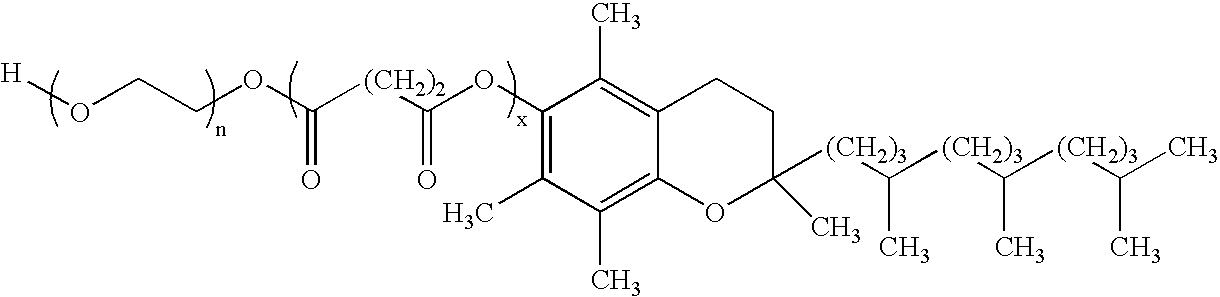
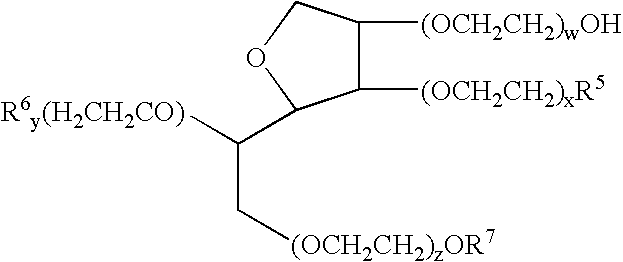
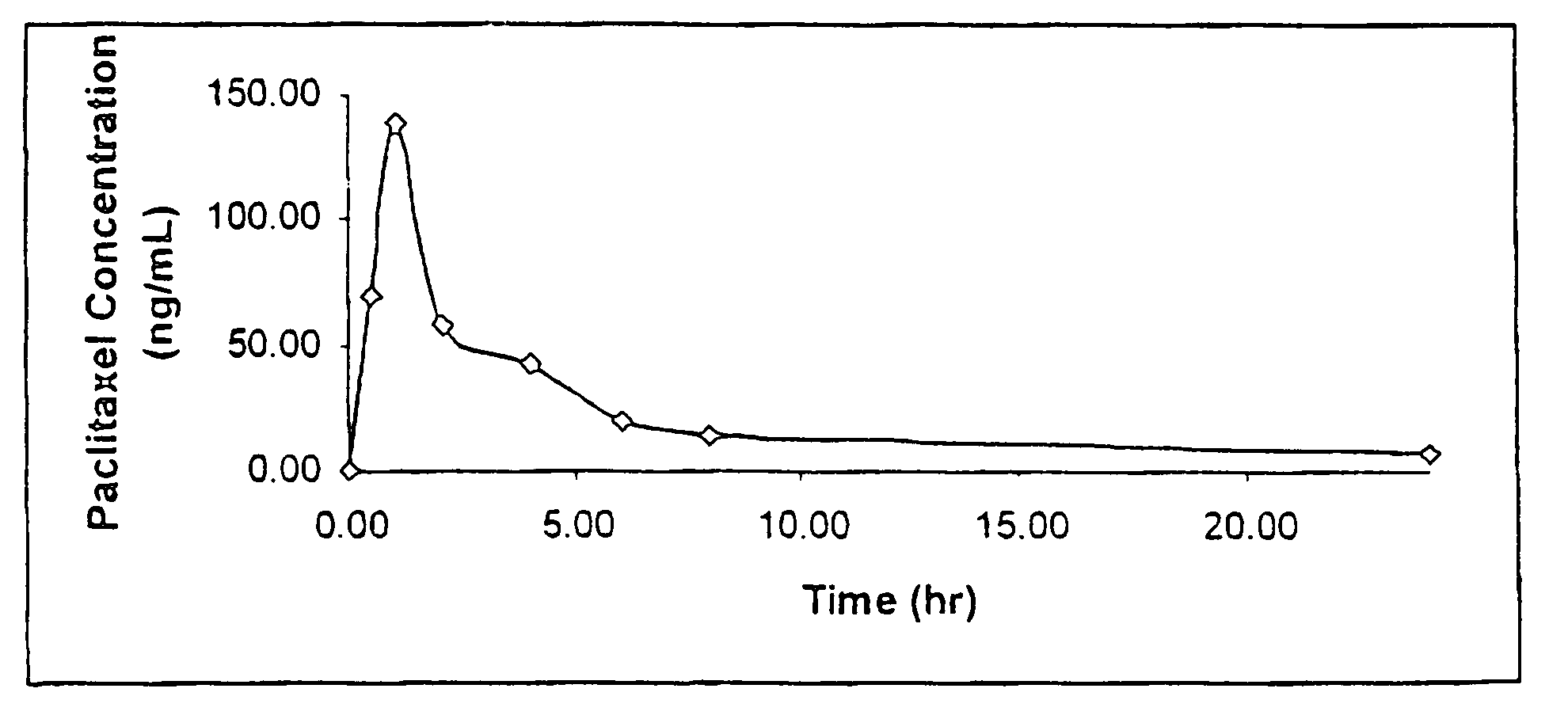
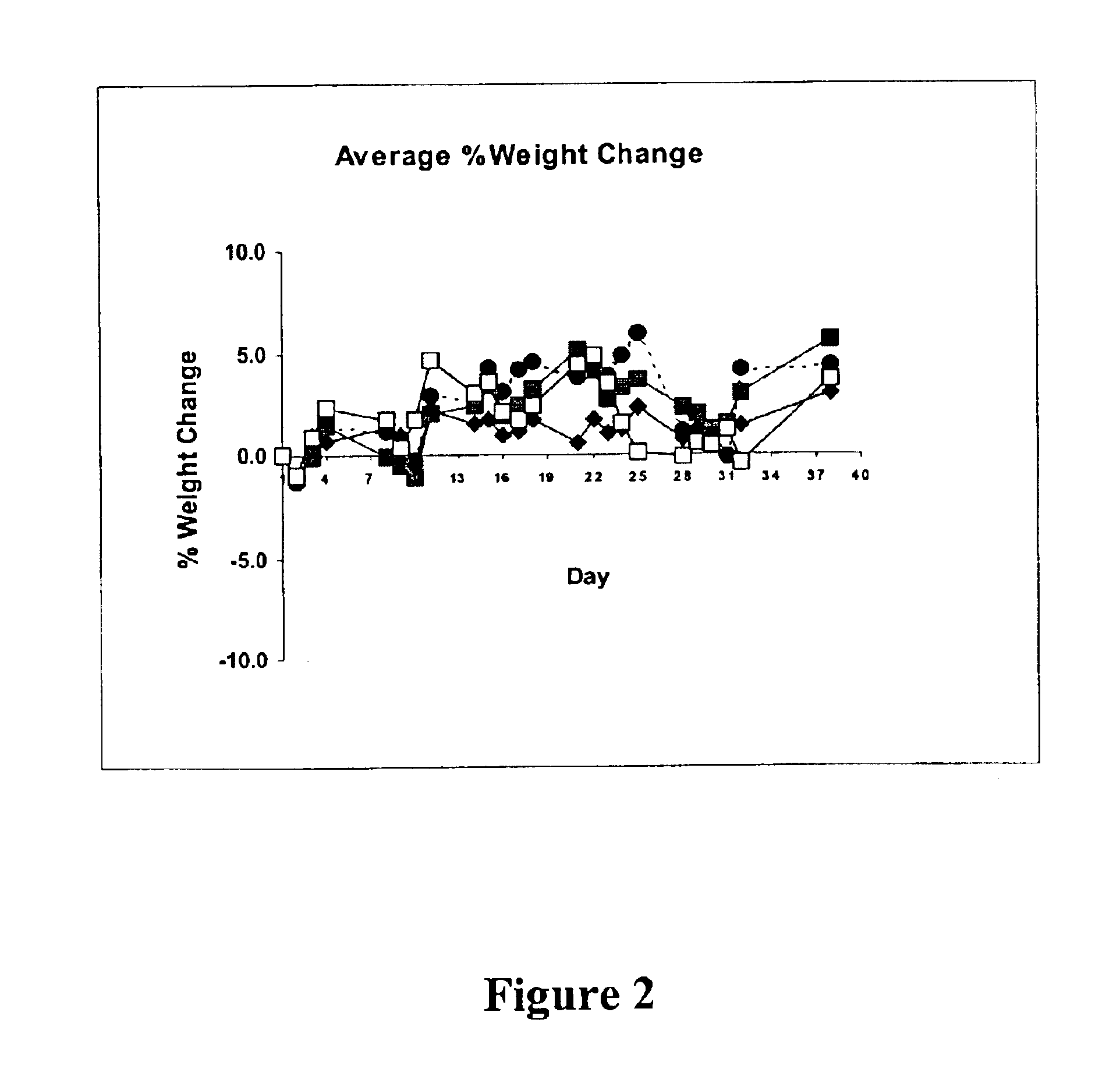
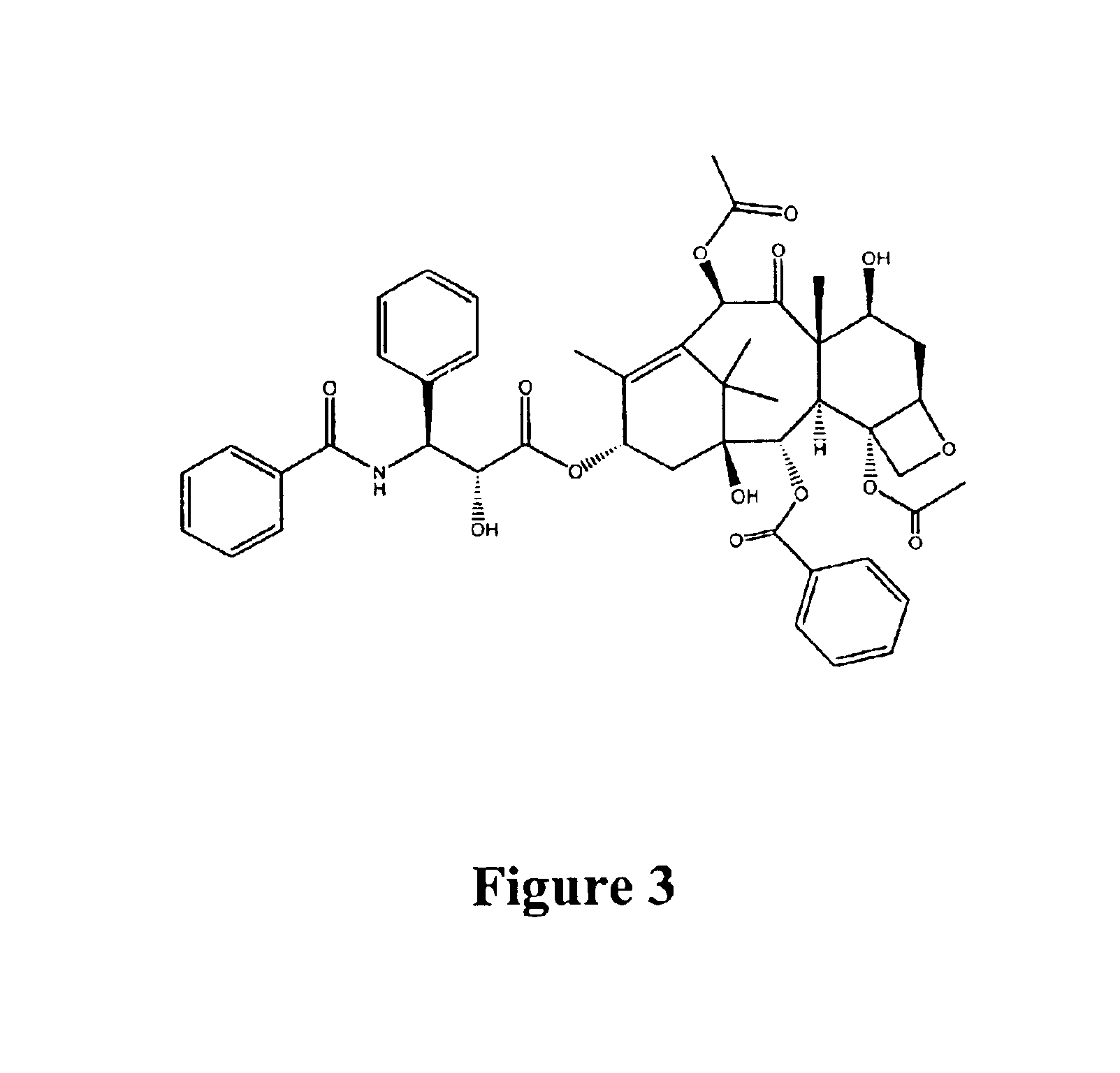
Patents
Literature
1001 results about "Auraptene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
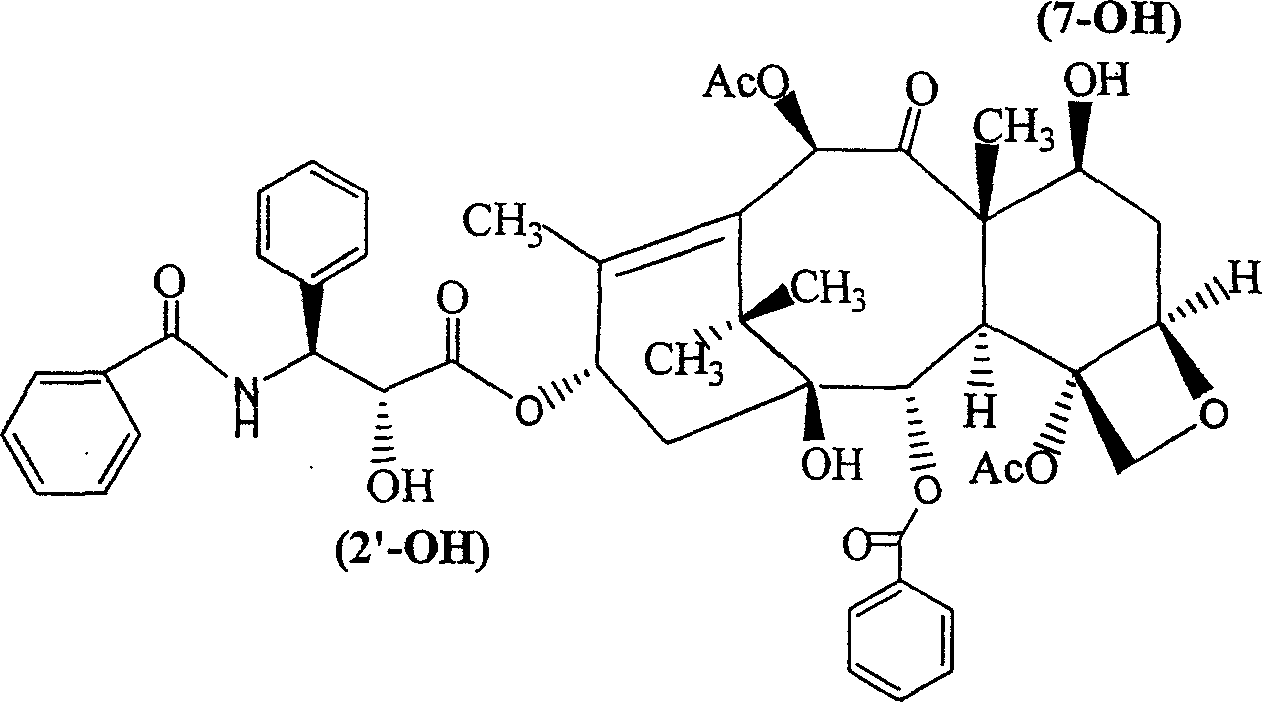
Auraptene is a natural bioactive monoterpene coumarin ether. It was first isolated from members of the genus Citrus. Auraptene has shown a remarkable effect in the prevention of degenerative diseases. Many studies have reported the effect of auraptene as a chemopreventative agent against cancers of liver, skin, tongue, esophagus, and colon in rodent models. The effect in humans is not yet known.
