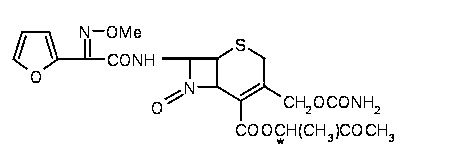
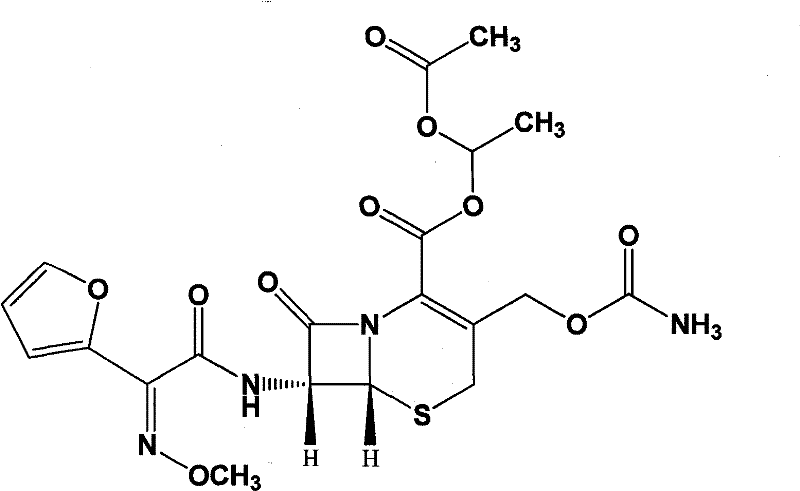
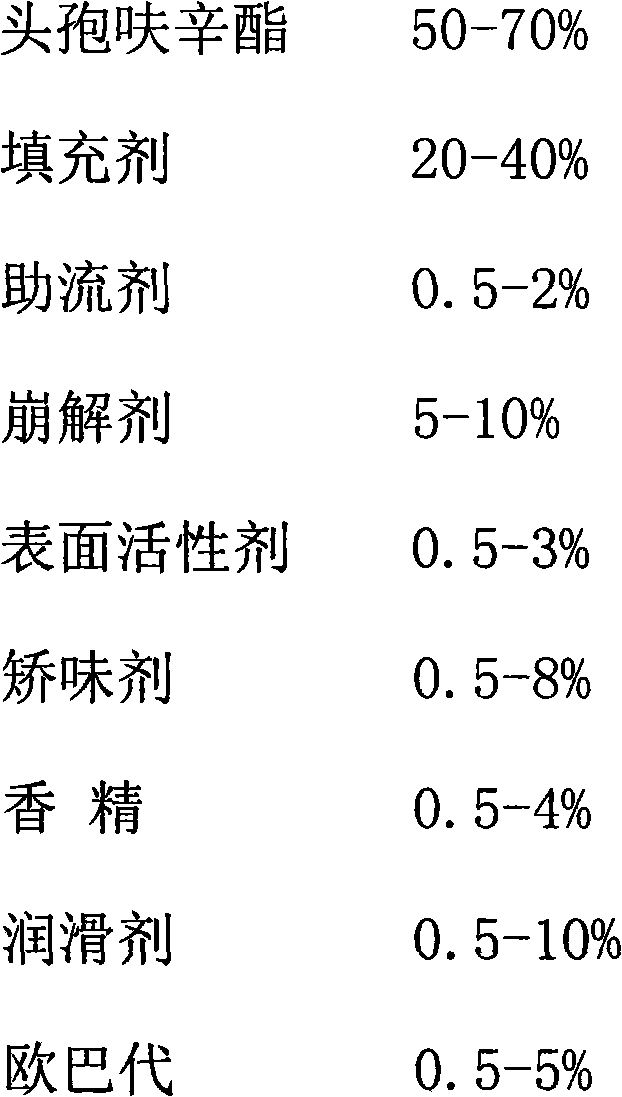
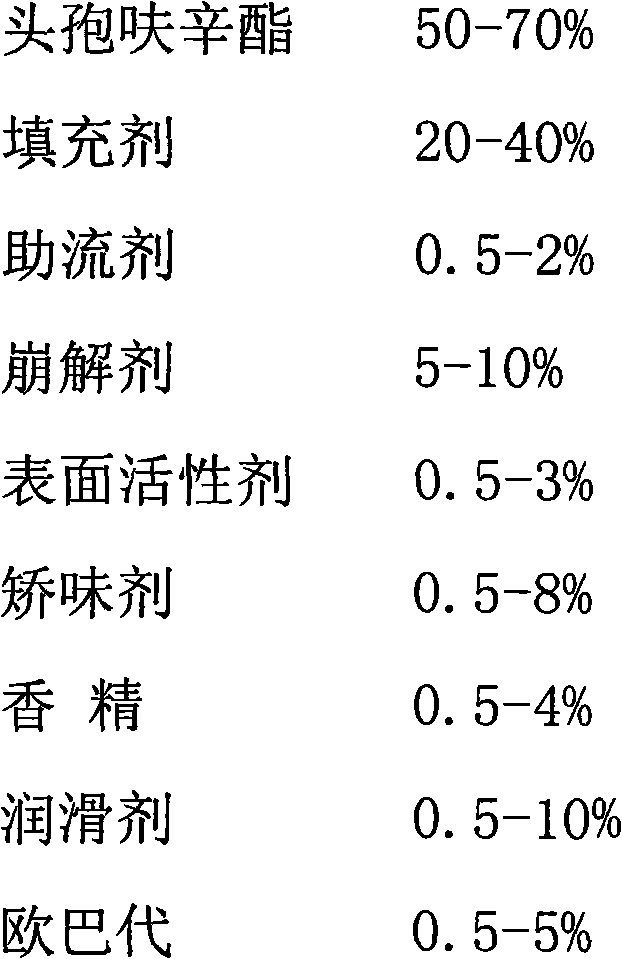
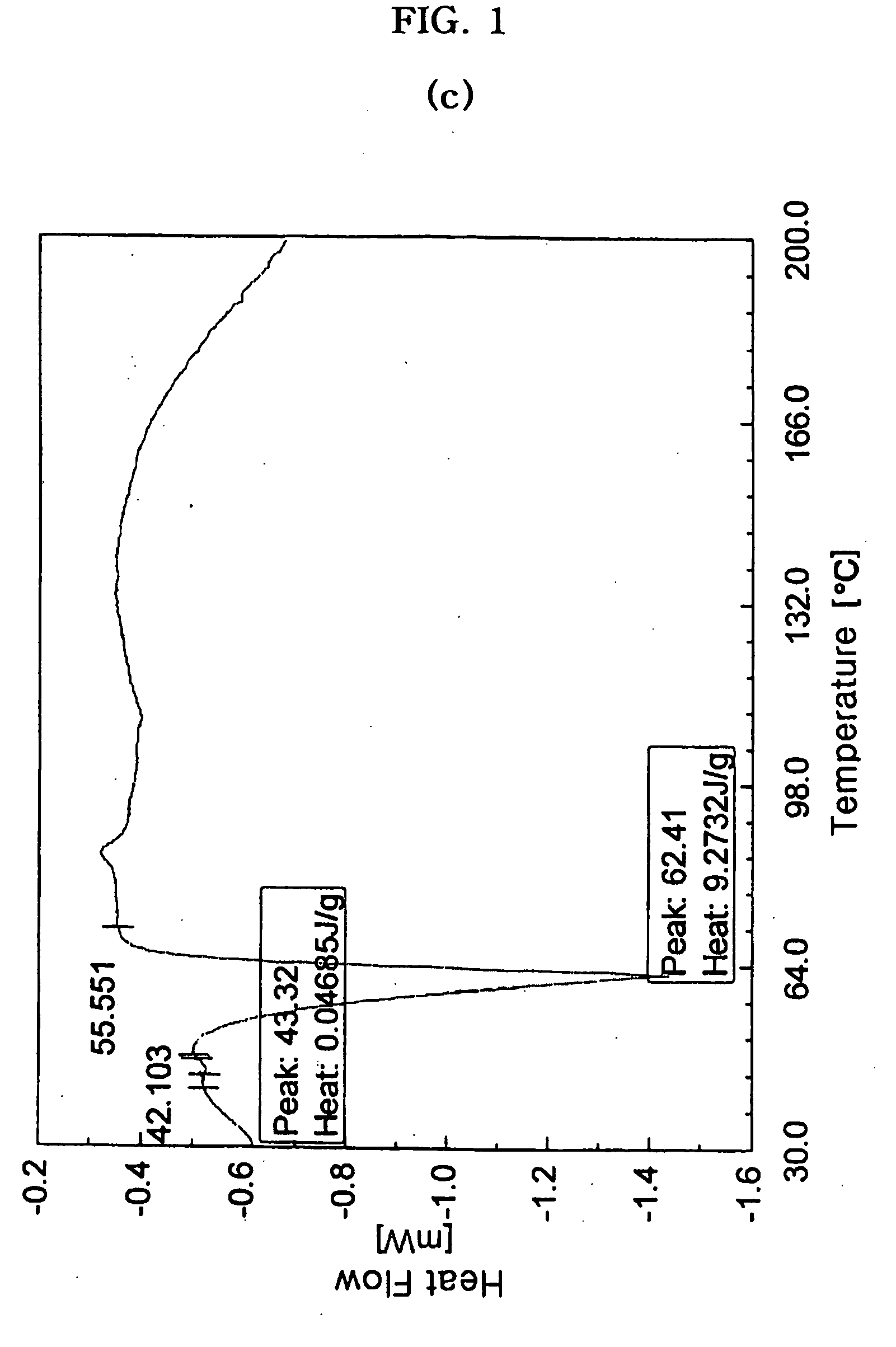
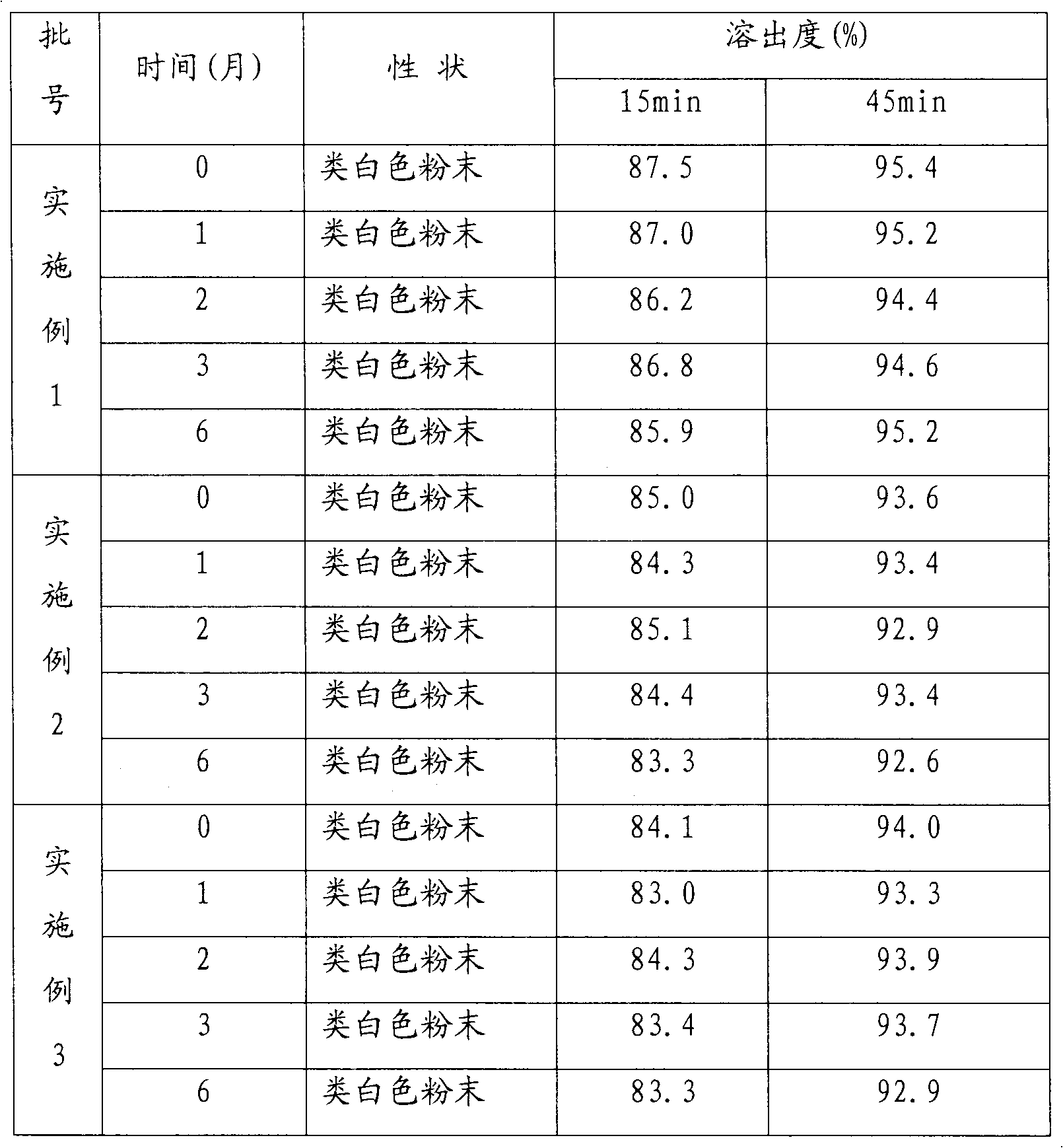
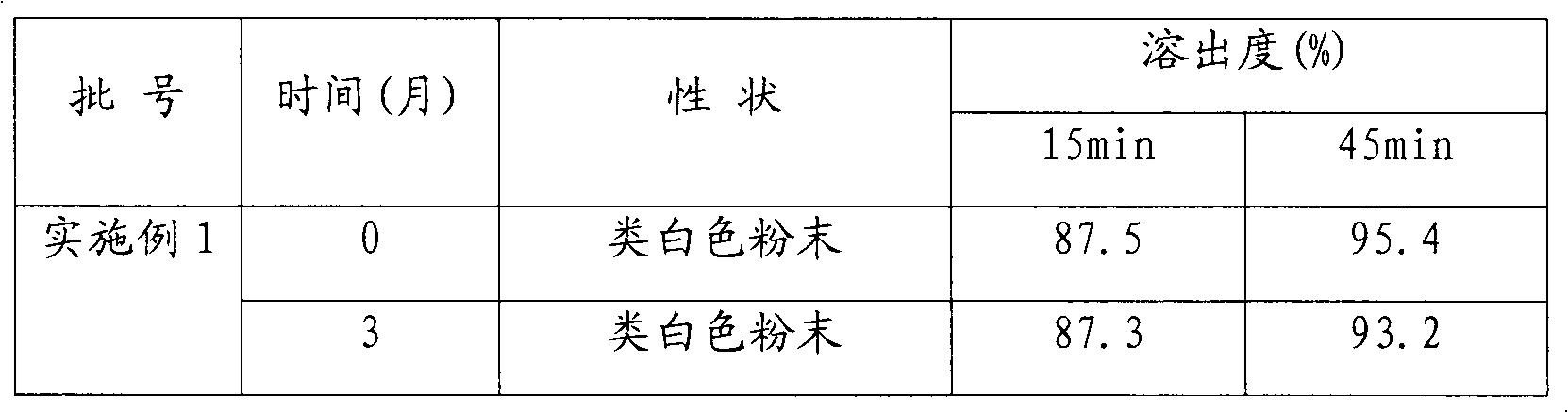
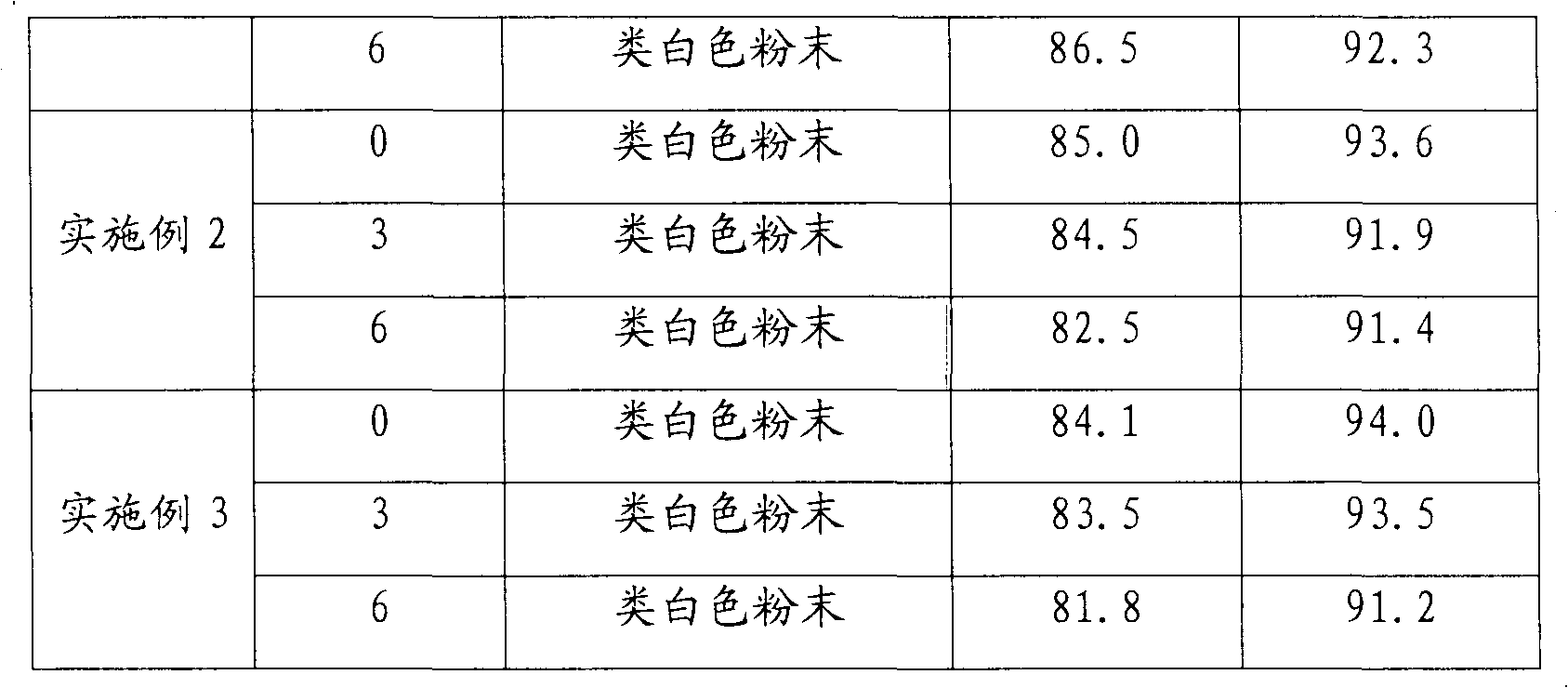
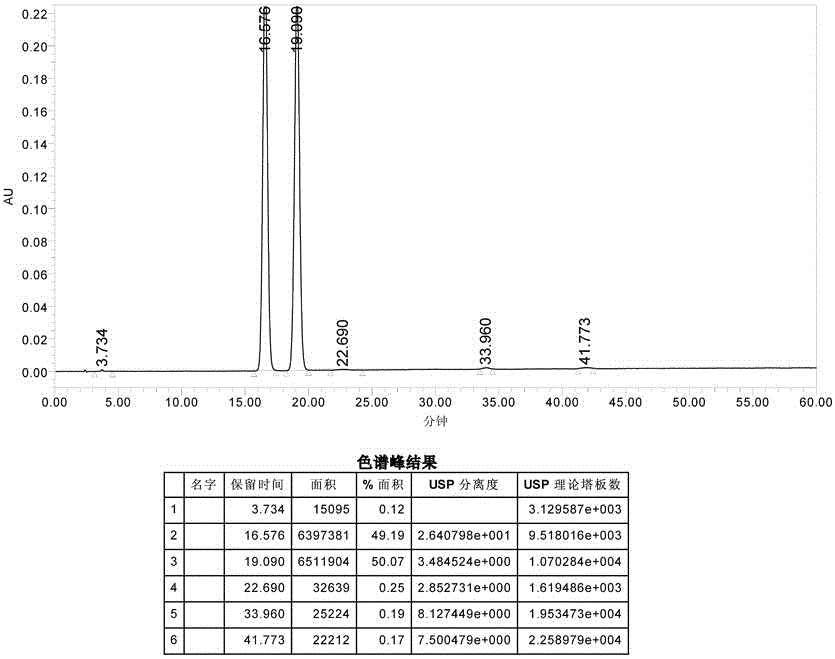
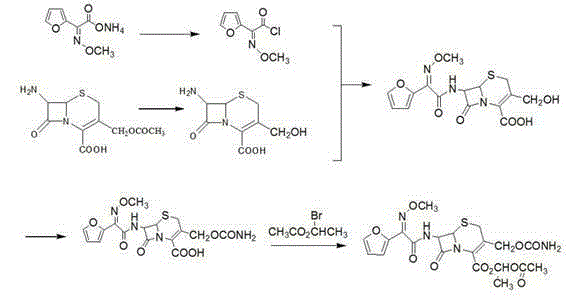
