Immunomodulator slow-release preparation and preparation method thereof
A technology of sustained-release tablets and sustained-release layers, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve the problems of affecting the therapeutic effect, low blood drug concentration, toxic and side effects, etc., and prolong the action time. , Simple prescription, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
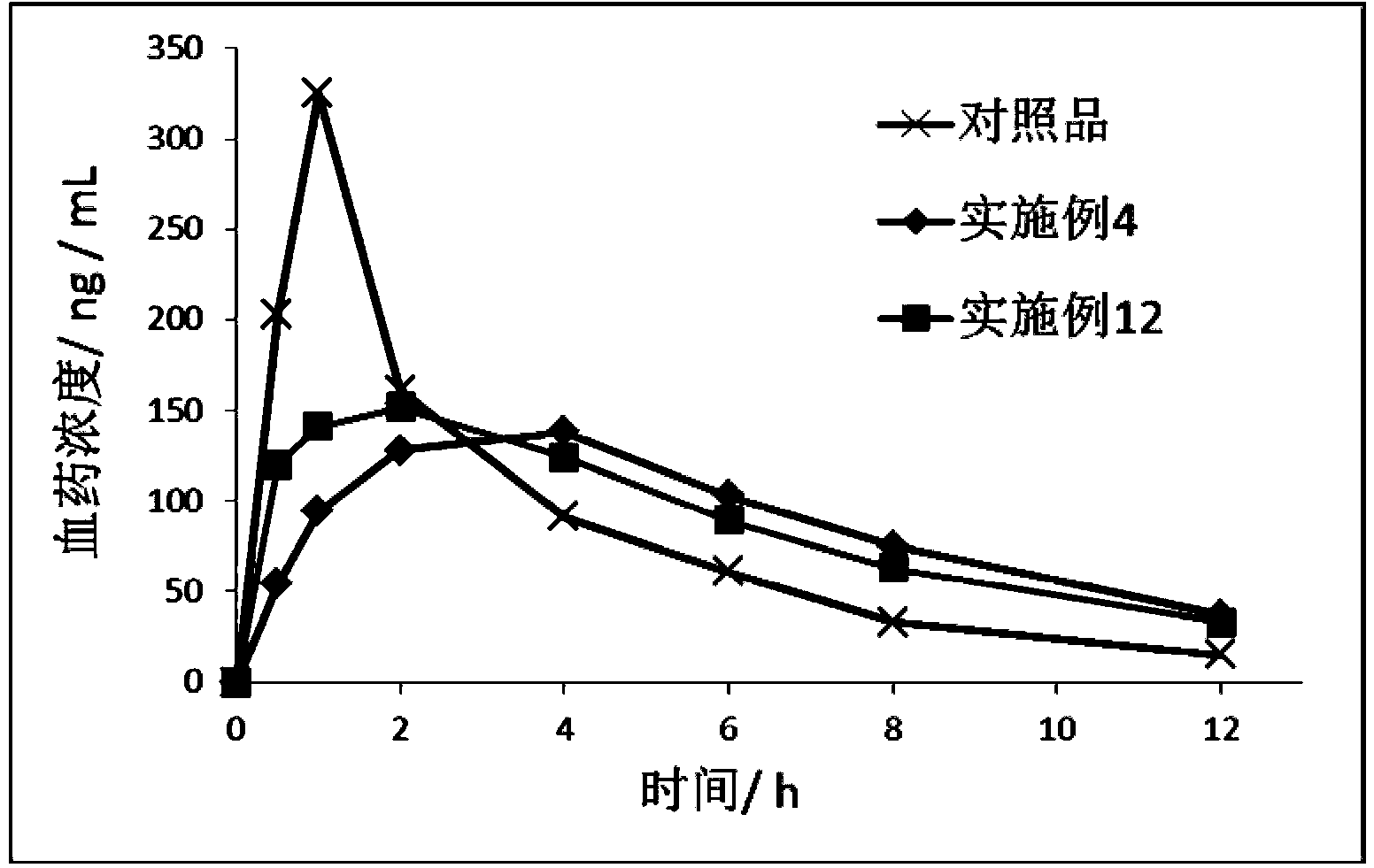
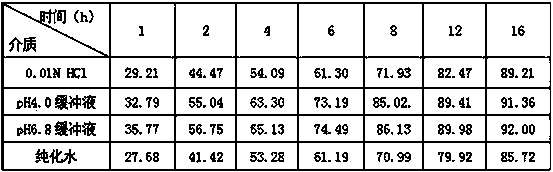
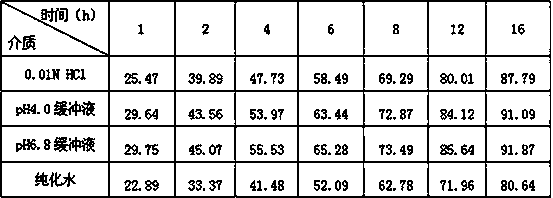
Image
Examples
Embodiment 1
[0136] A lenalidomide sustained-release tablet, comprising the following components (calculated in 1000 tablets):
[0137] Lenalidomide 25.00 g
[0138] Hypromellose (3000mPa·s-8000 mPa·s)
[0139] (sustained release matrix material) 200.00 g
[0140] Povidone (adhesive) 25.00 g
[0141] Microcrystalline cellulose (filler) 112.50 g
[0142] Mannitol (bulking agent) 100.00 g
[0143] Povidone (solubilizer) 25.00 g
[0144] Talc powder (glidant) 5.00 g
[0145] Magnesium stearate (lubricant) 7.50 g
[0146] Opadry II (film coating material) 15.00 g
[0147] The method for preparing the above-mentioned lenalidomide sustained-release tablet comprises the following steps:
[0148] (1) According to the prescription, lenalidomide fine powder, slow-release matrix material, adhesive, filler and solubilizer are passed through a 40-mesh sieve, and the glidant and lubricant are passed through a 80-mesh sieve;
[0149] (2) Accurately weigh lenalidomide, sustained-release matrix ma...
Embodiment 2
[0157] A lenalidomide sustained-release tablet, comprising the following components (calculated in 1000 tablets):
[0158] Lenalidomide 25.00 g
[0159] Hypromellose (12000mPa·s-21000 mPa·s)
[0160] (sustained release matrix material) 150.00 g
[0161] Pregelatinized starch (binder) 100.00 g
[0162] Calcium hydrogen phosphate (filler) 635.00 g
[0163] Povidone (solubilizer) 10.00 g
[0164] Talc powder (glidant) 50.00 g
[0165] Magnesium stearate (lubricant) 30.00 g
[0166] Opadry II (film coating material) 30.00 g
[0167] The method for preparing the above-mentioned lenalidomide sustained-release tablet is the same as that in Example 1.
[0168]
Embodiment 3
[0170] A lenalidomide sustained-release tablet, comprising the following components (calculated in 1000 tablets):
[0171] Lenalidomide 25.00 g
[0172] Carbomer (sustained release matrix material) 180.00 g
[0173] Povidone (adhesive) 15.00 g
[0174] Compressible starch (filler) 52.50 g
[0175] Hydroxypropyl-β-cyclodextrin (solubilizer) 65.00 g
[0176] Micronized silica gel (glidant) 1.50 g
[0177] Magnesium stearate (lubricant) 1.50 g
[0178] Opadry II (film coating material) 12.00 g
[0179] The method for preparing the above-mentioned lenalidomide sustained-release tablet is the same as that in Example 1.
[0180]
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com