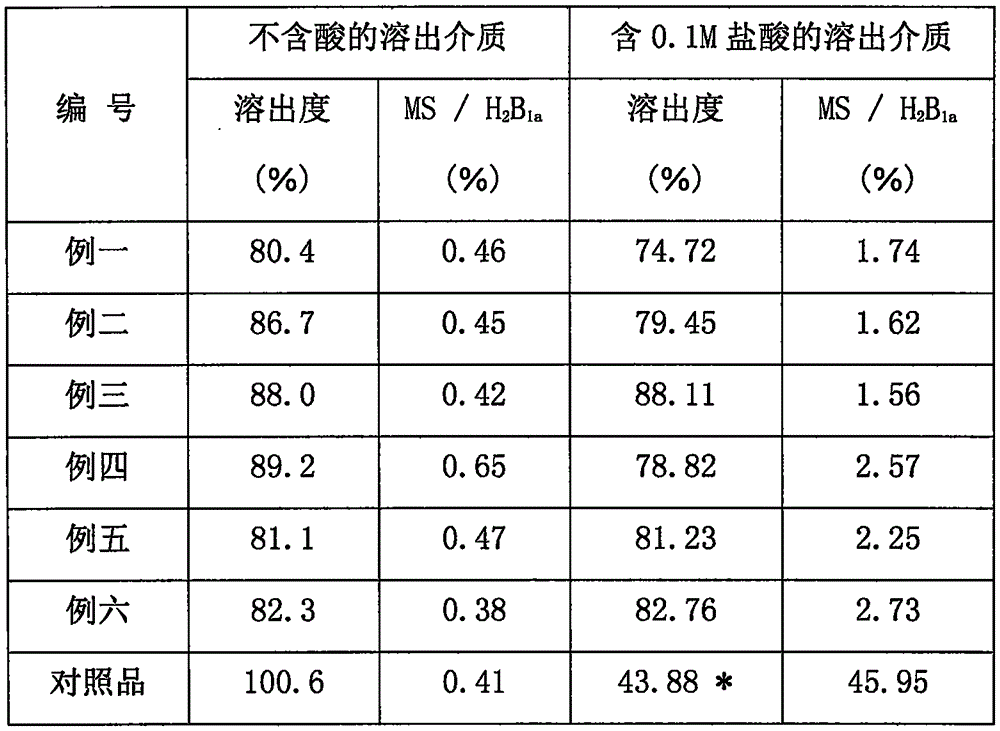
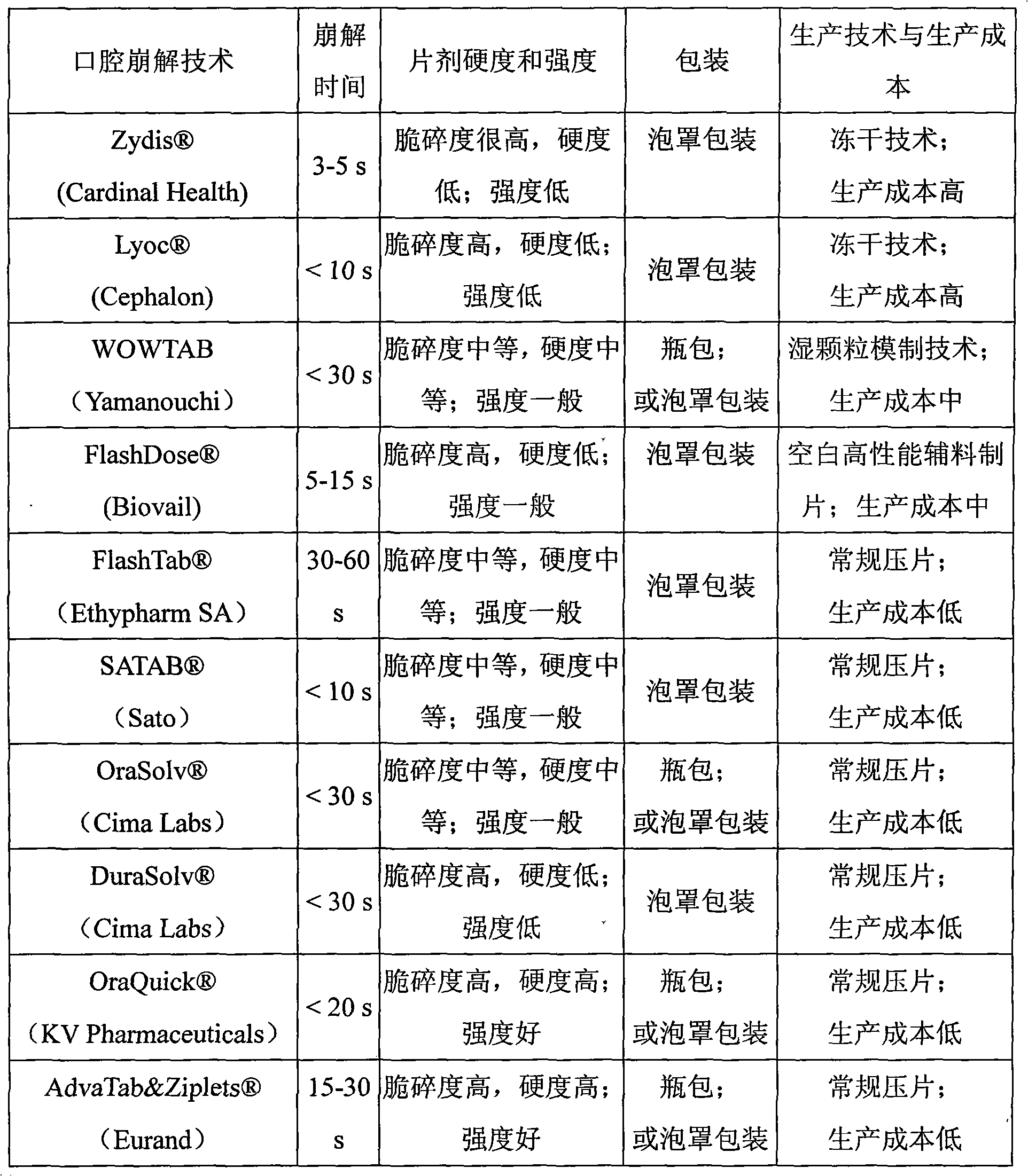
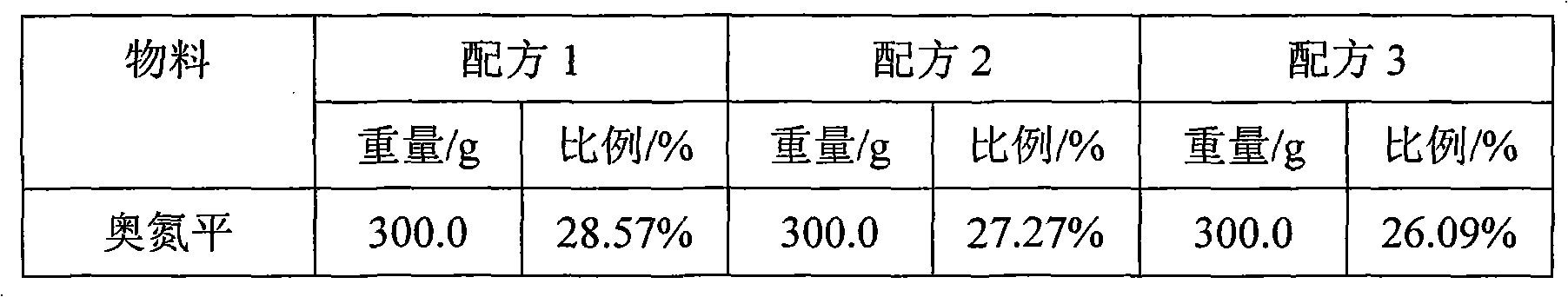
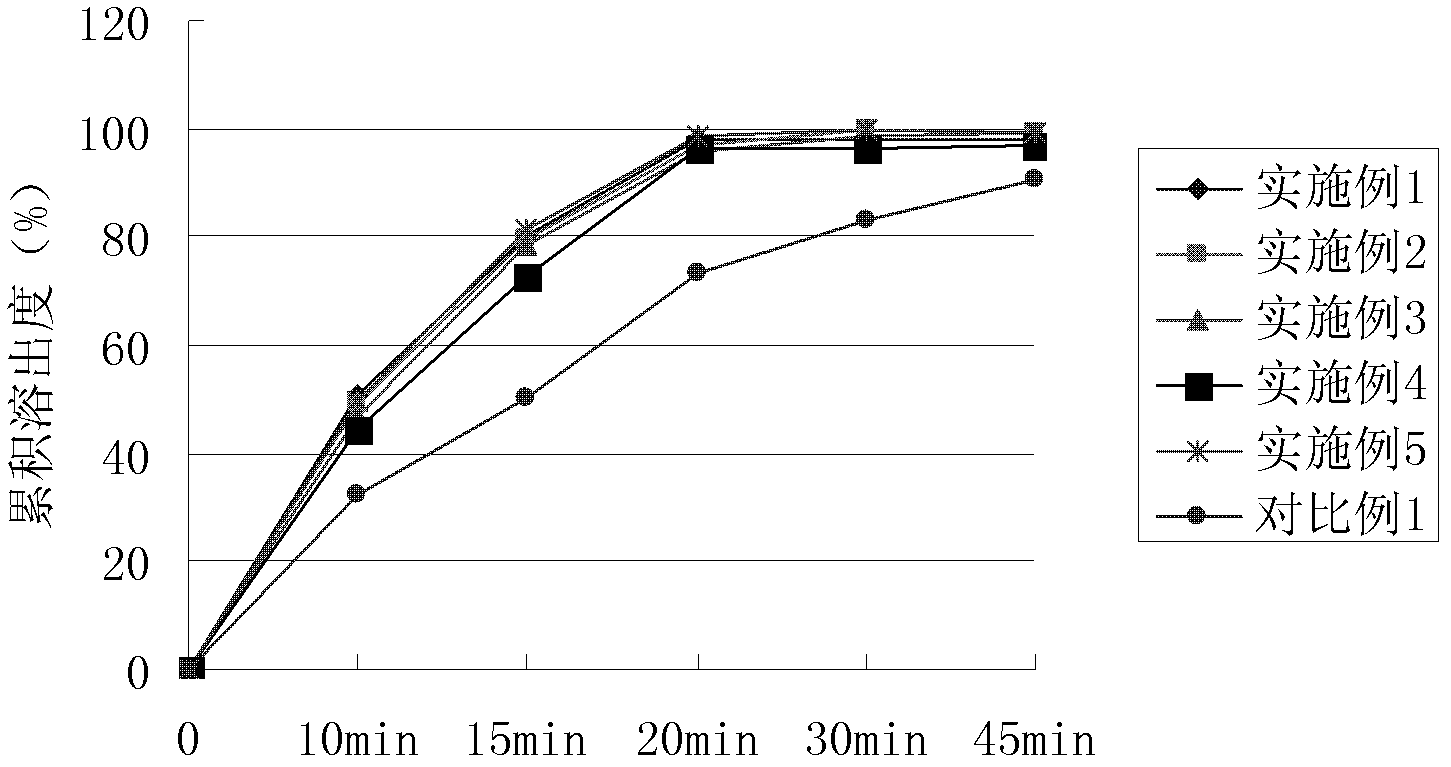
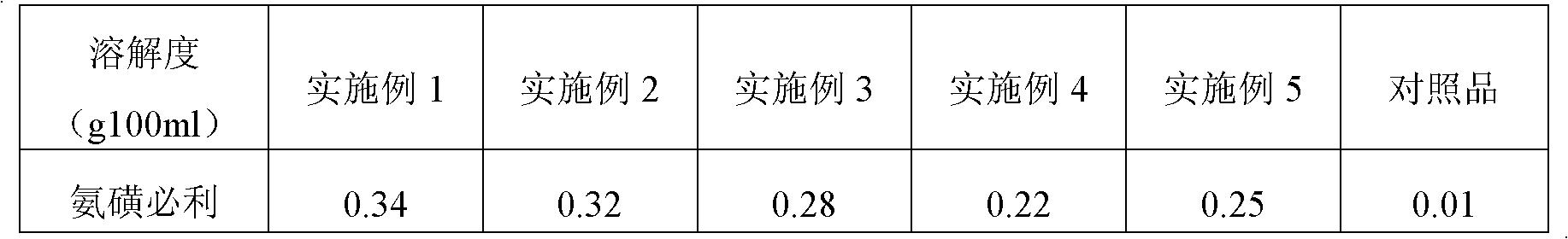
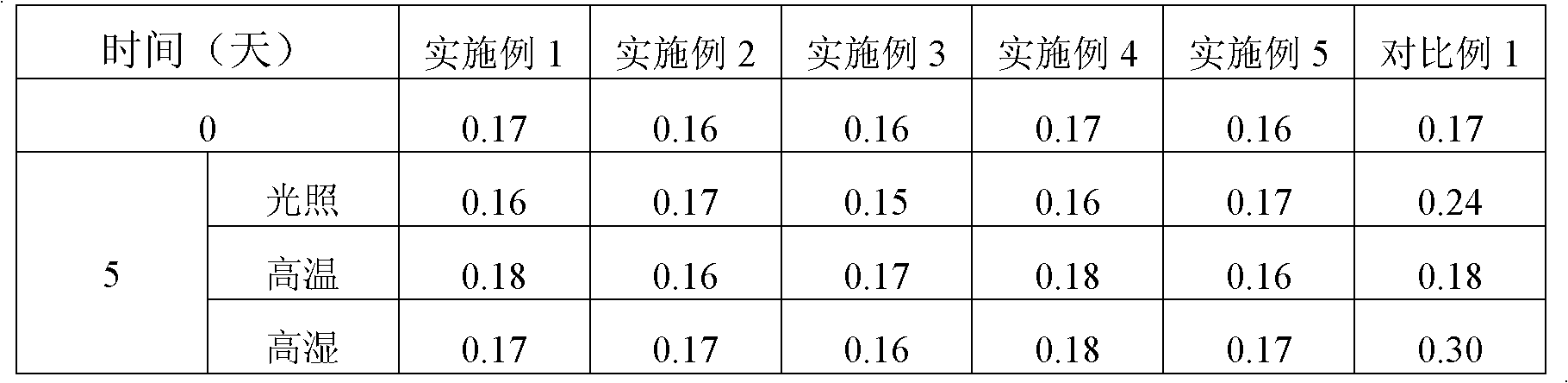
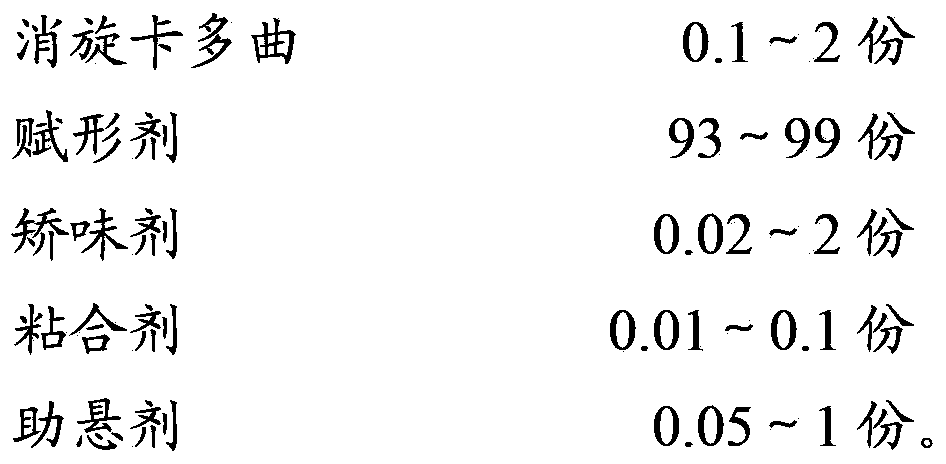Patents
Literature
641results about How to "Mask bitterness" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coating compositions for bitterness inhibition
InactiveUS7294347B2Mask bitternessImprove palatabilityPowder deliveryInksPH-sensitive polymersPh independent
The present invention discloses coating compositions with taste masking property, comprising a blend of pH sensitive polymers and optionally a pH independent polymer or a blend of the pH sensitive polymer and pH independent polymer used for taste masking of highly bitter drugs. The pH sensitive polymers used comprise the acid soluble polymers and the enteric polymers. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said coating compositions is disclosed. The concomitant use of the polymers inhibits the release of the bitter drug at the pH of saliva. The said coating compositions deliver substantial amount of the bitter drug immediately with improved palatability.
Owner:COUNCIL OF SCI & IND RES
TS-1 granules and preparation method thereof
ActiveCN101843621AAvoid interactionImprove stabilityPharmaceutical non-active ingredientsGranular deliverySolubilityPotassium oxonate
The invention belongs to the technical field of medicaments, and relates to TS-1 granules for treating advanced gastric cancer and a preparation method thereof. The method comprises the following steps of: preparing active medicaments tegafur, gimeracil and potassium oxonate into a cyclodextrin inclusion compound; and sieving the inclusion compound, uniformly mixing the sieved inclusion compound and pharmaceutically acceptable auxiliary materials and performing wet granulation or dry granulation to prepare the granules. The granules have the advantages of increasing the water solubility and stability of the medicaments, covering the bitter taste of the tegafur, prolonging the action time, improving the medication compliance of patients with cancer, greatly improving the bioavailability of the medicaments and reducing the toxic or side effect of the medicaments.
