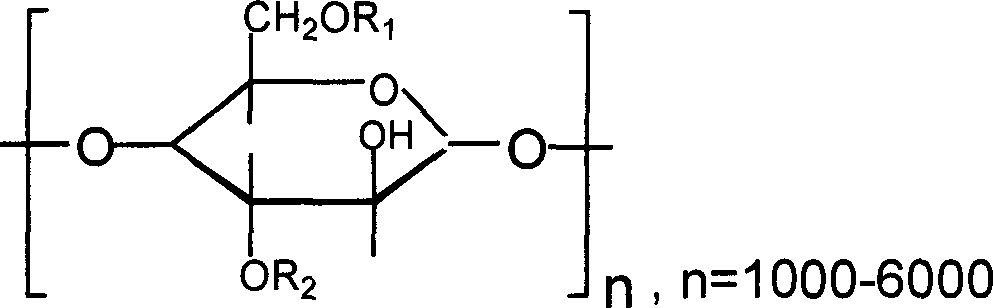
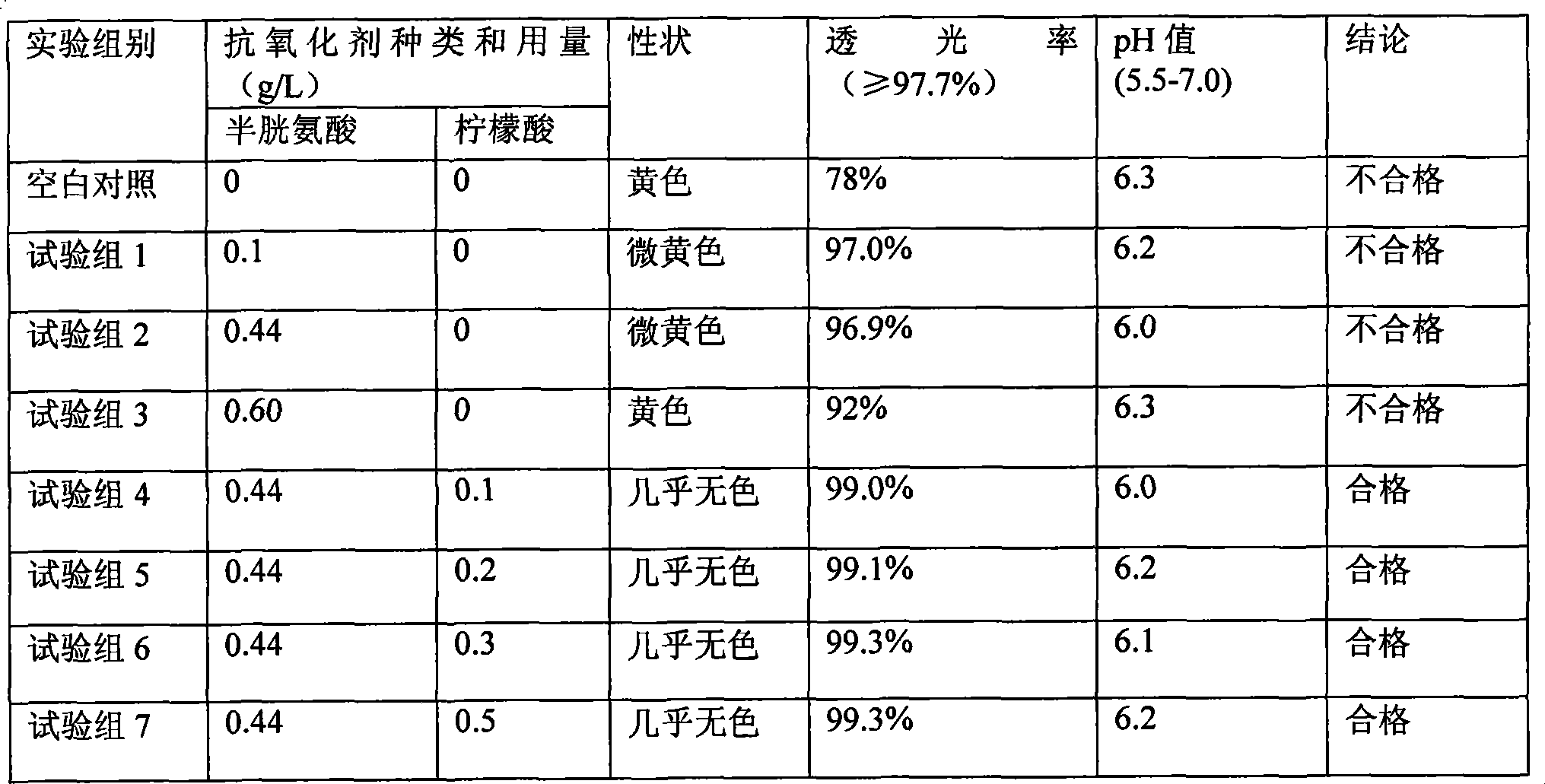
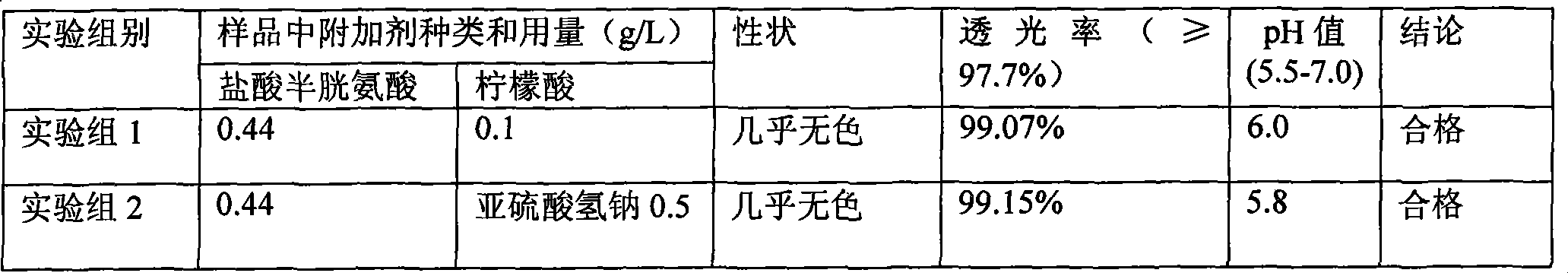
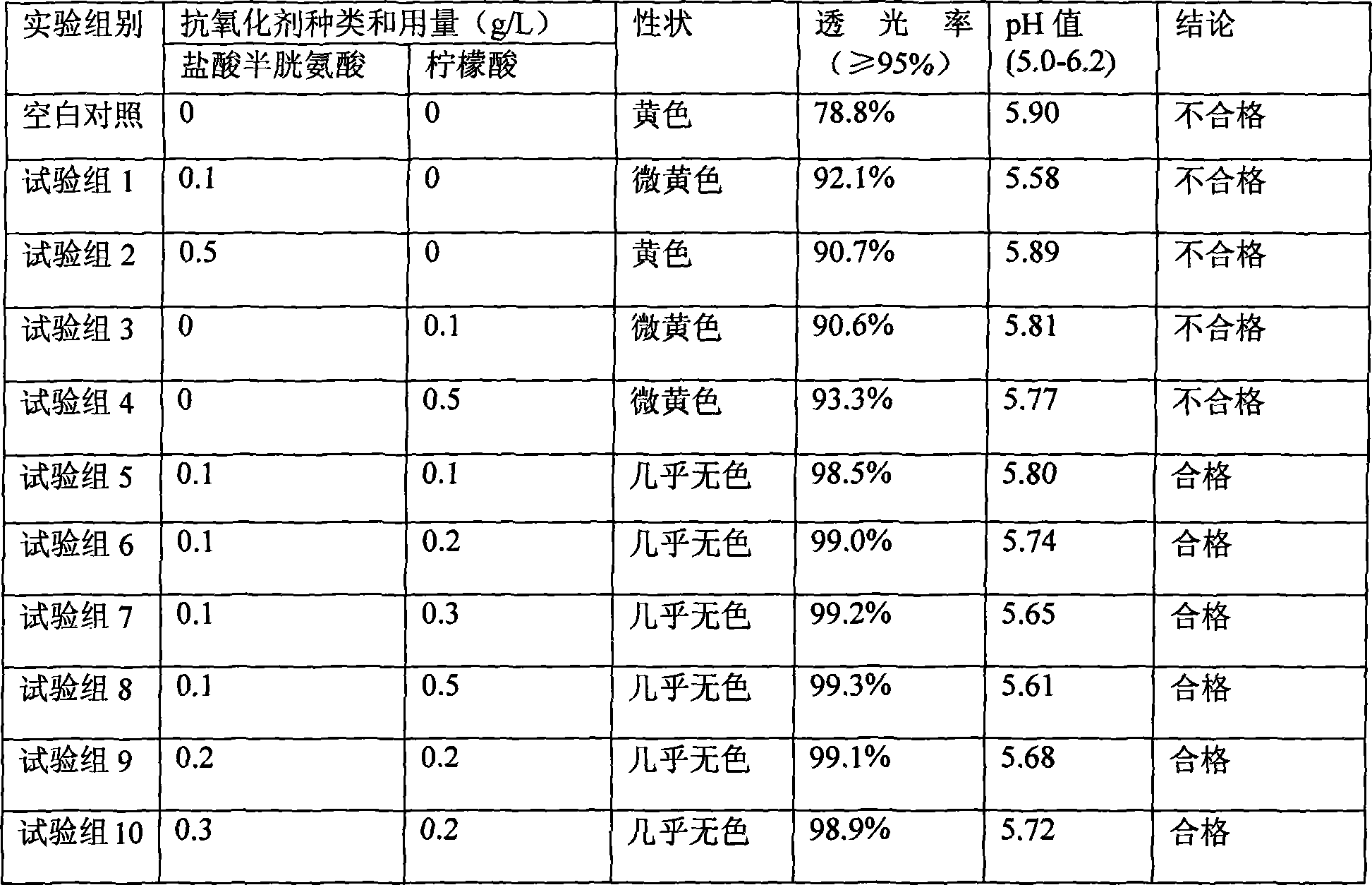
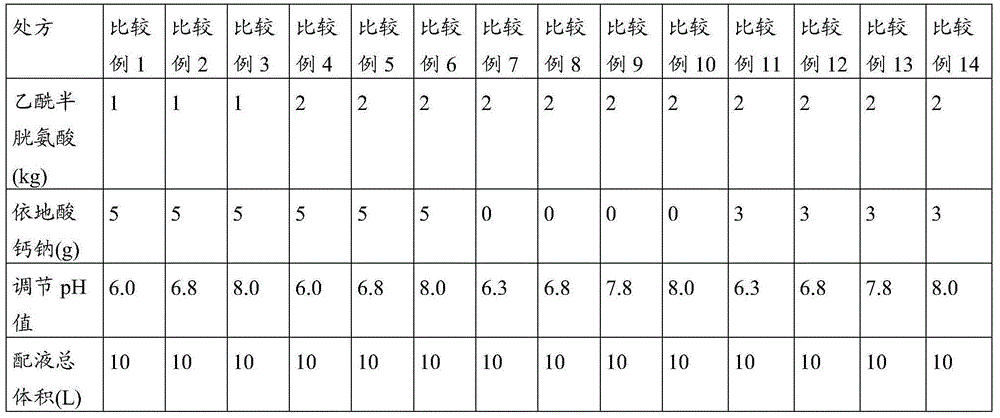
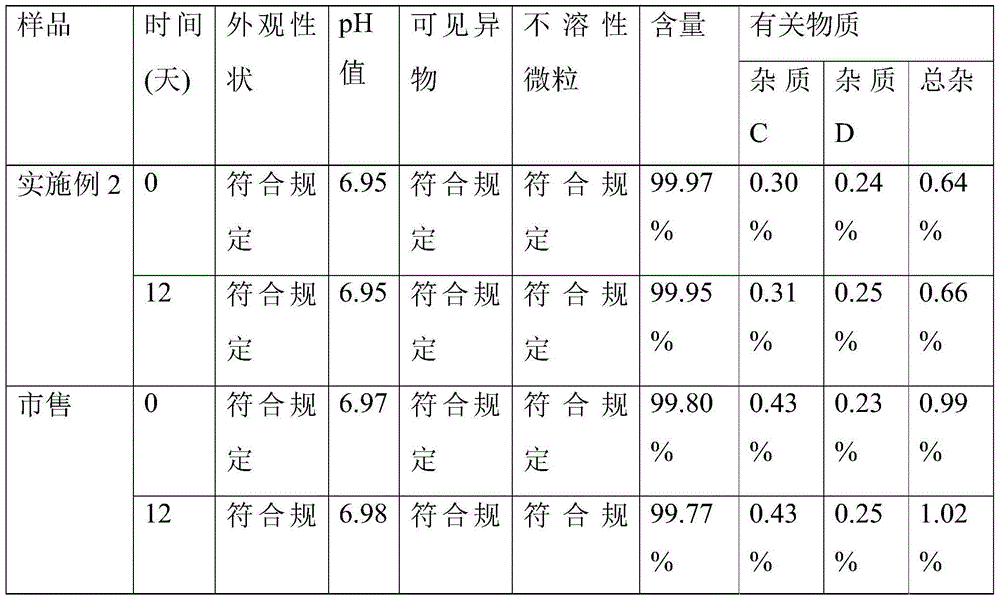
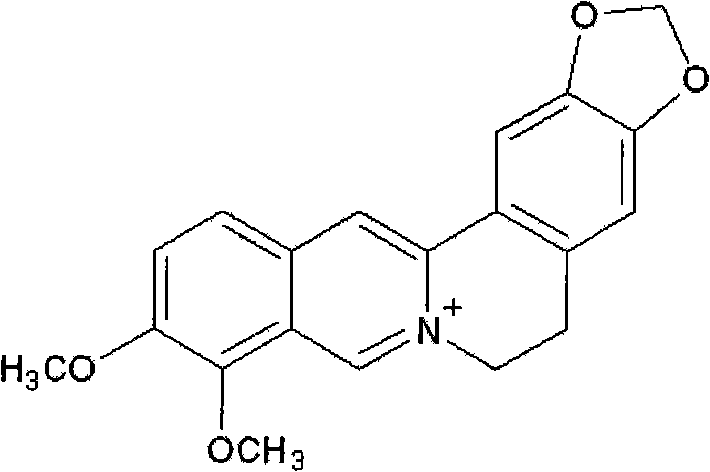
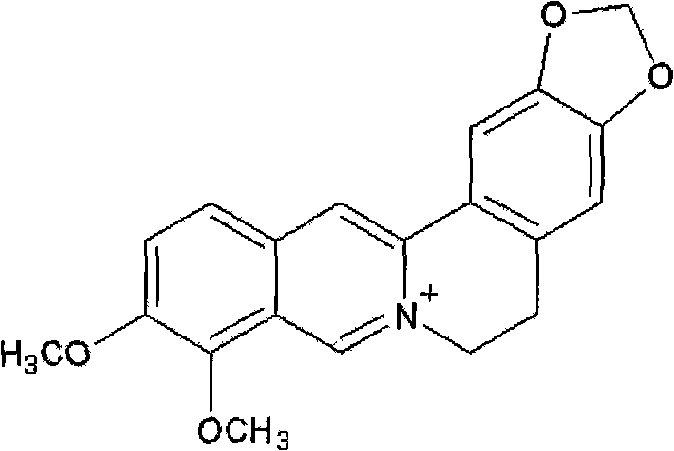
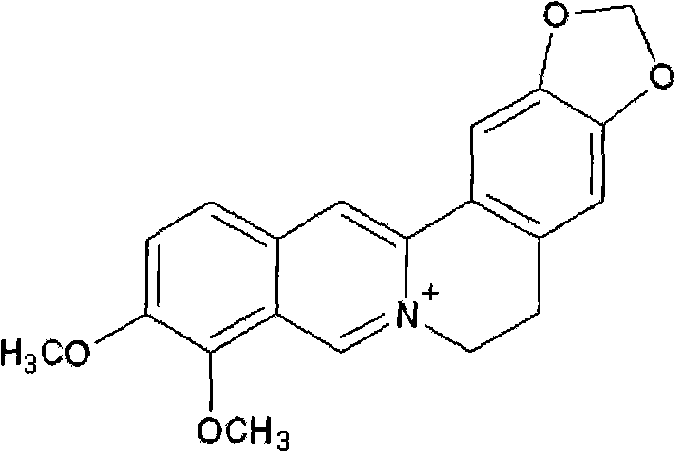
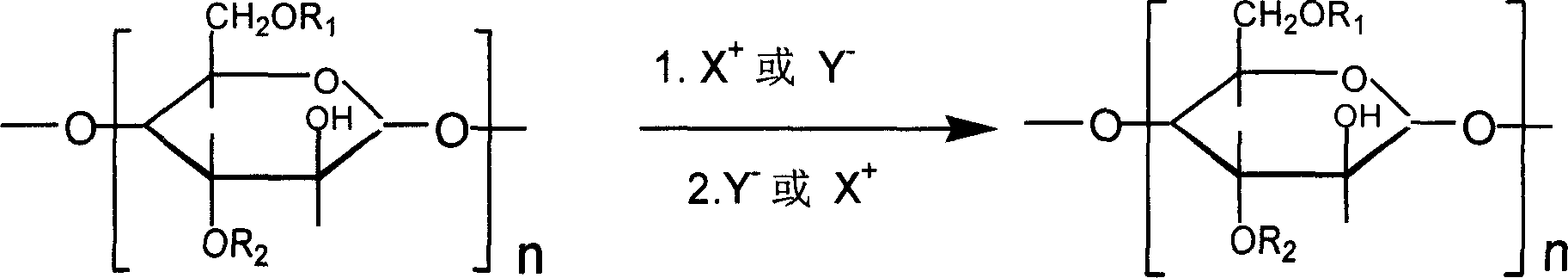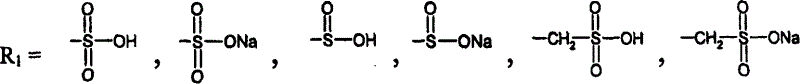Patents
Literature
110 results about "Cysteic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
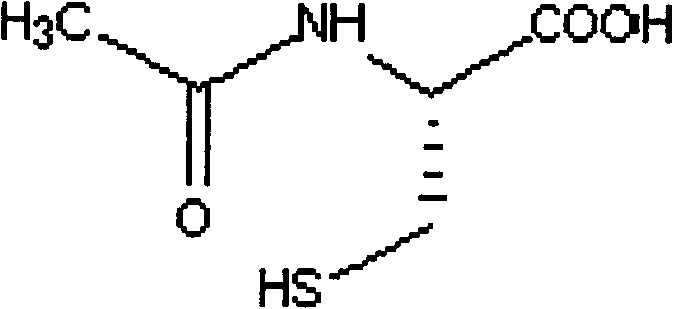
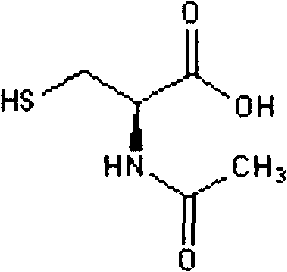
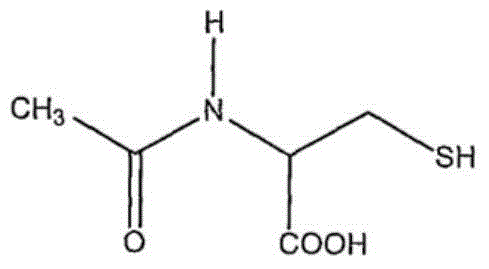
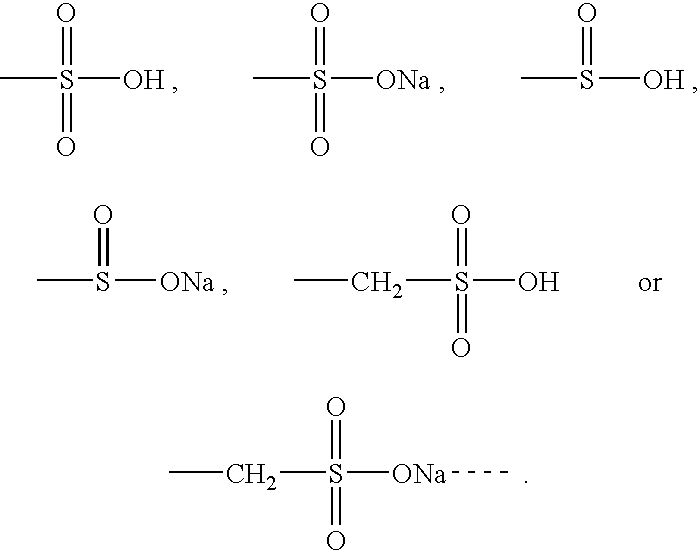
Cysteic acid also known as 3-sulfo-l-alanine is the organic compound with the formula HO₃SCH₂CH(NH₂)CO₂H. It is often referred to as cysteate, which near neutral pH takes the form ⁻O₃SCH₂CH(NH₃⁺)CO₂⁻.
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Therapeutic compounds
InactiveUS20040048923A1Improve pharmacological activityGood curative effectBiocideOrganic compound preparationDocetaxel-PNPTreatment effect
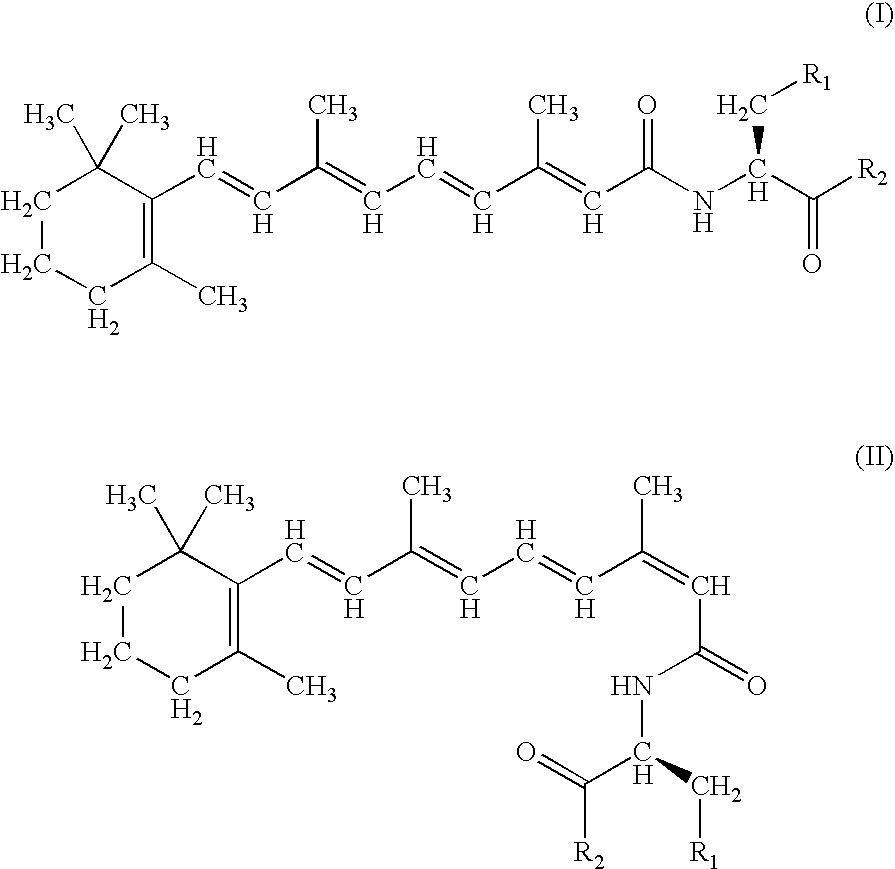
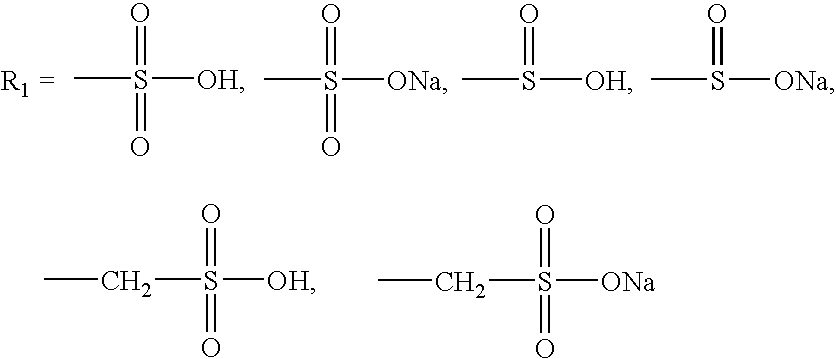
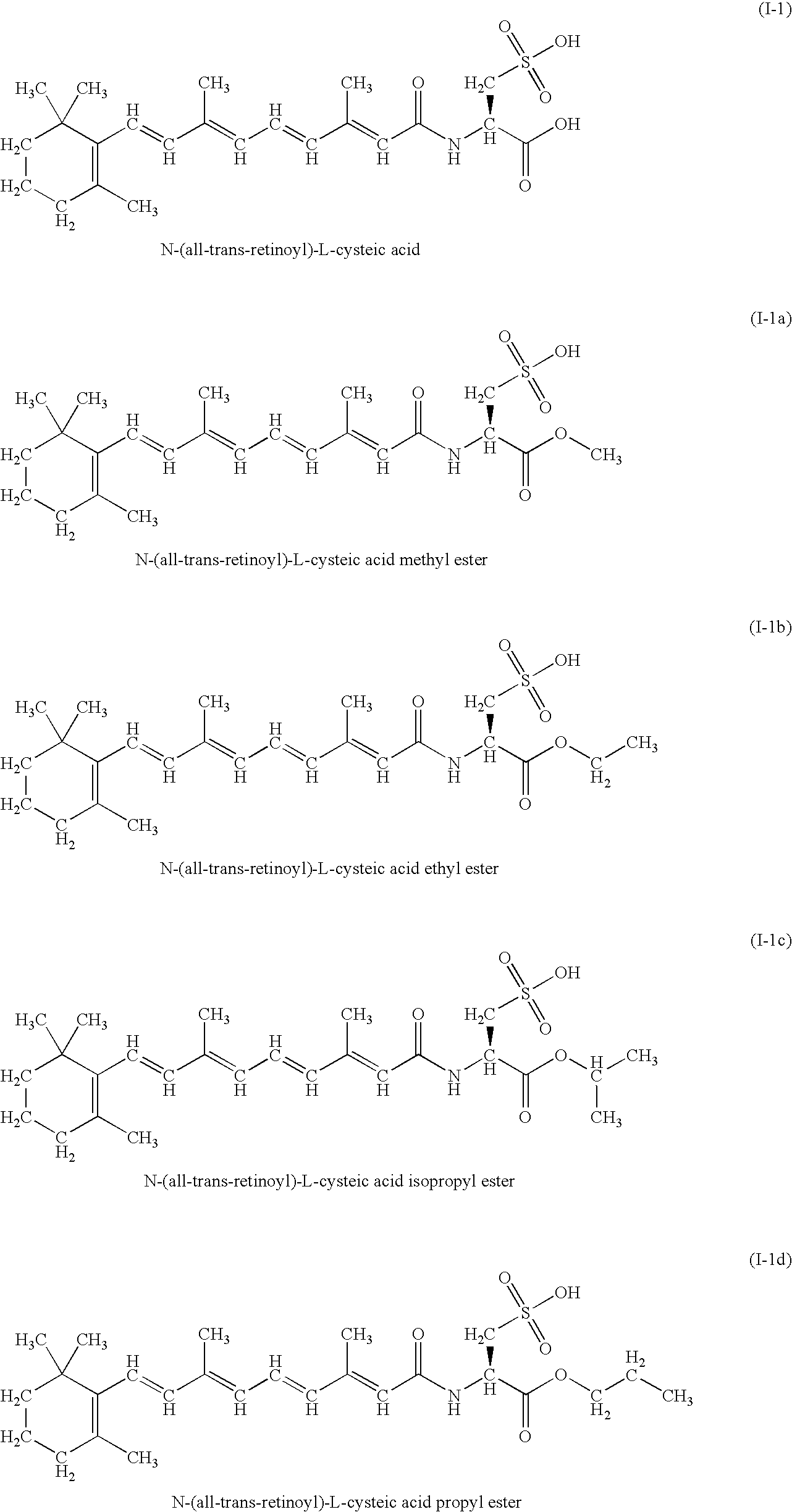
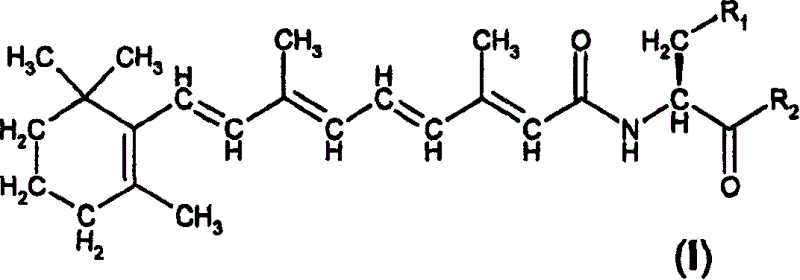
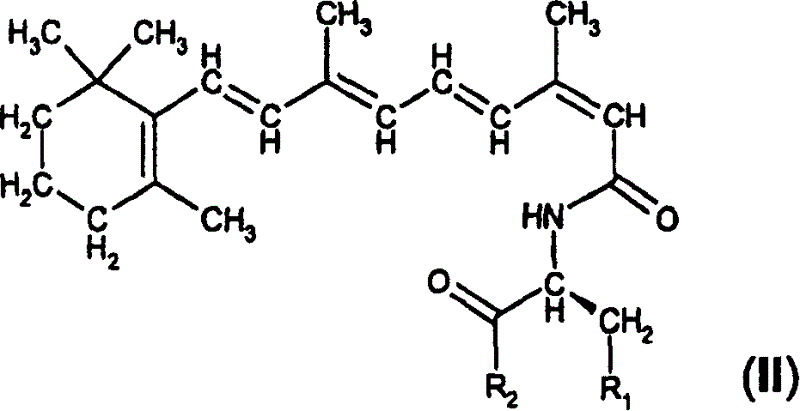
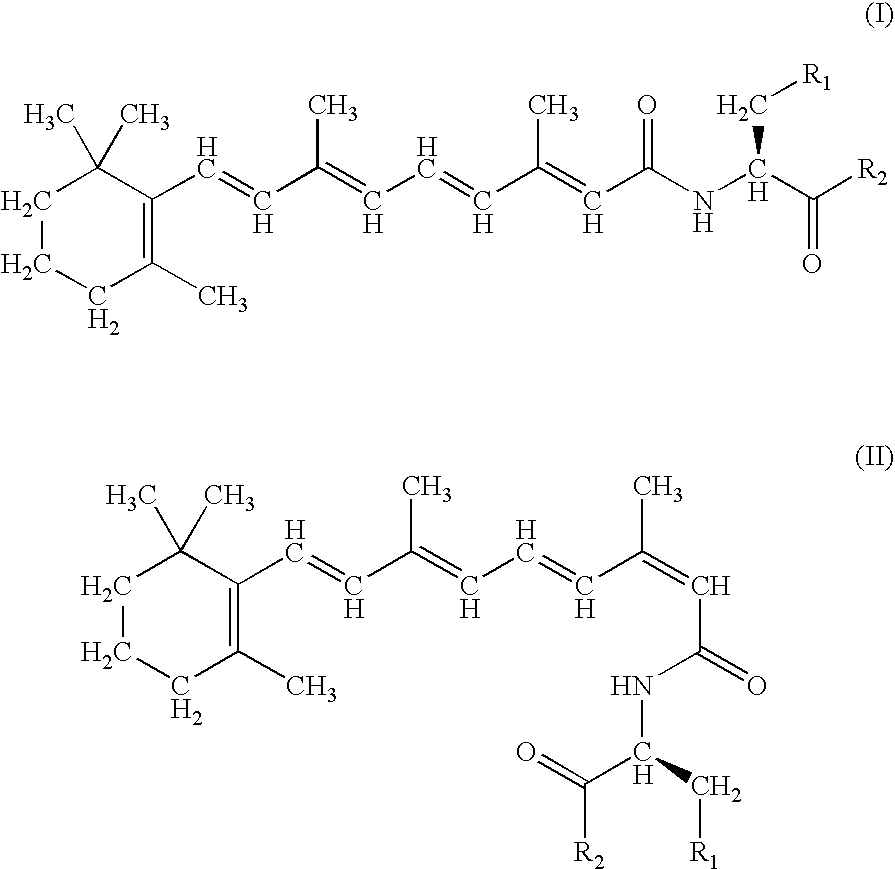
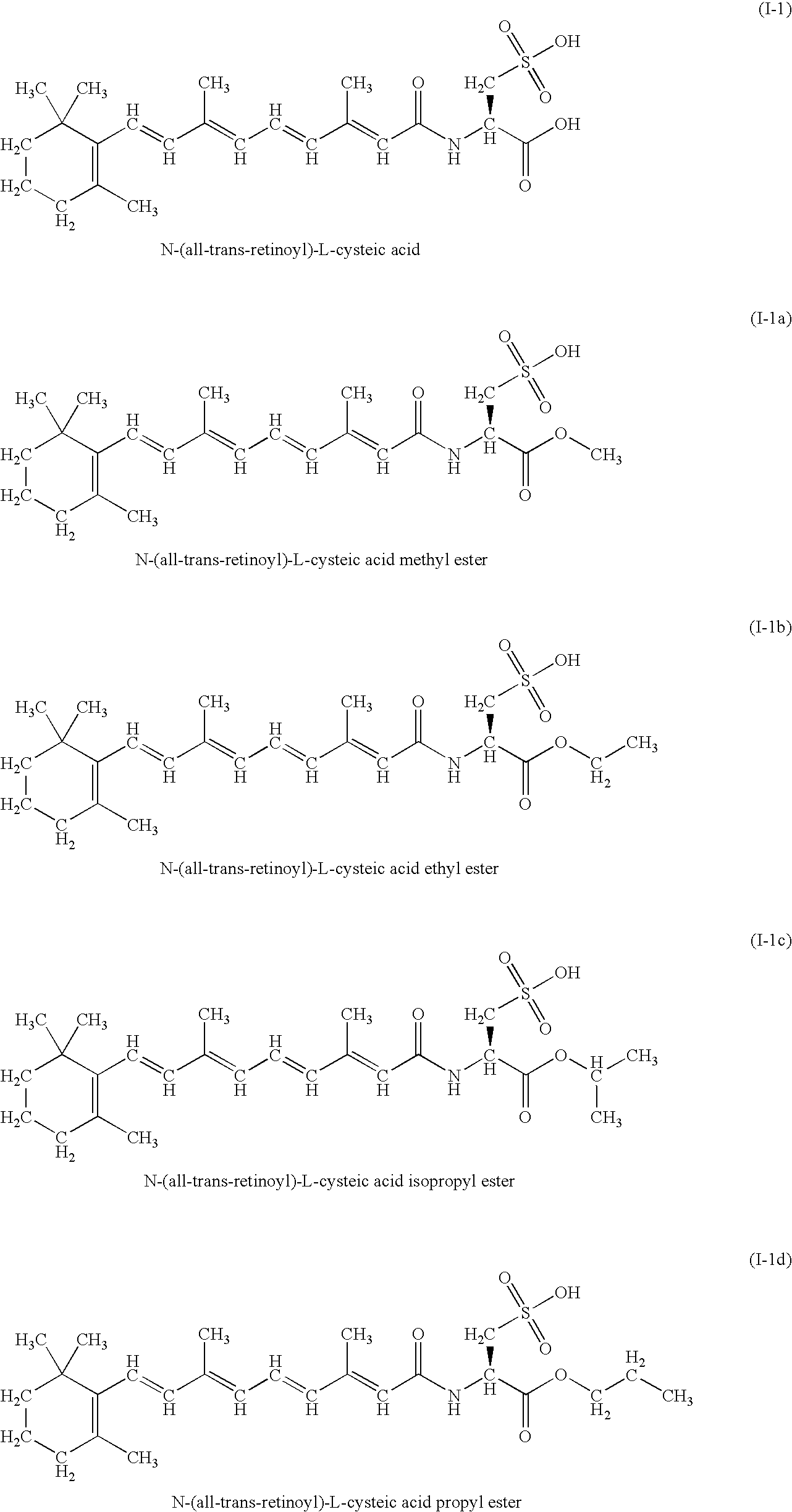
A group of new compounds, N-(all-trans-Retinoyl)-L-cysteic acid, N-(13-cis-Retinoyl)-L-cysteic acid, N-(all-trans-Retinoyl)-L-cysteinesulfinic acid, N-(13-cis-Retinoyl)-L-cysteinesulfinic acid, N-(all-trans-Retinoyl)-L-homocysteic acid, N-(13-cis-Retinoyl)-L-homocysteic acid, and sodium salts of these compounds, including sodium salts of their esters and amides, is shown to exhibit therapeutic effects per se, and which compounds in combination with cytotoxic compounds, such as docetaxel, paclitaxel, doxorubicin and mitoxantrone, exhibit a synergistic effect. These compounds make it possible to manufacture new formulations of poorly soluble pharmaceutical compounds, and the present invention discloses a process of manufacturing water-soluble formulations of such compounds, exemplified by docetaxel, and paclitaxel, exhibiting enhanced pharmacological activity, and formulations of water-soluble pharmaceuticals exemplified by doxorubicin and mitoxantrone, exhibiting improved therapeutic efficacy.
Owner:VIVESTO AB
