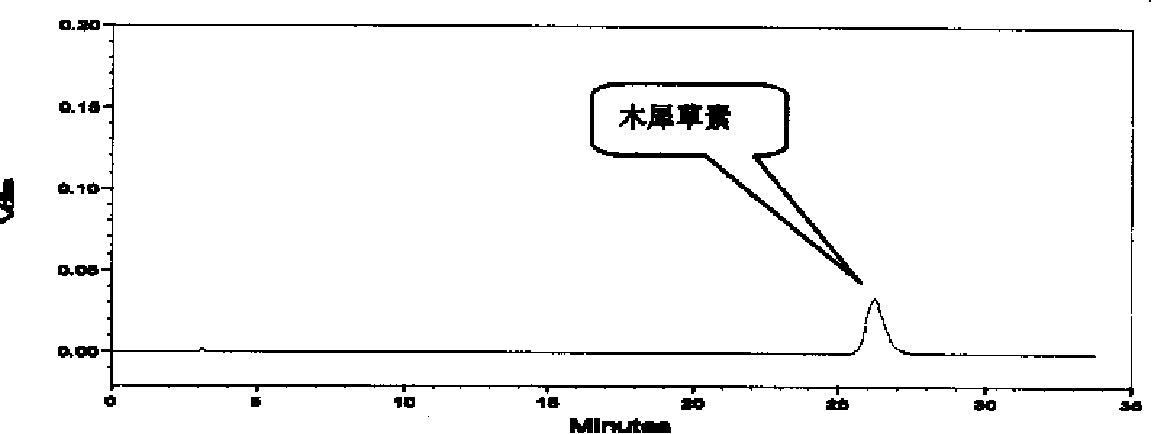
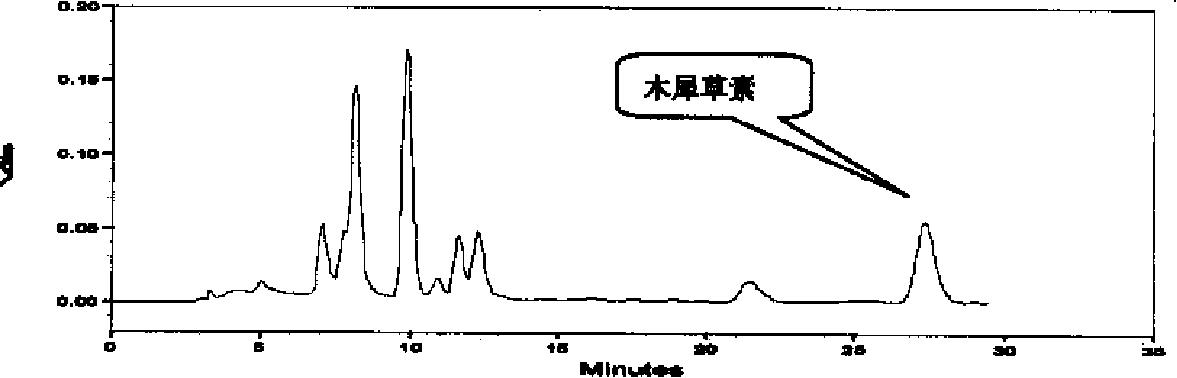
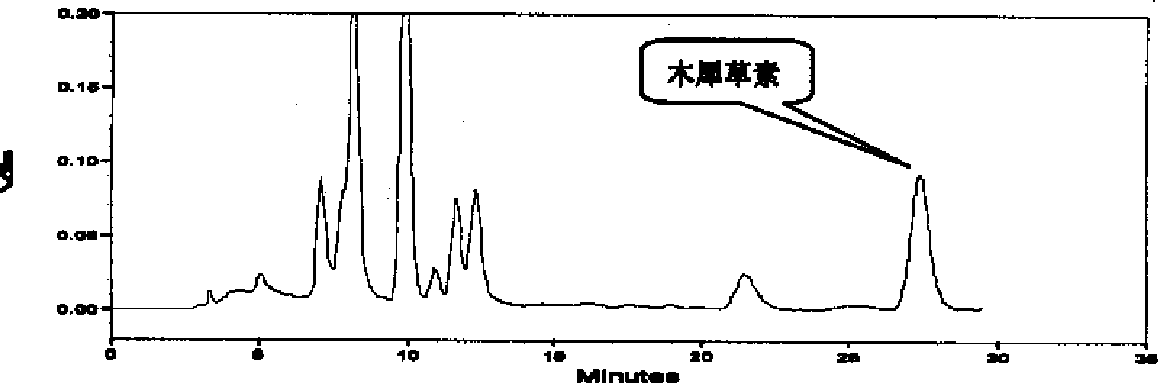
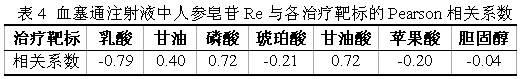
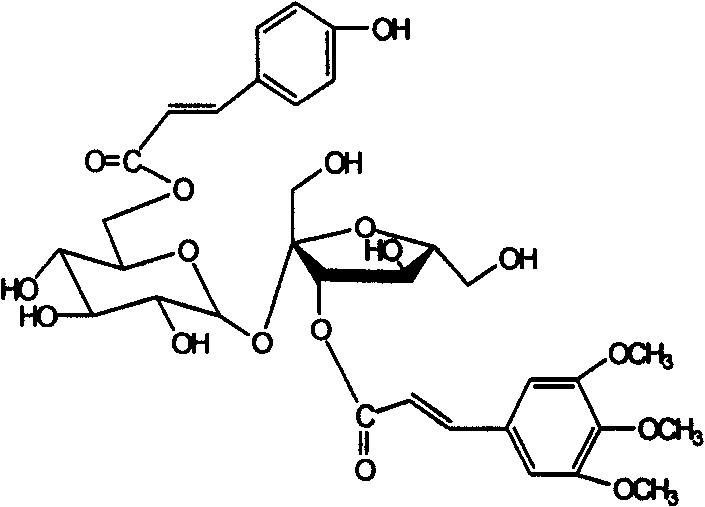
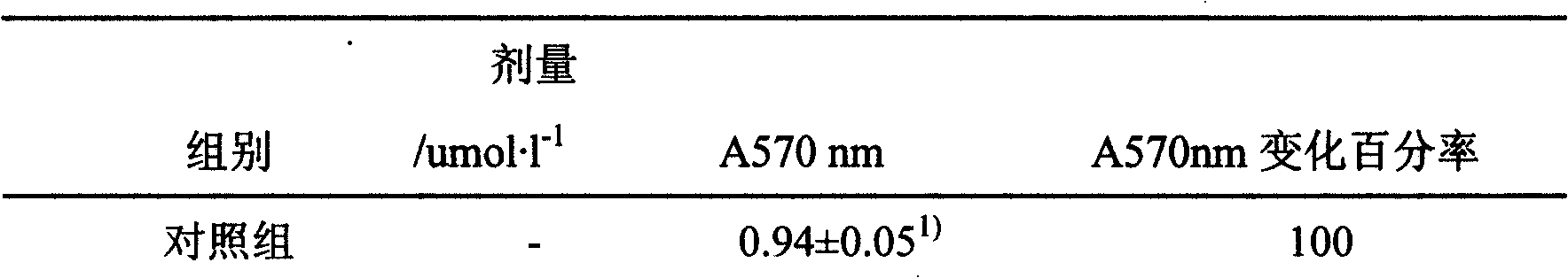
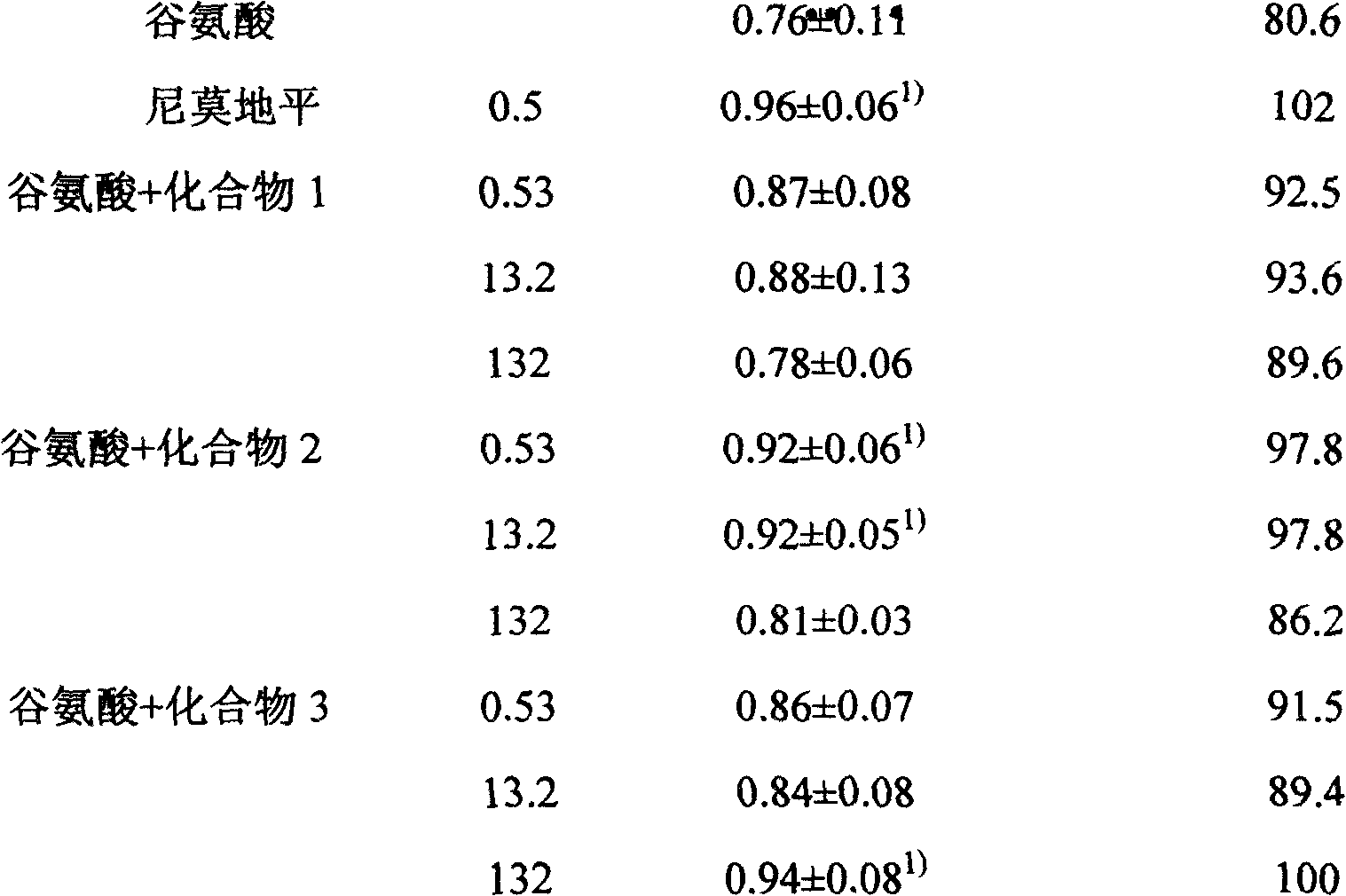
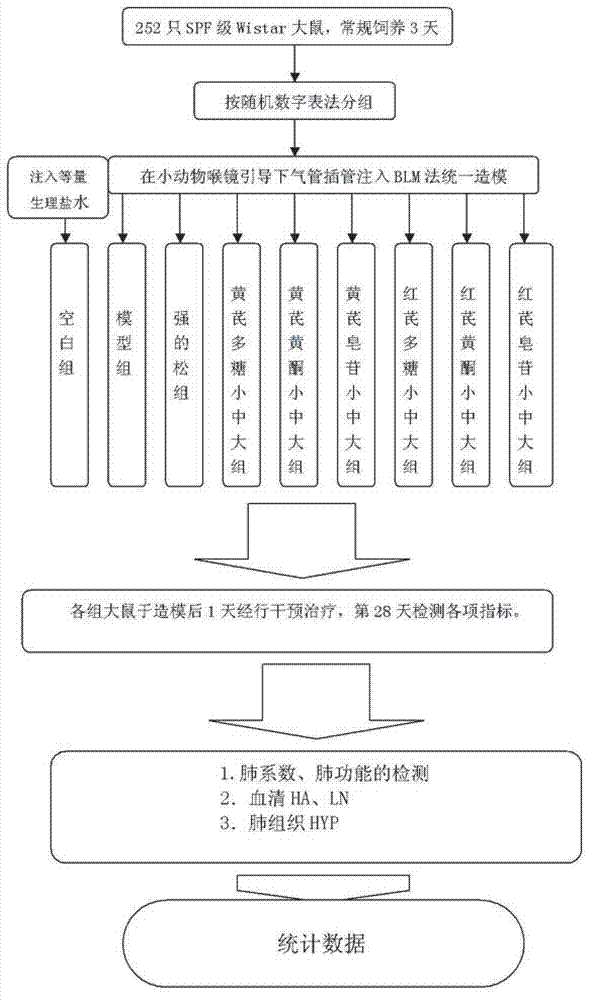
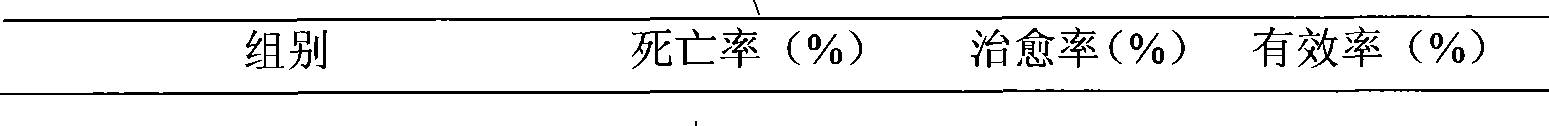Patents
Literature
41 results about "Dose group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dracocephalum moldavica extract and dracocephalum moldavica dropping pills, and method of preparing the same
InactiveCN101219161AEasy to operateEasy to usePill deliveryCardiovascular disorderClinical efficacyDracocephalum moldavica
The invention relates to a moldavica dragonhead extract, a moldavica dragonhead dropping pill and a production method thereof, wherein, the moldavica dragonhead extract contains higher total flavonoids and luteolin and the production method of the moldavica dragonhead extract is simply operated. Pharmacodynamics test result indicates that the moldavica dragonhead dropping pill acquired from the invention has excellent curative effect to rat ischemia myocardial injury; each dose group of the moldavica dragonhead dropping pill has varying degrees of protection; Composite salviae dropping pill has similar effect with the middle-dose group of moldavica dragonhead dropping pill; all dose groups of the moldavica dragonhead dropping pill have better curative effect than Yixing Badiranjibuya Keli at ischemia myocardial; dosages of middle-dose group of moldavica dragonhead dropping pill and low-dose group of moldavica dragonhead dropping pill are only 50 percent and 25 percent of the dosage of Yixing Badiranjibuya Keli respectively, thereby greatly improving the compliance of sufferers. The moldavica dragonhead dropping pill acquired from the invention is a novel preparation of convenient use and good compliance, thus bringing into play better clinical curative effect of the moldavica dragonhead, an age-old Uighur medicinal material. The production method of the moldavica dragonhead dropping pill can be carried out easily.
Owner:XINJIANG INST OF MATERIA MEDICA
Traditional Chinese medicine pharmacokinetics-pharmacodynamics combined analysis method
InactiveCN102183608AMulti-targetMultiple therapeutic pathwaysComponent separationEndogenous metabolismMetabolite
The invention provides a traditional Chinese medicine pharmacokinetics-pharmacodynamics combined analysis method, which determines various endogenous metabolites in blood samples of a blank control group, a model group and a model treatment group by a GC-MS method, uses an abnormally-changed metabolite group of the model group as a biomarker group, uses an effect index with obvious callback of the abnormally-changed metabolite group after traditional Chinese medicine intervention as an efficacy marker group, determines the contents of various drugs in the blood samples of a model animal after single dose by a LC-MS method, draws a plasma concentration-time curve, calculates the pharmacokinetic parameters of each component; determines the amount of the endogenous efficacy marker group in the blood samples of the model animal single dose group by a GC-MS method, draws a time-effect curve, performs PK-PD combined analysis by combining with the plasma concentration-time curve and parameters. The invention reveals the efficacy substance base of traditional Chinese medicine in the treatment of cardiovascular diseases, and provides an effective method for traditional Chinese medicine pharmacokinetics-pharmacodynamics combined analysis.
