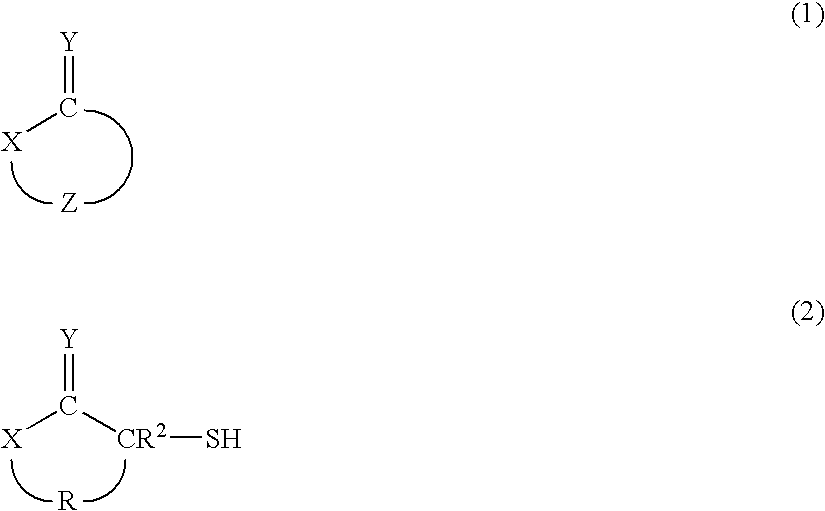Patents
Literature
323 results about "Acetylcysteine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
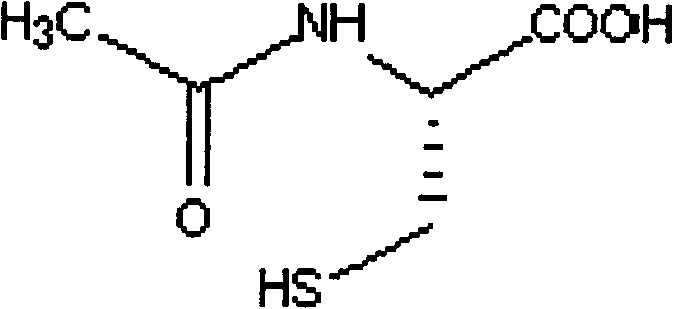
This medication is used to prevent liver damage from acetaminophen overdose..
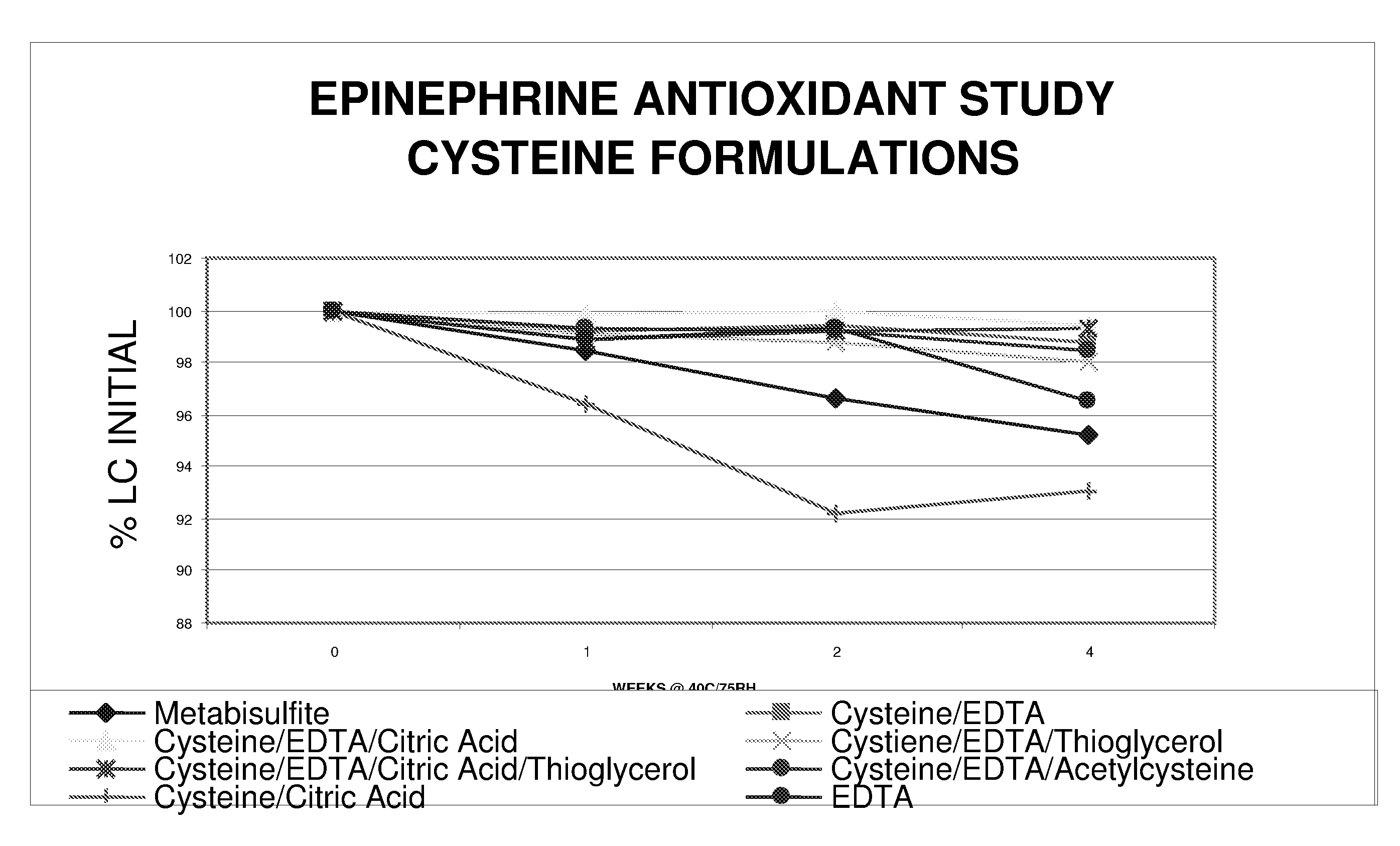
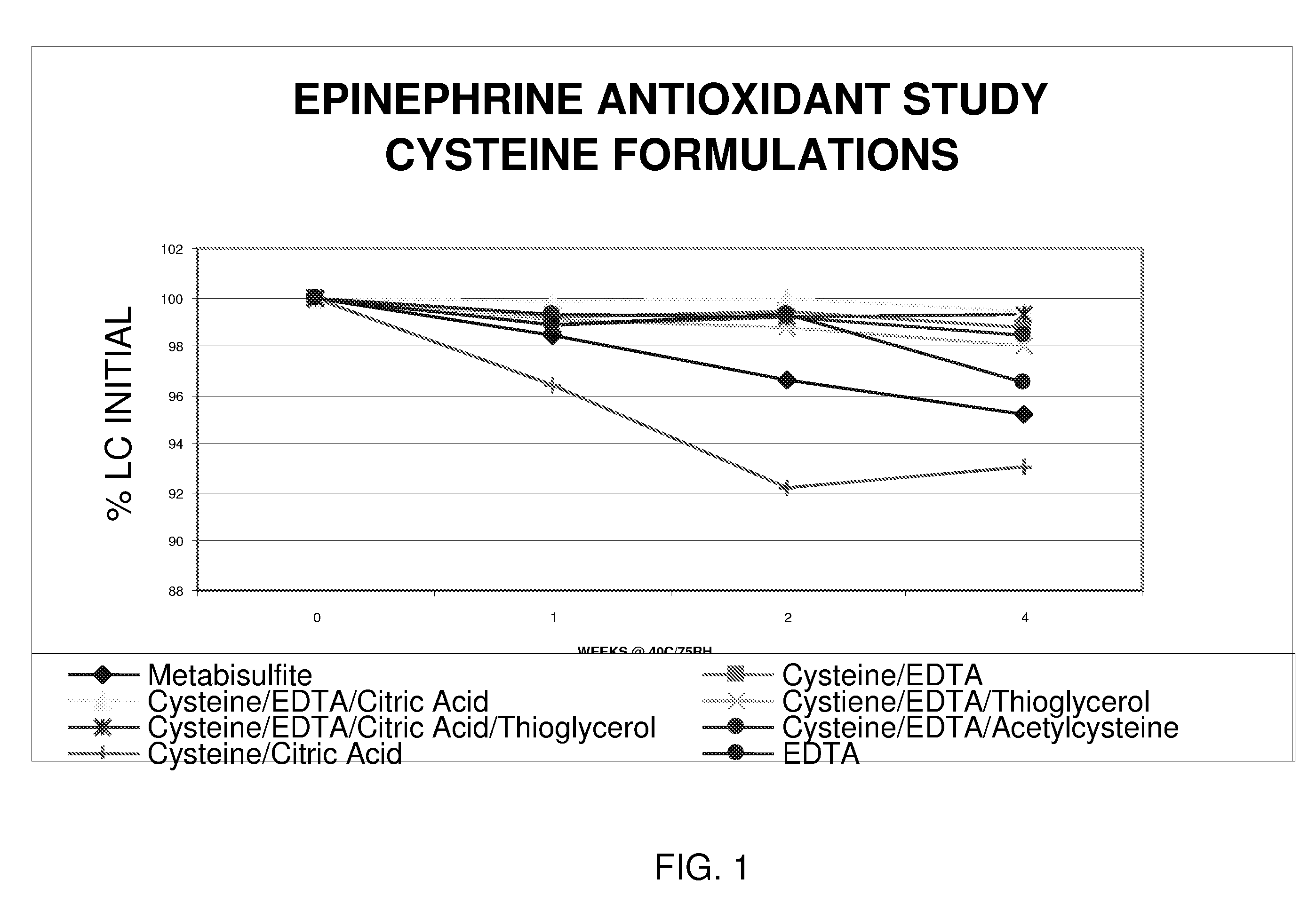
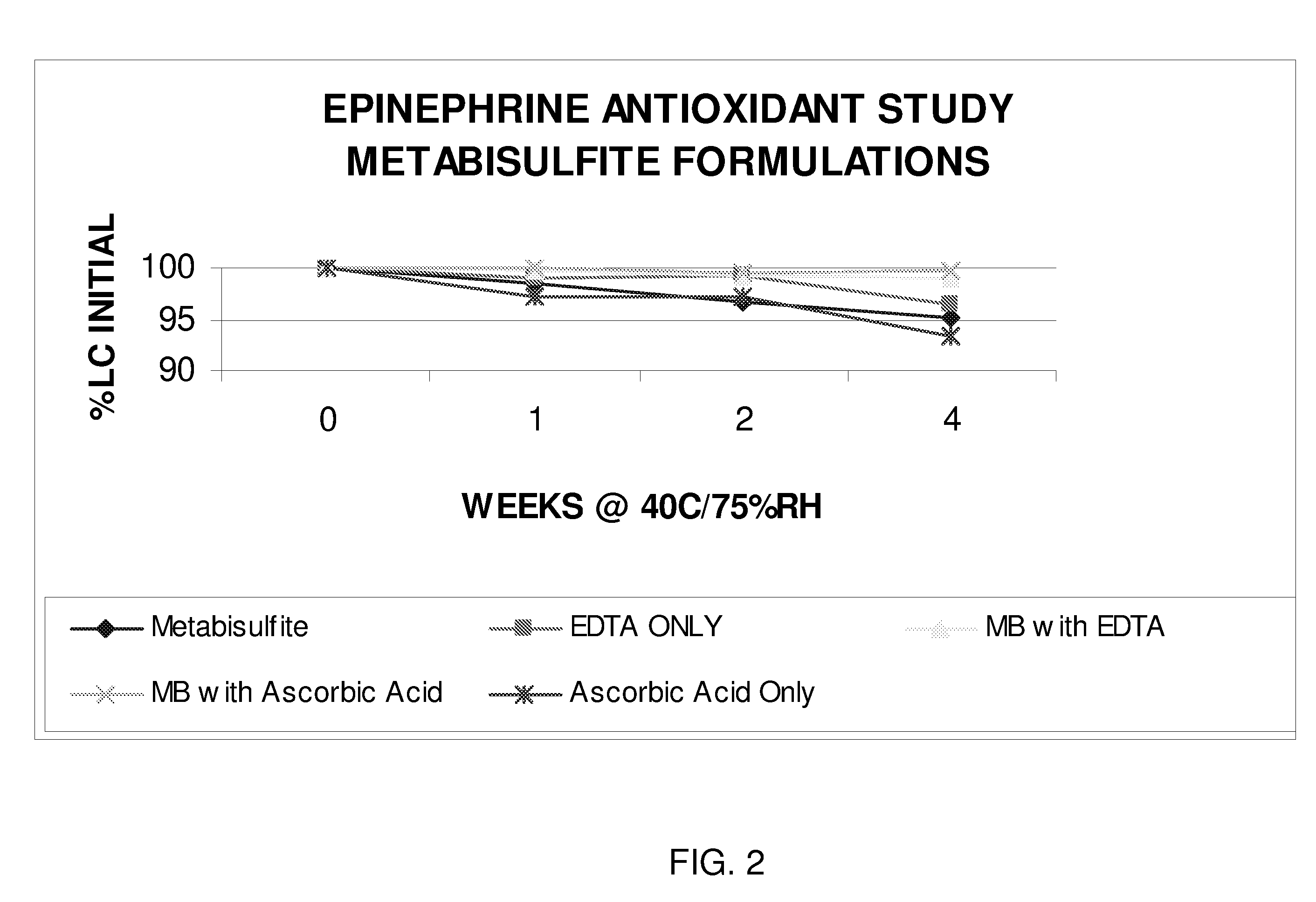
Epinephrine formulations
InactiveUS20080269347A1Improve stabilityBiocideOrganic active ingredientsCardiorespiratory arrestAntioxidant
The present invention generally concerns an epinephrine formulation that has enhanced stability. In particular embodiments, the formulation is an injectable formulation. In specific aspects, the formulation comprises epinephrine, EDTA, and one or more of an antioxidant such as cysteine, citric acid, acetylcysteine, or thioglycerol. The formulations are suitable for any medical condition that is in need of epinephrine, although in specific embodiments the medical condition is anaphylaxis, asthma, or cardiac arrest.