Synthesis of soluble amphoteric starch
Synthesis of amphoteric soluble starch is carried out by taking starch as raw material, taking any kind from sodium chloroacetate, butanedioic anhydride and 3-chlorine-2-cysteic acid as anion reagent, taking any kind from 3-chlorine-2-hydroxypropyl trimethyl-ammonia chloride and N-(2,3-epoxide)trimethyl-ammonia chloride as cation reagent, catalyzing by composite catalyst, synthesizing the final product at 30-150 degree for 1-8hrs by semi-drying method. It' simple, cheap and efficient, has no after-treatment and waste ejection.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439036AInhibition of oxidative decomposition reactionsQuality assuranceOrganic active ingredientsMetabolism disorderAntioxidantTryptophan
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-V) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 2.89 of arginine hydrochloride, 2.46 of histidine hydrochloride, 3.79 of leucine, 1.70 of isoleucine, 3.33 of lysine hydrochloride, 2.83 of phenylalanine, 1.97 of threonine, 1.36 of valine, 1.06 of methionine, 0.39 of tryptophan, 3.24 of glycine, 1.88 of alanine, 1.00 of proline, 0.11 of tyrosine, 0.67 of serine, 0.44 of cysteine hydrochloride, 1.15 of aspartic acid, 1.97 of glutamic acid, 50 of xylitol, 0.10 to 0.30 of citric acid and injection water with proper amount. The pharmaceutical composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test and a quality test, results show that the pharmaceutical composition is as stable as or more stable than like products (18AA-V) which are sold in the markets and contain sulfites.
Owner:福州凯瑞医药咨询有限公司
Cysteic acid derivatives of Anti-viral peptides
InactiveUS20090088377A1Easy to processEasy to purifyPeptide/protein ingredientsVirus peptidesFeline immunodeficiency virusImmunodeficiency virus
This invention relates to C34 peptide derivatives having improved aqueous solubility that are inhibitors of viral infection and / or exhibit antifusogenic properties. In particular, this invention relates to C34 derivatives having inhibiting activity against human immunodeficiency virus (HIV), respiratory synctial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) with long duration of action for the treatment of the respective viral infections.
Owner:CONJUCHEM
Homocysteine dry chemical detection strip and preparation method thereof
InactiveCN101592656ASensitive and goodImprove featuresMaterial analysis by observing effect on chemical indicatorBiological testingReagent stripChemical reaction