Owner:鲁南新时代生物技术有限公司
Coating compositions for bitterness inhibition
InactiveUS20050281874A1Improve protectionMask bitternessPowder deliveryInksPH-sensitive polymersBitter taste
The present invention discloses coating compositions with taste masking property, comprising a blend of pH sensitive polymers and optionally a pH independent polymer or a blend of the pH sensitive polymer and pH independent polymer used for taste masking of highly bitter drugs. The pH sensitive polymers used comprise the acid soluble polymers and the enteric polymers. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said coating compositions is disclosed. The concomitant use of the polymers inhibits the release of the bitter drug at the pH of saliva. The said coating compositions deliver substantial amount of the bitter drug immediately with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Cyclodextrin-included florfenicol quick-release water-soluble powder preparation and preparation method thereof
InactiveCN102160854AGood water solubilityImprove solubilityAntibacterial agentsPowder deliveryCyclodextrinDissolution
The invention relates to a cyclodextrin-included florfenicol quick-release water-soluble powder preparation and a preparation method thereof. The preparation is prepared by stirring florfenicol, cyclodextrin and water at a uniform speed and including by using an inclusion technology. The preparation can be directly dissolved in water to be taken by livestock, and has the characteristics of high medicine dissolution rate, high dissolubility, high bioavailability, good absorption and the like; and the medicine (florfenicol) is included in a carrier material, the bitterness of the florfenicol can be masked, and the intake or drinking of animals cannot be influenced by the bitterness of the medicine.
