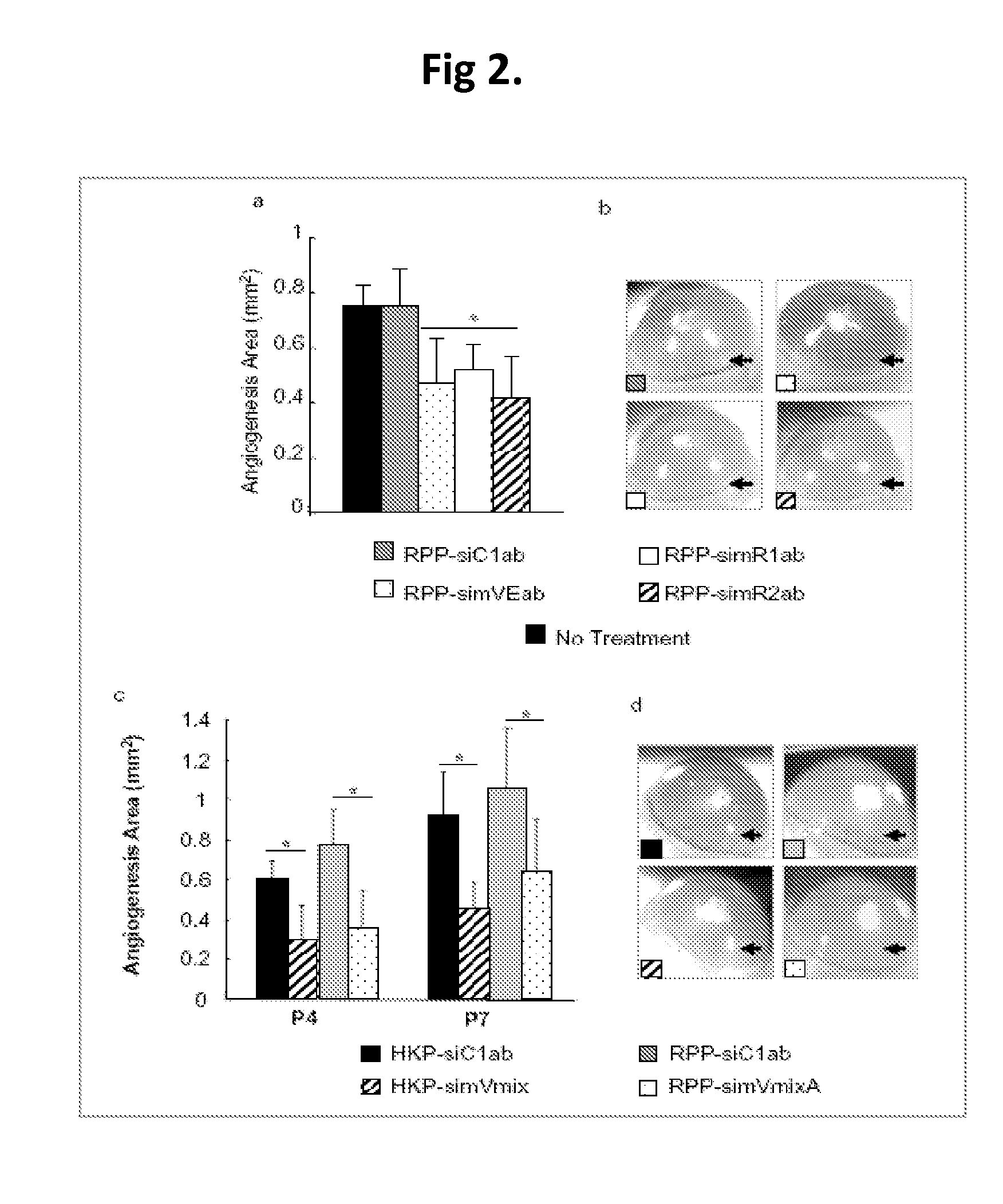
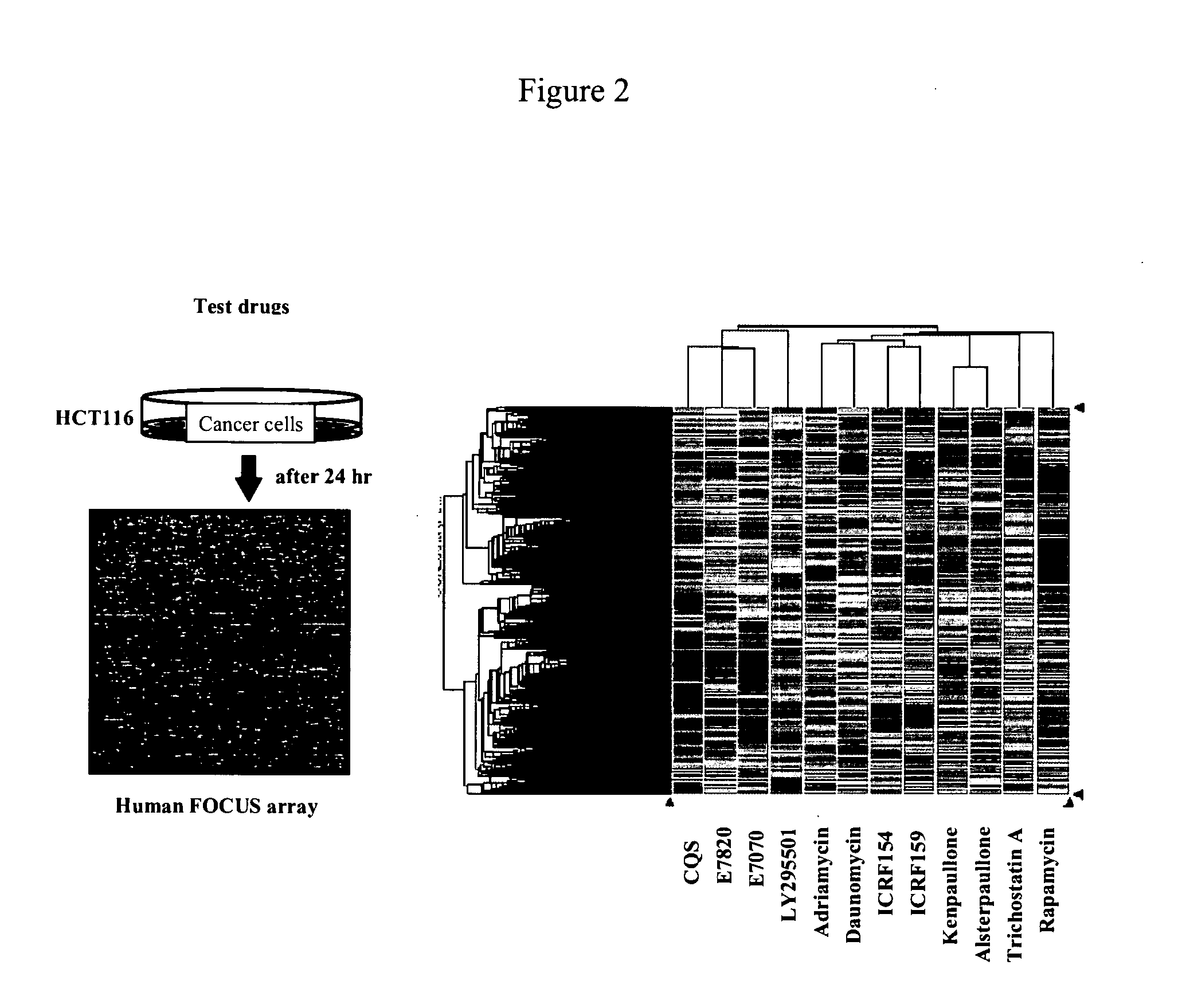
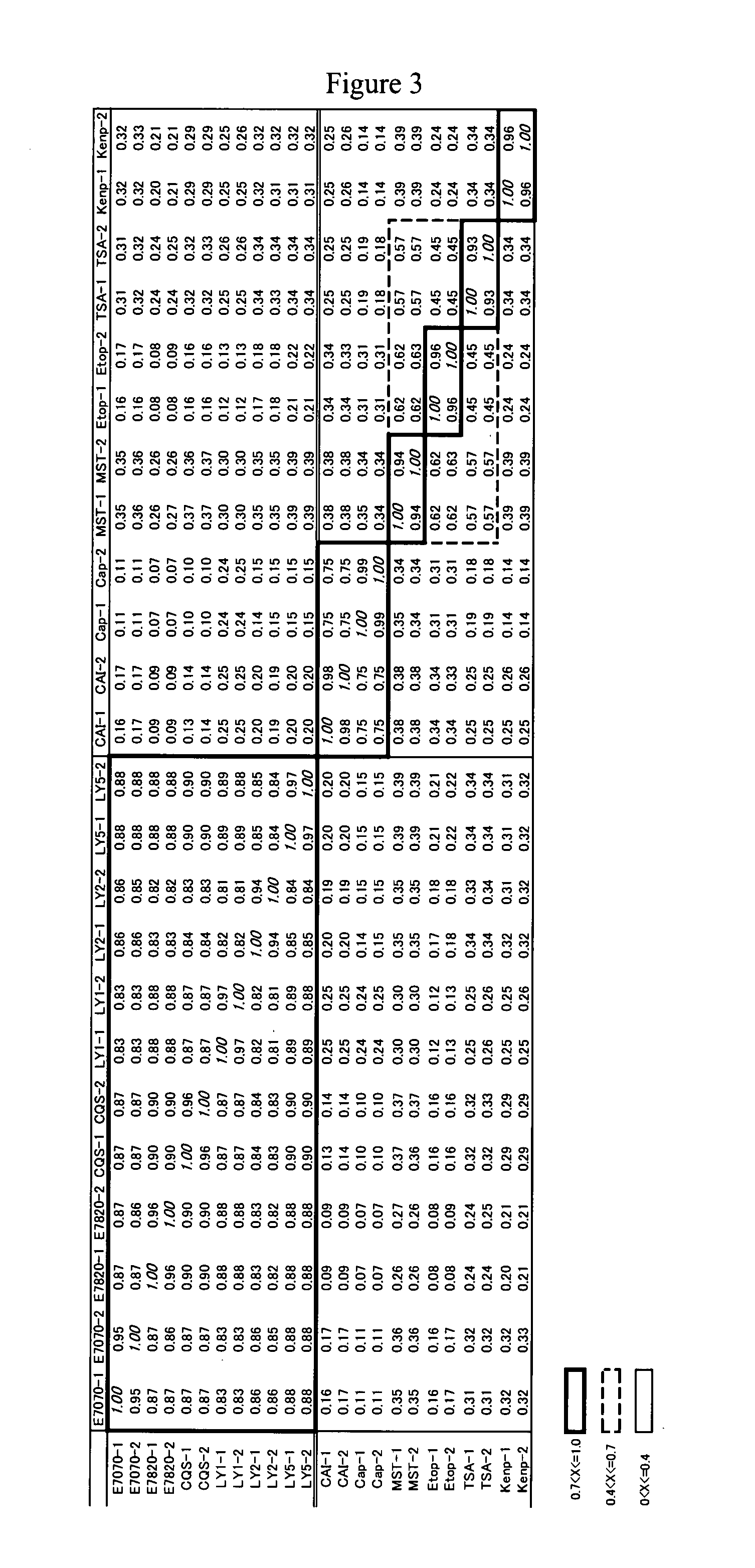
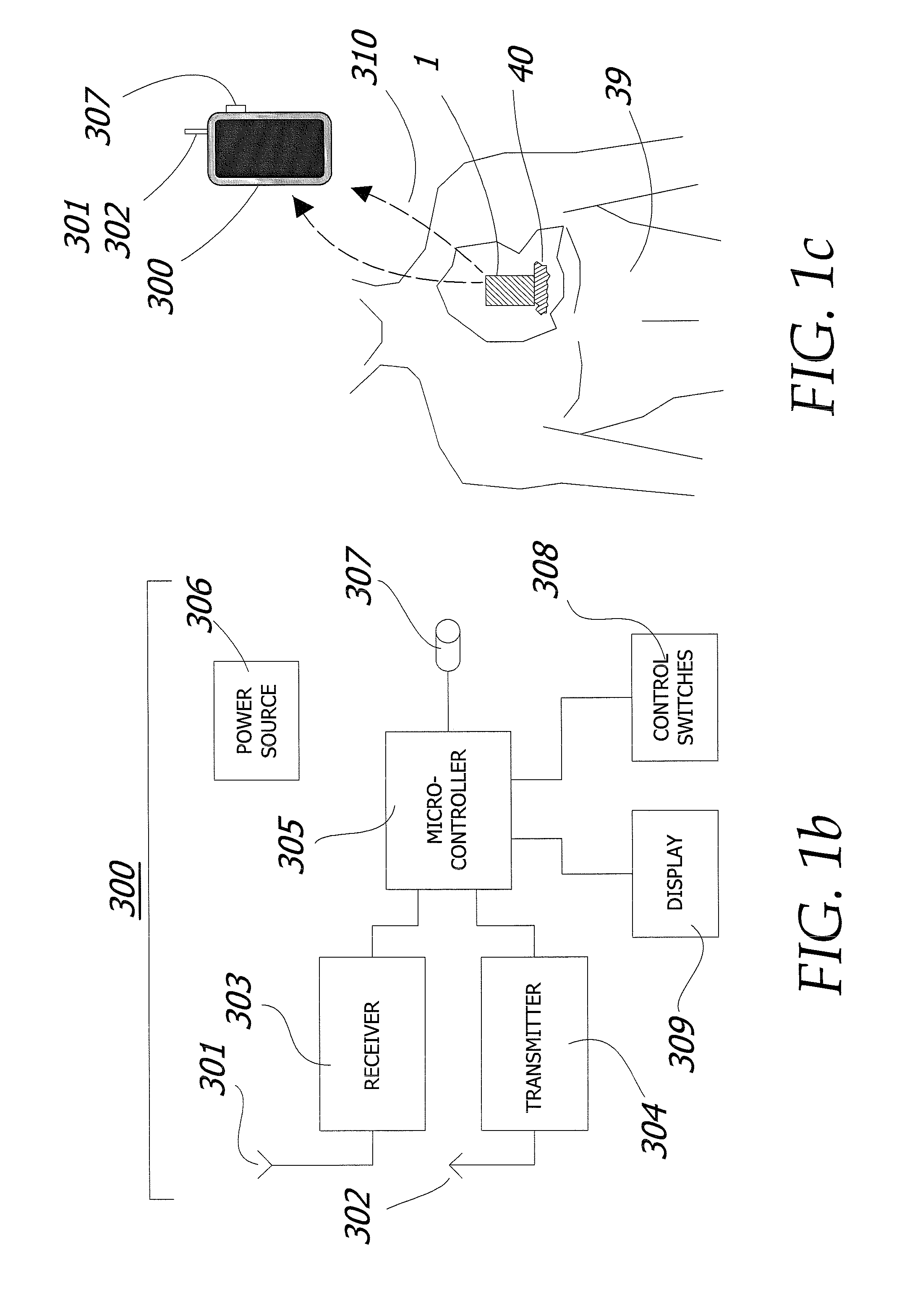
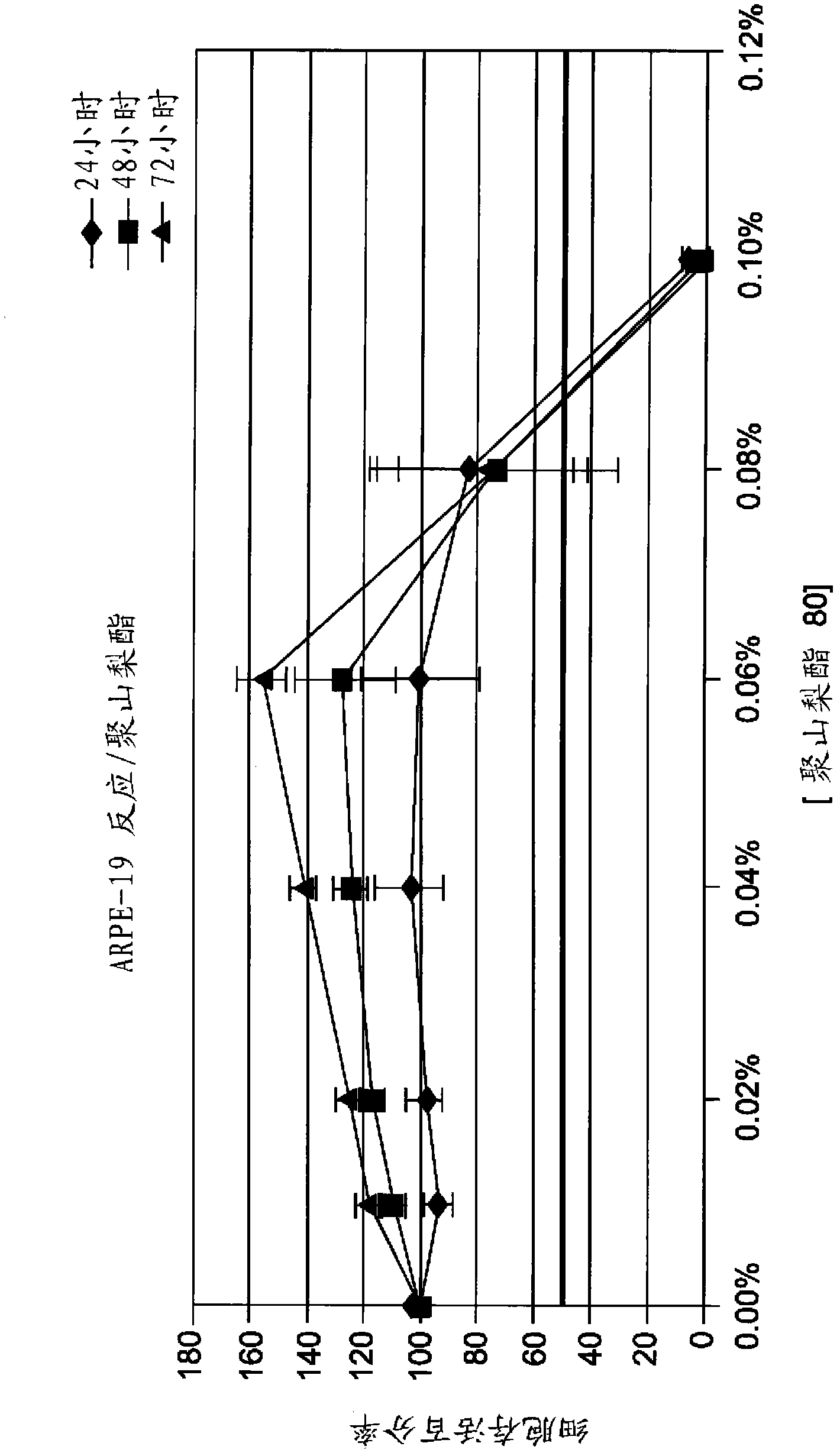
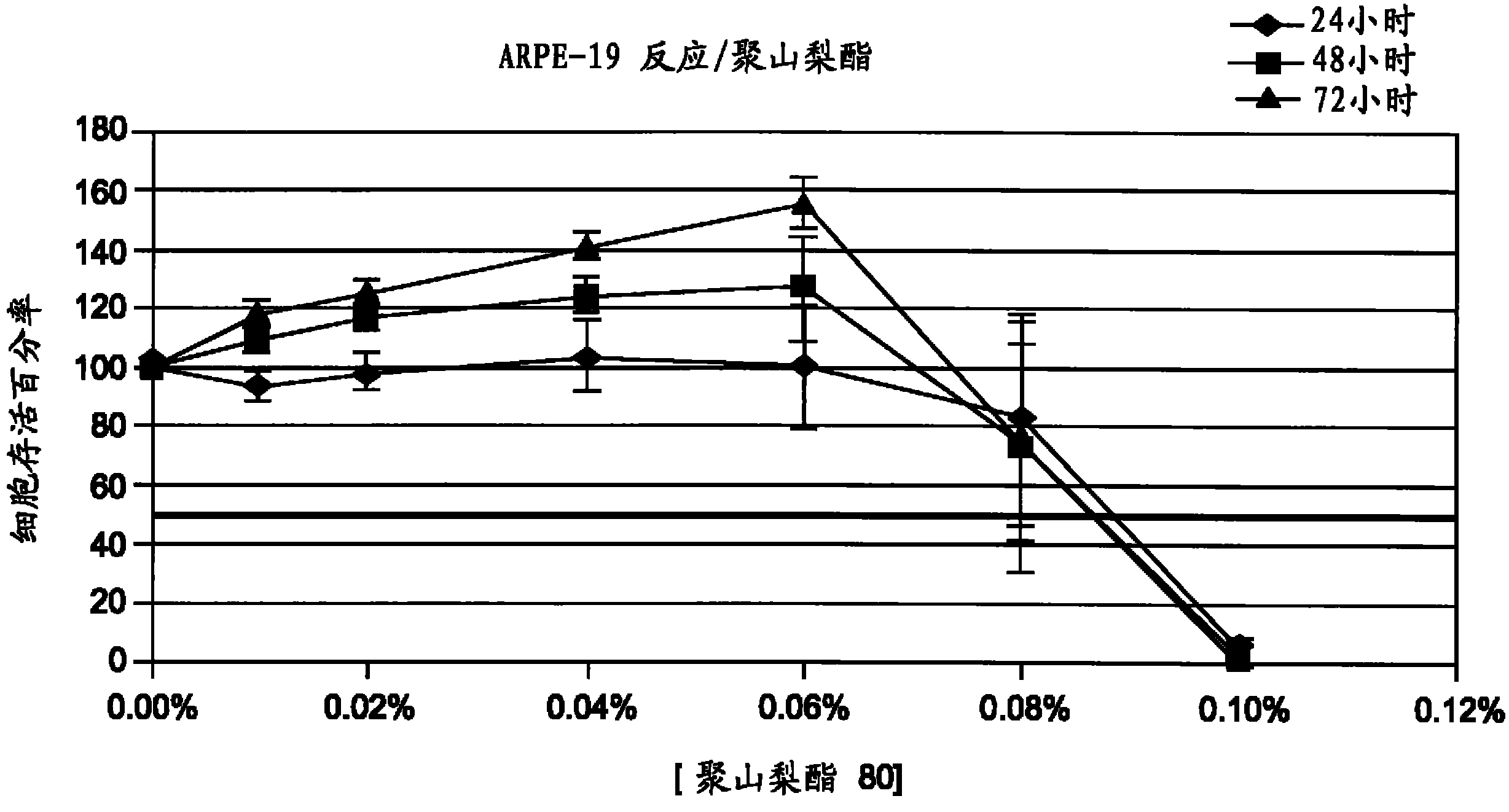
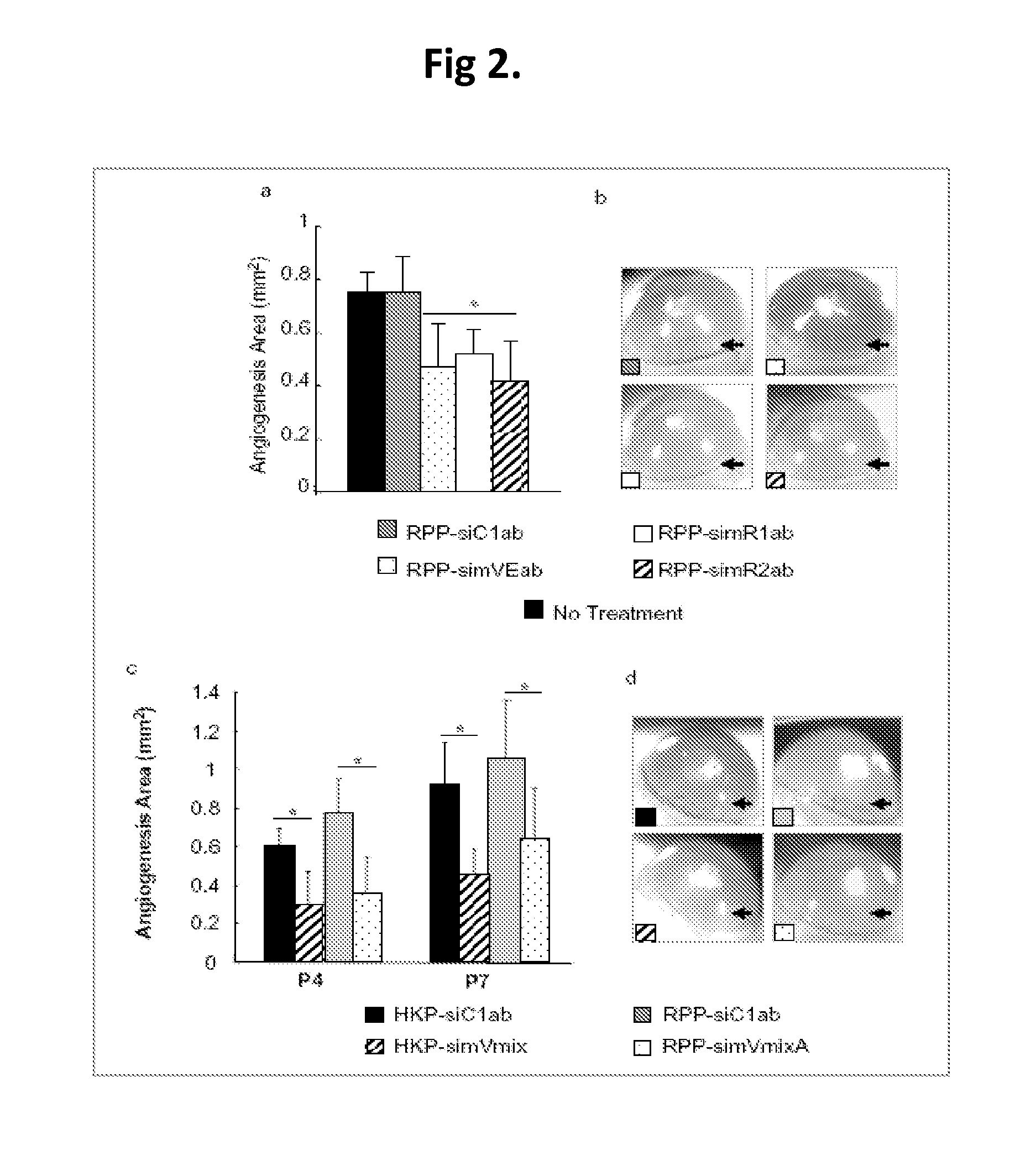
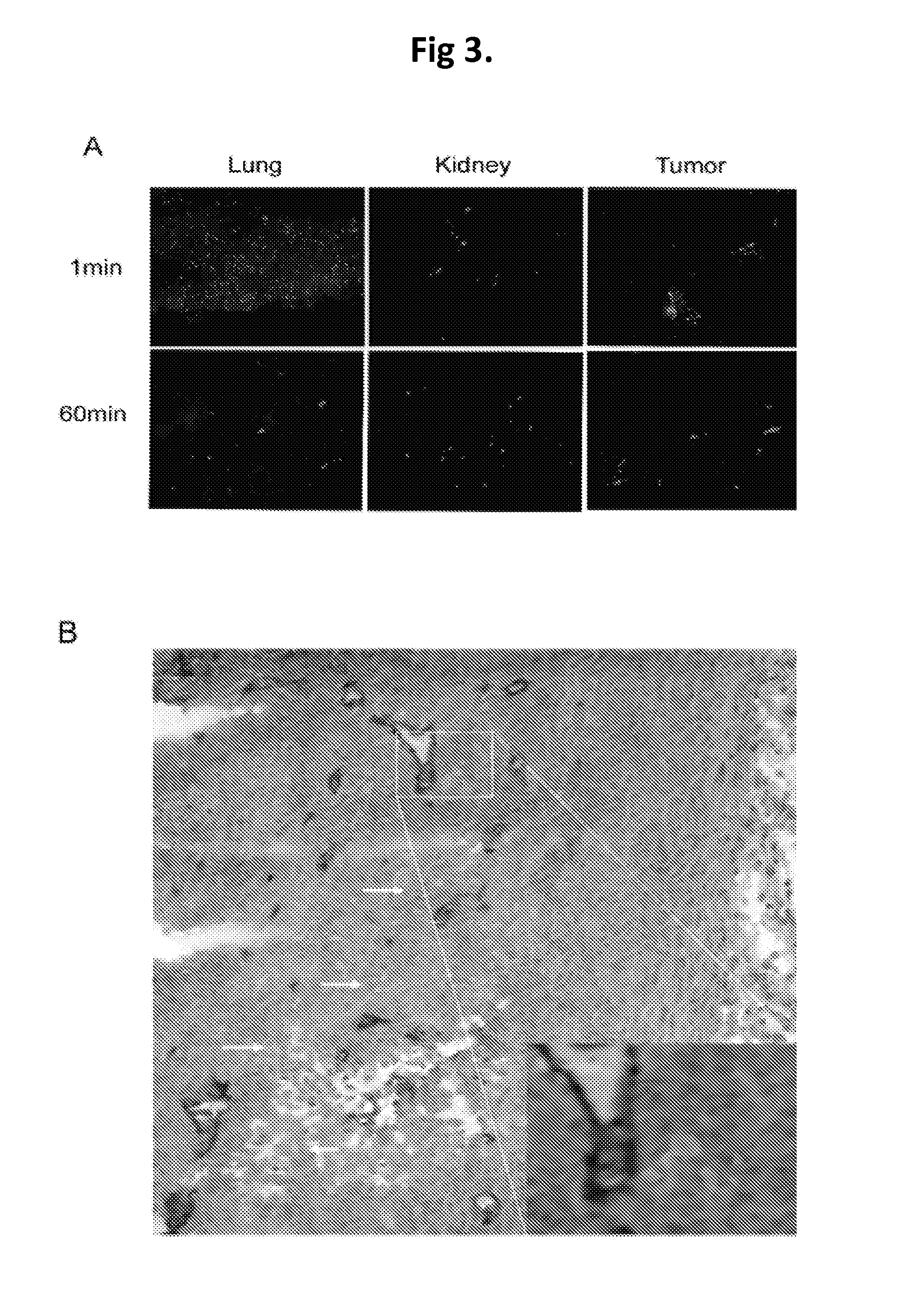
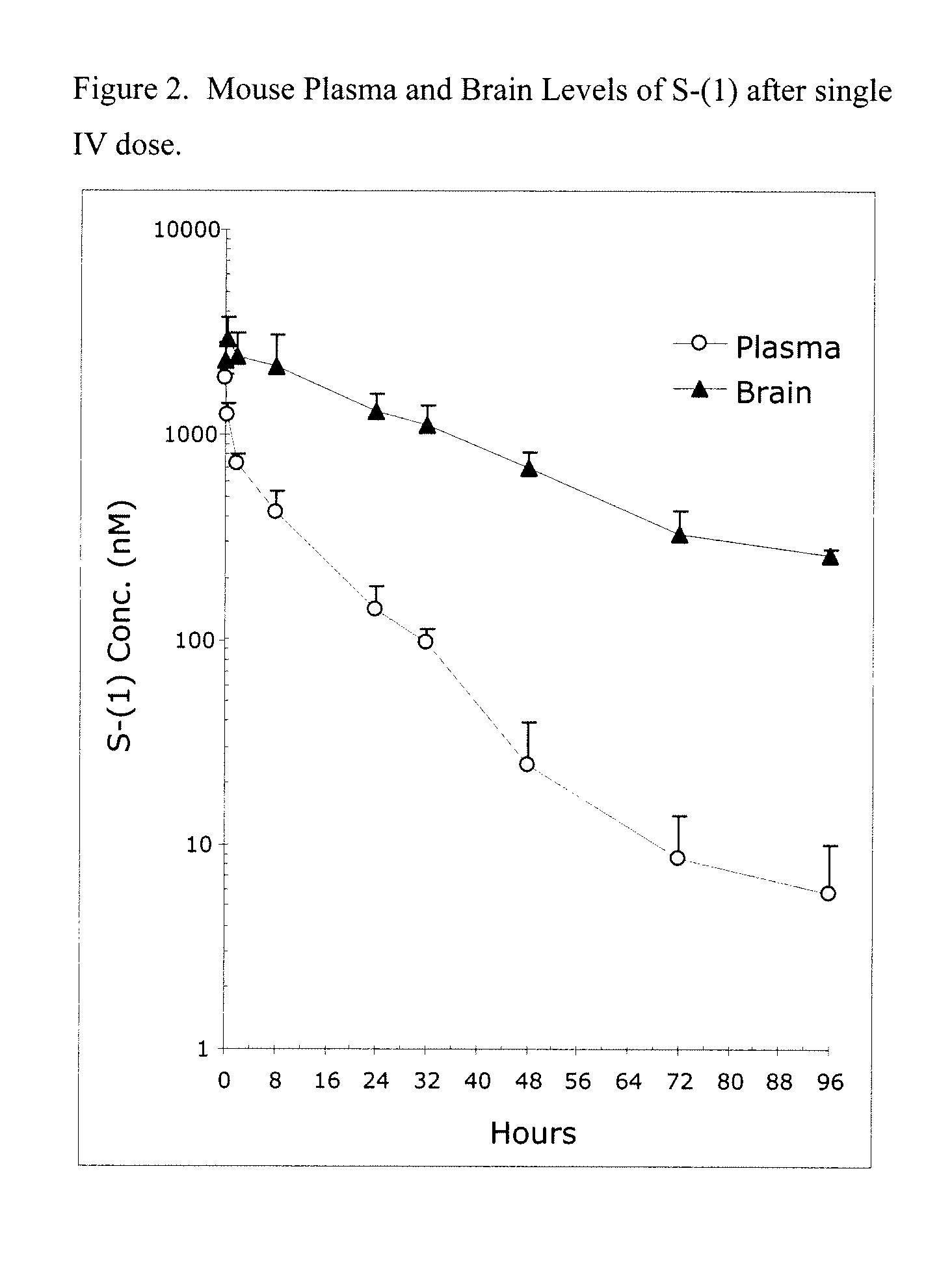
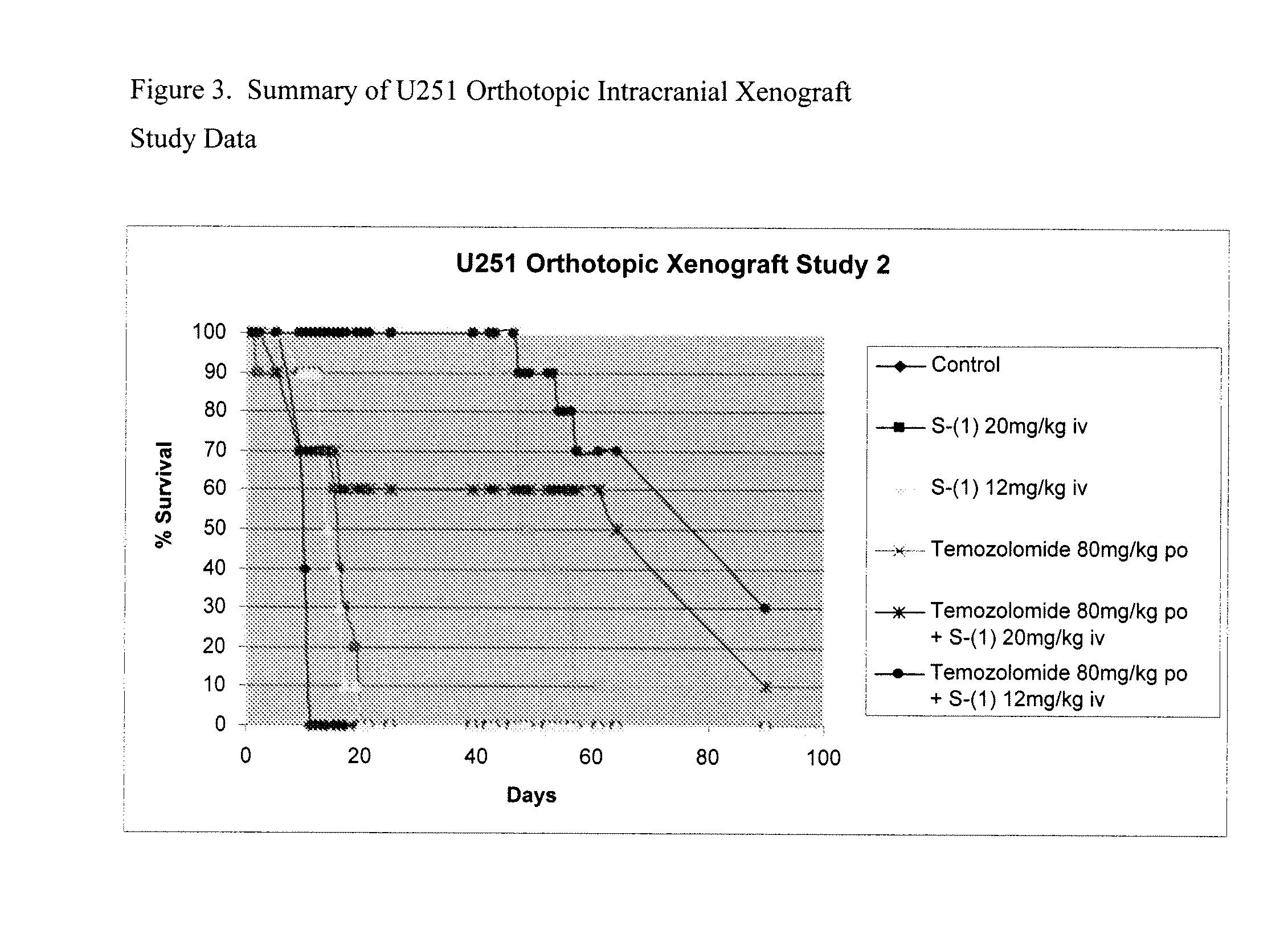
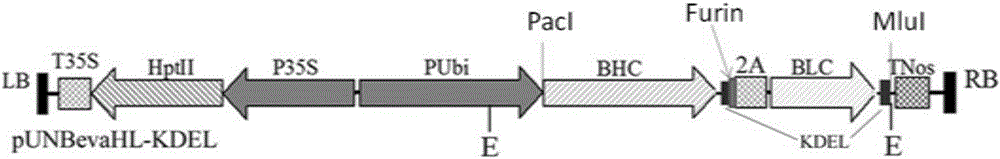
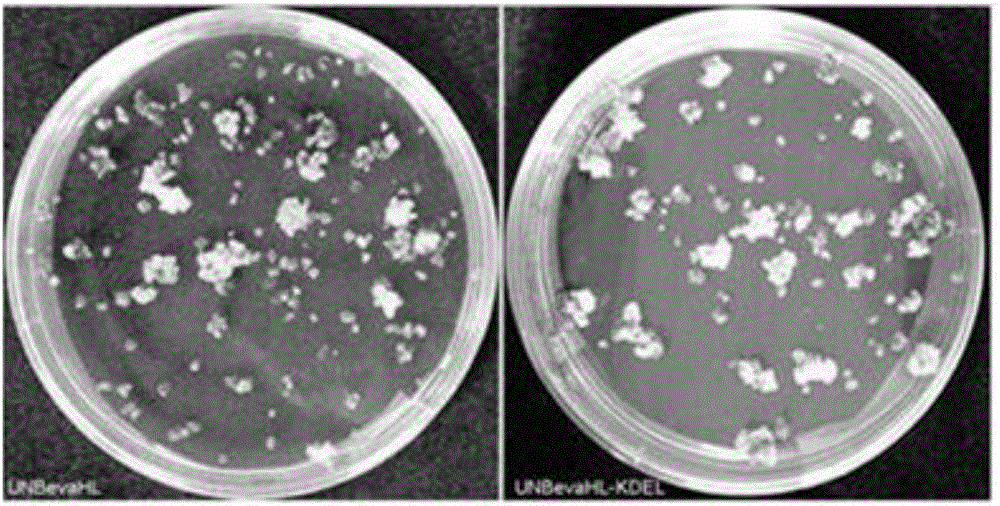
Patents
Literature
147 results about "Bevacizumab" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is a man-made antibody (IgG1) used to treat kidney, cervical, ovarian, colon, and rectal cancer. Bevacizumab is also used to treat lung cancer (non-small cell type), certain types of brain tumors, and cancer found in the fallopian tube or lining of the abdominal wall (peritoneal).
