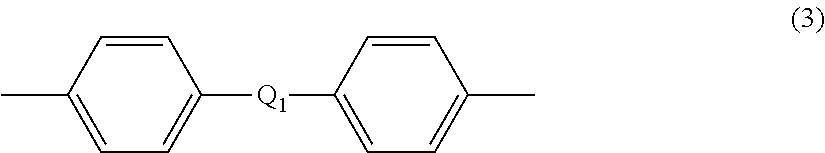
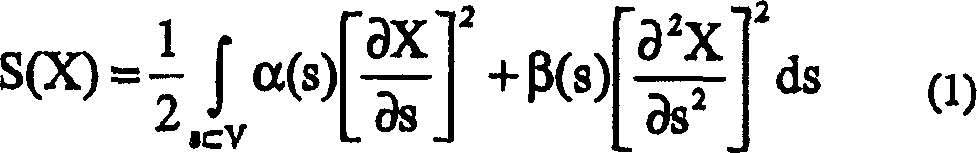Patents
Literature
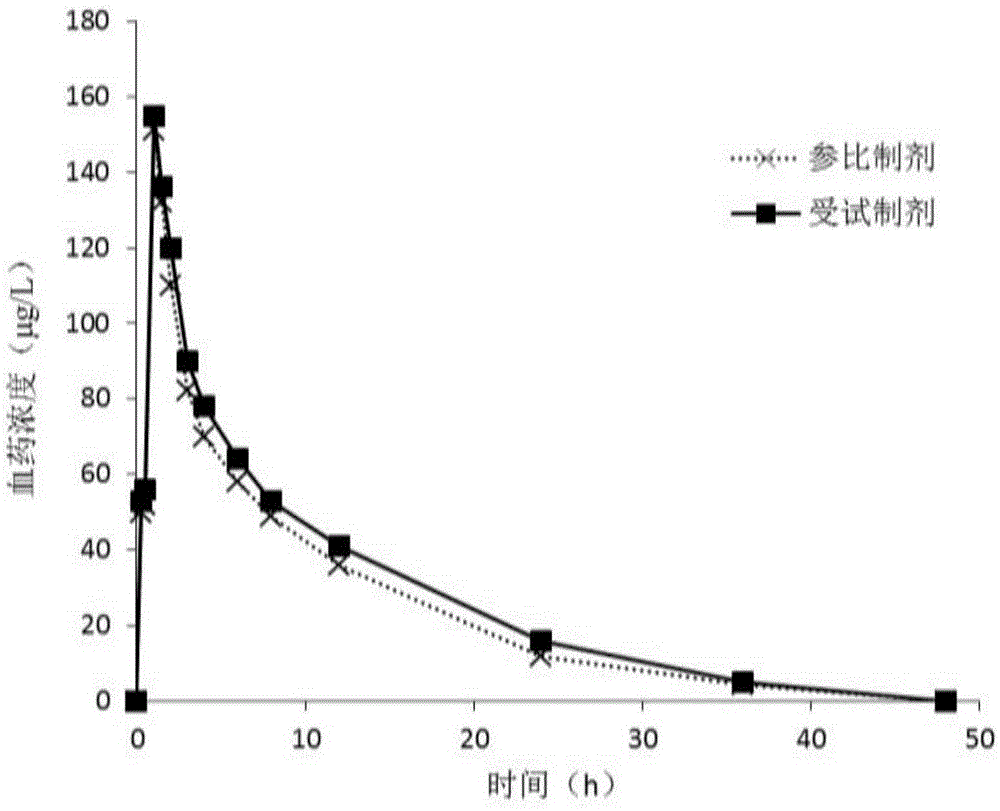
34 results about "Time to peak" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
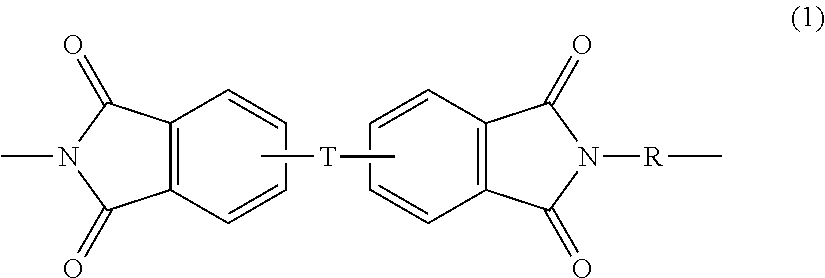
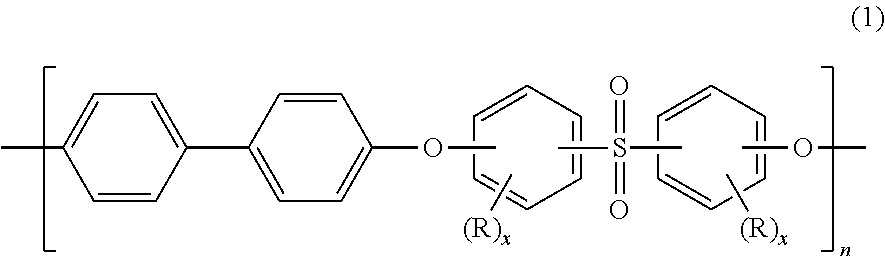
Reinforced thermoplastic articles, compositions for the manufacture of the articles, methods of manufacture, and articles formed therefrom
A composition for the manufacture of a porous, compressible article, the composition comprising a combination of: a plurality of reinforcing fibers; a plurality of polyimide fibers; and a plurality of polymeric binder fibers; wherein the polymeric binder fibers have a melting point lower than the polyimide fibers; methods for forming the porous, compressible article; and articles containing the porous, compressible article. An article comprising a thermoformed dual matrix composite is also disclosed, wherein the composite exhibits a time to peak release, as measured by FAR 25.853 (OSU test), a 2 minute total heat release, as measured by FAR 25.853 (OSU test), and an NBS optical smoke density of less than 200 at 4 minutes, determined in accordance with ASTM E-662 (FAR / JAR 25.853).
Owner:SABIC GLOBAL TECH BV
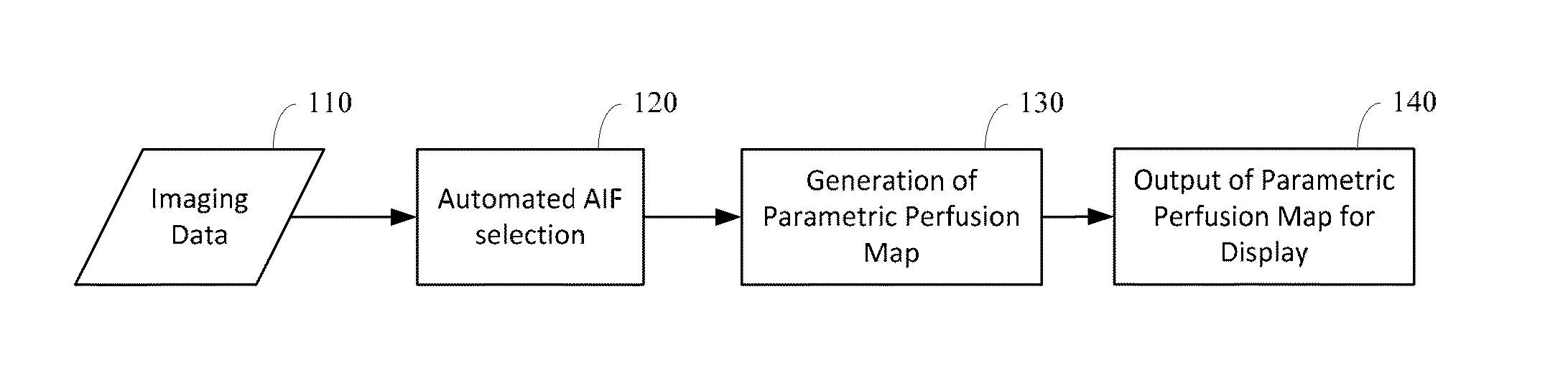
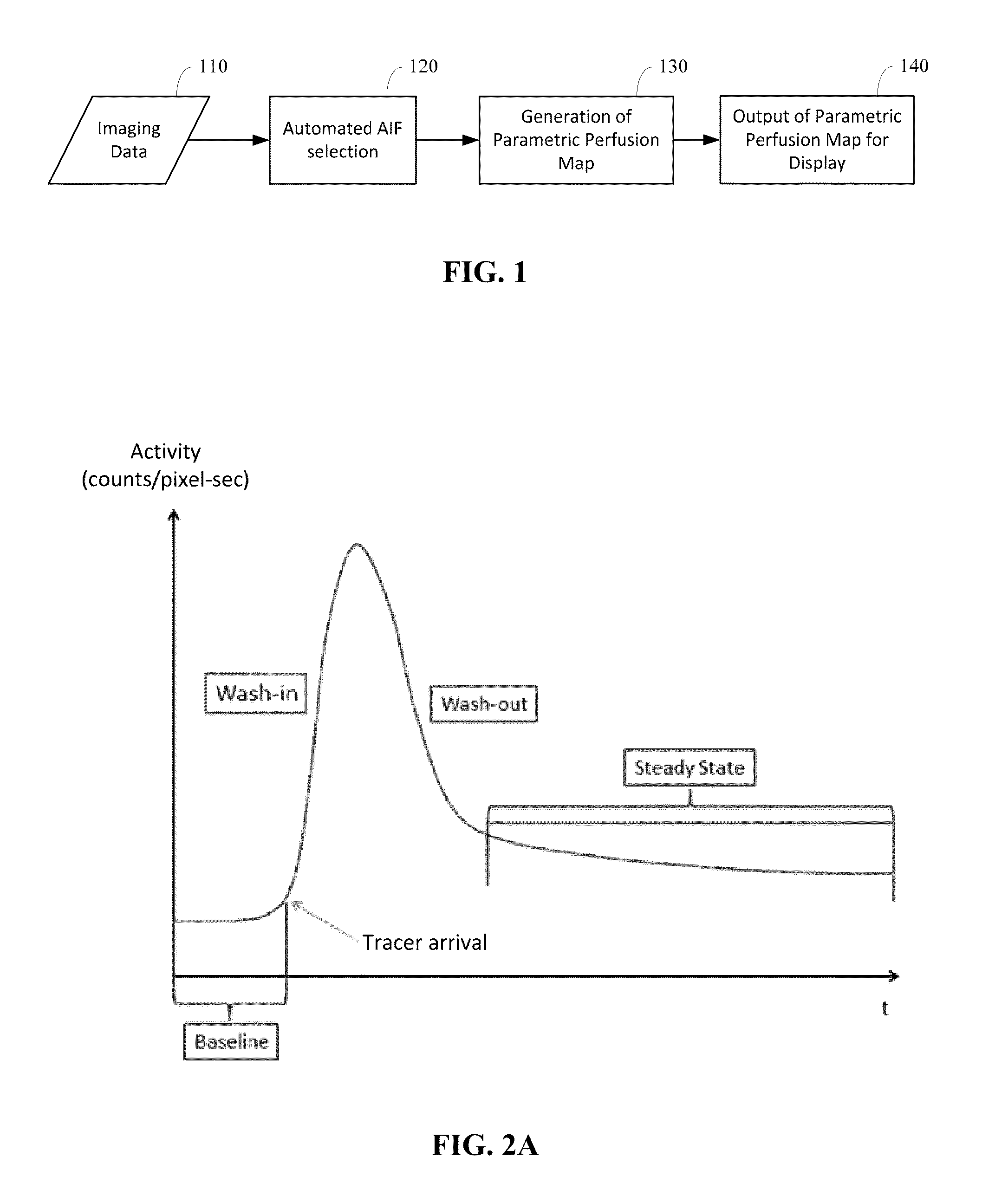
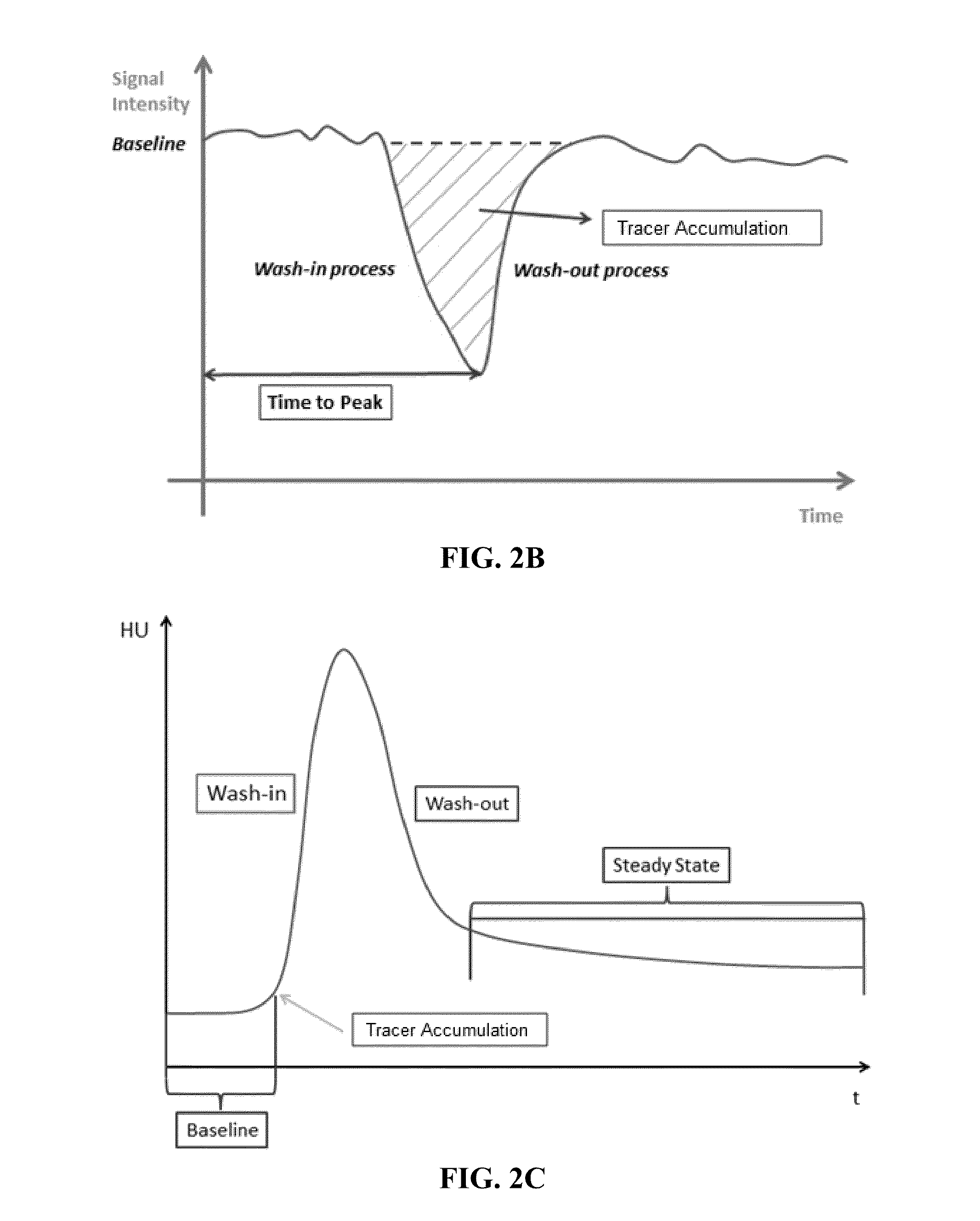
Automated determination of arterial input function areas in perfusion analysis
InactiveUS20140163403A1Well formedMagnetic measurementsCatheterImaging modalitiesArterial input function
Automatic arterial input function (AIF) area determination is provided that can be used to facilitate the generation of parametric maps for perfusion studies based on various imaging modalities and covering a variety of tissues. Automatic AIF determination can be accomplished by extracting characteristic parameters such as maximum slope, maximum enhancement, time to peak, time to wash-out, and wash-out slope. Characteristic parameter maps are generated to show relationships among the extracted characteristic parameters, and the characteristic parameter maps are converted to a plurality of two-dimensional plots. Automated segmentation of non-AIF tissues and determination of AIF areas can be accomplished by automatically finding peaks and valleys of each phase of AIF areas on the plurality of two-dimensional plots.
Owner:TEXAS A&M UNIVERSITY
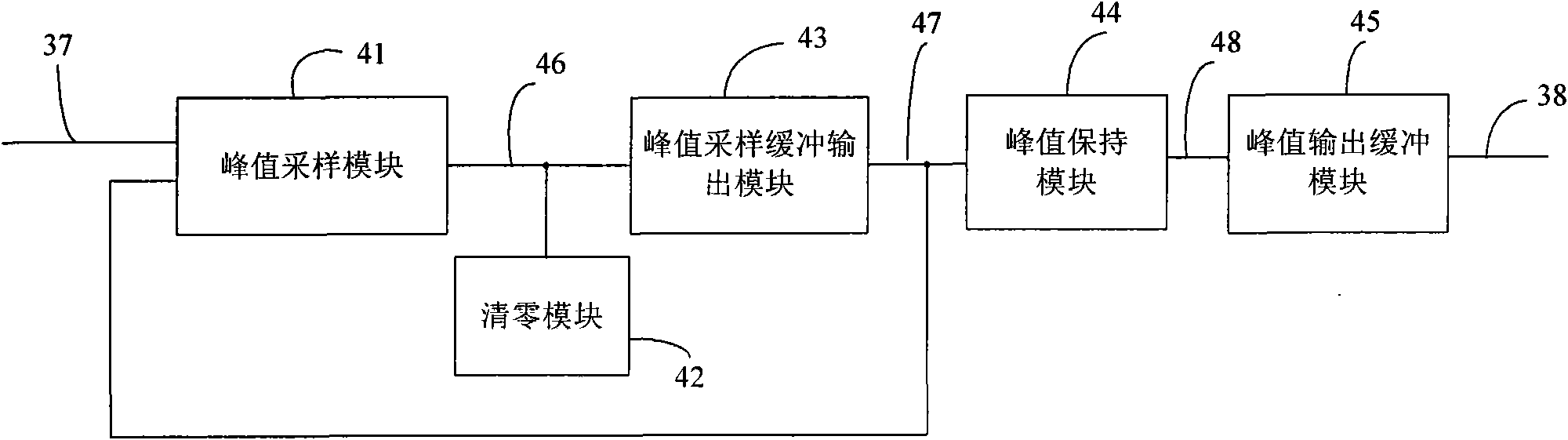
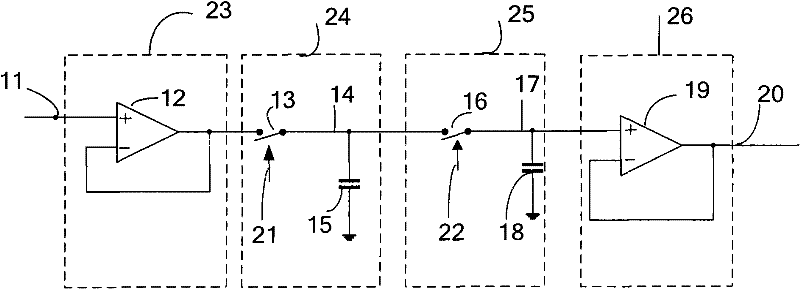
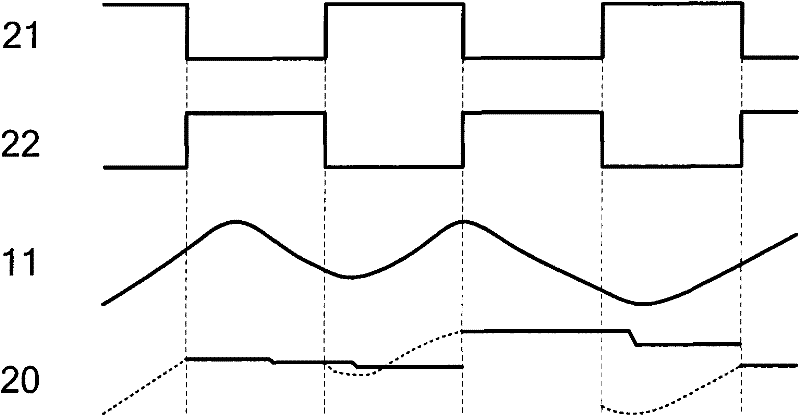
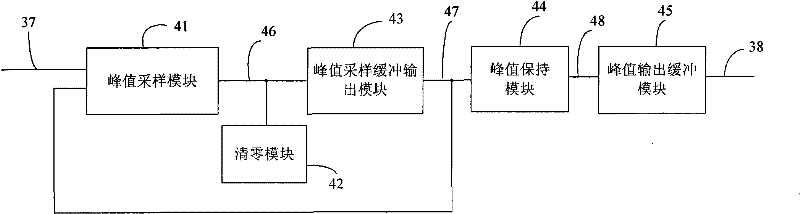
Peak sampling hold circuit, peak sampling hold method and application
InactiveCN101615432AAvoid accumulationImprove load driving capabilityElectric analogue storesPeak valueComputer science
The invention discloses a peak sampling hold circuit and a peak sampling hold method. The peak sampling hold circuit comprises a peak sampling module, a peak sampling buffer input module, a peak hold module, a peak output buffer module and a zero clearing module; the peak sampling module samples peak voltage of all time points of input signals; the peak sampling buffer output module follows the peak voltage and enhances load driving capability of the peak voltage; the peak hold module holds the voltage output by the peak sampling buffer output module; the peak output buffer module enhances the output load capability of the peak hold module; the zero clearing module carries out clearing in time to peak voltage of last period output by the peak sampling module after the peak hold module outputs voltage so as to be convenient to carry out peak sampling in next period. The peak sampling hold circuit and a peak sampling hold method can better reappear the peak of the input signal and can apply the peak sampling hold circuit to a power system.
