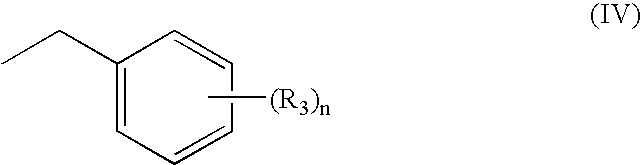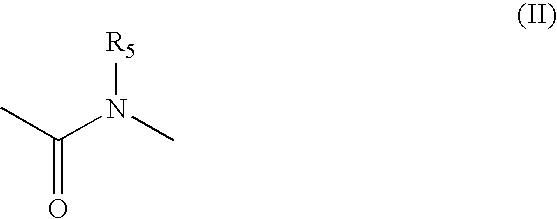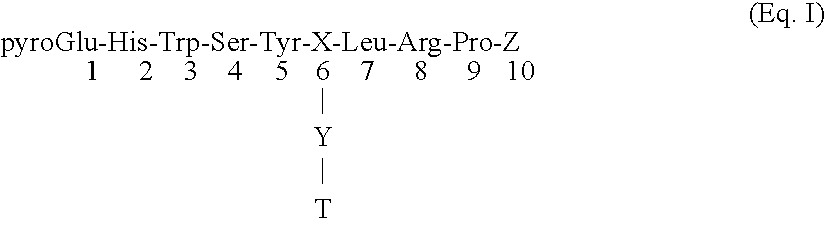Patents
Literature
113 results about "Melphalan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain types of cancer (such as multiple myeloma, ovarian).
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
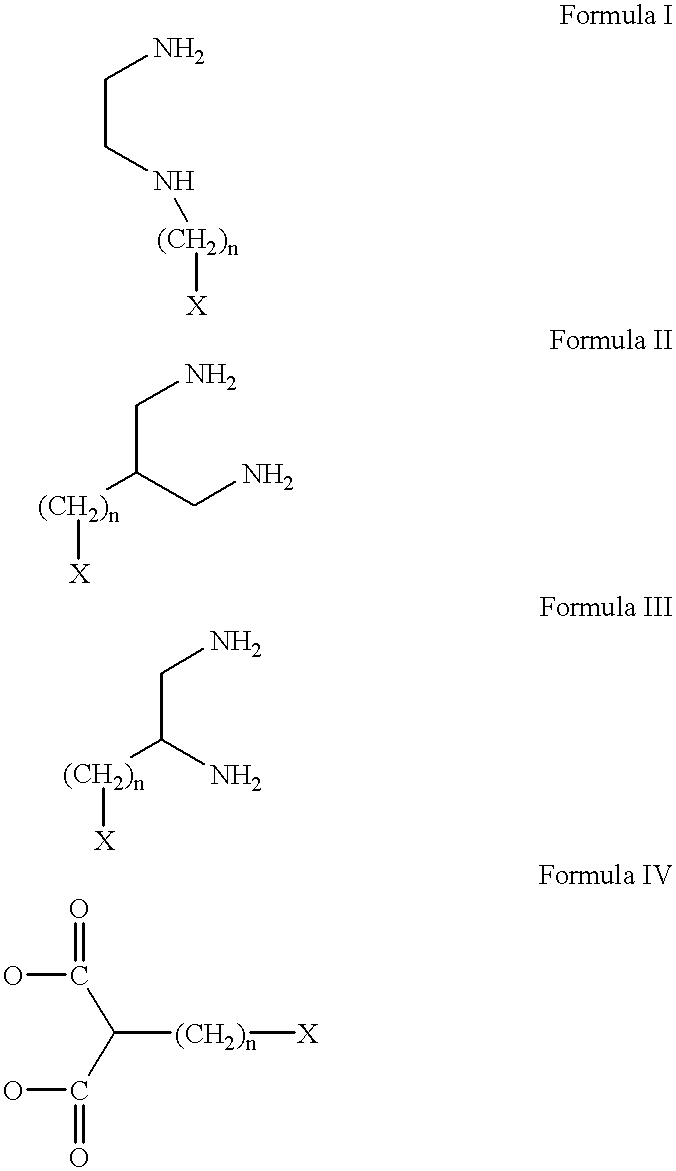
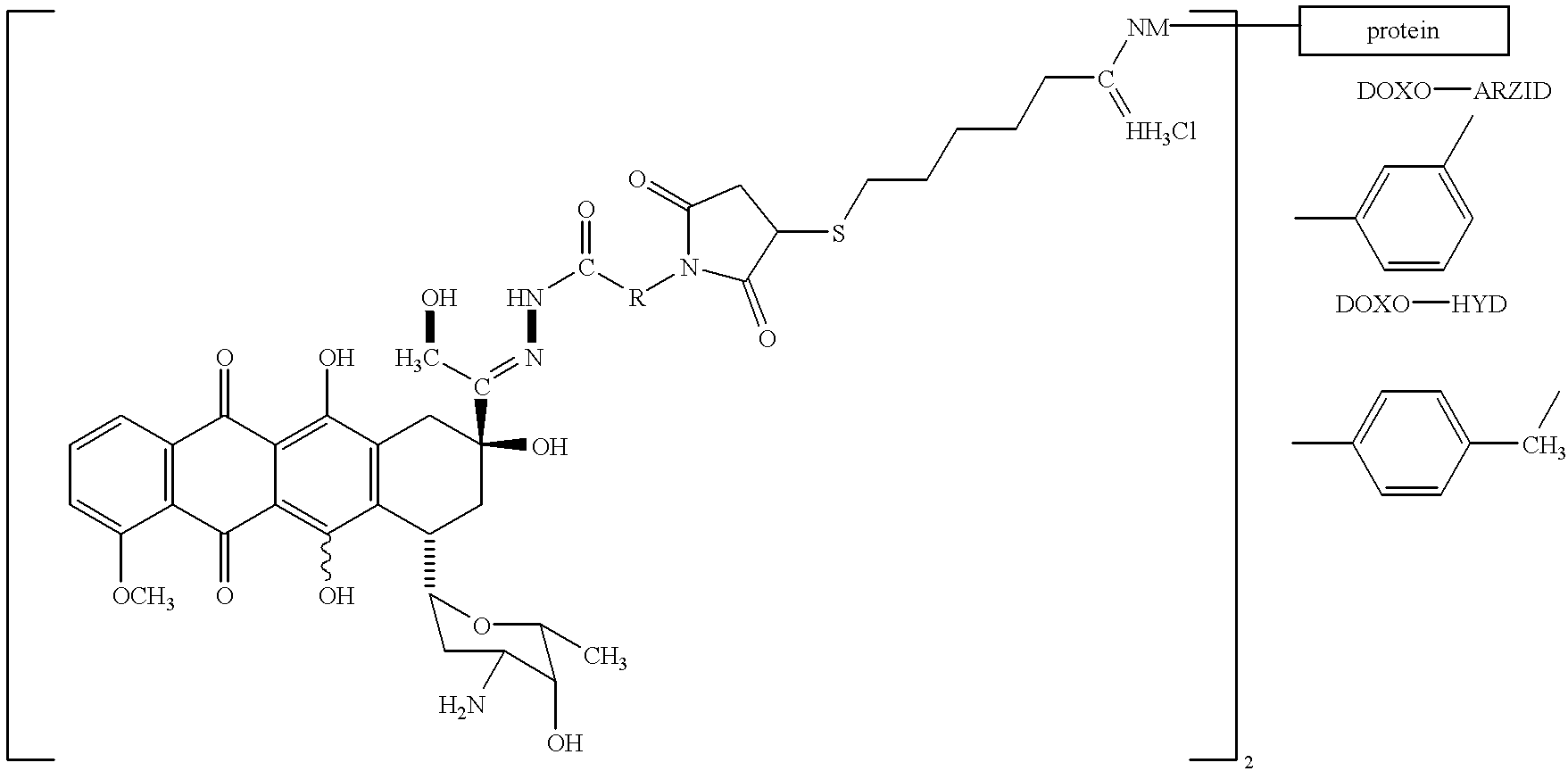
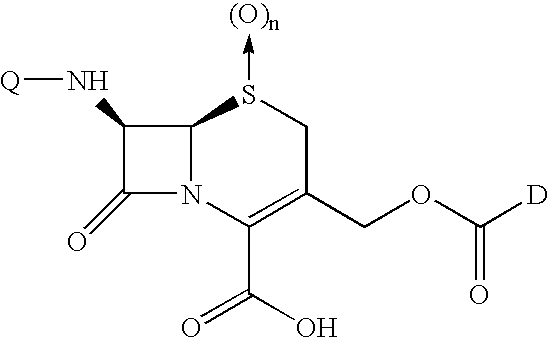
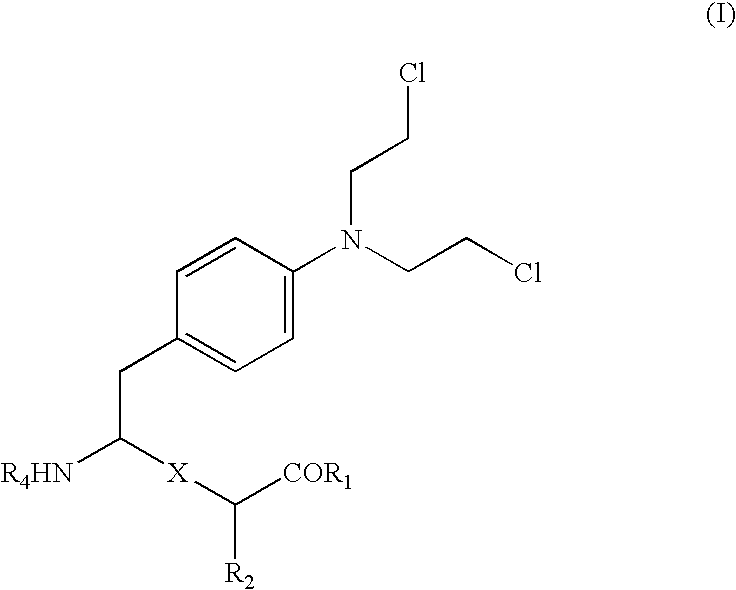
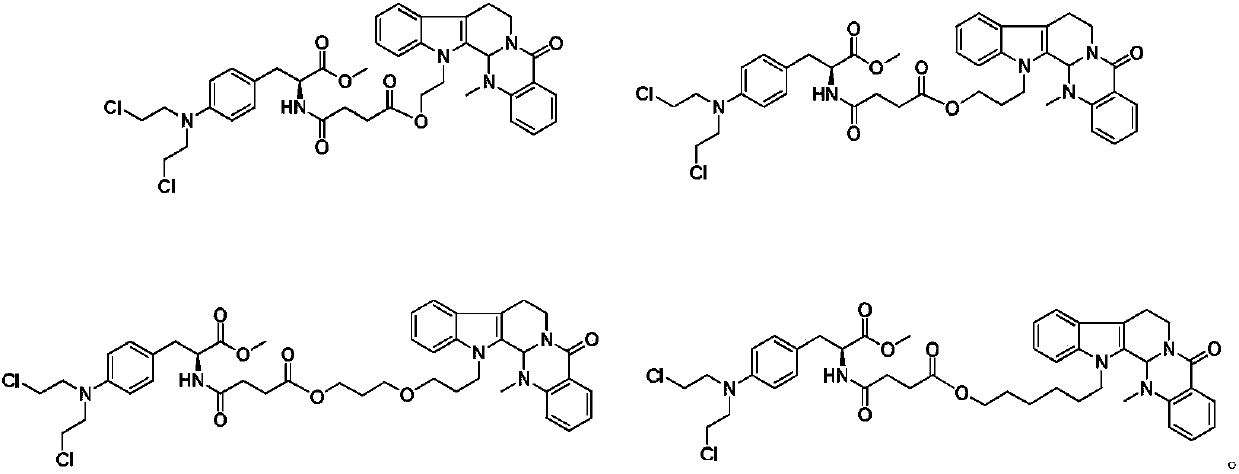
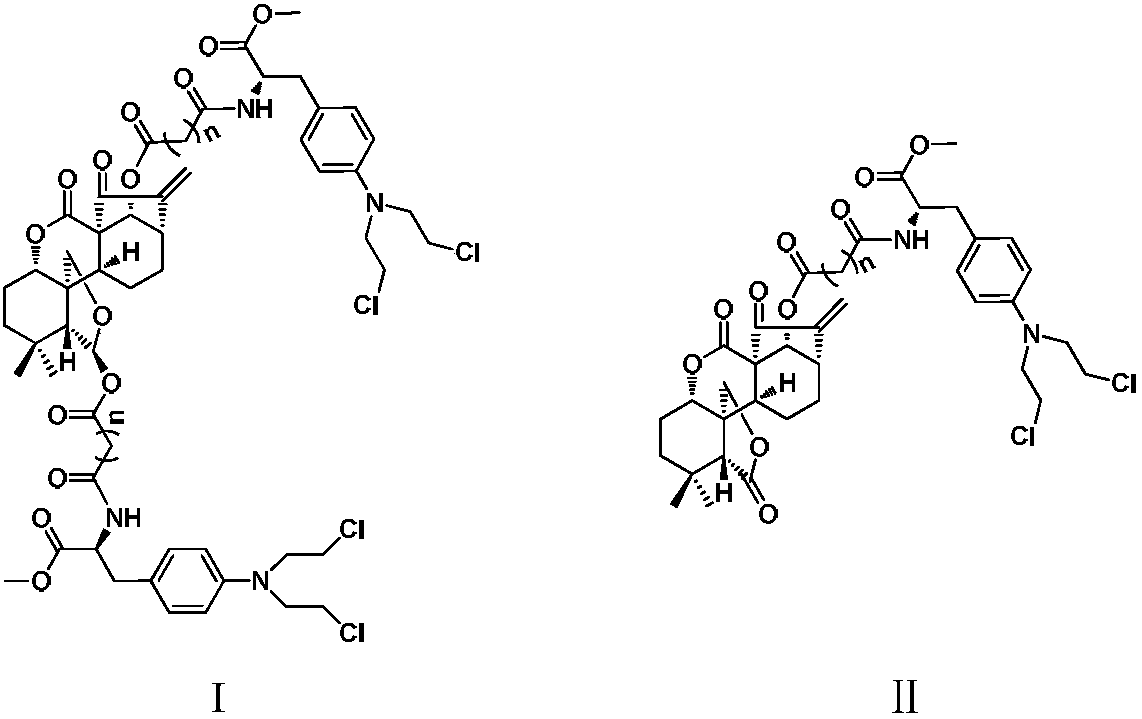
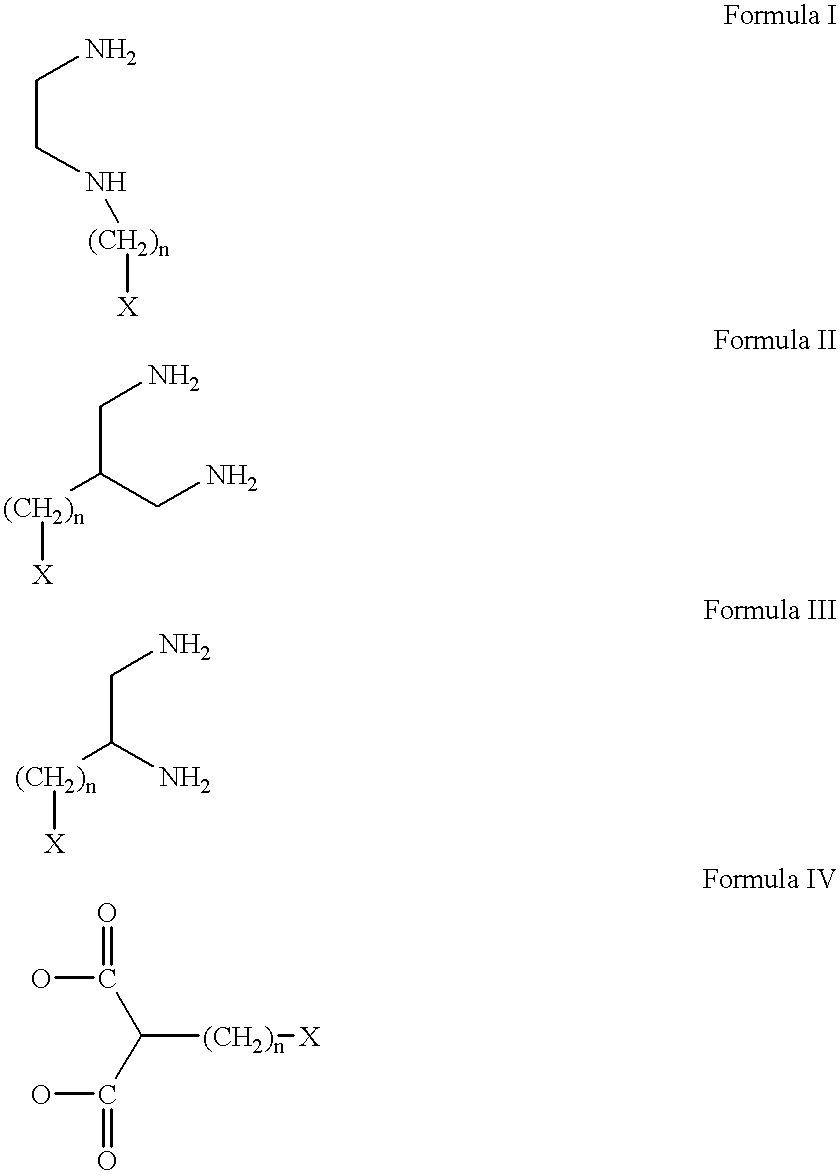
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Melphalan prodrugs
InactiveUS20050214310A1High specificity of actionImprove stabilityNanomedicineAntibody ingredientsSolubilityTherapeutic window
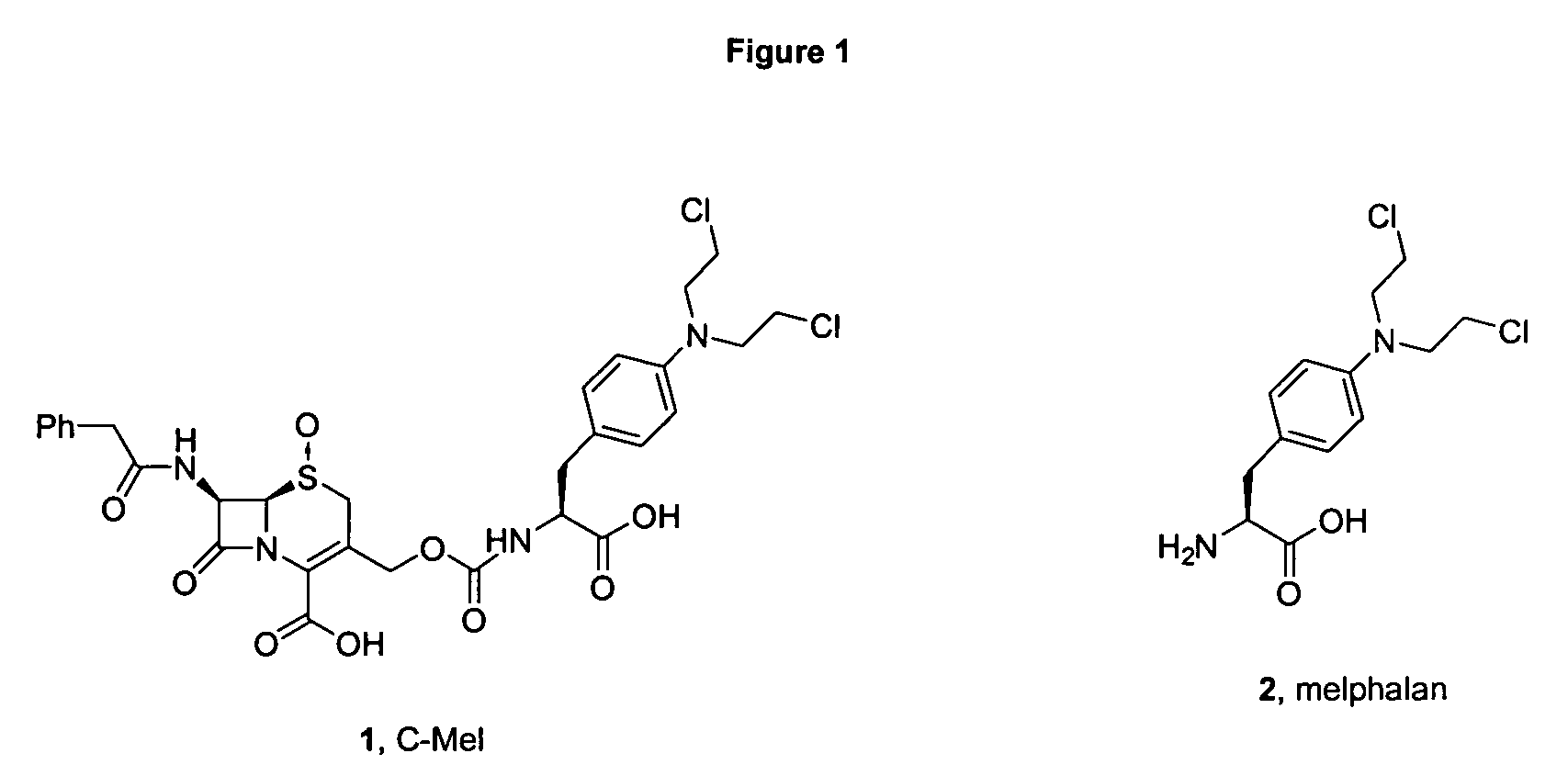
Shown and described are the synthesis of more potent forms of C-Mel, a prodrug used in Antibody-Directed Enzyme Prodrug Therapy, that releases the clinically used anticancer alkylating agent melphalan extracellularly. Shown and described are the synthesis of a variety of melphalan analogues with the intention to promote facile intracellular drug access. Esters, amides, and peptides of melphalan are shown. Cephalosporin prodrugs of the most interesting melphalan derivatives were synthesized and evaluated for potency, toxicity, therapeutic window, plasma stability, and solubility.