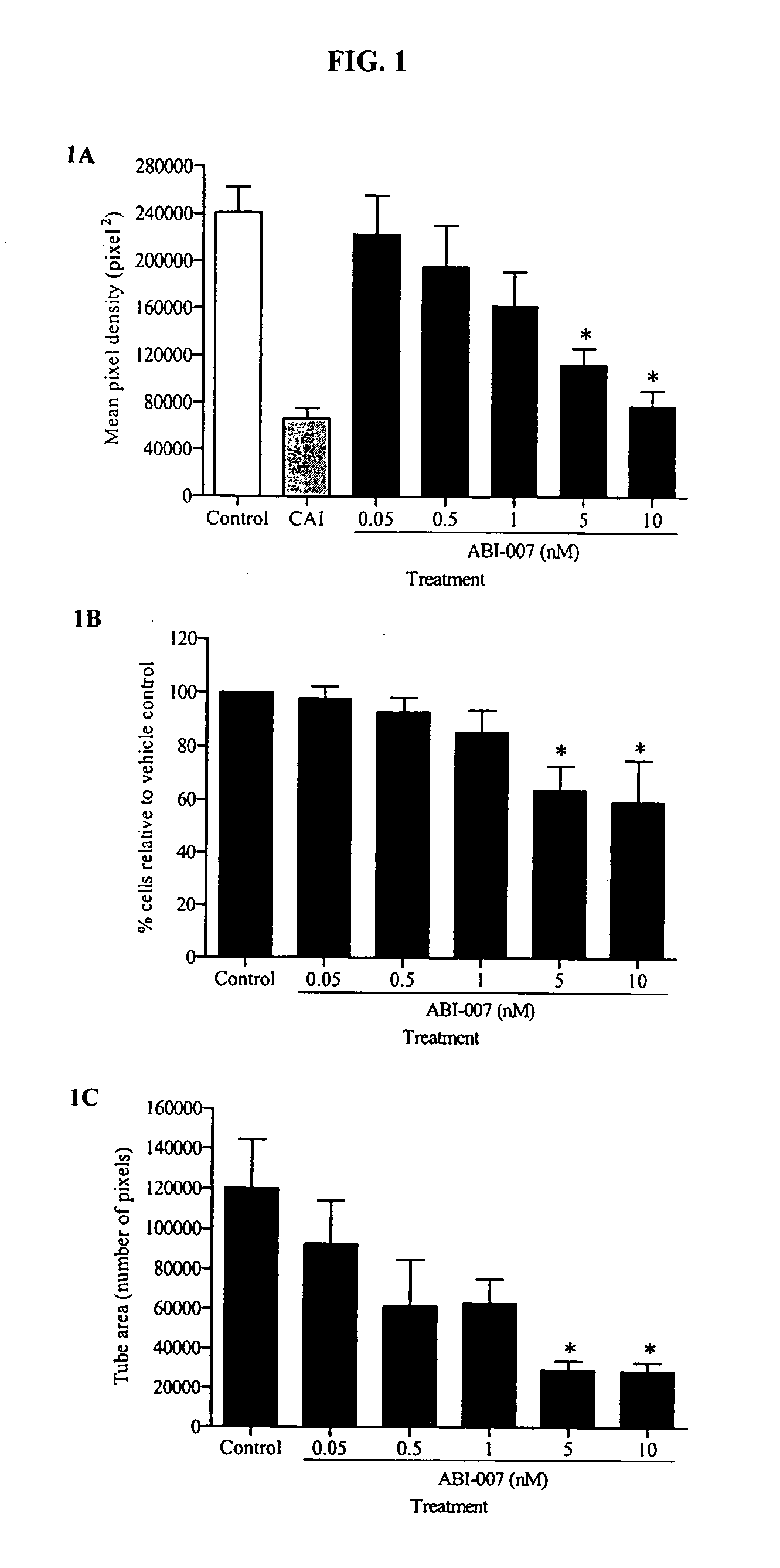
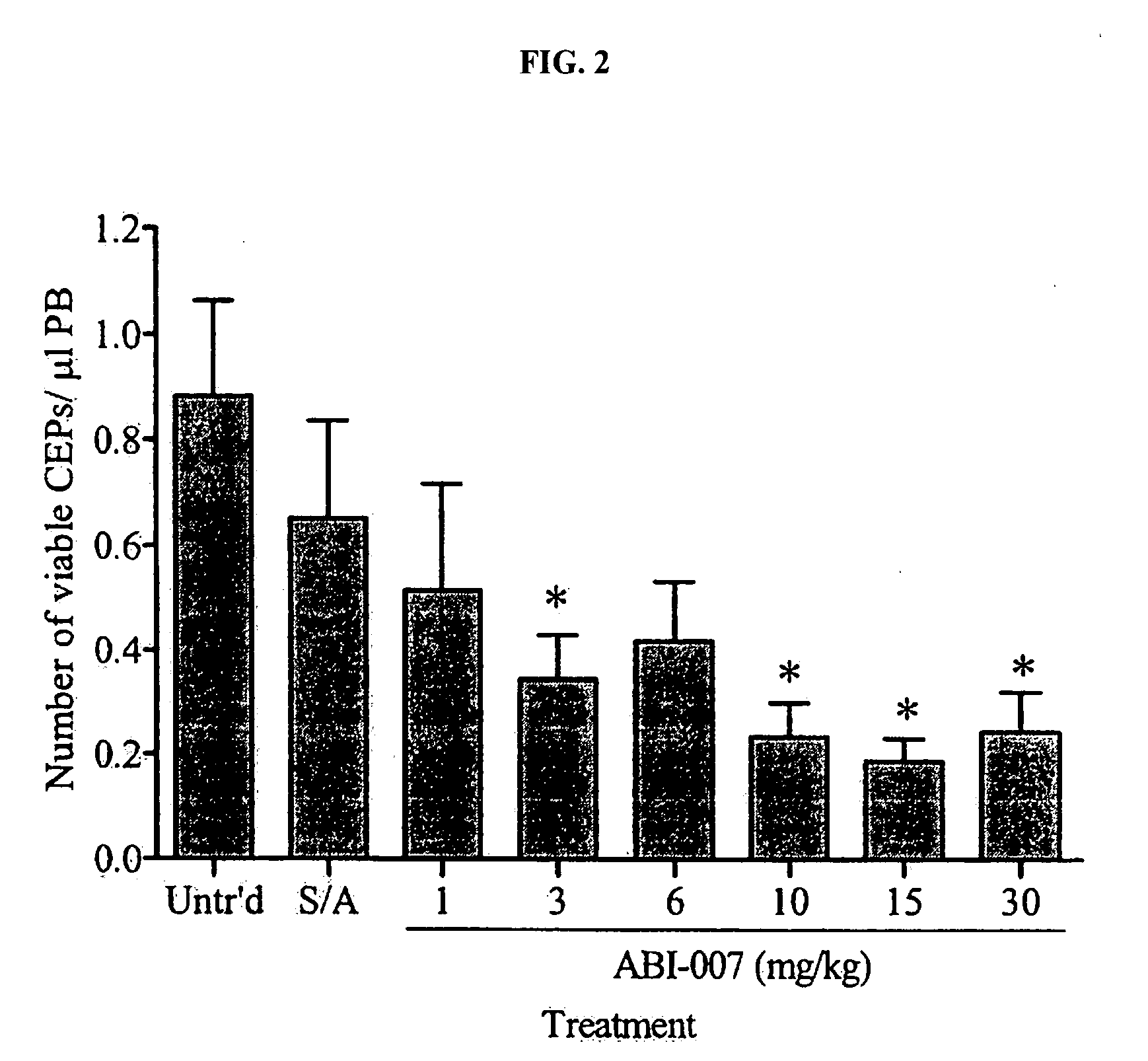
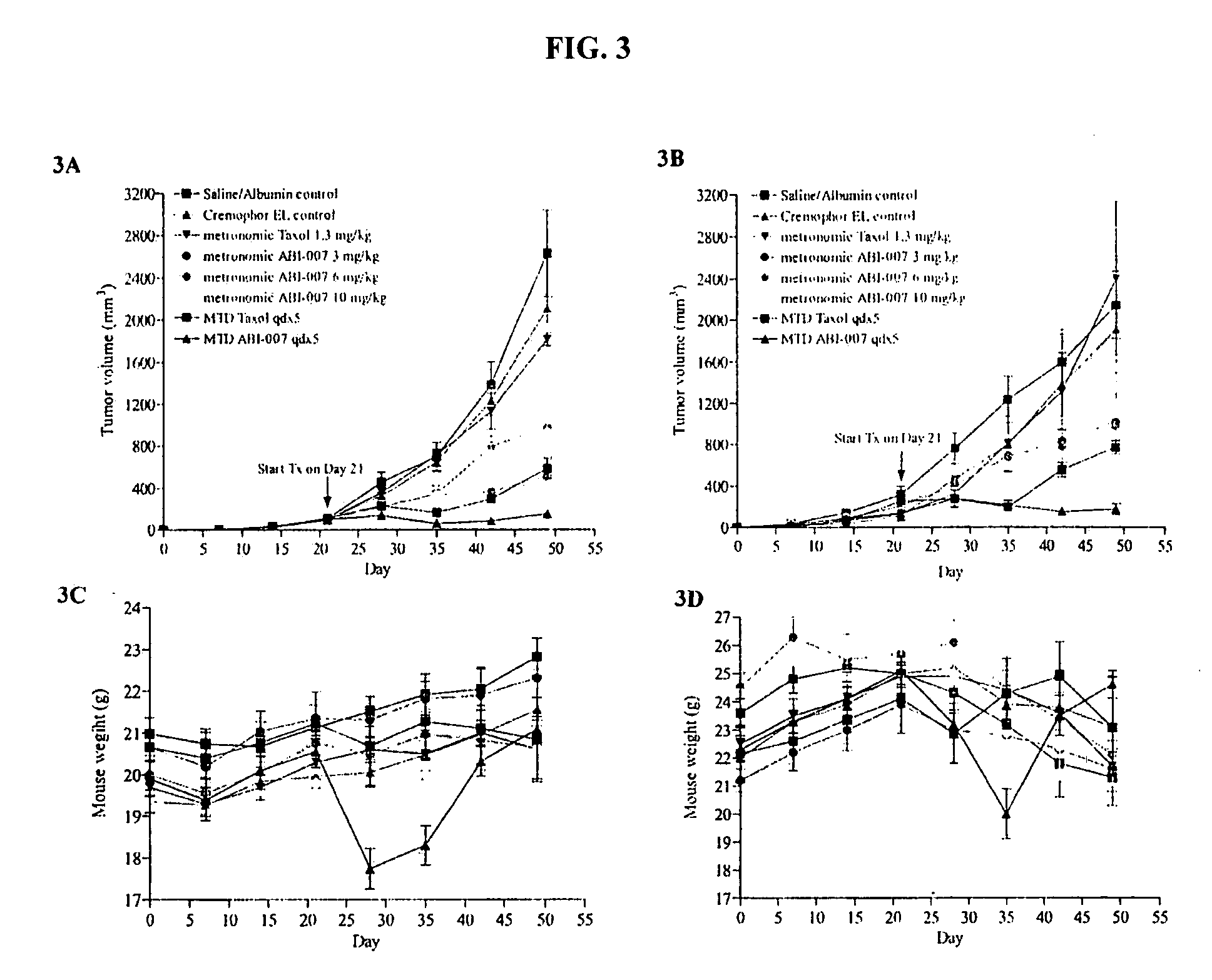
Nanoparticles of paclitaxel and albumin in combination with bevacizumab against cancer
InactiveUS20100112077A1Organic active ingredientsHeavy metal active ingredientsBevacizumab InjectionAnti vegf antibody
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents (such as an anti-VEGF antibody), or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
Treatment of Asthma and Chronic Obstructive Pulmonary Disease With Anti-proliferate and Anti-inflammatory Drugs
InactiveUS20080175887A1Promote absorptionOrganic active ingredientsPowdered material dispensingDiseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a method for treatment of respiratory disorders such as asthma, chronic obstructive pulmonary disease, and chronic sinusitis, including cystic fibrosis, interstitial fibrosis, chronic bronchitis, emphysema, bronchopulmonary dysplasia and neoplasia. The method involves administration, preferably oral, nasal or pulmonary administration, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues).
Owner:LUTONIX INC
Formulation for paclitaxel
A pharmaceutical formulation is provided for delivering paclitaxel in vivo comprising: water and micelles comprising paclitaxel and a pharmaceutically-acceptable, water-miscible solubilizer forming the micelles, the solubilizer selected from the group consisting of solubilizers having the general structuresR1COOR2, R1CONR2, and R1COR2,wherein R1 is a hydrophobic C3-C50 alkane, alkene or alkyne and R2 is a hydrophilic moiety. The solubilizer is selected such that it does not have a pKa less than about 6.
