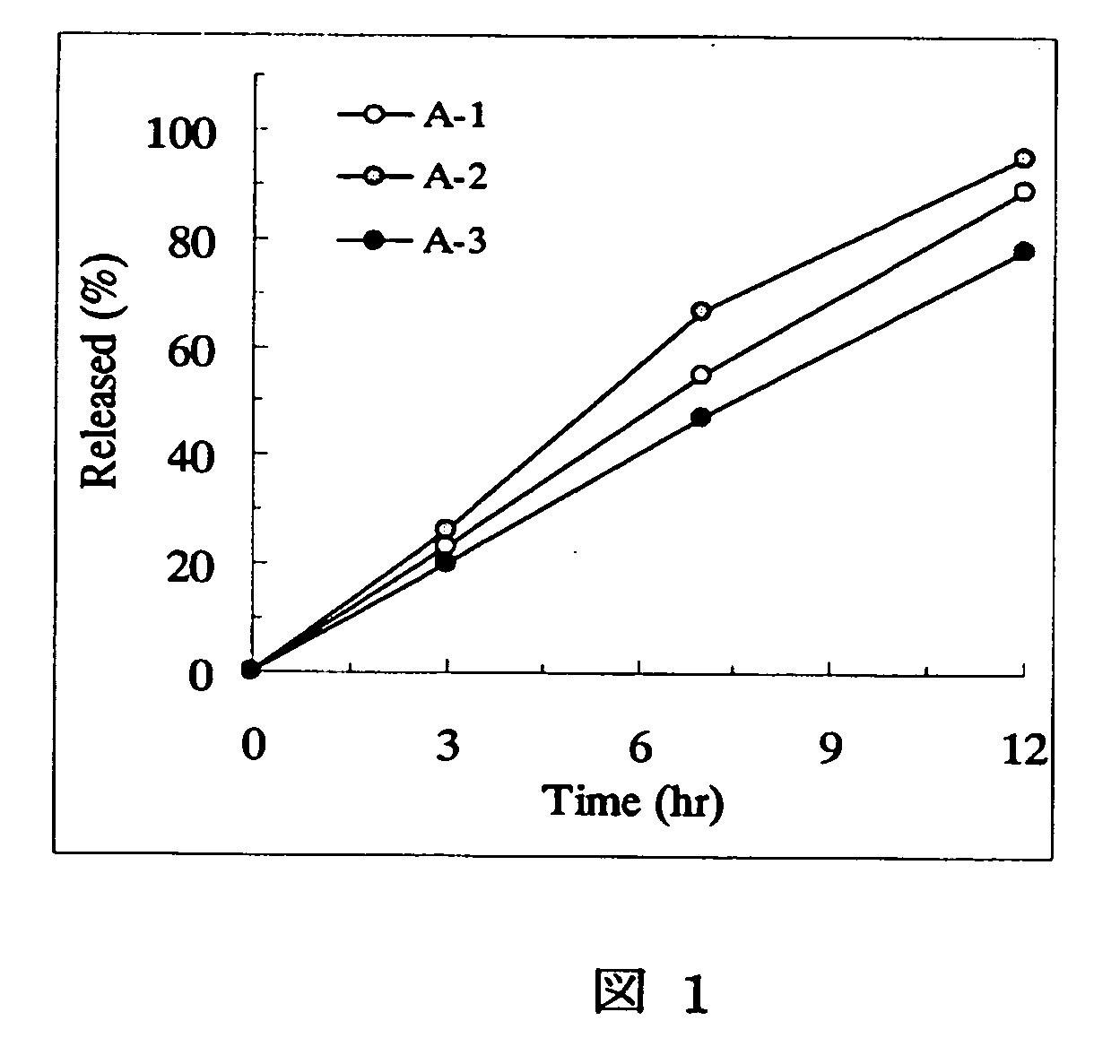
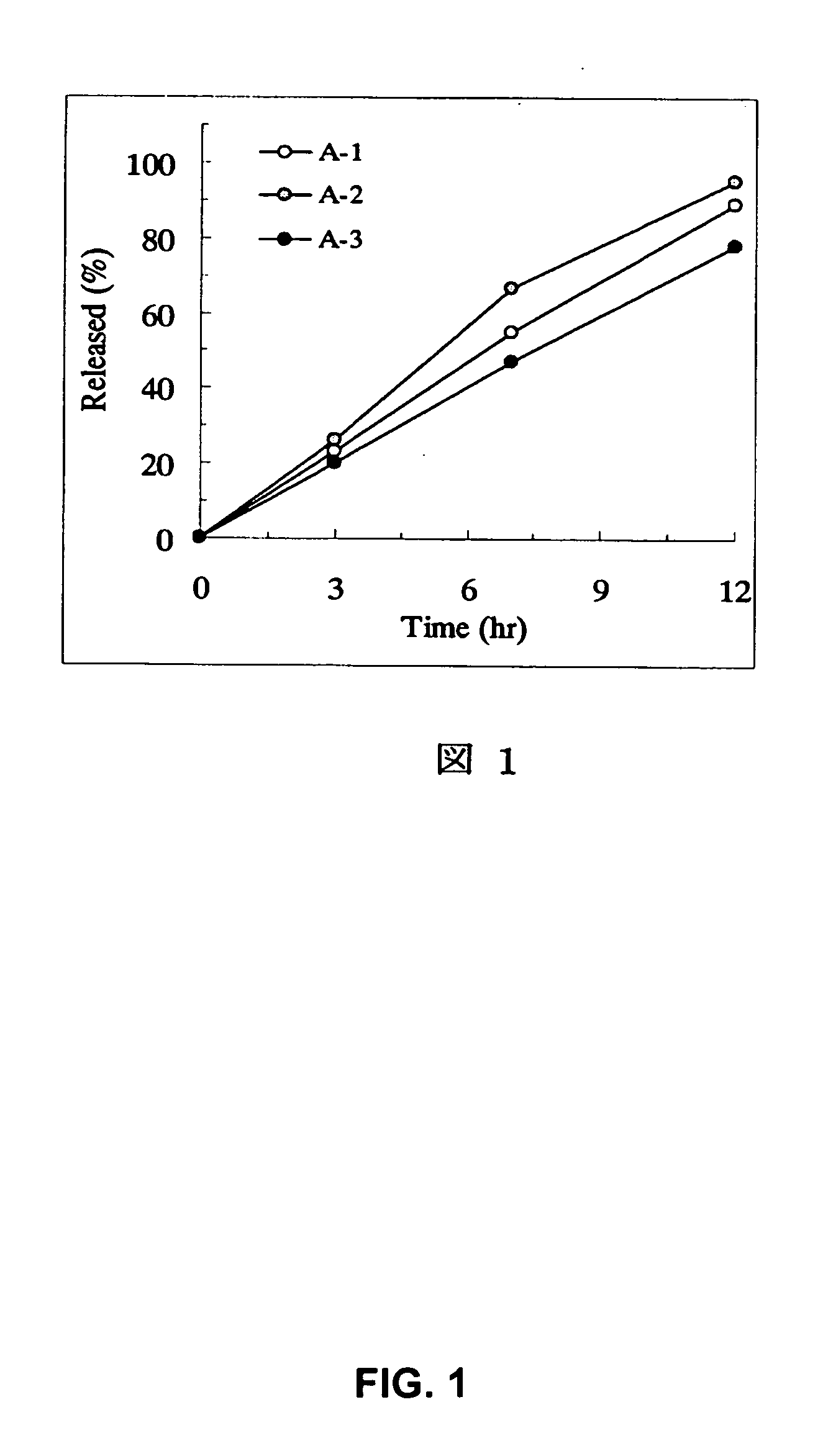
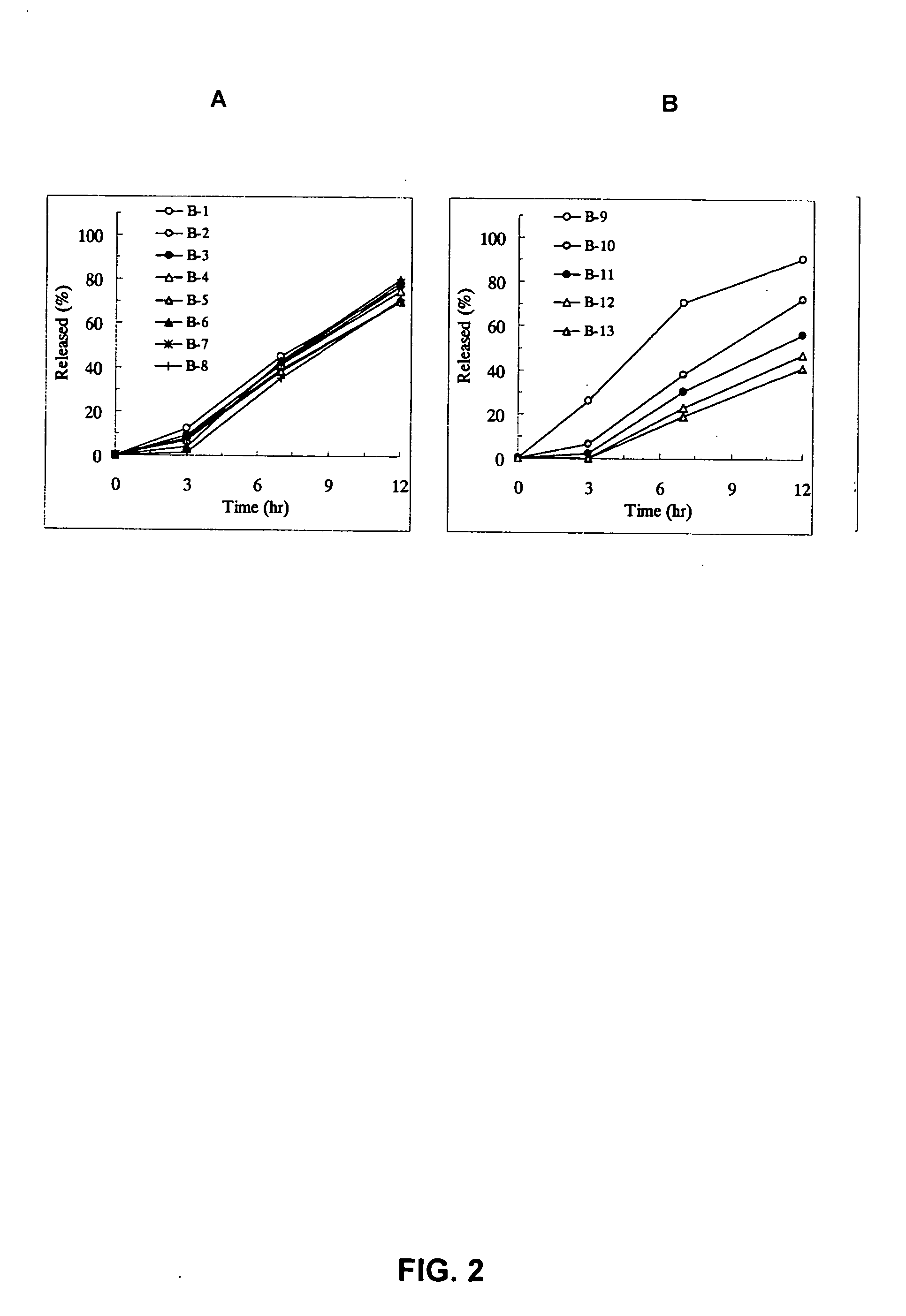
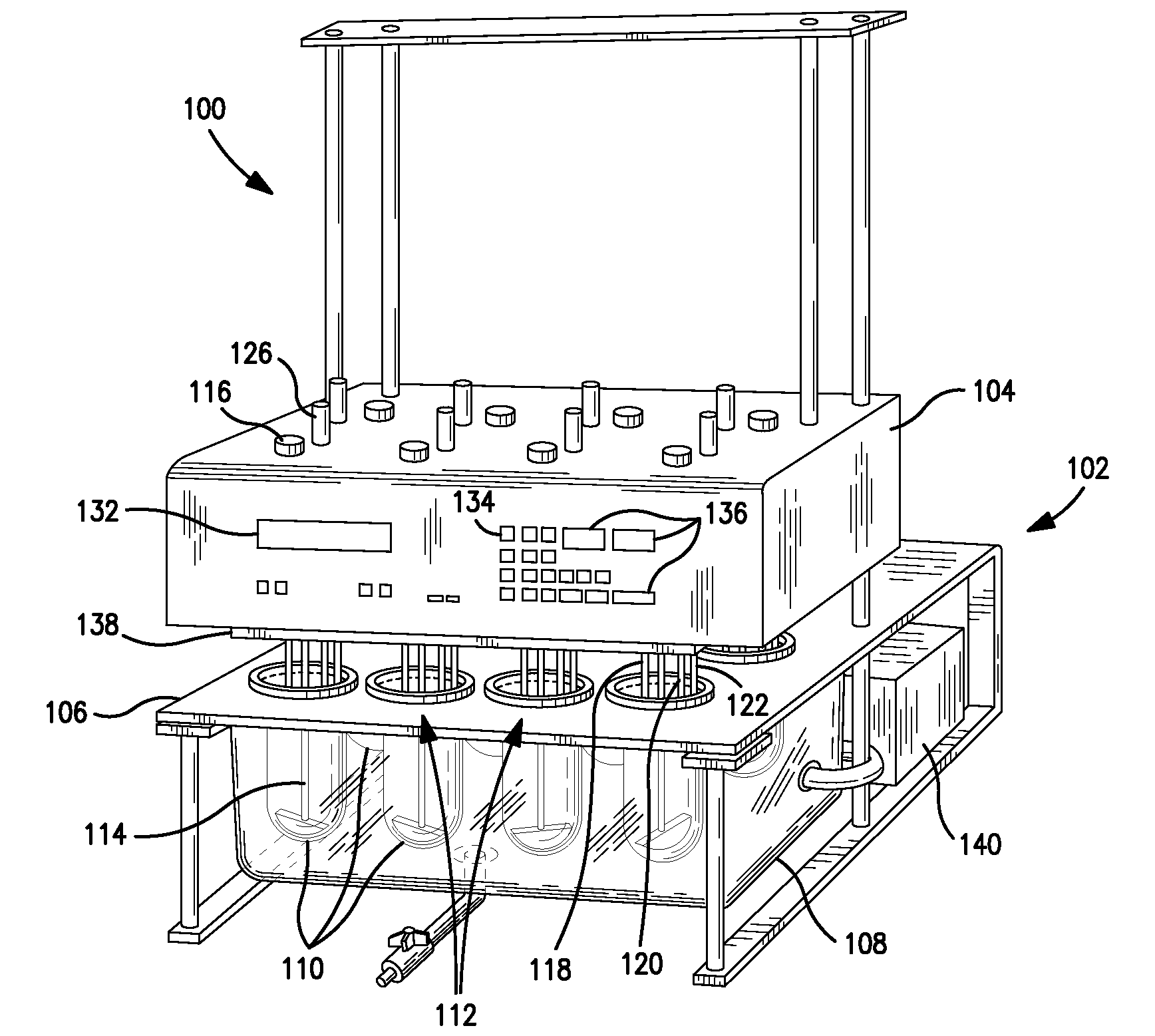
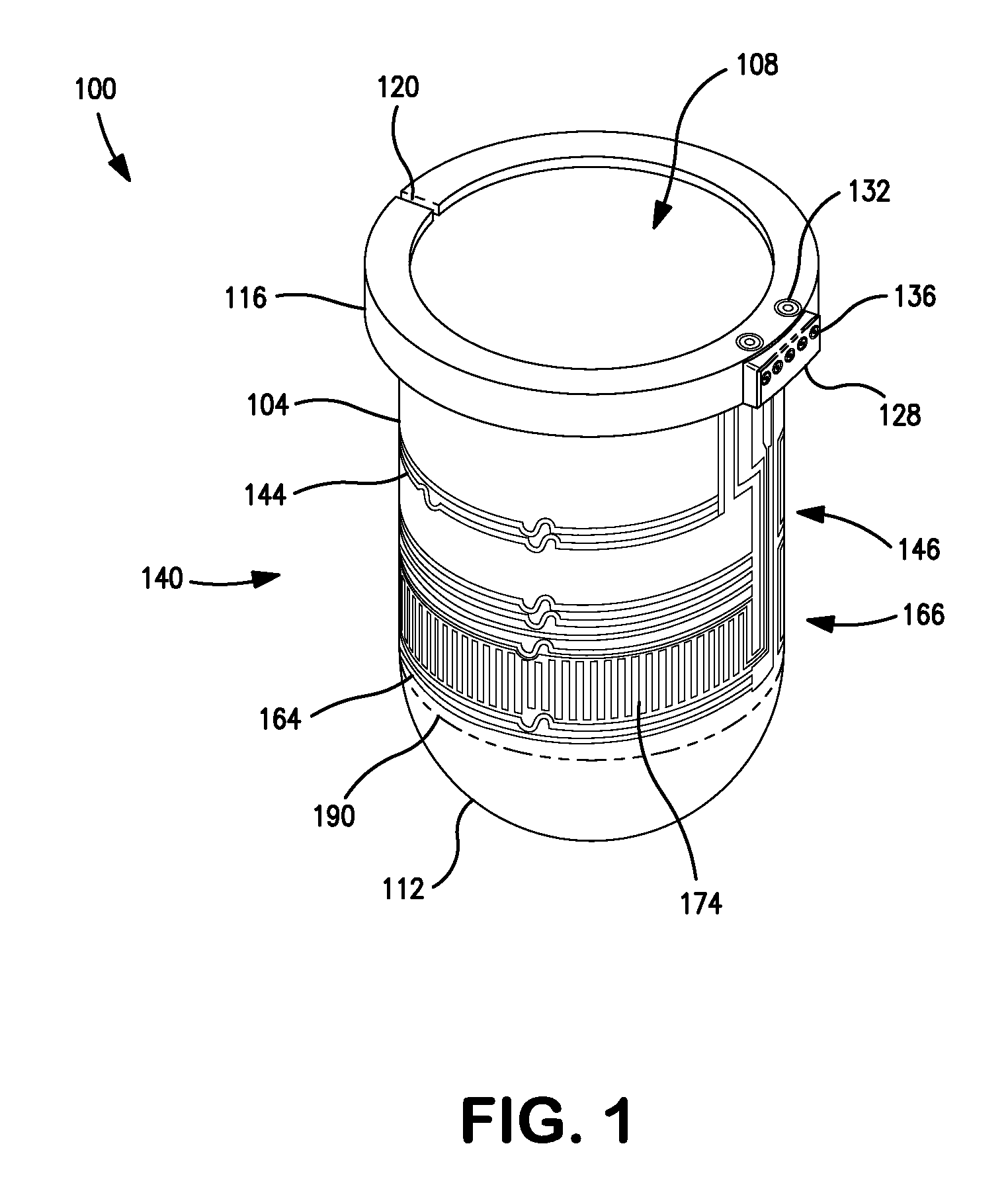
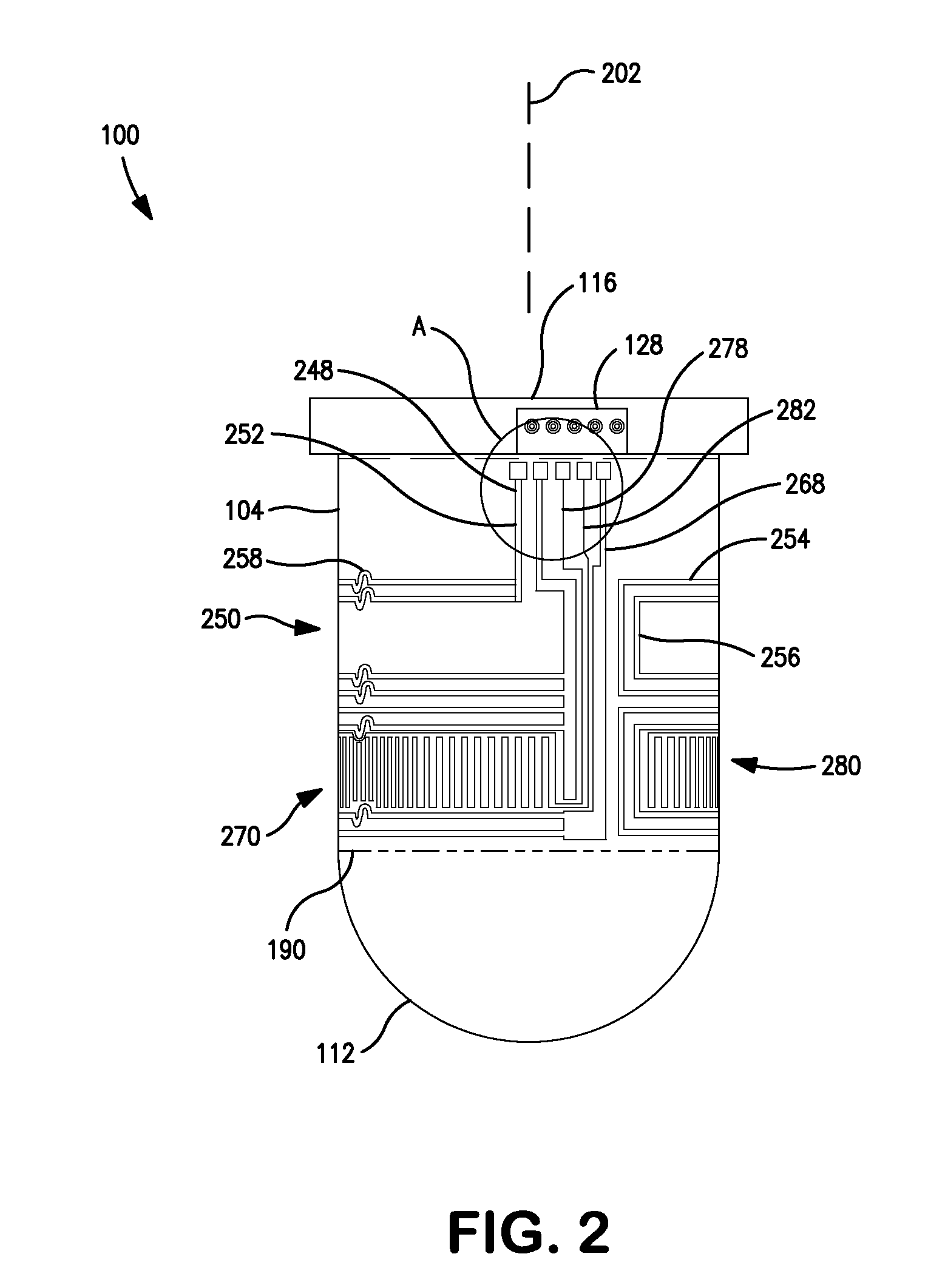
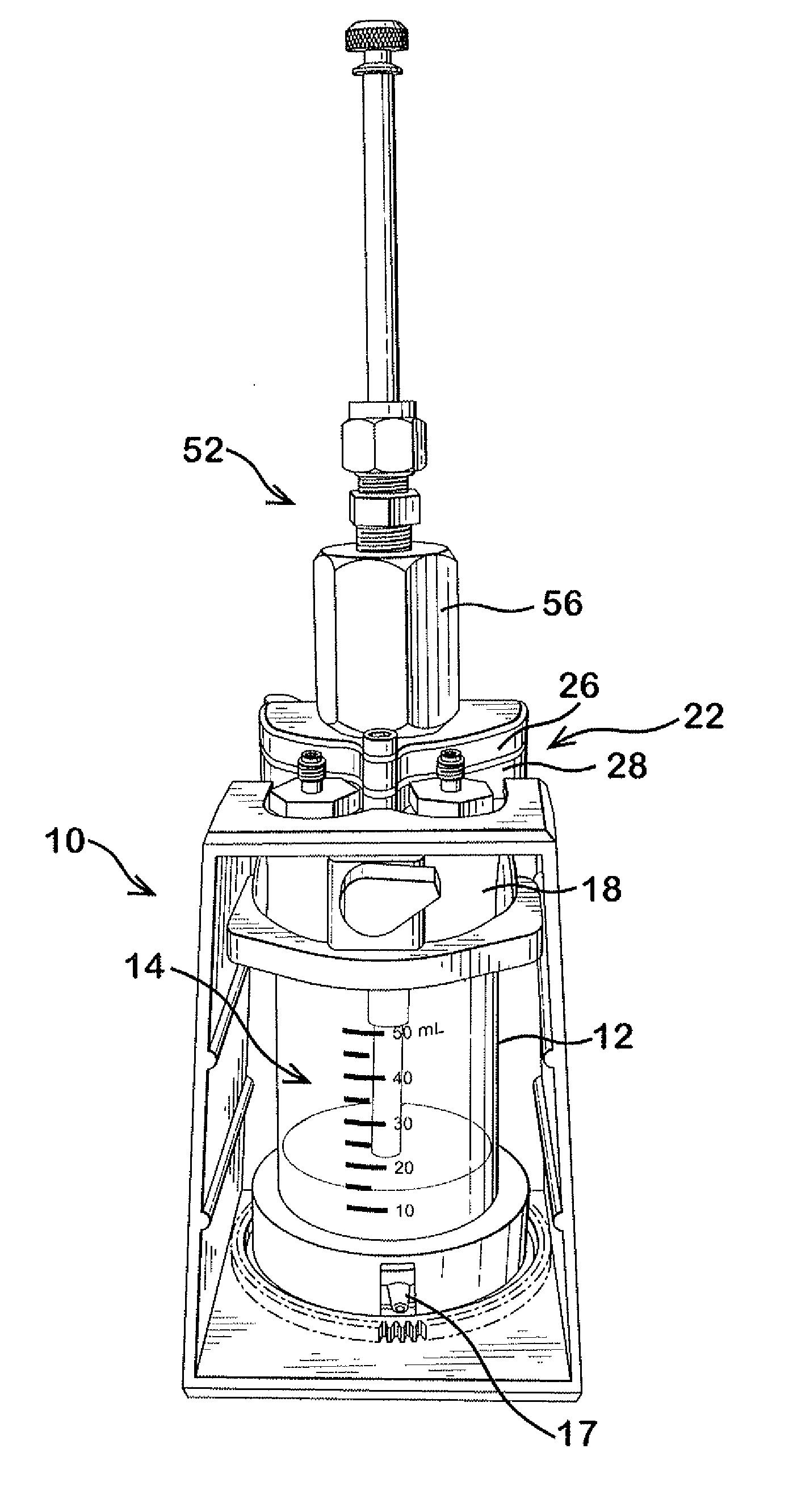
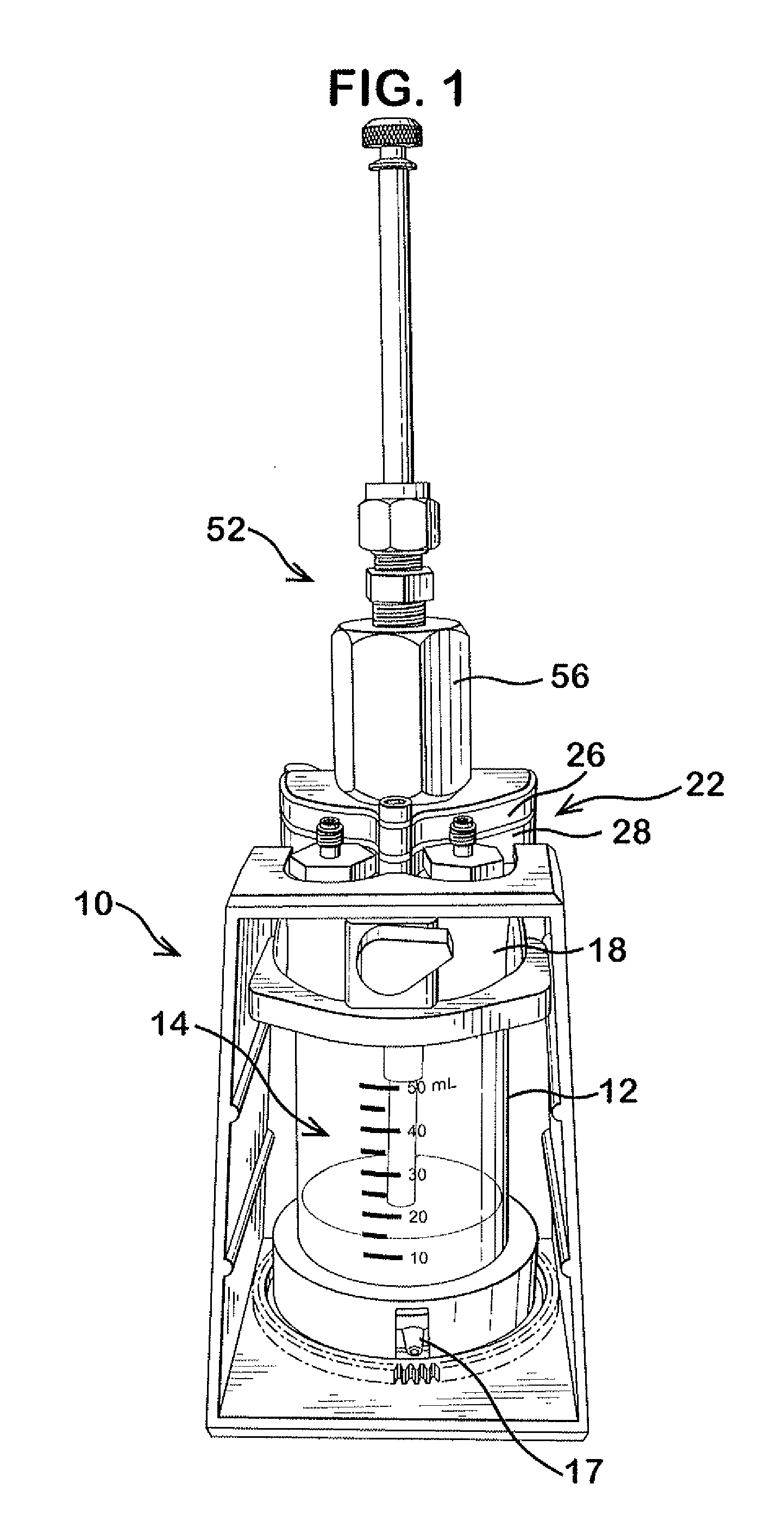
Patents
Literature
59 results about "Dissolution testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
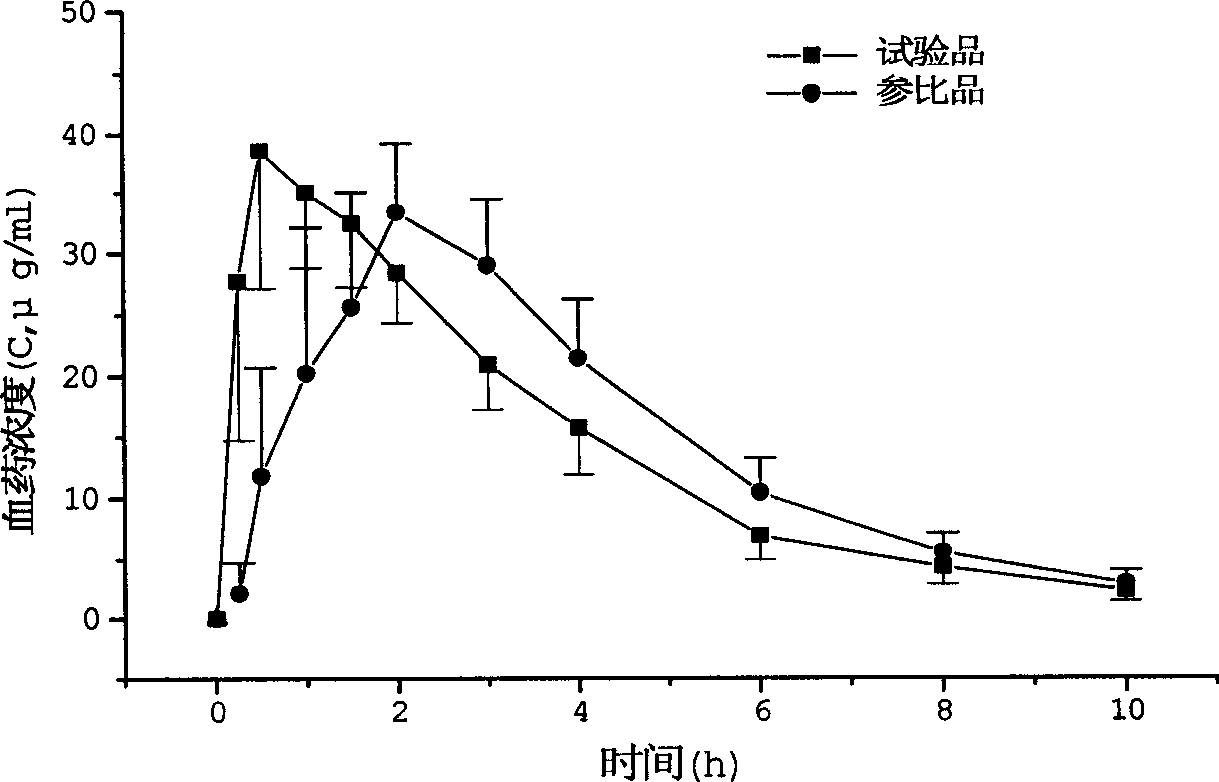
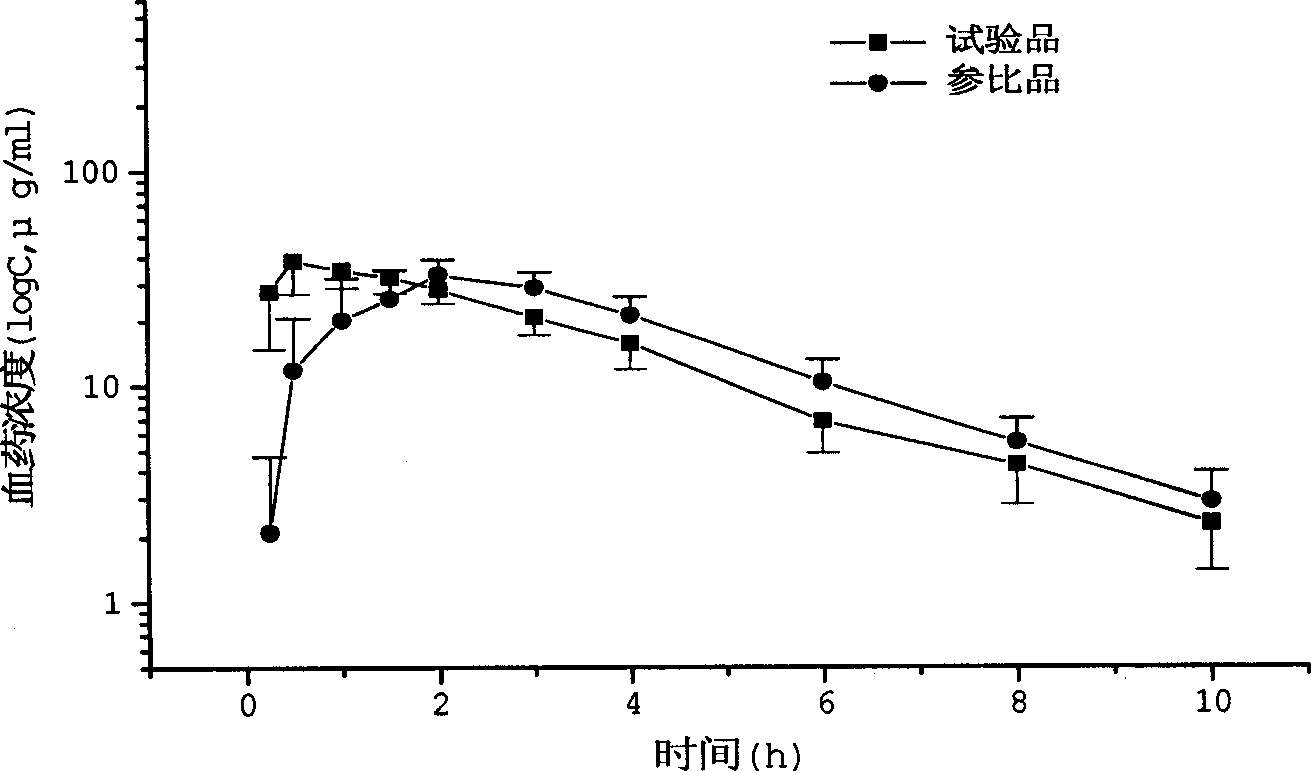
In the pharmaceutical industry, drug dissolution testing is routinely used to provide critical in vitro drug release information for both quality control purposes, i.e., to assess batch-to-batch consistency of solid oral dosage forms such as tablets, and drug development, i.e., to predict in vivo drug release profiles. There are three typical situations where dissolution testing plays a vital role: (i) formulation and optimization decisions: during product development, for products where dissolution performance is a critical quality attribute, both the product formulation and the manufacturing process are optimized based on achieving specific dissolution targets. (ii) Equivalence decisions: during generic product development, and also when implementing post-approval process or formulation changes, similarity of in vitro dissolution profiles between the reference product and its generic or modified version are one of the key requirements for regulatory approval decisions. (iii) Product compliance and release decisions: during routine manufacturing, dissolution outcomes are very often one of the criteria used to make product release decisions.