Owner:SEATTLE GENETICS INC
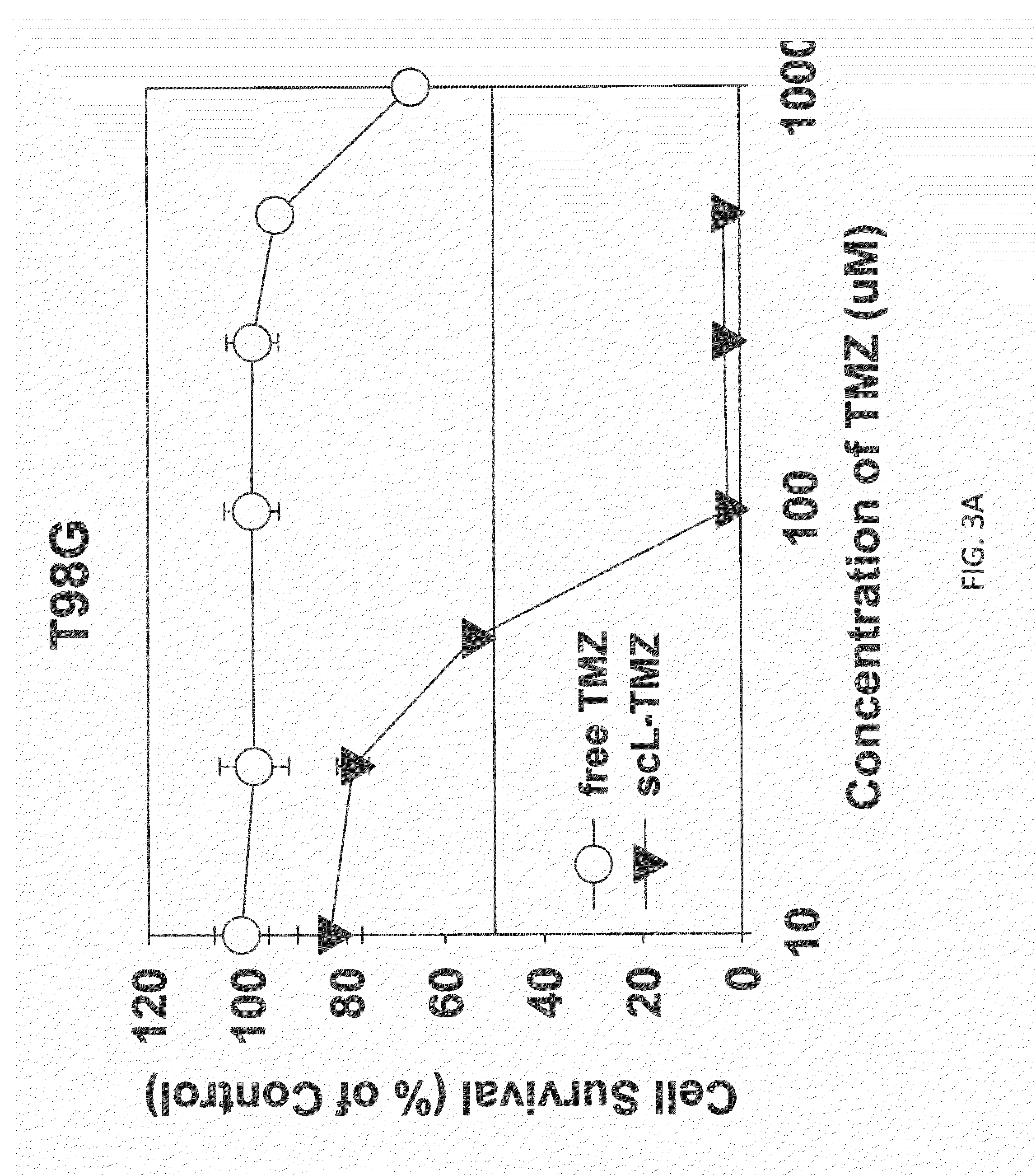
Targeted liposomes
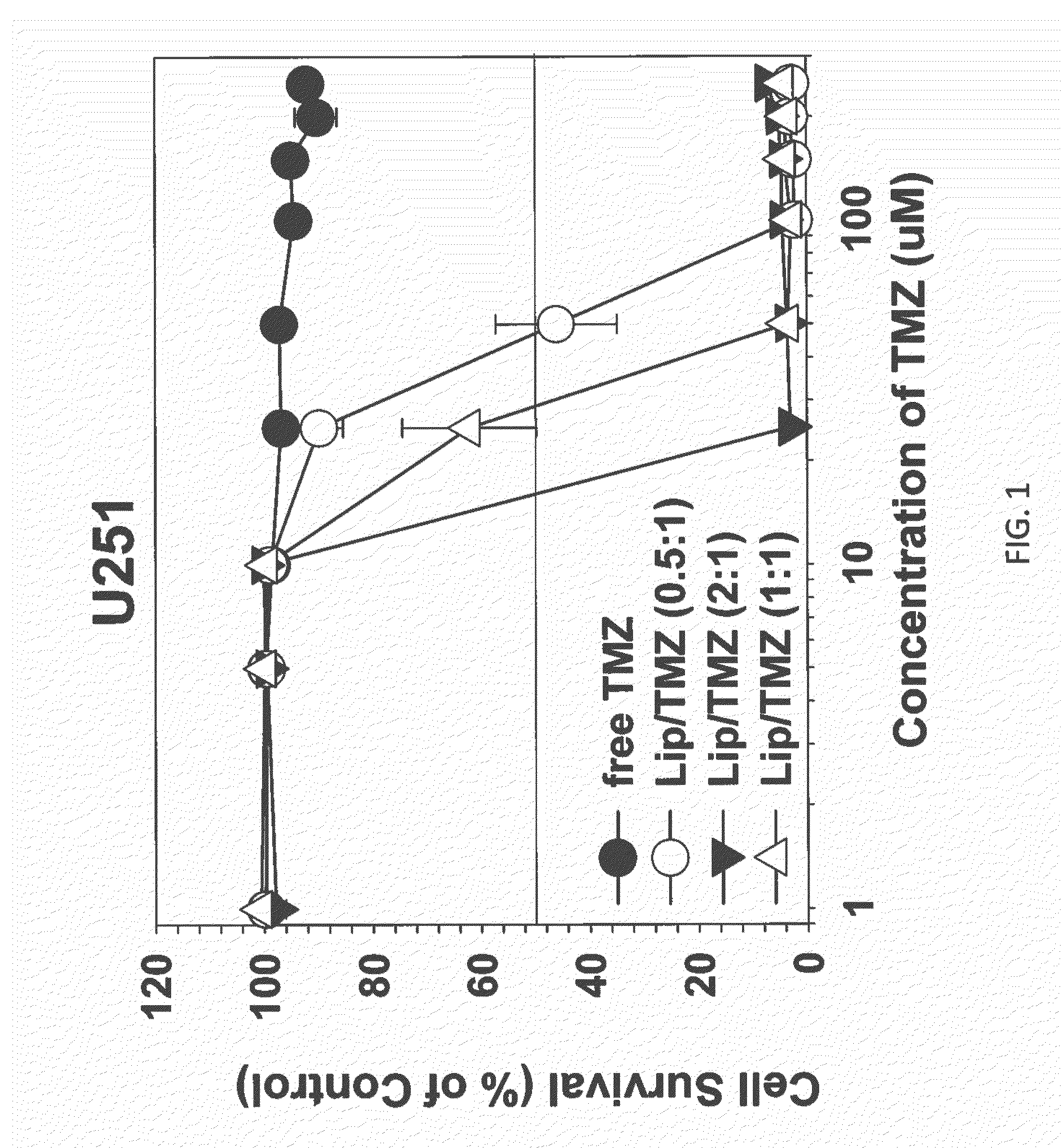
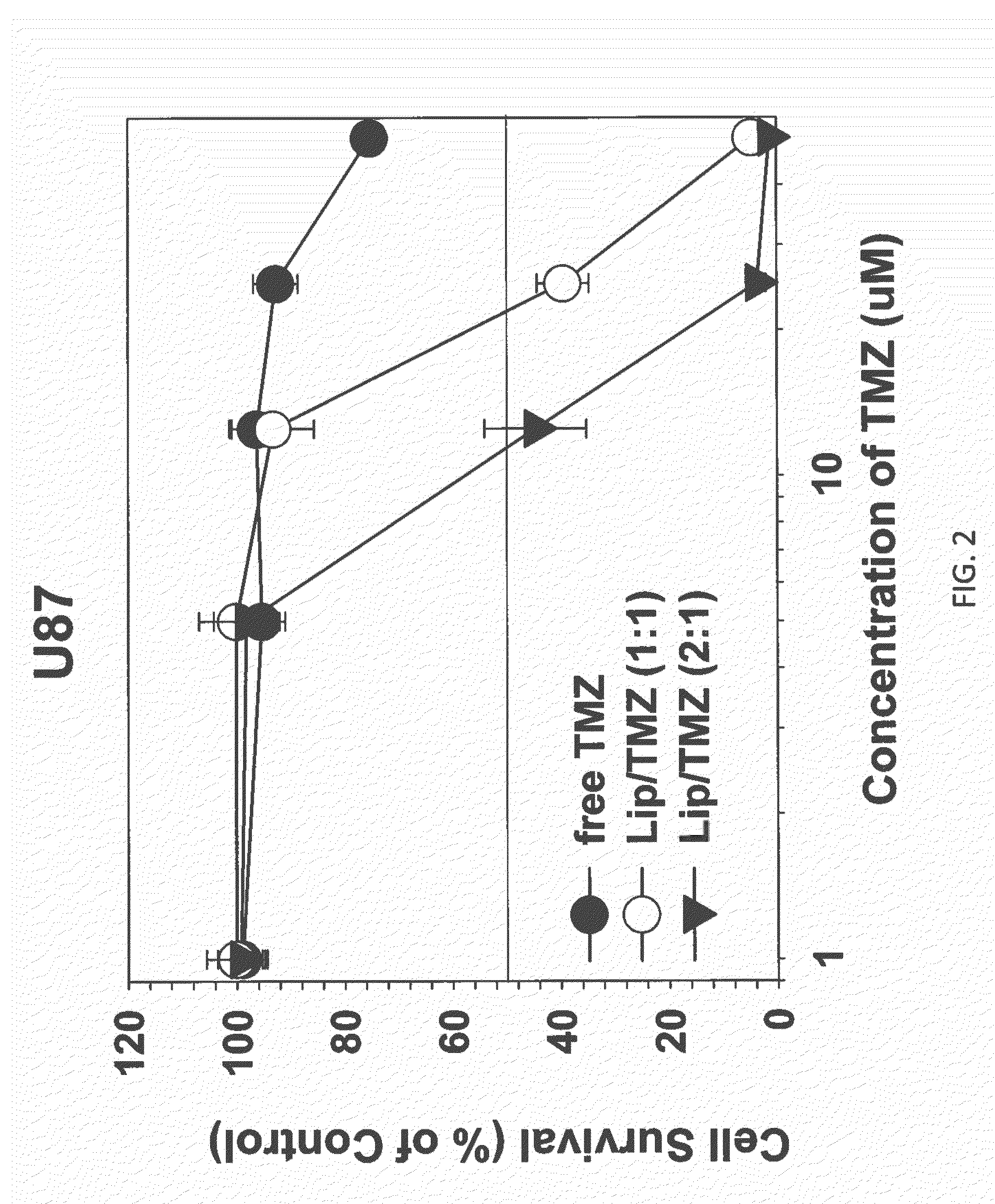
The present invention is in the field of drug delivery, and specifically, cationic liposome-based drug delivery. In embodiments, this invention provides methods of making ligand-targeted (e.g., antibody- or antibody fragment-targeted) liposomes useful for the delivery of liposomes to tumors, including brain tumors. In embodiments, the liposomes deliver temozolomide across the blood-brain barrier for treatment of primary or metastatic brain tumors. Additional cancers that can be treated with the liposomes include neuroendocrine tumors, melanoma, prostate, head and neck, ovarian, lung, liver, kidney, breast, urogenital, gastric, colorectal, cervical, vaginal, angiosarcoma, liposarcoma, rhabdomyosarcoma, choriocarcinoma, pancreatic, retinoblastoma and other types of cancer. In another embodiment the liposomes deliver melphalan for the treatment of multiple myeloma, other tumors of the blood or other solid tumors. In still other embodiments the liposomes can deliver other drugs such as pemetrexed or irinotecan for treatment of cancer or drugs including atropine for treatment of organophosphate poisoning.