Owner:ZHEJIANG UNIV
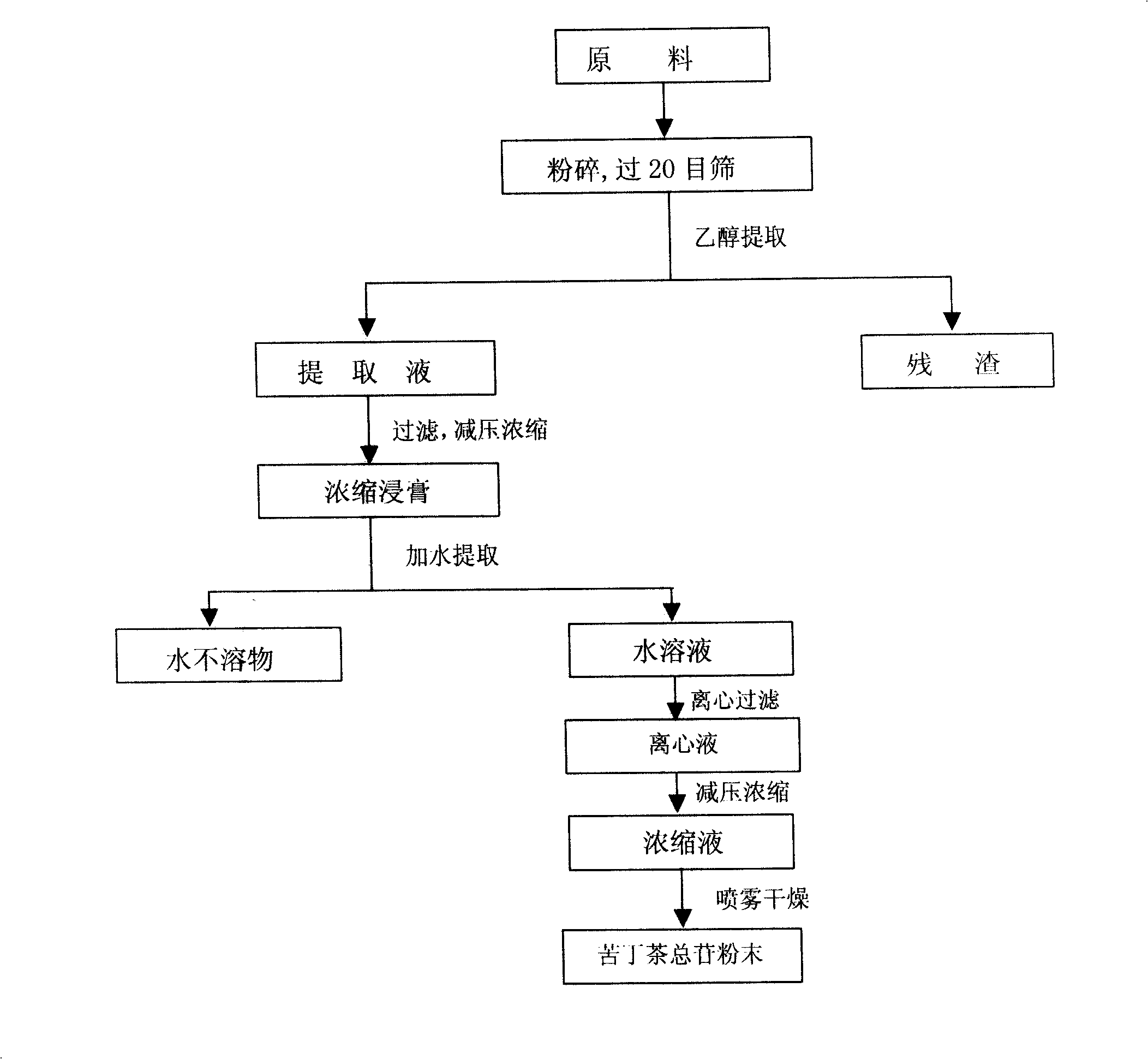
Applications of lectuce tea total glycosides and method of preparing the same
InactiveCN101254222AThe adsorption purification cycle savesAvoid fat removalSugar derivativesDigestive systemAlanine aminotransferaseAlcoholic hepatitis
The invention relates to the application of Ilex latifolia total glycosides and a preparation method thereof. Studies finds that the Ilex latifolia total glycosides can reduce the content of malonaldehyde (MDA) and triglyceride (TG) in liver, increase the content of reduced glutathione (GSH) in each dose group of liver, reduce the content of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in liver, and has alcoholic hepatitis and fatty liver resisting activity. Compared with the prior art, the invention provides a new application of Ilex latifolia total glycosides in preparing drugs and / or functional food for preventing and / or treating alcoholic hepatitis and fatty liver; the invention also provides a preparation method of the Ilex latifolia total glycosides with the advantages of safe and simple process, saved cost, high efficiency, and easy quality control; and the invention further provides a preparation of the Ilex latifolia total glycosides.
Owner:海南阿苷饮品有限公司
Traditional Chinese medicine cancer toxin prescription for treating liver cancer and application thereof in pharmacy
ActiveCN102357202AInduced growthInhibit expressionAnthropod material medical ingredientsAntineoplastic agentsAnticarcinogenic EffectOncology
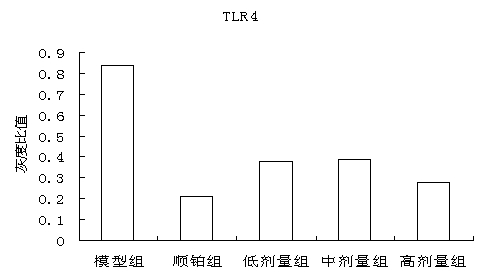
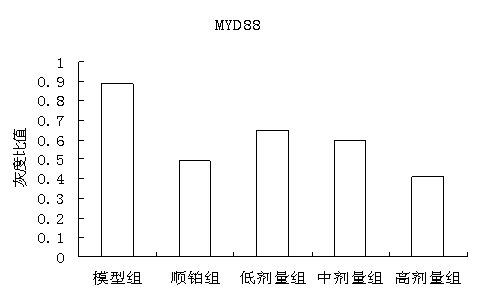
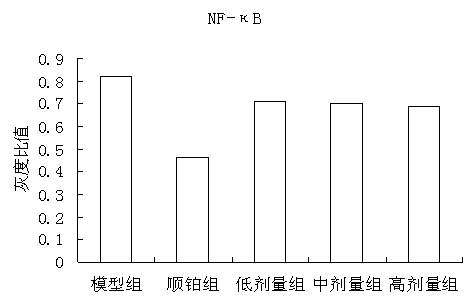
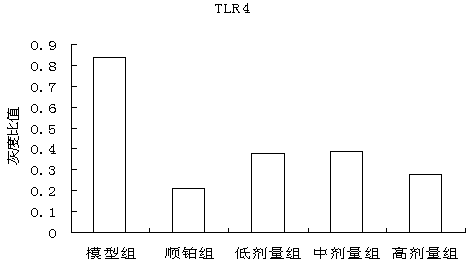
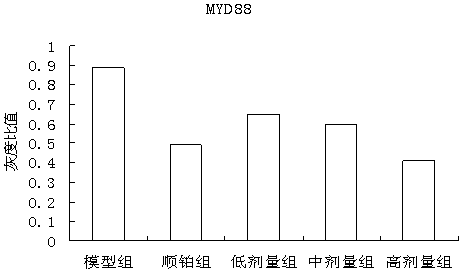
The invention discloses a traditional Chinese medicine cancer toxin prescription for treating liver cancer and application thereof in the preparation of medicaments for resisting liver cancer. The cancer toxin prescription is prepared in accordance with the cancer toxin theory proposed by traditional Chinese medicine master Professor Zhou Zhongying, and is prepared from hedyotidis herba, muscardine silkworm, centipede, akebia fruit, pseudostellaria root, dwarf lilyturf root and rhizoma pleionis according to a certain weight ratio. The prescription has the main functions of eliminating pathogens, eliminating cancer and detoxicating and the auxiliary function of strengthening healthy qi. By using mice with liver cancer H22 transplantation tumor as materials, the pharmacological experiment result shows the cancer resistance effect of the cancer toxin prescription: the tumor inhibition rates of a low-dose group, an intermediate-dose group and a high-dose group are respectively 24.5%, 42.8% and 21.1%, thereby showing the effect of inhibiting the growth of H22 tumor mass; the tumor mass cell apoptosis rates of the low-dose group, the intermediate-dose group and the high-dose group are respectively 47.16%, 60.52% and 57.59%, thereby showing the effect of inducing tumor cells to die; and the cancer toxin prescription can block the high expression of TLR4, block the MyD88 dependent pathway of TLR4 intracellular signal transduction and inhibit the expression of downstream NF-kB proteins. Experiment shows that the cancer toxin prescription can be used for preparing medicaments for resisting liver cancer.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Application of sibiricose A1, sibiricose A5 and tenuifoliside A in preparing tristimania treating products
The invention discloses a new medicinal application of sibiricose A1, sibiricose A5 and tenuifoliside A, in particular to an application of sibiricose A1, sibiricose A5 and tenuifoliside A in preparing tristimania treating products. The sibiricosee A1, the sibiricose A5 and the tenuifoliside A have a better treatment function for tristimania caused by various reasons; the sibiricose A5 and the tenuifoliside A have a certain protecting function for PC 12 cells damaged by glutamic acid; and the sibiricosee A1, the sibiricose A5 and the tenuifoliside A all can shorten the tail-hanging resting time and the swim resting time of a mouse. Compared with a mould group, a dose group has marked difference in statistics and dose dependence and shows a better anti-depression effect.
Owner:GENERAL HOSPITAL OF PLA
Application of effective parts of astragalus mongholicus and hedysarum polybotrys
InactiveCN102885878AIncreased lung coefficientImproved lung coefficientOrganic active ingredientsRespiratory disorderHedysarumAstragalus mongholicus
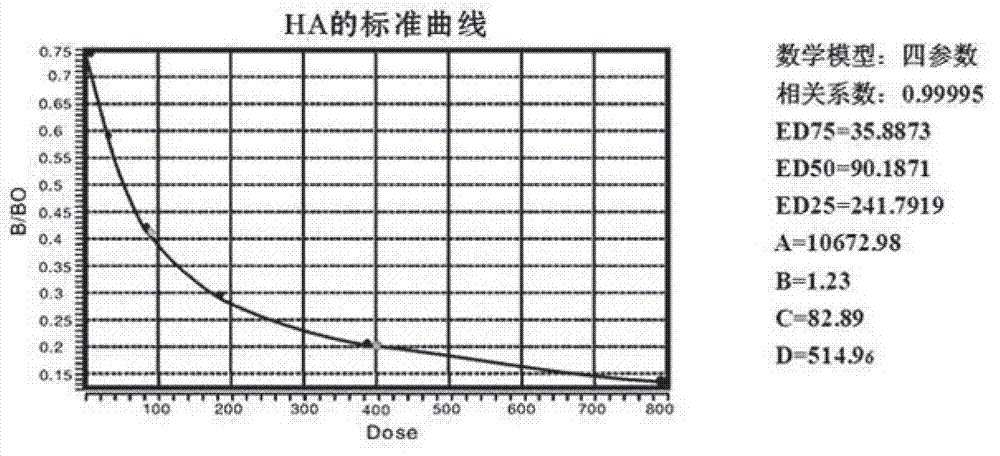
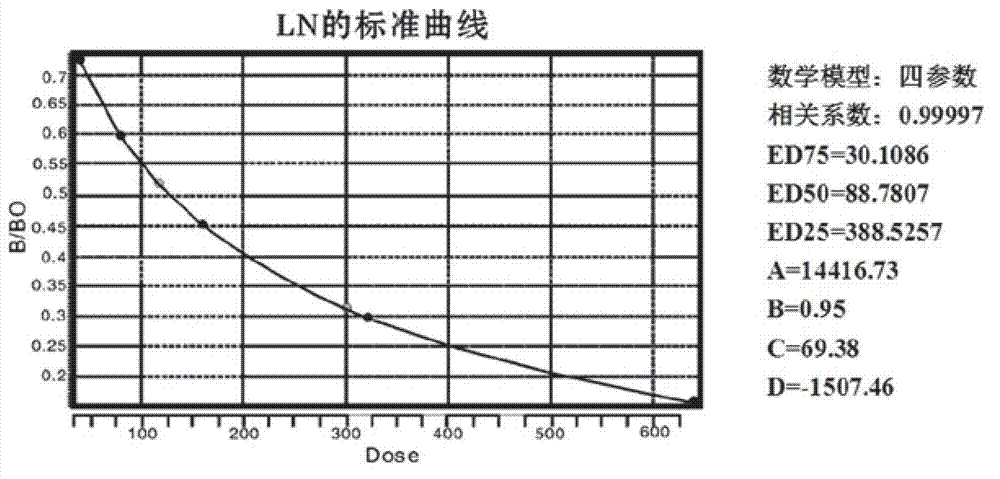
The invention belongs to the traditional Chinese medicine field and relates to the preparation of effective parts of astragalus mongholicus and hedysarum polybotrys and selection thereof for pulmonary fibrosis model intervention so as to obtain further study on the effective parts having the optimal curative effect. An interstitial pulmonary fibrosis model of rats is established through modeling; and compared with the normal rats, the rats having interstitial pulmonary fibrosis have obvious difference and pathologic change typical cases in various related detection indexes and pathologic changes. The various effective parts of astragalus mongholicus and hedysarum polybotrys are capable of suppressing various related indexes of the interstitial pulmonary fibrosis model of rats induced by bleomycin and preventing the development of interstitial pulmonary fibrosis, wherein low and medium dose groups of hedysarum polybotrys flavone have the optimal effect. The degree of the effect of the effective parts of astragalus mongholicus and hedysarum polybotrys on stopping the progress of interstitial pulmonary fibrosis is related to appropriate concentrations of equivalent dugs. Through comprehensive assessment, the hedysarum polybotrys flavone is better in influence on the lung function, HA, LN, HYP and the like of the rats having interstitial pulmonary fibrosis than other effective parts.