Owner:HANGZHOU SILAN MICROELECTRONICS
Reinforced thermoplastic articles, compositions for the manufacture of the articles, methods of manufacture, and articles formed therefrom
A composition for the manufacture of a porous, compressible article, the composition comprising a combination of: a plurality of reinforcing fibers; a plurality of polysulfone fibers; and a plurality of polymeric binder fibers; wherein the polymeric binder fibers have a melting point lower than the polysulfone fibers; methods for forming the porous, compressible article; and articles containing the porous, compressible article. An article comprising a thermoformed dual matrix composite is also disclosed, wherein the composite exhibits a time to peak release, as measured by FAR 25.853 (OSU test), a 2 minute total heat release, as measured by FAR 25.853 (OSU test), and an NBS optical smoke density of less than 200 at 4 minutes, determined in accordance with ASTM E-662 (FAR / JAR 25.853).
Owner:SABIC GLOBAL TECH BV
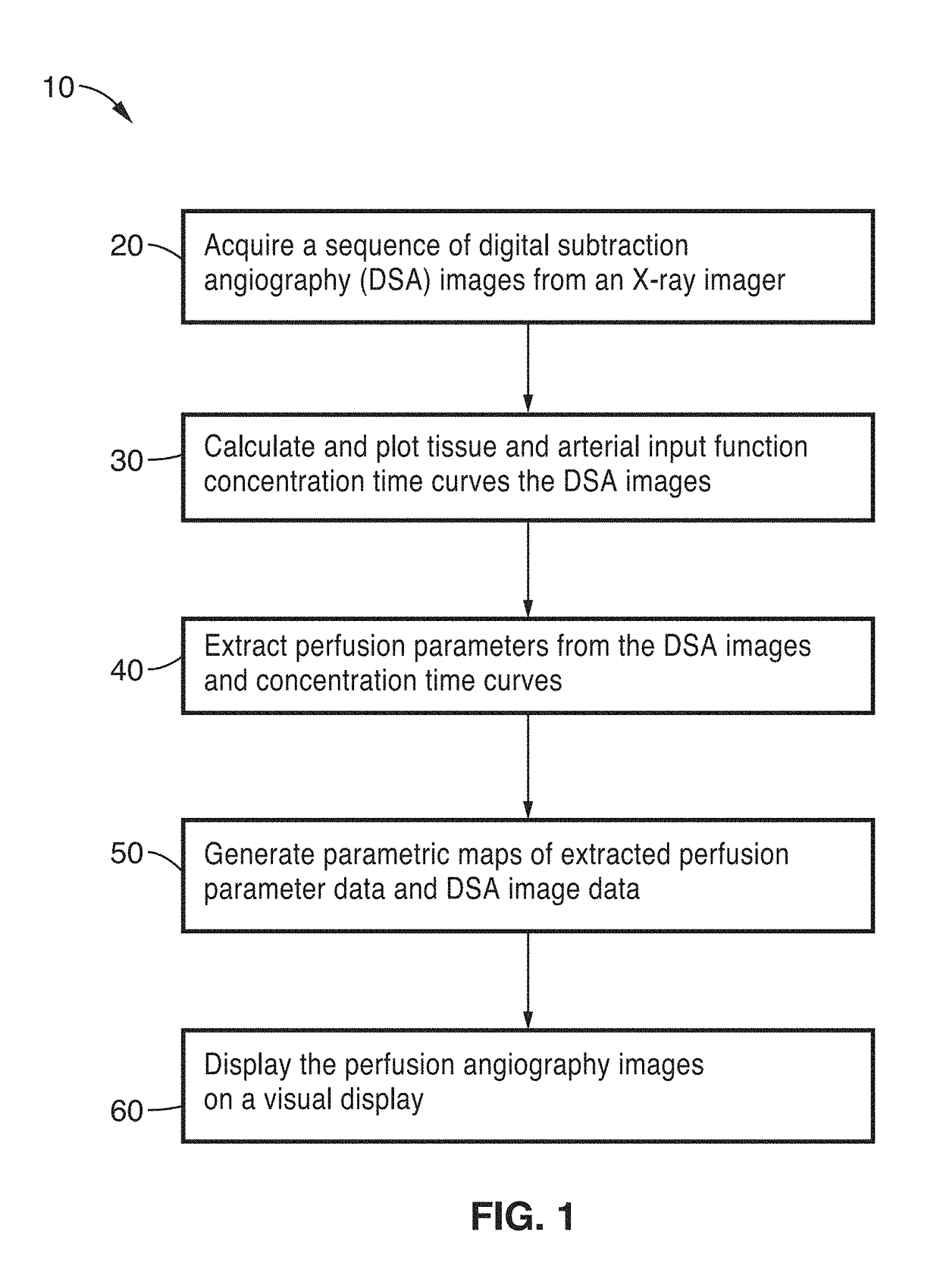
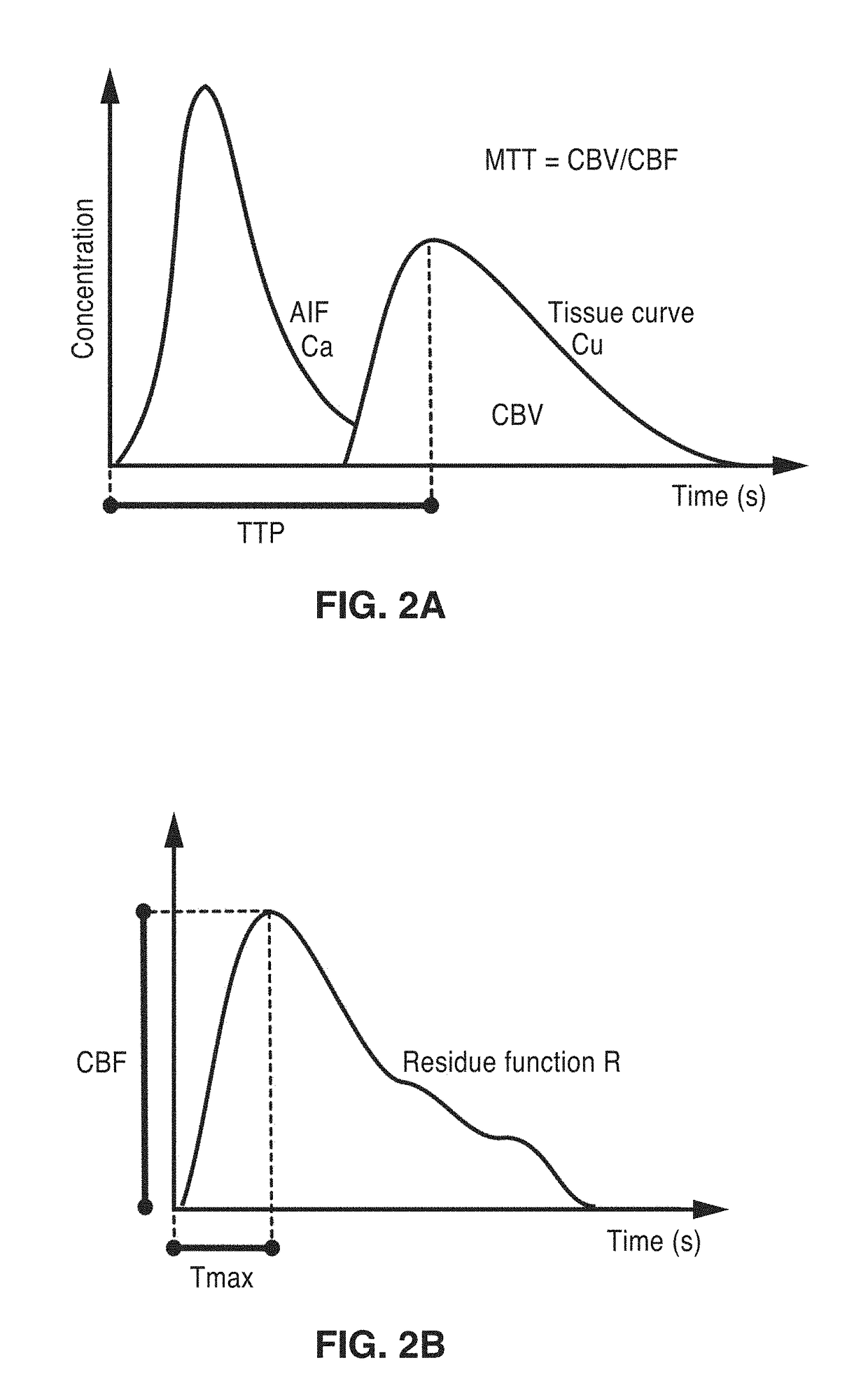
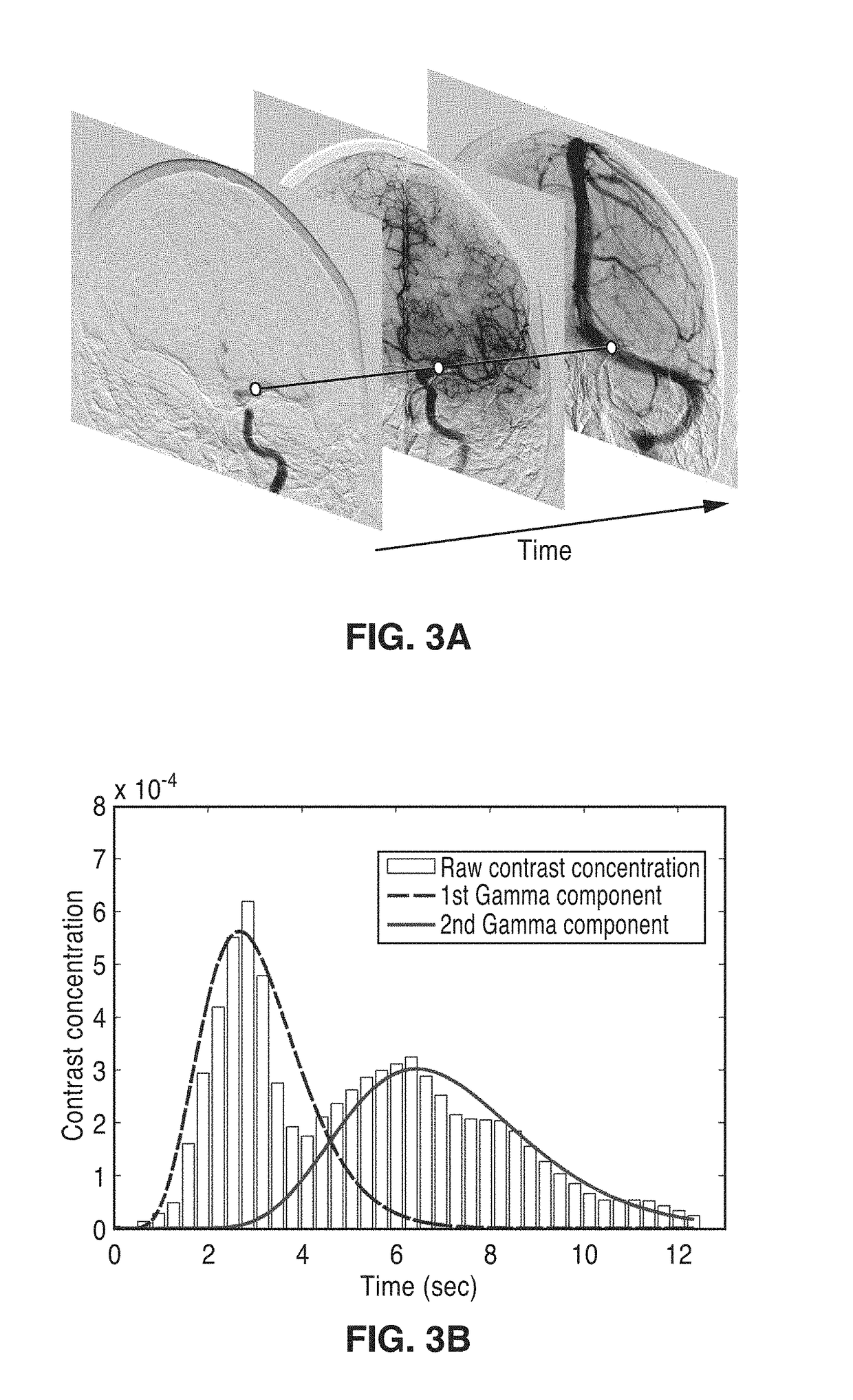
Perfusion digital subtraction angiography
ActiveUS20190015061A1Improve patient outcomesSustain viabilityImage enhancementImage analysisTime density curveVolumetric Mass Density
An apparatus and methodological framework are provided, named perfusion angiography, for the quantitative analysis and visualization of blood flow parameters from DSA images. The parameters, including cerebral blood flow (CBF) and cerebral blood volume (CBV), mean transit time (MTT), time-to-peak (TTP), and Tmax, are computed using a bolus tracking method based on the deconvolution of time-density curves on a pixel-by-pixel basis. Individual contrast concentration curves of overlapping vessels can be delineated with multivariate Gamma fitting. The extracted parameters are each transformed into parametric maps of the target that can be color coded with different colors to represent parameter values within a particular set range. Side by side parametric maps with corresponding DSA images allow expert evaluation and condition diagnosis.
Owner:RGT UNIV OF CALIFORNIA
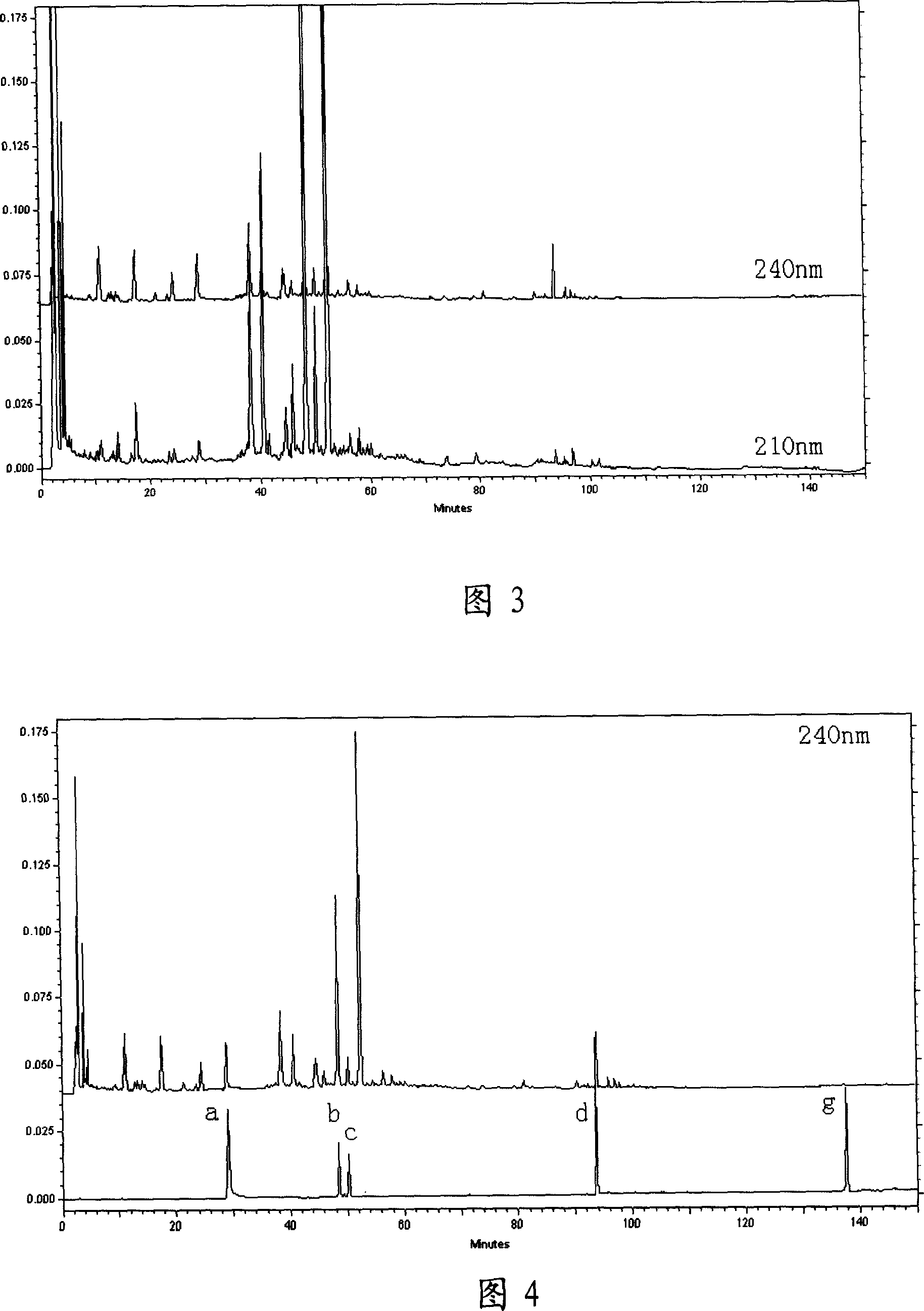
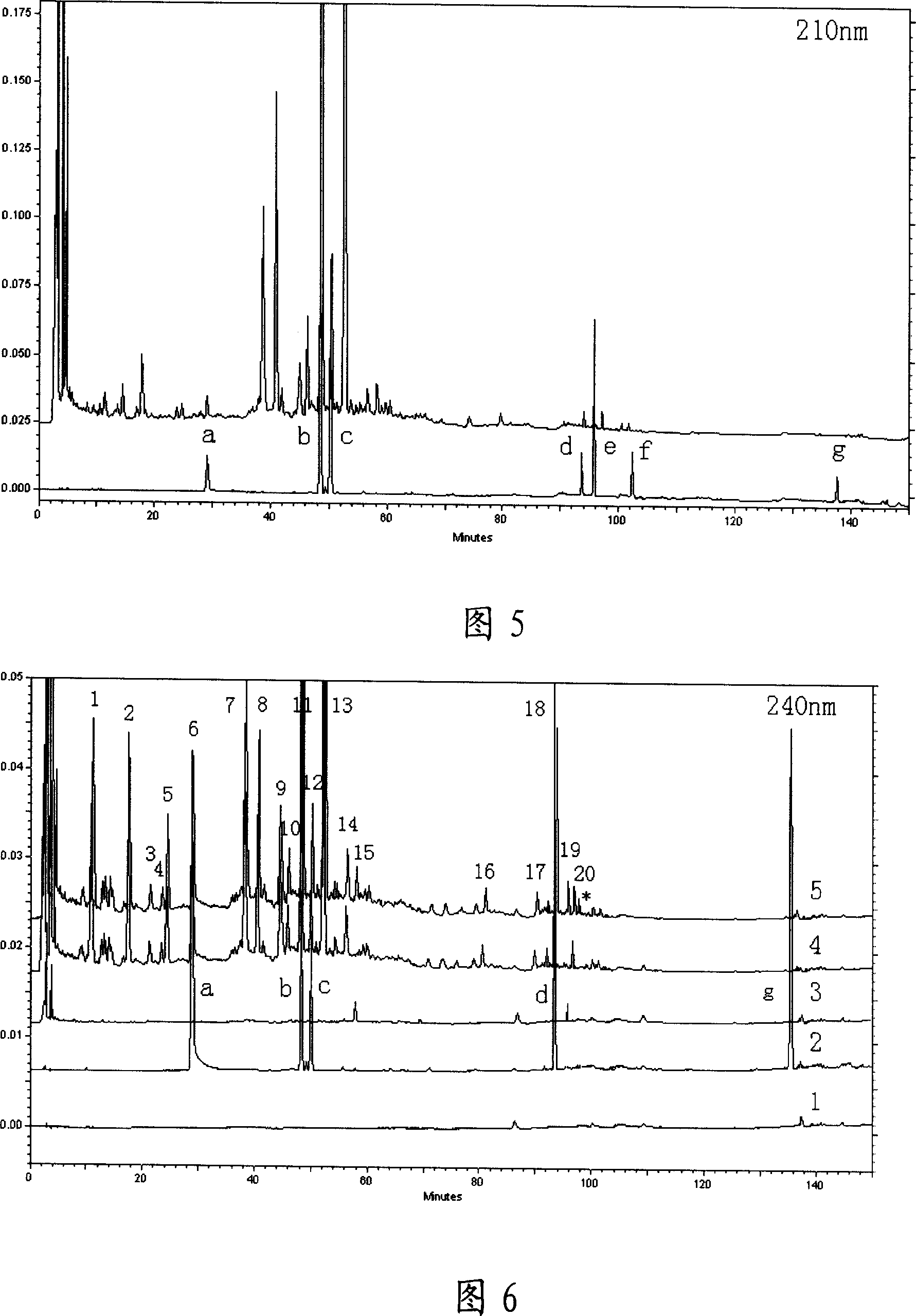
Fingerprint of Sinisan decoction and establishing method therefor
InactiveCN101008633AFully detectedReflect inner qualityComponent separationPreparing sample for investigationRetention timePeak area
This invention discloses one four inverse water boiling HPLC standard print spectrum establish method, which comprises the following steps: a, taking four inverse diffusion boiling powder and adding carbinol supersonic extracting, filtering and the filter liquid uses carbinol through micro hole filter film to get standard solution; b, extracting certain naringinase to dilute flow phase to process comparison resolution liquid; c, separately absorbing fin standard liquid and comparison liquid and adding high effective liquid phase color spectrum column to keep time and peak area value as one to compute standard relative keep time to peak proportion to get standard print spectrum.
