Patents
Literature
42 results about "Increase dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
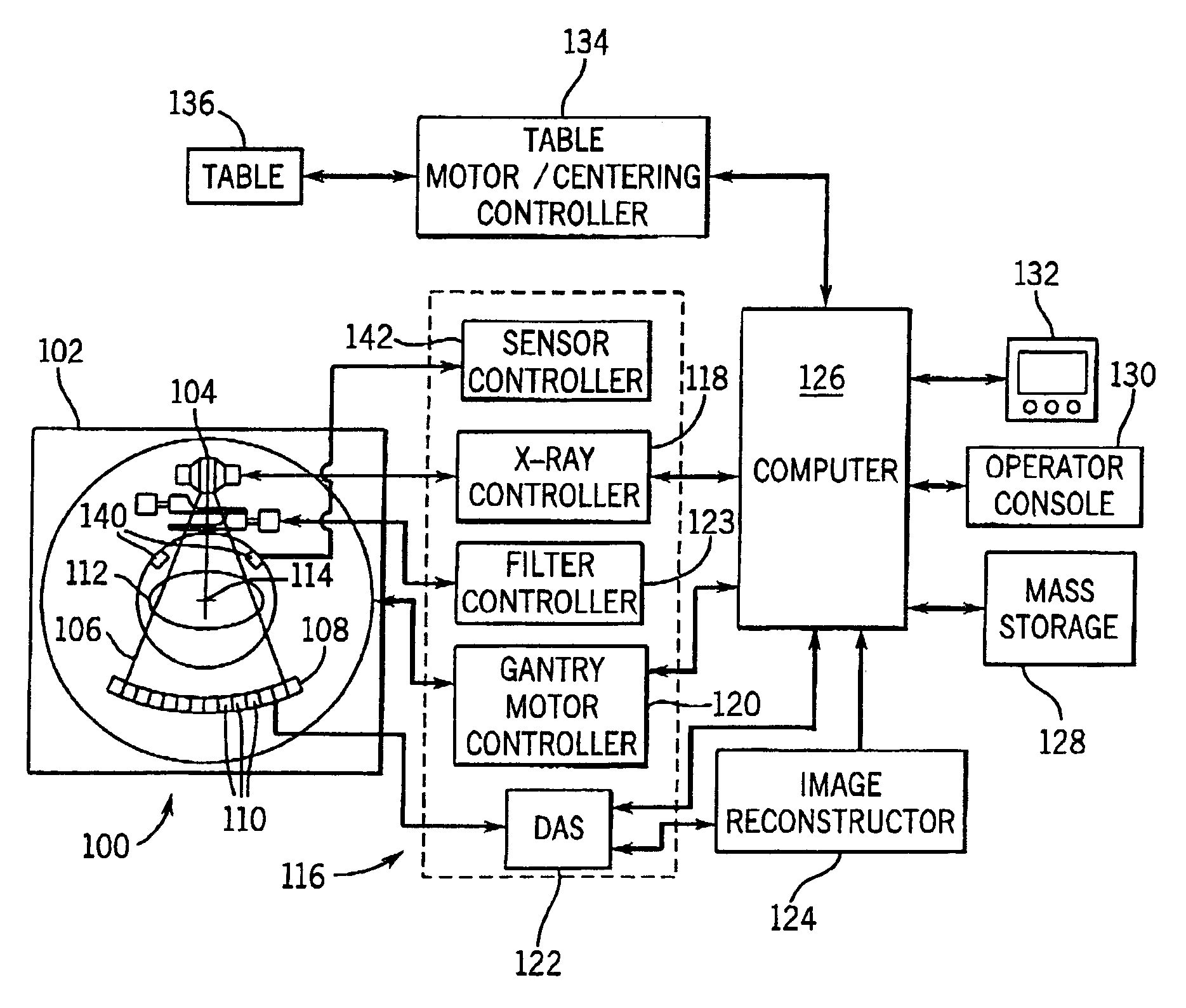
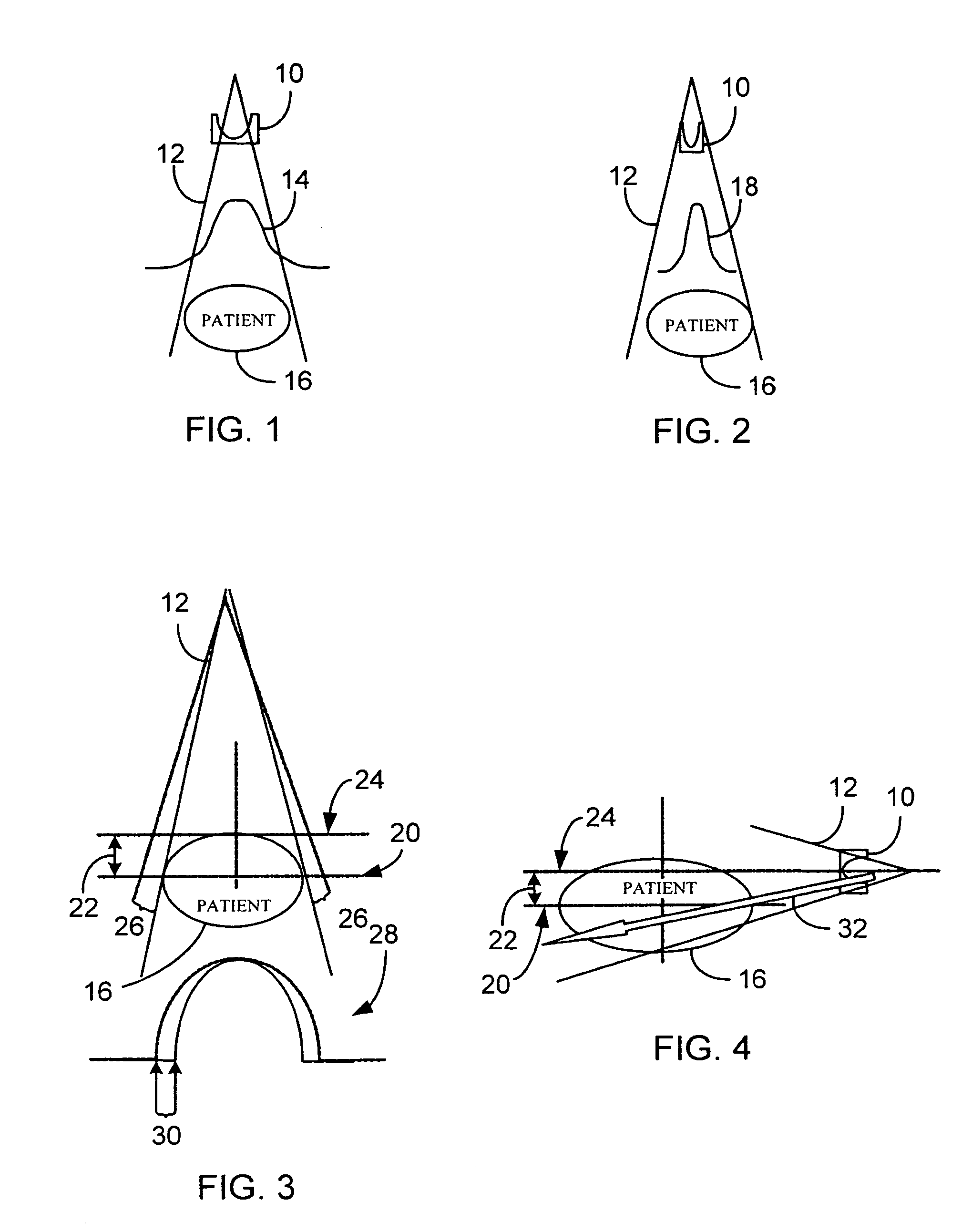
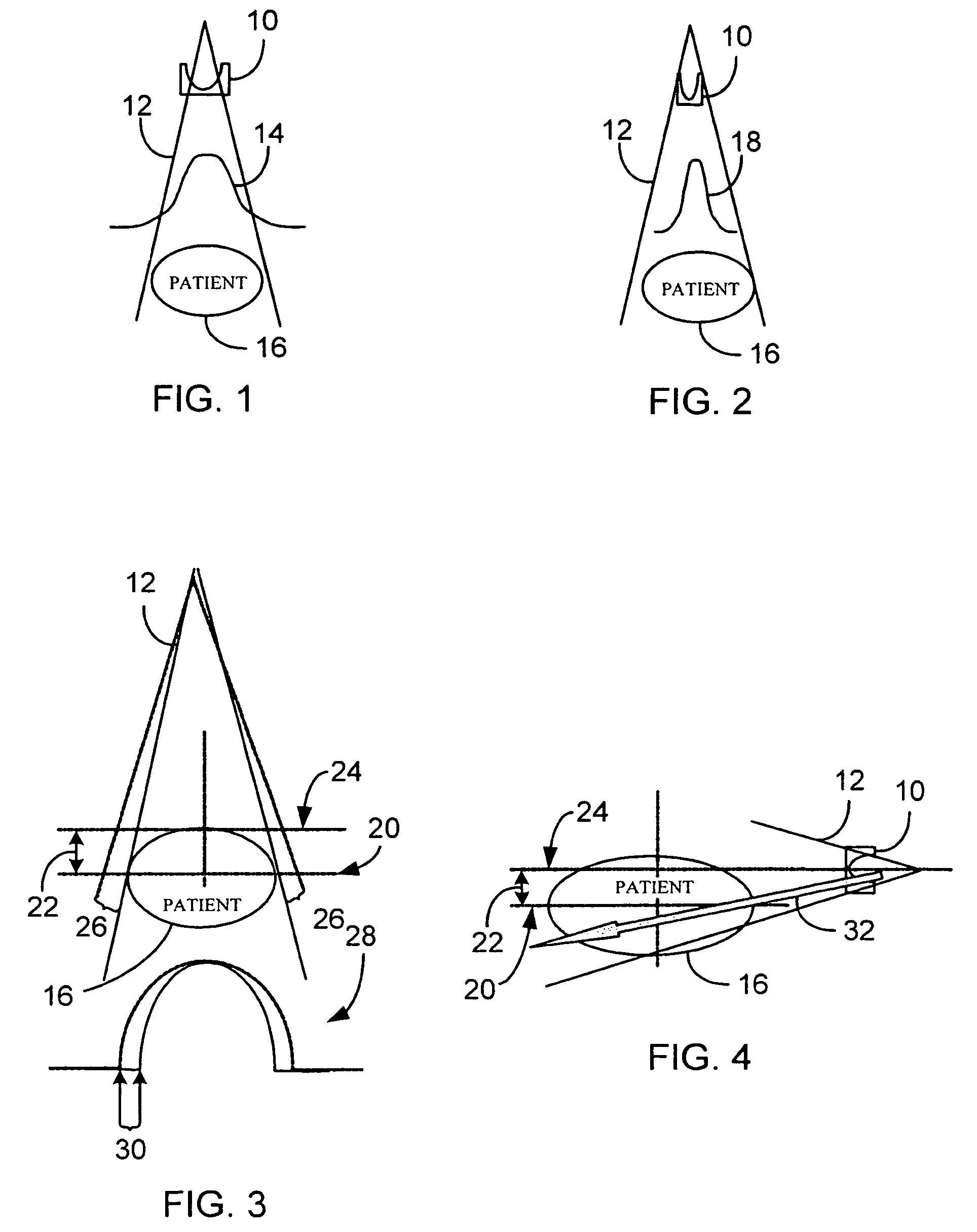
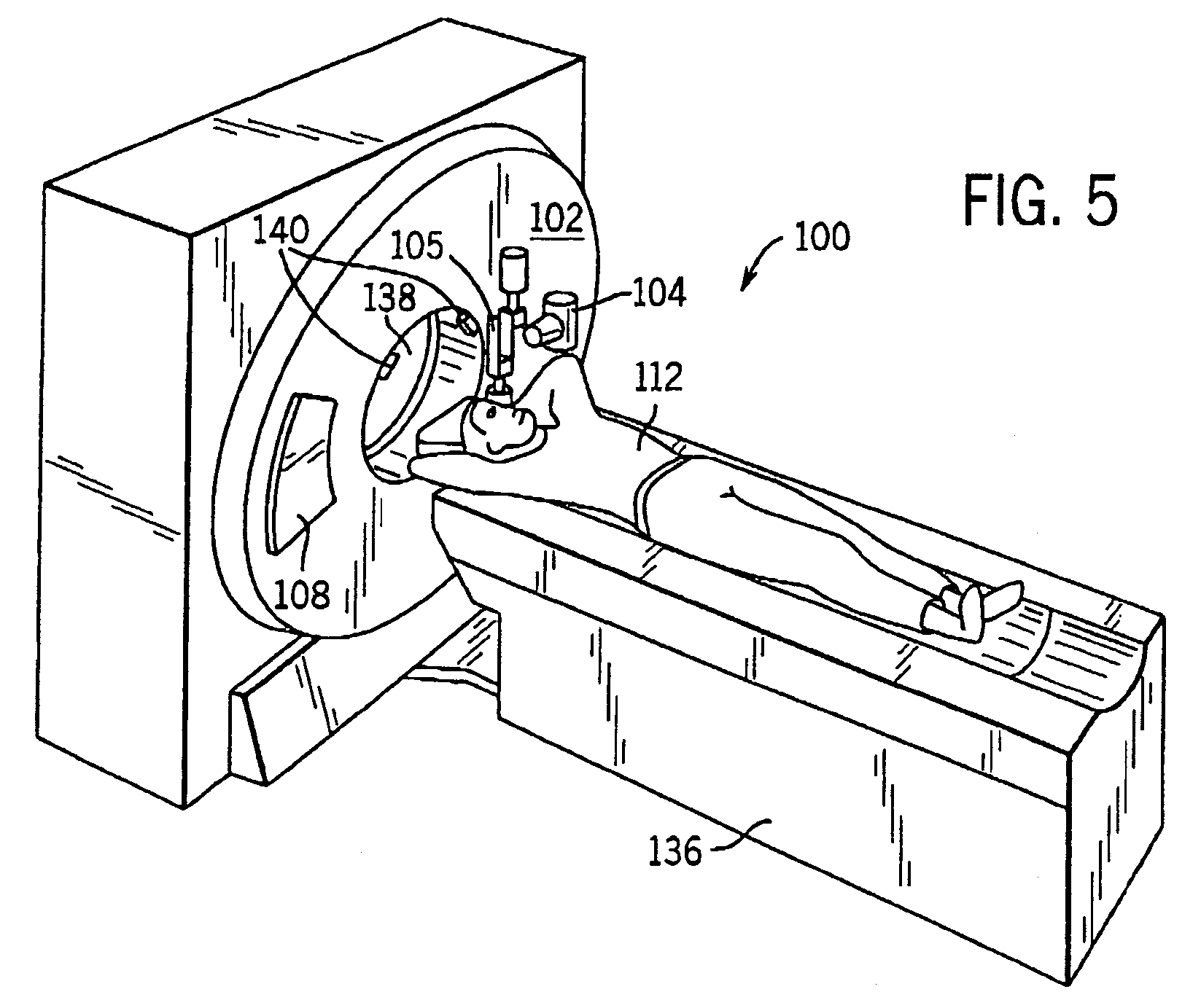
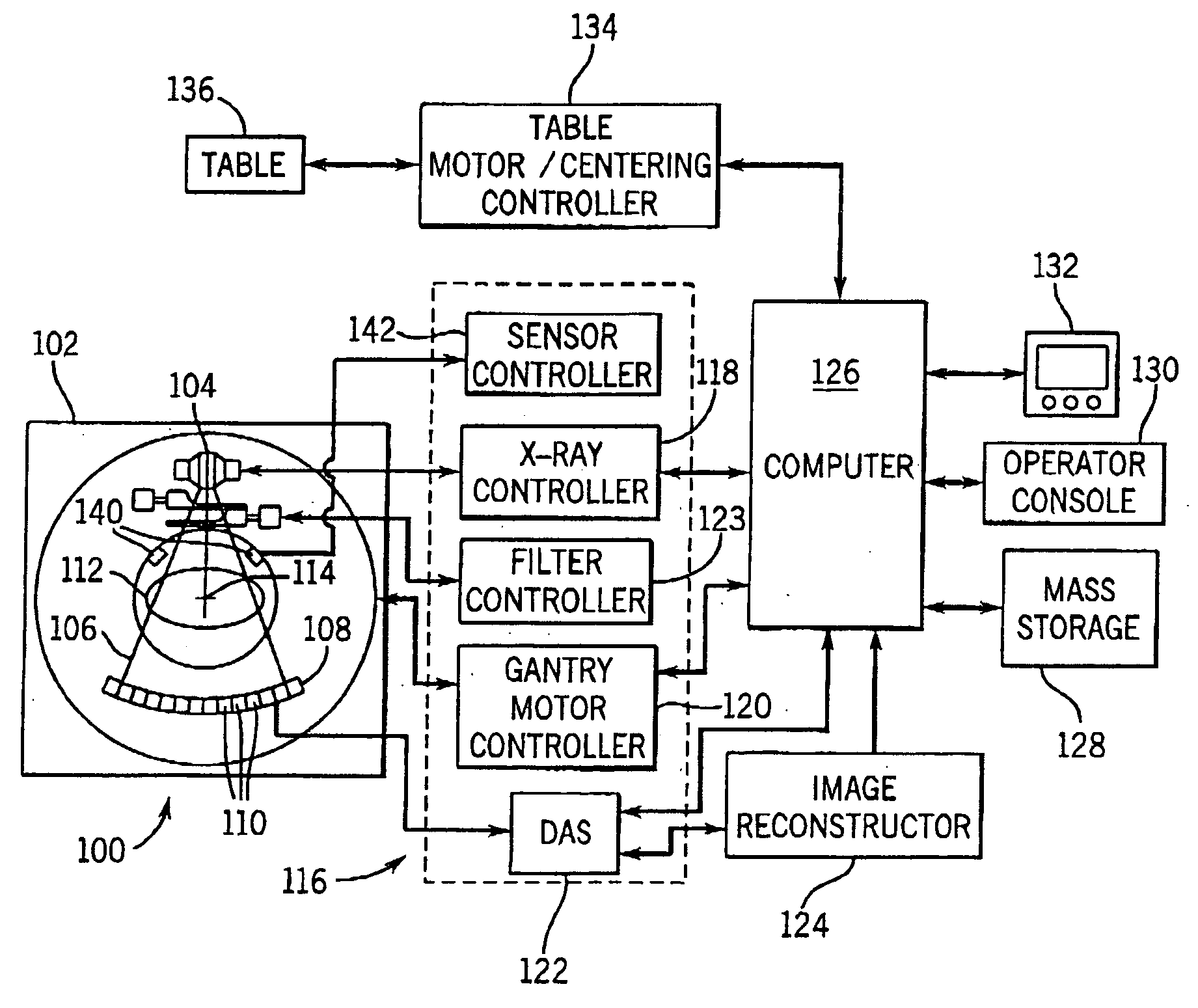
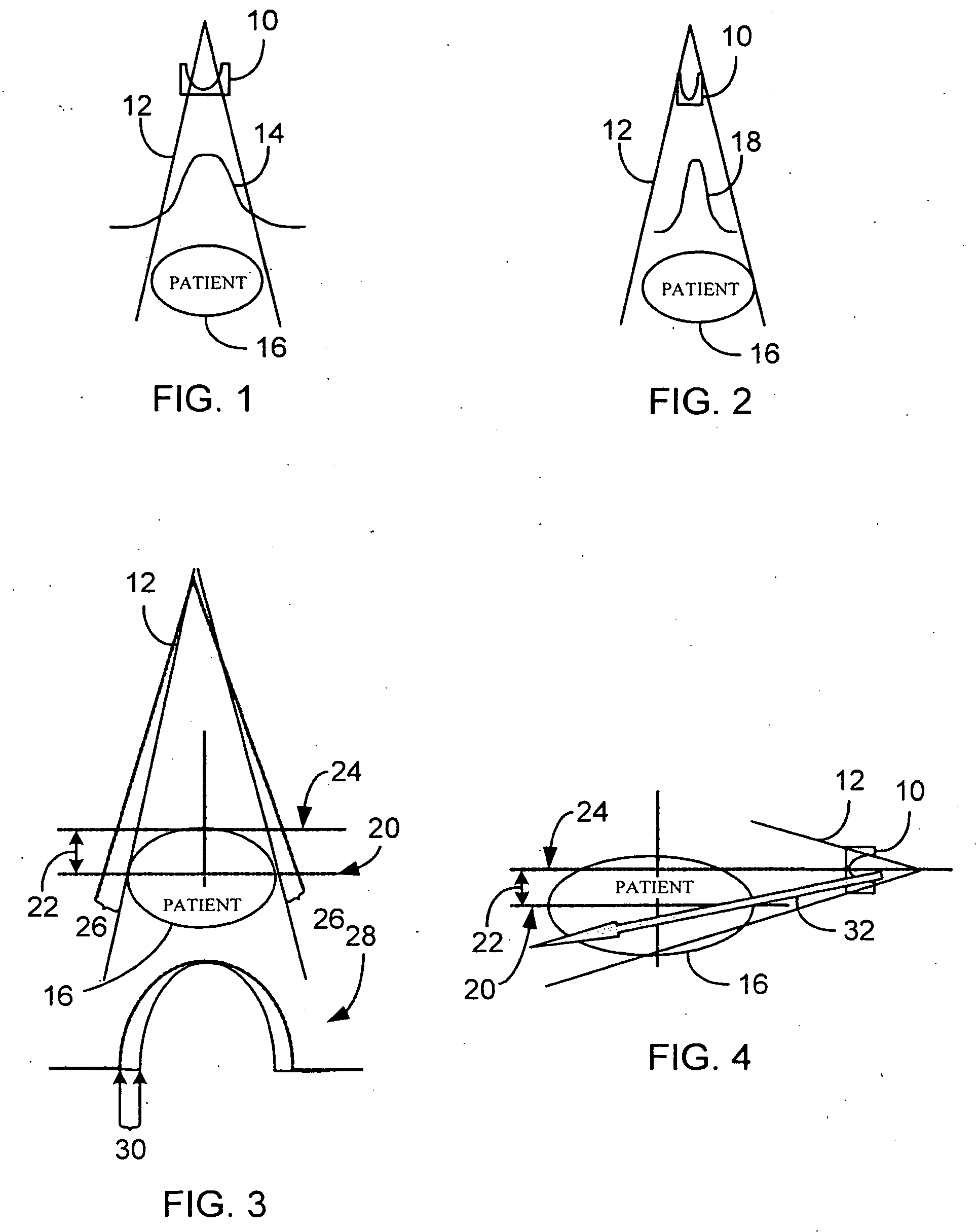
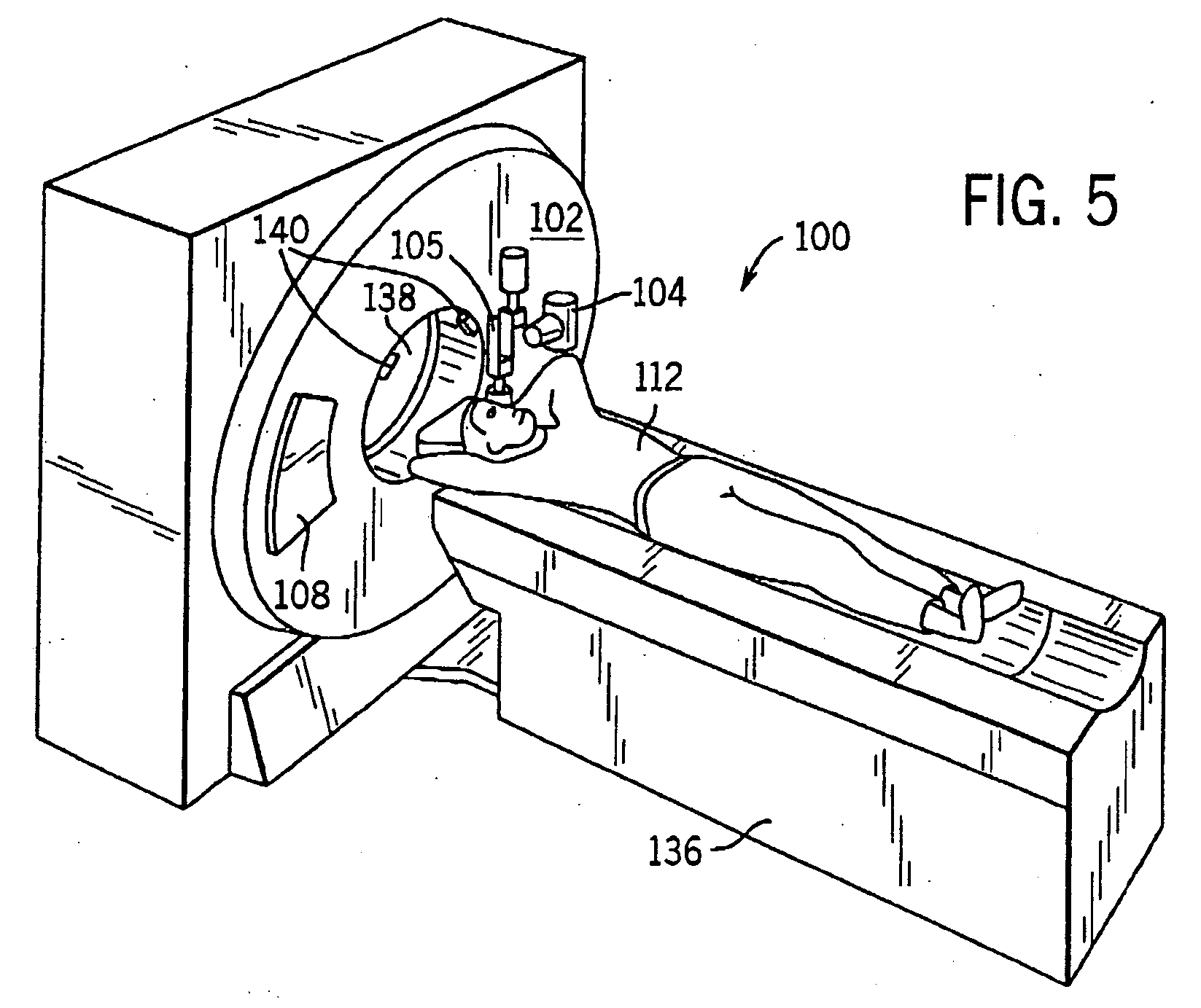
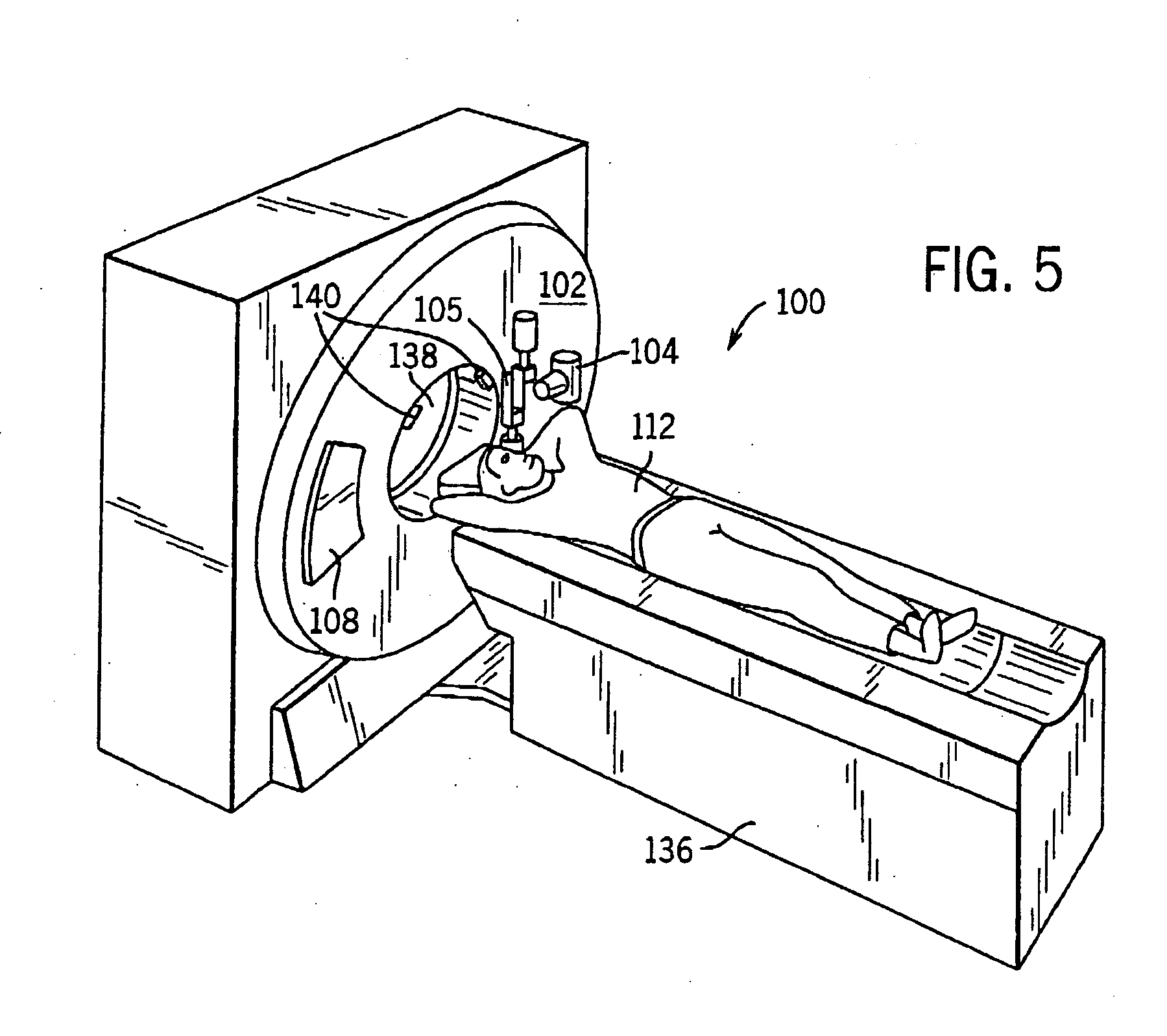
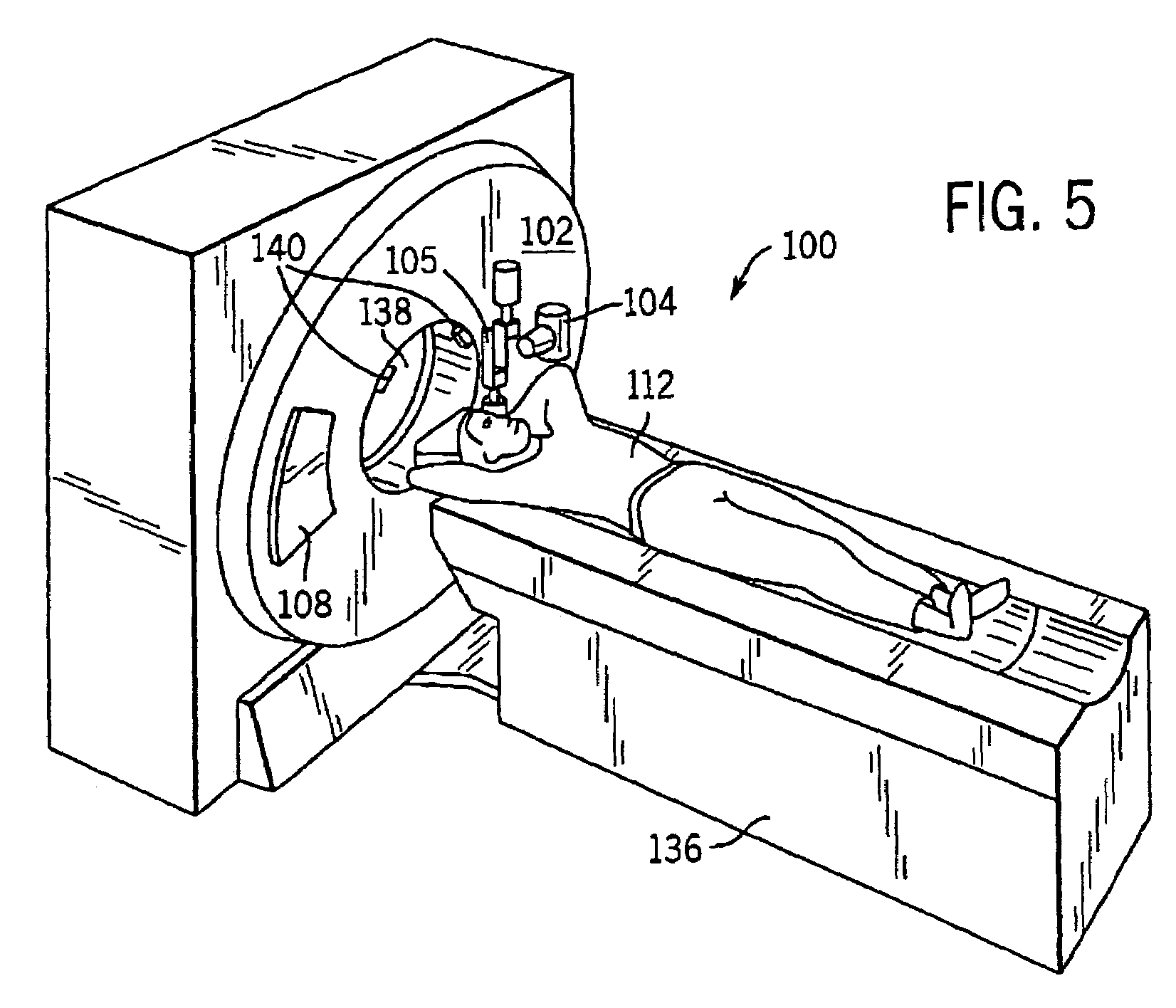
System and method of determining a user-defined region-of-interest of an imaging subject for x-ray flux management control
ActiveUS6990171B2Material analysis using wave/particle radiationRadiation/particle handlingUltrasound attenuationX-ray
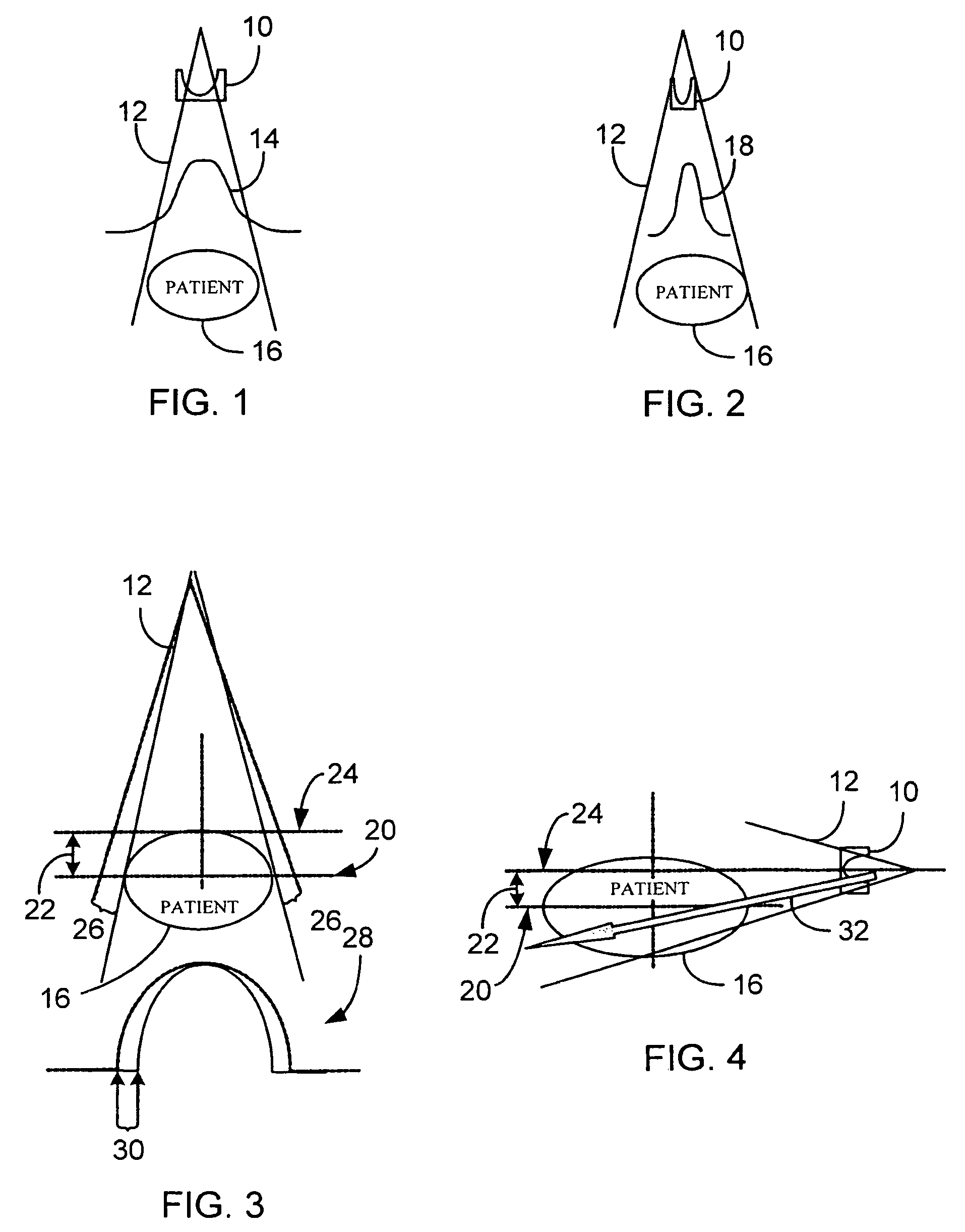
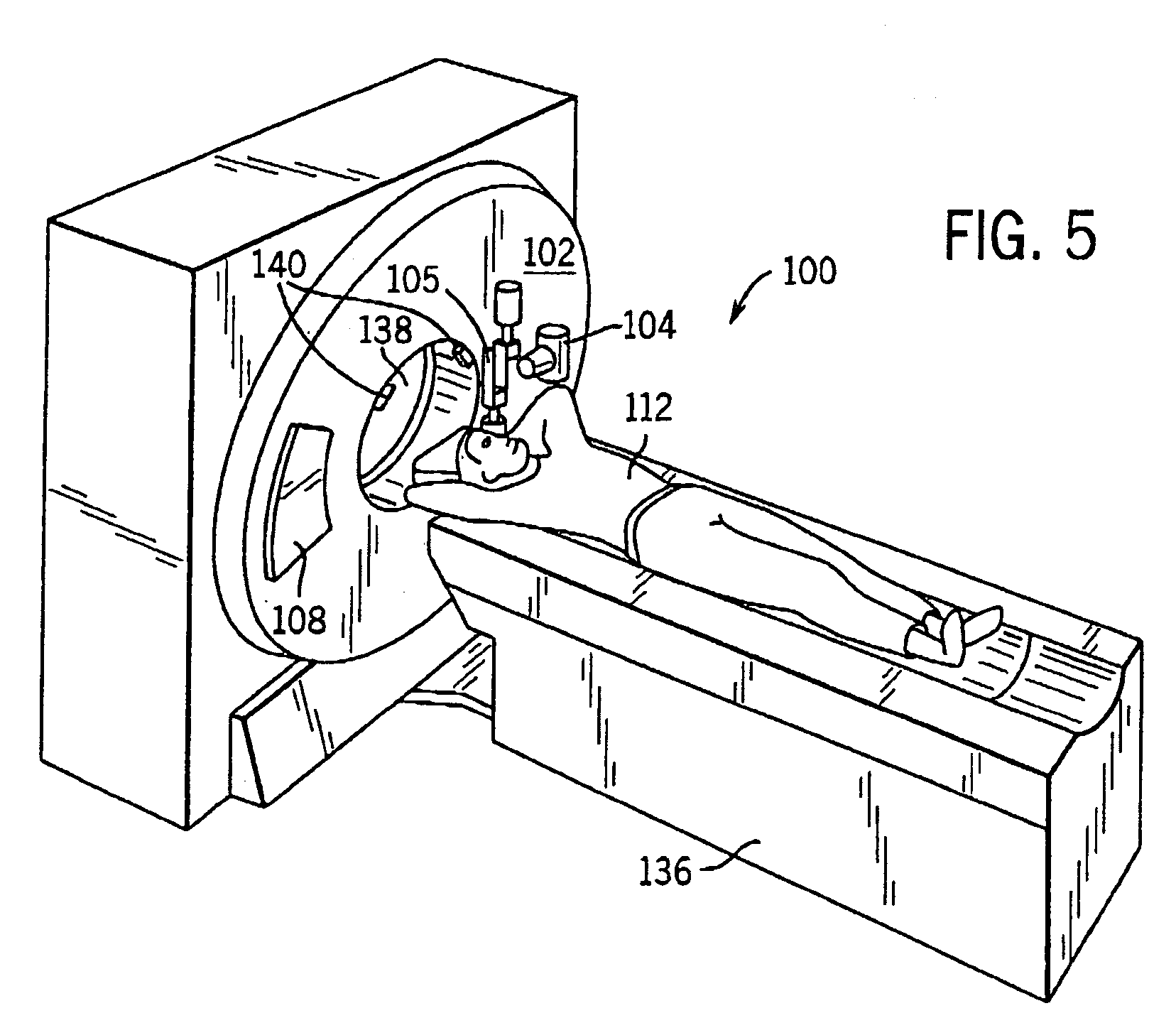
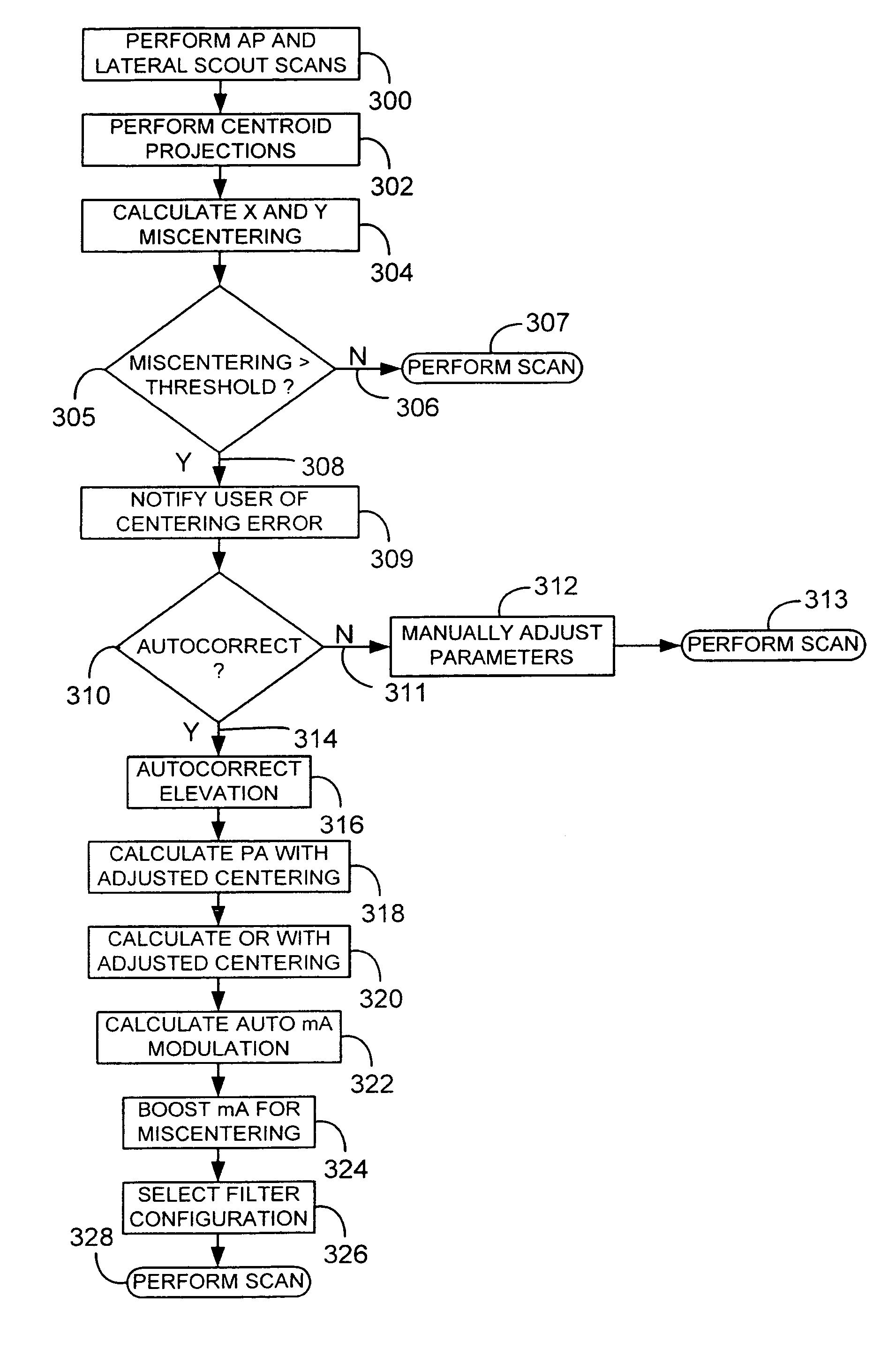
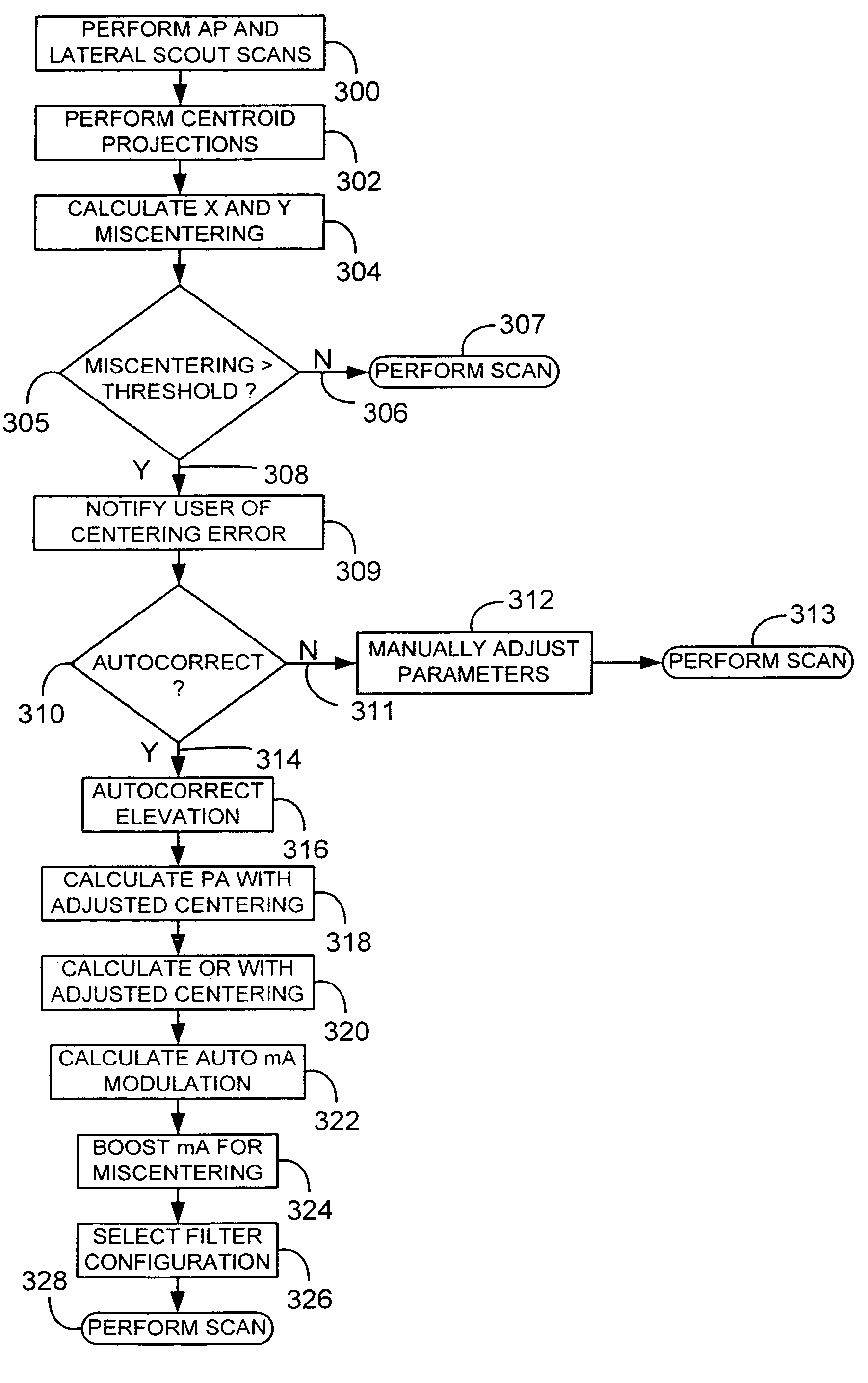
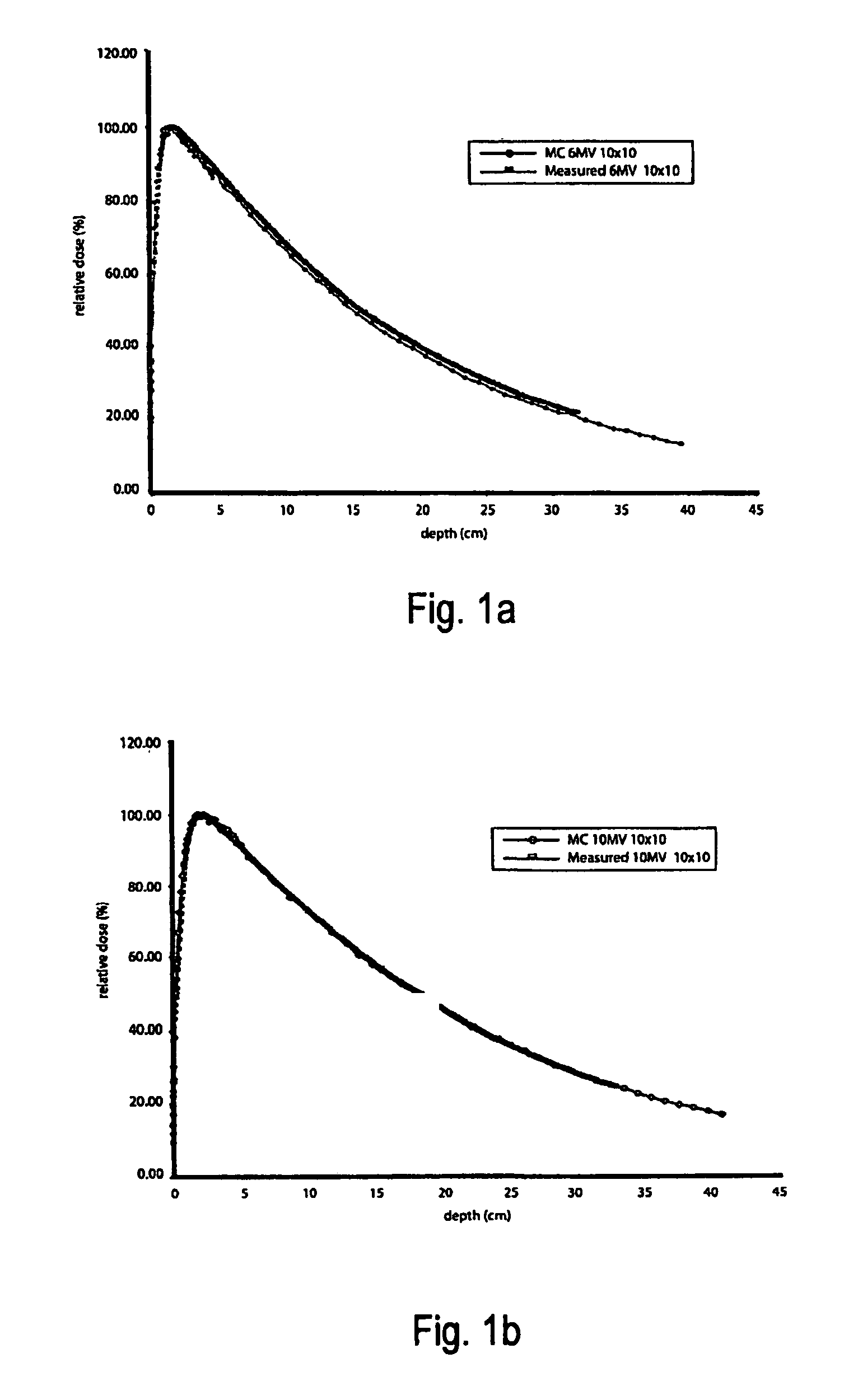
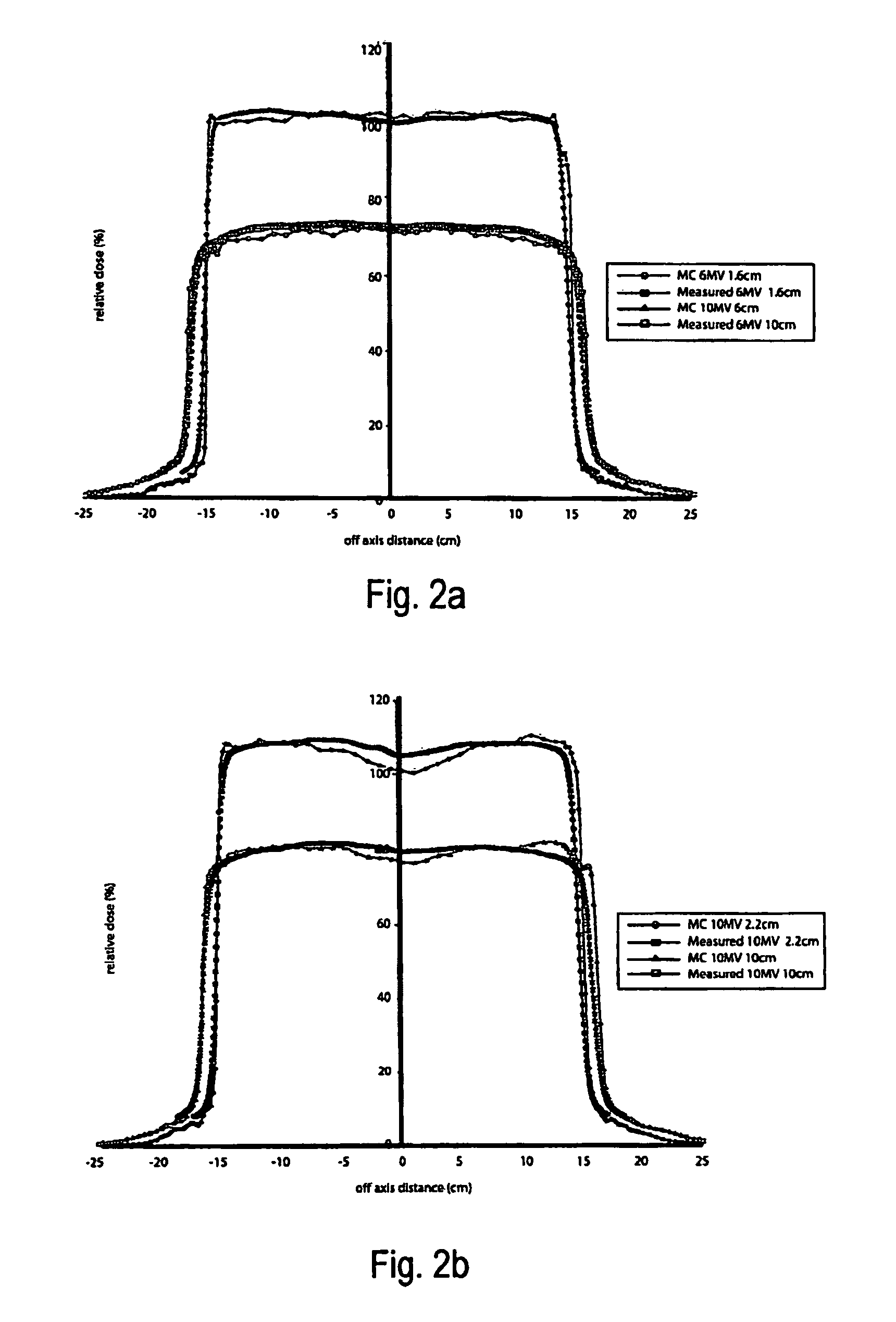
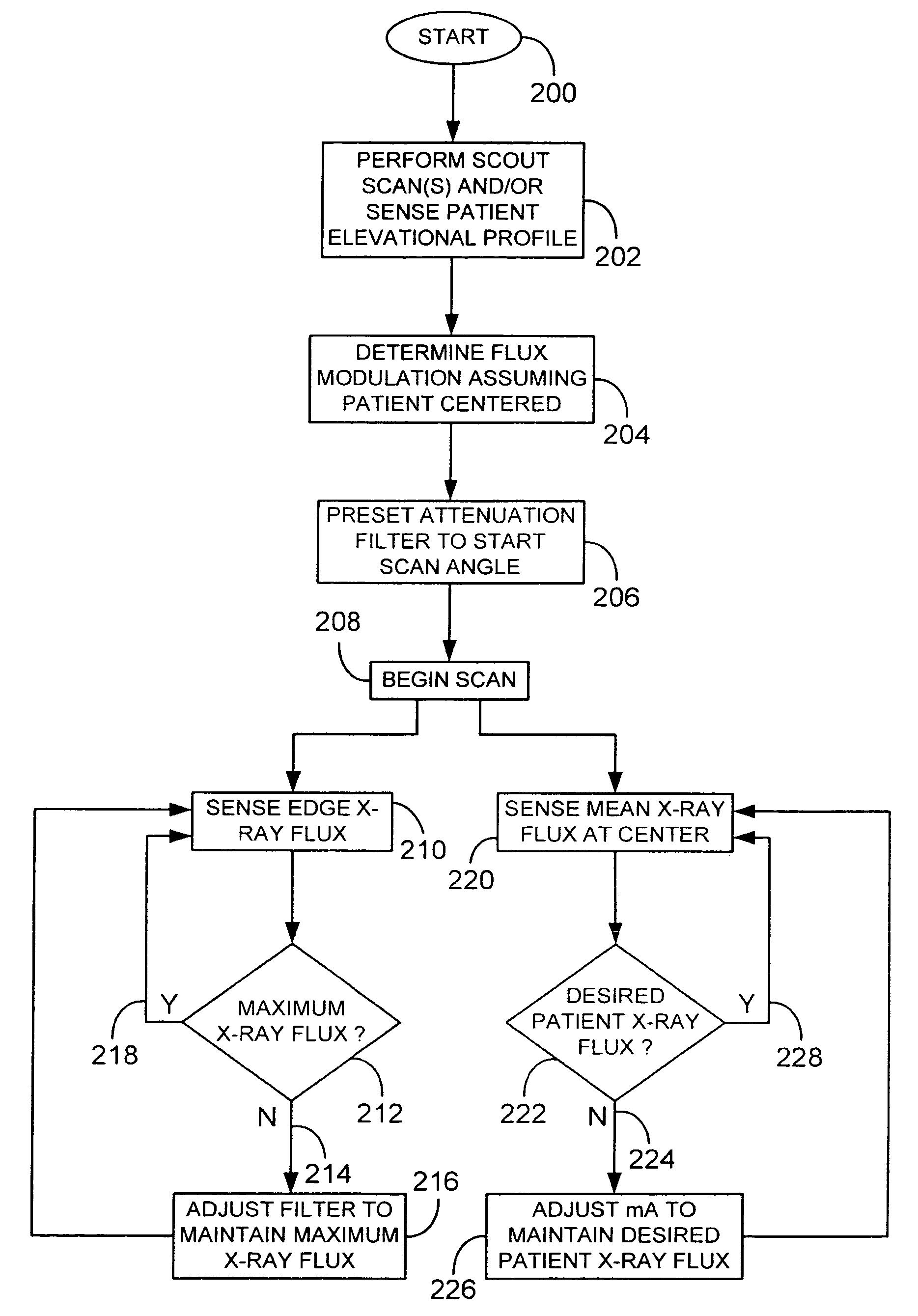
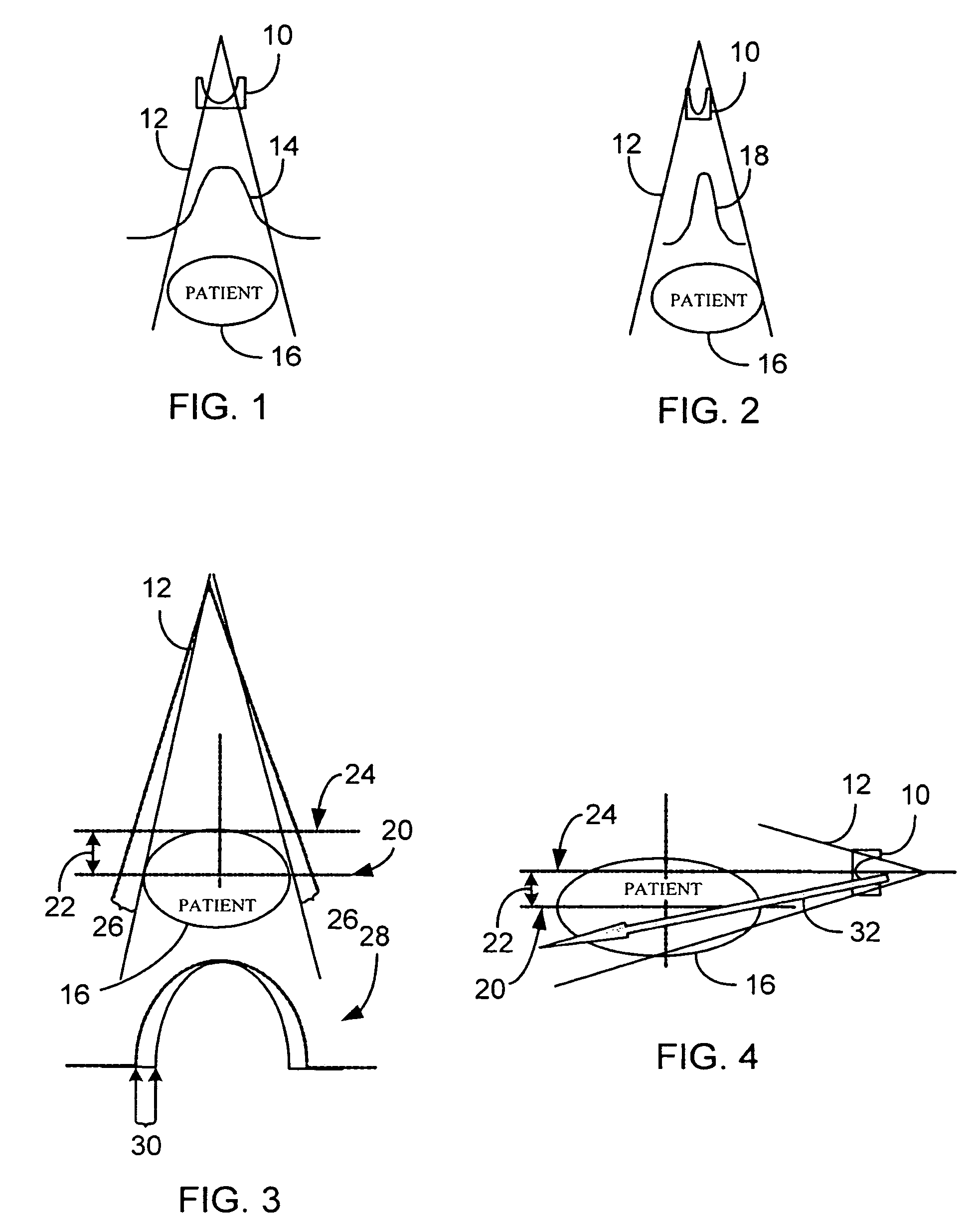
A system and method of diagnostic imaging is provided that includes positioning a subject in an imaging device, performing at least one scout scan, and marking a user-defined region-of-interest. An attenuation characteristic of an attenuation filter is then automatically adjusted based on the user-defined region-of-interest. The present invention automatically selects a proper attenuation filter configuration, corrects patient centering, and corrects noise prediction errors, thereby increasing dose efficiency and tube output.
Owner:GENERAL ELECTRIC CO