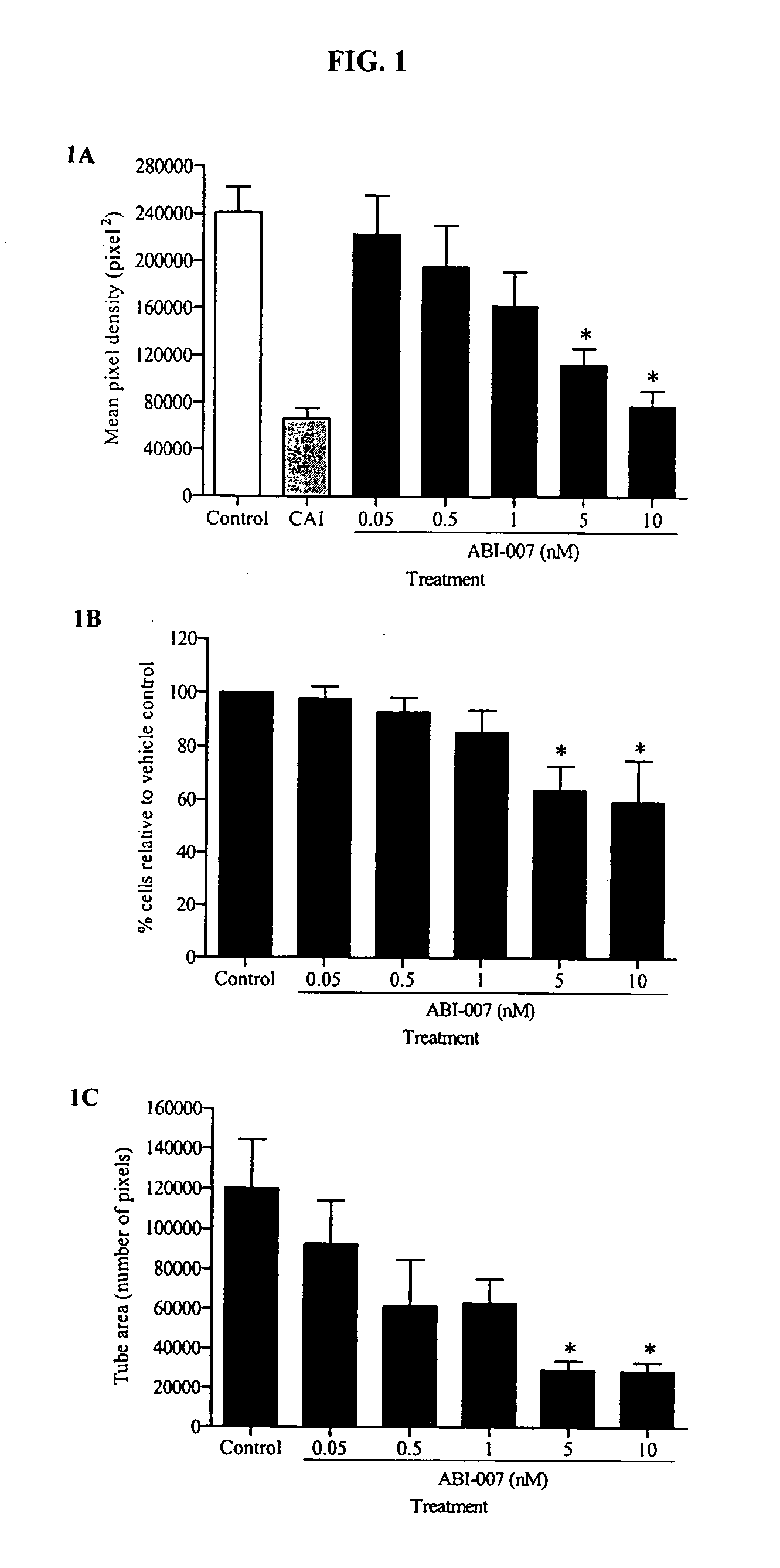
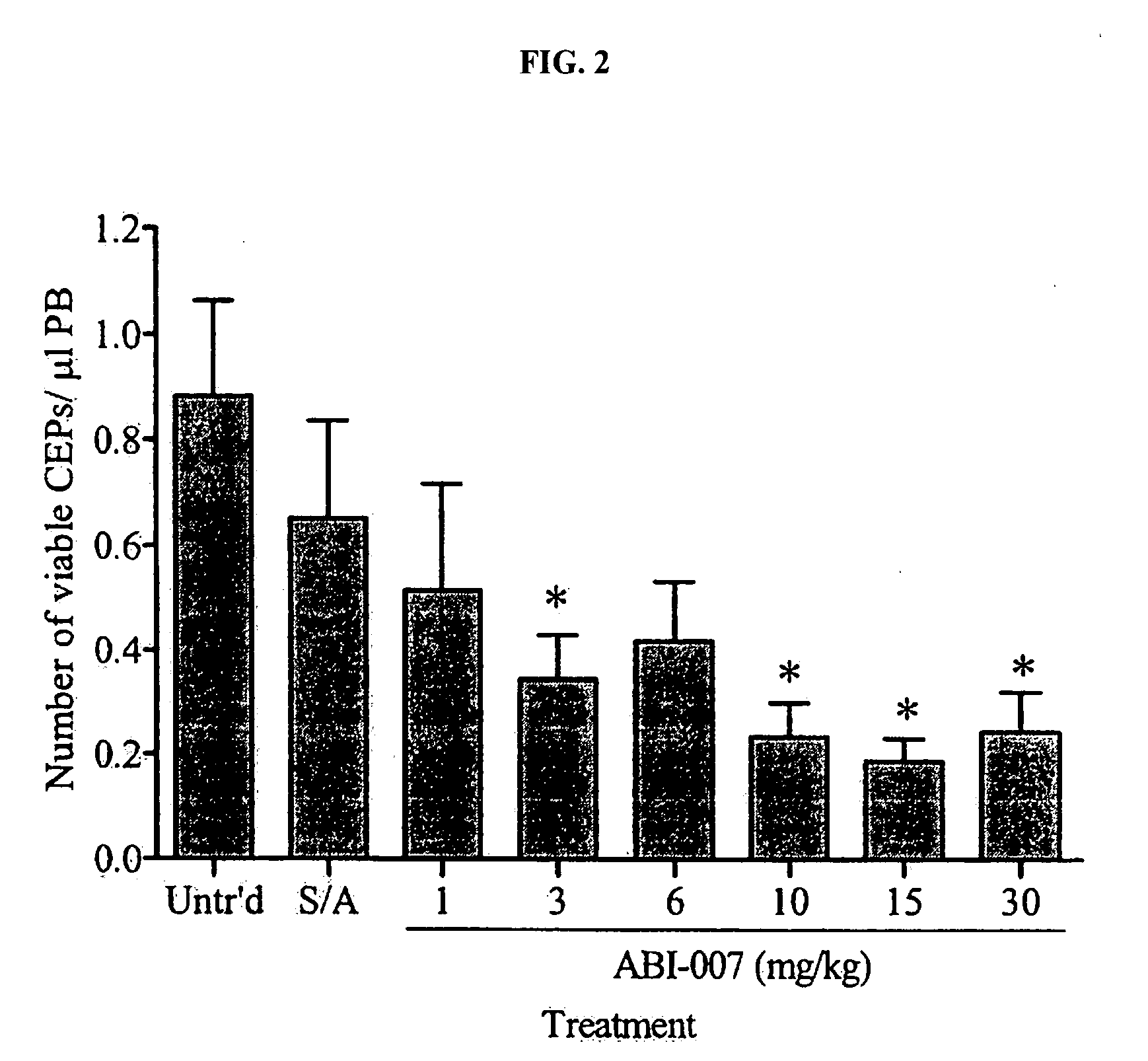
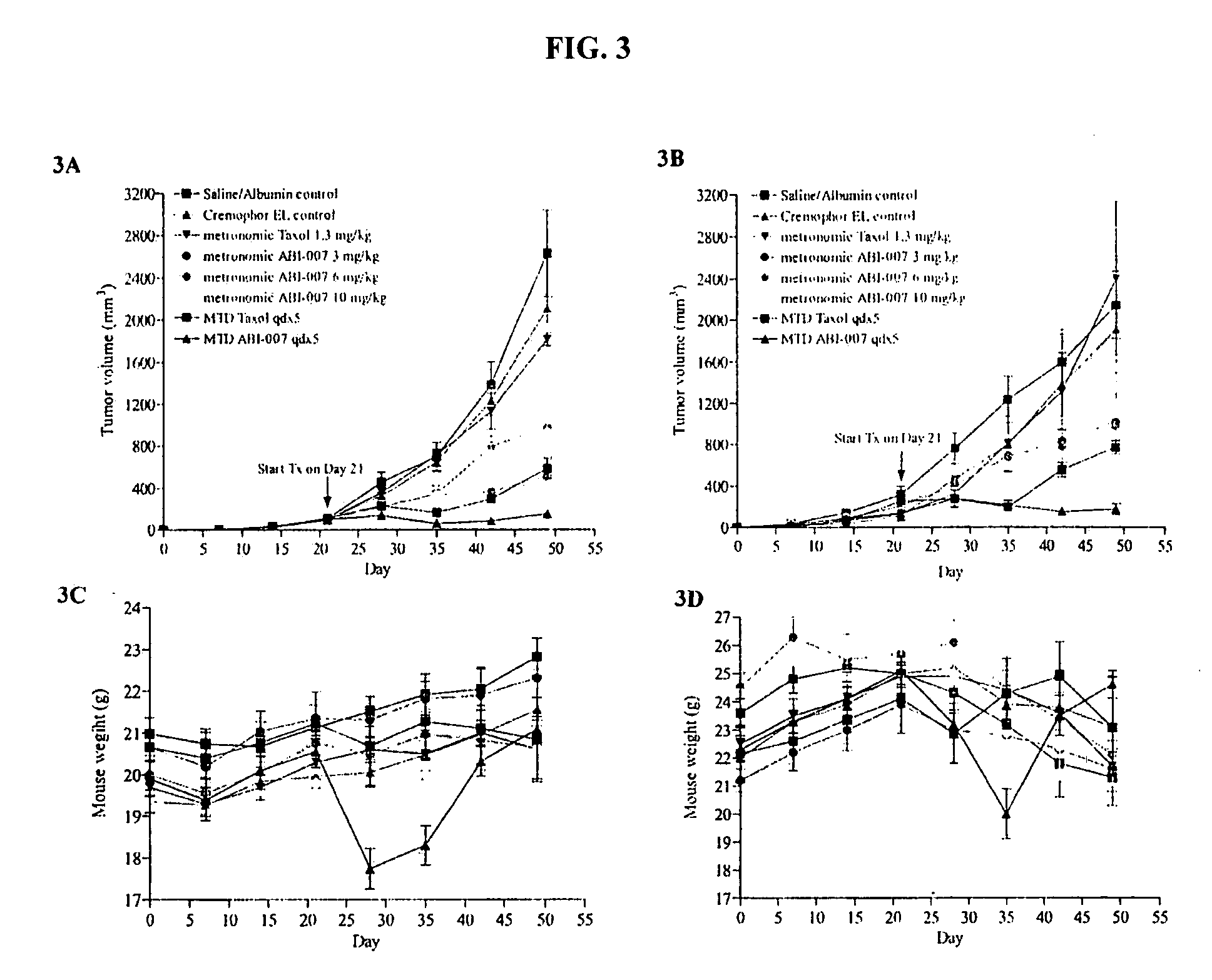
Nanoparticles of paclitaxel and albumin in combination with bevacizumab against cancer
InactiveUS20100112077A1Organic active ingredientsHeavy metal active ingredientsBevacizumab InjectionAnti vegf antibody
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents (such as an anti-VEGF antibody), or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
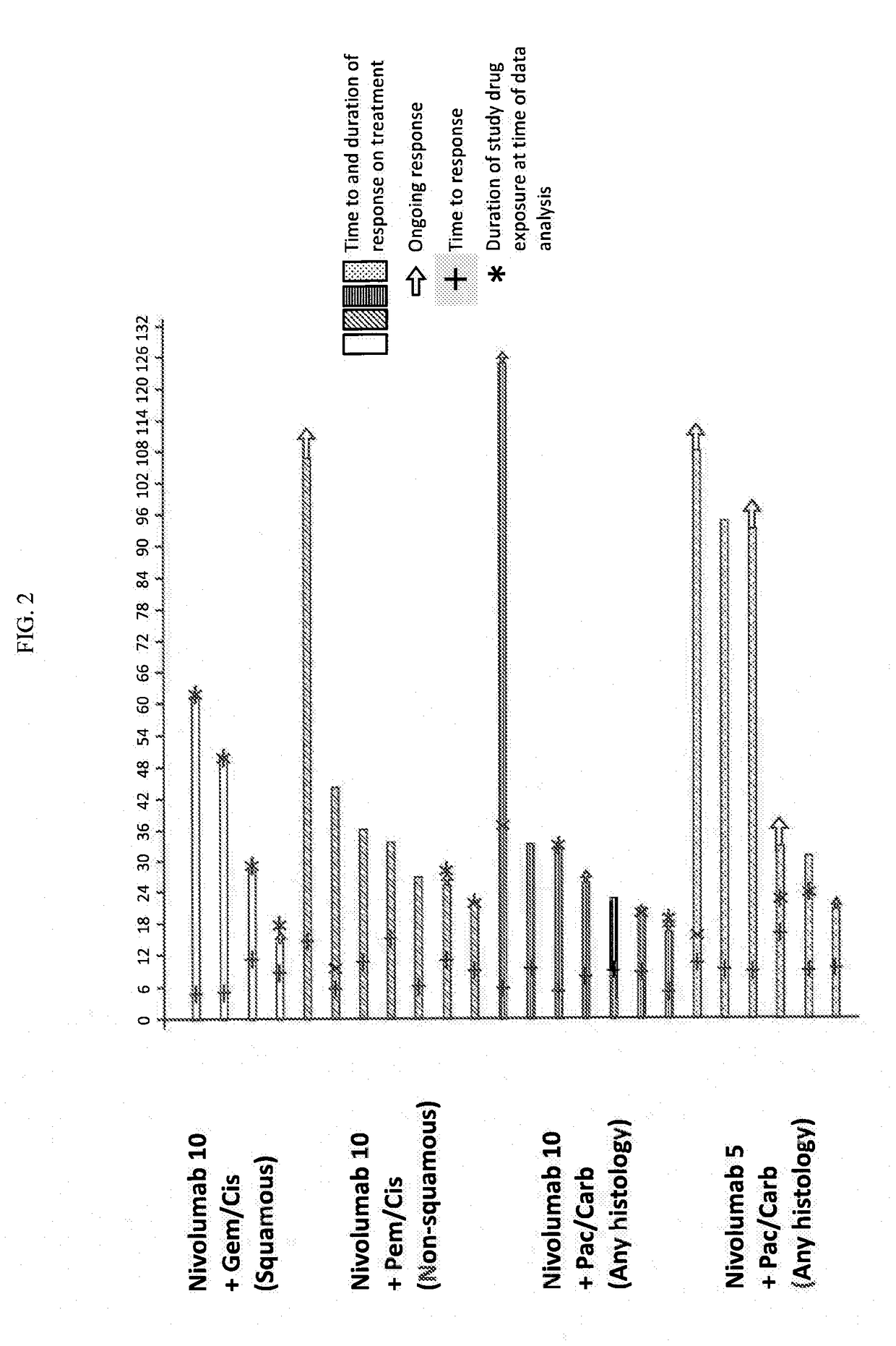
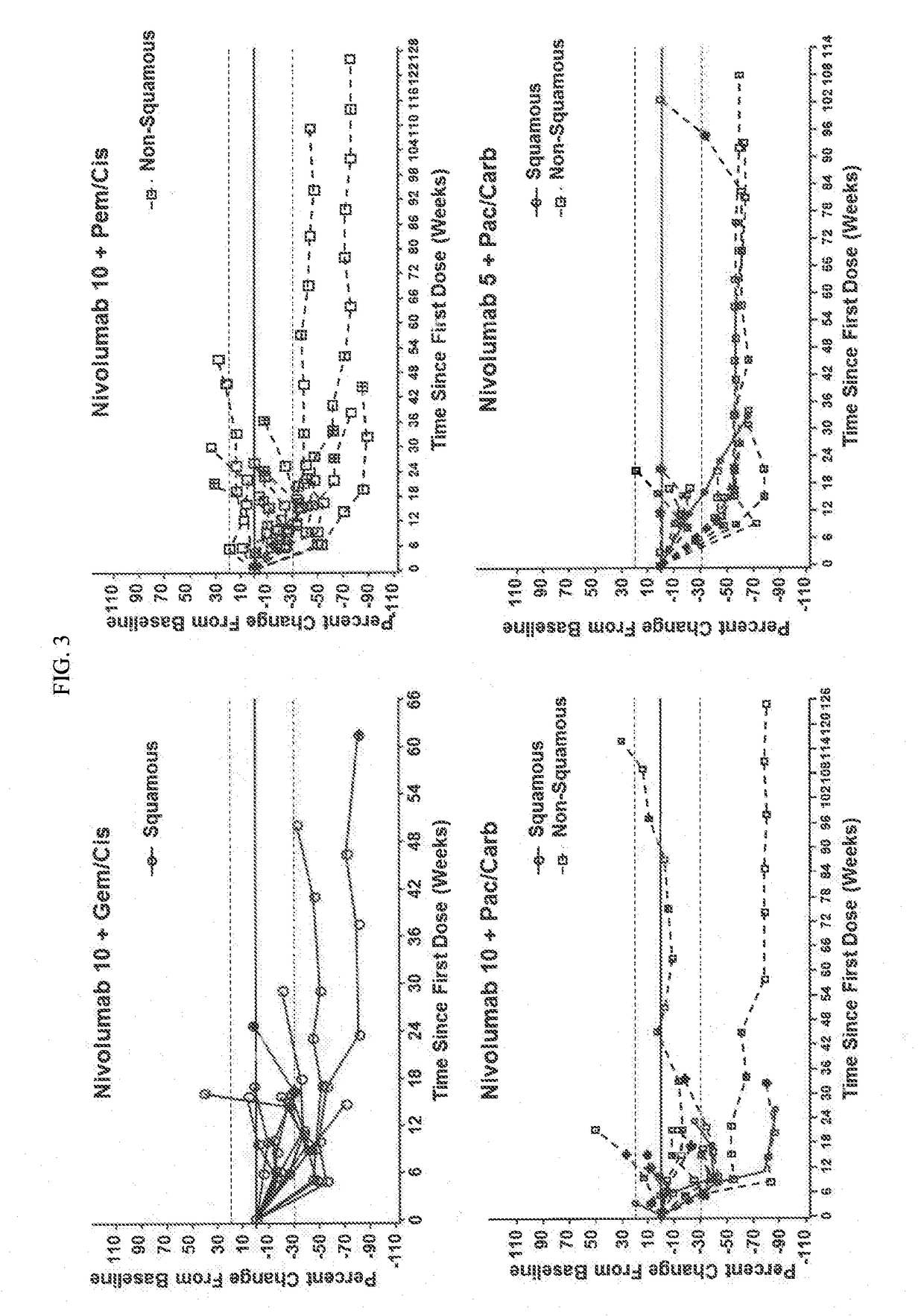
Treatment of lung cancer using a combination of an Anti-pd-1 antibody and another Anti-cancer agent
InactiveUS20170158776A1Durable clinical responseReliable responseHeavy metal active ingredientsImmunoglobulins against growth factorsAnticarcinogenTyrosine-kinase inhibitor
This disclosure provides a method for treating a subject afflicted with a lung cancer, which method comprises administering to the subject therapeutically effective amounts of: (a) an anti-cancer agent which is an antibody or an antigen-binding portion thereof that specifically binds to a Programmed Death-1 (PD-1) receptor and inhibits PD-1 activity; and (b) another anti-cancer agent. The other anti-cancer agent can be a platinum-based doublet chemotherapy, an EGFR-targeted tyrosine kinase inhibitor, bevacizumab, an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody, or any other therapy used to treat lung cancer in the art or disclosed herein.