Owner:HEILONGJIANG UNIV OF CHINESE MEDICINE
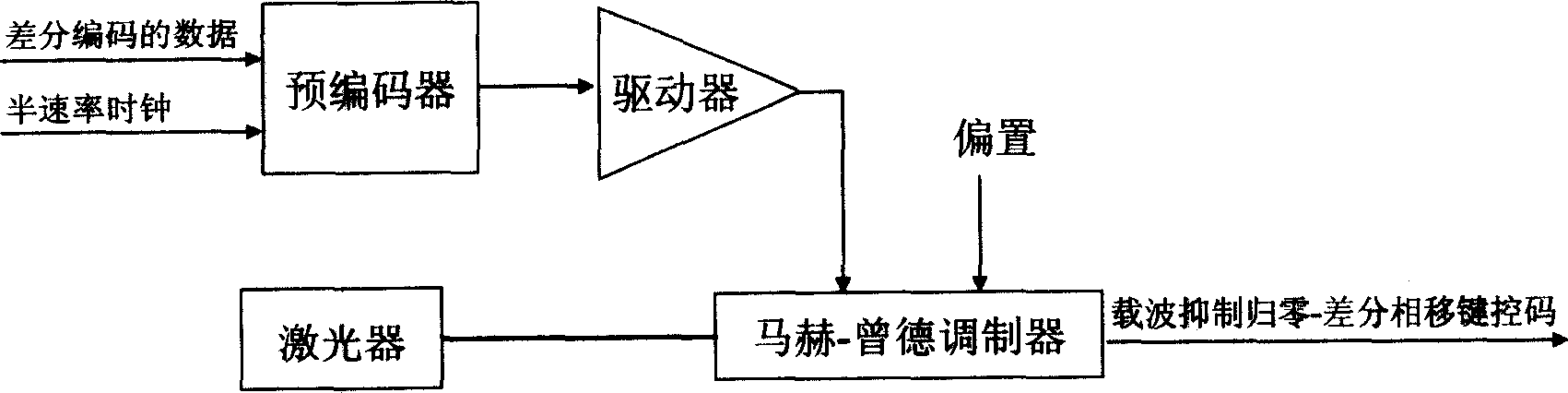
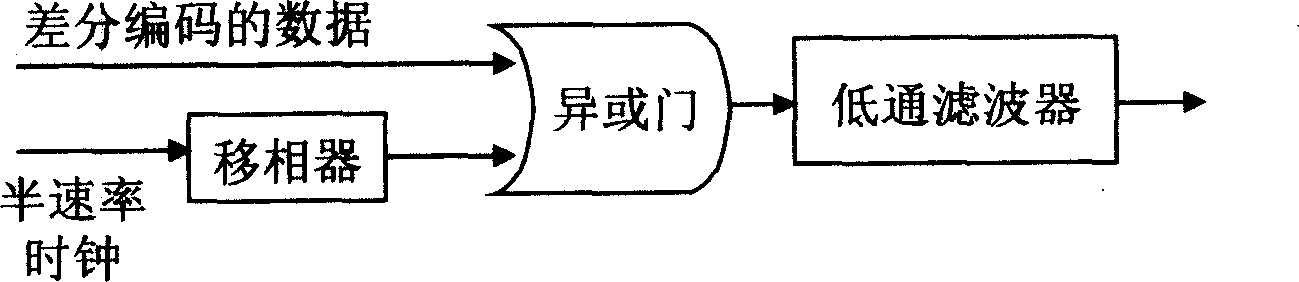
Single modulator realization method for CSRZ-DPSK
InactiveCN1845475AEasy to implementEasy to integrateElectromagnetic transmissionSmall amplitudeCarrier signal
The implementation method for a CSRZ-DPSK single modulator comprises: 1) taking code pre-treatment to the input data and synchronous semi-speed clock signal to form phase-modulated semi-speed clock signal; 2) driving the former signal to amplify the small-amplitude signal with V pi voltage as two times to peak-teak value; 3) mapping signal into light carrier by optical modulation to form CSRZ-DPSK sequence that jumps ' pi' phase when input '0' or not when input '1'. This invention can reduce cost and complexity.
Owner:SHANGHAI JIAO TONG UNIV
Pavement material with wide phase change temperature zone and preparation method thereof
The invention discloses a pavement material with a wide phase change temperature zone and a preparation method thereof. The pavement material comprises a shape-stabilized phase change material and asphalt, wherein a mass ratio of the shape-stabilized phase change material to the asphalt is 1:(5-20); the shape-stabilized phase change material is prepared by mixing tetradecane / carrier, pentadecane / carrier, hexadecane / carrier, heptadecane / carrier, octodecane / carrier, nonadecane / carrier, eicosane / carrier and polyethylene glycol / carrier; and a mass ratio is 1:1:(1-3):(1-3):(1-3):(1-3):1:1. The shape-stabilized phase change material of different phase change temperatures is doped into the asphalt, so that the asphalt concrete pavement temperature is regulated, the adaptability on the outside temperature change is enhanced, the temperature rise rate inside large-volume asphalt concrete is effectively reduced, time-to-peak force is delayed, the influence of freezing-thawing cycle and extremely high or low temperature on the concrete pavement performance is reduced, thermal cracks occurring in the asphalt concrete can be prevented, the durability of the material is improved, and the service life is prolonged.
Owner:ZHEJIANG OCEAN UNIV
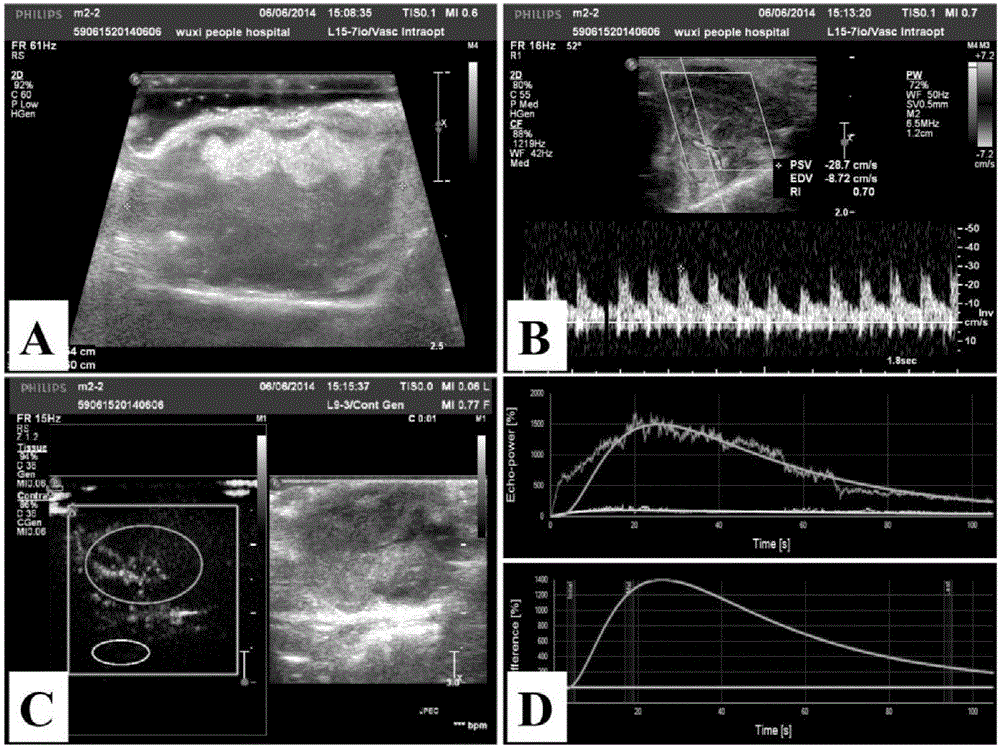
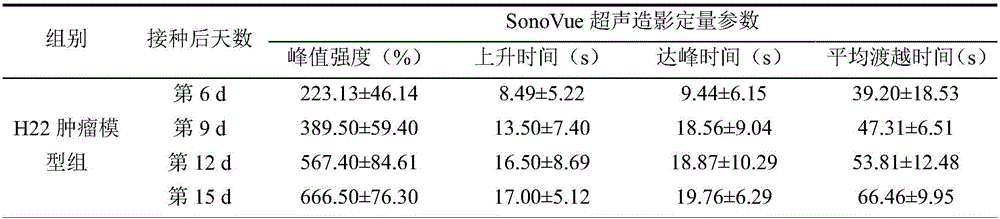
Examination indexes of SonoVue ultrasonic contrast technology in diagnosis and treatment of tumor angiogenesis mimicry
InactiveCN105879064AEchographic/ultrasound-imaging preparationsAbnormal tissue growthVascular ultrasound
The invention discloses examination indexes of a SonoVue ultrasonic contrast technology in diagnosis and treatment of tumor angiogenesis mimicry and belongs to the technical field of clinical tumor vessel ultrasonic examination. The situation that ultrasonic examination indexes including the rising time, the time to peak and the average transition time can be used for clinical diagnosis and treatment of tumor angiogenesis mimicry is found for the first time, a basis is provided for clinical early noninvasive diagnosis of tumor angiogenesis mimicry, the grade malignancy, the blood supply mode and other characters of a tumor of a patient can be clinically diagnosed easily, and a diagnosis basis is provided for drug selection or operation treatment.
Owner:JIANGNAN UNIV
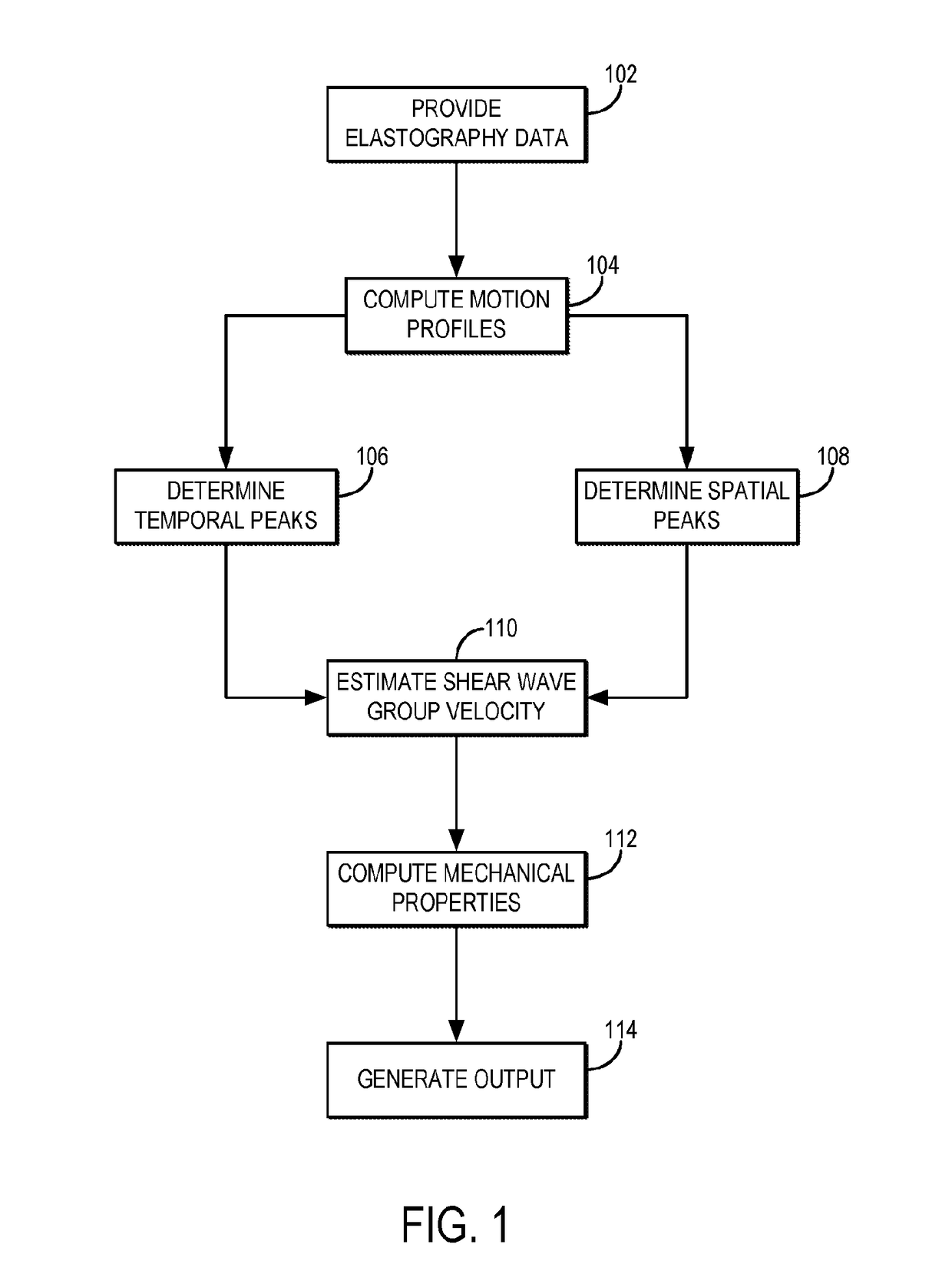
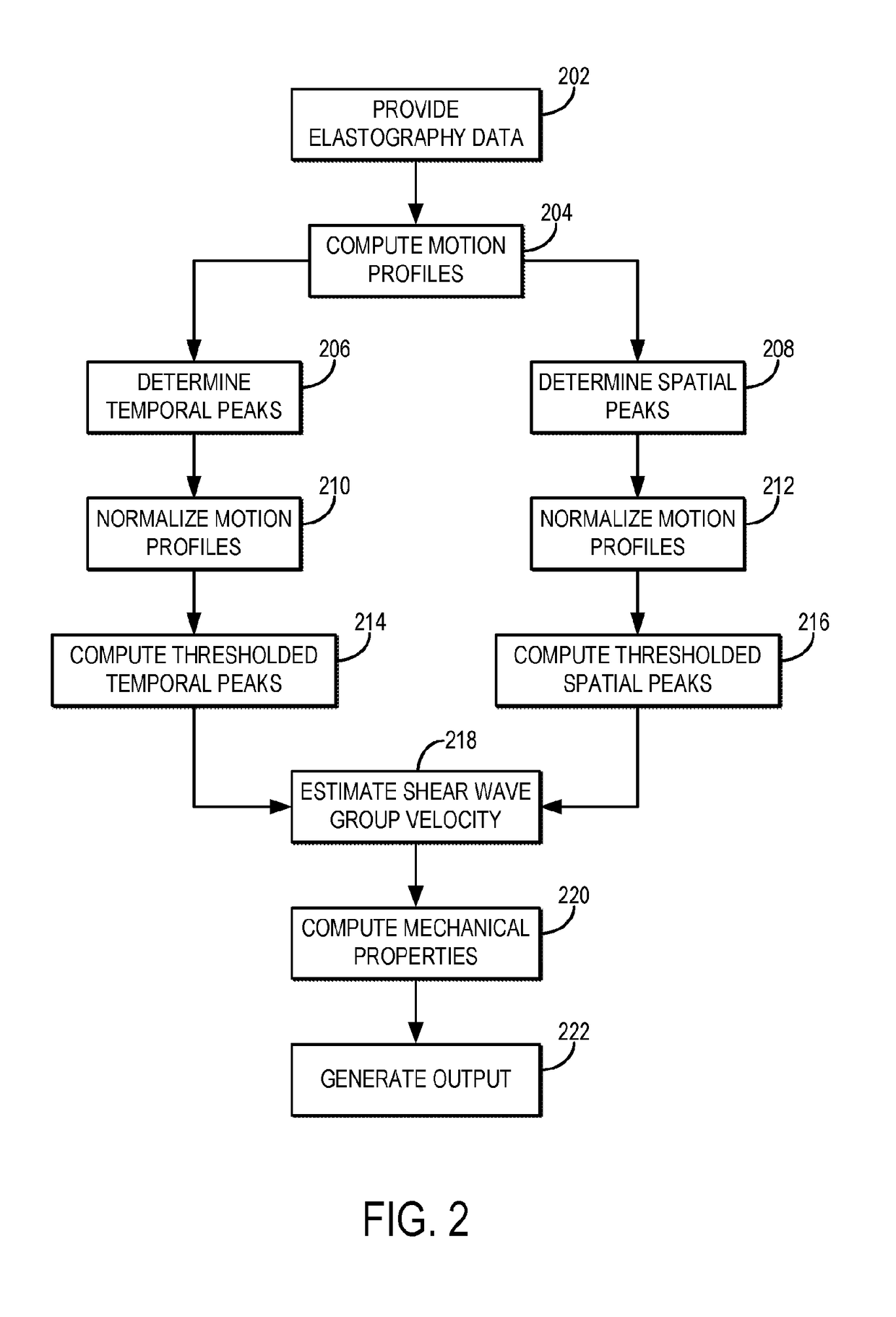
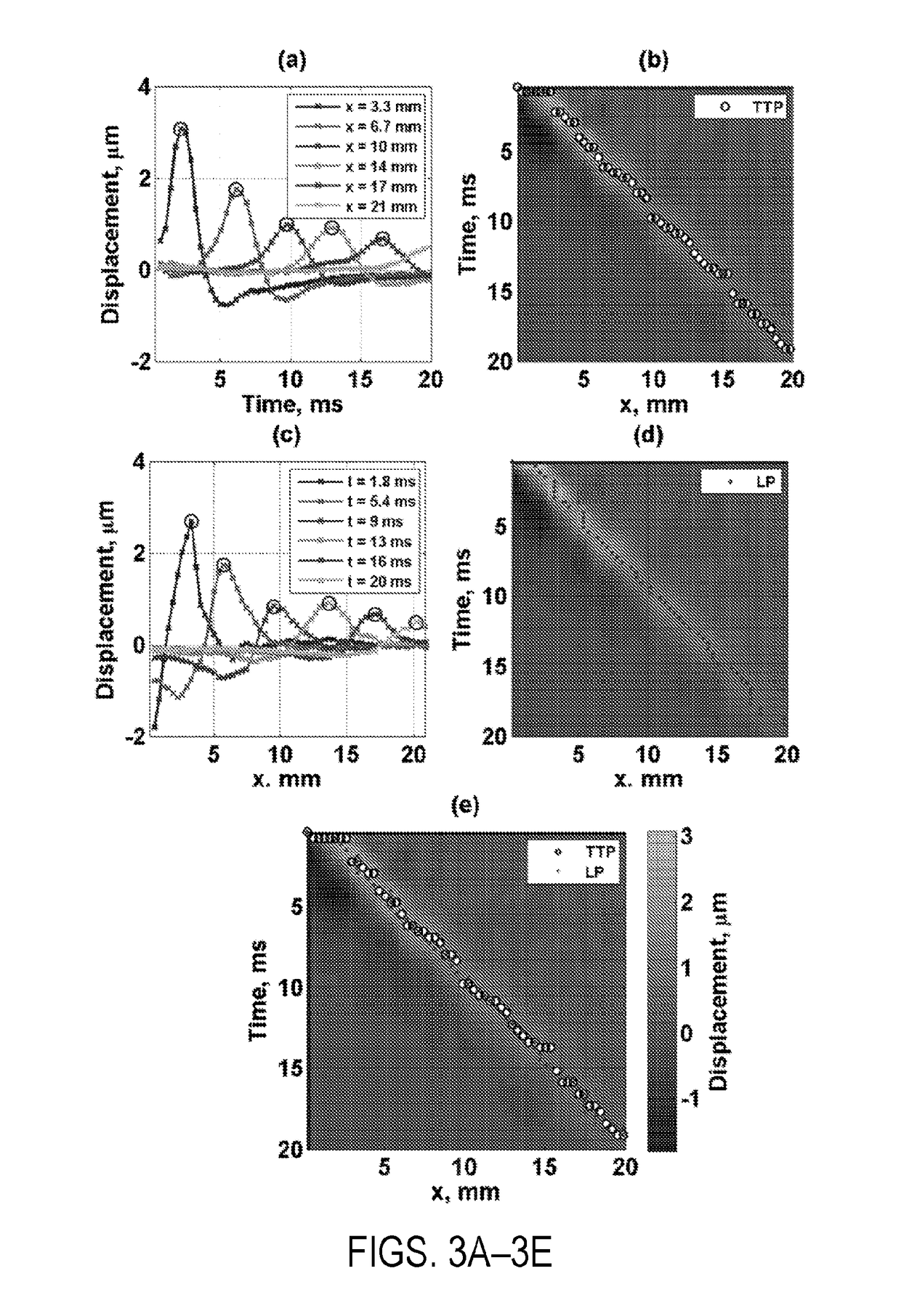