Owner:GANSU UNIV OF CHINESE MEDICINE
Method for detecting combination of polypeptide medicine and plasma proteins
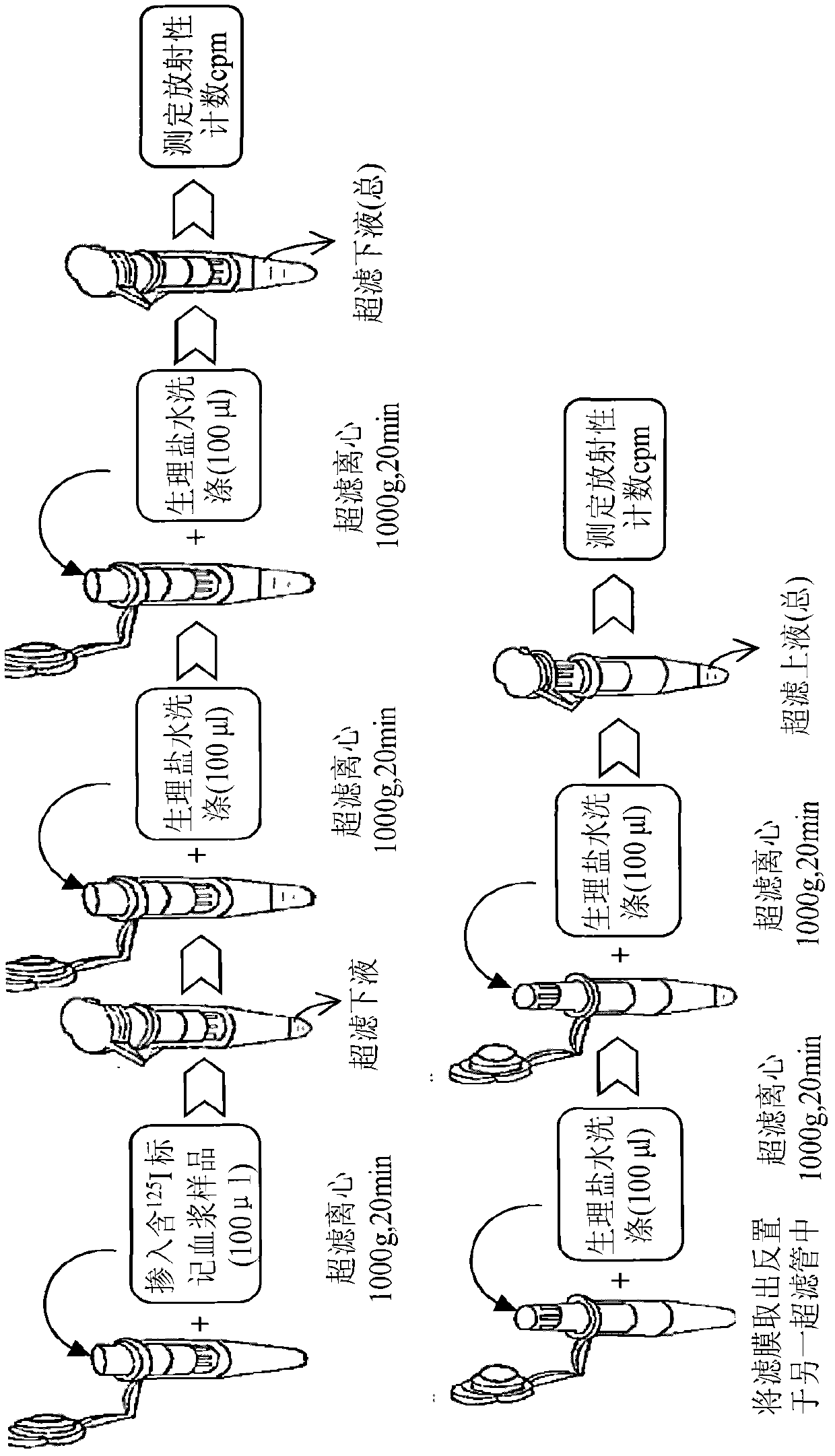
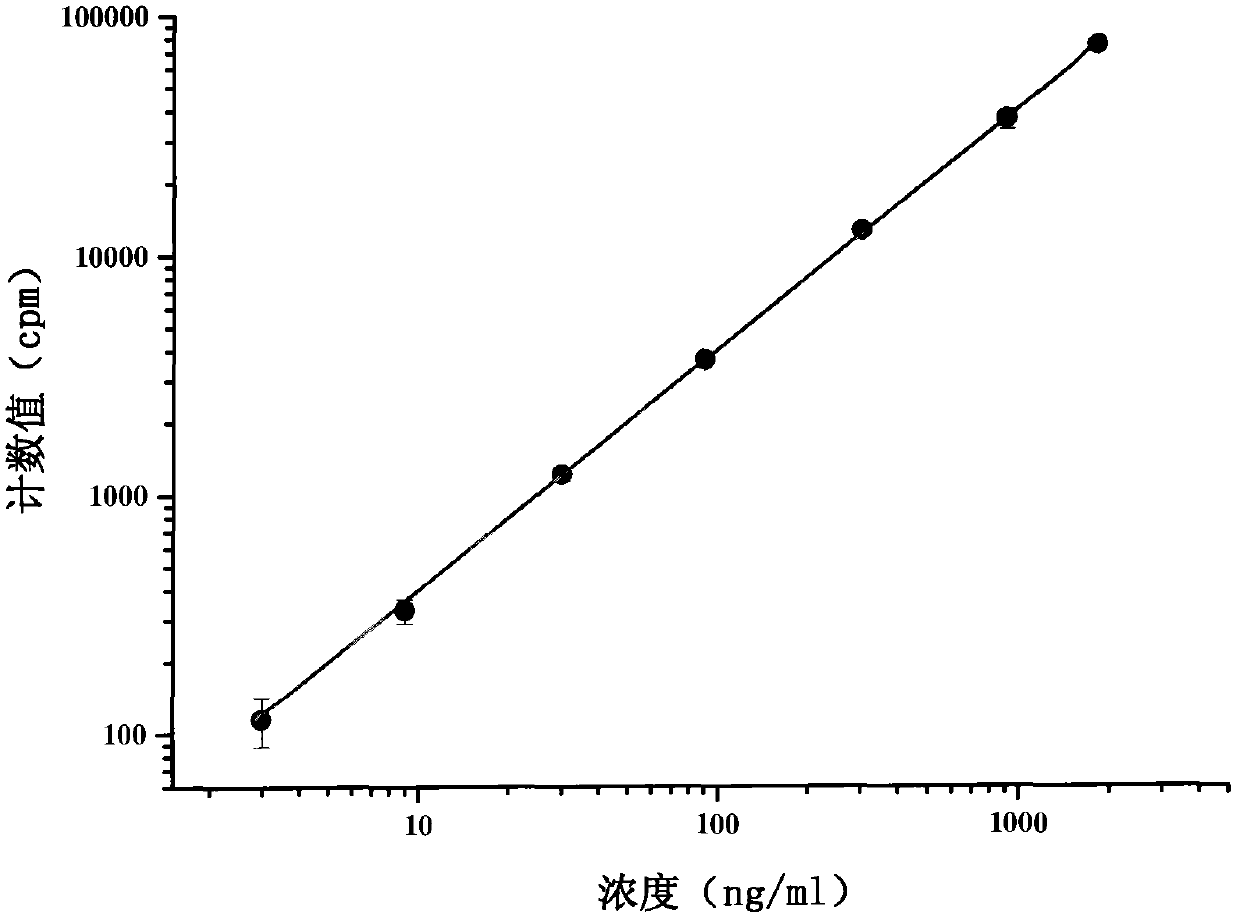
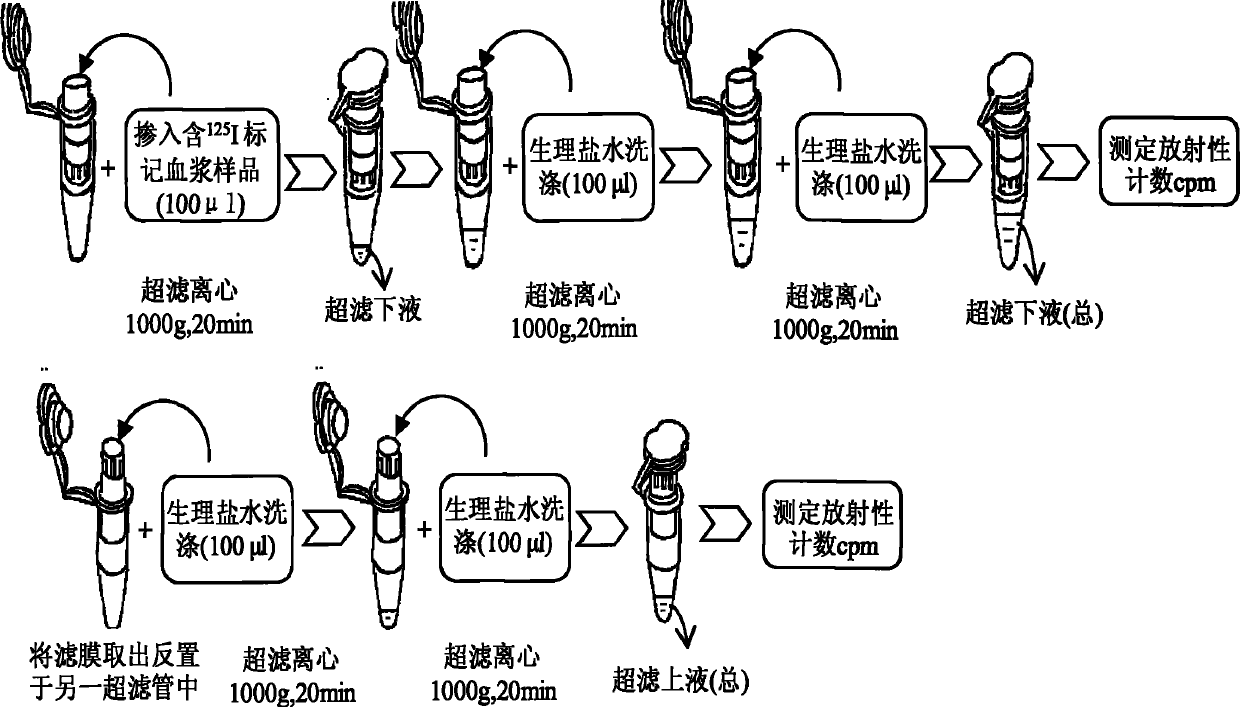
ActiveCN102565414AImprove adsorption capacityReduce adsorptionBiological testingUltrafiltrationHigh doses
The invention provides a method for detecting the combination of polypeptide medicine and plasma proteins. The method comprises the following steps: marking part of the polypeptide medicine through radioactive isotopes; preparing part of the marked polypeptide medicine into a series of polypeptide medicine plasma solutions with known concentrations, quantificationally detecting the radioactivity of the polypeptide medicine plasma solutions with the known concentrations respectively, and drawing standard curves corresponding to the concentrations of the polypeptide medicine plasma solutions respectively; and preparing part of the marked polypeptide medicine into to-be-detected polypeptide medicine plasma solutions with known concentrations, performing centrifugal ultrafiltration on the to-be-detected polypeptide medicine plasma solutions, and quantificationally detecting the radioactivity of filtered solutions and unfiltered solutions, wherein the concentrations of the to-be-detected polypeptide medicine plasma solutions can be set according to plasma concentration peak values after the administration of a low dose group, a medium dose group and a high dose group in a polypeptide medicine pharmacokinetics research, so as to set the three concentrations which are approximately consistent with peak concentrations or are different from the peak concentrations with the variation amplitudes less than 10 percent.
Owner:天津天诚新药评价有限公司 +1
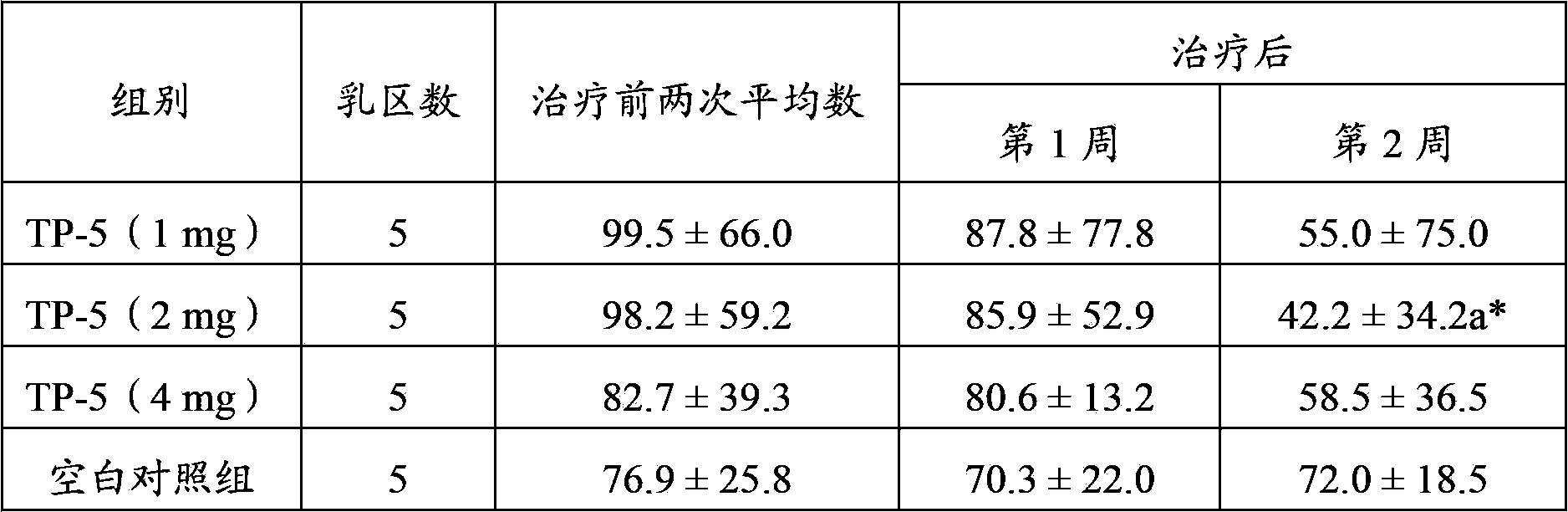
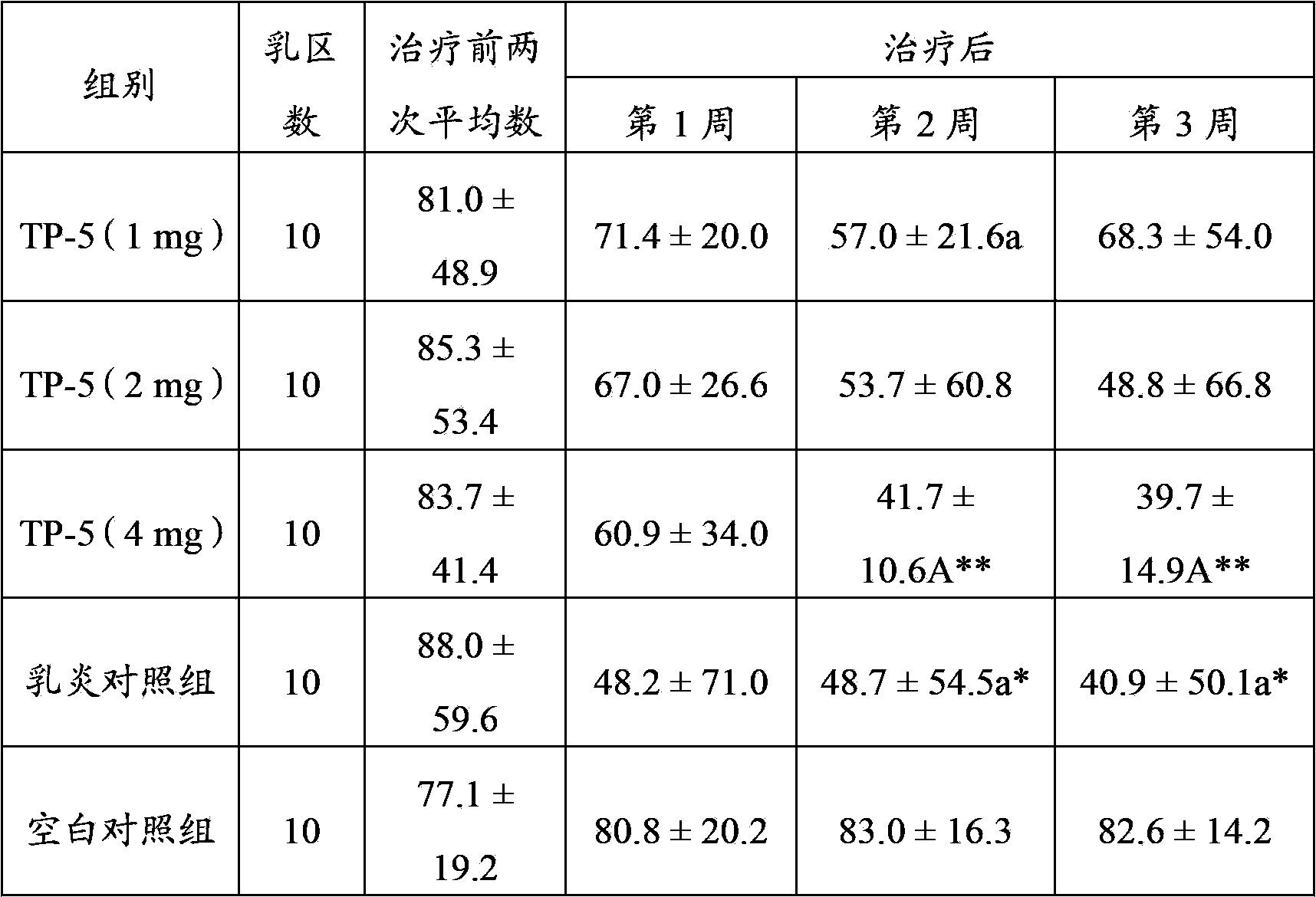
Application of thymopentin in preparing medicine used for treating mastitis
ActiveCN103386115AImproved ability to remove pathogenic bacteriaSwelling improvedPeptide/protein ingredientsSexual disorderAfter treatmentThymopentin
The invention relates to the field of medicine, and especially relates to an application of thymopentin in preparing medicine used for treating mastitis. As a result of subclinical mastitis efficacy test, infection mammary area numbers of thymopentin treatment groups, especially a high dose (4mg) group, have a reduction tendency after treatment. As a result of clinical mastitis efficacy test, through injection of thymopentin (TP-5) in breast lymph node for continuously 3 days, milk SCC of two TP-5 dose groups (10mg and 20mg) both have reduction tendencies, and cow breast swellings are substantially ameliorated in appearance. Therefore, medicines prepared with high-dose thymopentin (higher than 10mg) have significant effects against cow clinical mastitis. Therefore, the medicines prepared by using thymopentin have significant effects against subclinical mastitis and / or clinical mastitis.
Owner:ZHEJIANG HUAERCHENG PHARMA
Domestic sewage treatment apparatus
InactiveCN107935253AAffect ecologyGuaranteed Composite Emission StandardsWater treatment parameter controlFatty/oily/floating substances removal devicesSewage dischargeSewage sludge treatment
The invention relates to a domestic sewage treatment device, which includes a treatment box; two ends of the treatment box are respectively connected to a sewage inlet pipe and a clean water outlet pipe, and a first middle partition and a second middle partition are arranged at equal intervals in the treatment box. The first middle partition and the second middle partition divide the treatment box into a first-level treatment room, a second-level treatment room and a third-level treatment room. The aeration tube, two sets of dosing boxes are arranged on the top of the third-level treatment room, and the dosing box is connected with the third-level treatment room through the dosing pipeline; the invention is simply provided with a treatment box, and the first-level treatment room is set in the box 1, secondary treatment room and tertiary treatment room, the domestic sewage is treated three times to ensure the composite discharge standard of domestic sewage, and this device does not need special site settings, and any location with centralized sewage discharge can be used.