Owner:BRISTOL MYERS SQUIBB CO
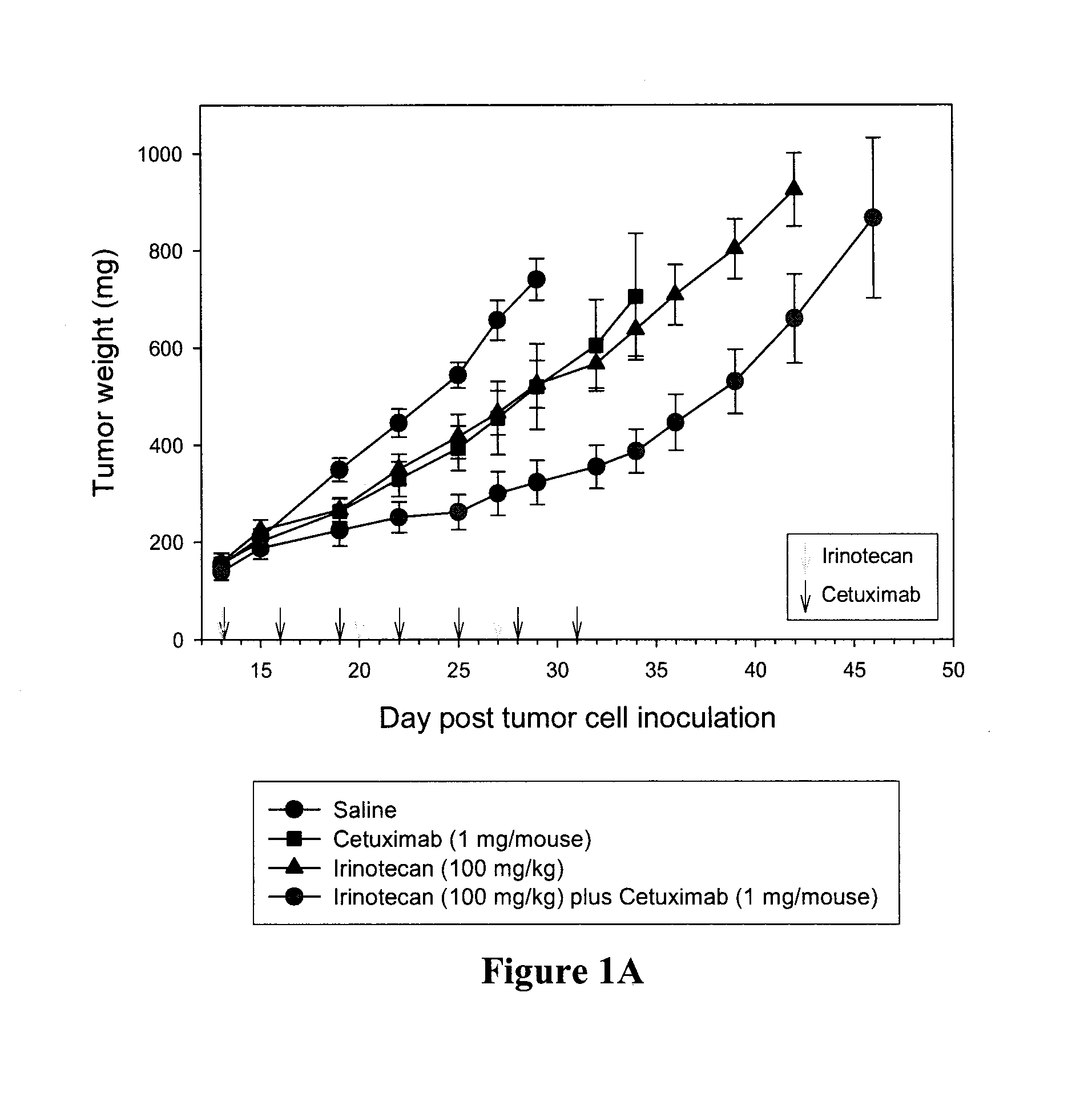
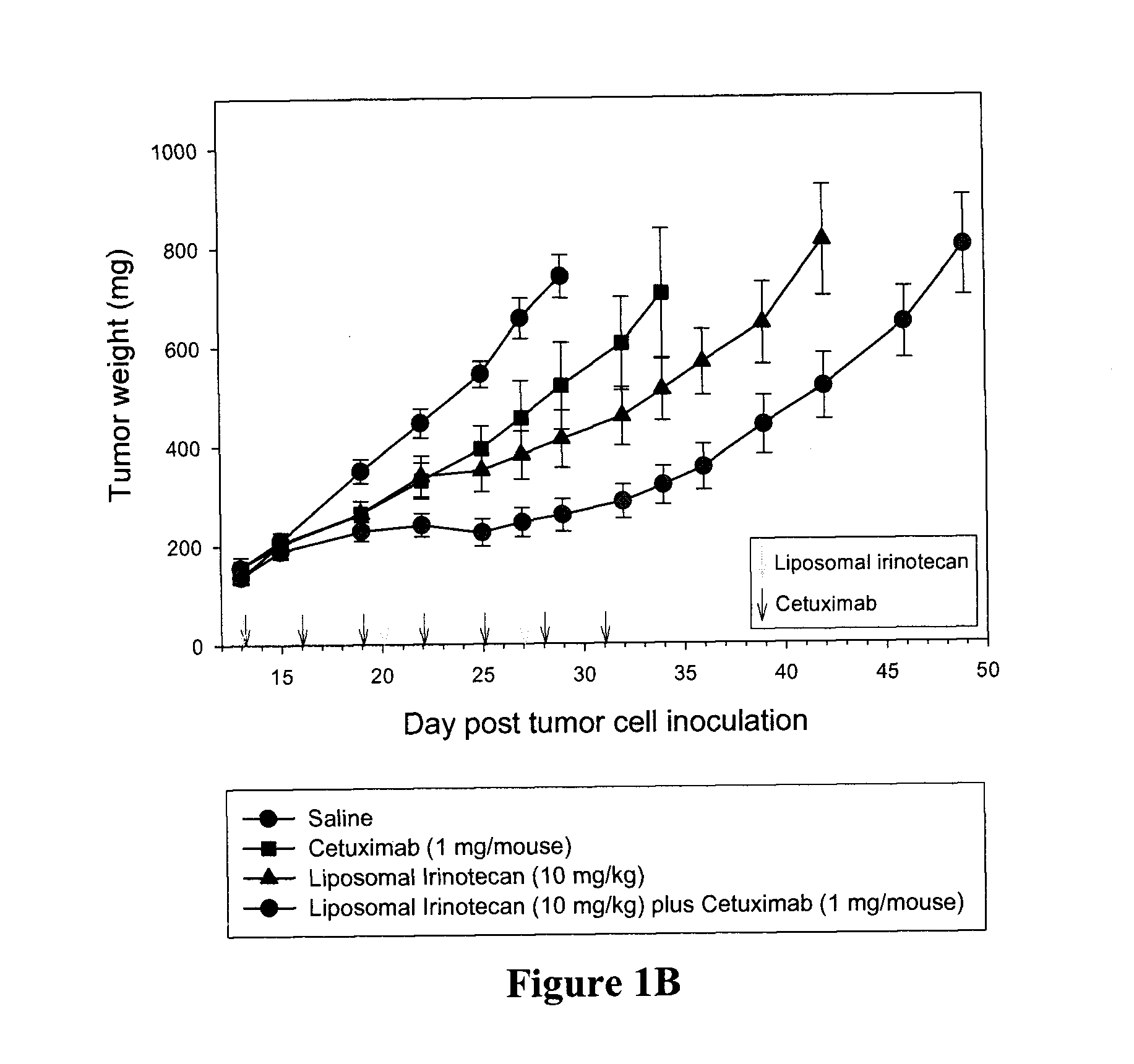
Combination methods and compositions
InactiveUS20110223241A1BiocideCarbohydrate active ingredientsBevacizumab InjectionTherapeutic effect
Compositions which comprise a liposomal water-soluble camptothecin and optionally a liposomal fluoropyrimidine in combination with a vascular epithelial growth factor (VEGF) inhibitor such as cetuximab or an epidermal growth factor receptor (EGFR) inhibitor such as bevacizumab are useful in achieving enhanced therapeutic effects for the treatment of cancer.
Owner:CELATOR PHARMA INC
Method of treating estrogen receptor (ER) -positive breast cancers with selective androgen receptor modulator (SARMS)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
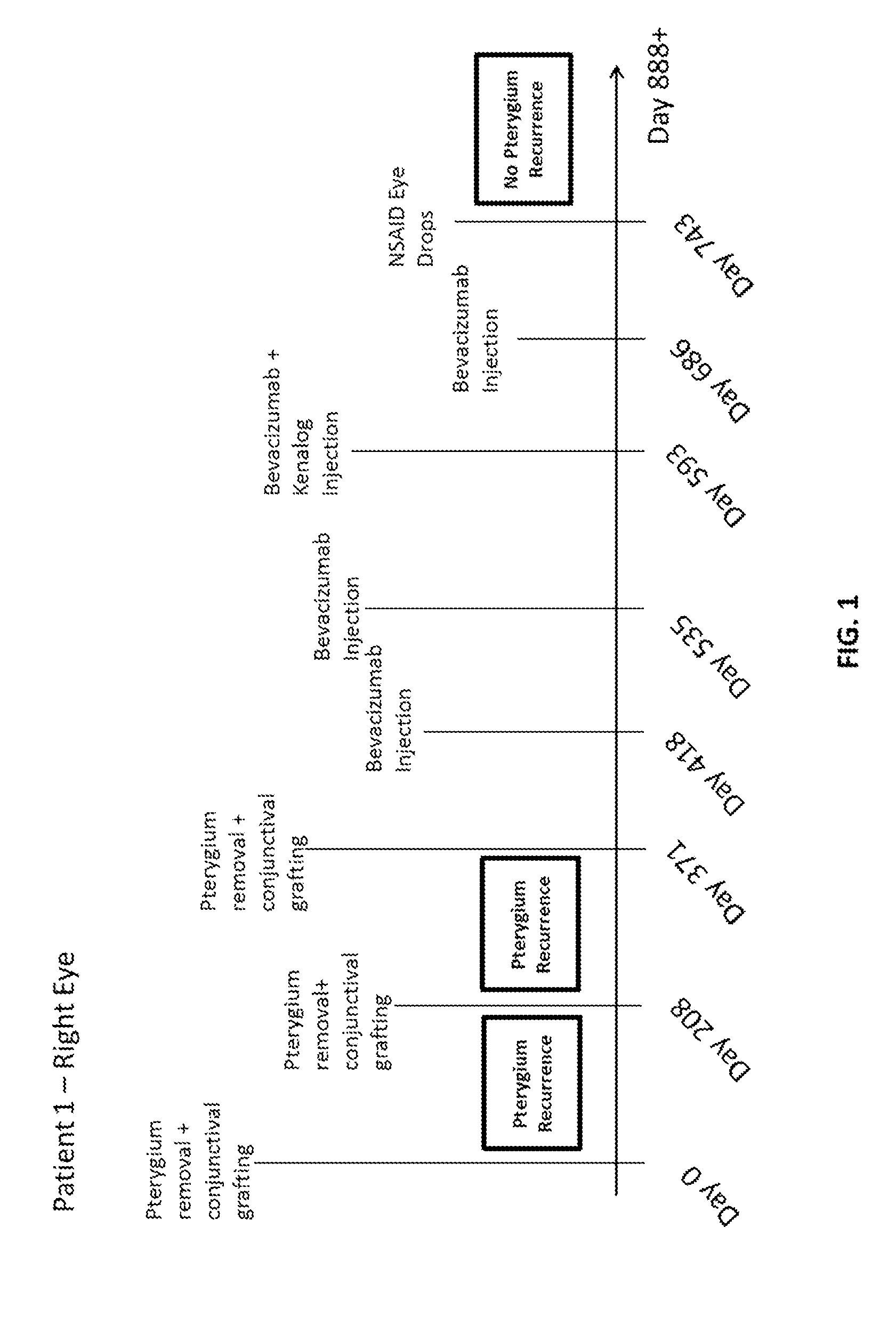
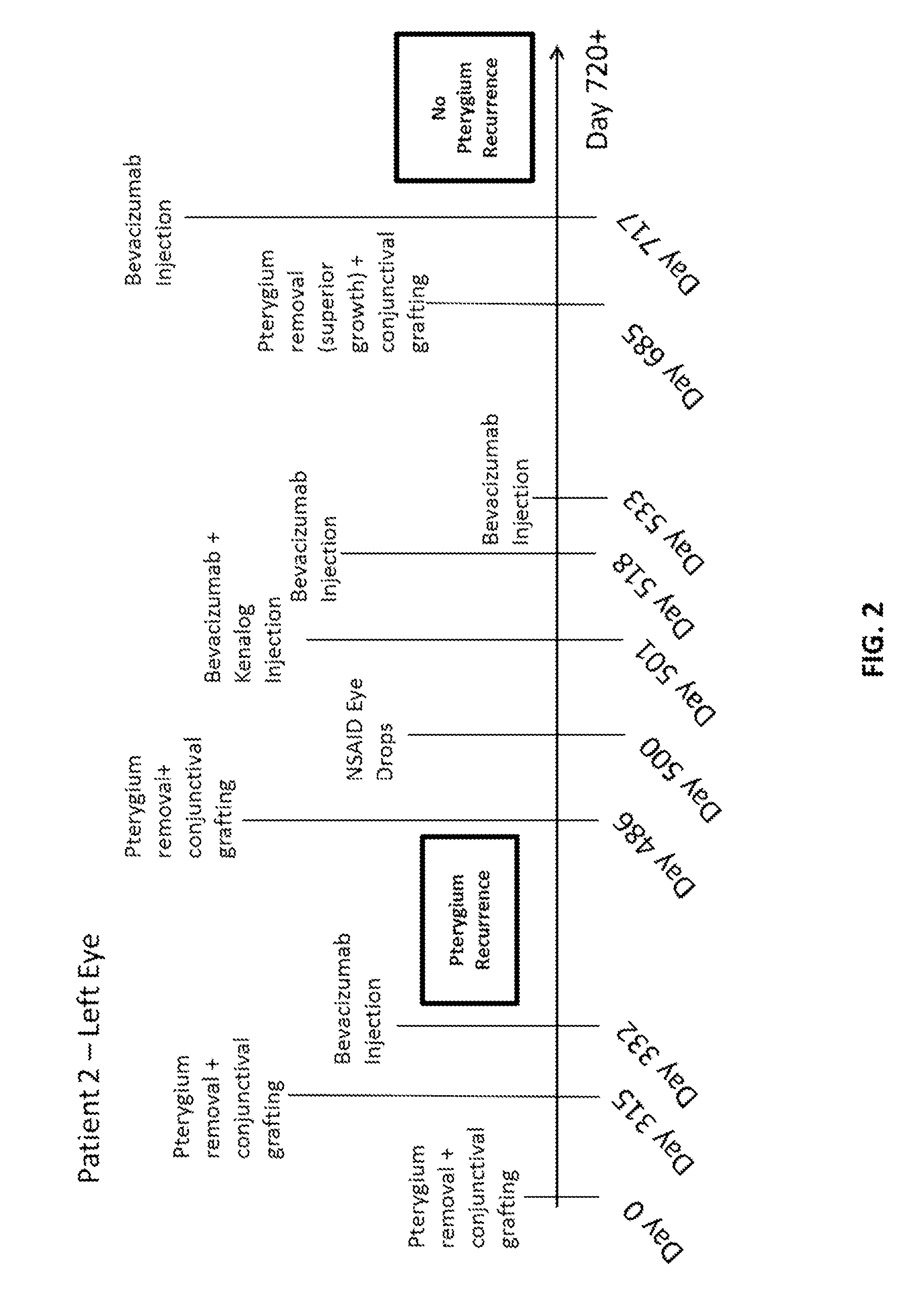
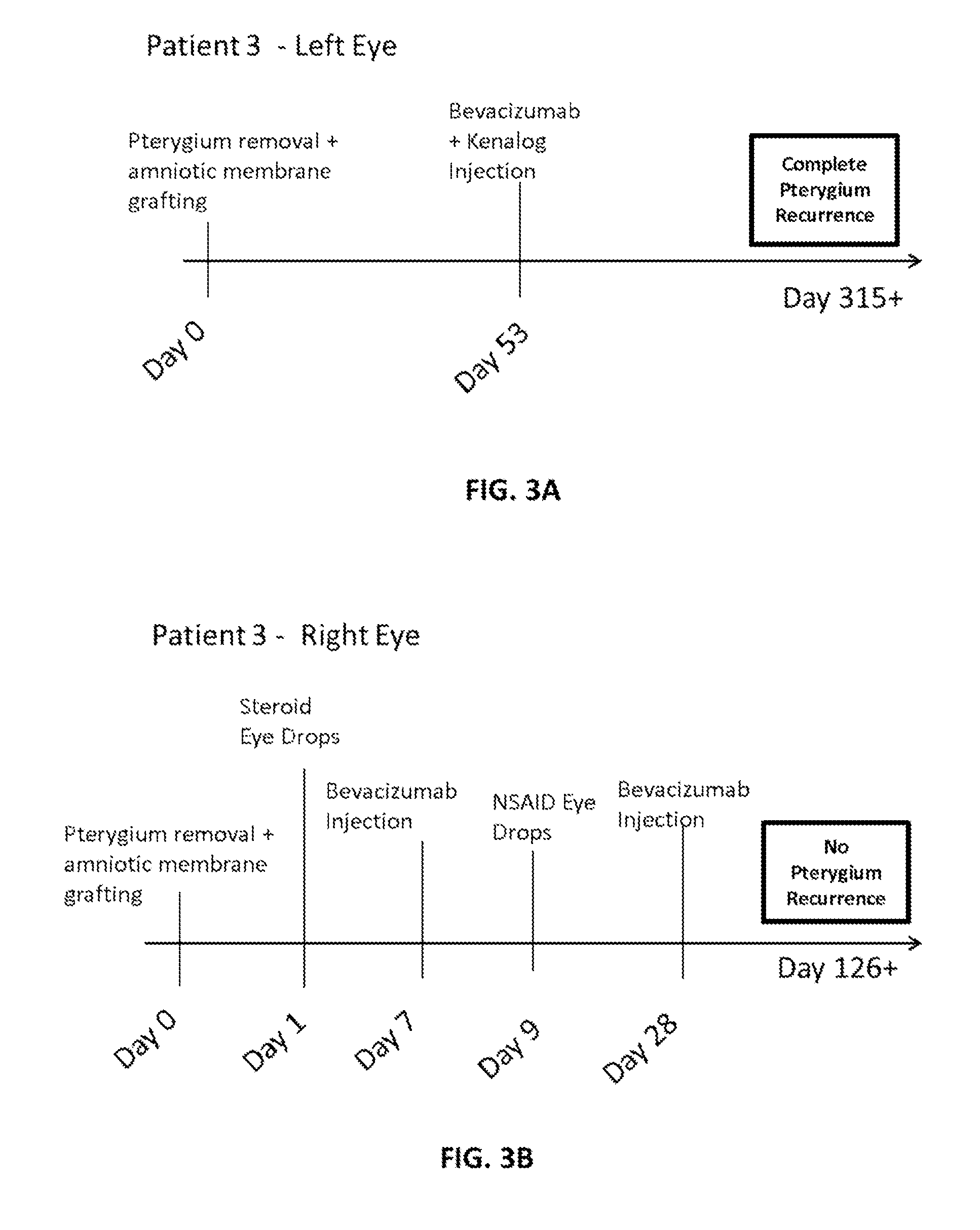
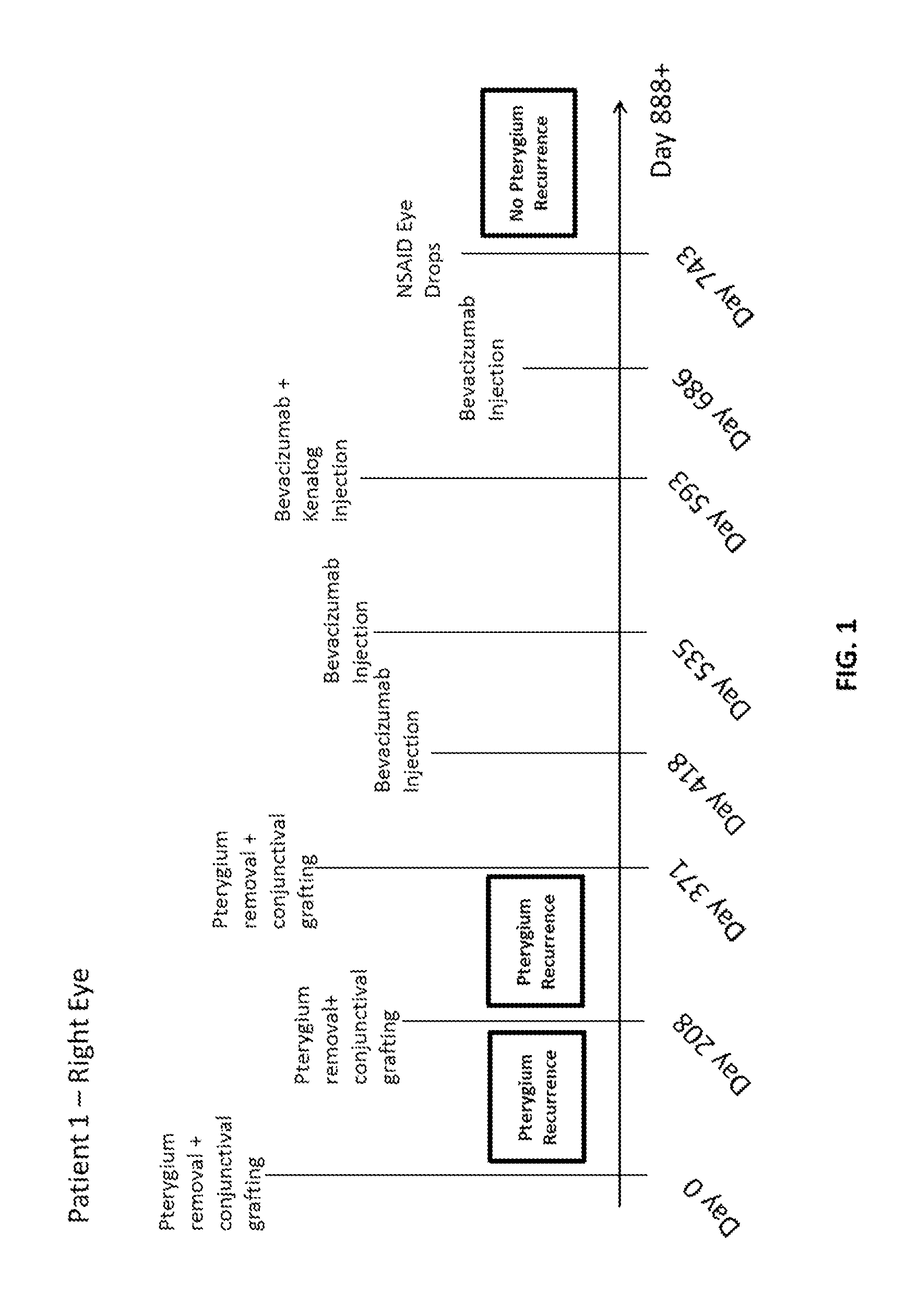
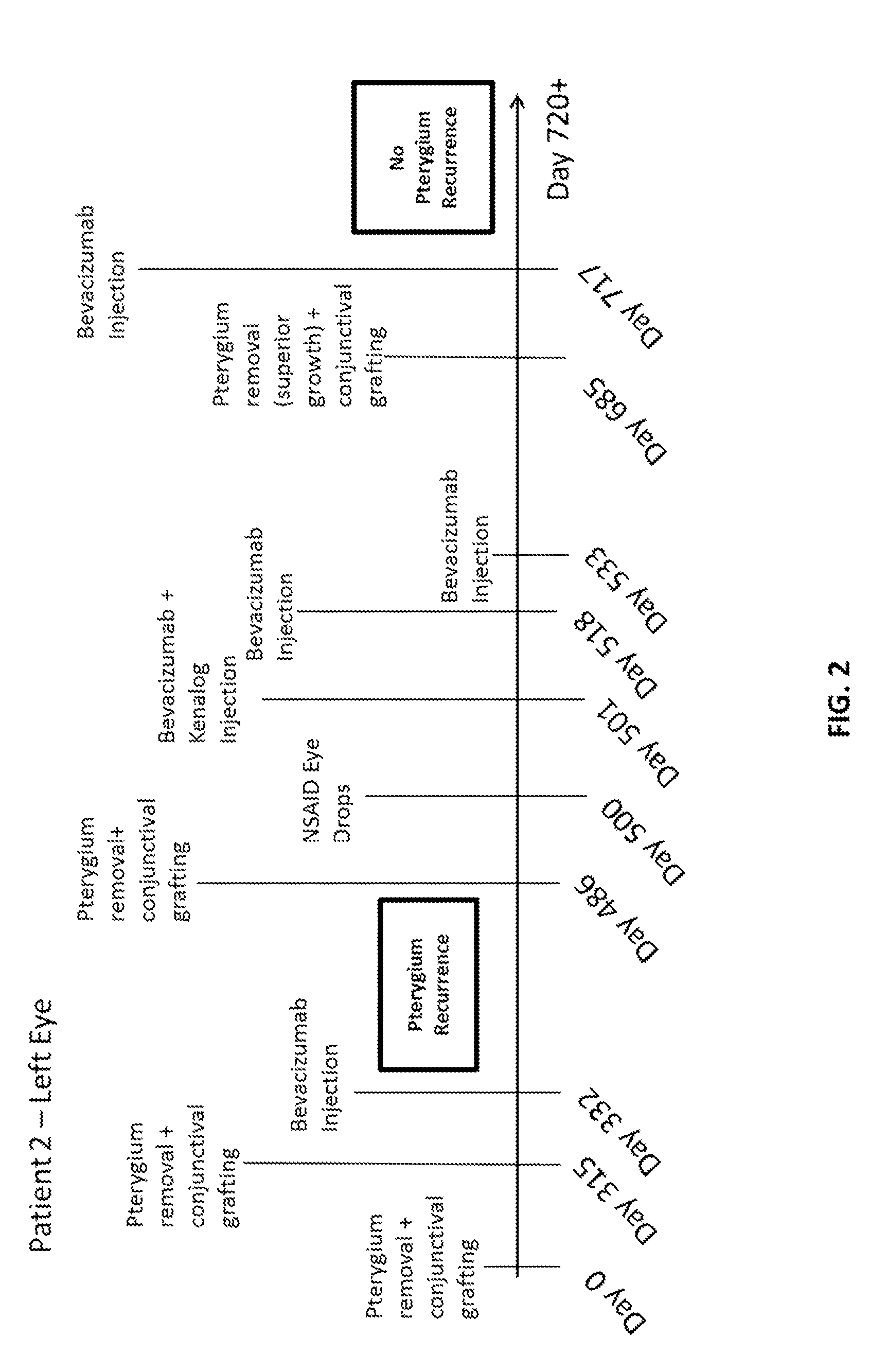
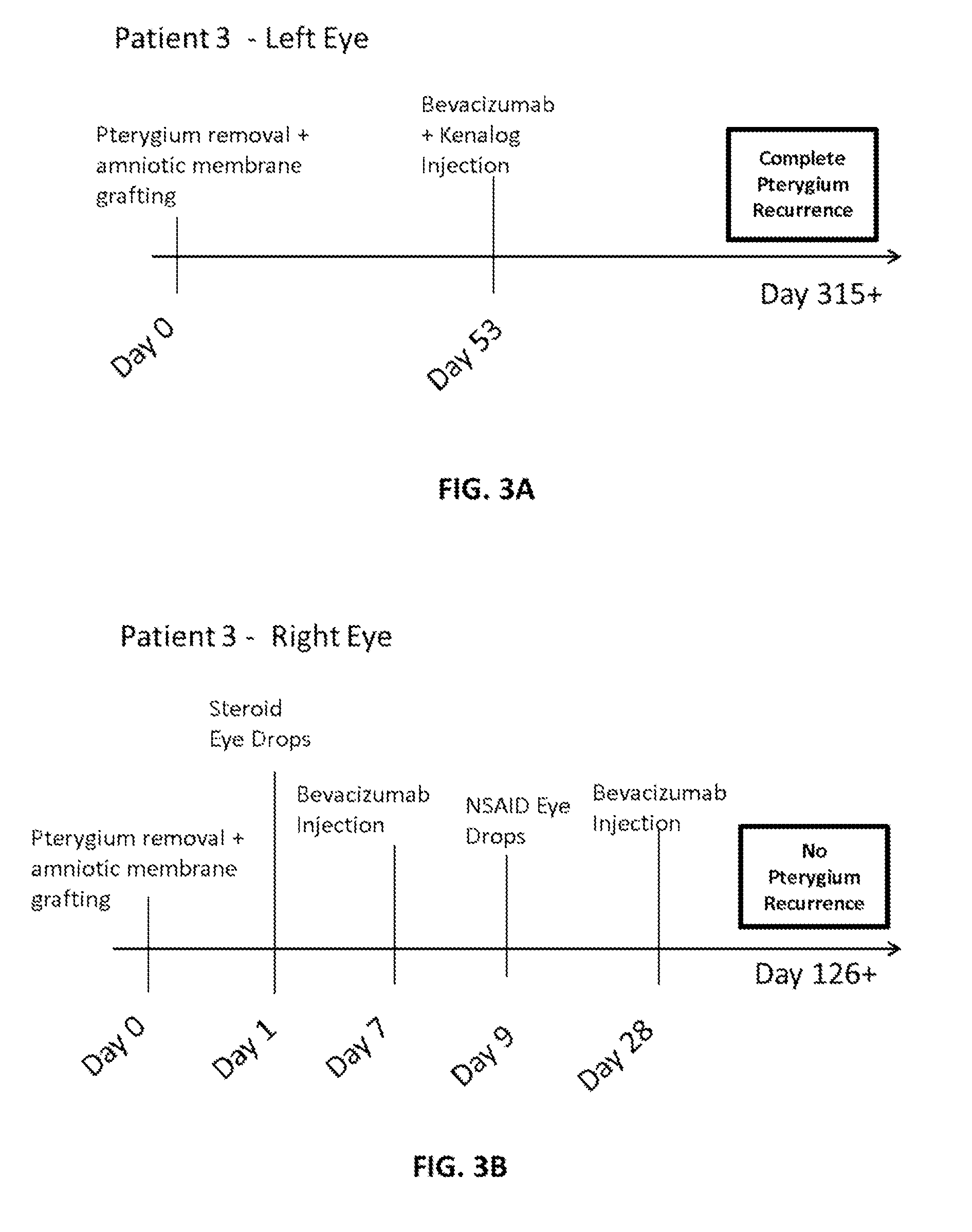
Methods of treating pterygium
Methods for treating pterygium recurrence following pterygiectomy, and for treating keloid recurrence, following surgical removal of the keloid, are disclosed. The methods include administering an anti-VEGF agent (e.g., antibody (e.g., bevacizumab) or small molecule inhibitor of VEGF signaling), or a combination therapy that includes co-administering an anti-VEGF agent, with an anti-inflammatory steroid and / or a non-steroidal anti-inflammatory drug (NSAID) to a subject.
Owner:PHAM RANDAL TANH HOANG
Method of treating androgen receptor (AR) -positive breast cancers with selective androgen receptor modulator (SARMS)
ActiveUS20140350102A1Treating and preventing and suppressing and inhibiting metastasisProlonged progression-free survivalBiocideOrganic chemistryToremifeneLymphatic Spread
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Compositions and Methods Using siRNA Molecules and siRNA Cocktails for the Treatment of Breast Cancer
ActiveUS20120071540A1Promotes tumorigenesisOrganic active ingredientsSugar derivativesAnticarcinogenNanoparticle
The present invention provides small interfering RNA (siRNA) molecules, compositions containing the molecules, and methods of using the molecules and compositions to treat breast cancer. In one aspect, a multi-targeted siRNAi cocktail is disclosed. The siRNA molecules may be encapsulated in nanoparticles to further enhance their anti-cancer activity. The compositions may also be used in combination with other anti-cancer agents, such as bevacizumab.