Owner:SUPERGEN
Oral pharmaceuticals formulation comprising paclitaxel, derivatives and methods of administration thereof
InactiveUS20040092428A1Improve bioavailabilityImprove oral bioavailabilityOrganic active ingredientsCyclic peptide ingredientsOral medicationBioavailability
The invention concerns excipients or combinations thereof suitable for preparing an oral formulation containing a pharmaceutical agent. More particularly, the invention is directed to stable, efficacious and bioavailable oral pharmaceutical formulations comprising paclitaxel, derivatives of paclitaxel and pharmaceutically acceptable salts thereof. The formulations of the invention increase bioavailability of paclitaxel when dissolved in the gastrointestinal system. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts of such derivatives to patients in need thereof. The formulations of the invention are particularly suitable for oral administration to mammals including humans.
Owner:TRANSFORM PHARMACEUTICALS INC
Anticancer compositions
Pharmaceutical dosage forms for anticancer drugs, and paclitaxel in particular, are described in which the active drug is formulated as storage stable self-emulsifying preconcentrate.
Owner:RTP PHARMA
Process for preparation of paclitaxel trihydrate and docetaxel trihydrate
A process for converting paclitaxel or docetaxel to the respective trihydrate characterized by very high purity, comprises dissolving either paclitaxel or docetaxel in a mixture of alkane and chlorinated alkane to provide a crude product of 65-75% assay and dissolving the crude product in an alkyl ketone, followed by addition of an alkane to provide a product of increased chromatographic purity; dissolving the product of increased chromatographic purity in an aliphatic nitrile, with addition of water to precipitate taxane trihydrate.
Owner:DABUR PHARM LTD
Oral formulation for paclitaxel
A pharmaceutical formulation is provided for delivering paclitaxel in vivo comprising: water and micelles comprising paclitaxel and a pharrnaceutically-acceptable, water-miscible solubilizer forming the micelles, the solubilizer selected from the group consisting of solubilizers having the general structureswherein R1 is a hydrophobic C3-C50 alkane, alkene or alkyne and R2 is a hydrophilic moiety. The solubilizer is selected such that it does not have a pKa less than about 6.
Owner:SUPERGEN
Pharmaceutical formulations comprising paclitaxel, derivatives, and pharmaceutically acceptable salts thereof
The invention concerns paclitaxel solubilizers and formulations thereof with a high propensity to dissolve paclitaxel. The formulations of the invention reduce or obviate the need for the disadvantageous excipient Cremophor(R) EL. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts or such derivatives to patients in need thereof. The formulations of the invention are suitable for parenteral, oral, local, or transdermal administration to mammals including humans, particularly for intravenous delivery.
Owner:TRANSFORM PHARMACEUTICALS INC
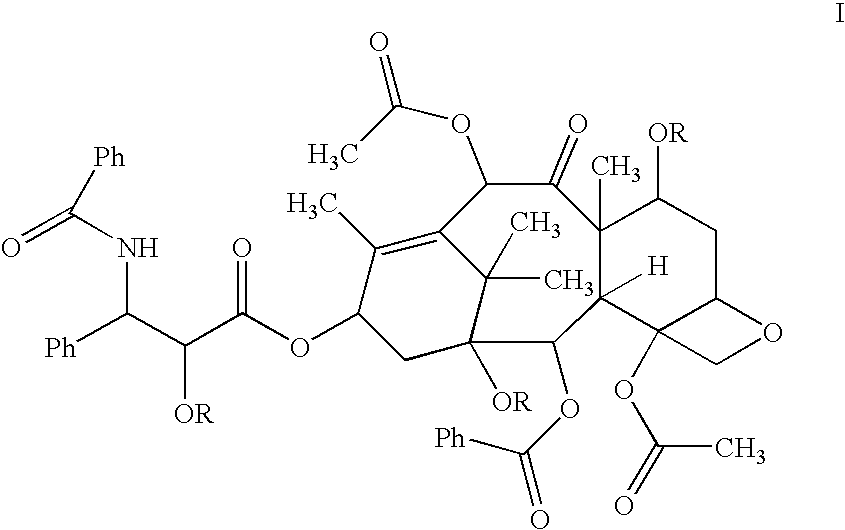
Taxol enhancer compounds
InactiveUS6924312B2High anticancer activityImprove efficiencyBiocideOrganic chemistryArylStructural formula
Disclosed is a compound represented by the Structural Formula (I): Y is a covalent bond, a phenylene group or a substituted or unsubstituted straight chained hydrocarbyl group. In addition, Y, taken together with both >C═Z groups to which it is bonded, is a substituted or unsubstituted aromatic group. Preferably, Y is a covalent bond or —C(R7R8)—.R1 and R2 are independently an aryl group or a substituted aryl group, R3 and R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R5-R6 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R7 and R8 are each independently —H, an aliphatic or substituted aliphatic group, or R7 is —H and R8 is a substituted or unsubstituted aryl group, or, R7 and R8, taken together, are a C2-C6 substituted or unsubstituted alkylene group.Z is ═O or ═S.Also disclosed are pharmaceutical compositions comprising the compound of the present invention and a pharmaceutically acceptable carrier or diluent. Also disclosed is a method of treating a subject with cancer by administering to the subject a compound of Structural Formula (I) in combination with taxol or an analog of taxol.
Owner:SYNTA PHARMA CORP
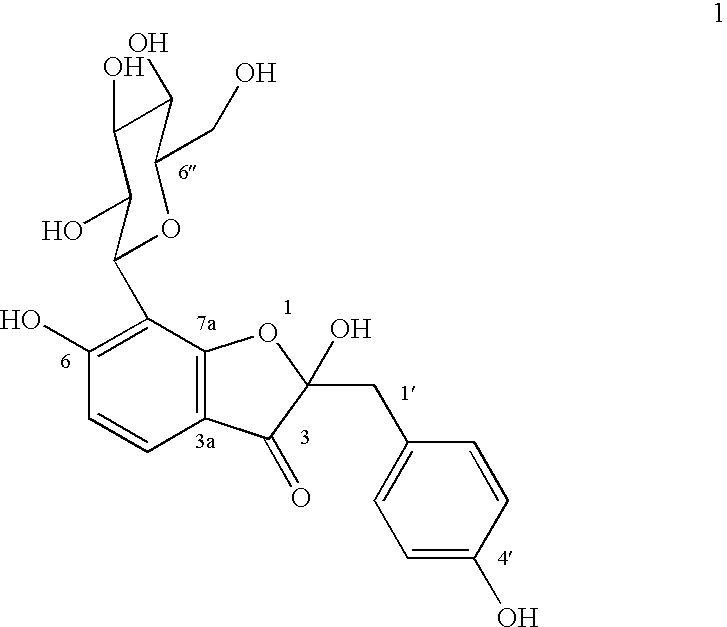
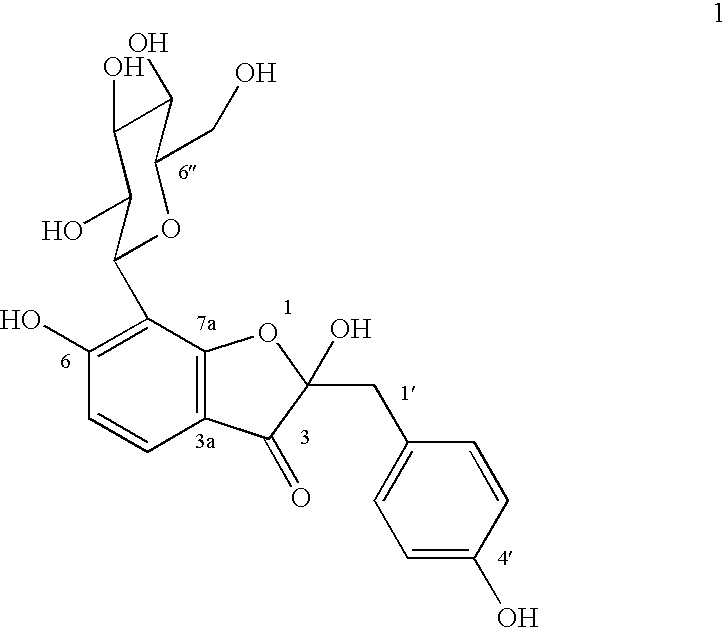
Glucopyranoside and process of isolation thereof from pterocarpus marsupium pharmaceutical composition containing the same and use thereof
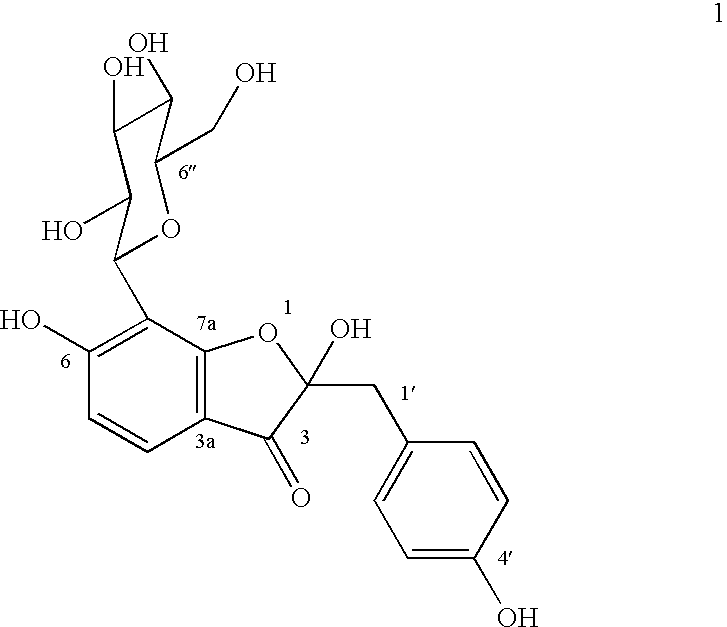
A novel glucopyranoside, 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H) benzofuran-7-C-beta-D-glucopyranoside of the formula 1isolated from Pterocarpus marsupium and to a process for the isolation thereof is disclosed. The invention also relates to a pharmaceutical composition containing 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H)benzofuran-7-C-beta-D-glucopyranoside and to method for the treatment of diabetes using said compound.
Owner:COUNCIL OF SCI & IND RES
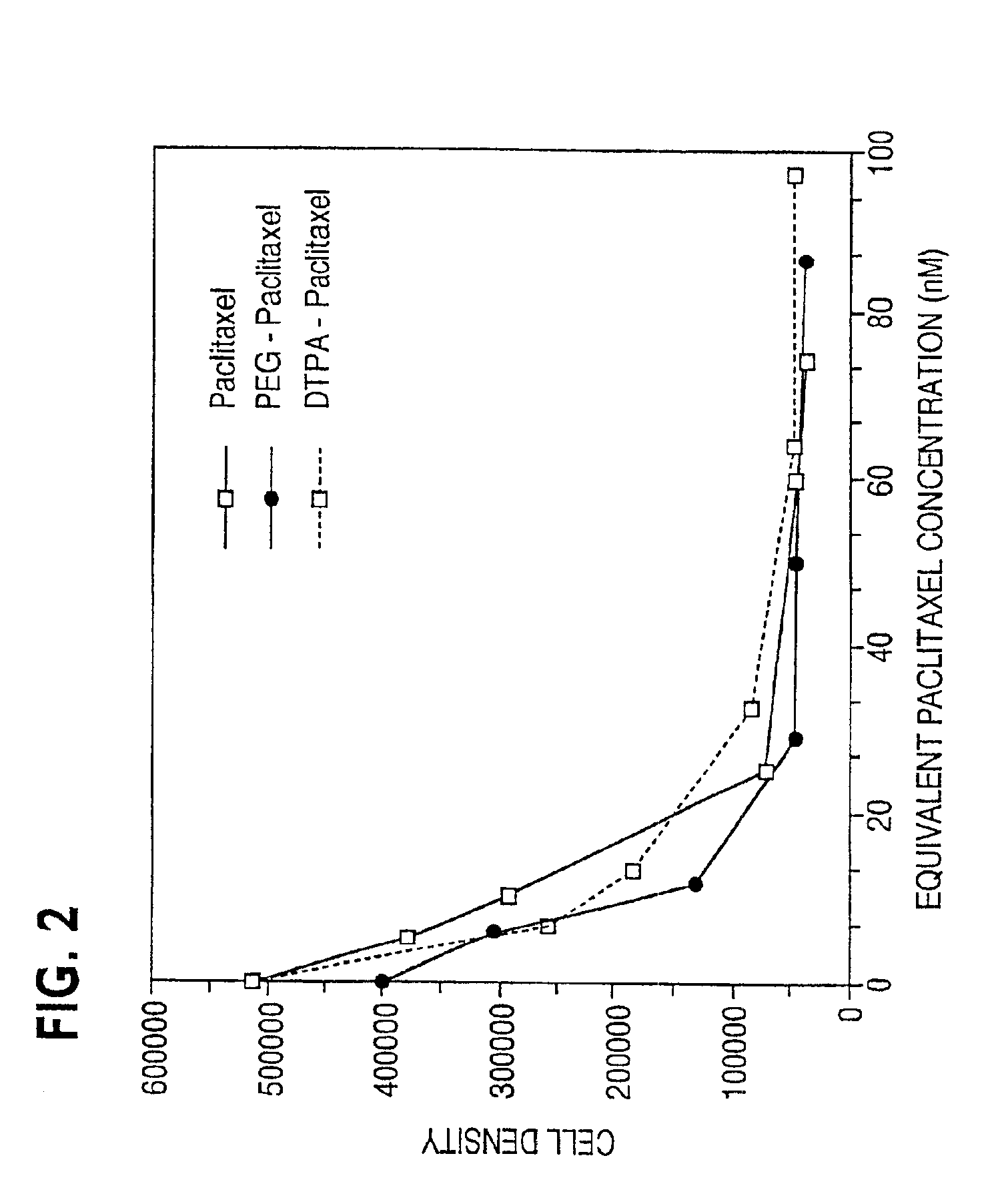
Water soluble paclitaxel derivatives
InactiveUS6884817B2Surprising antitumor activityImprove efficacyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Disclosed are water soluble compositions of paclitaxel and docetaxel formed by conjugating the paclitaxel or docetaxel to a water soluble polymer such as poly-glutamic acid, poly-aspartic acid or poly-lysine. Also disclosed are methods of using the compositions for treatment of tumors, auto-immune disorders such as rheumatoid arthritis. Other embodiments include the coating of implantable stents for prevention of restenosis.