The invention provides a homocysteine dry chemical detection strip and a preparation method thereof. The invention aims to provide a detection strip and a preparation method thereof for fast semiquantitatively / quantitatively detecting homocysteine. The strip is composed of an elongated upper support layer, an elongated lower support layer and test layers in the middle and is divided into a hand-held area and a test area. A diffusion layer, a filtration layer, an enzyme reagent layer and a colour development reagent layer are arranged in the test area from top to bottom. Various grid materials of synthetic fibre materials can be used to prepare the diffusion layer, various filterable materials for separating blood can be used to prepare the filtration layer, and various asymmetric synthetic membranes can be used to prepare the enzyme reagent layer and the colour development reagent layer. A loading hole is arranged in the upper support layer, a sample is sent from the loading pole to the diffusion layer, the sample then permeates into the filtration layer, the enzyme reagent layer and the colour development reagent layer, chemical reactions are performed in the enzyme reagent layer and the colour development reagent layer to change the colour, and the change of optical density in reaction process can be tested through a test pole of the lower support layer.
Owner:BEIJING HUAANFO BIOMEDICAL RES CENT +1
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439031ASolve the problem of trace oxygenSolve the oxygen redissolution problemOrganic active ingredientsMetabolism disorderAntioxidantArginine
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-II) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 1.50 of aspartic acid, 2.50 of glutamic acid, 1.90 of serine, 3.00 of histidine, 3.50 of glycine, 2.50 of threonine, 7.20 of alanine, 4.90 of arginine, 0.20 of tyrosine, 0.20 of cystine, 3.20 of valine, 2.50 of methionine, 0.85 of tryptophan, 3.50 of phenylalanine, 2.50 of isoleucine, 3.40 of leucine, 5.50 of lysine acetate, 2.90 of proline, 0.10 of cysteine hydrochloride and 0.20 of lemon acid. The composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test, a test result shows that the pharmaceutical composition containing 18 amino acids is as stable as or more stable than like products which are sold in the markets and contain sulfites.