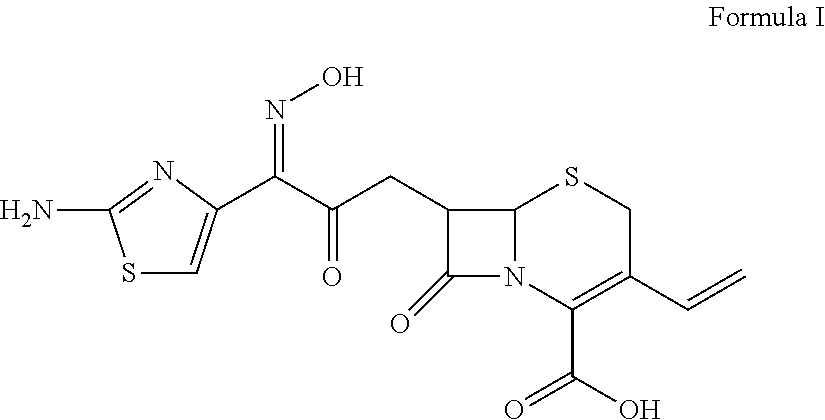
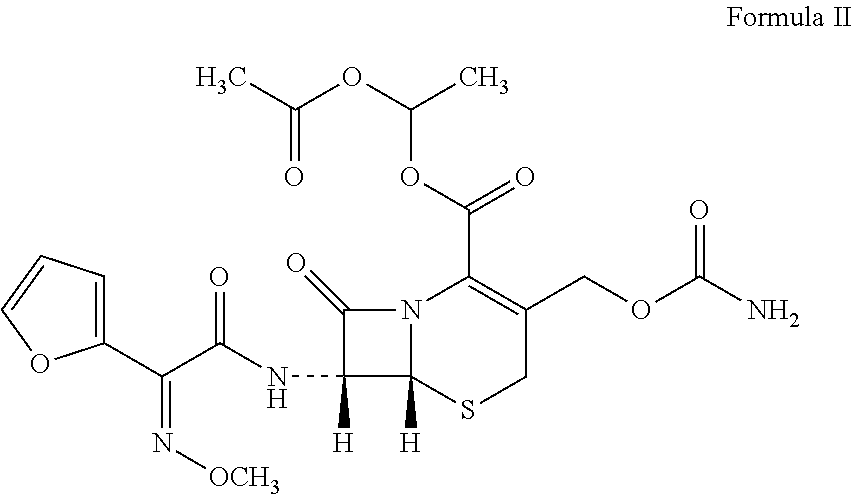
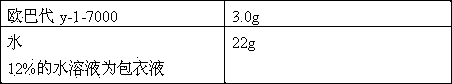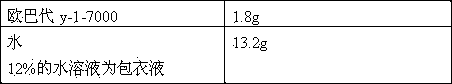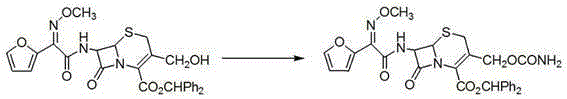Patents
Literature
92 results about "Cefuroxime axetil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cefuroxime is used to treat a wide variety of bacterial infections.
Pharmaceutical Composition
InactiveUS20030161888A1Great tasteImprove mouth "feelAntibacterial agentsPowder deliveryLipid formationOral medication
A composition comprising cefuroxime axetil in particulate form, the particles being coated with integral coatings of a lipid or mixture of lipids which are insoluble in water in which the composition further comprises a sweetener system and a texture modifier which serves to mask the bitter taste of cefuroxime axetil upon oral administration is disclosed.