Shear wave group velocity estimation using spatiotemporal peaks and amplitude thresholding
ActiveUS20190076126A1Health-index calculationOrgan movement/changes detectionVibration amplitudeClassical mechanics
Described here are systems and methods for estimating shear wave velocity from data acquired with a shear wave elastography system. More particularly, the systems and methods described here implement a spatiotemporal time-to-peak algorithm that searches for the times at which shear wave motion is at a maximum while also searching for the lateral locations at which shear wave motion is at a maximum. Motion can include displacement, velocity, or acceleration caused by propagating shear waves. A fitting procedure (e.g., a linear fit) is performed on a combined set of these temporal peaks and spatial peaks to estimate the shear wave velocity, from which mechanical properties can be computed. Motion amplitude thresholding can also be used to increase the number of points for the fitting.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
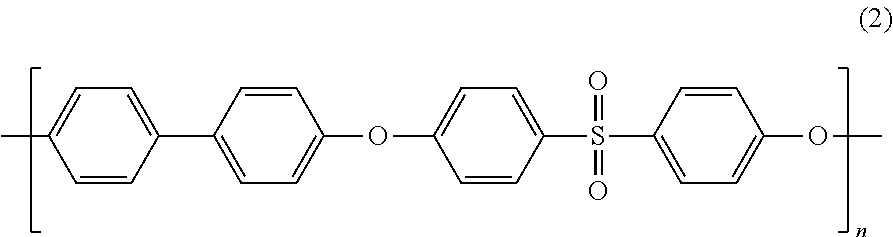
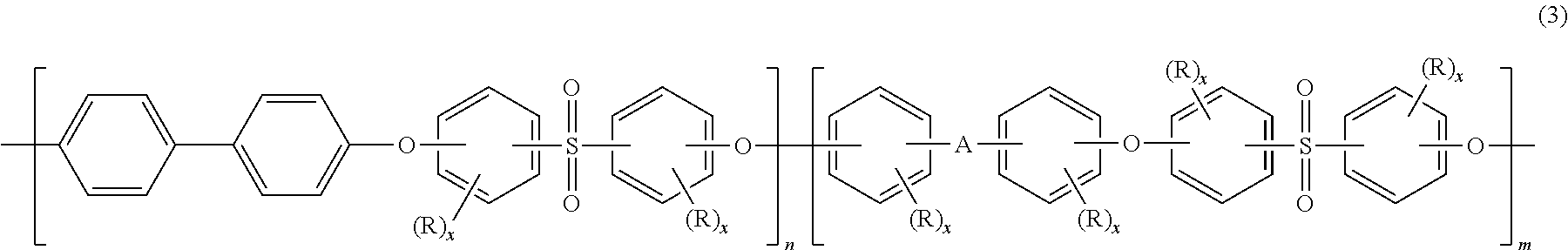
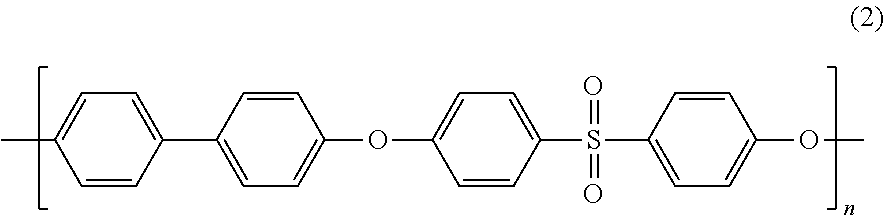
Blends of polyphenylene ether sulfone and silicone polyester carbonate
Disclosed herein is a composition comprising a polyphenylene ether sulfone and a resorcinol based silicone aryl polyester carbonate copolymer wherein greater than or equal to 50 mol % of copolymer repeating units are ester units derived from resorcinol, based on the total molar amount of all repeating units in the polymer and wherein the blend has less than 1 weight percent polycarbonate, based on the total weight of the composition, has FAR 25.853 peak heat release of less than 60 KW / m2 and time to peak heat release of greater than or equal to 120 seconds.
Owner:SABIC INNOVATIVE PLASTICS IP BV
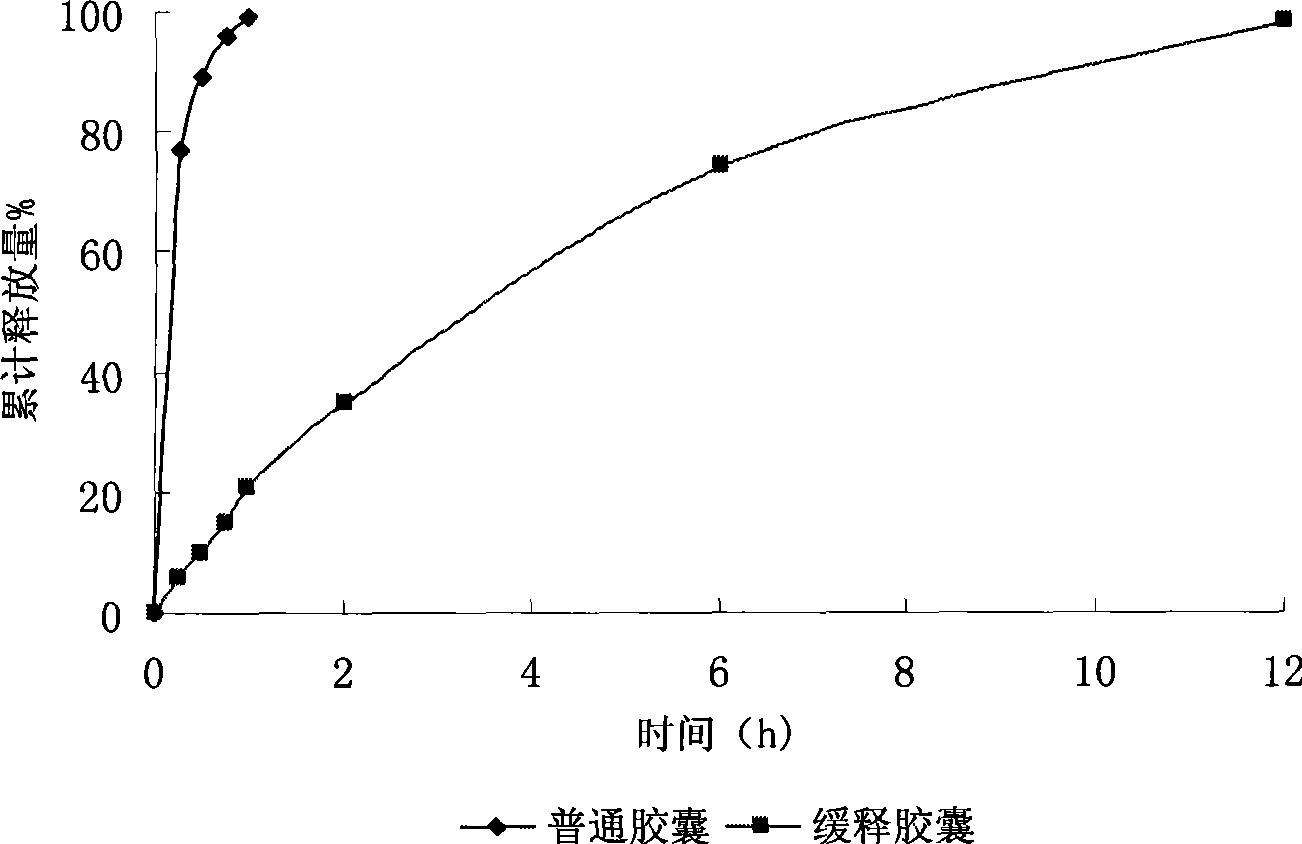
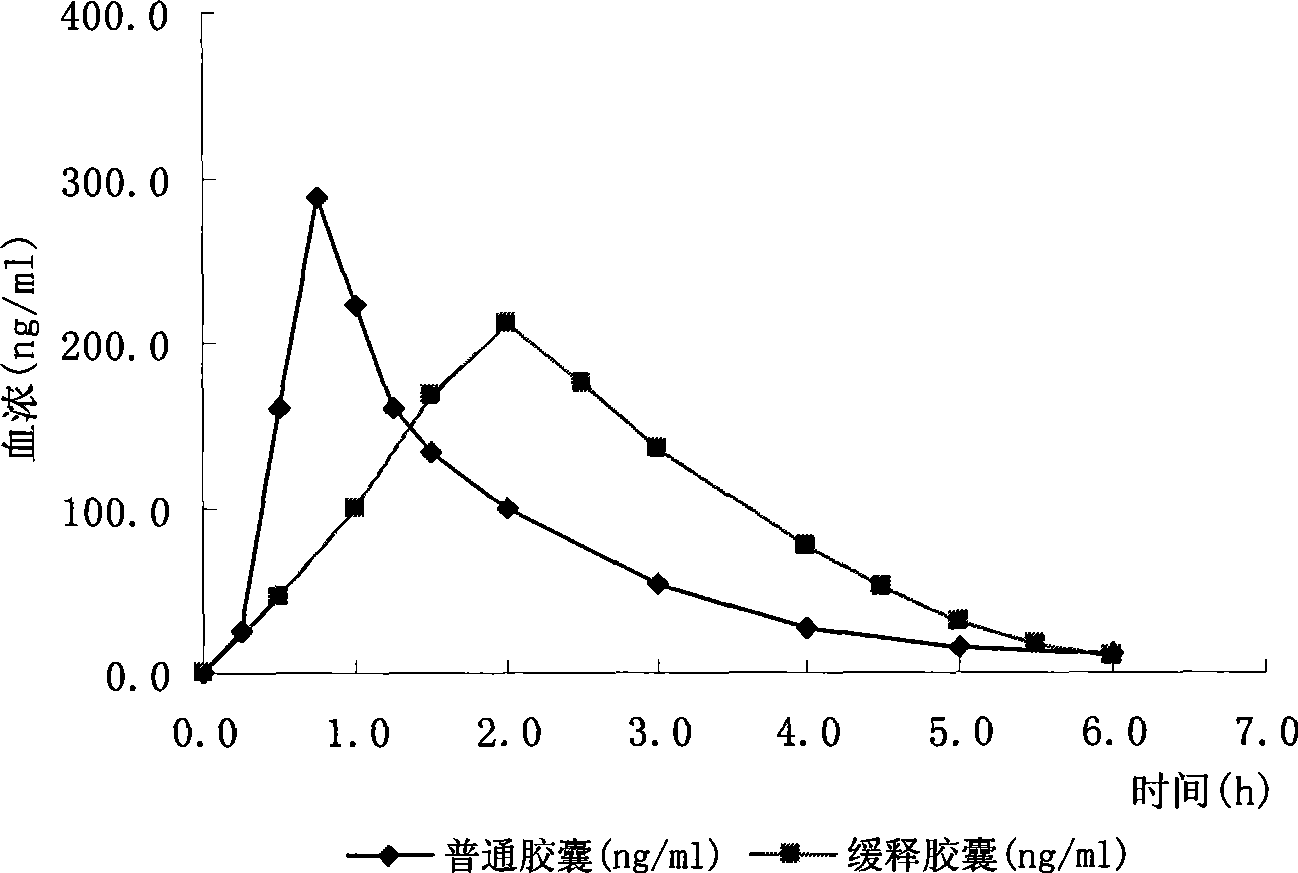
Trimebutine or its salt sustained release capsules and preparation method thereof
InactiveCN101361724ADigestive systemPharmaceutical delivery mechanismBlood concentrationCentrifugation
The invention discloses a slow release capsule of trimebutine or the salt thereof and a preparation method thereof. The slow release capsule of trimebutine or the salt thereof is prepared by adding the accessories of filler, adhesive and slow release materials and the like into the trimebutine, wherein, the trimebutine can be replaced by the maleate thereof or other medicinal salts. The slow release capsule of trimebutine or the salt thereof can be obtained through the following preparation processes: a pill core is first prepared by adopting microcrystalline cellulose or starch and the like through centrifugation, spray cementing, powder supply, polishing and other processes and coated with a medicated layer to prepare a medicated pill core, which is then coated with a slow release film to make a trimebutine slow release mini-pill, finally the slow release capsule of trimebutine or the salt thereof is obtained after filling the mini-pill in a capsule. Compared with the common preparation, the slow release capsule can continue with drug delivery for up to 12 hours, and has comparably stable blood concentration and long time to peak. And the preparation method of the capsule is characterized by good repeatability and easy operation and the like.
