Arbidol dry suspension and preparation method thereof
A technology of arbidol hydrochloride and suspension, applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of unwell children, poor patient compliance, etc. Short peak time, the effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
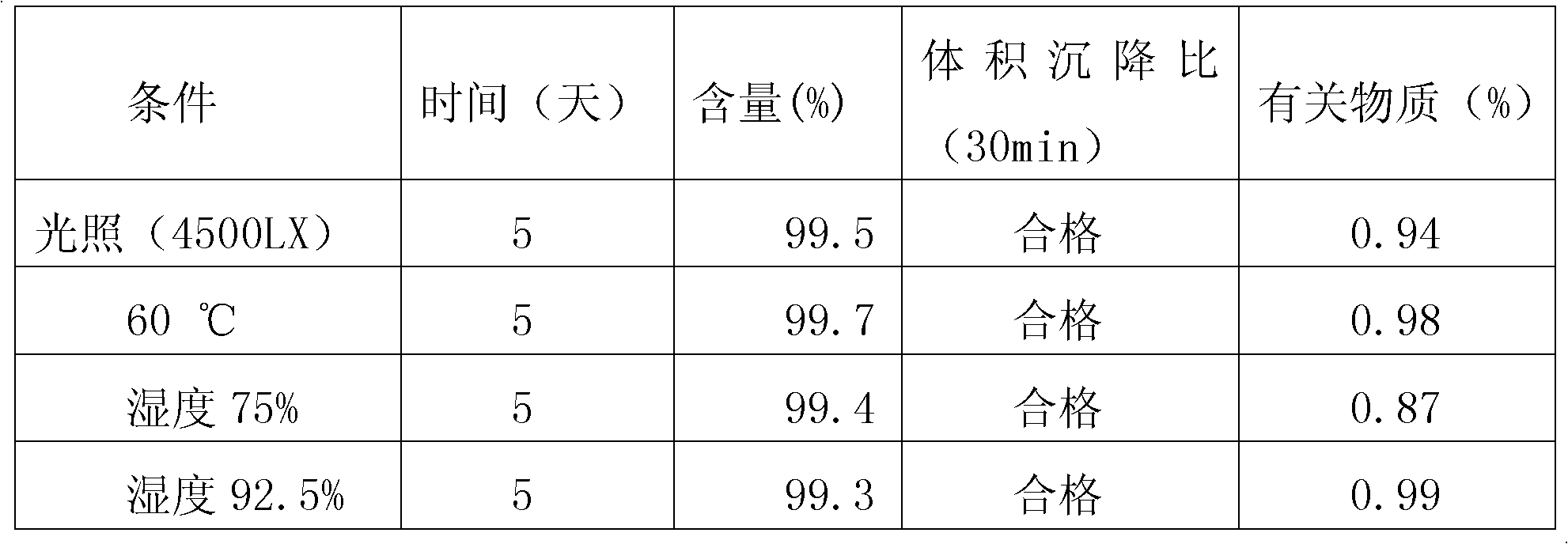
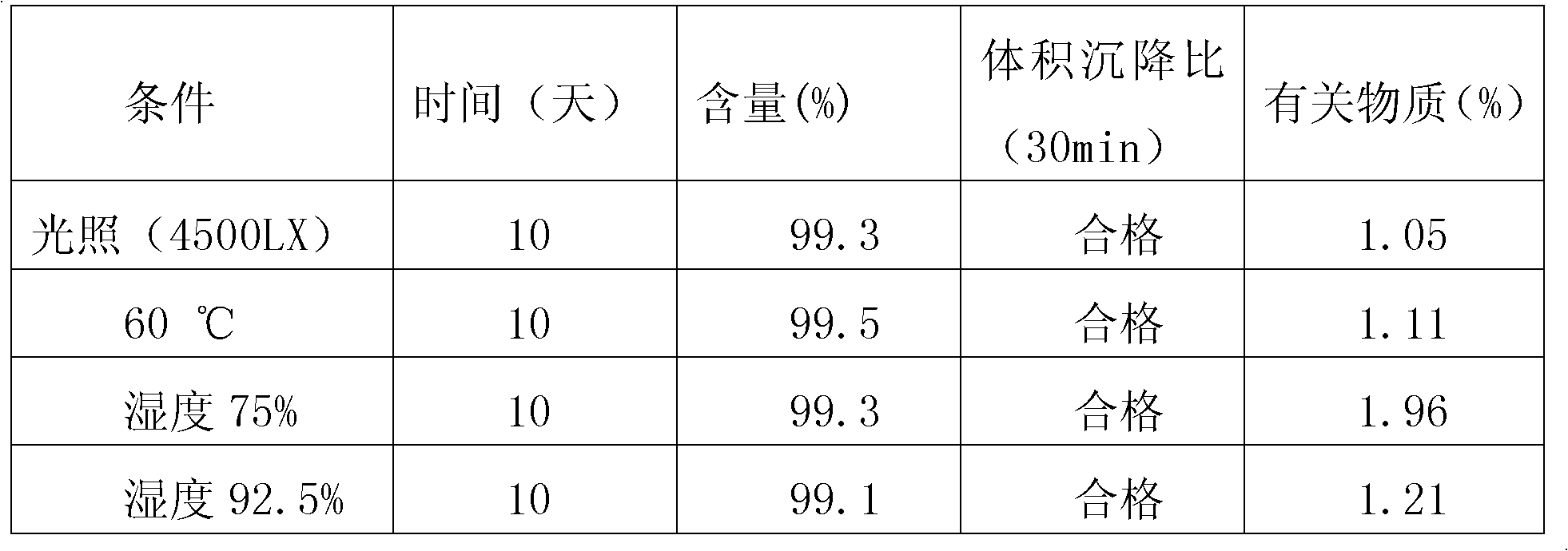
[0009] Example 1: A dry suspension of Arbidol, 100 g Arbidol hydrochloride, 100 g gum arabic, 50 g hydroxypropyl cellulose, 15 g xanthan gum, 2.0 g sodium lauryl sulfate, and citric Acid 20g, aspartame 50g, sucrose 656g, orange flavor 0.005g; Arbidol hydrochloride through a 100 mesh sieve, gum arabic, hydroxypropyl cellulose, xanthan gum, sodium lauryl sulfate, citric The acid, sucrose, and strawberry flavor were crushed and passed through a 100-mesh sieve; except for sucrose, the remaining components were thoroughly mixed, and then sucrose was added to mix evenly; then the soft material was moistened with 90% ethanol and passed through a 32-mesh sieve for granulation. Dry it at ℃, pass through a 38-mesh sieve and sizing. After passing the inspection, put it into an aluminum-plastic composite film bag to make 1000 bags, 1g each.
Embodiment 2
[0010] Example two, a dry suspension of Arbidol, 100 g of Arbidol hydrochloride, 100 g of gum arabic, 50 g of hydroxypropyl cellulose, 15 g of xanthan gum, 2 g of sodium lauryl sulfate, and citric acid 20g, sucrose 713g, orange flavor 0.005g; Arbidol hydrochloride through a 100 mesh sieve, gum arabic, hydroxypropyl cellulose, xanthan gum, sodium lauryl