Owner:郑飞雄
Peptide compositions and therapeutic uses thereof
ActiveUS9018352B2Promotes proliferationEasy SurvivalSenses disorderAntipyreticBinding siteAnimal subject
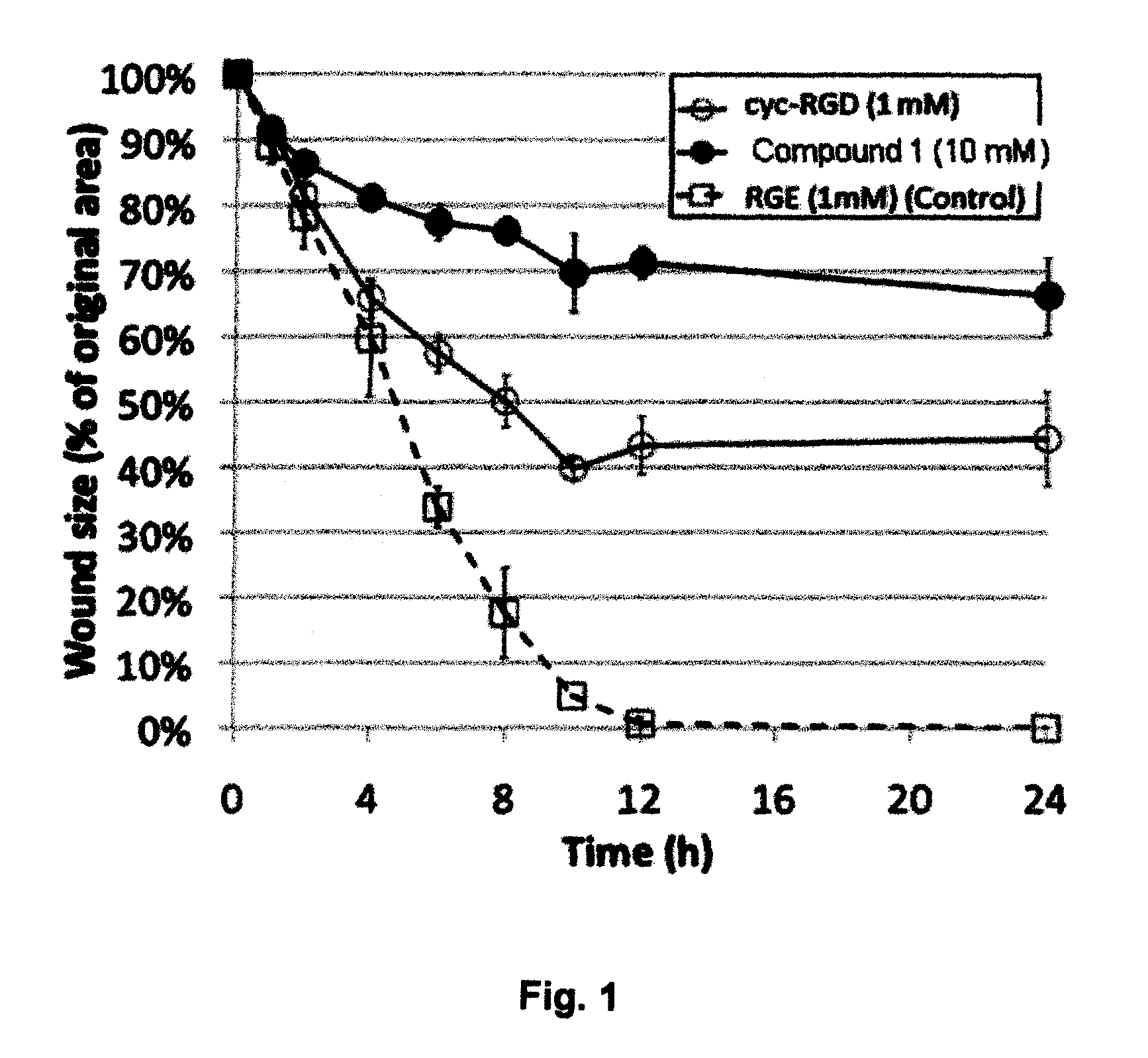
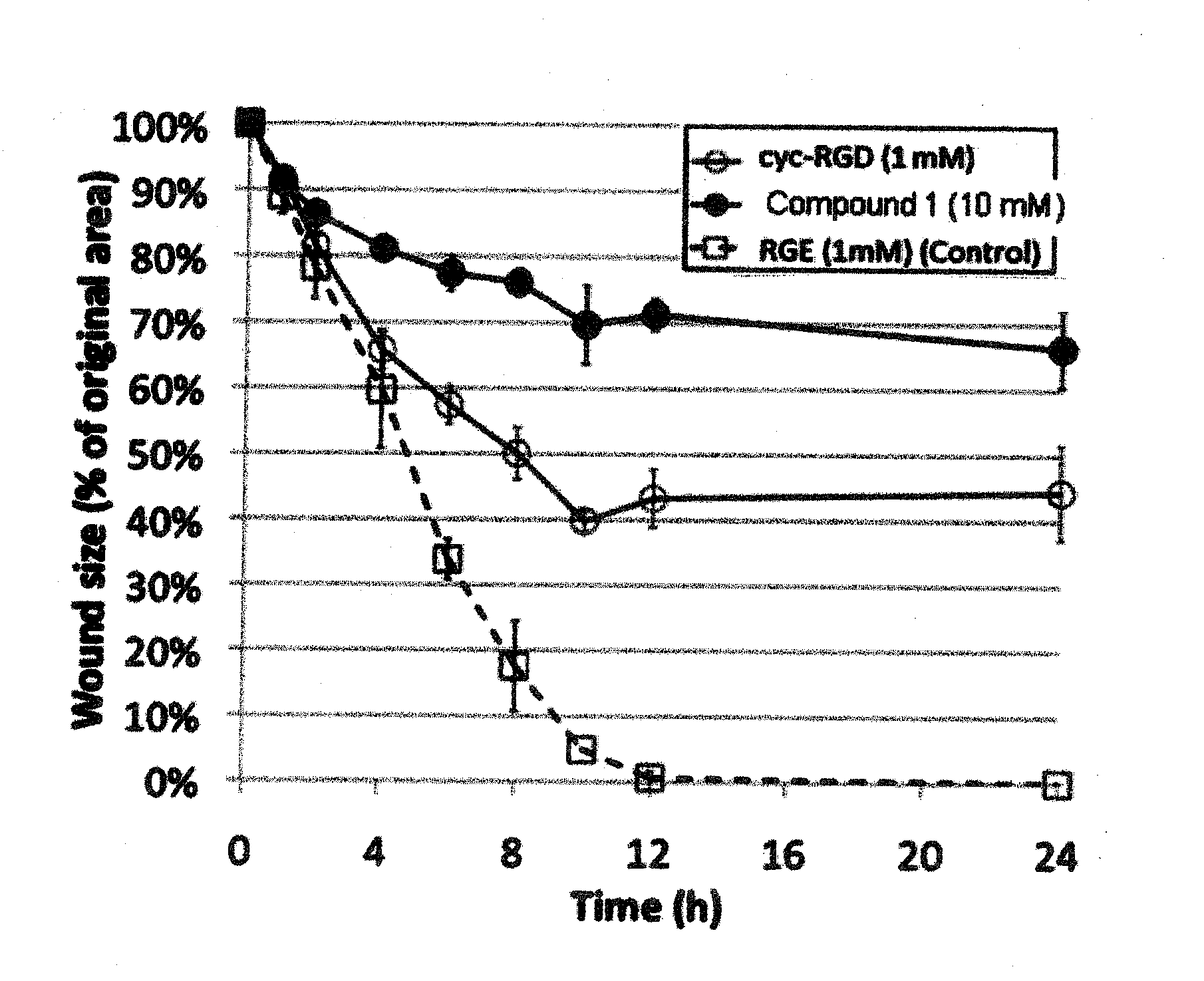
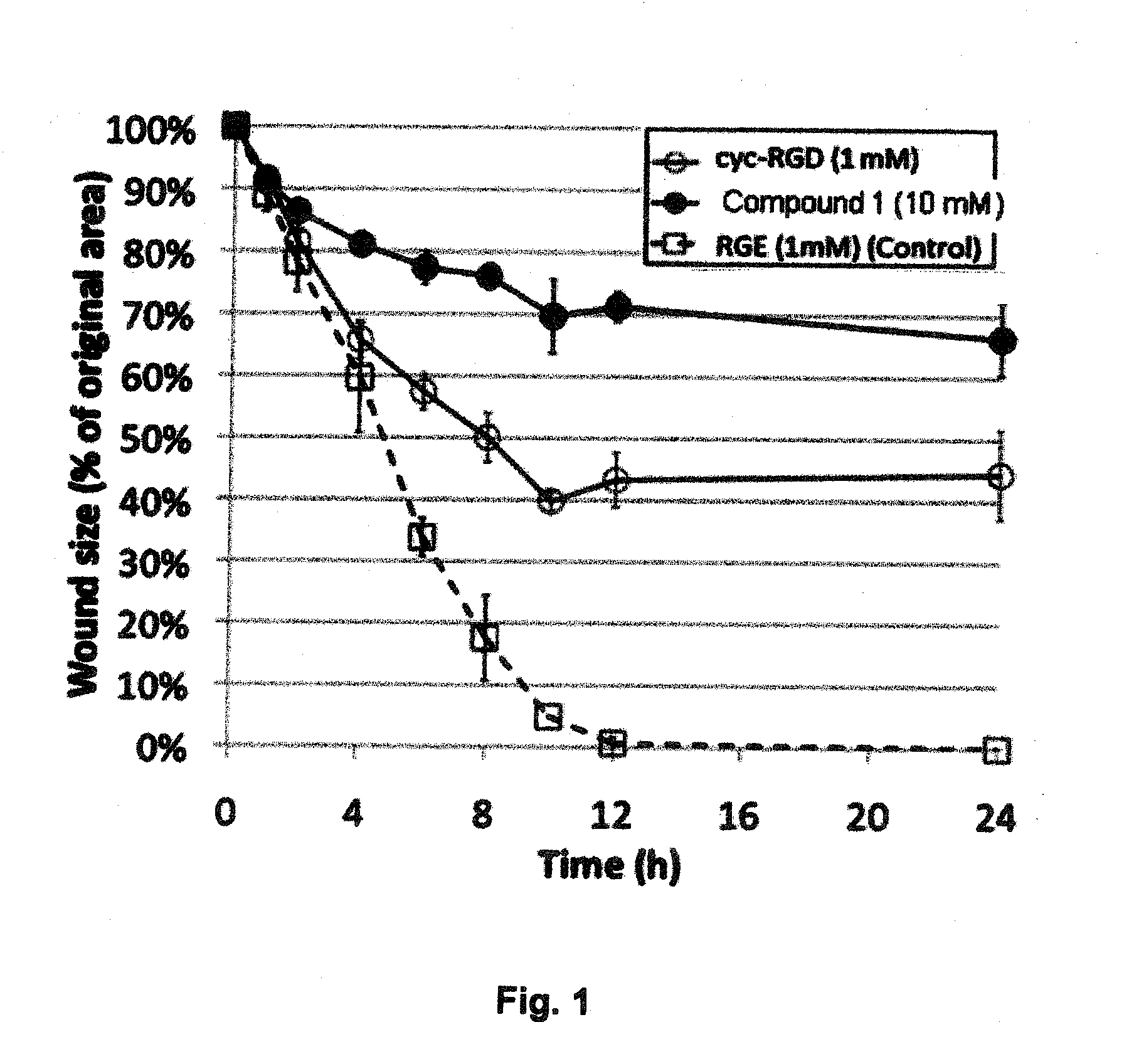
Compounds comprising R-G-Cysteic Acid (i.e., R-G-NH—CH(CH2—SO3H)COOH or Arg-Gly-NH—CH(CH2—SO3H)COOH) and derivatives thereof, including pharmaceutically acceptable salts, hydrates, stereoisomers, multimers, cyclic forms, linear forms, drug-conjugates, pro-drugs and their derivatives. Also disclosed are methods for making and using such compounds including methods for inhibiting cellular adhesion to RGD binding sites or delivering other diagnostic or therapeutic agents to RGD binding sites in human or animal subjects.
Owner:ALLEGRO PHARMA
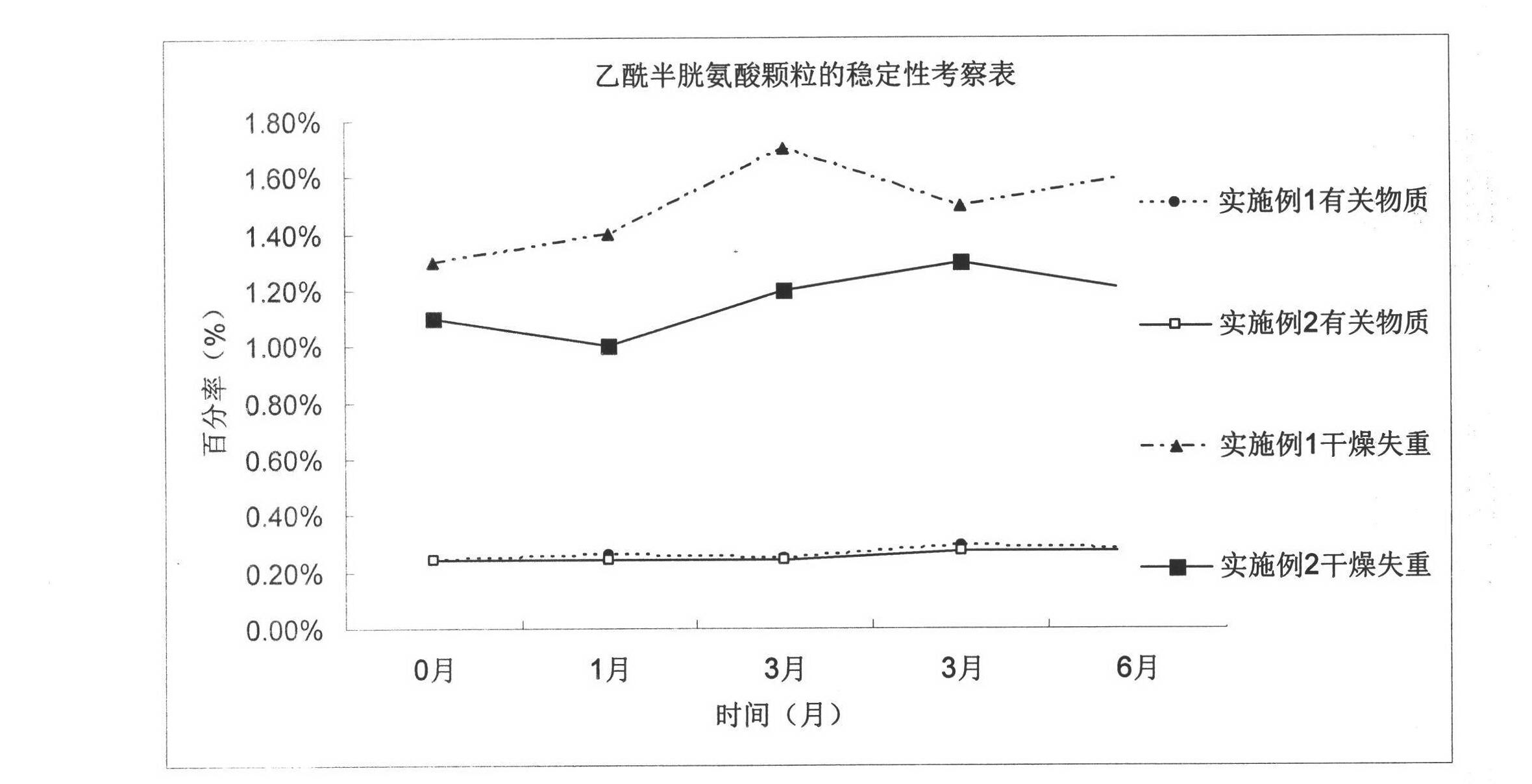
Acetylcysteine granule and preparation technology thereof
ActiveCN102144978AHumidity StableReduce adverse reactionsOrganic active ingredientsPharmaceutical non-active ingredientsMANNITOL/SORBITOLAcrylic resin
The invention discloses an acetylcysteine granule and a preparation method thereof, which belong to the new technical field of pharmaceutical preparation, and relates to a new composition of the acetylcysteine granule and the perpetration technology thereof. In the new composition and the preparation technology thereof, the granule mainly consists of acetylcysteine, acrylic resin, mannitol, aspartame and orange essence, and weight ratio of the main constituents is 10:1-50:2-200:0-1:0-1. After being coated with the acrylic resin, the acetylcysteine is mixed with the dry mannitol granule, the aspartame and the orange essence to prepare the acetylcysteine granule; and the prepared acetylcysteine granule has obviously improved stability in wet environment, and the taste of the acetylcysteine granule is greatly improved.