Sustained release pharmaceutical composition
InactiveUS20050100602A1Reduces adverse eventImprove featuresPowder deliveryBiocideTamsulosin hclJapanese Pharmacopoeia
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting, there is a problem to provide a sustained-release pharmaceutical composition in which efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and, when dissolution test is carried out according to Japanese Pharmacopoeia Dissolution Test Method 2, the tamsulosin release after 7 hours from the start of the dissolution is about 20 to about 85%.
Owner:ASTELLAS PHARMA INC
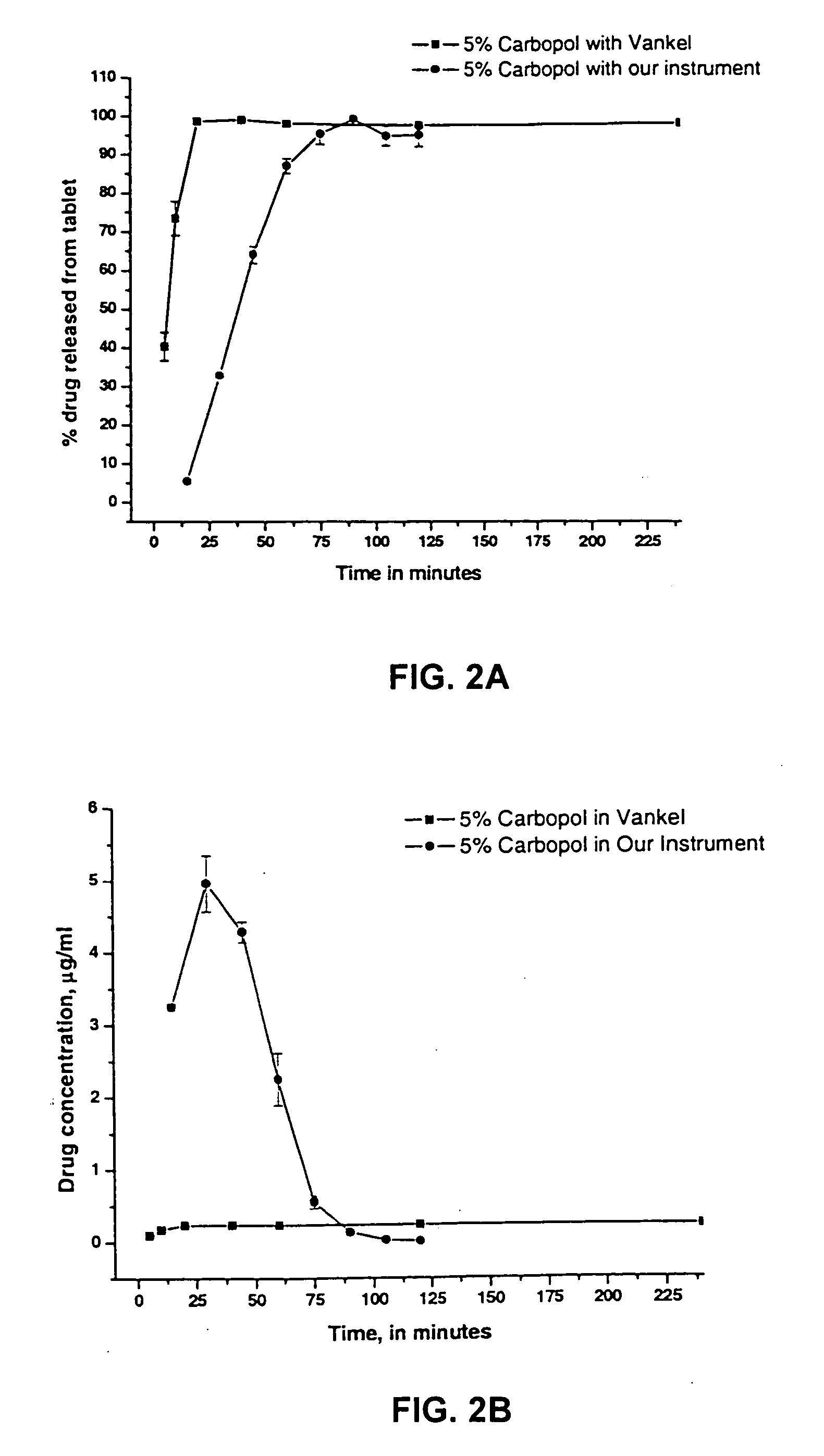
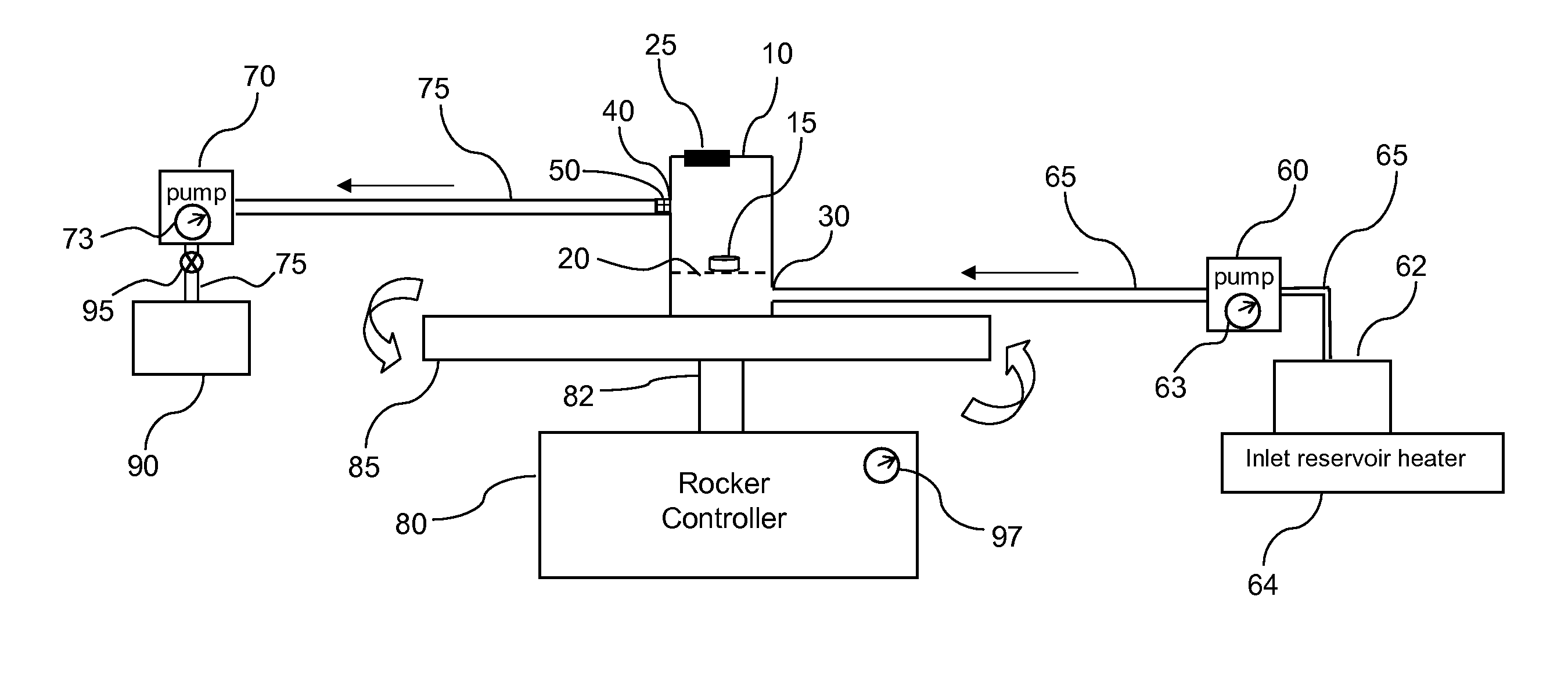
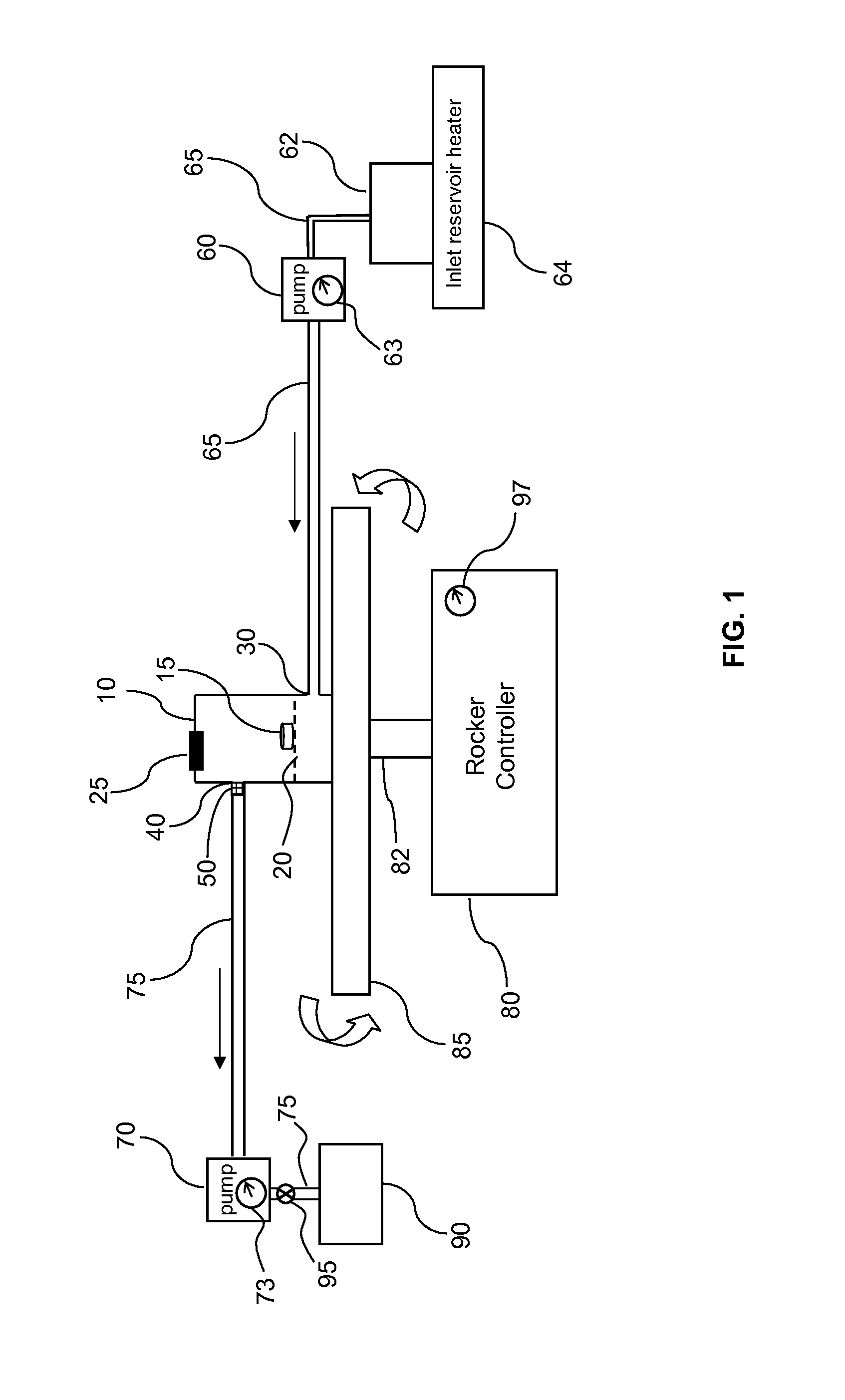
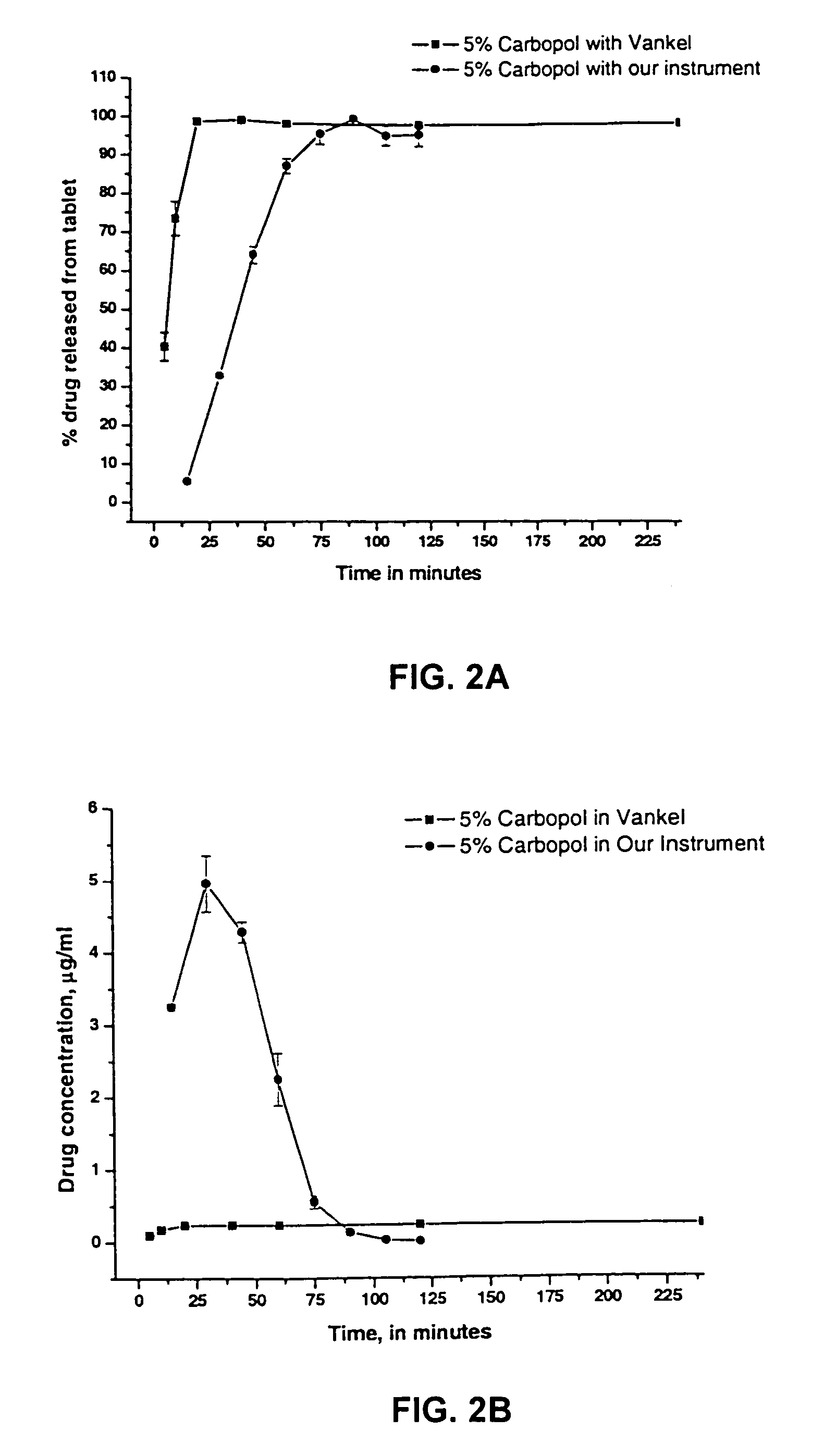
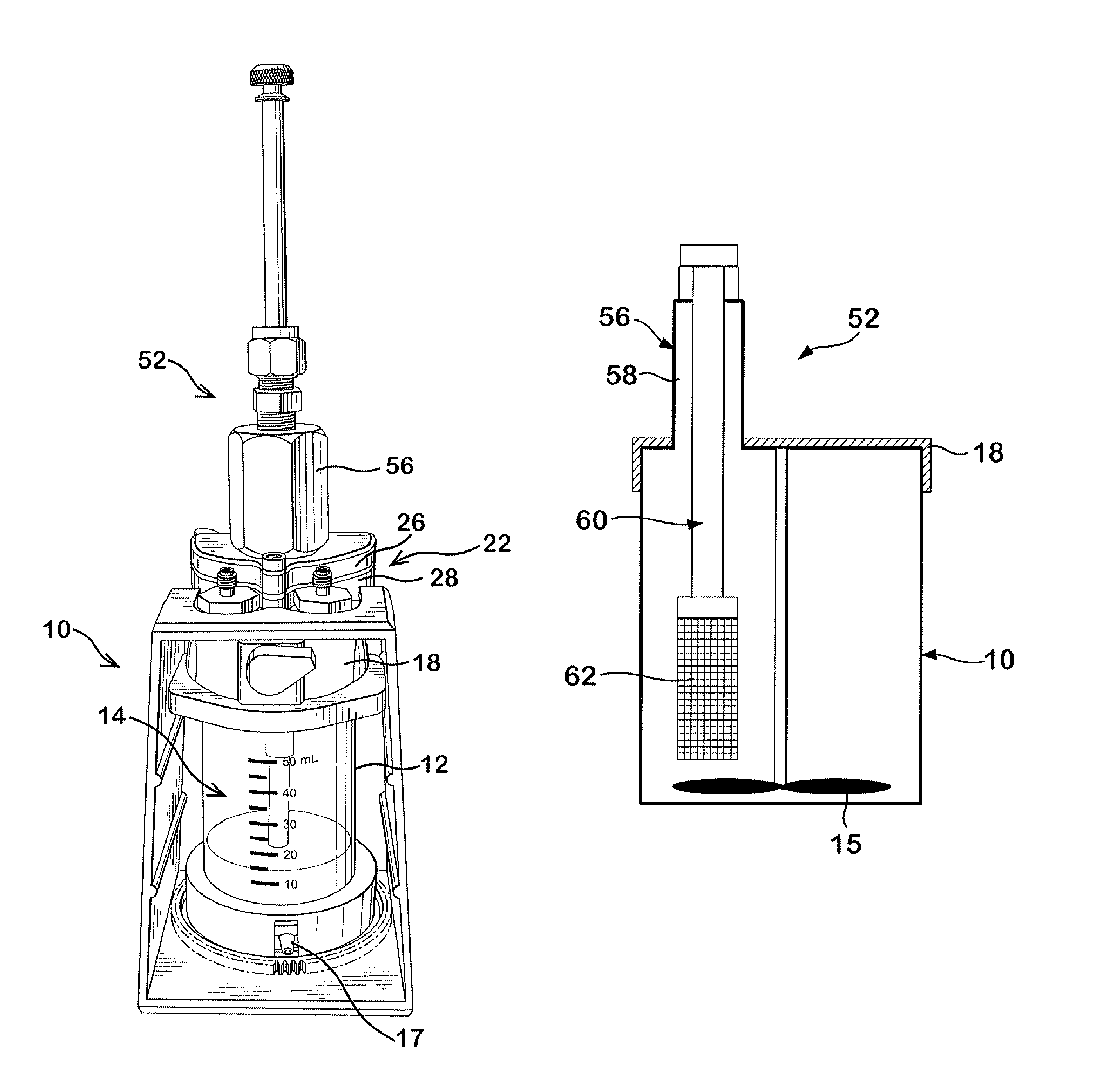
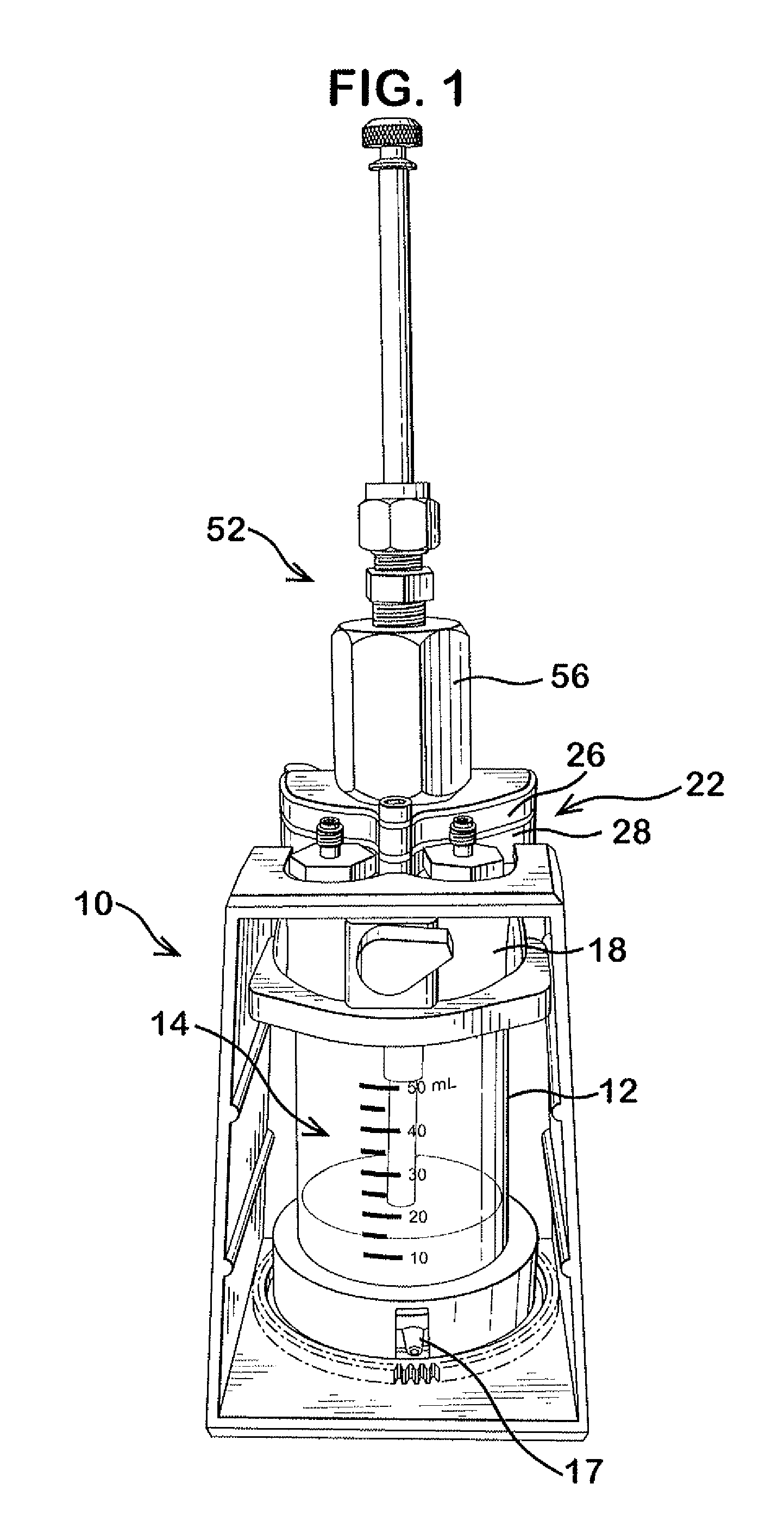
Dissolution testing with in-situ gravimetric volume measurement
InactiveUS20100107752A1Shaking/oscillating/vibrating mixersWeather/light/corrosion resistanceElectronic controllerControl signal
A dissolution test apparatus includes a vessel support member, weight sensors, a movable component, a media transport cannula, a pump, and an electronic controller. The vessel support member receives vessels. A weight sensor is located at each vessel site. Each weight sensor contacts a vessel and transmits a measurement signal indicative of the weight of the vessel and any contents therein. The movable component moves the media transport cannula toward a vessel site. The pump establishes media flow between the media transport cannula and the selected vessel. The controller communicates with the weight sensors and may also communicate with the pump. Based on the measurement signals received from the weight sensors, the electronic controller may calculate the volume of media in a given vessel. The electronic controller may also control media flows to or from the vessels by transmitting control signals to the pump assembly.