Owner:亚宝药业太原制药有限公司
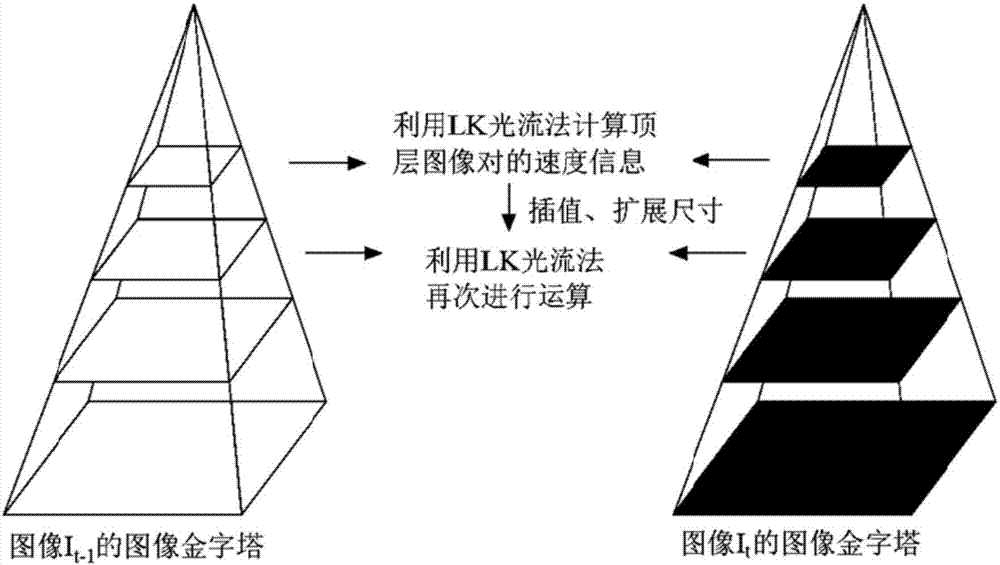
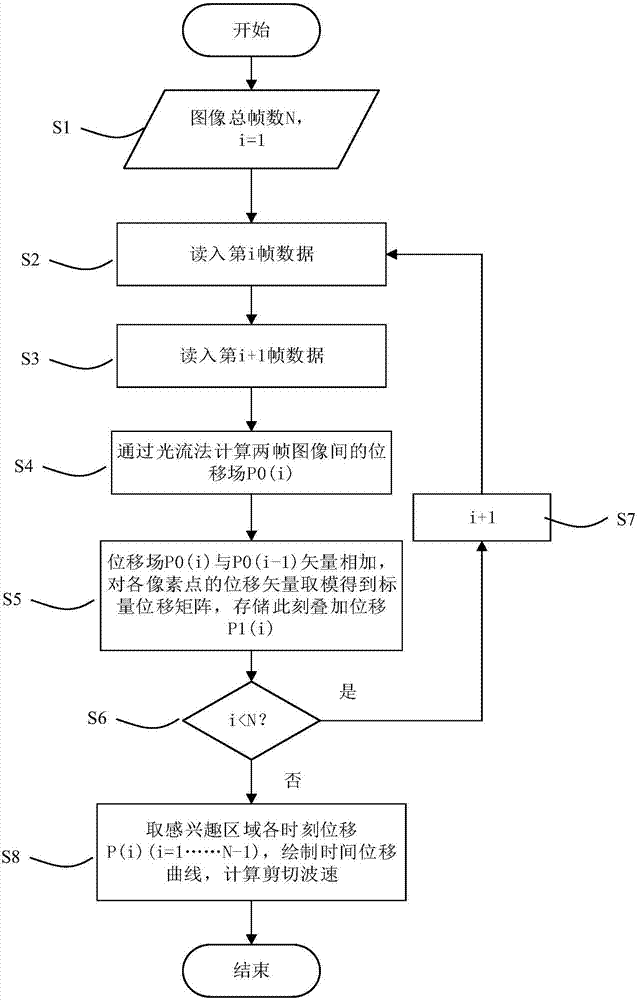
HIFU damage shear wave elastic characteristic estimation method based on LK optical flow
ActiveCN106214182AQuantitative estimation of elastic propertiesHigh sensitivityOrgan movement/changes detectionInfrasonic diagnosticsTreatment effectEngineering
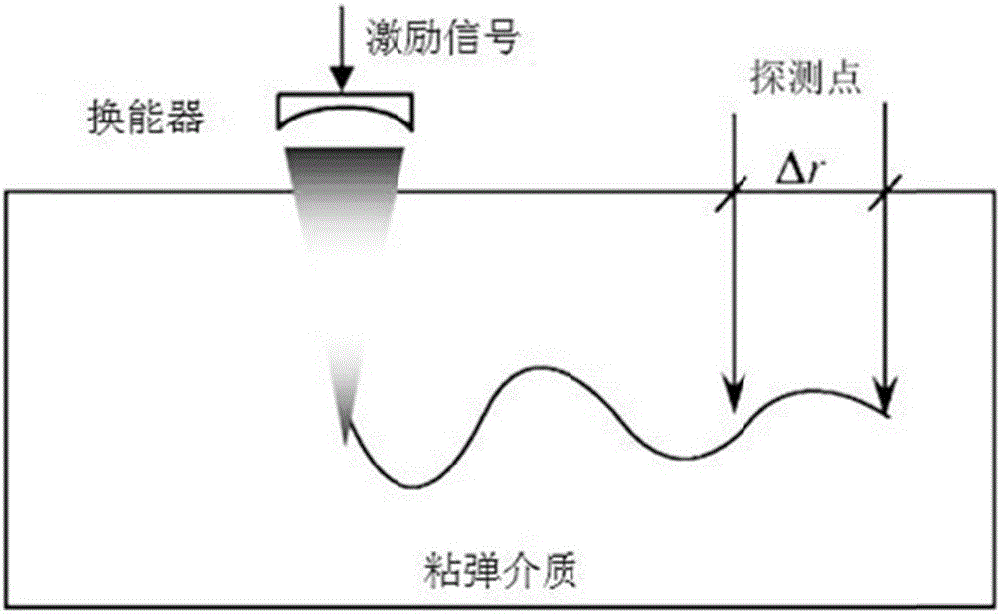
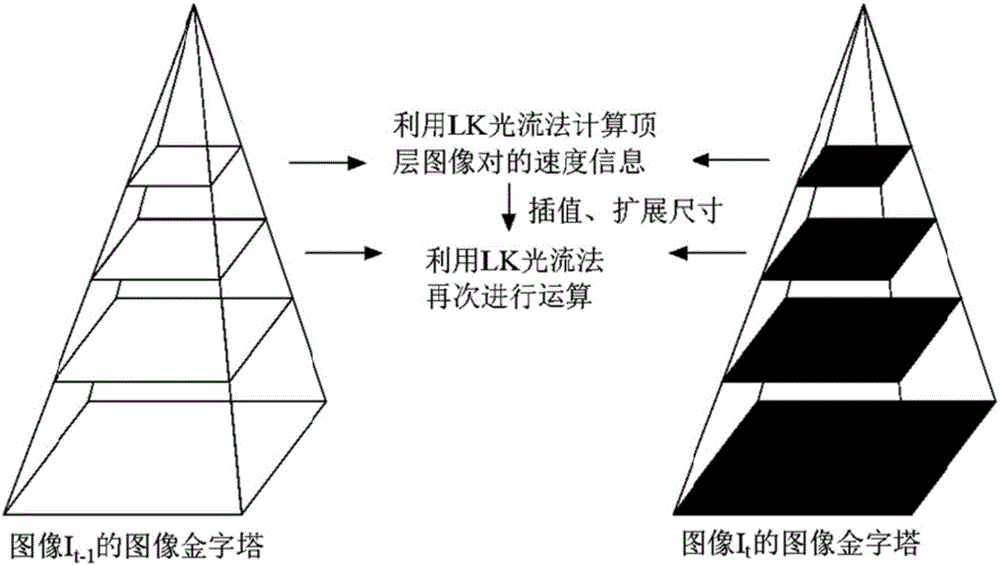
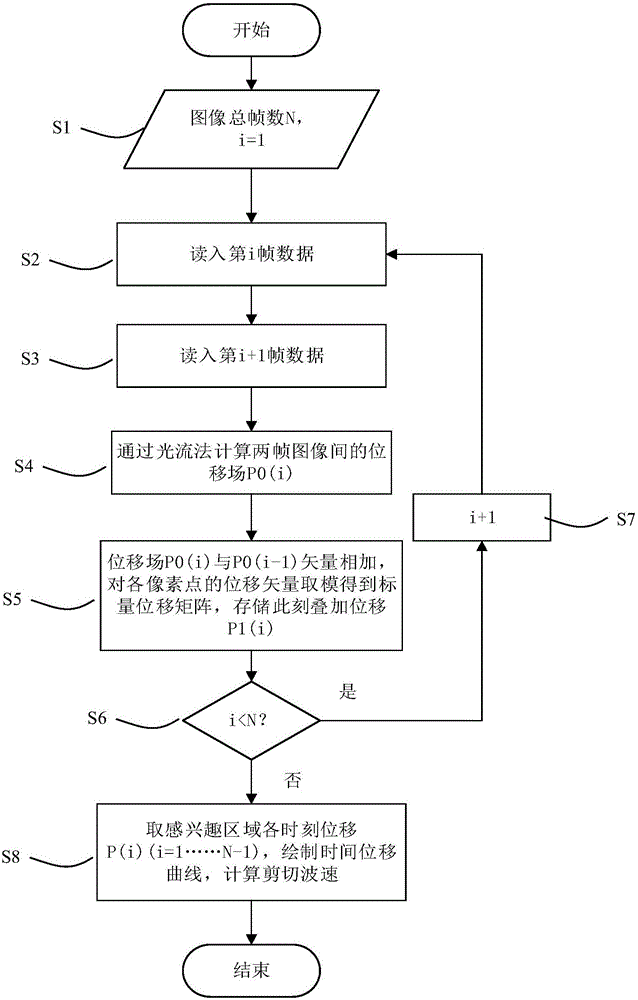
The invention discloses an HIFU damage shear wave elastic characteristic estimation method based on LK optical flow. A damage formation and vibration continuous image is obtained with a high-speed camera when HIFU acts on tissue phantom, a vibration displacement curve of damage is obtained with the Lucas-Kanade optical flow method of an image pyramid, the propagation speed of acoustic radiation force shear wave generated on damage under the action of HIFU is calculated with the time-to-peak (TTP) method, quantitative estimation of shear elasticity modulus of damage can be conducted according to the relation among medium density, shear wave velocity in medium and medium shear elasticity modulus, and then the elastic characteristic of target tissue during treatment is monitored in real time and treatment effect is evaluated.
Owner:永春县产品质量检验所福建省香产品质量检验中心国家燃香类产品质量监督检验中心福建
Active ingredient composition for treating alcoholic liver injury
ActiveCN102028701AClinical dosage is accurate, safe and effectiveEasy to useDigestive systemHeterocyclic compound active ingredientsSide effectHalf-life
The invention discloses an active ingredient composition for treating alcoholic liver injury, and relates to an active ingredient composition for treating liver injury. The invention solves the problems that the conventional traditional Chinese medicines and compound decoction thereof for treating liver injury are inconvenient to use, and the dosage and safety are difficult to control. The active ingredient composition for treating the alcoholic liver injury consists of 6,7-dimethoxybenzopyran-2-one, geniposide and parietic acid. The active ingredient composition for treating the alcoholic liver injury has the advantages that: the clinical medicinal dosage is accurate, safe and effective, the active ingredient composition is convenient to use, the treatment effect is improved, medicinal materials are saved, and toxic and side effects are reduced; and the 6,7-dimethoxybenzopyran-2-one, the geniposide and the parietic acid have liver-protecting and gut-benefiting components having the advantages of quick absorption (short time to peak), slow metabolism (longer half life of medicines), higher peak concentration and larger blood concentration area under a curve, so the 6,7-dimethoxybenzopyran-2-one, the geniposide and the parietic acid can serve as preferred active ingredients. The active ingredient composition provides a basis for subsequently preparing finished pharmaceutical products in various formulations.
Owner:王喜军
Automatic segmentation of tissues by dynamic change characterization
InactiveCN1914642ASplit possibleImage enhancementImage analysisDiagnostic dataAutomatic segmentation
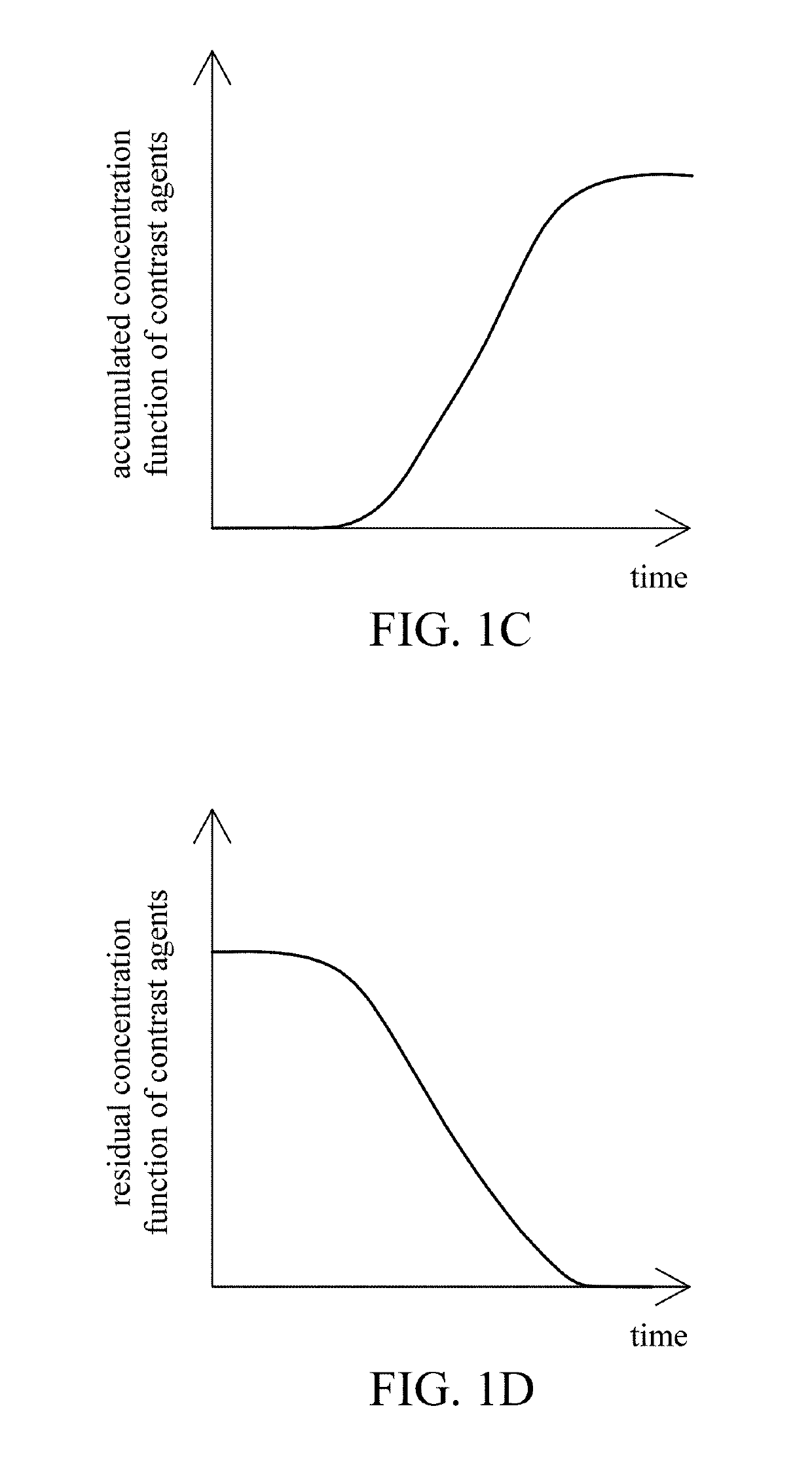
A reconstruction processor(24) reconstructs diagnostic data from a diagnostic imaging device, such as a CT scanner(10), starting before a contrast agent reaches a region of interest(50), as the concentration of contrast agent in the region of interest builds(52), and at a contrast agent peak(56). The plurality of images generated while the contrast agent concentration is building are aligned(78). A change map is generated indicative of a rate-of-change(62) gradient or a time-to-peak(64) for corresponding pixels or voxels of the images generated during the time the contrast agent is building to the peak. A segmentation processor(70) uses the change map in segmenting the diagnostic images generated without contrast agent or at the contrast agent concentration peak.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
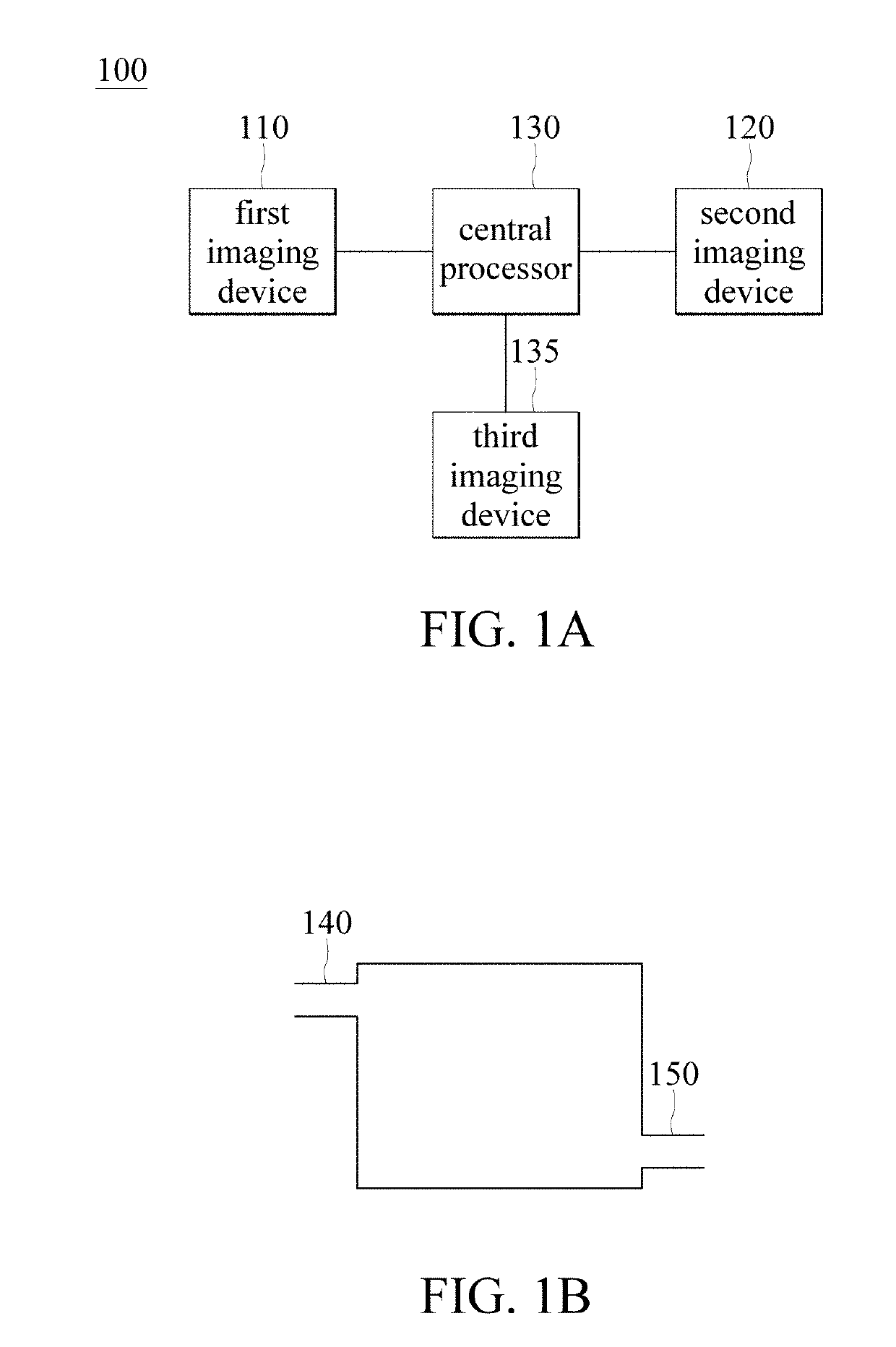
Brain imaging system and method
A brain imaging system includes a first imaging device, a second imaging device, a third imaging device and a central processor. The first imaging device captures a first brain image, and calculates a cerebral blood flow, a cerebral blood volume, a cerebral blood mean transit time and a first contrast agent time to peak. The second imaging device captures a second brain image, and calculates the cerebral blood flow, the cerebral blood volume, the cerebral blood mean transit time and a second contrast agent time to peak. The central processor generates an image of a vessel occlusion, infarction or ischemia region respectively according to the vessel occlusion, infarction or ischemia regions in the first brain image and in the second brain image. The third imaging device obtains a brain atrophy region according to the cerebral cortex volume calculated by the third imaging device.
