Application of recombinant human calcineurin B subunit
A drug delivery technology, bel-7402, is applied in the application field of recombinant human calcineurin B subunit, which can solve the problem of no killing or inhibition of liver cancer cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0038] Example 1 Establishment of BALB / c Nude Mice Bearing Human Hepatoma Cell Bel-7402 Subcutaneously Transplanted Tumor Model
[0039] Human liver cancer cell Bel-7402 was routinely cultured in vitro, digested and centrifuged to make a concentration of about 1×10 7 1 cell / mL cell suspension, 0.2 mL / only was inoculated subcutaneously in the right axilla of the mouse.
Embodiment 2B
[0040] Example 2 Establishment of BALB / c Nude Mice Bearing Human Hepatoma Cell Bel-7402 Orthotopic Transplantation Model
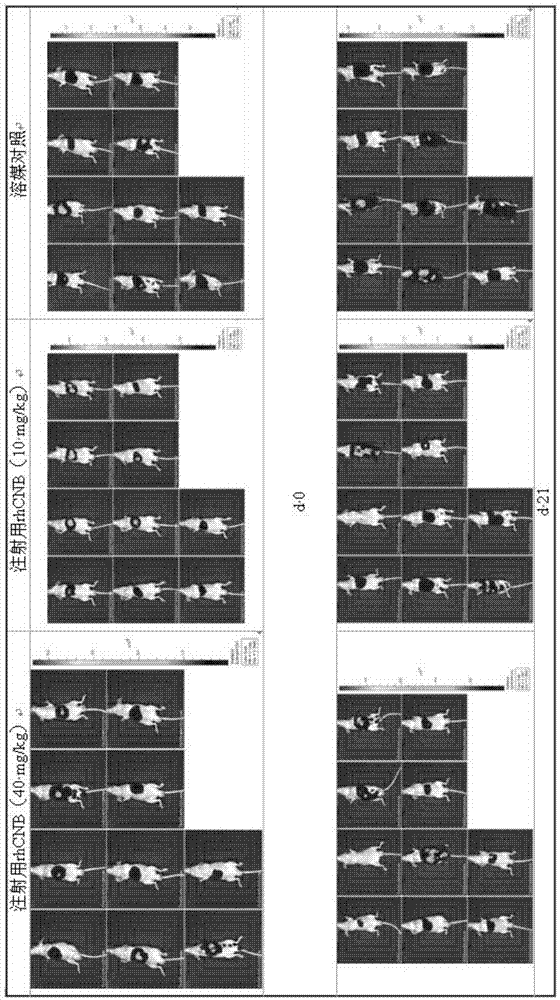
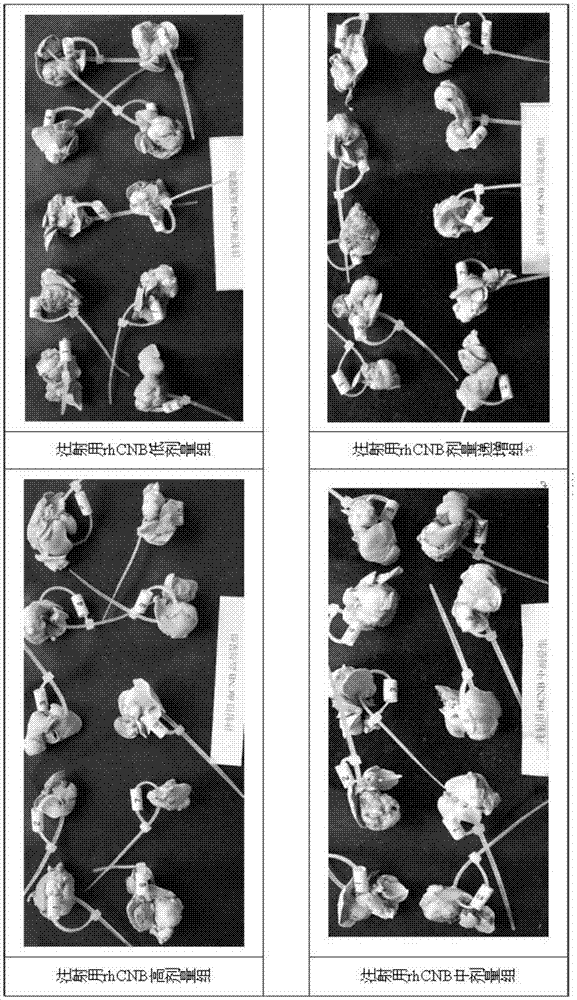
[0041] The well-grown subcutaneous xenografts were taken out and cut into tumor tissue pieces about 2×2×1 mm in size for later use. The animals to be inoculated were gas anesthetized with isoflurane and fed for 12 hours before the operation. After the animals were anesthetized, they were placed in a supine position, and the skin was cut horizontally at 2-5 mm below the xiphoid process layer by layer, with an opening of about 1 cm to expose the liver. Seven days after the operation, detection, grouping and drug administration were performed with a small animal in vivo imaging system.
Embodiment 3
[0042] Embodiment 3 animal grouping
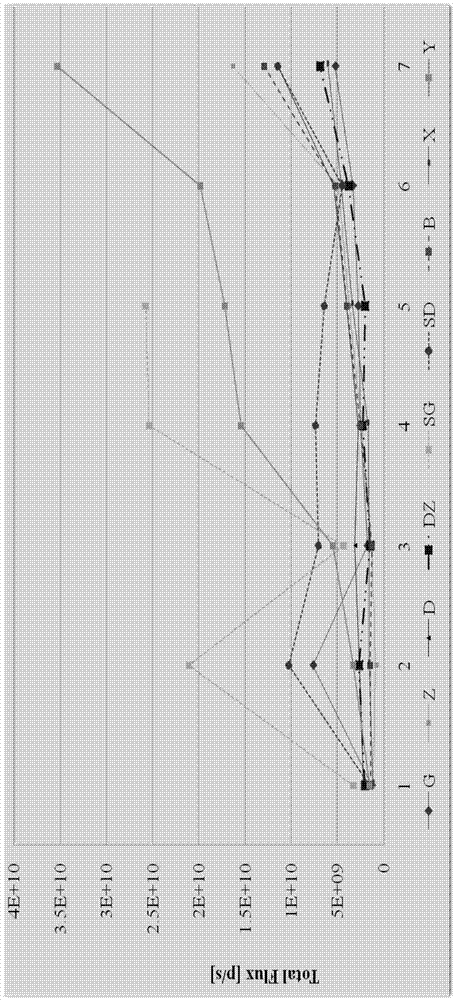
[0043] Take 90 inoculated nude mice and randomly divide them into rhCNB high-dose group for injection, rhCNB middle-dose group for injection, rhCNB low-dose group for injection, rhCNB dose-increasing group for injection and positive control rhCNB injection group according to bioluminescence detection data. Recombinant human interleukin-2 administration group, positive control cisplatin high-dose injection group, positive control cisplatin low-dose injection group, positive control hydroxycamptothecin injection group and vehicle control group Group, 10 in each group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com