Owner:UNION SPRINGS PHARMA
Composition of amino acid
ActiveCN101049500AImprove metabolic disordersNutritional support therapy works wellOrganic active ingredientsDipeptide ingredientsNutrition supportTryptophan
An amino acid composition with high nutrition supporting effect is composed of acetylcysteine, acetyltyrosine and tryptophan in weight ratio of (0.81-2): (0.81-2): (0.9-1.6).
Owner:BEIJING SHIQIAO BIOPHAM
Andrographolide derivatives and application of the same in pharmacy
The invention relates to an andrographolide derivative, which has the structure as shown in a general formula I: wherein, R1, R2 and R3 are the same or different hydrogen, substituted or non-substituted organic acid radical, inorganic acid radical, alkyl, aryl or heteroaryl, while at least one of R1, R2 and R3 is R-lipoic acid or S-lipoic acid or the mixture of R-lipoic acid and S-lipoic acid or the corresponding dihydrolipoic acid or acetylcysteine radical of R-lipoic acid or S-lipoic acid; the derivative has good anti-tumor effect, and the derivative can cause apoptosis of tumor cells, directly eliminate gram positive bacteria, staphylococcus aureus and sensitivities MRSA5676 and MRSA5677, inhibit the QS system of gram negative bacteria and pseudomonas aeruginosa and inhibit and damage the formation of the bio-film of pseudomonas aeruginosa; the product has prominent hypoplycemic effect and is suitable for the preparation of the medicines that can cure cancers, inflammations, diabetes mellitus and bacterial and viral infection.
Owner:JINAN UNIVERSITY
Herbal composition for treating hangovers
InactiveUS20080075710A1Alleviate and reduce effect of hangoverBiocidePeptide/protein ingredientsAdditive ingredientPotassium
An herbal composition is adapted to treat hangovers. In one embodiment, the herbal composition comprises potassium, thiamin (vitamin B-1), magnesium, silymarin and N-acetyl-L-cysteine (acetylcysteine), or any pharmaceutically acceptable salts thereof. In a method of treating hangovers, an herbal composition is provided that comprises potassium, thiamin, magnesium, silymarin and N-acetyl-L-cysteine. The herbal composition is placed in the body so as to alleviate or reduce the effects of a hangover.
Owner:LIQUID POTIONS
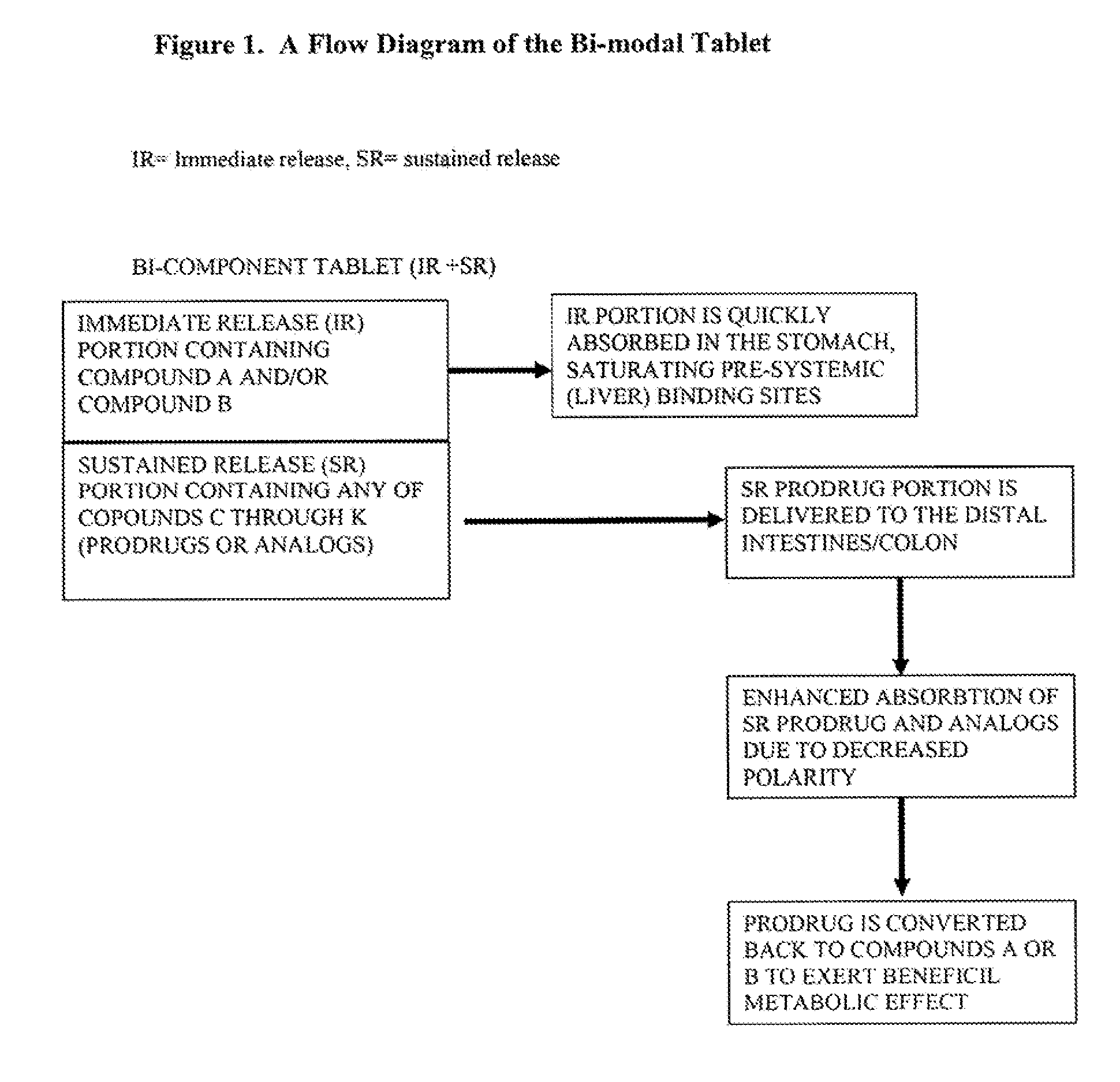
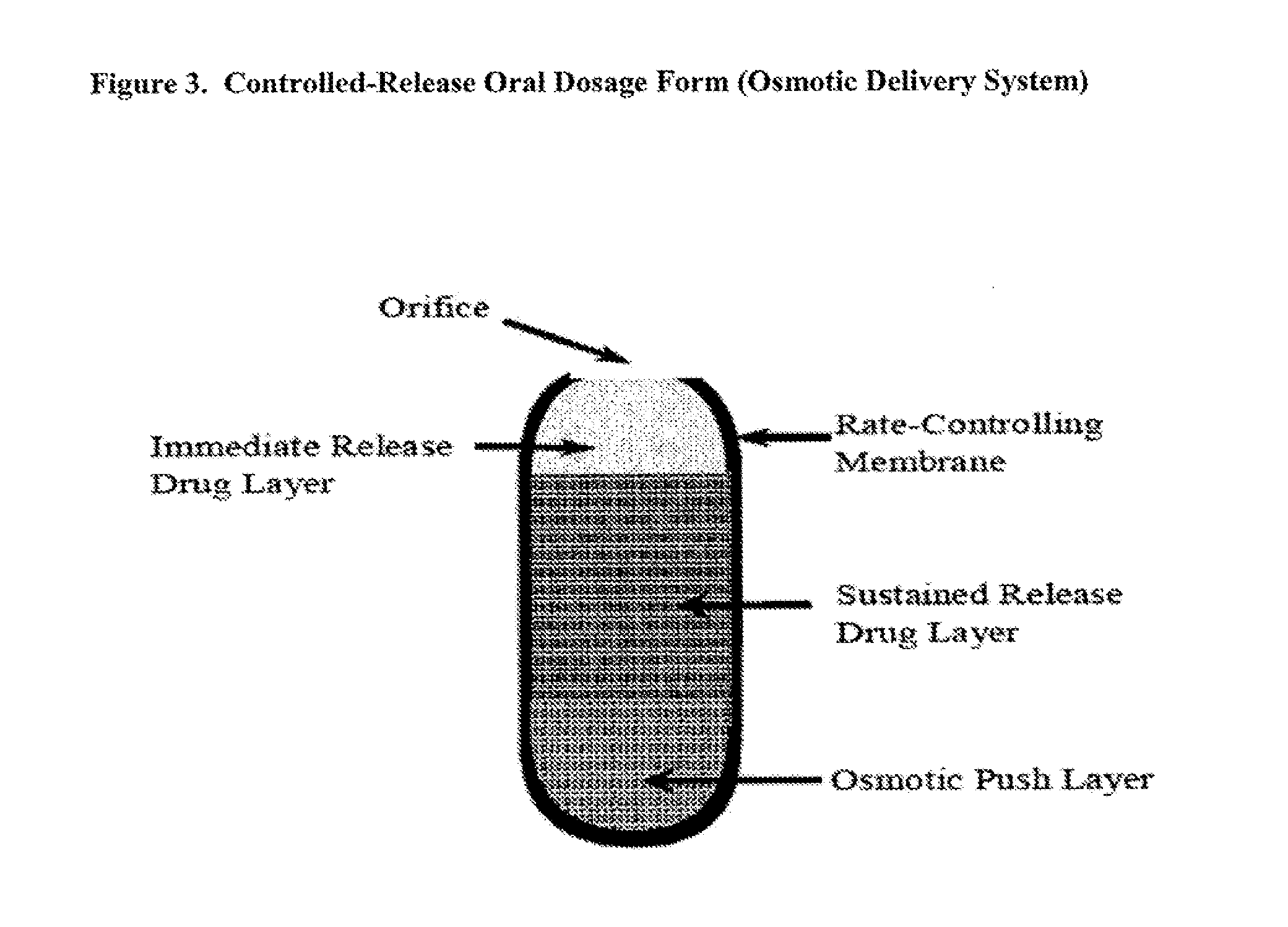
Controlled release of N-acetylcysteine (NAC) for reduction of systemic and/or vascular inflammation
The present invention provides a controlled-release composition which provides a therapeutically effective plasma concentration of N-acetylcysteine over prolonged period of time. The present invention also includes the use of the controlled-release composition, either alone or in combination with at least one additional active agent, for reduction of vascular inflammation marker and treatment of diseases, conditions, and / or symptoms associated with systemic and / or vascular inflammation in a patient. Furthermore, the present invention provides a process of making granules comprising N-acetylcysteine, or a salt, solvate, prodrug, and / or analog thereof.