Owner:A MOY LTD
Chlorpheniramine maleate compound and pharmaceutical composition thereof
ActiveCN103787958AShorten the timeShortened half-lifeOrganic active ingredientsOrganic chemistryChemical structureHalf-life
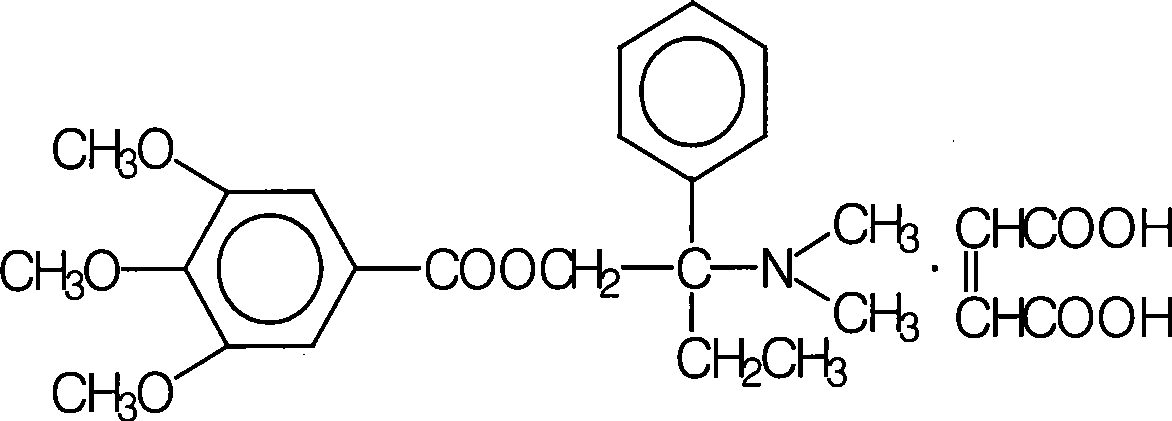
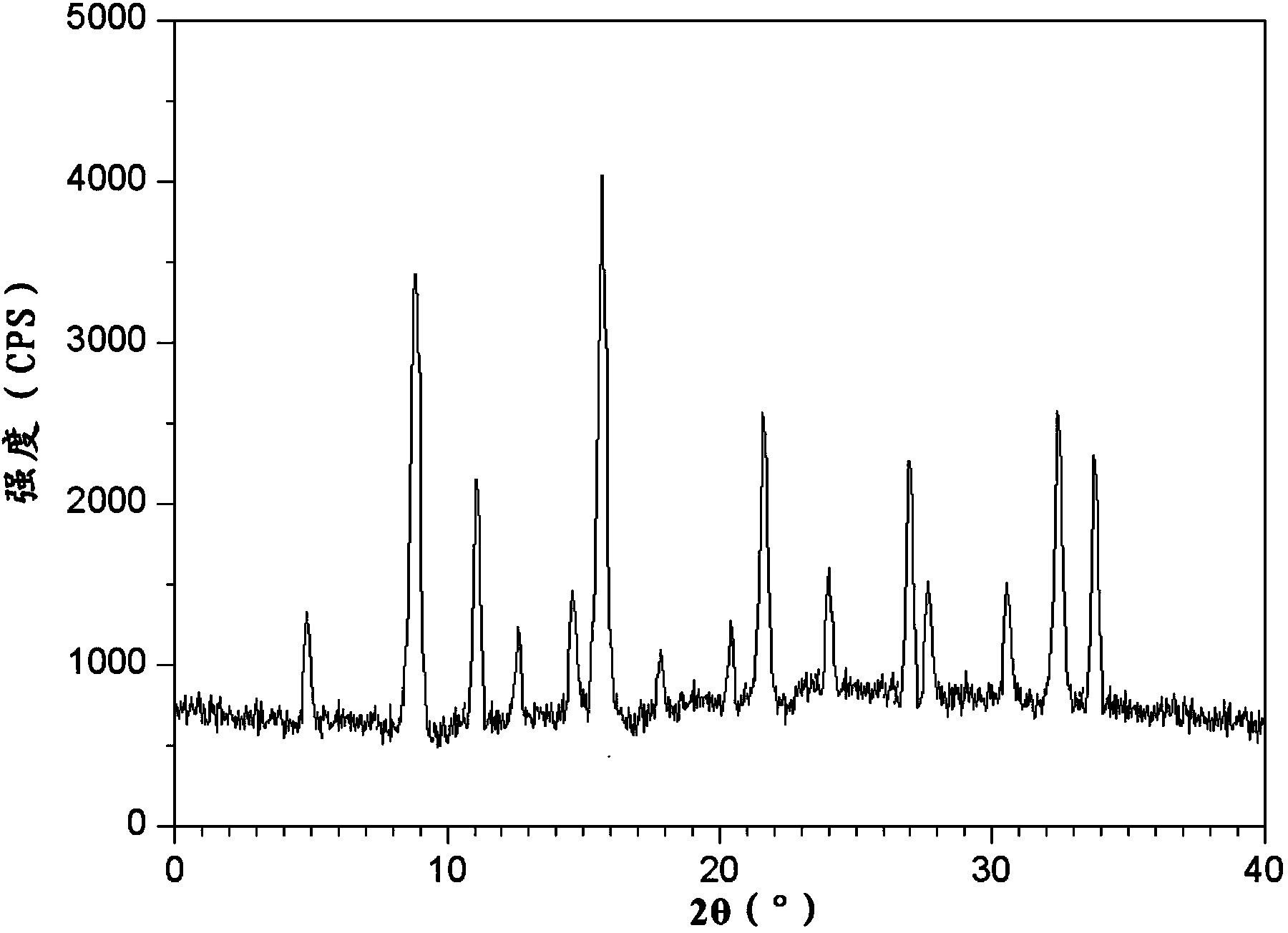
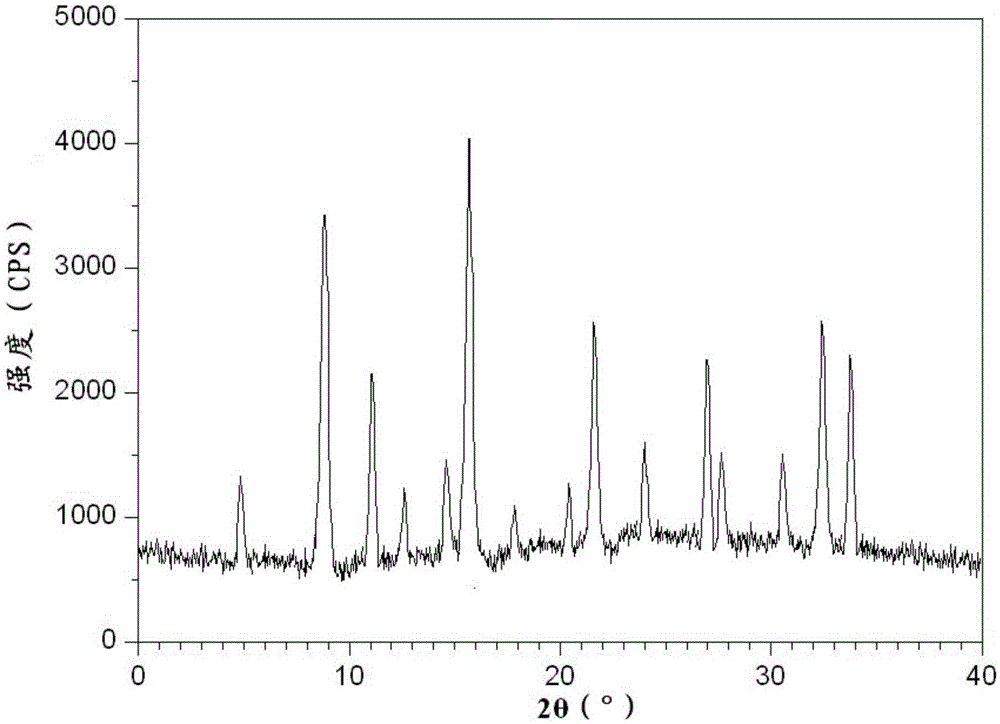
The invention belongs to the technical field of medicine, and particularly relates to a chlorpheniramine maleate compound and a pharmaceutical composition of the chlorpheniramine maleate compound. The chlorpheniramine maleate compound has a chemical structure formula seen in the formula (I) and is measured through an X-ray power diffraction measurement method, and an X-ray power diffraction pattern which is expressed by a diffraction angle of 2 theta+ / -0.2 degrees can be seen in the drawing 1. The chlorpheniramine maleate compound is a new crystal form compound different from existing chlorpheniramine maleate in the prior art. The time to peak and the half-life period of chlorpheniramine in pseudoephedrine hydrochloride and chlorphenamine maleate tablets prepared through the new crystal form compound are obviously shortened, the differences between the time to peak and the half-life period of the chlorpheniramine and the time to peak and the half-life period of ibuprofen and the differences between the time to peak and the half-life period of the chlorpheniramine and the time to peak and the half-life period of the pseudoephedrine hydrochloride are reduced, time for allowing concentration of active ingredients of the three types of medicine in blood to reach the effective concentration and time for allowing activity of the active ingredients of the three types of medicine to fade away are kept the same as much as possible, and therefore the pharmacological function of the pseudoephedrine hydrochloride and chlorphenamine maleate tablets is enhanced cooperatively.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Oil suspension containing doxycycline hydrochloride and flunixin meglumine and preparation method of oil suspension
The invention belongs to the technical fields of veterinary antibiotics and preparation methods of the antibiotics and particularly relates to an oil suspension injection containing doxycycline hydrochloride and flunixin meglumine and a preparation method of the oil suspension injection. The oil suspension injection is composed of crude drugs (a) of doxycycline hydrochloride and flunixin meglumine, a suspending aid (b), a surfactant (c) and an antioxidant (d). The oil suspension injection containing doxycycline hydrochloride and flunixin meglumine, provided by the invention, is prepared by using a dispersion method and is a light yellow slightly-thick oil suspension. The oil suspension injection containing doxycycline hydrochloride and flunixin meglumine, researched by the invention, is good in stability and biocompatibility and can be used as a veterinary long-acting injection, the absorption time and the elimination half life are prolonged, and the time to peak is delayed.
Owner:QINGDAO VLAND BIOTECH INC
Estimation method of HIFU damage shear wave elastic properties based on lk optical flow method
ActiveCN106214182BQuantitative estimation of elastic propertiesHigh sensitivityOrgan movement/changes detectionInfrasonic diagnosticsVolumetric Mass DensityEngineering
The invention discloses an HIFU damage shear wave elastic characteristic estimation method based on LK optical flow. A damage formation and vibration continuous image is obtained with a high-speed camera when HIFU acts on tissue phantom, a vibration displacement curve of damage is obtained with the Lucas-Kanade optical flow method of an image pyramid, the propagation speed of acoustic radiation force shear wave generated on damage under the action of HIFU is calculated with the time-to-peak (TTP) method, quantitative estimation of shear elasticity modulus of damage can be conducted according to the relation among medium density, shear wave velocity in medium and medium shear elasticity modulus, and then the elastic characteristic of target tissue during treatment is monitored in real time and treatment effect is evaluated.
Owner:永春县产品质量检验所福建省香产品质量检验中心国家燃香类产品质量监督检验中心福建
Reinforced thermoplastic articles, compositions for the manufacture of the articles, methods of manufacture, and articles formed therefrom
A composition for the manufacture of a porous, compressible article, the composition comprising a combination of: a plurality of reinforcing fibers; a plurality of polyimide fibers; and a plurality of polymeric binder fibers; wherein the polymeric binder fibers have a melting point lower than the polyimide fibers; methods for forming the porous, compressible article; and articles containing the porous, compressible article. An article comprising a thermoformed dual matrix composite is also disclosed, wherein the composite exhibits a time to peak release, as measured by FAR 25.853 (OSU test), a 2 minute total heat release, as measured by FAR 25.853 (OSU test), and an NBS optical smoke density of less than 200 at 4 minutes, determined in accordance with ASTM E-662 (FAR / JAR 25.853).
Owner:SABIC GLOBAL TECH BV
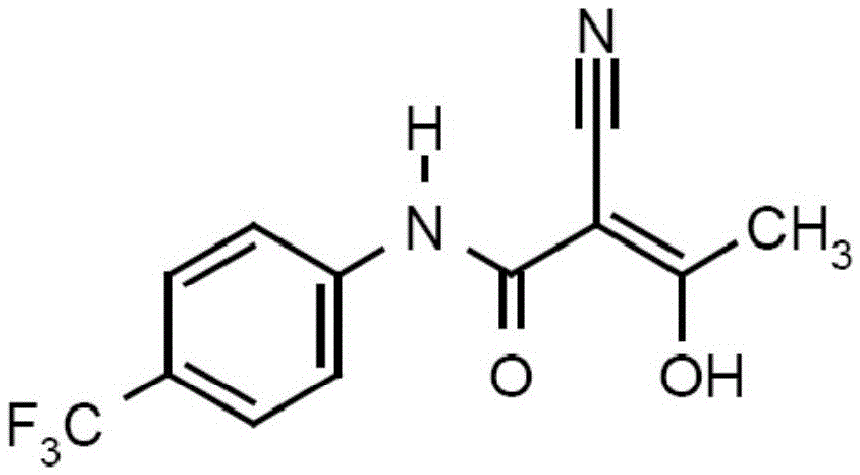
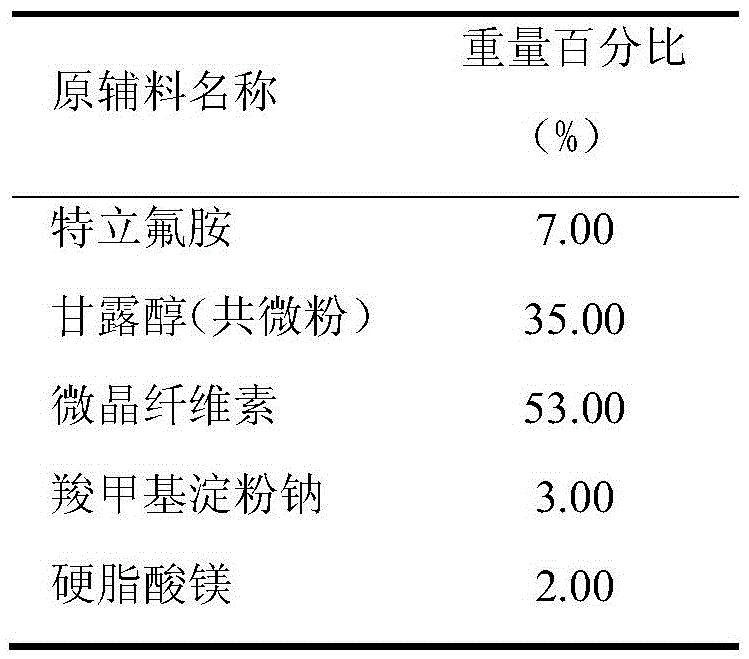
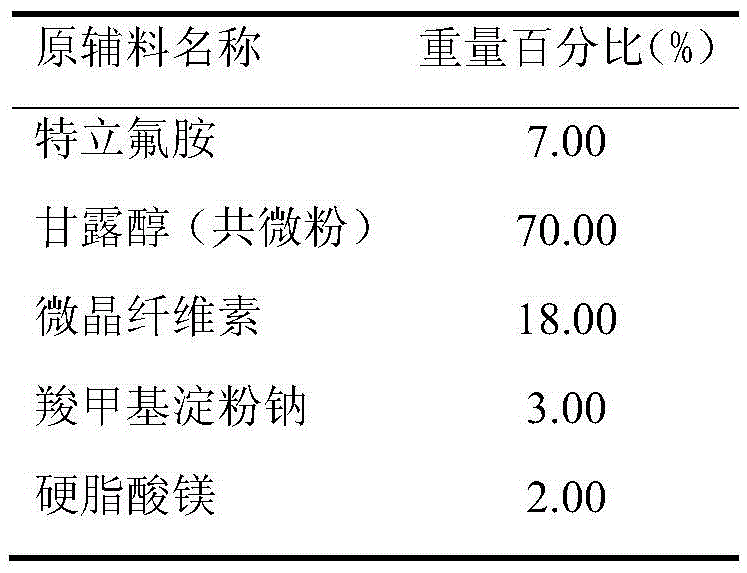
Teriflunomide dispersible tablet and preparation method thereof
InactiveCN106880608AThere are few varieties to chooseImprove securityNervous disorderPill deliveryMedicineDissolution
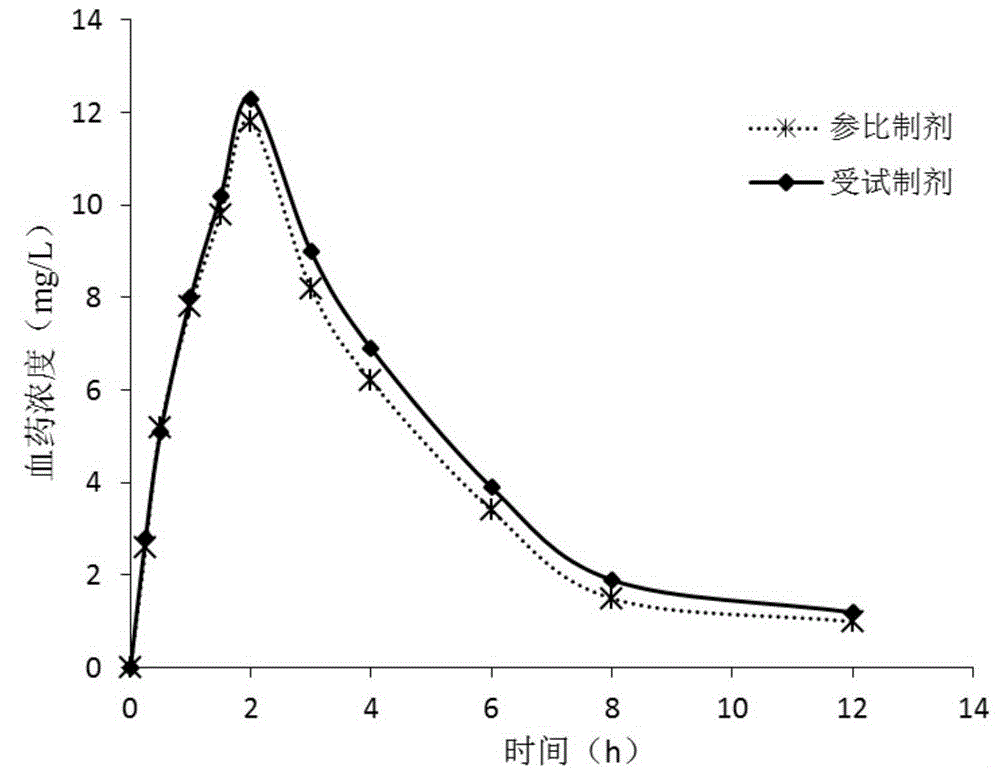
