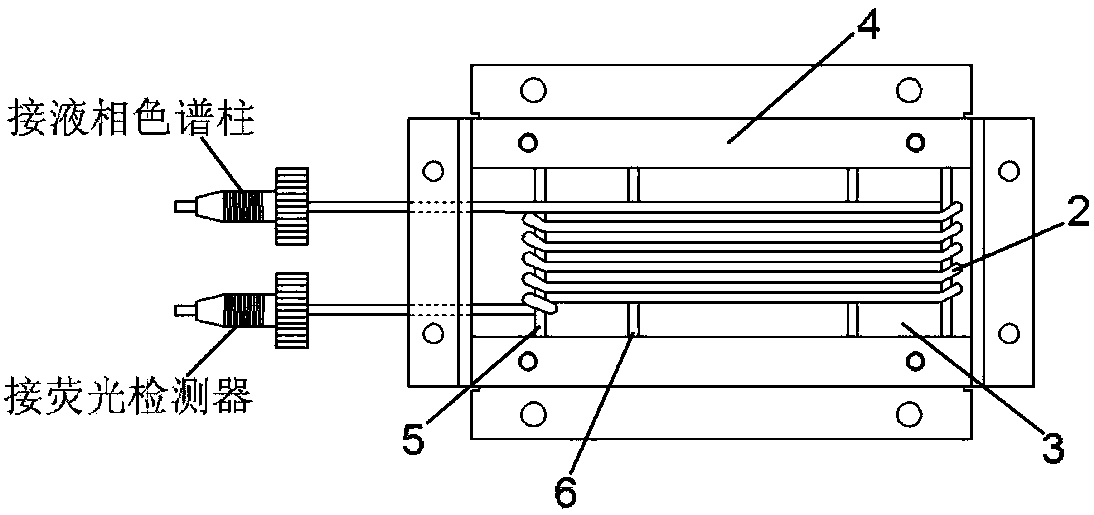
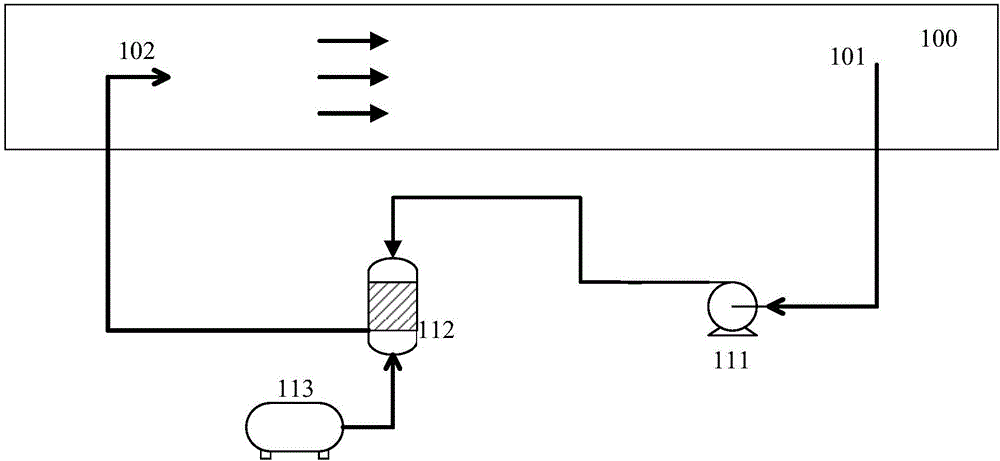
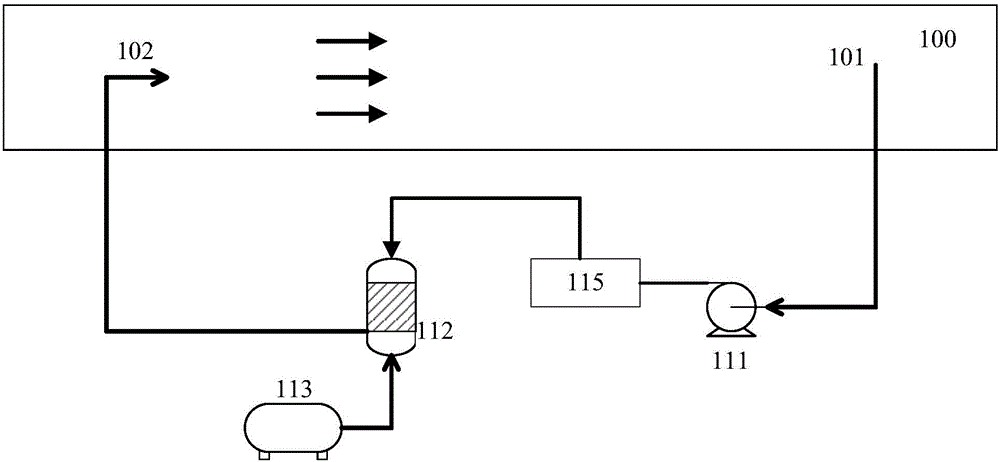
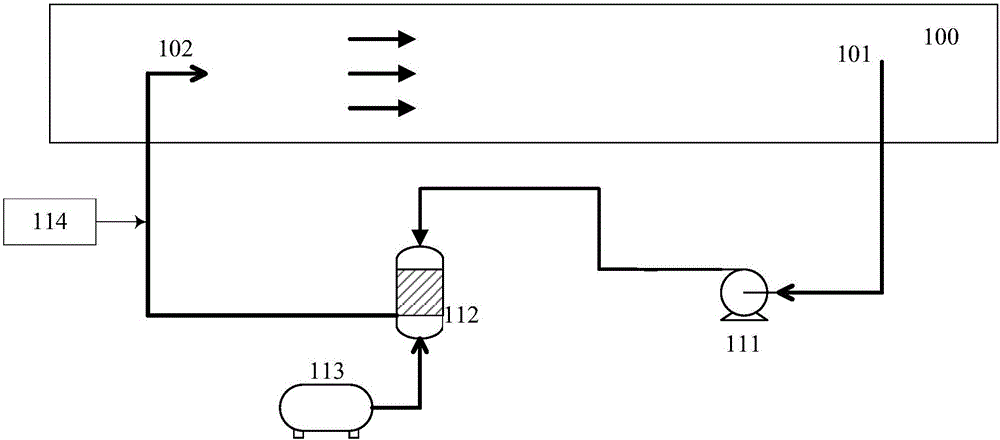
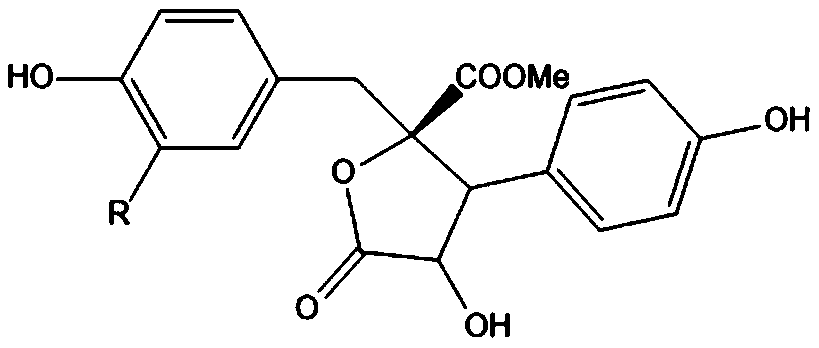
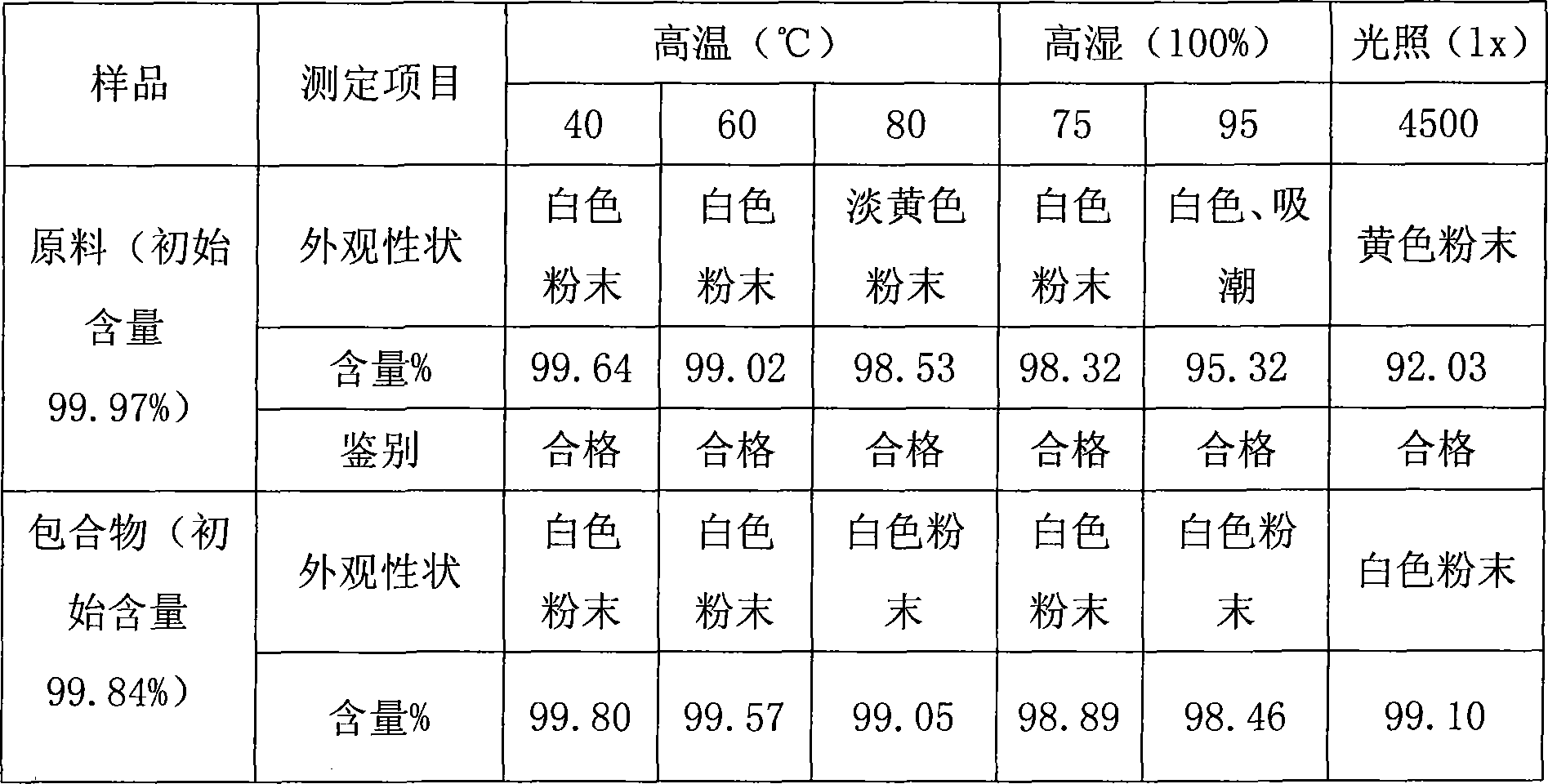
Patents
Literature
156results about How to "Short peak time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trimetazidine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN102885795AThe solution is not easy to cleanSimple preparation stepsOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazidine dihydrochloride sustained-release tablet and a preparation method thereof. The trimetazidine dihydrochloride sustained-release tablet comprises the following constituents in percentage by mass: 5-60% of trimetazidine dihydrochloride, 10-25% of sustained-release framework material, 1-8% of adhesive, 20-80% of filler, 0.1-5% of glidant and 0.2-3% of lubricant. According to the trimetazidine dihydrochloride sustained-release tablet, medicine can be slowly and uniformly released by adding the sustained-release framework material, so as to achieve regulation and control for a release speed, reduce the peak-valley ratio of the medicine, improve the efficacy, reduce the toxic and side effects of the medicine, reduce daily medicine-taking times and enhance the compliance of the patient on the medicine. The preparation method of the trimetazidine dihydrochloride sustained-release tablet disclosed by the invention is simple in process, does not need specially process production equipment, and is low in cost and good for batch amplification and industrialized production for products.
Owner:AC PHARMA CO LTD
Oral preparation containing andrographolide and preparation method thereof
InactiveCN103070843AImprove toleranceAvoid direct accessOrganic active ingredientsAntiviralsEnteric-coated granulesSide effect
The invention relates to an oral preparation containing andrographolide and a preparation method thereof, belonging to the field of medicines. The oral preparation containing andrographolide is in dosage forms of enteric-coated granules, enteric-coated tablets, enteric-coated capsules and enteric-coated dispersible tablets which are prepared by mixing andrographolide and pharmaceutically acceptable auxiliary materials. The prepared oral preparation can reduce incidence of adverse reactions and side effects thereof and improve the medication safety while ensuring the therapeutic effect, and uses the andrographolide more widely.
Owner:司鹏 +1
Method for detecting trace phosphine gas in water sample by gas chromatograph (GC)-cooperating pre-column twice cold trap enrichment method
InactiveCN102393429AShort peak timeGood peak shapeComponent separationChromatographic columnBoiling point
The invention provides a method for detecting trace phosphine gas in a water sample by a gas chromatograph (GC)-cooperating pre-column twice cold trap enrichment method, and aims to overcome the shortcomings of the existing various measurement methods. The method mainly comprises the following steps of: (1) cooling and enriching the phosphine gas and establishing a GC / NPD (nitrogen phosphorous detector) detection device; (2) extracting and dissolving the phosphine gas in lake water by a gas-liquid two-phase phase equilibrium method, and preparing a gas sample to be detected; (3) preprocessingthe gas sample; (4) extracting a certain volume of the gas sample into a gas absorption tube; (5) performing primary enrichment of the processed gas sample in a cold trap I, and removing the impuritygas with a relatively low boiling point; (6) heating the cold trap I so that the gas enters a cold trap II for secondary enrichment; (7) detecting the final enrichment product by a nitrogen phosphorous detector in a gas chromatograph through a chromatographic column; and (8) converting the measured area of a characteristic peak of the phosphine according to a certain formula to obtain the concentration of the phosphine in the water sample to be detected.
Owner:NANJING UNIV
Sodium rebeilazole for injection use
InactiveCN1410062AAvoid destructionImprove stabilityAntibacterial agentsOrganic active ingredientsDiseaseDuodenal ulcer
A medicine Leibeilazuona for injection contains Leibeilazuona, excipient, pH regulator and antioxidizing agent. It can be used for treating stomach ulcer, duodenal ulcer, erosive gastroesophageal reverse flor disease, pyloric halicobacterium, and zollinger-ellison syndrome. Its advantages are high biological utilization rate and quickly taking its effect.