Owner:SIRNAOMICS INC
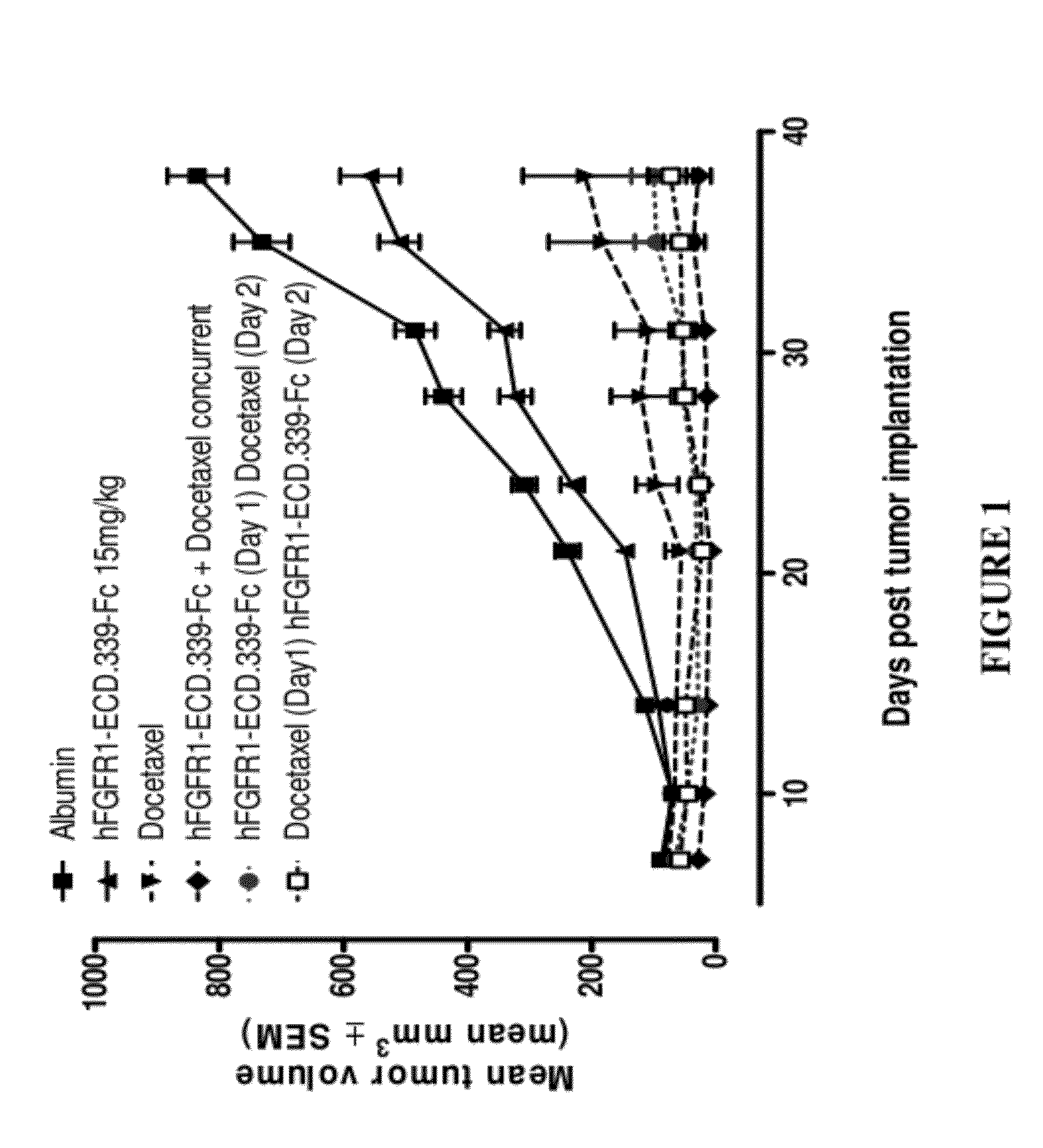
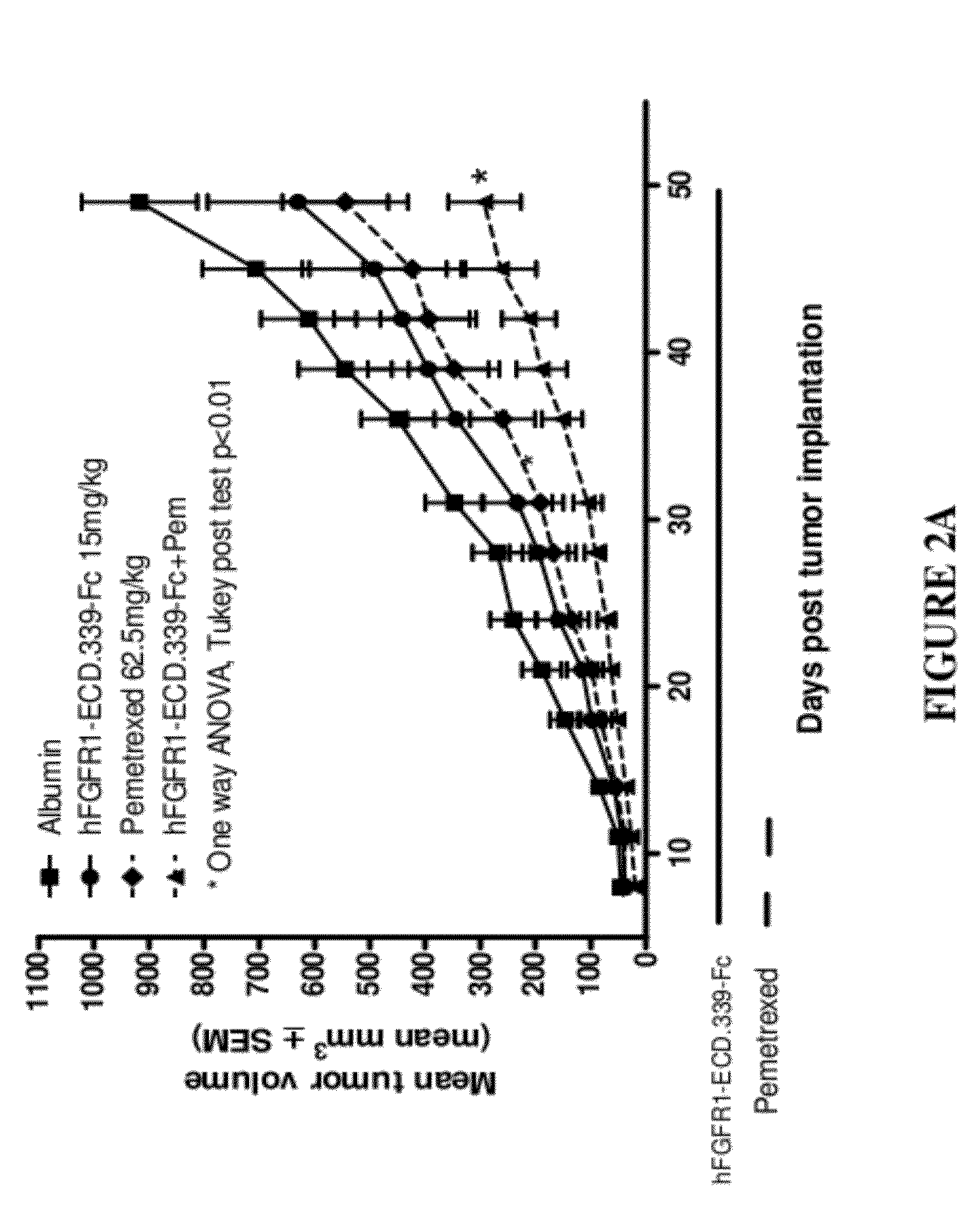
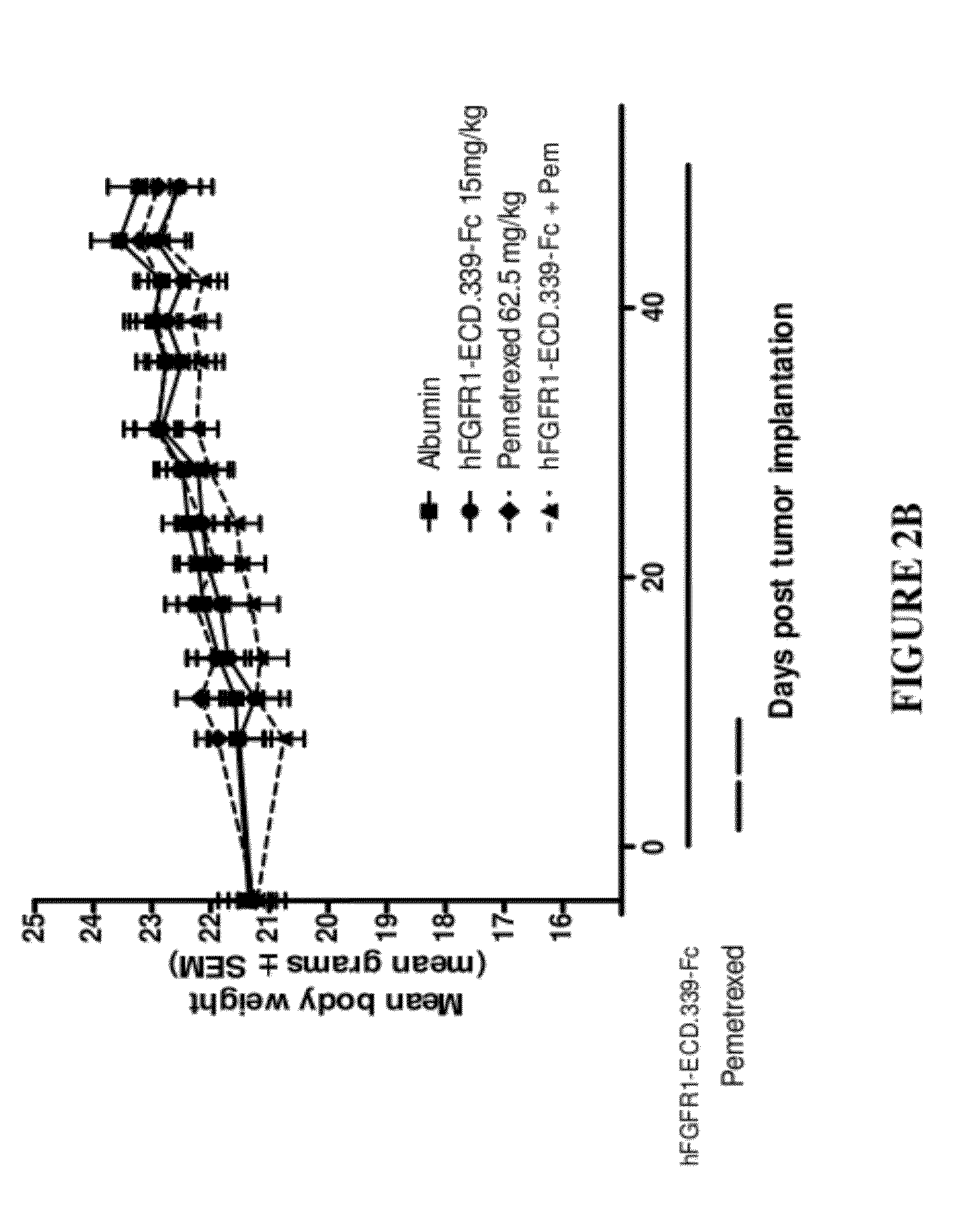
Fgfr1 extracellular domain combination therapies
InactiveUS20120237511A1Inhibit tumor cell growthOrganic active ingredientsHeavy metal active ingredientsCarboplatinDocetaxel
Methods of treating cancer comprising administering a fibroblast growth factor receptor 1 (FGFR1) extracellular domain (ECD) and / or an FGFR1 ECD fusion molecule in combination with at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are provided. Dosage packs comprising an FGFR1 ECD and / or an FGFR1 ECD fusion molecule and / or at least one additional therapeutic agent selected from docetaxel, paclitaxel, vincristine, carboplatin, cisplatin, oxaliplatin, doxorubicin, 5-fluorouracil (5-FU), leucovorin, pemetrexed, and bevacizumab are also provided. In some embodiments, a dosage pack comprises instructions for administering FGFR1 ECD and / or FGFR1 ECD fusion molecule with at least one additional therapeutic agent.
Owner:FIVE PRIME THERAPEUTICS
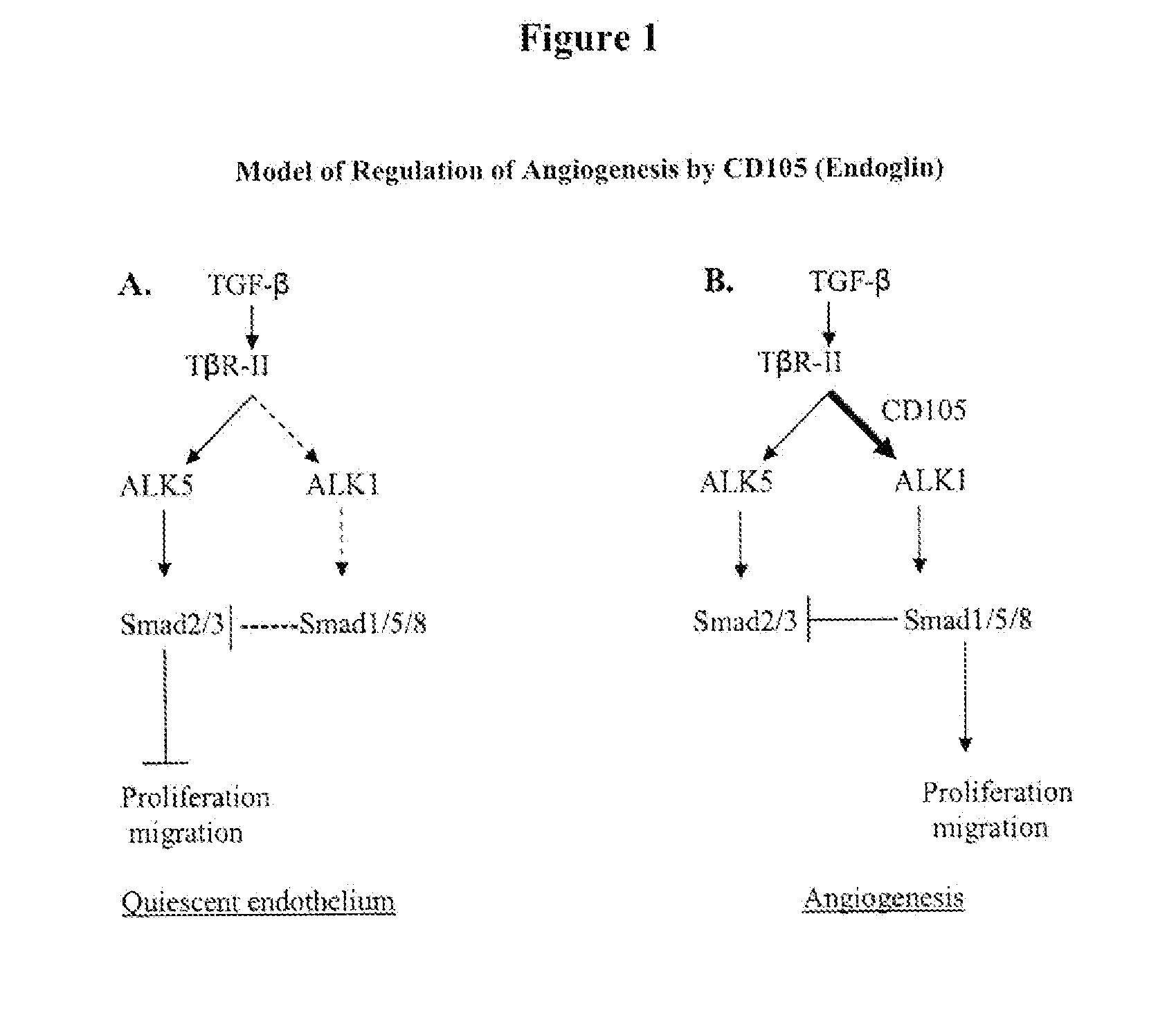
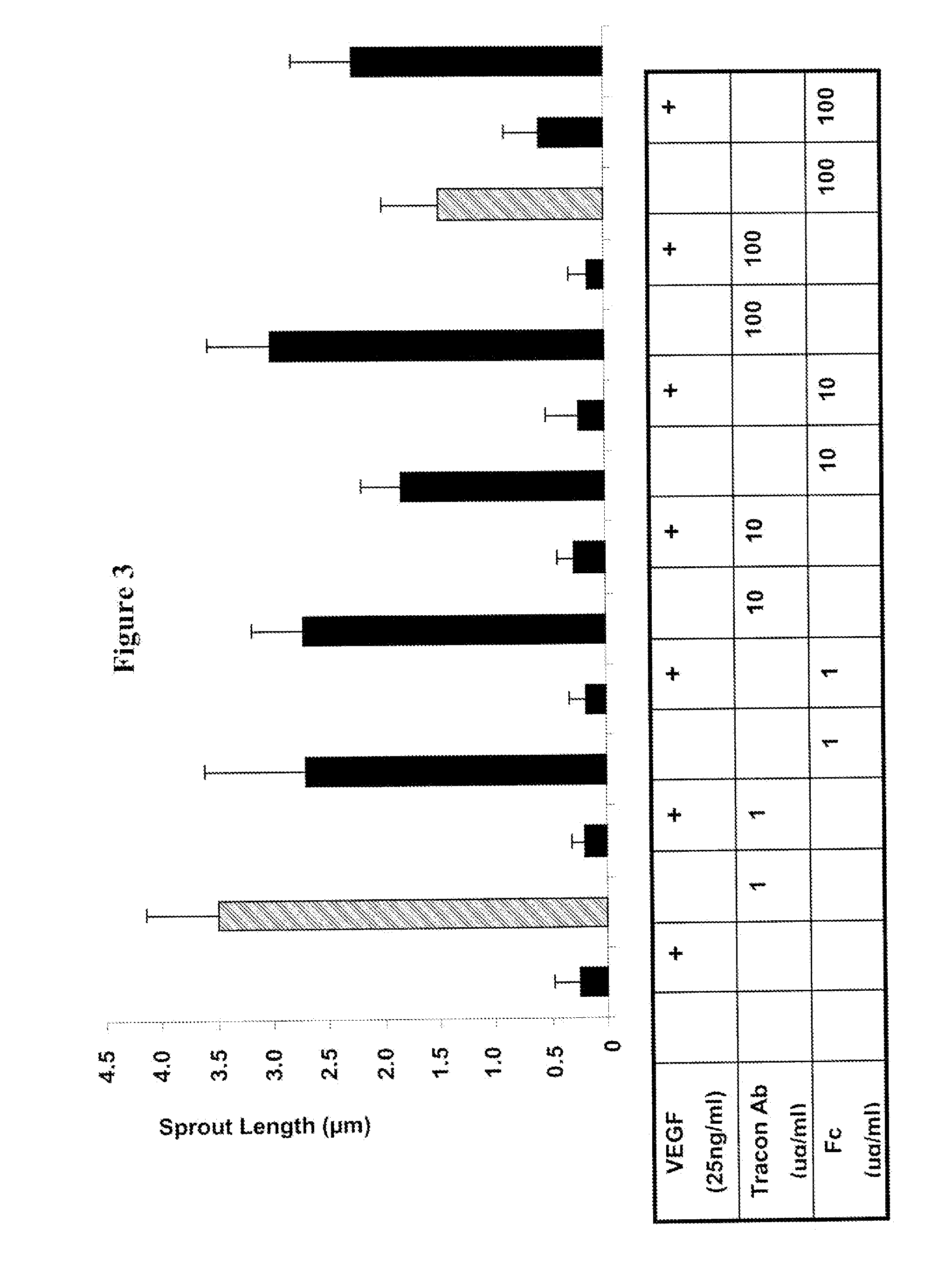
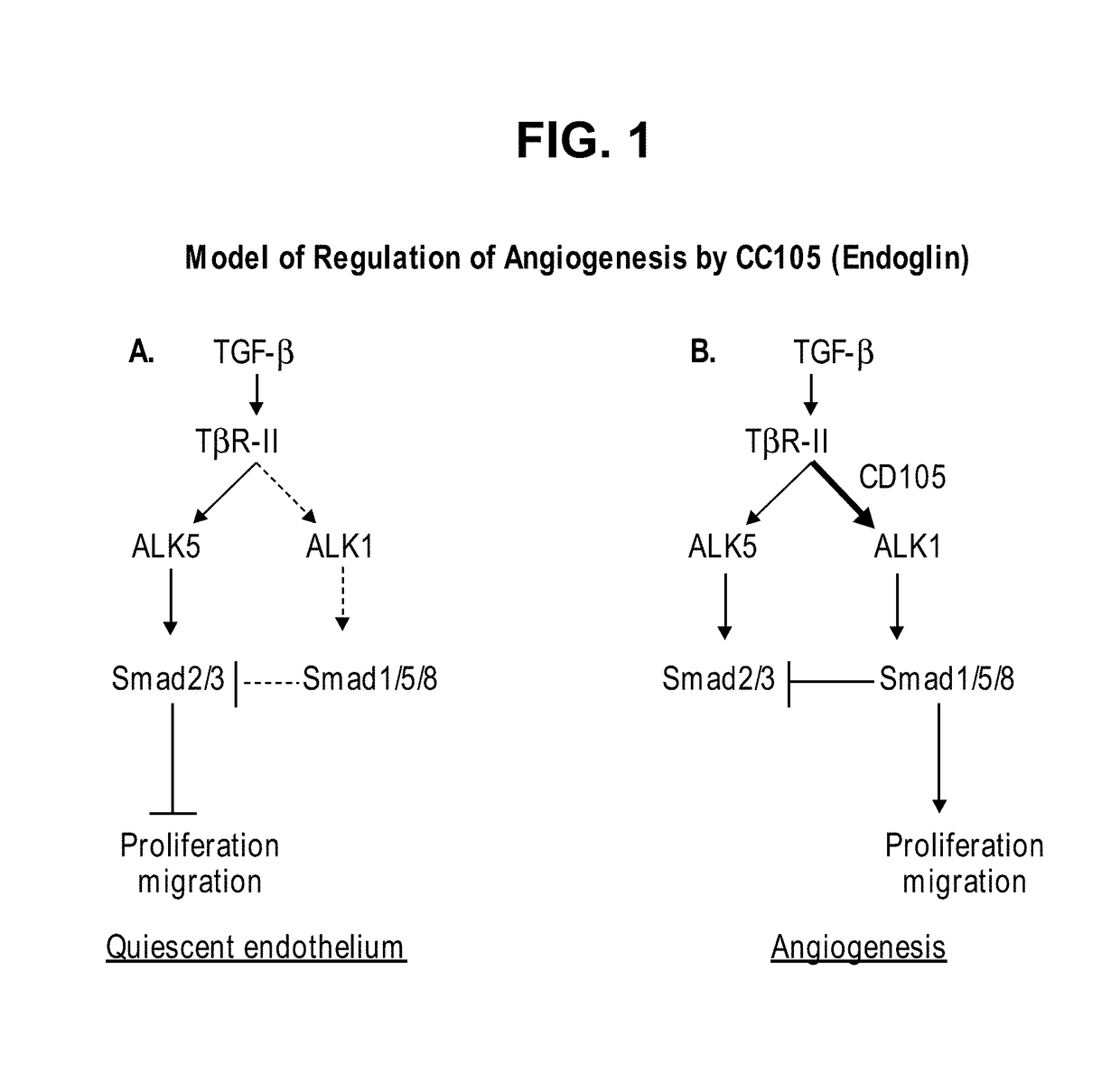
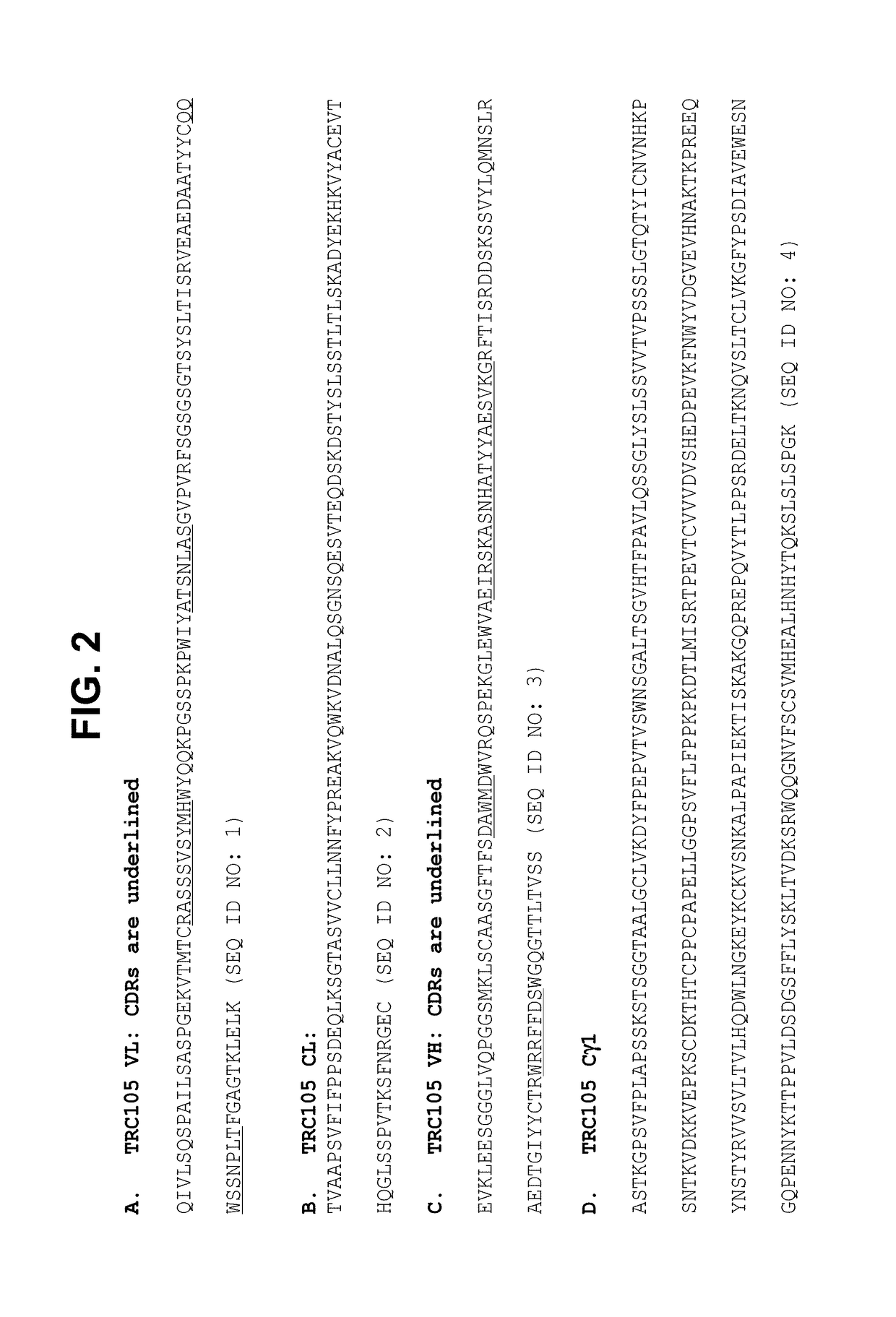
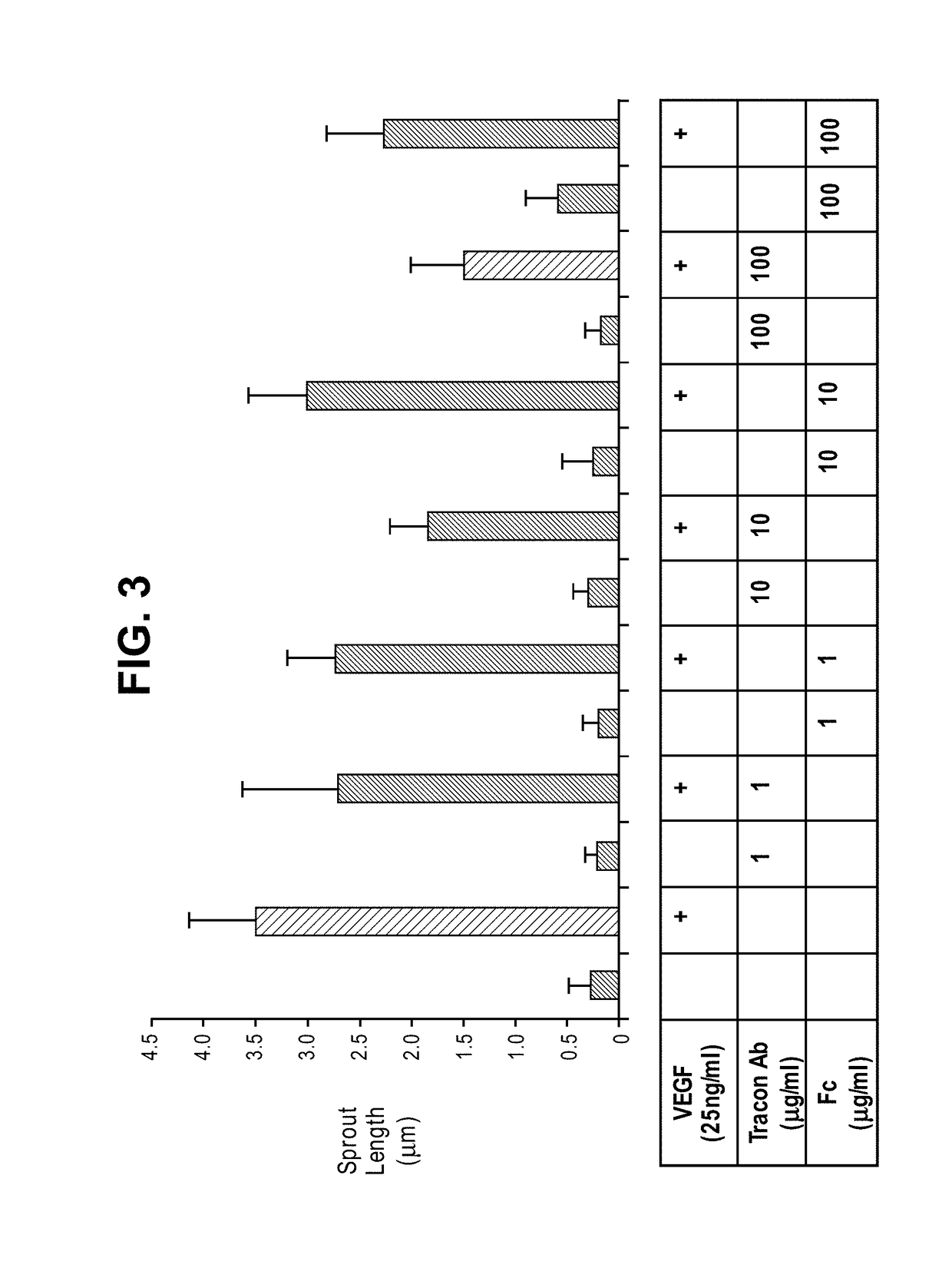
Combination therapy of cancer with Anti-endoglin antibodies and Anti-vegf agents
InactiveUS20120244147A1Improve the situationImprove survivalSenses disorderAntipyreticEndoglinBevacizumab Injection
The present application relates to compositions of chimeric anti-endoglin antibodies and anti-VEGF agents. Another aspect relates to the use of chimeric anti-endoglin antibodies and Bevacizumab. Another aspect relates to the use of the compositions to inhibit VEGF induced sprouting. Another aspect relates to the use of the compositions to inhibit angiogenesis.
Owner:HEALTH RES INC +1
Particles of paclitaxel and albumin in combination with bevacizumab against cancer
InactiveCN101573108AOrganic active ingredientsHeavy metal active ingredientsBevacizumab InjectionAnti vegf antibody
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents (such as an anti-VEGF antibody), or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
Methods of treating pterygium
Methods for treating pterygium recurrence following pterygiectomy, and for treating keloid recurrence, following surgical removal of the keloid, are disclosed. The methods include administering an anti-VEGF agent (e.g., antibody (e.g., bevacizumab) or small molecule inhibitor of VEGF signaling), or a combination therapy that includes co-administering an anti-VEGF agent, with an anti-inflammatory steroid and / or a non-steroidal anti-inflammatory drug (NSAID) to a subject.