Owner:NANJING ZENKOM PHARMA
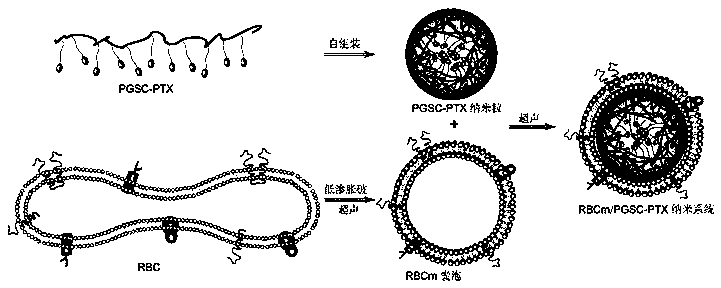
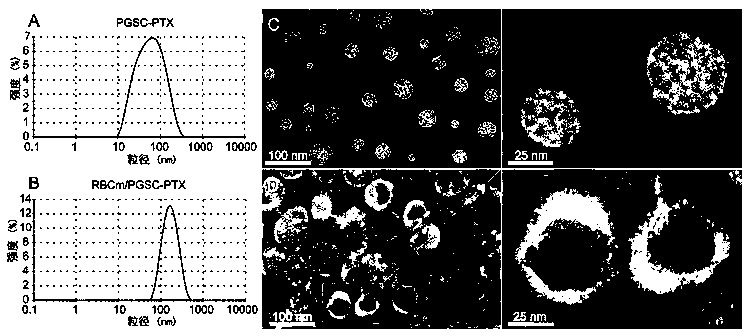
Preparation method and application of erythrocyte membrane-coated acid-sensitive polymer prodrug nano drug delivery system
InactiveCN105497912AEnhanced release propertiesNon-destructiveOrganic active ingredientsPharmaceutical non-active ingredientsErythrocyte membraneCarbocisteine
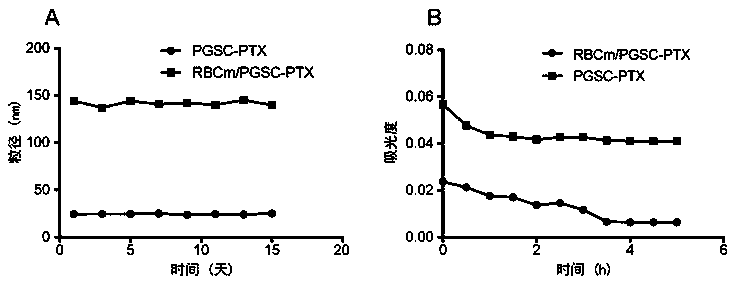
The invention discloses a preparation method and an application of an erythrocyte membrane-coated acid-sensitive polymer prodrug nano drug delivery system, wherein the polymer is formed by connecting sodium polyglutamate and carbocisteine through a peptide bond and then is bonded with an anti-cancer drug taxol to form the polymer prodrug; and the polymer prodrug is coated by an erythrocyte membrane to obtain an erythrocyte membrane-coated acid-sensitive polymer drug delivery system. The drug delivery system has the following characteristics that the particle size is about 100nm, and the shape is a sphere; the drug delivery system is relatively stable in a phosphate buffer and serum; the drug release character is relatively good in an acid environment; the toxicity is obviously reduced in cell experiments; the ingestion of the system by macrophage is obviously reduced; without destructive effect on erythrocyte, the system can be applied to intravenous injection; the circulating time of the system in blood is remarkably longer than that of polymer; and the system has an obvious inhibiting effect on the tumor growth of lung cancer.
Owner:EAST CHINA NORMAL UNIV
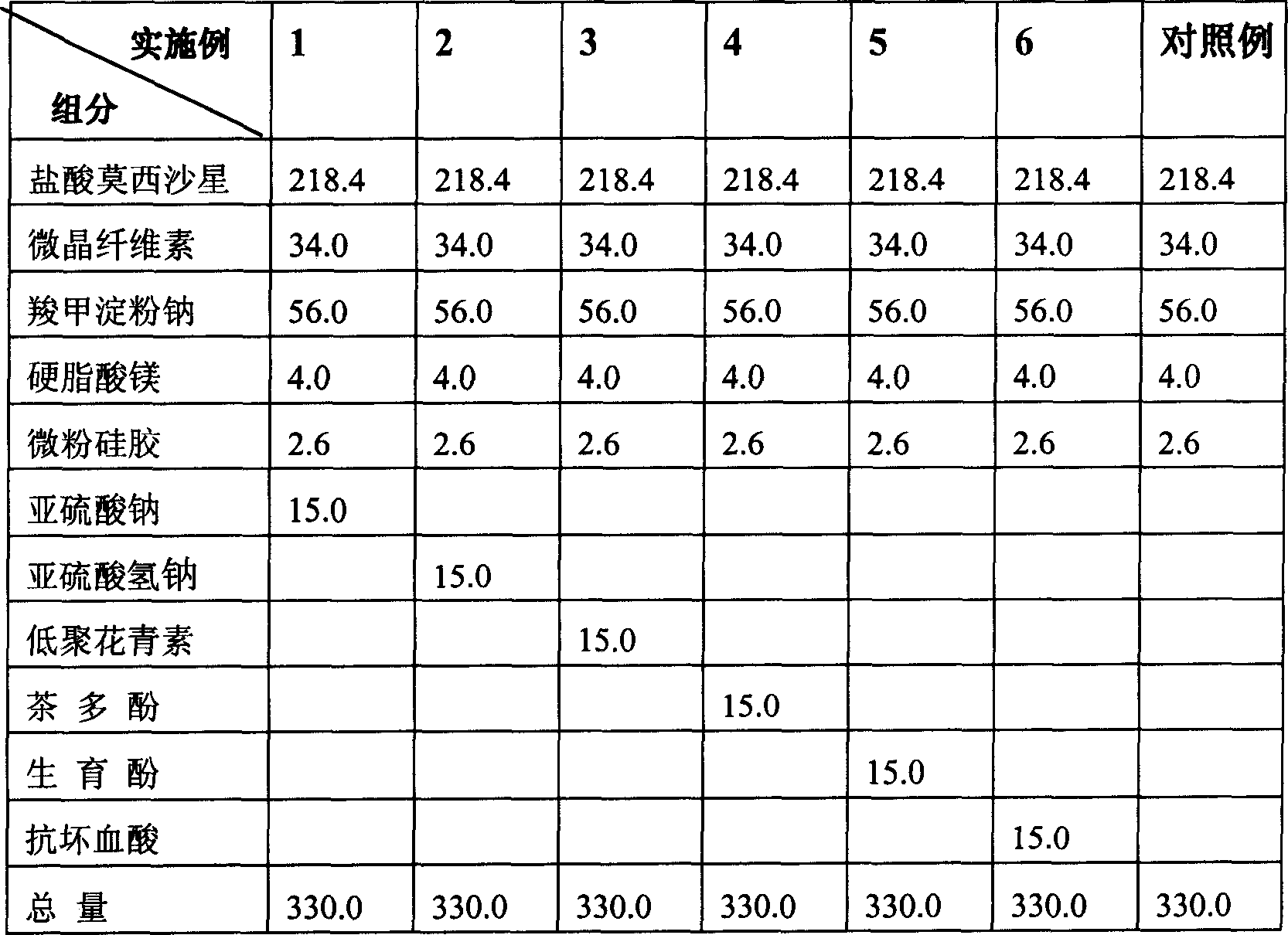
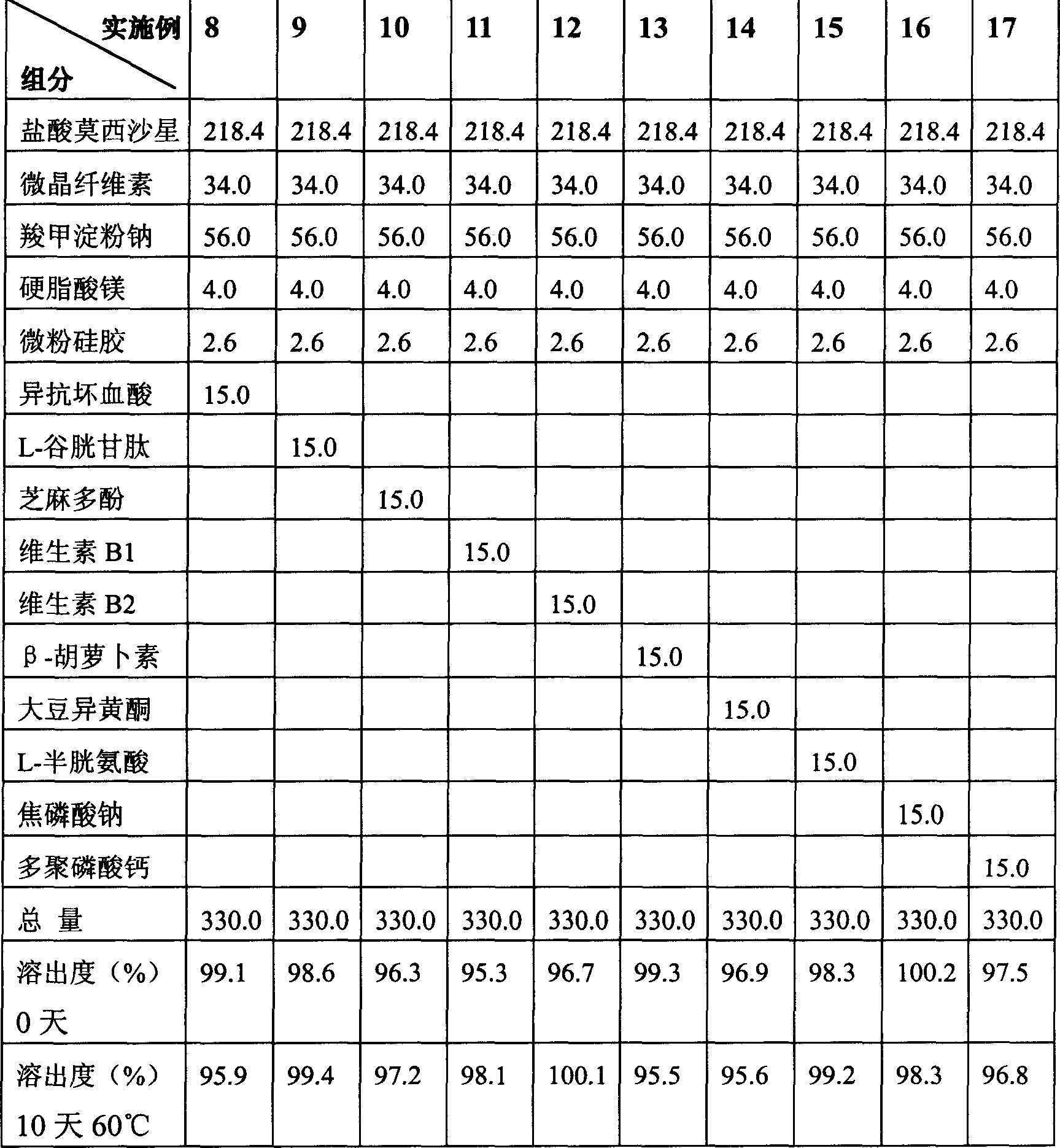
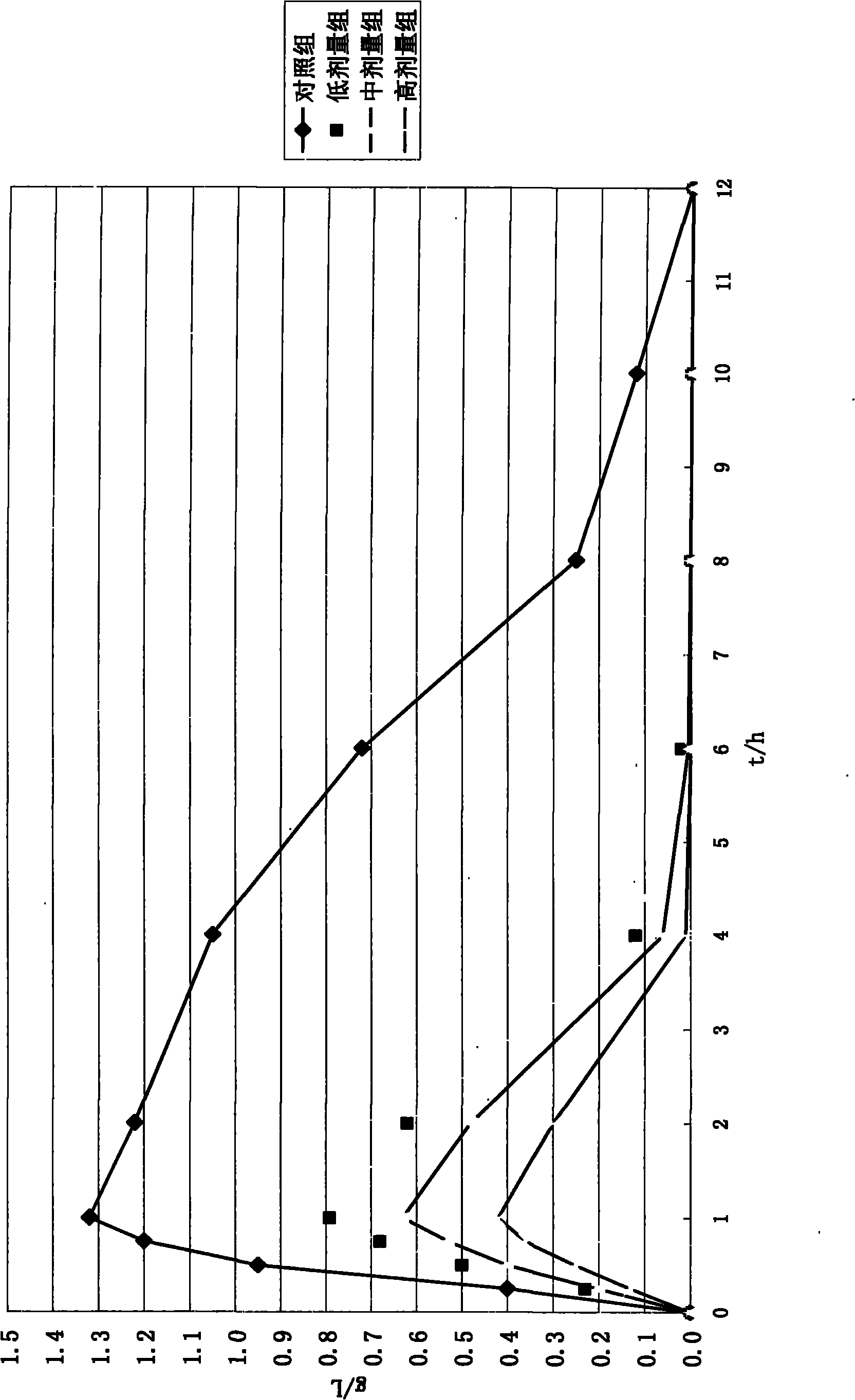
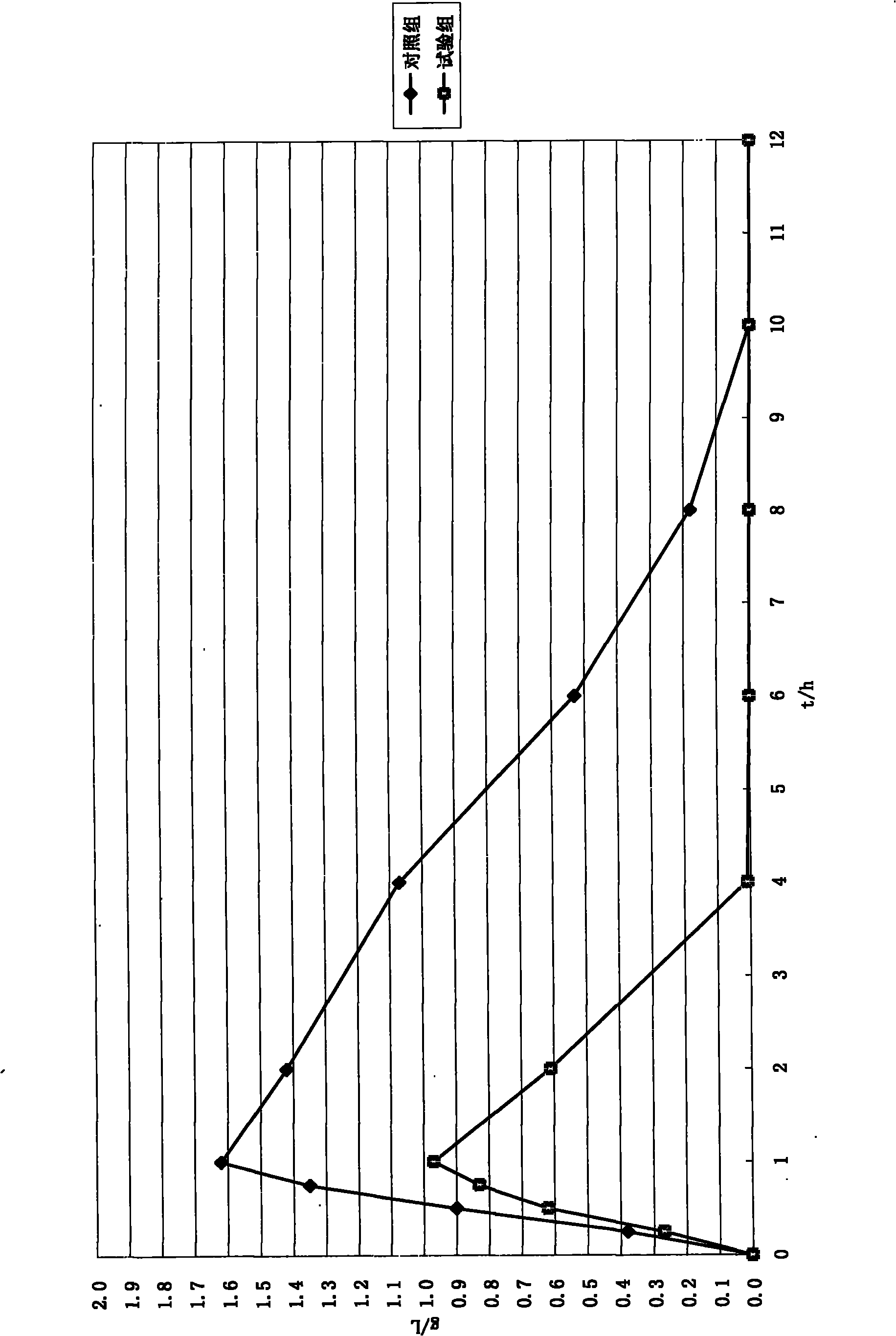
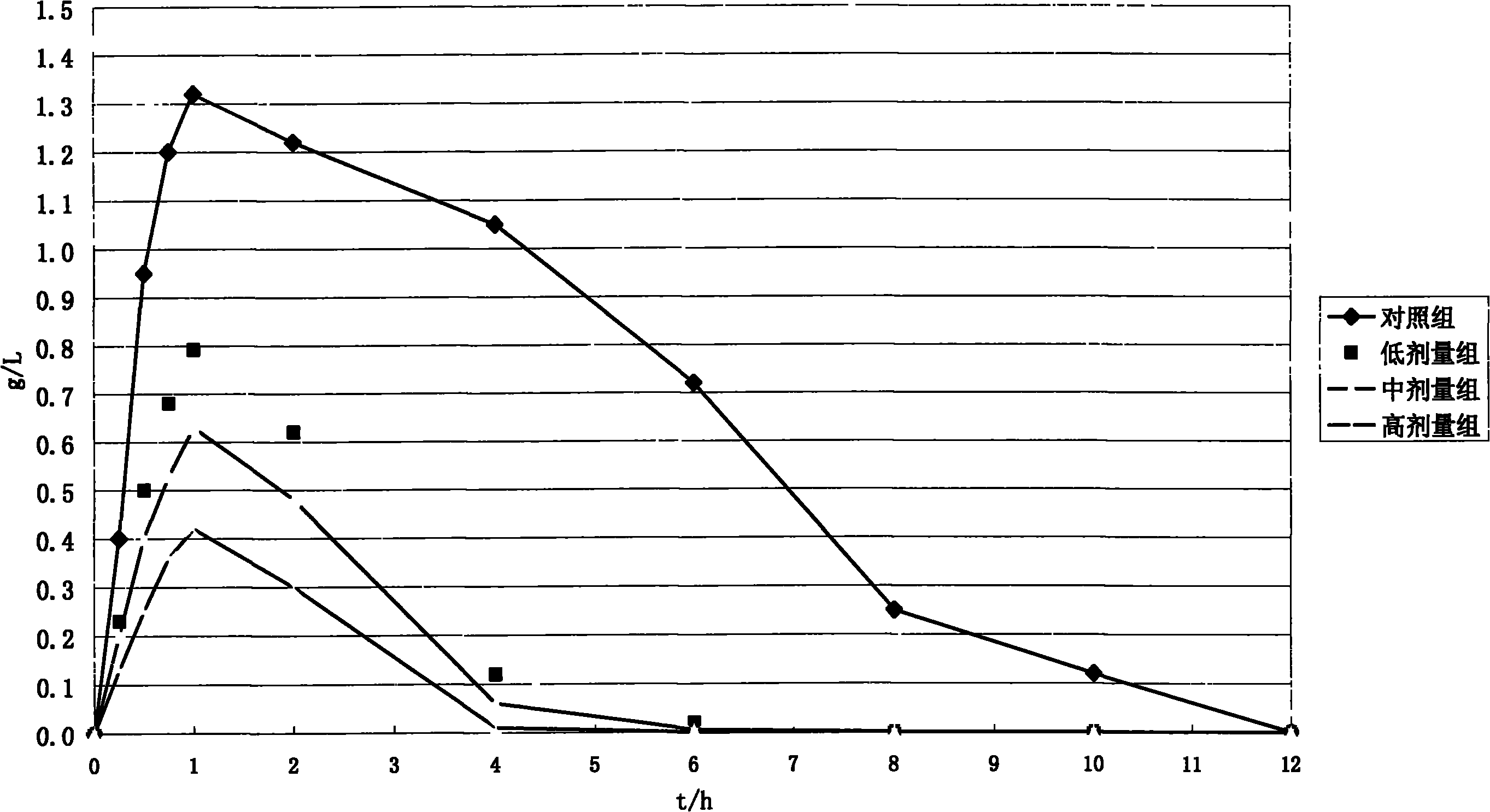
Moxifloxaci gelatin capsule and preparation process thereof
ActiveCN1736379APlay an inhibitory roleEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsBeta-CaroteneGreen Tea Polyphenols
The invention discloses a moxifloxacin gelatin capsule, whose contents comprises moxifloxacin or its salts and / or its hydrate and additive, the additive is at least one selected from the following substances: sodium sulfite, sodium acid sulfite, low-molecular proanthocyanidin, L-glutathion, sesame polyphenols, green tea polyphenols, tocopherol, ascorbic acid, isoascorbic acid, vitamin B1, vitamin B2, beta-carotene, soybean isoflavones, L-sulfo-aminolactic acid, pyrophosphoric acid, polyphosphoric acid and their medicinal salts.
Owner:JIANGSU TIANYISHI PHARMA
Composite for disintoxicating and sobering
The invention discloses a composite (B) for disintoxicating and sobering, which is prepared by glucose and fructose, fruit glucose, honey, L-cysteine, L-alanine, L-ornithine, L-glutamine, L-carnitine according to a certain weight range. In order to enable the effect better, one or more of taurine, L-asparaginic acid or L-aspartate, vitamin B1, vitamin B6, caffeine, L-arginine, L-glutamic acid, L-proline, phaseomannite, vitamin B2, nicotinic acid, folic acid, vitamin B12 and pantothenic acid are also added. The composite can be prepared into liquid preparation and electuary. The composite of the invention can be prepared into food and health food. The invention can quickly disintoxicate, sober, eliminate alcoholism symptom and continued effect of alcohol on a human body, and has no side effect.