Owner:AGILENT TECH INC
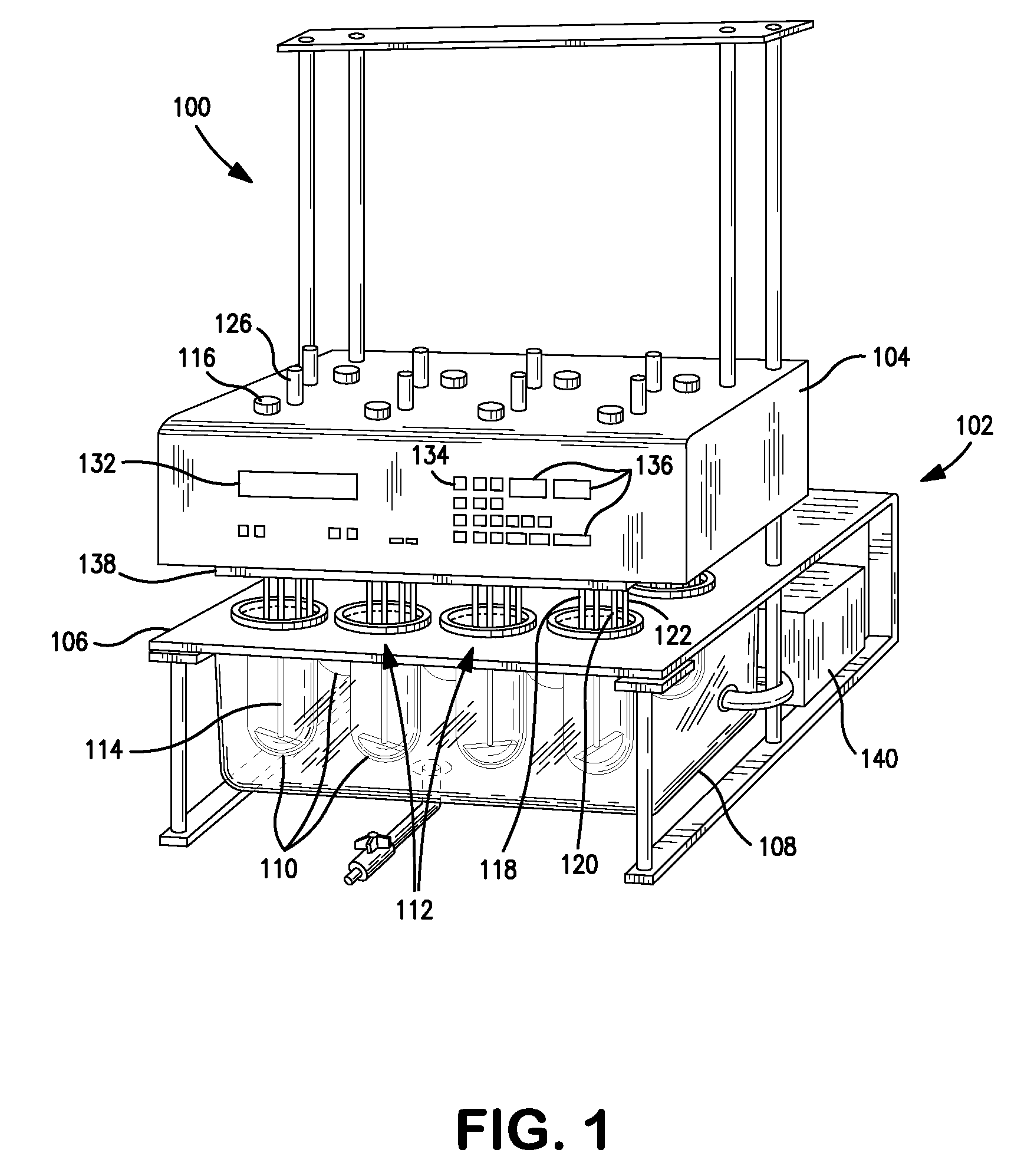
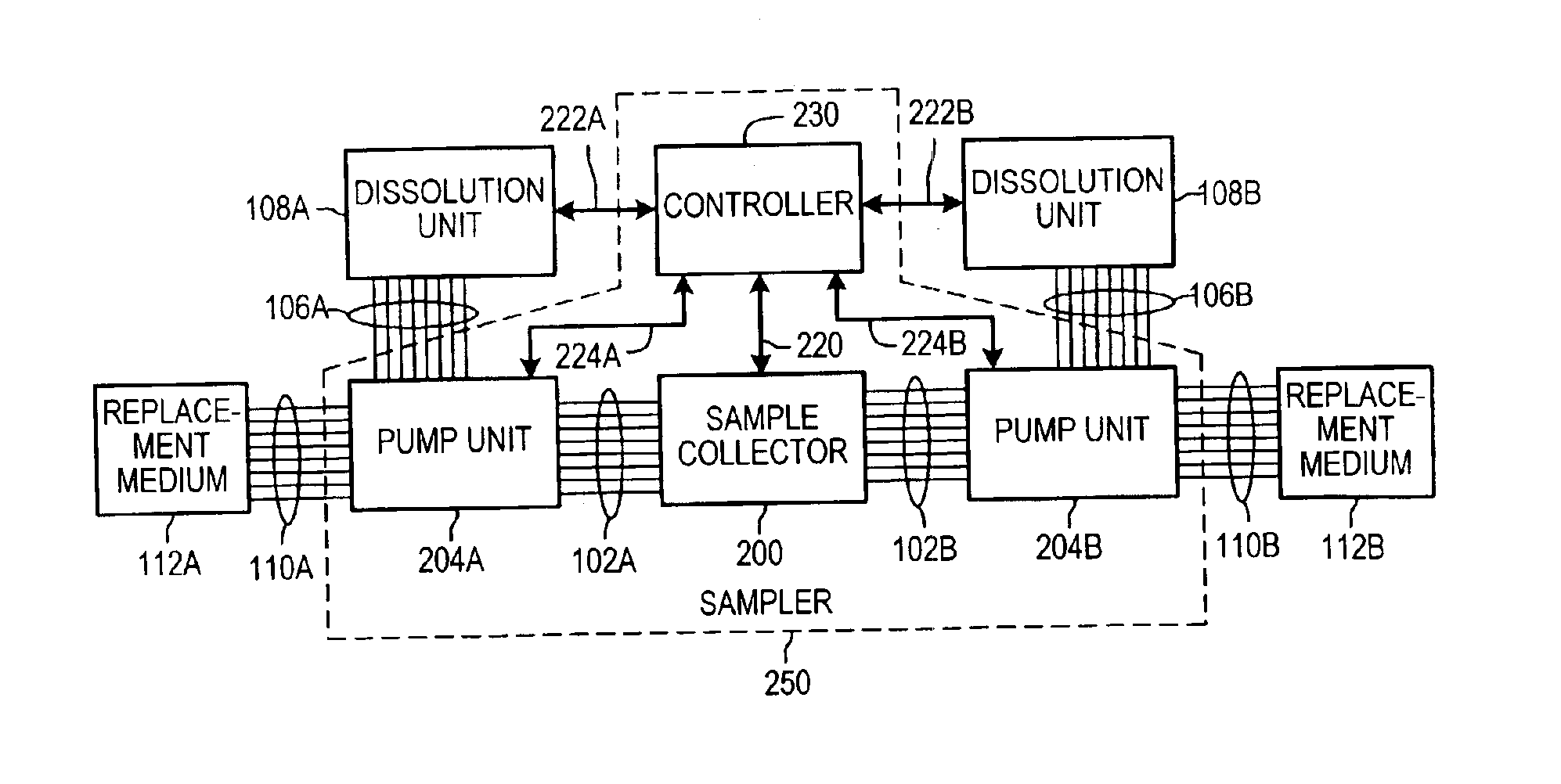
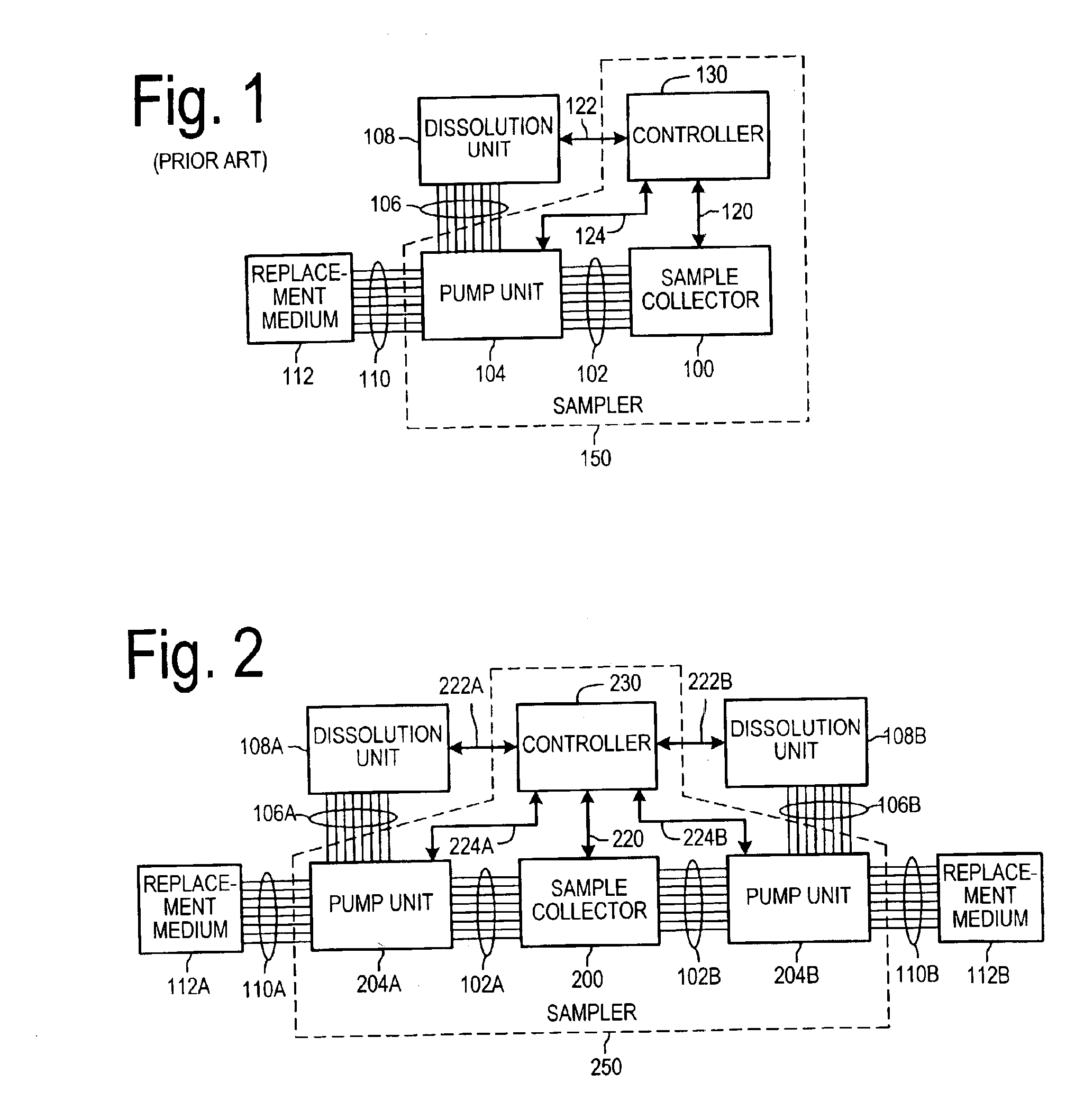
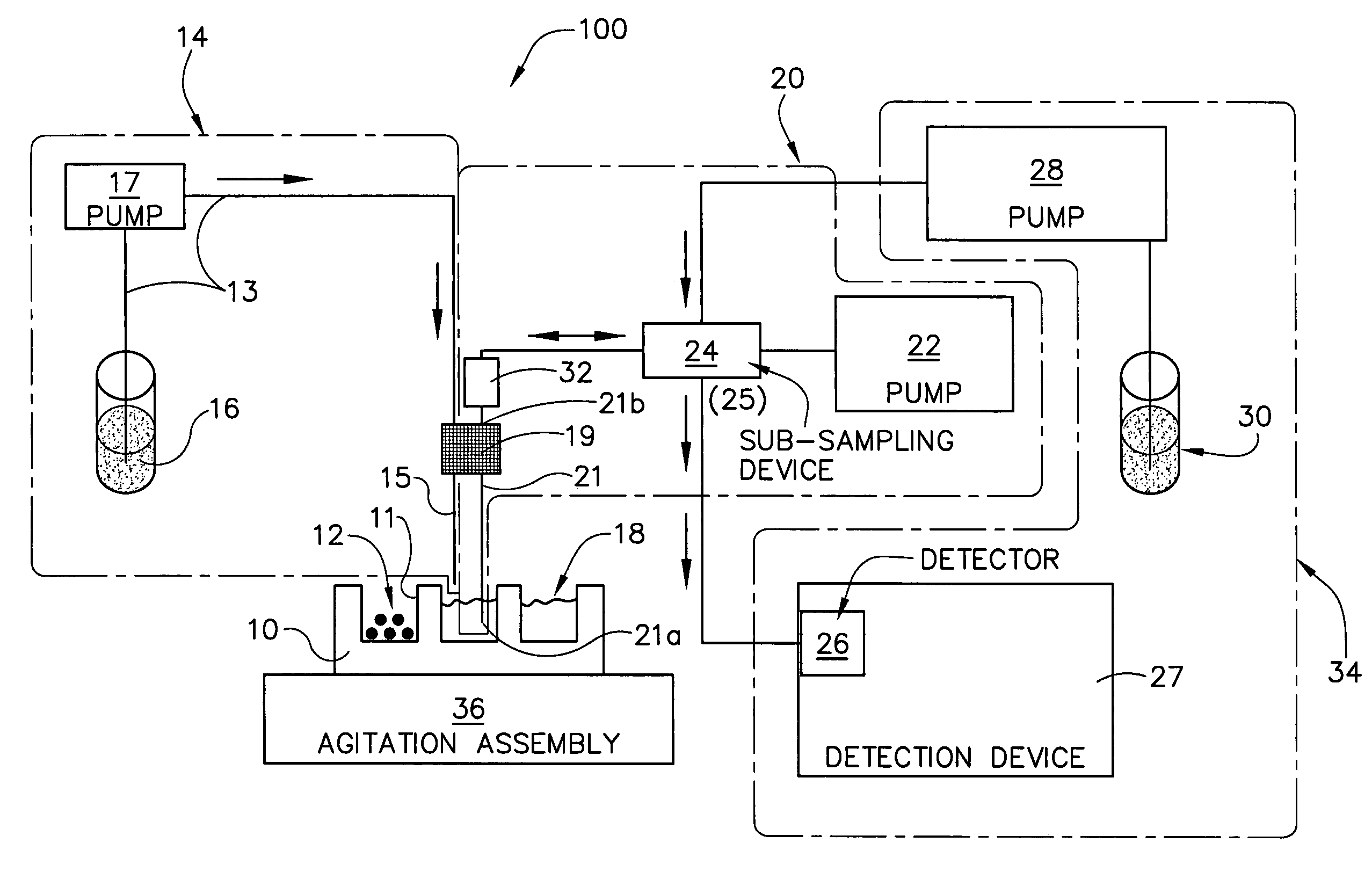
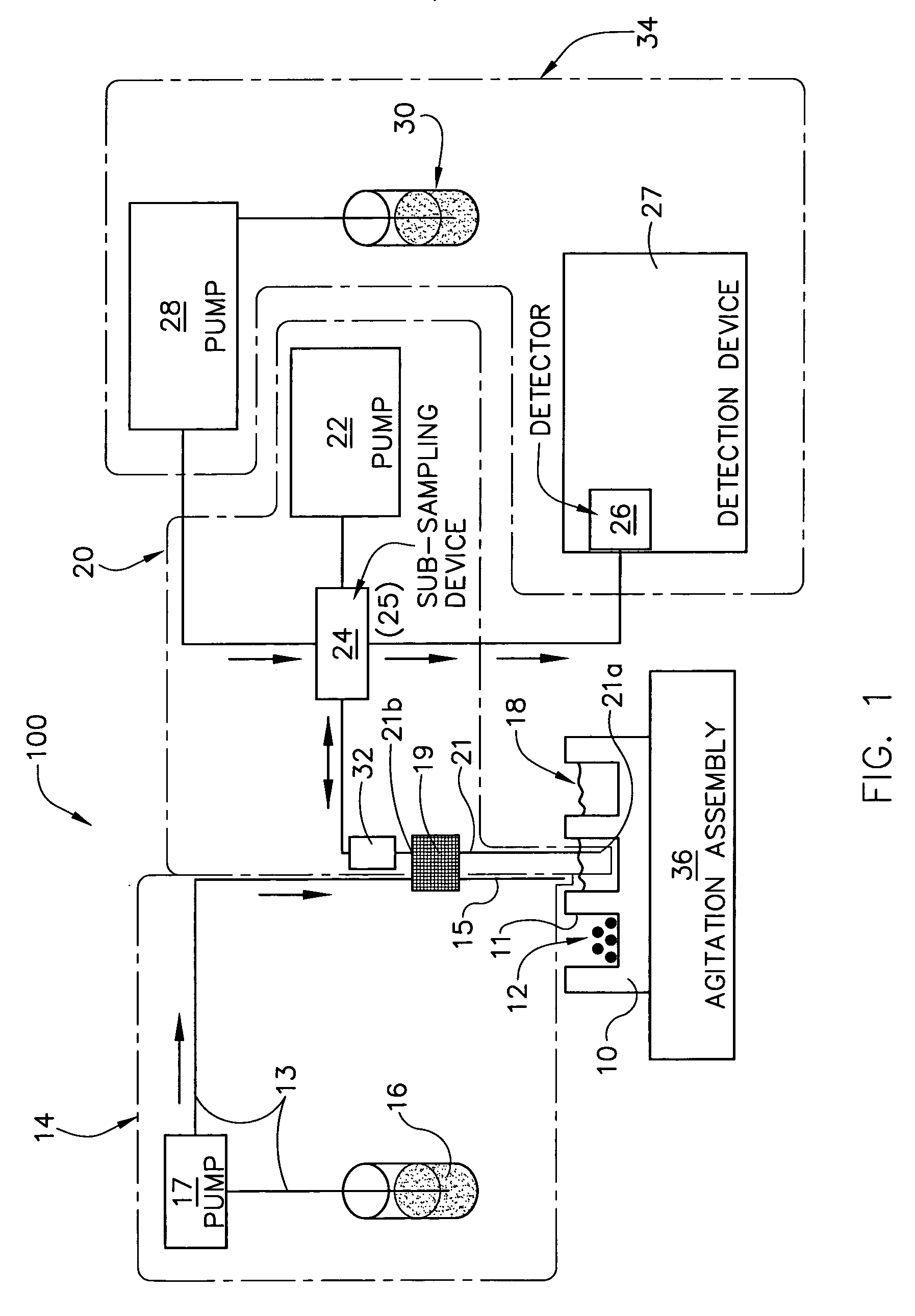
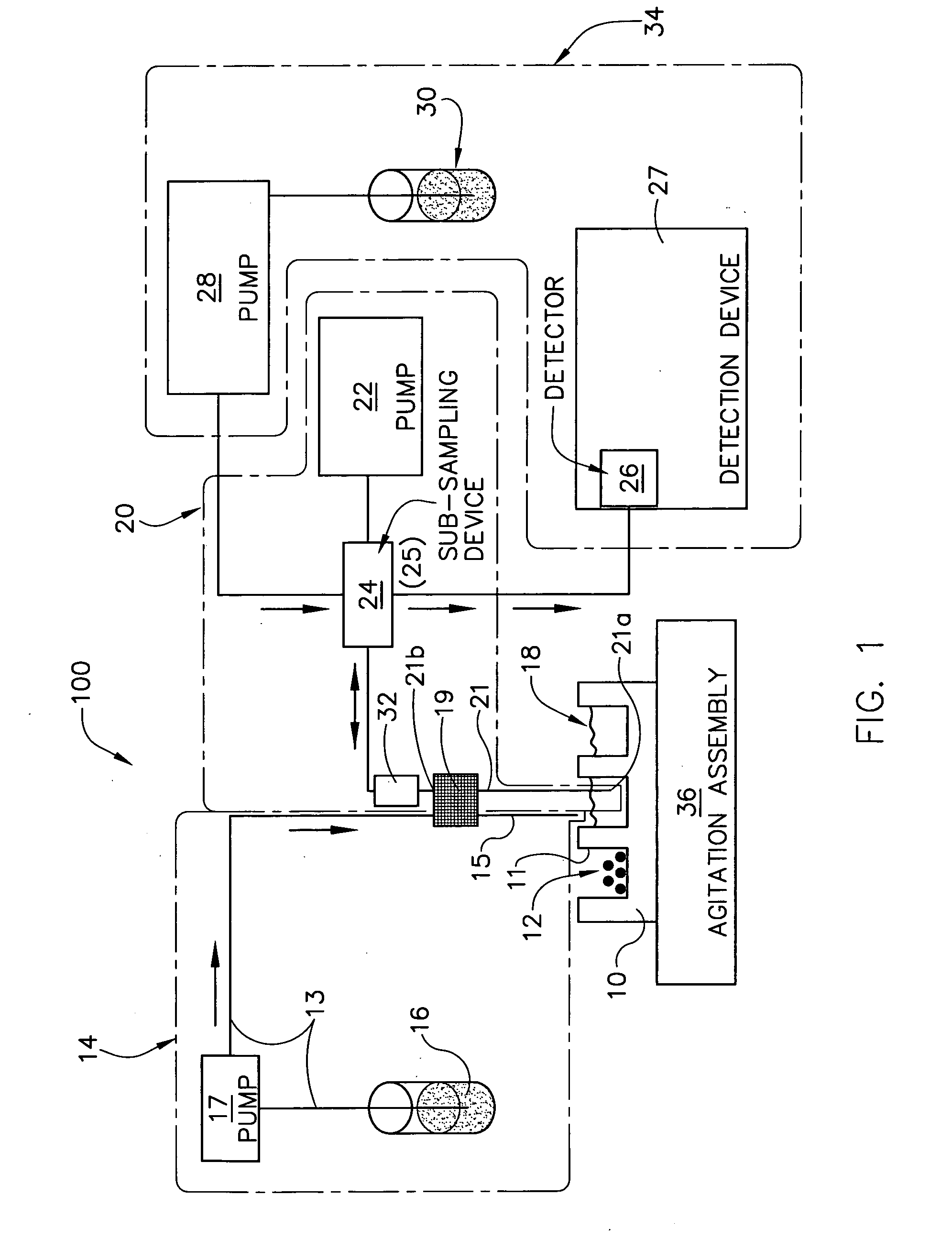
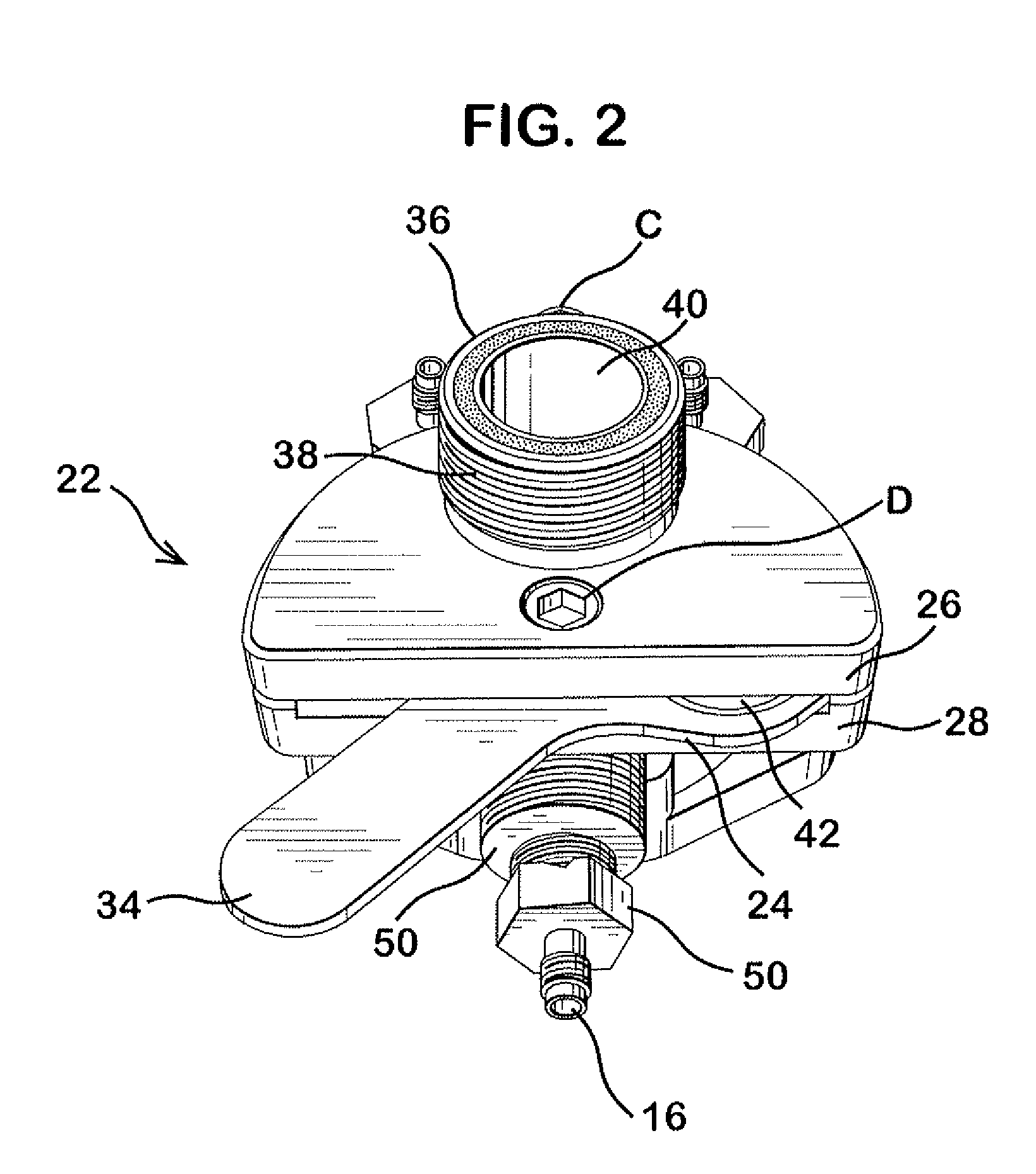
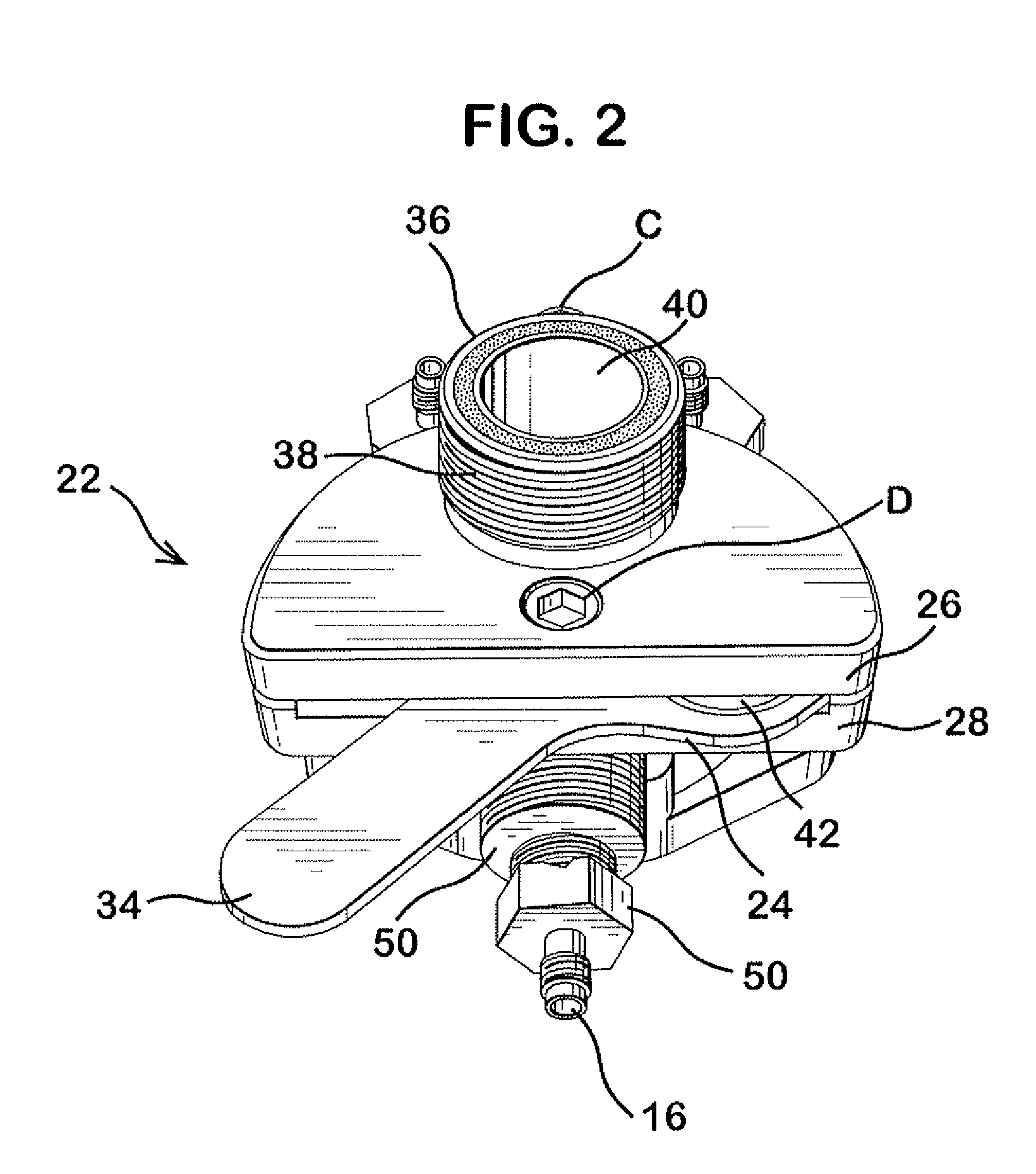
Dissolution test sampling
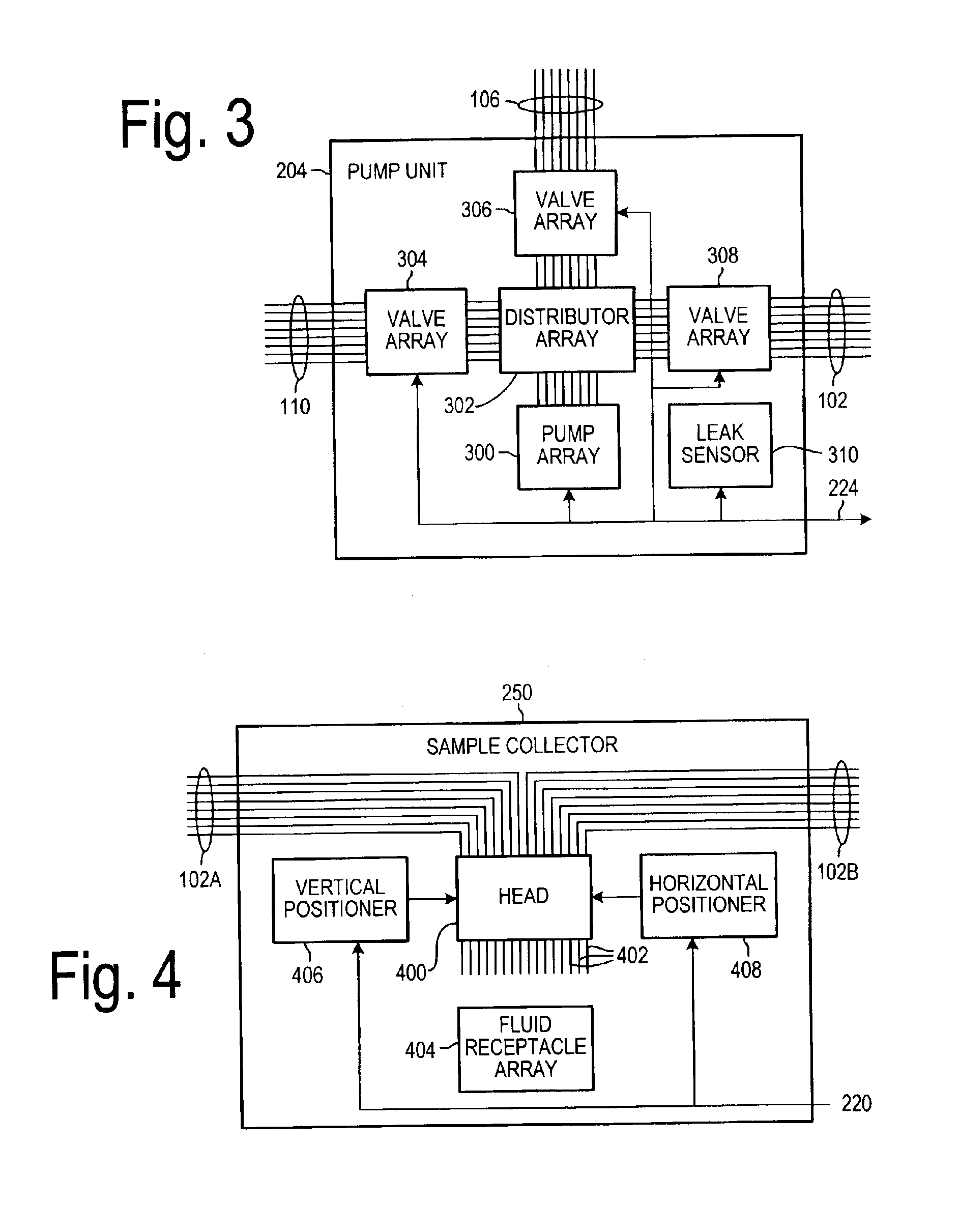
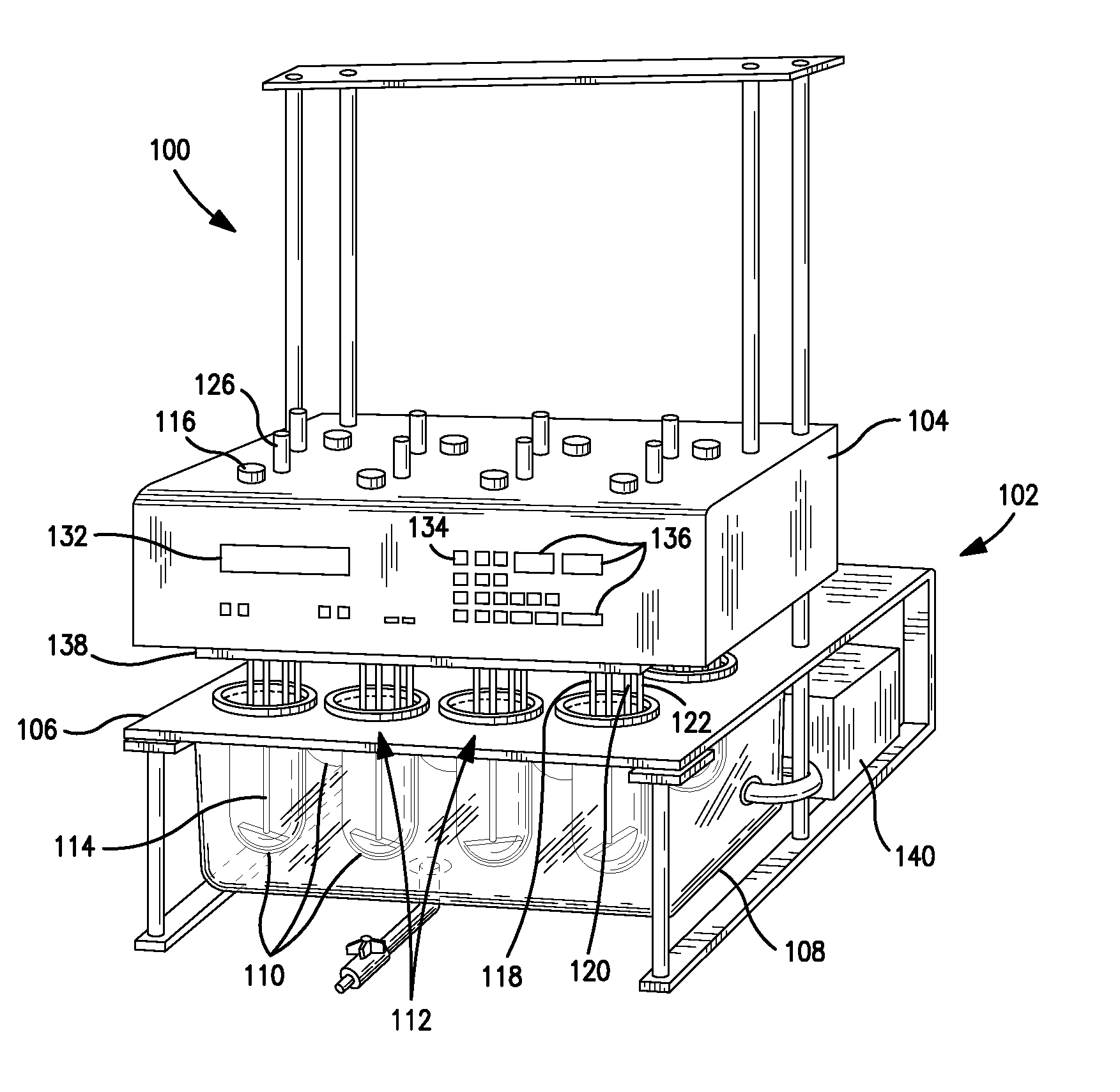
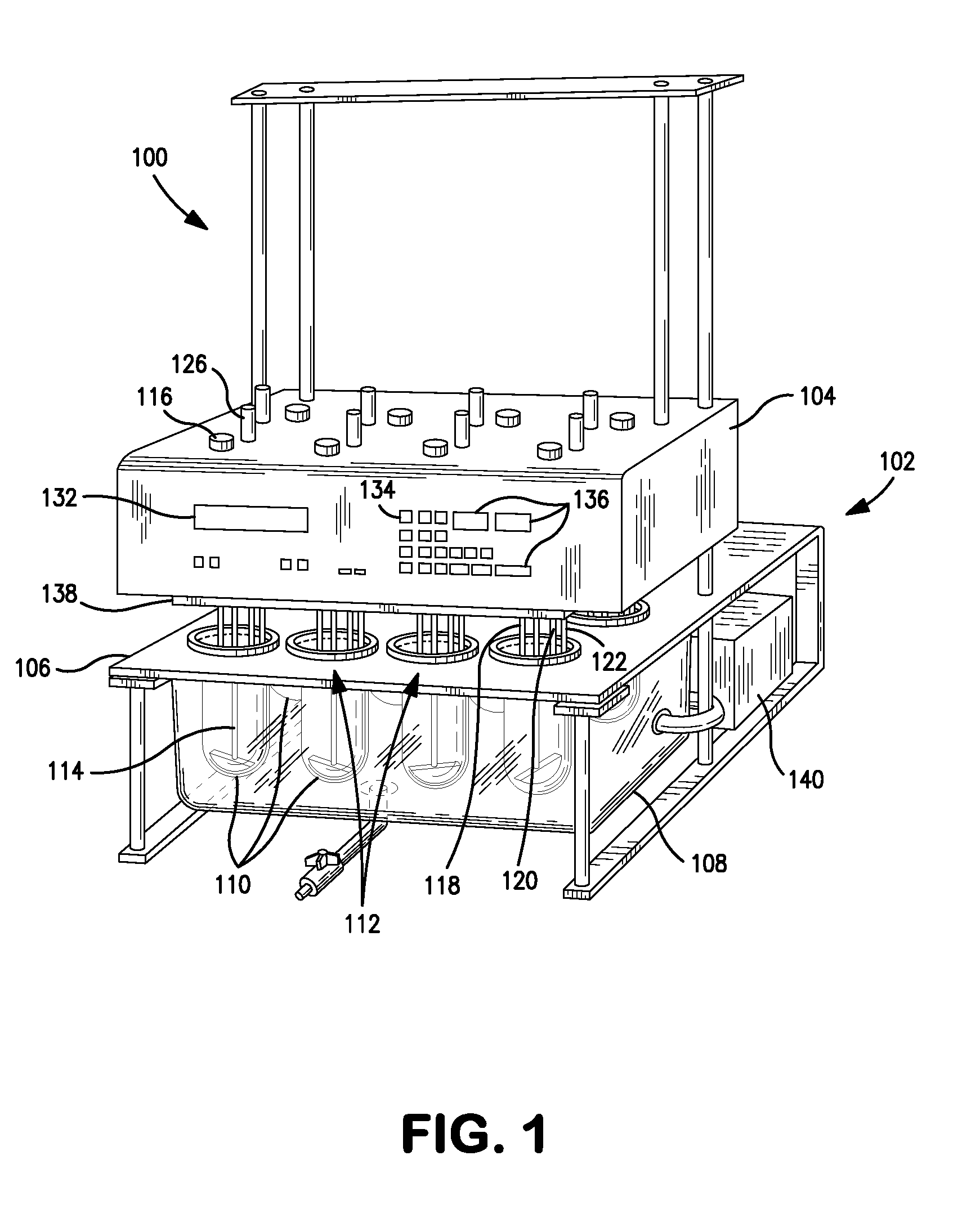
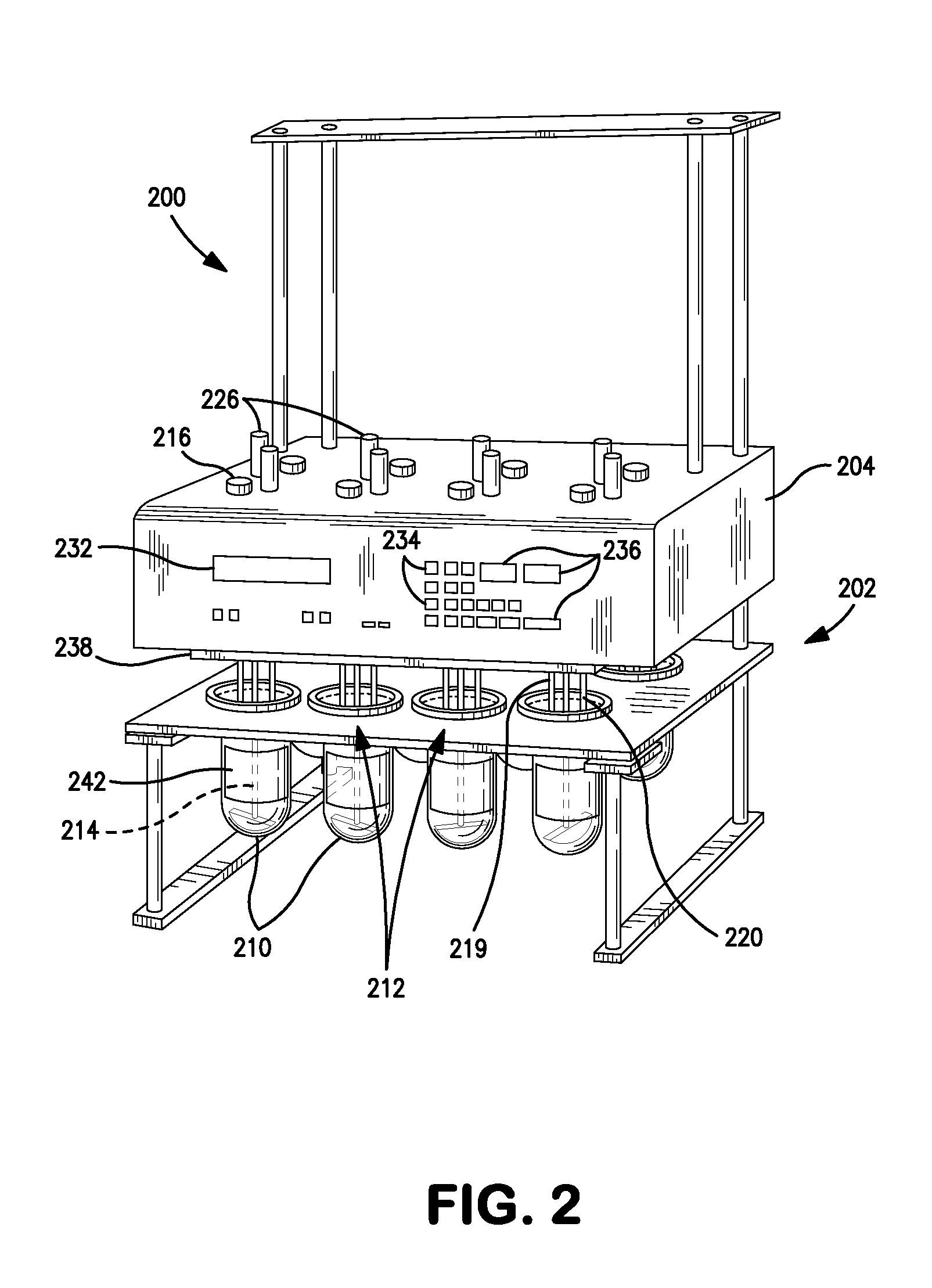
InactiveUS6948389B2Simple methodWithdrawing sample devicesSurface/boundary effectPump chamberDistributor
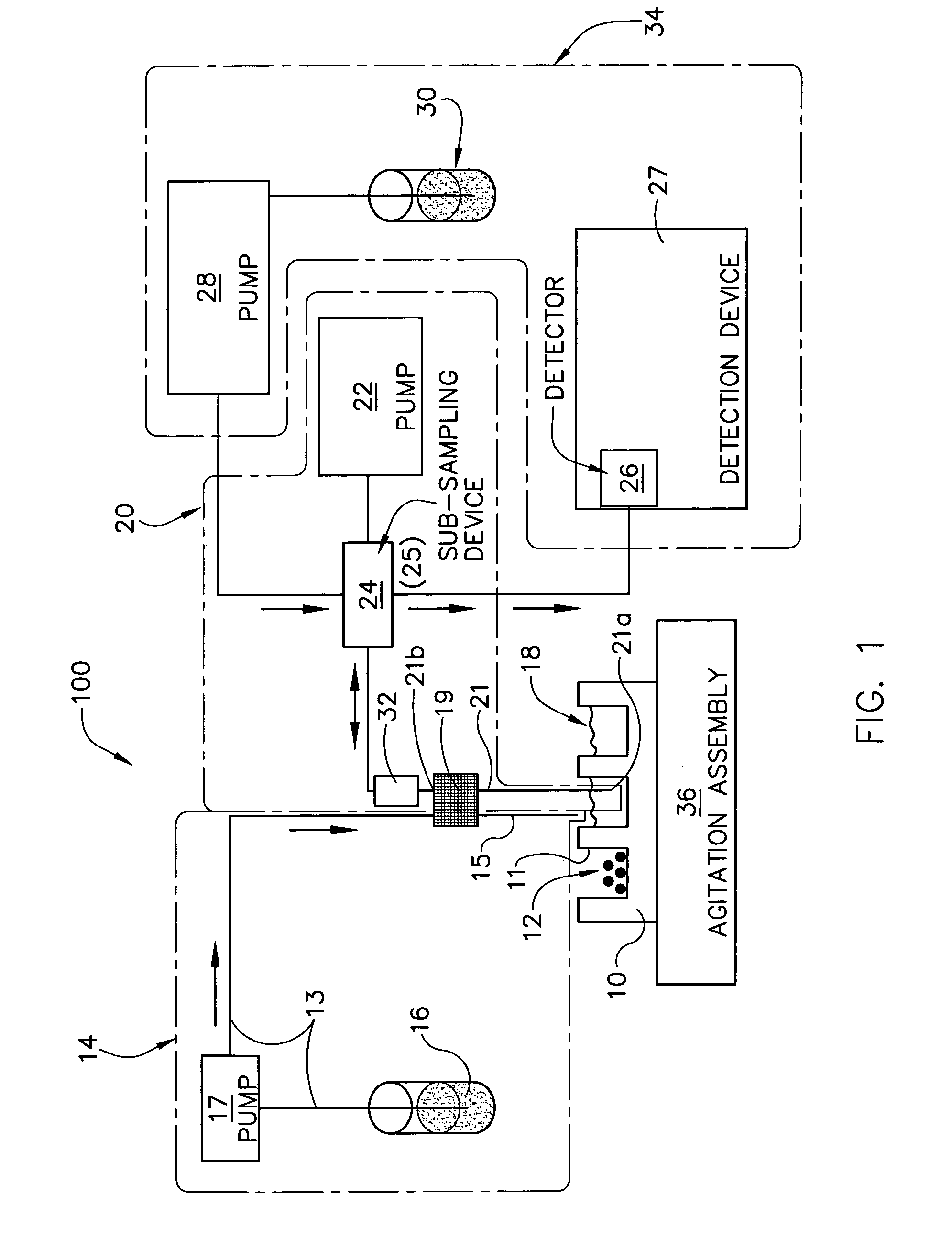
Improved sampler apparatus and methods for dissolution testing include a controller that determines whether several different dissolution testing methods can be performed concurrently by the sampler and controls the sampler in response to the determination; fluid delivery apparatus adapted to pierce a septum and to deliver fluid to or from a collection receptacle through a first aperture, and to permit air flow to or from the collection receptacle through a second aperture; a syringe pump including a distributor having apertures through which fluid flows into and out of the pump chamber, the piston of the syringe pump being moveable adjacent the apertures; and / or a leak sensor responsive to the presence of spilled fluids.
Owner:DISTEK
Dissolution testing with infrared temperature measurement
A dissolution test apparatus may include a vessel support member, a sensor support member, infrared temperature sensors, and an electronic controller. The vessel support member may have apertures for receiving vessels. The infrared temperature sensors are mounted at the sensor support member. Each infrared temperature sensor is positioned proximate to a respective vessel mounting site to receive infrared radiation emitted by media contained in a vessel mounted at the vessel mounting site, and is configured to transmit a measurement signal indicative of the temperature of the media. The electronic controller communicates with the infrared temperature sensors and is configured to receive and process the measurement signals transmitted from the infrared temperature sensors. The electronic controller may be configured to control media temperature in the vessels.
Owner:AGILENT TECH INC
Methods and systems for dissolution testing
InactiveUS7024955B2Durable systemWithdrawing sample devicesSurface/boundary effectSmall sampleDrug candidate
Methods and systems for determining a dissolution profile of a sample material, and for solubilization screening of a library defined by an array comprising multiple sample materials are disclosed. The methods and systems are particularly advantageous for sampling and evaluation of very small samples, and can be advantageously applied in connection with evaluation of drug candidates.
Owner:UNCHAINED LABS
Dissolution testing with in-situ gravimetric volume measurement
InactiveUS7938032B2Shaking/oscillating/vibrating mixersWeather/light/corrosion resistanceElectronic controllerControl signal