Owner:GEORGETOWN UNIV
Melphalan freeze-dried powder injection
InactiveCN101584669AImprove stabilityLow content of related substancesOrganic active ingredientsPowder deliveryFreeze-dryingMedicine
The invention relates to a melphalan freeze-dried powder injection and a preparing method thereof. The prepared melphalan freeze-dried powder injection is used for treating multiple myeloma, oophoroma, polycythemia vera, local malignant melanoma and soft tissue sarcoma. The melphalan freeze-dried powder injection contains melphalan, utilizes the mixed solvent composed of the tert-butyl alcohol and the injection water in the preparation process, wherein the concentration of the melphalan in the mixed solvents is 10-25 mg / ml; and the volume ratio of the solvents is: 5-50% of tert-butyl alcohol and the balance of injection water. The preparation process comprises the following steps: measuring tert-butyl alcohol, adding injection water, mixing evenly, cooling to 2-15 DEG C, heat preserving, adding melphalan, stirring and dripping 0.5 mol hydrochloric acid to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing plug, out box, tying and packing after quality test qualification.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
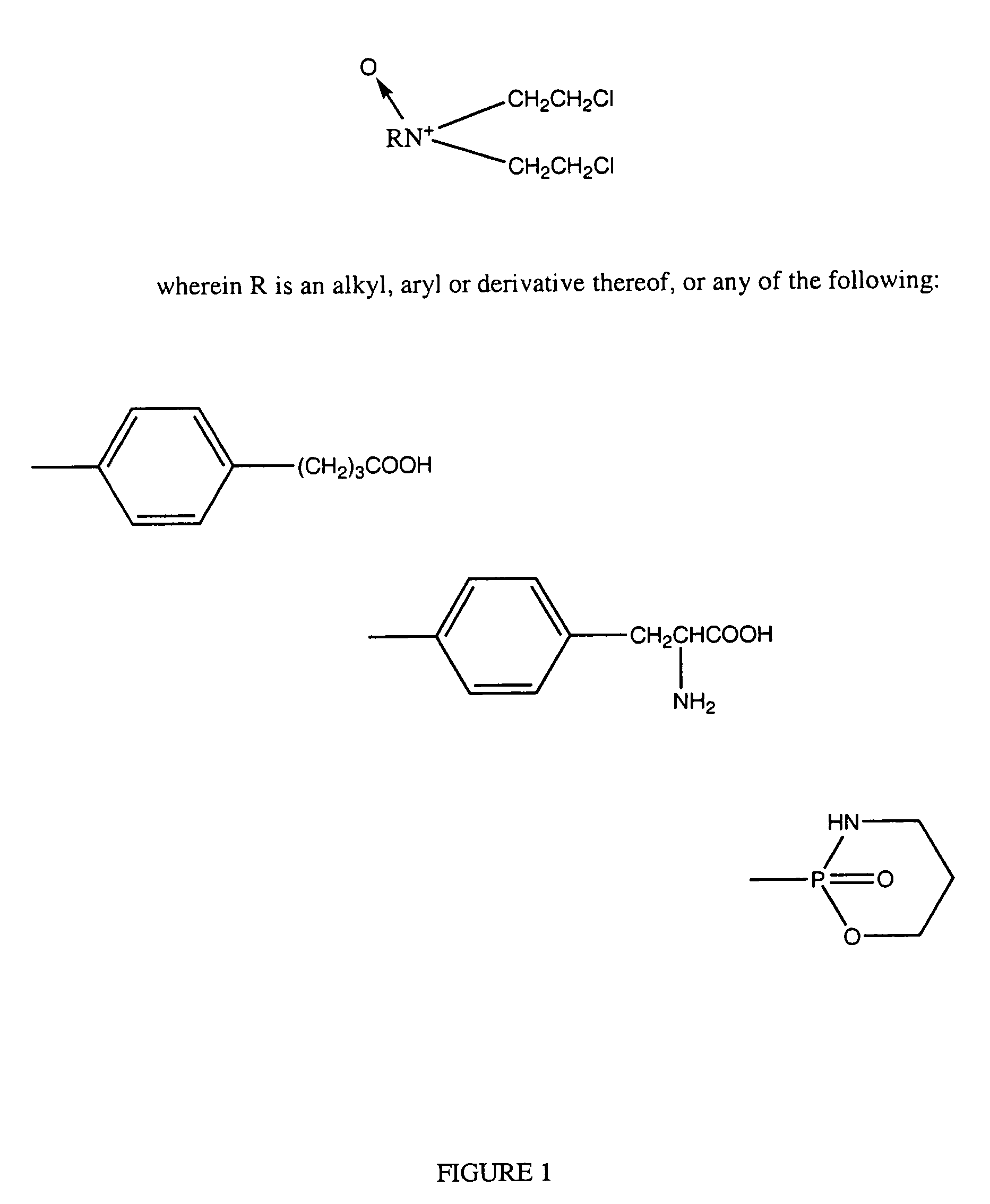
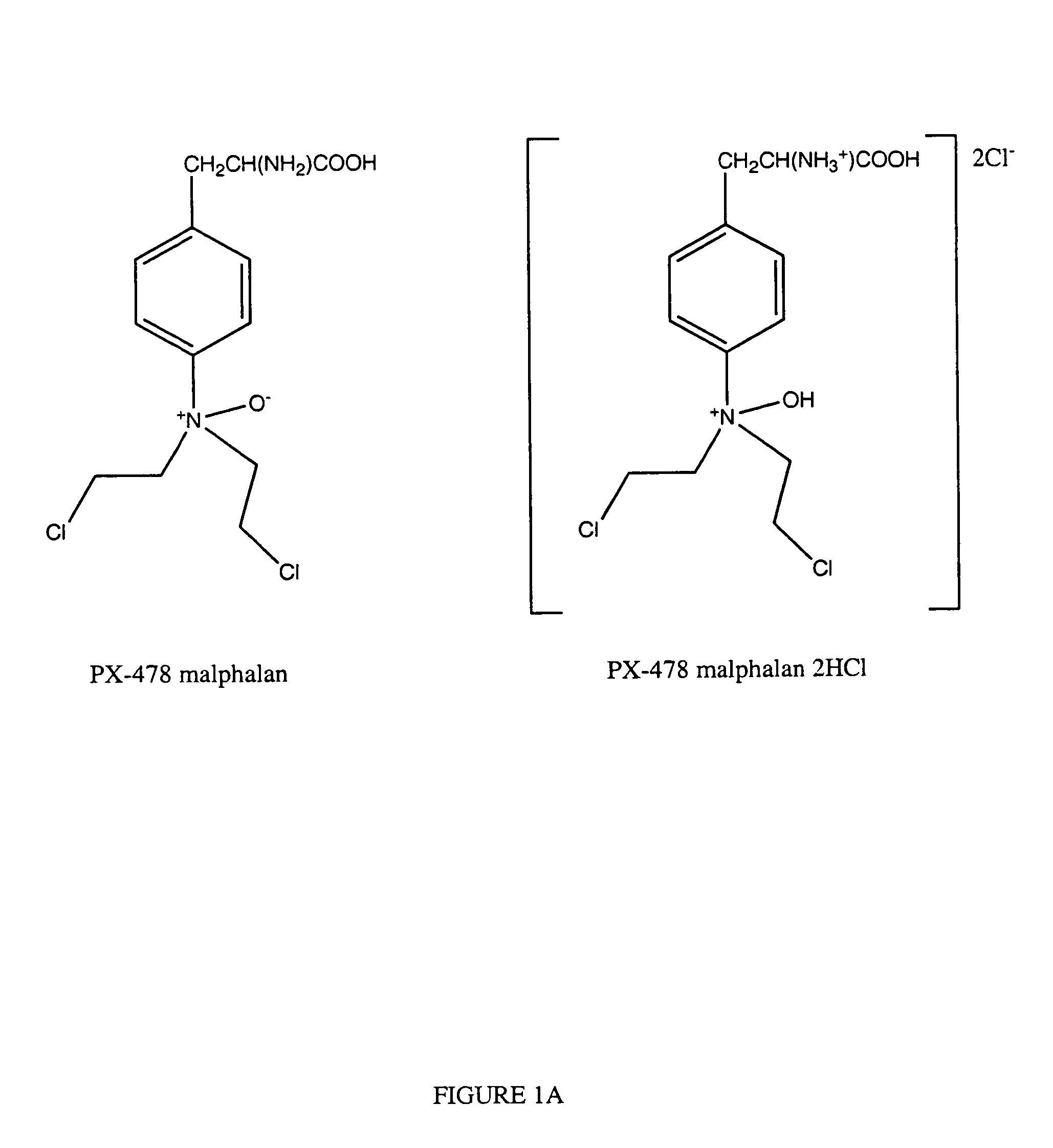
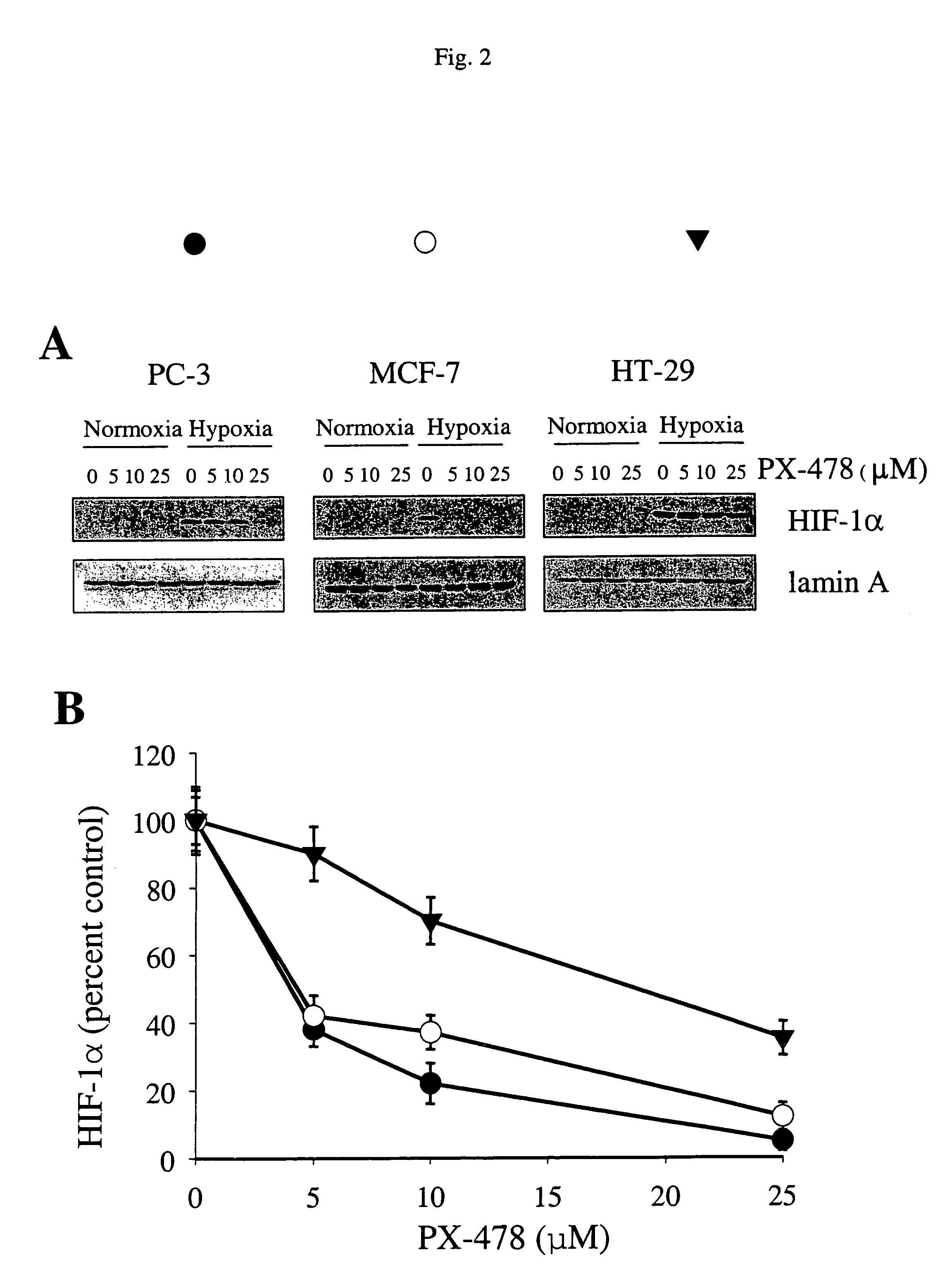
N-oxides and derivatives of melphalan for treating diseased states associated with hypoxia inducible factor