Owner:GUANGDONG YANGBLE BIOPHARMLS
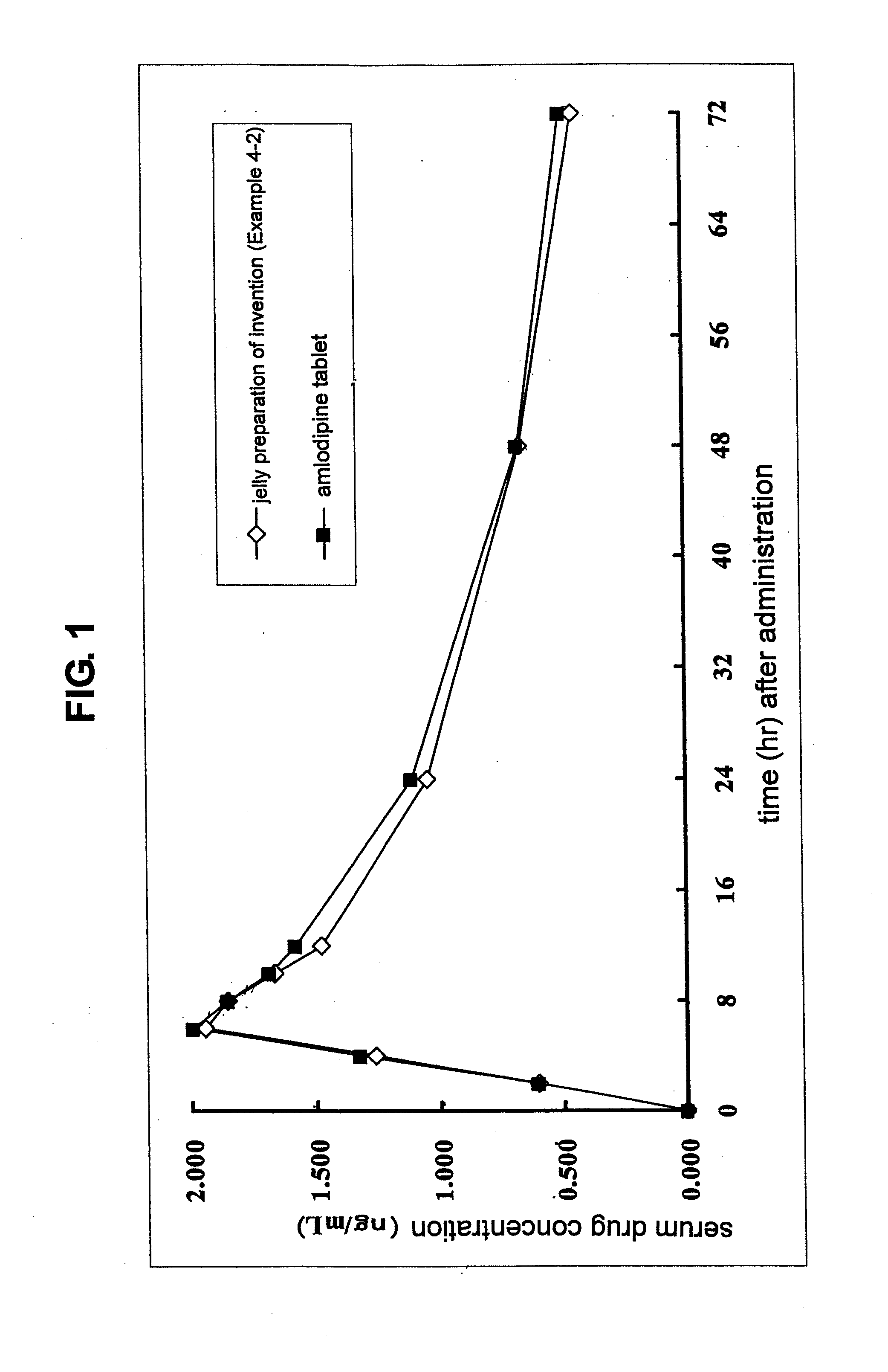
Aqueous oral preparation of stable amlodipine
InactiveUS20110294860A1Masking of bitter tasteEasy to carryBiocideOintment deliveryAmlodipineSURFACTANT BLEND
The invention provides a stable and rapidly disintegrable aqueous oral preparation (liquid or jelly preparation) of amlodipine. The liquid preparation comprises an anionic surfactant having a sulfuric acid group or a sulfonic acid group as a stabilizer in an aqueous solution of amlodipine, at pH 5-7, while the jelly preparation further comprises a gelling agent, a fine powder solid, and a gelling regulator.
Owner:MEDRX CO LTD
Method for processing acid and hot bitter bamboo shoot
InactiveCN1653946AReduce and mask bitternessMask bitternessFruit and vegetables preservationConfectioneryBamboo shootSugar
The present invention relates to food production, is especially the production process of sour and hot bitter bamboo shoot, and solves the technological problem of utilizing the resource of bitter bamboo shoot to produce delicious food product in simple process and low cost. The production process of sour and hot bitter bamboo shoot includes the following steps: slicing bitter bamboo shoot into shreds or slices; boiling in water with salt for 40-60 min; draining and cooling; mixing with sugar and pressurizing for 1-2 days; mixing with chicken bouillon, pickled chili chip and vinegar, sealing in can; sterilizing; etc.
Owner:余学军
Preparing method for pomelo wine
ActiveCN104450411AAvoid inhibitionRetain flavor substancesAlcoholic beverage preparationBiotechnologyPectinase
The invention discloses a preparing method for pomelo wine. According to the method, water, sugar, honey, acid, pectinase, yeast cell wall, nutrimental agents and yeast are added into a crushed pomelo and mixed uniformly to be fermented at the temperature of about 18 DEG C, an odor absorber is added when the alcoholic strength is about 6% vol, after fermentation is finished, wine liquid is taken and stored in the environment with the temperature of about 2 DEG C, wine sediment is removed after 60 days, then the wine liquid is stored for about 90 days, then the wine liquid is placed in the environment with the temperature of about minus 4 DEG C for 3 days, clarification is carried out, and the wine liquid is filtered and sterilized after being placed in the environment with the temperature of about minus 4 DEG C for 4 days. According to the method, the skin can also be fermented for generating the pomelo wine, the inhibiting effect on the fermenting effect from special ingredients in the pomelo skin is overcome, and the pomelo wine with the special taste can be brewed, and has the dense lemon scent; the way of adding the odor absorber to the special fermenting process is fished out, bitterness of the pomelo can be covered up, flavor compounds in the pomelo wine are effectively preserved, the scent of the pomelo wine is more complex, and the mouthfeel is enhanced.
Owner:广州市顺昌源酒业有限公司
Manufacturing method of freeze-dried fruit slices
The invention belongs to the technical field of food, and particularly relates to a manufacturing method of freeze-dried fruit slices. The manufacturing method comprises the following steps: raw material selection, cleaning, primary freeze-drying, slicing, soaking, secondary freeze-drying and packaging. According to the manufacturing method, the freeze-drying is carried out before slicing, thereby reducing the loss of the fruit juice in the slicing process, and greatly maintaining the nutrients and fruity odor in the fruit. The slicing and other treatment processes are carried out under the condition of weak respiration of fruit tissue cells, so that the enzyme activity is lowered, thereby lowering the influence of the fruit metabolism on the fruit quality in the working process. The freeze-drying is carried out twice, thereby greatly reducing the loss of nutrients and odor, maximally removing the water in the fruit and prolonging the preservation period of the fruit slices.
Owner:四川中兴绿丰农业发展有限公司
Masked berberine hydrochloride micro pill and its prepn
InactiveCN1931137AMask bitternessImprove complianceAntibacterial agentsOrganic active ingredientsSucrosePlasticizer
The masked berberine hydrochloride micro pill is coated berberine hydrochloride micro pill, and the berberine hydrochloride micro pill is prepared with berberine hydrochloride and supplementary material through an extruding and centrifugal pelletizing process, a centrifugal laminating process or a rolling process in a sugar coating pot. The supplementary material is selected from microcrystalline cellulose, starch, cane sugar and dextrin, and accounts for 15-90 % of the micro pill. The coating material includes filming material, plasticizer and antiagglomerant. The masked berberine hydrochloride micro pill may be further prepared into granule, dispersed tablet, dry suspension, bolus or other preparation forms. The present invention has the bitter of berberine hydrochloride masked and thus raised patient's compliance, and is especially suitable for infant and old persons with dysphagia.
Owner:SUNSTONE TANGSHAN PHARM CO LTD
Hypoglycemic powder and its preparation method
InactiveCN101057662ALong-term curative effect is stableImprovement in physical characteristicsFood preparationPEARBalsam
The invention discloses an anti-hypelipidemic powder mainly for diabetes patient and the preparing method. The raw material essentially comprises balsam pear powder, union powder, black soybean powder, duck wheat powder and pumpkin powder. The early stage curative effect of this product is apparent and the medium-long term curative effect is stable.
Owner:刘丹
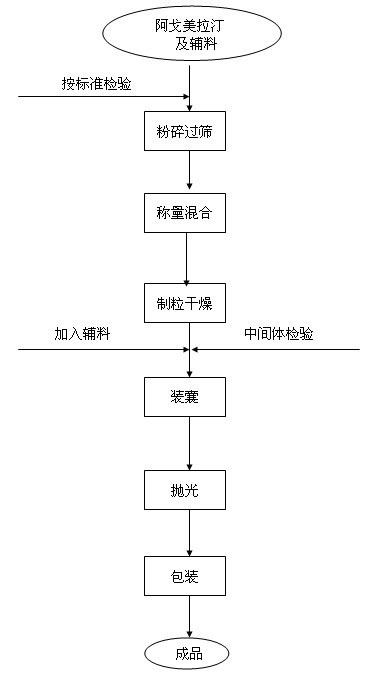
Stable Agomelatine capsule medicine composition
InactiveCN101991559AMask the smellMask bitternessOrganic active ingredientsNervous disorderMannitolBioavailability
The invention discloses a stable Agomelatine capsule medicine composition which is characterized in that 1000 capsules comprise the following components of 15-30g of Agomelatine, 70-140g of mannitol, 0.5-1g of superfine silica powder and a proper amount of 10 percent pre-gelatinized starch. The invention also relates to a preparation method of an Agomelatine capsule. The Agomelatine capsule prepared by adopting the formula and the preparation method provided in the invention has the advantages of good flowability, good dissolution rate, small content uniformity, high bioavailability and good treatment effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of buckwheat flavone microcapsule and its product
InactiveCN102334685AHigh mechanical strengthHigh activityFood preparationPolygonum fagopyrumSolubility
The invention provides a preparation method of a buckwheat flavone microcapsule, which comprises the following steps: 1) pretreating: drying buckwheat grain, buckwheat shell, buckwheat husk or buckwheat stem leaf, sieving after crushing for standby to obtain buckwheat powder; 2) extracting; 3) separating and purifying; 4) preparing a capsule emulsion: uniformly mixing a core material, a wall material and an emulsifier to prepare a buckwheat flavone microcapsule emulsion; 5) spray drying to obtain the buckwheat flavone microcapsule. The invention has the advantages that: 1, the activity of an active component flavone substance in buckwheat is protected; 2, the buckwheat flavone is prevented from damage of gastric acid, has certain targeting after microencapsulation, and has good enteric solubility but insolublility in stomach; 3, the bitter taste of buckwheat is covered; 4, the release speed is controlled, the flavone substance can not be stored in vivo, and the concentration of the flavone substance is rapidly increased in vivo after taking and rapidly decreasing due to metabolism degradation effect, the microencapsulation enables a slow release function and releases functional factors in certain time and scope.
