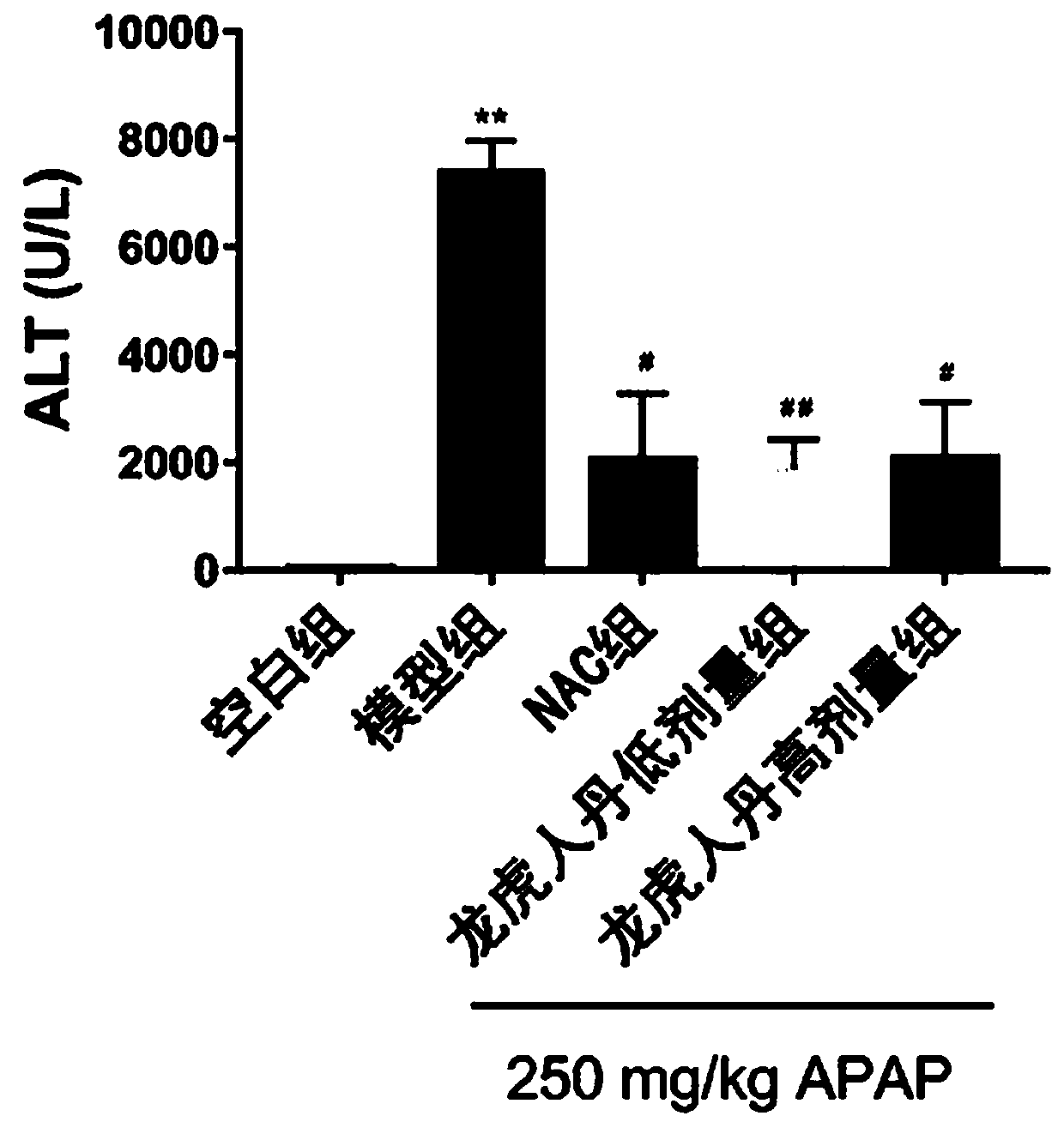
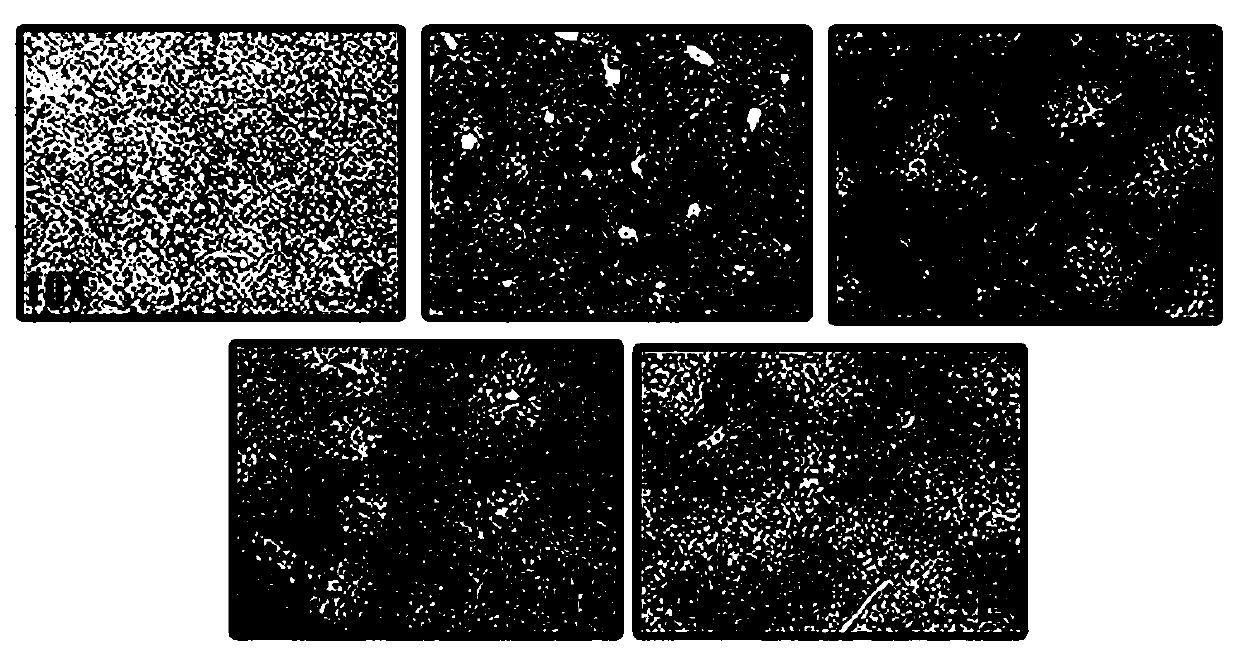
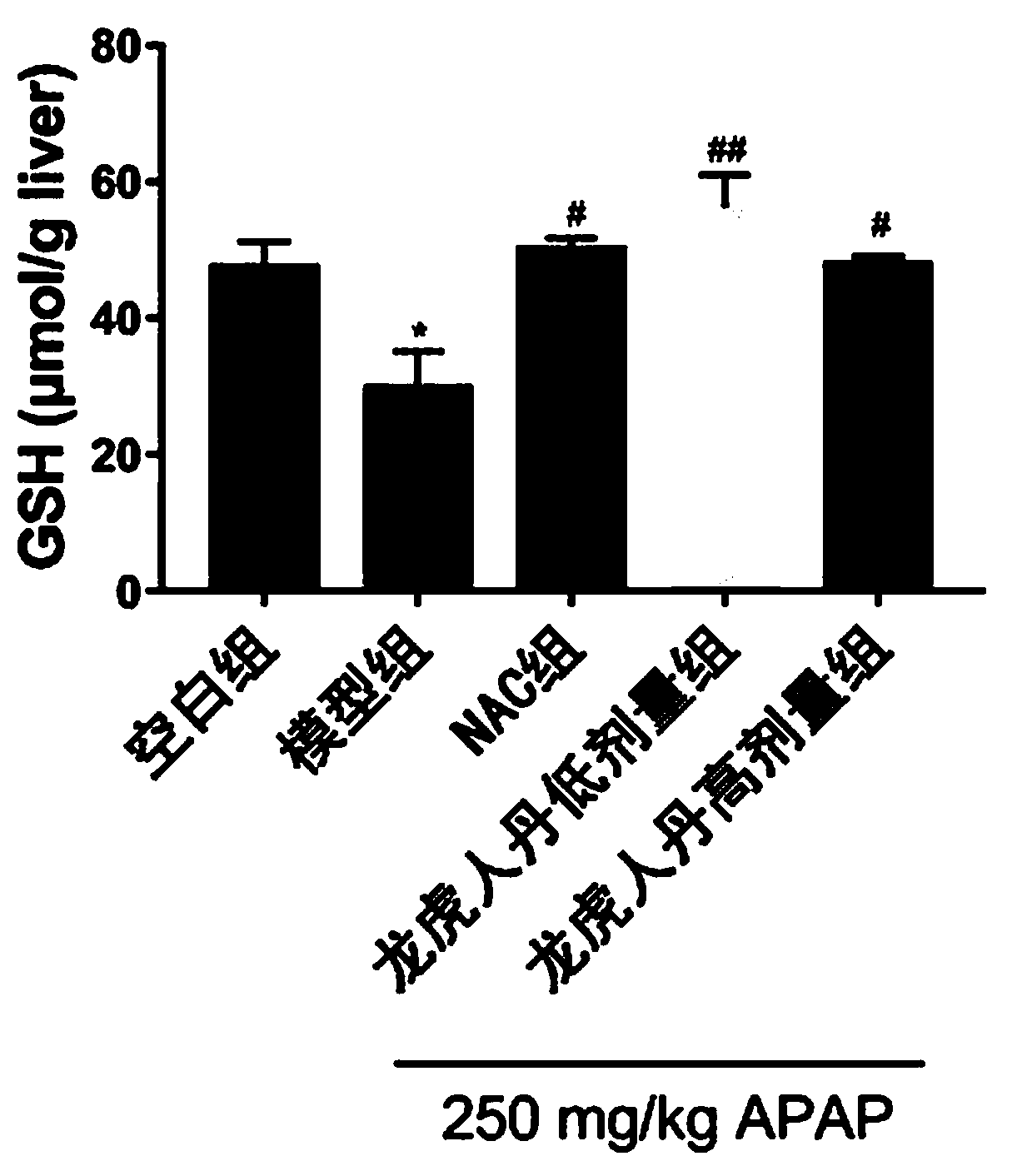
Application of Long Hu Ren Dan in prevention and treatment of drug-induced liver injury
A technology for drug-induced liver injury and acute liver injury, applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve problems such as restrictions on wide-scale application, scarcity of donors, and time urgency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] Specific experimental process:
[0015] 1. Animals
[0016] Thirty healthy SPF-grade C57BL / 6 male mice, 6-8 weeks old, were provided by the Guangdong Experimental Animal Center, animal production license number: SCXK (Guangdong) 2018-0034, experimental unit use license number: SYXK ( ) 2017-0125. 6 birds / cage, group breeding, free access to water and food.
[0017] 2. Drugs
[0018] Longhu Rendan (produced by Shanghai Zhonghua Pharmaceutical Co., Ltd.), acetaminophen, NAC (produced by Shanghai McLean Biochemical Technology Co., Ltd.), carboxymethylcellulose sodium (CMC-Na, Sangon Bioengineering (Shanghai) Co., Ltd. Co., Ltd.), PBS powder (Beijing Dingguo Changsheng Biotechnology Co., Ltd.).
[0019] 3. Main reagents and instruments
[0020] Anti-GCLC (PROTEINTECH GROUP), Anti-EndoG (Affinity Biosciences), Anti-β-actin (Beijing Quanshijin Biotechnology Co., Ltd.), ALT detection kit, GSH detection kit (Nanjing Jiancheng Bioengineering Research Institute Co., Ltd. ), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com