following components (calculated in 1000 tablets):
[0065]
[0066] The method for preparing the above-mentioned trimetazidine hydrochloride sustained-release tablet comprises the following steps:
[0067] (1), grinding and sieving: grind trimetazidine hydrochloride into fine powder, get trimetazidine hydrochloride fine powder, hypromellose (80000mPa s-120000mPa s), povidone, micro Crystalline cellulose is passed through a 40-mesh sieve, and micronized silica gel and magnesium stearate are passed through an 80-mesh sieve;
[0068] (2), weighing: Accurately weigh 35.00g of trimetazidine hydrochloride, 40.00g of hydroxypropylmethylcellulose (80000mPa s-120000mPa s), 16.00g of povidone, 107.60g of microcrystalline cellulose, micropowder 0.40g of silica gel, 2.00g of magnesium stearate, 10.00g of Opadry (film coating material is hypromellose) for later use;
[0069] (3), mixing: put the weighed tri...
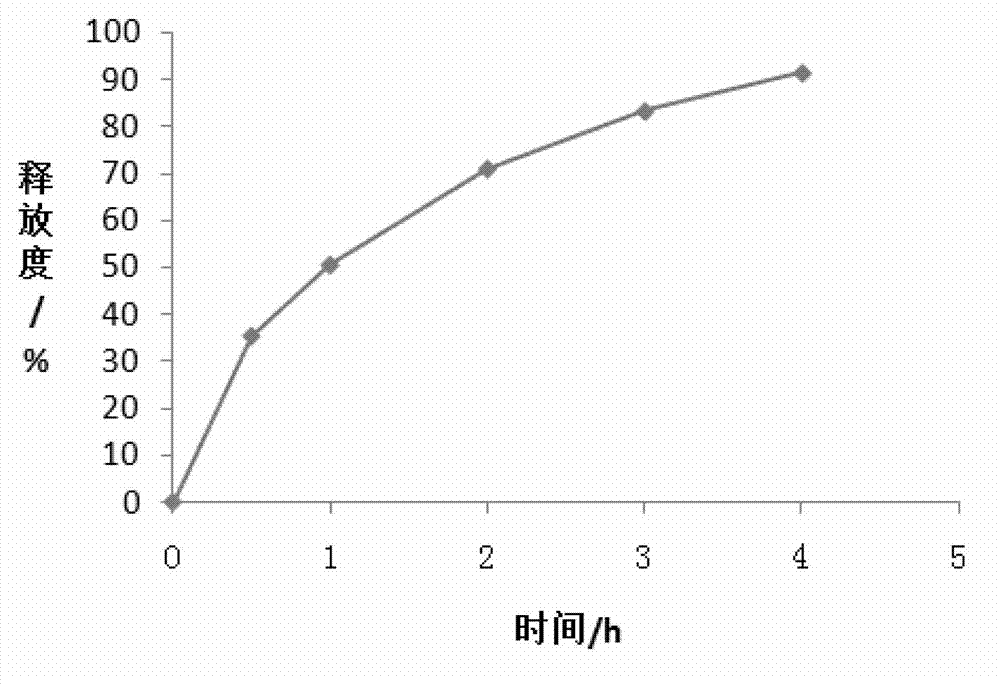
Embodiment 3
[0075] A trimetazidine hydrochloride sustained-release tablet, comprising the following components (calculated in 1000 tablets):
[0076]
[0077]
[0078] The method for preparing the above-mentioned trimetazidine hydrochloride sustained-release tablet comprises the following steps:
[0079] (1), grinding and sieving: grind trimetazidine hydrochloride into fine powder, take trimetazidine hydrochloride fine powder, glyceryl behenate, hydroxypropyl methylcellulose, povidone, pregelatinized starch Cross 40 mesh sieves, get micropowder silica gel and magnesium stearate and cross 80 mesh sieves;
[0080] (2), weighing: Accurately weigh 35.00g of trimetazidine hydrochloride, 30.00g of glyceryl behenate, 16.00g of hydroxypropyl methylcellulose (3000mPa s-5600mPa s), 10.00g of povidone, Pregelatinized starch 117.60g, micropowder silica gel 0.40g, magnesium stearate 2.00g. Opadry (film coating material is hydroxypropyl methylcellulose) 10.00g spare;
[0081] (3), mixing: the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com