Owner:PG TXL COMPANY
Taxol enhancer compounds
Disclosed is a compound represented by the Structural Formula (I): Y is a covalent bond, a phenylene group or a substituted or unsubstituted straight chained hydrocarbyl group. In addition, Y, taken together with both >C═Z groups to which it is bonded, is a substituted or unsubstituted aromatic group. Preferably, Y is a covalent bond or —C(R7R8)—.R1 and R2 are independently an aryl group or a substituted aryl group, R3 and R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R5–R6 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R7 and R8 are each independently —H, an aliphatic or substituted aliphatic group, or R7 is —H and R8 is a substituted or unsubstituted aryl group, or, R7 and R8, taken together, are a C2–C6 substituted or unsubstituted alkylene group.Z is ═0 or ═S.Also disclosed are pharmaceutical compositions comprising the compound of the present invention and a pharmaceutically acceptable carrier or diluent.Also disclosed is a method of treating a subject with cancer by administering to the subject a compound of Structural Formula (I) in combination with taxol or an analog of taxol.
Owner:SYNTA PHARMA CORP
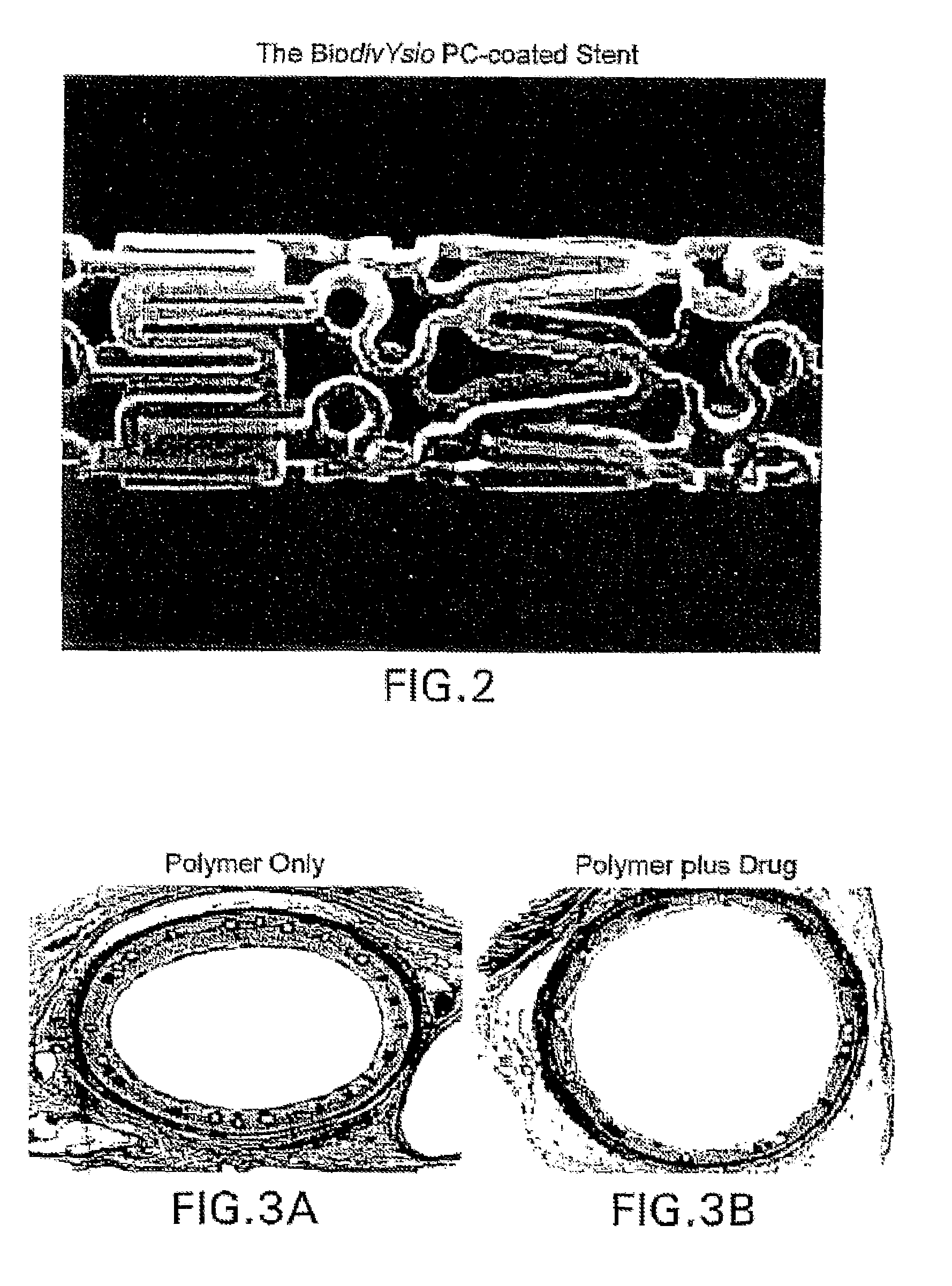
Medical stent provided with a combination of melatonin and paclitaxel
InactiveUS20080050413A1Inhibiting SMC proliferationStentsKetone active ingredientsSmooth musclePercent Diameter Stenosis
A stent is provided with a composition which includes melatonin and paclitaxel for use in treating smooth muscle cell proliferation, such as stenosis and preventing restenosis in vascular vessels.
Owner:BLUE MEDICAL DEVICES
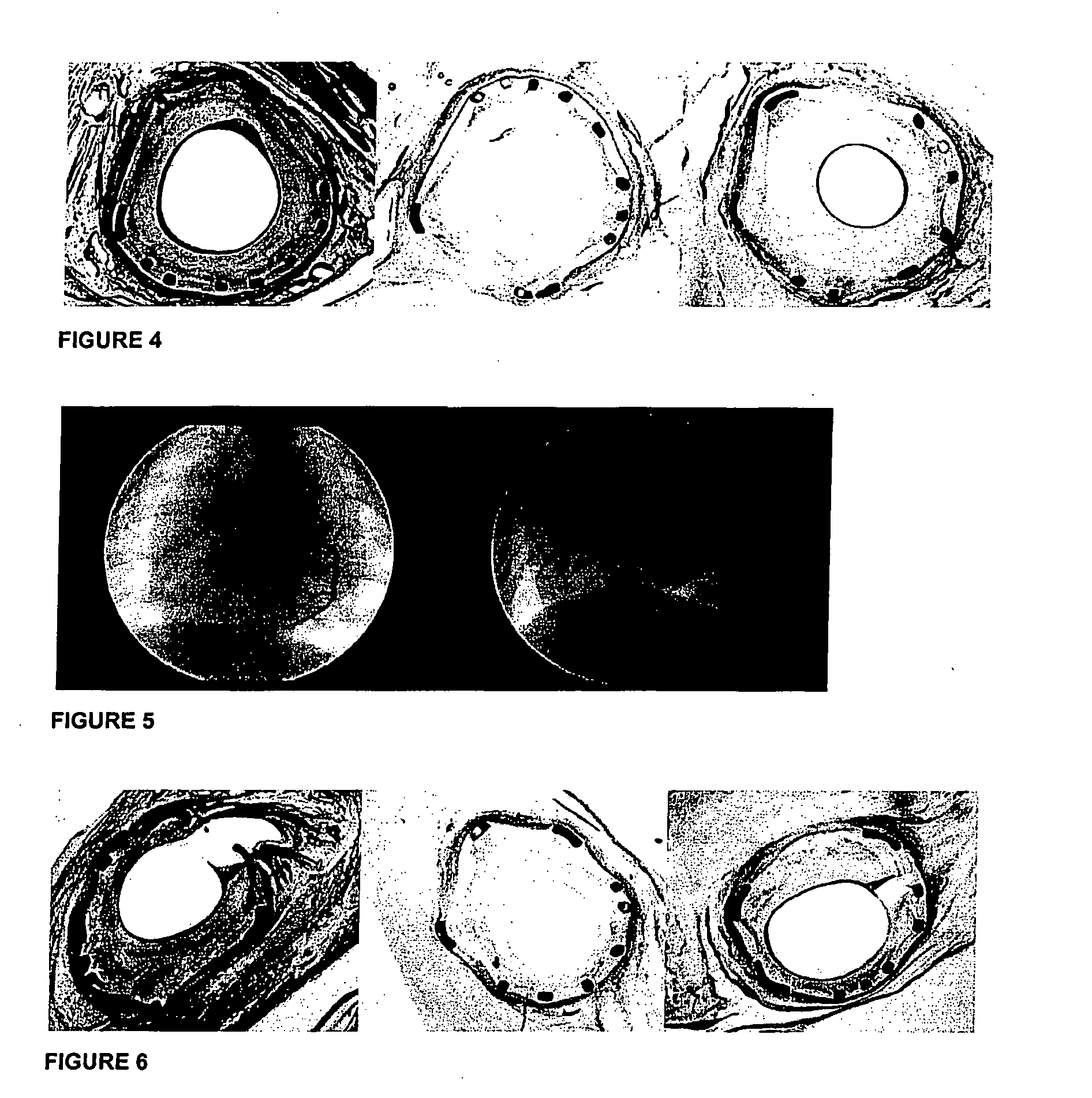
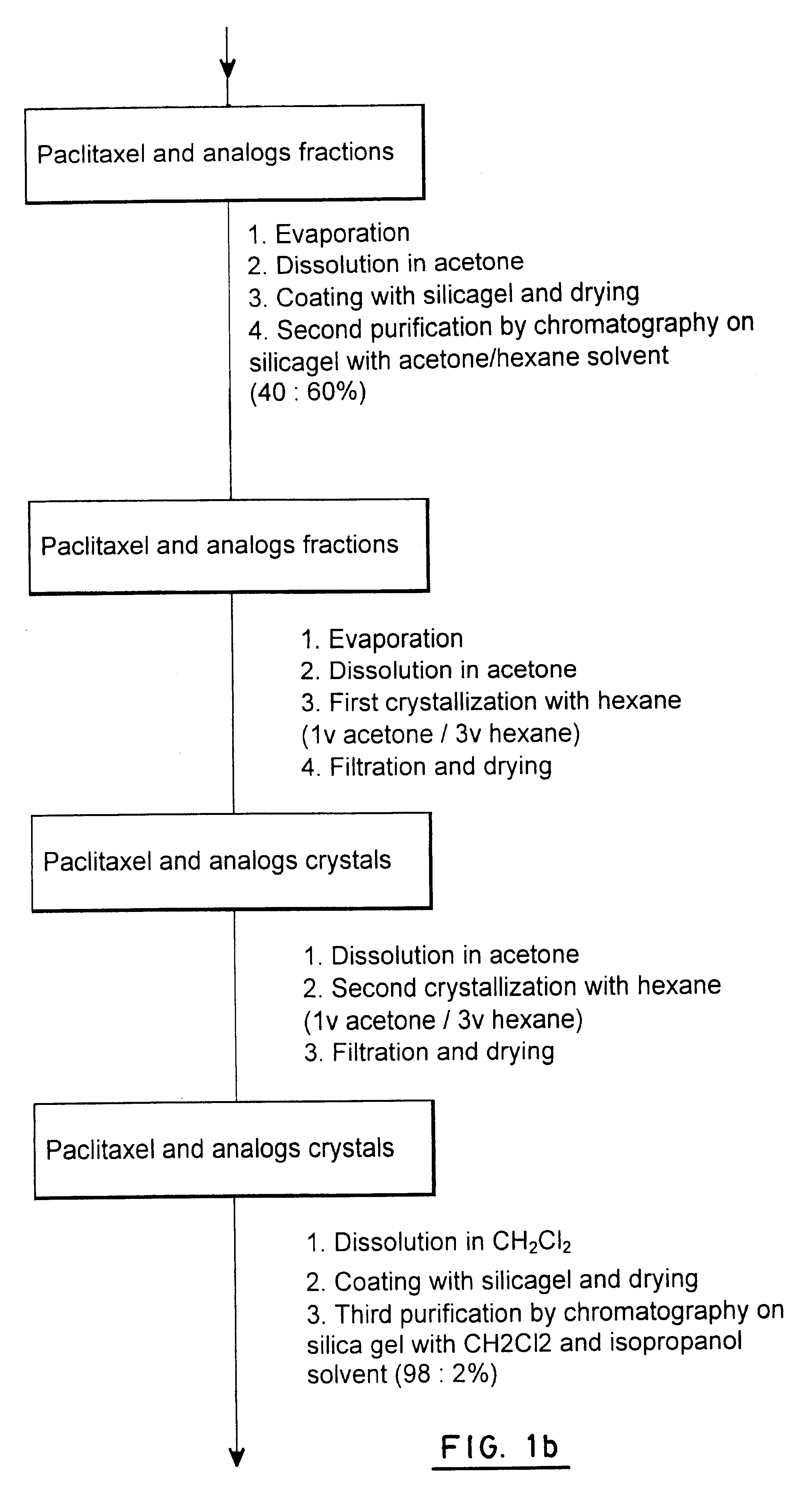
Process for extraction and purification of paclitaxel from natural sources
A process for the extraction and purification of Paclitaxel from a natural source of taxanes, comprising extracting Paclitaxel with an organic solvent from a natural source of taxanes, and treating the raw material with a base or an acid to obtain a biomass by precipitation. The biomass is isolated and dried, and resin and natural pigments are removed. The biomass is then dissolved in acetone and at least one non-polar solvent is added, until a Paclitaxel-enriched oily phase is obtained. The Paclitaxel-enriched oily phase is then treated with a base or an acid to obtain a second biomass, which is recovered by precipitation and dried. A solution of the second biomass in a volatile solvent is chromatographically purified at least once and crystallized.