Owner:SHAANXI UNIV OF SCI & TECH
Tilmicosin micro-capsule preparation and preparation method thereof
InactiveCN102688220AMask bitternessProlong the action timeAntibacterial agentsOrganic active ingredientsWaxAcrylic resin
The invention relates to a tilmicosin micro-capsule preparation, and in particular relates to a tilmicosin micro-capsule preparation and a preparation method of the tilmicosin micro-capsule preparation, belonging to the technical field of veterinary medicine. The tilmicosin micro-capsule preparation comprises an inner core layer and a coating layer, wherein the inner core layer comprises a tilmicosin raw material and macromolecule auxiliary materials, the macromolecule auxiliary materials are selected from one or more than one of 12-carbon fatty acid-18-carbon fatty acid, paraffins and vegetable wax; and the coating layer comprises an inner coating layer and an outer coating layer, wherein the material of the inner coating layer is one or more than one of starch, calcium carbonate and calcium hydrophosphate, and the material of the outer coating layer is acrylic resin. The tilmicosin micro-capsule preparation disclosed by the invention is tasteless, enteric, and good in palatability. According to the invention, the tilmicosin is packed by the macromolecule auxiliary materials, a layer of enteric coating layer is sprayed on the surfaces of the tilmicosin grains in the process of rolling circle when the materials are prepared, and the strong bitter taste and odor of the tilmicosin can be completely covered due to the double-layer package, and the preparation method is simple in technology, and low in cost.
Owner:HUZHOU AIBAOLAI ANIMAL PHARMA
Beverage precursor and process for the manufacture thereof
InactiveUS20080020069A1Short timeImprove efficiencyBiocideFruit and vegetables preservationFood gradeCatechin
Owner:CONOPCO INC D B A UNILEVER
Racecadotril granules and preparation technology thereof
InactiveCN104224724AImprove granulation effectMask bitternessOrganic active ingredientsDigestive systemRacecadotrilSucrose
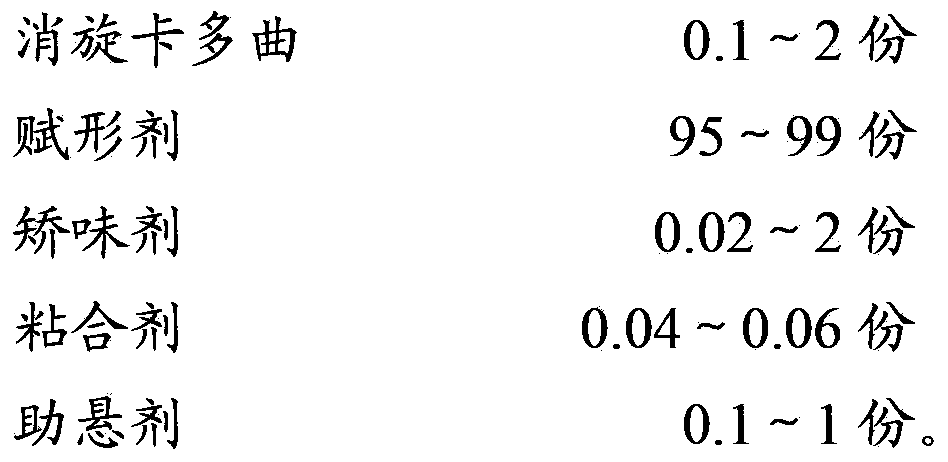
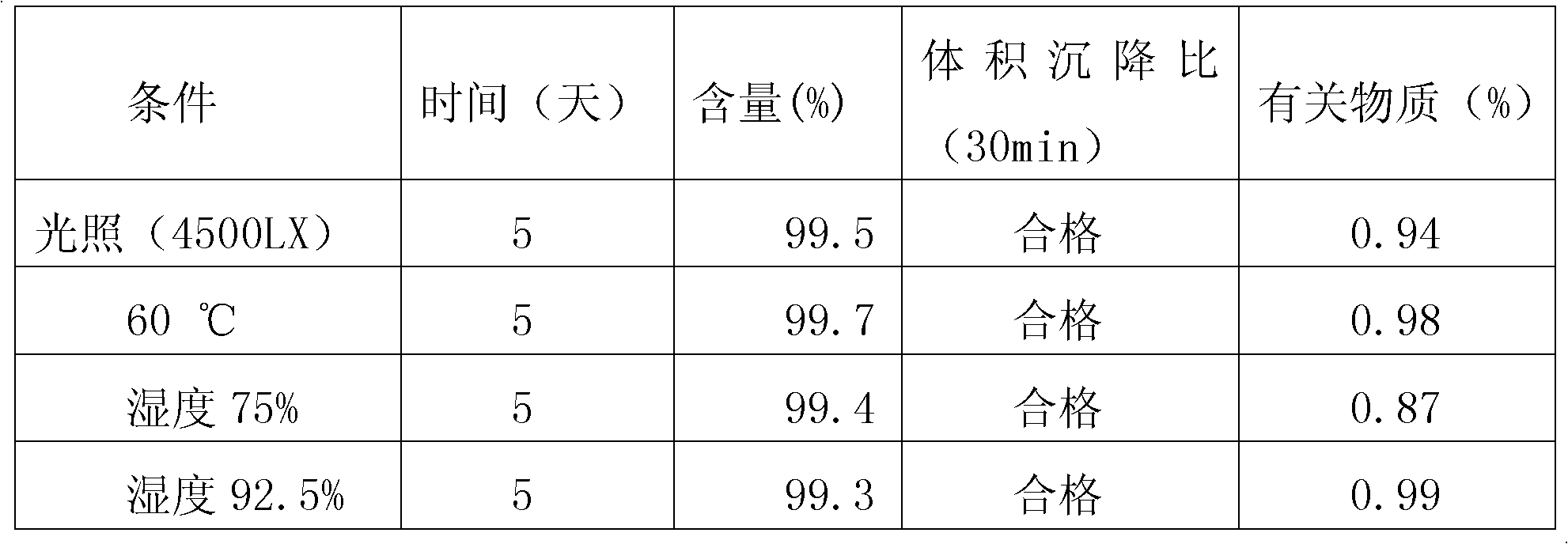
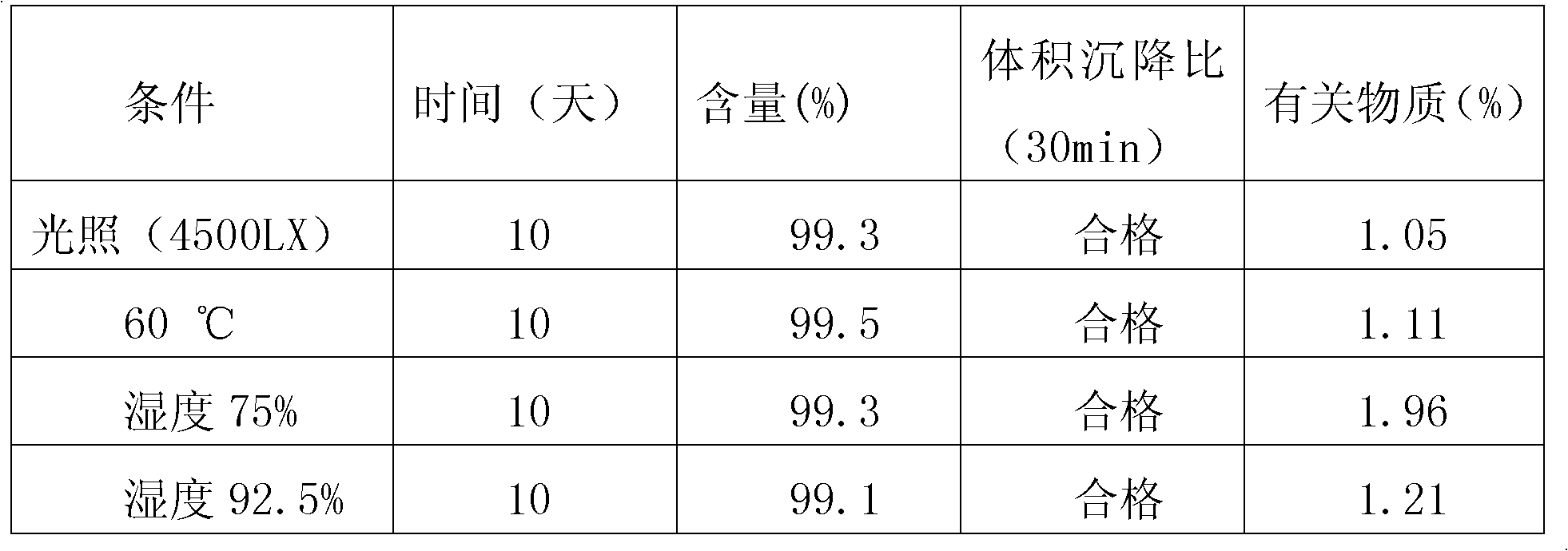
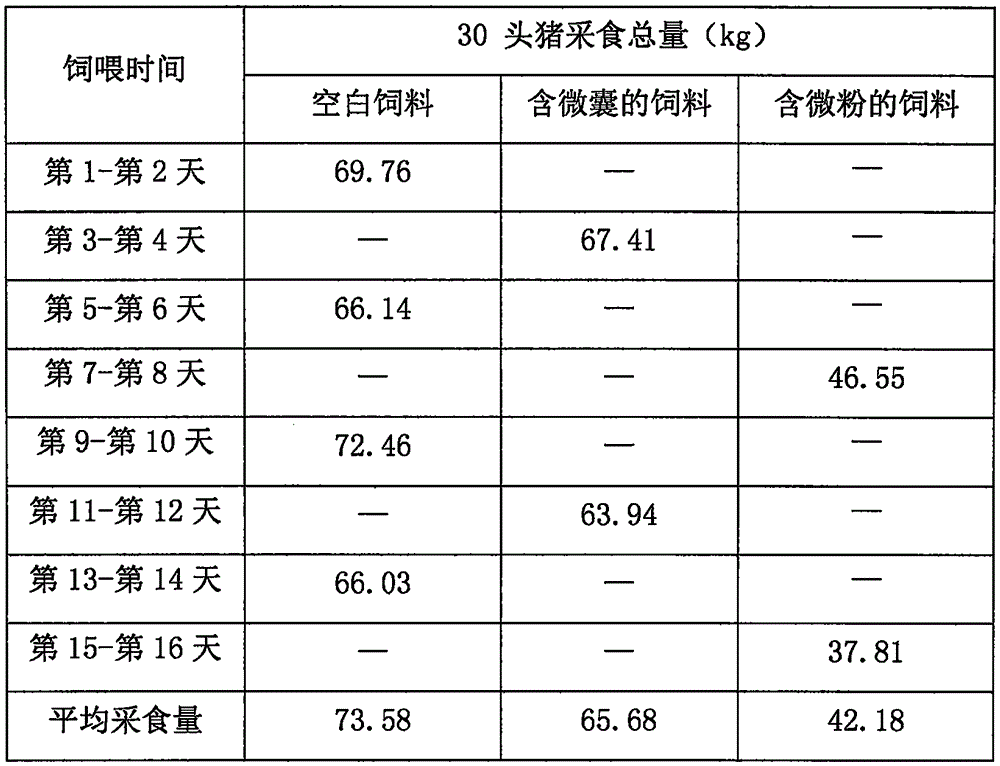
The invention relates to the field of medicinal preparations, and particularly relates to racecadotril granules and a preparation method thereof. The racecadotril granules are prepared from the following raw materials by weight: 0.1-2 parts of racecadotril, 93-99 parts of an excipient, 0.02-2 parts of a corrigent, 0.01-0.1 part of an adhesive and 0.05-1 part of a suspending agent. The racecadotril granules are prepared by suspending racecadotril in the adhesive and dissolving partial sucrose in the adhesive, so that bitter taste of a drug can be covered. The prepared racecadotril granules have relatively good granulation effect, and increase compliance of children.
Owner:BEIJING HANMI PHARMA CO LTD
Azithromycin enteric dry suspension and preparation method thereof
InactiveCN101991544AMask bitternessAvoid stimulationAntibacterial agentsOrganic active ingredientsGastric mucosaCoating drugs
The invention relates to an azithromycin enteric dry suspension and a preparation method thereof. The dry suspension comprises the following components in percentage by weight: 4 to 10 percent of azithromycin, 0.3 to 0.5 percent of isolated coating material, 1.6 to 3.2 percent of enteric coating material, 75 to 90 percent of flavoring agent and 3 to 6 percent of suspending agent. The azithromycin enteric dry suspension solves the problem that the azithromycin stimulates gastric mucosa in the background art, and provides an azithromycin preparation which is dissolved, released and absorbed in intestinal tracks. The azithromycin enteric dry suspension also solves the problem that the basic remedy and a capsule shell in an azithromycin enteric capsule generate cross-linking reaction, and masks the bitterness of the azithromycin through a taste masking technique, so the azithromycin enteric dry suspension is suitable for medicaments for children.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Arbidol dry suspension and preparation method thereof
ActiveCN102000030AImprove complianceMask bitternessOrganic active ingredientsAntiviralsSodium cyclamateSodium sulfate