Owner:PHAM RANDAL TANH HOANG
Fully human anti-VEGF (Vascular Endothelial Growth Factor) monoclonal antibody and preparation method as well as application thereof
ActiveCN102167740AAntibody affinity is highInhibition of proliferative abilityDigestive systemImmunoglobulins against growth factorsPhage antibodiesAntiendomysial antibodies
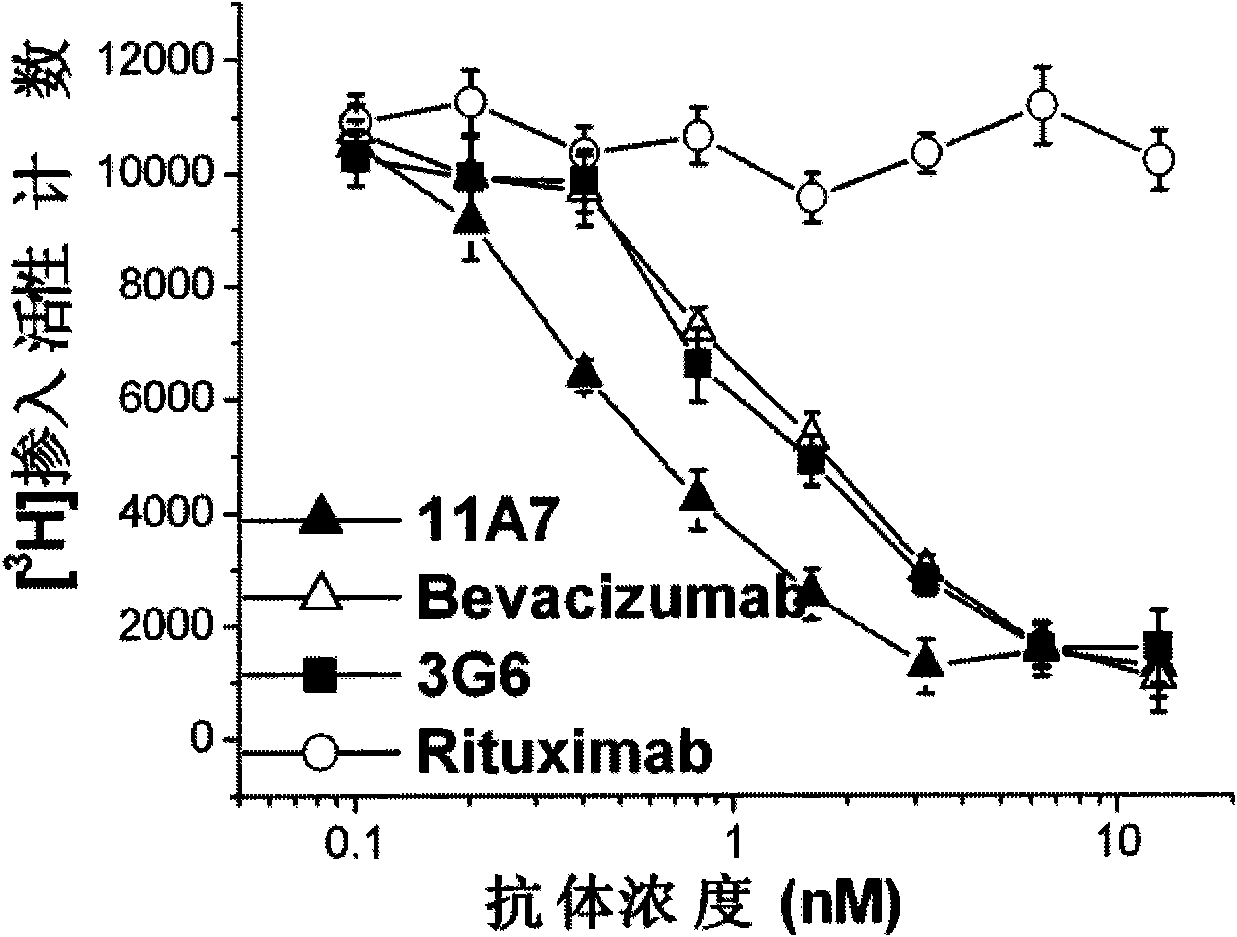
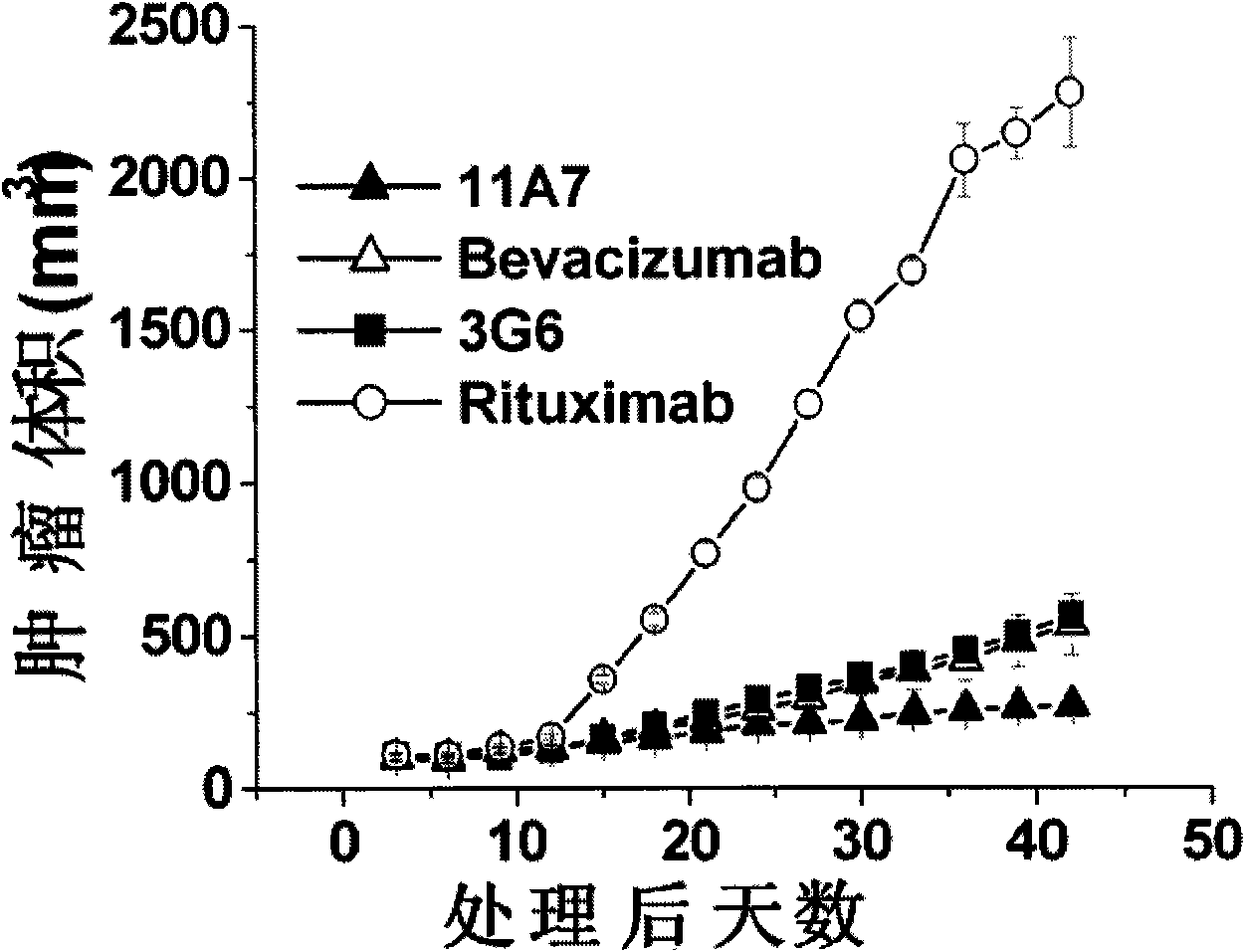
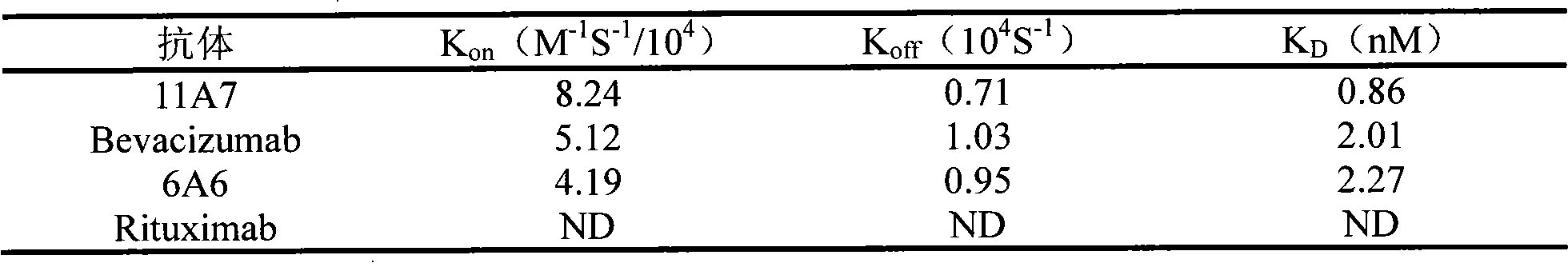
The invention relates to the field of biotechnology, in particular to a fully human anti-VEGF (Vascular Endothelial Growth Factor) monoclonal antibody and a preparation method as well as application thereof. In the invention, a large-capacity natural human phage antibody library is constructed; a fully human anti-VEGF antibody 11A7 is screened from the library; an amino acid sequence of a heavy chain variable region of the antibody is shown as SEQ ID NO: 6; and an amino acid sequence of a light chain variable region of the antibody is shown as SEQ ID NO: 8. The invention also discloses the preparation method of the 11A7 antibody, a nucleotide sequence encoding the 11A7 antibody, a nucleic acid sequence-containing expression vector and a host cell. Compared with a humanized antibody bevacizumab, the 11A7 antibody has higher affinity of the antibody and higher capacity of suppressing proliferation of tumor cells and can be used for obviously suppressing growth of tumors and preparing anti-tumor medicaments.
Owner:YUEHAI BIOPHARM SHAOXING LTD SHAOXING CITY
Novel Use of Sulfonamide Compound in Combination with Angiogenesis Inhibitor
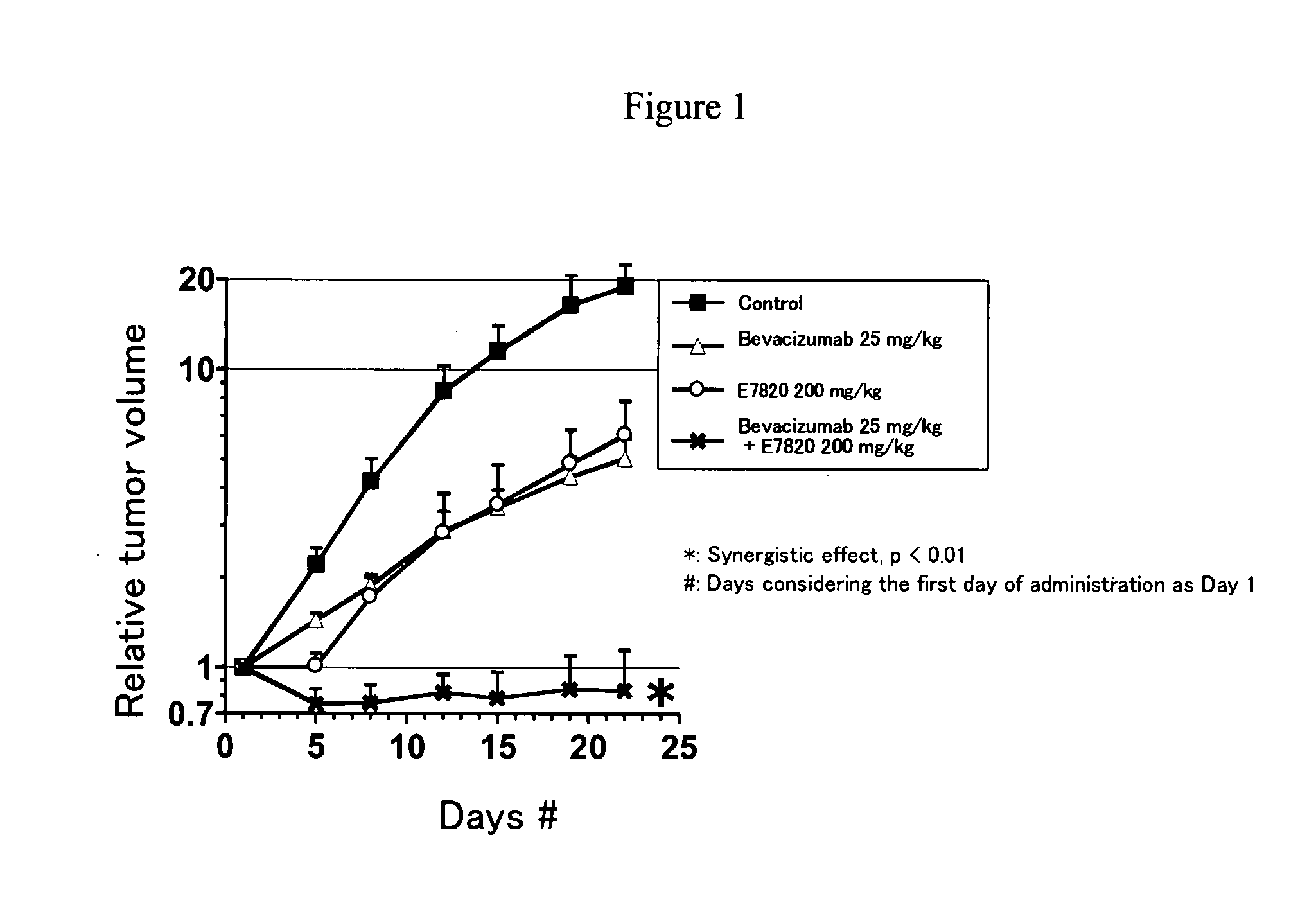
InactiveUS20080286282A1Remarkable anti-tumor effectSynergistic effectBiocideImmunoglobulins against growth factorsBevacizumab InjectionAngiogenesis growth factor
The present invention relates to a pharmaceutical composition, a kit and a method for treating cancer and / or a method for inhibiting angiogenesis, comprising a sulfonamide compound in combination with Bevacizumab.
Owner:EISIA R&D MANAGEMENT CO LTD
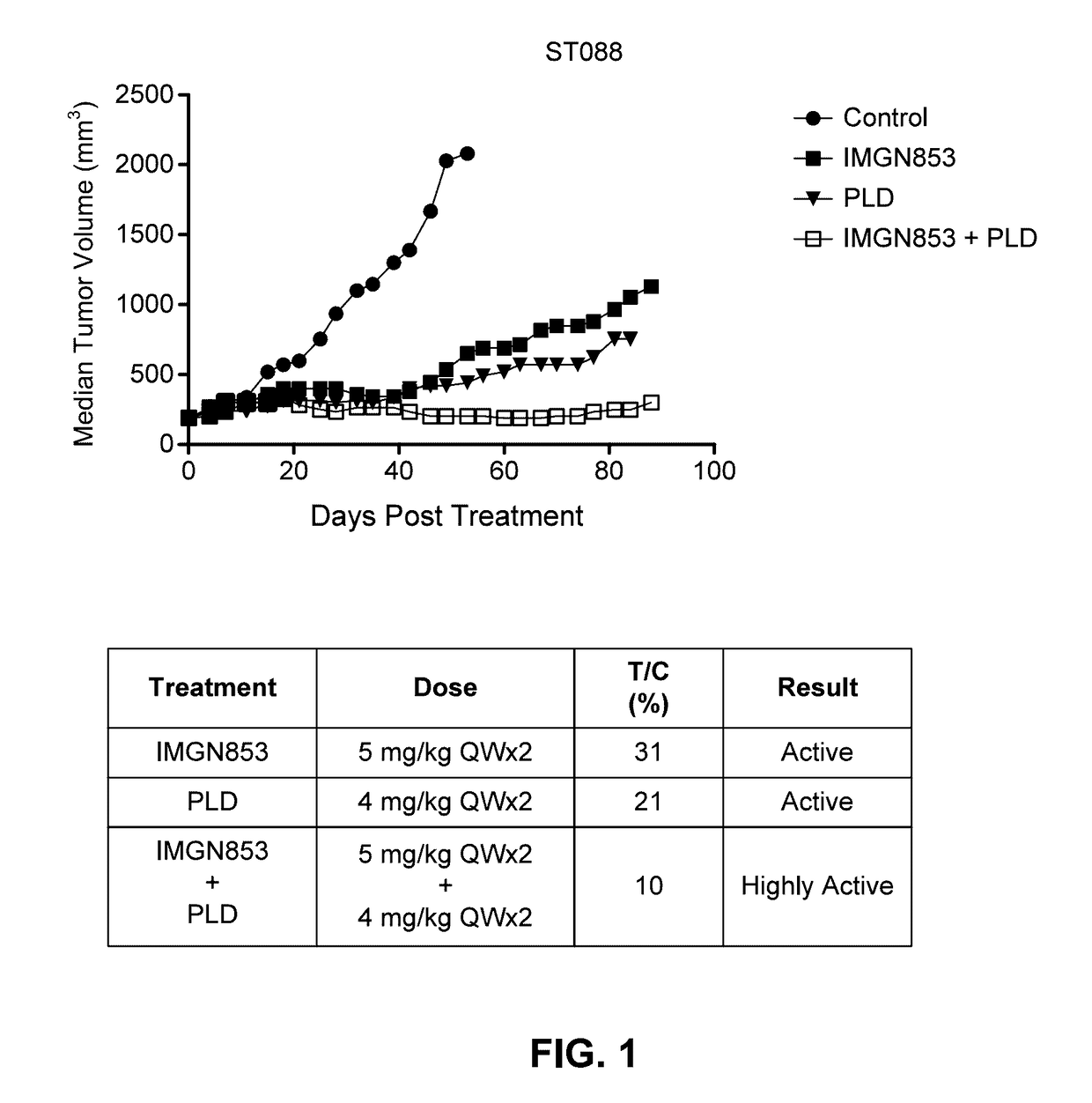
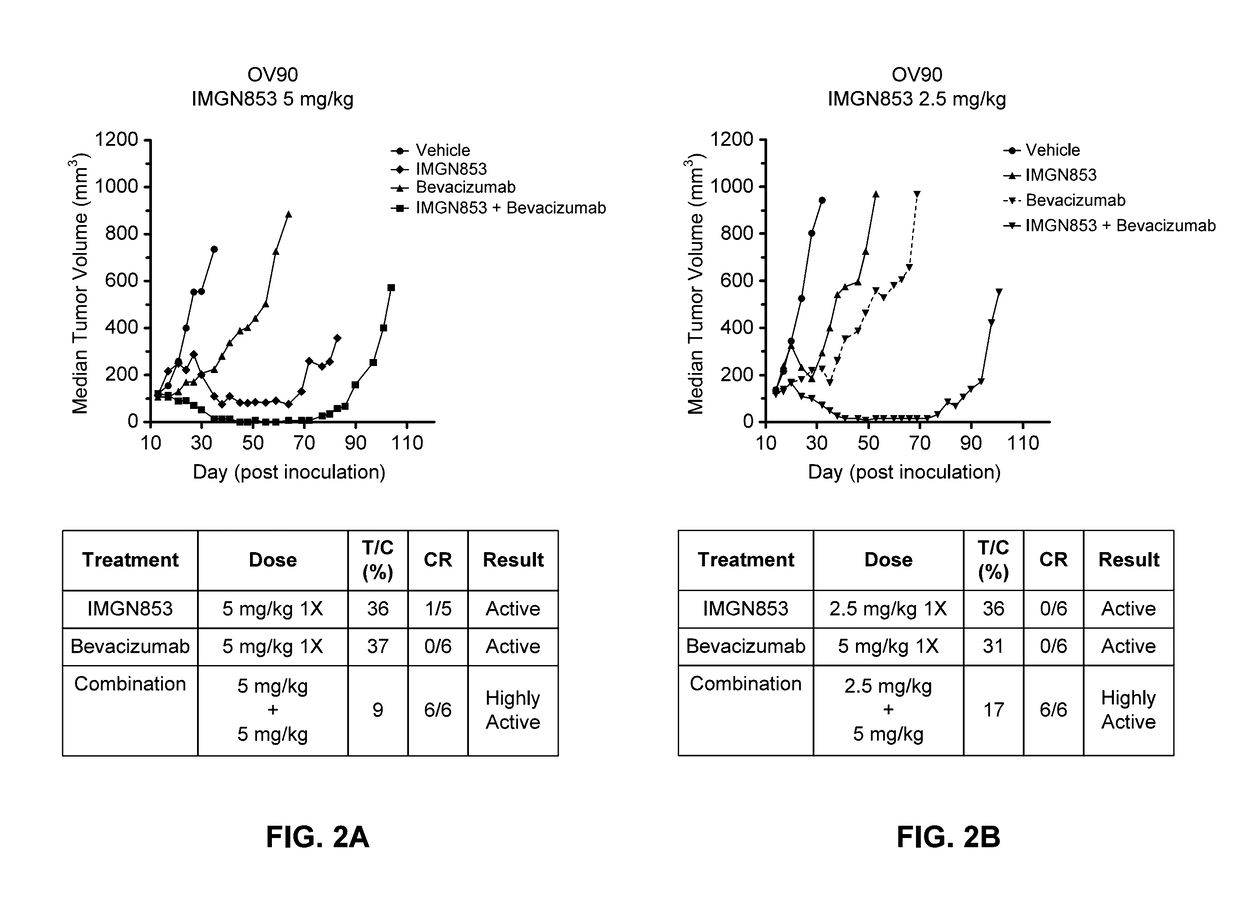
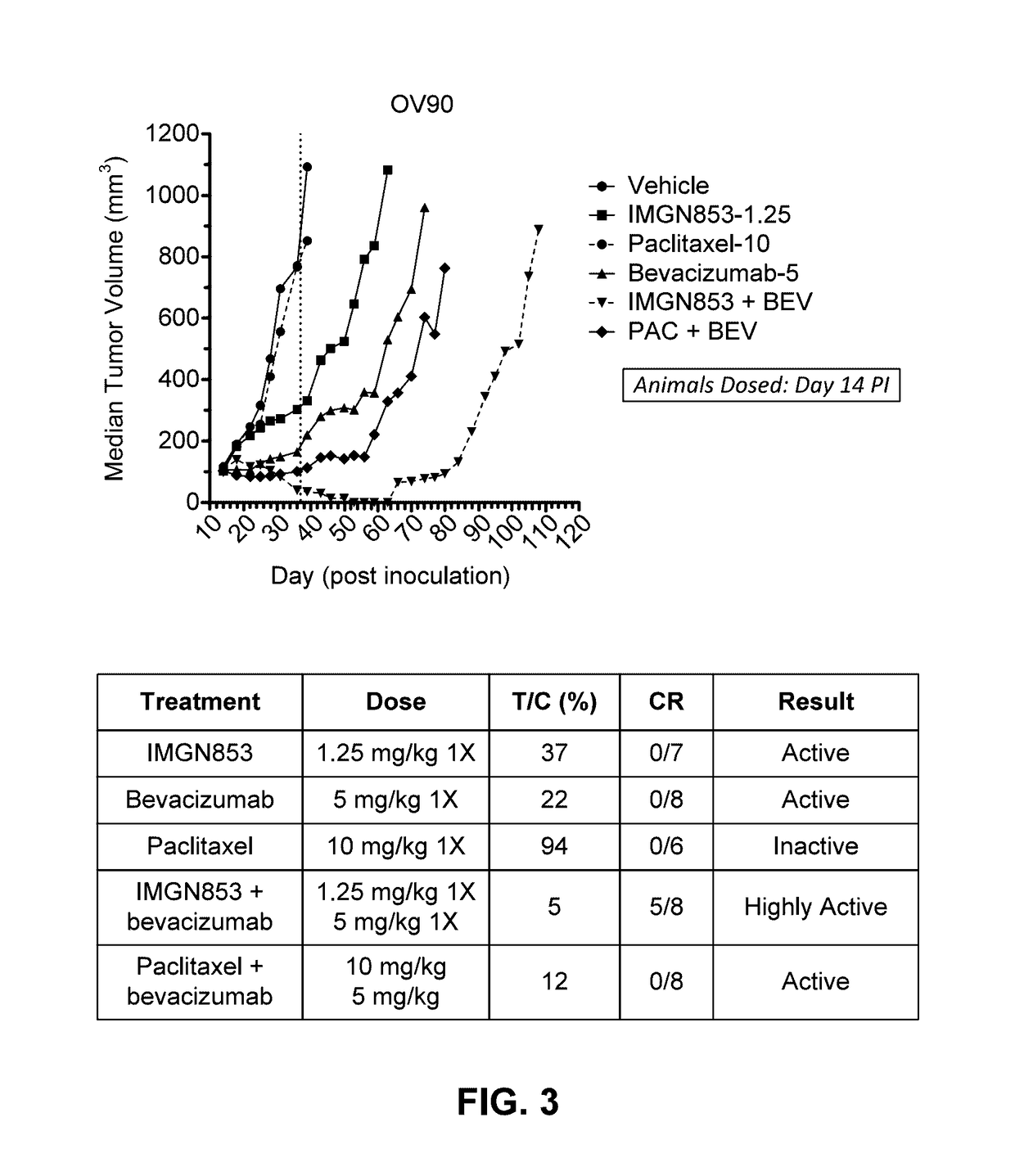
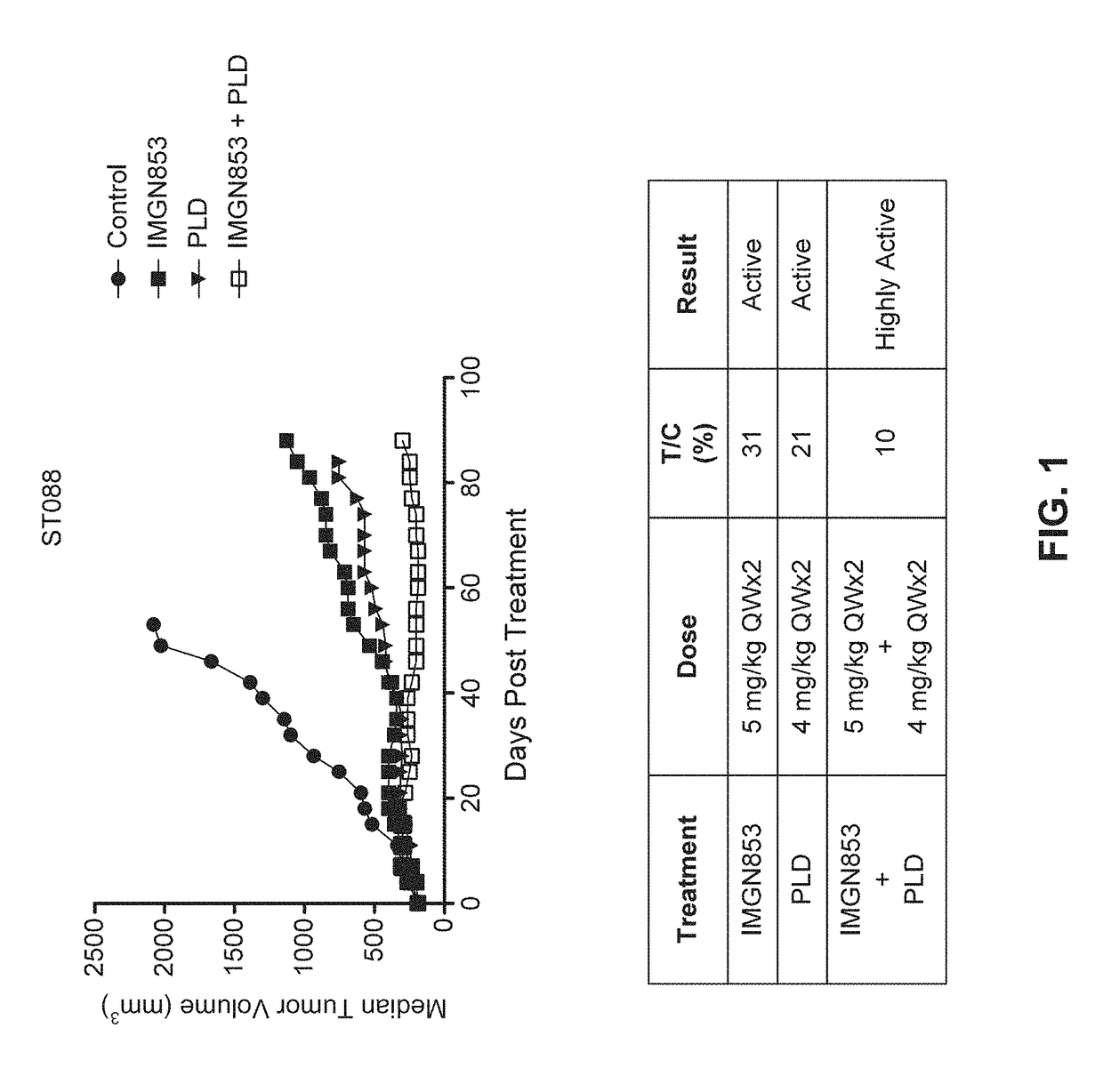
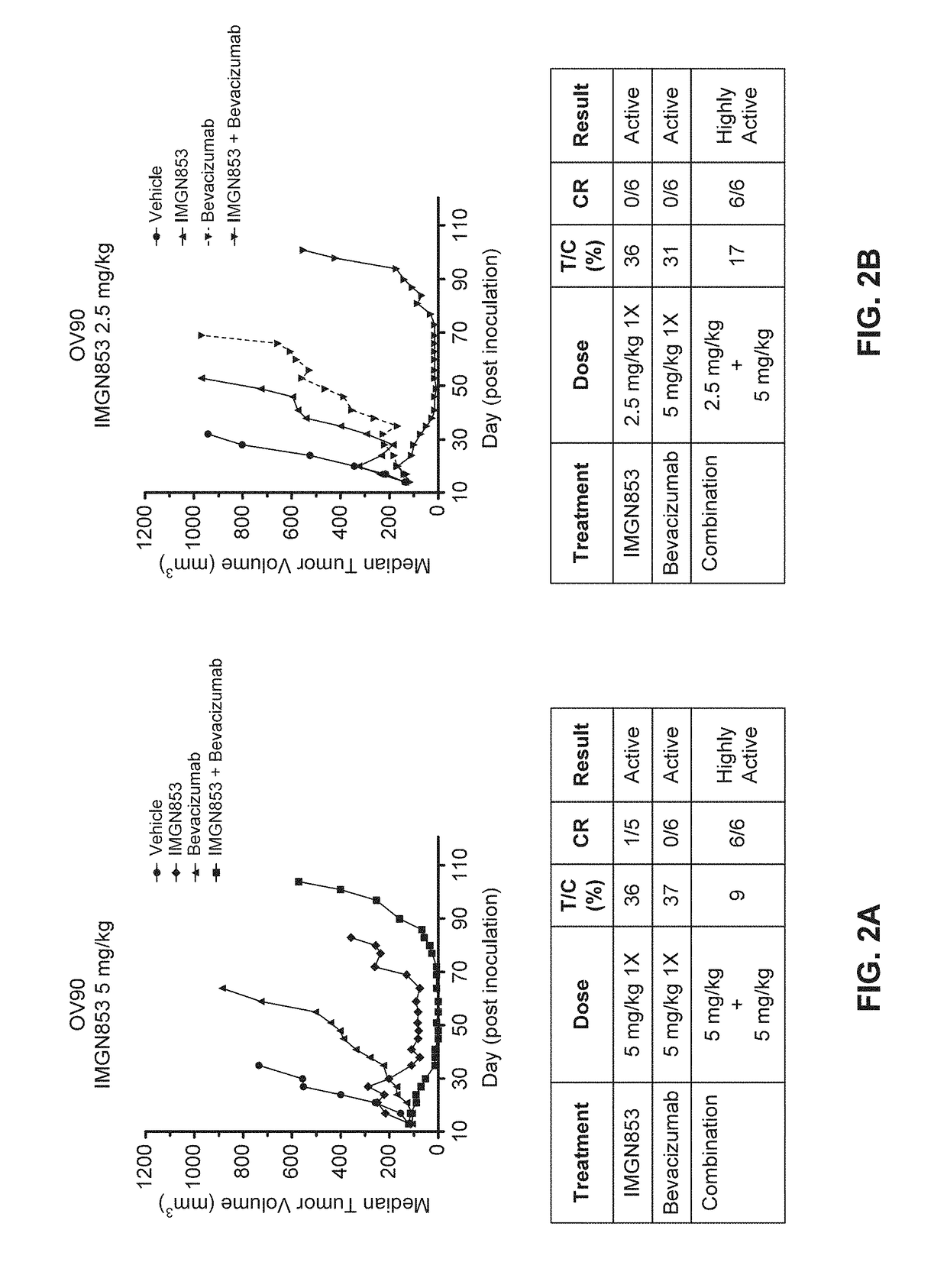
Therapeutic Combinations Comprising Anti-FOLR1 Immunoconjugates
ActiveUS20170095571A1Synergistic efficacyImprove efficacyHeavy metal active ingredientsOrganic active ingredientsClinical efficacyCurative effect
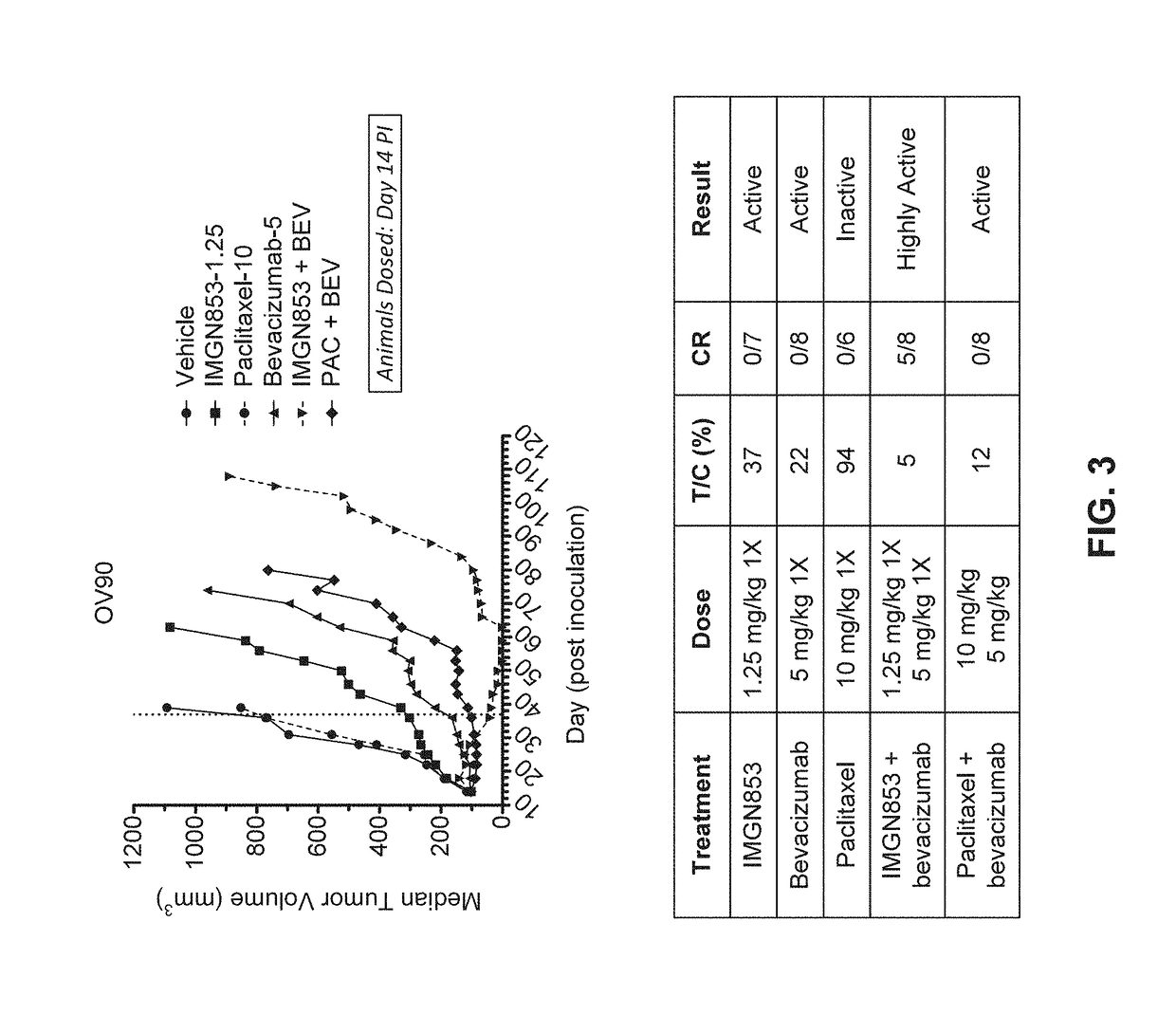
Therapeutic combinations of immunoconjugates that bind to FOLR1 (e.g., IMGN853) with anti-VEGF agents (e.g., bevacizumab), a platinum-based agent, and / or doxorubicin are provided. Methods of administering the combinations to treat cancers, e.g., ovarian cancers, with greater clinical efficacy and / or decreased toxicity are also provided.