This invention relates to compounds which are N-oxides and derivatives thereof, as well as their use to treat HIF related diseases. These compounds have the general formula set out below and are used to treat a variety of diseases associated with HIF:wherein R is an alkyl, aryl, arakyl or derivatives thereof such as CH3OCH2CH2—, CH3CH2OCH2CH2—, C6H5OCH2CH2—, C6H5CH2—, CH3(CH2)3OCH2CH2Cl; or any one of the following:
Owner:PROLX PHARMA +1
Combinations of therapeutic agents for treating melanoma
ActiveUS20130330421A1Less side effectsRemarkable effectHeavy metal active ingredientsOrganic active ingredientsCarboplatinDocetaxel
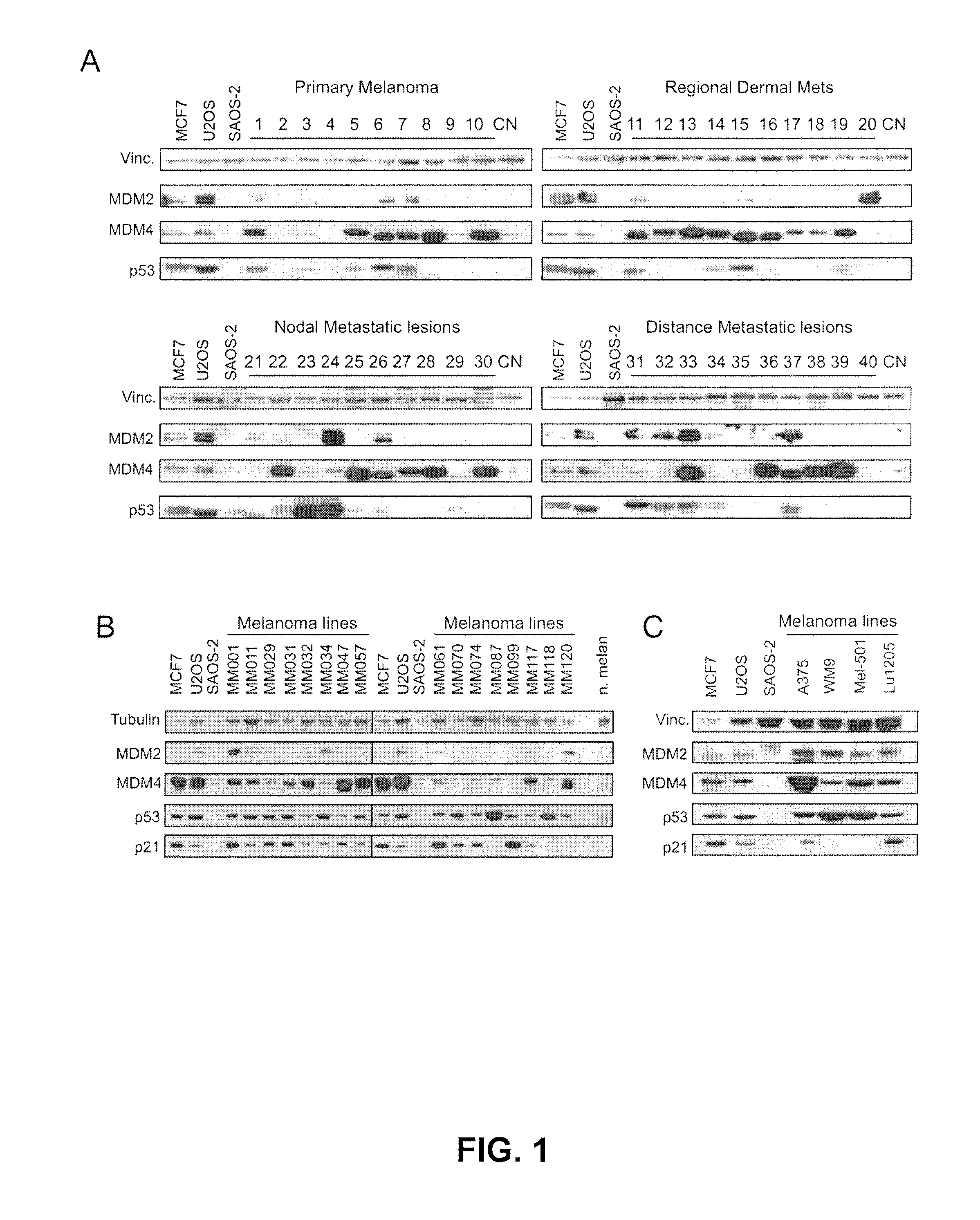
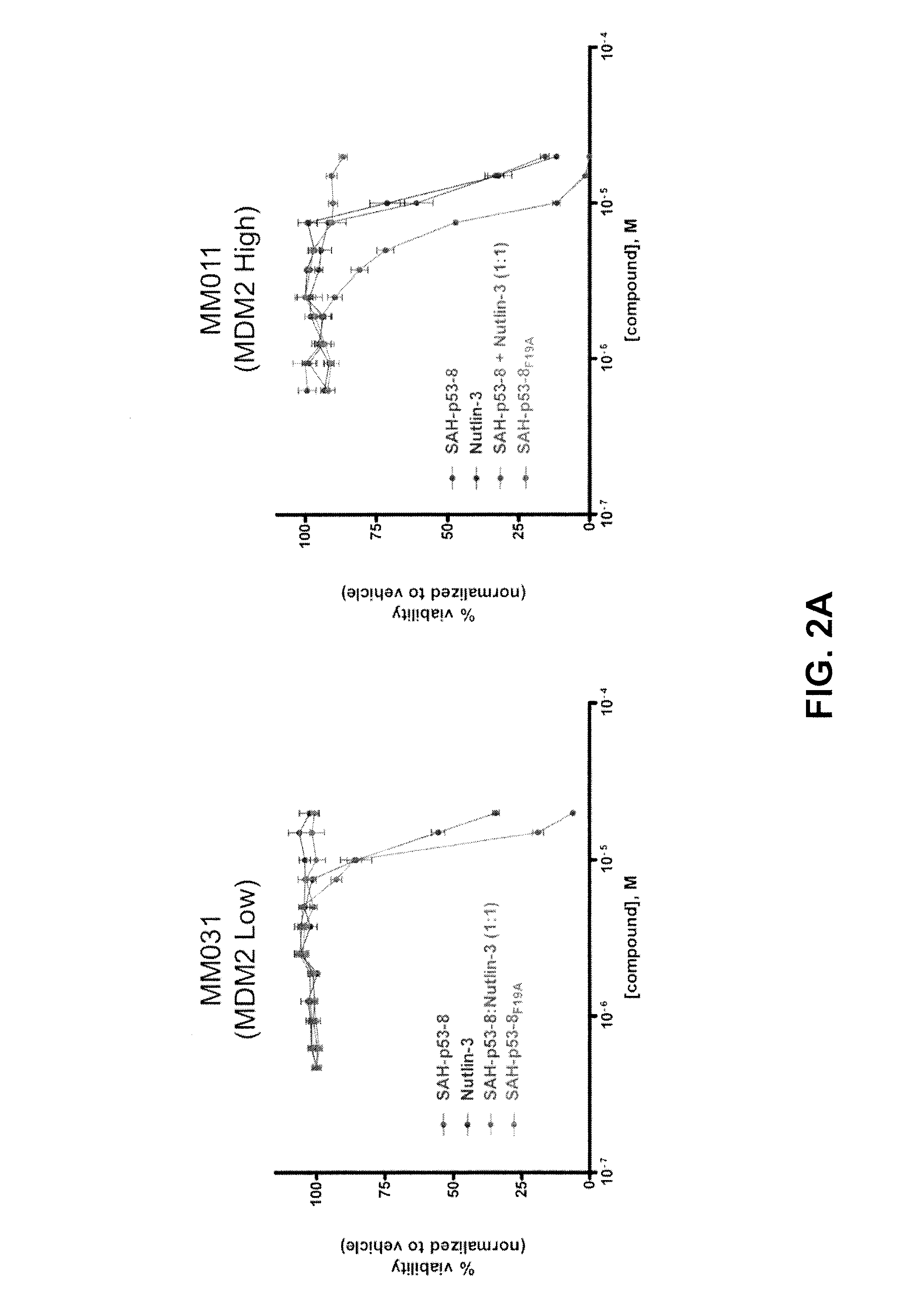
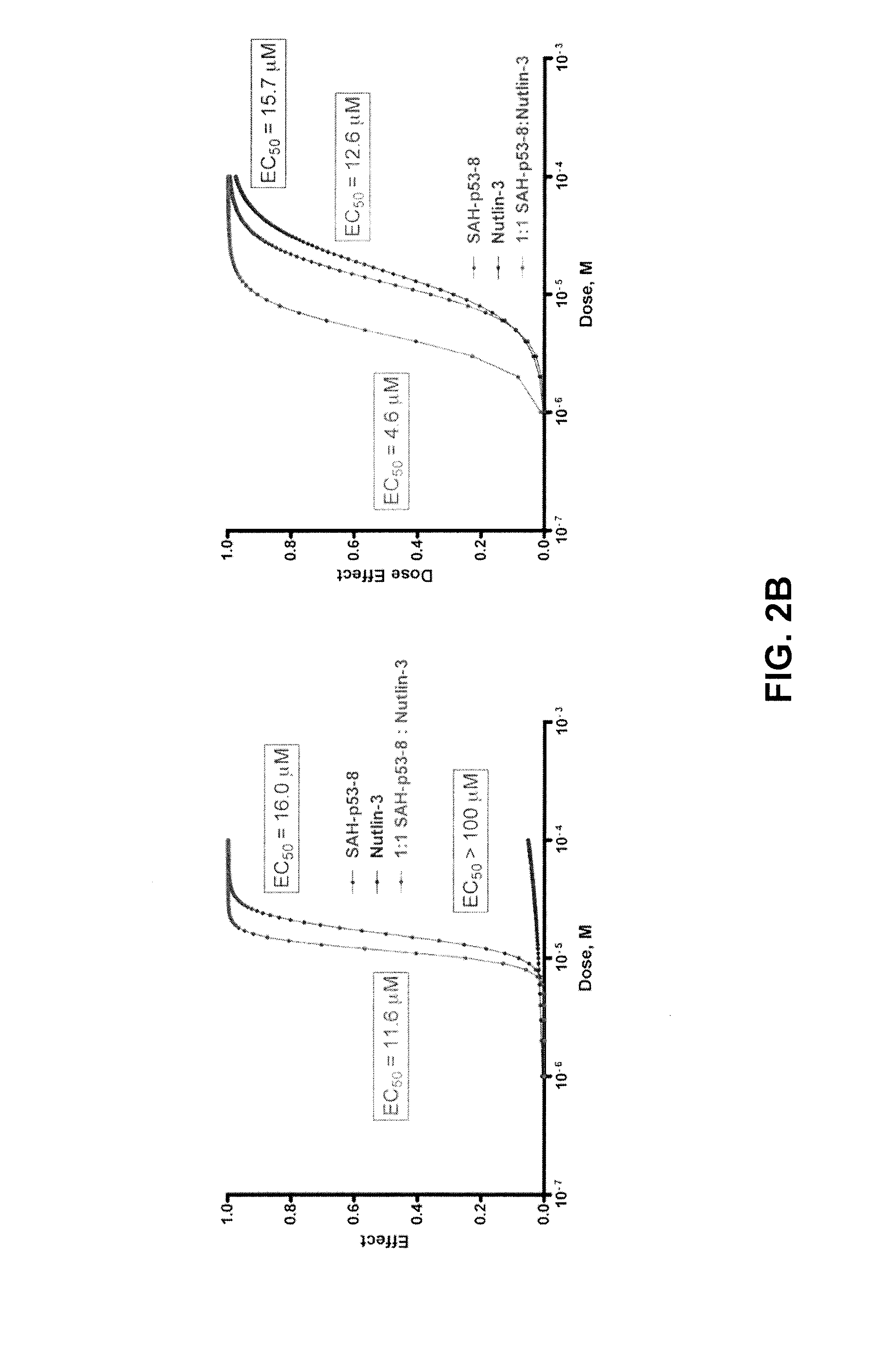
The present disclosure relates to the field of oncology, more particularly to the field of melanoma. Provided are methods of treating melanoma, particularly advanced cutaneous melanoma, with a combination of pharmaceutical agents comprising MDM4-specific antagonists (such as an inhibitor of the MDM4-p53 interaction or a molecule that decreases MDM4 protein stability) or MDM4-MDM2 dual inhibitors (i.e., molecules that disrupt the interactions between p53 and MDM2 and p53 and MDM4) and one or more chemotherapeutic agents such as for example alkylating agents (i.e., Dacarbazine (DITC) or melphalan), alkylating-like agents (i.e., cisplatin or carboplatin) or mitotic inhibitors (taxanes docetaxel or paclitaxel) and PI3K-AKT, B-RAF and MEK inhibitors. Further provided are pharmaceutical formulations of MDM4-specific antagonists (be it an inhibitor of the MDM4-p53 interaction or a molecule that decreases MDM4 protein stability) or MDM4-MDM2 dual inhibitors (i.e., molecules that disrupt the interactions between p53 and MDM2 and p53 and MDM4) and a pharmaceutical formulation of one or more chemotherapeutic agents such as for example alkylating agents (i.e., Dacarbazine (DITC) or melphalan), alkylating-like agents (i.e., cisplatin or carboplatin) or mitotic inhibitors (taxanes docetaxel or paclitaxel) and B-RAF and MEK inhibitors.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
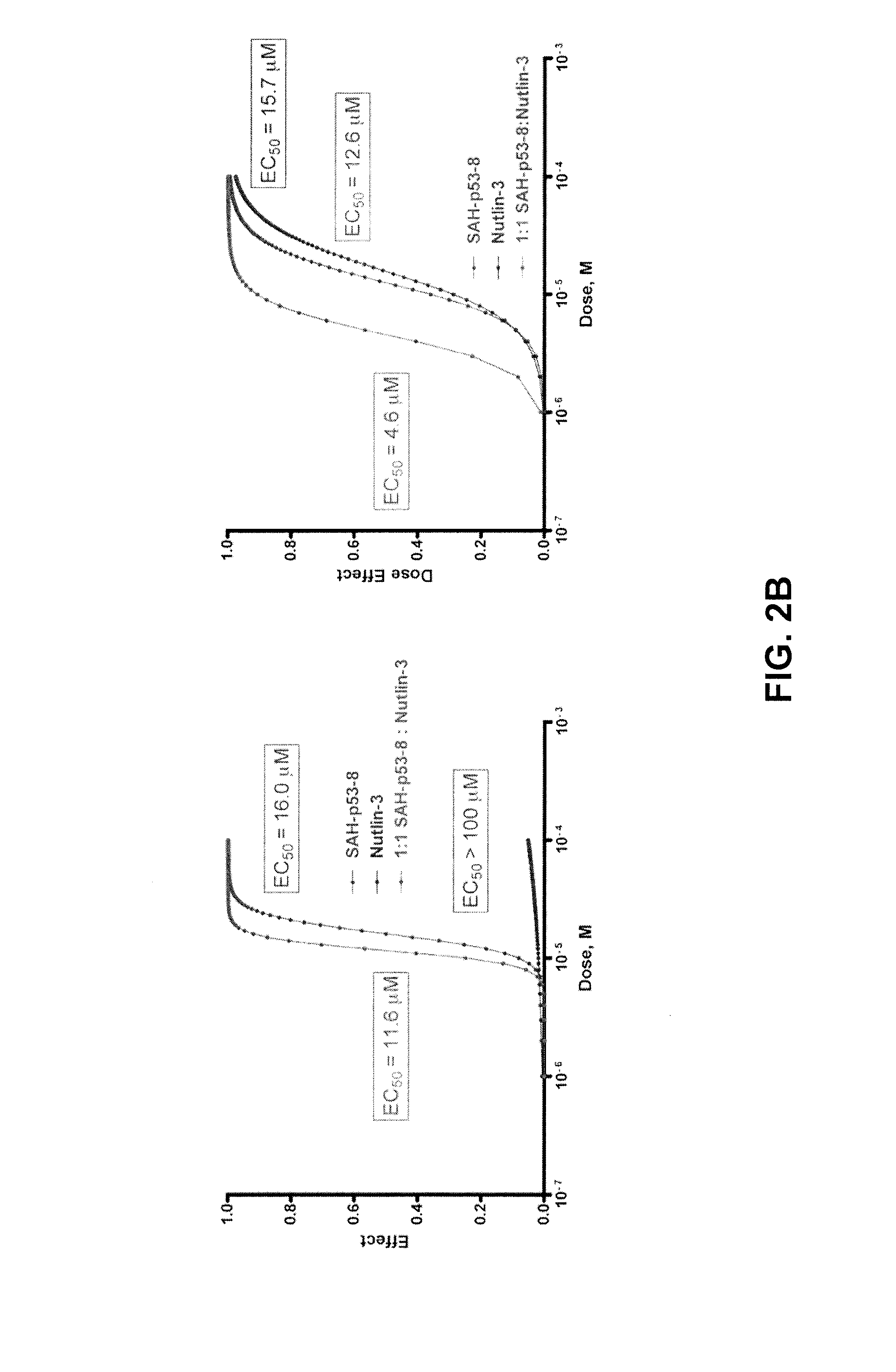
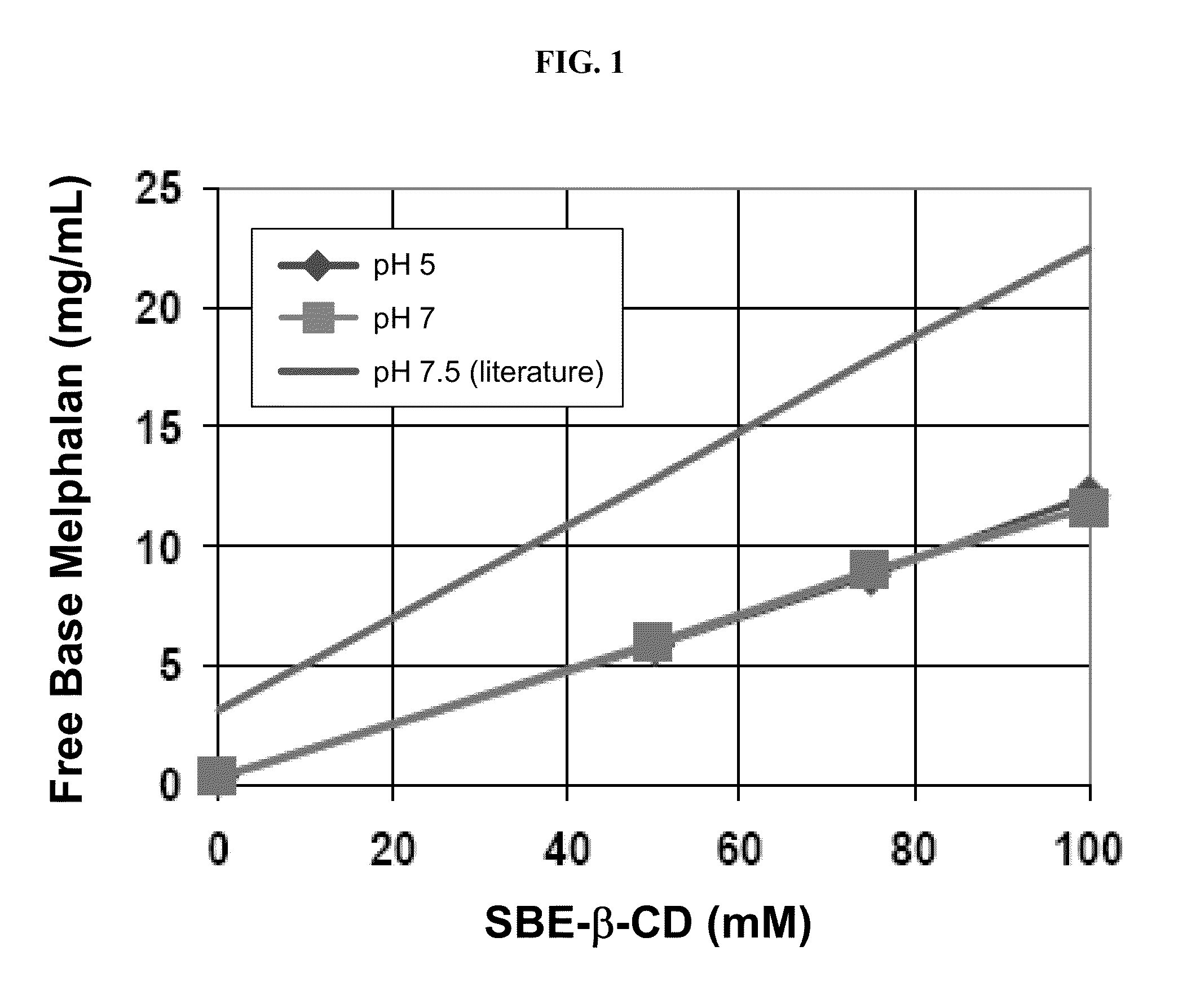
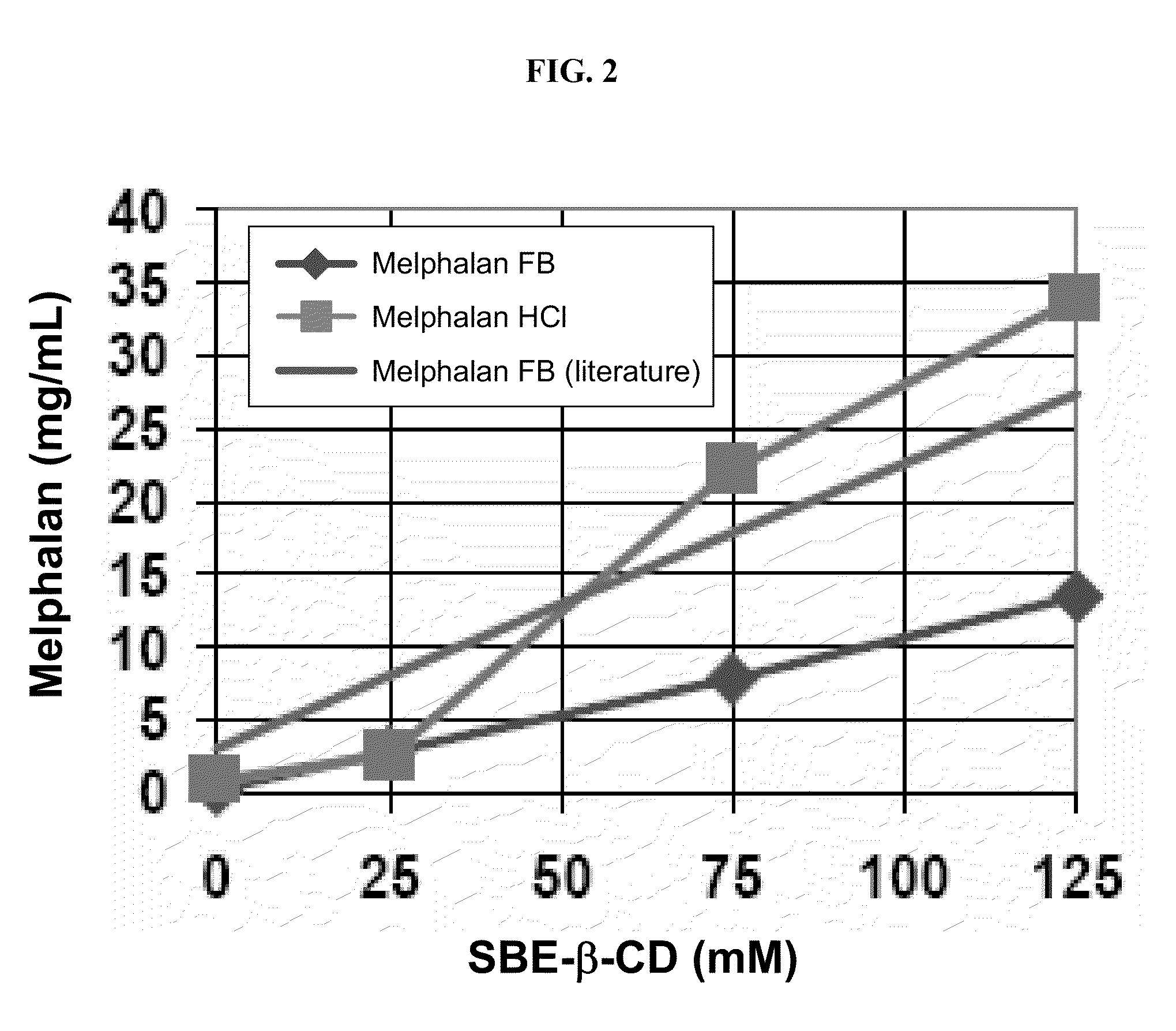
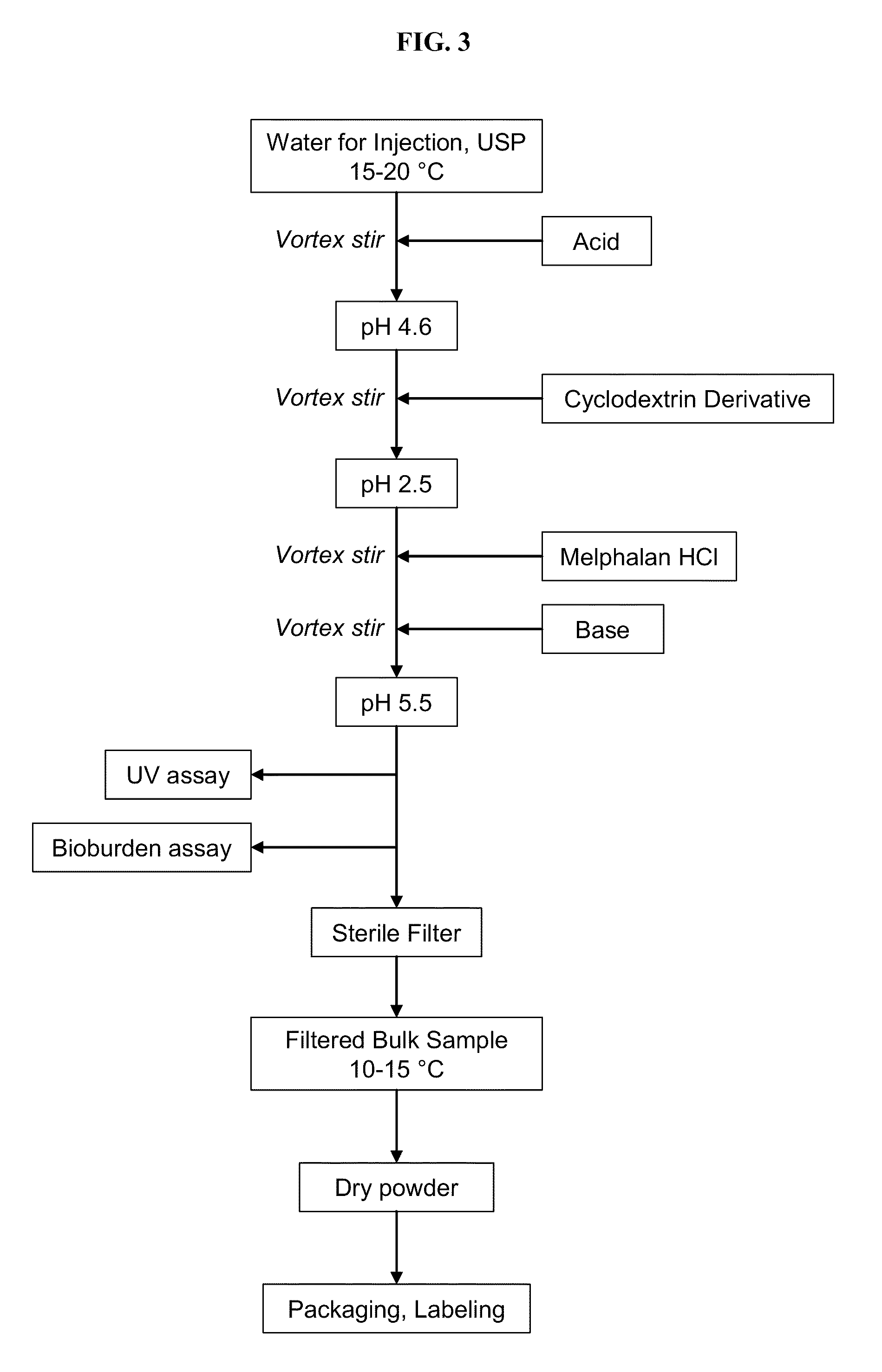
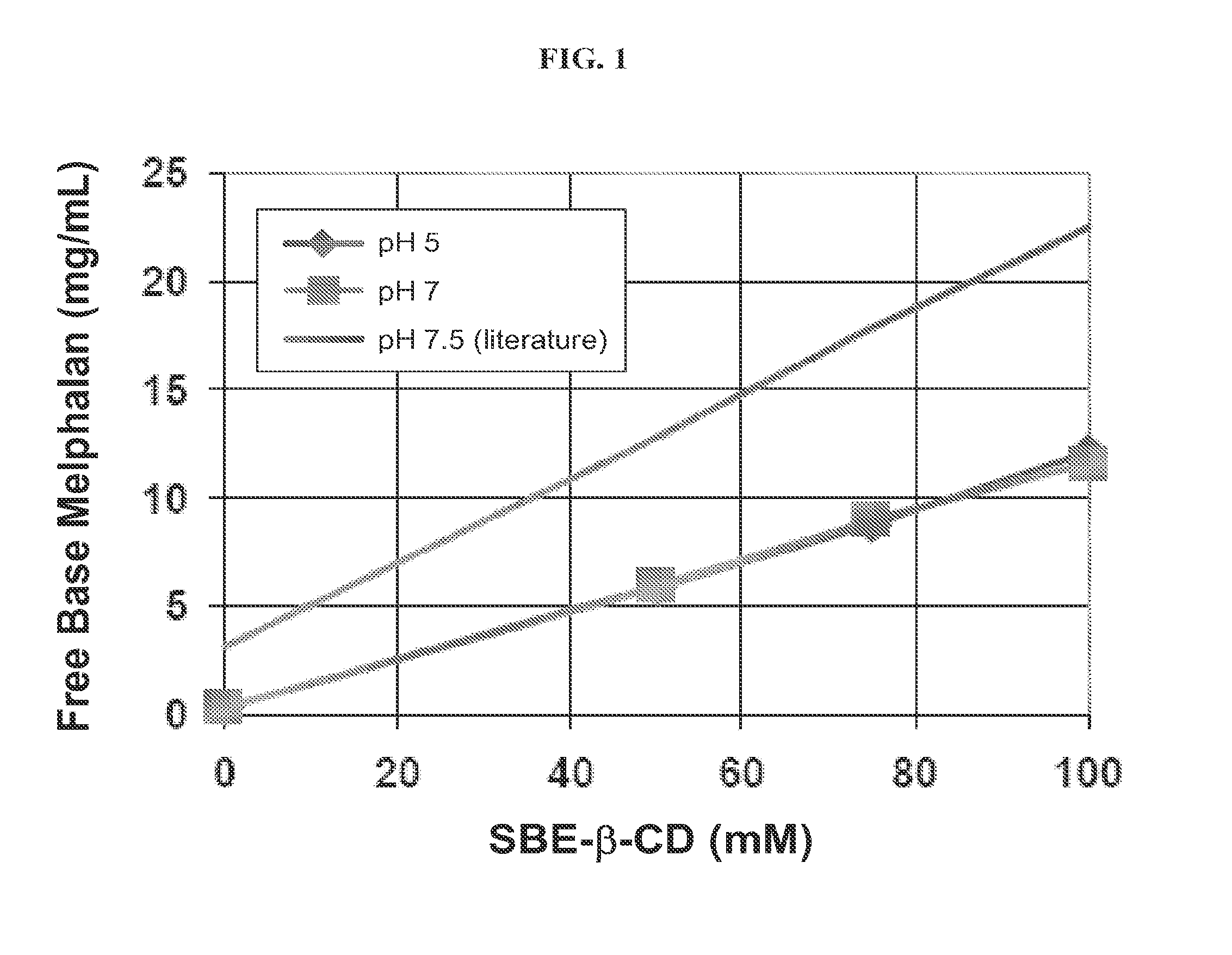
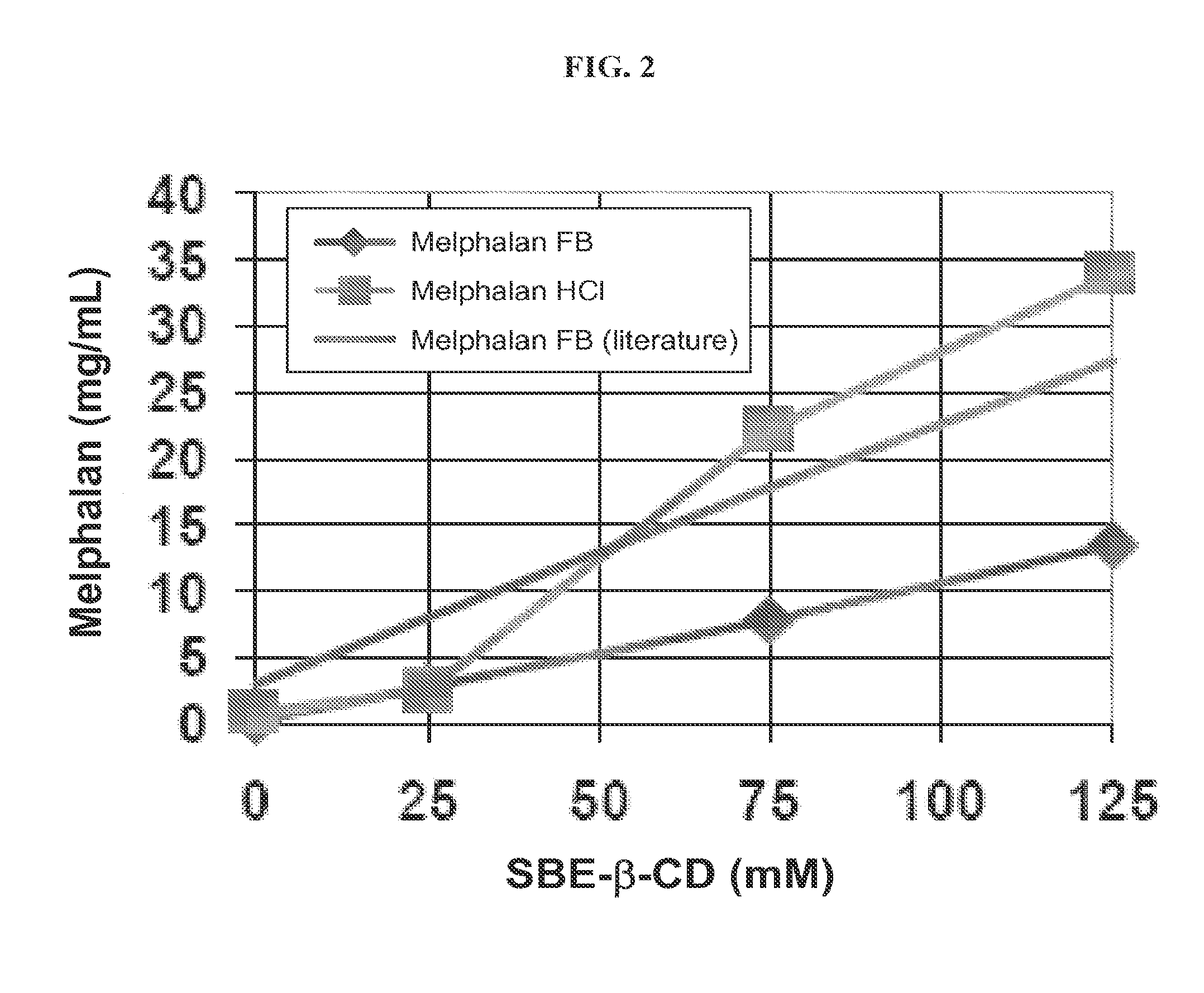
Injectable Melphalan Compositions Comprising a Cyclodextrin Derivative and Methods of Making and Using the Same
InactiveUS20100311838A1Minimize toxicologyMinimize side-effect profileBiocidePeptide/protein ingredientsCyclodextrin derivativeMelphalan
The present invention is directed to pharmaceutical compositions comprising melphalan and a cyclodextrin derivative, and methods of making and using the same.