Owner:FERNANDEZ MATILDE IBANEZ +1
Preparation method of high-purity cefuroxime acid
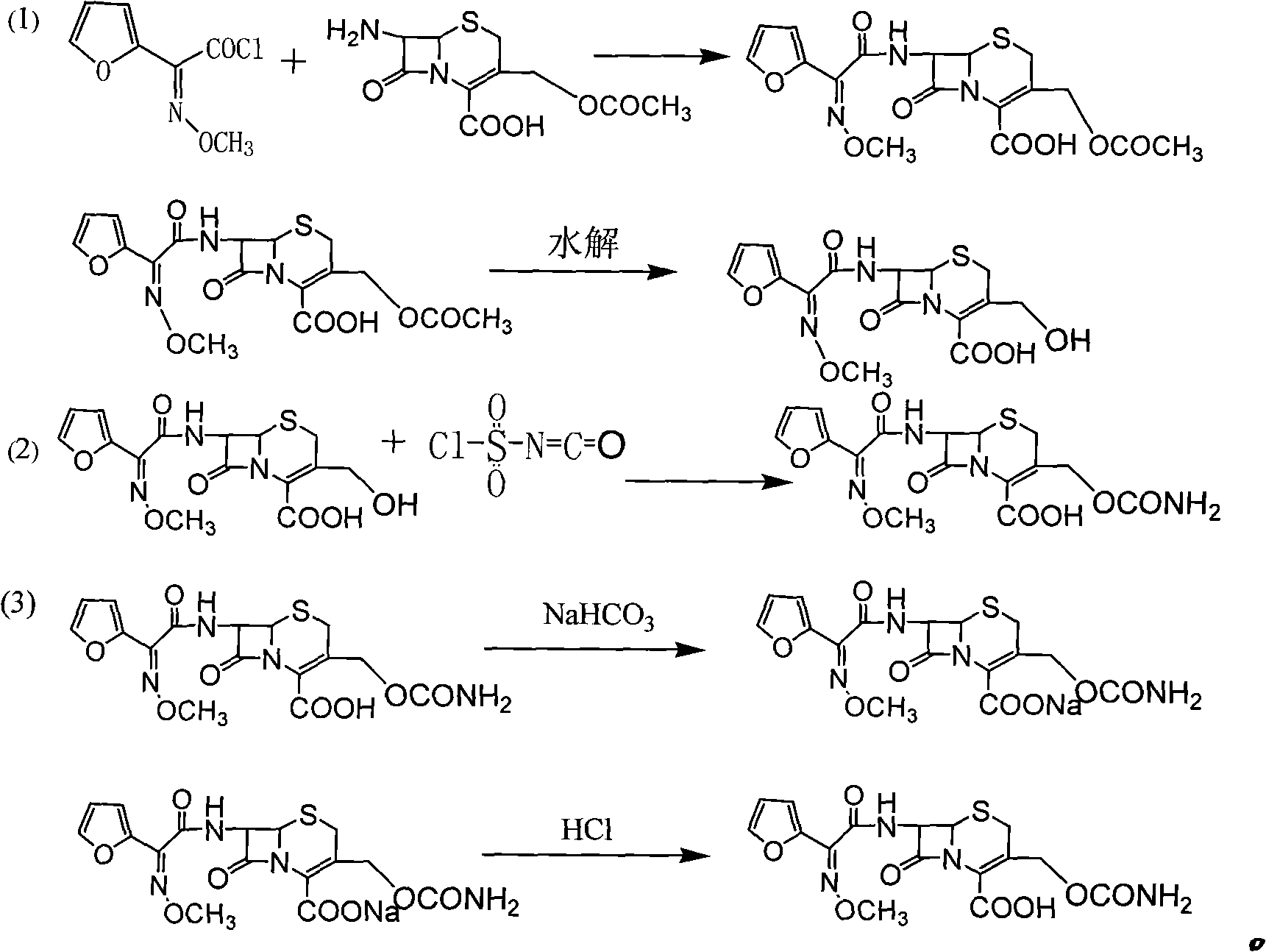
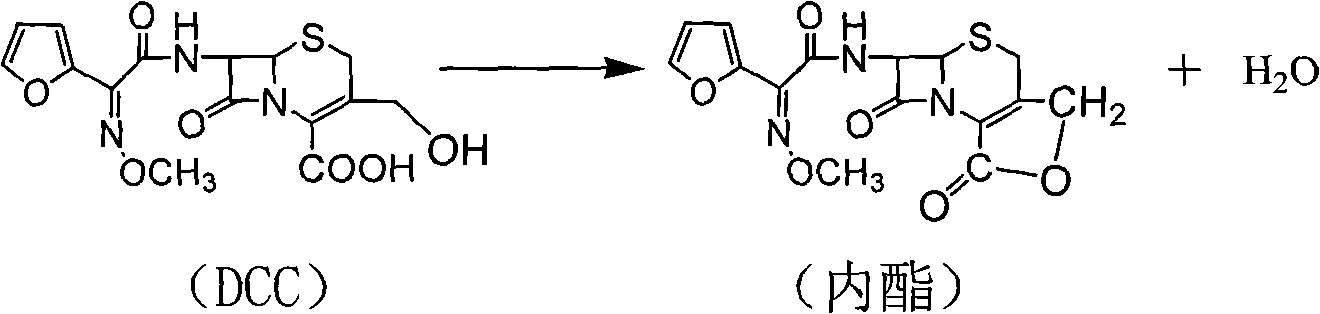
The invention discloses a preparation method of high-purity cefuroxime acid which is an intermediate for synthesizing second-generation cephalosporins cefuroxime sodium and cefuroxime axetil. The preparation method comprises the following steps: based on 7-aminocephalosporanic acid (7-ACA) as a raw material, carrying out an N-acylation reaction on the 7-ACA and furoyl acetylcholine at the 7-position; at a low temperature, hydrolyzing 3-acetyl with a sodium hydroxide solution, crystallizing, filtering and drying so as to obtain the intermediate 3-deformamido cefuroxime acid (DCC); quantitatively adding the DCC in a tetrahydrofuran solvent, dropwise adding chlorosulfonyl isocyanate for a nucleophilic addition reaction so as to generate chlorosulfonyl cefuroxime acid, and adding purified water for hydrolysis so as to prepare a cefuroxime acid reaction liquid; adding sodium bicarbonate for salifying; removing by-reactant lactone and other unsaponifiable impurities in the reaction liquid with a ternary compound extracting agent of dichloromethane, ethyl acetate and tetrahydrofuran, layering, and adding hydrochloric acid in a water phase for acidification; adding the ternary compound extracting agent to extract and separate out the cefuroxime acid; and removing water-soluble impurities, crystallizing and filtering a distilled organic phase, and then drying so as to obtain the high-purity cefuroxime acid with the purity of more than or equal to 99%.
Owner:四平市精细化学品有限公司
Method for determining 10 kinds of antibiotics in water environment through combination of sample pre-treatment technology and HPLC-MS
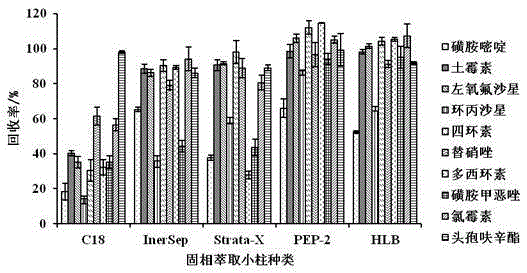
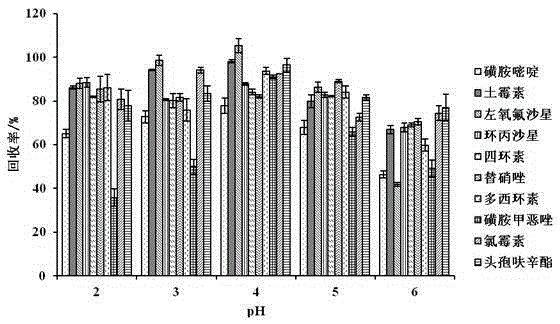
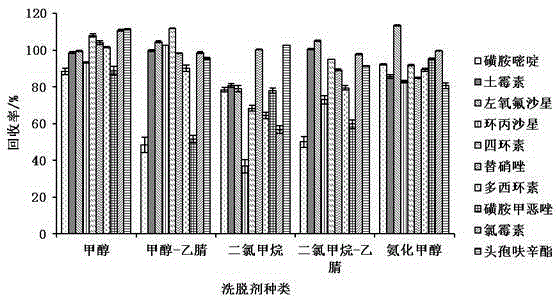
The present invention relates to a method for determining 10 kinds of antibiotics in a water environment through combination of a sample pre-treatment technology and HPLC-MS, and belongs to the field of detection of safety of trace organic contaminant residue in the water environment. The method is characterized in that a water sample is separated and enriched through combination of solid phase extraction and dispersive liquid-liquid microextraction (SPE-DLLME), and then an ultra-high performance liquid chromatography-mass spectrometry instrument (UPLC-MS / MS) is adopted as a detection tool to directly determine the contents of 10 kinds of common antibiotics in the water environment (drinking water, tap water, river water, sewage treatment plant influent and effluent), wherein the 10 kinds of the common antibiotics respectively are sulfadiazine, sulfamethoxazole, oxytetracycline, tetracycline, doxycycline, ciprofloxacin, levofloxacin, chloramphenicol, cefuroxime axetil and tinidazole. According to the present invention, the water sample pre-treatment method and the instrument detection conditions are investigated and optimized, and the optimal SPE-DLLME-UPLC-MS / MS method is established and is successfully applied for the real sample determination; and compared with the traditional method, the method of the present invention has advantages of high sensitivity, high extraction recovery rate, wide application objects, environmental protection, and the like.