Owner:CHAICHEM PHARMA INT
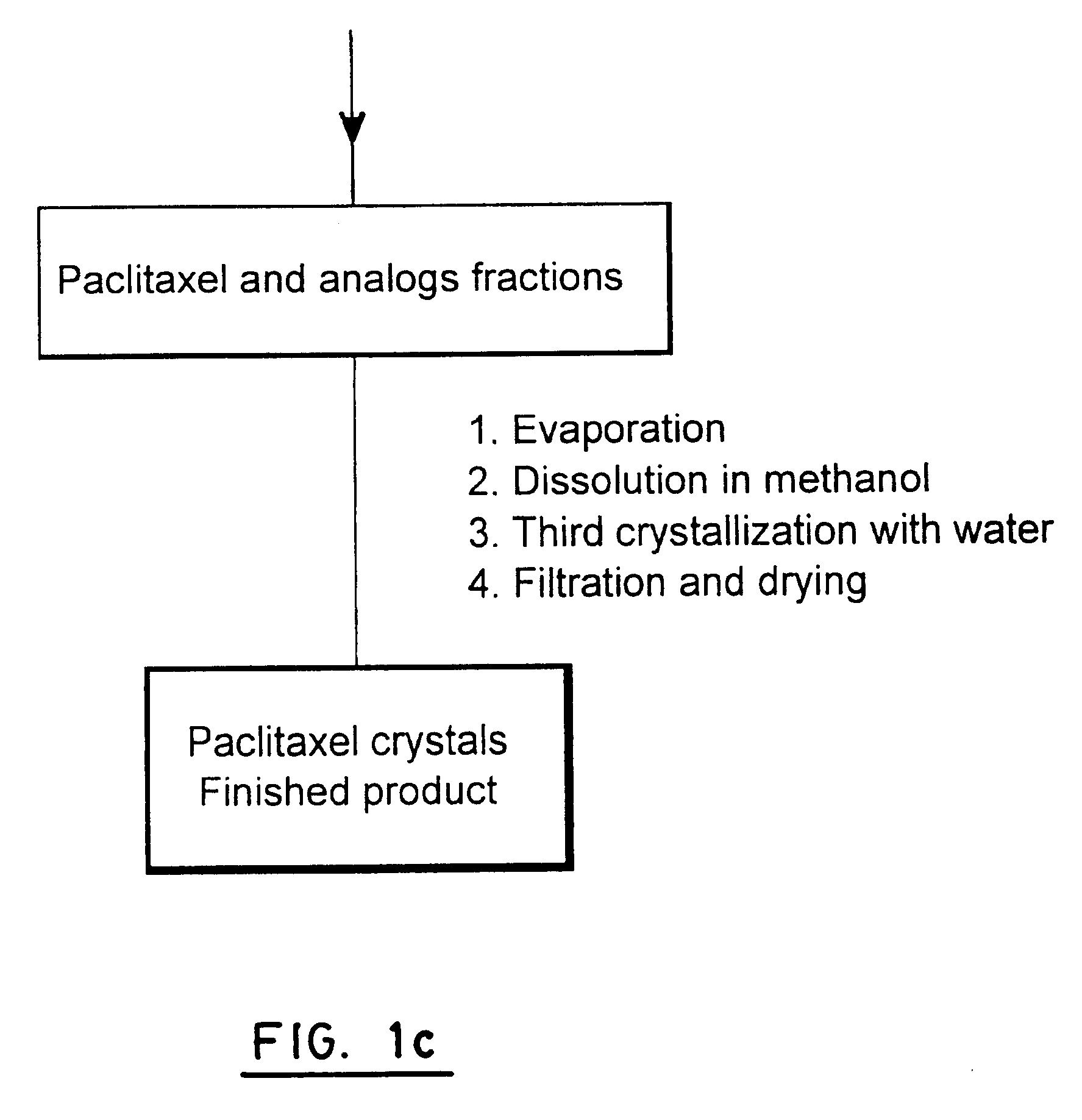
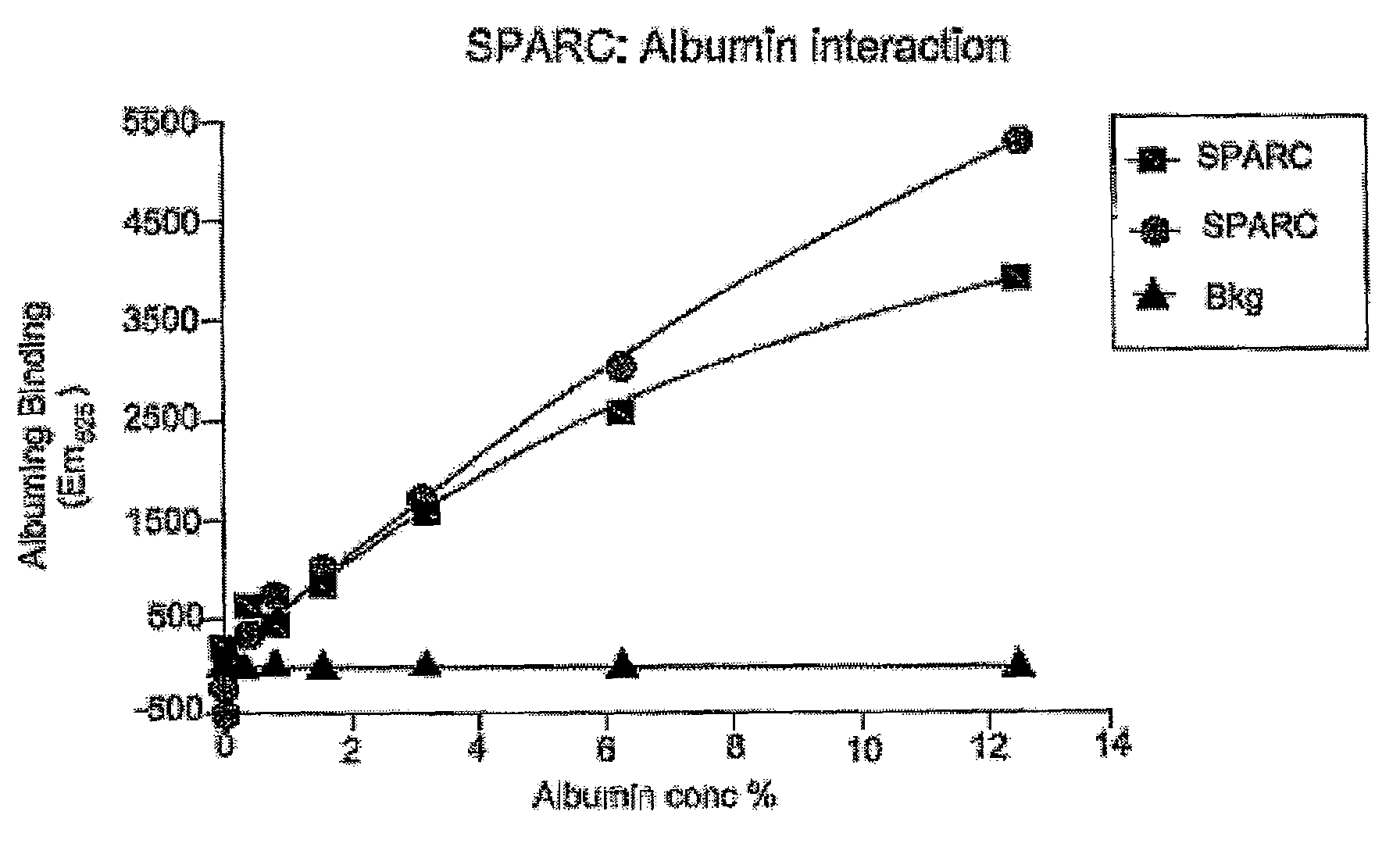
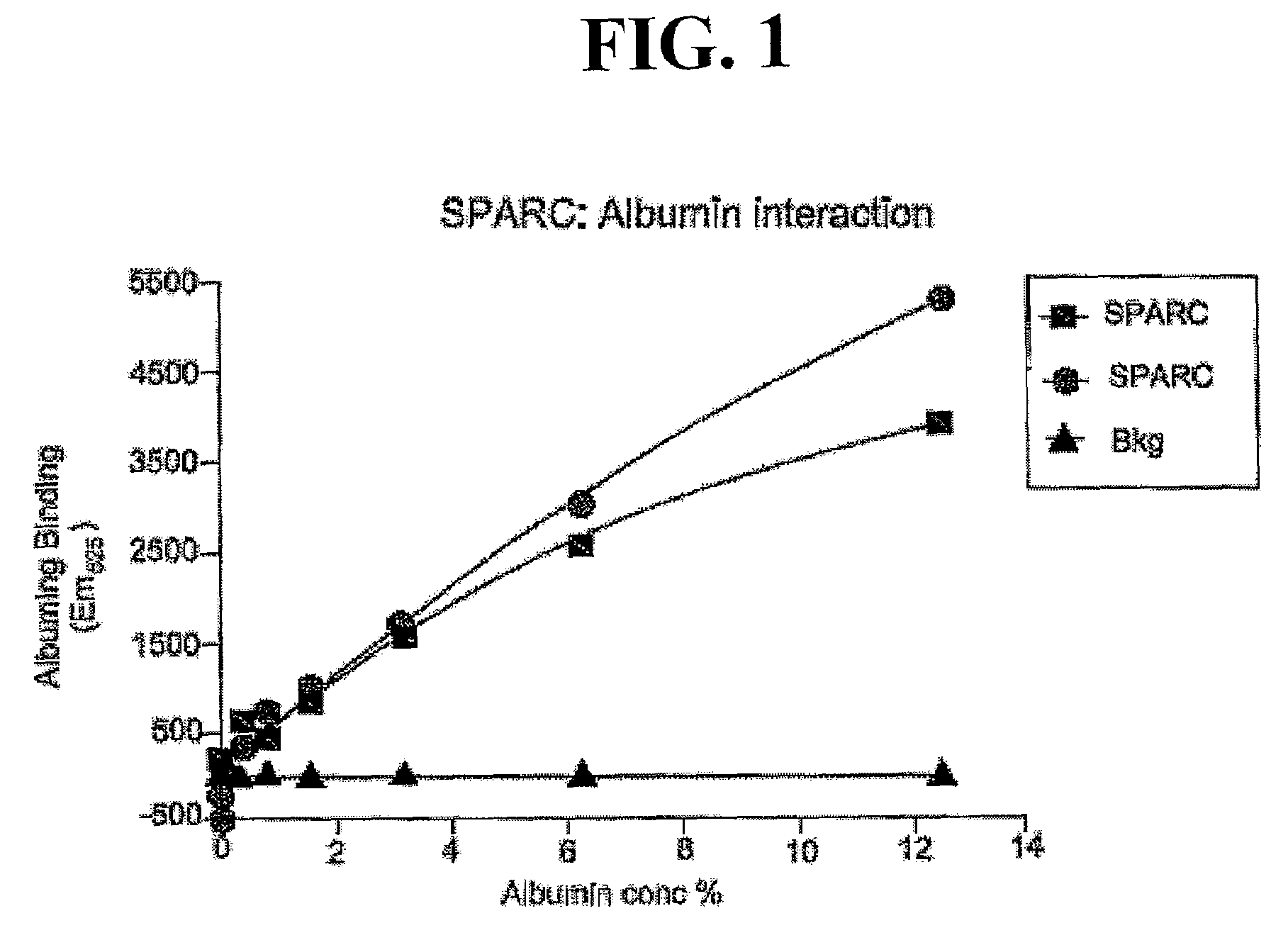
Sparc and methods of use thereof
InactiveUS20080255035A1Organic active ingredientsPeptide/protein ingredientsOncologyCombination therapy
The invention provides methods of treating a mammalian tumors comprising combination therapy with SPARC polypeptides, an angiogenesis inhibitor and paclitaxel. The invention provides also methods of treating a mammalian tumors comprising combination therapy with SPARC polypeptides and paclitaxel. Further, the invention produces kits and methods to predict therapy responses.
Owner:ABRAXIS BIOSCI LLC
Use of nano structured lipid carrier drug feeding system
InactiveCN101129335AIncrease intakeGood curative effectPowder deliveryOrganic active ingredientsLipid formationMonoglyceride
The invention discloses an application of nanometer structure lipid carrier administration system in the antineoplastic drug to reverse the multiple-drug tolerance of tumour cell, which comprises the following parts: solid lipid material, liquid lipid material and antineoplastic drug, wherein the rate of the liquid lipid (such as oleic acid) is 0-30wt%; the solid lipid is selected from monoglyceride; the liquid lipid is oleic acid; the antineoplastic drug is Paclitaxel or adriablastina. The invention has highly effective cell uptaking and cytolymph condensing function with packing molecular target in the antineoplastic drug of cell, which avoids P-glucoprotein in the drug tolerant cytolymph from identifying the antineoplastic drug to reduce exclusion.
Owner:ZHEJIANG UNIV
Composition for solubilization of paclitaxel and preparation method thereof
ActiveUS8075917B2Improve solubilityPromote absorptionBiocideCosmetic preparationsMonoglycerideIntestinal walls
The present invention relates to a paclitaxel composition and the preparation methods thereof to solubilize paclitaxel wherein said composition comprises 4 to 90% by weight of at least one selected from the monoglycerides, 0.01 to 90% by weight of at least one oil and 0.01 to 20% by weight of paclitaxel. Also the present invention relates to a paclitaxel composition including emulsifiers and the preparation methods thereof to solubilize paclitaxel wherein said composition comprises 4 to 90% by weight of at least one selected from the monoglycerides, 0.01 to 90% by weight of at least one emulsifier and 0.01 to 20% by weight of paclitaxel. The composition of the present invention is an effective paclitaxel delivery system since the composition solubilizes paclitaxel, does not form aggregates after being dispersed in water, adsorbs well on the intestinal wall, and therefore has high bioavailability.