Owner:TIARA PHARMA
Unifying mechanism and methods to prevent cancer and neurodegenerative diseases
InactiveUS20050164911A1Grow moreInhibition formationBiocideNervous disorderCancer preventionMetabolite
The present invention relates to methods for preventing the development of cancer or neurodegenerative diseases by administering N-Acetylcysteine (NAC), melatonin, or a combination thereof. The present invention also relates to methods for diagnosing cancer and / or neurdegenerative disease by detecting or determining the amount of dopamine metabolites, 4-CE, 2-CE, methylation of CE or CE-Q conjugates.
Owner:PREVENTION
N-Acetylcysteine Compositions and Methods for Treating Acute Exacerbations of Inflammatory Lung Disease
The present invention relates to N-acetylcysteine compositions and methods for treating inflammation and redox imbalance in acute exacerbations of inflammatory lung disease.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Culture media for hepatocyte culture and liver organ preparation
The invention provides a proliferation culture medium and differentiation culture medium for hepatocyte culture and liver organ preparation. The proliferation culture medium and the differentiation medium both take a culture medium for growth of mammalian cells as a basic culture medium, and an agent for supplementing L-glutamine, a pH value modifier for maintaining the pH values of the culture media stable, a primary cell culture antibiotic, a serum substitute, N-acetylcysteine, arbitrary nicotinamide and any one or more of a BMP inhibitor, a Wnt agonist, a growth factor, a Rock signaling pathway inhibitor, a P38 signal path inhibitor, a Notch signal path inhibitor, dexamethasone, BMP7 and a cAMP activator are added into the culture media. By using the culture media for culturing liver cells, functional liver organs can be obtained.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
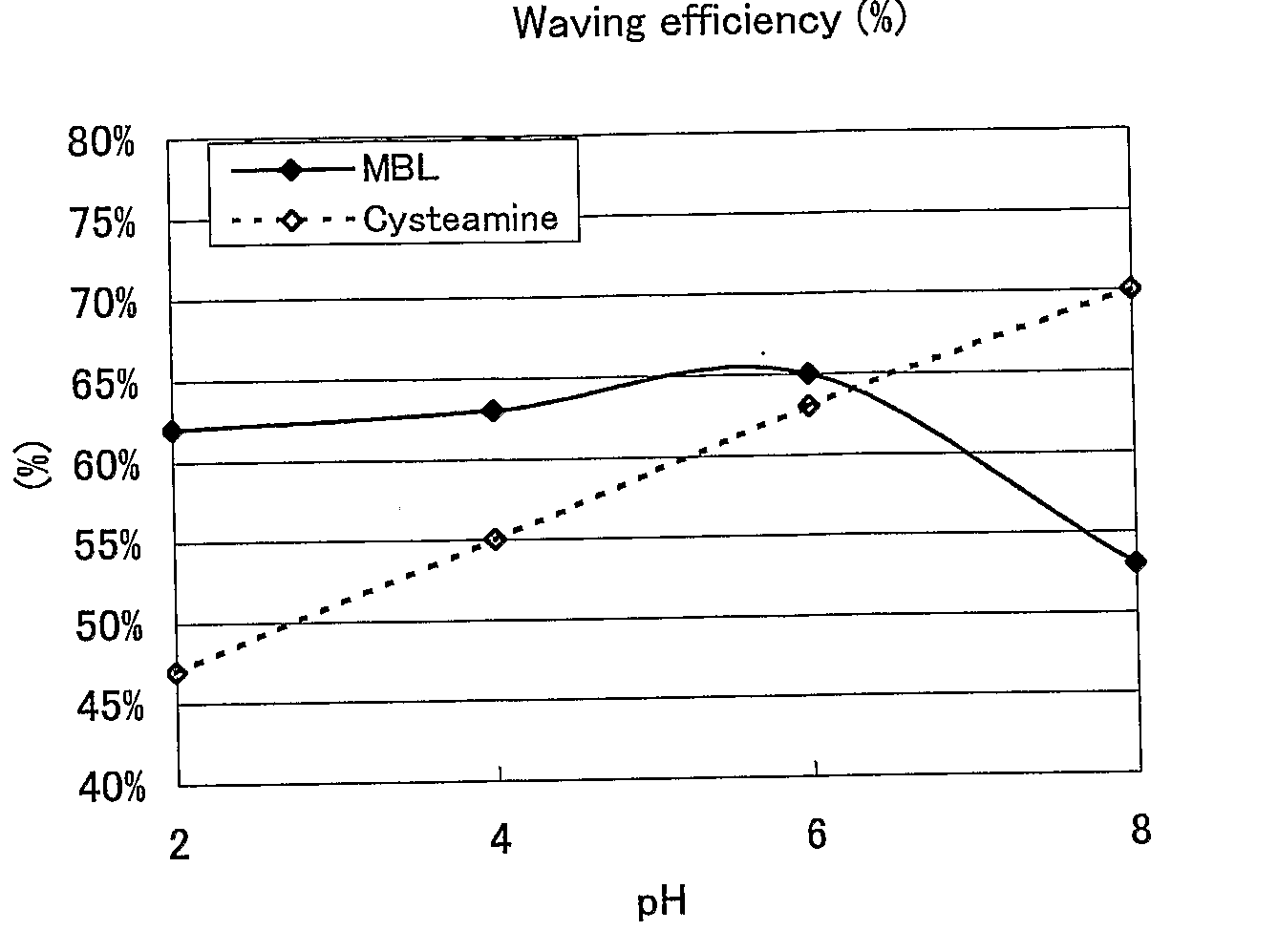
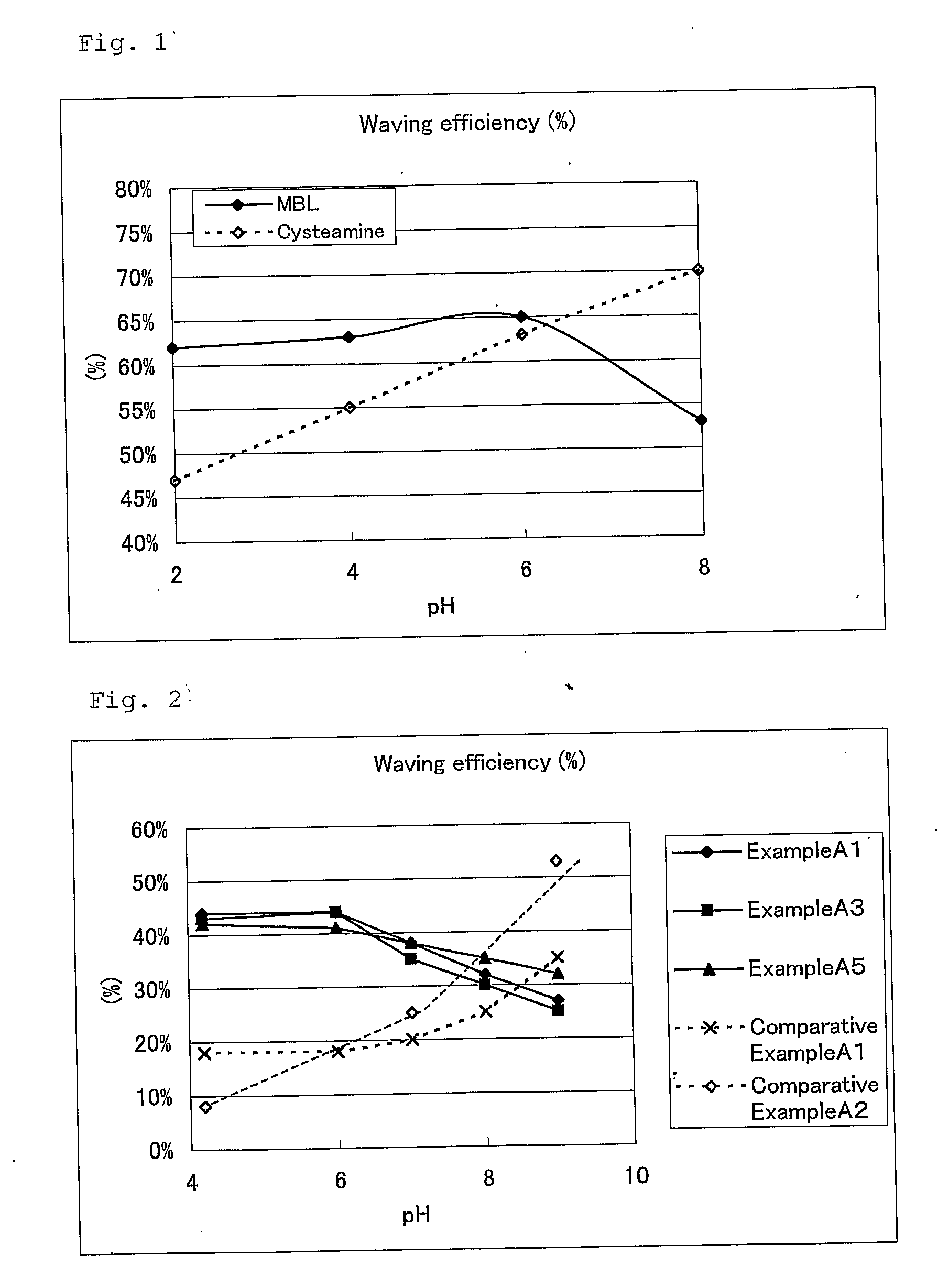
Hair Processing Agent And Method For Permanent Waving Hair
ActiveUS20080085251A1Improve performanceGood lookingCosmetic preparationsHair cosmeticsThiolactic acidCysteamine
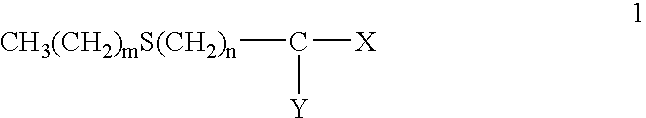
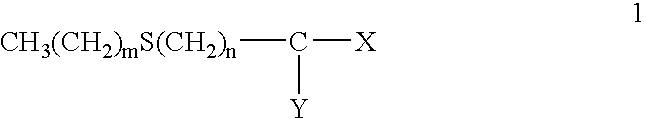
Provided are hair processing agents capable of permanent waving hair even at a neutral to weakly acidic pH range that causes less irritation to the skin, and hair processing agents in which an unpleasant odor is masked. Hair processing agents contain at least one compound represented by the formula (2). Hair processing agents contain a compound of the formula (2) and at least one compound (ii) selected from thioglycolic acid, thiolactic acid, cysteine, acetylcysteine, cysteamine, acylcysteamine, salts thereof and ester derivatives thereof. Hair processing agents contain a compound of the formula (2), a surfactant and water, and are emulsified. Hair processing agents contain a compound of the formula (2) and a specific perfume. wherein X is a structure selected from —O—, —S—, —NH— and —NR1—; R1 is an alkyl group of 1 to 6 carbon atoms; Y is an oxygen atom or a sulfur atom; in the formula (1), Z is a divalent organic residue having at least one mercapto group; in the formula (2), R is a divalent organic residue optionally having a mercapto group; and R2 is a hydrogen atom or an alkyl group of 1 to 6 carbon atoms.