A dissolution test apparatus includes a vessel support member, weight sensors, a movable component, a media transport cannula, a pump, and an electronic controller. The vessel support member receives vessels. A weight sensor is located at each vessel site. Each weight sensor contacts a vessel and transmits a measurement signal indicative of the weight of the vessel and any contents therein. The movable component moves the media transport cannula toward a vessel site. The pump establishes media flow between the media transport cannula and the selected vessel. The controller communicates with the weight sensors and may also communicate with the pump. Based on the measurement signals received from the weight sensors, the electronic controller may calculate the volume of media in a given vessel. The electronic controller may also control media flows to or from the vessels by transmitting control signals to the pump assembly.
Owner:AGILENT TECH INC
Arginine ibuprofen tablet and preparation method thereof
ActiveCN101390844AFast-acting antipyreticFast-acting analgesiaOrganic active ingredientsAntipyreticMedicineArginine
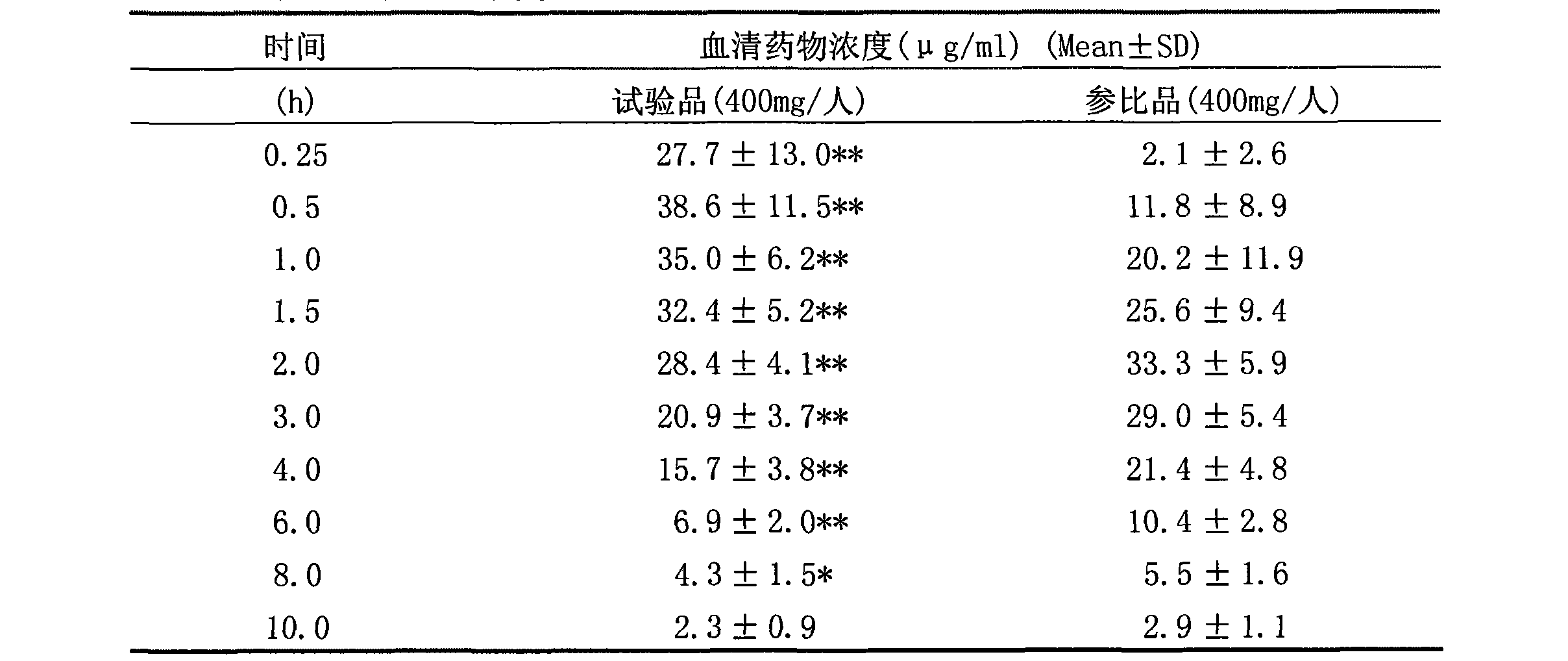
The invention relates to an arginine ibuprofen tablet which is antipyretic and analgesic, and a preparation method. The arginine ibuprofen tablet contains arginine ibuprofen and auxiliary material by weight proportion: 30%-90% of arginine ibuprofen, 10%-70% of auxiliary material and 0-10% of coating material. The preparation method adopts raw material direction compression or granule preparation; the prepared tablet can be coated or not be coated. The arginine ibuprofen tablet has short response time. As indicated in dissolution test, the dissolution rate is more than 95% in 20 minutes. 20 male volunteers take two preparations of arginine ibuprofen tablets (equal to 400 mg of ibuprofen) and ibuprofen tablets (400mg) for relative bioavailability research; the peak time of arginine ibuprofen tablet is 0.5h and the peak time of ibuprofen tablet is 2.0h. The test indicates that the arginine ibuprofen tablet has quick absorption and short peak time. Besides, the arginine ibuprofen tablet overcomes the pungent taste of arginine ibuprofen syrup and granules and can be taken by patients conveniently, so as to have the advantages of convenient carrying and low cost.
Owner:TIANJIN MEDICAL UNIV
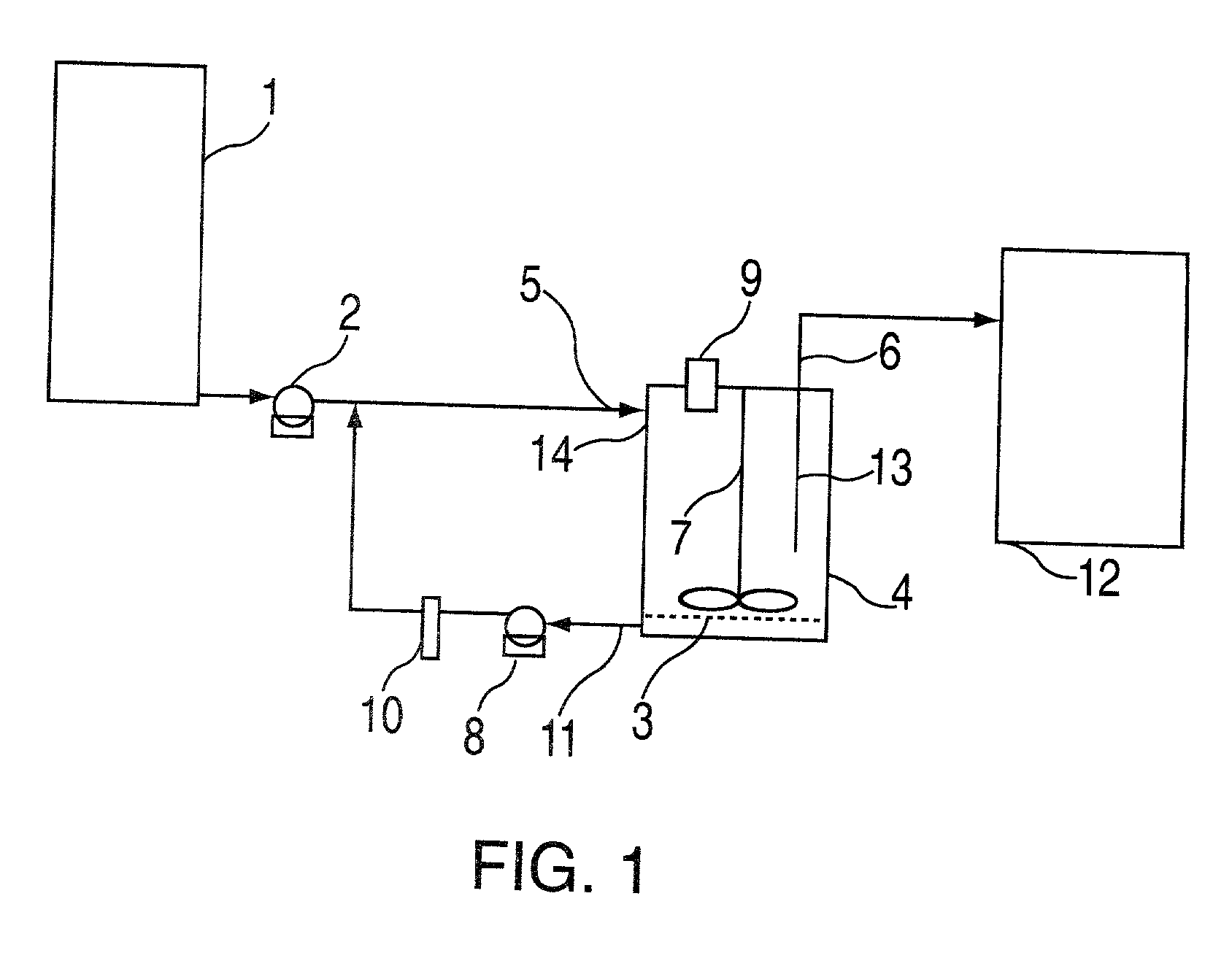
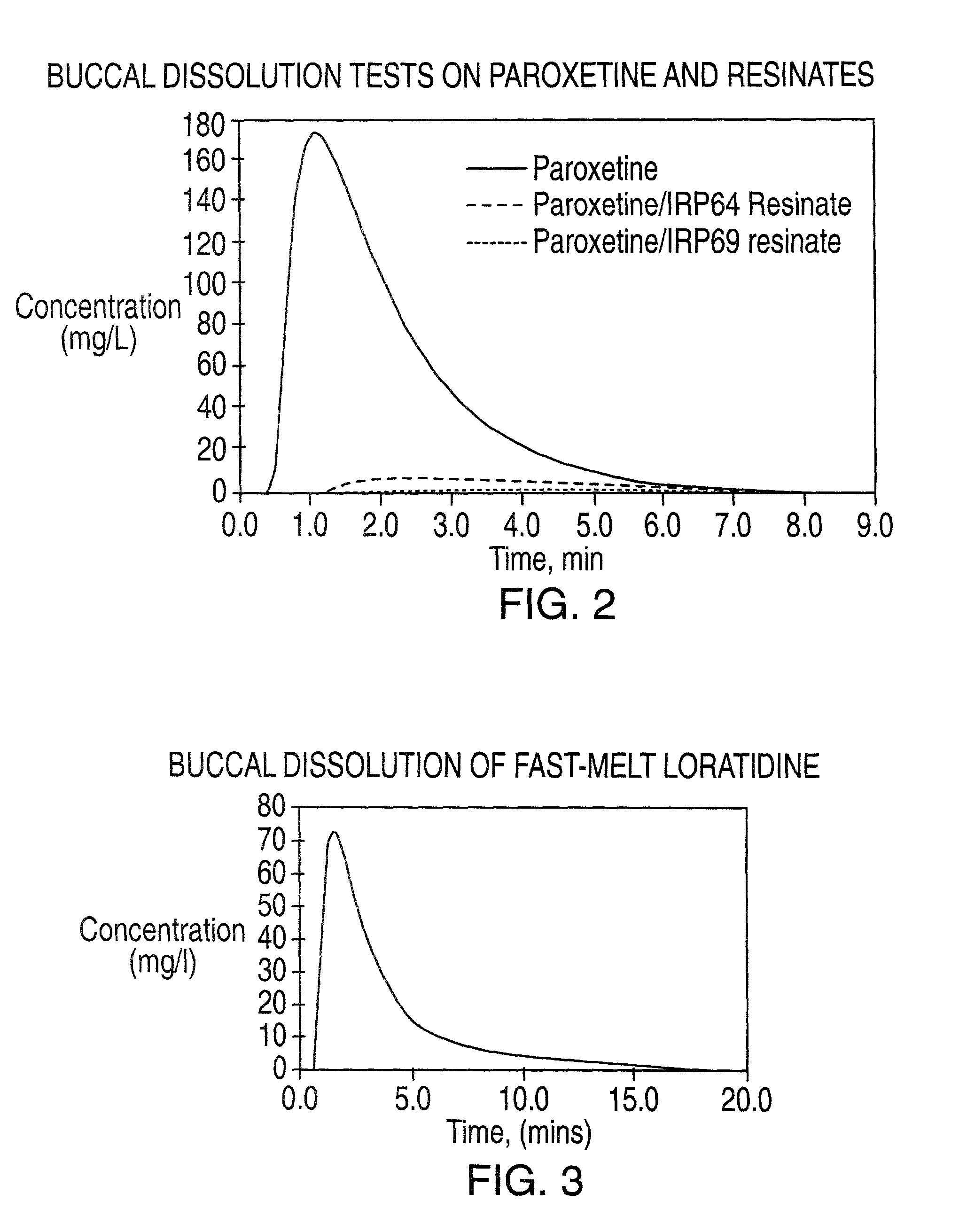
Dissolution testing of solid dosage forms intended to be administered in the oral cavity
The invention is a method and device for determining dissolution of a solid compound within the oral cavity. The device models dissolution within the oral cavity with a flow-through cell containing a solid compound and physiological amounts of simulated saliva. The device supplies and removes the simulated saliva at rates similar to production and loss of saliva within the oral cavity. The simulated saliva interaction with the solid compound mimics saliva interaction with a solid compound within the oral cavity. Dissolution of solid compound is determined from simulated saliva collected from the flow-through cell outflow.