Owner:DAE HWA PHARMA
Controlled local delivery of chemotherapeutic agents for treating solid tumors
InactiveUSRE37410E1Reduced bioavailabilityShort half-lifePowder deliveryCosmetic preparationsIn vivoExtended time
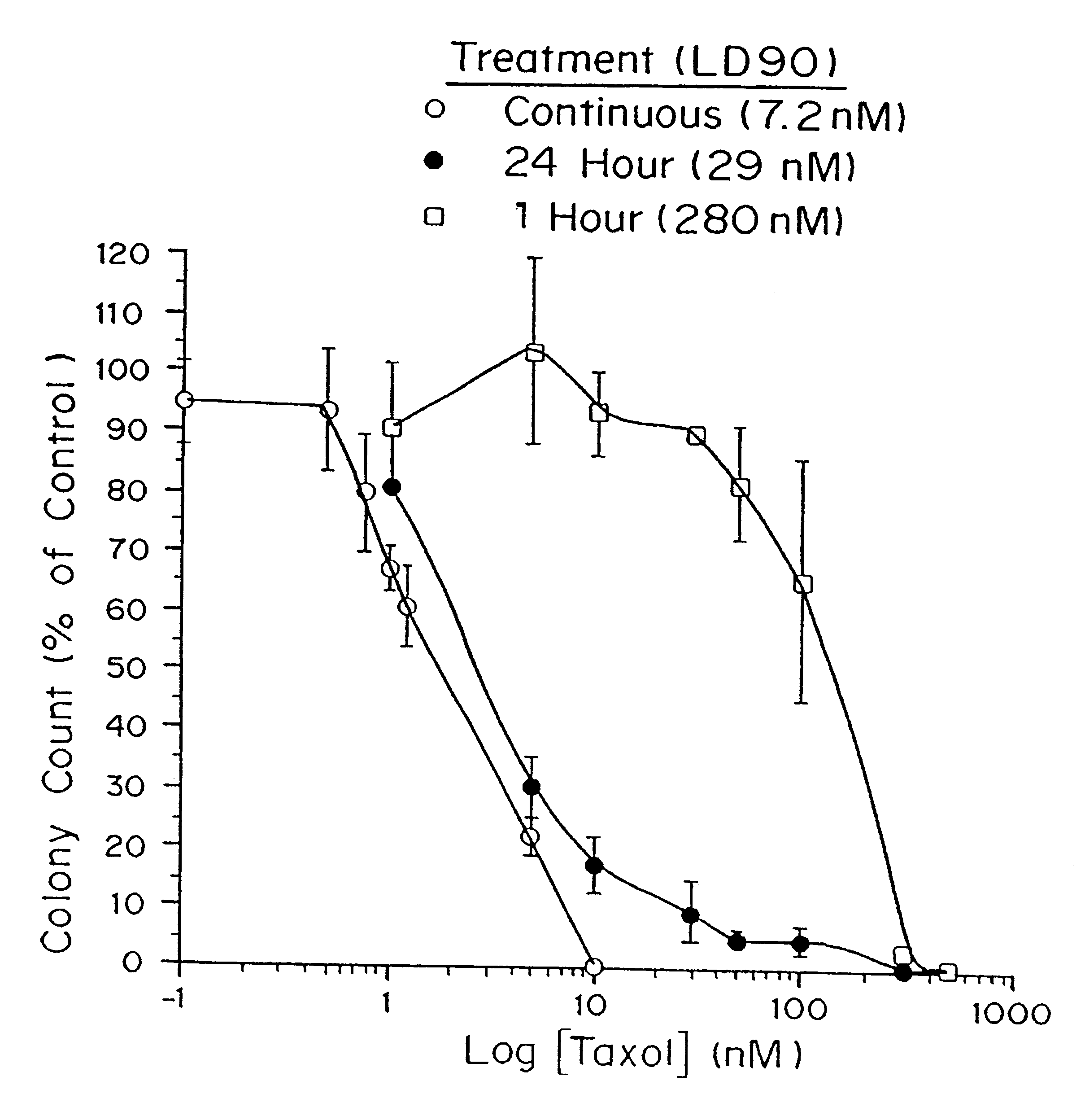
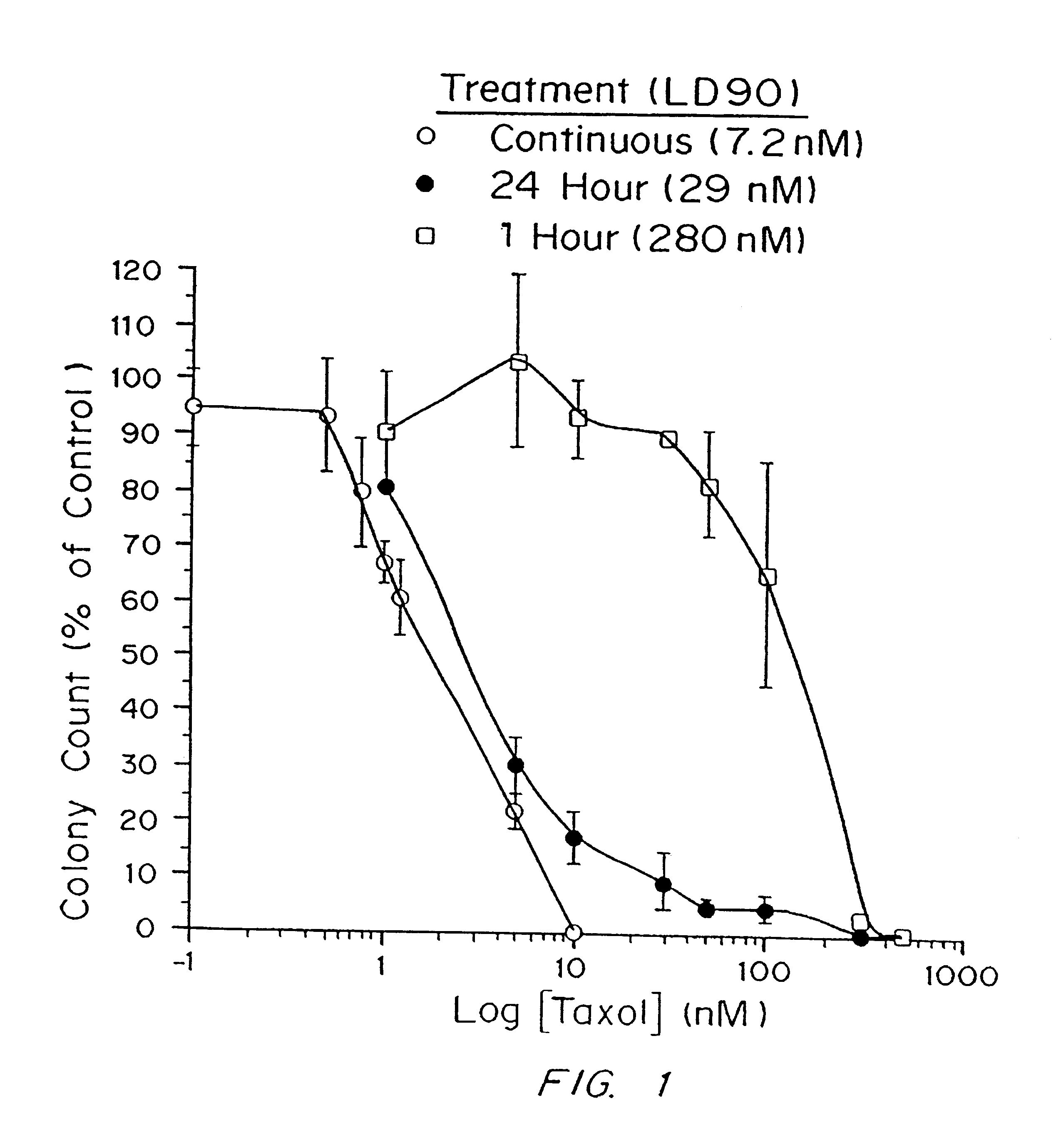
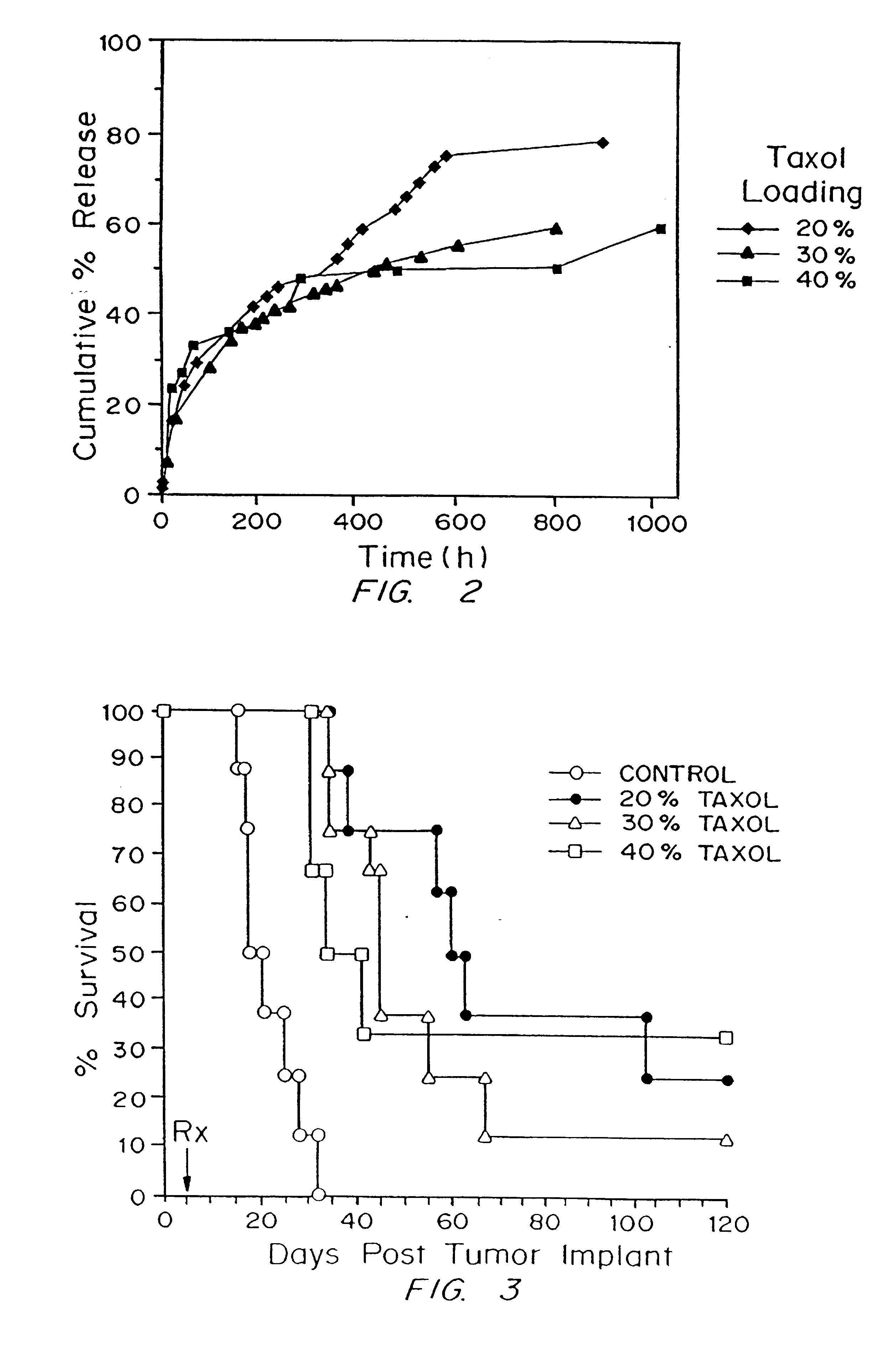
A method and devices for localized delivery of a chemotherapeutic agent to solid tumors, wherein the agent does not cross the blood-brain barrier and is characterized by poor bioavailability and / or short half-lives in vivo, are described. The devices consist of reservoirs which release drug over an extended time period while at the same time preserving the bioactivity and bioavailability of the agent. In the most preferred embodiment, the device consists of biodegradable polymeric matrixes, although reservoirs can also be formulated from non-biodegradable polymers or reservoirs connected to implanted infusion pumps. The devices are implanted within or immediately adjacent the tumors to be treated or the site where they have been surgically removed. The examples demonstrate the efficacy of paclitaxel and camptothecin delivered in polymeric implants prepared by compression molding of biodegradable and non-biodegradable polymers, respectively. The results are highly statistically significant.
Owner:MASSACHUSETTS INST OF TECH +1
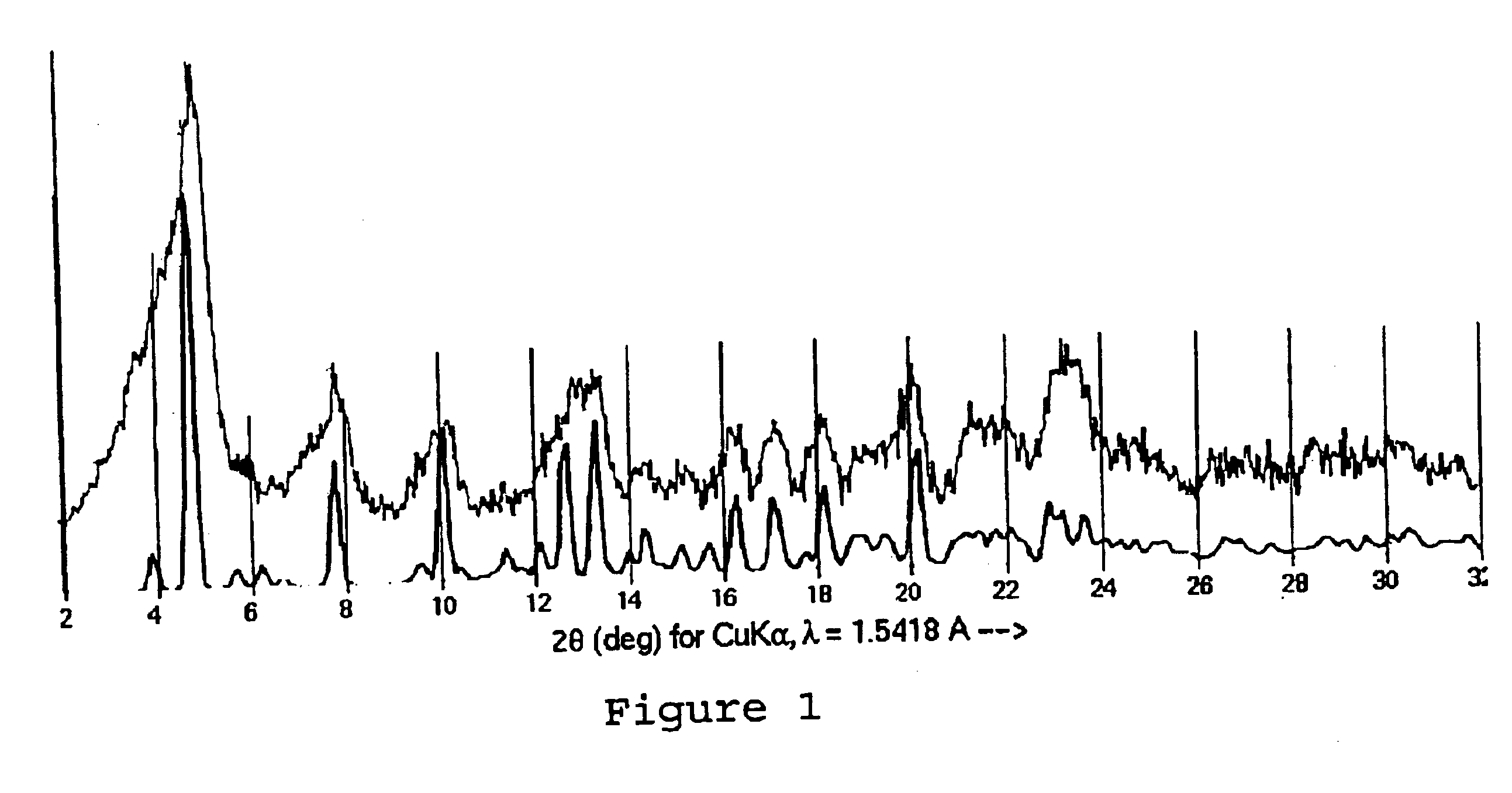
Paclitaxel solvates
Biologically active crystalline solvates of paclitaxel are precipitated using polar, aprotic, organic solvents. A pharmaceutical composition is also disclosed, as well as the preparation of the novel solvates and their uses as anti-tumor agents.