Owner:SUZHOU SHUYUE CARBON ADSORBENT CO LTD
Effective component extracted from bupleurum Chinese and application of antidepression activity thereof
The invention discloses a preparation method and medicinal application of CHB which is an effective component of bupleurum Chinese and particularly relates to an application of CHB in preparation of a product for treating depression. The CHB which is the effective component of the bupleurum Chinese has a good effect on treating depression caused by various factors, has a certain protection effect on a U251 cell damaged by corticosterone and has the effects of shortening the tail-suspending immobility time and the swimming immobility time of a mouse and improving the decrease of the body temperature of the mouse due to reserpine. Each dose group is significantly and statistically different from the model group, is dose-dependent and has a good antidepressant function.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
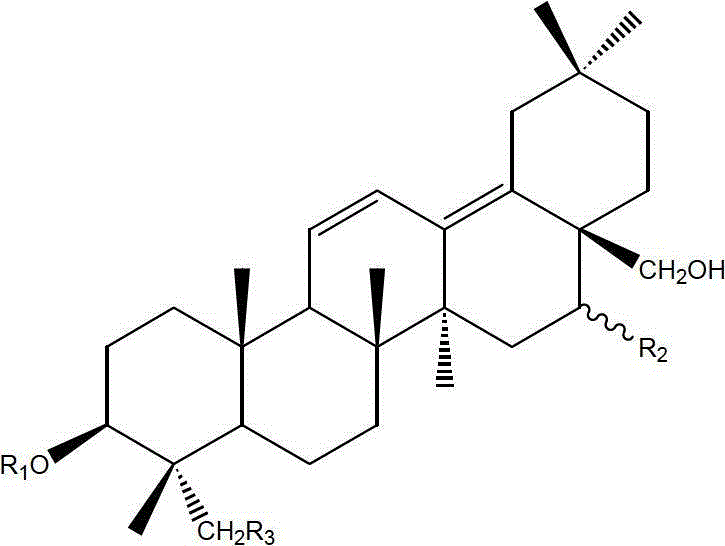
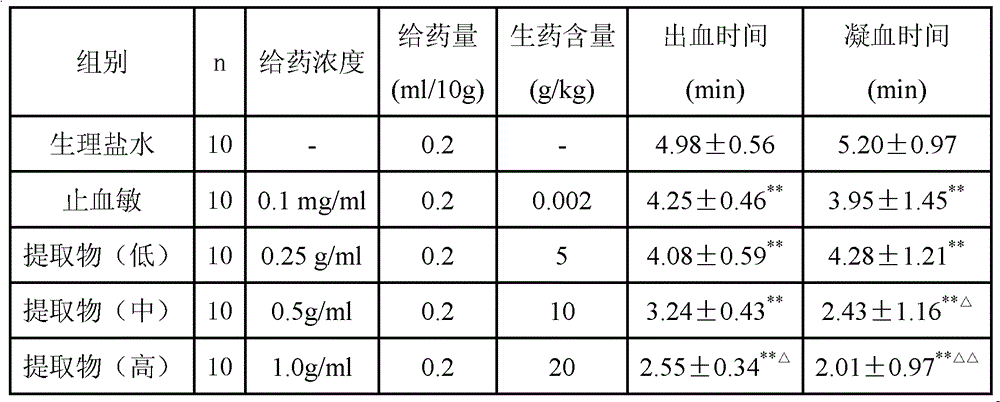
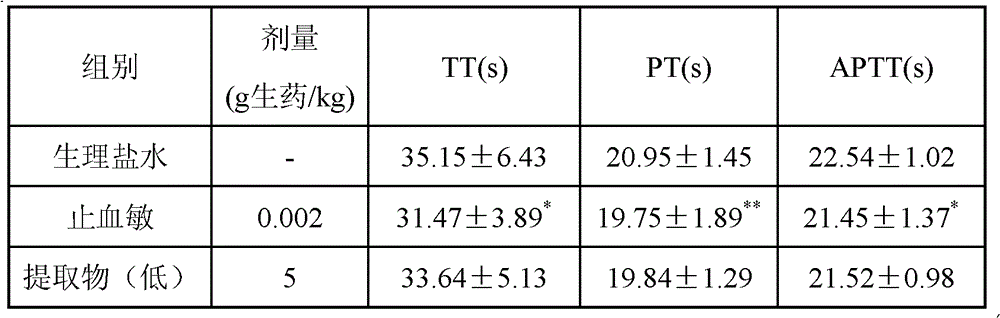
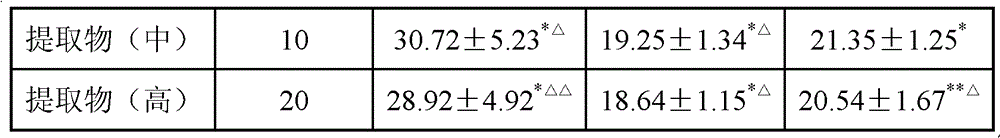
Chinese medicinal composition for treating thrombopenia as well as preparation method and application of composition
ActiveCN103054976AReduce bleedingShorten the timeBlood disorderExtracellular fluid disorderChronic idiopathic thrombocytopenic purpuraLithospermum
The invention discloses a Chinese medicinal composition for treating thrombopenia as well as a preparation method and application of the composition. The Chinese medicinal composition is prepared from madder and lithospermum in a mass ratio of (1:3)-(3:1). The composition is prepared by preparing and then uniformly mixing refined madder extract and refined lithospermum extract. Pharmacological and pharmacodynamic experimental results show that the composition of different dose groups can remarkably shorten the bleeding and clotting time of mice, the high or medium dose group can remarkably shorten the thrombin time (TT), the prothrombin time (PT) and the activated partial thromboplastin time (APTT) of chronic idiopathic thrombocytopenic purpura (ITP) model rats, and the high or medium dose group has an effect of remarkably improving the platelets of the rats. Thus, the Chinese medicinal composition can be used for preparing a medicinal preparation for treating the thrombopenia, and particularly can be used for preparing a medicinal preparation for treating immune thrombocytopenia.
Owner:SHANGHAI UNIV OF T C M
Polynucleotide microcapsule for treating diseases of livestock and poultry and preparation method thereof
InactiveCN101474165AProlong the action timeNo side effectsOrganic active ingredientsSugar derivativesDiseaseFiber
The invention relates to a polynucleotide microcapsule for treating animal disease and a preparation method thereof, belonging to the technical field of veterinary medicament preparation. Polyinosinic acid and polycytidylic acid are weighted and are added into preheated sodium chloride-phosphate buffer solution, and polynucleotide solution is prepared; the polynucleotide solution is added into ethyl cellulose to prepare soft wood and dry powder is prepared; the dry powder and magnesium stearate are added into ice-bath acetone to obtained a mixture, the mixture is stirred and slowly added into whiteruss containing 2%Span-80 surface active agent; and polynucleotide microcapsule is obtained by a method of in-liquid drying. The invention effectively prolongs the acting time of medicament within human body, decrease the applied time of the medicament and achieves the aims of long acting and sustained release. Experiments prove that the dead rate of the high dose group or the medium dose group of the polynucleotide microcapsule is obviously lower than that of an infectious control group. The cure rate is high, both are 80%, and the effective rate of the two groups are 100%. Experiments prove that the medicament of the invention is safe.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Immunological enhancement composition and application thereof
ActiveCN113413455AImprove growth performanceImprove proliferative abilityHydrolysed protein ingredientsFood processingAntagonismImmunity
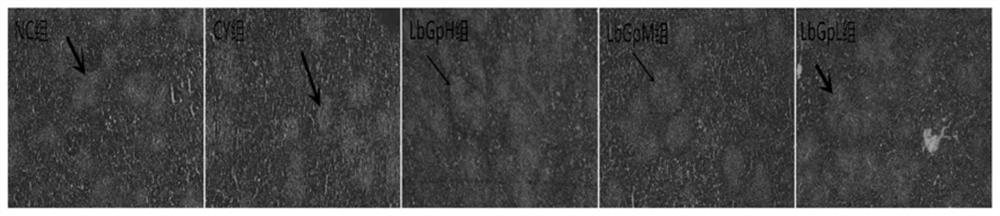
The invention provides an immunological enhancement composition and application thereof. The immunological enhancement composition comprises lycium barbarum glycopeptide LbGp4 and auxiliary materials; the mass ratio of the lycium barbarum glycopeptide to the auxiliary materials is 5: 1, and the purity of the lycium barbarum glycopeptide is greater than or equal to 90%. CY is adopted to induce chicks to generate an immunosuppression state, the antagonism of LbGp4 on immunosuppression is studied, and the result shows that the LbGp4 can remarkably improve the growth performance of the chicks induced by CY to generate immunosuppression, remarkably promote spleen development and accelerate the recovery process of spleen injury caused by CY; the multiplication capacity of CY-induced immunosuppression chick peripheral blood lymphocytes is remarkably improved, and the effect is the best when the LbGp4 medium dose group (5mg / kg.d) is adopted. According to the results, the immunological enhancement composition provided by the invention can be applied to preparation of the medicine for improving the immunity of the chicks.
Owner:宁夏农林科学院动物科学研究所
Application of recombinant human calcineurin B subunit
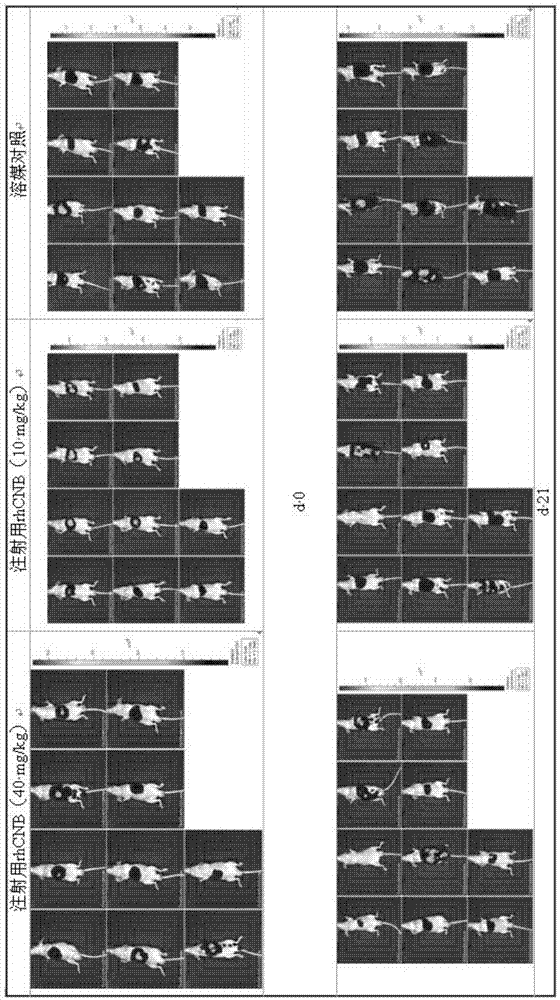
InactiveCN105435215AGood control effectEnhanced inhibitory effectPeptide/protein ingredientsHydrolasesPositive controlHigh doses
The invention relates to the field of proteins, in particular to application of recombinant human calcineurin B subunit, and mainly provides application of rhCNB to preparation of a medicine for killing and / or inhibiting a liver cancer cell Bel-7402. After continuous medication for 6 times, medication groups of rhCNB for injection with doses of 10 mg / kg, 20 mg / kg and 40 mg / kg all have a relatively good inhibiting effect on the growth of human liver cancer cell Bel-7402 solid tumors. Subsequent testing results show that the effects of high-dose, middle-dose and increasing-dose medication groups are superior to that of a low-dose medication group, and the treating effect of rhCNB for injection is equivalent to those of recombinant human interleukin-2 for injection and hydroxycamptothecine for injection in a solvent control group. The results of d7 testing after final medication show that the high-dose group has the optimum controlling effect (p is smaller than 0.01) on tumor cell proliferation, which is superior to those of other dose groups and a positive control group, and equivalent to those of a hydroxycamptothecine medication group and the increasing-dose group (p is smaller than 0.05).
Owner:HAIKOU QILI PHARMA
Components and process of lead-free ultra-low sodium glass tube
The invention relates to a chemical batching component and a process of a lead-free ultra-low-sodium glass tube, in particular to a chemical batching component and a process of a lead-free ultra-low-sodium energy-saving lamp tube, and belongs to the technical field of energy-saving lamp manufacturing. It consists of SiO2, AL2O3, B2O3, K2O, Na2O, S2O, BaO, CaO, MgO, Li2O. It is characterized in that their components are as follows: SiO2 accounts for 67-68%, AL2O3 accounts for 1.5-2.0%, B2O3 accounts for 1-1.5%, K2O accounts for 10-11%, Na2O accounts for 3.5-4.0%, S2O accounts for 1.2- 2%, BaO 8-9%, CaO 2-3%, MgO 0-0.5%, Li2O 1.5-2.0%. It is characterized in that their technological process is: weighing of raw materials→stirring→feeding→melting, clarifying and homogenizing in an electric melting furnace→drawing tube forming→finely cutting round mouth of glass tube→inspecting and packing→storage. The invention has the advantages of good chemical stability, large luminous flux, high resistivity, low ultraviolet intensity, long service life, anti-aging and the like.