Owner:SHENYANG PHARMA UNIVERSITY
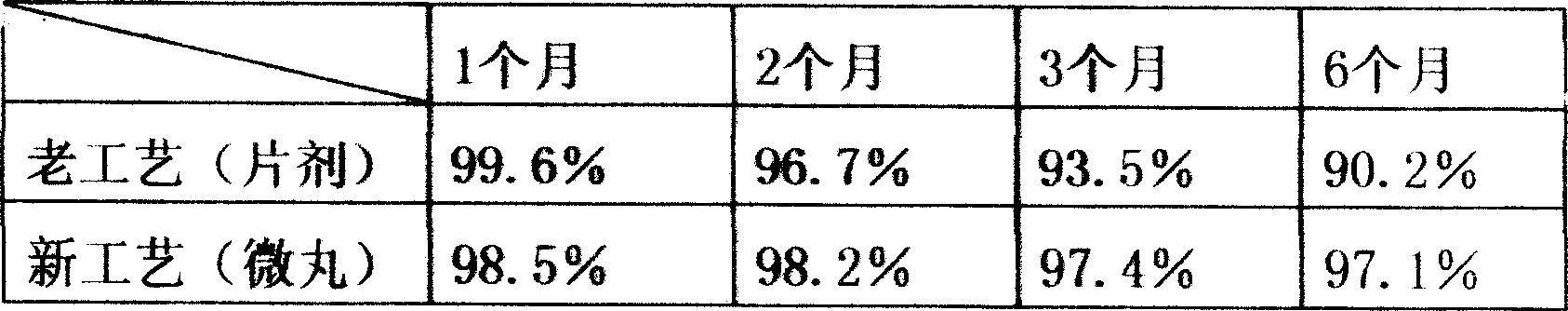
Cefuroxime axetil taste masking pellet and method of preparing the same
The invention relates to a cefuroxime axetil taste masking pellet and the preparation method. The invention contains cefuroxime axetil pill and a taste masking coating layer wrapping the pill. The invention overcomes the shortages of bitter taste with traditional cefuroxime axetil tablet, provides a preparation method of cefuroxime axetil taste masking pellet which masks bitterness, releases drug rapidly and has stable quality.
Owner:莫敬柱
Cefuroxime axetil odor-masking pellet and preparation method
InactiveCN101590021ADisintegrates quicklyImprove solubilityAntibacterial agentsOrganic active ingredientsMedicineBitter taste
The invention relates to a cefuroxime axetil odor-masking pellet and a preparation method thereof. The cefuroxime axetil odor-masking pellet consists of an uncoated pellet containing the cefuroxime axetil and an odor-masking layer coating outside the uncoated pellet. The cefuroxime axetil odor-masking pellet overcomes the defect of bitter taste of the prior cefuroxime axetil tablets, and provides a novel cefuroxime axetil preparation which masks bitter taste, releases medicament rapidly and has stable quality.
Owner:湖南中南科伦药业有限公司 +1
Cefuroxime axetil composition and preparation method thereof
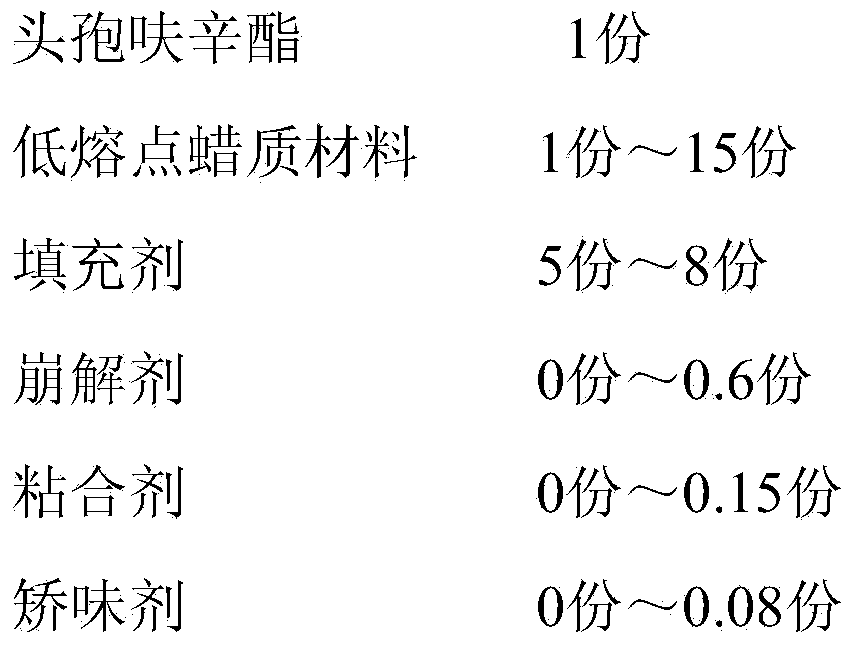
The invention discloses a cefuroxime axetil composition which comprises the following components in parts by weight: 1 part of cefuroxime axetil, 1-15 parts of a low-melting-point wax material, 5-8 parts of filler, 0-0.6 part of a disintegrating agent, 0-0.15 part of a binder and 0-0.08 part of a corrigent. The invention further discloses a preparation method of the cefuroxime axetil composition. The cefuroxime axetil composition obtained can better cover bitter taste of the medicine and is good in dissolution rate, so that the medication compliance of patients is further improved.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Direct compression process for cefuroxime axetil dispersible tablets
InactiveCN101703448AHigh dissolution rateImprove bioavailabilityAntibacterial agentsOrganic active ingredientsDissolutionStearic acid
The invention relates to a direct compression process for cefuroxime axetil dispersible tablets, which is characterized in that: the following raw materials and auxiliary materials are sieved through a 40 mesh sieve and are mixed for thirty minutes in a mixing tank, and then the mixed powder materials are directly compressed by a high speed rotary tablet press to form the cefuroxime axetil dispersible tablets; and based on 125 grams of cefuroxime axetil, the raw materials and the auxiliary materials for producing 1000 dispersible tablets comprise 25 grams of cefuroxime axetil, 4 to 10 grams of stearic acid, 210 to 250 grams of microcrystalline cellulose, 28 to 35 grams of croscarmellose sodium, 2 to 4 grams of silicon dioxide and 2 to 4 grams of sodium dodecyl sulfate. The direct compression process for the cefuroxime axetil dispersible tablets improves the dissolution rate of the cefuroxime axetil dispersible tablets and improves the bioavailability and the healing effect of the medicament, wherein the dissolution rate reaches 99.5 percent at the time when the tablets dissolve for 45 minutes. The direct compression process simplifies the production process, shortens the production period and saves energy.
Owner:山东淄博新达制药有限公司
Cephalofruxin ester liposome, its preparation and medicinal composition containing it
ActiveCN1939305APromote dissolutionThe drug works quicklyAntibacterial agentsPharmaceutical non-active ingredientsMedicineCholesterol
A liposome of cefuroxime axetil used to prepare the tablet with high target performance to the gastrointestinal mucosa cells is prepared from cefuroxime axetil, soybean lecithin and chlosterol in weight ration of 1:2:2. Its preparing process is also disclosed.