Owner:克科
Retinol derivatives, their use in the treatment of cancer and for potentiating the efficacy of other cytotoxic agents
A group of new compounds, N-(all-trans-Retinoyl)-L-cysteic acid, N-(13-cis-Retinoyl)-L-cysteic acid, N-(all-trans-Retinoyl)-L-cysteinesulfinic acid, N-(13-cis-Retinoyl)-L-cysteinesulfinic acid, N-(all-trans-Retinoyl)-L-homocysteic acid, N-(13-cis-Retinoyl)-L-homocysteic acid, and sodium salts of these compounds, including sodium salts of their esters and amides, is shown to exhibit therapeutic effects per se, and which compounds in combination with cytotoxic compounds, such as docetaxel, paclitaxel, doxorubicin and mitoxantrone, exhibit a synergistic effect. These compounds make it possible to manufacture new formulations of poorly soluble pharmaceutical compounds, and the present invention discloses a process of manufacturing water-soluble formulations of such compounds, exemplified by docetaxel, and paclitaxel, exhibiting enhanced pharmacological activity, and formulations of water-soluble pharmaceuticals exemplified by doxorubicin and mitoxantrone, exhibiting improved therapeutic efficacy.
Owner:OASMIA PHARMA AB
Powder injection of compound glycyrrhizic acid glycosides and preparation method thereof
The present invention relates to compound glycyrrhizin type powder injection and the preparation method thereof, in particular to compound glycyrrhizin for injection, compound glycyrrhizic acid mono-ammonium S powder injection and the preparation method thereof, which are characterized in that the powder injection consisting of glycyrrhizin (or mono-ammonium glycyrrhizinate), glycine and cysteine hydrochloride that are taken as the active ingredient and the bearer acceptable in medicine and the preparation method are included in the prescription; wherein, the bearer acceptable in medicine contains dextran. The compound glycyrrhizin type powder injection of the present invention can be preserved at room temperature, thus remarkably improving the stability of the medicine, better guaranteeing safety and significance of the medicine and effectively decreasing the storage and transportation cost in production and transportation processes of the medicine.
Owner:TIBET WEIXINKANG MEDICINE CO LTD
Powderd injecta of compounded glycyrrhizin and preparing method
InactiveCN101023954AFull shapeUniform colorPowder deliveryOrganic active ingredientsSulfite saltSodium calciumedetate
The present invention relates to a compound glycyrrhizin powder injection preparation and its preparation method. It is made up by using monoammonium salt glycyrrhizinate, cysteine L-hydrochloride, glycine, anhydrous sodium sulfite, calcium sodium egtazate sodium hydroxide solution and sterile injection water through a certain preparation process.