Owner:IMMUNOGEN INC
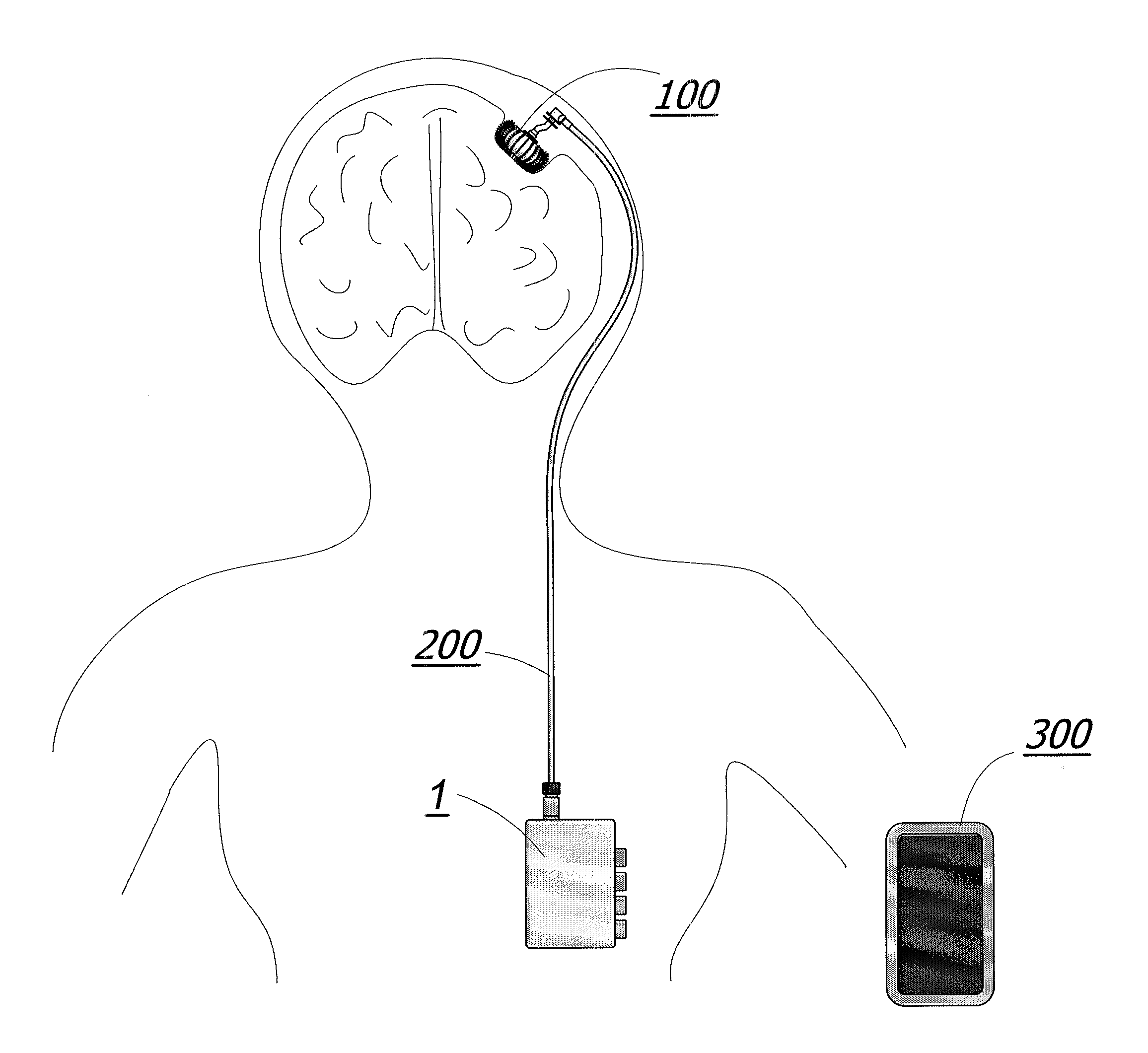
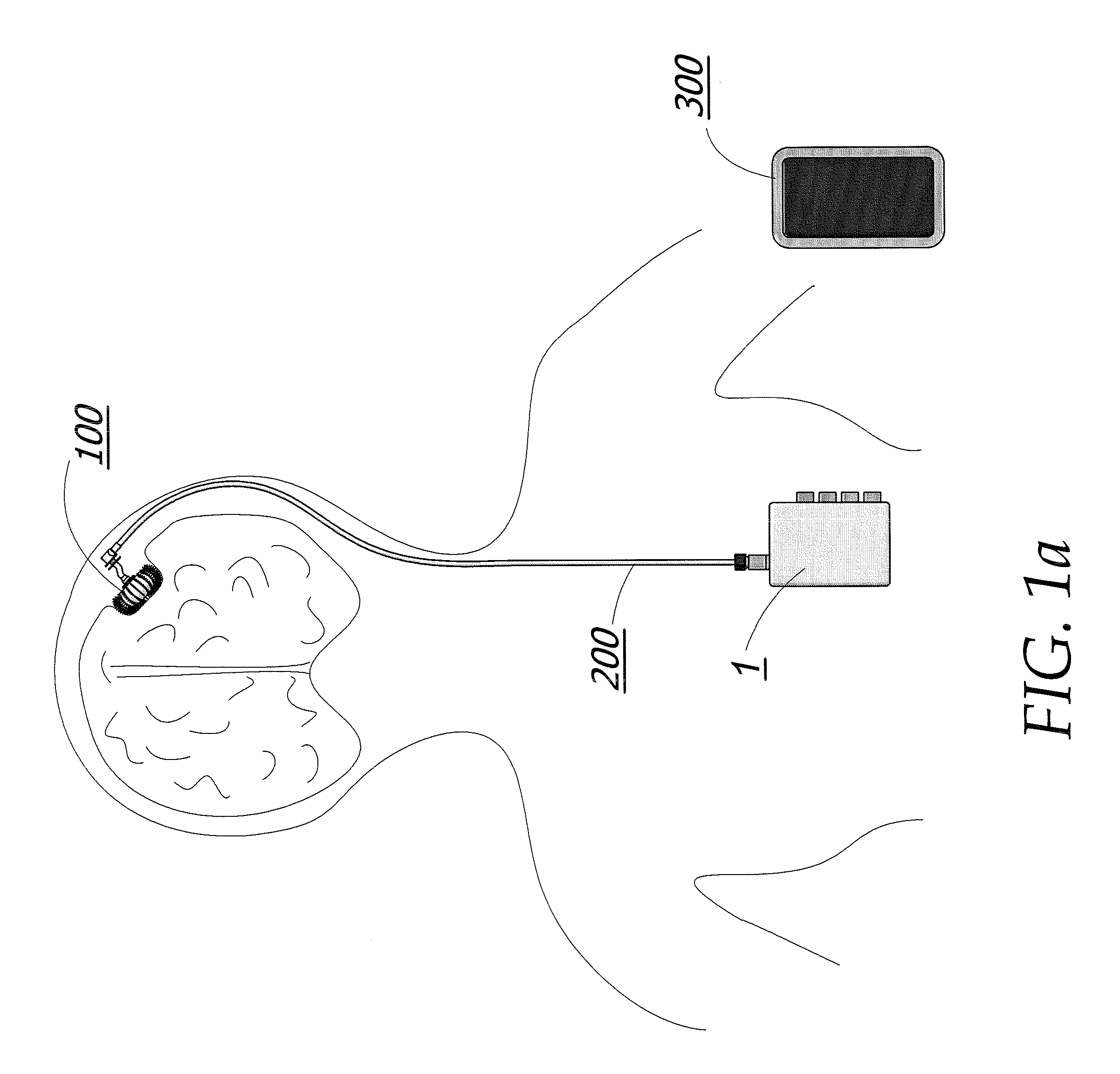
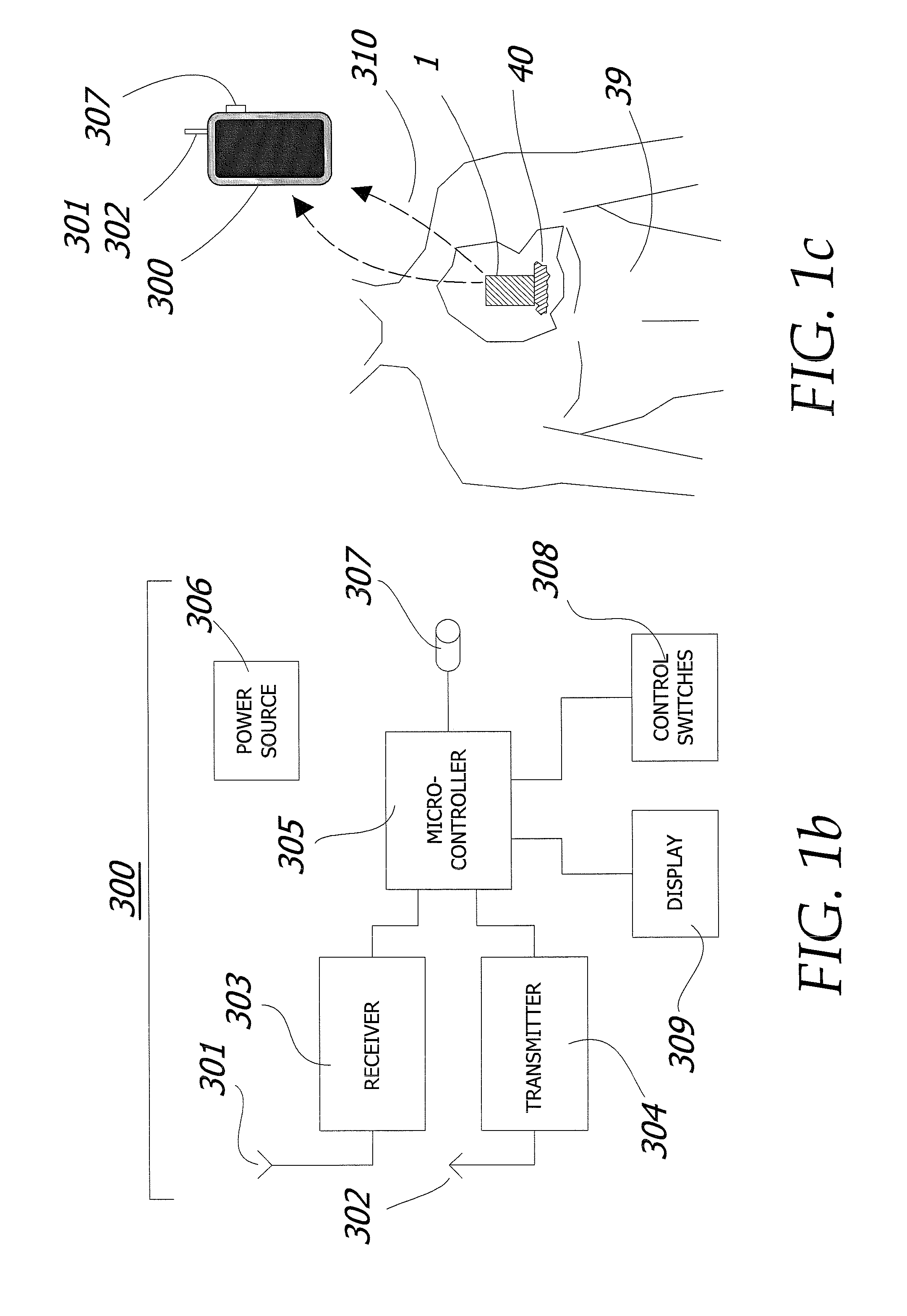
Enhanced method for delivering bevacizumab (avastin) into a brain tumor using an implanted magnetic breather pump
ActiveUS20110092960A1Optimization mechanismReduce eliminatePharmaceutical delivery mechanismMedical devicesBevacizumab InjectionDouble wall
A magnetically controlled pump is implanted into the brain of a patient and delivers a plurality of medicating agents mixed with Avastin at a controlled rate corresponding to the specific needs of the patient. The current invention comprises a flexible double walled pouch that is formed from two layers of polymer. The pouch is alternately expanded and contracting by magnetic solenoid. When contracted, the medicating agent Avastin is pushed out of the pouch through a plurality of needles. When the pouch is expanded, surrounding cerebral fluid is drawn into the space between the double walls of the pouch from which it is drawn through a catheter to an analyzer. In cases where a tumor resection is not performed, an intratumoral catheter will be implanted. Cerebral fluid drawn from the patient is analyzed. The operation of the apparatus and hence the treatment is remotely controlled based on these measurements and displayed through an external controller.
Owner:COGNOS THERAPEUTICS INC
Preparation method of long-acting sustained-release microspheres containing bevacizumab
InactiveCN102988301AGood volatilization effectReduce evaporation rateSenses disorderAntibody ingredientsMicrosphereWater soluble drug
The invention discloses a preparation method of long-acting sustained-release microspheres containing bevacizumab, which is a preparation method of the sustained-release microspheres formed by encapsulating water-soluble drug protein bevacizumab in a degradable biomedical polymer material. The microspheres are prepared by a W / O / W (water-in-oil-in-water) solvent evaporation method, which comprises the steps of: dispersing bevacizumab and a solution thereof and alginate as inner water phases into a solution which uses the degradable biomedical polymer material as an oil phase to form colostrum; dispersing the colostrum into an outer water phase which is water liquid containing emulsifier to form multiple emulsion; and stirring, distilling in reduced pressure, centrifuging, washing and drying to obtain the bevacizumab sustained-release microspheres. According to the long-acting sustained-release microspheres containing bevacizumab prepared by the method, the encapsulation efficiency of the water-soluble protein drug can be effectively improved, the drug protein activity is not influenced, the releasing time of water-soluble protein can be effectively prolonged, and the sustained release period can be 2-3 months, even longer, so that the number of injection times can be reduced. The preparation method is convenient for clinical application.
Owner:WENZHOU MEDICAL UNIV
Enhanced method for delivering bevacizumab (avastin) into a brain tumor using an implanted magnetic breather pump
ActiveUS8323270B2Easy to useMaximize efficiencyPharmaceutical delivery mechanismMedical devicesBevacizumab InjectionDouble wall
A magnetically controlled pump is implanted into the brain of a patient and delivers a plurality of medicating agents mixed with Avastin at a controlled rate corresponding to the specific needs of the patient. The current invention comprises a flexible double walled pouch that is formed from two layers of polymer. The pouch is alternately expanded and contracting by magnetic solenoid. When contracted, the medicating agent Avastin is pushed out of the pouch through a plurality of needles. When the pouch is expanded, surrounding cerebral fluid is drawn into the space between the double walls of the pouch from which it is drawn through a catheter to an analyzer. In cases where a tumor resection is not performed, an intratumoral catheter will be implanted. Cerebral fluid drawn from the patient is analyzed. The operation of the apparatus and hence the treatment is remotely controlled based on these measurements and displayed through an external controller.
Owner:COGNOS THERAPEUTICS INC
Method for treating atrophic age related macular degeneration
InactiveCN102159246AOrganic active ingredientsSenses disorderBevacizumab InjectionDry age-related macular degeneration
The present invention relates to compositions and methods for treating dry age related macular degeneration (dry AMD) by administration to an intraocular location of an anti-neovascular agent (such as bevacizumab) in either a liquid or solid polymeric vehicle (or both), such as a biodegradable hyaluronic acid or PLGA (or PLA).
Owner:ALLERGAN INC
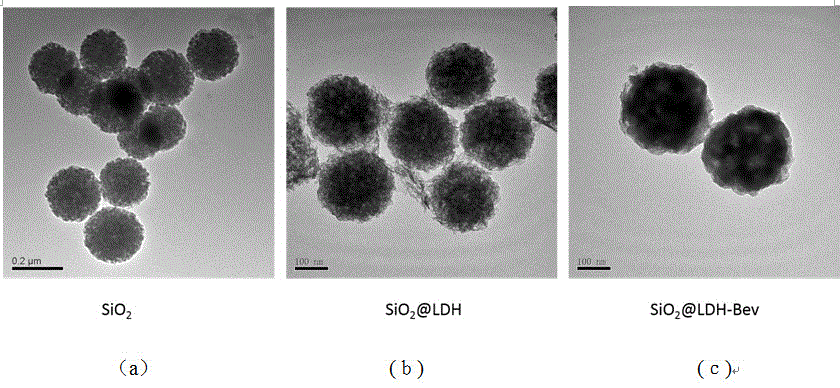
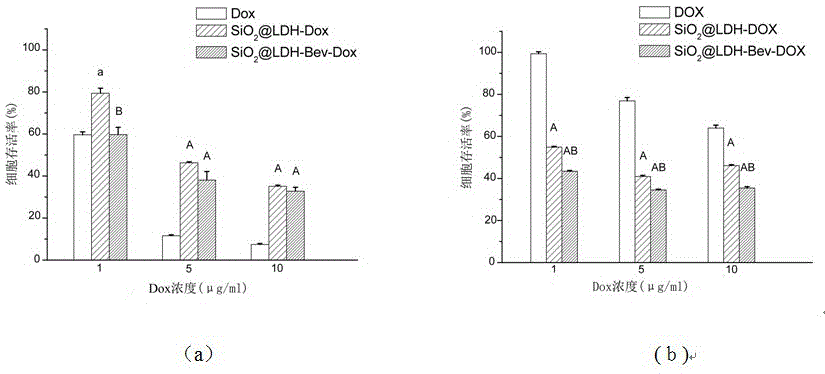
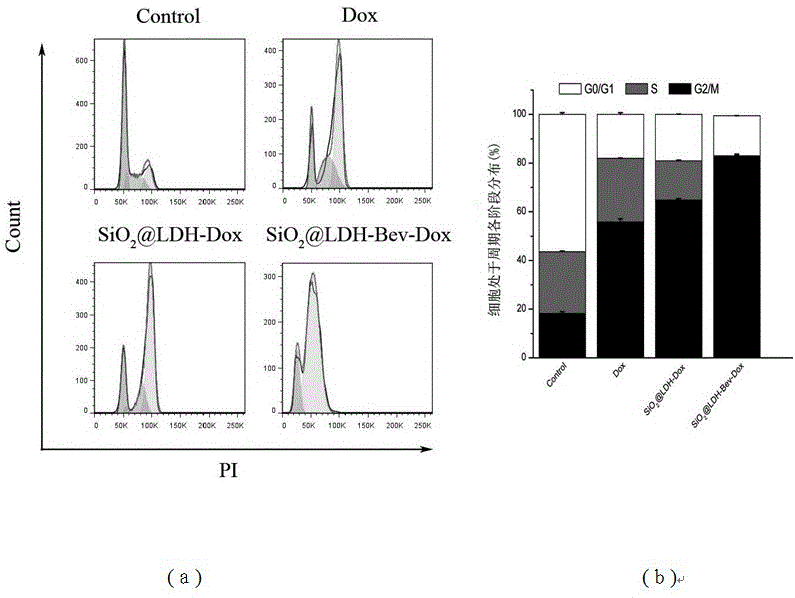
Nanometer drug carrier (bevacizumab medicated-SiO2@LDH (sodium dioxide @ double hydroxide)) with active tumor targeting function, preparation method and application
InactiveCN105999262AInorganic non-active ingredientsAntibody ingredientsSynthesis methodsTumor targeting
The invention relates to a nanometer drug carrier (bevacizumab medicated-SiO2@LDH (sodium dioxide @ double hydroxide)) with an active tumor-targeting function, a preparation method and an application. The nanometer drug carrier uses mesoporous silica as a core, an outer layer is coated with magnesium-aluminum LDH, and the surface is connected with bevacizumab to form a nanometer compound. The nanometer drug carrier can be carried with a treatment drug; the purpose of tumor-targeting treatment is realized by recognizing the bevacizumab specificity and combining with the characteristics of VEGF (vascular endothelial growth factor) which is excessively secreted by tumors. The nanometer drug carrier has the advantages that the safety, active targeting property and drug carrying effect are higher; the synthesis method is simple, the cost is economic, and the application prospect in tumor-targeting treatment is broad.