Owner:CSPC OUYI PHARM CO LTD
Rapidly disintegrating sustained release cefuroxime axetil composition
InactiveUS6932981B2Increased blood levelsGood curative effectPharmaceutical non-active ingredientsMicrocapsulesMethacrylateControlled release
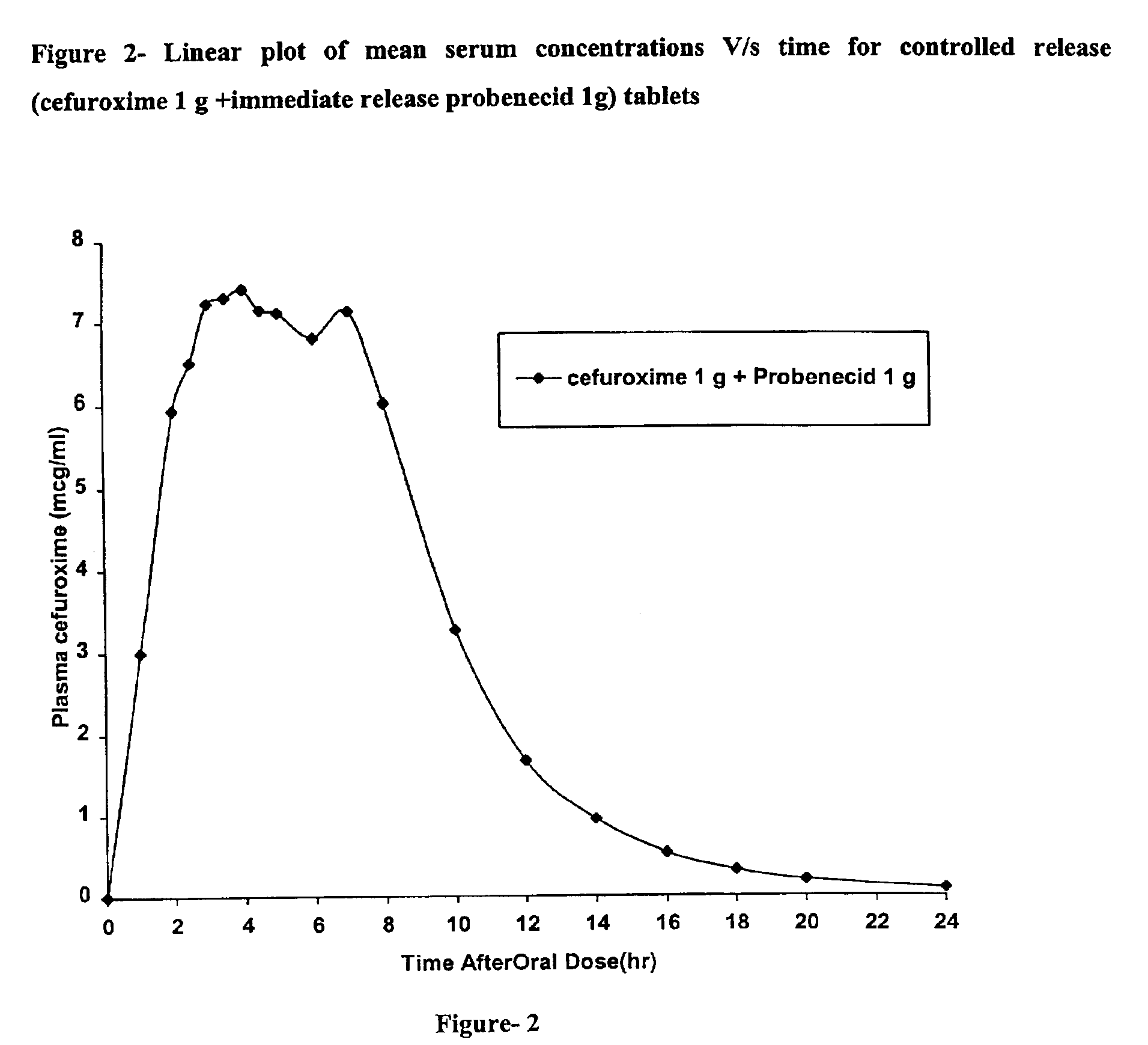
A fast disintegrating controlled release oral composition comprising a core material containing cefuroxime axetil present as controlled release form, the cefuroxime axetil being provided with an outer coating of a copolymer selected from aqueous dispersions of enteric methacrylic acid and methacrylic acid esters anionic copolymers having carboxyl group as the functional group or mixtures thereof and an inner coating of a sustained-release copolymer selected from aqueous dispersions of acrylate and methacrylate pH independent copolymers having quaternary ammonium group as a functional group or mixtures thereof, and optionally probenecid. Additionally, the coating composition may contain plasticizers. The composition is suitable for once daily administration.
Owner:LUPIN LABORATORIES LTD
Preparation method of cefuroxime axetil
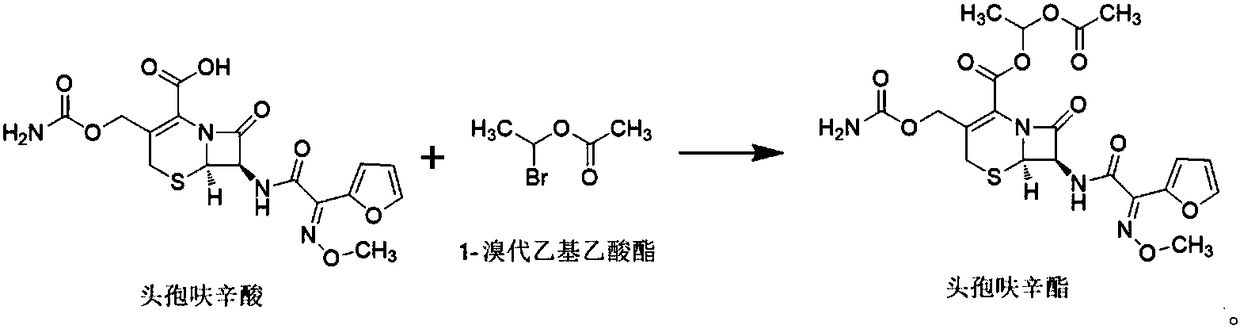
The invention discloses a preparation method of cefuroxime axetil. The method comprises the following steps: completely dissolving cefuroxime acid in dimethylformamide, and carrying out esterification reaction with 1-bromethylacetate under the catalytic action of cupric chloride; and hydrolyzing with ethyl acetate and sodium chloride solution, extracting, carrying out vacuum distillation, crystallizing with cyclohexane, carrying out vacuum filtration, and drying to obtain high-purity cefuroxime axetil. The cupric chloride, which has the advantages of no toxicity, no harm and high catalytic efficiency, is preferably used as the catalyst; the cyclohexane for crystallization is easy to recover and reutilize, thereby lowering the production cost; and meanwhile, the method has the advantages of mild and controllable reaction conditions, short production cycle and lower energy consumption, and is suitable for industrial production.
Owner:GUANGDONG LIGUO PHARMACY
Pharmaceutical composition of cefuroxime axetil for suspension and preparation method thereof
InactiveCN102440960AImprove bitternessEasy to acceptAntibacterial agentsOrganic active ingredientsUse medicationSucrose
The invention relates to a pharmaceutical composition of cefuroxime axetil for suspension for pharmaceutical purposes and a preparation method thereof. The pharmaceutical composition of the cefuroxime axetil for suspension disclosed by the invention comprises 100 parts by weight of cefuroxime axetil (calculating according to the weight of cefuroxime), 50-250 parts by weight of cane sugar and 400-650 parts by weight of stearic acid. The invention further provides the method for preparing the composition. According to the pharmaceutical composition for suspension disclosed by the invention, the bitter taste of the cefuroxime axetil is effectively covered up; and the pharmaceutical composition is applied to adults, more importantly, the problem for difficultly taking medicines of children, old people and patients suffering from dysphagia is solved, and the compliance of medicine taking patients is greatly increased.
Owner:SHANDONG LUKANG PHARMA +1
Cefuroxime axetil dispersible tablet
InactiveCN103845298ASimple manufacturing processProcess stabilityAntibacterial agentsOrganic active ingredientsBitter tasteDrug product
The invention provides a cefuroxime axetil dispersible tablet. The cefuroxime axetil dispersible tablet prepared by the invention can be released within a short time; the formula is reasonable; bitter taste of the cefuroxime axetil dispersible tablet is masked; the mouth feel of the cefuroxime axetil dispersible tablet is improved; the medicinal compliance is improved; furthermore, the preparation process is simple and feasible, strong in operability and steady in process; the bioavailability and the curative effect of the cefuroxime axetil dispersible tablet disclosed by the invention are increased.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH +1
Dispersible tablet of cefuroxime axetil
InactiveCN102697747AReduce dosageShorten dispersion timeAntibacterial agentsOrganic active ingredientsSurface-active agentsMedical prescription
The invention provides a dispersible tablet of cefuroxime axetil, wherein the preparation formula includes cefuroxime axetil, loading agents, flow aid, disintegrating agents, surface-active agents, flavoring agents, lubricant, essence, and coating powder. According to the invention, specific disintegrating agents are adopted, meanwhile, the use amount of the disintegrating agents is not increased, the dispersion time of the dispersible tablet is shortened, and the dissolution rate of the dispersible tablet is improved; and meanwhile, flavoring agents and essence are added in the formula to mask bitter of the dispersible tablet of cefuroxime axetil, so that the mouth feel of a patient taking the dispersible tablet of cefuroxime axetil can be improved.
Owner:GUANGZHOU NANXIN PHARMA
Cefuroxime axetil pharmaceutical composition and preparation method thereof
ActiveCN104586854AHigh dissolution rateGood dissolution effectAntibacterial agentsOrganic active ingredientsPharmaceutical industryFluidized bed
The invention provides a cefuroxime axetil pharmaceutical composition and a preparation method thereof. The method comprises the following steps: fully fluidizing cefuroxime axetil with specific particle size distribution in a fluidized bed; slowly spraying molten refractory materials on the surface of cefuroxime axetil through a spraying device, and fully enveloping the surface of the cefuroxime axetil; and adding other auxiliary materials, mixing evenly, and preparing a cefuroxime axetil dry suspension or granule. The preparation process can be finished in the fluidized bed; a hot melt pelletizer or a spray dryer is not used; and industrialized mass production of the modern pharmaceutical industry is facilitated.