Owner:RESONAC CORP
Specific culture medium for lung tumor organ and stentless 3D culturing method
ActiveCN110592022AStrong cell stemnessRetain heterogeneityCulture processCell culture active agentsY-27632HEPES
The invention discloses a specific culture medium for a lung tumor organ and a stentless 3D culturing method. The specific culture medium is prepared from the following components: FBS, double antibody, N-2, Noggin, B-27, EGF, FGF-10, Y-27632, A 83-01, SB202190, N-acetylcysteine, HEPES, Glutamax, IGF-1, hydrocortisone and Advanced DMEM / F12. The culturing method comprises the following steps: adding a tumor cell into a low serum culture medium, re-suspending the tumor cell, inoculating the tumor cell into a culture vessel, adding the specific culture medium into the culture vessel, changing thespecific culture medium once a day, and performing culturing until an organoid is formed. According to the culture medium and culturing method, a tumor organoid can quickly generate, can be stably cultured for a long time, is regular in spheroid form and has uniform and controllable size, and the heterogeneity of a tumor tissue of a patient can be well maintained in vitro.
Owner:浙江弘瑞医疗科技有限公司
Medicinal composition containing dimeticone/simethicone
InactiveCN101596181AImprove clarityImprove effectivenessOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseAcetylcysteine
The invention discloses a medicinal composition containing dimeticone / simethicone. The composition is mainly prepared from the following raw material medicaments in portion by weight: 1 to 1,000 portions of acetylcysteine or pharmaceutically acceptable salt thereof, and 1 to 500 portions of the dimeticone / simethicone. The composition can be prepared into various pharmaceutically acceptable preparations; and the composition can be applied to gastrointestinal endoscopy and treatment or an adjuvant drug administered before imaging examination, and compared with the acetylcysteine or the dimeticone or simethicone which is in single use at the same dosage, the composition has stronger defrothing effect, and stronger grume removal effect, so that the composition can effectively improve the visual definition of examination and treatment, reduce misdiagnosis and missed diagnosis, and improve effectiveness of diagnosis and treatment, contributes to early diagnosis discovery of digestive tract diseases, and has wide application prospect.
Owner:重庆健能医药开发有限公司
Compound amino acid injection composition suitable for liver disease patient
InactiveCN101120918AReduce contentIncrease contentDigestive systemPharmaceutical delivery mechanismAntioxidantArginine
The present invention comprises a compound amino acid injection and the preparation method. The characteristics of the injection are as follows. The prescription comprises most human essential amino acid and non essential amino acid. Content of branched chain amino acid (BCAA) in each amino acid is high, and content of the aromatic amino acid (AAA) is low. Content of arginine is high. The prescription comprises the acetylcysteine. The compound amino acid injection is prepared under strict control of aerobic environment, which makes the product in accordance with quality requirement without any antioxidant. The production is applicable in the patients with hepatic disease, which is in accordance with specific requirement for AAA restriction. No antioxidant is added in the prescription, which can effectively reduce incidence rate of adverse reaction in clinical application.
Owner:费森尤斯卡比华瑞制药有限公司
Differentiation of bone marrow cells into neuronal cells and uses therefor
The present invention relates to methods of inducing differentiation of mammalian bone marrow stromal cells into neuronal cells by contacting marrow stromal cells with a neuronal differentiation-inducing compounds. Neuronal differentiation-inducing compounds of the invention include anti-oxidants such as, but not limited to, beta-mercaptoethanol, dimethylsulfoxide, butylated hydroxyanisole, butylated hydroxytoluene, ascorbic acid, dimethylfumarate, and n-acetylcysteine. Once induced to differentiate into neuronal cells, the cells can be used for cell therapy, gene therapy, or both, for treatment of diseases, disorders, or conditions of the central nervous system.
Owner:PHILADELPHIA HEALTH & EDUCATION CORP +1
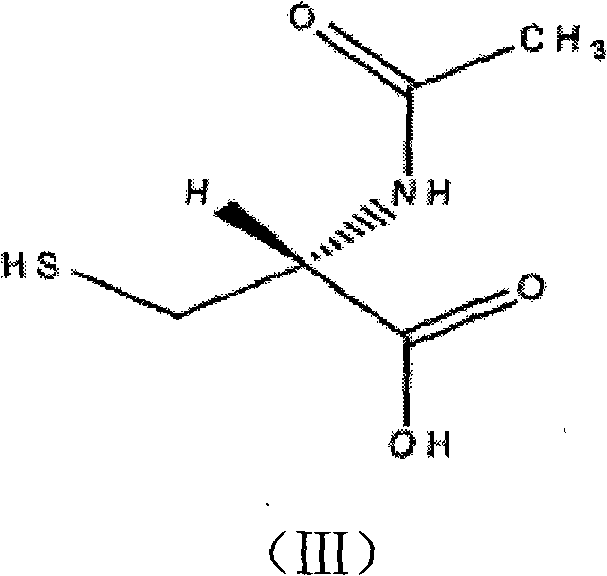
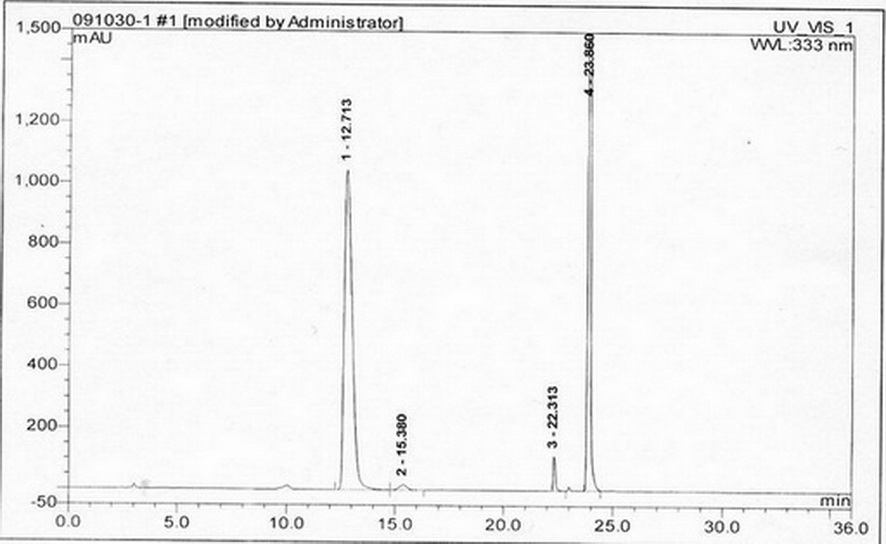
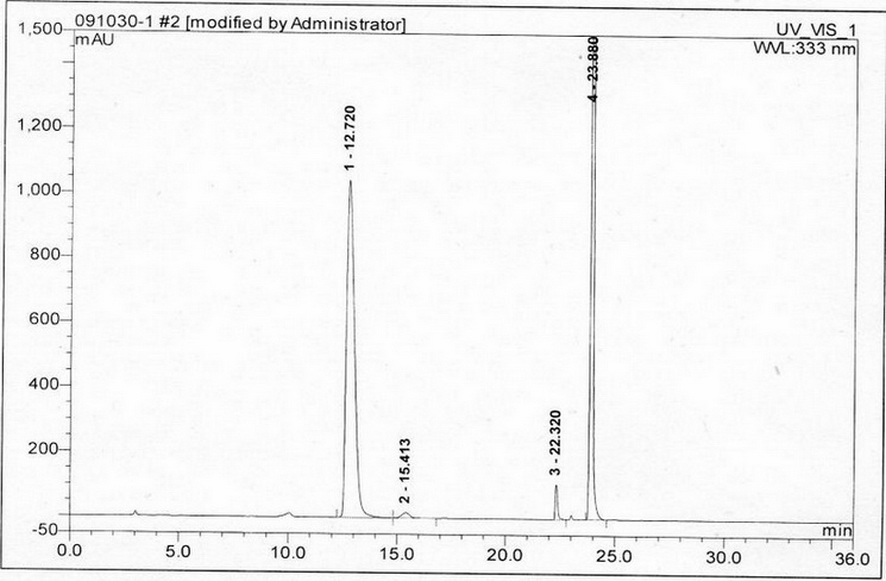
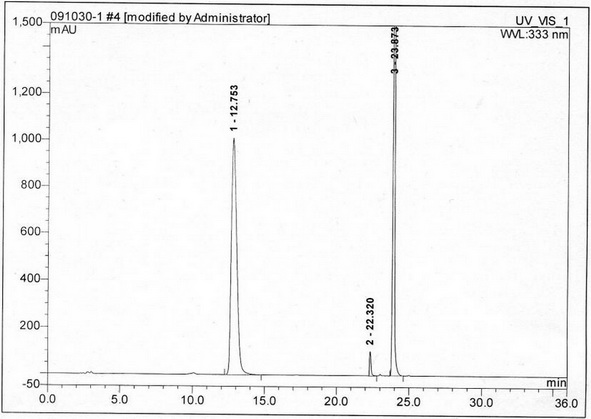
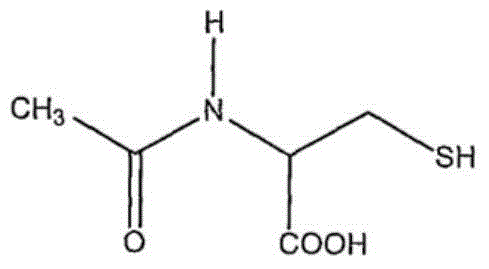
Method for separating and measuring acetylcysteine enantiomers
ActiveCN101968470AStrong UV Absorbing PropertiesEasy to measureComponent separationPhosphateEnantiomer
The invention provides a method for separating and measuring acetylcysteine enantiomers. In the method, before acetylcysteine is added in a chromatographic column, a derivatization reagent is used to perform derivatization, wherein the derivatization reagent is N(alpha)-(5-fluoro-2,4-dinitrophenyl)-L-amino acid compound. The method for separation and measurement combines the high performance liquid chromatography (HPLC) or high performance liquid chromatography-mass spectrum (HPLC-MS), and the used chromatographic column uses octadecylsilane chemically bonded silica as filler. The method for separation and measurement comprises the following steps: (1) taking acetylcysteine or a preparation with acetylcysteine, dissolving in low-concentration acid solution, adjusting the pH value of the mixed solution to 6.0-8.0 with alkaline solution to obtain a sample solution for testing; (2) mixing the acetylcysteine solution with 1mol / L of carbonate solution, adding N(alpha)-(5-fluoro-2,4-dinitrophenyl)-L-amino acid compound to mix evenly; (3) reacting the solution obtained by the step (2) at 40-60 DEG C in a dark place, adding hydrochloric acid solution after the reaction; (4) adding the solution obtained by the step (3) in a drying solution with the prestored phosphorus pentoxide and potassium hydroxide, adding phosphate buffer solution-acetonitrile, dissolving residues through ultrasonic treatment, filtering to obtain filtrate which is used as a testing solution; and (5) adopting HPLC or HPLC-MS to separate and measure the testing solution prepared by the step (4).