Owner:黄振华
Arbidol dry suspension and preparation method thereof
ActiveCN102000030AImprove complianceMask bitternessOrganic active ingredientsAntiviralsSodium cyclamateSodium sulfate
The invention discloses an arbidol dry suspension and a preparation method thereof, and belongs to the medical industry. The arbidol dry suspension comprises Arbidol hydrochloride, a suspending aid, a diluent, a lubricating agent, a flavoring agent and a pH regulator, wherein the suspending aid is one or more of Arabic gum, tragacanth, sodium alga acid, povidone, hydroxy propyl cellulose and xanthan gum; the diluent is saccharose, mannitol and microcrystalline cellulose; the lubricating agent is lauryl sodium sulfate and sodium lauryl sulphate; the flavoring agent comprises a sweetener and an aromatizer, the sweetener is mannitol, saccharose, sodium cyclamate and aspartame, and the aromatizer is orange essence, banana essence, strawberry essence, and pineapple essence; and the pH regulator is citric acid and tartaric acid. The preparation method comprises the following steps of: sieving the arbidol hydrochloride, and respectively crushing and sieving the suspending aid, the diluent, and the lubricating agent; fully mixing the components except the diluent, and then adding the diluent for uniform mixing; and wetting by using ethanol to prepare a soft material, drying, and packaging into an aluminum-plastic composite membrane bag. After the arbidol is prepared into the dry suspension, the bitter of the arbidol is effectively masked, and the compliance of the patient is greatly improved.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Method for detecting quality of senile coughand asthmatablets
InactiveCN106198837ARaise quality standardsQuality assuranceComponent separationThin-layer chromatographyEnvironmentally friendly
The invention relates to the method for detecting the quality of senile cough and cough tablets. The method comprises the following steps: qualitatively distinguishing the active ingredients including radix astragaliseuhedysari, bighead atractylodes rhizome, radix saposhnikoviae, licoriceroot,herbaepimedii andfructuspsoralea in asenile cough and cough tabletpreparation by using a thin layer chromatography, and determining the contents of icariin, psoralen and isopsoralenin the preparation by a UPLC method. The detection method provided by the invention has the advantages of simplifying the sampling steps, having simple operation and high specificity, shortening the content determination time, simultaneously detecting multi-index components, having stabledetection baseline, good resolution, high detection accuracy and good stability, improving efficiency, and being relatively economic and environmentally friendly.
Owner:ZHUZHOU QIANJIN PHARMA
Research for realizing quick classification and identification of chemical components in ixeris sonchifolia hance injection based on UPLC-Q-TOF-MS technology
ActiveCN104359968AEasy to separateSolve key problemsComponent separationMaterial analysis by electric/magnetic meansOrganic acidChemical compound
The invention discloses a research for realizing quick classification and identification of chemical components in an ixeris sonchifolia hance injection based on a UPLC-Q-TOF-MS technology, and aims to take flavonoids, organic acids, amino acids and nucleosides in the ixeris sonchifolia hance injection as research objects to realize quick classification and identification of the chemical components in the ixeris sonchifolia hance injection based on a UPLC-Q-TOF-MS technical platform. The research comprises the following steps: firstly, performing information integration on components of flavonoids, organic acids, amino acids and nucleosides in the ixeris sonchifolia hance injection to discover and summarize a rule for diagnosing fragments and neutral losses of the four types of substances; meanwhile, performing mass spectrographic analysis on reference substances of different types of compounds by adopting the UPLC-Q-TOF-MS technology, and performing verification; and constructing a method for realizing quick classification and identification of chemical components in the ixeris sonchifolia hance injection by using a method for diagnosing fragments and neutral losses as a screening and identifying tool.