Owner:SHIJIAZHUANG NO 4 PHARMA
Cefuroxime axetil hot-melt coating composition and preparation method of composition
ActiveCN103877027AMask bitternessImprove complianceAntibacterial agentsPowder deliveryPharmaceutical drugBitter tastes
The invention discloses a cefuroxime axetil hot-melt coating composition and a preparation method of the composition. The cefuroxime axetil hot-melt coating composition can effectively cover up the bitter taste of the medicine, so that the delivery compliance of the patient is improved. Meanwhile, the medicine of the cefuroxime axetil hot-melt coating composition has high dissolution rate in aqueous solvents of different pH values, and the bioavailability of the medicine is high. The composition disclosed by the invention can be further used for preparing a dry suspension or granules, thereby facilitating delivery of infants and children unable to swallow. The composition disclosed by the invention is simple in preparation method, free from any solvents, uniform in medicine content, short in heated time, less in energy consumption and easy to realize continuous production on a large scale.
Owner:GUANGDONG PHARMA UNIV
Cefuroxime axetil granule and process for the preparation thereof
InactiveCN1909889AImprove bioavailabilityImprove stabilityAntibacterial agentsOrganic active ingredientsOral medicationSucrose
A cefuroxime axetil granule composition, comprising non-crystalline cefuroxime axetil solid dispersion or substantially amorphous cefuroxime axetil, fatty acid sucrose ester, methacrylic acid-ethyl acrylate copolymer and a disintegrant, which is used in masking Cefuroxime axetil has desirable performance characteristics in terms of bitter taste, and has high bioavailability and stability of cefuroxime axetil, so it can be advantageously used for oral administration of cefuroxime axetil.
Owner:HANMI PHARMA
Cefuroxime oral antibacnterial composition
InactiveCN1742735AExcellent in vitro sterilizationImprove antibacterial propertiesAntibacterial agentsHeterocyclic compound active ingredientsCefuroxime.oralSulbactam Pivoxil
The present invention discloses an oral antimicrobial medicine composite composed of cefuroxime axetil and sulbactam pivoxil. The weight ratio range of cefuroxime axetil (by cefuroxime) and sulbactam pivoxil (by sulbactam) is 1:1 to 15:1, the optimum weight ratio range is 2:1.
Owner:夏中宁
Method and device for preparing superfine amorphousn cefuroxime axetil
InactiveCN101284839ASmall and precise structural featuresSmall heat and mass transferAntibacterial agentsOrganic chemistryMicroreactorDesolvation
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Cefuroxime axetil composition and preparation method thereof
ActiveCN103816123AMask bitternessImprove complianceAntibacterial agentsPowder deliveryPharmaceutical drugBitter tastes
The invention discloses a cefuroxime axetil composition and a preparation method thereof. The cefuroxime axetil composition can effectively cover bitter taste of a cefuroxime axetil medicine so as to improve the delivery compliance of the patient. Meanwhile, the medicine containing the cefuroxime axetil composition has higher dissolution rate and high bioavailability. The composition disclosed by the invention can be further used for preparing dry suspensions or granules which are more beneficial for infants, children and patients without the ability of swallowing to take. The composition disclosed by the invention is simple in preparation method, free from any solvents, uniform in medicine content, short in heating time, small in energy consumption and easy to realize continuous large-scale production.
Owner:GUANGDONG PHARMA UNIV
Amorphous cefuroxime axetil and preparation process therefore
InactiveUS20060020130A1Less spaceShorten the time of precipitationAntibacterial agentsPowder deliveryBioavailabilitySolvent
A novel process for the preparation of amorphous cefuroxime axetil particles and the amorphous cefuroxime axetil particles therefrom are disclosed in the invention. Specifically, the invention is implemented by means of antisolvent recrystallization to prepare the cefuroxime axetil in an amorphous form; particularly, the amorphous ultrafine or even nanosized cefuroxime axetil with a controllable particle size and a narrow particle size distribution. The cefuroxime axetil according to the invention can used to enhance bioavailability, since it is in an amorphous form and has a controllable particle size and a narrow particle size distribution.
Owner:BEIJING UNIV OF CHEM TECH +1
Cefuroxime axetil granule and process for the preparation thereof
InactiveUS20090175952A1Highly desirable performanceImprove bioavailabilityAntibacterial agentsOrganic active ingredientsOral medicationSucrose
A cefuroxime axetil granule composition comprising a non-crystalline cefuroxime axetil solid dispersion or a substantially amorphous cefuroxime axetil, sucrose fatty acid ester, methacrylic acid-ethylacrylate copolymer and a disintegrating agent has highly desirable performance characteristics in terms of masking the bitterness of cefuroxime axetil, as well as high bioavailability and stability of cefuroxime axetil, and thus, can be advantageously used for oral administration of cefuroxime axetil.
Owner:HANMI PHARMA
Cefuroxime axetil oral liquid and preparation method thereof
ActiveCN101890023AImprove bioavailabilityImprove onset timeAntibacterial agentsOrganic active ingredientsCross-linkCefuroxime.oral
The invention discloses a cefuroxime axetil oral liquid, comprising the following components in parts by weight: 280-350 parts of cefuroxime axetil, 50-100 parts of talcum powders, 10-50 parts of cross-linked polyvinylpyrrolidone and 3-4 parts of hydroxypropyl methyl cellulose. The invention also discloses a preparation method of the oral liquid. The cefuroxime axetil oral liquid of the inventionhas good stability; and in addition, the dissolution within 15 minutes is higher than 80% of that of labeled amount and the dissolution within 45 minutes is higher than 90% of that of the labeled amount.
Owner:HAINAN RIZHONGTIAN PHARMA
Cefuroxime axetil lipid microsphere solid preparation
InactiveCN102091044AHigh dissolution rateImprove stabilityAntibacterial agentsOrganic active ingredientsLipid formationSide effect
The invention provides a cefuroxime axetil lipid microsphere solid preparation which is prepared from the following raw and auxiliary materials in parts by weight: 1 part of cefuroxime axetil, 3-11 parts of arabic gum, 0.4-8 parts of deoxysodium cholate and 1.5-10 parts of polysorbate 80. The cefuroxime axetil lipid microsphere solid preparation provided by the invention has the advantages of improved dissolution rate, improved bioavailability, high stability and increased quality stability, is suitable for being degraded in vivo, and does not have toxicity and immunogenicity. Especially, a large amount of test data proves that the preparation provided by the invention can be used as a medicament carrier to improve the medication index, improve the product quality of the preparation, reduce toxic side effects, reduce medicament doses and the like.
Owner:HAINAN MEIDA PHARMA
Stable taste masked formulations of cephalosporins
A stable taste masked, pharmaceutical composition comprising a plurality of coated, non-disintegrating discrete dosage units, said units comprising of a core comprising one or more cephalosporins such as cefuroxime axetil and cefpodoxime proxetil and one or more coating layers. Cefuroxime axetil is in α-crystalline and amorphous forms, where at least 30% of the Cefuroxime axetil is in the α-crystalline form, wherein the particle size distribution of the α-crystalline form being such that 100% of the particles have a particle size below 250μ. The ratio of the crystalline fraction to the amorphous fraction ranges from 0.3:0.7 to 0.99:0.01. The particle size of cefpodoxime proxetil is such that 90% of the particles are below 15μ. The process of preparation of coated, non-disintegrating pellets comprising the steps of reducing the particle size of the one or more cephalosporins, blending with the other excipients, wet granulation, extrusion, spheronization, drying and screening to obtain pellets, said pellets being further coated with one or more layers of film coating to achieve taste masking.
Owner:LUPIN LTD
Cefuroxime axetil pharmaceutical composition prepared by direct compression method
InactiveCN107569466ASolve the technical problems of poor liquidityAvoid poor compressibilityAntibacterial agentsOrganic active ingredientsMedicineFiller Excipient
The invention discloses a cefuroxime axetil pharmaceutical composition prepared by powder direct compression method, wherein the weight ratio of all components in the preparation is 38.8-63.1% of cefuroxime axetil (in terms of cefuroxime), 10.7 to 35.5% of filler, 1.5 to 15% of disintegrant, 0.3 to 3.5% of wetting agent and 0.3 to 2.0% of glidant, the single composition weight is 400 to 600mg. Theinvention solves the problems of poor raw material flowability and instability of wet granulation, avoids the problem of dissolution caused by multiple compression and comminution on the material inthe dry granulation process, effectively reduces the energy consumption in the production process, and is more benefit to enlarge production.