Owner:湖北新生源生物工程有限公司
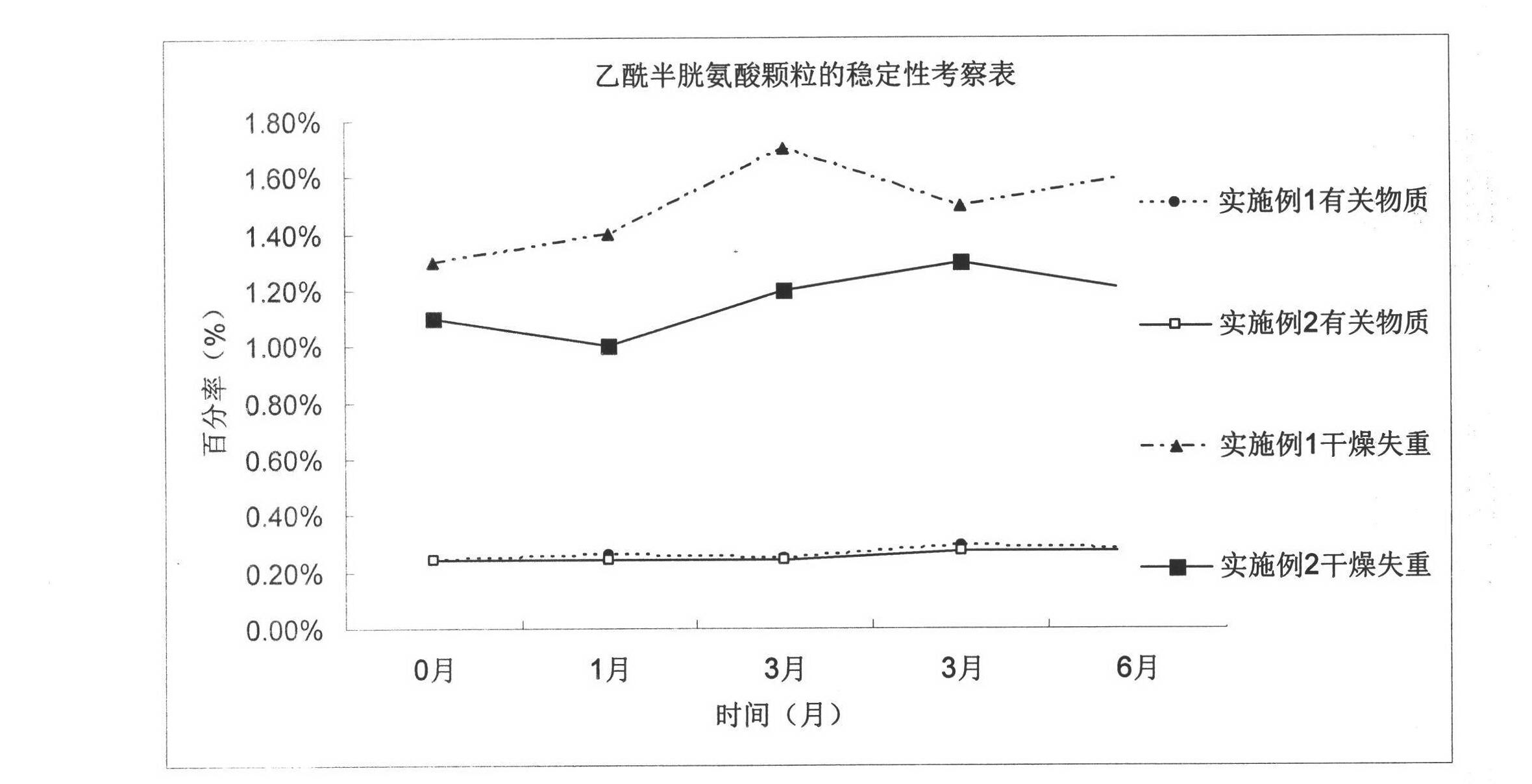
Acetylcysteine granule and preparation technology thereof
ActiveCN102144978AHumidity StableReduce adverse reactionsOrganic active ingredientsPharmaceutical non-active ingredientsMANNITOL/SORBITOLAcrylic resin
The invention discloses an acetylcysteine granule and a preparation method thereof, which belong to the new technical field of pharmaceutical preparation, and relates to a new composition of the acetylcysteine granule and the perpetration technology thereof. In the new composition and the preparation technology thereof, the granule mainly consists of acetylcysteine, acrylic resin, mannitol, aspartame and orange essence, and weight ratio of the main constituents is 10:1-50:2-200:0-1:0-1. After being coated with the acrylic resin, the acetylcysteine is mixed with the dry mannitol granule, the aspartame and the orange essence to prepare the acetylcysteine granule; and the prepared acetylcysteine granule has obviously improved stability in wet environment, and the taste of the acetylcysteine granule is greatly improved.
Owner:NANJING ZENKOM PHARMA
Acetylcysteine gargle for treating dental ulcers, and preparation method thereof
InactiveCN103027891ARich varietyPrevent ulcersOrganic active ingredientsDigestive systemOral ulcersEfficacy
The invention discloses an acetylcysteine gargle for treating dental ulcers, and a preparation method thereof. The preparation method comprises the following steps: cooling a small amount of purified water by mass percent; adding the acetylcysteine and then stirring and mixing; under a stirring action, regulating the pH value until the acetylcysteine is dissolved; adding a solution stabilizer, a corrigent and a corrosion remover and mixing; adding poloxamer and then stirring till dissolving; statically placing an obtained mixture; filtering the mixture; and adding the water to a filtrate, thereby obtaining the acetylcysteine gargle. The acetylcysteine gargle prepared by using the method has the functions of quickly relieving pain and preventing / treating the ulcers, and especially has the medicinal efficacy of inhibiting the continuous ulcer expansion and promoting the ulcer wound healing for patients accepting radiotherapy, chemotherapy and bone marrow transplantation. The method provided by the invention has the advantages of simple preparation process and easiness in industrial scale production realization. The acetylcysteine gargle provided by the invention has the advantages of high stability, single and clear active ingredients and good quality controllability, thereby enriching the drug category for adjuvant therapy of clinical cancer. Therefore, a good selection for the nursing service of the patients accepting the radiotherapy is provided.
Owner:TIANJIN KUNJIAN BIOLOGICAL PHARMA
Liquid foundation added with pearl powder
InactiveCN101695471AWhitening effect hasCosmetic preparationsBody powdersAdditive ingredientGlycerol
The invention discloses liquid foundation added with pearl powder. The liquid foundation consists of polydimethylsiloxane, hexadecanol / octadecanol, isononyl isononanoate, glycerol, pearl powder, isopropyl palmitate, butanediol, dimer diol carbonate, acetylcysteine / collagen amino acids, spermaceti stearyl alcohol / coconut-oil-based glucoside, acrylic acid (ester) / C10-30 alkanol acrylate crosslinked polymers, triethanolamine, phenoxyethanol, ferroferric oxide and deionized water which are heated, mixed and then homogenized. As nanometer pearl powder containing a plurality of amino acids and trace elements is added to pressed powder, the liquid foundation not only can improve complexion and cover flaws, but also keeps effective components in pearls from being destroyed so as to help to the absorption of the effective components in the pearls, provides skin with nutrient components, fades pigment, inhibits the generation of melanin, achieves the aim of really whitening the skin while resisting water and sweat, and has the characteristics of lasting make-up and easy make-up removal.
Owner:HAINAN JINGRUN PEARL BIOTECH
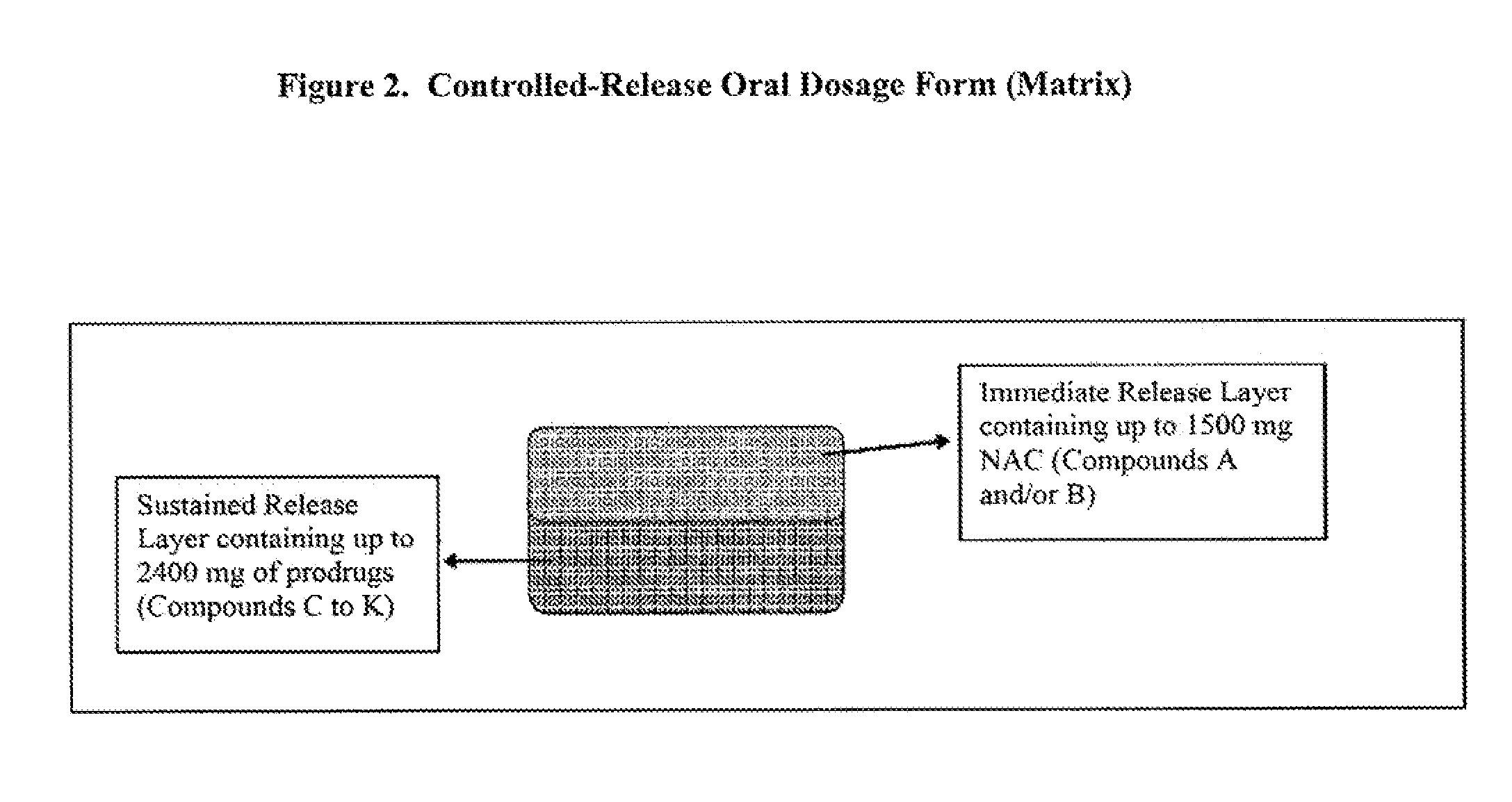
Oral formulations
InactiveUS20120093939A1Powder deliverySalicyclic acid active ingredientsLower Gastrointestinal TractImmediate release
Disclosed are pharmaceutical compositions comprising immediate release and sustained release formulations of 5-aminosalicylic acid, or a pharmaceutically acceptable salt or ester thereof, and / or N-acetylcysteine, or a pharmaceutically acceptable salt or ester thereof, for release in the lower gastrointestinal tract.
Owner:ALTHEUS THERAPEUTICS
Culture medium and culture method for colorectal cancer organoids
ActiveCN111394314ALow costReduce the risk of contaminationCell dissociation methodsCulture processPenicillinOncology
The present invention provides a culture medium and a culture method for colorectal cancer organoids. The culture medium comprises a basic culture medium Advanced DMEM / F12, specific additive factors and sterile water; wherein a mass ratio of the basic culture medium Advanced DMEM / F12 to the sterile water is 99:1; and the specific additive factors comprise B27 without vitamin A, N-acetylcysteine, EGF, Noggin, R-spondin 1, Wnt3a, CHIR99021, thiazovivin, Gastrin I, a penicillin-streptomycin mixture and Primocin. The culture medium is used to culture the colorectal cancer organoids, can maintain morphological structures and genetic characteristics of primary tissues, effectively reduces risks of microbial contamination in colorectal cancer culture, and improves success rate and survival rate of colorectal cancer organoid culture.