System and method of determining a center of mass of an imaging subject for x-ray flux management control
InactiveUS7068751B2Optimize radiation exposureAccurately tailoredMaterial analysis using wave/particle radiationRadiation/particle handlingUltrasound attenuationX-ray
A system and method of diagnostic imaging is provided that includes positioning a subject in a scanning bay, comparing a center of mass of the subject to a reference point, and repositioning the subject in the scanning bay to reduce a difference in position between the center of mass of the subject and the reference point. The present invention automatically selects a proper attenuation filter configuration, corrects patient centering, and corrects noise prediction errors, thereby increasing dose efficiency and tube output.
Owner:GENERAL ELECTRIC CO
System and method of x-ray flux management control
ActiveUS7068750B2Material analysis using wave/particle radiationRadiation/particle handlingUltrasound attenuationX-ray
A system and method of diagnostic imaging is provided that includes determining a position of a subject in a scanning bay and tailoring x-ray attenuation such that the specific position of the subject is taken into consideration. The present invention automatically selects a proper attenuation filter configuration, corrects patient centering, and corrects noise prediction errors, thereby increasing dose efficiency and tube output.
Owner:GENERAL ELECTRIC CO
System and method of x-ray flux management control
ActiveUS20050089135A1Optimize radiation exposureMaterial analysis using wave/particle radiationRadiation/particle handlingUltrasound attenuationX-ray