The invention discloses an arbidol dry suspension and a preparation method thereof, and belongs to the medical industry. The arbidol dry suspension comprises Arbidol hydrochloride, a suspending aid, a diluent, a lubricating agent, a flavoring agent and a pH regulator, wherein the suspending aid is one or more of Arabic gum, tragacanth, sodium alga acid, povidone, hydroxy propyl cellulose and xanthan gum; the diluent is saccharose, mannitol and microcrystalline cellulose; the lubricating agent is lauryl sodium sulfate and sodium lauryl sulphate; the flavoring agent comprises a sweetener and an aromatizer, the sweetener is mannitol, saccharose, sodium cyclamate and aspartame, and the aromatizer is orange essence, banana essence, strawberry essence, and pineapple essence; and the pH regulator is citric acid and tartaric acid. The preparation method comprises the following steps of: sieving the arbidol hydrochloride, and respectively crushing and sieving the suspending aid, the diluent, and the lubricating agent; fully mixing the components except the diluent, and then adding the diluent for uniform mixing; and wetting by using ethanol to prepare a soft material, drying, and packaging into an aluminum-plastic composite membrane bag. After the arbidol is prepared into the dry suspension, the bitter of the arbidol is effectively masked, and the compliance of the patient is greatly improved.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Green tea-bitter gourd slices and preparation method and application thereof
The invention discloses a green tea-bitter gourd slices, and a preparation method and application thereof. The preparation method comprises the following steps of: pre-treating, debitterizing and hardening bitter gourds, and maintaining the bitter gourds green; crushing and sieving green tea, heating and digesting the green tea with deionized water, filtering the green tea, adding xylitol and high fructose syrup in filtrate, and adjusting the pH value of the filtrate to be 6-7, thus obtaining green tea soup; soaking the treated bitter gourd slices in the green tea soup to enable ingredients such as tea polyphenol, the xylitol and the high fructose syrup in the tea soup to permeate in the bitter gourd slices; and draining and drying the soaked bitter gourd slices which are fished out, thusobtaining the green tea-bitter gourd slices. The green tea-bitter gourd slices prepared by the preparation method disclosed by the invention are 0.2cm to 0.8 cm thick, brownish green, semi-ring-shaped, full in appearance, and free from crystals on the surface; the taste of the tea-bitter gourd slices is slightly bitter and then sweet, rich in tea aroma, and mild in bitter gourd flavour; and the total content of the tea polyphenol is 8840-11510 mg / kg. The green tea-bitter gourd slices are convenient to store, and can be eaten by chewing or drunk by being mixed with warm water or boiling water.
Owner:GUANGDONG IND TECHN COLLEGE
Medicinal core substance and micro-capsule prepared by taking corn cob flour as core material
InactiveCN106822033AImprove stabilityImprove bioavailabilityPharmaceutical non-active ingredientsMicrocapsulesEmulsionDrug carrier
The invention provides a medicinal micro-capsule taking corn cob flour as a core material and a preparation method of the medicinal micro-capsule. The preparation method comprises the following steps that 1, a core substance is prepared: a medicinal solvent is dissolved to prepare a medicinal solution, or a medicine and assistants such as an emulsifier are combined to prepare an emulsion; the medicinal solution or the emulsion and the corn cob flour are mixed, sufficiently stirred and dried to obtain the core substance taking the corn cob flour as a medicinal carrier; 2, bonding and fixing are performed: a bonding agent is dissolved by a solvent to obtain a bonding agent solution; the bonding agent solution and the core substance are mixed, sufficiently stirred, dried and sieved to obtain the secure core substance containing the bonding agent; 3, coating is performed: the core substance is coated with the core material to prepare the medicinal micro-capsule taking the corn cob flour as the core material, wherein coating methods include an air suspension method, a spray drying method, a pan coating method and a painting method; the painting coating method is preferable.
Owner:中农华威生物制药(湖北)有限公司
Taste masking spill-resistant formulation
InactiveUS20050143471A1Suppress bitternessIncrease sweetnessOrganic active ingredientsBiocidePolyethylene glycolFood flavor
The invention relates to a spill-resistant pharmaceutical composition, comprising a spill-resistant formulation with taste masking concentrations of polyethylene glycol (PEG) and diphenhydramine, which is less bitter, sweeter and has better overall flavor than current pharmaceutical compositions, while maintaining advantageous spill-resistant properties.