Owner:ACCURATE INT BIOTECHNOLOGY (GUANGZHOU) CO LTD
Method for separating and extracting human subcutaneous adipose-derived mesenchymal stem cells and special culture medium for extraction
InactiveCN102965337AHigh purityIncreased proliferationSkeletal/connective tissue cellsWater bathsPrimary cell
The invention discloses a method for separating and extracting human subcutaneous adipose-derived mesenchymal stem cells and a special culture medium for extraction. The method comprises the following steps of: cutting up the human subcutaneous adipose tissue, centrifuging at 4 DEG C to remove big adipose tissue, and adding mixed I type and II type collagenase for water-bath digestion; suspending with the special culture medium for extraction, and performing water bath at 37 DEG C; filtering the collected cells, adding the special culture medium for extraction, and culturing primary cells for 5-7 days; culturing the cells after primary culture with a common culture medium, and optionally performing continuous culture; and adding certain concentration of fetal bovine serum, recombinant fibroblast growth factor, epidermal growth factor, insulin, acetylcysteine and thyronine, wherein the special culture medium for extraction includes a low-sugar dulbecco's modified eagle's medium. Through the invention, a great quantity of adipose-derived mesenchymal stem cells can be extracted from subcutaneous adipose, and the intracellular stem cells subjected to primary culture have higher purity and stronger activity.
Owner:SOUTHEAST UNIV
Exfoliative cell sap base preserving fluid, method thereof for flaking and kit
ActiveCN104642300ASimple and fast operationEasy to prepareMicrobiological testing/measurementDead animal preservationPotassiumMonopotassium phosphate
The invention discloses an exfoliative cell sap base preserving fluid, a method thereof for flaking and a kit. The exfoliative cell sap preserving fluid comprises the following components by massic volume percentage: 3.8-4.2% of paraformaldehyde, 0.8-1.5% of N-methyl-N-cyclohexyl-2-amino-3,5-bromhexine, 0.8-1.5% of glacial acetic acid, 0.1-0.2% of acetylcysteine, 0.75-0.85% of sodium chloride, 0.01-0.03% of potassium chloride, 0.12-0.15% of disodium hydrogen phosphate, 0.025-0.03% of monopotassium phosphate, and the balance of water; and pH is regulated to 7.2-7.8. The exfoliative cell sap base preserving fluid is small in shrinking to the exfoliative cell, and suitable for storage for a long time; the cell coated by the mucus in the sample is successfully separated, the positive cell stacking and loss are avoided, and the effective cell number is improved; and the preserving fluid can be used for observing whether the occult blood symptom is existent.
Owner:SHANGHAI YU KANG HOSPITAL
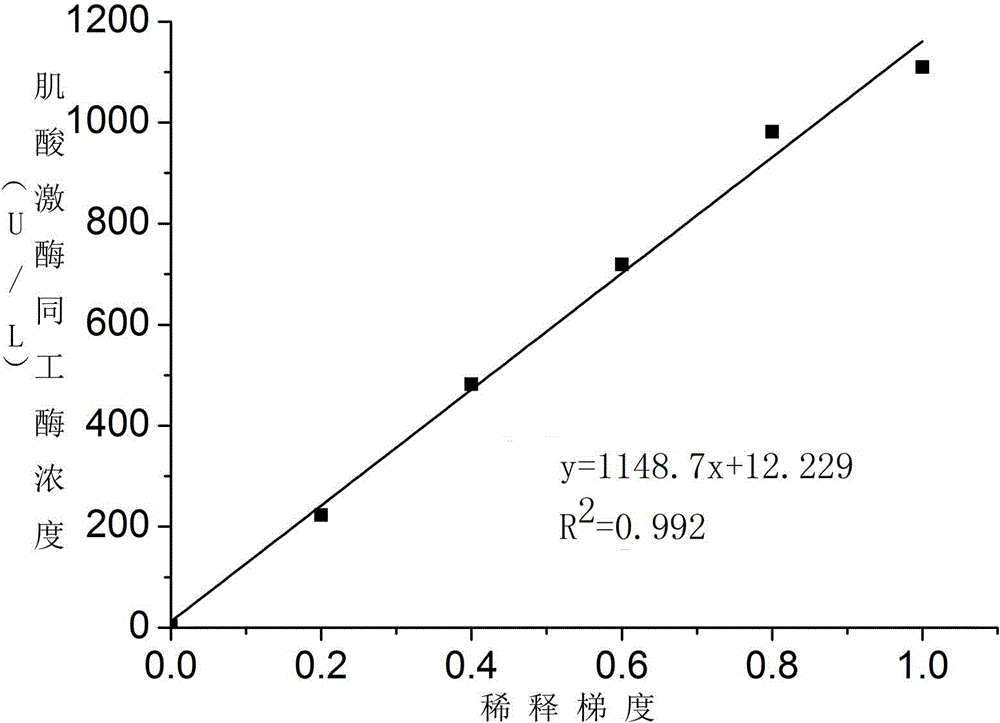
Creatine kinase isoenzyme detection reagent
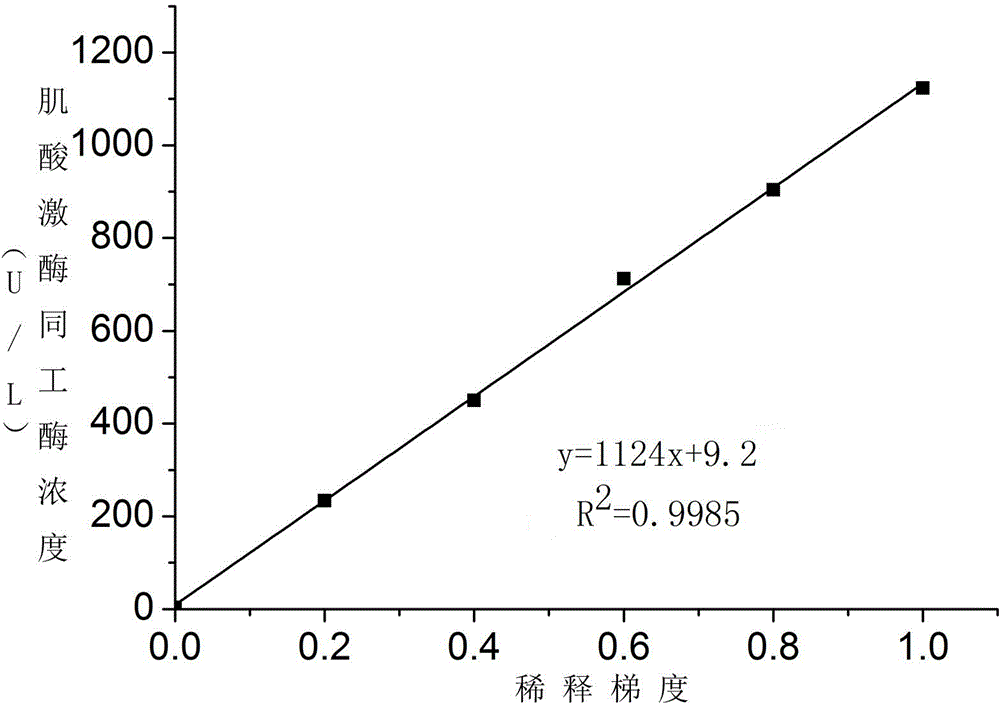
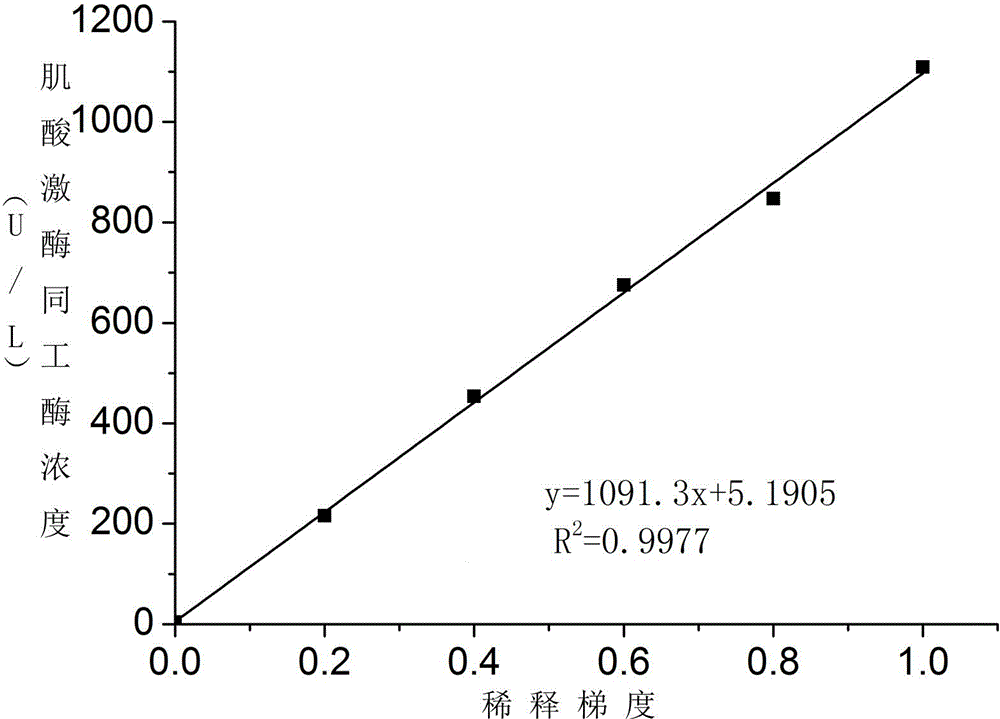
ActiveCN104374925AHigh activityEfficient removalBiological testingSodium acetateAntiendomysial antibodies
The invention discloses a creatine kinase isoenzyme detection reagent. The reagent consists of a reagent R1 and a reagent R2 according to the volume ratio of 4 to 1, wherein the reagent R1 consists of an imidazole buffer liquid, glucose, nano particles, N-acetylcysteine, sodium ethylene diamine tetracetate, adenosine diphosphate, cozymase I, ribonucleotide, pyruvate decarboxylase, glucose 6-phosphate dehydrogenase, hexokinase, a goat anti-human CK-M polyclonal antibody and trehalose; the reagent R2 consists of an imidazole buffer liquid, phosphocreatine, sodium dodecyl benzene sulfonate and a preservative. The reagent disclosed by the invention is a creatine kinase isoenzyme detection reagent which is stable, accurate, and high in anti-interference, and has very high clinical application value.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Culture medium for stomach cancer organs and culture method
ActiveCN111411083ALow costReduce the risk of contaminationCell dissociation methodsCulture processPenicillinValproic Acid
The invention provides a culture medium for stomach cancer organs and a culture method. The culture medium comprises a basic culture medium 1640, specific addition factors and sterile water, wherein the mass ratio of the basic culture medium 1640 to the sterile water is 99:1; and the specific addition factors comprise B27 without vitamin A, N-acetylcysteine, EGFs (epidermal growth factors), Noggin, R-spondin 1, Wnt3a, CHIR99021, thiazovivin, Gastrin I, valproic acid, a penicillin and streptomycin mixed liquid, amphotericin B and Primocin. The culture medium can be adopted to culture the stomach cancer organs, morphology structures and gene characteristics of primary tissue can be maintained, the risk of microorganism pollution in stomach cancer culture can be effectively reduced, and the success rate and the survival rate of stomach cancer organ culture can be increased.
Owner:ACCURATE INT BIOTECHNOLOGY (GUANGZHOU) CO LTD
Inhalant acetylcysteine solution and preparation method of inhalant acetylcysteine solution
ActiveCN104800854AGood quality assuranceImprove securityOrganic active ingredientsPharmaceutical delivery mechanismMedicineCysteic acid
The invention belongs to the field of a medicine preparation and relates to an inhalant acetylcysteine solution and a preparation method of the inhalant acetylcysteine solution. Physiological seawater is added into the solution, so that the sensitization of the acetylcysteine solution is effectively reduced.