Owner:IDAHO STATE UNIVERSITY
Methods and systems for dissolution testing
Methods and systems for determining a dissolution profile of a sample material, and for solubilization screening of a library defined by an array comprising multiple sample materials are disclosed. The methods and systems are particularly advantageous for sampling and evaluation of very small samples, and can be advantageously applied in connection with evaluation of drug candidates.
Owner:FREESLATE
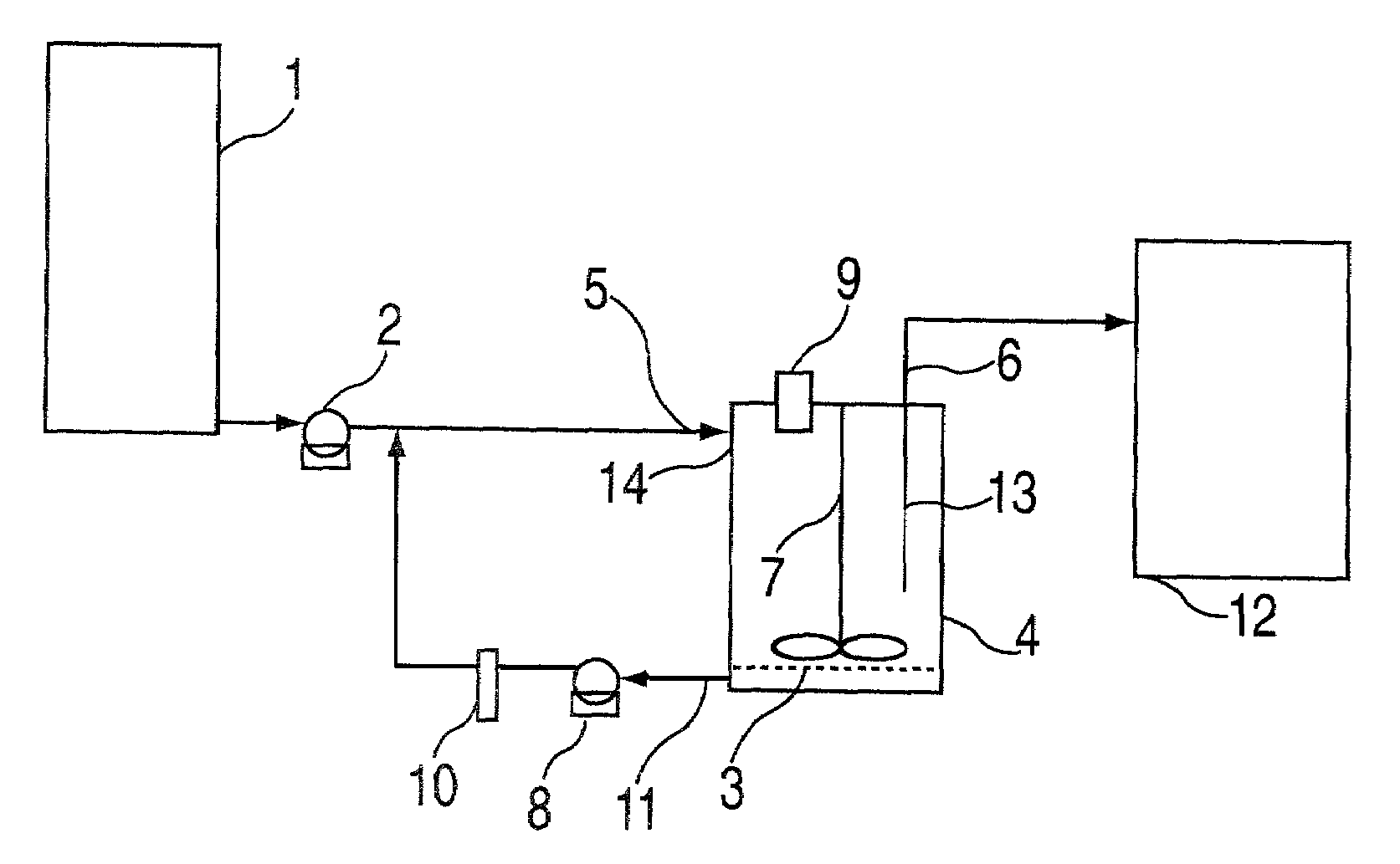
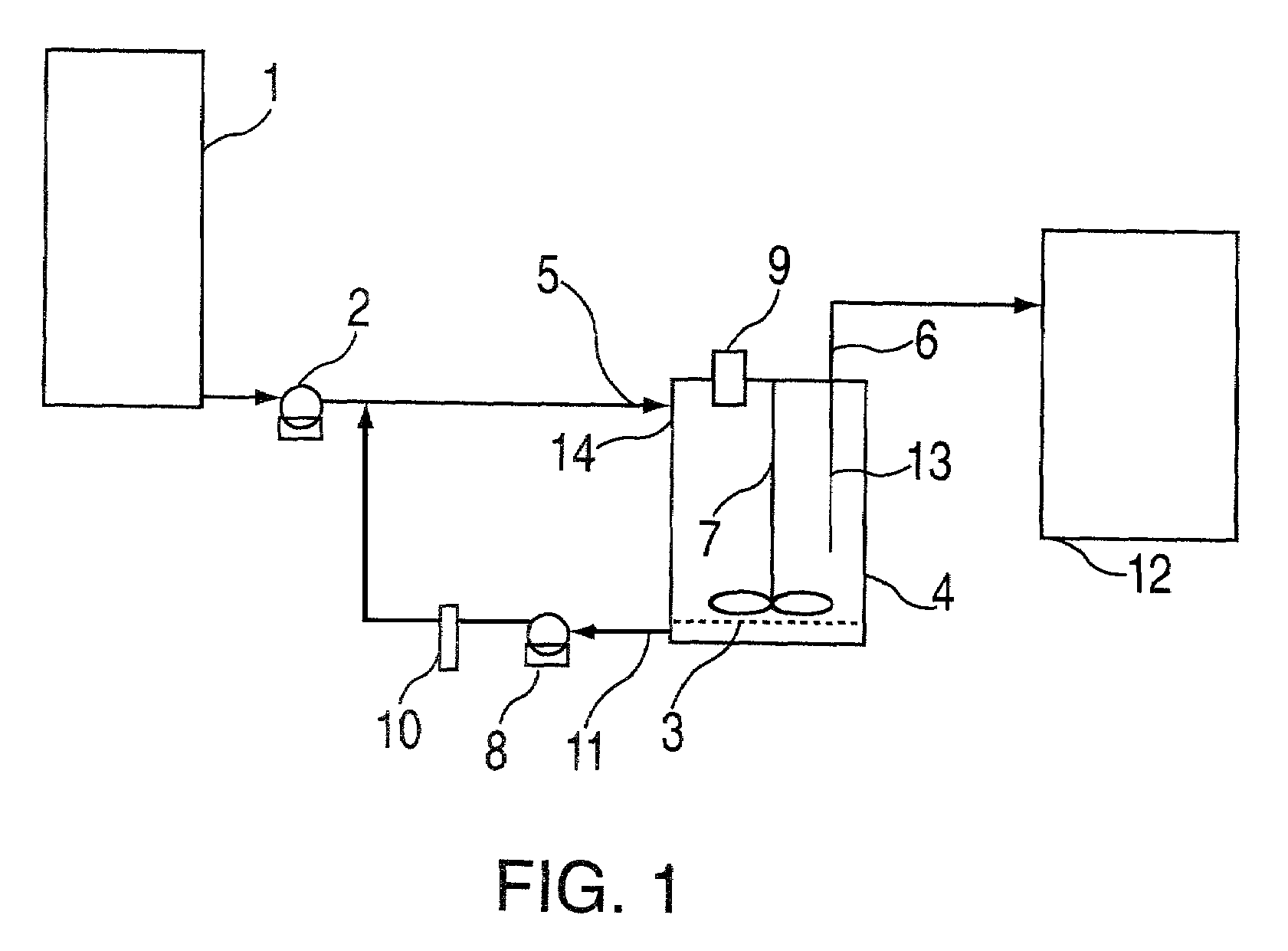
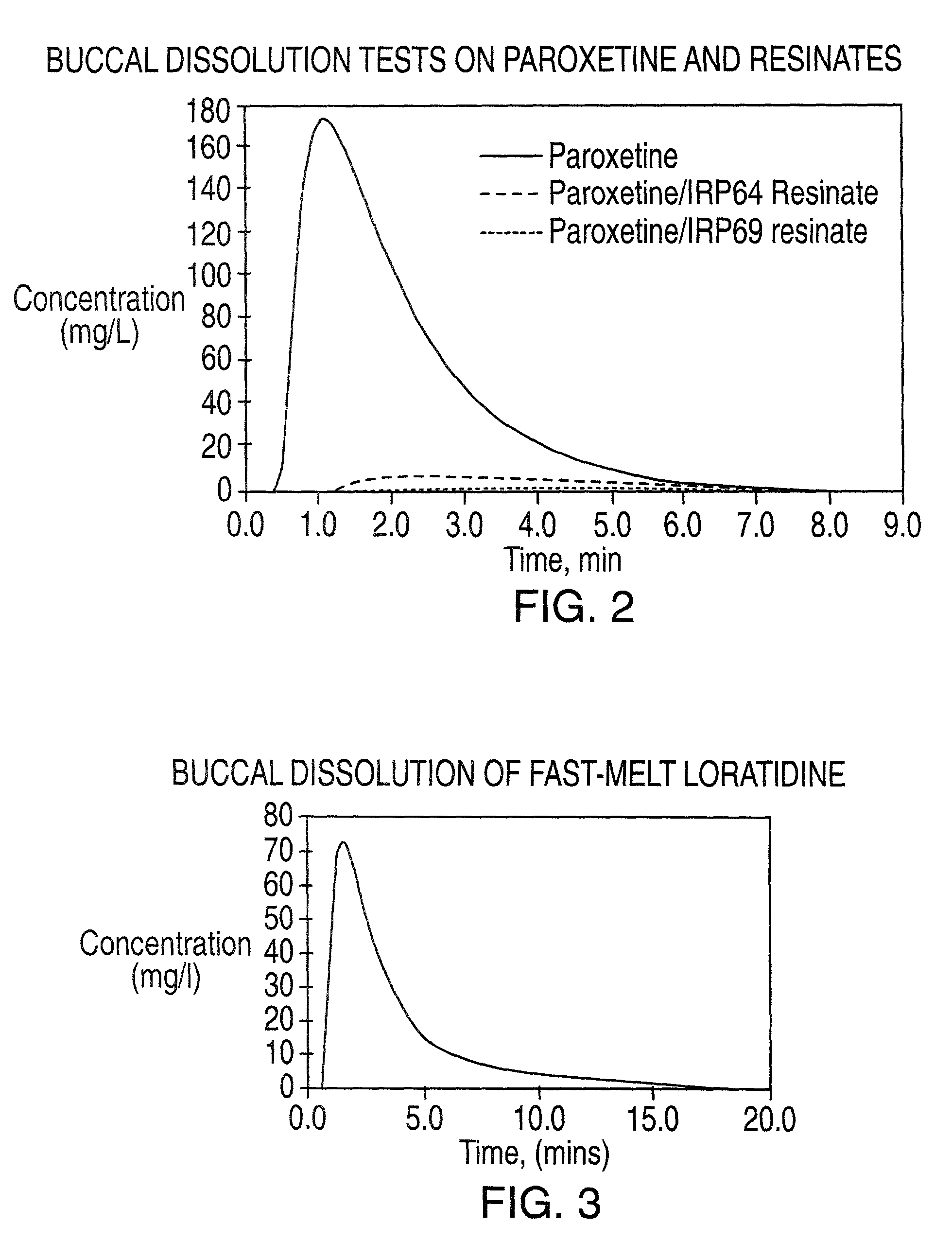
Buccal dissolution of active substances
InactiveUS20030087457A1Short stayReduce flow rateAnalysis using chemical indicatorsWithdrawing sample devicesOral agentsOral administration
Equipment and method of use for in vitro buccal dissolution testing. The invention is particularly useful for evaluating the effect of taste-masking in oral dosage forms.
Owner:ROHM & HAAS CO
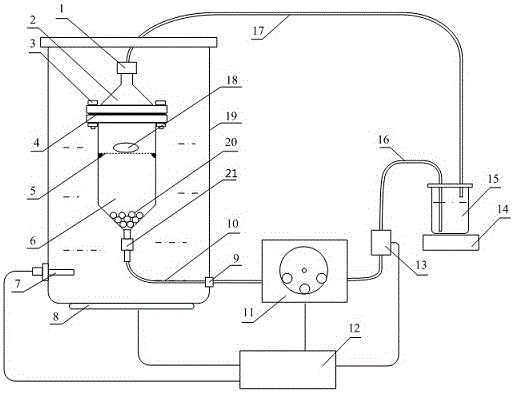
Simple type test device for dissolution rate of flowing pool
InactiveCN105784952ASimple structureEasy to operateTesting medicinal preparationsPeristaltic pumpMicrosphere
The invention discloses a simple type test device for a dissolution rate of a flowing pool. The simple type test device comprises the flowing pool, a filter, a multichannel peristaltic pump, a heating pool, a solvent storage bottle and a controller, wherein an upper opening of the flowing pool is in sealing connection with one opening of the filter; the other opening of the filter is communicated with the solvent storage bottle through a hose a; a lower opening of the flowing pool is connected with one end of a hose b; the other end of the hose b is connected with an outlet of the multichannel peristaltic pump; an inlet of the multichannel peristaltic pump is communicated with the solvent storage bottle through a hose c; the flowing pool is arranged in the heating pool. The simple type test device can work in an open or closed form; the heating temperature and the solvent flow rate are controlled; the simple type test device has the characteristics of simple structure, convenience in operation, low cost, and the like and is fit for determining the releasing rate of various drug sustained-release preparations, such as drug release brackets, implants, microspheres, microchips, lipidosome, of institutions of colleges, research institutions, pharmaceutical factory, and the like.
Owner:YANBIAN UNIV
Methods and systems for dissolution testing
Methods and systems for determining a dissolution profile of a sample material, and for solubilization screening of a library defined by an array comprising multiple sample materials are disclosed. The methods and systems are particularly advantageous for sampling and evaluation of very small samples, and can be advantageously applied in connection with evaluation of drug candidates.