sulfate, citric acid, sucrose, orange flavor respectively After crushing, pass through a 100-mesh sieve; except for sucrose, the remaining components are thoroughly mixed, and then sucrose is added and mixed uniformly; then the soft material is wetted with 80% ethanol, granulated through a 20-mesh sieve, dried at 40°C, and passed through a 40-mesh sieve Whole grains, after passing the inspection, put them into aluminum-plastic composite film bags to make 1000 bags, 1g per bag.
[0011] Example two, a dry arbidol suspension, taking 100g arbidol hydrochloride, 90g hydroxypropyl cellulose, 30g tragacanth, 2.5g sodium laurate, 2.5g tar...
Embodiment 3
[0012] Example three, a dry arbidol suspension, taking 100 g arbidol hydrochloride, 120 g acacia, 55 g povidone, 2.5 g sodium lauryl sulfate, 19.5 g citric acid, 680 g sucrose, Microcrystalline cellulose 20g, aspartame 3g, banana flavor 0.005g; Arbidol hydrochloride through a 100 mesh sieve, acacia, povidone, sodium lauryl sulfate, citric acid, sucrose, microcrystalline Cellulose, aspartame and banana essence were crushed and passed through a 100-mesh sieve; except for sucrose and microcrystalline cellulose, the remaining components were thoroughly mixed, then sucrose and microcrystalline cellulose were added and mixed uniformly; then 90% ethanol Wet the soft material, pass through a 32-mesh sieve, granulate, dry at 45°C, and pass through a 38-mesh sieve for granulation. After passing the inspection, put it into an aluminum-plastic composite film bag to make 1000 bags, 1g each.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com