Owner:WUHAN WUYAO SCI & TECH
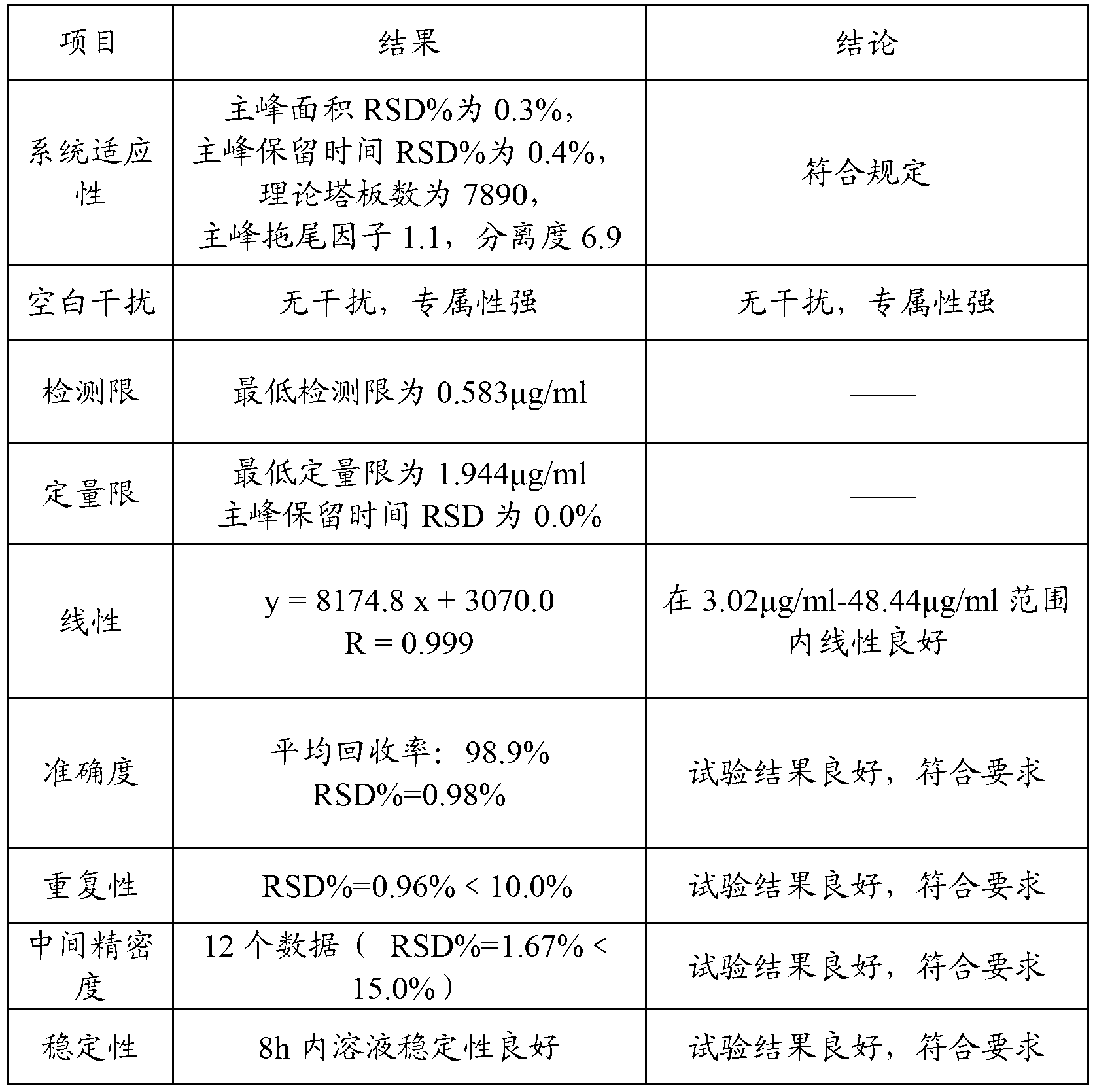
Method for detecting acetylcysteine and related substances thereof
InactiveCN101699281AGood acid and alkali toleranceGood practical valueComponent separationUltraviolet detectorsPhosphate
The invention relates to a method for detecting acetylcysteine and related substances thereof. The method adopts a high performance liquid chromatography, wherein the chromatographic condition is that styrene-divinylbenzene copolymer type superpolymer chromatographic column, namely MKF-RP-MH is 4 to 10 mu meters and (100-300)*(8.0-4.0) millimeters (I.D.); the volume ratio of phosphate buffer (pH is between 2 and 5) with mobile phase compositions of 0.01-0.05 mol / L to acetonitrile is 80-95:20-5; the flowing speed is between 0.8 and 1.2 mL / min; the detecting wavelength detected by an ultraviolet detector is 210nm; and the sample size is between 10 and 20 mu liters. The method does not use ionpairreagent, overcomes the defects that ODS column detecting method does not have acid resistance or low organic phase resistance when detecting the acetylcysteine.
Owner:NANJING UNIV OF TECH
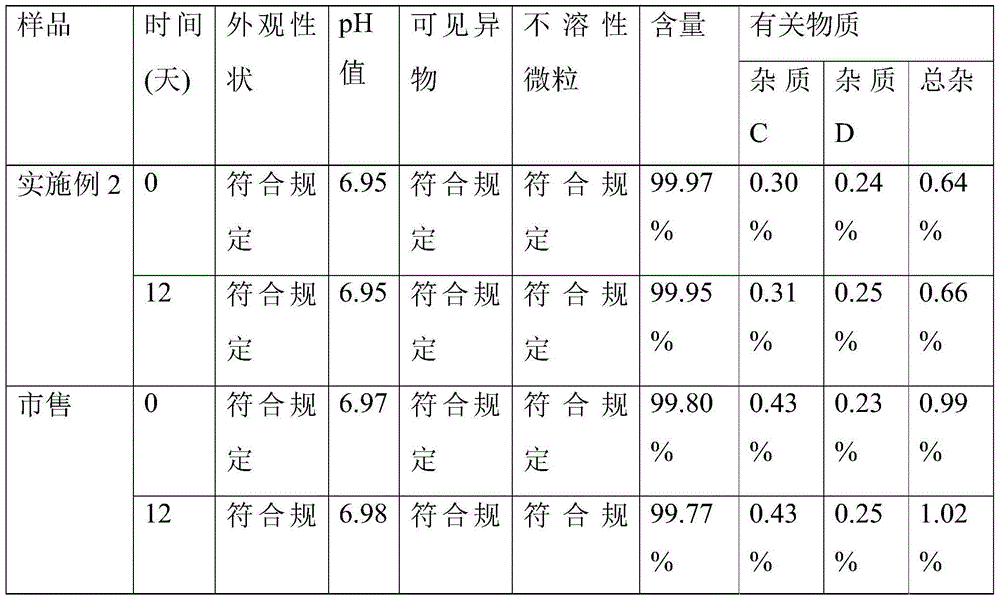
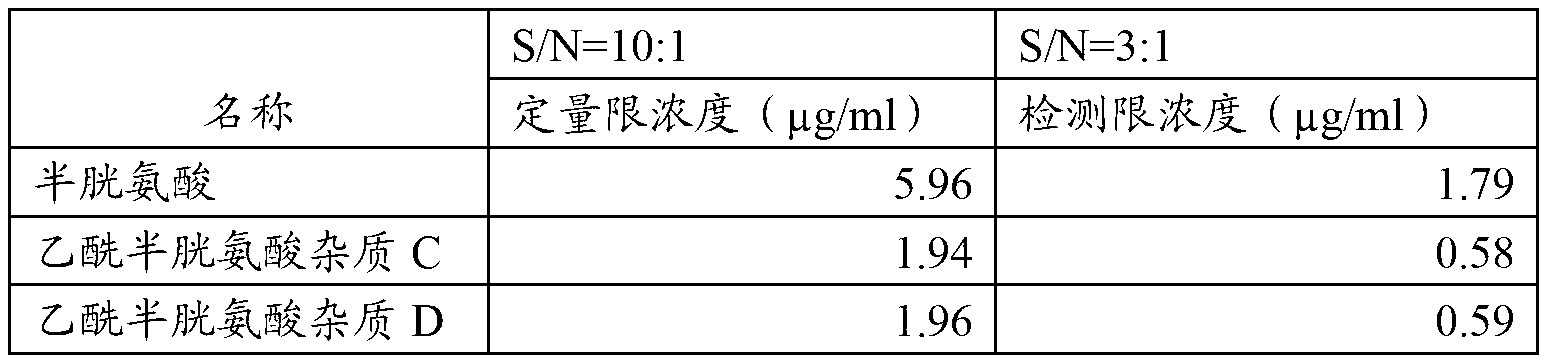
Quantitative detection method of acetylcysteine related substances in organ preservation solution
The invention provides a quantitative detection method of acetylcysteine related substances in an organ preservation solution. The quantitative detection method is characterized by comprising the steps of: (1) determination of the limits of three acetylcysteine related substances in a preparation; (2) methodology validation of the detection method and determination of the acetylcysteine related substances in the preparation. The quantitative limits of the three related substances are determined by adopting an HPLC (High Performance Liquid Chromatography) detection method with reference to allowed limit values of active ingredient impurities in the preparation and an existing process of the preparation; the methodology validation is carried out on the detection method in the aspects of specificity, linearity, sample injection recovery rate, repeatability, durability and the like to prove that direct injection detection results of a stock solution of the organ preservation solution A are good. The quantitative detection method abandons an inapplicable self-control quantitative method, can be used for realizing the quantitative determination of the acetylcysteine related substances in the organ preservation solution by using an external standard method for the first time and effectively controlling the quality of the organ preservation solution, and is beneficial to the guidance of process study and the improvement of the product safety.
Owner:HUAREN PHARMACEUTICAL CO LTD
Acetylcysteine effervescent tablet and preparation method and application thereof
ActiveCN102233139AFast disintegrationGreat tasteEmulsion deliverySodium bicarbonateEffervescent tablet
The invention discloses an acetylcysteine effervescent tablet, a preparation method and an application thereof; the effervescent tablet comprises the following pharmaceutical components by weight: 2-6 parts of acetylcysteine, 3.2-13 parts of organic acids, 3-9.5 parts of sodium bicarbonate, 1.5-13.5 parts of filling agents, 3-4 parts of anhydrous alcohol, 0.2-0.6 parts of PVPK30, 0.05-0.25 parts of glycerol, 0.05-0.35 parts of sodium chloride, 0.1-0.75 parts of essence, 0.1-0.4 parts of sweetening agents, and 0.15-0.8 parts of lubricants; the effervescent tablet has low hygroscopicity, and has no sticking and picking problem during tabletting in environment with humidity of less than 55%; and the effervescent tablet is applicable to the preparation of a preoperative adjuvant drug for digestive endoscope examinations and treatment or imaging examinations.