Owner:盐城市旭源节能灯管件有限公司
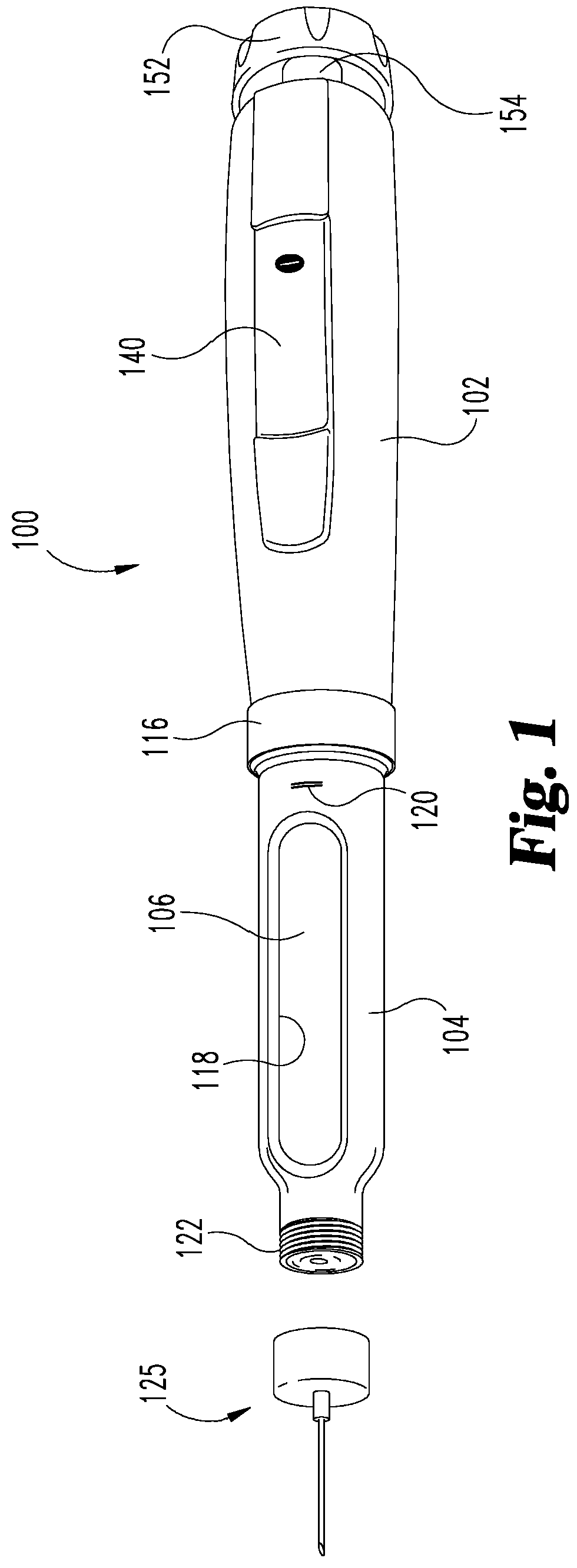
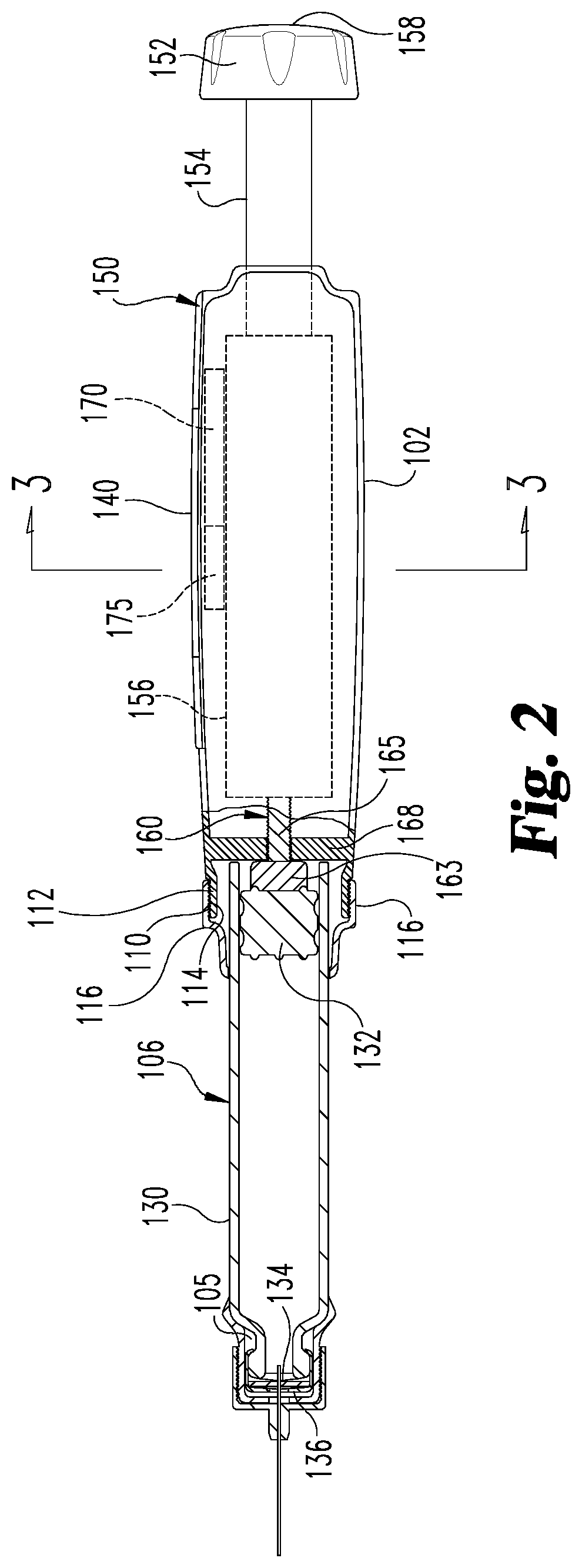
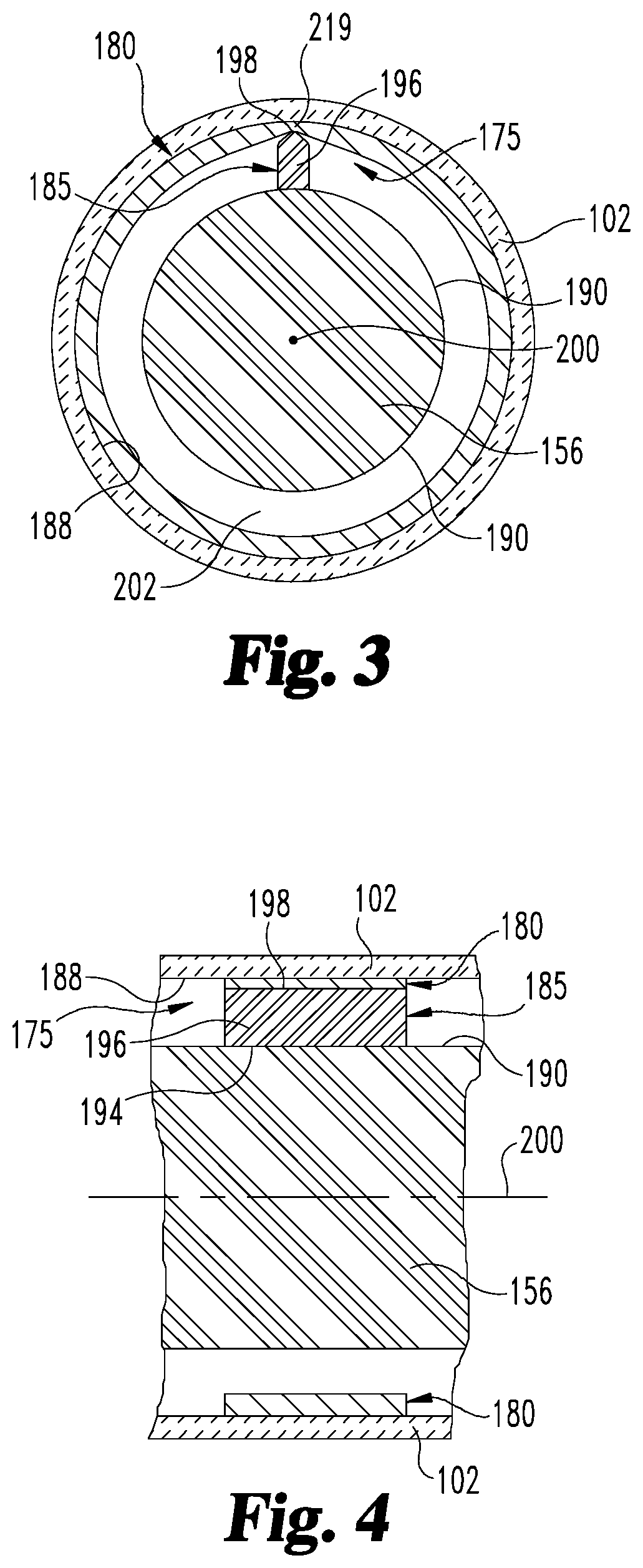
Medication delivery device with sensing system
A medication delivery device with a sensing system to determine at least one of a dose set and a dose delivery. The sensing system is operable to detect relative rotational positions of first and second members of the device which are indicative of at least one of an amount of a dose set and an amount of a dose delivered by operation of the device, and generate outputs correlated to such relative rotational positions. The system includes a wiper coupled to the first member, and a sensing band coupled to the second member for physically contacting the wiper as the second member rotates relative to the first member. A controller electrically communicates with the sensing system to determine, based on the generated outputs of the sensing system, at least one of the amount of the dose set and the amount of the dose delivered by operation of the device.
Owner:ELI LILLY & CO
Method of Estimating Effect of Test Chemical on Living Organisms
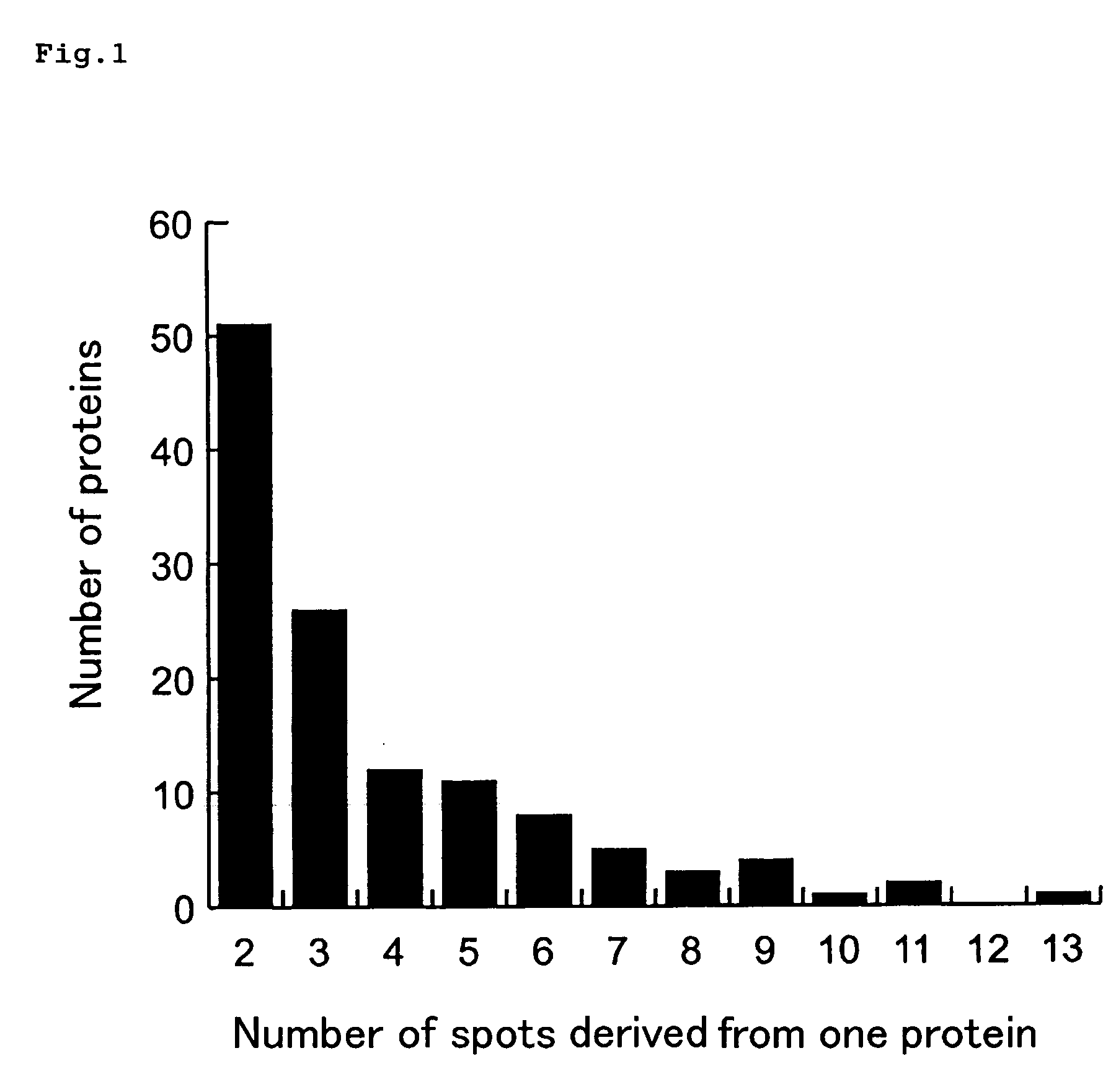
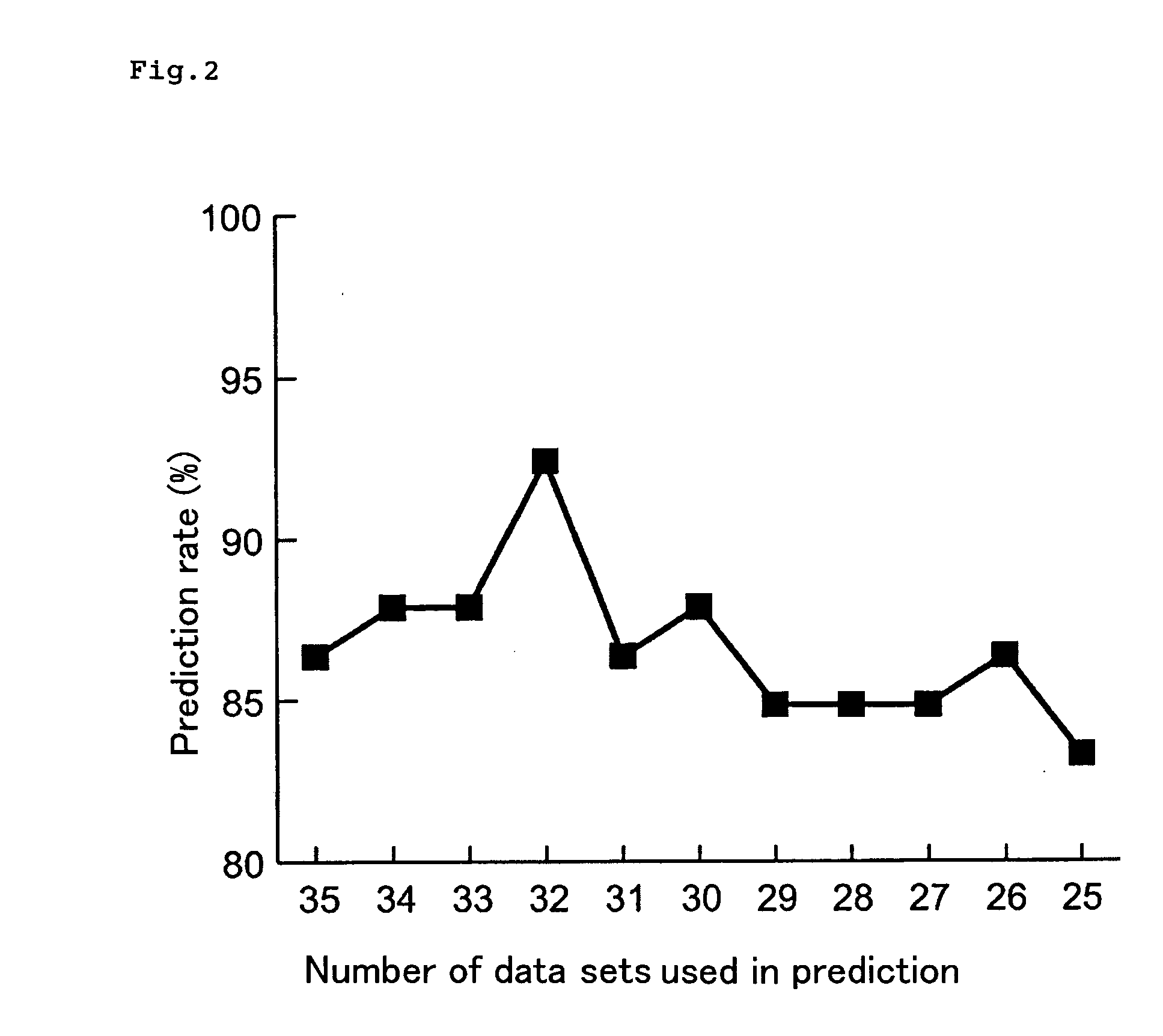
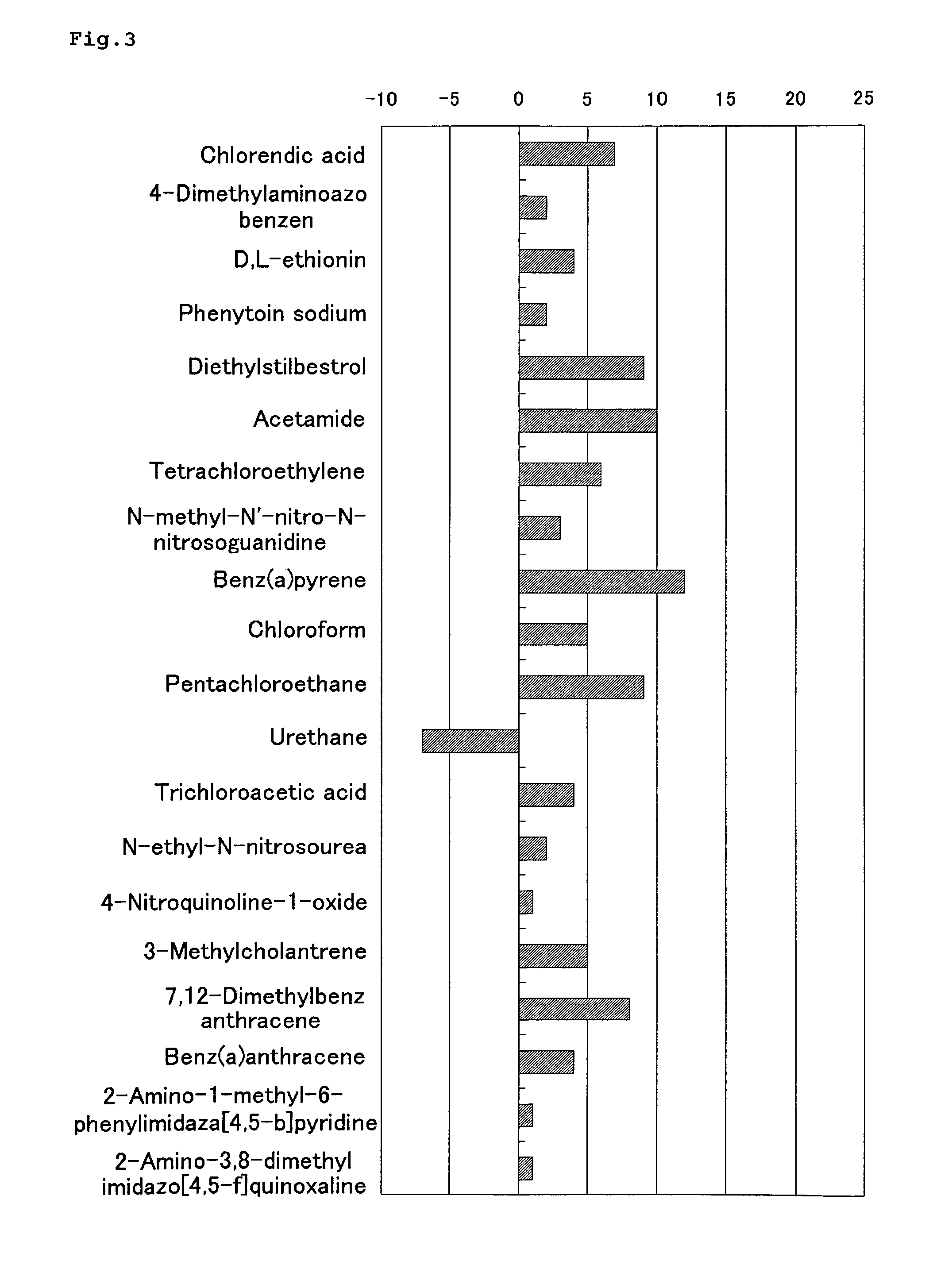
InactiveUS20090134028A1Guaranteed predictive effectThe effect is accurateElectrolysis componentsVolume/mass flow measurementBiological bodyDefinite period
A method of predicting an effect of a test chemical on living organisms, which comprises:a step of administering a plurality of chemicals whose effects on living organisms are known, to respective chemical dosed groups, administering a test chemical to a test chemical dosed group, collecting proteins from each group after a definite period of time, separating the proteins by two-dimensional gel electrophoresis, measuring the signal intensities of spots of the proteins and modified proteins formed therefrom, and calculating a signal intensity ratio of at least two spots, anda step of comparing the signal intensity ratio of test chemical dosed group with the signal intensity ratio of each chemical dosed group.