Owner:TONGHUA HUAXIA PHARMA
Drug complexes for preventing and curing schemic cerebrovaseular disease as well as preparation method thereof
InactiveCN101181256AReduce doseReduce cerebral edemaHydroxy compound active ingredientsCardiovascular disorderDiseaseBULK ACTIVE INGREDIENT
The invention provides a pharmaceutical composition for the prevention and treatment of ischemic cerebrovascular diseases and the preparation method thereof, which contains active ingredients and carriers, wherein the active ingredients consist of fumalic acid or the pharmaceutically acceptable salt and borneol with the weight ratio of 100: 0.5 to 10. The invention can be prepared into the pharmaceutical formulations which are applicable to the clinical application, and the formulations include: granules, capsules, tablets, dropping pills, suppositories and injections. The invention reveals the neuro-protective effect of the composition of fumalic acid or the salt and borneol for transient total cerebral ischemia, and the synergy of the two drugs is played by the combination of the two; compared with single fumalic acid, the absorption half-life is prolonged, the time for reaching peak is faster, the dosage of fumalic acid is reduced and the safety of the drugs is increased. The invention has the advantages of the complementary and synergy of pharmacological effects, instant effects, prolonged effective time, small dosage and precise efficacy when being taken as the drug for the prevention and treatment of ischemic cerebrovascular diseases.
Owner:SUN YAT SEN UNIV
Ion chromatography detection method for six negative ions in cigarette paper
ActiveCN105784908AImprove extraction efficiencyEasy extractionComponent separationIon chromatographyFormate
The invention discloses an ion chromatography detection method for six negative ions in cigarette paper.Ultrapure water is adopted as an extracting agent, IonPac AS19 negative ion exchange columns are separation columns, chromatographic conditions are optimized in a targeted mode, and the ion chromatography is adopted for achieving quantitative analysis of lactate, acetate, formate, chloridion, phosphate radicals and citrate in cigarette paper samples.A result shows that the recycling rates of the six negative ions range from 91.0% to 109.5%, and the relative standard deviation ranges from 0.82% to 6.26%.The method is accurate, simple, reliable and applicable to quantitative determination of relative components, particularly six negation ions in the cigarette paper samples.
Owner:CHINA TOBACCO GUANGDONG IND
Separation and detection method for astaxanthin in haematococcus pluvialis extract
InactiveCN106501395AGood miscibilityImprove pretreatment efficiencyComponent separationHaematococcus pluvialis extractAstaxanthin
The invention relates to a separation and detection method for astaxanthin in a haematococcus pluvialis extract. The separation and detection method comprises the following steps of 1 extraction of carotenoid in the haematococcus pluvialis extract; 2 enzymolysis of the carotenoid; 3 astaxanthin separation and detection through a normal-phase high performance liquid analysis method, wherein the liquid-phase on-device conditions comprise the detection wavelength is 474 nm, a chromatographic column is Luna 3micro Silica(2), the chromatographic column temperature ranges from 20 DEG C to 25 DEG C, the flow speed is 1-1.2 ml / min, a flow phase is prepared from n-hexane and acetone according to the volume ratio of 75%:25%-90%:10%, and isocratic elution is conducted. According to the separation and detection method, the pretreatment efficiency is high, the intersolubility between the extracting reagent acetone and the astaxanthin is good, the enzymolysis time is shorter than the saponification time, and the efficiency is high; the high performance liquid chromatography on-device conditions are good; isocratic elution is achieved, a base line is easier to stabilize, the peak shape is good, the separation degree is high, and more isomers can be separated out; the peak flowing time is short, and the method is not prone to be influenced by outside light and heat and more suitable for large-scale detection.
Owner:QINGDAO SAMUELS INDAL & COMML
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
ActiveCN102319225BSmooth releaseImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMagnesium stearate
The invention belongs to the field of sustained release medicament preparations, and particularly relates to a trimetazidine hydrochloride sustained release tablet and a preparation method thereof. The trimetazidine hydrochloride sustained release tablet is prepared from 40 to 45 parts of trimetazidine hydrochloride, 100 to 200 parts of polyoxyethylene, 100 to 200 parts of dextrin, 60 to 100 parts of 3-10 percent ethyl cellulose solution and 3 to 5 parts of magnesium stearate through material mixing, soft material preparing, drying, tabletting and other steps. In the trimetazidine hydrochloride sustained release tablet, the polyoxyethylene serves as an auxiliary material, and the sustained release tablet is prepared from the medicaments by a method of direct tabletting or tabletting aftergranulating. The drug dissolution of the trimetazidine hydrochloride sustained release tablet reaches about 90 percent 6 hours later, so the sustained release tablet is only required to be taken twice a day; therefore, the sustained release tablet has the advantages of releasing drug slowly and uniformly to reduce release rate and postpone peak time, reducing the number of administration times per day, improving the compliance of patients to the medicament and the like. Furthermore, the preparation method of the invention is simple and easy to operate.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
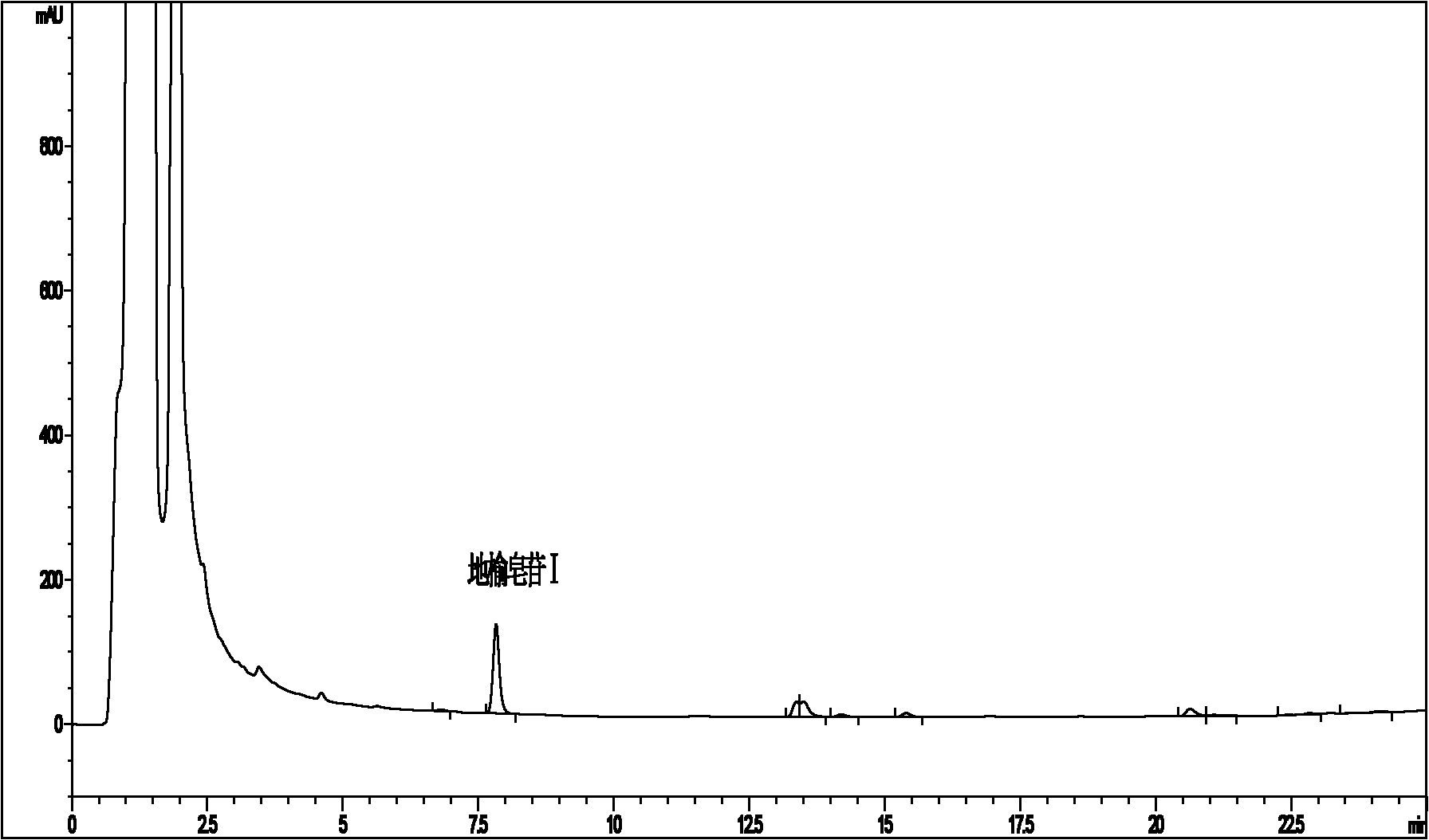
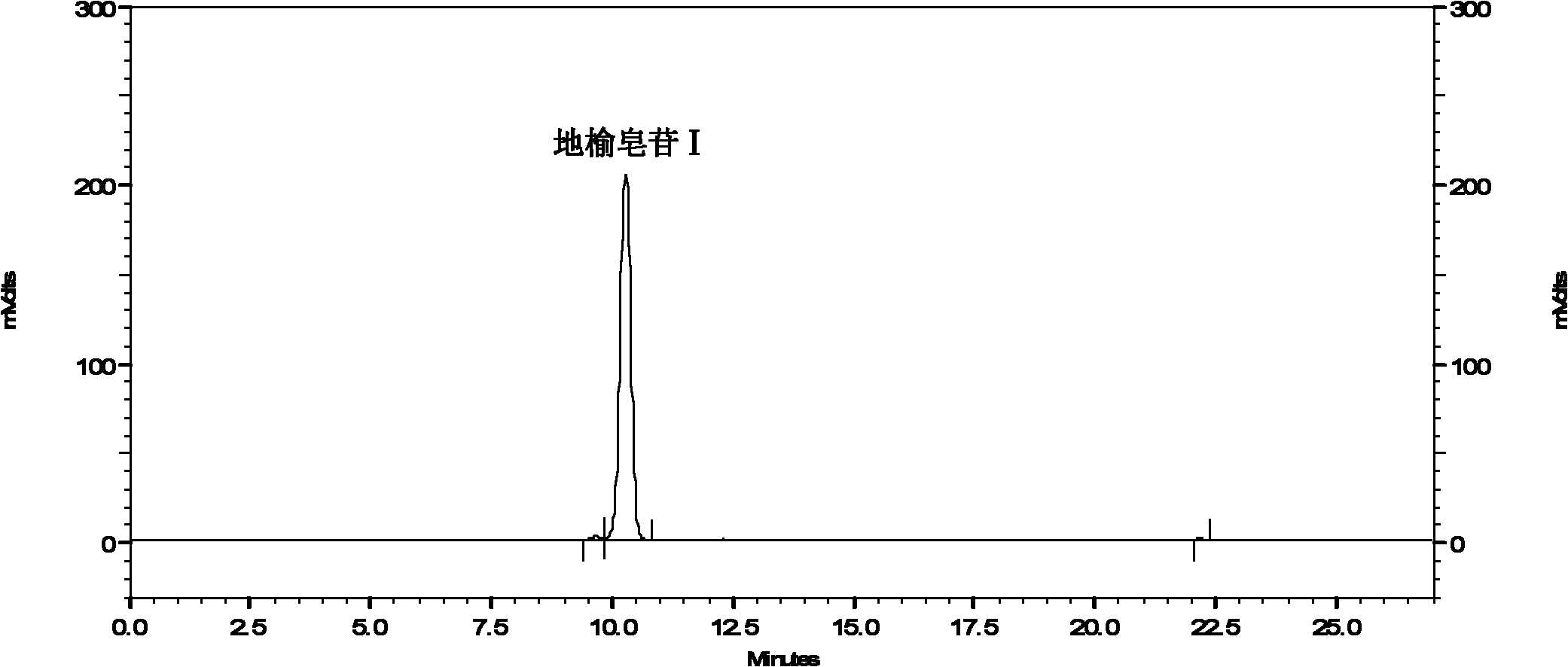
Content determination method of sanguisorbin I
InactiveCN102721773AShort peak timeRapid determinationComponent separationBlood disorderChemistryPeak area
The invention discloses a content determination method of sanguisorbin I. The method comprises the following steps of: (1) respectively dissolving a standard product of sanguisorbin I and a sample containing sanguisorbin I into an ethanol aqueous solution to respectively prepare a standard solution and a sample solution; and (2) respectively injecting the standard solution and the sample solution which have equal volumes into a high-efficiency liquid-phase chromatograph, and then measuring and calculating the peak area of the standard solution and the sample solution to acquire the content of sanguisorbin I in the sample. The invention provides a rapid and accurate method for measuring the content of sanguisorbin I in a medical herb of garden burnet and extractives or preparations thereof.