Owner:重庆健能医药开发有限公司
Pharmaceutical composition for reducing homosysteine
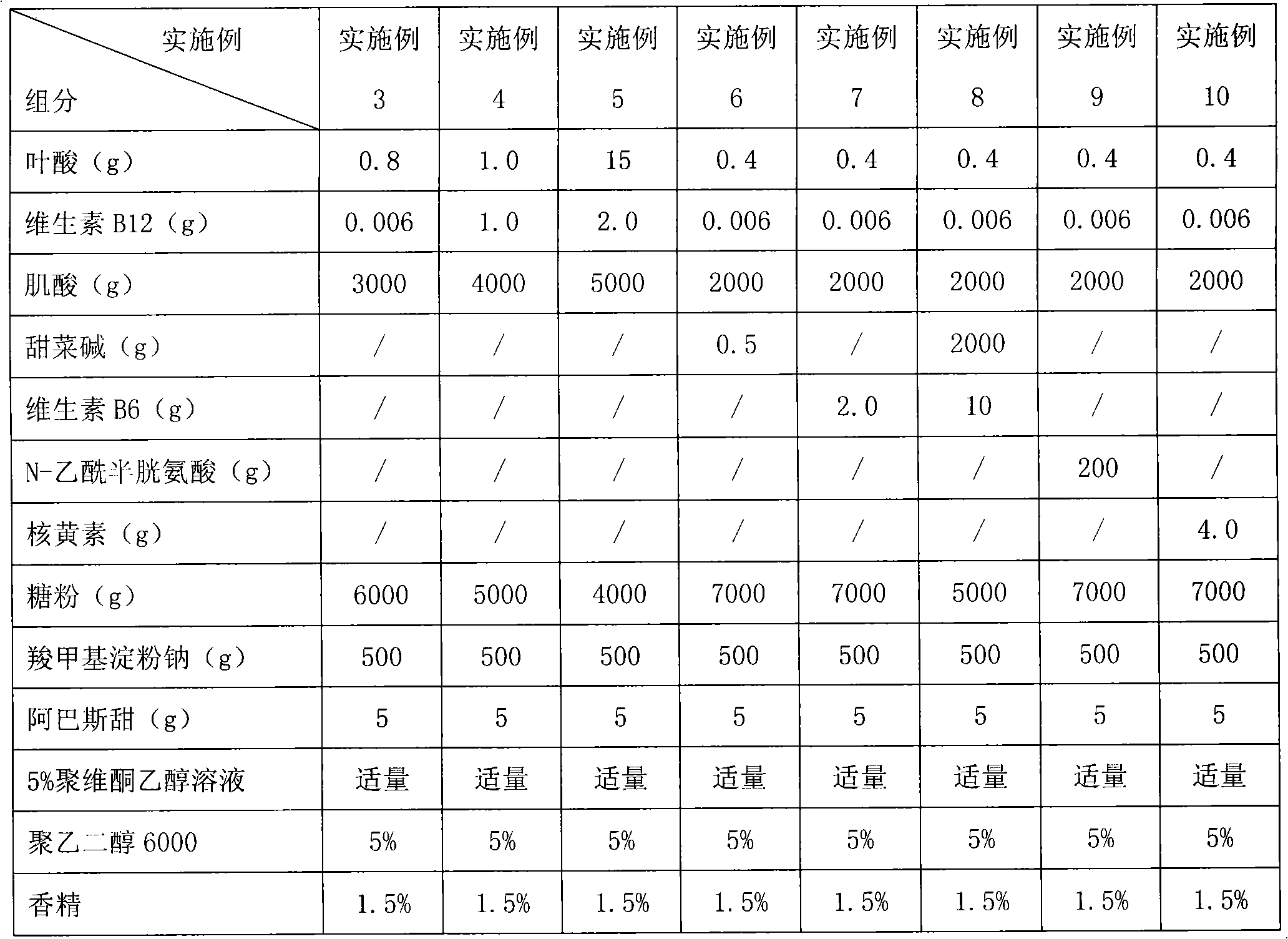
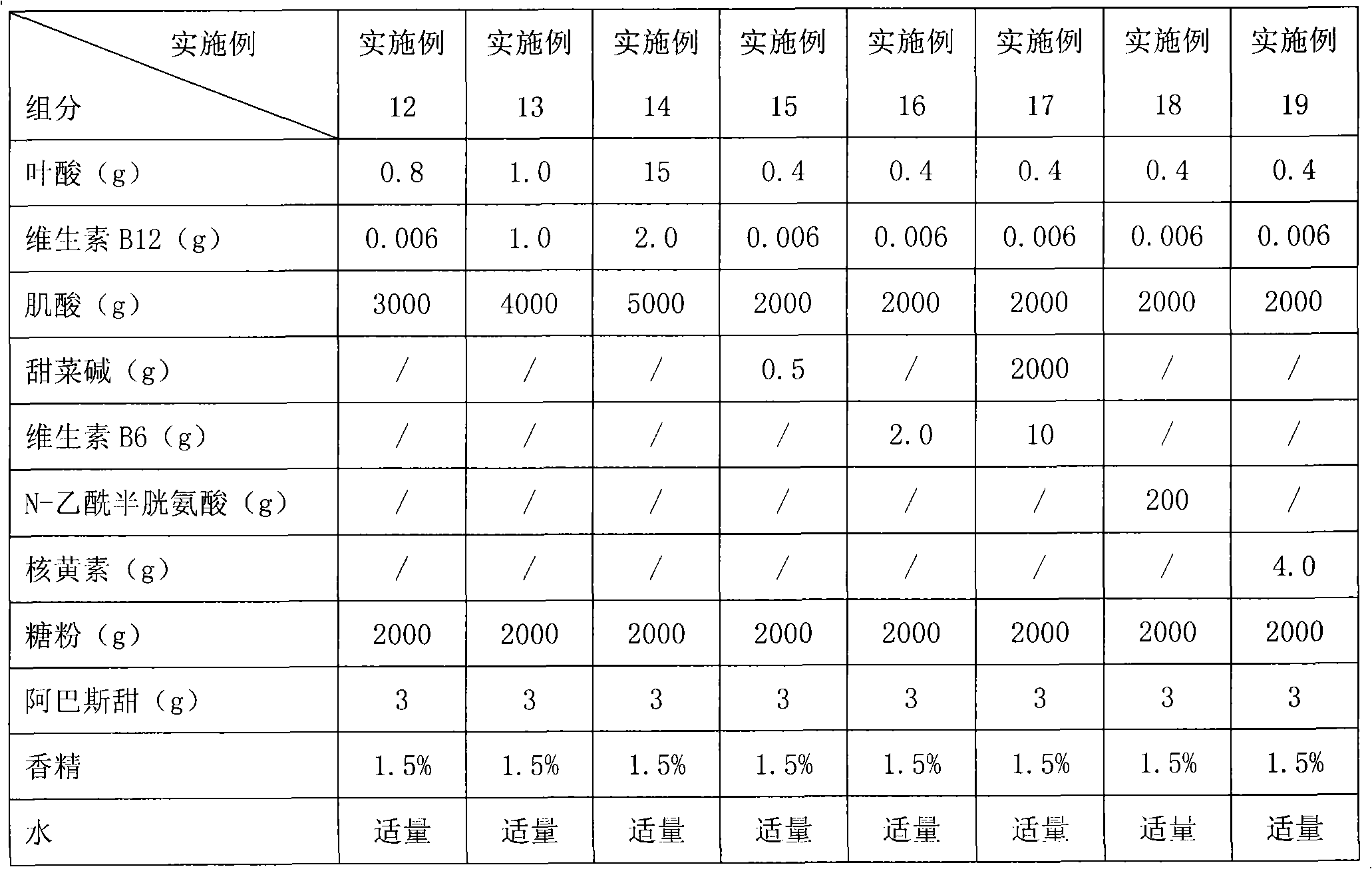
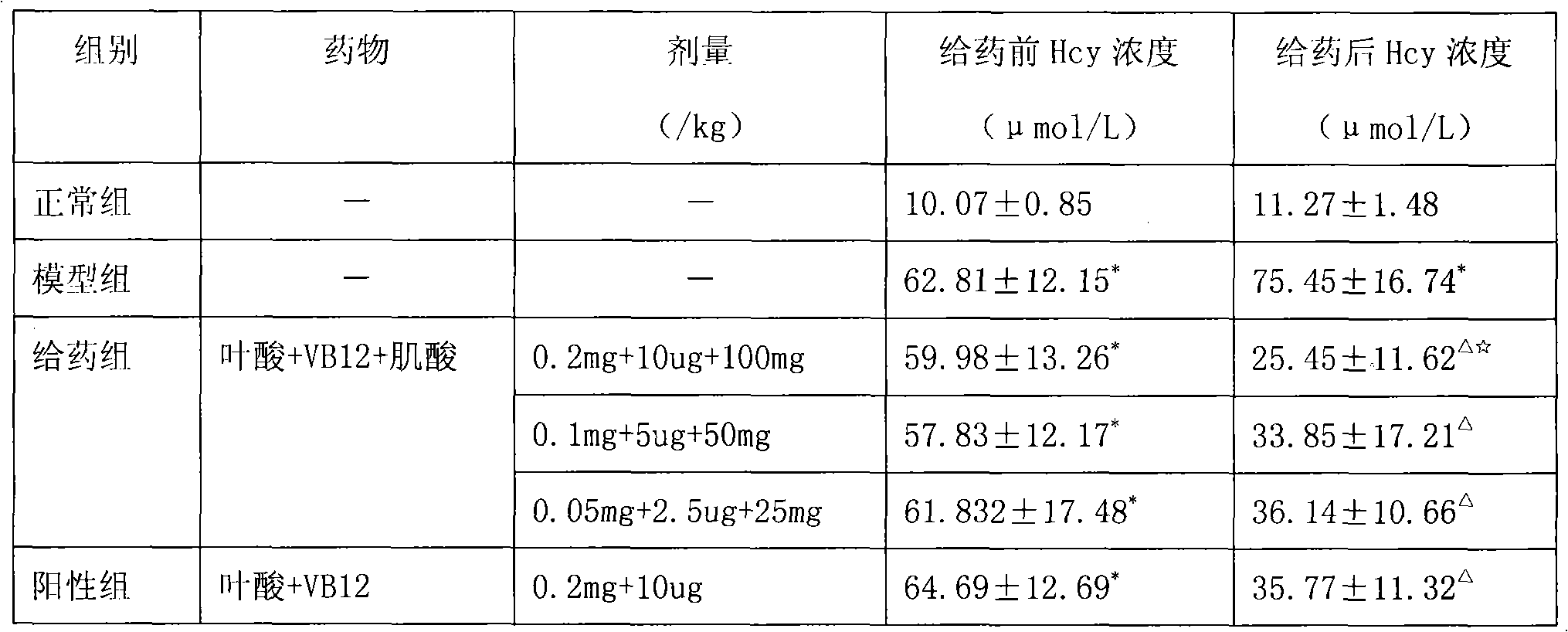
ActiveCN101406478ALower homocysteineEffective therapeutic effectOrganic active ingredientsCardiovascular disorderBetaineVitamin B12
The invention relates to a pharmaceutical composition for reducing homocysteine, which comprises the following components: 1) an officinal dosage of folic acid or pharmaceutical salts thereof; 2) an officinal dosage of vitamin B12 or pharmaceutical salts thereof; 3) an officinal dosage of creatine or pharmaceutical salts thereof; and 4) pharmaceutical carriers or excipients. The composition further comprises one or more than one of betaine, VB6, N-acetylcysteine, and riboflavin. The invention also relates to application of the pharmaceutical composition in preparing drugs for reducing the homocysteine as well as the application thereof in preparing the drugs for treating life bodies suffered from hyperhomocysteinemia. The pharmaceutical composition belongs to the field of pharmacy.
Owner:SHENZHEN AUSA PHARMA
Protectant Combinations for Reducing Toxicities
Pharmaceutical compositions and methods of preventing or reducing hearing or balance loss and damage to ear cells in patients who have been exposed to toxic levels of noise and other toxic insults are provided. These methods comprise administering an effective amount of a protectant combination or composition comprising two or more protectants selected from the group of methionine protectant agents, N-acetylcysteine, carnitine, magnesium ions, lipoic acid, ebselen, glutathione, and glutathione ester. These protectant combinations can be administered prior to, simultaneously with, or subsequently to exposure to noise other toxic insults.
Owner:SOUTHERN ILLINOIS UNIVERSITY
Injection acetylcysteine powdery medicinal composition and its making method
InactiveCN101028252AImprove stabilityLower serum bilirubinOrganic active ingredientsPowder deliveryDicarbonateSodium bicarbonate
An acetylcysteine composition in the form of powder injection with high solubility and stability is prepared from acetylcysteine and pH regulate chosen from sodium dicarbonate, sodium carbonate, sodium hydroxide and bisodium dicarbonate in weight ratio of 1: (0.25-1). Its preparing process is also disclosed.
Owner:何晶
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com