A system and method of diagnostic imaging is provided that includes determining a position of a subject in a scanning bay and tailoring x-ray attenuation such that the specific position of the subject is taken into consideration. The present invention automatically selects a proper attenuation filter configuration, corrects patient centering, and corrects noise prediction errors, thereby increasing dose efficiency and tube output.
Owner:GENERAL ELECTRIC CO
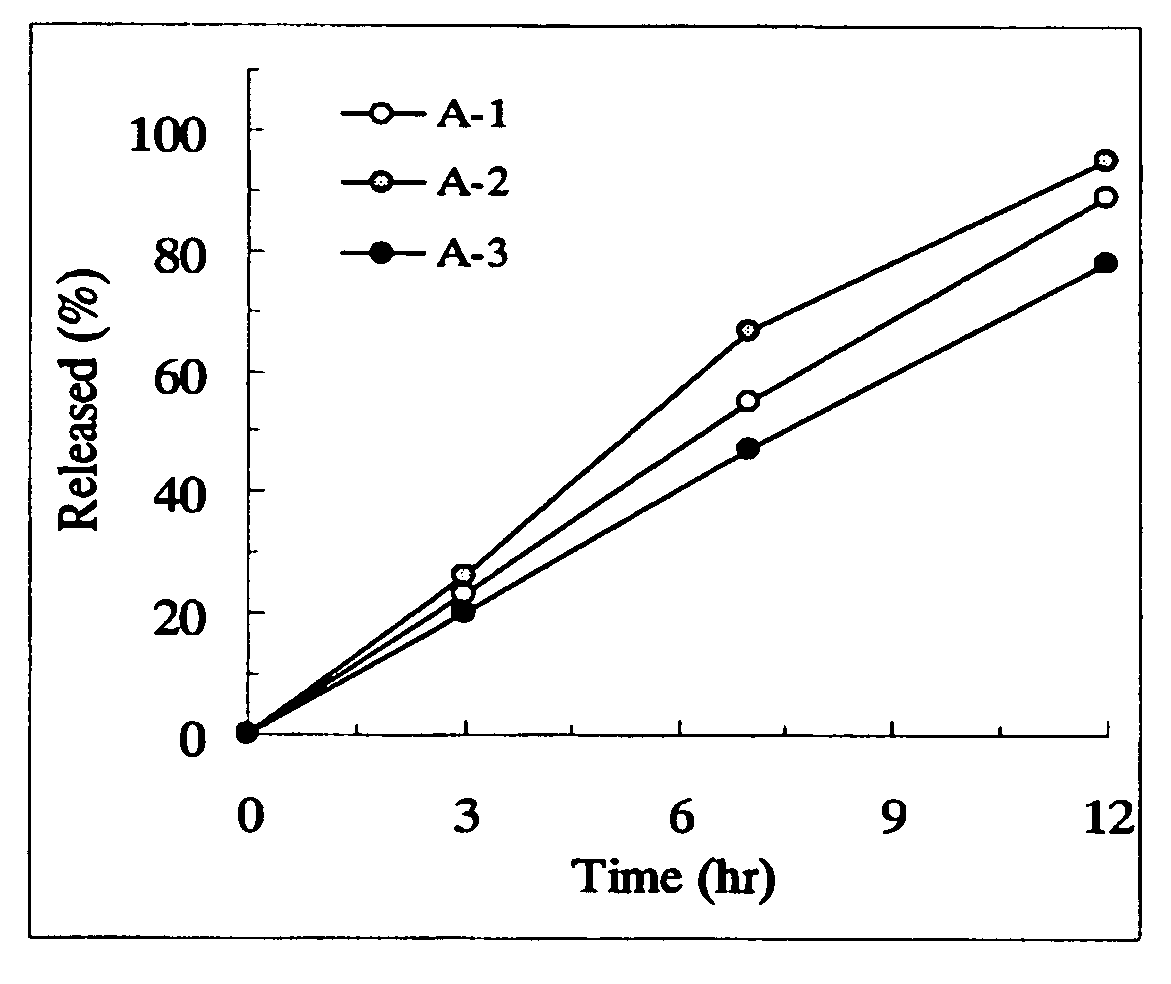
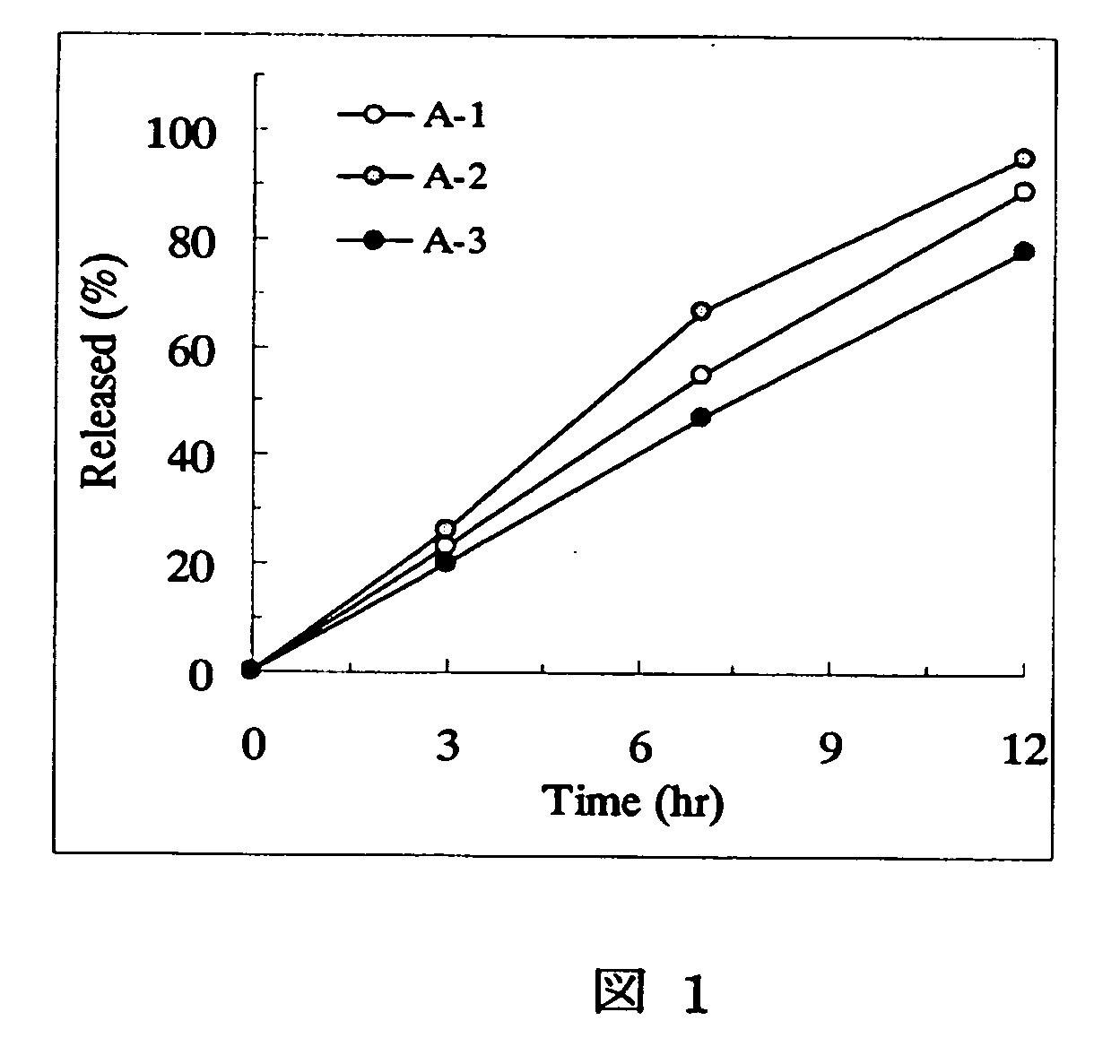
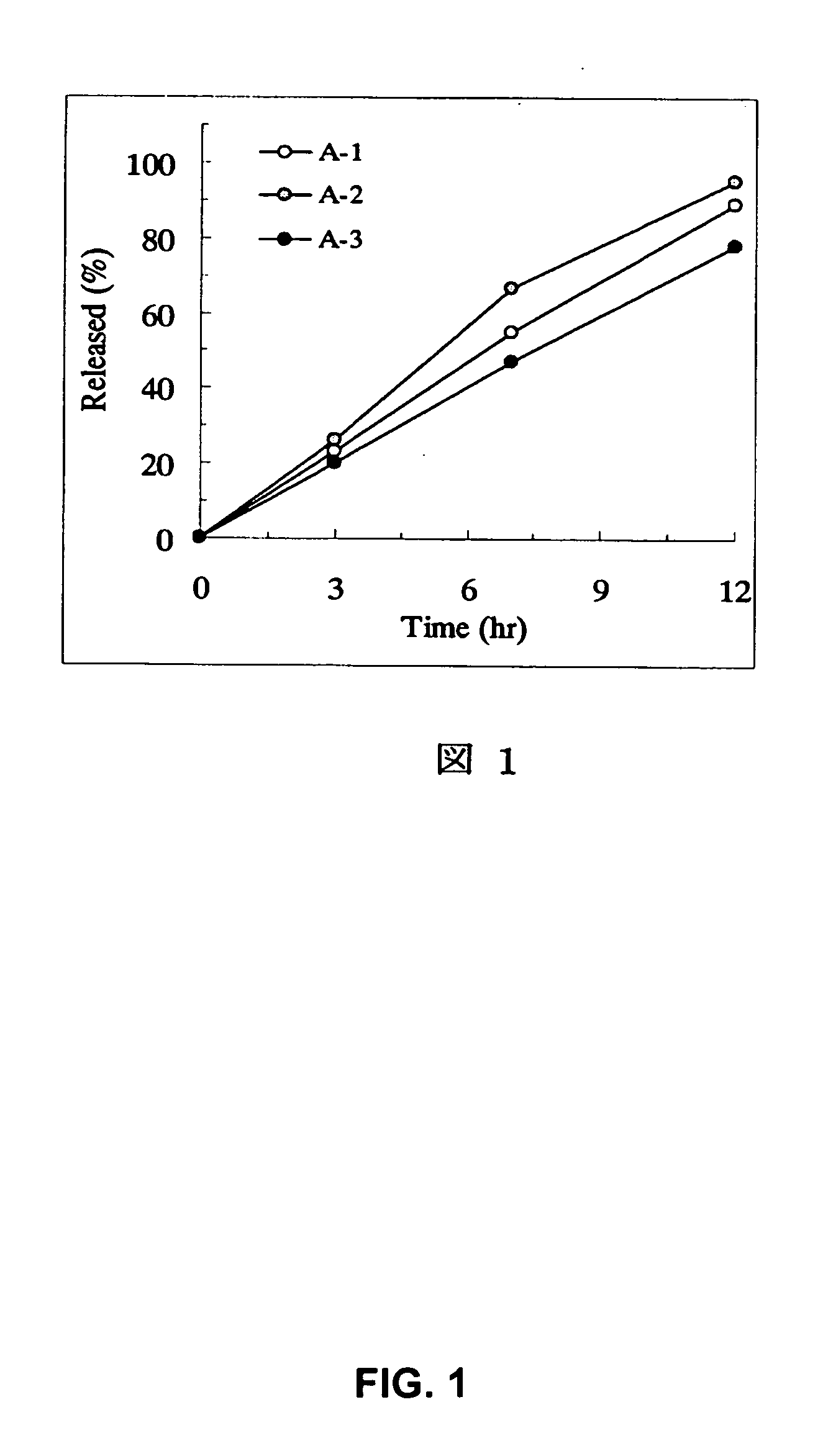
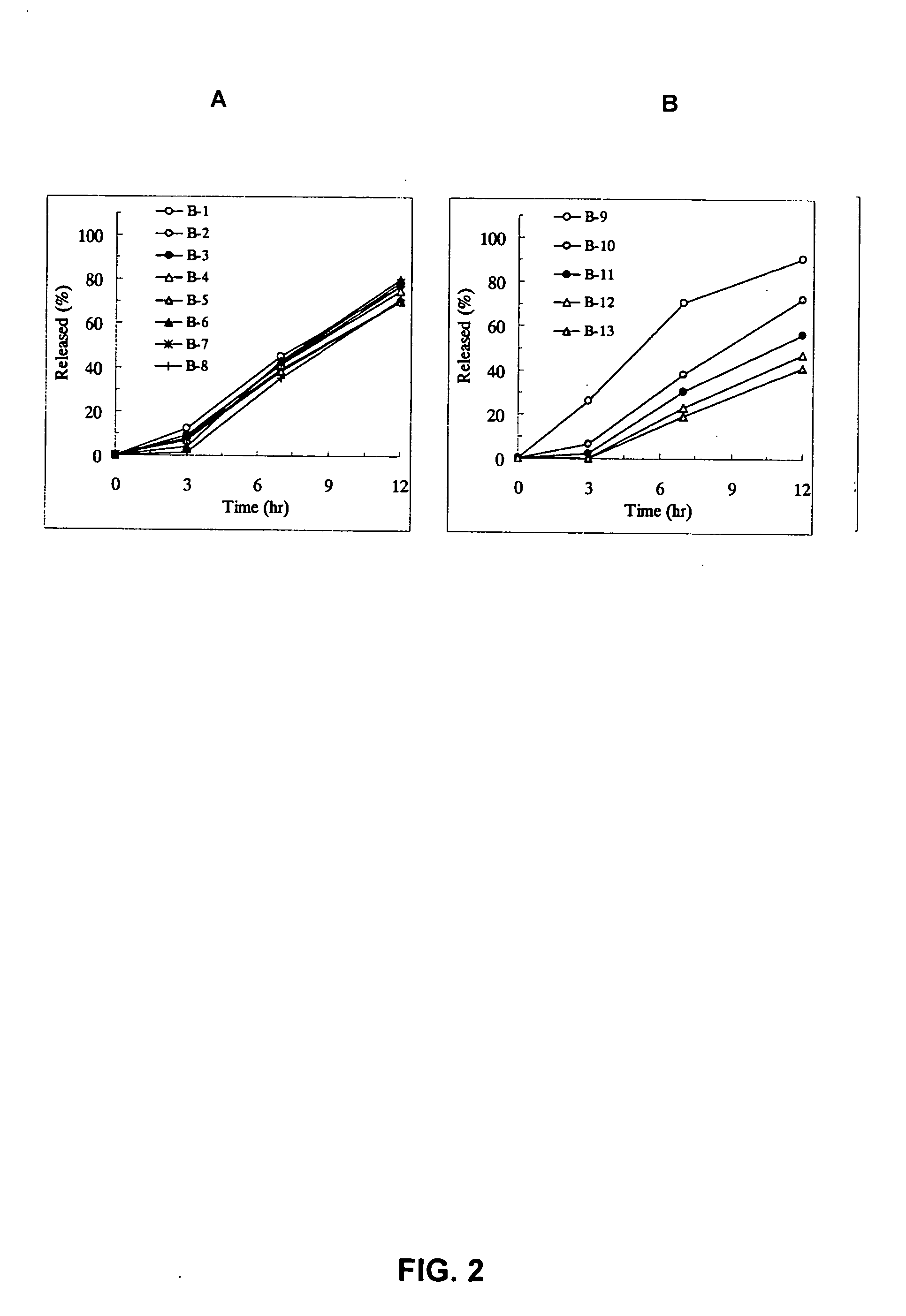
Sustained release pharmaceutical composition
InactiveUS20050100603A1Equivalent and even more efficacyReduce the adverse eventsPowder deliveryBiocideTamsulosin hclSustained release drug
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting at present, it is needed to provide a sustained-release pharmaceutical composition in which the efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited in the dosage and it is also needed to provide a method for administration of tamsulosin hydrochloride in which the adverse reactions accompanied by therapy or prevention on the basis of an α1 receptor blocking action are reduced. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and the ratio (Cmin / Cmax ratio) of the plasma tamsulosin concentration at 24 hours after the administration of the preparation per os (Cmin) to the maximum plasma tamsulosin concentration after the administration (Cmax) is about 0.4 or more.