Owner:CHENGDU DIAO PHARMA GROUP
Aflatoxin and sulfanilamide drug light derivatization device
ActiveCN107807112AImprove overall lifespanIncrease power consumptionComponent separationFluorescence/phosphorescenceSulfur drugUltraviolet lights
The invention relates to an flatoxin and sulfanilamide drug light derivatization device. An array ultraviolet LED is taken as an excitation light source, and an excitation wavelength is between 280 nmand 380 nm. A fine internal-diameter pipeline which allows ultraviolet light to pass is taken as a derivatization reaction tank, the derivatization reaction tubes are arranged in a linear mode, the internal diameter of the derivatization reaction tube is 0.15-0.5 millimeters, a length is 0.5-12 meters, and the volume of the derivatization reaction tank is 100-1000 [mu]L. The fluorescence intensity of flatoxin B1 is increased by 6.5 times by the light derivatization device, and the light derivatization device is suitable for requirements of derivatization after high performance liquid chromatography columns and flow injection analysis derivatization.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Black and odorous water body dissolved oxygen enhancing method and oxygen dissolving method and device
ActiveCN106186288AImprove water qualityImprove self-cleaning abilityTreatment using aerobic processesSustainable biological treatmentProduct gasOxygen
The invention discloses a black and odorous water body dissolved oxygen enhancing method and an oxygen dissolving method and device. The black and odorous water body dissolved oxygen enhancing method includes the following steps that firstly, a pressure enhancement device is used for pumping river water from the downstream of a target river way; secondly, the river water obtained in the first step and oxygen in a gas oxygen generation device are mixed in an oxygen dissolving device till saturation is reached, all oxygen is dissolved in the water, and no bubbles are formed; thirdly, the river water where oxygen is dissolved in the second step is conveyed to the upstream of the target river way. The method achieves comprehensive treatment with the five technologies of point source control, flowing water circulation, displacement purification, ecological enhancement and ecological restoration.
Owner:南京宇行环保科技有限公司
Method for detecting content of beta-lactoglobulin in dairy product
InactiveCN102539575ASolve for quick extractionResolve accuracyComponent separationGradient elutionChromatography column
The invention provides a method for detecting the content of beta-lactoglobulin in a dairy product. The method comprises the following steps of: extracting beta-lactoglobulin from the dairy product; and measuring the content of the beta-lactoglobulin by adopting a high performance liquid chromatography method. According to the high performance liquid chromatography method, a C18 chromatographic column is adopted, gradient elution is performed, and the detection wavelength is 210-230 nanometers; an elution solution consists of a mobile phase A and a mobile phase B; the mobile phase A is an ultrapure aqueous solution of trifluoroacetic acid with a volume fraction of 0.05 to 0.15, and the mobile phase B is a mixed solution of acetonitrile, ultrapure water and trifluoroacetic acid; the volume ratio of the mobile phase A to the mobile phase B is 80: 20 to 82: 18; and in the mobile phase B, the volume ratio of the acetonitrile to the ultrapure water is 320: 100 to 480: 100, and the volume of the ultrapure water to trifluoroacetic acid is 100: 0.4 to 100: 0.6. The method is low in cost, short in target peak apperance time, high in separation degree and accuracy, and is favorable for detection of the content of beta-lactoglobulin in the dairy product; the peak shape is acute and symmetric; and the influence from environment change is low.
Owner:上海德诺产品检测有限公司
Dispersible tablet containing metformin and glibenclamide and preparation method thereof
InactiveCN101167731AShort peak timeLower blood sugar concentrationMetabolism disorderSulfonylurea active ingredientsGlibenclamideMetformin
The invention relates to dispersible tablets containing diabetosan and glibenclamide. The dissolving rate of glibenclamide is improved by making colloidal powder or solid dispersion powder of glibenclamide before making tablets. The dispersible tablets can disintegrate quickly, and the dissolving rates of diabetosan and glibenclamide of the dispersible tablets are significantly faster than that of common preparation.
Owner:林海平
Application of aspergillus terreus secondary metabolite-butyrolactone in preparation of medicament for treating diabetes
InactiveCN109999024AImprove inflammationReduce generationOrganic active ingredientsMetabolism disorderSecondary metaboliteEthyl acetate
The invention provides an application of aspergillus terreus secondary metabolite-butyrolactone in preparation of a medicament for treating diabetes, and belongs to the technical field of microbial medicaments. The aspergillus terreus secondary metabolite-butyrolactone comprises butyrolactone I, wherein the butyrolactone I is extracted according to the following method: fermenting aspergillus terreus OUCMDZ-2739 inoculated in fermentation broth for 25-35 days at 20-30 DEG C; filtering the fermentation broth after fermentation with denim to separate filtrate and mycelium, respectively extracting with ethyl acetate, mixing the obtained ethyl acetate extracts, and concentrating to obtain ethyl acetate solution extract; and further purifying the ethyl acetate solution extract to obtain the butyrolactone I. By the adoption of the application of the aspergillus terreus secondary metabolite-butyrolactone in the preparation of the medicament for treating diabetes, the medicament can inhibit activity of alpha-glucosidase, regulate a composition of intestinal flora, metabolize more short-chain fatty acids, reduce a uric acid level, maintain synthesis of insulin, avoid rising of a blood sugarlevel, and achieve the purpose of relieving type 2 diabetes.