Owner:XINMA MEDICINE SCI TECH SHENYANG
Acetylcysteine pharmaceutical composition and preparation method thereof
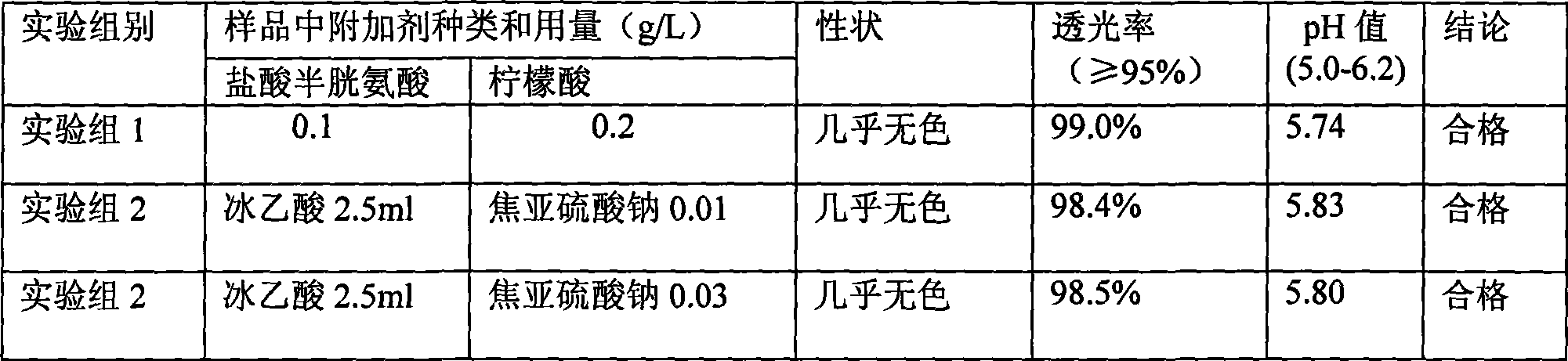
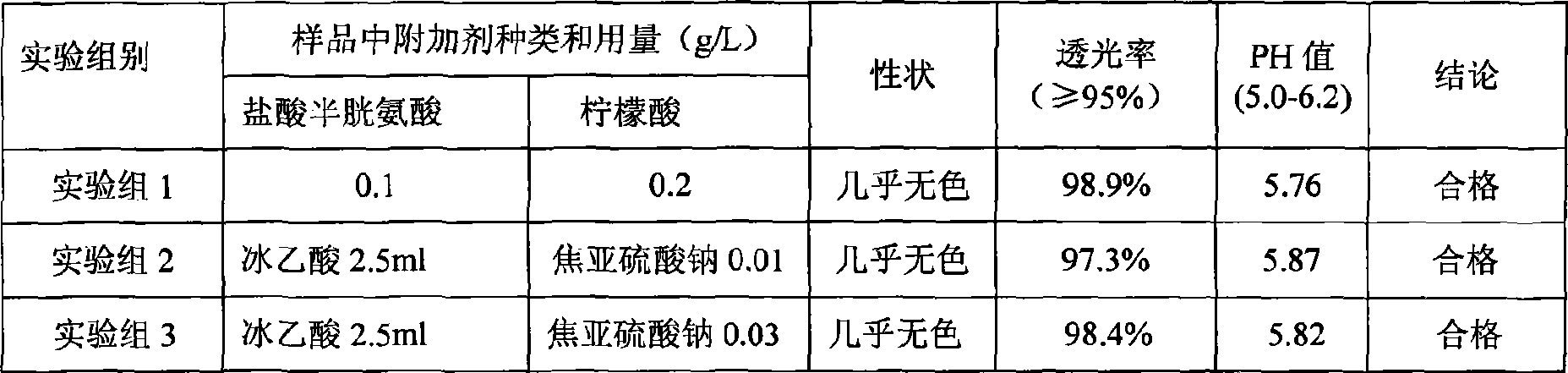
InactiveCN106692124AImprove stabilityQuality improvementOrganic active ingredientsDigestive systemCysteic acidAqueous solution
The invention aims to provide a more stable acetylcysteine pharmaceutical composition and a preparation method thereof. The acetylcysteine pharmaceutical composition comprises acetylcysteine and medically acceptable excipients, and the aqueous solution of the composition has a pH value of 7.0-7.5.
Owner:TIBET WEIXINKANG MEDICINE CO LTD
N-acetylcysteine pharmaceutical composition and preparation method thereof
InactiveCN102743369ASolve easy aggregationSolve the problem of not easy to mixOrganic active ingredientsDigestive systemSucroseAdditive ingredient
The invention discloses an N-acetylcysteine pharmaceutical composition and a preparation method thereof. The pharmaceutical composition comprises an active component and excipients, wherein the active component is N-acetylcysteine and the excipients are cane sugar, sweet orange essence, aspartame, sunset yellow and flow aid. The preparation method is as follows: first, granulating the excipients of cane sugar, sweet orange essence, aspartame and sunset yellow and drying the obtained granules and then carrying out size stabilization to the dried granules; and then mixing with the active component N-acetylcysteine and the flow aid to obtain the granular N-acetylcysteine pharmaceutical composition. The granular N-acetylcysteine pharmaceutical composition provided by the invention is simple in preparation, wherein the raw material N-acetylcysteine is more stable, difficult to be destroyed, and is easy to mix with other excipients.
Owner:苏州朗易生物医药研究有限公司
Natural vegetable hair-dyeing agent and preparation method thereof
InactiveCN108158940ASafe hair coloringLong-lasting safe dyeingCosmetic preparationsHair cosmeticsSide effectMonoglyceride
The invention discloses a natural vegetable hair-dyeing agent and a preparation method thereof. The natural vegetable hair-dyeing agent is prepared from a hair-dyeing cream agent I and a hair-dyeing cream agent II, wherein the hair-dyeing cream agent I is prepared from the following components in parts by weight: 65-75 parts of deionized water, 7-10 parts of cetyl alcohol, 5-8 parts of cysteine hydrochloride, 3-5 parts of octadecyl trimethyl ammonium chloride, 3-5 parts of propylene glycol, 3-5 parts of natural vegetable melanin, 3-5 parts of isopropyl palmitate, 2-3 parts of jojoba oil, 2-3 parts of monoglyceride stearate, 1-2 parts of lanolin and 0.5-1 part of essence. The natural vegetable hair-dyeing agent does not relate to oxidizing agents and reducing agents, does not stimulate thescalp and is not allergic, and the safety is superior to that of a permanent hair-dyeing agent. The hair-dyeing effect can be kept for one month or more in a lasting manner, and the washing-resistantdegree is superior that of a semipermanent hair-dyeing agent. The natural vegetable hair-dyeing agent is safe and healthy, has no side effect and does not stimulate the scalp.
Owner:广州市骄子日化有限公司
Inhalant acetylcysteine solution and preparation method of inhalant acetylcysteine solution
ActiveCN104800854AGood quality assuranceImprove securityOrganic active ingredientsPharmaceutical delivery mechanismMedicineCysteic acid
The invention belongs to the field of a medicine preparation and relates to an inhalant acetylcysteine solution and a preparation method of the inhalant acetylcysteine solution. Physiological seawater is added into the solution, so that the sensitization of the acetylcysteine solution is effectively reduced.
Owner:WUHAN WUYAO SCI & TECH
Method for detecting acetylcysteine and related substances thereof
InactiveCN101699281AGood acid and alkali toleranceGood practical valueComponent separationUltraviolet detectorsPhosphate
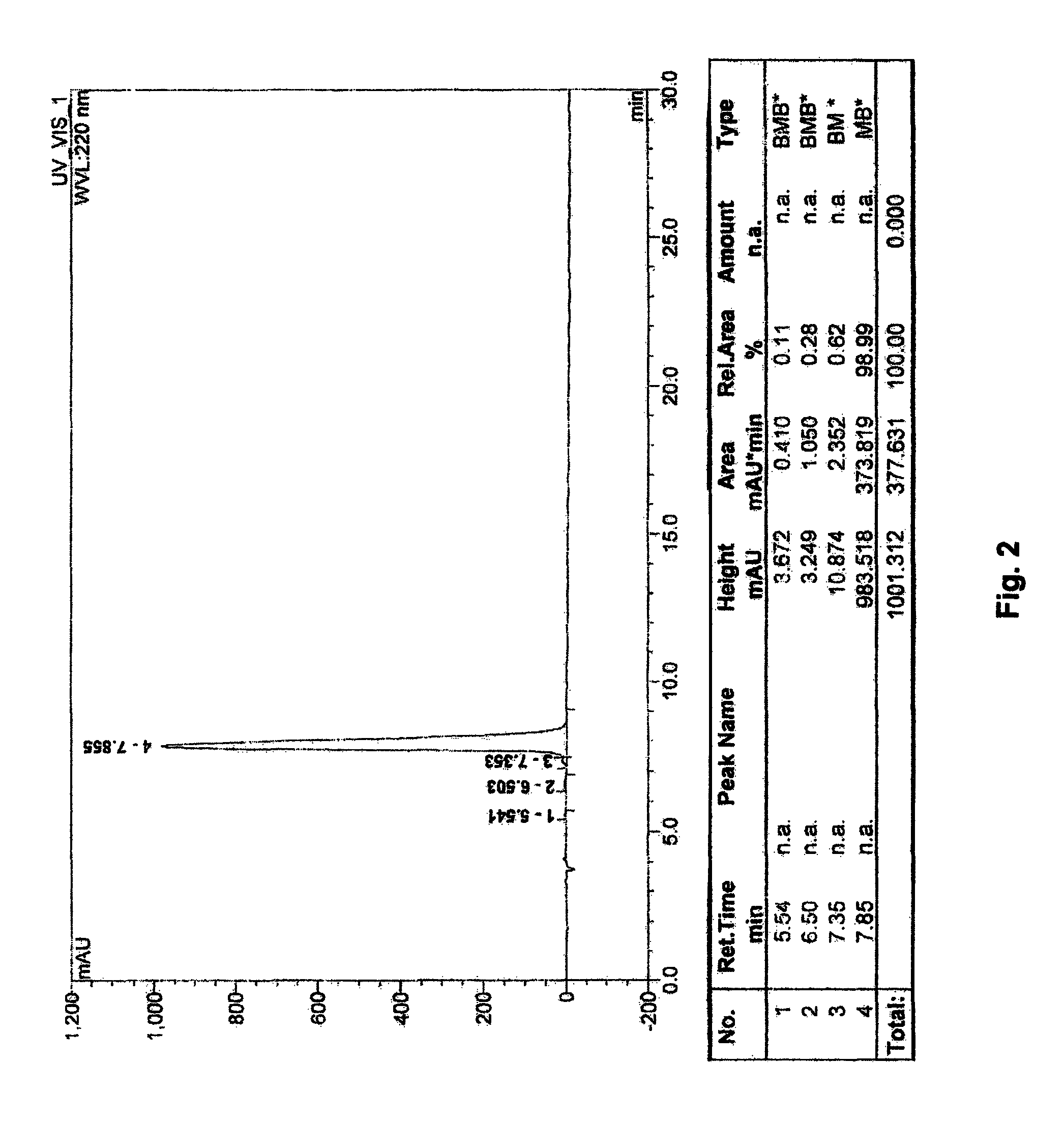
The invention relates to a method for detecting acetylcysteine and related substances thereof. The method adopts a high performance liquid chromatography, wherein the chromatographic condition is that styrene-divinylbenzene copolymer type superpolymer chromatographic column, namely MKF-RP-MH is 4 to 10 mu meters and (100-300)*(8.0-4.0) millimeters (I.D.); the volume ratio of phosphate buffer (pH is between 2 and 5) with mobile phase compositions of 0.01-0.05 mol / L to acetonitrile is 80-95:20-5; the flowing speed is between 0.8 and 1.2 mL / min; the detecting wavelength detected by an ultraviolet detector is 210nm; and the sample size is between 10 and 20 mu liters. The method does not use ionpairreagent, overcomes the defects that ODS column detecting method does not have acid resistance or low organic phase resistance when detecting the acetylcysteine.
Owner:NANJING UNIV OF TECH
Natural hair dye by taking coptis extract as dye
InactiveCN101559029ADoes not damage hairSmooth hairCosmetic preparationsHair cosmeticsSide effectHair dyes
The invention discloses an application of a coptis extract used as plant dye in hair dye. The hair dye comprises hair processing agent and a dye preparation, wherein the processing agent is prepared in such as way that thickening agent and water are added into thioglycollic acid amine, sodium thiosulfate, cysteine homologous compound and a derivative and salt thereof or the penetrating agent of clove, ginger and azone; and the dye preparation is prepared in such as way that the coptis extract is used as the plant dye, and the thickening agent and the water are added. During hair dyeing, the hair processing agent and the dye preparation are used together. The product is safe, has no poison and no side effect, and ensures that the dyed hair has smooth and nature hair quality and nature and stable color and luster.
Owner:闫征
Compound glycyrrhizin lyophilized powder injection and preparation method thereof
InactiveCN103860483APowder deliveryOrganic active ingredientsDipotassium hydrogen phosphateLiquid content
The present invention relates to a compound glycyrrhizin lyophilized powder injection and a preparation method thereof, particularly to compound glycyrrhizin for injection, a compound monoammonium glycyrrhizinate S lyophilized powder injection for injection, and a preparation method thereof. According to the present invention, the prescription contains a lyophilized powder injection comprising glycyrrhizin (monoammonium glycyrrhizinate) adopted as an active component, glycine, cysteine hydrochloride and a disodium hydrogen phosphate-sodium dihydrogen phosphate or dipotassium hydrogen phosphate-potassium dihydrogen phosphate pH buffer, and a preparation method of the lyophilized powder injection; and the drug liquid content is stable during the compound glycyrrhizin lyophilized powder injection preparation process, the finished product can be stored at a room temperature, the injection stability is significantly improved, the safety and the effectiveness of the injection are well ensured, and the warehousing transportation cost during the injection production storage and transportation process is effectively reduced.
Owner:北京藏卫信康医药研发有限公司
Injection acetylcysteine powdery medicinal composition and its making method
InactiveCN101028252AImprove stabilityLower serum bilirubinOrganic active ingredientsPowder deliveryDicarbonateSodium bicarbonate
An acetylcysteine composition in the form of powder injection with high solubility and stability is prepared from acetylcysteine and pH regulate chosen from sodium dicarbonate, sodium carbonate, sodium hydroxide and bisodium dicarbonate in weight ratio of 1: (0.25-1). Its preparing process is also disclosed.