Owner:TARO PHARMA US INC
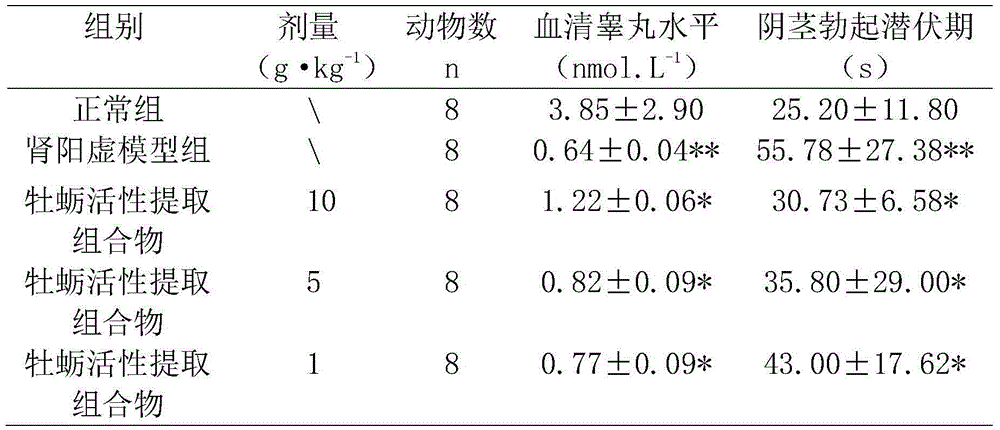
Preparing method of oyster active extract having functions of producing sperm to tonify the kidney and improving sexual function
InactiveCN104887713AKeep intactReduce lossesSexual disorderMolluscs material medical ingredientsSpermatorrheaSexual function
The invention discloses a preparing method of oyster active extract having functions of producing sperm to tonify the kidney and improving sexual function. The oyster active extract has the functions of producing the sperm to tonify the kidney and improving the sexual function. A composition mainly comprising the oyster active extract as a main active ingredient further comprises Epimedium herb, astragalus root, root of hairy asiabell, fruit of Chinese wolfberry, common Macrocarpium fruit, prepared rehmannia root, thinleaf milkwort root-bark, rhizome of oriental water plantain, licorice root and Indian bread. The preparing method includes special debitterizing, papain hydrolysis, fermentation-based deodorization via activated carbon and yeast, secondary debitterizing via beta-cyclodextrin embedding with mycose, decoloring via diatomite, color optimizing via bamboo leaf flavonoid and neutral sodium phytate, and taste optimizing via compound flavoring (comprising glucose, fructo-oligosaccharide and xylitol). The preparing method has the advantages that the process is advanced, the formula is scientific and reasonable, the nutrients are complete, the fishy smell is slight, the composition has natural flavor and the functions of producing the sperm to enhance the kidney, resisting fatigue, improving the sexual function and enhancing the body immunity, and the composition assists in treating symptoms, such as impotence, spermatorrhea and premature ejaculation, caused by deficiency of kidney-yang or deficiency of kidney-yin.
Owner:广西北部湾海皇生物科技有限公司
Novel Olanzapine orally disintegrating tablet
ActiveCN102178657APrevent oxidationAvoid degradationOrganic active ingredientsNervous disorderParticulatesOrally disintegrating tablet
The invention relates to a novel Olanzapine orally disintegrating tablet. The tablet comprises (I) a stabilizing Olanzapine coating particulate and (II) an additive, wherein the stabilizing Olanzapine coating particulate contains: (i) a neutral core; (ii) an active layer surrounding the neutral core; and (iii) a protective layer surrounding the active layer. The invention also relates to a preparation method of the stabilizing Olanzapine coating particulate and the novel Olanzapine orally disintegrating tablet containing the stabilizing Olanzapine coating particulate.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
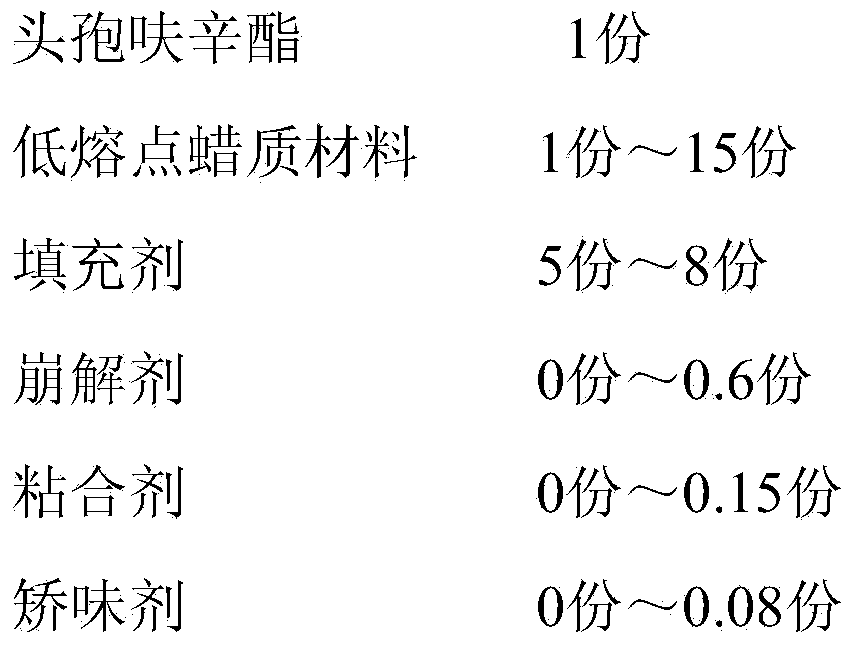
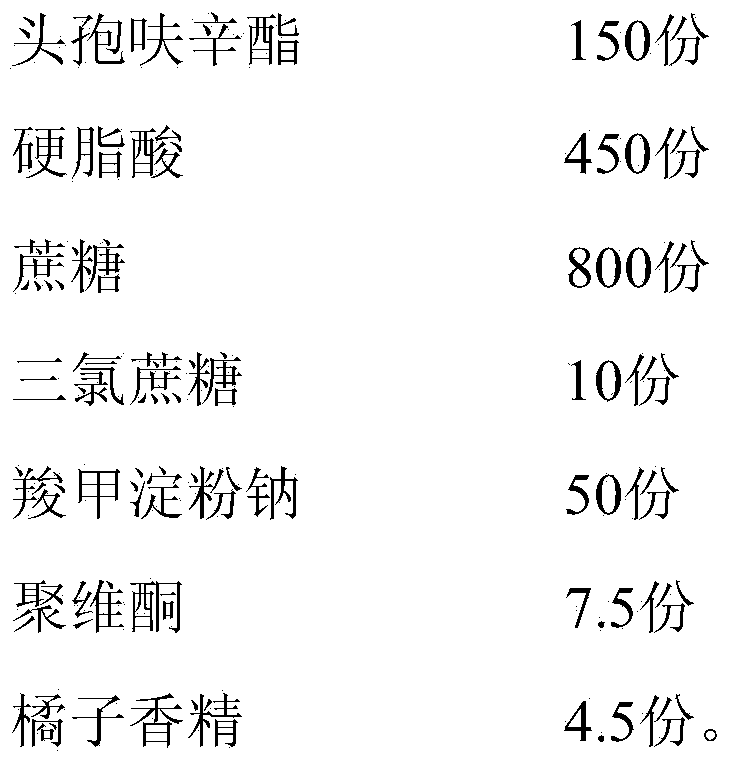
Cefuroxime axetil composition and preparation method thereof
The invention discloses a cefuroxime axetil composition which comprises the following components in parts by weight: 1 part of cefuroxime axetil, 1-15 parts of a low-melting-point wax material, 5-8 parts of filler, 0-0.6 part of a disintegrating agent, 0-0.15 part of a binder and 0-0.08 part of a corrigent. The invention further discloses a preparation method of the cefuroxime axetil composition. The cefuroxime axetil composition obtained can better cover bitter taste of the medicine and is good in dissolution rate, so that the medication compliance of patients is further improved.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Method for producing multi-cored molded article