Owner:嘉兴市爵拓科技有限公司
Countercurrent chromatographic separation process for reducing fixed phase
InactiveCN1506680AAchieving dynamic reductionShort peak timeComponent separationAlkaneChromatographic separation
The present invention relates to countercurrent chromatographic process for separating active component from plant extractive. The present invention features the separated compounding of fixed phase and flowing phase constituting the solvent system. The fixed phase is one of alkane, chlorine-containing organic solvent, ethyl acetate, fatty alcohol and fatty ketone or their combination; and the flowing phase is one of chlorine-containing organic solvent, fatty alcohol and water or their combination; with the fixed phase and the flowing phase being of different matter. The present invention has shortened active component monomer peak displaying time, improves the peak shape of the active component monomer, and may be used in industrial production to raise the yield of active component monomer and raise production efficiency.
Owner:TEA RES INST CHINESE ACAD OF AGRI SCI
Detection method for 2-hydroxypropyl nortadalafil
InactiveCN109387583AEvenly distributedStable proton concentrationComponent separationTest sampleStandard methods
The invention discloses a detection method for 2-hydroxypropyl nortadalafil, wherein the method comprises the following steps: (1) preparation of a test sample: taking a sample, carrying out ultrasonic extraction for 10 min, adding methanol to make up the lost weight, shaking evenly, filtering with an organic phase type filter membrane, taking subsequent filtrate, and diluting for standby application; (2) preparation of a blank solution: treating without addition of the sample and according to the same method for preparing the test sample, and preparing the blank solution; (3) preparation of standard working curve solutions: taking standard solutions, adding negative sample blank matrix extract solutions, making the volume constant, and shaking evenly to be used as a series of standard working solutions for standby application; and (4) high performance liquid chromatography-tandem mass spectrometry detection: detecting a liquid chromatography tandem mass spectrometry in a positive ionmode of an ESI source, collecting by a multi-reaction monitoring (MRM) way, and quantifying by an external standard method. The pretreatment steps of the detection method are simple to operate, the detection process has strong specificity and high sensitivity, and the detection efficiency is greatly improved.
Owner:广西东盟食品药品安全检验检测中心
Method for preparing norfloxacin capsule
InactiveCN101502496APromote dissolutionShort peak timeAntibacterial agentsOrganic active ingredientsSolubilityNorfloxacin
The invention relates to a method for preparing norfloxacin capsules, which comprises the following steps: (1) evenly stirring norfloxacin and beta-cyclodextrin by the substance ratio of norfloxacin to Beta-cyclodextrin being 1:1, then, adding water and evenly grinding the mixture into paste for 6 to 10 hours at the temperature of 20 to 35 DEG C until the norfloxacin / Beta-cyclodextrin inclusion compound is formed; (2) tiling and drying the inclusion compound at the temperature of 45 to 60 DEG C; (3) pulverizing the dried inclusion compound, granulating the ground medicine through a 80-mesh sieve and drying at the temperature of 45 to 60 DEG C until the water content is lower than 9%; and (4) measuring the norfloxacin content of the granules, converting and filling a No.0 empty capsule with the granules to ensure that each granule contains 0.1g of norfloxacin, and finally packaging to obtain the norfloxacin capsules. The product of the invention has good water-solubility and improves the bioavailability and the stability.
Owner:WUHAN POLYTECHNIC
Method of preparing high-purity polypeptide or analogue thereof
ActiveCN108101959ALow costSimple technical solutionPeptide preparation methodsStationary phaseFormate
The invention discloses a method of preparing a high-purity polypeptide or an analogue thereof. The method includes the steps of: preparing a purified AMP416 water solution through high performance liquid chromatography, and collecting a fraction of a target peak. Chromatographic conditions are described as follows: a stationary phase is C18, a mobile phase A is a phosphate, a formate or an acetate water solution and a mobile B is methanol or acetonitrile, wherein volume ratio of the mobile phase A to the mobile phase B is (80-100) : (0-20). The method can high-effectively separate and purifythe AMG416 at high yield and high product purity, so that the method has excellent industrial application potential.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Efficient liquid chromatogram detection method of L-leucine
ActiveCN108535385AShort peak timeAccurate measurementComponent separationMonopotassium phosphateColumn temperature
The invention discloses an efficient liquid chromatogram detection method of L-leucine, and relates to the technical field of column chromatographic methods. Chromatographic conditions are that the chromatographic column is an amino-bonded silica gel column, the mobile phase is a monopotassium phosphate aqueous solution and acetonitrile with the volume percentage being 37%:63%-42%:58%, the concentration of the monopotassium phosphate aqueous solution is 0.1-0.2 mol / L, ammonia water is contained in the monopotassium phosphate aqueous solution and accounts for 0.5 vt% of the monopotassium phosphate aqueous solution, and the pH value of the monopotassium phosphate aqueous solution is 4.2+ / -0.02; the flow rate of the mobile phase is 0.7-1.0 mL / min, the detection wavelength is 210 nm, and the column temperature of a chromatographic column is 30 DEG C; efficient liquid chromatogram analysis is conducted, and the L-leucine content in conversion fluid is measured. Accordingly, the L-leucine content can be measured rapidly and accurately, it is ensured that the quality is controllable, the error is reduced, the cost is reduced, operation is easy, the detection time is short, the accuracy ishigh, and the efficiency is improved.
Owner:JING JING PHARMA
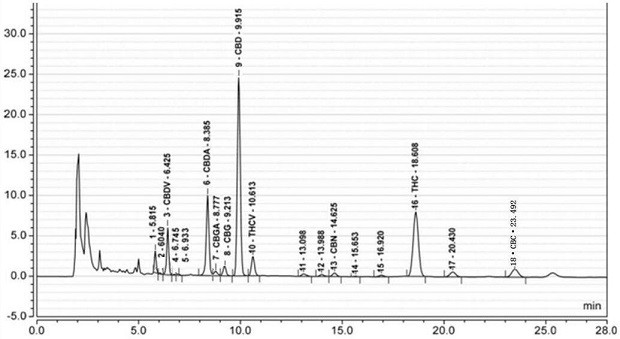
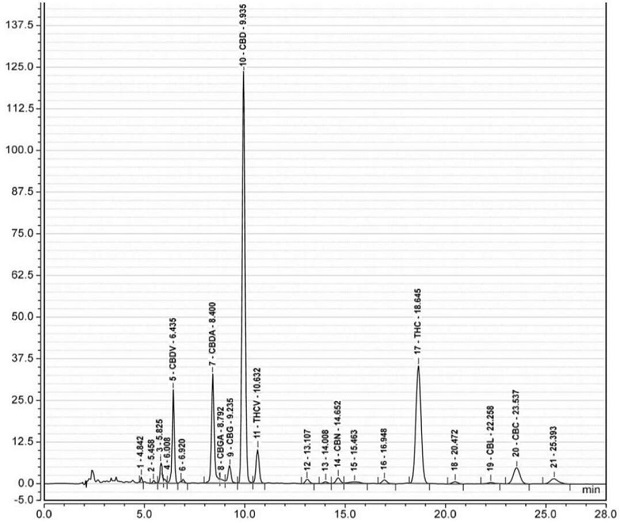
Method for detecting cannabinoid in industrial hemp floral leaves and extracts thereof by high performance liquid chromatography
The invention discloses a method for detecting cannabinoid in industrial hemp floral leaves and extracts thereof by high performance liquid chromatography. The method is characterized by comprising the following steps: (1) preparing floral leaf extraction solution: carrying out ultrasonic extraction on floral leaf powder by using a first extraction solvent, carrying out centrifugal separation, andfiltering to obtain floral leaf extracting solution; (2) preparing an extract sample: carrying out ultrasonic extraction on the floral leaf extracting solution by utilizing a second extracting solvent, and filtering to obtain an extract detection sample; (3) preparing a standard working solution: diluting a cannabinoid standard substance into the standard working solution by using methanol; and (4) high performance liquid chromatography determining: carrying out sample introduction detection on the extract detection sample and the standard working solution by using high performance liquid chromatography to obtain a standard curve, and calculating the cannabinoid component content of the extract detection sample according to the standard curve by using high performance liquid chromatography data processing software. The method has the advantages of short peak appearance time, short detection time, high detection efficiency and accurate and stable detection result, and is suitable for industrial detection.
