Bioabsorbable self-expanding endolumenal devices
a bioabsorbable, endolumenal technology, applied in the field of implantable medical devices, can solve the problems of adverse interaction with the metallic implant, negative impact a subsequent procedure may have on the patient, and strong magnetic field produced by the magnetic resonance imaging (mri) machine, and achieve the effect of enhancing tissue compliance of the devi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0108] This example describes the preparation of an extrudate made of a bio-absorbable polymer suitable for use in the present invention. The extrudate is subsequently extruded into a tubular shape for further processing into a device of the present invention.
[0109] A tri-block co-polymer of poly(glycolide) and poly(trimethylenecarbonate) having a weight to weight (w / w) ratio of 67% poly(glycolide) to 33% poly(trimethylenecarbonate) was acquired from Davis and Geck / United States Surgical (Manati, Puerto Rico—Lot #:01I01). This bioabsorbable PGA / TMC co-polymer, commonly referred to as polyglyconate, was provided with certification to its co-polymer ratio.
[0110] Upon receipt, approximately 25 mg of the PGA / TMC co-polymer was dissolved in 25 ml of hexafluoroisopropanol (HFIP). The produced dilute solution was found to possess an inherent viscosity (IV) of 1.41 dl / g when measured using an AUTOVISC™ I automated viscometer operating at 30 degrees centigrade.
[0111] Approximately 6 mg of...
example 2
[0114] This example describes the formation of a construction of a bioabsorbable polymeric material suitable for further processing into a device of the present invention. The construction was extruded into a tubular shape with a 0.5 inch, 24:1, screw extruder (Randcastle Extrusion Systems, Inc., Cedar Grove, N.J.). The extruder had a three-stage screw.
[0115] The process was begun by heating approximately 200 grams of the PGA / TMC block co-polymer of Example 1 overnight under vacuum at 130 degrees centigrade to dry the co-polymer. The dried co-polymer was then placed into the extruder.
[0116] The extruder was programmed to provide a temperature profile that achieves a melt temperature between 205 degrees and 210 degrees centigrade with a die temperature between 205 degrees and 210 degrees centigrade. The extruder melted and pumped the polymer through a Genca (Clearwater, Fla.) tubing die designed to produce a draw ratio of about 5:1 with a draw ratio balance of about 1.00.