Owner:BRISTOL MYERS SQUIBB CO
Compositions, systems, and kits for administering zotarolimus and paclitaxel to blood vessel lumens
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Method for preparing taxol micro ball anti-cancer medicine
InactiveCN1561988AIncrease concentrationGrowth inhibitionPowder deliveryOrganic active ingredientsCancer cellMicrosphere
Owner:山东居仁生物医药技术研究所
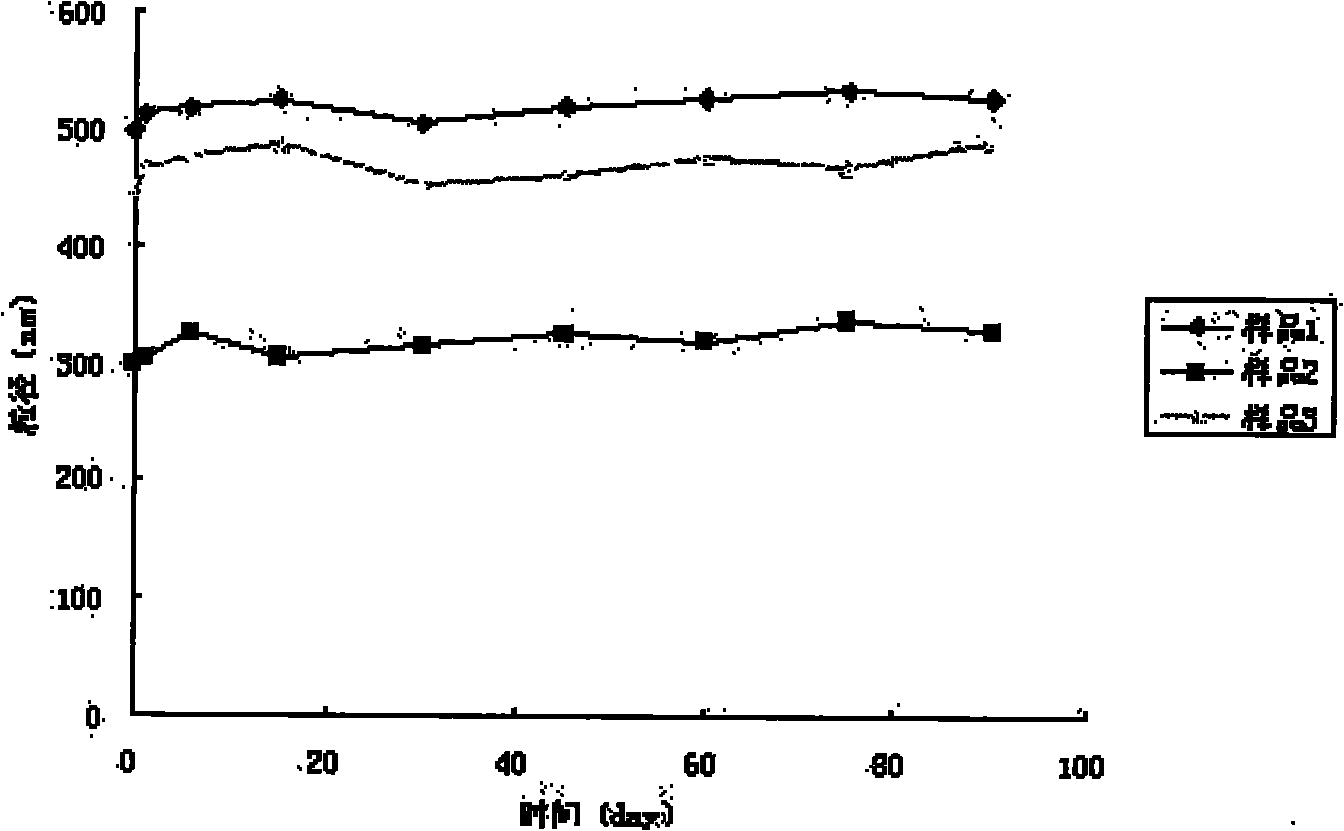
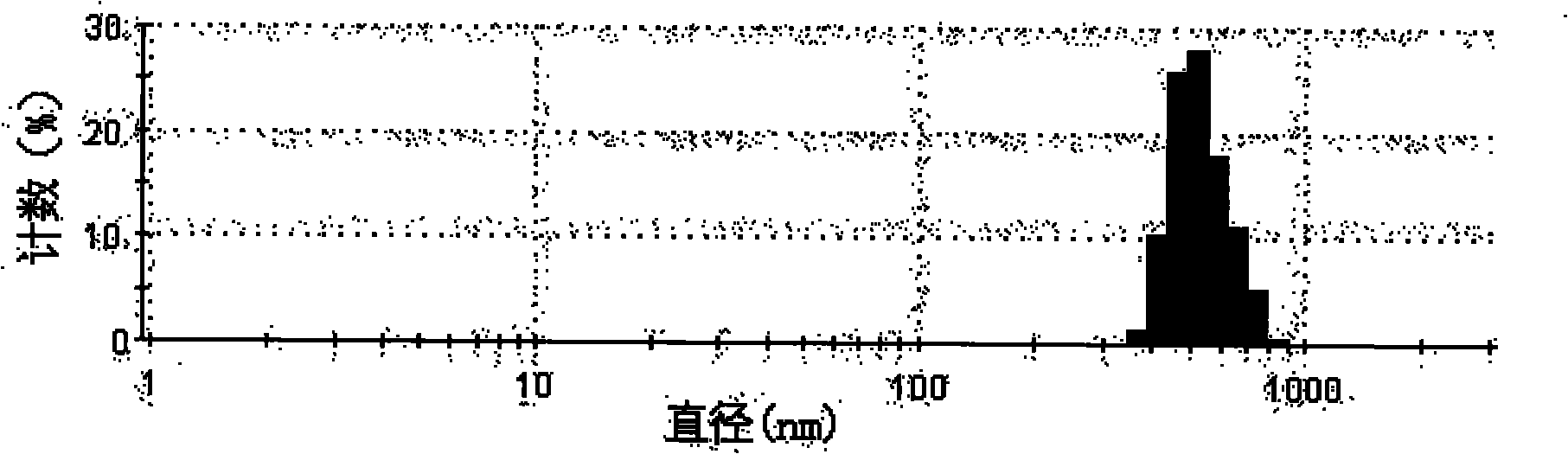
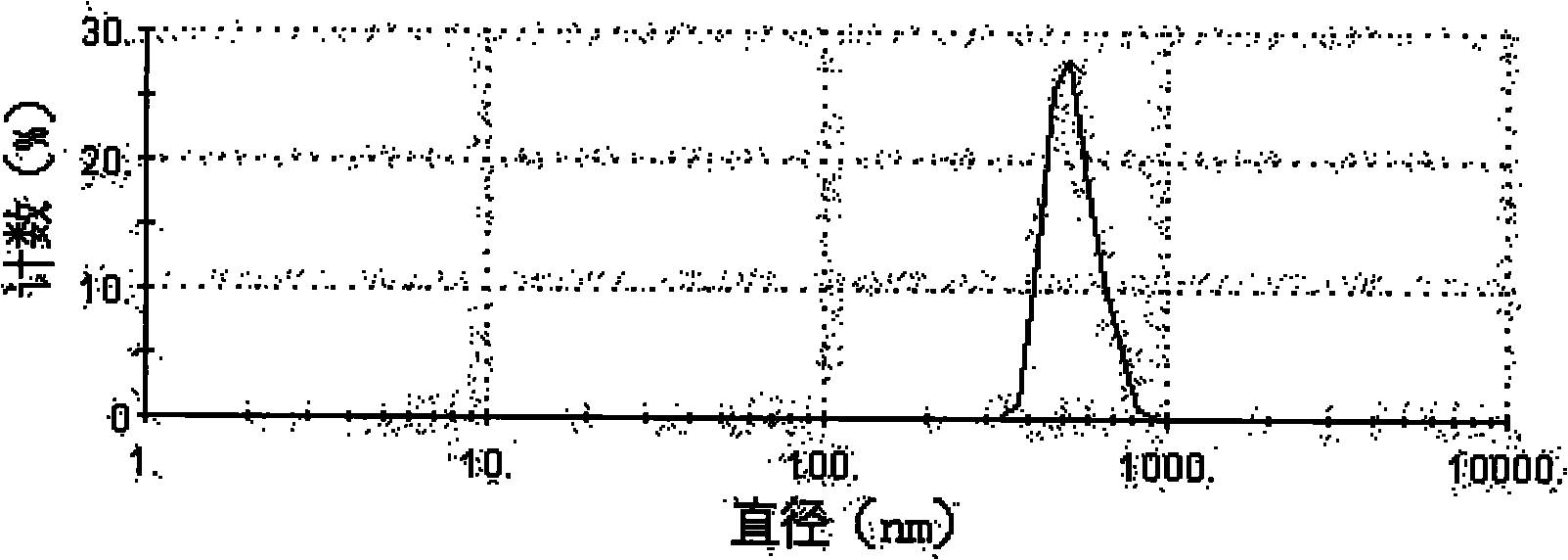
Taxol nanoparticle composition and preparation method thereof
The invention discloses a taxol nanoparticle composition and a preparation method thereof, belonging to the technical field of taxol preparations. The invention relates to taxol nanoparticle composition and nanosuspension, the high drug-loading taxol nanoparticle composition and nanosuspension is prepared by taking a surface stabilizer as a carrier, wherein the effective average particle size of the composition is less than 1000 nm; the suspension is subjected to freeze drying or spray drying to obtain the taxol nanoparticle preparation which can be intravenously injected or taken orally. The method of the invention is reliable, easy to operate and low in energy consumption, and avoids interference of organic solvent; meanwhile, the obtained taxol nanoparticle composition has higher bioactivity and can give full play to the curative effect of taxol.
Owner:无锡纳生生物科技有限公司
Chemotherapeutic microemulsion compositions of paclitaxel with improved oral bioavailability
InactiveUS7115565B2Rapid and efficient absorptionImprove bioavailabilityBiocideNervous disorderMonoglycerideSolvent
Pharmaceutical compositions suitable for oral administration comprising paclitaxel, a solvent, a surfactant, a substituted cellulosic polymer, and optionally but preferably a P-glycoprotein inhibitor. The composition may further comprise a diglyceride or mixture of diglyceride and monoglyceride. The composition generates a supersaturated paclitaxel microemulsion upon contact with water resulting in improved oral bioavailability of paclitaxel.
Owner:PHARMACIA & UPJOHN CO
Method for treating cancer based on level of a nucleoside transporter
The present invention provides methods and compositions for treating cancer by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a nucleoside analog (e.g., gemcitabine) based upon levels of a nucleoside transporter (e.g., hENT1).
Owner:ABRAXIS BIOSCI LLC
Tricyclic compounds having antimitotic and/or antitumor activity and methods of use thereof
The present invention provides tricyclic compounds, pharmaceutically acceptable salts, prodrugs, solvates, or hydrates thereof, having antimitotic activity, anti-multidrug resistance activity, for example P-glycoprotein inhibition, and antitumor activity, and which inhibit paclitaxel sensitive and resistant tumor cells. Also provided are methods of utilizing these compounds for treating tumor cells and inhibiting mitosis of cancerous cells.
Owner:DUQUESNE UNIVERSITY
Pharmaceutical formulations comprising paclitaxel, derivatives, and pharmaceutically acceptable salts thereof
The invention concerns paclitaxel solubilizers and formulations thereof with a high propensity to dissolve paclitaxel. The formulations of the invention reduce or obviate the need for the disadvantageous excipient Cremophor® EL. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts or such derivatives to patients in need thereof. The formulations of the invention are suitable for parenteral, oral, local, or transdermal administration to mammals including humans, particularly for intravenous delivery.
Owner:TRANSFORM PHARMACEUTICALS INC
Method for treating cancer based on mutation status of k-ras
The present invention provides methods and compositions for treating cancer by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and / or b) a therapeutic agent (e.g., gemcitabine) based upon K-ras mutation status.