Owner:滇麻生物科技(曲靖)有限公司
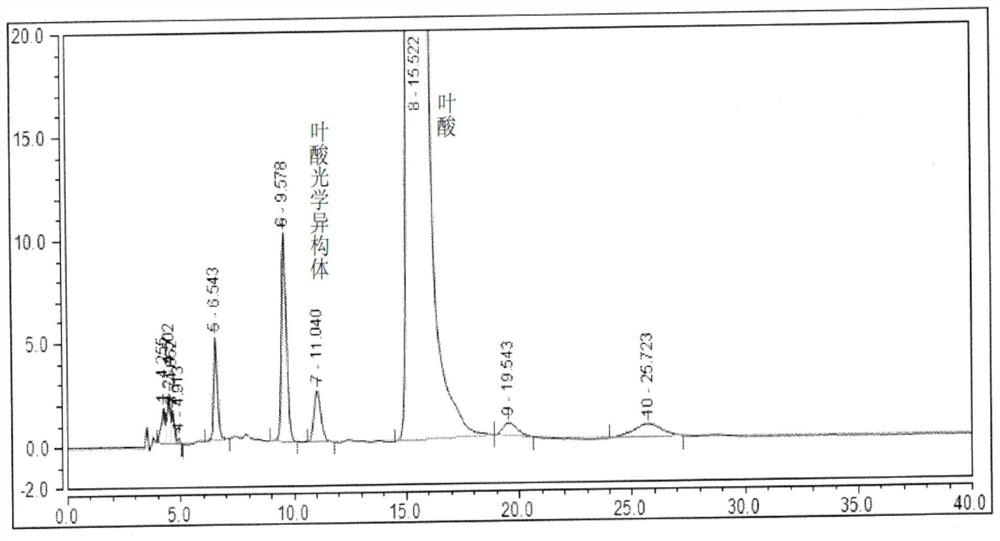
Method for separating and detecting folic acid and folic acid optical isomers in folic acid
ActiveCN112649524AEfficient separationValid identificationComponent separationEthylic acidOrganosolv
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to a method for separating and detecting folic acid and optical isomers thereof. A chromatographic column adopted by the method takes silica gel surface covalent bonding O-9-(tert-butylcarbamoyl) quinine as a filler, an organic solvent, organic acid and triethylamine are adopted for gradient elution. the folic acid and / or the folic acid optical isomer are / or is separated; the flow velocity of the mobile phase is 0.4-0.6 ml / min; the column temperature of the chromatographic column is 20-30 DEG C; the wavelength of the detector is set to be 280 + / -10nm; the used diluent is a mixed solution of tetrahydrofuran, methanol, water, acetic acid and triethylamine, and the contents of folic acid and optical isomers thereof are measured by using a peak area normalization method. According to the method, the peak appearing time of the folic acid and the optical isomer thereof is short, the operation can be finished within 20 minutes, peak patterns are symmetrical, the folic acid and the optical isomer thereof can be separated from various impurities to obtain the accurate content, and the method is simple, quick and effective.
Owner:CHONGQING HUABANGSHENGKAI PHARM
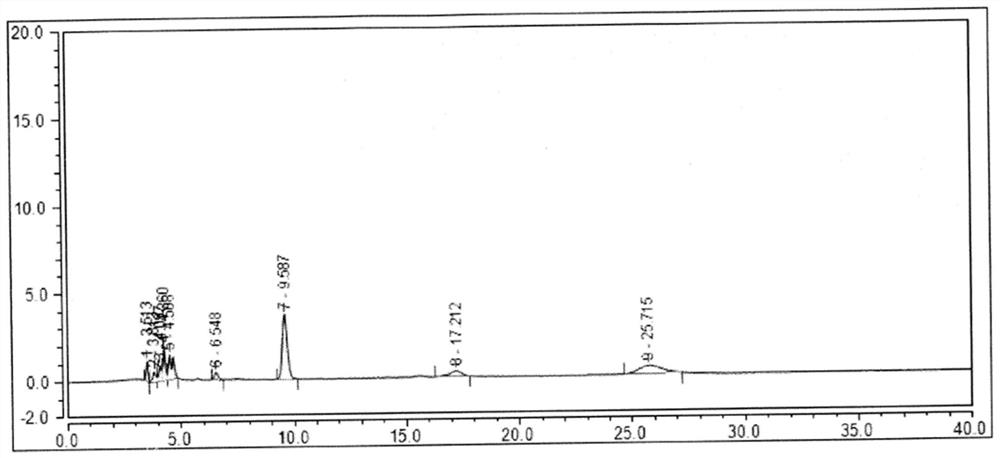
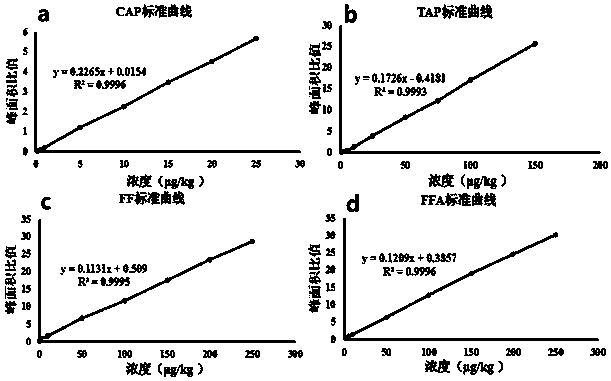
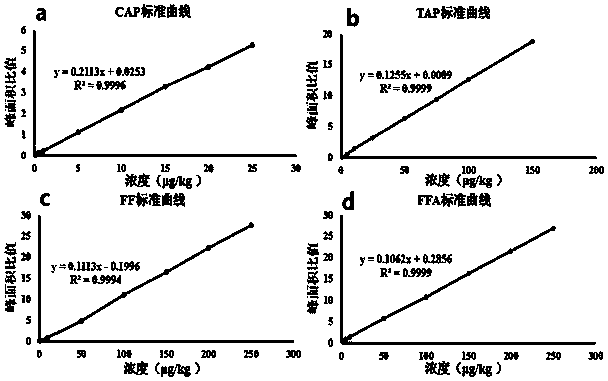
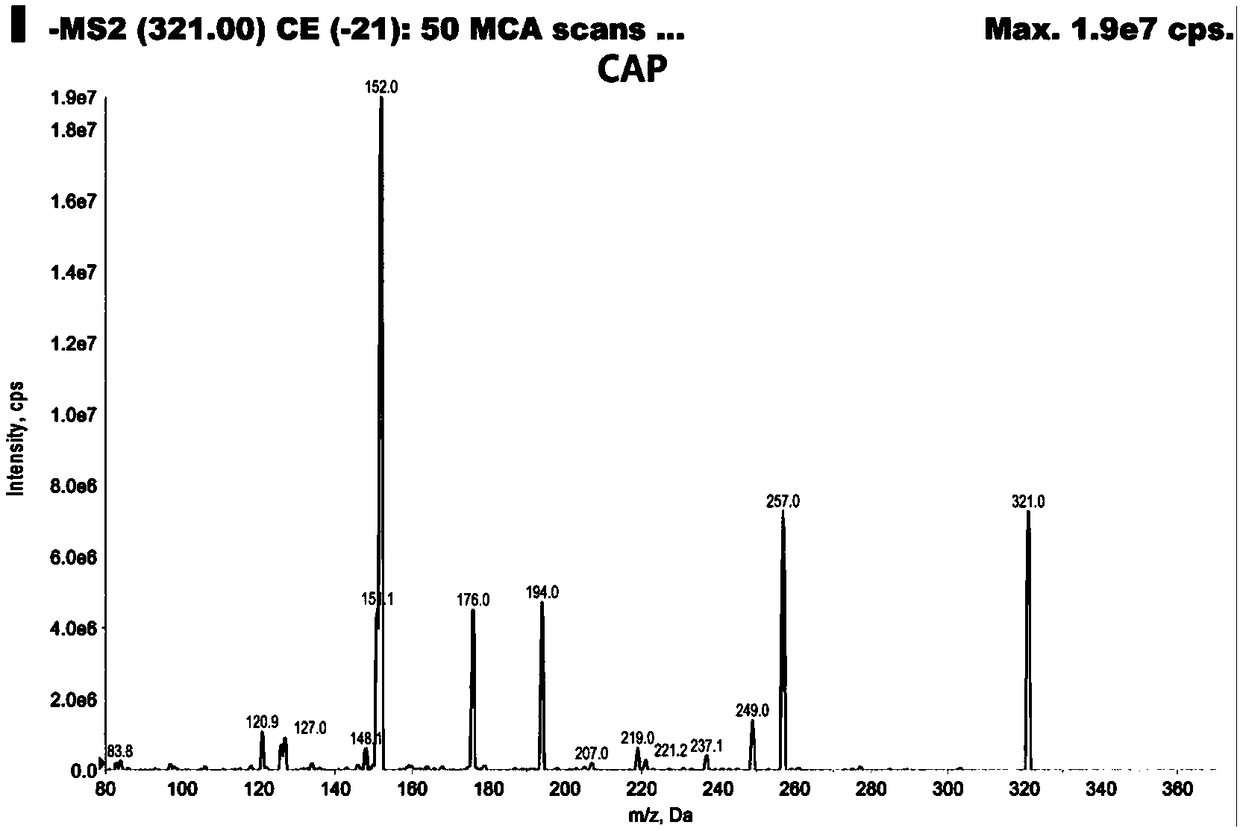
Analysis method for efficiently detecting multiple residues of chloramphenicol, thiamphenicol, florfenicol and metabolite florfenicol amine of florfenicol in eggs
InactiveCN108760938AHigh sensitivityShort peak timeComponent separationMetaboliteRelative standard deviation
The invention relates to the field of veterinary drug residue detection, in particular to an analysis method for efficiently detecting multiple residues of chloramphenicol, thiamphenicol, florfenicoland metabolite florfenicol amine of the florfenicol in eggs. An ultra-high performance liquid chromatography-tandem mass spectrum detection method for the multiple residues of the chloramphenicol, thethiamphenicol, the florfenicol and the metabolite florfenicol amine of the florfenicol in eggs is established for the first time; the appearance time of target compounds is short (1 min about), the sensitivity is high (the LOD of CAP, the LOD of TAP, the LOD of FF and the LOD of FFA are 0.03 microgram / kg, 0.3 microgram / kg, 0.1 microgram / kg and 0.4 microgram / kg in eggs respectively, and the LOQ ofthe CAP, the LOQ of the TAP, the LOQ of the FF and the LOQ of the FFA are 0.08 microgram / kg, 0.8 microgram / kg, 0.27 microgram / kg and 1.2 micrograms / kg in eggs respectively), the recovery rates when the adding concentrations of the CAP, the TAP, the FF and the FFA are LOQ, 0.5 MRL, 1.0 MRL and 2.0 MRL in eggs respectively are larger than or equal to 90.31%, 93.40%, 92.32% and 92.35% respectively,and the within-day relative standard deviation and the daytime relative standard deviation are lower than 4.33% and 5.77 % respectively. Meanwhile, the analysis method is simple in elution program, small in solvent consumption, higher in analysis efficiency, short in time consumption (each sample needs 4 min) and more suitable for application and popularization in mass sample analysis.