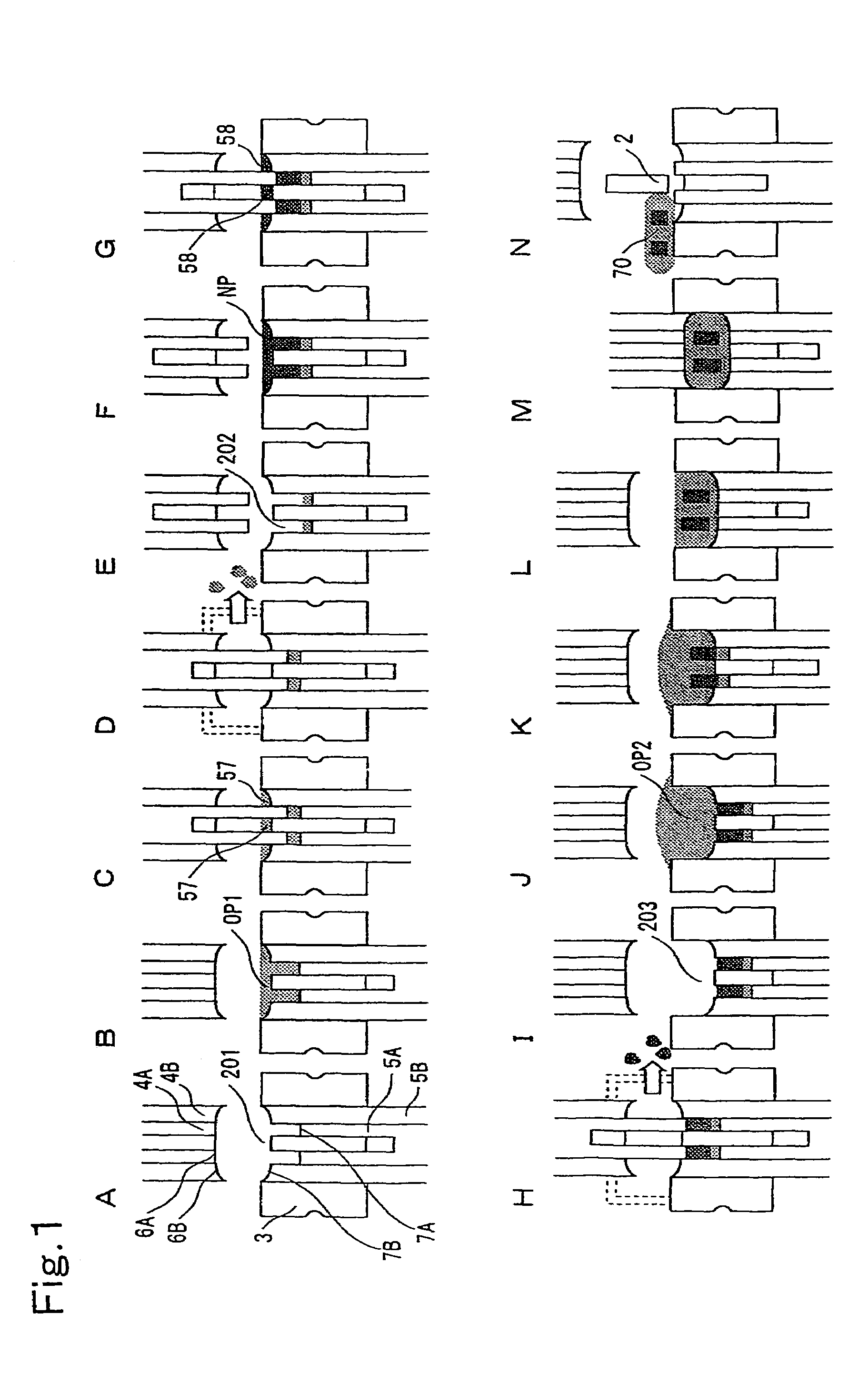
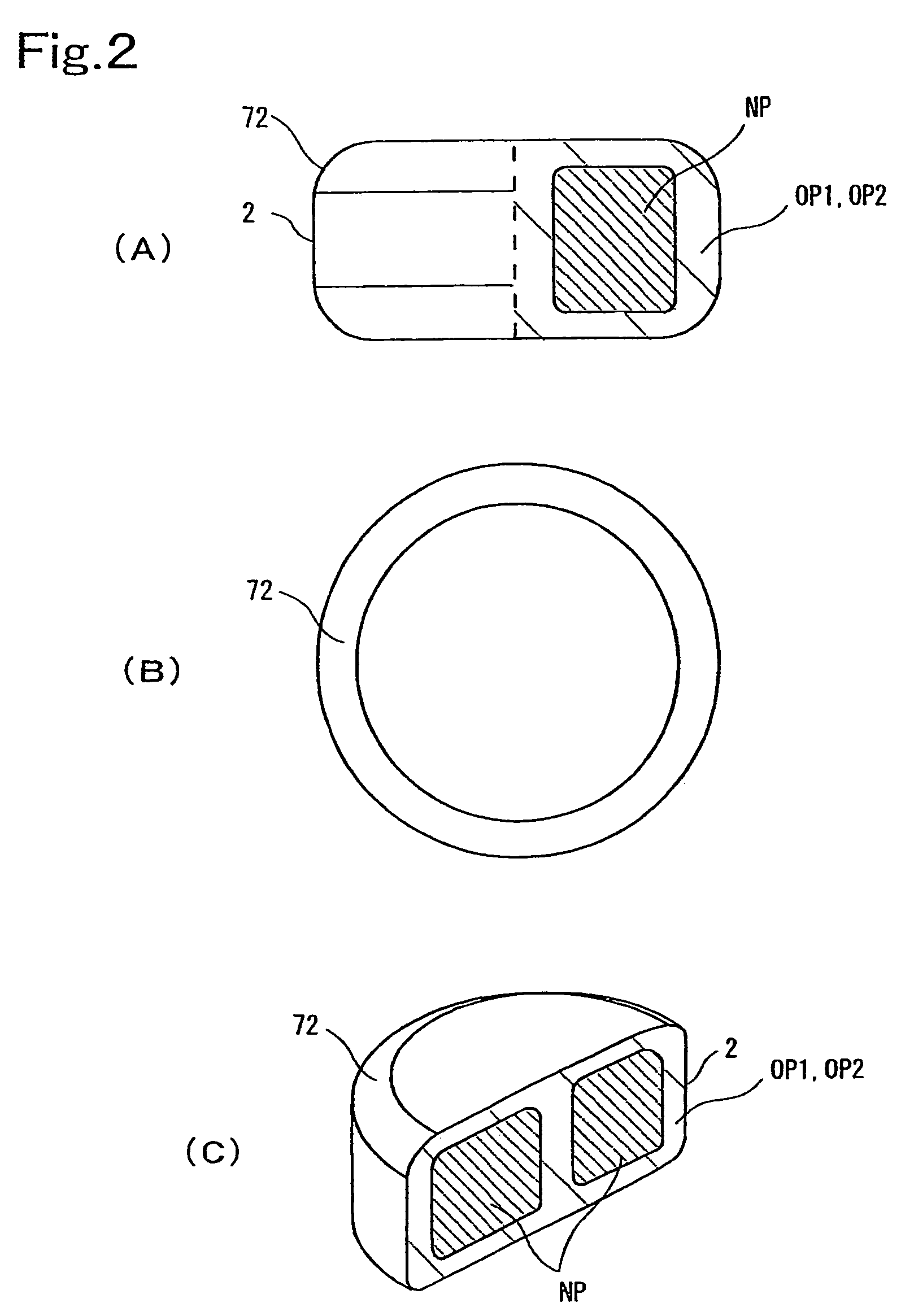
InactiveUS7132072B2Reduce probabilityImprove aestheticsConfectionerySweetmeatsCompression moldingShell molding
To manufacture a multi-core press-coated molded product efficiently and in a single step from molding materials, namely, powder or granular particles, a punch has been devised that is characterized in that the punch, consists of a center punch whose tip portion is split into two or more parts, and an outer punch enclosing the outer perimeter of the center punch, and whose tip portion fills the gap at the tip portion of the center punch, with both the center and outer punches being slidable and manipulatable for compression operation the method of manufacturing a multi-core press-coated molded product using the punch of the invention as compression molding means for at least the upper punch and preferably for both the upper and lower punches, and a rotary compression molding machine therefore is also discribed. This has led to the successful manufacture of a multi-core press-coated molded product in which a plurality of cores are arranged horizontally relative to the pressure applied surface of the molded product and further located at specific positions. When provided with a score line, the multi-core press-coated molded product can be made into a dividable molded product.
Owner:SANWAKAGUKU KENKYUSHO CO LTD
Roxhthromycin soft capsule and its preparing method
InactiveCN1709273AImprove bitternessMask bitternessOrganic active ingredientsRoxithromycinSolubility
The present invention relates to a roxithromycin soft capsule and its preparation method. In the invented roxithromycin soft capsule its liquid medicine contains roxithromycin with effective dose and medicinal auxiliary material. Said medicinal auxiliary material includes dilutent, antioxidant, solubilizing agent, solubility promoter and pH regulator. In the liquid medicine of every standard capsule 0.01-0.25g of roxithromycin is contained. Besides, said invention also provides the concrete steps of its preparation process.
Owner:秦引林
Common perilla and brown sugar stuffing sweet dumplings and production method thereof
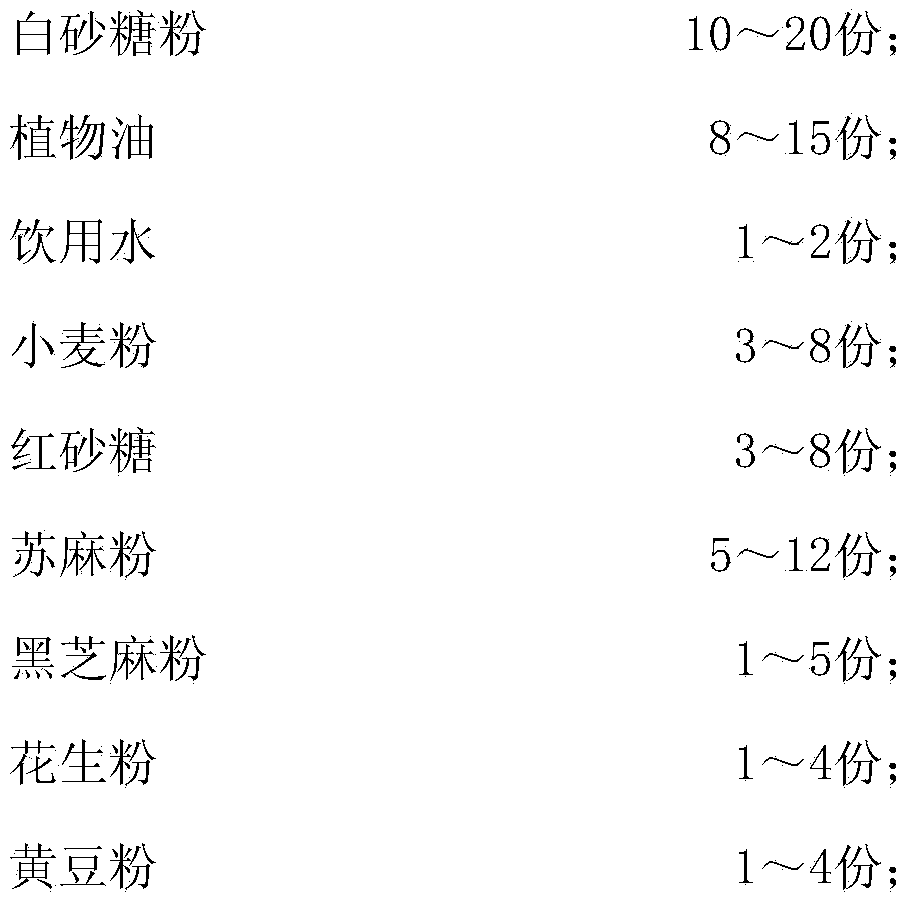
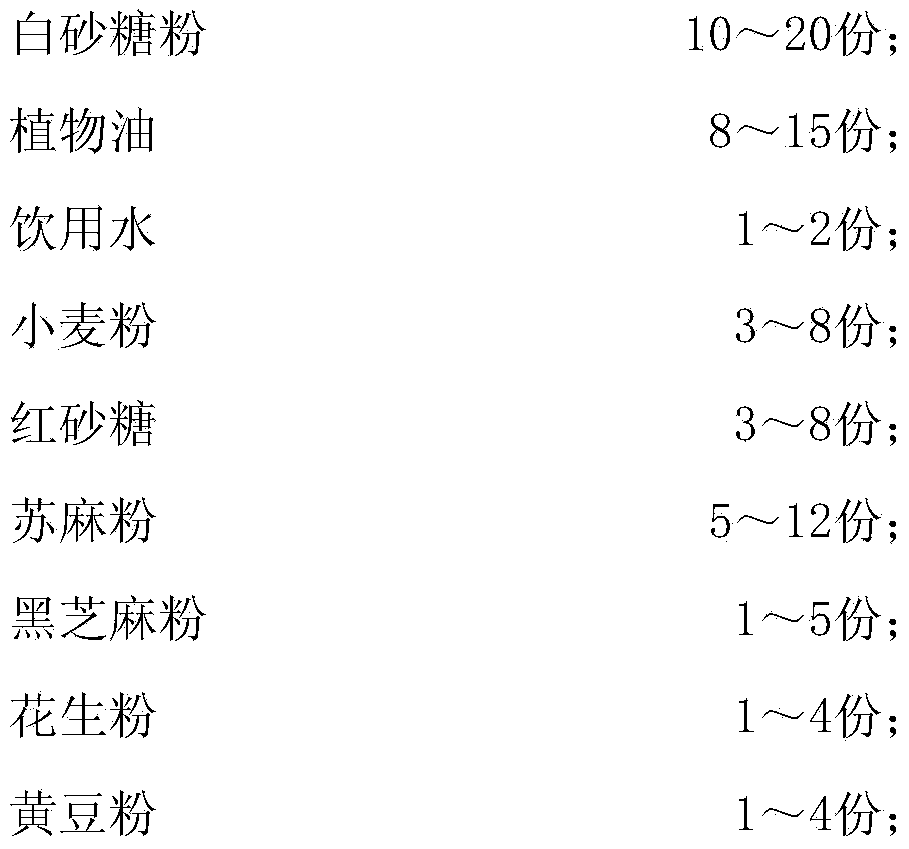
ActiveCN104207019AInhibition of reproductionGreat tasteFood coatingFood shapingMicroorganismTriticum turgidum
The invention discloses common perilla and brown sugar stuffing sweet dumplings and a production method thereof. The common perilla and brown sugar stuffing sweet dumplings consist of sweet dumpling stuffing and sweet dumpling wrappers, wherein the sweet dumpling stuffing comprises the components of white granulated sugar powder, plant oil, drinking water, wheat flour, brown sugar, common perilla powder, black sesame powder, peanut powder and soybean meal; and the sweet dumpling wrappers comprise the components of water-containing water-milled glutinous rice flour, cooked sticky rice roll, drinking water and konjaku flour. The production method of the common perilla and brown sugar stuffing sweet dumplings comprises the following steps: making the aqueous water-milled glutinous rice flour; preparing a sweet dumpling wrapper dough; preparing the stuffing; and making the sweet dumplings. If the prepared sweet dumplings are frozen quickly and preserved in a freezing manner, the preservation time of the sweet dumplings can be prolonged. Compared with the prior art, the common perilla and brown sugar stuffing sweet dumplings are rich and balanced in nutrition, have the effects of improving health, are good in color and mouthfeel, and are fine and smooth in taste. Moreover, the production method of the common perilla and brown sugar stuffing sweet dumplings is convenient and is easy in operation. The produced sweet dumplings are safer and more sanitary by means of inhibition of the breeding of microorganisms.