Owner:ASTELLAS PHARMA INC
Adjustable dosage syringe
The present invention is directed to determining, without repeated calculation by an operator, the volume of a non-solid composition remaining in the barrel of a syringe after discharge of a dose of the composition. The present invention provides a syringe barrel, and a plunger having a dose volume scale disposed along the longitudinal axis and orientated such that the numeric values increase in the direction from the proximal end of the plunger towards the discharge end. The dosage syringe also comprises an adjustable dose selector that may be moved to a volume mark of the scale corresponding to a desired dose to be delivered. The dose syringe barrel further comprises an indicator means that may be an opening in the barrel directly above the dose volume scale such that, when the plunger is in a fully retracted position, the window shows the volume of the full capacity of the syringe barrel and when the plunger is depressed, will show the remaining volume in the syringe.
Owner:MERIAL SAS
System and method of determining a user-defined region-of-interest of an imaging subject for x-ray flux management control
ActiveUS20050089136A1Material analysis using wave/particle radiationRadiation/particle handlingUltrasound attenuationX-ray
A system and method of diagnostic imaging is provided that includes positioning a subject in an imaging device, performing at least one scout scan, and marking a user-defined region-of-interest. An attenuation characteristic of an attenuation filter is then automatically adjusted based on the user-defined region-of-interest. The present invention automatically selects a proper attenuation filter configuration, corrects patient centering, and corrects noise prediction errors, thereby increasing dose efficiency and tube output.
Owner:GENERAL ELECTRIC CO
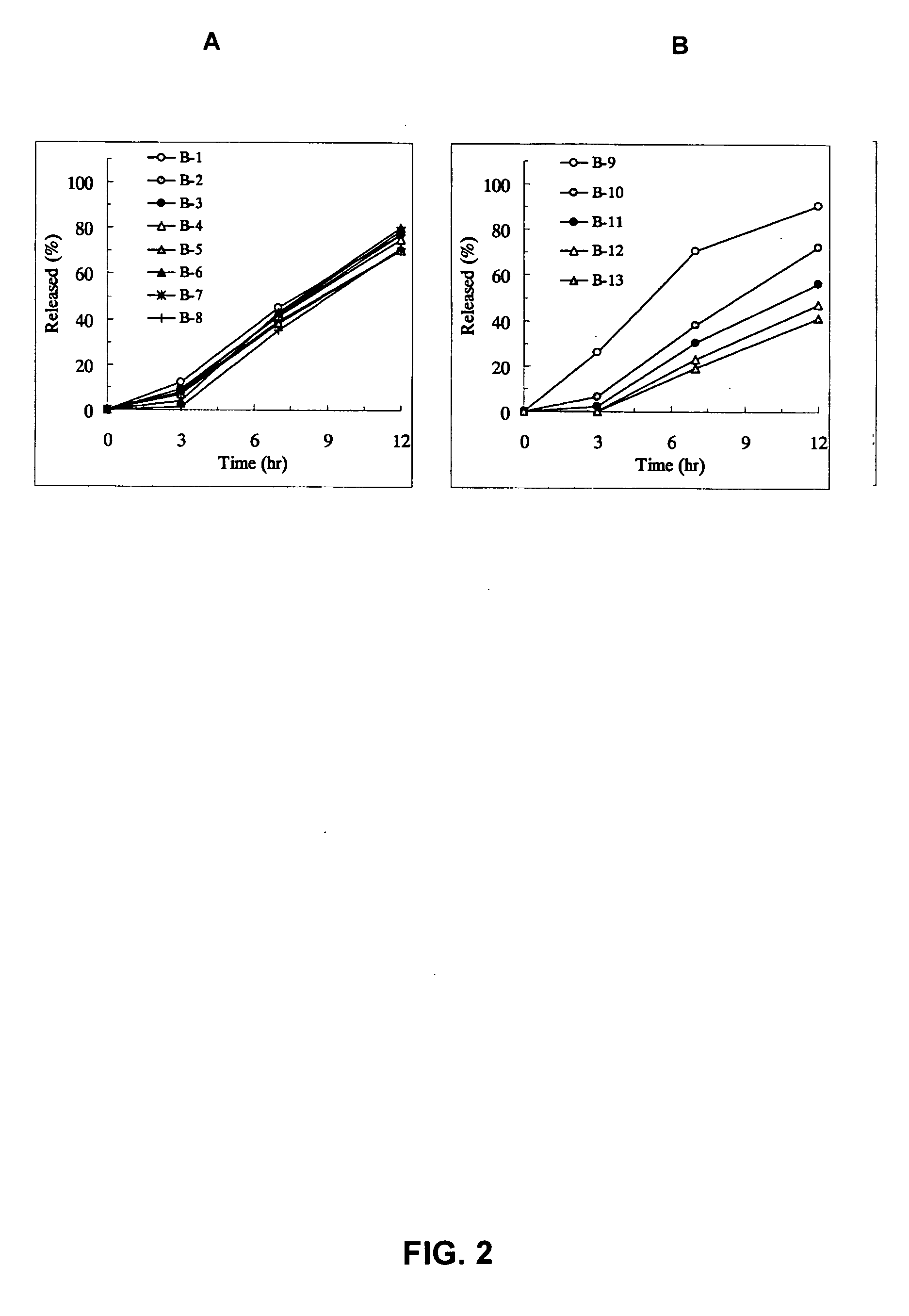
Sustained release pharmaceutical composition
InactiveUS20050100602A1Reduces adverse eventImprove featuresPowder deliveryBiocideTamsulosin hclJapanese Pharmacopoeia
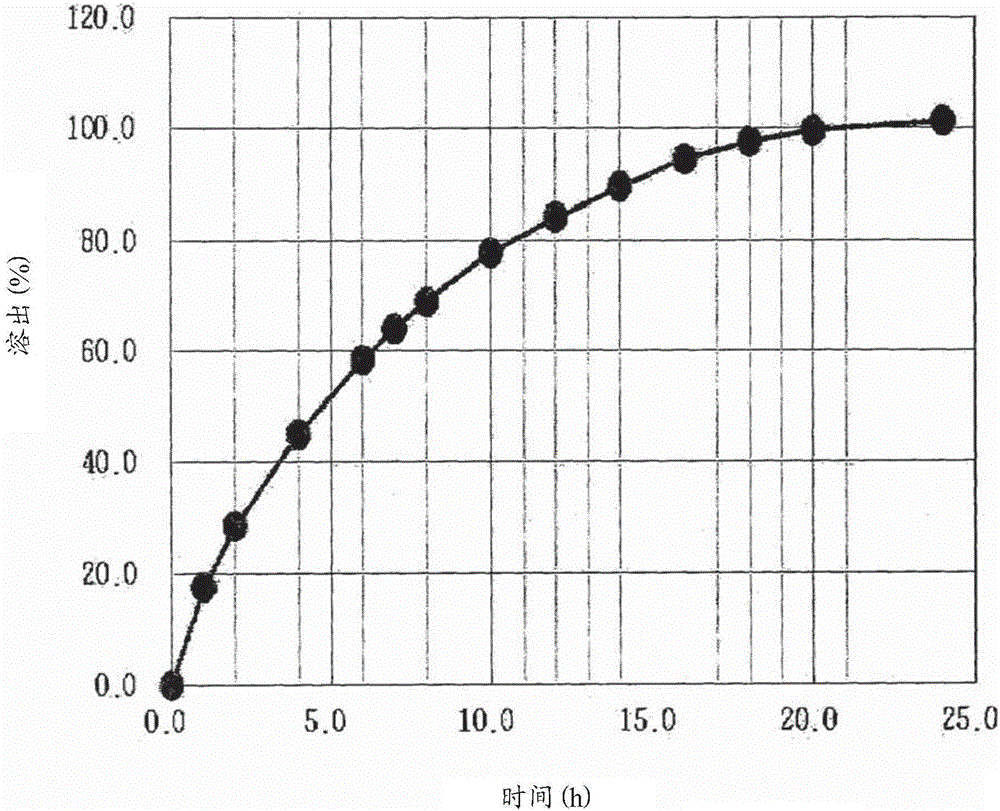
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting, there is a problem to provide a sustained-release pharmaceutical composition in which efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and, when dissolution test is carried out according to Japanese Pharmacopoeia Dissolution Test Method 2, the tamsulosin release after 7 hours from the start of the dissolution is about 20 to about 85%.
Owner:ASTELLAS PHARMA INC
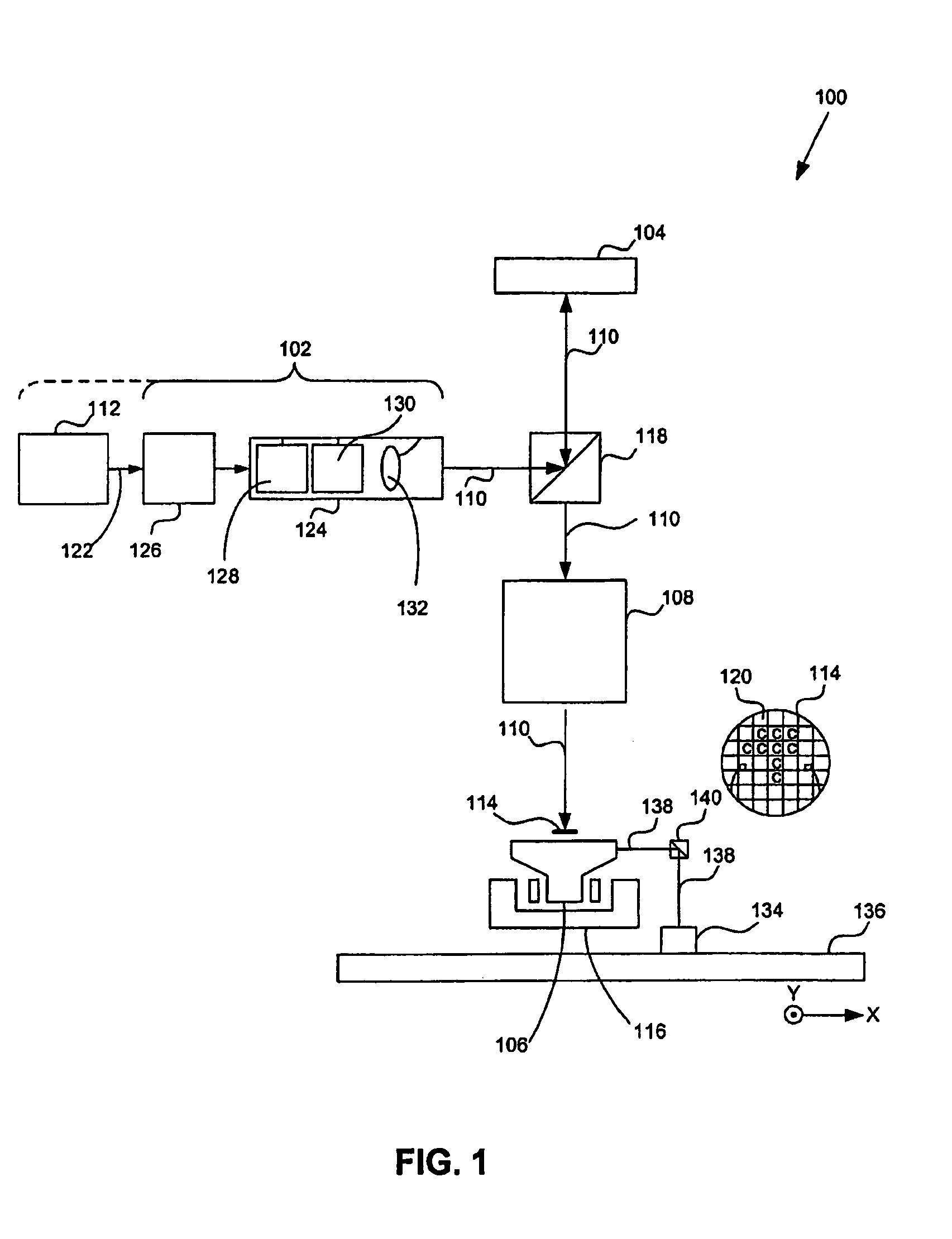
Lithographic apparatus and device manufacturing method
ActiveUS20050270515A1Increase radiation doseReduce the amount of compensationSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusLight beamMaximum dose
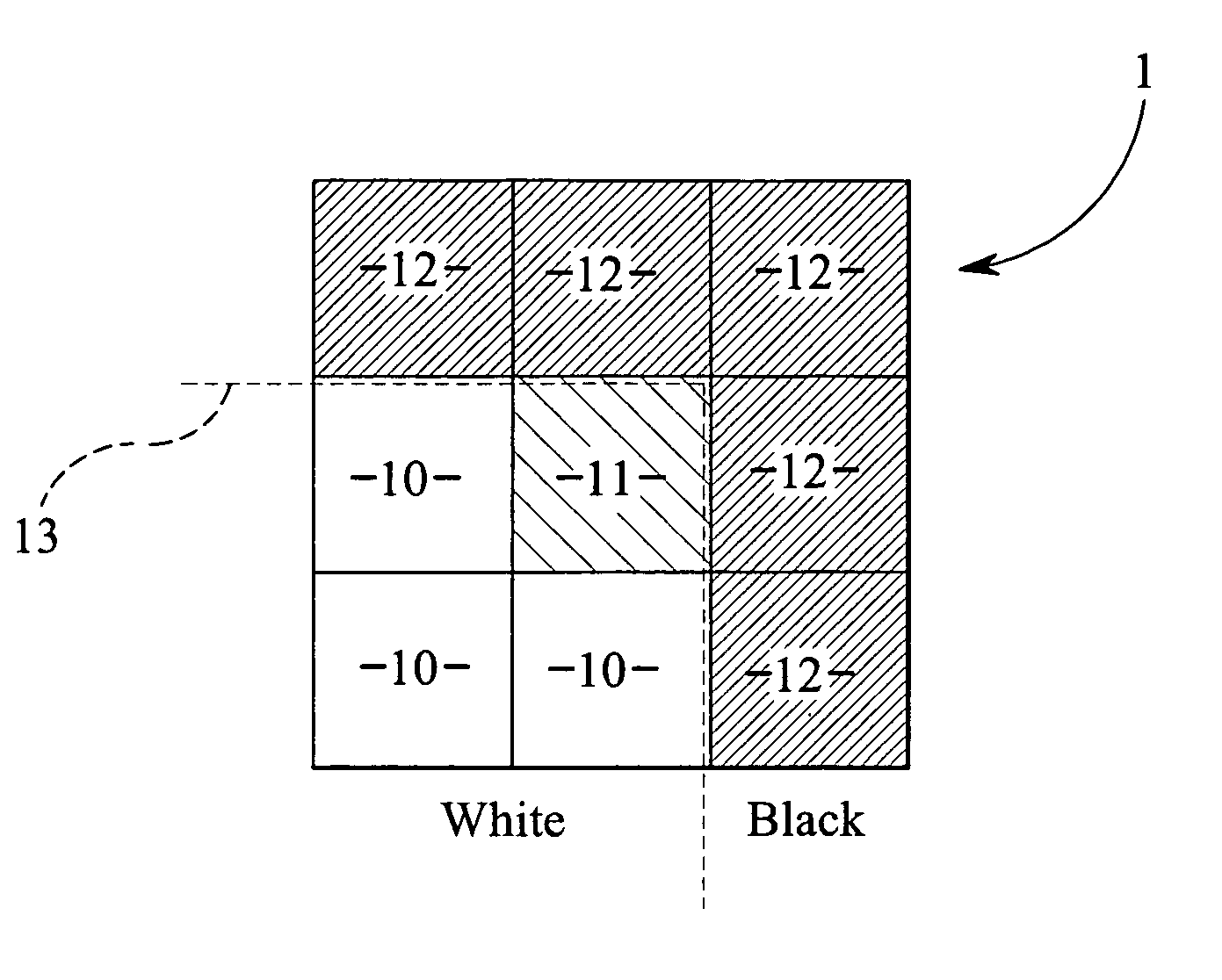
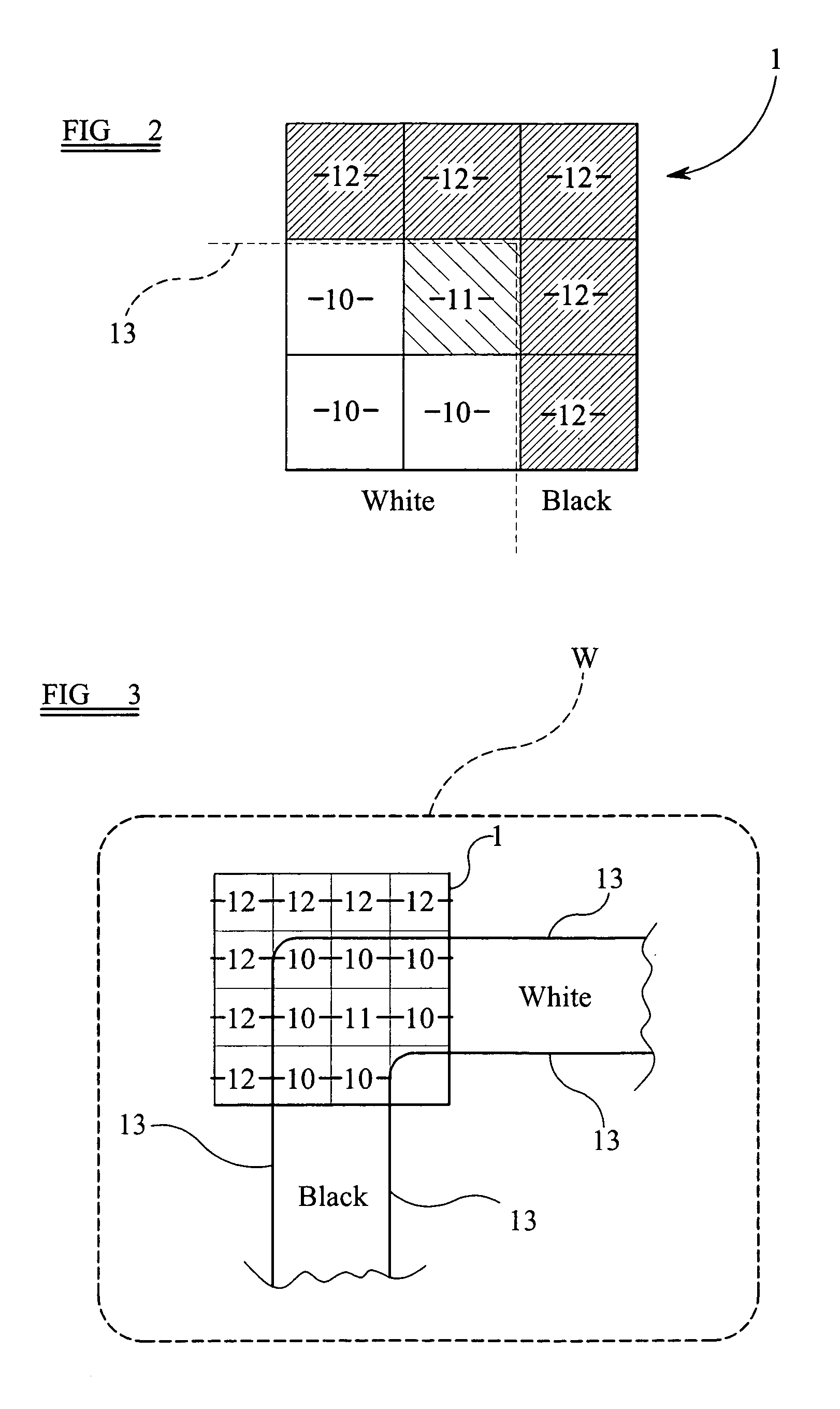
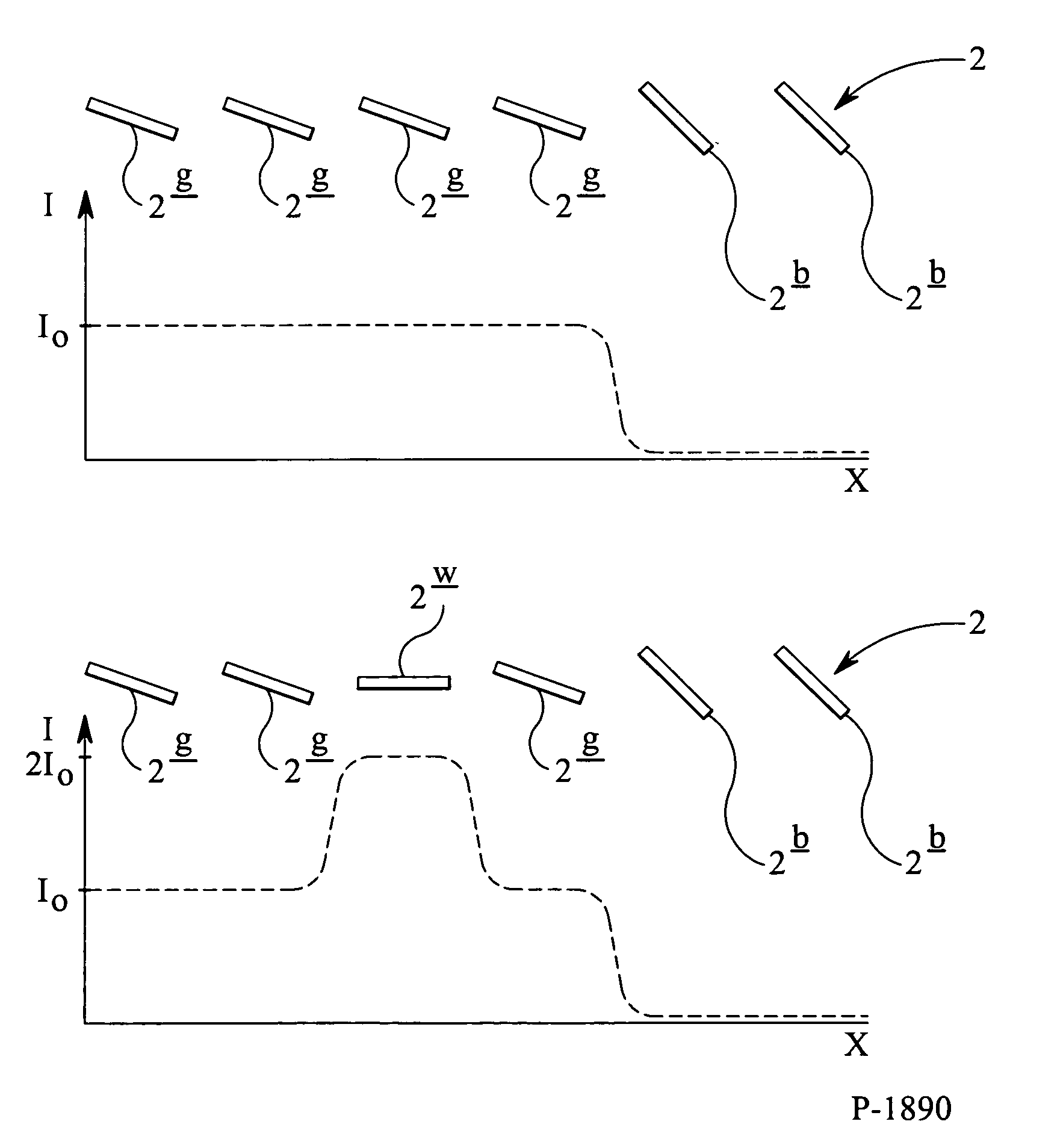
A system and method to manufacturing a device provide a substrate and perform at least one exposure step. Each exposure step projects a patterned beam of radiation, which was patterned using an individually controllable elements, onto a target portion of the substrate. The projected patterned beam includes a plurality of pixels. Each exposure step also: (a) ordinarily controls the elements, such that each pixel delivers a radiation dose no greater than a predetermined normal maximum dose to the target portion in the exposure step; and (b) exceptionally controls the elements, such that at least one selected pixel delivers an increased radiation dose, greater than the normal maximum dose. The increased dose may be delivered to compensate for the effect of a defective element at a known position in the array on a pixel adjacent a selected pixel. Alternatively, it may compensate for underexposure of the target portion at the location of a selected pixel resulting from exposure of that location to a pixel affected by a known defective element in another exposure step. The invention uses doses up to the predetermined normal maximum for normal printing, but reserves at least one increased dose for compensation purposes. Accordingly, even when a dead black pixel falls in the middle of a group of surrounding white pixels it can be compensated for by increasing the radiation dose delivered by one or more of those neighboring white pixels, above the normal fully white value.