Owner:CHEM EVALUATION & RES INST +2
Medicinal composition for treating diabetic encephalopathy as well as preparation method and application thereof
ActiveCN104857319APromote new lifeImprove the state of ischemia and hypoxiaNervous disorderMetabolism disorderTherapeutic effectBlood vessel
The invention provides a medicinal composition for treating diabetic encephalopathy. The medicinal composition is a preparation which is prepared from the following raw materials in parts by weight: 12-18 parts of kudzuvine root, 8-12 parts of coptis chinensis, 8-12 parts of scutellaria baicalensis, 12-18 parts of dendrobe, 2-4 parts of fel ursi and 2-4 parts of pseudo-ginseng. The invention also provides a preparation method and an application of the medicinal composition. The medicinal composition is remarkable in treatment effect on diabetic encephalopathy; the treatment effect of the medicinal composition is superior to cilostazol; and especially, the optimal treatment effect is achieved by a median dose group. The medicinal composition can be used for promoting angiogenesis by protecting capillaries which serve as blood brain barriers, improving the ischemia anaerobic condition of sea horses and regulating the activity of IGF-1 and PI3K / AKT / CREB signal channels of the sea horses.
Owner:CHENGDU HUASUN GRP INC LTD
Optimized universal biological analysis method by adding monkey serum
The invention discloses an optimized universal biological analysis method by adding monkey serum. The method comprises the following steps: preparing a universal coated antibody buffer solution, and carrying out incubating overnight; adding a closed buffer solution for incubation, adding two sets of same to-be-detected objects with certain gradients, and respectively adding the detection antibodyworking solution containing the monkey serum and the detection antibody working solution without the monkey serum into the to-be-detected objects; and comparing the background value and the sensitivity of each group of data after the data is processed by a microplate reader. According to the method, the problems that the sensitivity of the method is influenced and the detection of the exposure amount of animals in a low-dose group is not facilitated due to the fact that a general background value of a general method in monkey serum or monkey plasma is relatively high can be solved.
Owner:WUXI APPTEC SUZHOU
Itraconazole applied to treatment of malignant tumor or salt thereof and composition thereof
InactiveCN103044408AExpand anti-tumor spectrumSlow growth rateOrganic active ingredientsOrganic chemistryItraconazoleCytoplasm
The invention relates to itraconazole applied to treatment of gastric cancer, lung cancer and liver cancer, and a composition of the itraconazole. The daily dose of the itraconazole is 0.1-5000mg, and the itraconazole has good curative effect when the daily dose is 200mg. The itraconazole and the composition of the itraconazole disclosed by the invention have the beneficial effects that by subcutaneous injection of the itraconazole solution or the composition, the survival time of high and low-dose groups of SPF-grade Konmin mice that have liver cancer H22 can be prolonged. The itraconazole or the composition of the itraconazole has obvious inbibitional effect on Lewis lung cancer of mice in comparison with the control group according to the influence on subcutaneous tumor of the Lewis lung caner, the growth rate (P(0.01) of the tumor can be slowed down; the size (P(0.01) of the tumor is reduced; and the tumor control rate is respectively 43.0% and 45.6% after the treatment is ended. The itraconazole or the composition of the itraconazole influences positive staining of the human gastric cancer tissue VEGF (Vascular Endothelial Growth Factor) in cell cytoplasm, and the VEGF positive expression of a 0.9% sodium chloride solution group is obviously higher than that of the itraconazole group (P(0.05).
Owner:万礼 +1
Corn stigma anti-fatigue oral liquid and preparation method thereof
ActiveCN104172388AGood effectNo side effectsNatural extract food ingredientsFood ingredient functionsAcute toxicity testingPhysiology
The invention relates to corn stigma oral liquid with an anti-fatigue function, which is prepared by taking corn stigma, chrysanthemum and liquorice as raw materials, and subjecting the materials to extracting, filtering, mixing, sterilizing and filling. The corn stigma anti-fatigue oral liquid is administrated to mice in a high-dose group, a mediate-dose group, a low-dose group and a blank control group through gavage for 30 d, and the results show that by administrating the corn stigma oral liquid, the burden swimming time of the mice can be prolonged; the activity of lactic acid dehydrogenase is increased; the generation of urea nitrogen is reduced; and hepatic glycogen of the mice in different groups is improved to different extent. An acute toxicity test shows that LD 50 can not be detected, suggesting that the corn stigma oral liquid has no toxic or side effect. In a maximum dosing test, the mice are administrated with the corn stigma oral liquid in high, mediate and low doses three times a day, and the blank group is administrated with equal volume of saline through gavage. The mice are observed for 14 d after dosing, death does not occur, piloerection, moving restlessly, tail erecting, excited jumping, curling up, hyperactivity, slow response to stimulation or allergy does not occur, and symptoms such as stiffness, fremitus, ataxia do not occur either, suggesting that the maximum dose of the corn stigma oral liquid for the mice is 60 g / kg.
Owner:JILIN INST OF CHEM TECH
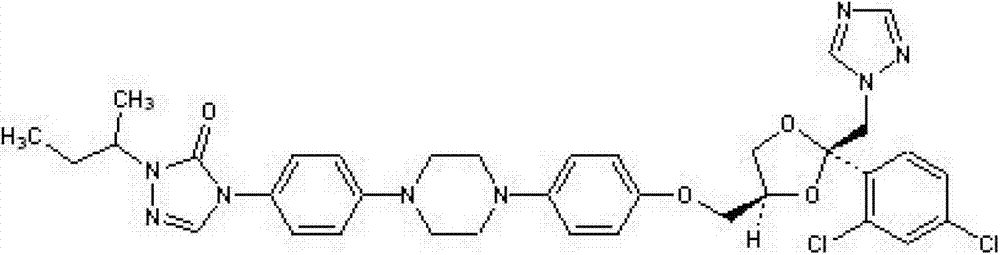
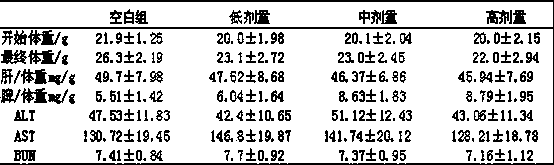
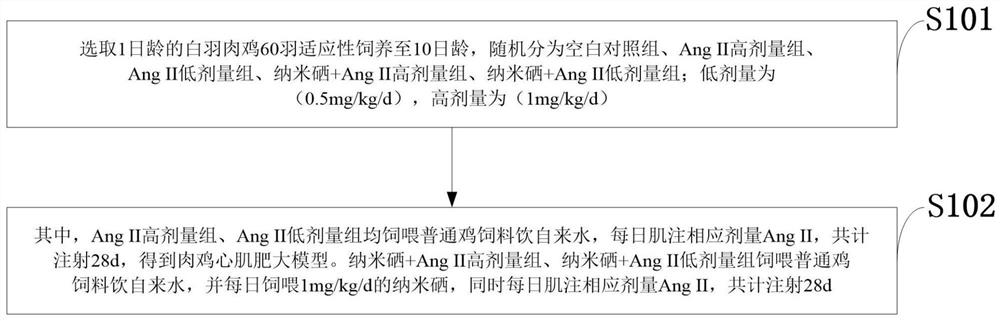
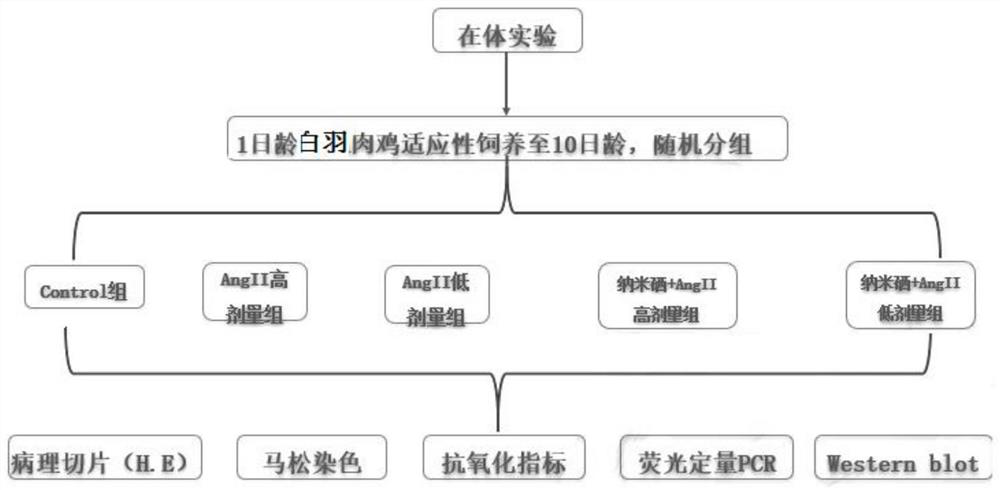
Drug for inhibiting myocardial hypertrophy and construction method of model
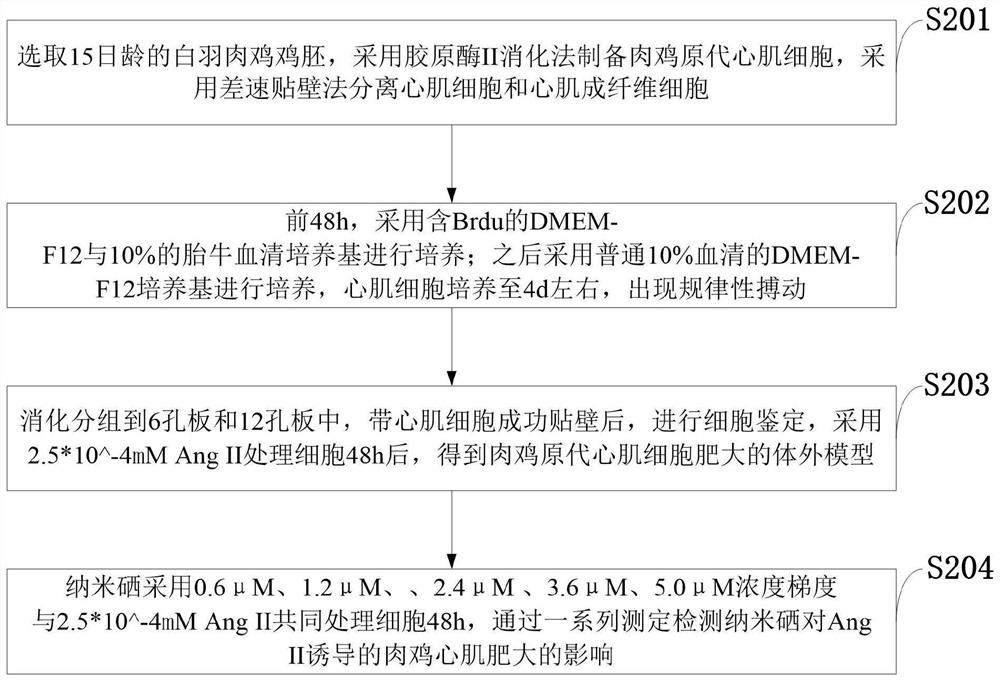
PendingCN114053298ACell dissociation methodsSkeletal/connective tissue cellsMechanism of actionPharmaceutical drug
The invention belongs to the technical field of animal model construction, and discloses a drug for inhibiting myocardial hypertrophy and a construction method of a model. 60 1-day-old white feather broilers are selected and adaptively fed to an age of 10 days, and the white feather broilers are randomly divided into a blank control group, an Ang II high-dose group, an Ang II low-dose group, a nano-selenium + Ang II high-dose group and a nano-selenium + Ang II low-dose group. 15-day-old white feather broiler embryos are selected, and broiler primary myocardial cells are extracted and treated with Ang II of 2.5 * 10 <-4 > mM for 48 hours to obtain a broiler myocardial hypertrophy in-vitro model. Then, the cells are co-treated for 48 hours by adopting nano-selenium with different concentrations and Ang II with the concentration of 2.5 * 10 <-4 > mM, and the action mechanism of the nano-selenium for relieving the myocardial hypertrophy of the broilers is explored. The method can be used for researching whether biogenic nano-selenium can inhibit myocardial hypertrophy by inhibiting generation of free radicals of organisms or not.
