Compound glycyrrhizin lyophilized powder injection and preparation method thereof
A technology for glycyrrhizin and injection, which is applied in the field of compound glycyrrhizin freeze-dried powder injection and its preparation, and can solve the problems of opalescence, strict requirements on storage conditions, and inability to fully meet the requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
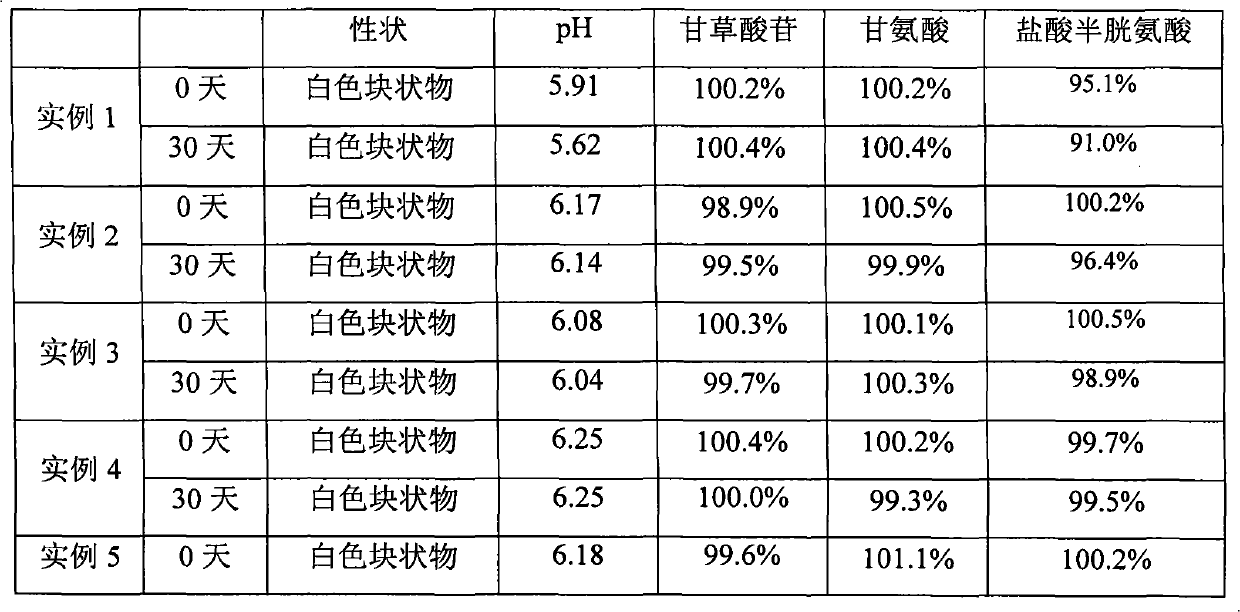
[0073] Prescription: monoammonium glycyrrhizinate S (calculated as glycyrrhizin) 400g, cysteine hydrochloride 200g, glycine 4000g.
[0074] Preparation method: Take an appropriate amount of water for injection and add it to the liquid mixing tank (barrel), add glycine to the liquid mixing tank (barrel), stir to dissolve; add glycyrrhizic acid monoammonium salt to the liquid mixing tank (barrel), stir until dissolved, add hydrochloric acid Dissolve cysteine with an appropriate amount of water for injection, add it into the liquid preparation tank (barrel) while stirring, adjust the pH value of the liquid medicine to the range of 5.5-7.5 with ammonia water, add 0.1% (g / ml) activated carbon for needles, and stir After 20-30 minutes, filter to remove charcoal, and rinse with appropriate amount of water. Calculate the dilute constant volume according to the batch size and prescription quantity, add water for injection to the constant volume (25000mL), stir for more than 10 minu...
Embodiment 2
[0076] Prescription: monoammonium glycyrrhizinate S (calculated as glycyrrhizin) 400g, cysteine hydrochloride 200g, glycine 4000g, anhydrous sodium sulfite 160g.
[0077] Preparation method: Take an appropriate amount of water for injection and add it to the liquid mixing tank (barrel), add glycine to the liquid mixing tank (barrel), stir and dissolve; add monoammonium glycyrrhizinate and anhydrous sodium sulfite to the liquid mixing tank (barrel), and stir until Dissolve, dissolve cysteine hydrochloride with an appropriate amount of water for injection, add it to the liquid preparation tank (barrel) while stirring, adjust the pH value of the liquid medicine to the range of 5.5 to 7.5 with ammonia water, add 0.1% (g / ml) needle Use activated carbon, stir for 20-30 minutes, filter to remove carbon, and rinse with appropriate amount of water. Calculate the dilute constant volume according to the batch size and prescription quantity, add water for injection to the constant vol...
Embodiment 3
[0079] Prescription: monoammonium glycyrrhizinate S (calculated as glycyrrhizin) 400g, cysteine hydrochloride 200g, glycine 4000g, disodium hydrogen phosphate 24.36g, sodium dihydrogen phosphate 13.73g.
[0080] Preparation method: Take an appropriate amount of water for injection and add it to the liquid mixing tank (barrel), add glycine to the liquid mixing tank (barrel), stir to dissolve; add glycyrrhizic acid monoammonium salt to the liquid mixing tank (barrel), stir until dissolved, add hydrochloric acid Dissolve cysteine with an appropriate amount of water for injection, add it to the liquid preparation tank (barrel) while stirring, add disodium hydrogen phosphate-sodium dihydrogen phosphate pH buffer, and adjust the pH value of the liquid to within the range of 5.5 to 7.5 with ammonia water , add 0.1% (g / ml) activated carbon for needles, stir for 20-30 minutes, filter to remove carbon, and rinse with appropriate amount of water. Calculate the dilute constant volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com