Owner:ABRAXIS BIOSCI LLC
Paclitaxol predrug of biodegradable polymer and its synthesis method
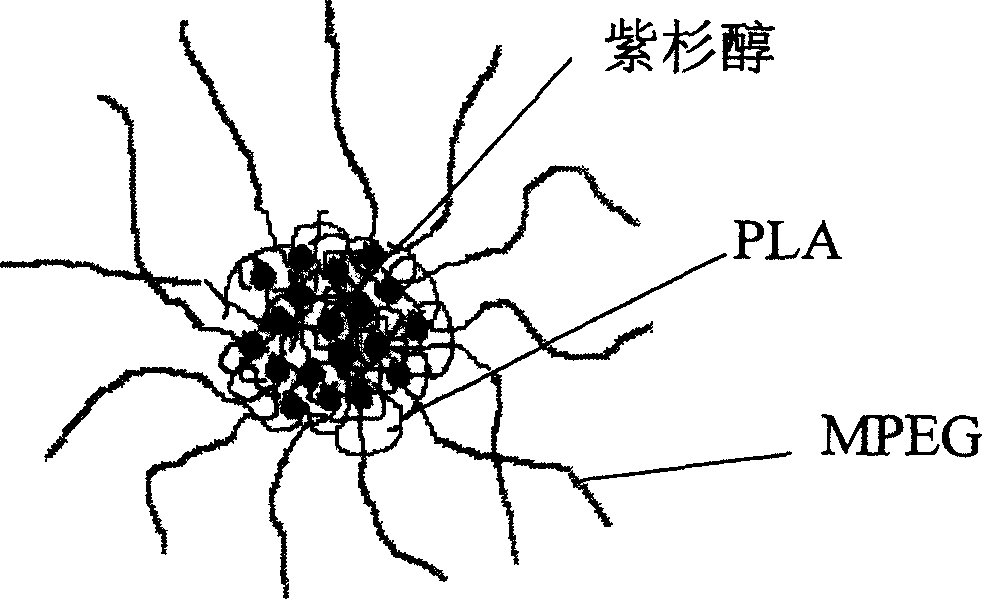
The invention provides a paclitaxol predrug of biodegradable polymer and its synthesis method, which is bond from polyoxyalkylene-aliphatic polyester blocked copolymer and paclitaxol, the synthesizing process comprises, carrying out ring-opening polymerization of aliphatic cyclic esters at the presence of polyethylene glycol (PEG), solvent and catalyst, obtaining polyethylene glycol - aliphatic polyester blocked copolymer, then converting its end hydroxyl into end carboxyl, reacting with paclitaxol for esterification at the presence of condensing agent, the obtained paclitaxol predrug has amphiphilic, thus can be prepared into water based preparation.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of polyene-containing taxol nanoparticle mixed micelle preparation and freeze-drying agent
InactiveCN101804021AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPowder deliveryMixed micelleFreeze-drying
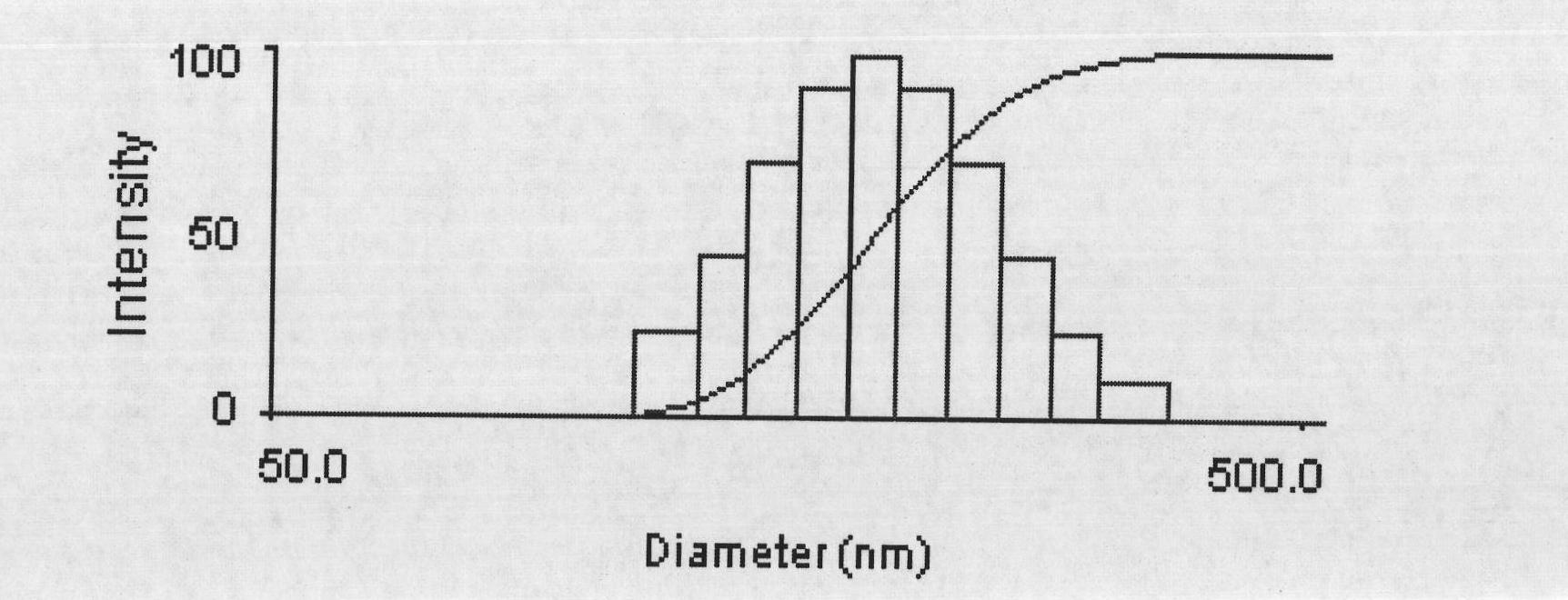
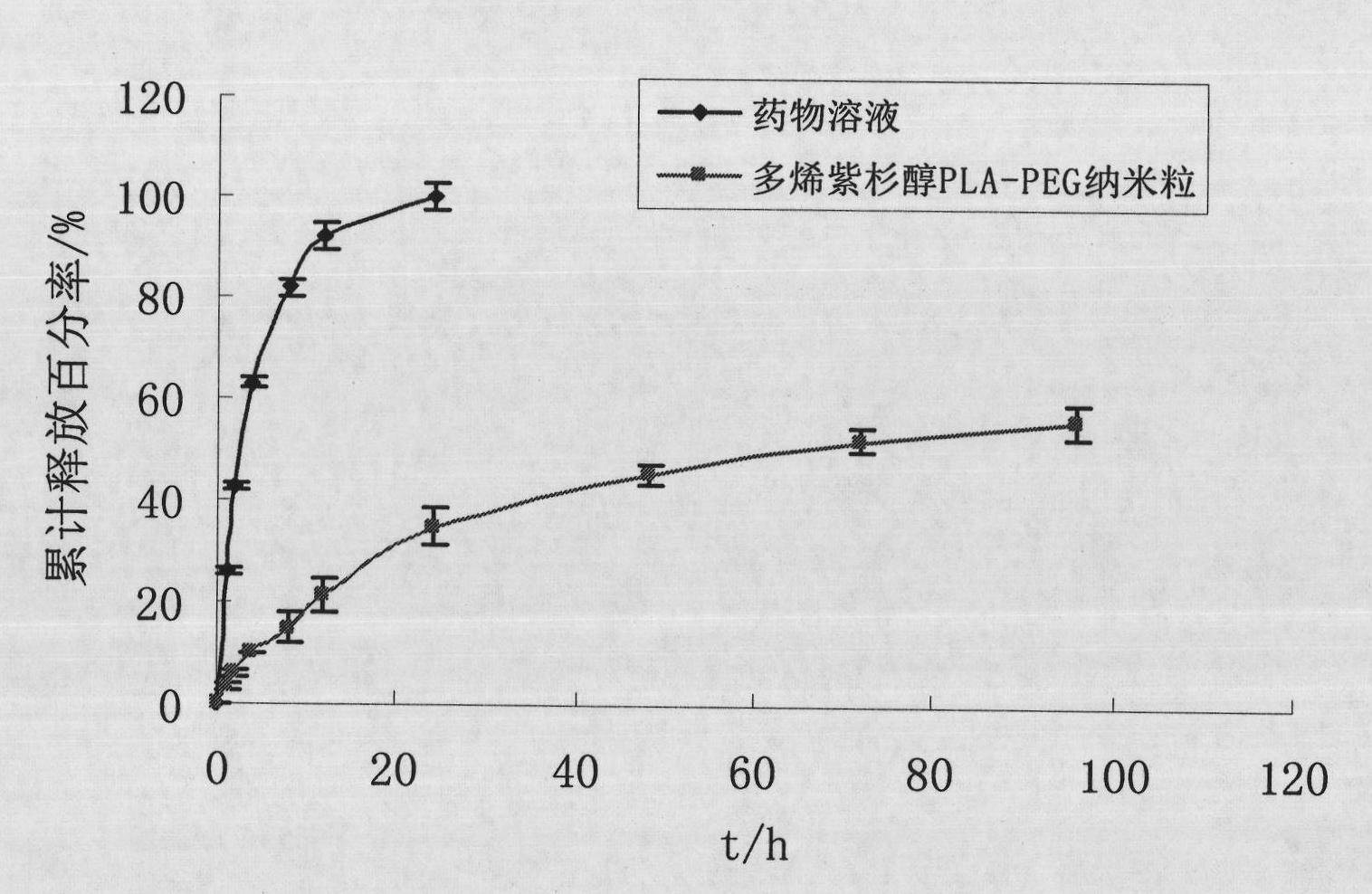
The invention discloses a preparation method of a polyene-containing taxol nanoparticle mixed micelle preparation and a freeze-drying agent, which prepares docetaxel PLA-PEG nanoparticles or micelle or nanoparticle mixed micelle through a modified solvent evaporation method, takes PLA-PEG copolymer as a carrier, and wraps docetaxel in a PLA hydrophobic core. When in use, the docetaxel PLA-PEG containing long cycle freeze-dried preparation only needs to be added with water and is dissolved, and uniform nanoparticle suspension, micellar solution or mixed micellar nanoparticle suspension can be prepared. The preparation method does not need tween-80 and ethanol solubilization, only takes the biodegradable PLA-PEG as the carrier, and does not contain any surfactant; and compared with the docetaxel injection on sale, the preparation can reduce the toxicity and the adverse reactions of the medicine, and improve the clinical application safety of the medicine.
Owner:SHANDONG UNIV
Mesoporous nano silicon ball compound targeting drug delivery system as well as preparation method and application thereof
InactiveCN104474555AOvercoming multidrug resistanceSignificant effectOrganic active ingredientsPowder deliveryMicrosphereFluorescence
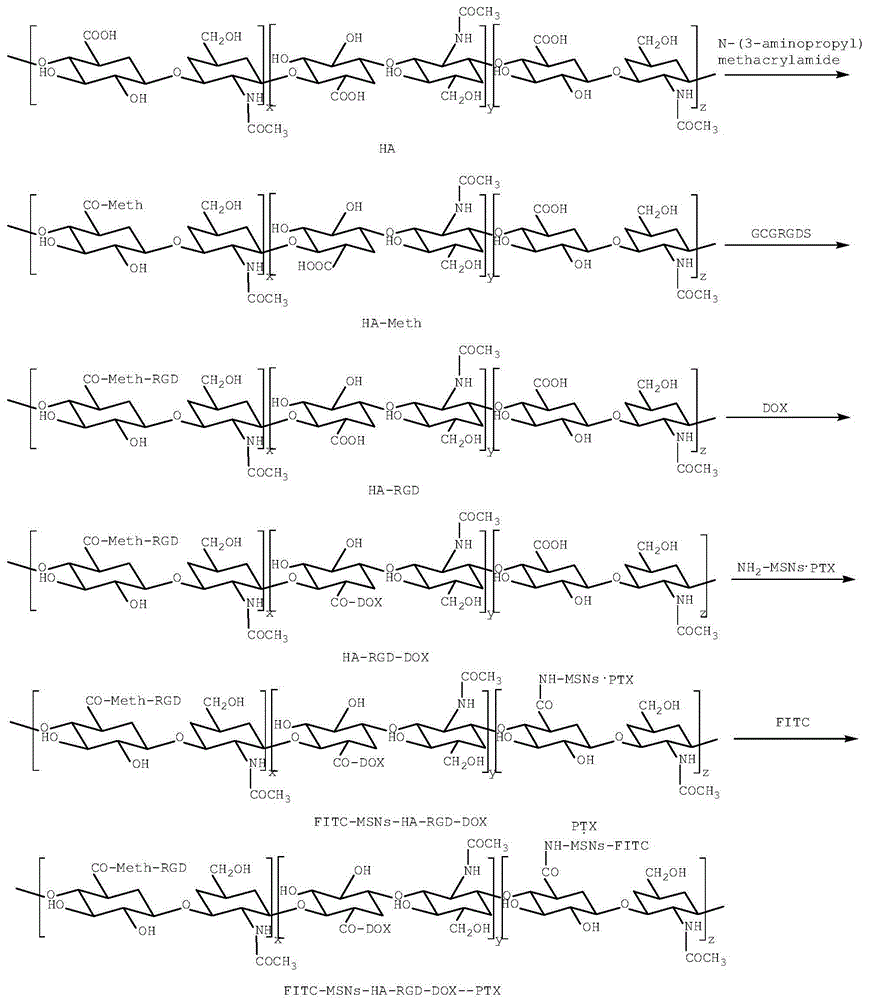
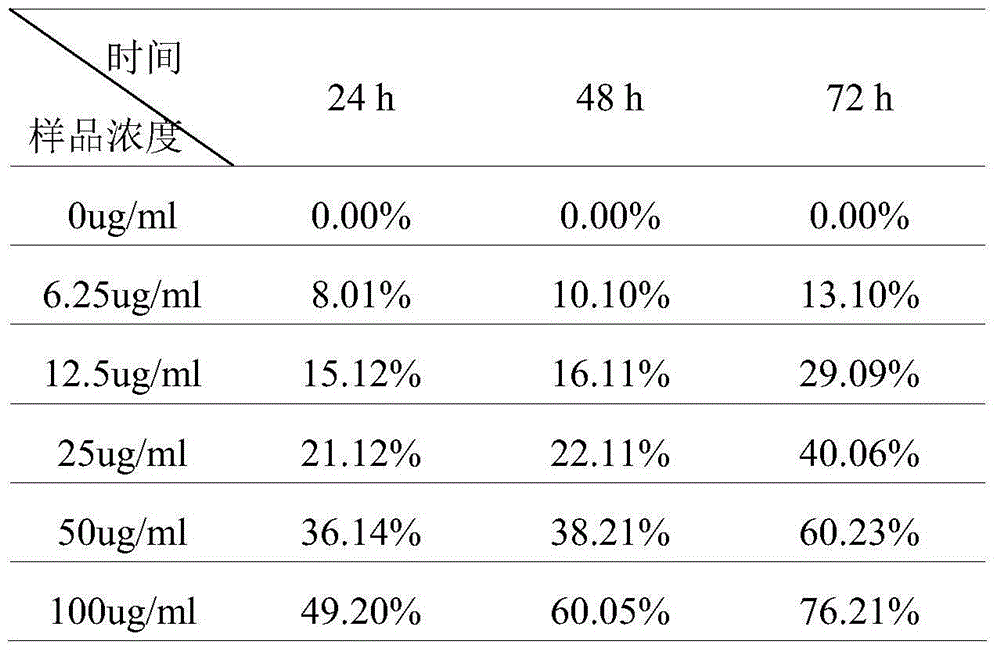
The invention relates to a mesoporous nano silicon ball compound targeting drug delivery system as well as a preparation method and application thereof. The preparation method of the mesoporous nano silicon ball compound targeting drug delivery system comprises the following steps: 1) preparing amino-functionalized drug loading mesoporous silicon dioxide microspheres; 2) preparing hyaluronic acid-hydrosulphonyl polypeptide-adriamycin (HA-RGD-DOX); 3) preparing mesoporous microsphere-hyaluronic acid-hydrosulphonyl polypeptide-adriamycin--paclitaxel (MSNs-HA-RGD-DOX_PTX); and 4) preparing fluorescent marker modified mesoporous microsphere-hyaluronic acid-hydrosulphonyl polypeptide-adriamycin--paclitaxel compound (MSNs-HA-RGD-DOX-PTX). The mesoporous nano silicon ball compound targeting drug delivery system has the beneficial effects that firstly multi-targeting synergistic drug delivery is realized, multiple tumour cells and tissues can be killed, and reversal drug resistance is good; secondly, blood stability is excellent; thirdly, invisibility, drug release degree and controlled release properties are good; fourthly, in vivo tracing function is good; and fifthly, the mesoporous nano silicon ball compound targeting drug delivery system has good general applicability.
Owner:WUHAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com