Owner:HUAZHONG AGRI UNIV
Application of Long Hu Ren Dan in prevention and treatment of drug-induced liver injury
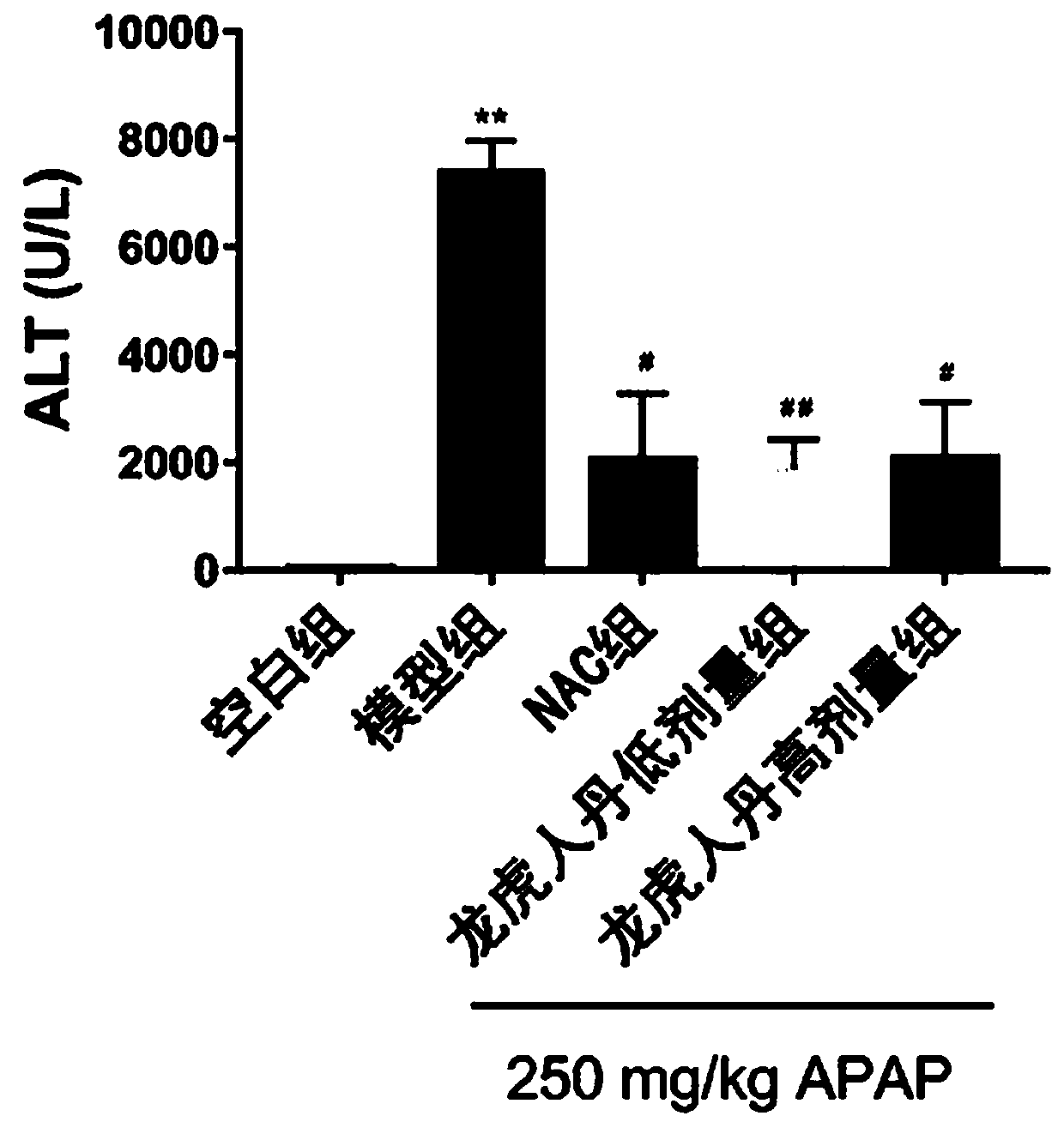
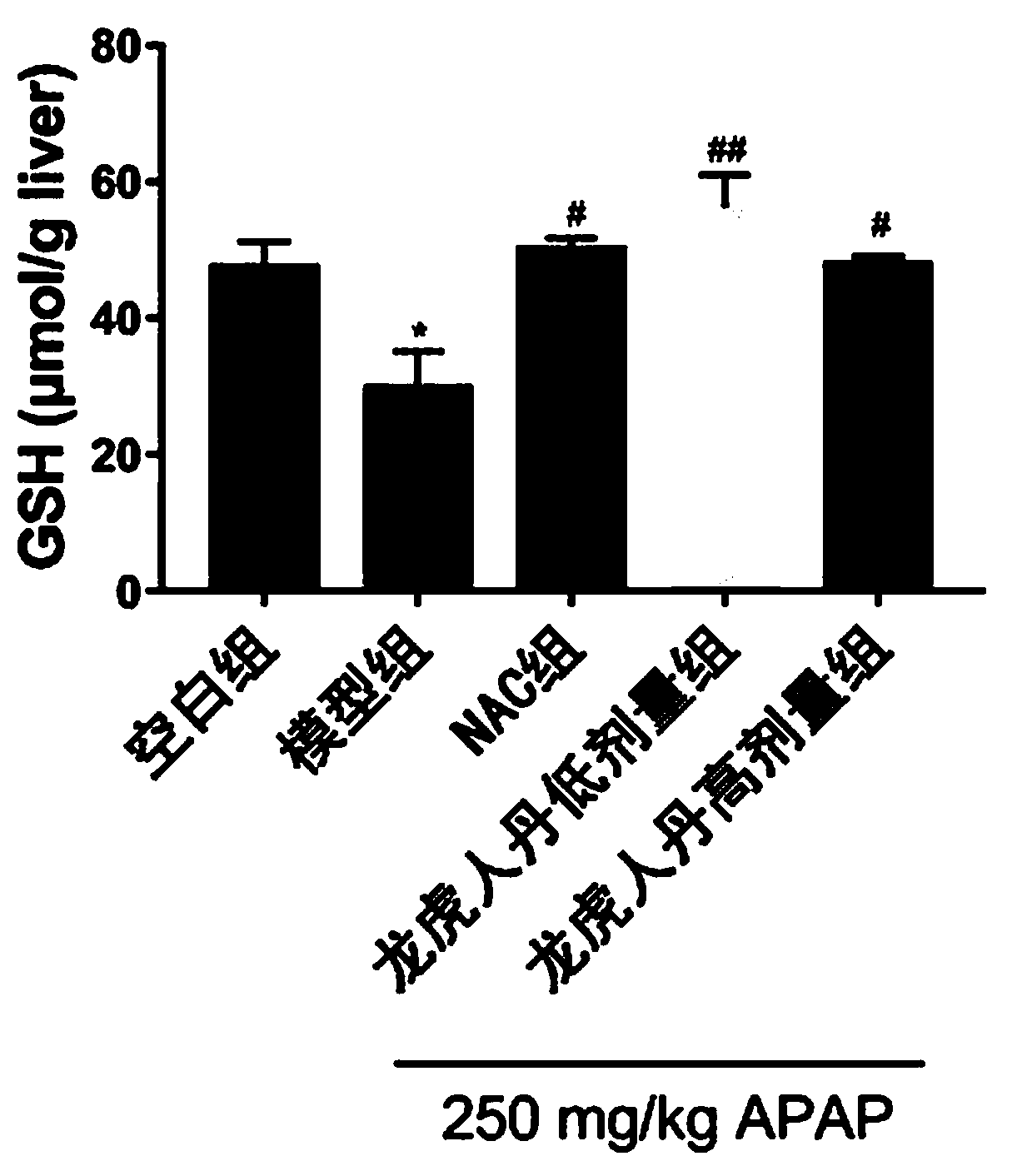
ActiveCN110638986AHydroxy compound active ingredientsDigestive systemAlanine aminotransferaseWestern blot
The invention discloses application of Long Hu Ren Dan in prevention and treatment of drug-induced liver injury. The invention relates to the following steps: randomly dividing 24 mice into an APAP model group, an N-acetyl-L-cysteine positive control group (50 mg / kg), an LHRD low-dose group and an LHRD high-dose group, and additionally taking 6 mice as a normal control group; and after 6 days of administration at fixed time, performing fasting for 22 hours, injecting 250 mg / kg APAP into the abdominal cavity, killing the mice after 6 hours, and taking serum and livers of the mice. A kit is adopted to detect the serum alanine aminotransferase (ALT) activity and the glutathione (GSH) content in the livers of each group of mice; hematoxylin-eosin (H & E) dyeing is adopted to observe morphological changes of liver tissues; and Western Blot is adopted to detect expression of glutamic acid-cysteine ligase catalytic subunits and endonuclease G in the liver tissues. A fluorescent probe DCFH-DAmethod is adopted to detect the expression of reactive oxygen species (ROS) in tissue cells. The conclusion is that the LHRD can improve the acute liver injury induced by excessive acetaminophen and enhance the oxidative stress resistance of the liver.
Owner:SHANGHAI ZHONGHUA PHARMA
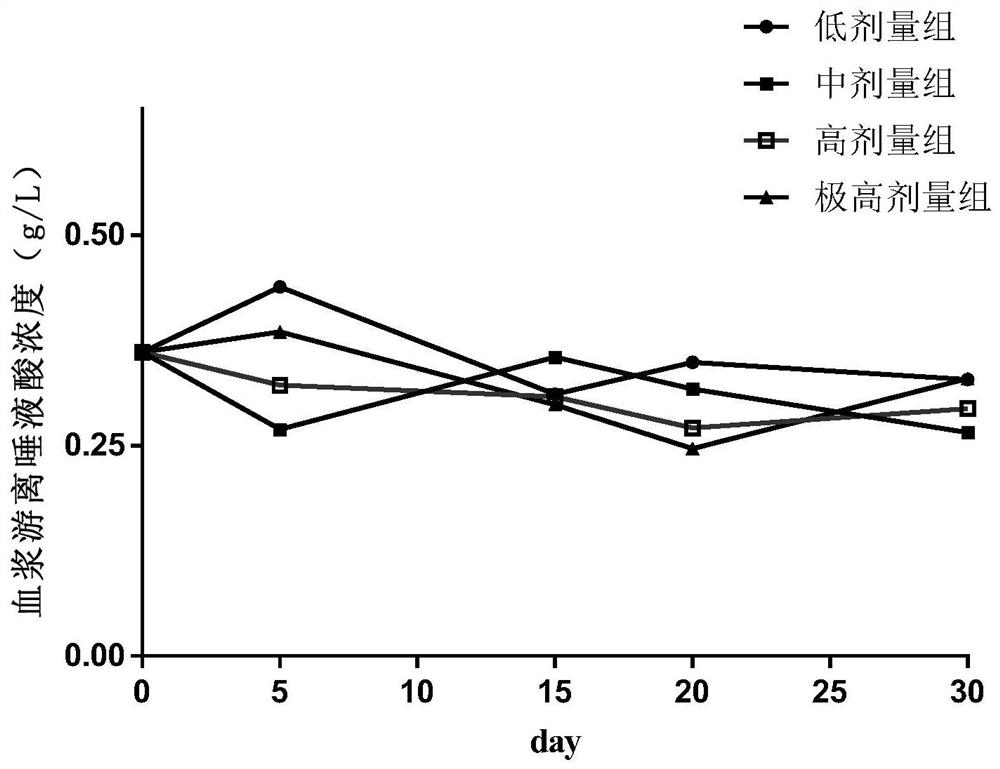
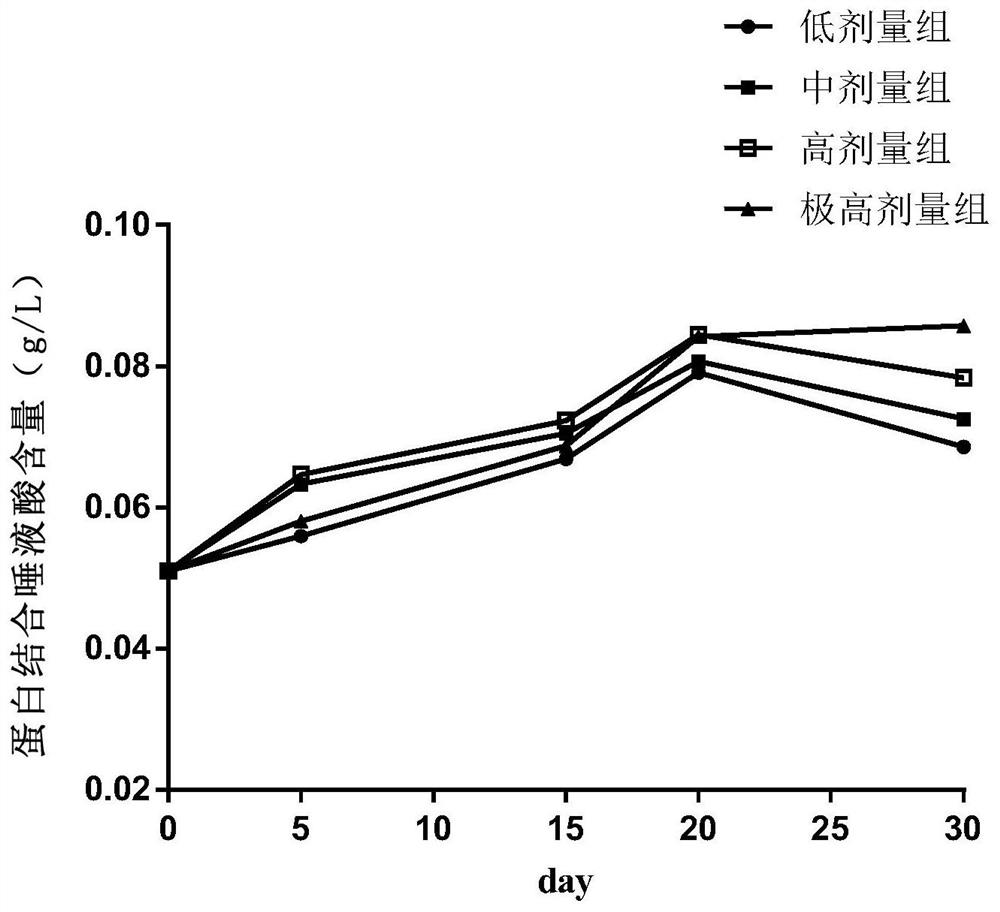
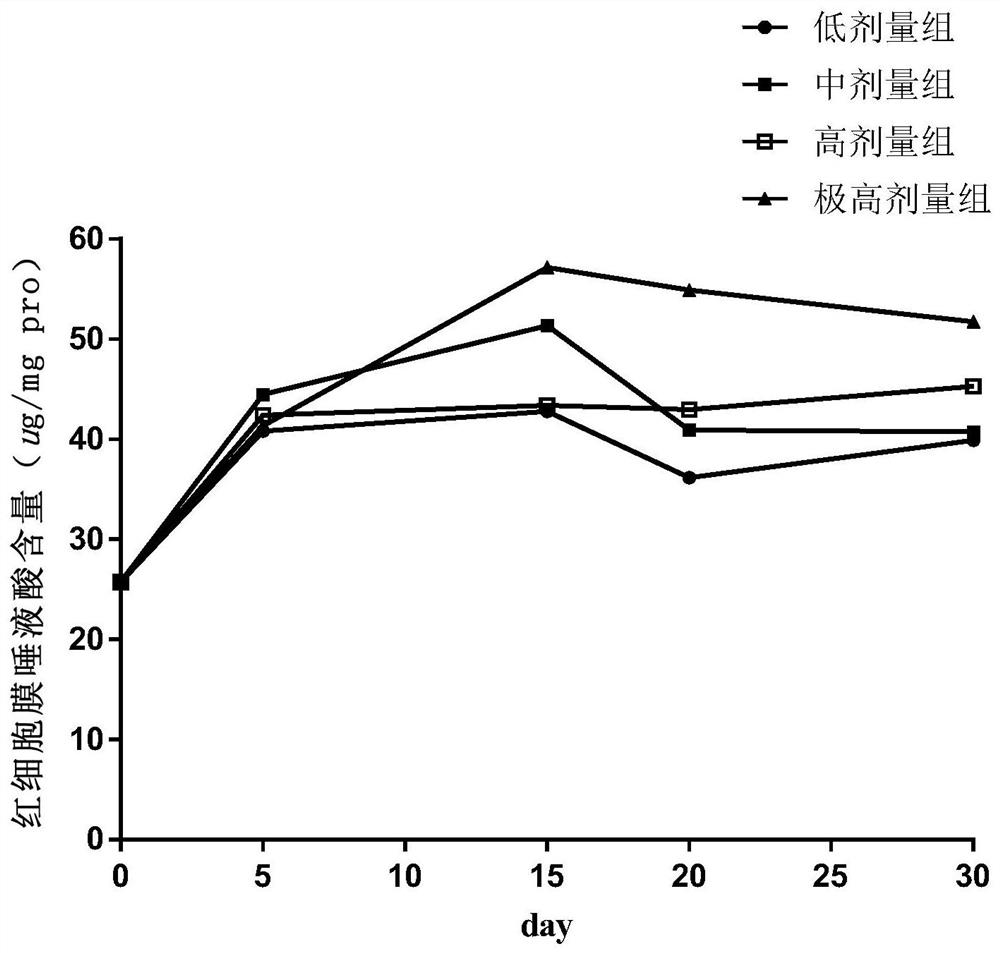
Food nutrition absorption and utilization evaluation method taking sialic acid as marker and application
The invention discloses a food nutrition absorption and utilization evaluation method taking sialic acid as a marker and application, and relates to detection and evaluation of food nutritional ingredients. The food nutrition absorption and utilization evaluation method comprises the following steps of: 1) dividing male ICR mice into four dose groups, namely a low dose group, a medium dose group,a high dose group and an extremely high dose group, and respectively and continuously carrying out intragastric administration on the mice; 2) collecting blood at different time points, separating theblood into plasma, precipitating plasma protein, and extracting an erythrocyte membrane; 3) detecting the content of free sialic acid, erythrocyte membrane sialic acid and protein-bound sialic acid in mouse blood; and 4) drawing a dose time concentration curve, and constructing an ordinary differential equation model of sialic acid in-vivo metabolism. An ordinary differential equation can be usedfor describing a simple and linear in-vivo metabolic process in pharmacokinetics, and a dynamic process and a change rule among studies are disclosed by establishing a relationship among variables. The food nutrition absorption and utilization evaluation method simplifies biological complexity, and can be used for evaluating and predicting the absorption and utilization of the sialic acid-containing food.