Owner:SICHUAN LONGWANG FOOD
Oral preparation containing amisulpride
ActiveCN102600132AMask bitternessImprove medication complianceNervous disorderMacromolecular non-active ingredientsDrugWater soluble
Owner:QILU PHARMA
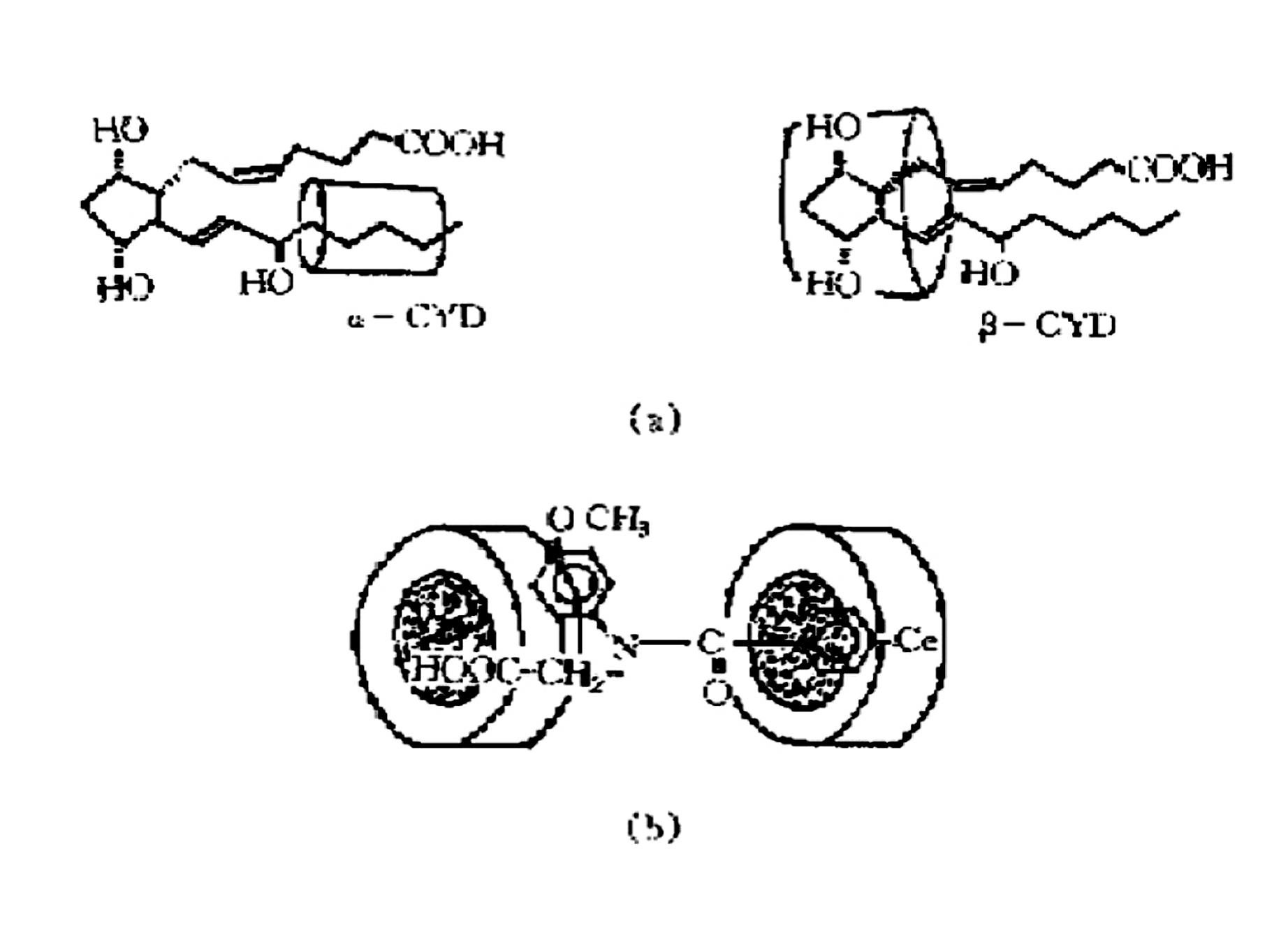
Compound medicine contg. famotidine cyclodextrin clathrate, and its prepn. method
InactiveCN1868473AMask bitternessImprove medication complianceOrganic active ingredientsDigestive systemMedicineFamotidine
A compound medicine taken orally is prepared from famotidine, cyclodextrin and antiacid through including the famotidine particles by cyclodextrin, and mixing the inclusion compound with antiacid.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Health characteristic non-standardized or standardized high cocoa dark chocolate with improved taste texture, melt, creaminess and reduced bitterness
InactiveUS20100278984A1Great tasteReduce bitternessMilk preservationDough treatmentMilk ChocolateAntioxidant
The present invention provides for a non-standardized dark chocolate composition to have up to 75% less saturated fat content than regular dark chocolate and have an improved healthier fat structure. A non-standardized and standardized dark chocolate include an antioxidant blend of; Virgin Coconut Oil, Vanilla Powder, Blackberry Powder, and Acai Berry Extract that creates a synergistic affect making the compositions have the rich and creamy taste, texture, melt and creaminess similar to milk chocolate. The final compositions have improved health characteristics, antioxidant content, and significantly less bitterness. Additionally, the method for preparing such non-standardized and standardized dark chocolate compositions is covered.
Owner:ANTIOXIDANT SUPERFOODS
Low-sodium and low-phosphate meat product
ActiveCN104223176AImprove flavorImprove yieldFood ingredient as taste affecting agentInorganic compound food ingredientsChemistryFlavor
The invention relates to a low-sodium and low-phosphate meat product which comprises a main material and auxiliary materials, wherein the main material is fresh minced meat and fresh meat loaves, and the auxiliary materials comprise lysine, arginine, potassium chloride, magnesium chloride, sodium chloride, water, monosodium glutamate, ginger powder, white granulated sugar, white pepper powder and sodium nitrite. The raw materials in the formula are used for making sausage, stewed meat or fried products respectively; sodium salt is partially replaced with sylvite and magnesium salt, so that the addition quantity of sodium chloride is reduced to lower than 1.75%, addition of sodium salt and phosphate is lower than corresponding national standard; astringency and bitter caused by addition of sylvite and magnesium salt can be hidden effectively through collaborative application of lysine and arginine, and the flavor and water conservation of products can be improved; and indexes including the color and luster, flavor, tissue state, taste and the like of the meat product are close to or better than like products in the market.
Owner:HEFEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com