Owner:CYDEX PHARMACEUTICALS INC
Combinations of therapeutic agents for treating melanoma
ActiveUS9408885B2Less side effectsRemarkable effectHeavy metal active ingredientsOrganic active ingredientsCarboplatinDocetaxel
The present disclosure relates to the field of oncology, more particularly to the field of melanoma. Provided are methods of treating melanoma, particularly advanced cutaneous melanoma, with a combination of pharmaceutical agents comprising MDM4-specific antagonists (such as an inhibitor of the MDM4-p53 interaction or a molecule that decreases MDM4 protein stability) or MDM4-MDM2 dual inhibitors (i.e., molecules that disrupt the interactions between p53 and MDM2 and p53 and MDM4) and one or more chemotherapeutic agents such as for example alkylating agents (i.e., Dacarbazine (DITC) or melphalan), alkylating-like agents (i.e., cisplatin or carboplatin) or mitotic inhibitors (taxanes docetaxel or paclitaxel) and PI3K-AKT, B-RAF and MEK inhibitors. Further provided are pharmaceutical formulations of MDM4-specific antagonists (be it an inhibitor of the MDM4-p53 interaction or a molecule that decreases MDM4 protein stability) or MDM4-MDM2 dual inhibitors (i.e., molecules that disrupt the interactions between p53 and MDM2 and p53 and MDM4) and a pharmaceutical formulation of one or more chemotherapeutic agents such as for example alkylating agents (i.e., Dacarbazine (DITC) or melphalan), alkylating-like agents (i.e., cisplatin or carboplatin) or mitotic inhibitors (taxanes docetaxel or paclitaxel) and B-RAF and MEK inhibitors.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
Melphalan derivatives and their use as cancer chemotherapeutic drugs