Owner:SHIJIAZHUANG NO 4 PHARMA
Preparation method of cefuroxime axetil
InactiveCN105131016ALow impurity contentGood colorOrganic chemistryBulk chemical productionCephalosporanic AcidsTrichloroacetyl isocyanate
The invention relates to a preparation method of cefuroxime axetil. The preparation method comprises the following steps: (1) reacting de-ammoniated formyl cephalosporanic acid with diphenyl diazomethane to generate acetyl-diphenyl methyl cephalosporanate; (2) reacting diphenyl methyl de-ammoniated formyl cephalosporanate with trichloroacetic isocyanate to generate diphenyl methyl cefuroxime ester; (3) hydrolyzing diphenyl methyl cefuroxime ester to obtain cefuroxime acid; (4) reacting cefuroxime acid with 1-acetoxyl-bromoethane to generate cefuroxime axetil. During the preparation process, the carboxyl group is protected by diphenyl diazomethane, the generation of impurities is reduced, and the product quality is improved.
Owner:JIANGSU QINGJIANG PHARMA
Pharmaceutical compositions
A solid oral dosage composition of cefuroxime axetil comprising a tablet inside a capsule, the capsule serving to mask the bitter taste of the drug upon oral administration. This tablet-in-a-capsule format is bioequivalent to the commercial film-coated tablet.
Owner:UNILAB PHARMA TECH
Preparations of effervescent formulations comprising second and third generation cephalosporin and uses thereof
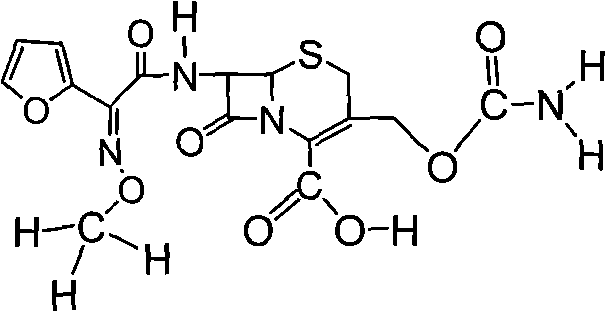
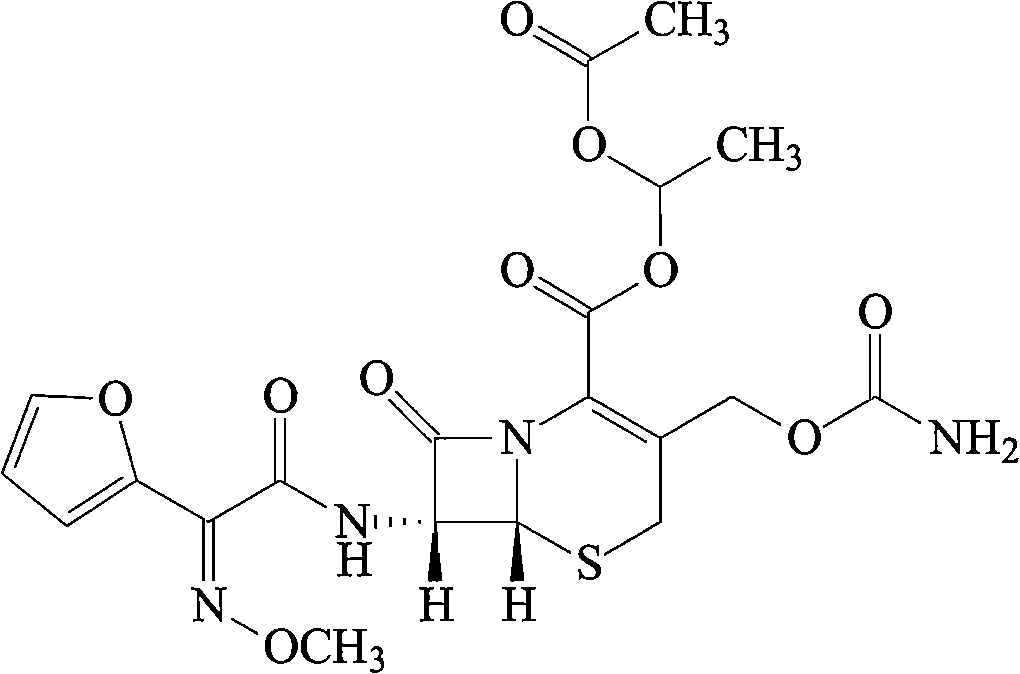
The invention relates to effervescent pharmaceutical dosage forms including cefdinir as the active agent, and their preparation. The invention also relates to effervescent formulations including ceftibuten and / or its pharmaceutically acceptable salts, hydrates, solvates, esters, amorphous and crystal forms and / or a combination thereof. The invention also relates to pharmaceutical compositions including (Z)-3-Carboxymethyl-7-(2-(2-furyl)-2-methoxyiminoacetylamino)-3-sefem-4-carboxylic acid which is named cefuroxime axetil or any pharmaceutically acceptable derivative thereof, and the use of these compositions in the treatment of bacterial infections. Lastly, the invention relates to pharmaceutical formulations including a third generation cephalosporin together with clavulanic acid and / or derivatives thereof as the active agents.
Owner:BILGIC MAHMUT
Crystalline cefuroxime axetil preparation method
InactiveCN108586493AEasy to manufactureEasy to purifyOrganic chemistryOrganic solventCefuroxime axetil
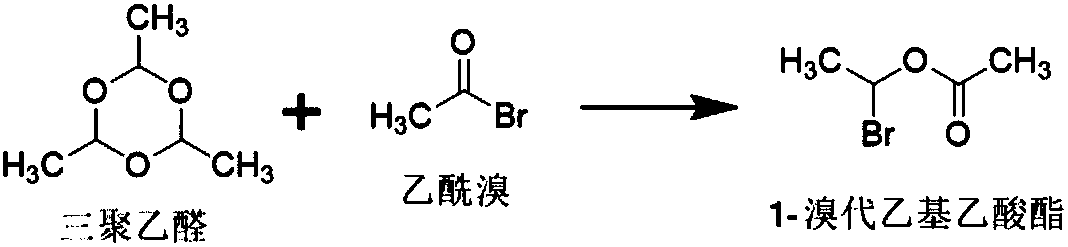
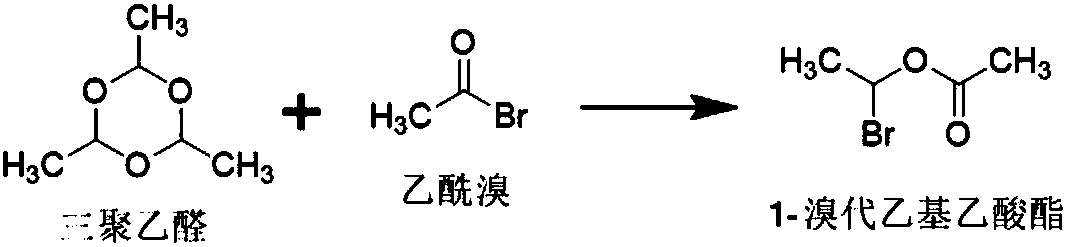
The invention relates to a crystalline cefuroxime axetil preparation method, which comprises: 1) carrying out a reaction on paraldehyde and acetyl bromide at a temperature of -10-10 DEG C under the action of a catalyst to obtain 1-bromoethyl acetate; 2) adding cefuroxime acid and a catalyst to the mixed solution of an organic solvent and water, adjusting the temperature to -20-5 DEG C, adding the1-bromoethyl acetate prepared in the step 1) to the mixed solution in a dropwise manner to obtain the crystalline cefuroxime axetil. According to the present invention, the method improves the traditional process, simplifies the process, improves the product quality, does not use the extraction solvent dichloromethane, greatly reduce the production cost, and improves the reaction efficiency and the purity of the final product.
Owner:BENGBU BBCA MEDICINE SCI DEV
Cefuroxime axetil dispersible tablet and its preparation method
InactiveCN102488668AMask bitternessImprove bioavailabilityAntibacterial agentsOrganic active ingredientsCross-linkSilicon dioxide
The invention belongs to the medicine preparation field, more specifically relates to a Cefuroxime axetil dispersible tablet and its preparation method, the dispersible tablet comprises the following raw materials: Cefuroxime axetil, magnesium stearate, microcrystalline cellulose PH-102, aspartame, cross linked sodium carboxymethyl cellulose, essence, silica and sodium dodecyl sulfate. The method comprises the following steps: passing the above raw materials through a sieve with 40 meshes, mixing the aspartame and microcrystalline cellulose PH-102 with equal weight for 5 minutes, then adding all the cross linked sodium carboxymethyl cellulose for mixing for 5 minutes, placing the mixed powder and residual raw materials in a mixed tank, mixing for 20 minutes, tabletting by a high speed rotary tablet machine to obtain the Cefuroxime axetil dispersible tablet. The dissolution rate of the prepared Cefuroxime axetil dispersible tablet can reach more than 90% after 15 minutes, 99.5% after 45 minutes, thereby the biological availability and curative effect of the medicines can be fully enhanced.
Owner:山东淄博新达制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com