Owner:厦门市燕之屋丝浓食品有限公司
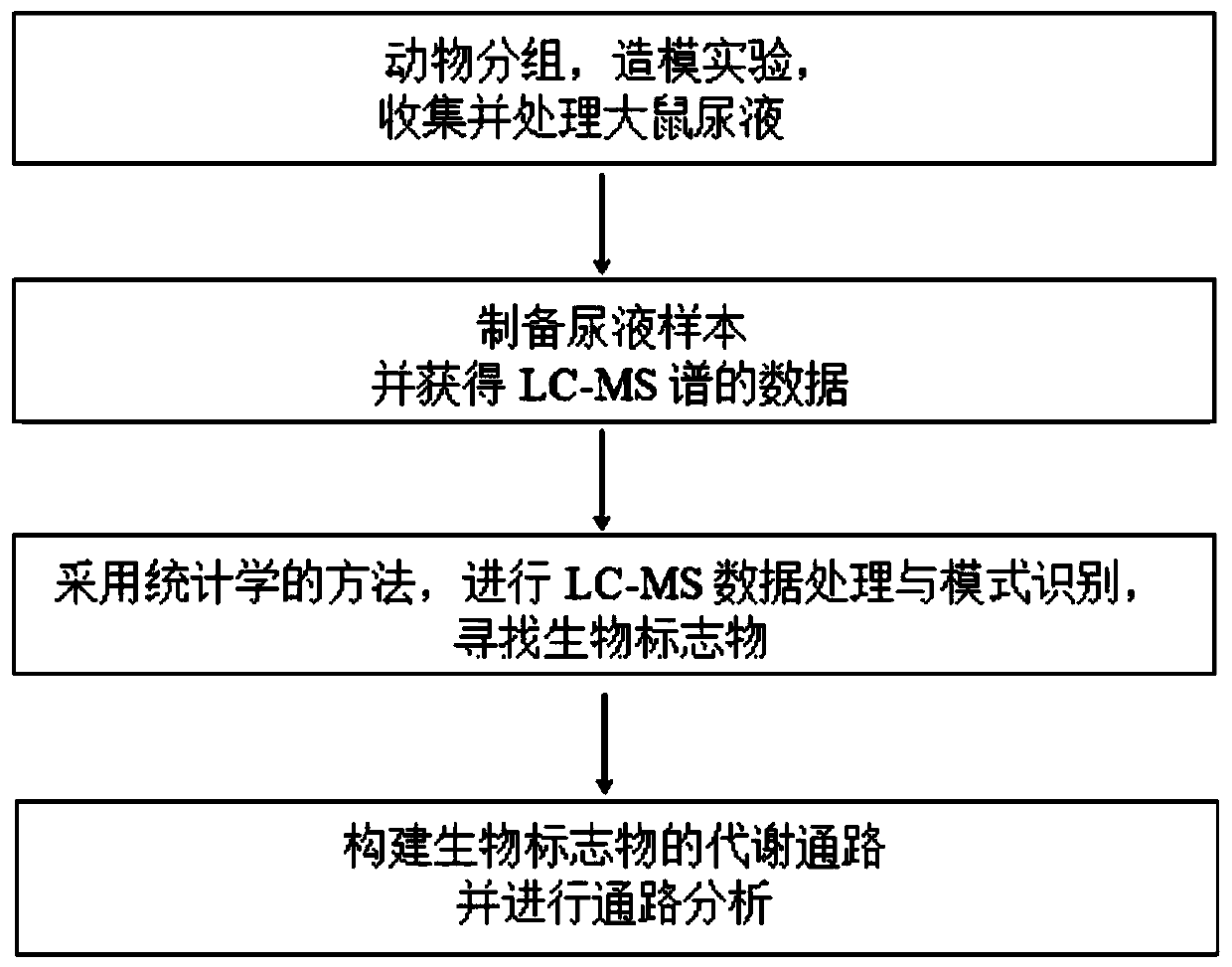
A method for constructing a rat model for anti-oxidative aging drug screening based on metabolomics analysis
The invention discloses a constructing method of a rat model for screening drugs for resisting oxidative aging based on metabolomics analysis. The constructing method comprises the following steps: detecting and analyzing a collected urine sample by adopting an LC-MS (Liquid Chromatography-Mass Spectrometry) spectrum; carrying out clustering analysis on rat urine metabolites from six groups: a normal control group, a model group, a positive control group, an ellagic acid high dose group, a medium dose group and a low dose group by adopting PCA (Principal Component), PLS (Partial Least Square)-DA (Discriminant Analysis) and OPLS-DA (Orthogonal Partial Least Square-Discriminant Analysis) and other technologies, and screening out a potential biomarker; carrying out signal pathway analysis by applying a KEGG (Kyoto Encyclopedia of Genes and Genomes) database; annotating a metabolite molecule and analyzing related enzyme or transport protein and related properties thereof by using an HMDB (Human Metabolome Database); carrying out visual mapping on a metabolic pathway by Met PA network software; constructing the metabolic pathway of rat urine metabolomics for improving D-type galactose-induced aging by ellagic acid; and providing a metabolomics idea for screening, evaluating, researching and developing new drugs for resisting the oxidative aging.
Owner:WUHAN UNIV
Traditional Chinese medicine cancer toxin prescription for treating liver cancer and application thereof in pharmacy
ActiveCN102357202BInduced growthInhibit expressionAnthropod material medical ingredientsAntineoplastic agentsAnticarcinogenic EffectOncology
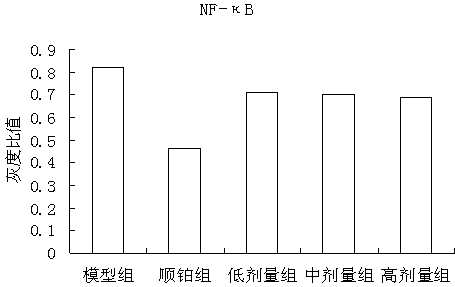
The invention discloses a traditional Chinese medicine cancer toxin prescription for treating liver cancer and application thereof in the preparation of medicaments for resisting liver cancer. The cancer toxin prescription is prepared in accordance with the cancer toxin theory proposed by traditional Chinese medicine master Professor Zhou Zhongying, and is prepared from hedyotidis herba, muscardine silkworm, centipede, akebia fruit, pseudostellaria root, dwarf lilyturf root and rhizoma pleionis according to a certain weight ratio. The prescription has the main functions of eliminating pathogens, eliminating cancer and detoxicating and the auxiliary function of strengthening healthy qi. By using mice with liver cancer H22 transplantation tumor as materials, the pharmacological experiment result shows the cancer resistance effect of the cancer toxin prescription: the tumor inhibition rates of a low-dose group, an intermediate-dose group and a high-dose group are respectively 24.5%, 42.8% and 21.1%, thereby showing the effect of inhibiting the growth of H22 tumor mass; the tumor mass cell apoptosis rates of the low-dose group, the intermediate-dose group and the high-dose group are respectively 47.16%, 60.52% and 57.59%, thereby showing the effect of inducing tumor cells to die; and the cancer toxin prescription can block the high expression of TLR4, block the MyD88 dependent pathway of TLR4 intracellular signal transduction and inhibit the expression of downstream NF-kB proteins. Experiment shows that the cancer toxin prescription can be used for preparing medicaments for resisting liver cancer.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
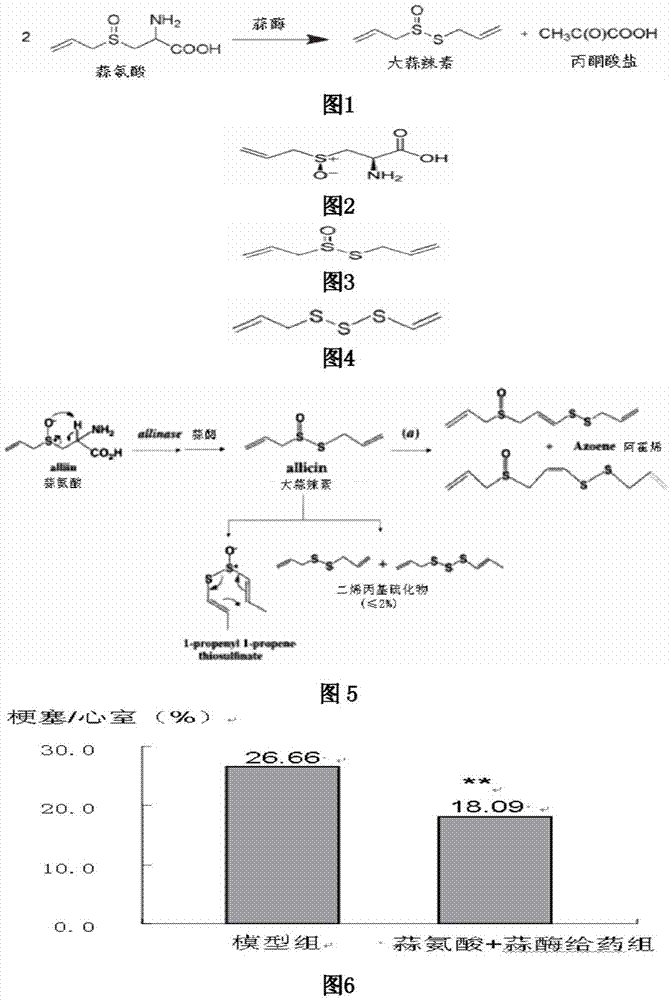
Application of allicin multi-layer enteric-coated tablets as medicine for the treatment of myocardial infarction
ActiveCN104491849BSignificant dose-dependent relationshipEffective controlOrganic active ingredientsPeptide/protein ingredientsDiseaseDrug treatment
Owner:XINJIANG AILEXIN PHARMA
Experimental method of Penthorum chinense Pursh on effect of normal sexual functions of male rats
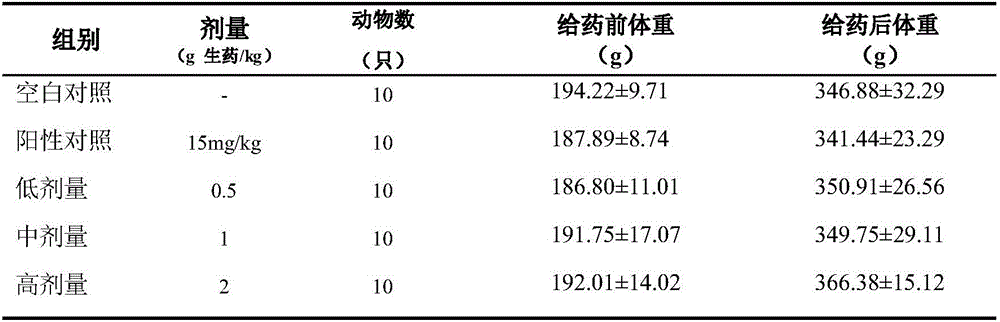
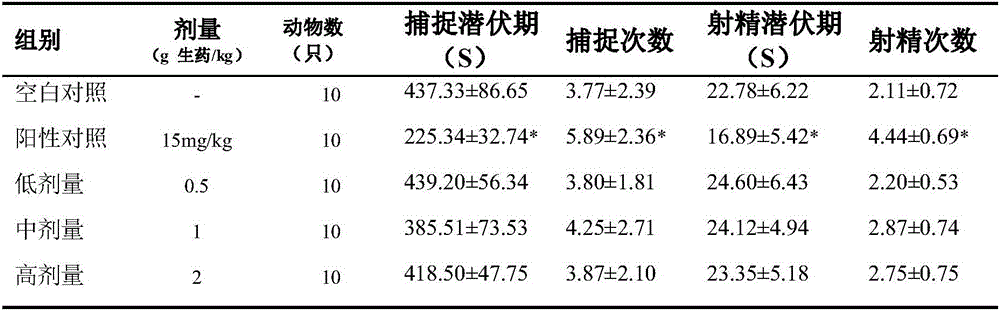
InactiveCN105833297AImprove mating abilityMating ability as usualCompounds screening/testingPenisPositive control
The invention discloses an experimental method for the influence of C. chinensis on the normal sexual function of male rats. The rats are randomly divided into a blank control group, a positive control group of 15 mg / kg Viagra and three dosage groups of C. kg, 1.0g crude drug / kg, 2.0g crude drug / kg, continuous gavage for 14 days, the test was carried out to determine the effect of C. chinensis on the mating ability of rats, the weight of sexual organs, the erectile function of penis, and the sperm of normal male rats. The research results of the present invention suggest that Viagra may improve the mating ability of male rats, but three doses of C. chinensis were administered orally for 28 days continuously, and the behavior of the animals was as usual, and the general condition was good. The mating ability, sexual organ index, penile erectile function, and sperm survival rate of animals in each experimental group had no obvious promoting or inhibiting effects, and there was no significant difference compared with the blank control group. Therefore, Chahuangcao has no obvious effect on the normal sexual function of male rats, and it is safe for long-term application.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Disposable quantitative dosing group cup
Owner:张健 +1
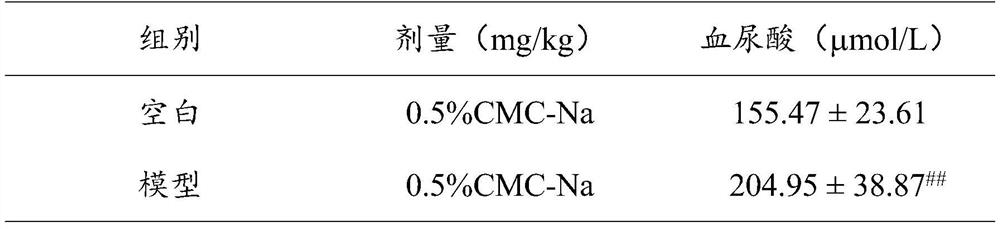
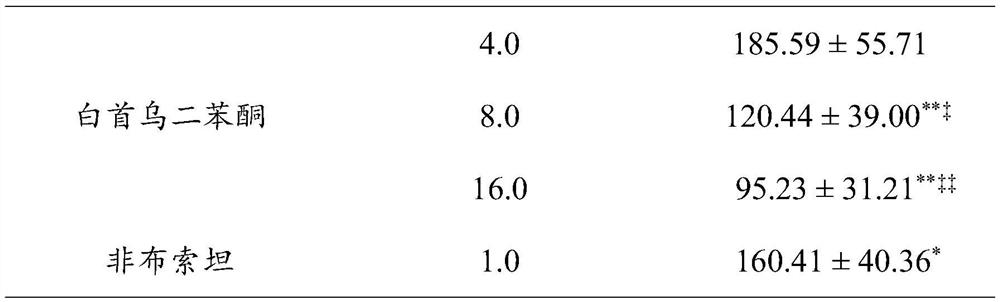
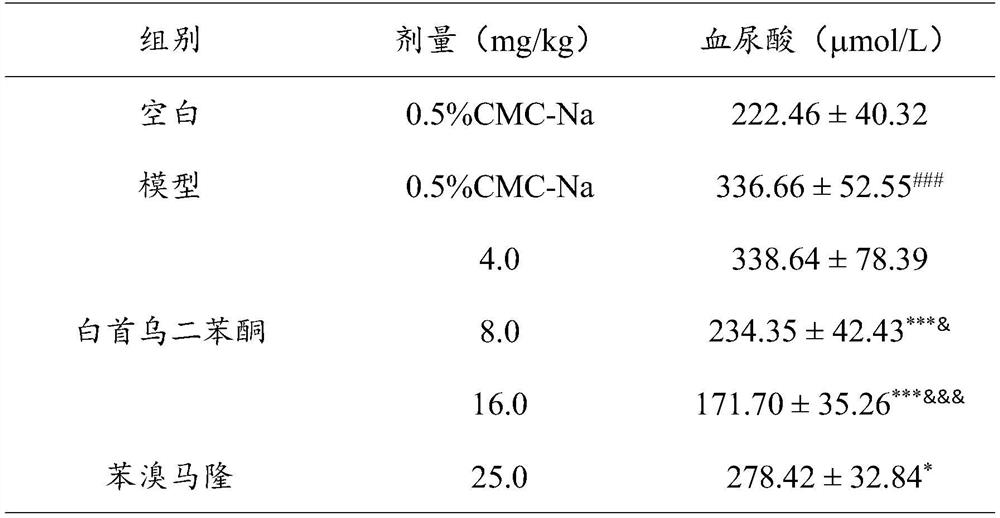
Application of Radix Polygoni Multiflori Benzophenone in the Preparation of Uric Acid-lowering Drugs
ActiveCN112807292BLower blood uric acid levelsGood effect of lowering uric acidOrganic active ingredientsSkeletal disorderPotassium oxonateBenzbromarone
The invention provides the application of Radix Polygonum benzophenone in the preparation of uric acid-lowering medicine, which belongs to the technical field of medicine. The test results showed that Baishouwu benzophenone could significantly reduce the blood uric acid level in mice with hyperuricemia induced by potassium p-oxonate, and its potency was stronger than that of the positive drug febuxostat 1mg / kg dose group, the difference was significant. Statistically significant; at the same time, the 8.0mg / kg and 16.0mg / kg dose groups of Radix Polygonum benzophenone can significantly reduce the blood uric acid level of mice with uric acid-induced hyperuricemia, and there is a certain dose-dependence, Compared with the positive drug, its potency is stronger than that of the positive drug benzbromarone 25mg / kg dose group, and the difference is statistically significant. The application of the Radix Polygoni Multiflori benzophenone provided in the present invention in the preparation of uric acid-lowering medicines has achieved good uric acid-lowering effects and is safe and has no sequelae.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com