The invention refers to new alkylating di- and tripeptides based on a melphalan unit, and one or two additional amino acids or amino acid derivatives, which can be used in the treatment of carcinogenic diseases. Further, the invention refers to a a pharmaceutical composition comprising the alkylating peptides of the invention.
Owner:ONCOPEPTIDES
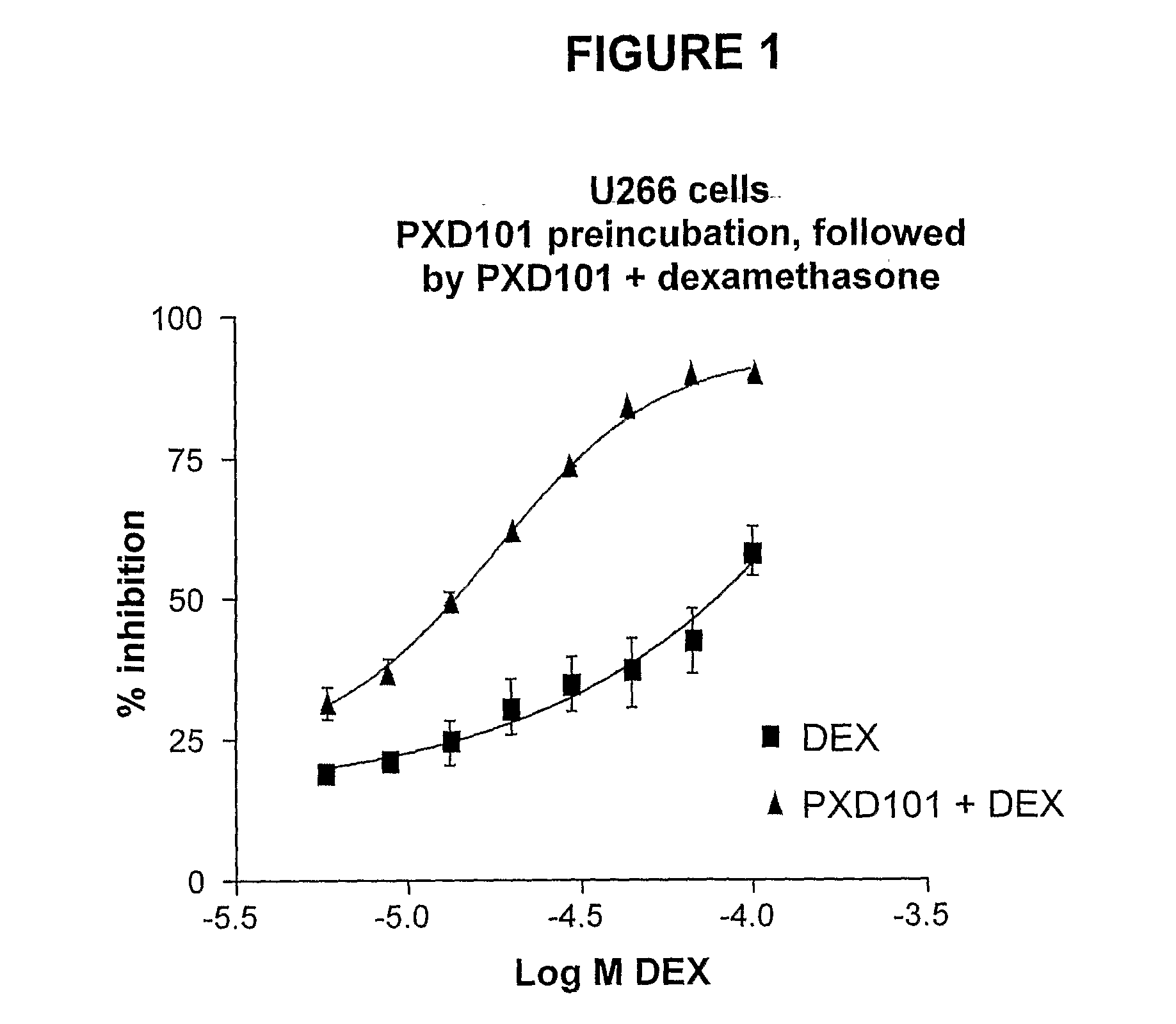
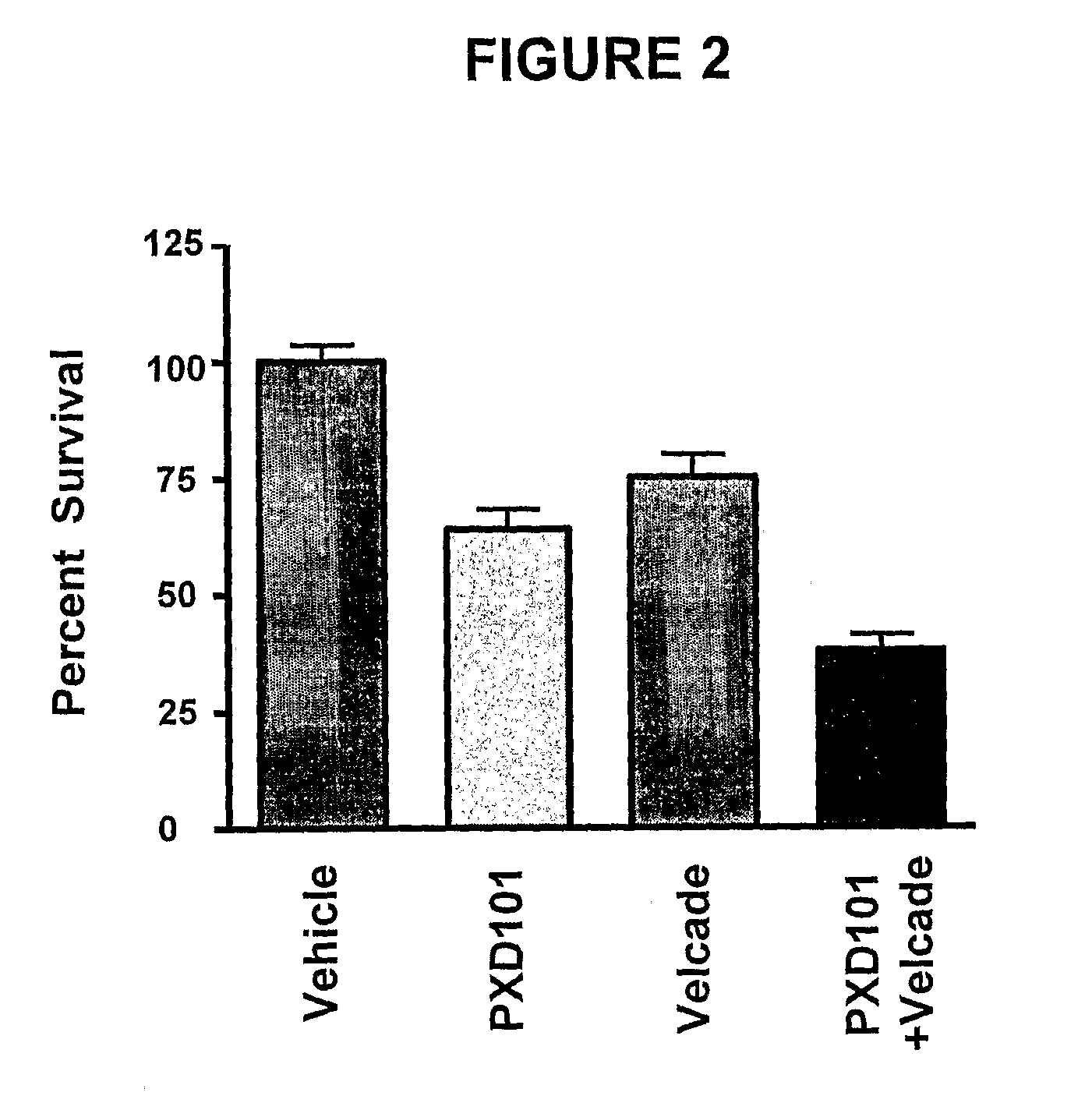
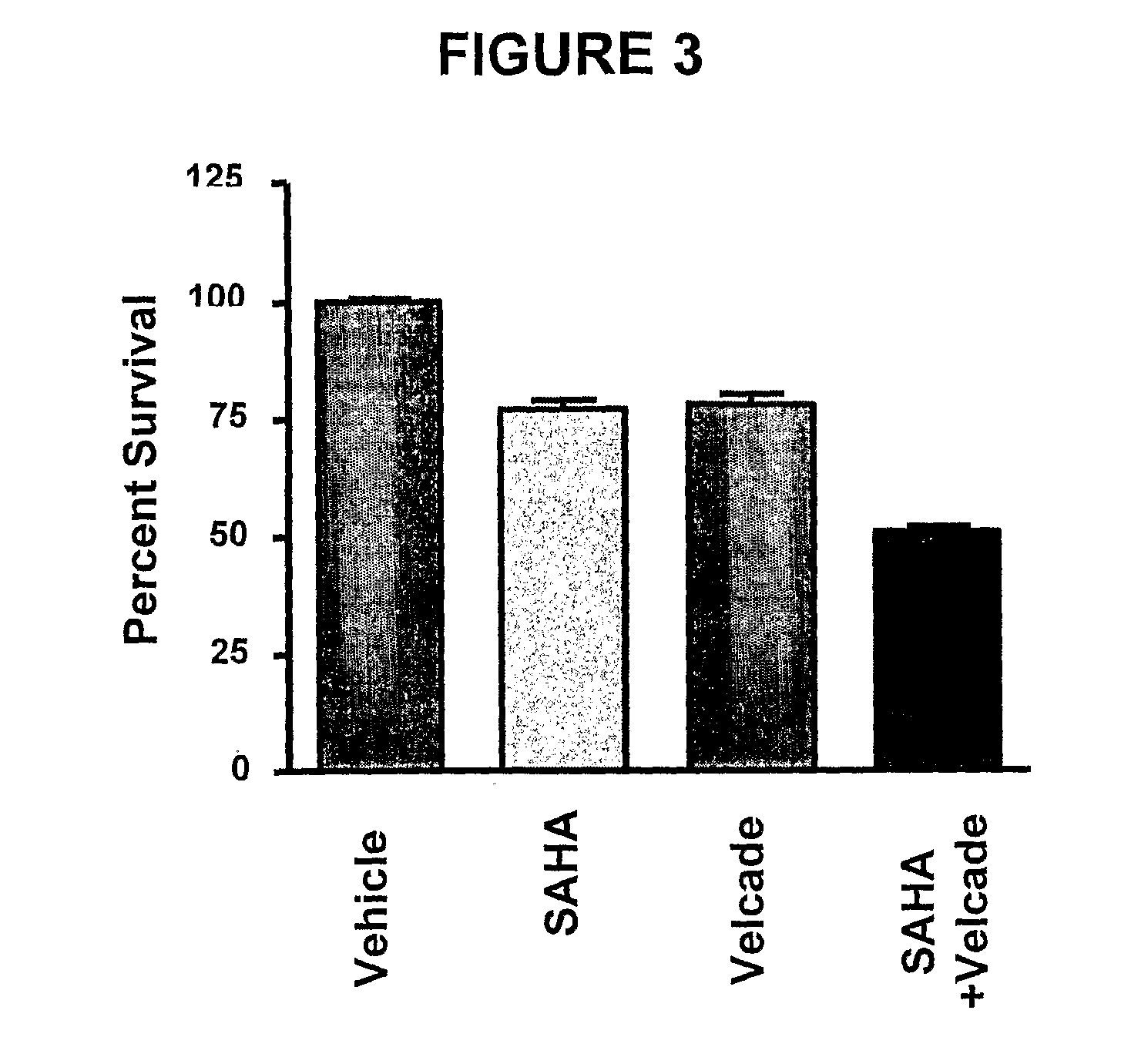
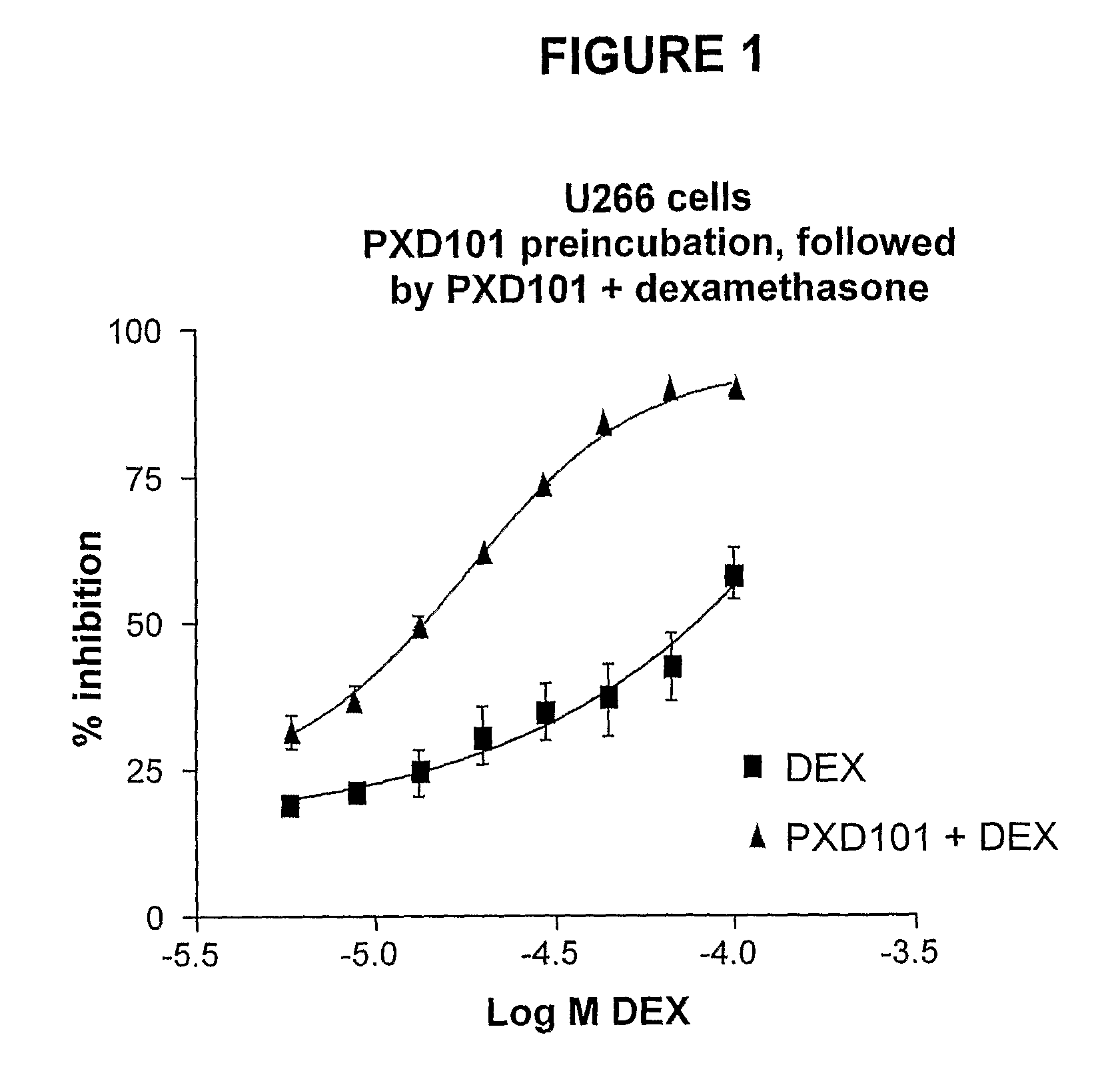
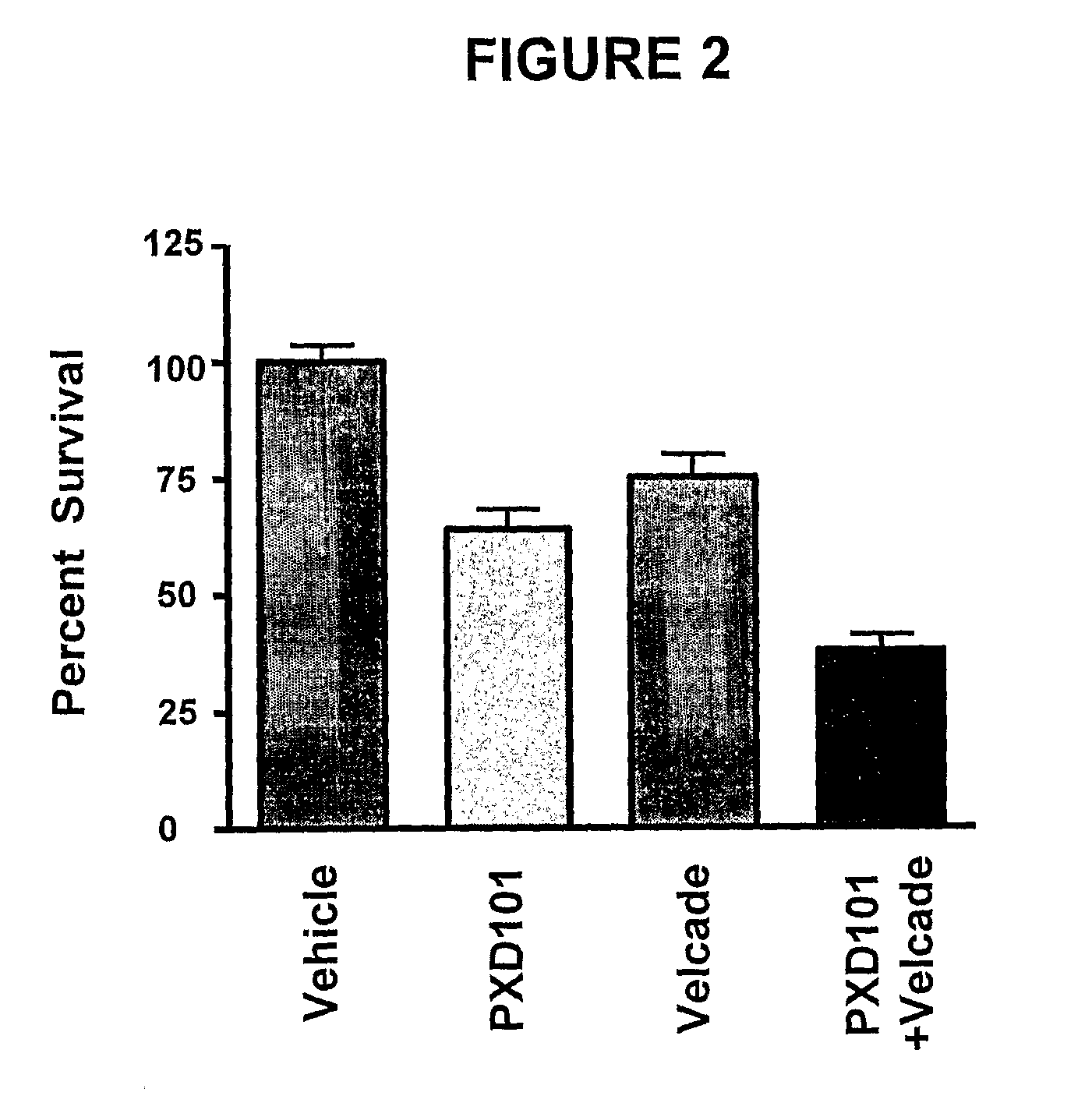
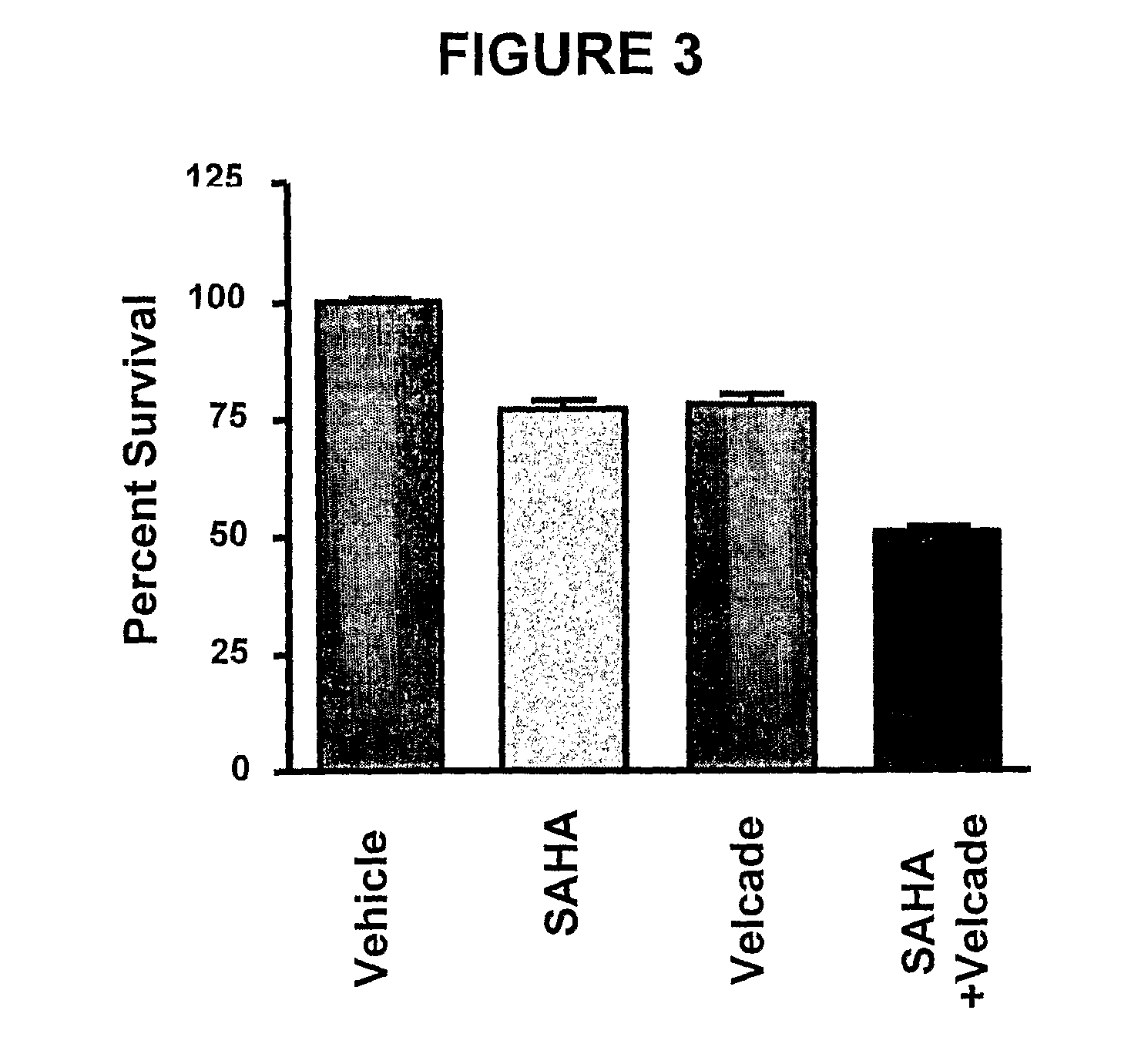
Histone Deacetylase (Hdac) Inhibitors (Pxd101) for the Treatment of Cancer Alone or in Combination With Chemotherapeutic Agent
ActiveUS20080274120A1Reduced characteristicsReduced viabilityBiocidePeptide/protein ingredientsVincristineLeukemia
The present invention relates generally to methods for treating cancer. In one respect, the present invention relates to a method of treating a hematological cancer (e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof a therapeutically effective amount of a histone deacetylase inhibitor, for example, a histone deacetylase (HDAC) inhibitor as described herein, for example, PXD-101. In another respect, the present invention relates to a method of treating cancer (e.g., solid tumour cancer, e.g., rectal cancer, colon cancer, ovarian cancer; hematological cancer, e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof, a first amount of a histone deacetylase (HDAC) inhibitor, for example, a histone deacetylase inhibitor as described herein, for example, PXD-101, and a second amount of an other chemotherapeutic agent, for example, an other chemotherapeutic agent selected from: an antibody against VEGF, Avastin®, an antibody against CD20, rituximab, bortezomib, thalidomide, dexamethasone, vincristine, doxorubicin, and melphalan, wherein the first and second amounts together comprise a therapeutically effective amount.
