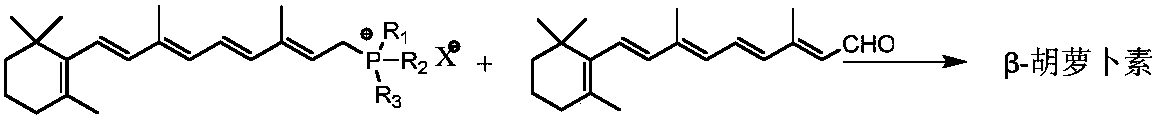
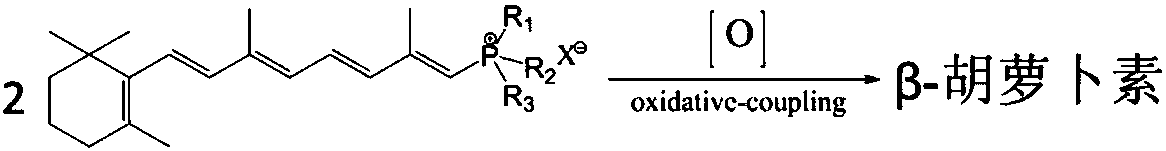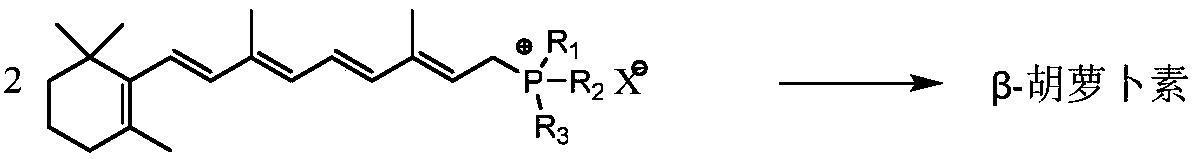Synthetic method for beta-carotene
A technology of carotene and synthesis method, applied in the field of synthesis of beta-carotene, can solve problems such as slow reaction rate and low product yield, and achieve the effects of easy handling, simple operation and improved catalytic oxidation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Coupling reaction prepares β-carotene
[0038] Take 90g of vitamin A acetate oil (2.213 million IU / g, 0.208mol) and 48.4g of aniline (0.52mol) and dissolve it in 1100ml of ethanol to prepare a vitamin A acetate-ethanol solution. Add 218.2g triphenylphosphine (0.832mol) and 2300ml ethanol respectively in the three-necked flask, cool down to below 10°C, slowly drop 31.0g concentrated sulfuric acid (0.316mol) into the dropping funnel, and control the dropping temperature below 10°C, After the dropwise addition, stirring was continued for 2h. Add vitamin A acetate-ethanol solution and react at 25°C for 24 hours to obtain vitamin A triphenylphosphine chloride solution. Then concentrate under reduced pressure, then add 200ml of acetone to dissolve, put it in the refrigerator for 24h to crystallize, filter, wash and dry the crystals to obtain vitamin A triphenylphosphine sulfate (the same below).
[0039] Vitamin A triphenylphosphine sulfate (1.0mmol), VO(acac)...
Embodiment 2
[0041] Take 90g of vitamin A acetate oil (2.213 million IU / g, 0.208mol) and 48.4g of aniline (0.52mol) and dissolve it in 1100ml of ethanol to form a vitamin A acetate-ethanol solution. Add 218.2g triphenylphosphine (0.832mol) and 2300ml ethanol respectively in the three-necked flask, cool down to 10°C, slowly add 51.5ml concentrated hydrochloric acid (mass concentration 37.5%, 0.624mol) dropwise through the dropping funnel, and control the dropping temperature to 10°C, after the dropwise addition, continue to stir for 2h. Add vitamin A acetate-ethanol solution and react at 25°C for 24 hours to obtain vitamin A triphenylphosphine chloride solution. Then concentrate under reduced pressure, then add 200ml of acetone to dissolve, put it in the refrigerator for 24h to crystallize, filter the crystals, wash, and dry to obtain vitamin A triphenylphosphine hydrobromide (the same below).
[0042] Vitamin A triphenylphosphine hydrochloride (1.0mmol), VO(OAc) 2 (0.1mmol), 4A molecular...
Embodiment 3
[0044] Take 90.96g vitamin A alcohol (2.9497 million IU / g, 0.281mol) and 15.4ml pyridine (0.191mol) and dissolve it in 500ml methanol to make vitamin A alcohol-methanol solution. Add 77.4g (0.295mol) of triphenylphosphine and 1000ml methanol respectively in the three-necked flask, cool down to 0°C, slowly add 45.4ml HBr (mass concentration is 47%, 0.393mol) dropwise through the dropping funnel, and control the dropping temperature to 0°C, continue to stir for 2h after the dropwise addition, add vitamin A alcohol-methanol solution, and react at 10°C for 24h. Then concentrate under reduced pressure, then add 200ml of acetone to dissolve, put it in the refrigerator for 24h to crystallize, filter the crystals, wash, and dry to obtain vitamin A triphenylphosphine hydrobromide (the same below).
[0045] Vitamin A triphenylphosphine hydrobromide (1.0mmol), NH 4 VO 3 (0.1mmol), 4A molecular sieve (0.3g), 12-crown-4 (2.0mmol) and KOH (2.0mmol) were added in tetrahydrofuran (20ml), an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com