Owner:ASML NETHERLANDS BV
Methods and Compositions for Treating Lactose Intolerance
InactiveUS20080126195A1Improve actionEncouraging growthOrganic active ingredientsBiocideLactose intoleranceEnvironmental health
The invention provides methods and compostions for treating lactose intolerance. In embodiments, the invention provides methods and composition for decreasing symptoms of lactose intolerance by administering to an individual suffering from lactose intolerance increasing doses of lactose using a protocol such that at the end of treatment the individual's symptoms of lactose intolerance are decrease and such that symptoms remain decreased after a period of time.
Owner:RITTER PHARMA
Unfiltered radiation therapy
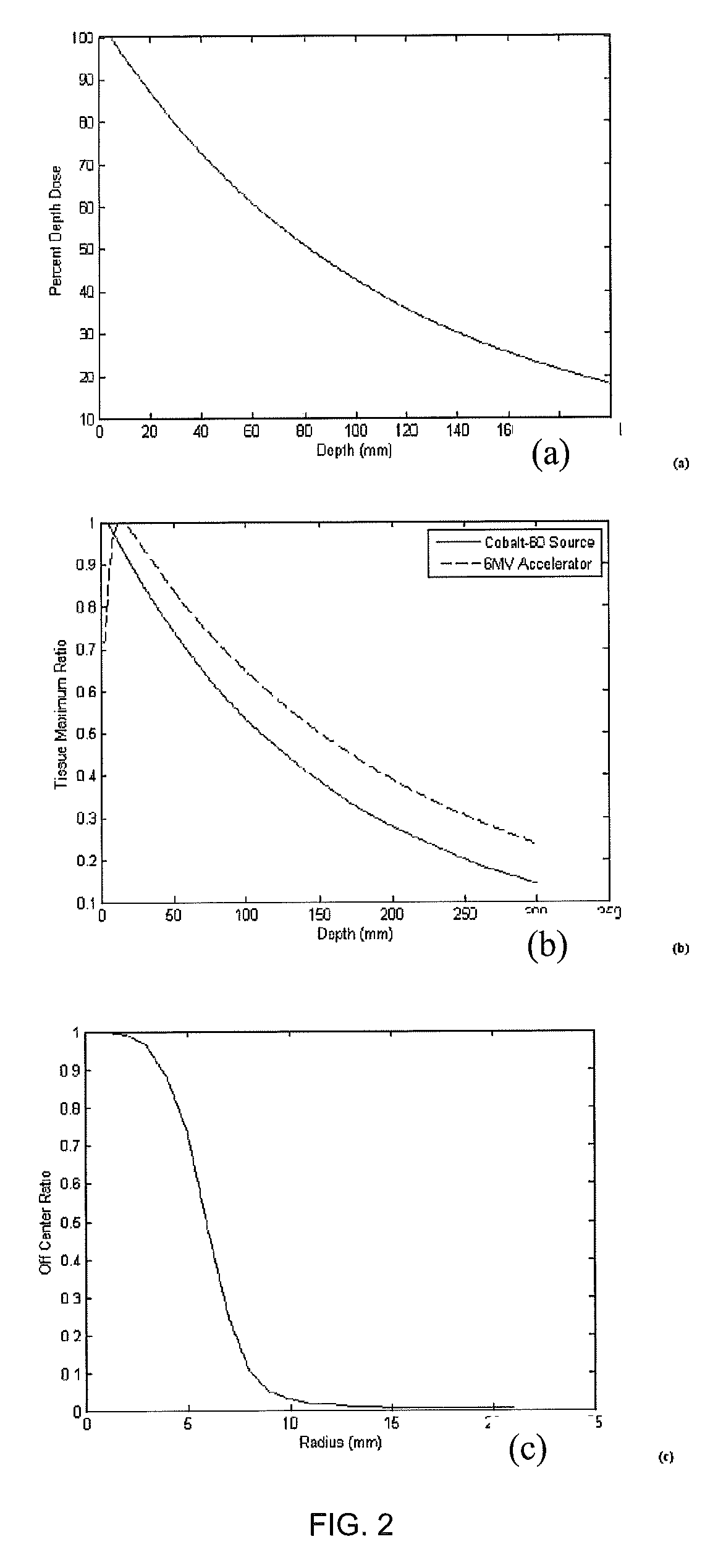
ActiveUS9370672B2Reduce doseIncrease dose rateRadiation/particle handlingX-ray/gamma-ray/particle-irradiation therapyDose rateUnfiltered
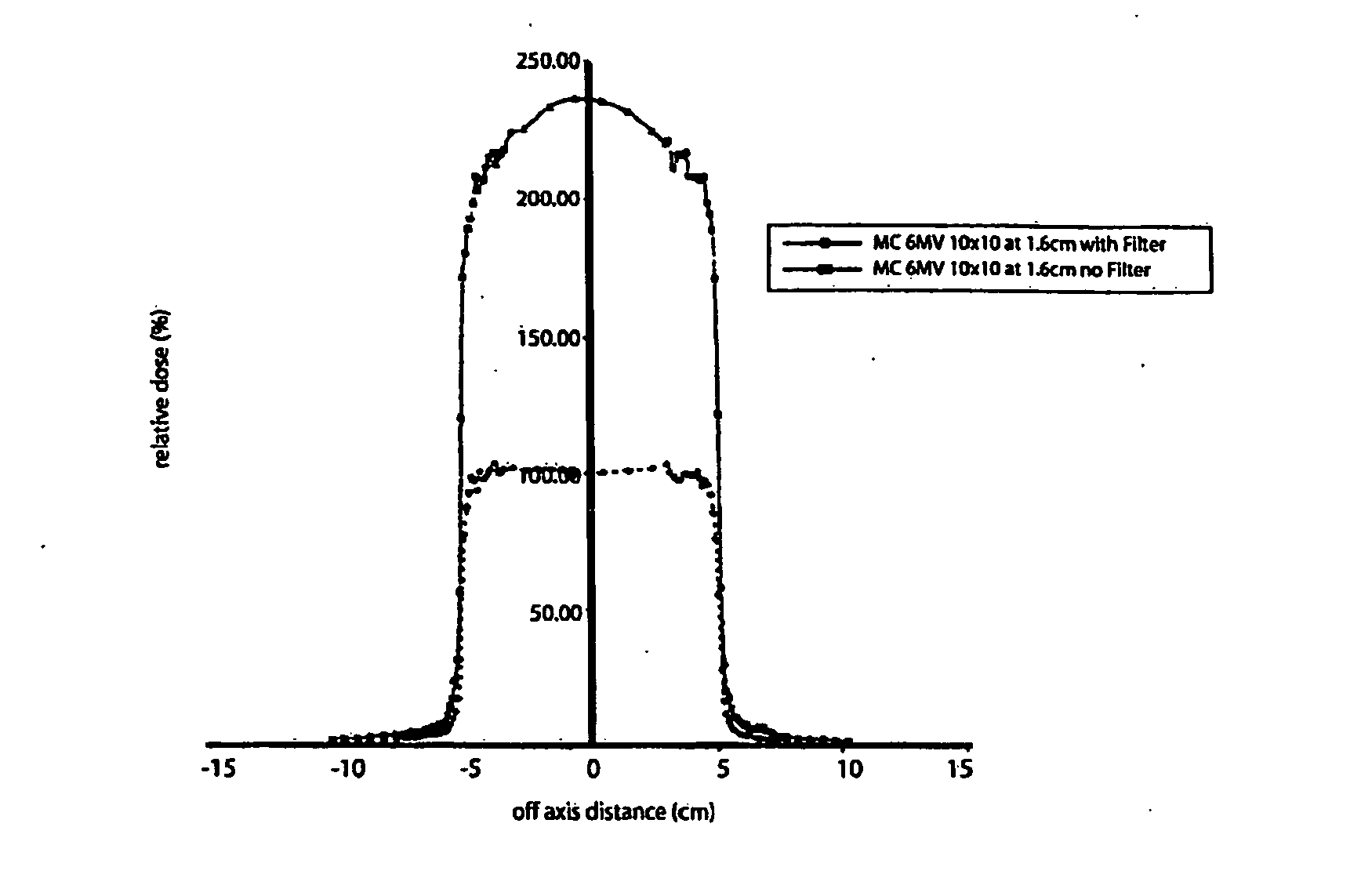
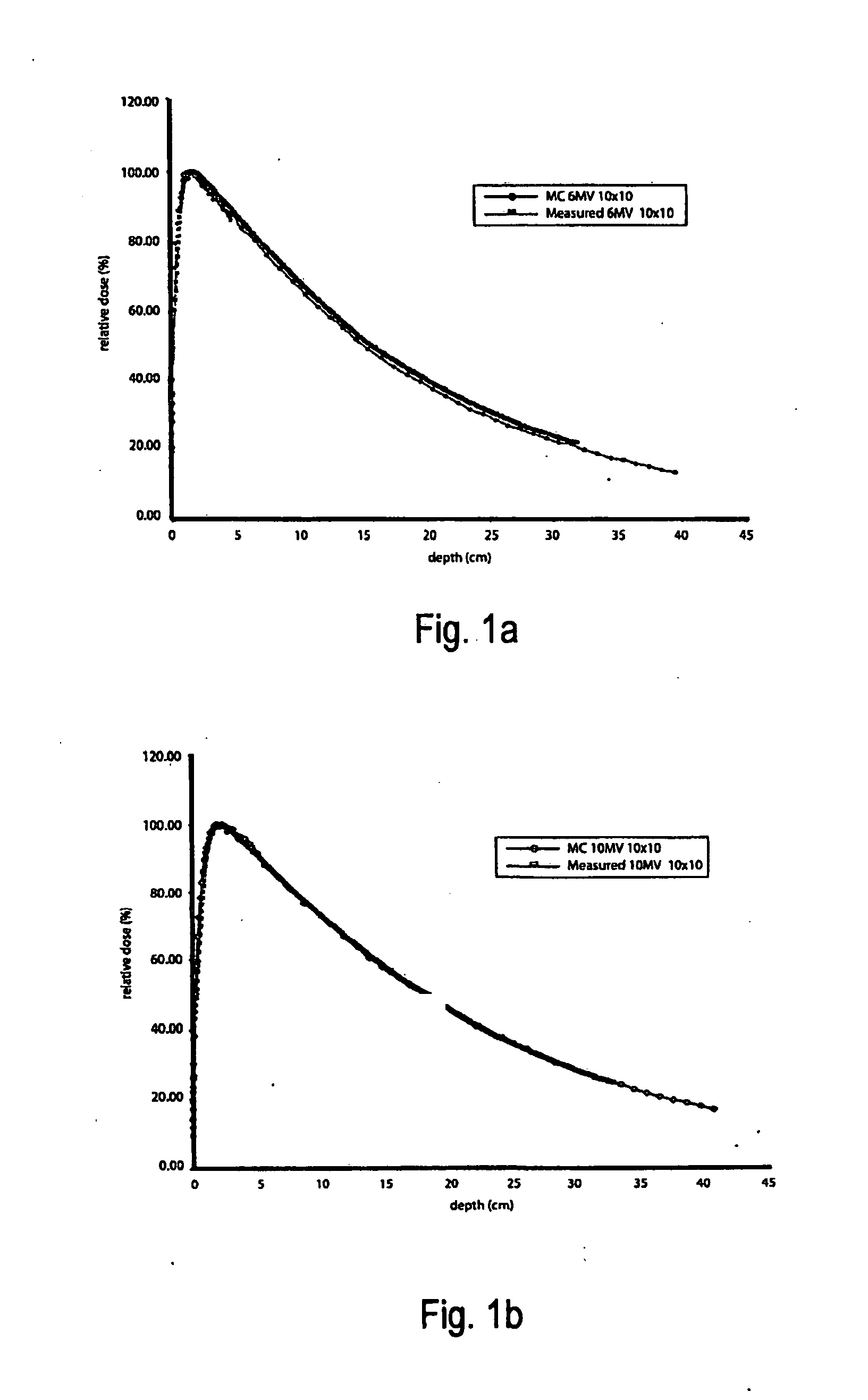
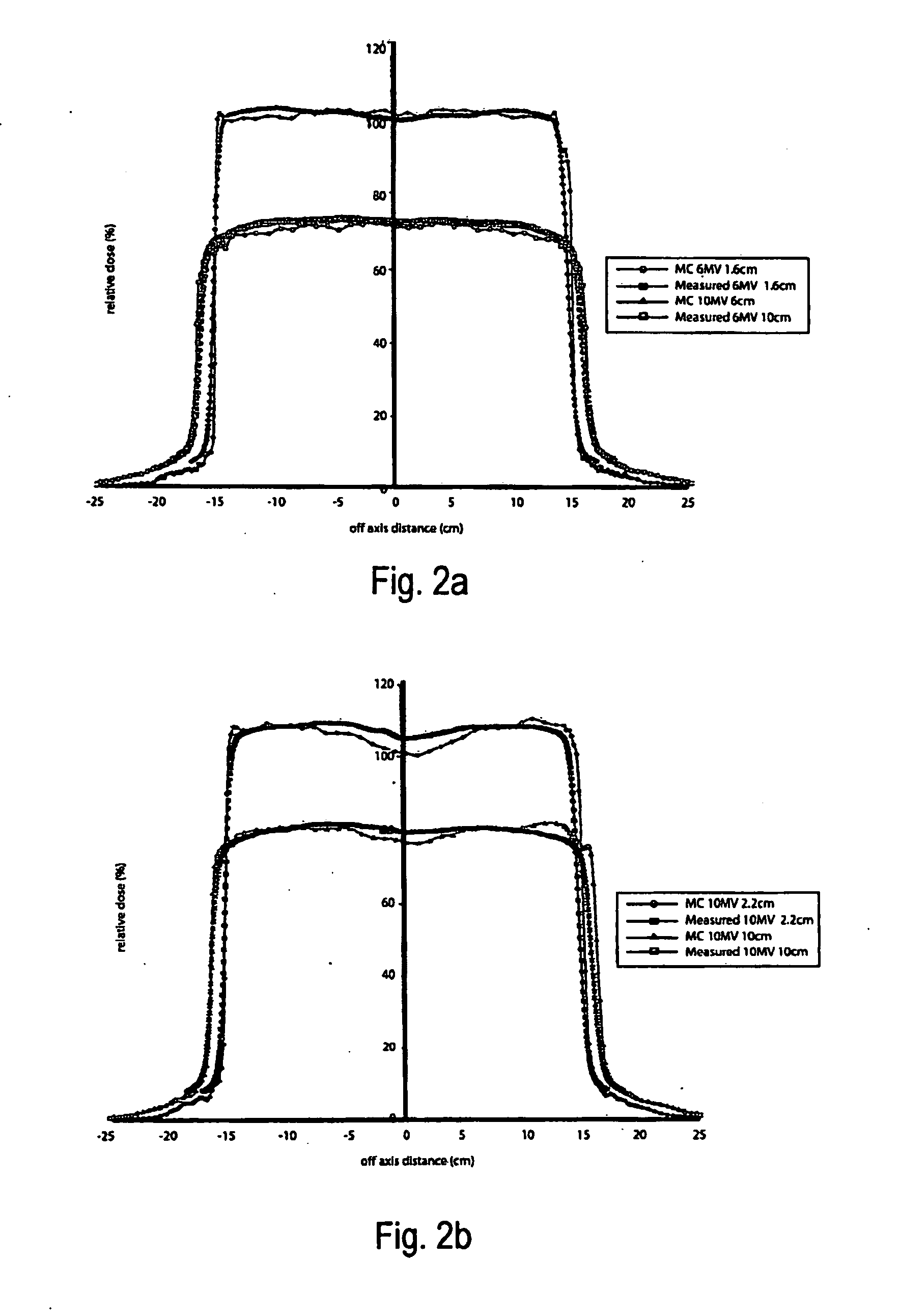
This is a new technique in IMRT and 3D conformal gamma radiation dose delivery using a linear accelerator with no flattening filter. The technique improves patient radiation therapy by reducing radiation scattered to surrounding normal tissue and reducing electron contamination. It increases dose rate to shorten treatment time. Linear accelerators have for decades come with a photon flattening filter to make the photon profile of planar fluence to make the dose distribution more uniform. These filters, however, resulted in fluence attenuation and contamination of the beam. Now in the age of techniques such as intensity modulated radiation therapy (IMRT) the function of the flattening filter becomes redundant. The flattening filter now merely reduces the efficiency of the beam by reducing the fluence and increasing scattered radiation. Our technique involves removal of the flattening filter for complex treatments. It uses inverse planning along with multi-leaf collimators to shape the dose distribution.
Owner:TOLEDO THE UNIV OF
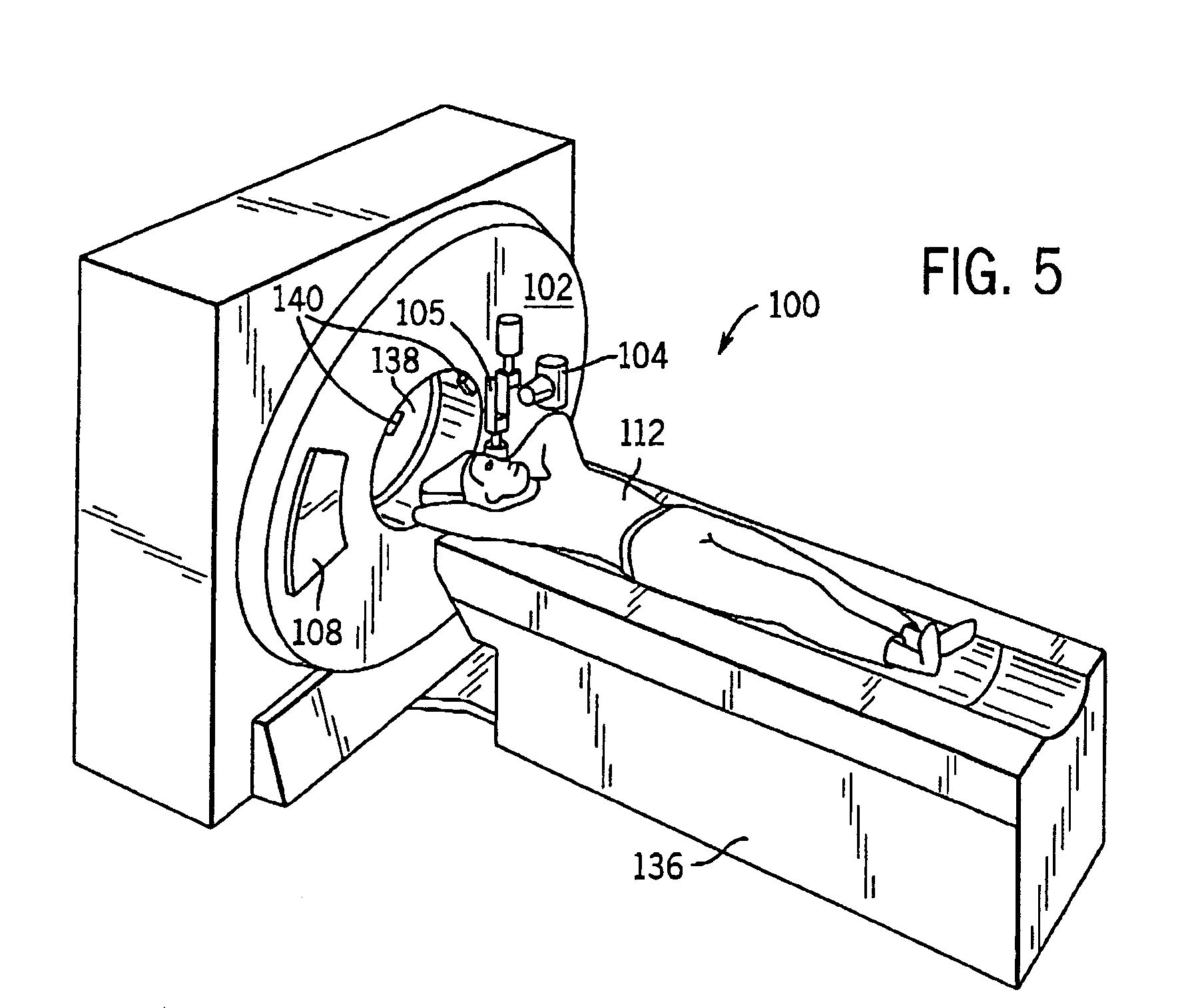
System and method of collecting imaging subject positioning information for x-ray flux control
ActiveUS7313217B2Material analysis using wave/particle radiationRadiation/particle handlingUltrasound attenuationRadiology
A system and method of diagnostic imaging is provided that includes positioning a subject in an imaging device, collecting positioning information of the subject from at least one sensor disposed in proximity of the imaging device, and determining a relative position of the subject within the imaging device from at least the position information. The present invention automatically selects a proper attenuation filter configuration, corrects patient centering, and corrects noise prediction errors, thereby increasing dose efficiency and tube output.