Owner:YANGZHOU UNIV
Rapid and efficient detection method of brassinosteroid
InactiveCN105116060ASimple extraction processHigh extraction rateComponent separationFiltrationDerivatization
The present invention provides a rapid and efficient detection method of brassinosteroid. The rapid and efficient detection method comprises: adopting fresh grape fruits as a material, rapidly grinding into powder with liquid nitrogen, adding 80-95% methanol, isotope internal standard 2H3 brassinosteroid and 2H3 brassin sterone, carrying out low temperature ultrasonic extraction, standing for 2-5h, filtering, taking the filtration residue, adding 80-95% methanol, leaching for 1-2 h, filtering, merging the two filtrates, carrying out physical adsorption to remove the water, sequentially loading onto a C18 column, a sephadex LH-20 column and a silica column, eluting with methanol to obtain an enriched sample liquid, adding phenylboronic acid, carrying out a derivatization reaction, cooling, concentrating to achieve a dry state, redissolving with acetonitrile so as to be used for liquid chromatography determination, selecting acetonitrile and water as the mobile phase, and collecting the target peak, wherein a volume ratio of the acetonitrile to water is (6:4)-(9:1), and the elution way is isocratic elution. The method of the present invention has characteristics of rapidness, efficiency, stable baseline and good brassinosteroid separation.
Owner:ZHEJIANG WANLI UNIV
Method for determining content of chloroethanol in gelatin hollow capsule
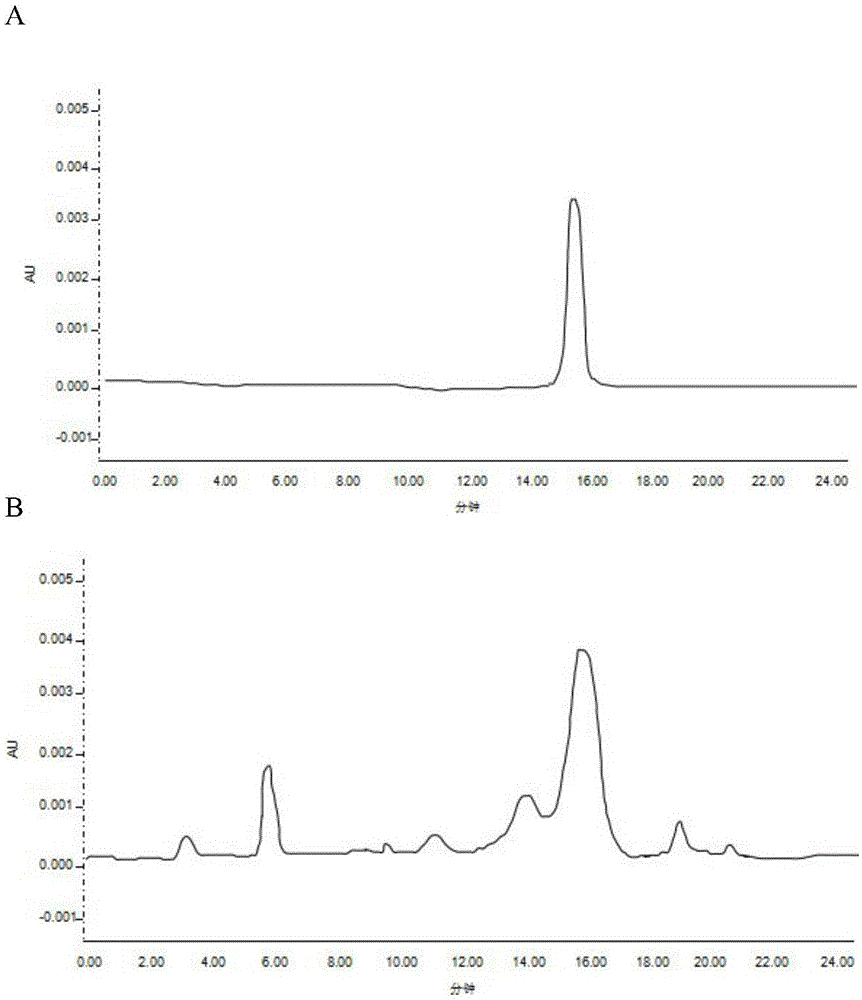
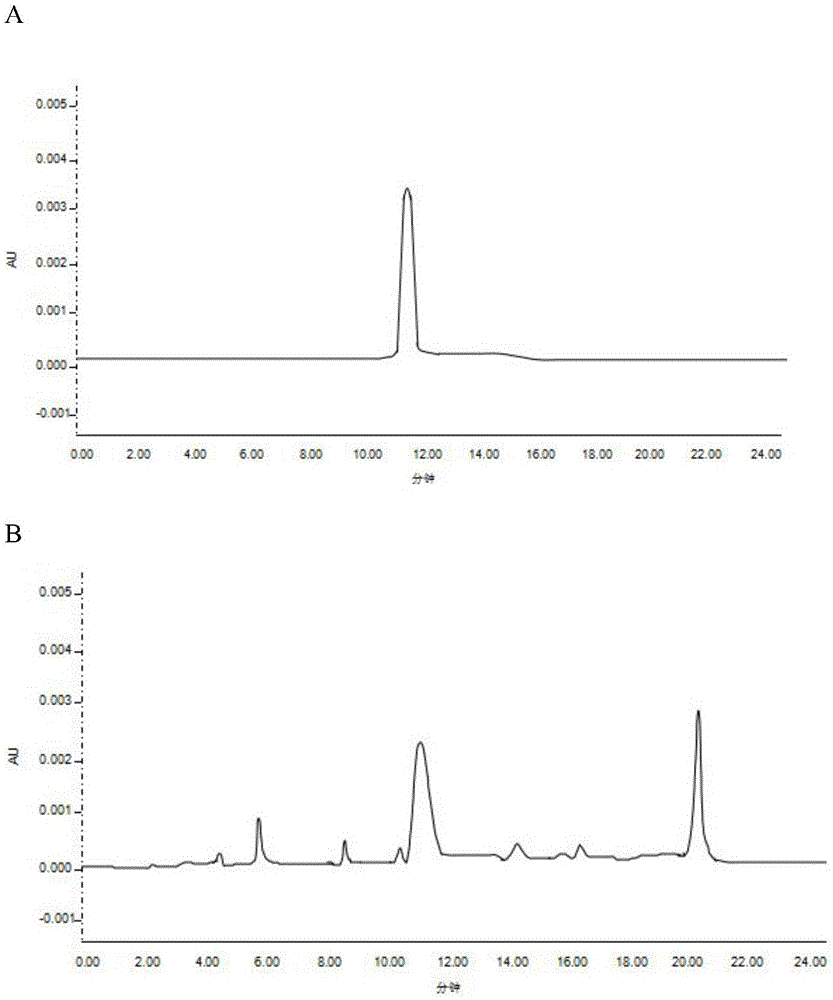
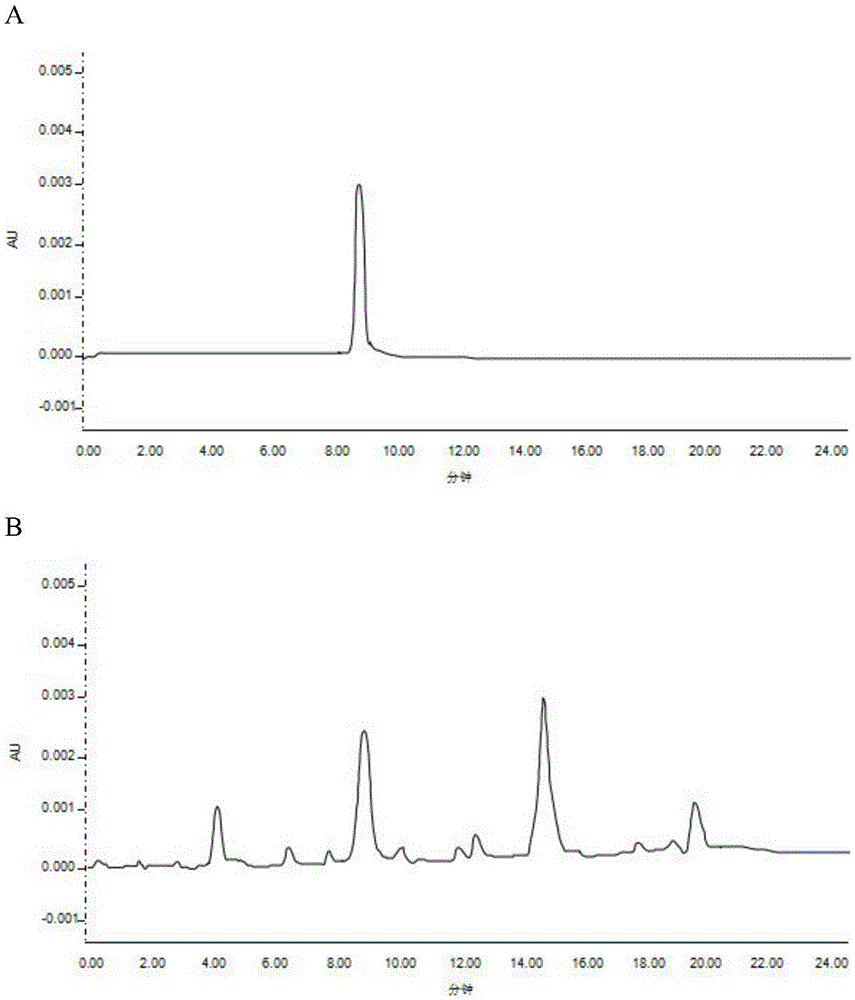
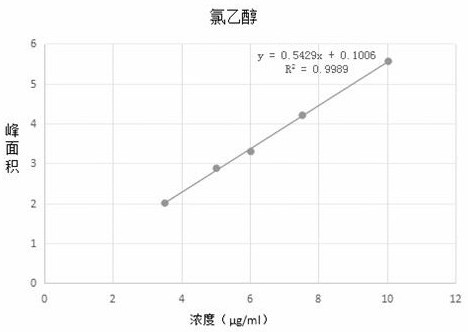
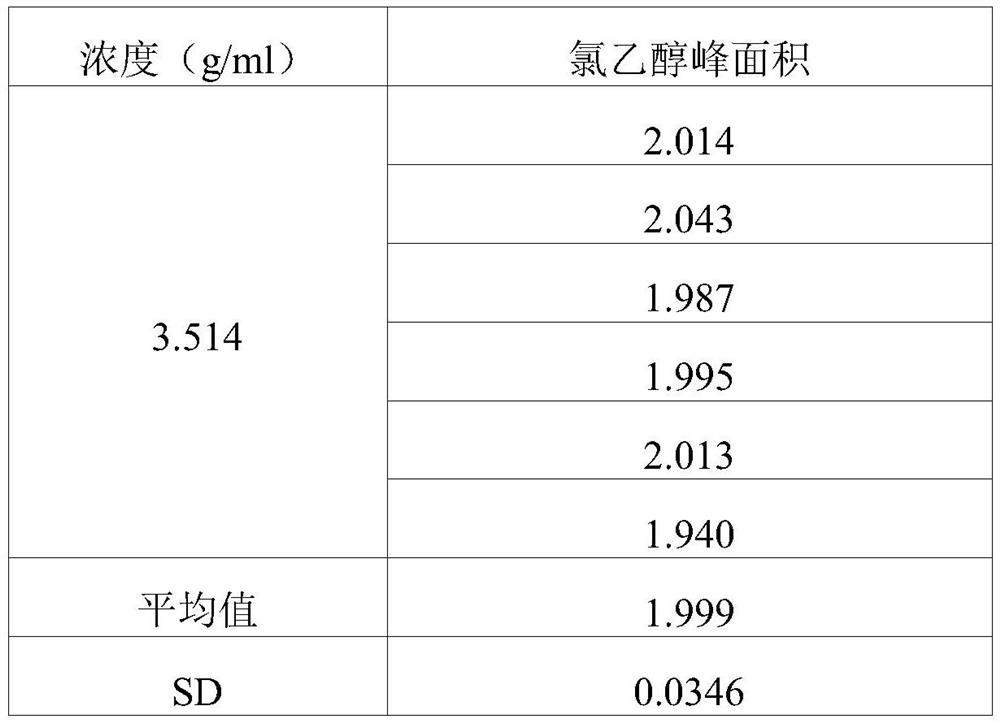
PendingCN113552230AHigh penetration rateReduce use costComponent separationGas liquid chromatographicEngineering
The invention discloses a method for determining the content of chloroethanol in a gelatin hollow capsule, which comprises the following steps: step 1, preparing a test solution, taking a proper amount of gelatin hollow capsule, cutting into pieces, weighing, placing in a headspace bottle, precisely adding n-hexane, sealing, dipping overnight, taking the subsequent filtrate as the test solution, and preparing a blank sample by using the n-hexane to perform a blank test; step 2, preparing a reference substance solution, taking a proper amount of a chloroethanol reference substance, precisely weighing, dissolving with n-hexane, and quantitatively diluting chloroethanol to prepare a solution; and step 3, determination: taking the test solution, the blank sample and the reference solution prepared in the step 1 and the step 2, and carrying out gas chromatograph detection on the test solution, the blank sample and the reference solution. The detection method provided by the invention is verified by a test, and a result shows that the method is strong in specificity, good in precision and high in accuracy, and the content of chloroethanol can be rapidly measured.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Enrofloxacin quick-release pellet and preparation method thereof
ActiveCN110075082AImprove bioavailabilityAddressing palatability issuesAntibacterial agentsOrganic active ingredientsDrugEnrofloxacin
