Cefuroxime axetil oral liquid and preparation method thereof
A technology for oral nitroxide axetil and cefuroxime axetil, which is applied in the field of antibiotic drugs, can solve the problems of affecting the efficacy of preparations, low dissolution rate, uneven particle size, etc., and achieves improved bioavailability and onset time, mixing High uniformity and uniform particle size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Cefuroxime axetil capsules
[0028] Cefuroxime axetil capsules, including the following ingredients and their weight:
[0029] Cefuroxime axetil 310g (equivalent to C 16 H 16 N 4 O 8 S 250.0g)
[0030] Talc 70.0g
[0031] PVPP 30.0g
[0032] 2wt% HPMC aqueous solution 200g
[0033] After mixing the above-mentioned API and talc, pass through an 80 mesh sieve, mix with PVPP, pass through a 60 mesh sieve, add 2% hydroxypropyl methylcellulose (HPMC) aqueous solution, granulate at 30 mesh, dry at 60 degrees, 24 mesh Sieve whole granules, fill capsules (0 # Capsules), a total of 1000 capsules are made, each capsule is 0.25gC 16 H 16 N 4 O 8 S.
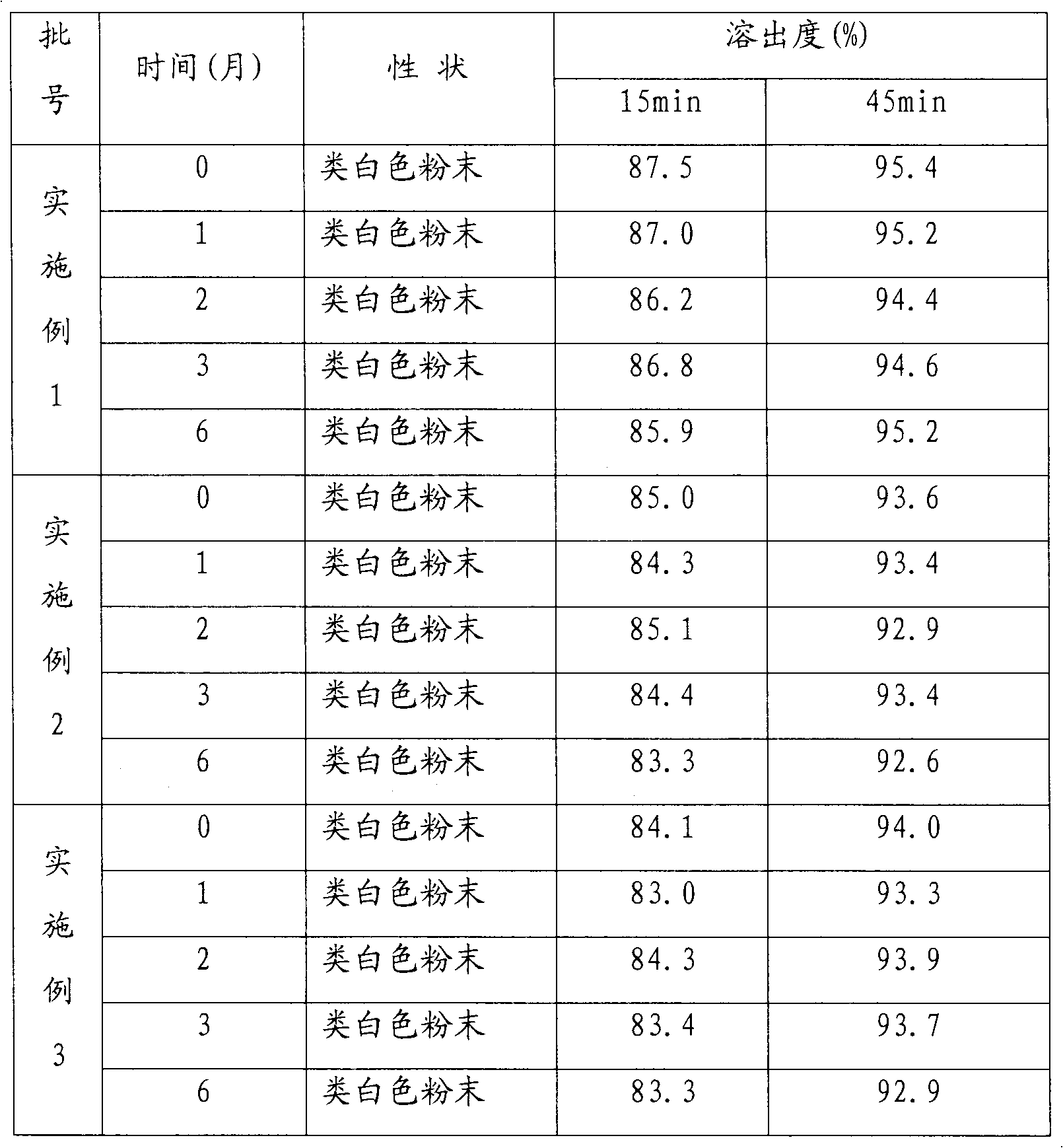
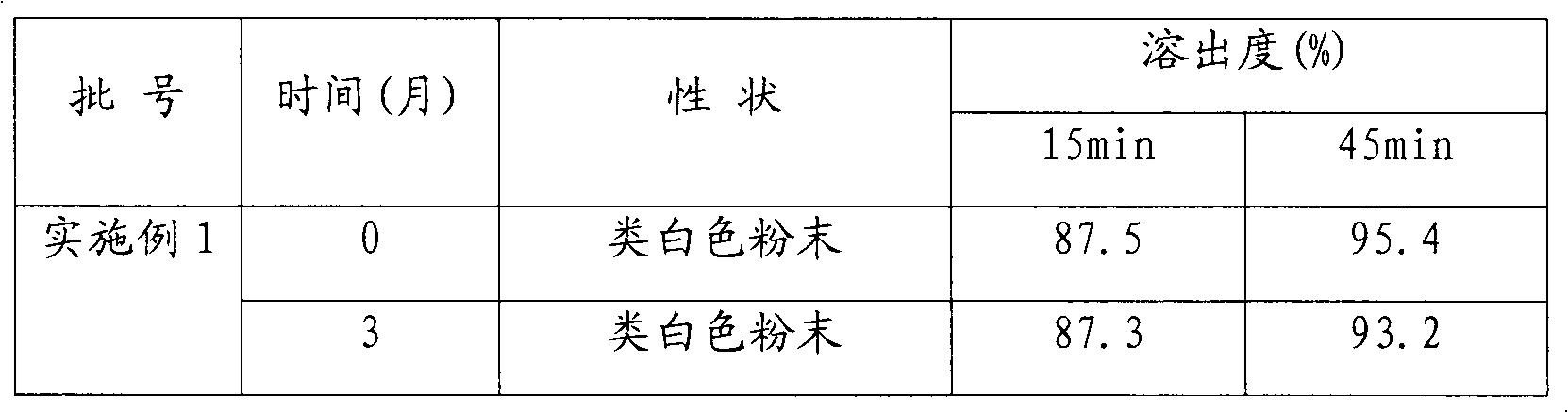
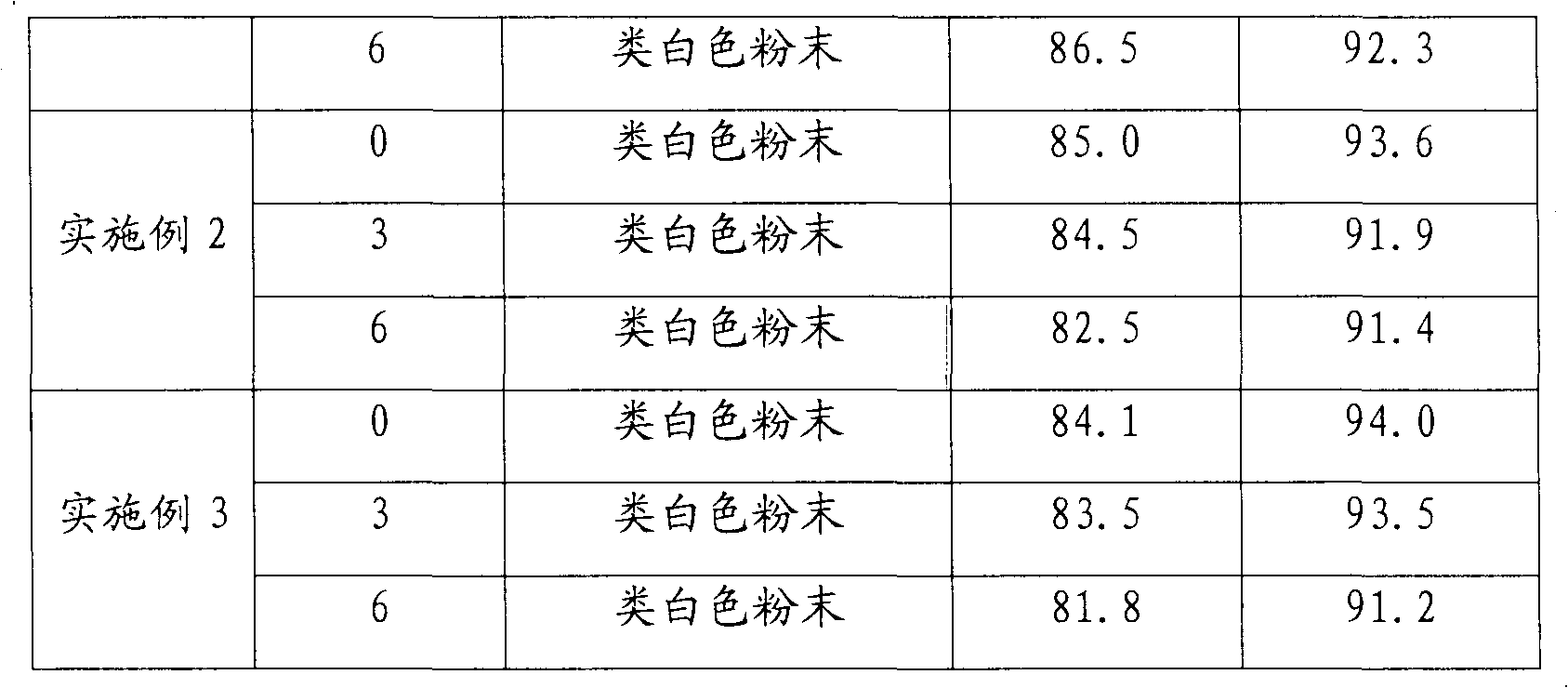
[0034] Using the conventional dissolution test method, the dissolution rate of cefuroxime axetil capsules was 87% in 15 minutes and 95% in 45 minutes.
Embodiment 2
[0035] Example 2 Cefuroxime axetil tablets
[0036] Cefuroxime axetil tablets, including the following weights of various ingredients:
[0037] Cefuroxime axetil 280g
[0038] Talc 50.0g
[0039] PVPP 15.0g
[0040] 5wt% HPMC aqueous solution 150g
[0041] After mixing the above-mentioned crude drug and talc, pass through an 80 mesh sieve, mix with PVPP, pass through a 60 mesh sieve, add 2% hydroxypropyl methylcellulose (HPMC) aqueous solution, granulate at 30 mesh, dry at 60 degrees, 24 mesh Sieve and granulate, and then obtain 1000 cefuroxime axetil tablets according to the conventional tableting method.
[0042] Using conventional dissolution testing methods, the dissolution rate of cefuroxime axetil tablets reached 85% in 15 minutes and 93% in 45 minutes.
Embodiment 3
[0043] Example 3 Cefuroxime axetil dispersible tablet
[0044] Cefuroxime axetil tablets, including the following weights of various ingredients:
[0045] Cefuroxime axetil 350g
[0046] Talc 100.0g
[0047] PVPP 50.0g
[0048] 3wt% HPMC aqueous solution 180g
[0049] After mixing the above-mentioned crude drug and talc, pass through an 80 mesh sieve, mix with PVPP, pass through a 60 mesh sieve, add 2% hydroxypropyl methylcellulose (HPMC) aqueous solution, granulate at 30 mesh, dry at 60 degrees, 24 mesh Sieve and granulate, and make 1,000 cefuroxime axetil dispersible tablets according to conventional methods.
[0050] Using the conventional dissolution test method, the dissolution rate of cefuroxime axetil dispersible tablets was 84% in 15 minutes and 94% in 45 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com