Owner:TONGJI UNIV
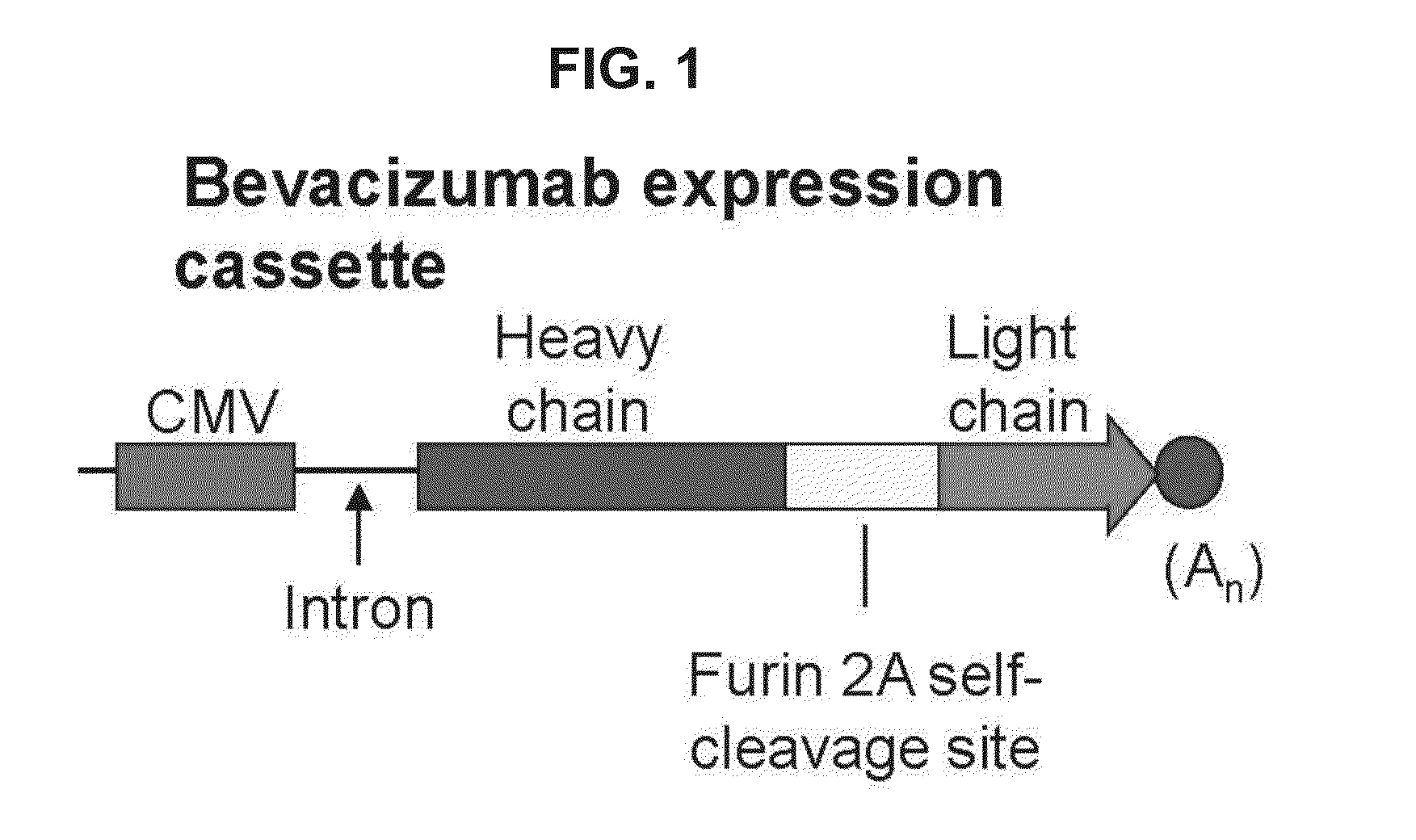
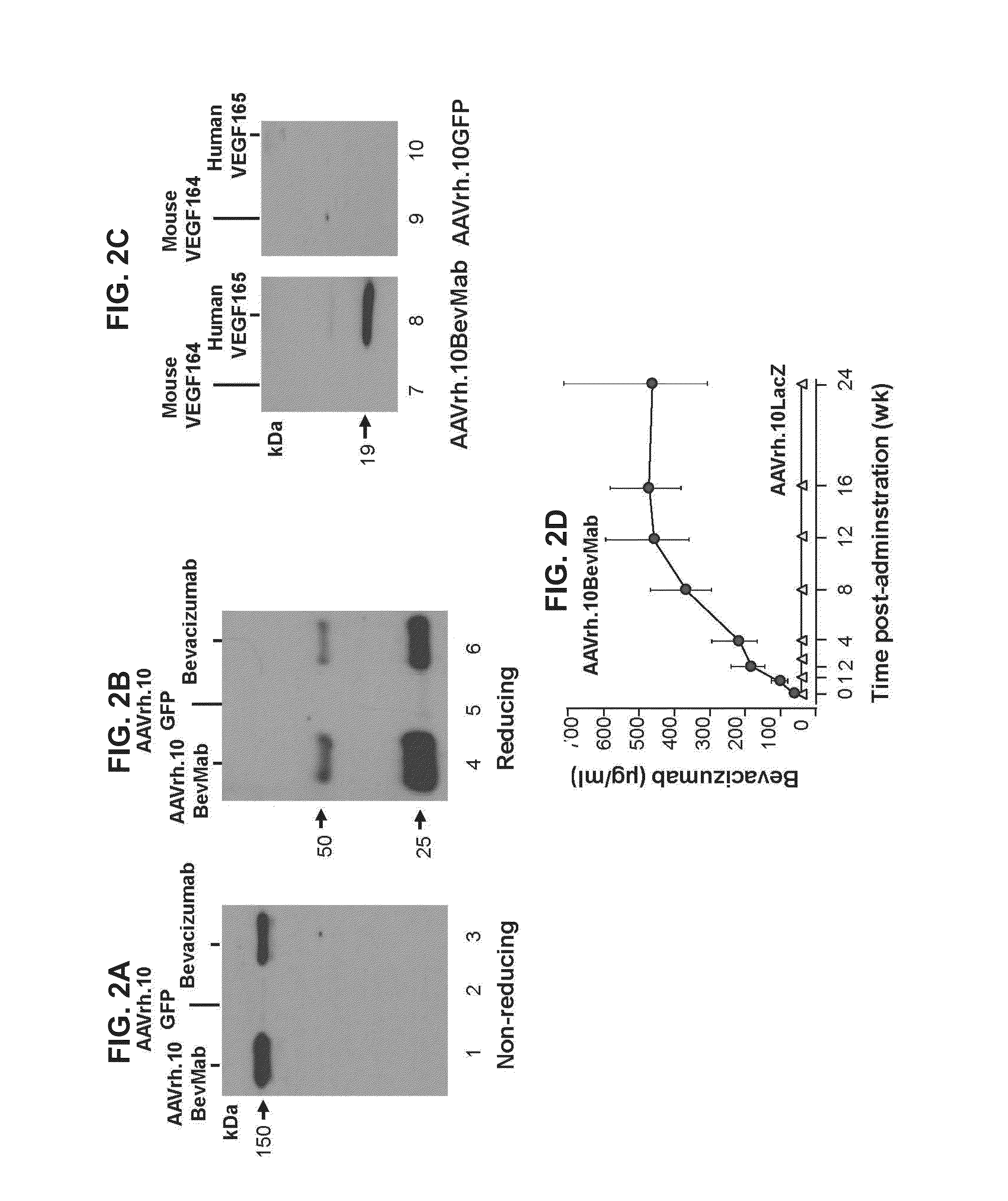
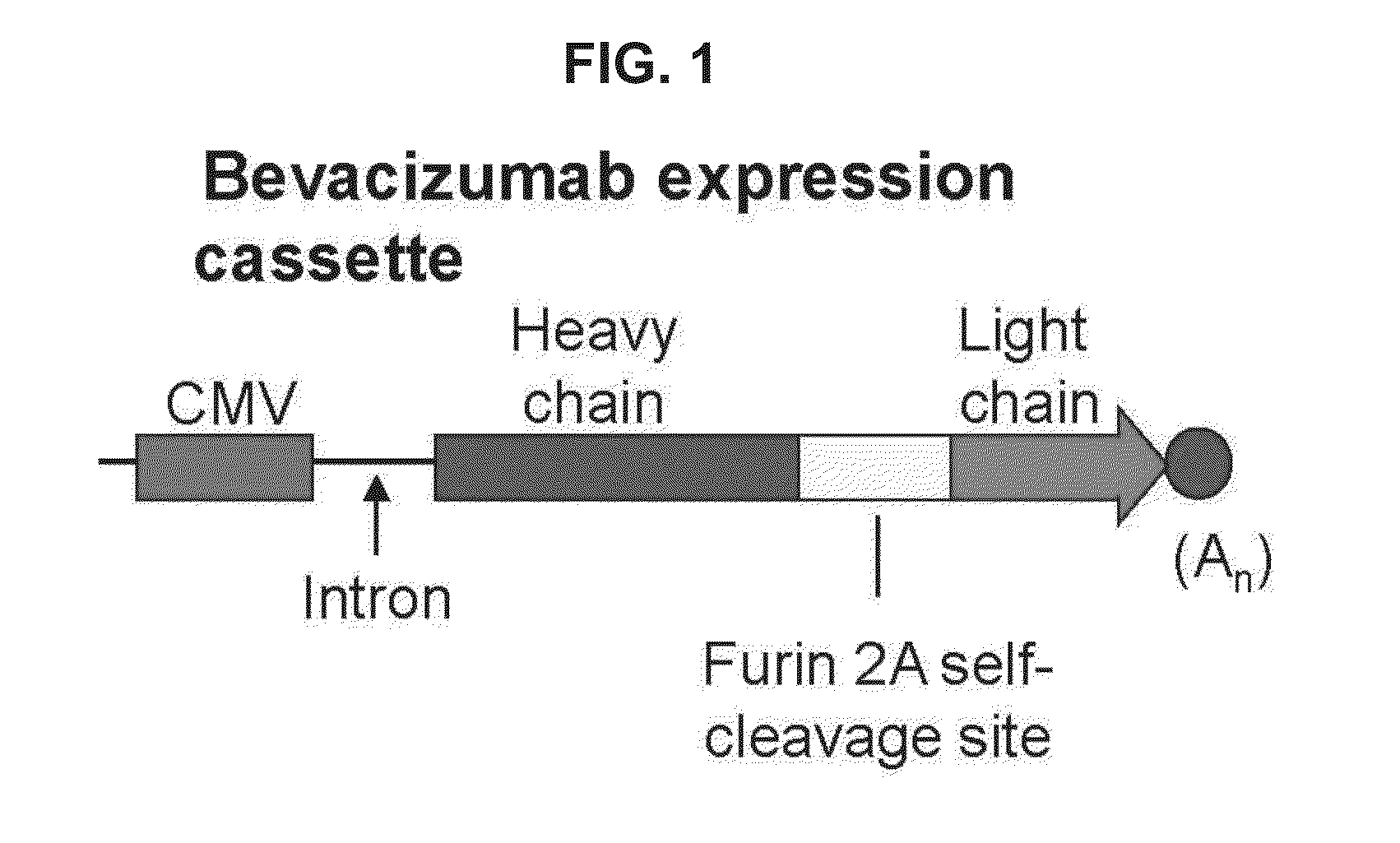
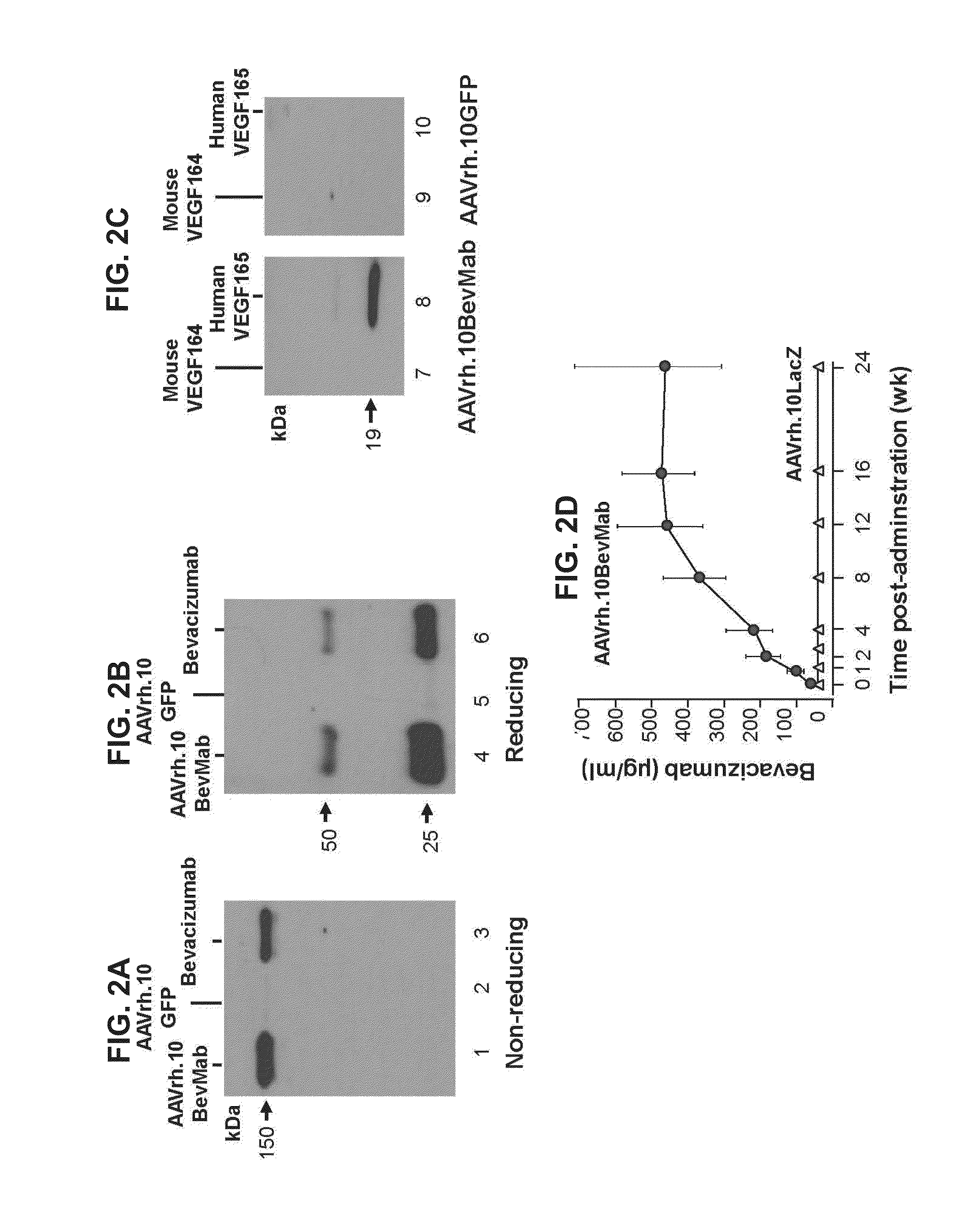
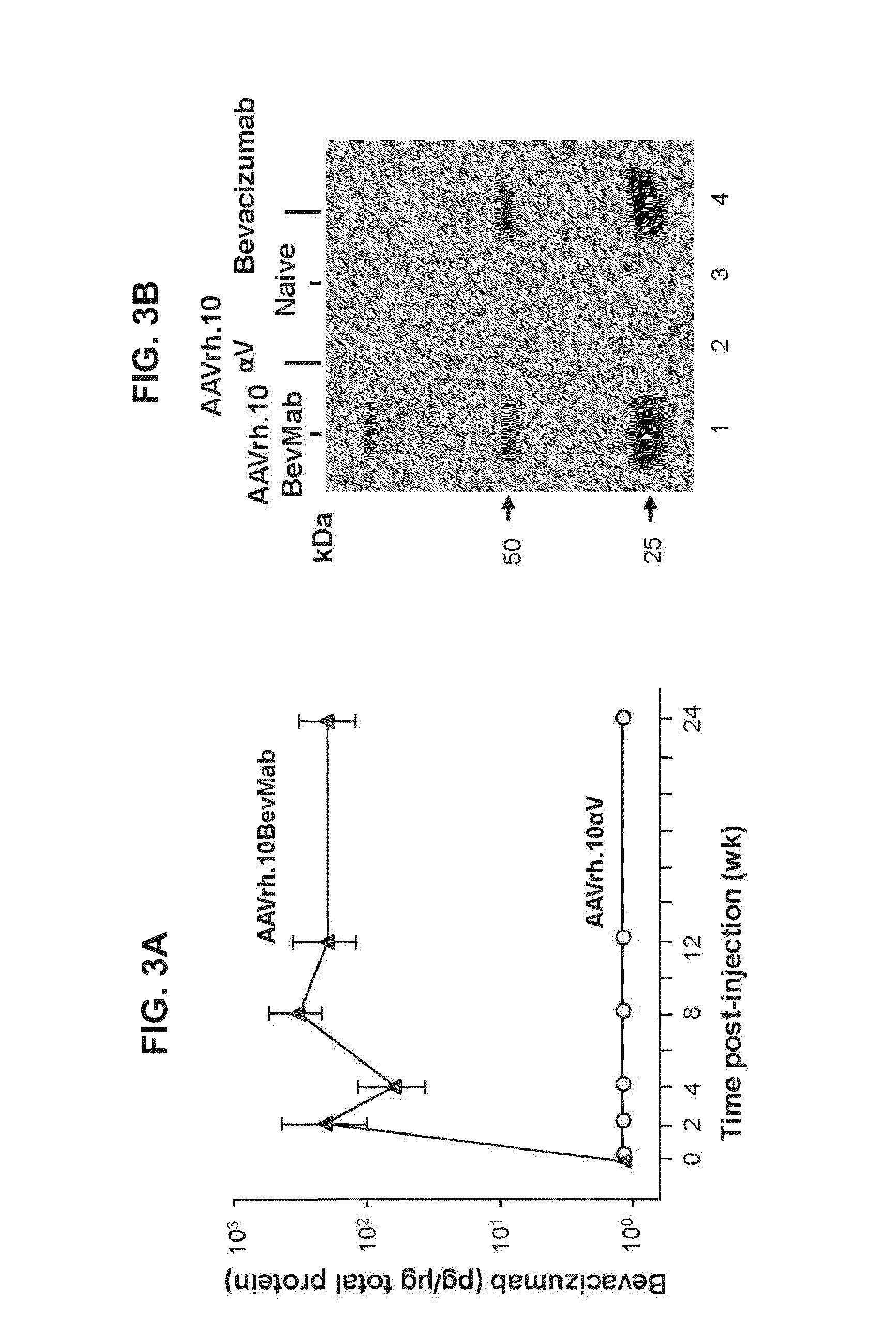
Virus-mediated delivery of bevacizumab for therapeutic applications
InactiveUS20130090375A1Reduction in total area of neovascularizationReduce neovascularizationOrganic active ingredientsSenses disorderBevacizumab InjectionOphthalmology
The invention provides a method of inhibiting ocular neovascularization in a mammal by administering a composition comprising a bevacizumab-encoding adeno-associated virus (AAV) vector directly to the eye of the mammal.
Owner:CORNELL UNIVERSITY
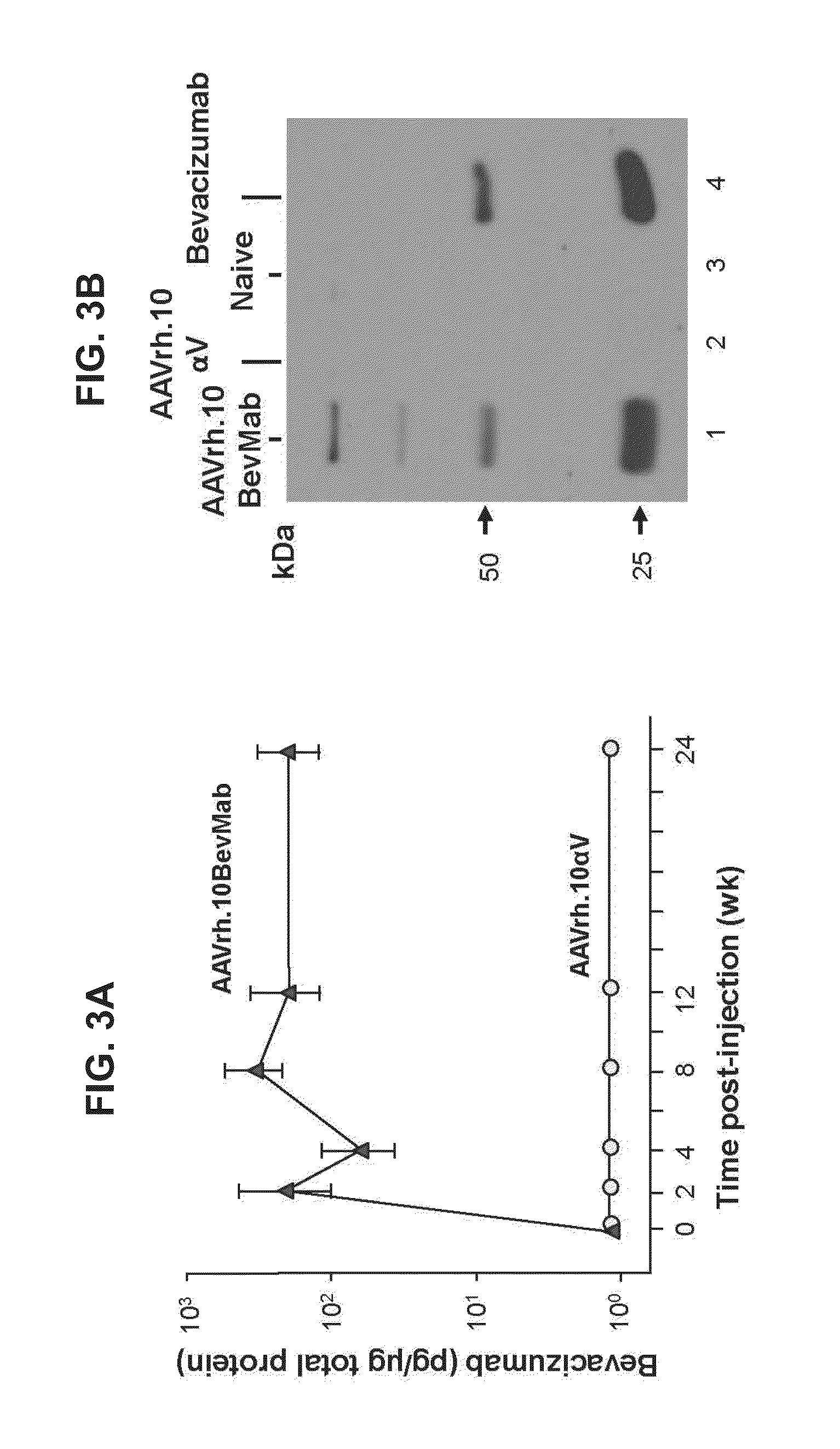
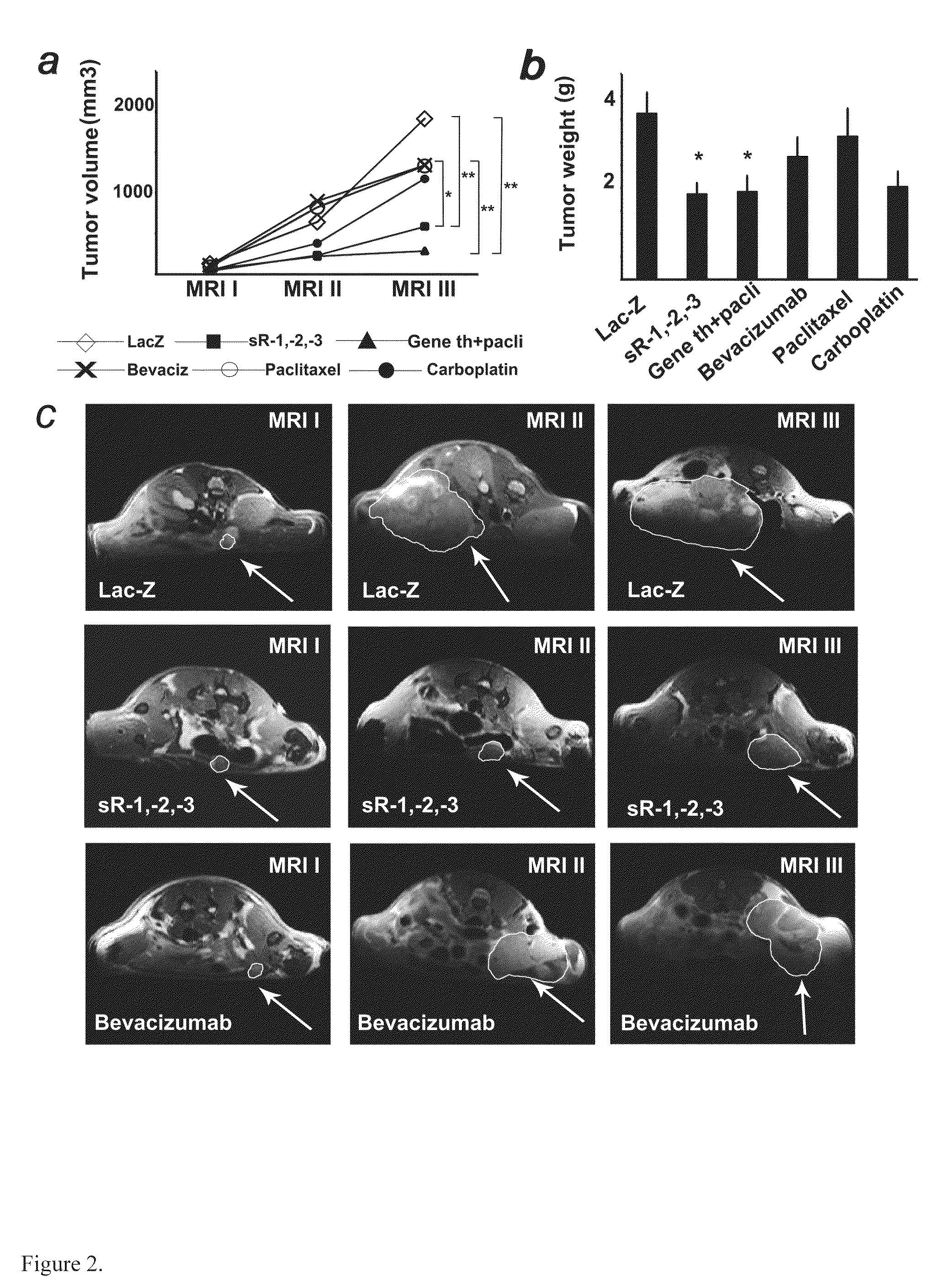
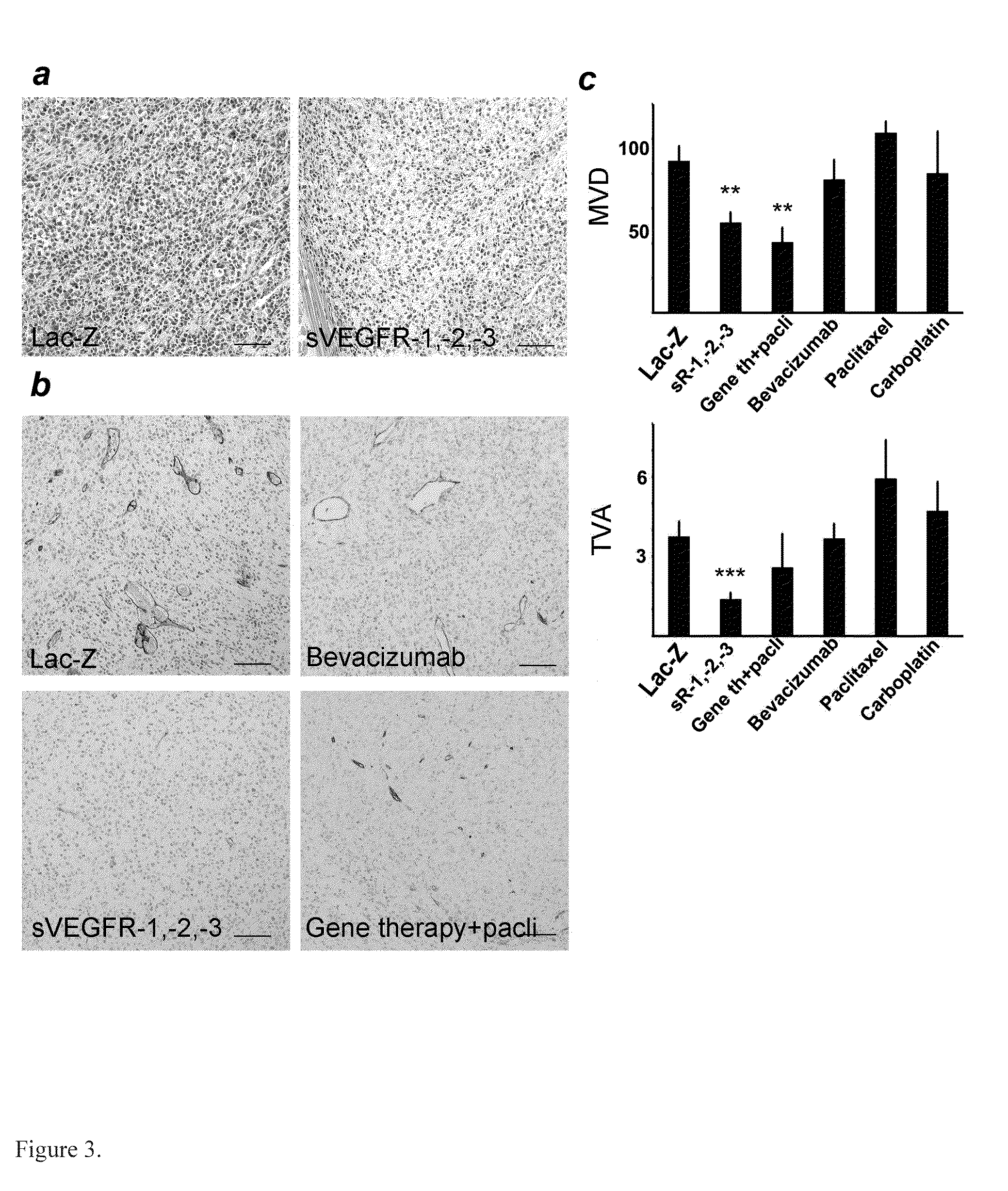
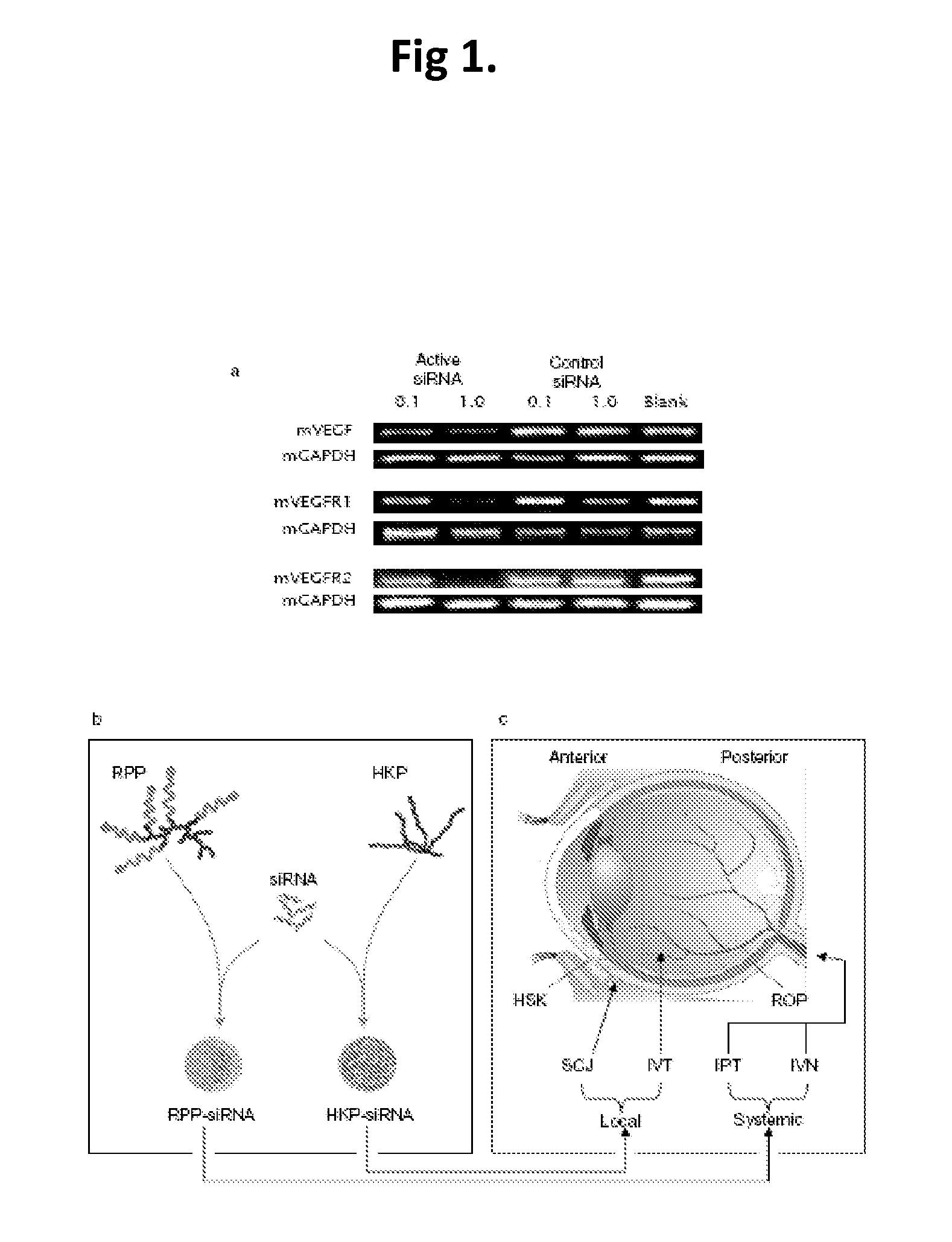
Anti-Angiogenic Gene Therapy With Soluble VEGF Receptors -1, -2 and -3 Together With Paclitaxel Prolongs Survival Of Mice With Human Ovarian Carcinoma
InactiveUS20140057851A1Good treatment effectPoor prognosisOrganic active ingredientsPeptide/protein ingredientsCarboplatinWilms' tumor
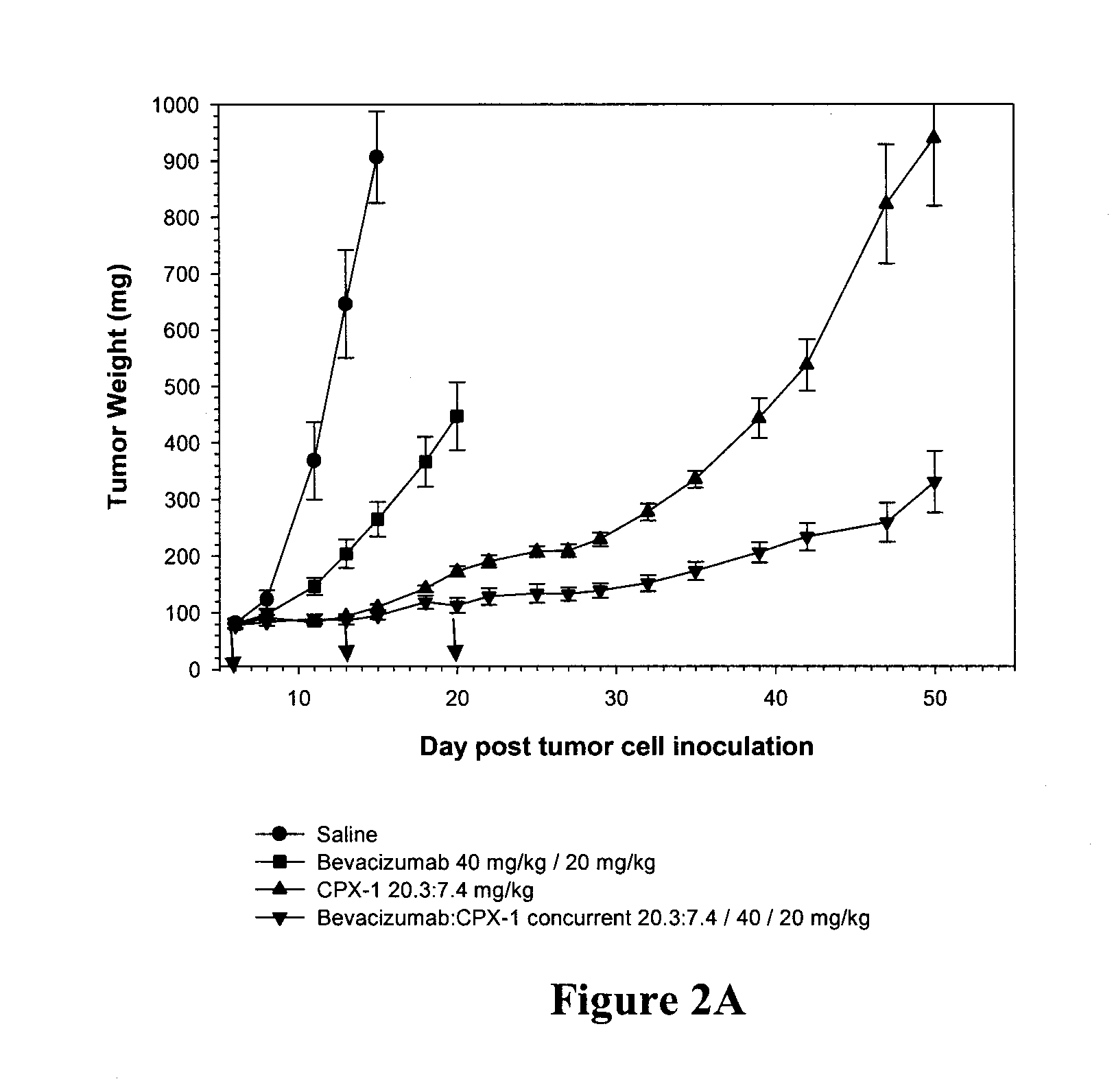
Anti-angiogenic gene therapy with a combination of soluble Vascular Endothelial Growth Factors (sVEGFR) improves the efficacy of chemotherapy with paclitaxel for reducing ovarian cancer mean tumor volume (in cubic millimetres) as measured using magnetic resonance imaging. The study groups were: AdLacZ control, combination of AdsVEGFR-1, -2 and -3, combination of AdsVEGFR-1, -2, -3 and paclitaxel, bevacizumab monotherapy, paclitaxel monotherapy and carboplatin monotherapy. Effectiveness was assessed by survival time and surrogate measures such as sequential MRI, immunohistochemistry, microvessel density and tumor growth. Antiangiogenic gene therapy combined with paclitaxel significantly prolonged the mean survival compared to the controls and all other treatment groups (p=0.001). Tumors of the mice treated by gene therapy were significantly smaller than in the control group (p=0.021). The mean vascular density and total vascular area were also significantly smaller in the tumors of the gene therapy group (p=0.01).