Owner:何晶
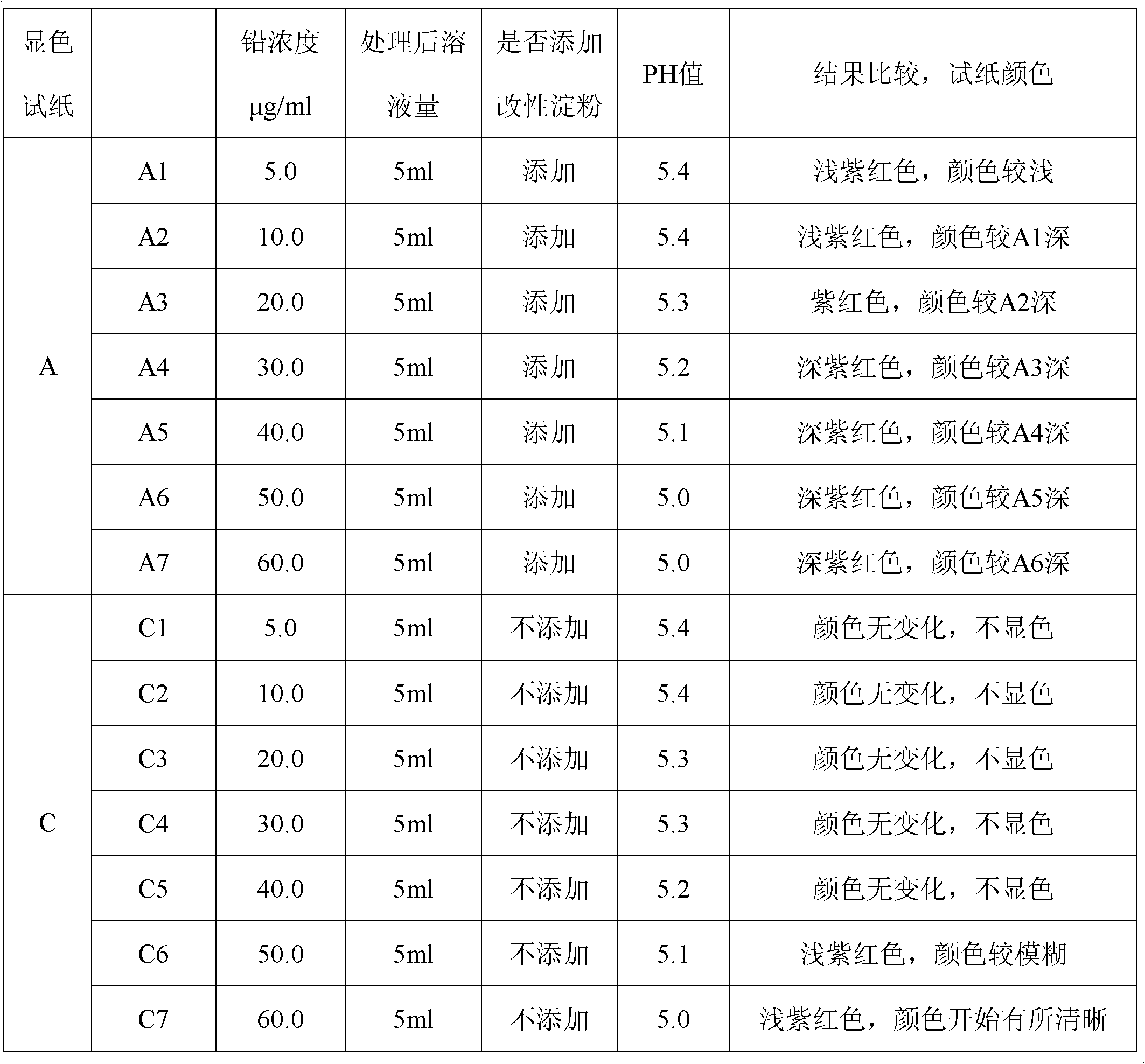
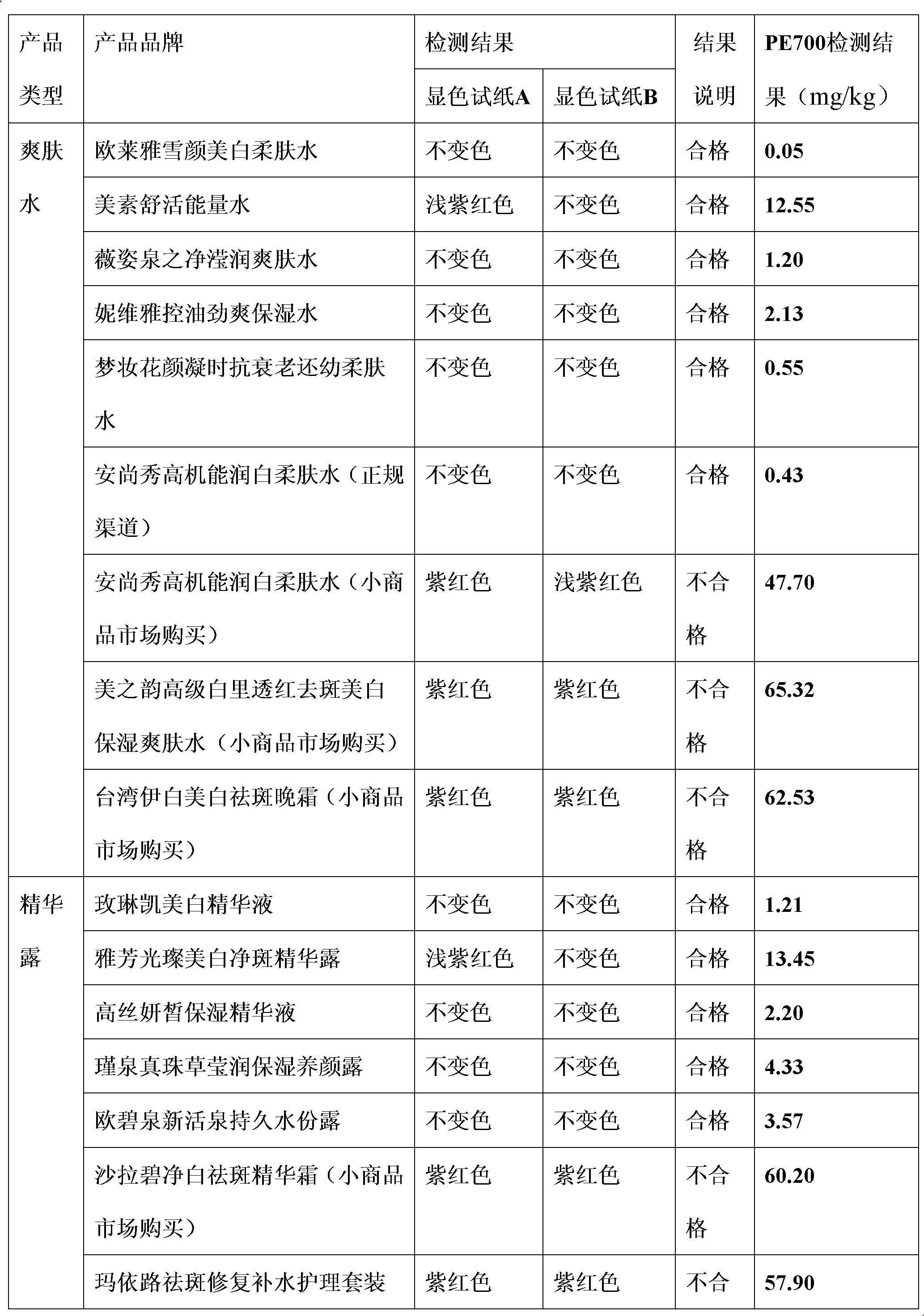
Qualitative and semiquantitative lead detection test paper and application thereof
InactiveCN102565051AImprove detection accuracyQuick monitoringMaterial analysis by observing effect on chemical indicatorEthylene diamineLow speed
The invention discloses qualitative lead detection test paper and semiquantitative lead detection test paper and application thereof. The qualitative lead detection test paper comprises developing test paper A which is obtained by the steps of soaking low-speed quantitative filter paper in a developer solution A, taking the low-speed quantitative filter paper out, and drying the low-speed quantitative filter paper at low temperature; the developer solution A is prepared from xylenol orange, thiourea, aminothiopropionic acid, modified starch and deionized water; and in the developer solution A, the concentration of the xylenol orange is 30 to 50mg / kg, the concentration of thiourea is 1 to 10mg / kg, the concentration of the aminothiopropionic acid is 1 to 10mg / kg, and the modified starch is 1 to 5mg / kg. The difference between the semiquantitative lead detection test paper and the qualitative lead detection test paper is that: a developer solution B which is used by the semiquantitative lead detection test paper contains ethylene diamine tetraacetic acid disodium salt at the concentration of 23.43mg / L except substances contained in the developer solution A. The qualitative lead detection test paper and the semiquantitative lead detection test paper can be used for qualitative and semiquantitative detection of lead in water cosmetics.
Owner:CHINA JILIANG UNIV
Therapeutic compounds
A group of new compounds, N-(all-trans-Retinoyl)-L-cysteic acid, N-(13-cis-Retinoyl)-L-cysteic acid, N-(all-trans-Retinoyl)-L-cysteinesulfinic acid, N-(13-cis-Retinoyl)-L-cysteinesulfinic acid, N-(all-trans-Retinoyl)-L-homocysteic acid, N-(13-cis-Retinoyl)-L-homocysteic acid, and sodium salts of these compounds, including sodium salts of their esters and amides, is shown to exhibit therapeutic effects per se, and which compounds in combination with cytotoxic compounds, such as docetaxel, paclitaxel, doxorubicin and mitoxantrone, exhibit a synergistic effect. These compounds make it possible to manufacture new formulations of poorly soluble pharmaceutical compounds, and the present invention discloses a process of manufacturing water-soluble formulations of such compounds, exemplified by docetaxel, and paclitaxel, exhibiting enhanced pharmacological activity, and formulations of water-soluble pharmaceuticals exemplified by doxorubicin and mitoxantrone, exhibiting improved therapeutic efficacy.
Owner:VIVESTO AB
Compound monoammonium glycyrrhizinate S pharmaceutical composition and method for preparing frozen powder injection
The invention belongs to the technical field of pharmaceutical preparations, in particular to a compound monoammonium glycyrrhizinate S pharmaceutical composition and a method for preparing a frozen powder injection. The composition is the monoammonium glycyrrhizinate S frozen powder injection and comprises the following components: monoammonium glycyrrhizinate S, cysteine hydrochloride, glycine, anhydrous sodium sulphite, sodium citrate, sodium chloride, sodium hydroxide and water for injection. The frozen powder injection is more stable and safer.
Owner:九瑞健康股份有限公司
Infusion solution for peripheral intravenous administration
There is provided an infusion solution for peripheral intravenous administration comprising a multichamber vessel constructed in a connectable manner, which separately houses a sugar solution containing sugars, electrolytes and vitamin B1, and an amino acid solution containing amino acids, electrolytes and a sulfite, whereby the vitamin B1 and amino acids are stably maintained. In the infusion solution for peripheral intravenous administration, the sugar solution contains calcium gluconate, contains no greater than 1.4 g / L sodium lactate, has a pH of 4.0-4.7 and has a titratable acidity of 2-4, the amino acid solution contains 4.0-8.0 g / L sodium lactate, contains no calcium gluconate, has a pH of 6.8-7.2, contains free L-cysteine as cysteine and contains 0.05-0.2 g / L sodium hydrogen sulfite, and after the sugar solution and amino acid solution have been mixed, the infusion solution has a pH of 6.5-7.1 and a titratable acidity of 5-10.
Owner:AJINOMOTO CO INC
Compositions and methods for inhibiting cellular adhesion or directing diagnostic or therapeutic agents to rgd binding sites
ActiveUS20110182989A1Avoid blindnessAlleviate macular tractionSenses disorderAntipyreticBinding siteCysteic acid
Compounds comprising R-G-Cysteic Acid (i.e., R-G-NH—CH(CH2—SO3H)COOH or Arg-Gly-NH—CH(CH2—SO3H)COOH) and derivatives thereof, including pharmaceutically acceptable salts, hydrates, stereoisomers, multimers, cyclic forms, linear forms, drug-conjugates, pro-drugs and their derivatives. Also disclosed are methods for making and using such compounds including methods for inhibiting cellular adhesion to RGD binding sites or delivering other diagnostic or therapeutic agents to RGD binding sites in human or animal subjects.
Owner:ALLEGRO PHARMA
Compound monoammonium glycyrrhizate S injection pharmaceutical composition as well as preparation method and application thereof
InactiveCN108553415AImprove stabilityAvoid it happening againOrganic active ingredientsDigestive systemSulfite saltDisodium Edetate
The invention relates to the technical field of medicines, in particular to a compound monoammonium glycyrrhizate S injection pharmaceutical composition as well as a preparation method and applicationthereof. The pharmaceutical composition is prepared from 400-600g of monoammonium glycyrrhizinate S, 300-500g of cysteine hydrochloride, 4.0-6.0kg of glycine, 400-650g of anhydrous sodium sulfite, 40-60g of disodium edetate, 1.0-3.0kg of sodium chloride and 100-200g of activated carbon; finally, fixing the volume to 180-450L by using water for injection. The preparation method of the pharmaceutical composition comprises the steps of weighing, concentrating, diluting, carrying out sterile filtration, filling and sealing. The compound monoammonium glycyrrhizate S injection pharmaceutical composition can be used for treatment of abnormal liver function caused by acute and chronic and persistent hepatitis, not only improves medicament stability, but also can avoid insoluble particles from generating in medicament, and is good in safety, better in efficacy and more remarkable in curative effect.
Owner:JILIN BAINIAN HANKE PHARM CO LTD
Amphiphilic chitosan-macadamia oil nano microcapsules and preparation method and application thereof
ActiveCN108992476AReduce volatilityReduce photothermal instabilityCosmetic preparationsDispersion deliveryWater insolubleStearic acid
The invention discloses an amphiphilic chitosan and its preparation. The amphiphilic chitosan is stearic acid, N-acetyl-L-cysteine grafted chitosan, and has a molecular weight of 10 to 300 kDa. The amphiphilic chitosan of the invention is used as a nano microcapsule embedding wall material, a hydrophobic substance can be embedded to form a water-soluble nano microcapsule solid, or can be used as the embedding wall material of a hydrophilic substance to form water-insoluble nano-capsules. The invention also discloses amphiphilic chitosan-macadamia oil nano microcapsules and a preparation methodand an application thereof. The nano microcapsule is formed by embedding a core material by the wall material, the core material is macadamia oil, and the wall material is the amphiphilic chitosan provided by the invention. The nano-microcapsules provided by the invention have the advantages of high embedding rate, good dispersibility and uniformity, and the macadamia oil has reduced volatility through embedding of the amphiphilic chitosan, and the stability and bioavailability are significantly improved.
Owner:AGRI PRODS PROCESSING RES INST CHINESE ACAD OF TROPICAL AGRI SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com