Cefuroxime axetil composition and preparation method thereof
A technology of cefuroxime axetil and its composition, which is applied in the field of pharmaceutical preparations, can solve problems such as unfavorable clinical use, bitter taste, and poor drug compliance, and achieve the effects of improving taste and drug compliance, simple preparation method, and reducing bitterness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
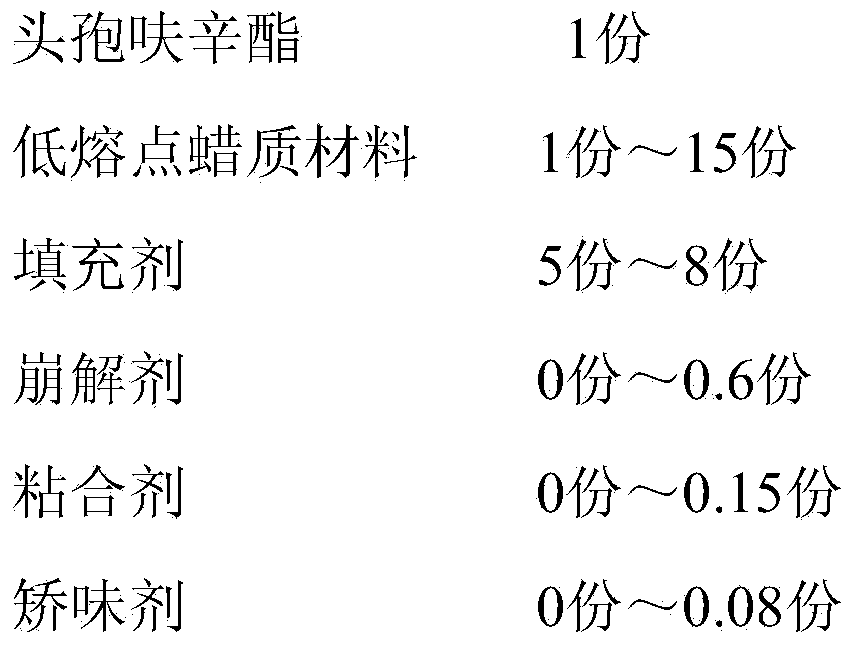
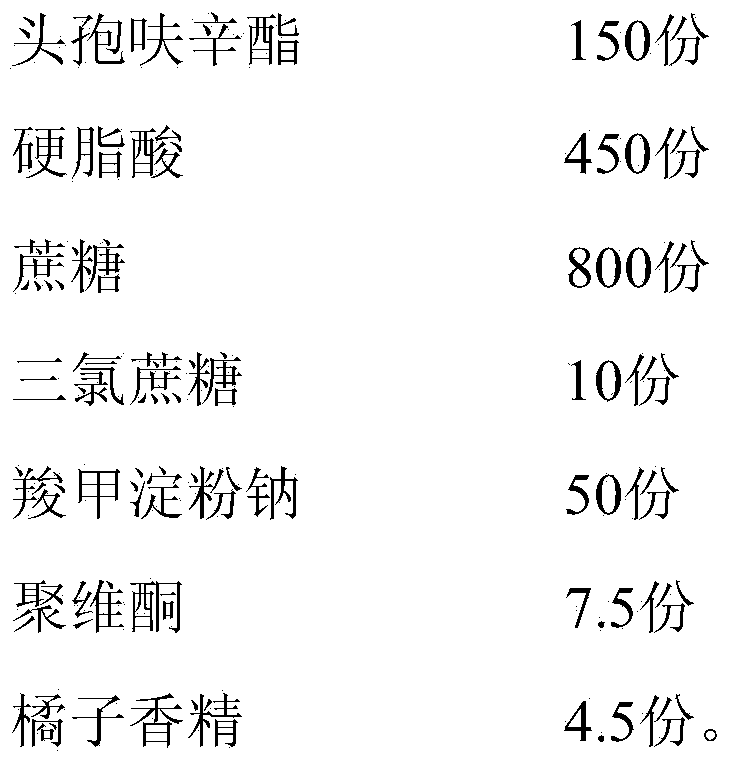
[0049] A cefuroxime axetil granule, by weight, comprises the following components:
[0050] Table 1. Embodiment a group of distribution ratio
[0051] components
Weight (parts)
cefuroxime axetil
150
450
800
10
50
Povidone
7.5
4.5
[0052] A preparation method of cefuroxime axetil granules, comprising the following steps (the amount of each component is as described in Table 1):
[0053] 1) Heat and melt stearic acid;
[0054] 2) Evenly disperse the cefuroxime axetil after sieving and dispersion in the molten stearic acid, stir and disperse evenly to make a suspension;
[0055] 3) Atomize and spray the suspension into the condensation chamber through compressed air, control the temperature of the condensation chamber below 10°C, wrap cefuroxime axetil in the condensation chamber, and solidify to form an incl...
Embodiment 2
[0060] A cefuroxime axetil granule, by weight, comprises the following components:
[0061] Table 2. Embodiment two groups distribution ratio
[0062] components
Weight (parts)
cefuroxime axetil
150
stearic acid
150
1000
15
45
Povidone
10
8
[0063] A preparation method of cefuroxime axetil granules, comprising the following steps (the consumption of each component is as described in Table 2):
[0064] 1) Heat and melt stearic acid;
[0065] 2) Uniformly disperse cefuroxime axetil in molten stearic acid to form a suspension;
[0066] 3) Add sorbitol, aspartame, carboxymethyl starch sodium and povidone when the temperature is gradually lowered to the beginning of solidification, control the stirring speed to 2rps~6rps, the shear speed to 2rps~30rps, and then shear and granulate;
[0067] 4) After the granules...
Embodiment 3
[0069] A cefuroxime axetil granule, by weight, comprises the following components:
[0070] Table 3. Embodiment three groups distribution ratio
[0071]
[0072]
[0073] A preparation method of cefuroxime axetil granules, comprising the following steps (the amount of each component is as described in Table 3):
[0074] 1) Mix stearic acid, cefuroxime axetil, mannitol, acesulfame potassium, carboxymethyl starch sodium and povidone evenly with stirring and shearing;
[0075] 2) Turn on the heating, stir while heating, control the heating temperature to 70°C, adjust the stirring speed to 2rps-6rps, the shearing speed to 2rps-30rps, and the shearing granulation time to 3min-15min;
[0076] 3) After the granules are cooled to room temperature, they are sieved through 14 and 80 mesh sieves. The sieved granules between 14 mesh and 80 mesh are added with orange essence and mixed evenly to obtain cefuroxime axetil granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com