The invention provides a teriflunomide dispersible tablet and a preparation method thereof. The dispersible tablet consists of teriflunomide, which serves as an active ingredient, and a filler, a disintegrating agent and a lubricating agent which are used as pharmaceutical adjuvants. The teriflunomide dispersible tablet prepared by the invention can be completely disintegrated rapidly, so that a time to peak is shortened; a dissolution rate can reach 90% or above; the dispersible tablet is convenient to take, rapid in efficacy release and high in bioavailability; and the preparation method is simple in process, easy for control, convenient to operate and suitable for industrial production.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Sofosbuvir dispersible tablet and preparation method thereof
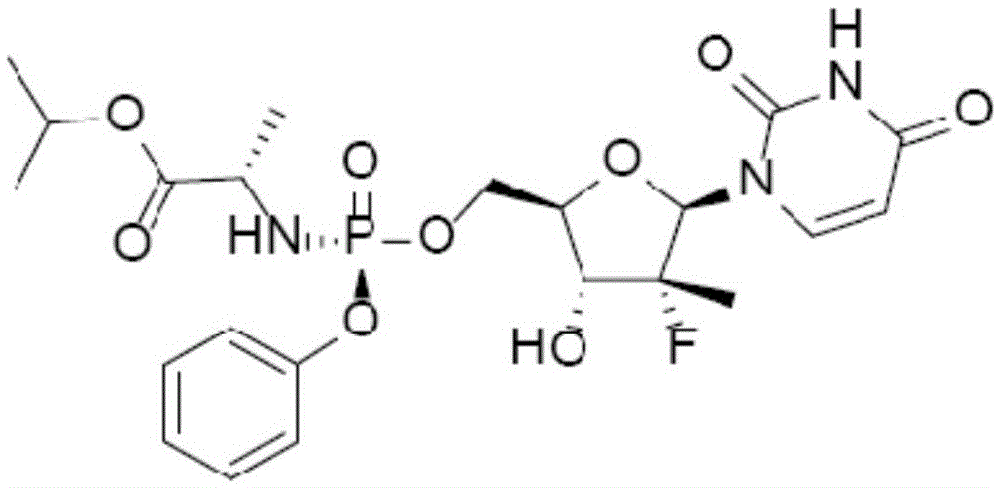
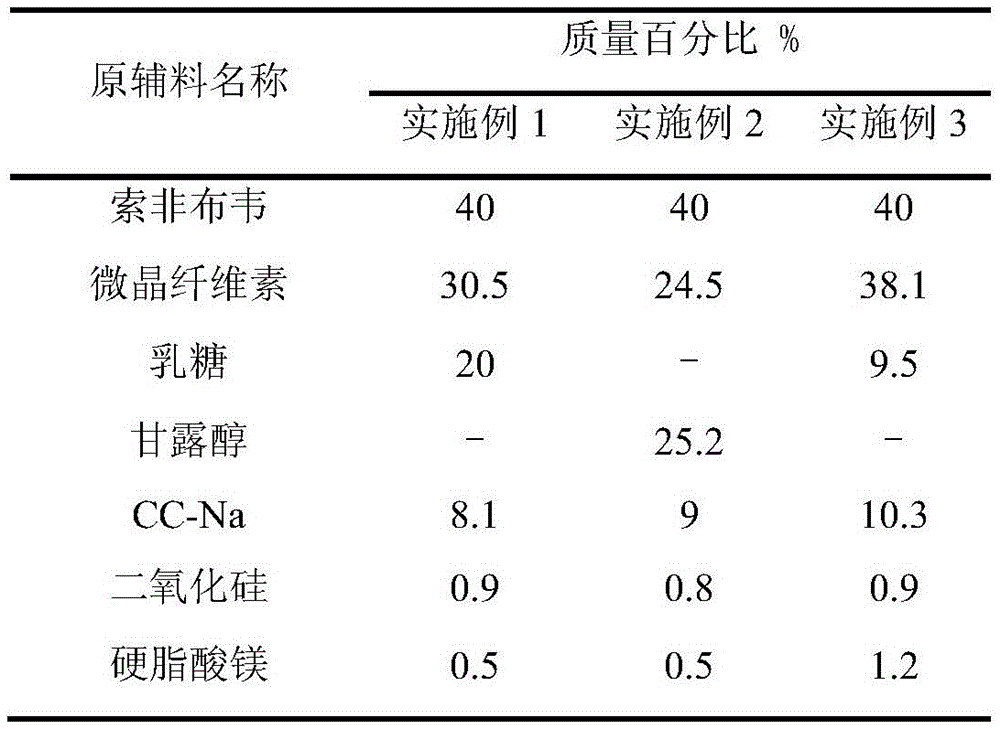
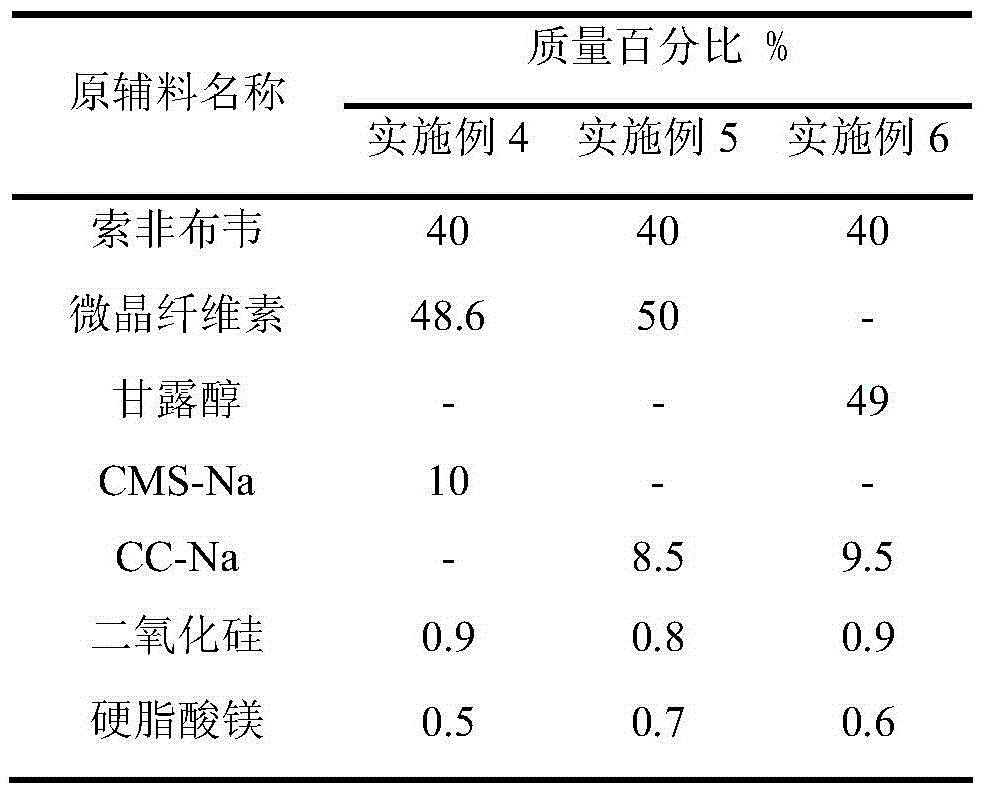
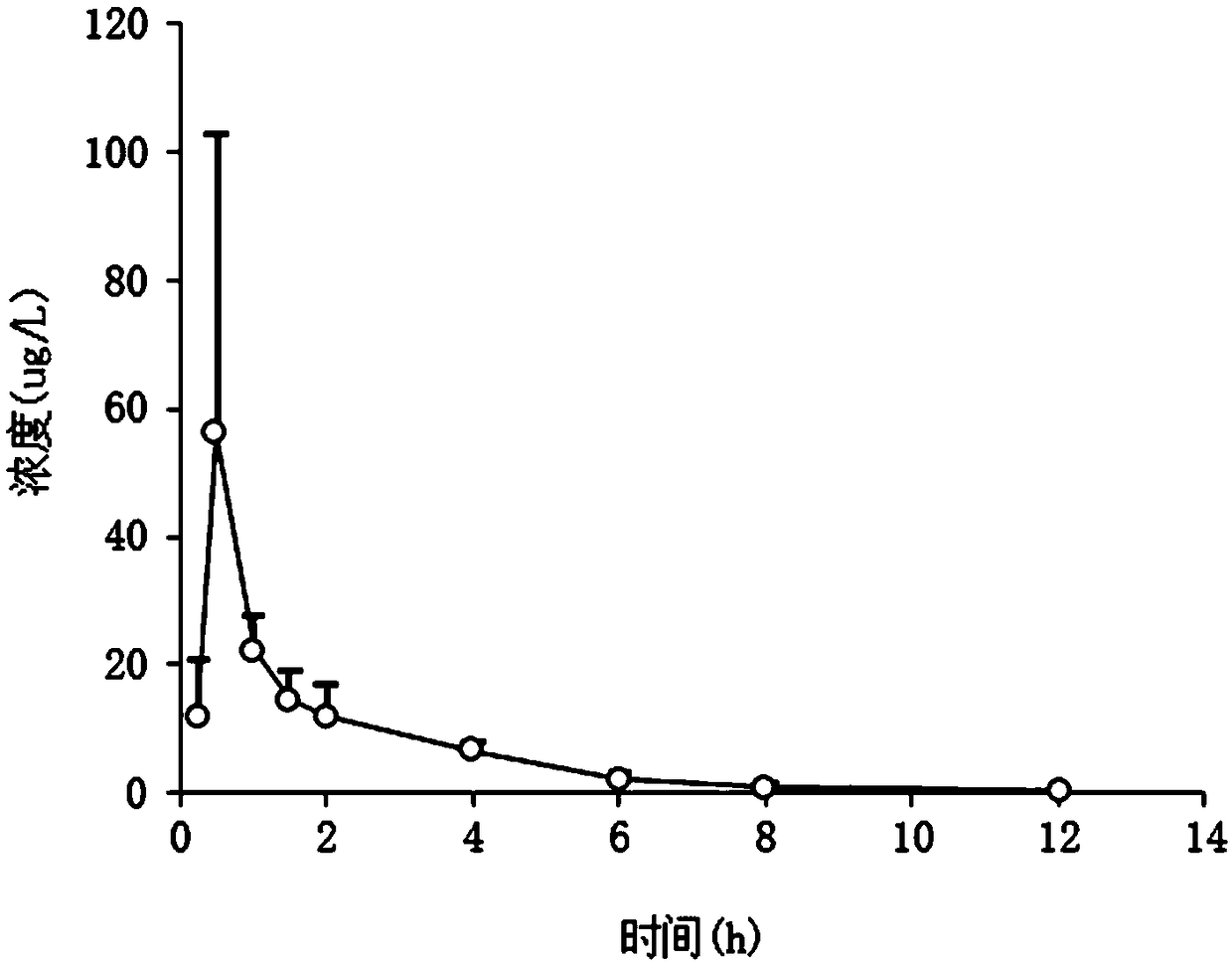
InactiveCN106880609APromote absorptionEasy to takeOrganic active ingredientsDigestive systemAdjuvantTableting
The invention discloses a sofosbuvir dispersible tablet and a preparation method thereof. The dispersible tablet consists of the following components in percentage by mass: 30-70% of sofosbuvir, which serves as a raw material, and 40-90% of adjuvants, wherein the adjuvants include a filler, a disintegrating agent and a lubricating agent; and the preparation method comprises processes of weighing the raw material and the adjuvants, mixing the raw material and the adjuvants and tableting an obtained mixture. The dispersible tablet prepared by the invention can be completely disintegrated rapidly, so that a time to peak is shortened and drug absorbency; the dispersible tablet has the characteristics of being convenient to take, rapid in efficacy release, high in bioavailability and the like; and the preparation method is simple in process, easy for control, convenient to operate and suitable for industrial production.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Construction method of tree shrew model for evaluating pharmacokinetics and pharmacodynamics of anti-influenza drugs
InactiveCN109453155AGood drug treatment predictabilityDetox InhibitionOrganic active ingredientsAntiviralsHalf-lifeBlood plasma
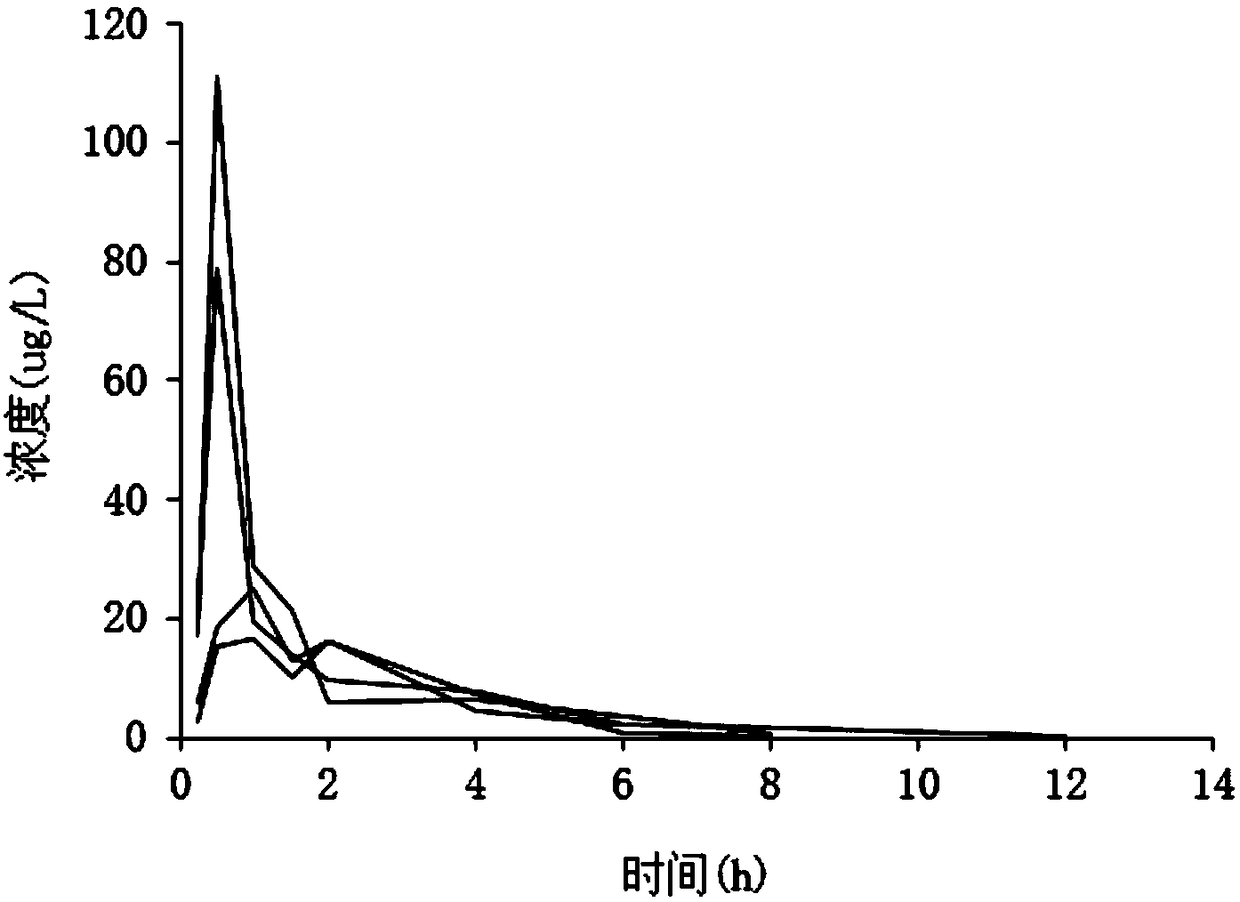
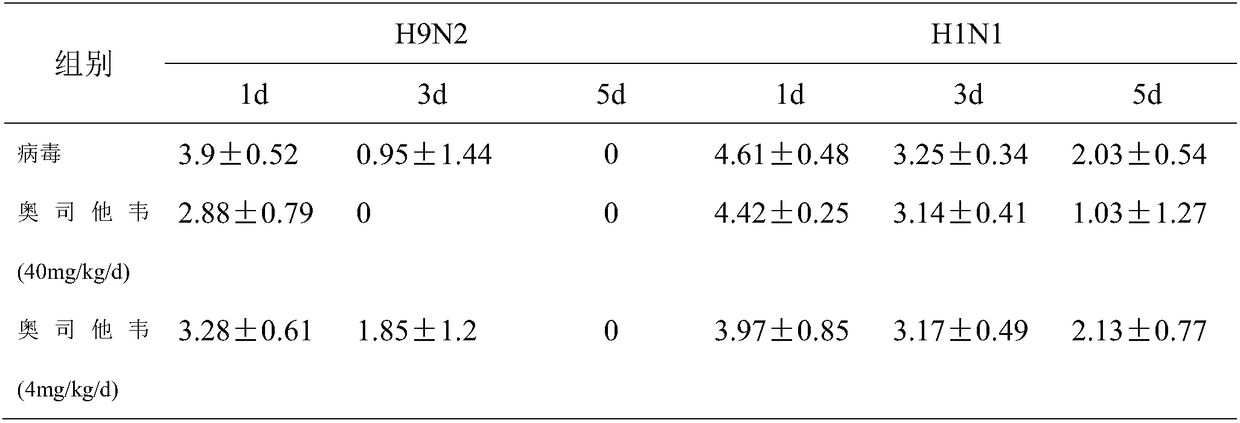
The invention provides a construction method of a tree shrew model for evaluating pharmacokinetics and pharmacodynamics of anti-infuenza drugs. The method adopts tree shrew as a platform to evaluate the drug effect of oseltamivir for treating the tree shrew infected by different influenza viruses. The drug plasma clearance half-time period and time to peak of the tree shrew after taking the oseltamivir are similar to that of mice, and the detoxification and corresponding inflammation response of H9N2 influenza viruses in the tree shrew body can be reduced by virtue of the treatment with the oseltamivir, so that the tree shrew can be used as an influenza animal model with the drug treatment foreseeability.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +3
A kind of chlorpheniramine maleate compound and its pharmaceutical composition
ActiveCN103787958BShorten the timeImprove efficacyOrganic active ingredientsOrganic chemistryChemical structureHalf-life
The invention belongs to the technical field of medicine, and particularly relates to a chlorpheniramine maleate compound and a pharmaceutical composition of the chlorpheniramine maleate compound. The chlorpheniramine maleate compound has a chemical structure formula seen in the formula (I) and is measured through an X-ray power diffraction measurement method, and an X-ray power diffraction pattern which is expressed by a diffraction angle of 2 theta+ / -0.2 degrees can be seen in the drawing 1. The chlorpheniramine maleate compound is a new crystal form compound different from existing chlorpheniramine maleate in the prior art. The time to peak and the half-life period of chlorpheniramine in pseudoephedrine hydrochloride and chlorphenamine maleate tablets prepared through the new crystal form compound are obviously shortened, the differences between the time to peak and the half-life period of the chlorpheniramine and the time to peak and the half-life period of ibuprofen and the differences between the time to peak and the half-life period of the chlorpheniramine and the time to peak and the half-life period of the pseudoephedrine hydrochloride are reduced, time for allowing concentration of active ingredients of the three types of medicine in blood to reach the effective concentration and time for allowing activity of the active ingredients of the three types of medicine to fade away are kept the same as much as possible, and therefore the pharmacological function of the pseudoephedrine hydrochloride and chlorphenamine maleate tablets is enhanced cooperatively.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Peak sampling hold circuit, peak sampling hold method and application
InactiveCN101615432BAvoid accumulationImprove load driving capabilityElectric analogue storesComputer scienceTime to peak
The invention discloses a peak sampling hold circuit and a peak sampling hold method. The peak sampling hold circuit comprises a peak sampling module, a peak sampling buffer input module, a peak hold module, a peak output buffer module and a zero clearing module; the peak sampling module samples peak voltage of all time points of input signals; the peak sampling buffer output module follows the peak voltage and enhances load driving capability of the peak voltage; the peak hold module holds the voltage output by the peak sampling buffer output module; the peak output buffer module enhances the output load capability of the peak hold module; the zero clearing module carries out clearing in time to peak voltage of last period output by the peak sampling module after the peak hold module outputs voltage so as to be convenient to carry out peak sampling in next period. The peak sampling hold circuit and a peak sampling hold method can better reappear the peak of the input signal and can apply the peak sampling hold circuit to a power system.