[0117] Th...
example 3
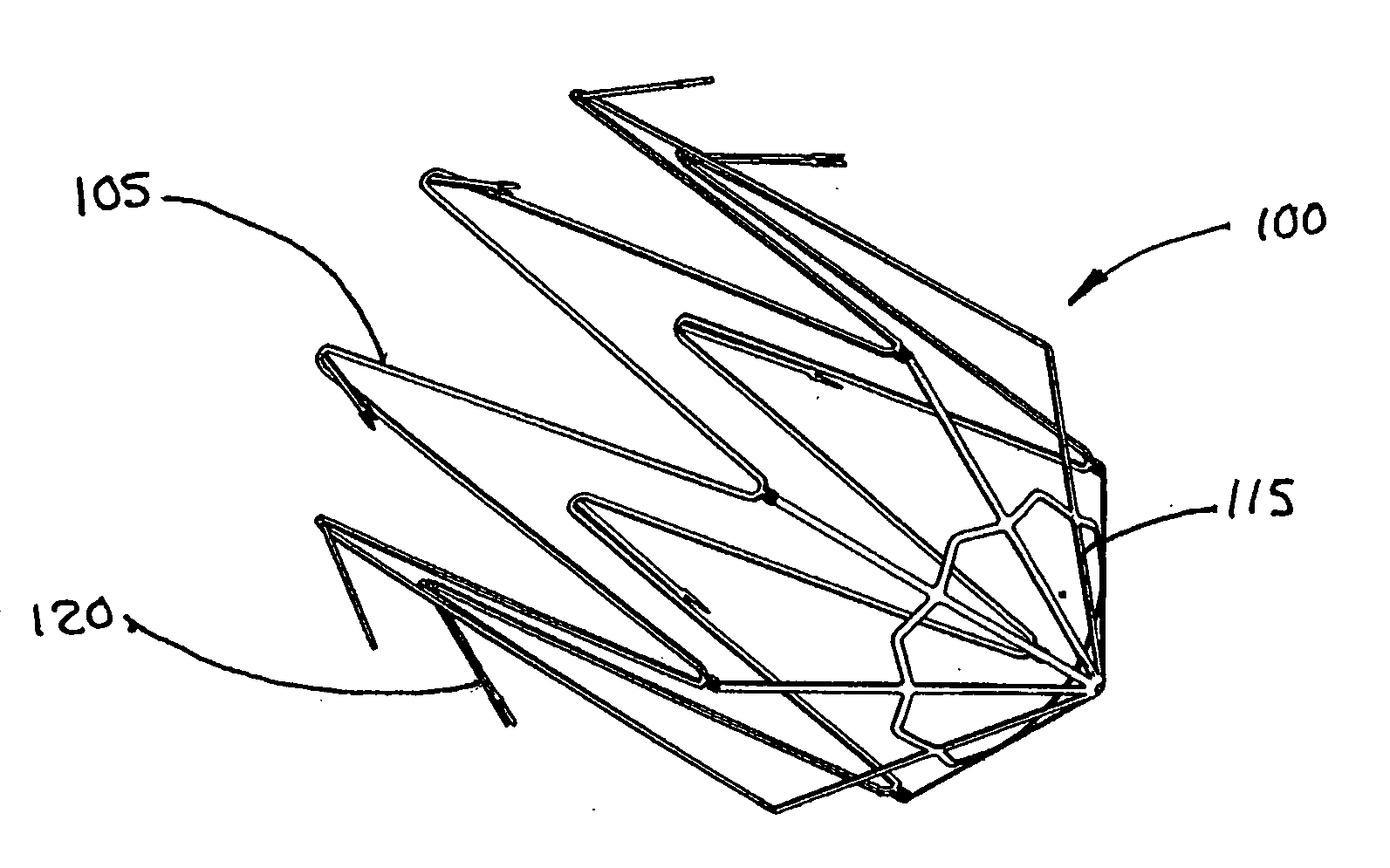
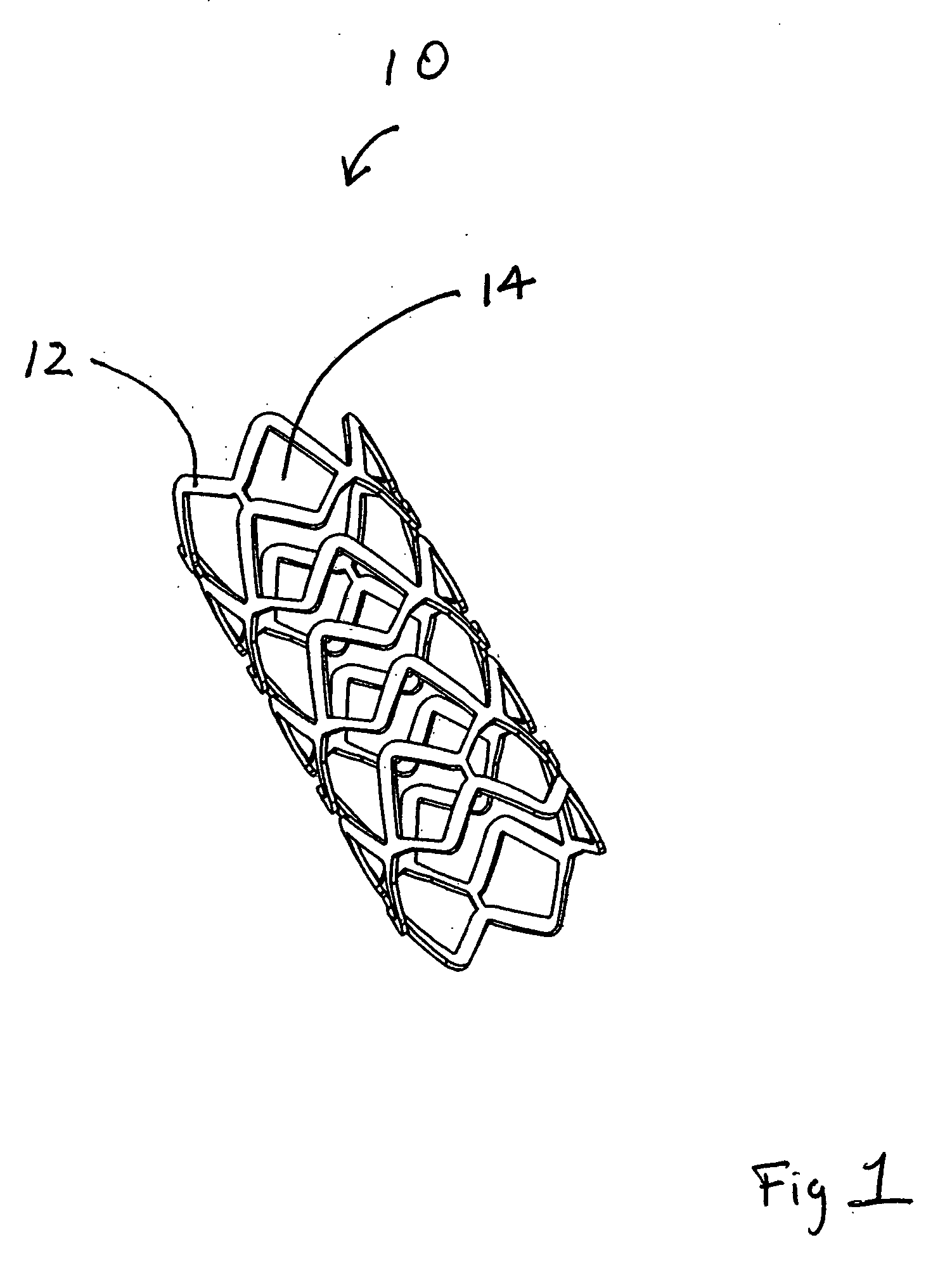
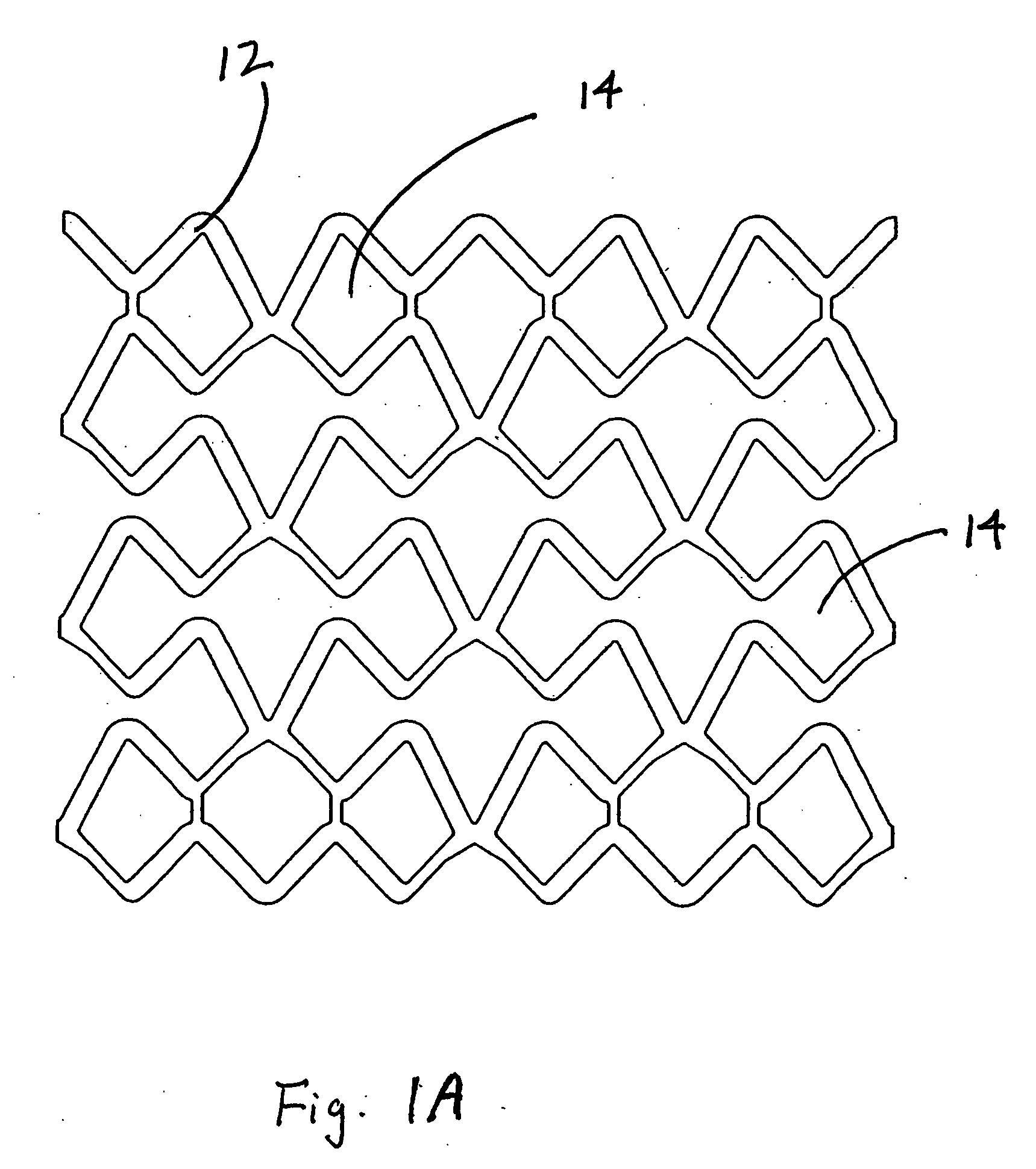
[0120] This example describes forming fenestrations (14) in the tubular construction (16) of Example 2 with an excimer laser. When the fenestrations were formed in the construction, a device of the present invention (10) comprising an integral self-expanding, non-elongating, bioabsorbable framework (12) was formed. The finished device once sterilized can serve as a bioabsorbable support for a body conduit.
[0121] A 248 nm gas excimer laser (available from J. P. Sercel Associates, Inc., Hollis, N.H., system JPSA 100-01-INV024, model IX1000) was used to form a series of fenestrations (14) in the tubular construction (16). The laser was set to produce a 110 milli-joules light beam, repeatedly pulsed at 200 Hz. The energy density at the device was 2 Joules per square centimeter.
[0122] The laser was fitted with attachments that permitted a chuck for holding and rotating a mandrel to be attached to the laser. The chuck was driven by a rotary servomotor. A base tube for a device of the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com