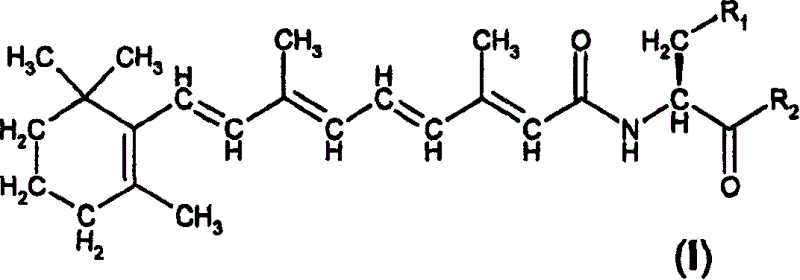
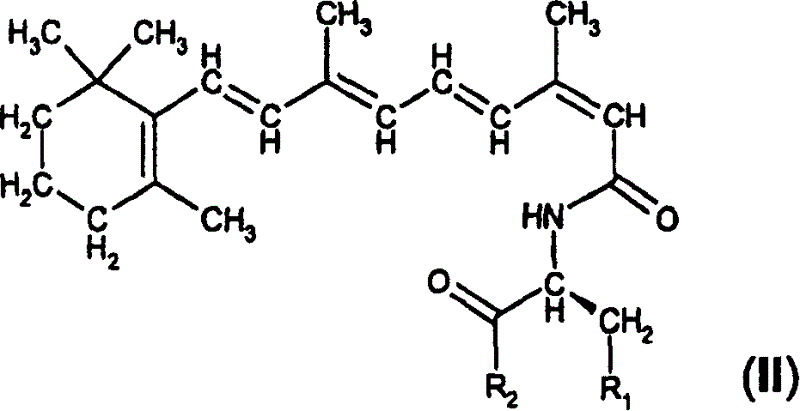
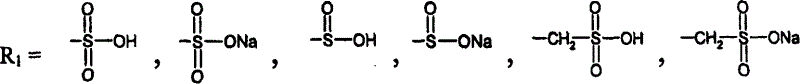Retinol derivatives, their use in the treatment of cancer and for potentiating the efficacy of other cytotoxic agents
A technology of solvents and substances, applied in the field of new compounds, can solve problems such as complex preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1. N-retinoyl-L-cysteine and amides and N-retinoyl-L-homo Synthesis of Cysteines and Amides
[0139] Synthesis of L-cysteine and L-homocysteine. Thionyl chloride (3mmol, 0.219ml) was added to 50ml of a suitable alcohol under stirring, and L-cystesulfonic acid monohydrate (1mmol, 187mg) or L-homocystesulfonic acid (1mmol , 183 mg), the mixture was refluxed for 48 hours. The alcohol was evaporated under reduced pressure, and the residue was recrystallized from ethanol. Yields between 85-95% were obtained for all esters.
[0140] Synthesis of L-cysteinesulfonic acid amides and L-homocystesulfonic acid amides. 10ml of an aqueous solution of the appropriate amine (28% NH 3 aqueous solution or 40% methylamine or dimethylamine in water) was added to 5 ml of an aqueous solution (1 mmol) of the methyl ester of the appropriate acid and the resulting solution was allowed to stand for 2-7 days. The solvent and excess amine were removed under reduced pressure and...
Embodiment 2
[0177] Example 2. Effect of compound I-1a in human breast cancer MDA-MB-231 cell line culture Cytotoxicity evaluation related to the final concentration of compound I-1a in the culture
[0178] A stock solution (1 mg / ml) of compound I-la was prepared by dissolving the dry matter in saline. From this solution, working solutions of different concentrations of Compound I-la in MEM containing 5% FBS were prepared by serial dilution for addition to the cultures.
[0179] On day 1 after inoculation, MDA-MB-231 cell cultures were treated with drug solution. Aliquots of working solution (2 μL) of different concentrations of compound I-1a were added to 200 μL of culture, so that the final concentration of compound I-1a in the culture was at 10 -9 -10 -7 between mol / L. In control cultures, 2 μL of medium containing 5% FBS was added as a solvent control.
[0180] After continuous culture for 2 days, the number of viable cells in the culture was counted, and the degree of growth i...
Embodiment 3
[0187] Example 3. Effect of compound I-1b in human breast cancer MDA-MB-231 cell line culture Cytotoxicity evaluation involving the final concentration of compound I-1b in the culture
[0188] A stock solution (1 mg / ml) of Compound I-1b was prepared by dissolving the dry matter in saline. From this solution, working solutions of different concentrations of compound I-lb in MEM containing 5% FBS were prepared by serial dilution for addition to the cultures.
[0189] On day 1 after inoculation, MDA-MB-231 cell cultures were treated with drug solution. Aliquot working solutions (2 μL) of different concentrations of compound I-1b were added to 200 μL of culture, so that the final concentration of compound I-1b in the culture was at 10 -9 -10 -7 between mol / L. In control cultures, 2 μL of medium containing 5% FBS was added as a solvent control. After continuous culture for 2 days, the number of living cells in the culture was counted, and the degree of growth inhibition of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com