Owner:FREESLATE
Dissolution test equipment
ActiveUS20120034704A1Transportation and packagingWithdrawing sample devicesFiltrationBiomedical engineering
Apparatus and method for dissolution testing of active substances in various dosage forms is provided. The apparatus has filtration cells equipped and configured to simulate bodily functions, operate continuously and facilitate testing various types of dosage forms including, but not limited to, tablets, capsules and those having non-disintegrating substrates.
Owner:DDP SPECIALTY ELECTRONICS MATERIALS US 8 LLC
Dissolution testing of solid dosage forms intended to be administered in the oral cavity
The invention is a method and device for determining dissolution of a solid compound within the oral cavity. The device models dissolution within the oral cavity with a flow-through cell containing a solid compound and physiological amounts of simulated saliva. The device supplies and removes the simulated saliva at rates similar to production and loss of saliva within the oral cavity. The simulated saliva interaction with the solid compound mimics saliva interaction with a solid compound within the oral cavity. Dissolution of solid compound is determined from simulated saliva collected from the flow-through cell outflow.
Owner:IDAHO STATE UNIVERSITY
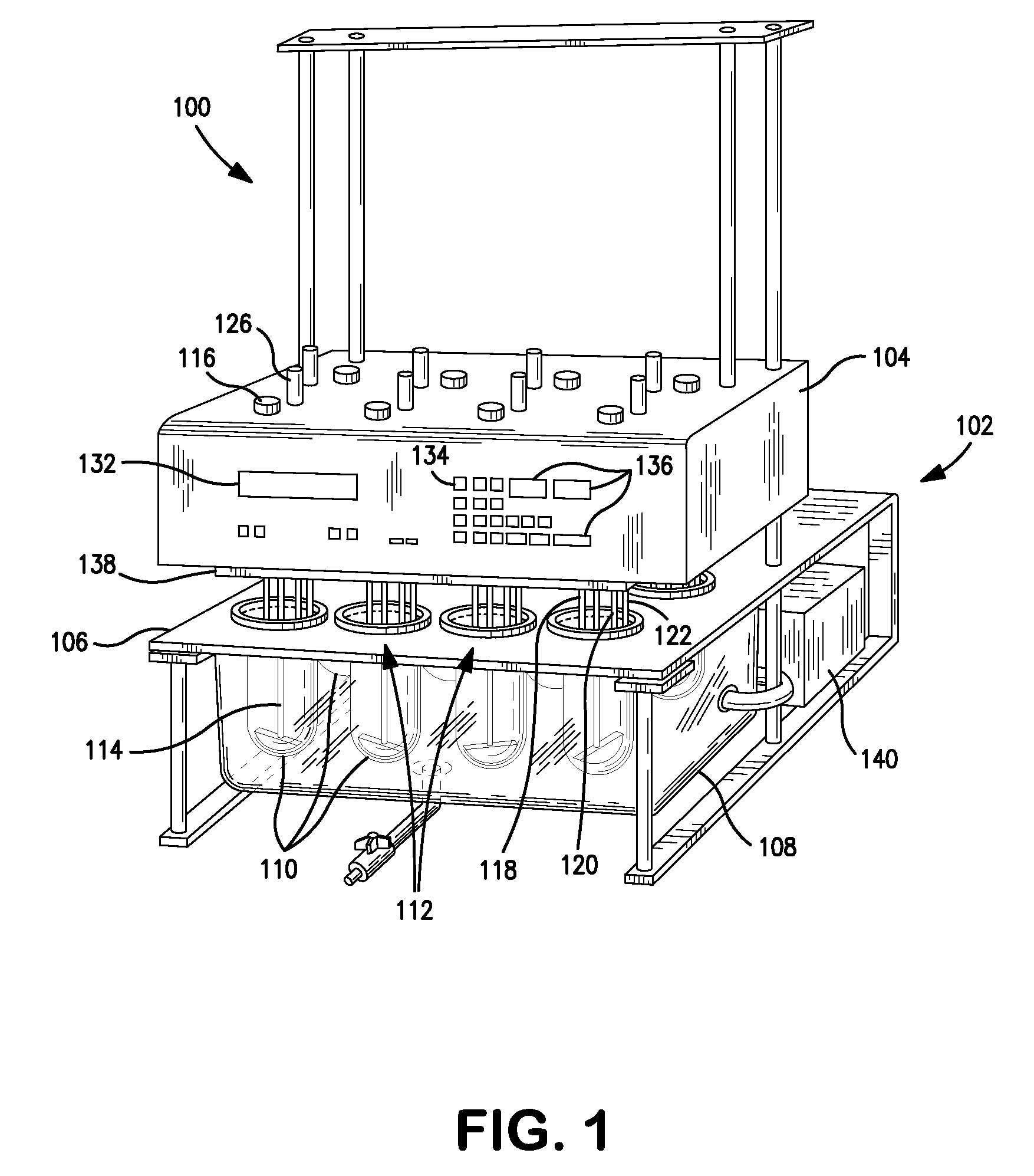
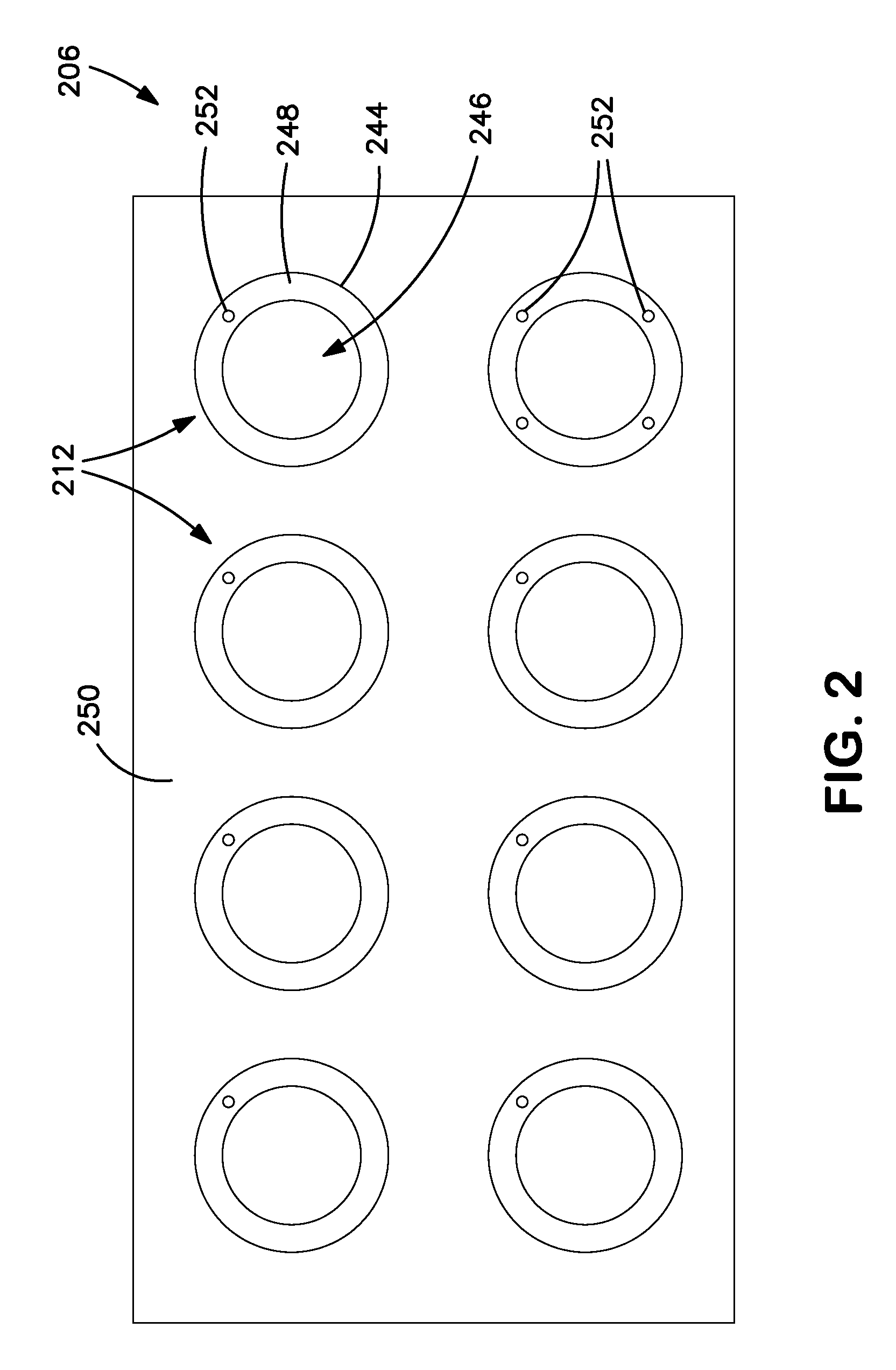
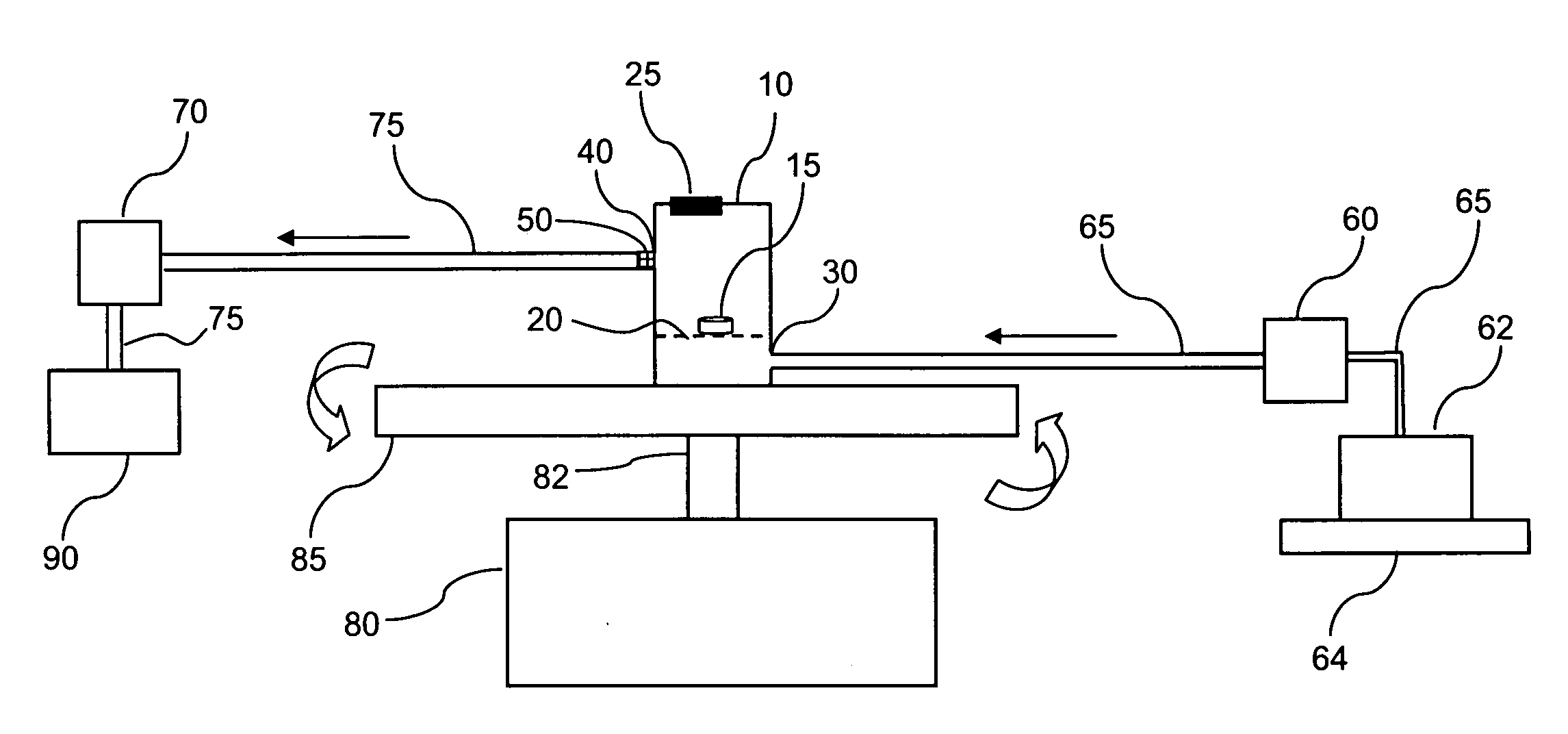
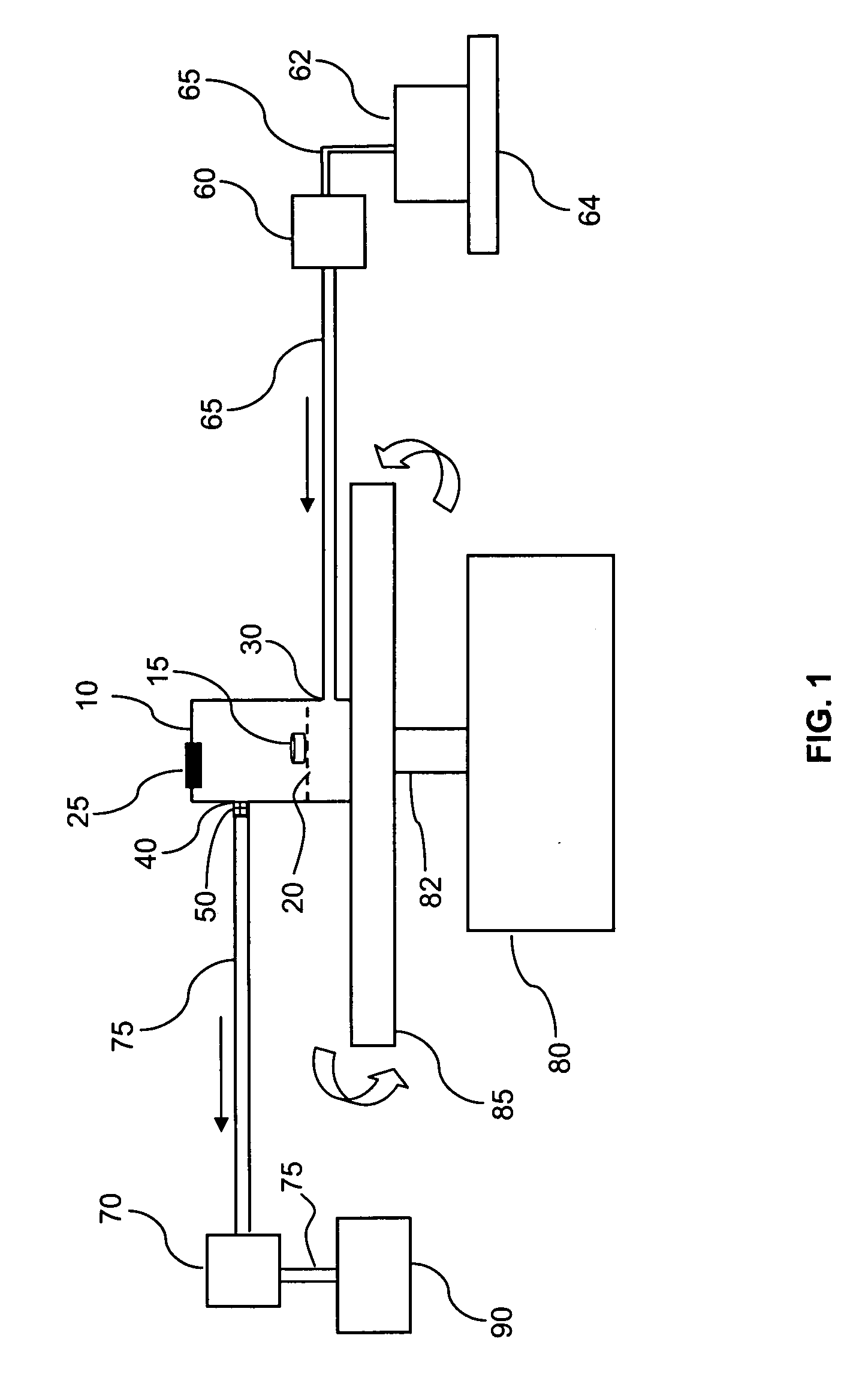
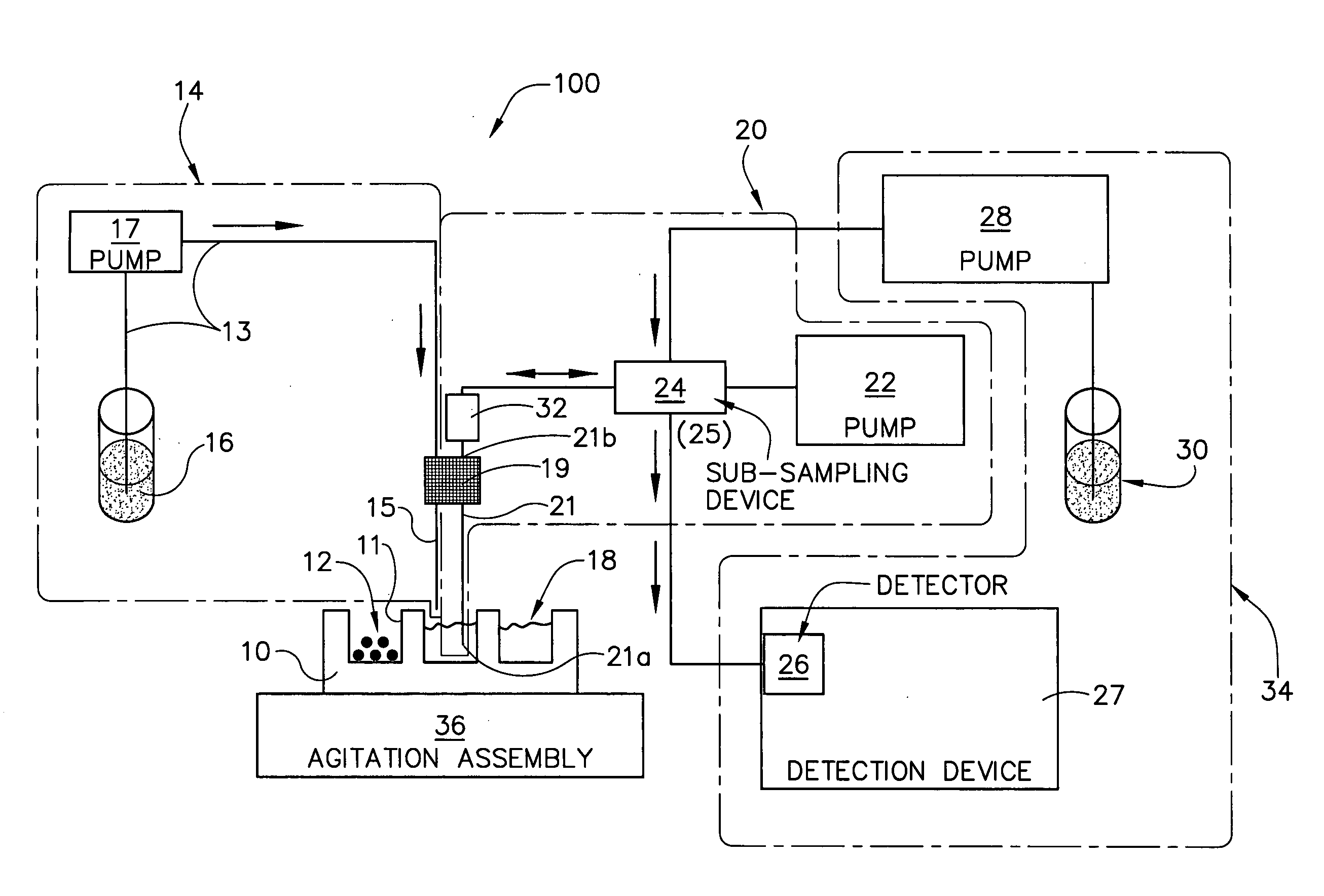
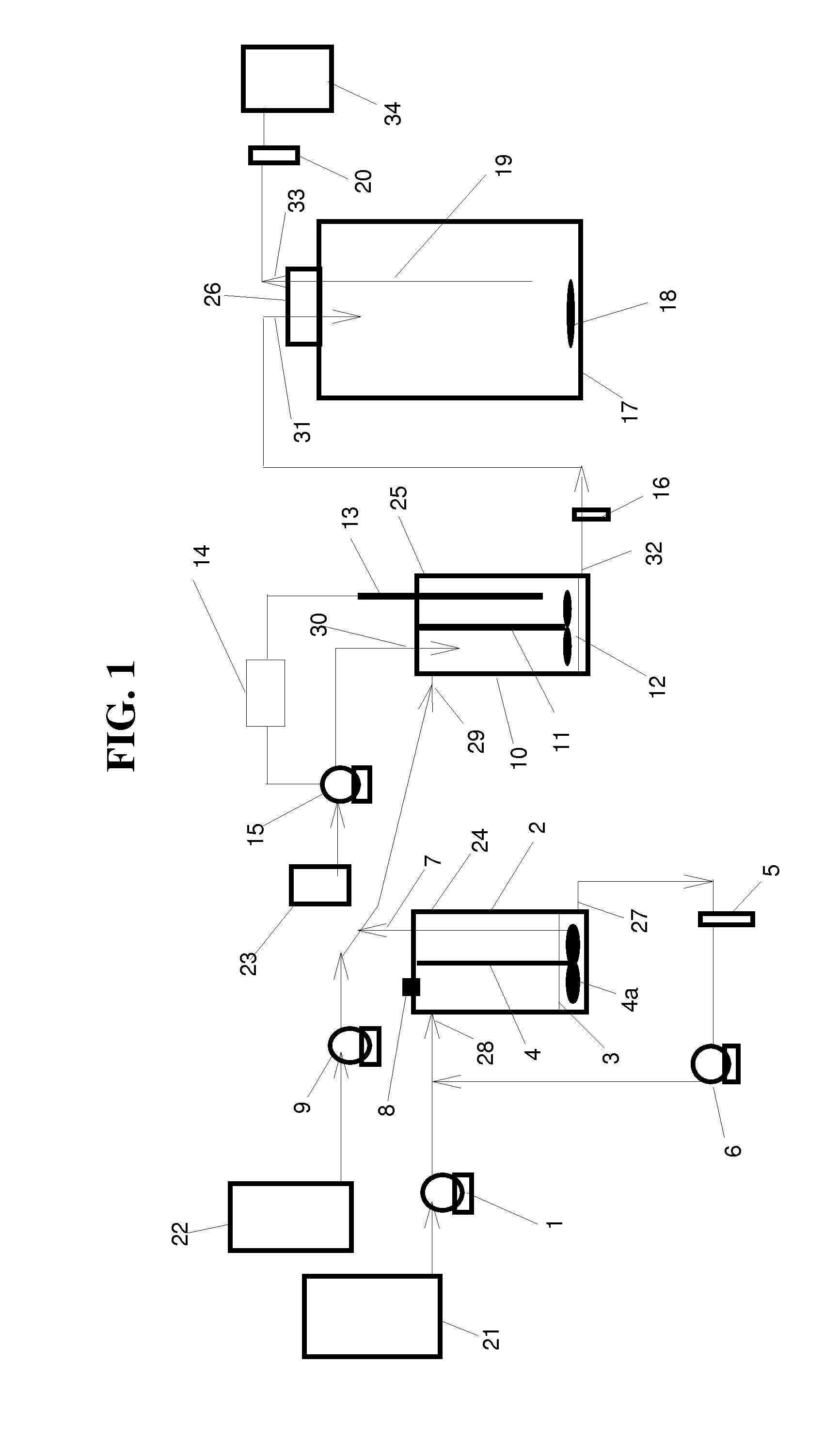
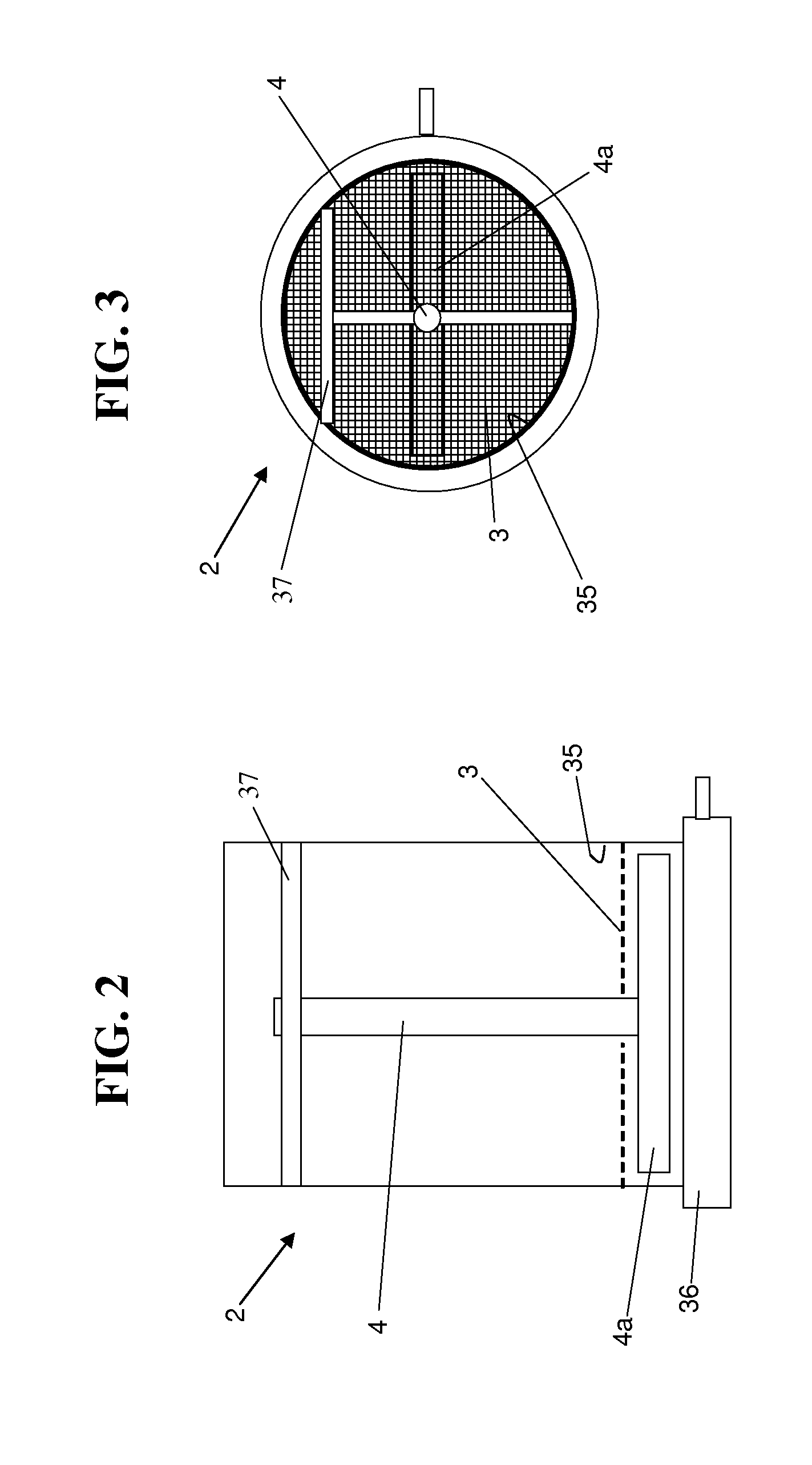
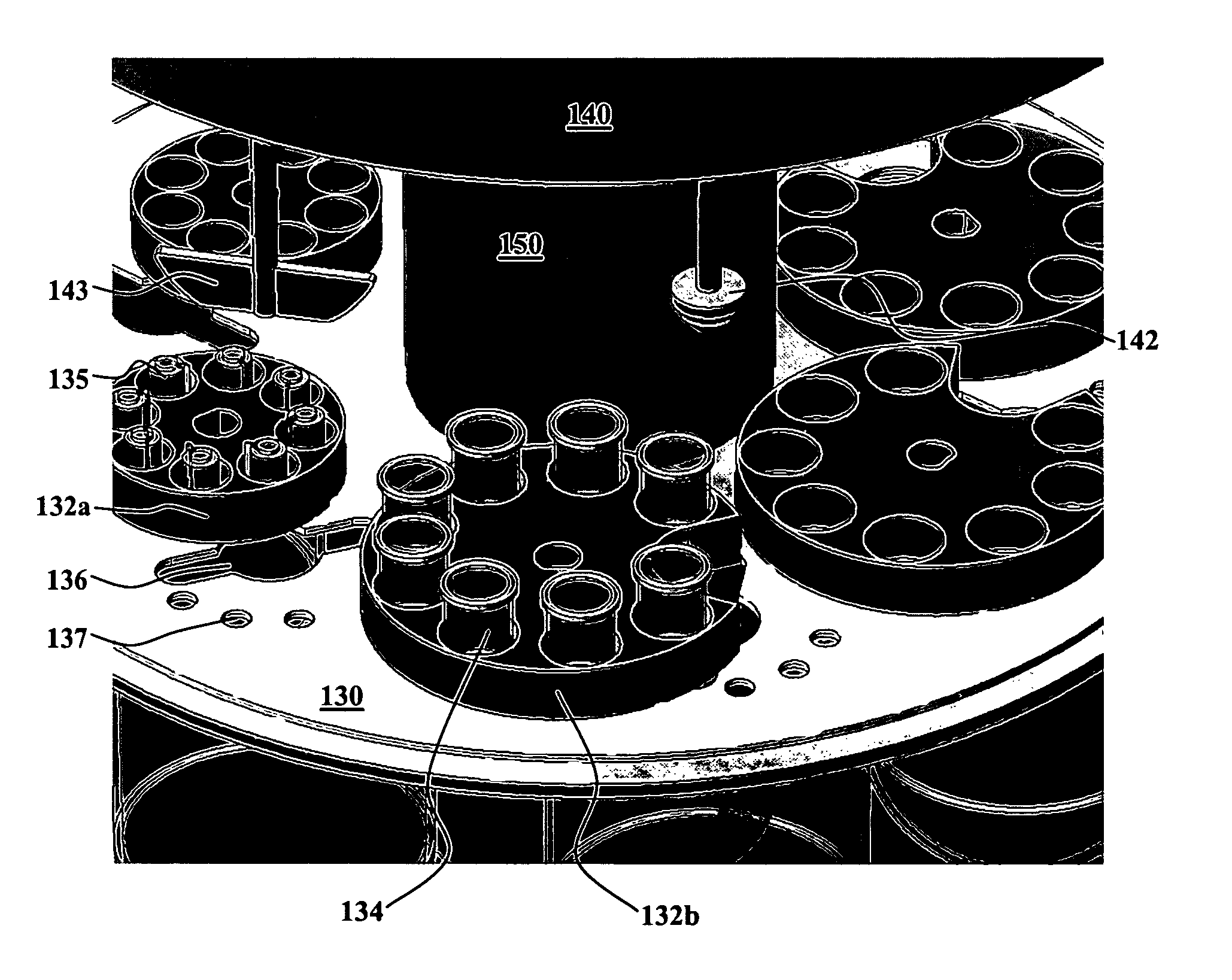
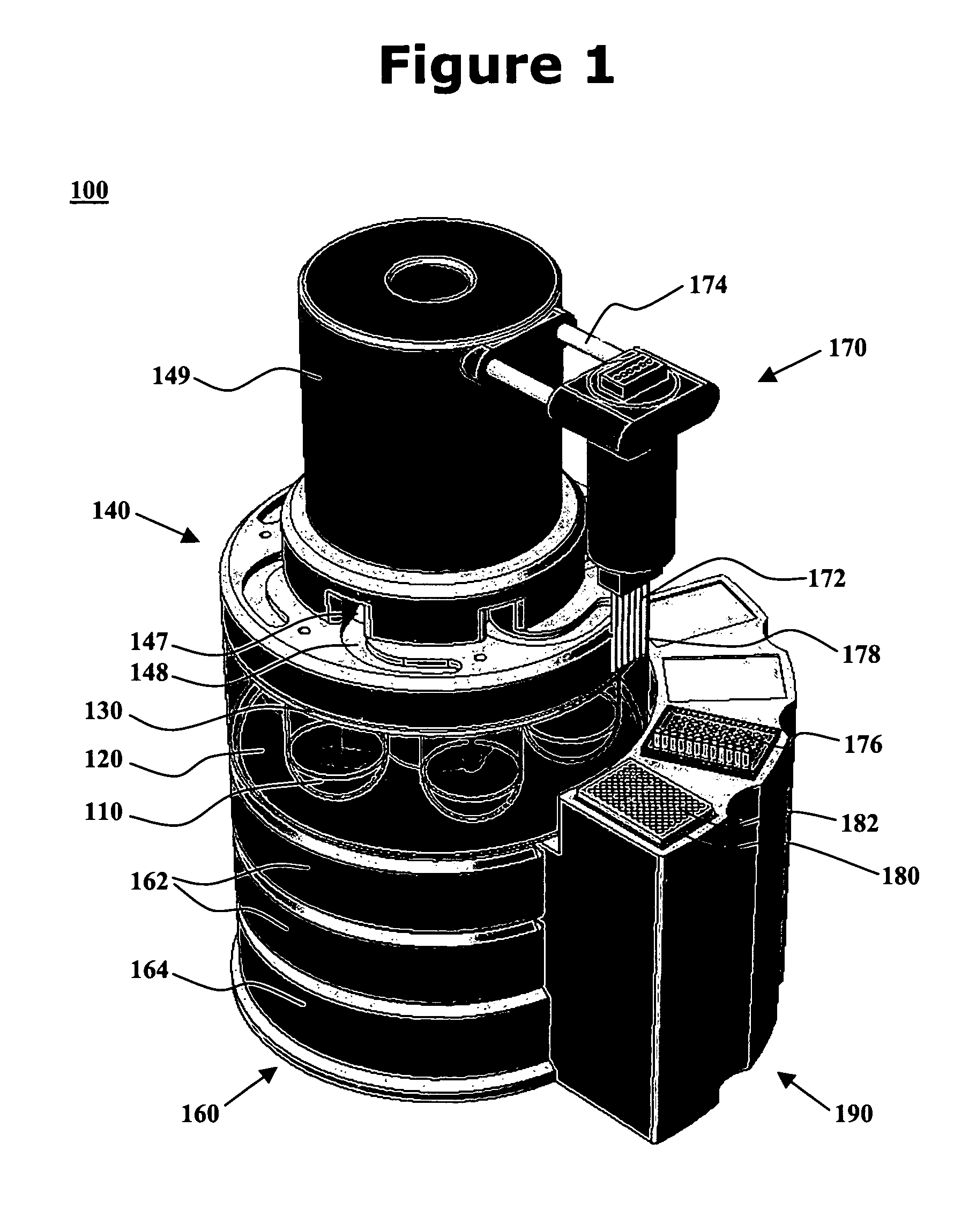
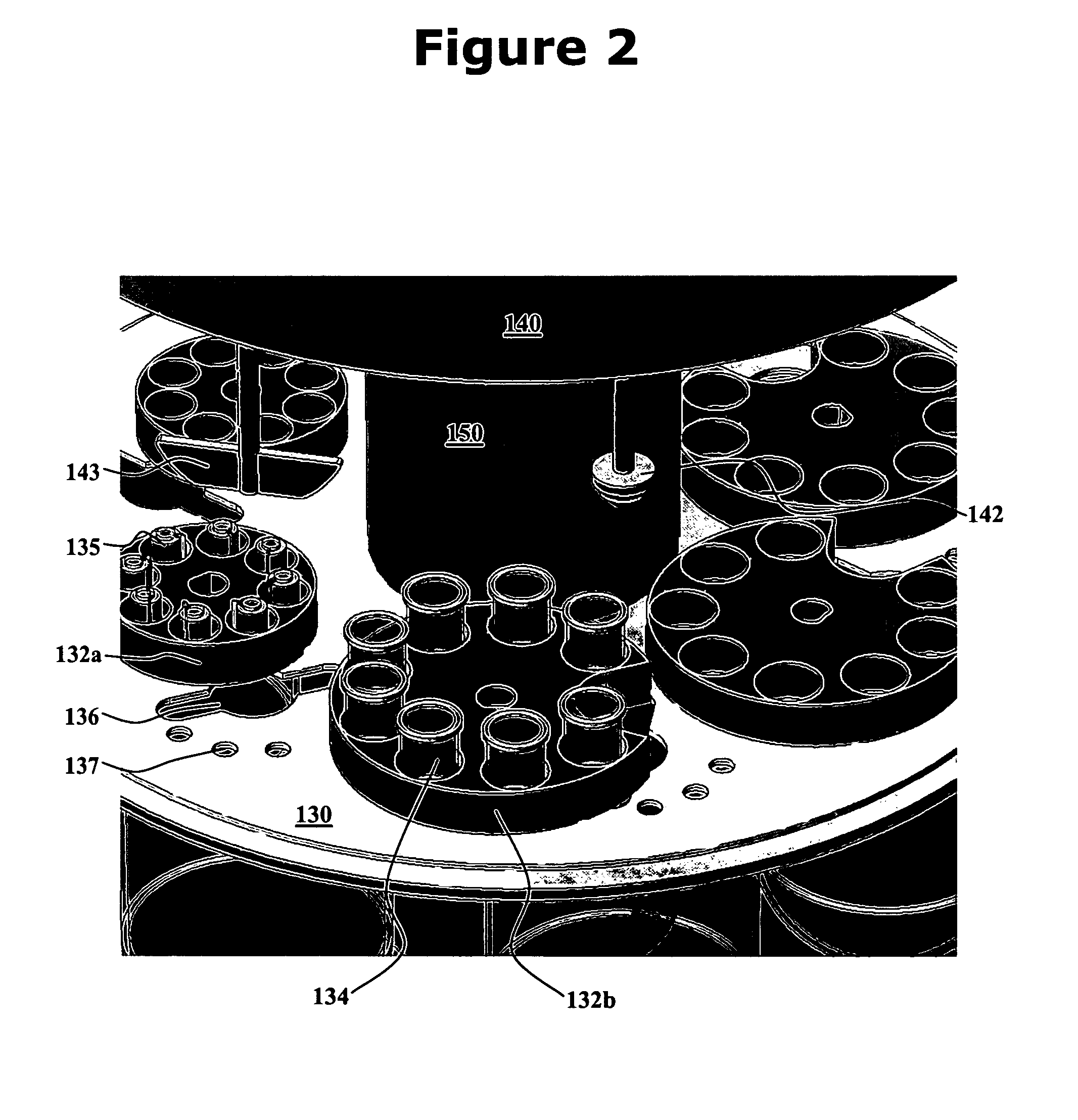
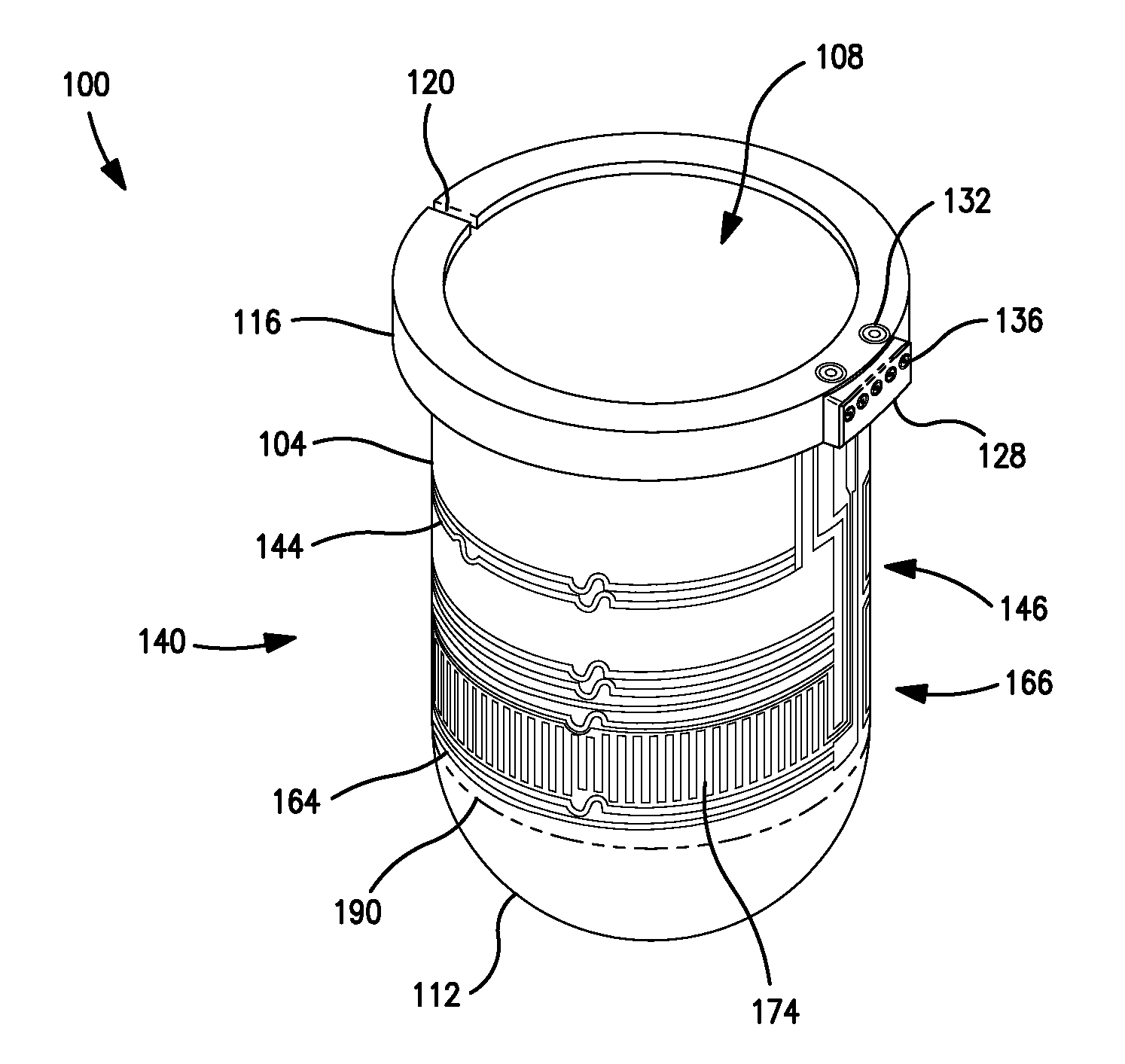
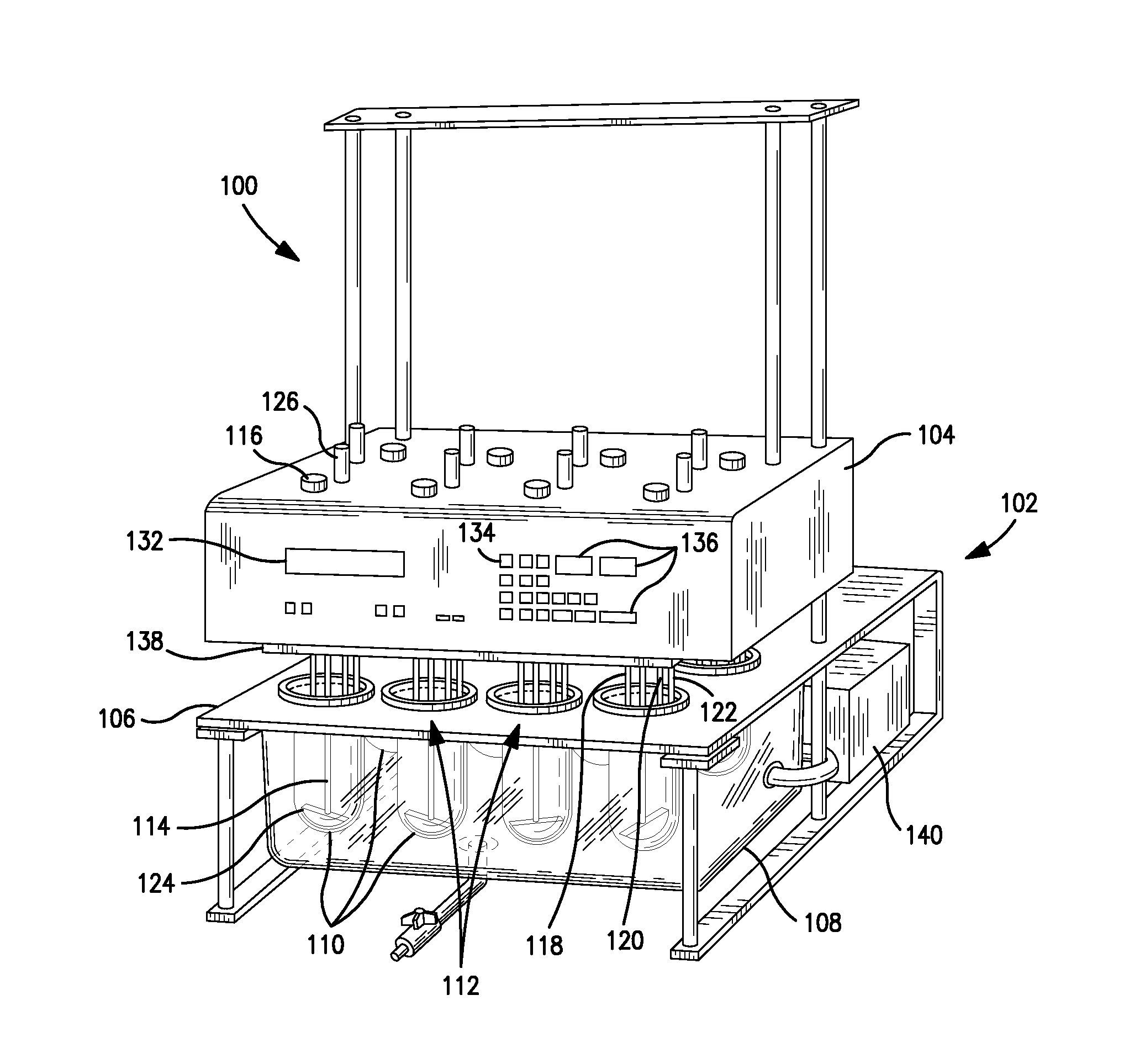
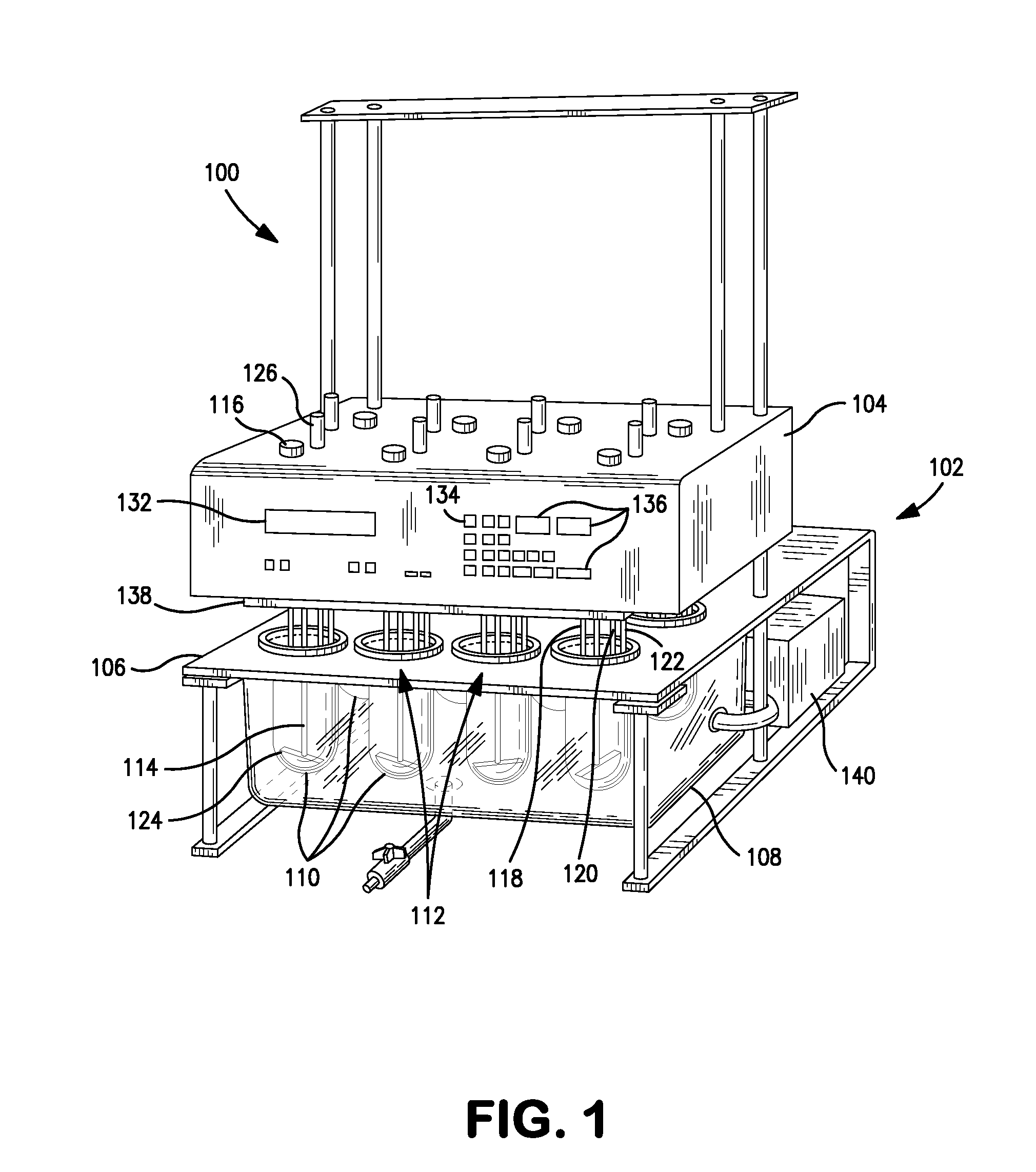
Integrated dissolution processing and sample transfer system
ActiveUS8158059B2Analysis using chemical indicatorsPreparing sample for investigationTransfer systemPipette
The invention provides a system and method for dissolution testing. The system includes multiple dissolution vessels and a dose carrier positioned above the dissolution vessels. The dose carrier holds multiple removable carousels that receive individual doses for dissolution tested. Carousels that receive tablets or sinkers typically have a first configuration, while carousels that receive baskets typically have a second configuration. The two different configurations of carousels are interchangeable on the same dose ring. The system further includes a drive head positioned above the dose carrier, the drive head having a basket arbor and a mixing paddle removably and interchangeably attached. A pipettor integral with the system transfers sample aliquots having volumes in the range of 50 μl to 1 ml from the dissolution vessels to wells of an external receptacle.