Owner:GENERAL ELECTRIC CO
Methods and compositions for treating lactose intolerance
Owner:RITTER PHARMA
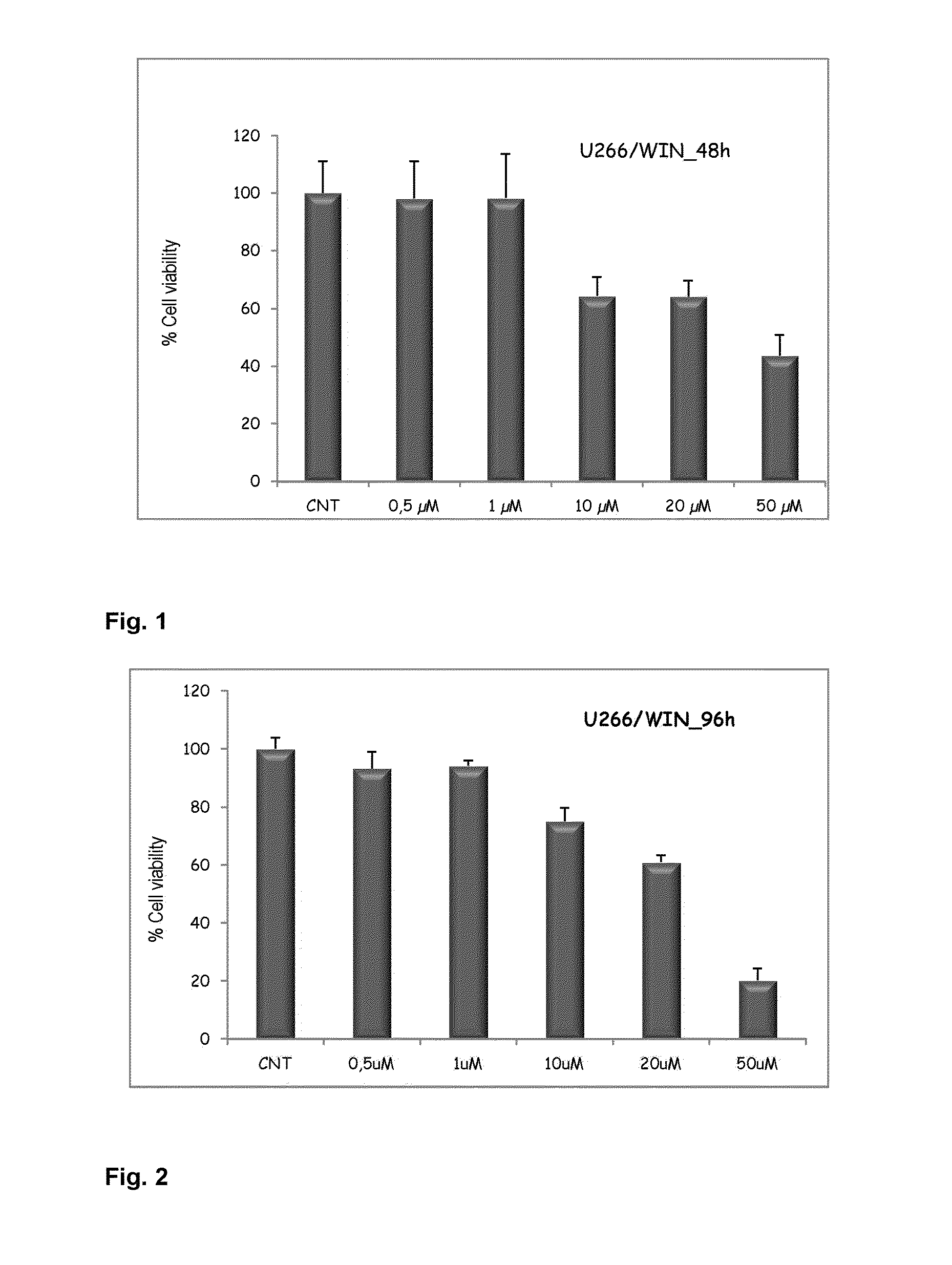
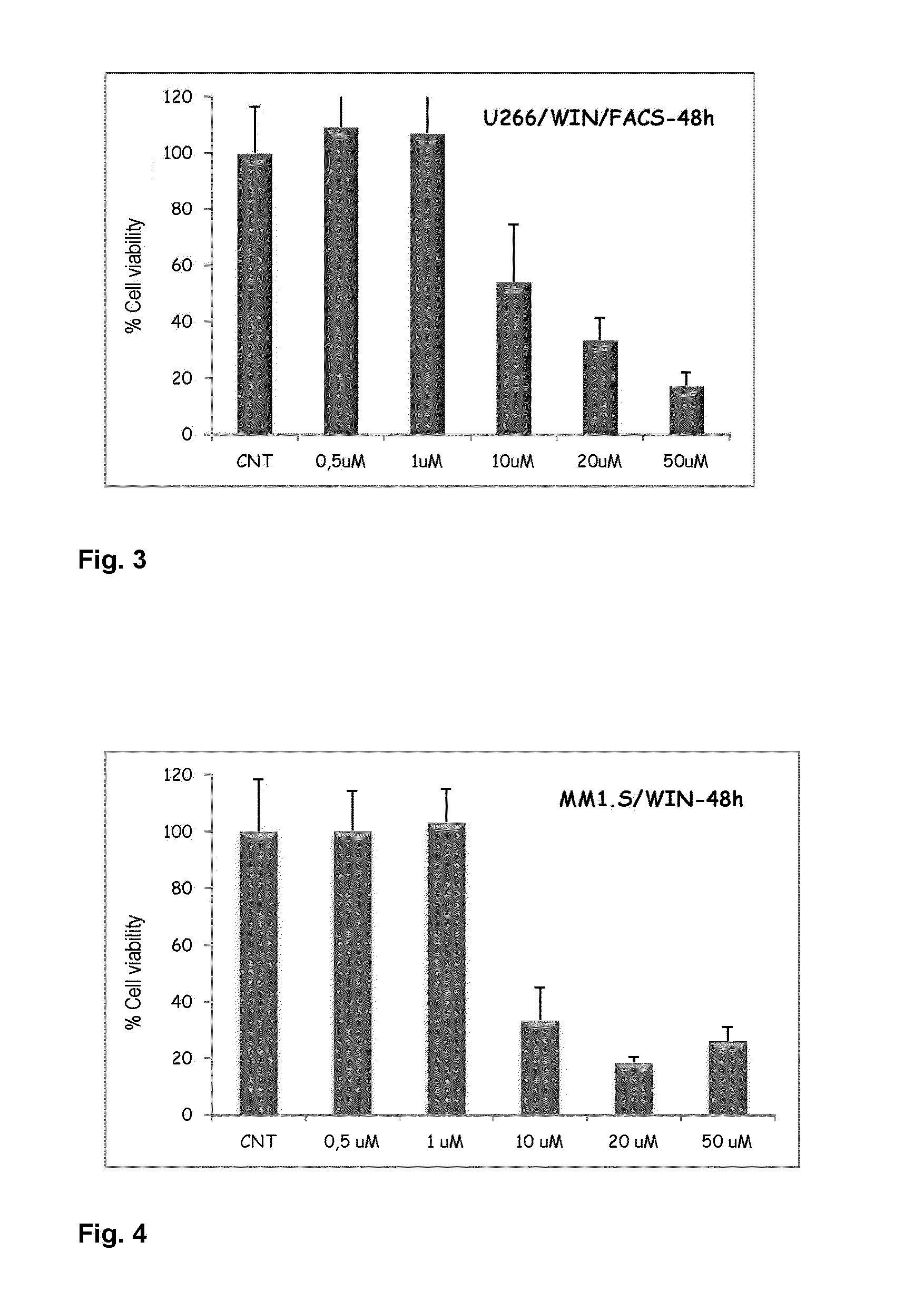
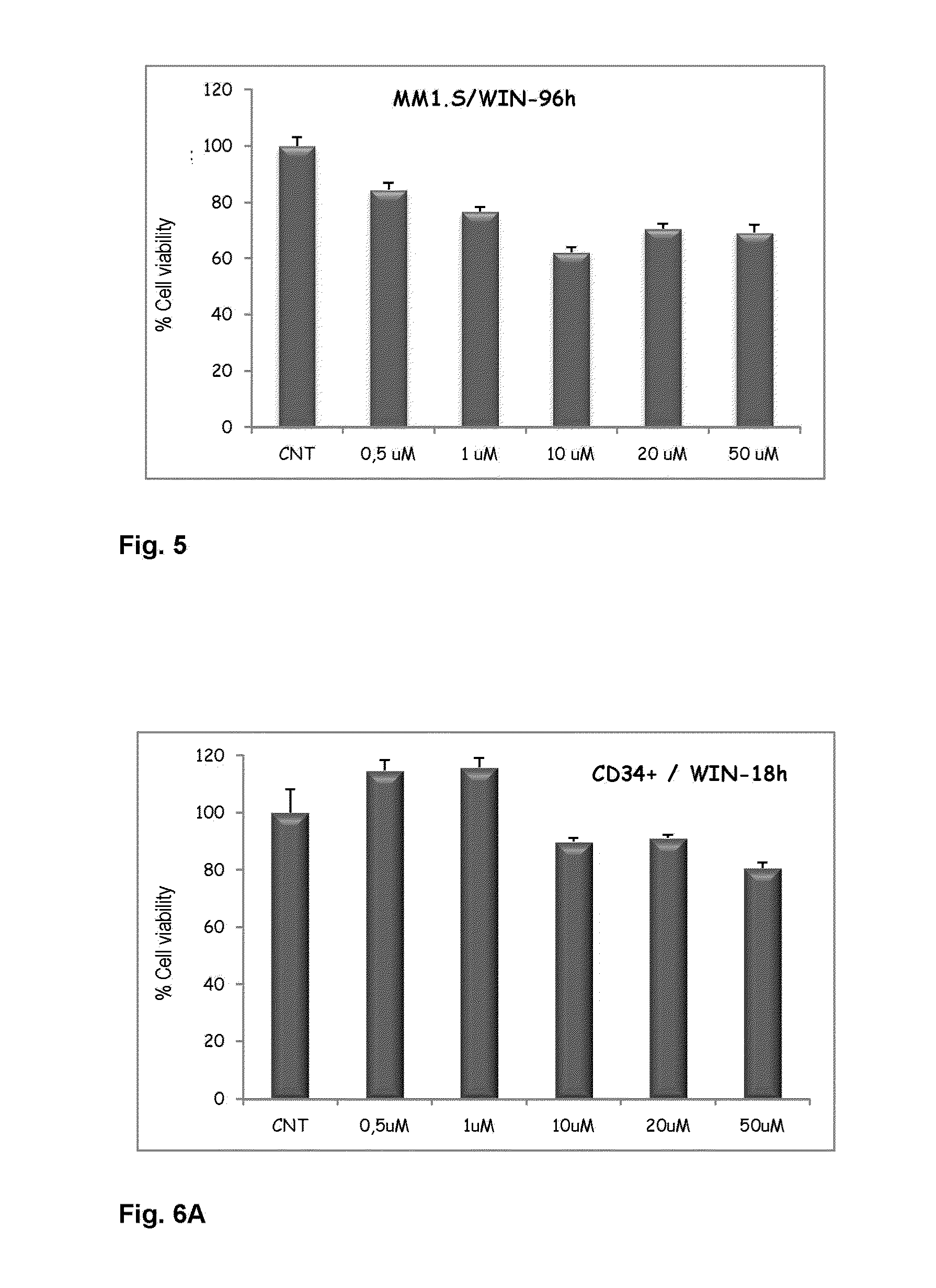
Agents for treating multiple myeloma
The present invention relates to the use of compounds of a cannabinoid nature for inhibiting viability with increasing doses of myeloma cell lines. Furthermore, said compounds have been shown not to affect CD34+ cells (normal hematopoietic progenitors) in terms of viability and proliferation. For this reason, the invention paves the way for the use of compounds of a cannabinoid nature as a promising therapy against multiple myeloma and related diseases.
Owner:SERVICIO ANDALUZ DE SALUD (SAS)
Unfiltered Radiation Therapy
ActiveUS20110033028A1Reduces efficiency of beamReduce the impactRadiation/particle handlingX-ray/gamma-ray/particle-irradiation therapyDose rateIntensity modulation
This is a new technique in IMRT and 3D conformal gamma radiation dose delivery using a linear accelerator with no flattening filter. The technique improves patient radiation therapy by reducing radiation scattered to surrounding normal tissue and reducing electron contamination. It increases dose rate to shorten treatment time. Linear accelerators have for decades come with a photon flattening filter to make the photon profile of planar fluence to make the dose distribution more uniform. These filters, however, resulted in fluence attenuation and contamination of the beam. Now in the age of techniques such as intensity modulated radiation therapy (IMRT) the function of the flattening filter becomes redundant. The flattening filter now merely reduces the efficiency of the beam by reducing the fluence and increasing scattered radiation. Our technique involves removal of the flattening filter for complex treatments. It uses inverse planning along with multi-leaf collimators to shape the dose distribution.
Owner:TOLEDO THE UNIV OF
Lithographic apparatus and method utilizing dose control
ActiveUS7123348B2Increase radiation doseReduce the amount of compensationSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusLight beamMaximum dose
A system and method are used to manufacture a device using at least one exposure step. Each exposure step projects a patterned beam of radiation onto a substrate. The patterned beam includes a plurality of pixels. Each pixel delivers a radiation dose no greater than a predetermined normal maximum dose to the target portion in the exposure step and / or at least one selected pixel delivers an increased radiation dose, greater than the normal maximum dose. The increased dose may be delivered to compensate for the effect of a defective element at a known position in the array on a pixel adjacent a selected pixel or compensate for underexposure of the target portion at the location of a selected pixel resulting from exposure of that location to a pixel affected by a known defective element in another exposure step.
Owner:ASML NETHERLANDS BV
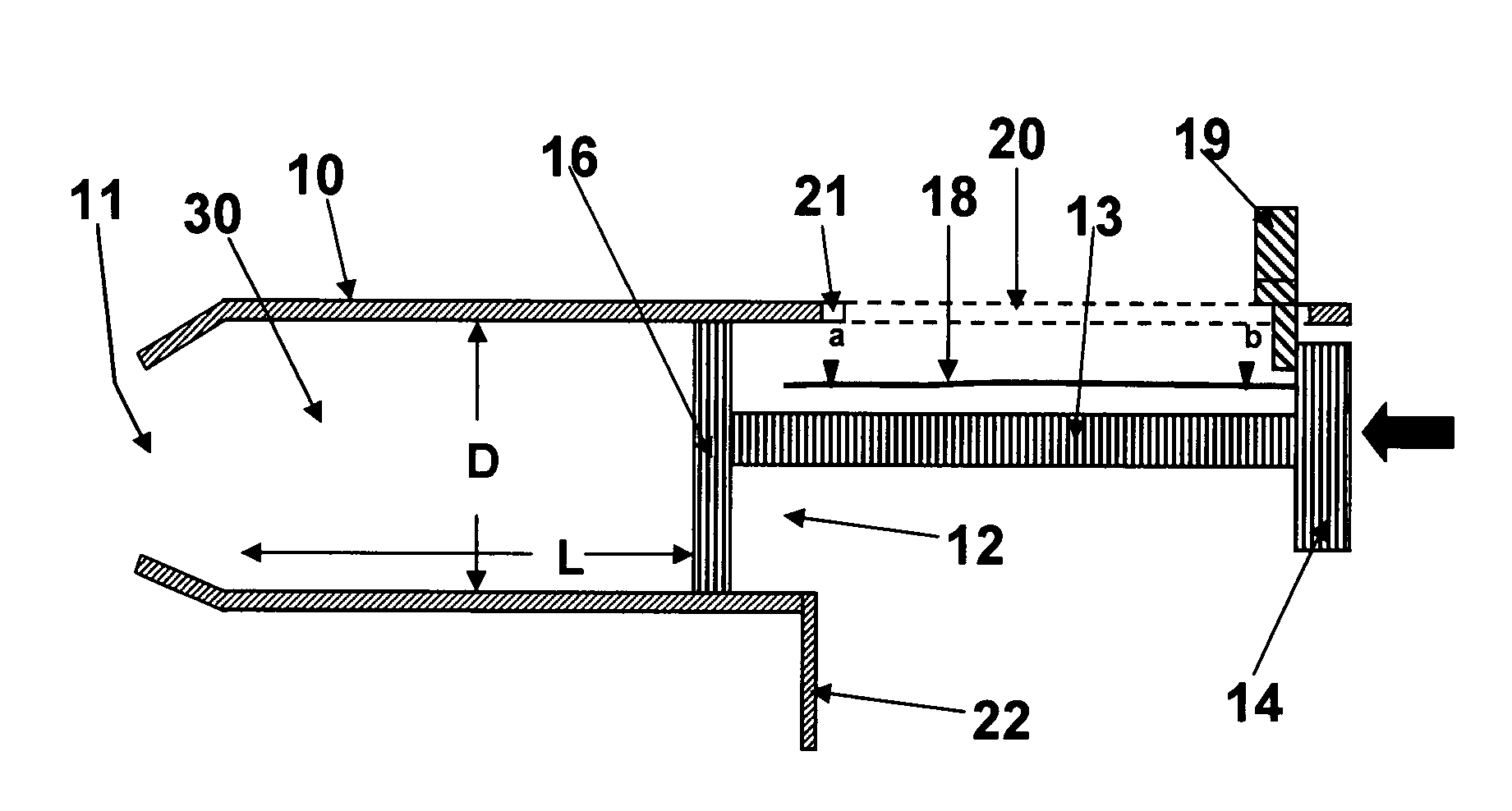
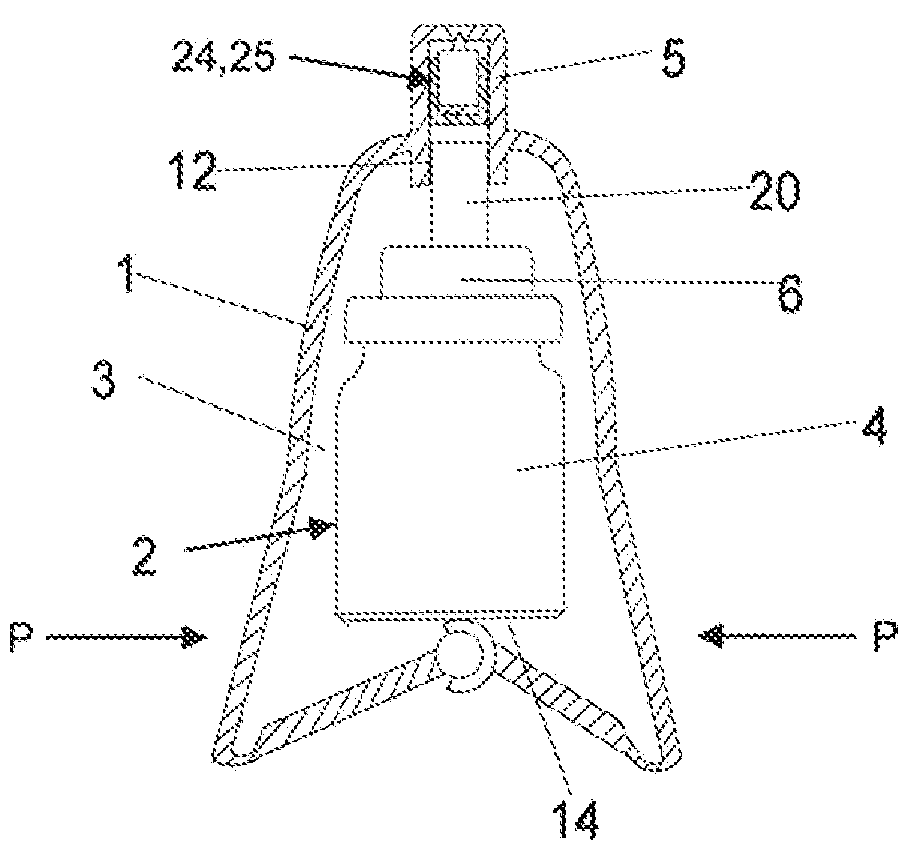
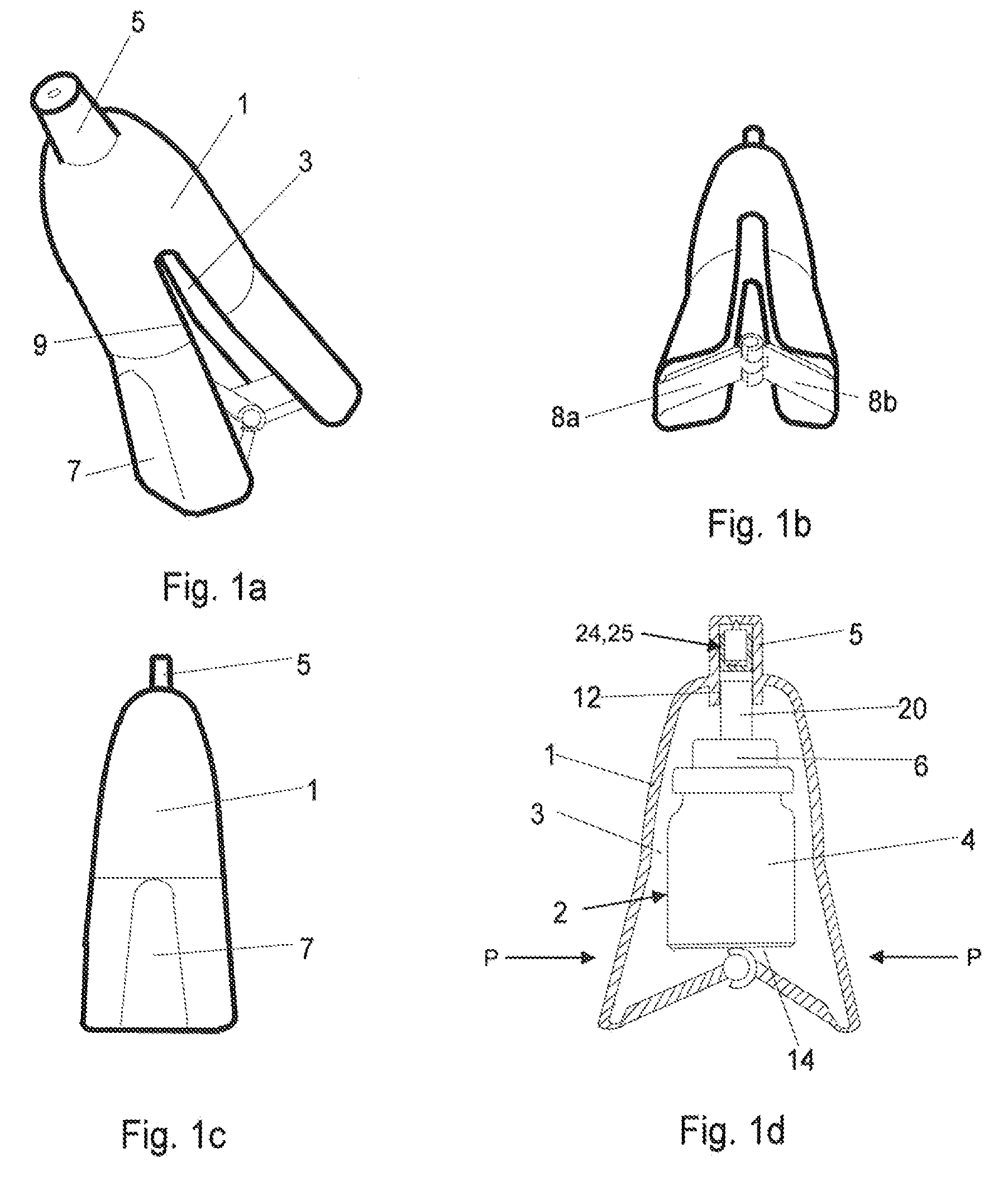
Spray device and nozzle closure
InactiveUS20090235923A1User with disadvantageMinimize residual amountWriting implementsMetal-working apparatusEvaporationSpray nozzle
A spray device including a casing and a spray bottle. A closing element is arranged in a nozzle. The closing element substantially fills the nozzle in order to minimize residual amounts of a drug in the nozzle. The overall purpose is to protect the spray device from contamination and evaporation. A method for manufacturing with as few parts as possible a spray device that by usually manual action causes the spray bottle to effect a pumping movement which triggers a spray dose through the nozzle. The device is intended to increase dose accuracy and facilitate the actual pumping.
Owner:MEDUX AB
Spray device and nozzle closure
The invention relates to a spray device which comprises a casing (1 ) and a spray bottle (2) and which has in its nozzle (5) a closing element which at the same time substantially fills the nozzle in order to minimise residual amounts of, preferably, a drug 5 in the nozzle, the overall purpose being to protect the spray device from contamination and evaporation. The invention further relates to a method for manufacturing with as few parts as possible a spray device which by usually manual action causes the spray bottle (2) to effect a pumping movement which triggers a spray dose through the nozzle (5). The device is intended to increase dose accuracy and facilitate the actual pumping.