Owner:TRIZELL LTD
Methods of treating colon cancer using nanoparticle mtor inhibitor combination therapy
The present application provides methods of treating a colon cancer (such as advanced and / or metastatic colon cancer) in an individual, comprising administering to the individual: a) an effective amount of a composition comprising nanoparticles comprising an mTOR inhibitor (such as a limus drug, such as sirolimus or a derivative thereof) and an albumin, b) an effective amount of anti-VEGF antibody (such as bevacizumab), and c) a therapeutically effective FOLFOX regimen (such as FOLFOX4 or a modified FOLFOX6).
Owner:ABRAXIS BIOSCI LLC
Virus-mediated delivery of bevacizumab for therapeutic applications
InactiveUS20150182638A1Senses disorderPharmaceutical delivery mechanismBevacizumab InjectionOphthalmology
The invention provides a method of inhibiting ocular neovascularization in a mammal by administering a composition comprising a bevacizumab-encoding adeno-associated virus (AAV) vector directly to the eye of the mammal.
Owner:CORNELL UNIVERSITY
Compositions and methods using siRNA molecules and siRNA cocktails for the treatment of breast cancer
The present invention provides small interfering RNA (siRNA) molecules, compositions containing the molecules, and methods of using the molecules and compositions to treat breast cancer. In one aspect, a multi-targeted siRNAi cocktail is disclosed. The siRNA molecules may be encapsulated in nanoparticles to further enhance their anti-cancer activity. The compositions may also be used in combination with other anti-cancer agents, such as bevacizumab.
Owner:SIRNAOMICS INC
Therapeutic combinations comprising anti-FOLR1 immunoconjugates
ActiveUS10172875B2Improve efficacyOrganic active ingredientsHeavy metal active ingredientsClinical efficacyEfficacy
Owner:IMMUNOGEN INC
Anti-human VEGF antibodies with unusually strong binding afinity to human VEGF-a and cross reactivity to human VEGF-b
ActiveUS20150315270A1High homologyHigh affinityOrganic active ingredientsSenses disorderNeoplasmStrong inhibitor
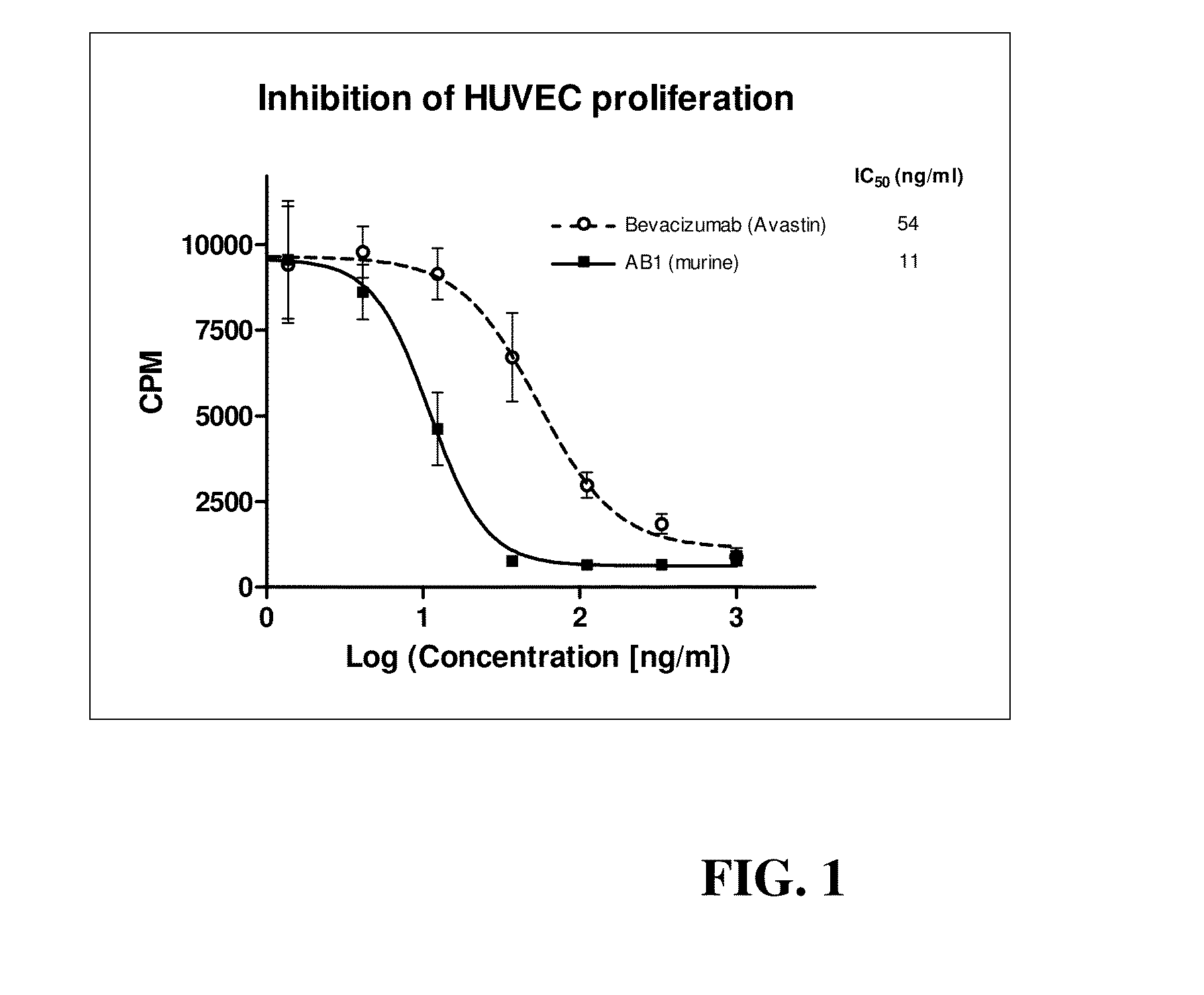
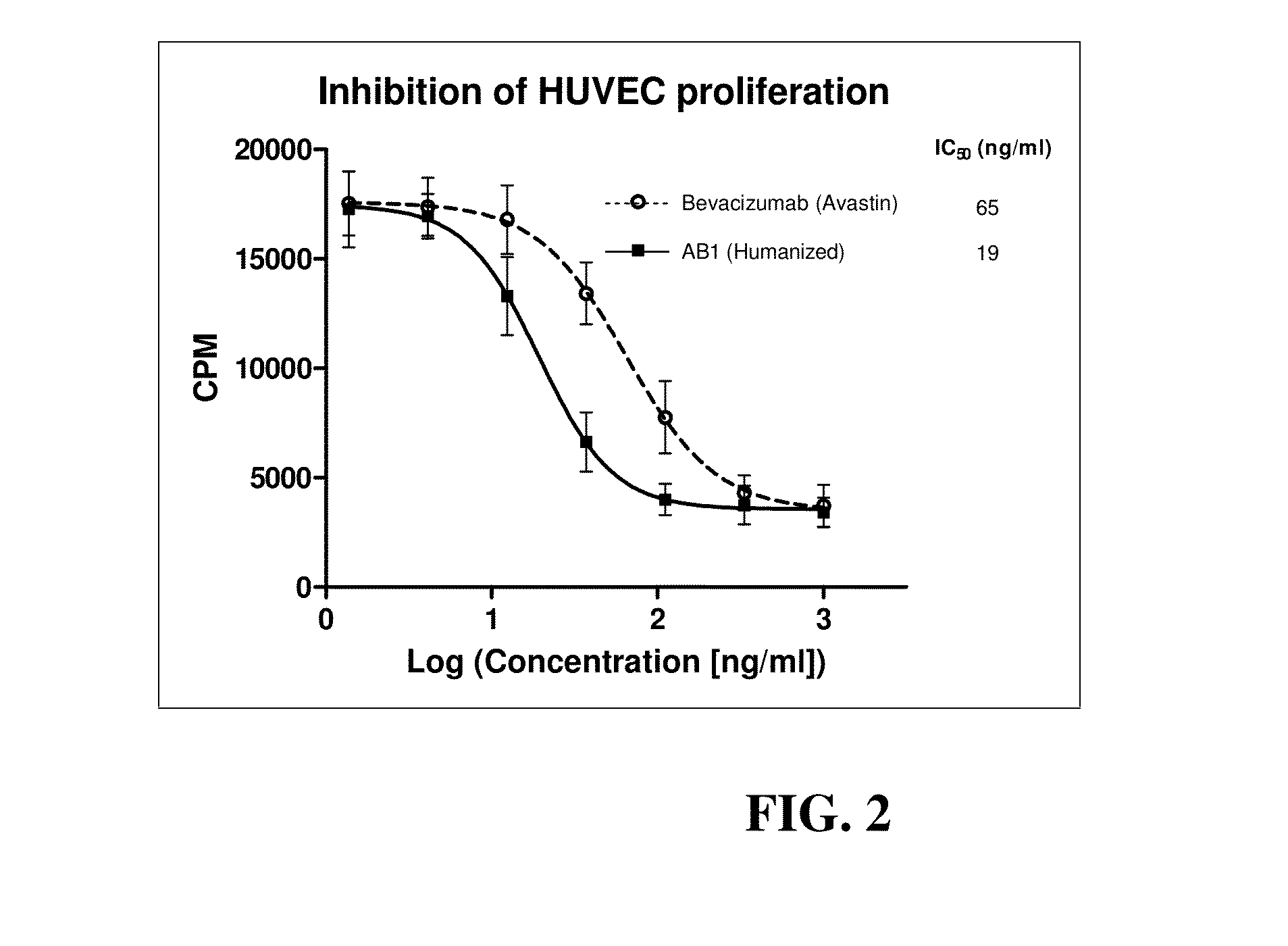
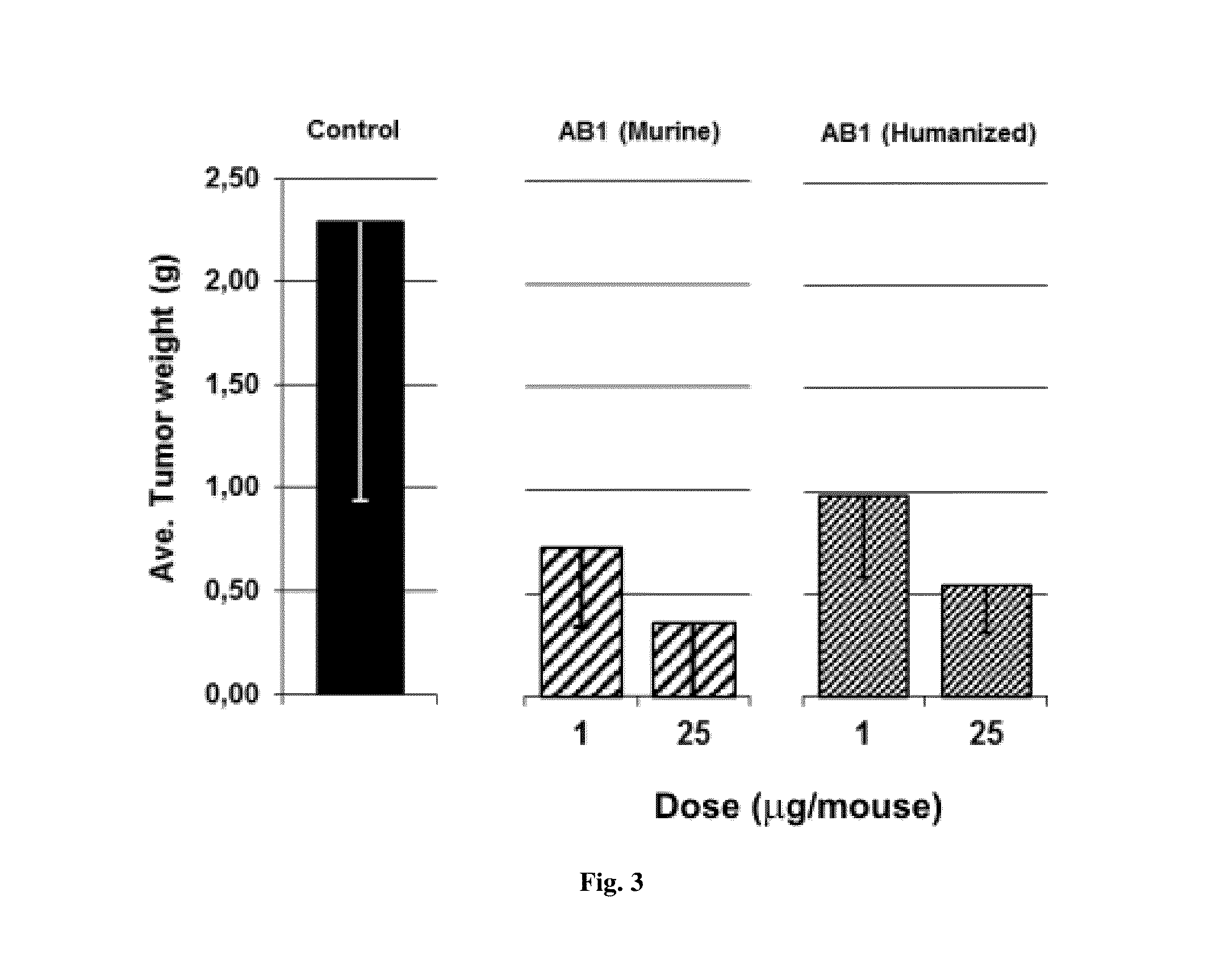
Murine and humanized anti-human VEGF antibodies and uses are disclosed. The anti-human VEGF antibodies of the invention have higher binding affinity for human VEGF-A, are stronger inhibitors of the VEGF-A induced proliferation of endothelial cells in culture as compared with anti-human VEGF antibodies in the art. Moreover, these antibodies cross react with human VEGF-B. The antibodies of the invention inhibit tumor growth in vivo in greater extent than Bevacizumab (Avastin™) when administered at the same dosage
Owner:CONSEJO NACIONAL DE INVESTIGACIONES CIENTIFICAS Y TECNICAS CONICET 99 +1
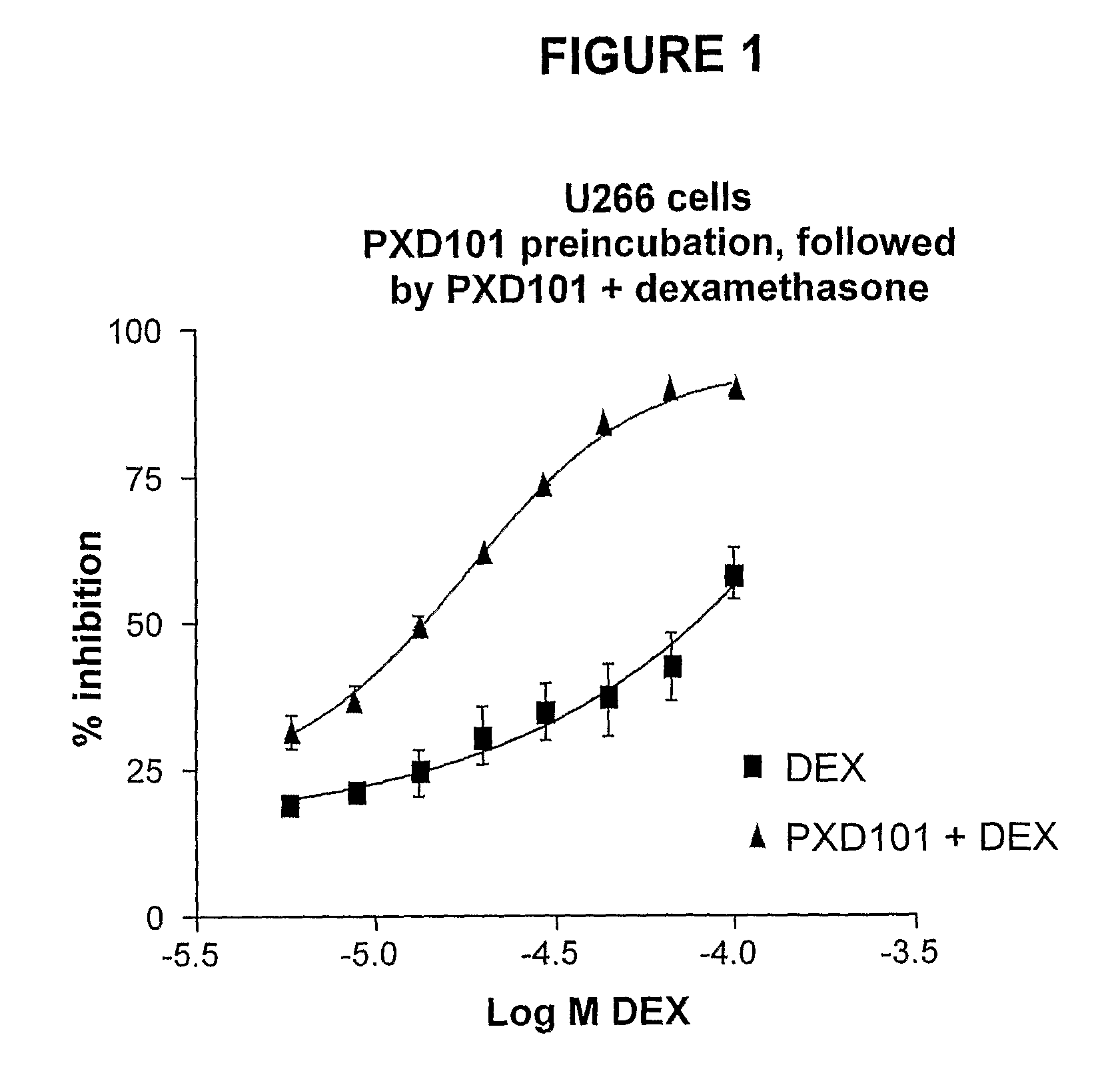
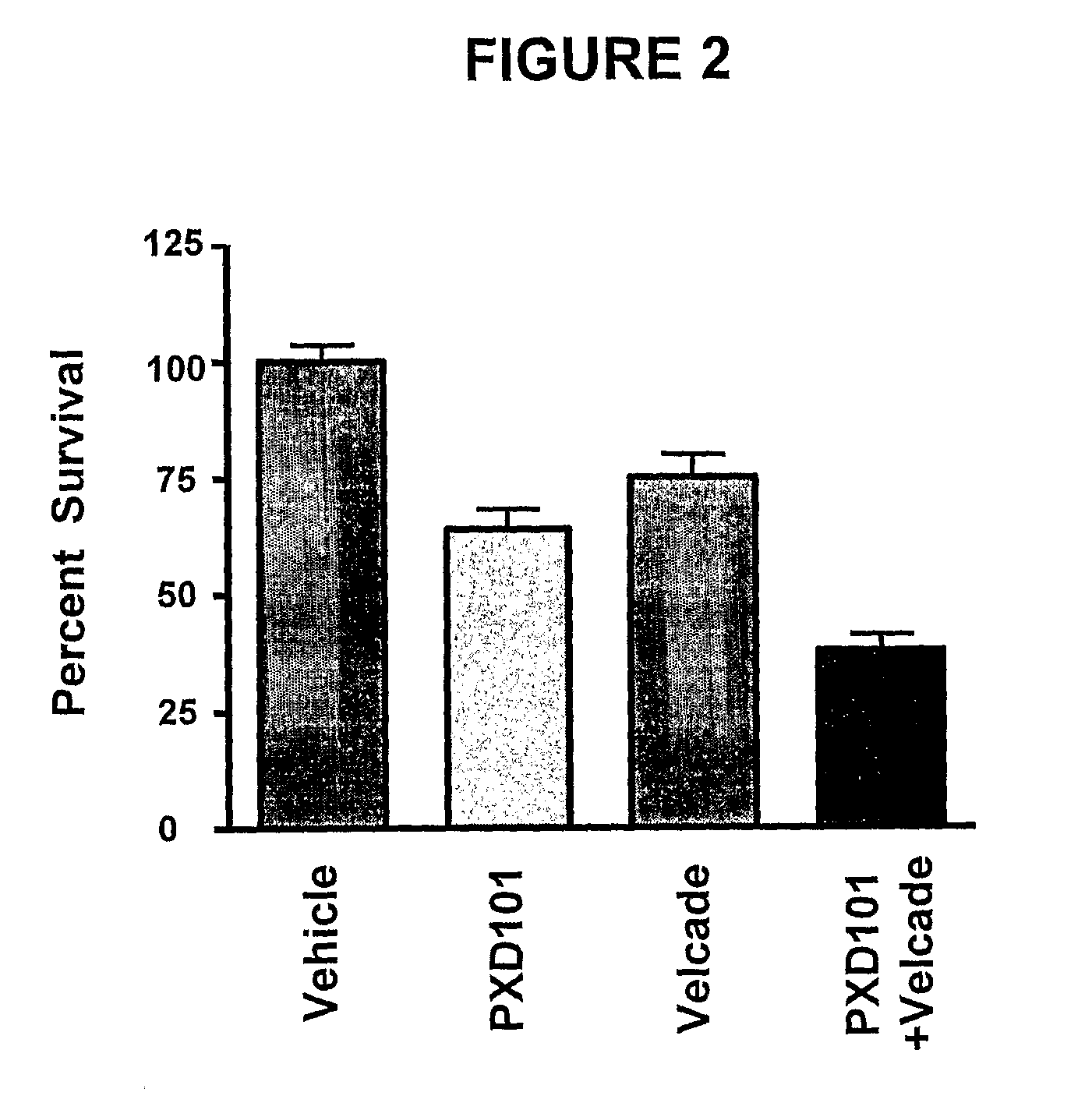
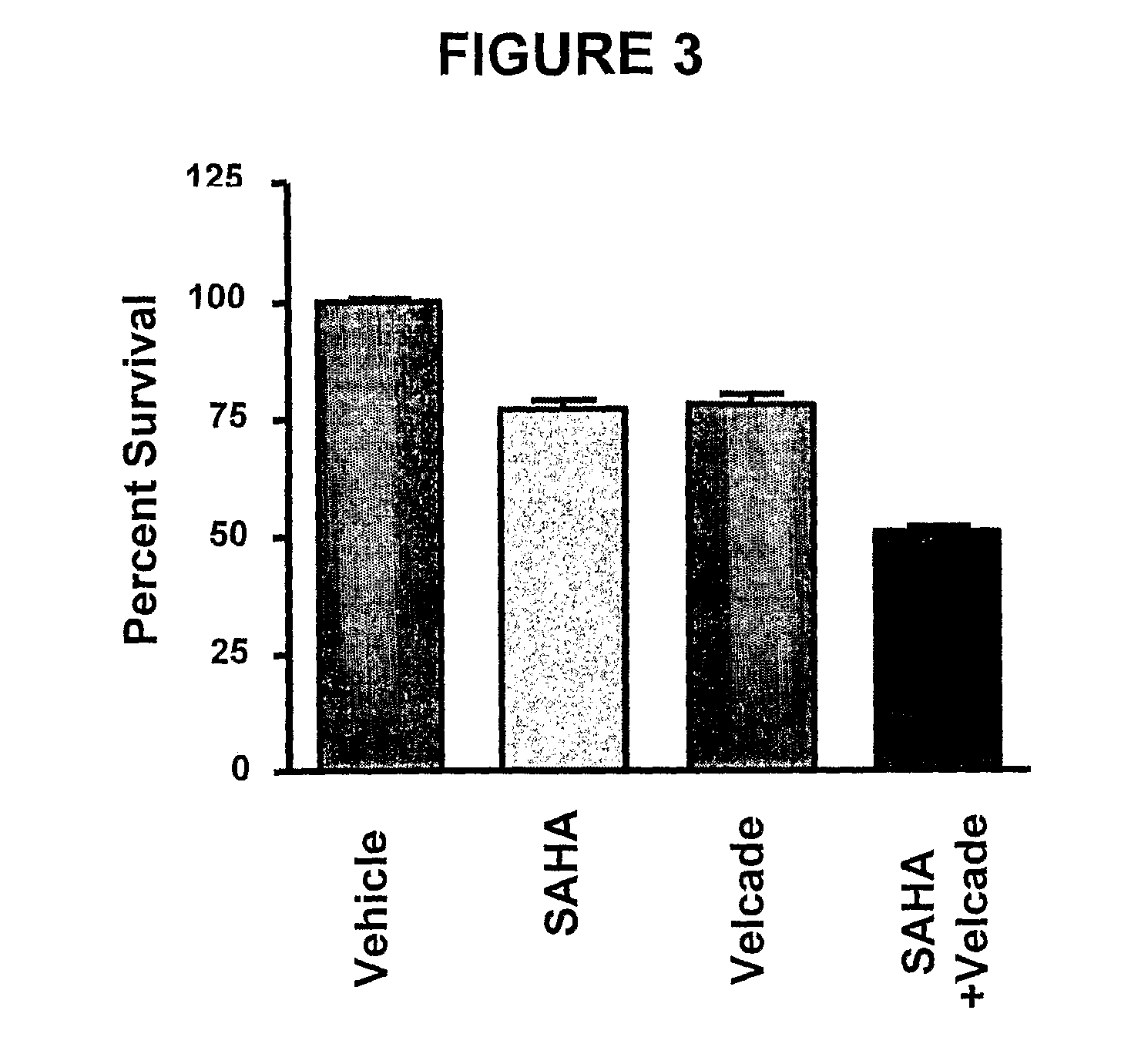
Histone deacetylase (HDAC) inhibitors (PXD101) for the treatment of cancer alone or in combination with chemotherapeutic agent
The present invention relates generally to methods for treating cancer. In one respect, the present invention relates to a method of treating a hematological cancer (e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof a therapeutically effective amount of a histone deacetylase inhibitor, for example, a histone deacetylase (HDAC) inhibitor as described herein, for example, PXD-101. In another respect, the present invention relates to a method of treating cancer (e.g., solid tumor cancer, e.g., rectal cancer, colon cancer, ovarian cancer; hematological cancer, e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof, a first amount of a histone deacetylase (HDAC) inhibitor, for example, a histone deacetylase inhibitor as described herein, for example, PXD-101, and a second amount of an other chemotherapeutic agent, for example, an other chemotherapeutic agent selected from: an antibody against VEGF, AVASTIN® (bevacizumab), an antibody against CD20, rituximab, bortezomib, thalidomide, dexamethasone, vincristine, doxorubicin, and melphalan, wherein the first and second amounts together comprise a therapeutically effective amount.
Owner:TOPOTARGET UK LTD
Taxane analogs for the treatment of brain cancer
ActiveUS20110318334A1High activityImprove solubilityBiocideOrganic active ingredientsBevacizumab InjectionMammal
Provided herein are compounds and methods for the treatment of brain cancer in a mammal, wherein the method comprises the administration to the mammal a compound that stabilizes tubulin dimers or microtubles at G2-M interface during mitosis but is not a substrate for MDR protein. In particular, the present application relates to the use of an orally effective abeo-taxane, alone or in combination with temozolomide or bevacizumab, for the treatment of brain cancer.
Owner:TAPESTRY PHARMACEUTICALS INC
Preparing method and medical application of optimized plant source recombination humanized bevacizumab
InactiveCN106222194AIncrease productionReduce plant-specific glycosylationVaccinesBiological material analysisPlant SourcesLung cancer
The invention belongs to the technical field of biological medicine, and particularly relates to a preparing method and medical application of optimized plant source recombination humanized bevacizumab. Heavy-chain and light-chain fusion protein of the recombinant antibody bevacizumab is expressed in plants, heavy chain and light chain are expressed in an proportion appropriate to 1:1 by adding 2A sequence to fusion protein, the proportion promotes assembly of a complete antibody obviously, and results show that the yield of antibodies expressed with the system is high. Meanwhile, due to the fact that a stable tetramer structure is formed through equal-proportion assembly of the heavy chain and light chain of the recombinant antibody, the number of unassembled polypeptides easy to degrade is reduced, and then pure and consistent monoclonal antibodies are obtained. The bevacizumab developed with a new method is applied to medicine to be used for treating breast cancer, lung cancer, spongioblastoma, kidney cancer, cervix uterus cancer, ovarian cancer, colon cancer and rectal cancer.
Owner:深圳麦客思鱼生物科技发展有限公司
Combination therapy of cancer with Anti-endoglin antibodies and Anti-vegf agents
InactiveUS20180057602A1Relieve symptomsIncreased apoptosisSenses disorderBoron compound active ingredientsBevacizumab InjectionAngiogenesis growth factor
The present application relates to compositions of chimeric anti-endoglin antibodies and anti-VEGF agents. Another aspect relates to the use of chimeric anti-endoglin antibodies and Bevacizumab. Another aspect relates to the use of the compositions to inhibit VEGF induced sprouting. Another aspect relates to the use of the compositions to inhibit angiogenesis.
Owner:TRACON PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com