Owner:SOTAX CORP
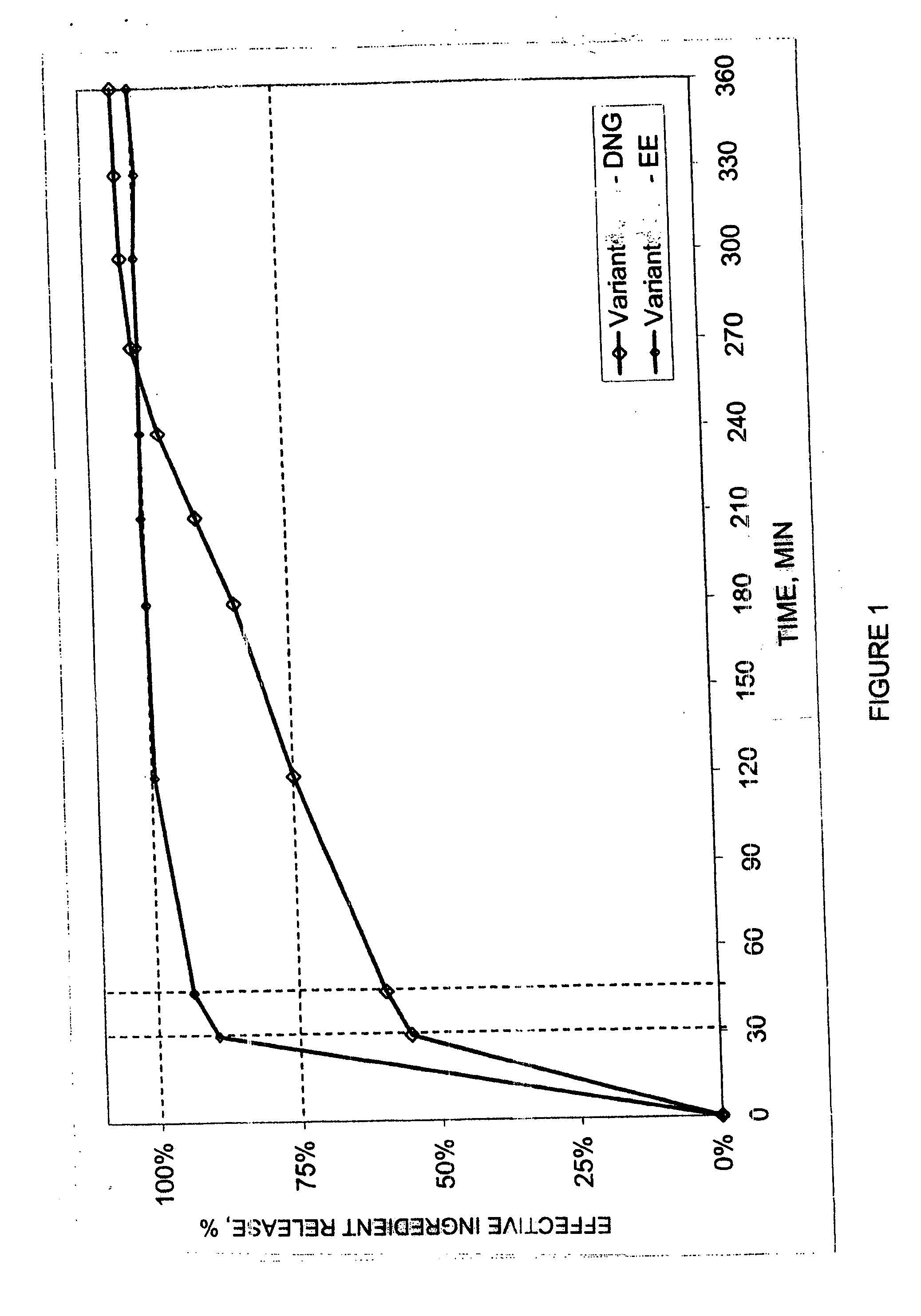
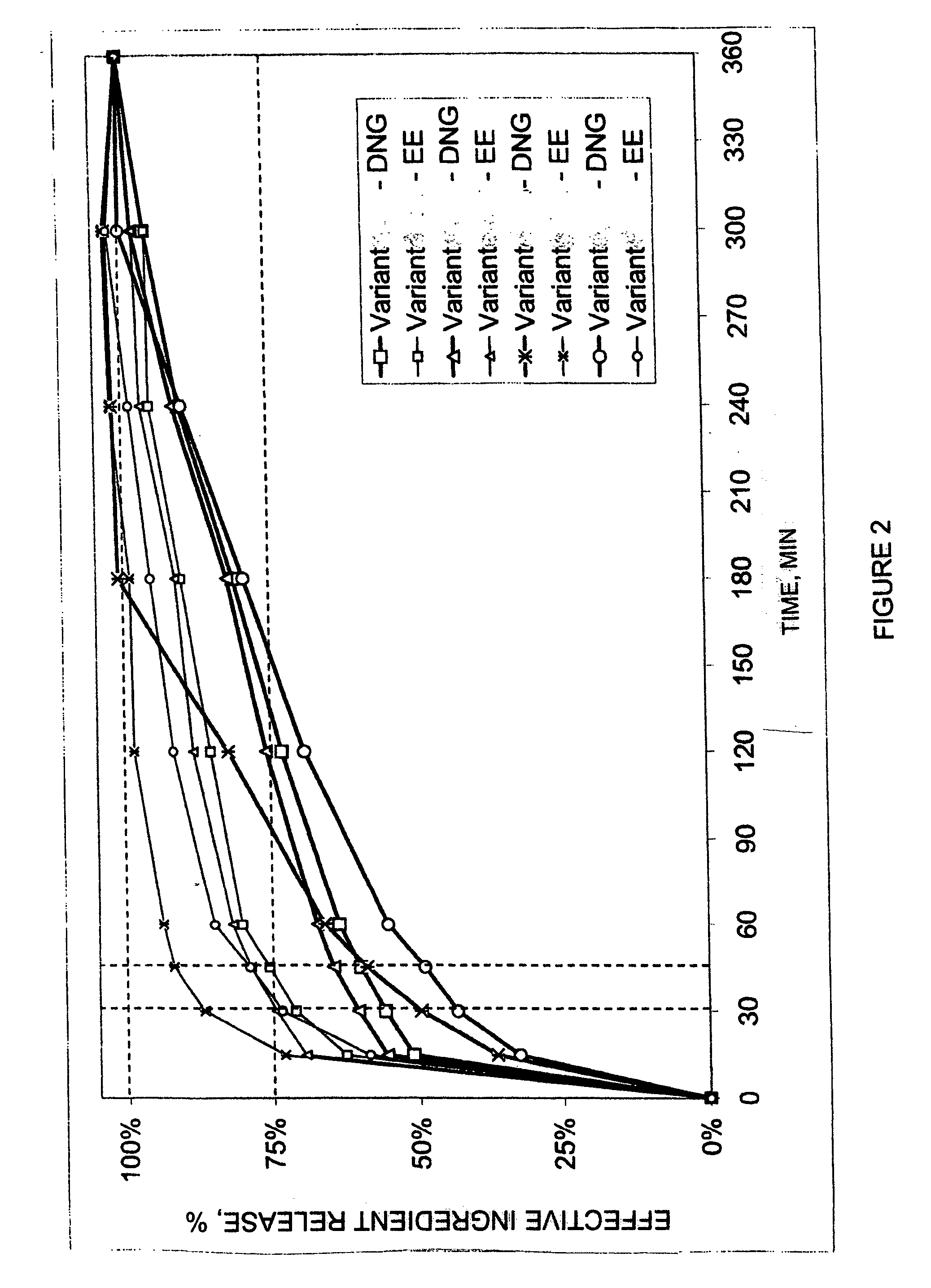
Pharmaceutical preparation for oral contraception
InactiveUS20060183725A1Effective contraceptive actionEasy cycle controlBiocideOrganic active ingredientsOral medicationAdditive ingredient
The pharmaceutical preparation for oral contraception has 28 daily dosage units, of which at least 21 daily dosage units each contain from 1.5 mg to 2 mg of dienogest and from 0.015 mg to 0.02 mg of ethinyl estradiol together in a pharmaceutically acceptable carrier. Seven or fewer daily dosage units contain no effective ingredient. Each daily dosage unit can be a film tablet for oral administration, which has a tablet core and film coating on the tablet core. At least 30% of the dienogest is released from the tablet core preferably in a delayed manner after more than 30 minutes, while at least 70% of the dienogest and ethinyl estradiol are released from the film coating preferably in 30 minutes, as determined by a standard dissolution test.
Owner:SCHERING AG
Rapid measurement method of drug dissolution rate
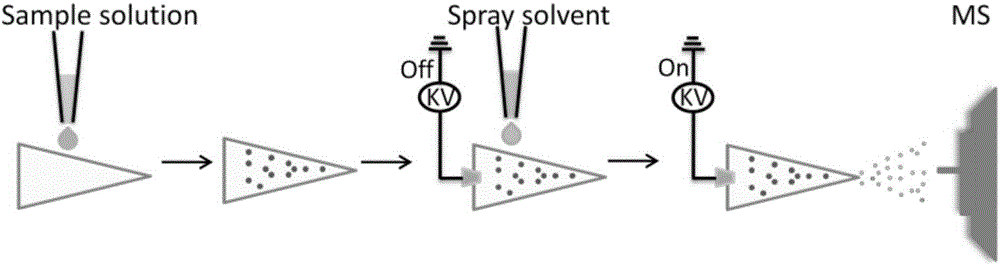
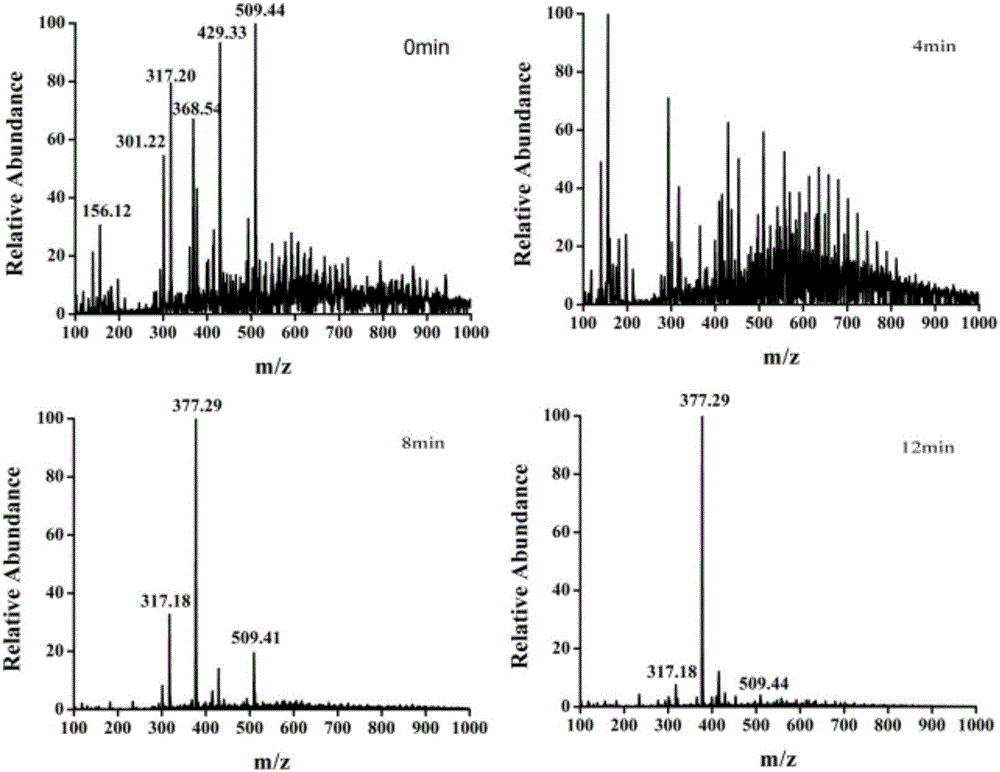
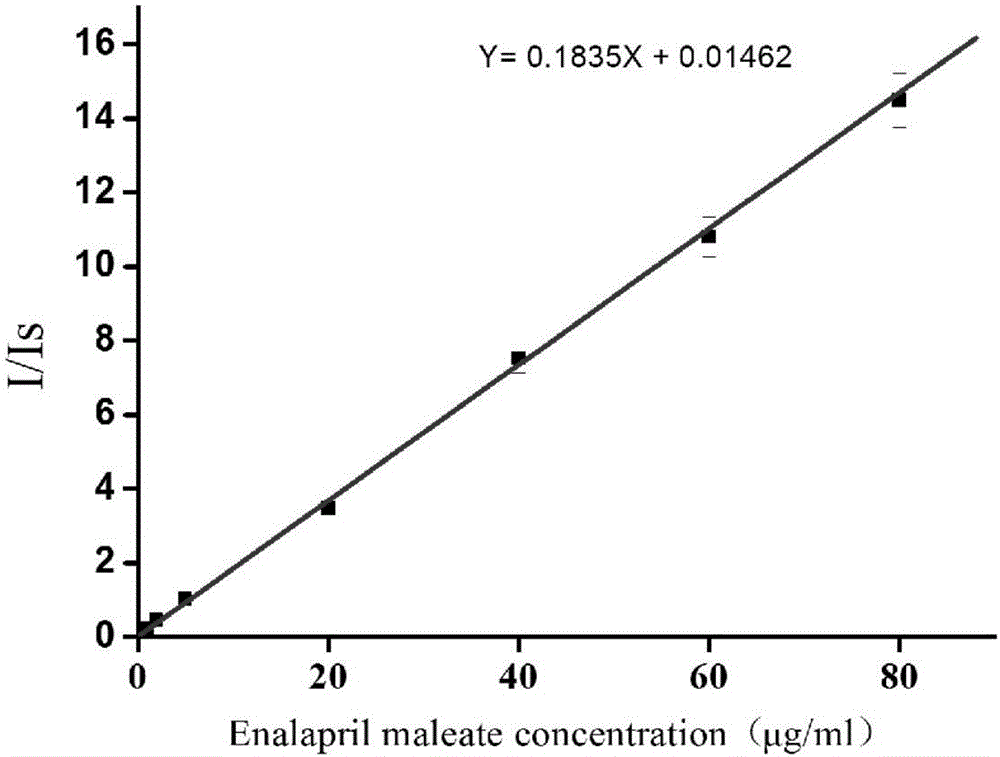
InactiveCN106370718AQuick analysisFix compatibility issuesMaterial analysis by electric/magnetic meansInternal standardMass Spectrometry-Mass Spectrometry
The invention discloses a rapid measurement method of a drug dissolution rate. A paper spray mass spectrometry MRM mode is used as a quantitative mode, and is used for measurement of the drug dissolution rate. Measurement of the dissolution rate comprises the steps of: firstly, shearing qualitative filter paper into a triangle; mixing dissolution liquid with corresponding internal standard solution according to a volume ratio of 1 to (0.1 to 10); dropping the obtained mixed solution on the triangle paper piece; carrying out air-drying; applying an external voltage of 3 to 5kv to the sample paper piece; dropping a spray solvent acetonitrile onto the paper piece to carry out spray detection. The invention provides mass spectrometry, so that for tolerance of involatile buffer salt, dissolution liquid of a drug can be directly analyzed without suffering from the pre-treatment process, and thus, a new method is provided for rapid measurement of the drug dissolution rate.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Coal gangue leaching and immersion testing device under multi-field coupling effect
ActiveCN108318660ASimple structureReasonable structureEarth material testingPermeability/surface area analysisSaline waterData information
The invention discloses a coal gangue leaching and immersion testing device under a multi-field coupling effect. The coal gangue leaching and immersion testing device comprises an inner barrel, an outer barrel, a leaching solution barrel and a leaching solution collector, wherein the inner barrel is divided into a leaching solution collection region and a leaching region for filling a testing material through filtering and penetration plates; a liquid inlet plate capable of bearing pre-set pressure is arranged above the leaching region and a liquid inlet hole is formed in the liquid inlet plate; the leaching solution collection region is connected with the leaching solution collector; the leaching solution barrel is connected with the liquid inlet hole of the liquid inlet plate through a pipeline; quantitative saline water is filled into the outer barrel; the inner barrel is arranged in the outer barrel; a semiconductor chilling plate is arranged in the outer barrel. The testing devicedisclosed by the invention has the advantages of simple structure, reasonable structure and convenience for utilization; the testing device can be used for simulating a leaching and immersion test ofgoaf solid filler under the multi-field coupling effect of a stress field, a seepage field, a temperature field, an acid-alkali field and the like; a collected leaching solution is used for carryingout a heavy-metal element dissolution testing experiment, and basic data information is provided for solving the environment protection problem of solid filling in a coal mine goaf.
Owner:CHINA UNIV OF MINING & TECH
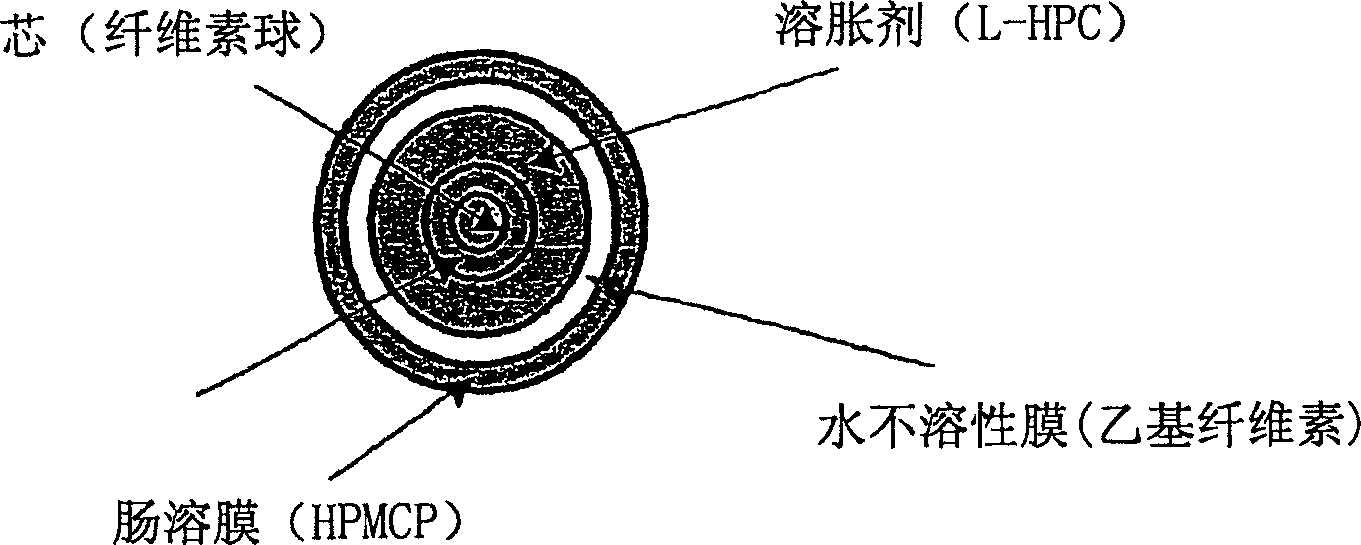
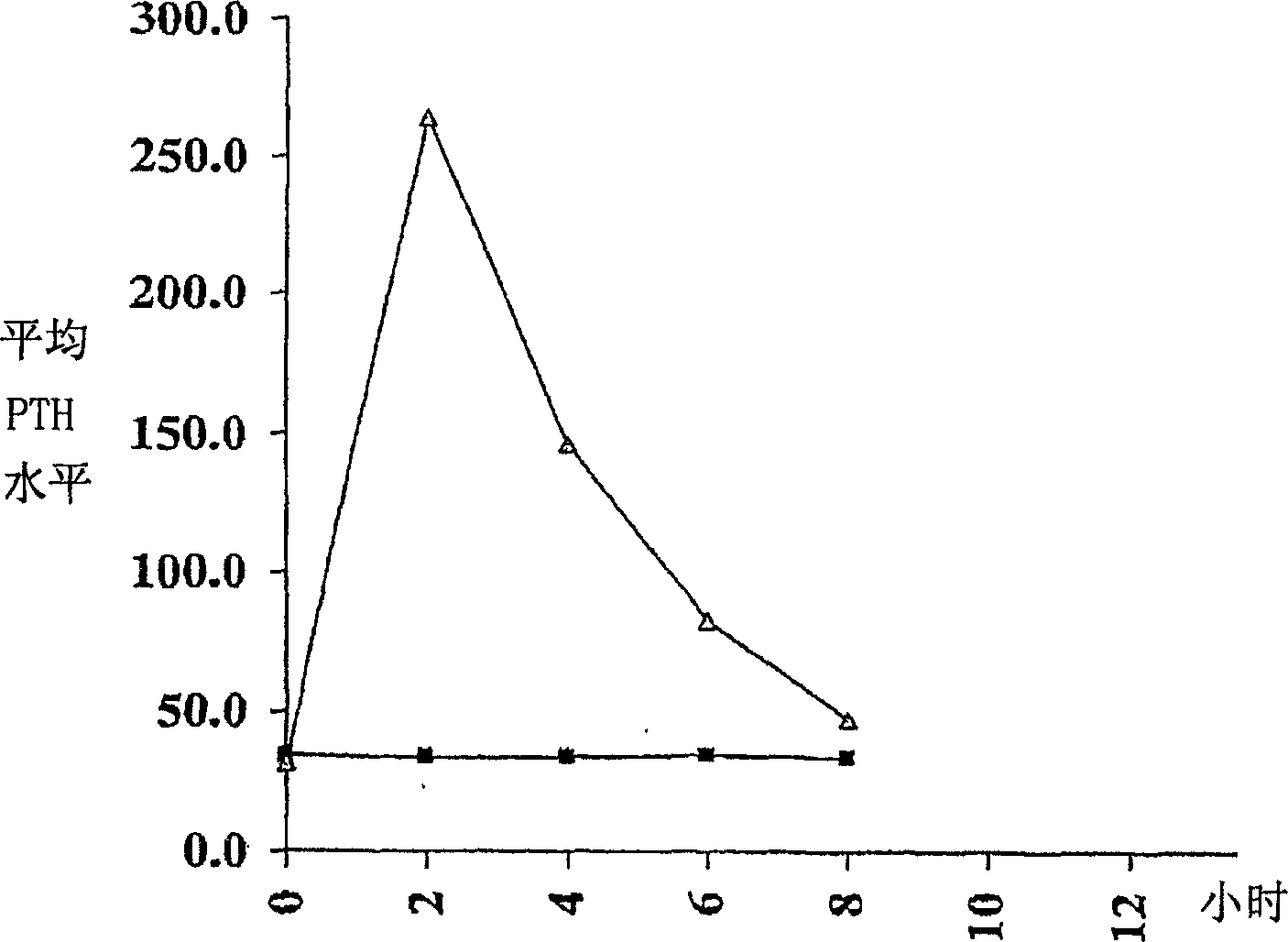
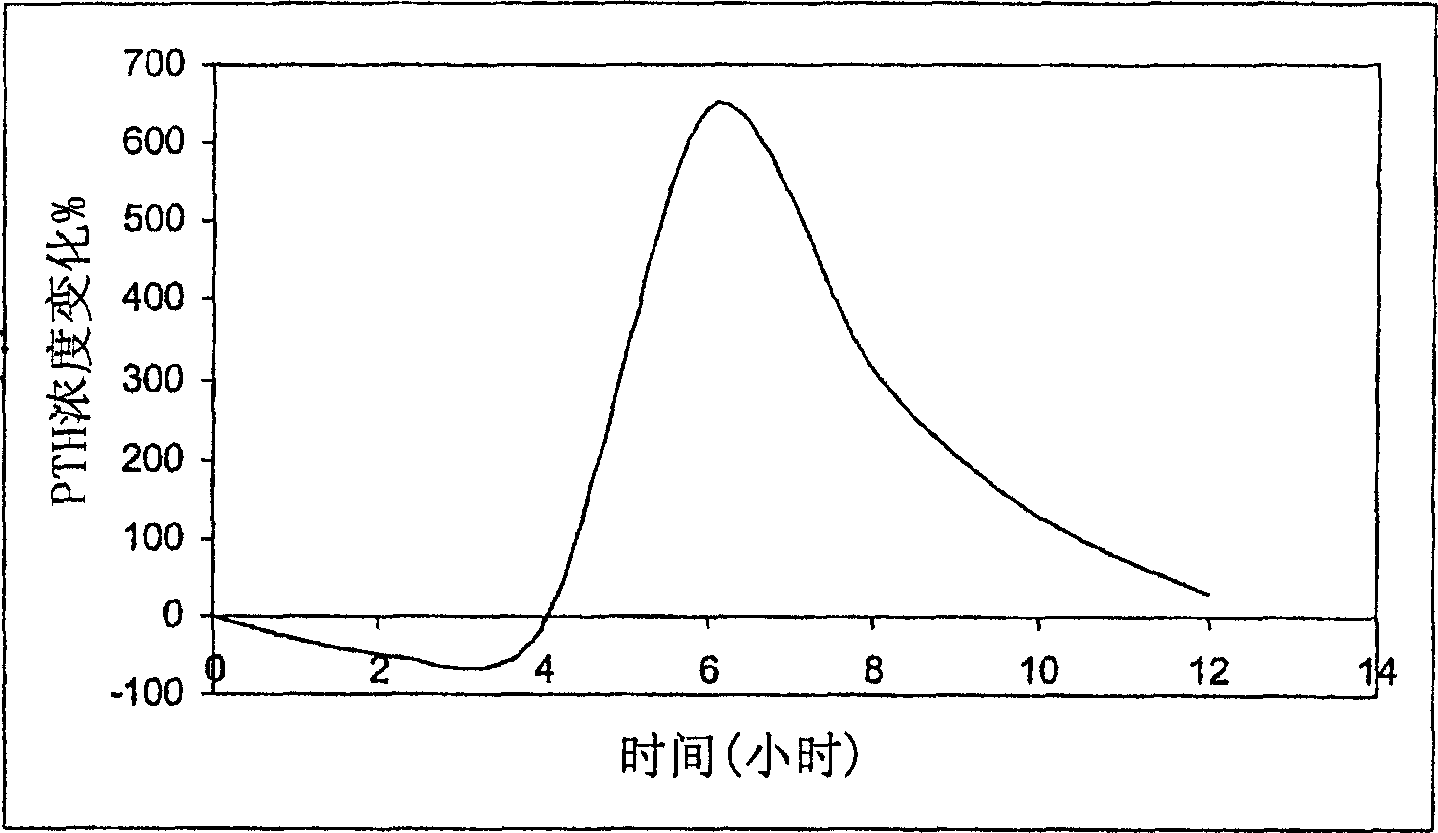
Parathyroid hormone (pth) containing pharmaceutical compositions for oral use
A pharmaceutical composition for oral administration comprising PTH, wherein the in vitro release of PTH-when tested in a dissolution test of pharmacopoeia standard-is delayed with at least 2 hours and once the release starts, at least 90% w / w such as, e.g., at least 95% or at least 99% of all PTH contained in the composition is released within at the most 2 hours. The composition may also comprises a calcium containing compound and / or a vitamin, D. In particular, PTH is administered in combination with a calcium-containing compound for the treatment or prevention of bone-related diseases, so that I) an effective amount of a calcium-containing compound is administered to lower the plasma level of endogenous PTH, and II) an effective amount of PTH is administered to obtain a peak concentration of PTH once the endogeneous PTH level is lowered. This present a potential therapeutic or prophylactic regimen for bone-related disorders including osteoporosis.
