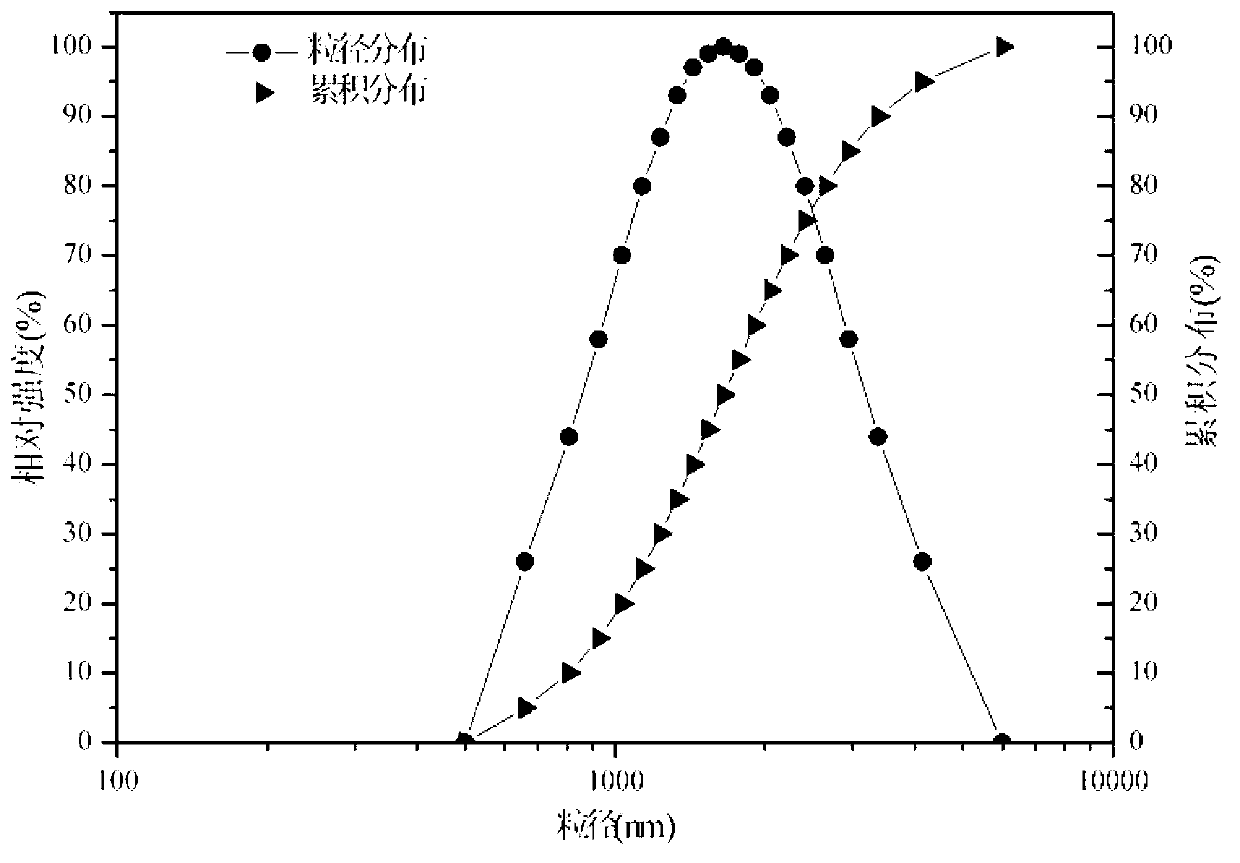
Preparation method of long-acting sustained-release microspheres containing bevacizumab
A technology of bevacizumab and slow-release microspheres, which is applied in the direction of antibody, anti-tumor drug, block delivery, etc., can solve the problem of disputes about the time interval of repeated drug injection, and achieve convenient clinical use, protection of activity, and improvement of The effect of encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of the long-acting slow-release microsphere containing bevacizumab comprises the following steps:
[0038] (1) Prepare a bevacizumab solution with a concentration of 200mg / ml;
[0039] (2) Take the bevacizumab solution prepared in step (1), add sodium alginate, and prepare a bevacizumab solution with a sodium alginate concentration of 15 mg / ml as the inner water phase; the prepared concentration is 150 mg / ml The dichloromethane solution of PLGA of ml, as oil phase, wherein, the polymerization ratio of PLGA is 75 / 25,3W; Prepare the polyvinyl alcohol aqueous solution containing 1000mg / ml calcium ion, the percentage composition of polyvinyl alcohol is 0.25%, as the external aqueous phase;
[0040] (3) Preparation of sustained-release microspheres by W / O / W double emulsion method
[0041] First, the sodium alginate solution as the inner water phase is dispersed into the organic solvent solution of the degradable biomedical polymer material as the oil...
Embodiment 2
[0047] The preparation method of the long-acting slow-release microsphere containing bevacizumab comprises the following steps:
[0048] (1) Concentrate the commercially available bevacizumab injection to obtain a bevacizumab solution with a concentration of 50 mg / ml;
[0049] (2) Take the bevacizumab solution prepared in step (1), add sodium alginate, and prepare a bevacizumab solution with a sodium alginate concentration of 10 mg / ml as the inner water phase; the prepared concentration is 300 mg / ml The dichloromethane solution of the PLGA of ml, as oil phase, wherein, the polymerization ratio of PLGA is 50 / 50, 10W;
[0050] Prepare polyvinyl alcohol aqueous solution containing 1000mg / ml calcium ion, the percentage composition of polyvinyl alcohol is 2%, as the external water phase;
[0051] (3) Preparation of sustained-release microspheres by W / O / W double emulsion method
[0052] First, the sodium alginate solution as the inner water phase is dispersed into the organic solv...
Embodiment 3
[0058] The preparation method of the long-acting slow-release microsphere containing bevacizumab comprises the following steps:
[0059] (1) Concentrate the commercially available bevacizumab injection to obtain a bevacizumab solution with a concentration of 500 mg / ml;
[0060] (2) Take the bevacizumab solution prepared in step (1), add sodium alginate, and prepare a bevacizumab solution with a sodium alginate concentration of 5 mg / ml as the inner water phase; the prepared concentration is 10 mg / ml ml of PLGA in dichloromethane, as the oil phase,
[0061] Prepare polyvinyl alcohol aqueous solution containing 1000mg / ml calcium ion, the percentage composition of polyvinyl alcohol is 15%, as the external water phase;
[0062] (3) Preparation of sustained-release microspheres by W / O / W double emulsion method
[0063] First, the sodium alginate solution as the inner water phase is dispersed into the organic solvent solution of the degradable biomedical polymer material as the oil ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com