The invention discloses an enrofloxacin quick-release pellet and relates to the field of veterinary medicines. The enrofloxacin quick-release pellet comprises the following components by weight percent: 10%-45% of enrofloxacin, 18%-86% of excipient, 1%-5% of disintegrating agent, 1%-2% of adhesive and 2%-30% of coating solution. The invention also relates to a preparation method for the enrofloxacin quick-release pellet. The enrofloxacin quick-release pellet is capable of covering the bitter taste of enrofloxacin and guaranteeing quick disintegration release of the preparation in gastrointestinal tracts. Compared with the preparation sold in the market, the enrofloxacin quick-release pellet has the advantages that the prescription is simplified, few sorts of ingredients are used, the quality problem of the preparation caused by uneven mixing of raw materials and ingredients in the preparation process is reduced, the preparation process is simple, the quality is stable and reliable andthe bioavailability of drugs in animal body is obviously increased.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Method for detecting pesticide residue quantity in radix ophiopogonis
InactiveCN108387655AHigh penetration rateReduce use costComponent separationReference productNitrogen
The invention relates to a method for detecting the pesticide residue quantity in radix ophiopogonis. The method comprises the following steps of (1) weighing radix ophiopogonis to be tested; performing treatment by using a QuEChERS pretreatment technology; preparing a first test product solution; (2) weighing a proper amount of ribonolactone and sorbitol; adding acetonitrile and water for dissolution; using the materials as an analysis protection agent; (3) measuring the first test product solution; blowing the solution to the near dry state by nitrogen; adding acetone for dissolution; addingthe analysis protection agent; performing uniform mixing; using the materials as a second test product solution; (4) weighing a pesticide reference product solution; respectively adding acetonitrileto prepare a first reference product solution; measuring and taking the first reference product solution; respectively adding acetone to prepare a second reference product solution; (5) using a testing method; (6) calculating the content of pesticide in the first test product solution and the second test product solution according to an outer standard method. The method has the characteristics ofhigh repeatability, high accuracy, low utilization cost and the like. The pesticide residue quantity in the radix ophiopogonis can be fast, effectively, simply and conveniently detected.
Owner:四川省食品药品检验检测院
Method for simultaneously detecting content of four components in Huangqi Guizhi Wuwu Granule
InactiveCN109521106AQuality is easy to controlEasy to separateComponent separationPattern recognitionSolvent
The invention discloses a method for simultaneously detecting the content of four components in Huangqi Guizhi Wuwu Granule. The four components are paeoniflorin, calycosin-7-glucoside, cinnamaldehydeand 6-gingerol. The method comprises the steps of extracting a to-be-detected sample by using an extraction solvent: 30-80% methanol, filtering extract and directly detecting by using a high-performance liquid chromatograph. Through the method disclosed by the invention, the content of the four components in the Huangzhi Guizhi Wuwu Granule can be simultaneously detected and the method has the advantages of high efficiency and accuracy and the like, so that the quality of the Huangqi Guizhi Wuwu Granule is more controllable.
Owner:JIANMIN PHARMA GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com