Owner:NYCOMED DANMARK AS
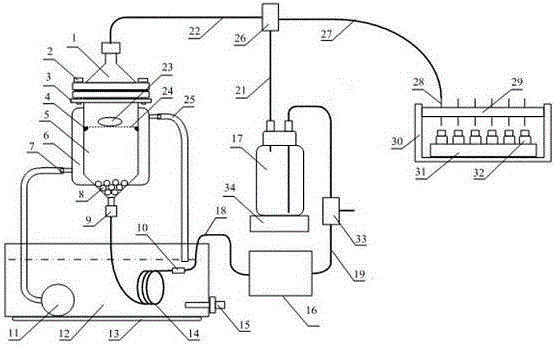
Full-automatic dissolvability testing device of flowing cell
InactiveCN105784953AAutomatic flow rate controlAutomatic control of heating temperatureTesting medicinal preparationsSolenoid valveSolvent based
The invention discloses a full-automatic dissolvability testing device of a flowing cell. The full-automatic dissolvability testing device at least comprises one set of flowing cells and a controller, wherein each flowing cell is provided with a columnar glass bottle; a constant-temperature water jacket layer is arranged on each glass bottle; a filter is arranged at the upper end opening of each glass bottle; each glass bottle is internally provided with a round bayonet and a stainless steel net; a solid preparation is put on the net; filter outlets of each set of flowing cells are commonly provided with a filter connection hose; a three-way solenoid valve is arranged at the other end of the filter connection hose; the three-way solenoid valve is provided with a hose for connecting a solvent storage bottle or a waste liquid collection bottle and a sample collector; a lower end opening of each glass bottle is connected with a stainless steel pipe, a liquid outlet hose, a piston pump and a connection hose in sequence; the other end of each connection hose is arranged in the solvent bottle; and each stainless steel pipe is arranged in a heating water tank. The full-automatic dissolvability testing device of the flowing cell has the advantages of automatically controlling heating temperature and solvent flow speed, automatically selecting a solvent base, automatically sampling, and can doing open type work and can also do sealed type work.
Owner:YANBIAN UNIV
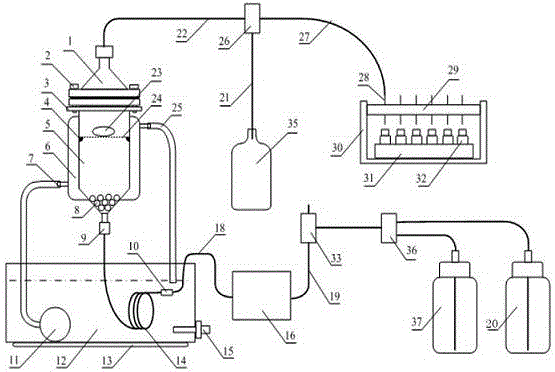
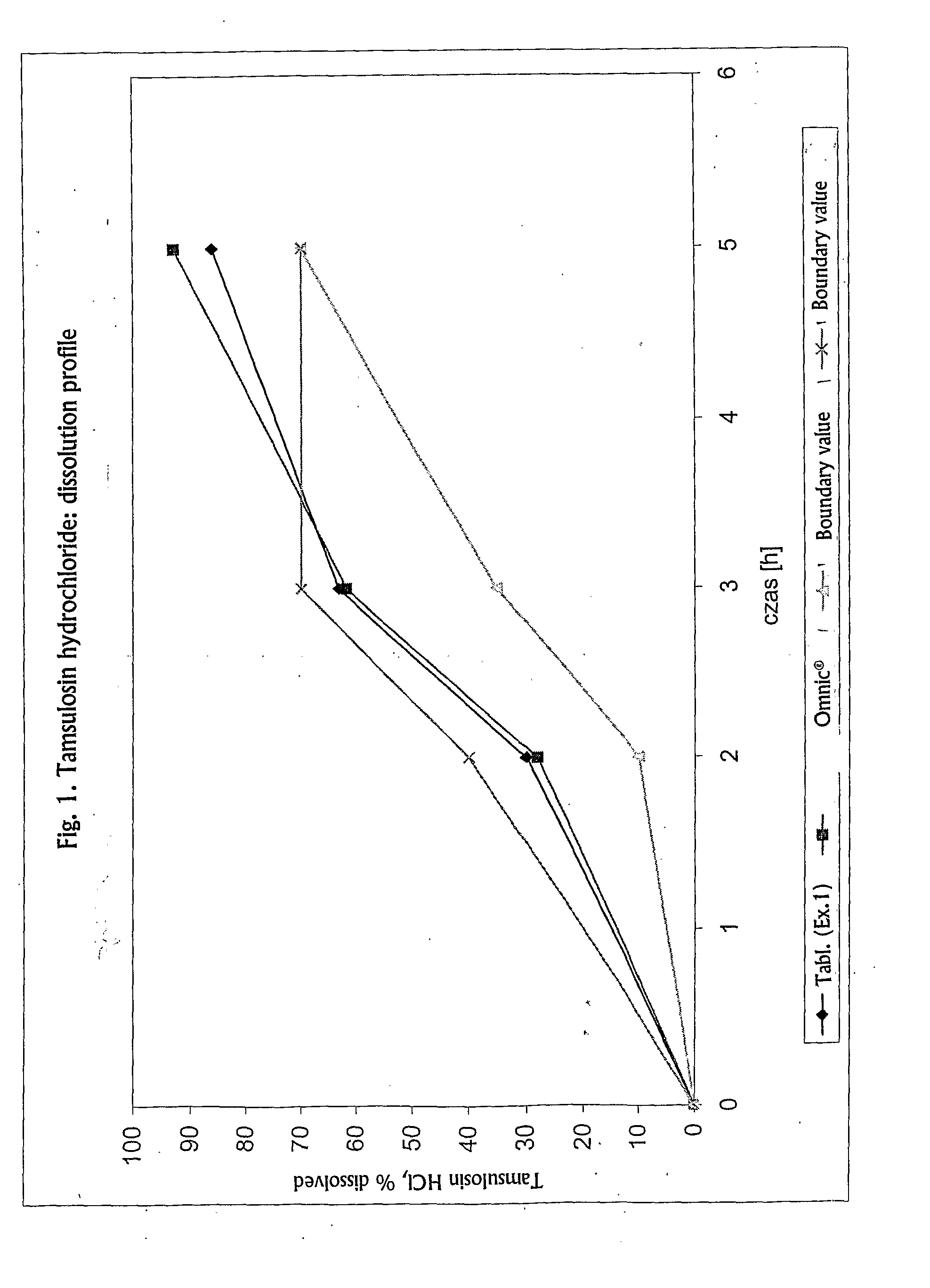
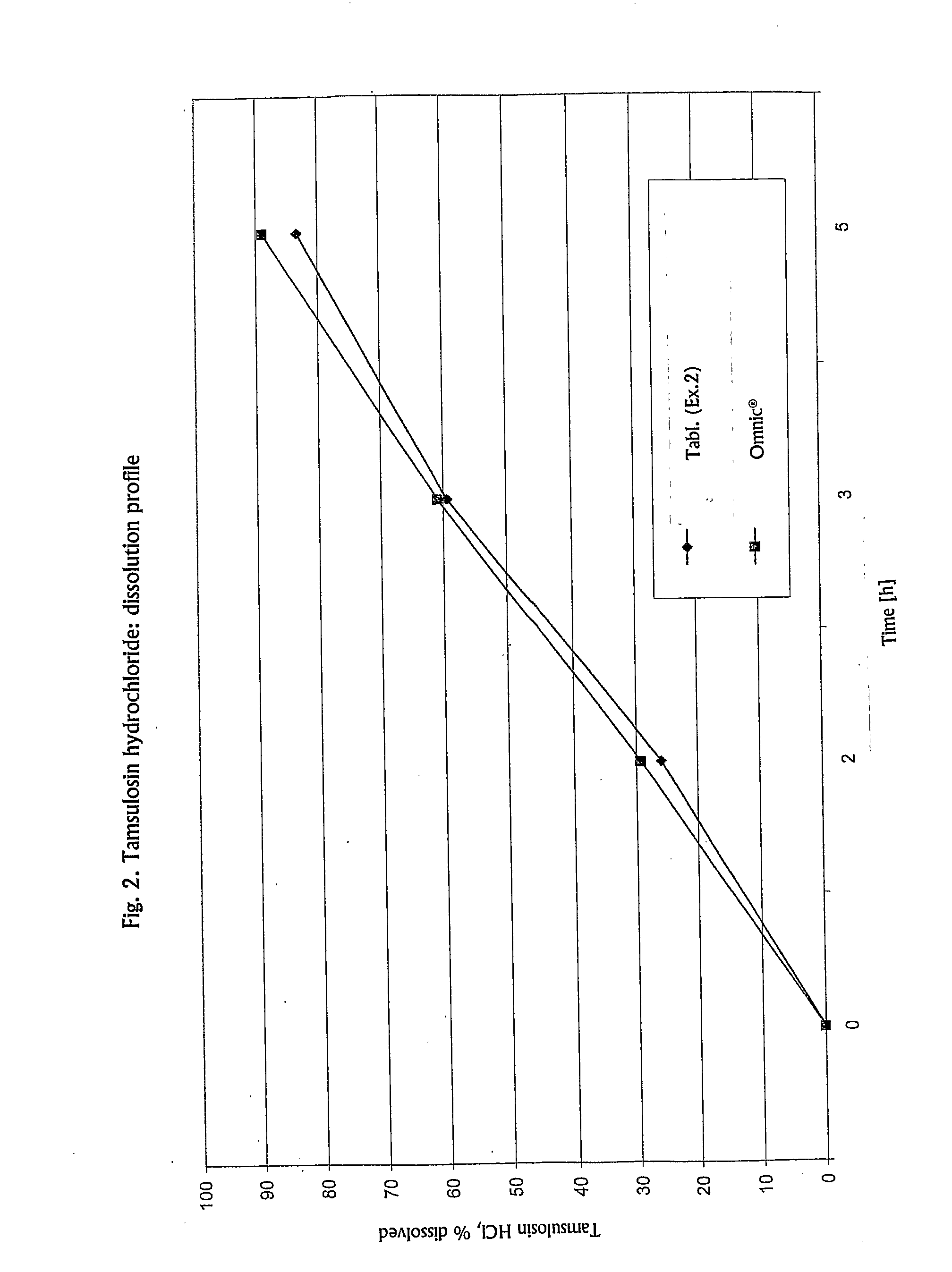
Controlled-Release Formulation Comprising Tamsulosin Hydrochloride
Taught herein is a solid, oral, controlled-release pharmaceutical composition of tamsulosin hydrochloride in the form of an enteric-coated tablet, wherein tamsulosin hydrochloride is homogenously dispersed within a matrix consisting of a mixture of a fatty component and a hydrophilic component, together with at least one diluent, and optionally other pharmaceutically acceptable excipients, exhibiting the following dissolution profile of tamsulosin hydrochloride, as measured in a Type II paddle apparatus in accordance with the dissolution testing method specified in the European Pharmacopoeia, i.e., at 37±0.5° C. and 100 rpm in a 0.1 N HCl buffer for 2 hours, followed by pH 7.2 buffer for the rest of the test: 10-40% dissolution during first 2 hours (in HCl), 35-70% dissolution after 3 h (in pH 7.2 buffer system), not less than 70% dissolution of the declared content after 5 h (in pH 7.2 buffer system).
Owner:INSTITUT FARMACEUTYCZNY
Test instrument for dissolution rate of drugs
ActiveCN106442904AGuaranteed credibilityIncrease support distanceTesting medicinal preparationsWater bathsInterference factor
The invention discloses a test instrument for dissolution rate of drugs and mainly relates to the technical field of drug inspection. The test instrument comprises a base, wherein four adjusting bolts are arranged at the edge of the base; a reference block is arranged on the base; a level bubble is arranged on the reference block; a cylindrical lug boss and a heating box are arranged at the top of the base; an annular water bath box is arranged on the cylindrical lug boss in a sleeving manner; an annular cover is arranged at the top of the annular water bath box; a lifting mechanism is arranged on the cylindrical lug boss; a cylindrical shell is arranged at the top of the lifting mechanism; a driving motor is arranged at the top of the cylindrical shell; a plurality of rotating shafts are annularly arrayed on the cylindrical shell; stirring paddles are arranged at the bottom of the rotating shafts; a dissolving cup is arranged in the annular water bath box. The test instrument has the beneficial effects of reducing the interference factor of the dissolution rate test, guaranteeing the reliability of the test result, shortening the time for correcting the test instrument for dissolution rate and improving the working efficiency.
Owner:上海安德盛实业有限公司
Direct vessel heating for dissolution testing
InactiveUS20100126980A1Surface/boundary effectOhmic-resistance heatingElectrical resistance and conductanceControl system
A dissolution test vessel configured for direct vessel heating includes a lateral wall, a resistive heating element, and a temperature-sensing element. The resistive heating element and the temperature-sensing element are bonded directly to the lateral wall, and may be formed by dispensing flowable materials onto the lateral wall. The resistive heating element includes a contiguous first heating element end, heating element section, and second heating element end. The temperature-sensing element includes a contiguous first sensing element end, sensing element section, and second sensing element end. The vessel may communicate with a heater control system, which may be provided with a dissolution test apparatus at which the vessel is mounted.
Owner:AGILENT TECH INC
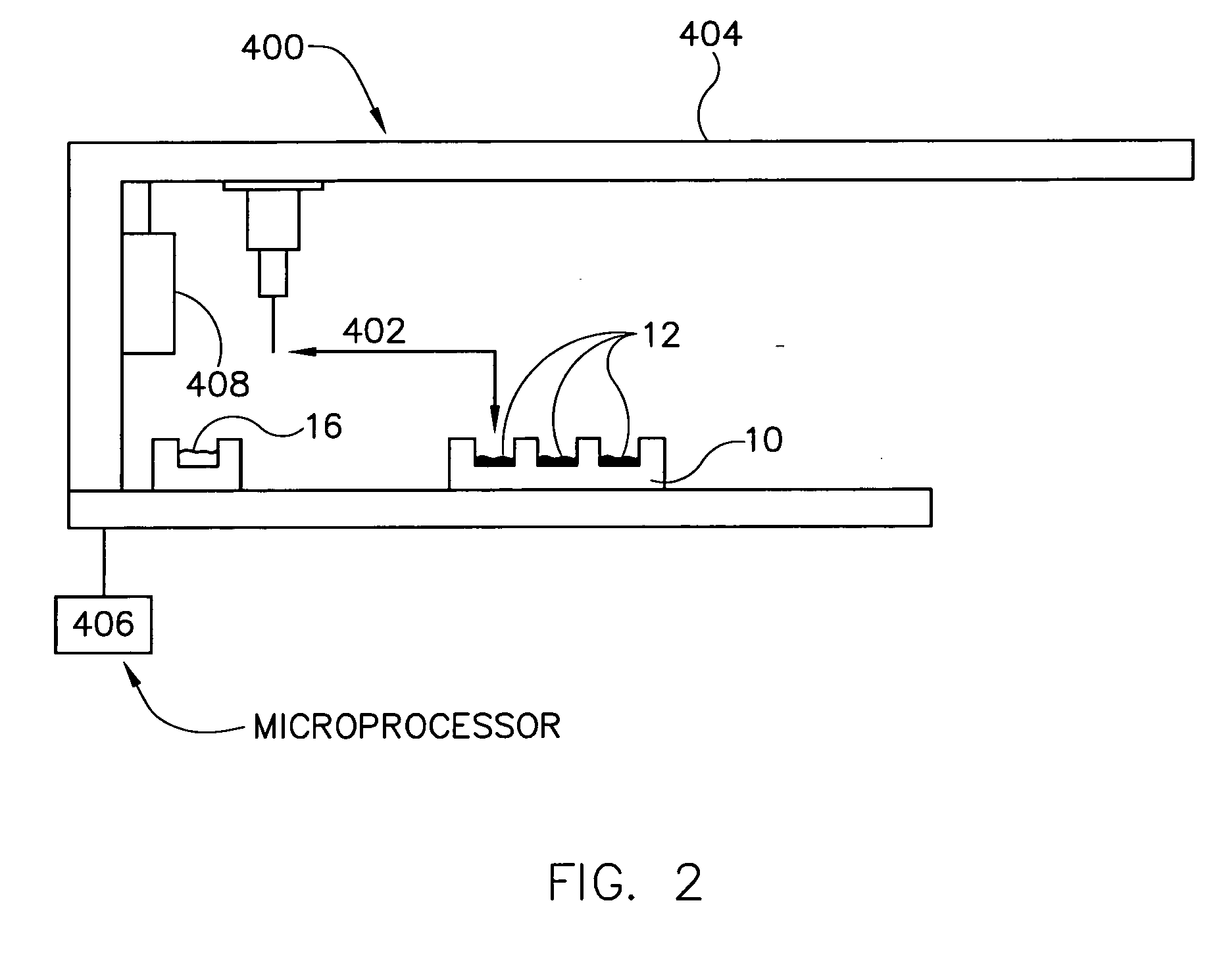
Delivery device for dispensing pharmaceutical dosage forms into dissolution testing apparatus
The present invention provides a dissolution test cell having a chamber and slide valve for dispensing pharmaceutical dosage forms, such as tablets, capsules, and powders, into the chamber without having to open the cell and expose the chamber and its contents to the ambient environment. The present invention also facilitates removal of a partially dissolved or non-disintegrating dosage form from the chamber and cell entirely, without exposing the interior of the cell and chamber. Dissolution test apparatus comprising at least one dissolution test cell in accordance with the invention is also provided, as well as methods for its operation to obtain Level A IVIVC dissolution results which accurately correlate with in vivo dissolution results are also provided.
Owner:DDP SPECIALTY ELECTRONICS MATERIALS US 8 LLC
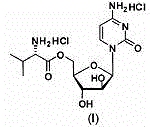
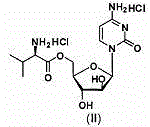
Pharmaceutical composition containing 5'-Ara-C-O-amino ester
The invention provides a composition for oral pharmaceuticals, using 5'-Ara-C-O-amino ester or its salt as an active ingredient. According to dissolution testing, a second method in the appendix XC of the second part of Chinese pharmacopoeia edition 2010, the dissolution of the active ingredient of the composition is higher than or equal to 80% in 15 min, by using 900 ml of water as a dissolving medium at a rotation speed of 50 rpm. Organic acids are added into a prescription of the composition, a certain ratio of the organic acids is maintained, and thus the stability of pharmaceutical composition is substantially improved. A short-term stability test presents no increase in impurity.
Owner:KUNMING JIDA PHARMA
Delivery device for dispensing pharmaceutical dosage forms into dissolution testing apparatus
The present invention provides a dissolution test cell having a chamber and slide valve for dispensing pharmaceutical dosage forms, such as tablets, capsules, and powders, into the chamber without having to open the cell and expose the chamber and its contents to the ambient environment. The present invention also facilitates removal of a partially dissolved or non-disintegrating dosage form from the chamber and cell entirely, without exposing the interior of the cell and chamber. Dissolution test apparatus comprising at least one dissolution test cell in accordance with the invention is also provided, as well as methods for its operation to obtain Level A IVIVC dissolution results which accurately correlate with in vivo dissolution results are also provided.
Owner:DDP SPECIALTY ELECTRONICS MATERIALS US 8 LLC
Method for detecting and evaluating in-vitro dissolution of enteric preparation
The invention relates to a method for detecting and evaluating in-vitro dissolution of an enteric preparation. The method is performed according to standards of Chinese Pharmacopoeia and comprises thesteps as follows: conducting a dissolution test in a simulated gastric fluid dissolution medium; then, transferring the enteric preparation in water or weakly acid dissolution medium for a dissolution test; besides, directly performing a dissolution test on the preparation in water or the weakly acid dissolution medium; comparing the difference between dissolution results of preparation subjectedto or not subjected to dissolution treatment in the simulated gastric fluid dissolution medium to determine the reliability of the enteric performance of the preparation. The method can provide datasupport for selection of multi-source reference preparations in generic drug consistency evaluation and provide reference for prescription screening and bioequivalence risk evaluation. More importantly, the method is used for detecting and evaluating in-vitro dissolution of the enteric preparation, so that the incidence rate of adverse events of clinical medication can be reduced.
Owner:BEIJING INST FOR DRUG CONTROL
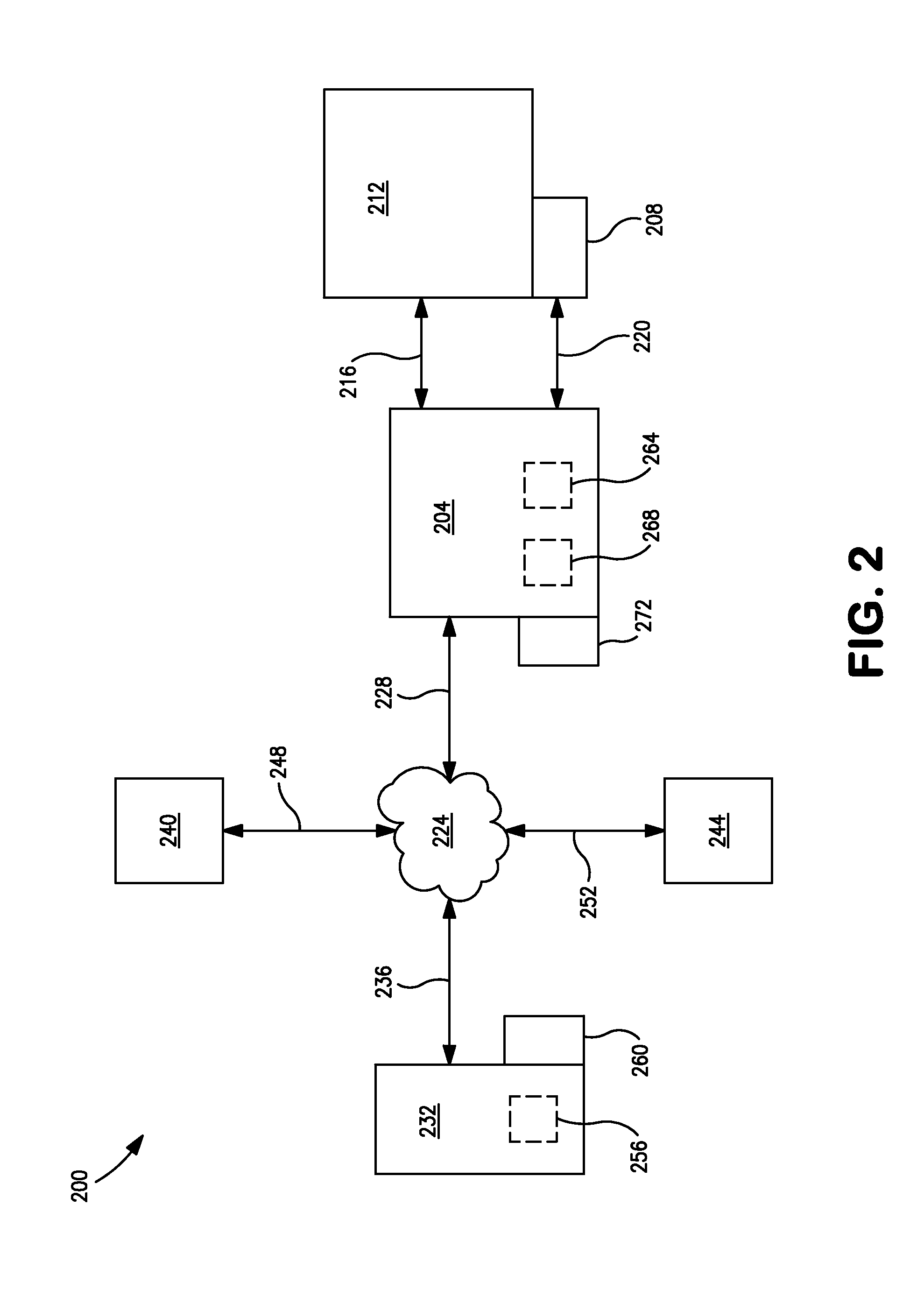
Systems and methods for acquiring and managing sensor data related to dissolution testing apparatus
In acquiring and managing measurement data relating to operating parameters of a dissolution tester, operating parameters are measured by operating one or more sensors. The measured operating parameters are transmitted from the sensors to a user computing device. It is then determined whether the measured operating parameters are in compliance or non-compliance with one or more standards, by comparing the measured operating parameters with a plurality of corresponding predefined values. The measured operating parameters and indications of compliance or non-compliance of each measured operating parameter are stored as a data record in a memory local or remote to the user computing device. The data record may be accessible by a computing device remote from the memory. The operating parameters may include, for example, shaft parameters.
Owner:AGILENT TECH INC
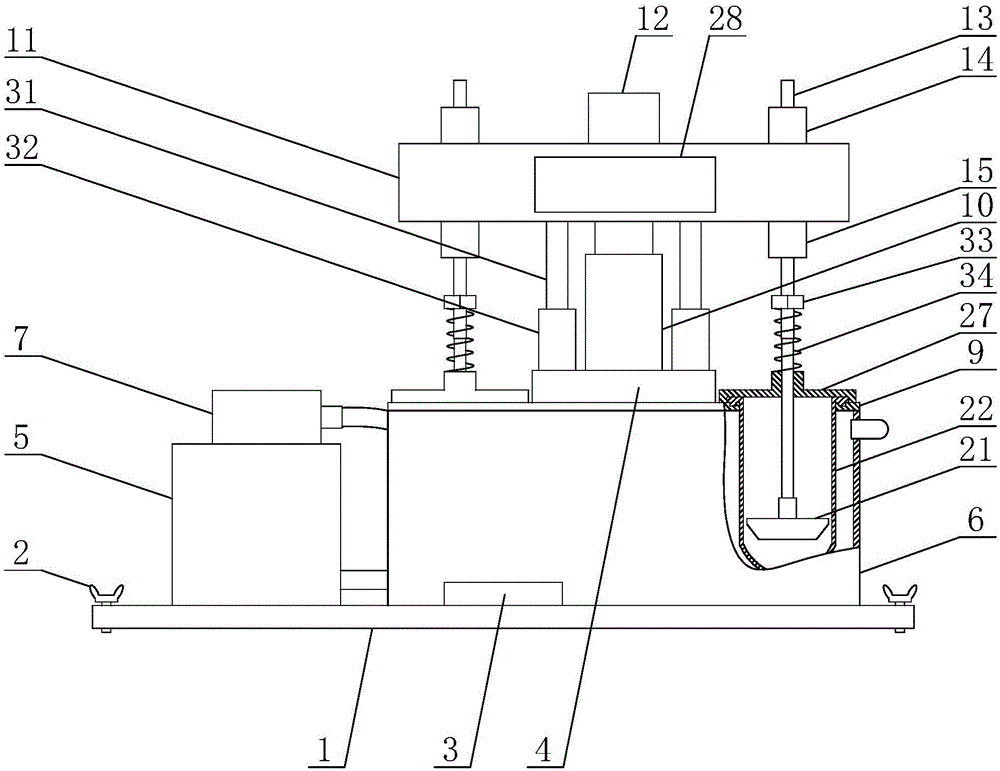
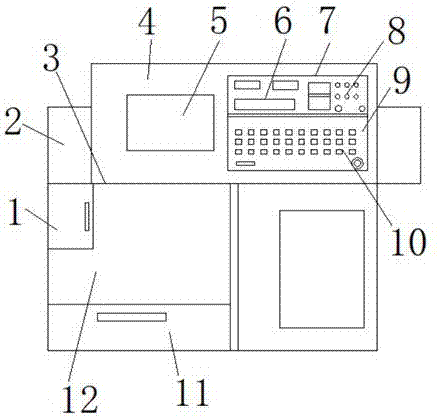
Lab operation platform suitable for food detection
InactiveCN107976218ARealize continuous integrated detectionImprove detection efficiencyMeasurement apparatus componentsEconomic benefitsEngineering
The invention discloses a lab operation platform suitable for food detection, and the platform comprises an operation equipment main body. The operation equipment main body comprises a storage room, and the upper end of the storage room is provided with dissolution testing equipment. The dissolution testing equipment comprises a box body, and the interior of the box body is provided with a dissolution tank. The upper end of the dissolution tank is provided with an electric push rod, and the lower end of the electric push rod is connected with a sealing cover. The interior of the dissolution tank is provided with a stirring device, and the inner side of the lower end of the dissolution tank is connected with a liquid pump through a liquid incoming pipe connector. The liquid pump is connected with metal detection equipment, and the metal detection equipment is connected with a second dissolution tank. The upper end of the second dissolution tank is connected with a reagent adding box, and the interior of the second dissolution tank is provided with a second stirring device. The second dissolution tank is connected with a liquid food integrated detector. The platform can achieve the continuous integrated detection of solid food, can achieve the detection of liquid food and dissolved solid food, is complete in detection function, is diversified in functions, is convenient and quickfor operation, greatly improves the food detection efficiency, and is remarkable in economic benefits.
Owner:尹大路
Buccal dissolution of active substances
InactiveUS7470545B2Analysis using chemical indicatorsWithdrawing sample devicesPhysiologyTaste masking
Owner:ROHM & HAAS CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com