Owner:MEDUX AB
Pharmaceutical composition containing pregabalin with improved stability and method for preparing same
InactiveCN106163566AImproved Dosing ConvenienceImprove complianceOrganic active ingredientsInorganic non-active ingredientsDiseaseExcipient
The present invention relates to a sustained release composition containing pregabalin or a pharmaceutically acceptable salt thereof using a gastroretentive drug delivery system (GRDDS), an oral sustained release formulation containing the composition, and a method for preparing the same. The sustained release composition and the formulation containing the same, according to the present invention, provide a gastroretentive drug delivery system in which a coating portion including sugars or derivatives thereof and a plasticizer is introduced on an external surface of pregabalin having a less stable structure to ensure stability and improve compatibility with an excipient, simultaneously, and the release rate is effectively controlled to increase dosing convenience, leading to increase the compliance of a subject to be administered, and thus the present invention can exhibit improved treatment or prevention effects on various mental disorders, such as neuropathic pain, epilepsy, and fibromyalgia, which could not be previously easily accomplished due to the characteristics of pregabalin.
Owner:YUNGJIN PHARM CO LTD
Method of preventing reduced feed intake in animals and treatment of disease conditions
InactiveUS20090053188A1Preventing a reduction in feed intake in an animalAvoid loweringBiocideBacterial antigen ingredientsDiseaseLaminitis
The present invention relates to a method for substantially preventing a reduction in feed intake in an animal which occurs when said animal is administered an antibiotic, the method comprising administering to an animal in need of prevention of a reduction in feed intake, increasing doses of a composition comprising one or more antibiotics. The present invention also relates to a method for treating laminitis and fermentative acidosis in an animal in need of said treatment, the method comprising administering increasing doses of a composition comprising one or more antibiotics. The present invention further relates to a method for treating equine grass sickness and pulpy kidney in an animal in need of said treatment, the method comprising administering increasing doses of a composition comprising one or more antibiotics.
Owner:PRECISION NUTRITION
Pharmaceutical formulations of naproxen for soft gel encapsulation and combinations thereof
ActiveUS20130011470A1Promotes consumer acceptanceImprove stabilityBiocidePeptide/protein ingredientsUnit sizeAdditive ingredient
An NSAID formulation for encapsulation into a soft gel capsule with increased stability, concentration and bioavailability of the NSAID. The preferred NSAIDS are naproxen, ibprofen, indomethacin and diclofenac, which are provided in both an acidic and basic form in the fill formulation. The pH values of fill formulations may be adjusted without additional process steps. A process for increasing the achievable concentration of an NASAID pharmaceutical ingredient in a fill composition for dosage units is also provided. The highly concentrated NASAID formulation permits a reduction in the fill volume or dosage unit size or an increase in concentration of the NSAID in each dosage it.
Owner:CATALENT ONTARIO LTD
Treatment of LFA-1 associated disorders with increasing doses of LFA-1 antagonist
A method is provided for reducing the occurrence of fever, headache, nausea and / or vomiting associated with administration of a therapeutic compound to a mammal in need therof, comprising administering to the mammal a first conditioning dose of a non-target cell depleting compound which binds to a cell surface receptor on a target mammalian cell; and administering a second therapeutic dose of the compound, wherein the second dose is higher than the first dose.
Owner:XOMA TECH LTD A BERMUDA +1
Application of recombinant human calcineurin B subunit
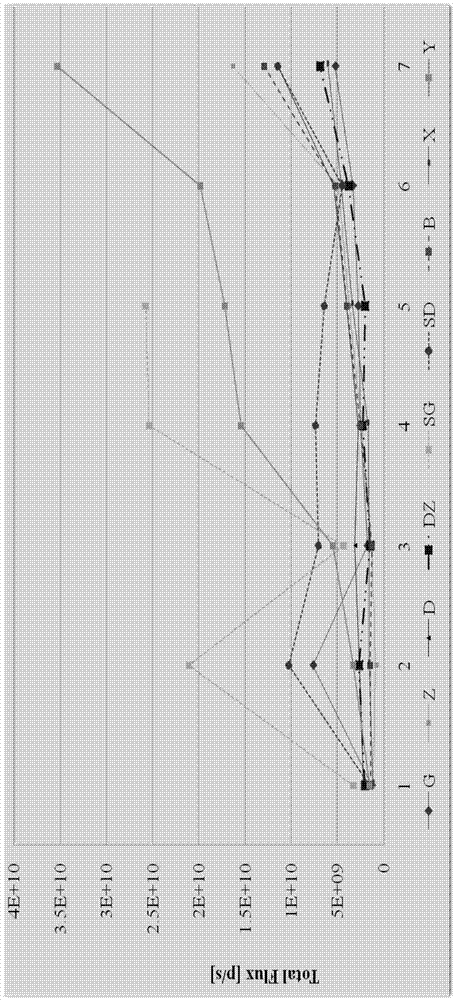
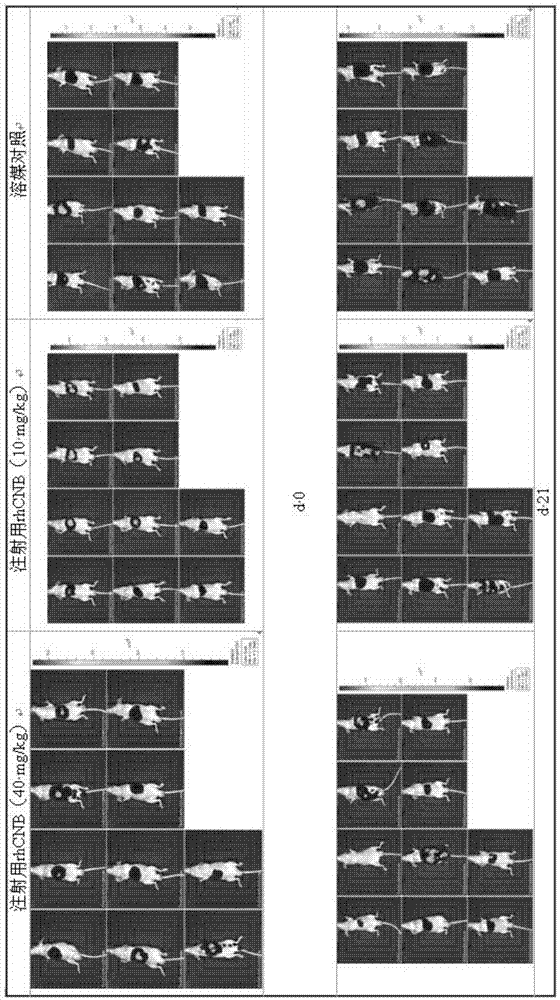
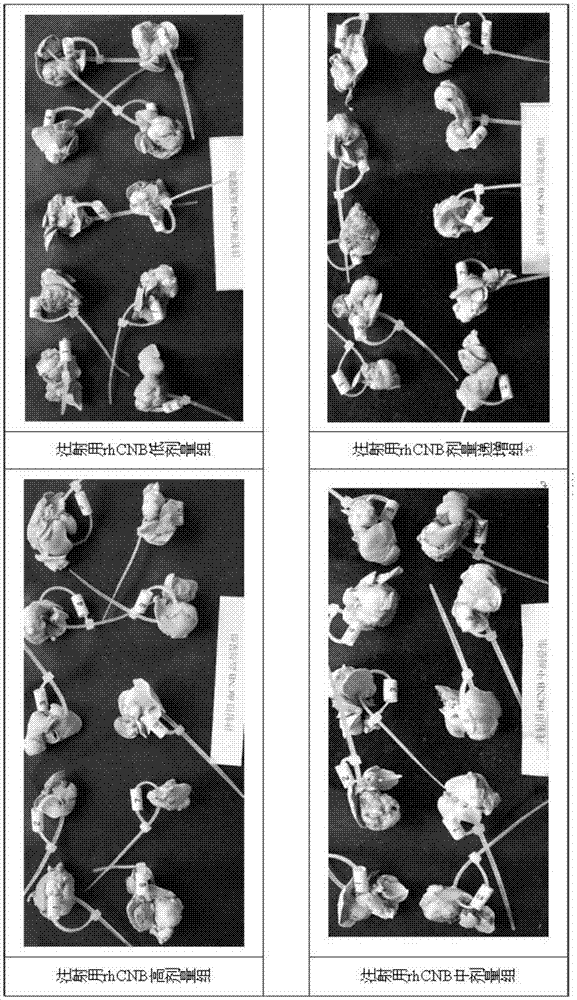
InactiveCN105435215AGood control effectEnhanced inhibitory effectPeptide/protein ingredientsHydrolasesPositive controlHigh doses
The invention relates to the field of proteins, in particular to application of recombinant human calcineurin B subunit, and mainly provides application of rhCNB to preparation of a medicine for killing and / or inhibiting a liver cancer cell Bel-7402. After continuous medication for 6 times, medication groups of rhCNB for injection with doses of 10 mg / kg, 20 mg / kg and 40 mg / kg all have a relatively good inhibiting effect on the growth of human liver cancer cell Bel-7402 solid tumors. Subsequent testing results show that the effects of high-dose, middle-dose and increasing-dose medication groups are superior to that of a low-dose medication group, and the treating effect of rhCNB for injection is equivalent to those of recombinant human interleukin-2 for injection and hydroxycamptothecine for injection in a solvent control group. The results of d7 testing after final medication show that the high-dose group has the optimum controlling effect (p is smaller than 0.01) on tumor cell proliferation, which is superior to those of other dose groups and a positive control group, and equivalent to those of a hydroxycamptothecine medication group and the increasing-dose group (p is smaller than 0.05).
Owner:HAIKOU QILI PHARMA
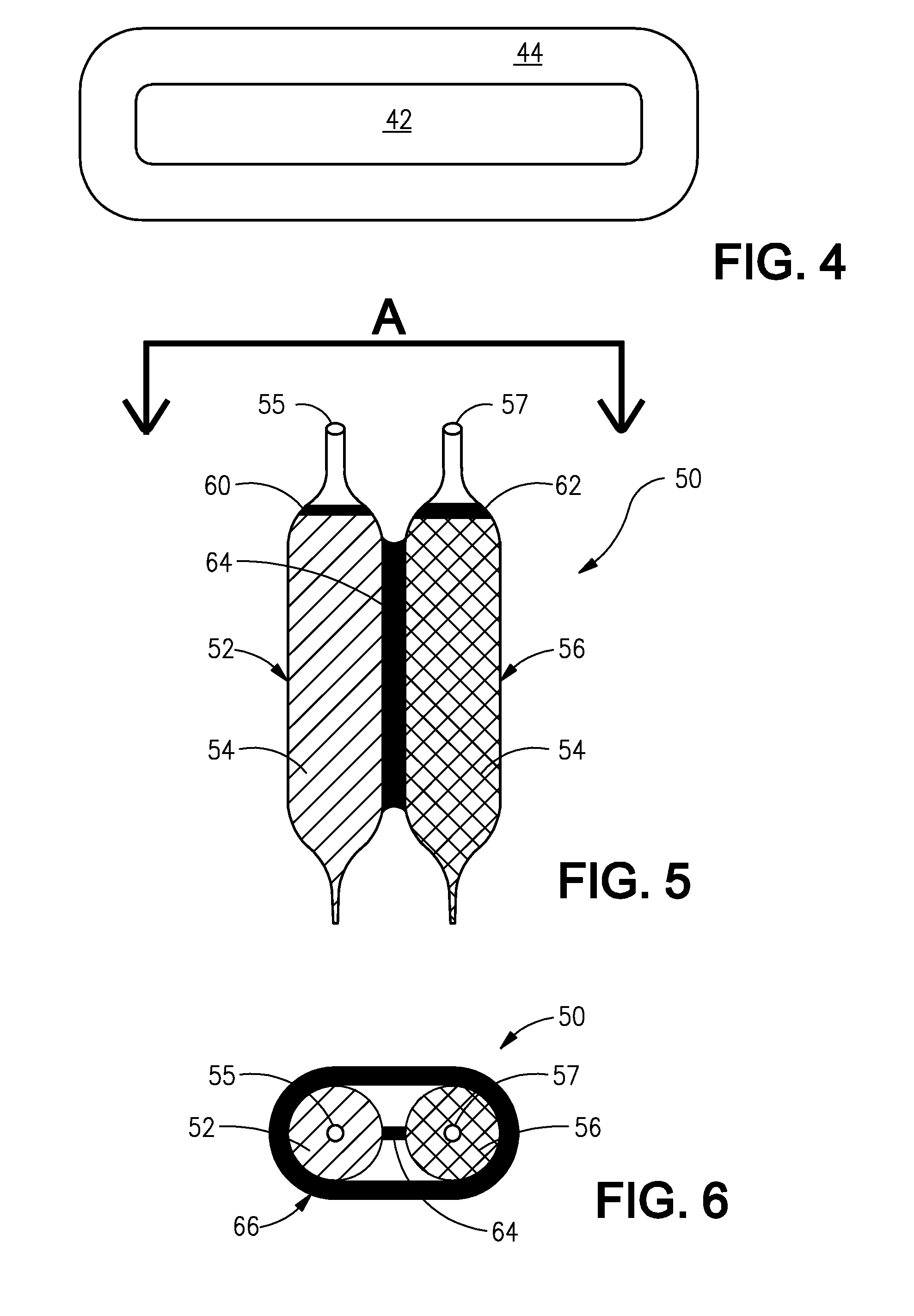
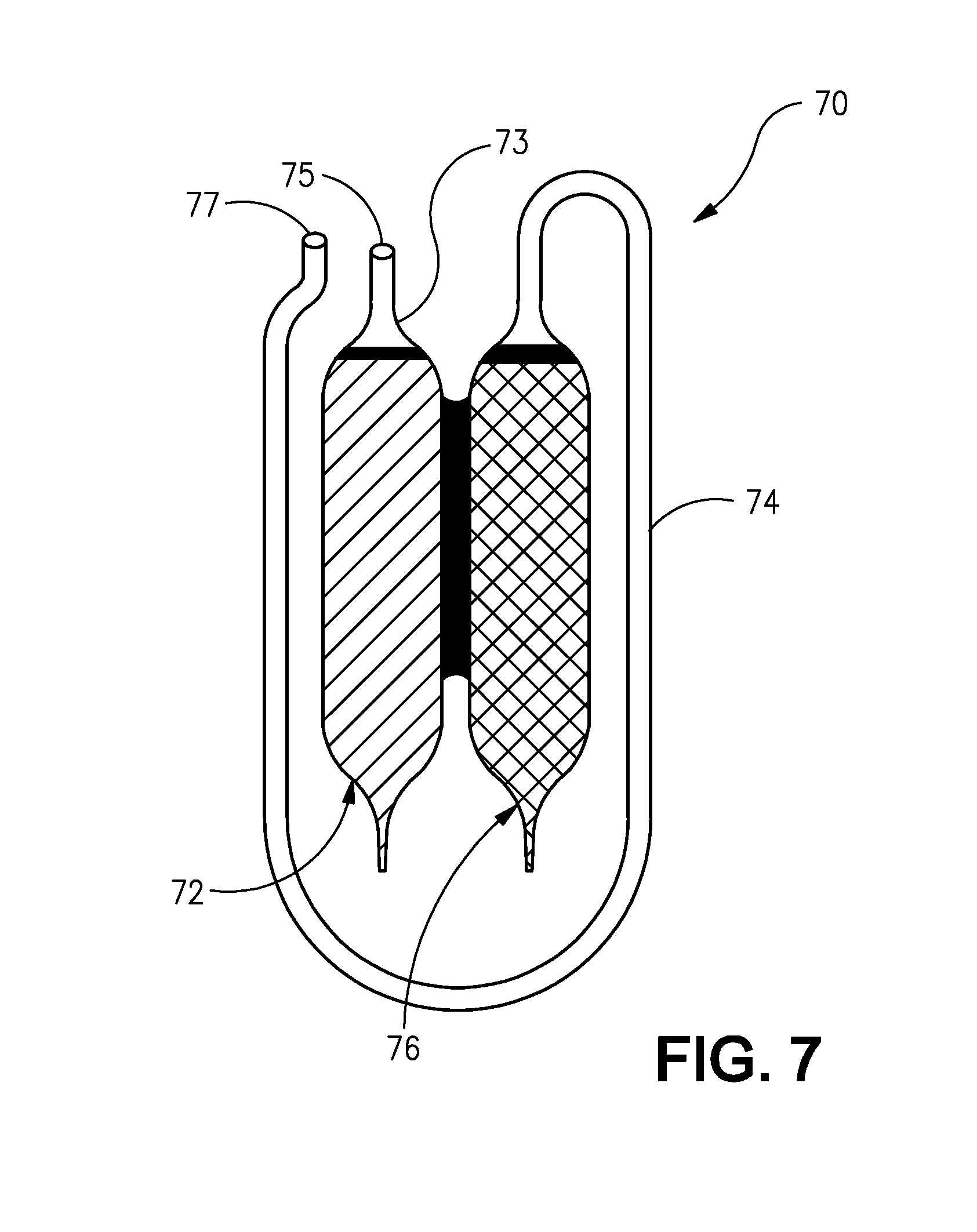
Sublingual immunotherapy with reduced oral itchiness
InactiveUS20140370086A1Consistent levelReduced and substantially no localized oral itchinessAllergen ingredientsPill deliveryCoated tabletsPatient compliance
Sublingual immunotherapy (SLIT) with reduced oral itchiness is disclosed. The improved sublingual immunotherapy combines a monotonically increasing dose of allergen, along with a constant dose of mast cell stabilizer, thereby substantially avoiding the oral itchiness and other uncomfortable adverse reactions typically experienced with SLIT, which can improve patient compliance. An antihistamine and / or a leukotriene inhibitor can also be added along with the mast cell stabilizer. Multi-layer and / or coated tablets, and flexible paired ampoules with special features to advantageously time the dose of the allergen relative to the dose of the mast cell stabilizer, have been provided to effectively administer the improved sublingual immunotherapy in a highly convenient manner. Methods, preparations, and apparatuses for administration of SLIT, apparatus for sequentially dispensing, methods for producing a sequence of allergen doses with reduced adverse reactions, methods for producing a stock solution for dilution, and methods for producing cooperative pre-filled vials are also disclosed.
Owner:HARISH ZIV
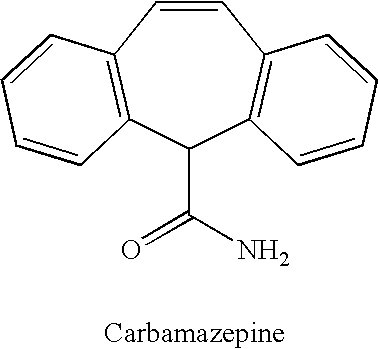
Methods for the treatment of bipolar disorder using carbamazepine
Carbamazepine, in extended release form, is useful in the treatment of patients suffering from bipolar disorder. In order to minimize the time it takes to reach efficacy, carbamazepine, in extended release form, can be administered to the patient at an initial daily dose which is then increased in daily increments until clinical efficacy is achieved.
Owner:VALIDUS PHARMA
System and methods of photon-based radiotherapy and radiosurgery delivery
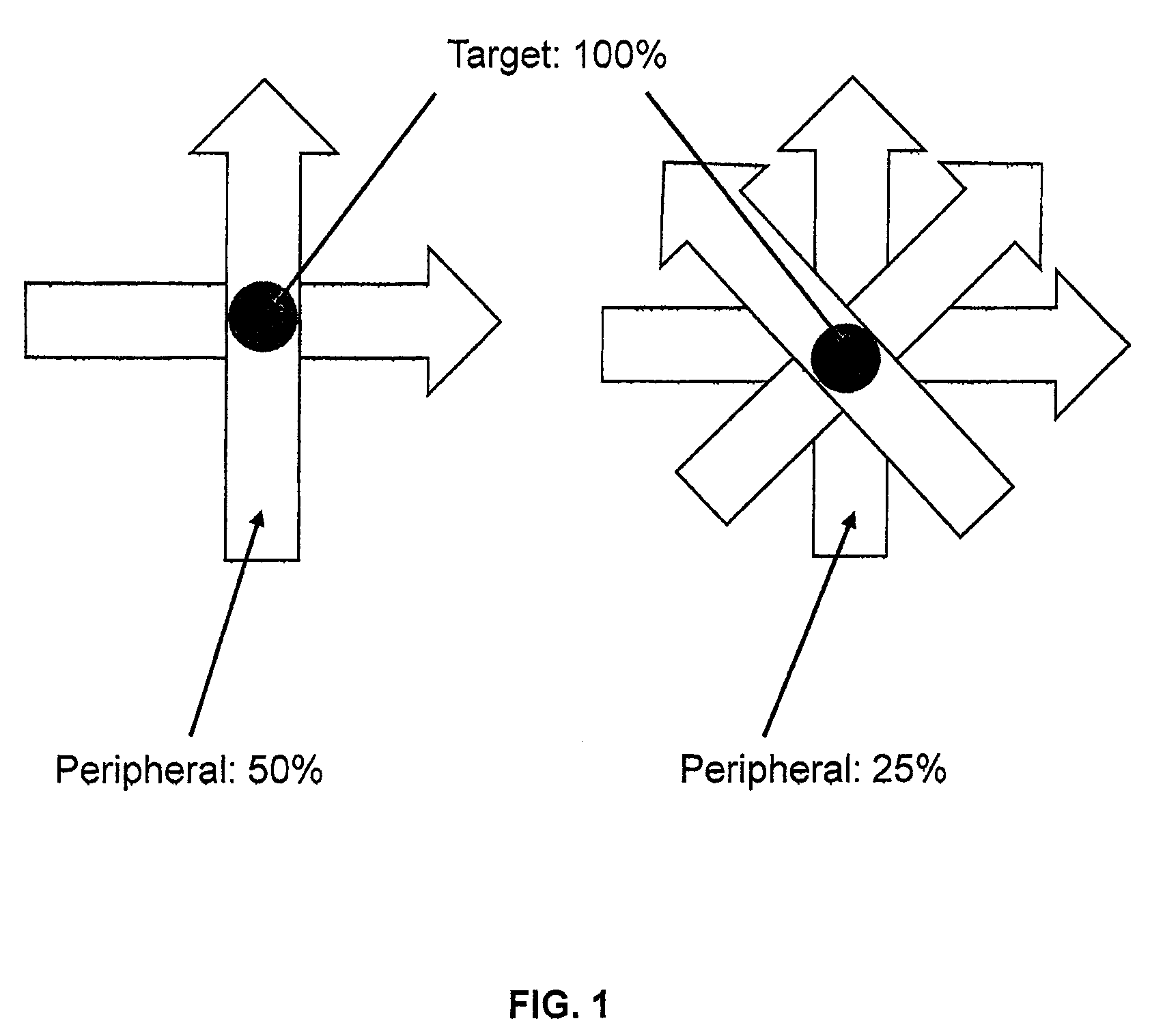
ActiveUS8835877B2Quality improvementIncrease ratingsEnergy based chemical/physical/physico-chemical processesChemical conversion by chemical reactionDose gradientBragg peak
Photon-based radiosurgery is widely used for treating local and regional tumors. The key to improving the quality of radiosurgery is to increase the dose falloff rate from high dose regions inside the tumor to low dose regions of nearby healthy tissues and structures. Dynamic photon painting (DPP) further increases dose falloff rate by treating a target by moving a beam source along a dynamic trajectory, where the speed, direction and even dose rate of the beam source change constantly during irradiation. DPP creates dose gradient that rivals proton Bragg Peak and outperforms Gamma Knife® radiosurgery.