Owner:HANGZHOU SILAN MICROELECTRONICS
A kind of immunomodulator sustained-release agent and preparation method thereof
ActiveCN103610658BProlong the action timeUniform and constant action timeOrganic active ingredientsPharmaceutical non-active ingredientsBlood concentrationImmune modulator
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
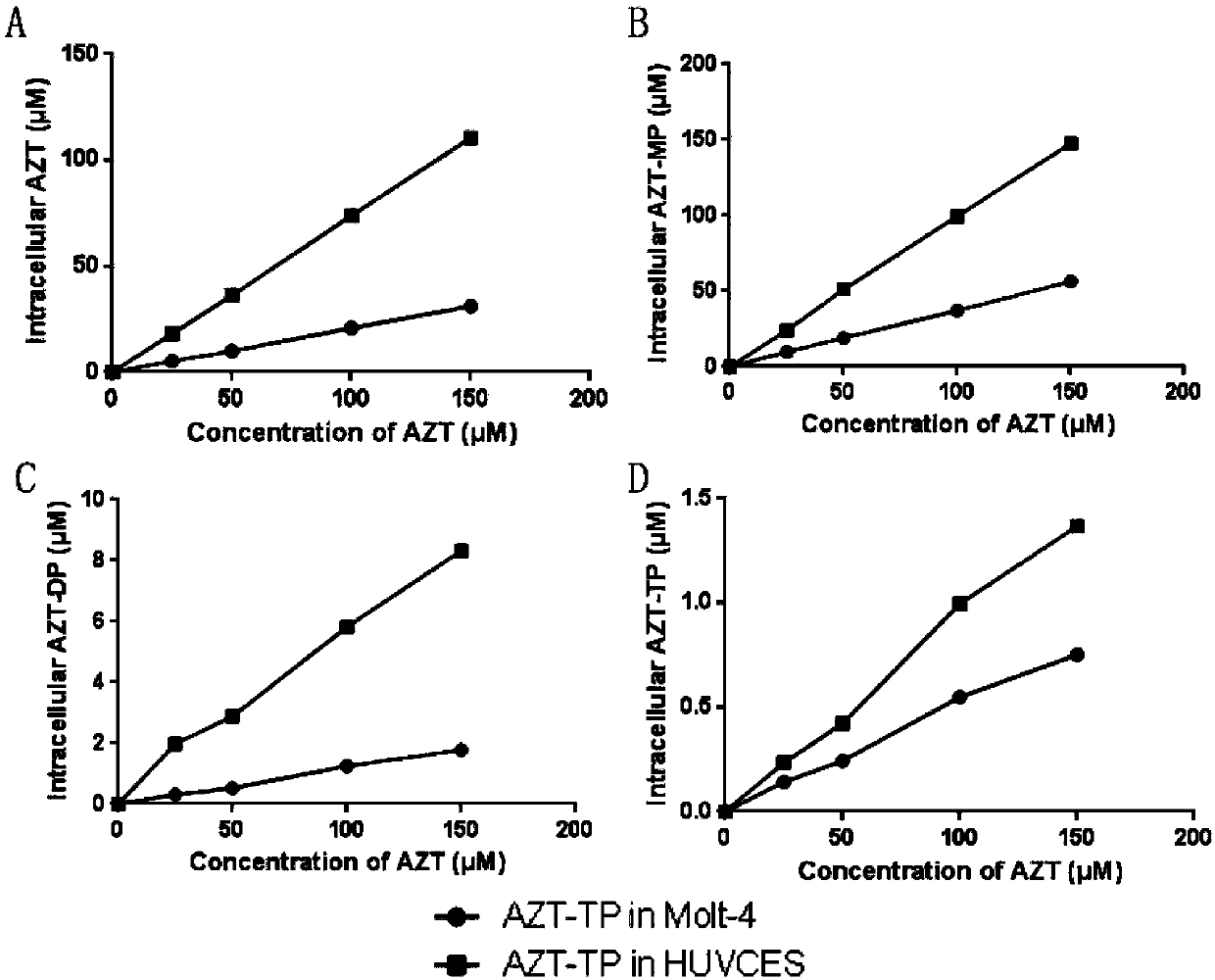
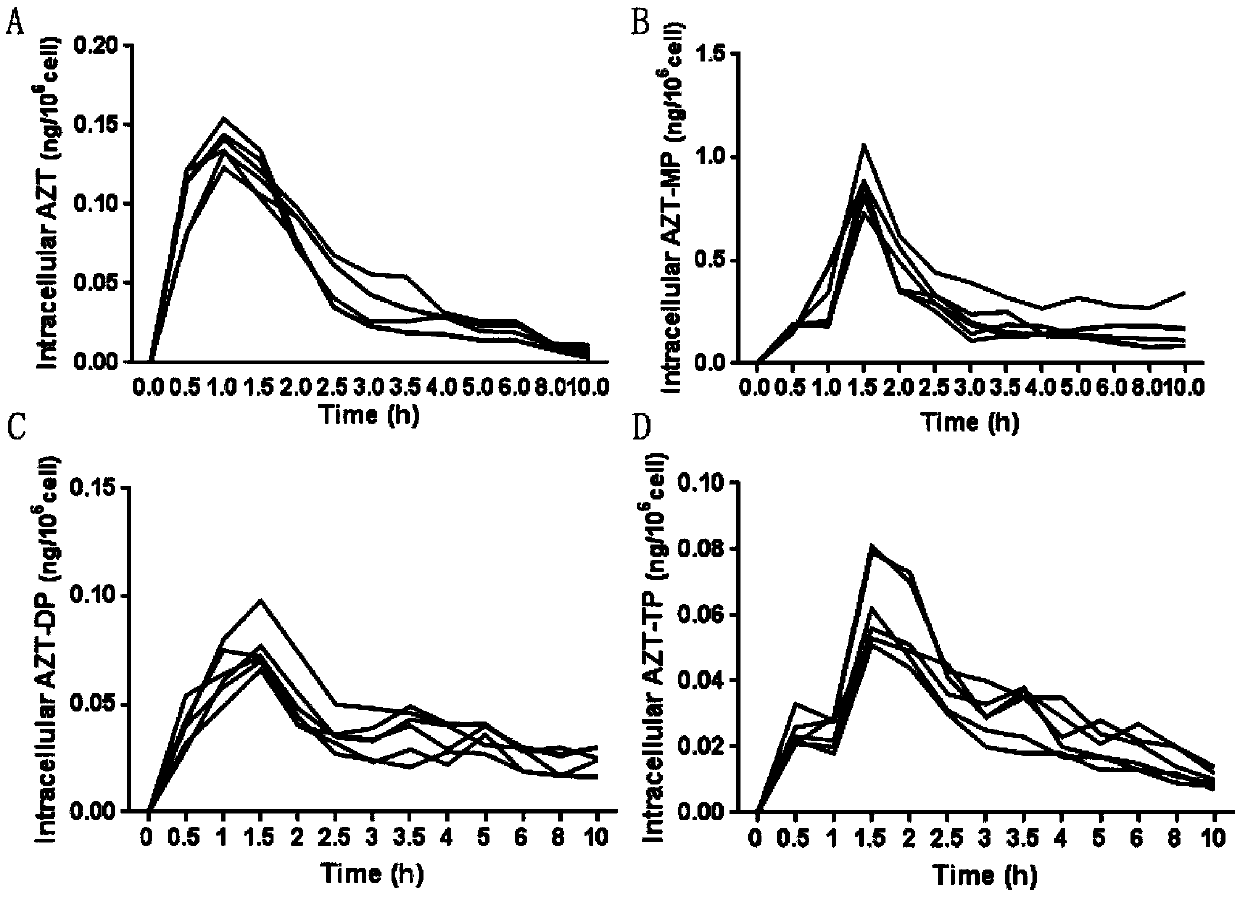
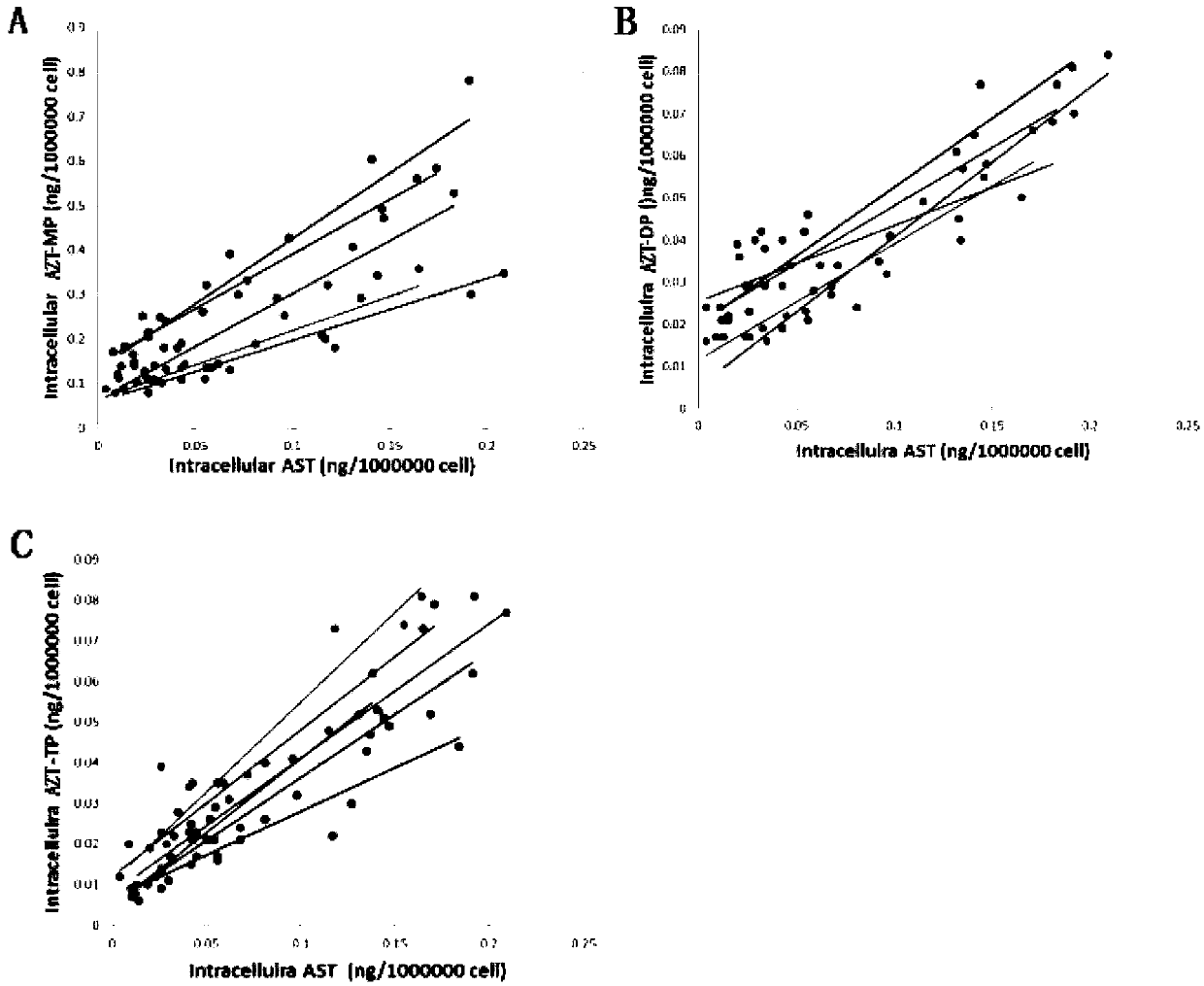
Drug effect estimation method for zidovudine
The invention relates to a drug effect estimation method for zidovudine. The method comprises the following steps of acquiring target cell drug peak concentration of the zidovudine, and obtaining target cell half-life concentration according to the target cell drug peak concentration; obtaining a relation between target cell drug concentration and drug administration time of the zidovudine according to target cell time to peak, the target cell drug peak concentration, target cell half-life time, the target cell half-life concentration and a first formula, wherein the first formula is (formula); and obtaining a relation between the target cell drug concentration and the drug administration time of a zidovudine phosphorylated product after 0.4 to 0.6 hour according to the relation between the target cell drug concentration and the drug administration time of the zidovudine. In this way, the drug effect of the zidovudine in target cells can be estimated by only acquiring the data of the target cell drug peak concentration.
Owner:余鹏
Active ingredient composition for treating alcoholic liver injury
ActiveCN102028701BFast absorptionShort peak timeDigestive systemHeterocyclic compound active ingredientsSide effectHalf-life
The invention discloses an active ingredient composition for treating alcoholic liver injury, and relates to an active ingredient composition for treating liver injury. The invention solves the problems that the conventional traditional Chinese medicines and compound decoction thereof for treating liver injury are inconvenient to use, and the dosage and safety are difficult to control. The active ingredient composition for treating the alcoholic liver injury consists of 6, 7-dimethoxybenzopyran-2-one, geniposide and parietic acid. The active ingredient composition for treating the alcoholic liver injury has the advantages that: the clinical medicinal dosage is accurate, safe and effective, the active ingredient composition is convenient to use, the treatment effect is improved, medicinal materials are saved, and toxic and side effects are reduced; and the 6,7-dimethoxybenzopyran-2-one, the geniposide and the parietic acid have liver-protecting and gut-benefiting components having the advantages of quick absorption (short time to peak), slow metabolism (longer half life of medicines), higher peak concentration and larger blood concentration area under a curve, so the 6, 7-dimethoxybenzopyran-2-one, the geniposide and the parietic acid can serve as preferred active ingredients. The active ingredient composition provides a basis for subsequently preparing finished pharmaceutical products in various formulations.
Owner:王喜军
Blends of polyphenylene ether sulfone and silicone polyester carbonate
Disclosed herein is a composition comprising a polyphenylene ether sulfone and a resorcinol based silicone aryl polyester carbonate copolymer wherein greater than or equal to 50 mol % of copolymer repeating units are ester units derived from resorcinol, based on the total molar amount of all repeating units in the polymer and wherein the blend has less than 1 weight percent polycarbonate, based on the total weight of the composition, has FAR 25.853 peak heat release of less than 60 KW / m2 and time to peak heat release of greater than or equal to 120 seconds.
Owner:SHPP GLOBAL TECH BV
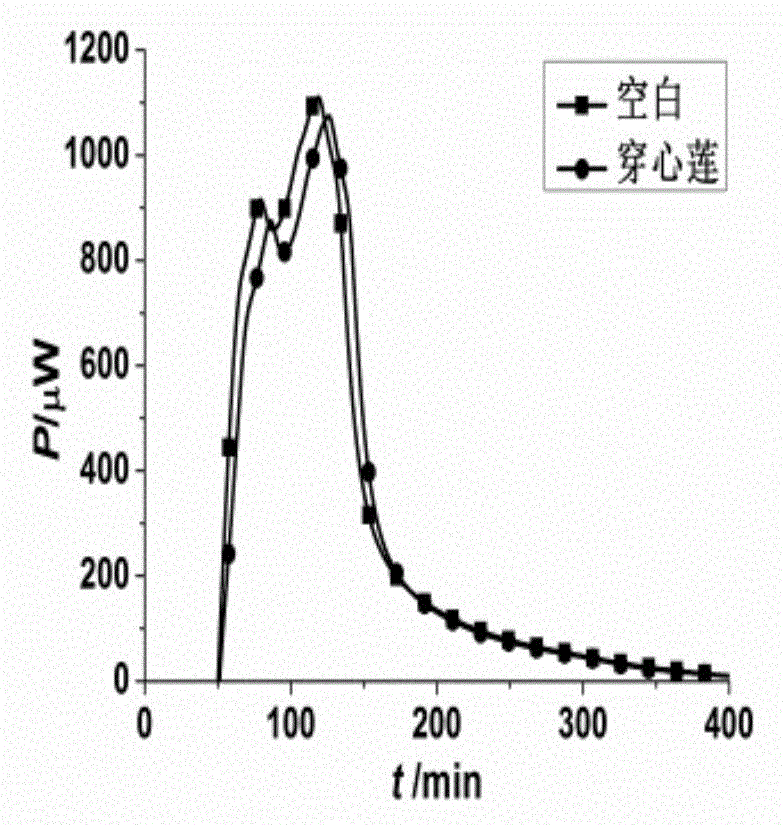
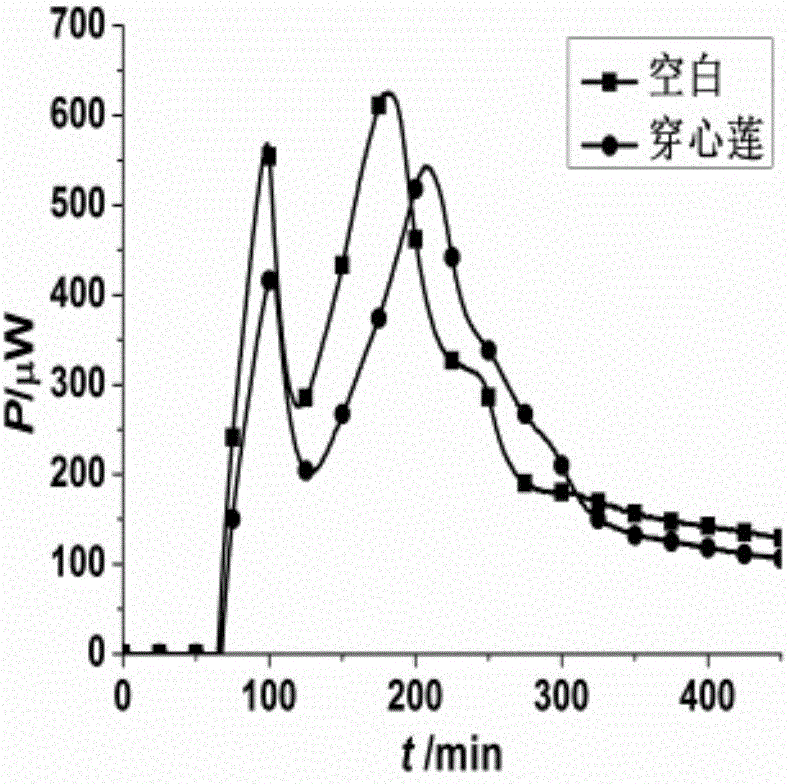
A method for evaluating the antibacterial activity of Andrographis paniculata
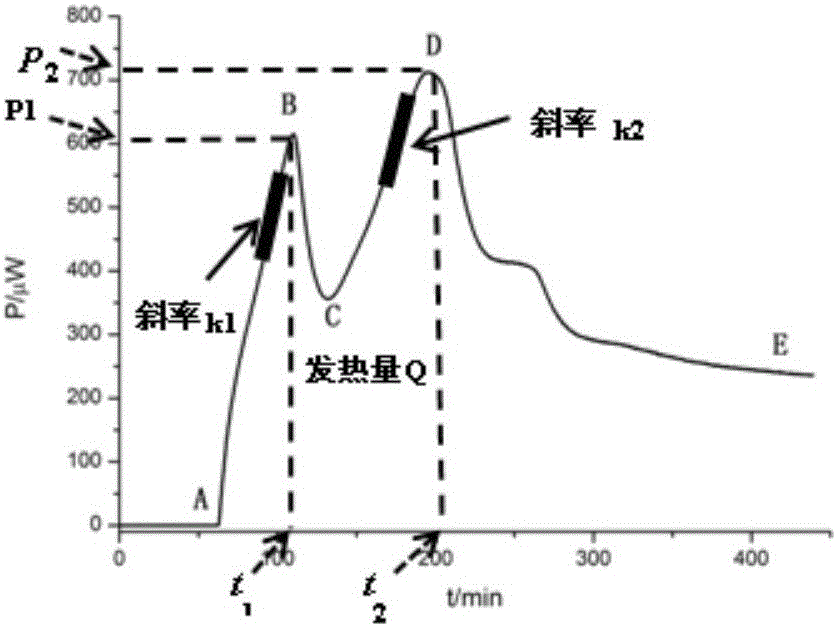
ActiveCN103792259BStrong specificityIncreased sensitivityMaterial heat developmentPharmacological interventionsAntibacterial activity
The invention discloses a method for evaluating antimicrobial activity of andrographis paniculata. According to the method, the antimicrobial activity of andrographis paniculata is evaluated by antibacterial rate I%, wherein the I% is calculated according to the following formula (1):I%=(t2-t0) / t0*100%(1), t0 refers to the time to peak of a second exponential growth phase in a growth and metabolism thermogram of bacteria which is not subjected to pharmacological intervention, t2 refers to the time to peak of a second exponential growth phase in a growth and metabolism thermogram of bacteria which is subjected to intervention of andrographis paniculata, and the concentration of a test solution of the andrographis paniculata interfering growth and metabolism of the bacteria ranges from 0.4mg / mL to 4 mg / mL, preferably 3mg / mL. The method provided by the invention can be used for qualitatively analyzing the antimicrobial activity of andrographis paniculata produced in different production areas, different preparation methods, different production batches or different growth time, and the like.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com