Owner:RGT UNIV OF CALIFORNIA +1
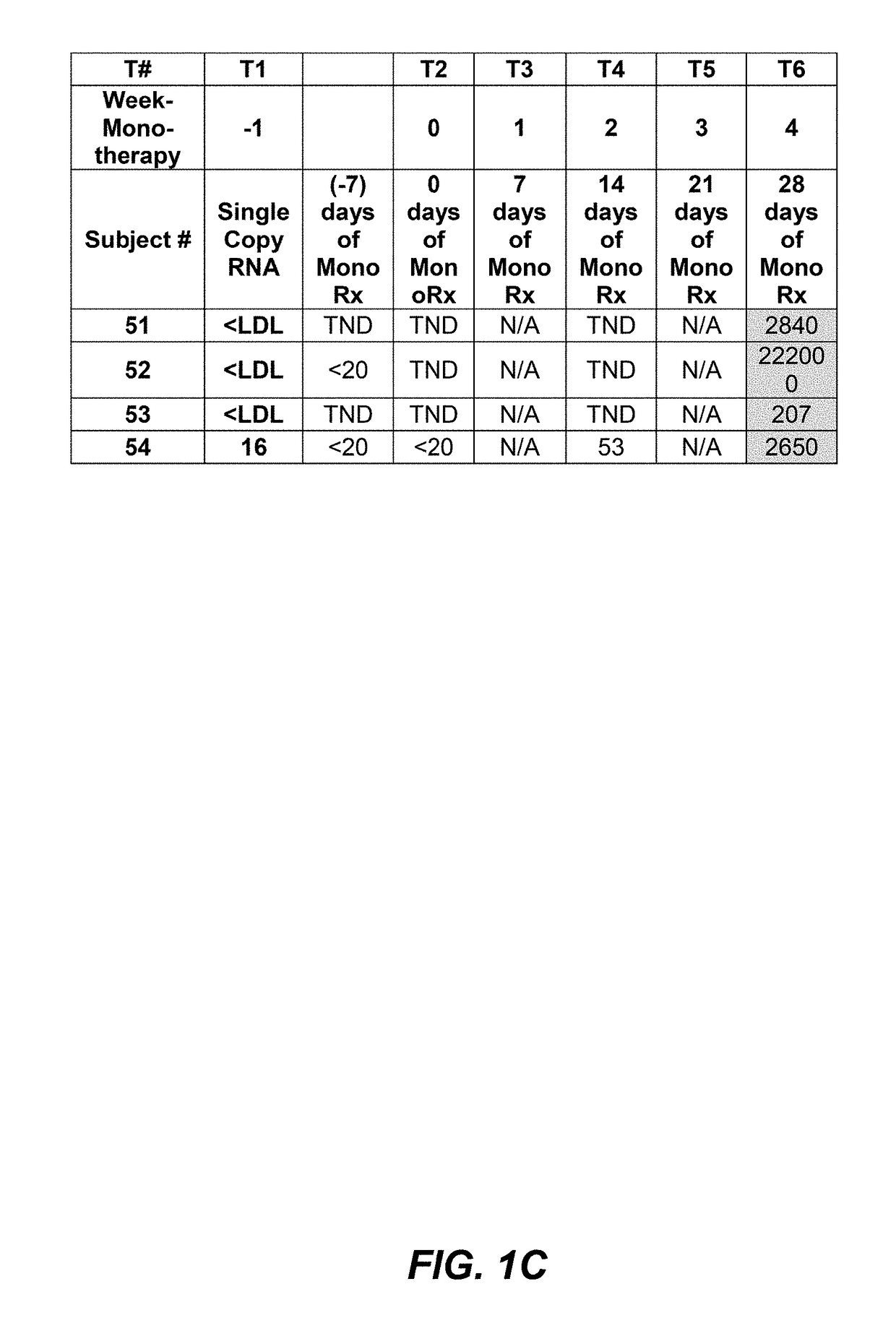
Screening methods for identifying and treating hiv-1 infected patient sub-populations suitable for long term Anti-ccr5 agent therapy
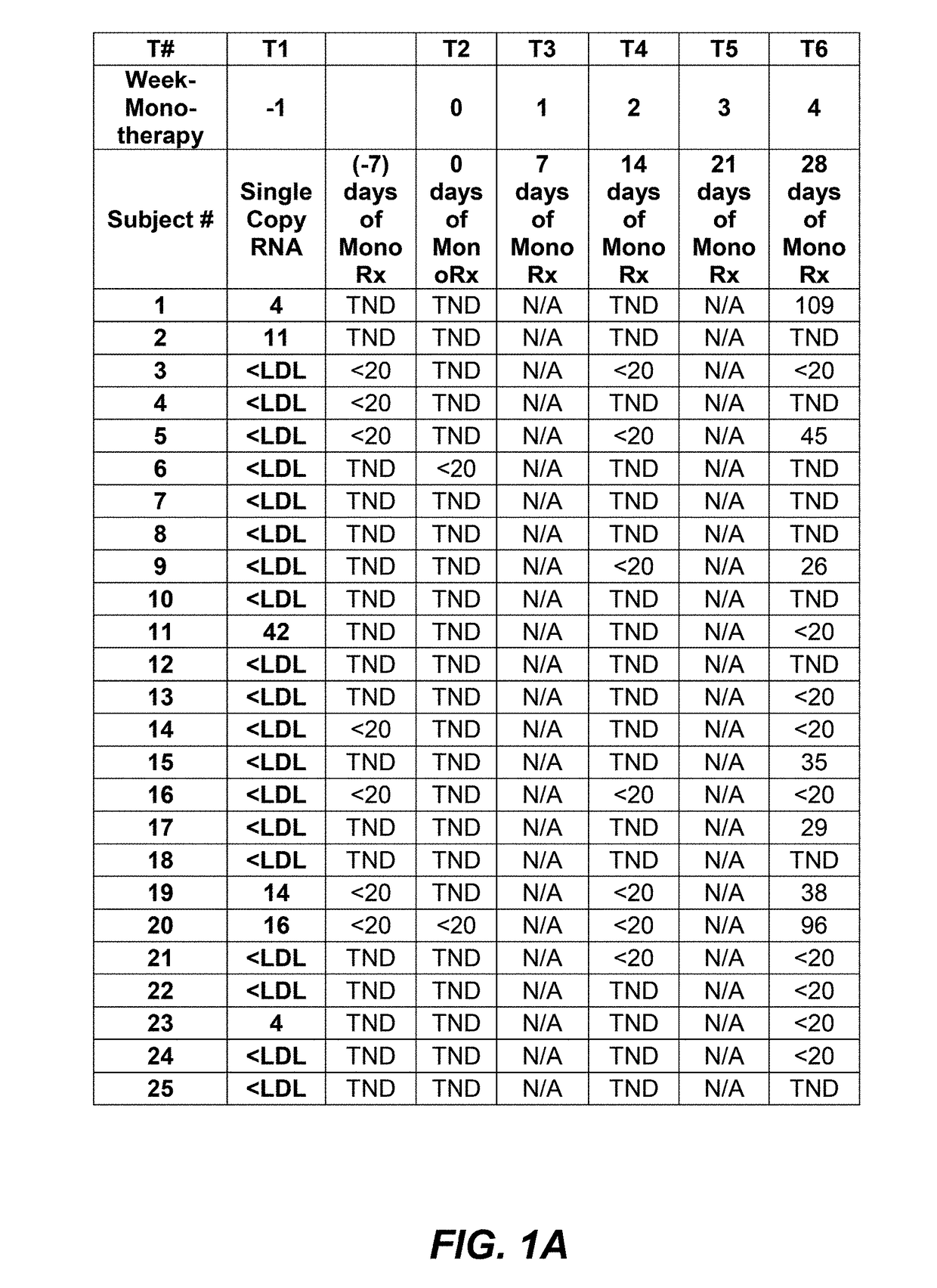
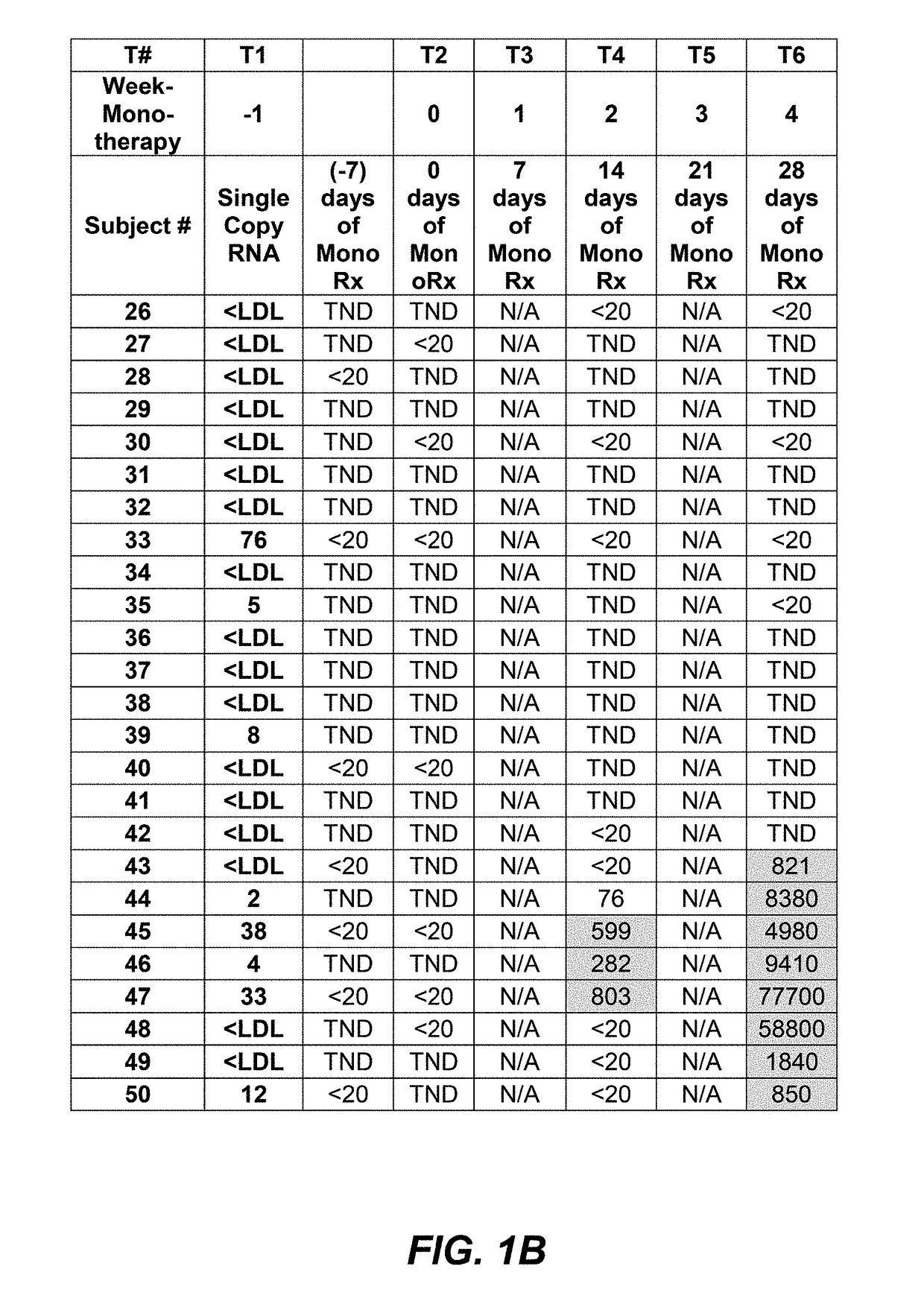
InactiveUS20190085086A1Great likelihoodEffective predictionBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsInfected patientMedicine
Certain R5 virus tropic HIV-1 subjects with viral load effectively conventionally controlled using HAART, i.e., subject having less than 50 viral copies / mL (<50 cp / mL), may be substantially more susceptible than others to effective monotherapy treatment using anti-CCR5 agents, e.g, PRO 140 mAbs. Certain HIV-1 subjects using PRO 140 monotherapy treatment may experience prolonged or unlimited time periods with actual undetectable viral loads, extremely low viral load counts ≤1 cp / mL, very low, or low levels, or at conventionally undetectable levels, during monotherapy. Increasing dose amounts of anti-CCR5 agents, e.g., PRO 140, from 350 mg to 525 mg or 700 mg, may beneficially suppress a subject's viral load count before, during, and / or maintain effective prolonged monotherapy and may shorten the period of time necessary to determine if a subject will respond positively to PRO 140 monotherapy to less than eight (8) weeks. This invention includes protocols, methods, and kits.
Owner:CYTODYN
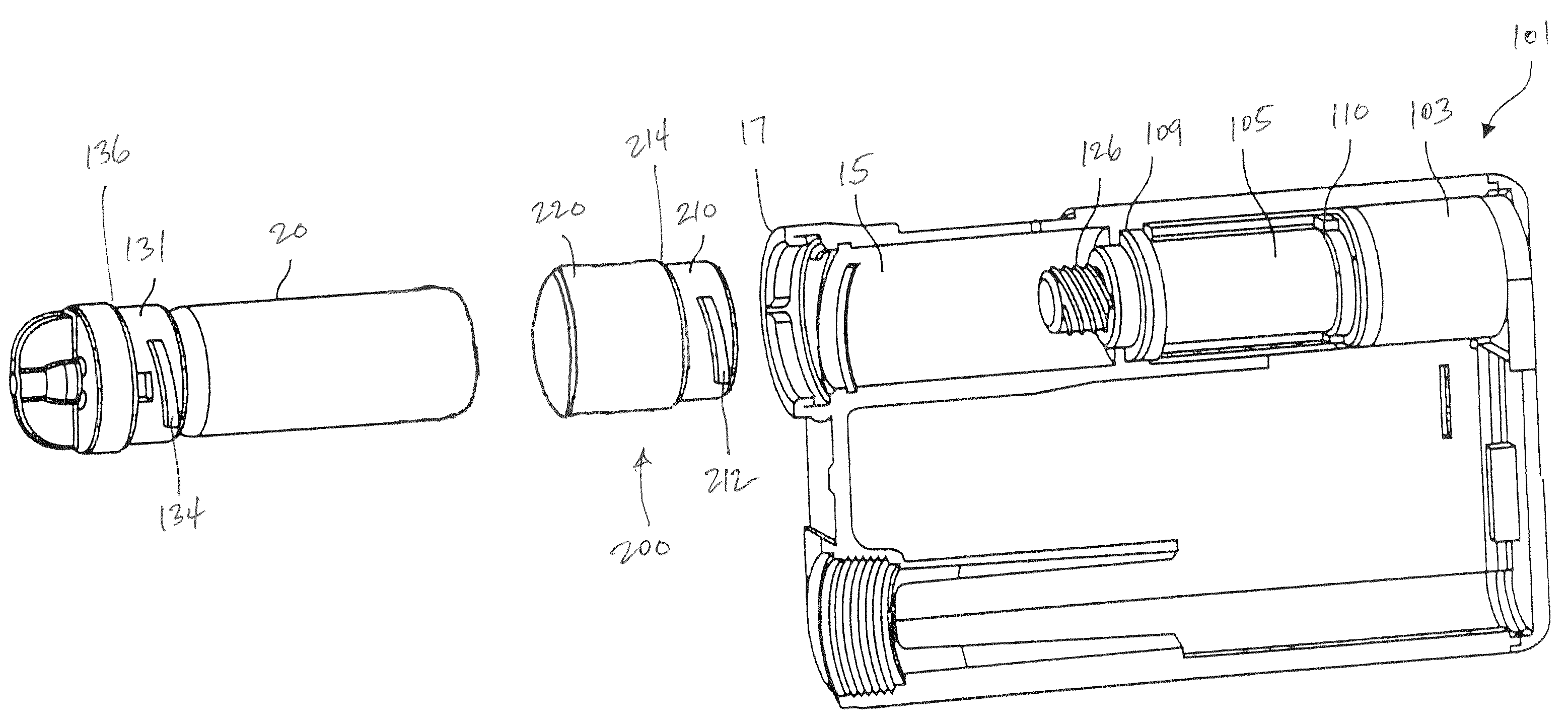
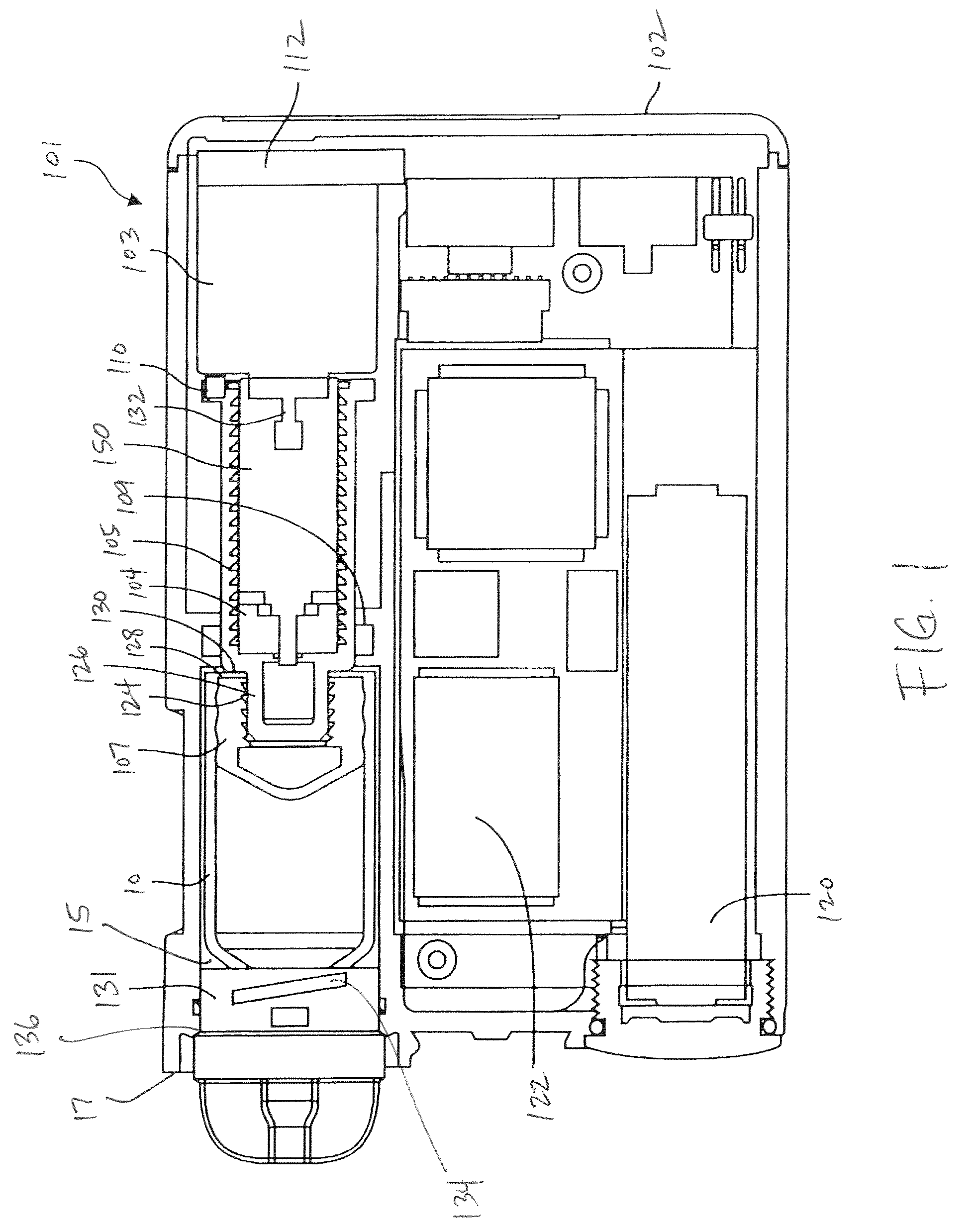
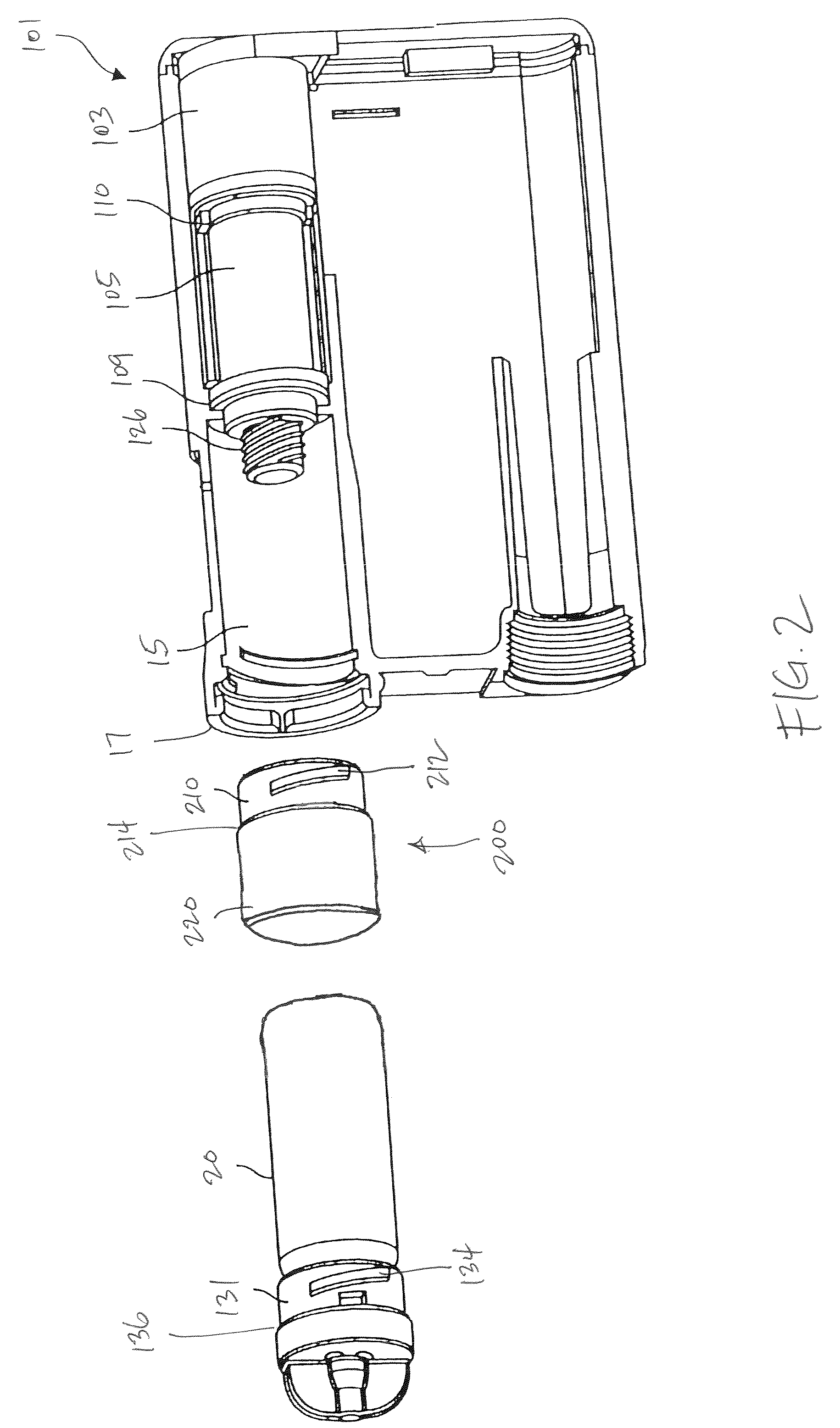
Reservoir compartment adapter for infusion device
The present invention provides a reservoir compartment adapter for use with a fluid delivery device. The adapter includes a first end adapted for coupling with a fluid delivery device, a second end adapted for coupling with a connector, and a structure between the first end and the second end including an interior space for receiving the reservoir, wherein an extended reservoir compartment for accommodating the reservoir is adapted to be formed when the first end is coupled to the fluid delivery device, and the reservoir is adapted to be secured in the extended reservoir compartment when the connector is coupled to the second end. The adapter allows a user of a delivery device accommodating reservoirs of a certain size to manage periods where increased medication dosage is needed without the burden of carrying a larger delivery device for accommodating reservoirs filled with the increased dosage.
Owner:MEDTRONIC MIMIMED INC
Plaque targeted sub-blistering dosimetry
InactiveUS20170216618A1Optimal and maximally efficacious dosingTreat psoriasisLight therapyLight treatmentDosimetry
A method for testing diseased skin for treatment of the diseased skin, comprising the steps of administering a plurality of increasing doses of phototherapy directly to regions of an area of diseased skin and analyzing the area of the diseased skin to assess the doses at which burning and blistering of the diseased skin occurs, determining a maximum dose of phototherapy that can be administered to the diseased skin based on the assessment of the doses at which the burning and the blistering of the diseased skin occurs, and treating the diseased skin.
Owner:PHOTOMEDEX
Application of total triterpenoids of Pteridocarpus in protecting chronic alcoholic liver injury
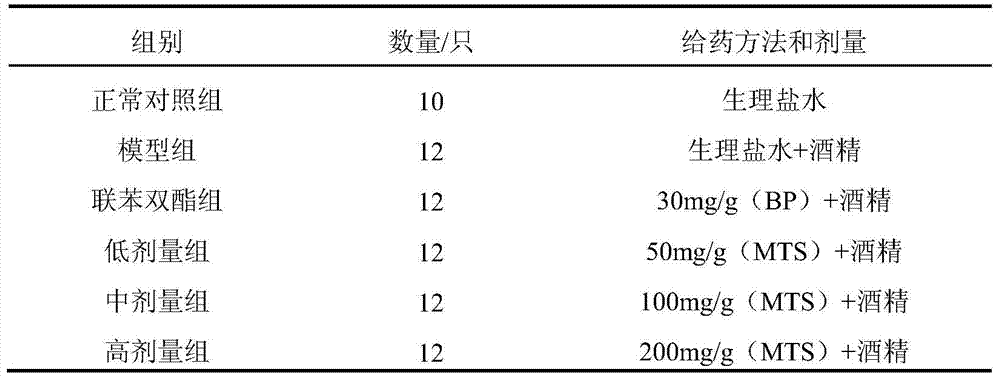
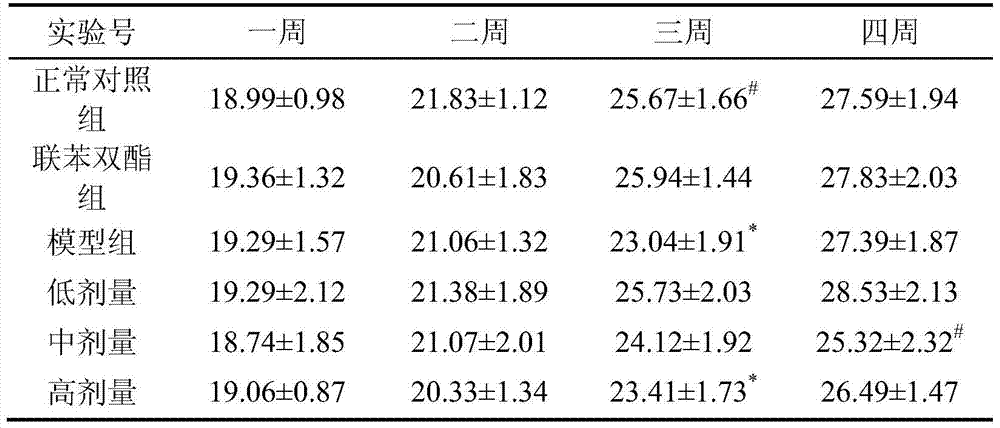
ActiveCN104922168BDamage reliefStrengthen the body's antioxidant capacityDigestive systemAntinoxious agentsPositive controlHigh doses
Owner:SHAANXI NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com