Owner:TOPOTARGET UK LTD
Solid tumor treating medicine composition
The present invention is solid tumor treating medicine composition and belongs to the field of medicine technology. The medicine composition contains melphalan in effective anticancer amount and medicinal supplementary material. The medicinal supplementary material is mainly biocompatible, degradable and absorbable polymer, and during the degradation and absorption of the polymer, melphalan is released slowly to local tumor part to lower the systemic toxic reaction and to maintain local medicine concentration. The present invention has enhanced treating effect. The solid tumor includes cerebral tumor, liver cancer, lung cancer, esophagus cancer, gastric cancer, etc.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
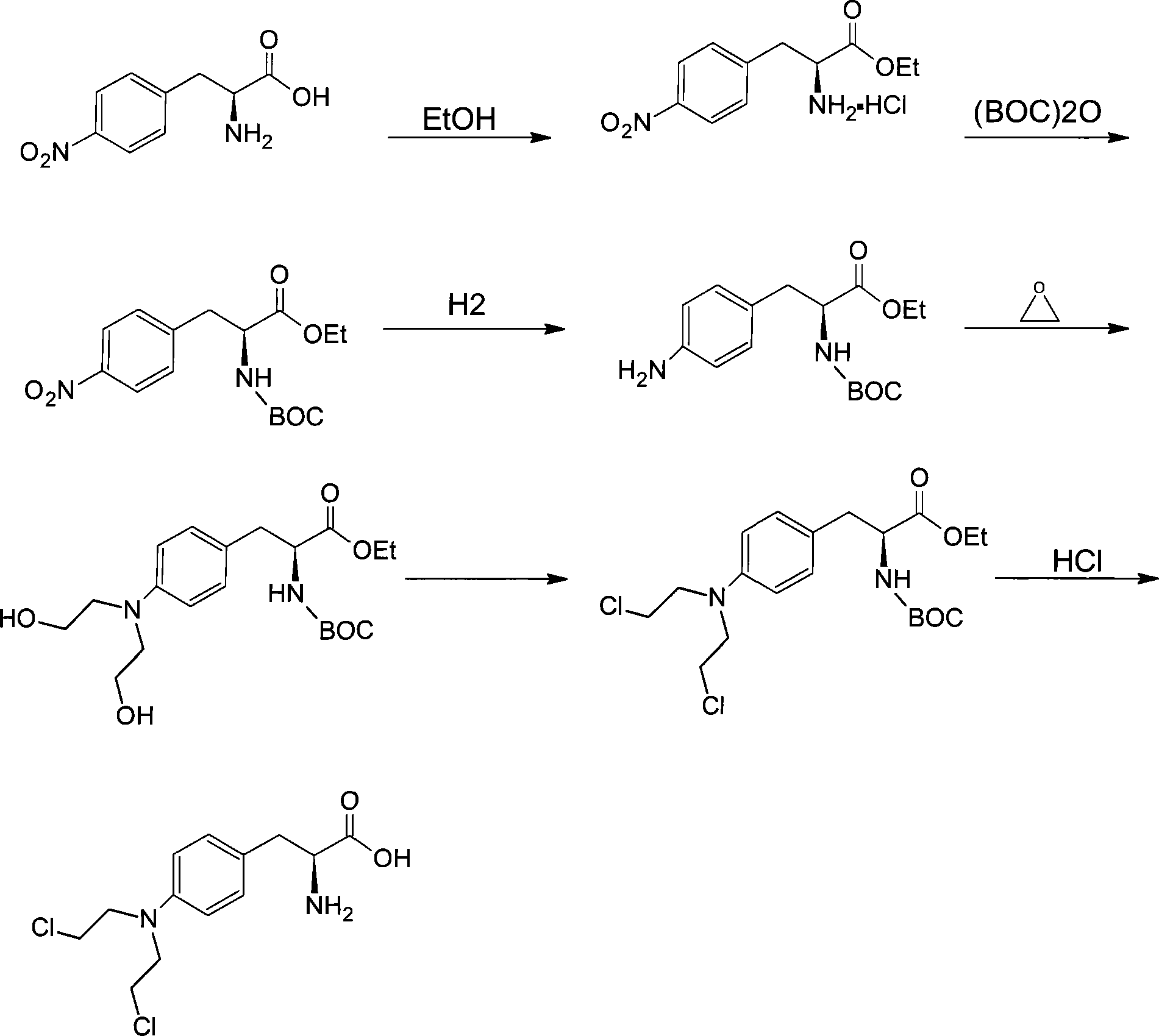
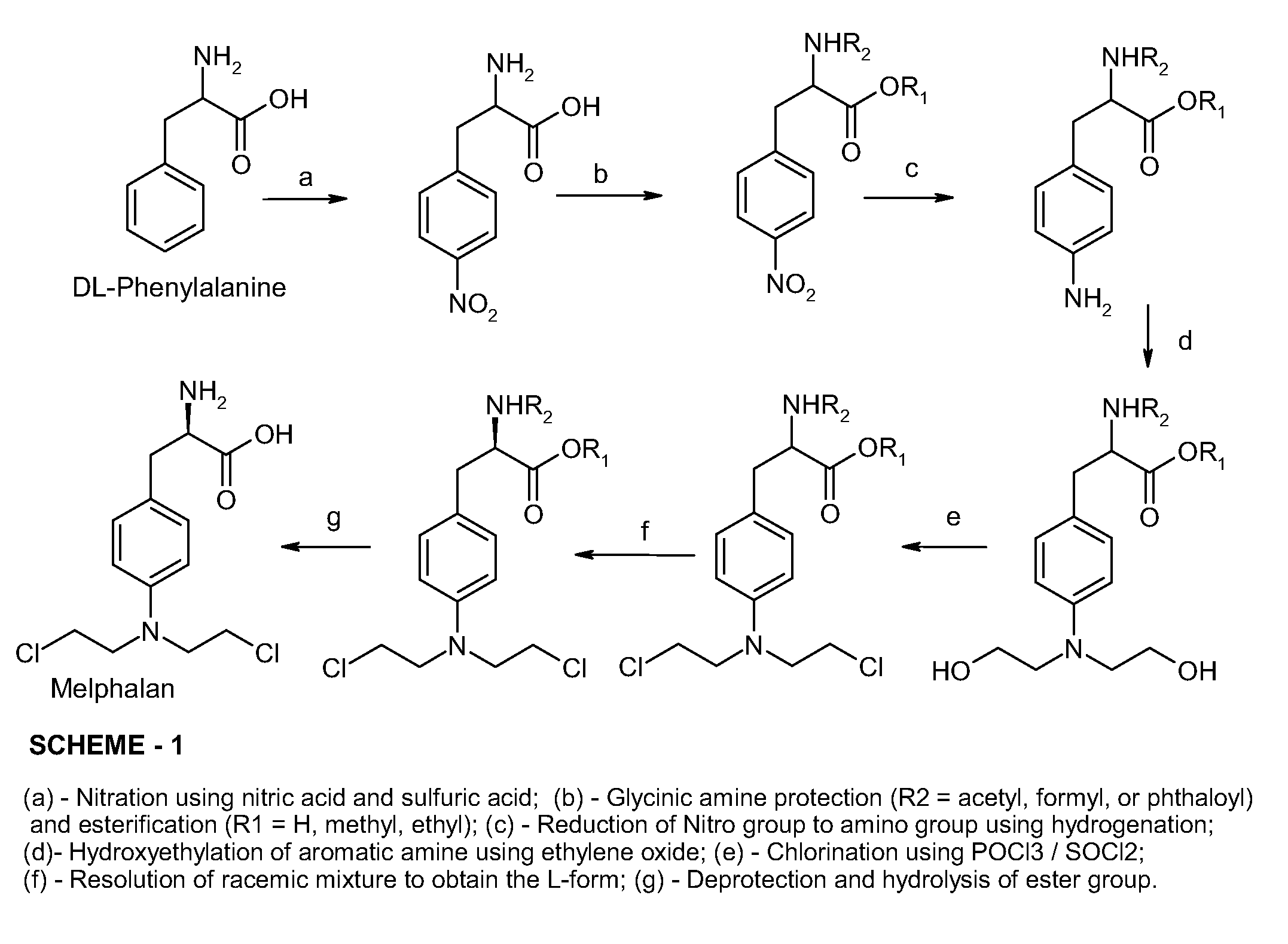
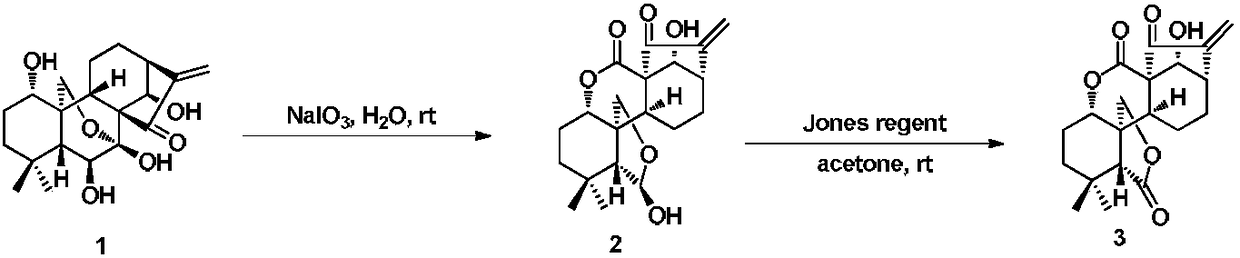
Technique for synthesizing antineoplastic melphalan
InactiveCN101100440AStable in natureFast reduction reactionOrganic compound preparationAmino-carboxyl compound preparationAlcoholEsterification reaction
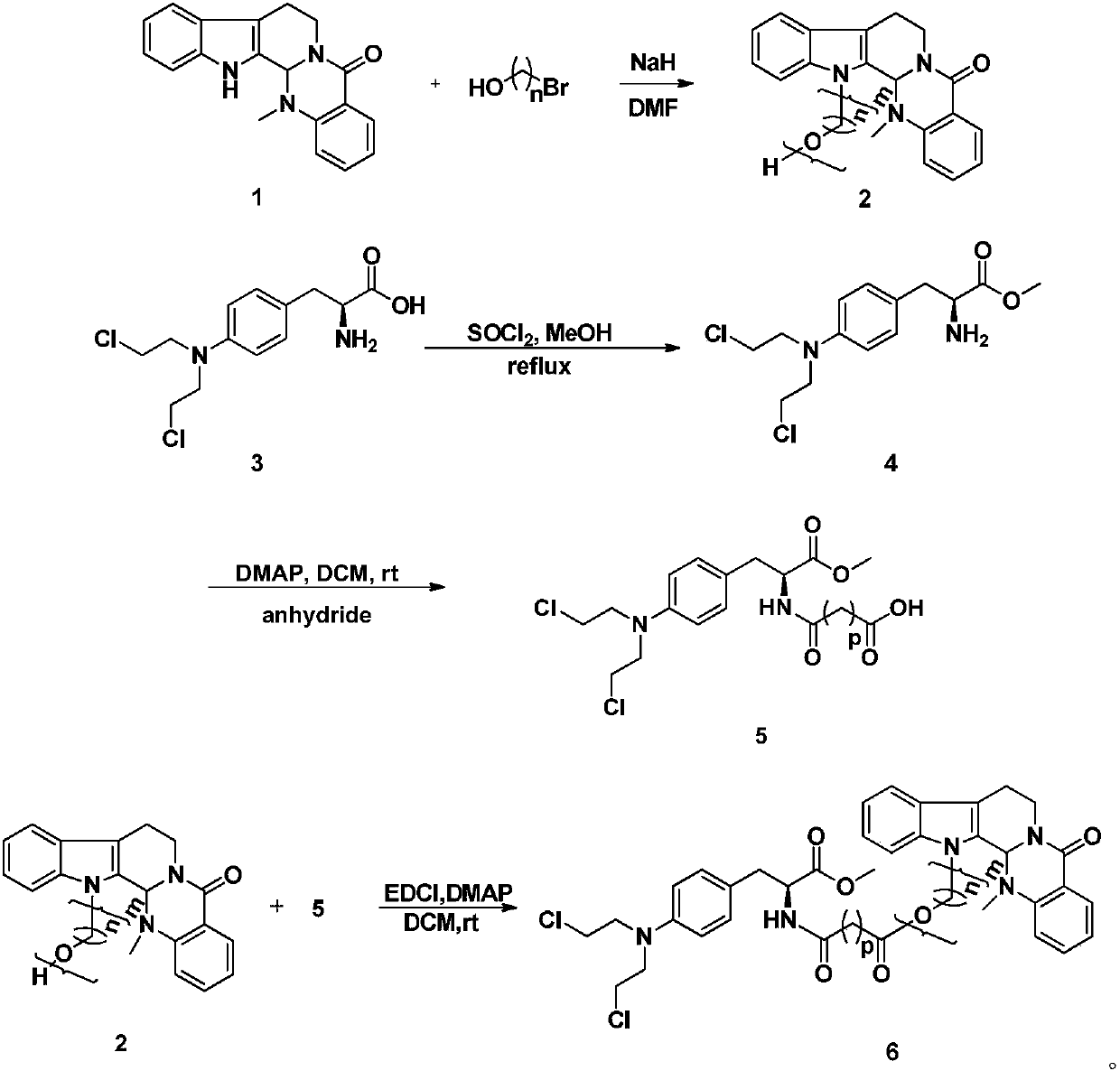
Synthesis of antineoplastic Melphalan is carried out by esterification reacting, amino-protection reacting, hydrogenation reducing, hydroxyethylation reacting, chlorination reacting, de-protection reacting, taking chlorinating agent, absolute alcohol protecting carboxyl and pyrocarbonic di-tert-butyl protecting amino, hydrolyzing by hydrochloric acid to obtain final product. It's cheap, efficient and non-toxic, has gentle reactive condition and can be used for industrial production. It's cheap, convenient, efficient and non-toxic and can be used for industrial production.
Owner:SUZHOU LEADER CHEM
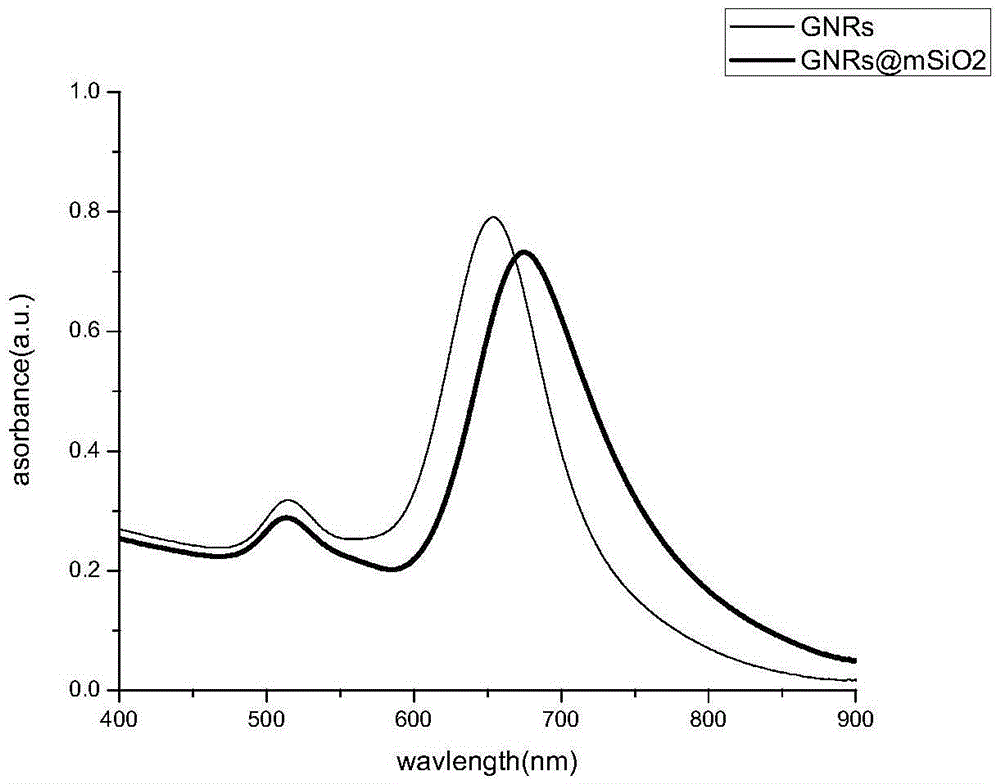
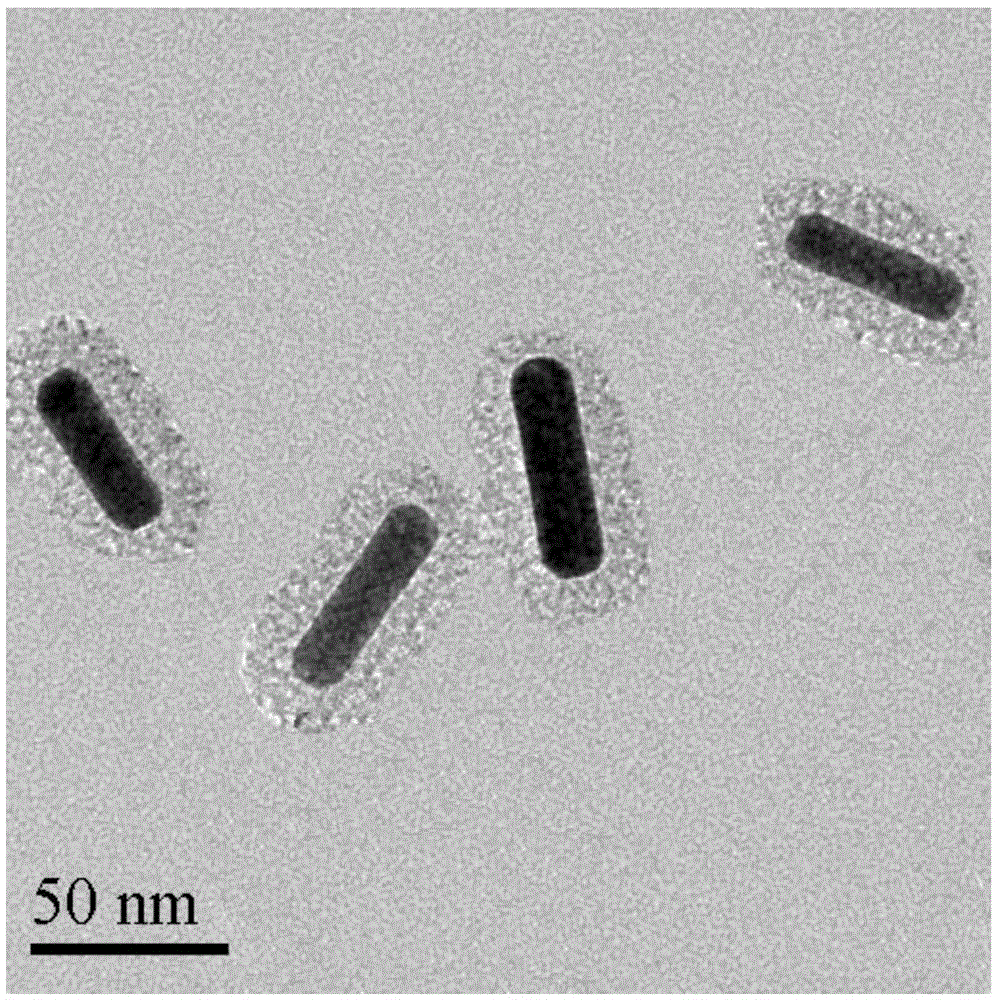
Preparation method of gold nano-composite targeting drug delivery system
InactiveCN105617392AAchieving Combined TherapyGood treatment effectOrganic active ingredientsEnergy modified materialsMesoporous silicaSilicon oxide
The invention discloses a preparation method of a gold nano-composite targeting drug delivery system. The preparation method comprises the following steps: sequentially adding a chloroauric acid solution and a silver nitrate solution into a hexadecyl ammonium bromide solution, mixing uniformly, then sequentially adding an ascorbic acid solution and a gold nano-particle seed solution, and centrifuging to obtain a gold nano-bar; adding ethyl orthosilicate to obtain a mesoporous silicon oxide nano-material; adding 3-aminopropyl triethylsilane to obtain aminated GNRs@mSiO2; adding a thionyl chloride solution of melphalan to obtain aminated GNRs@mSiO2-MEL; and adding HA-RGD to obtain GNRs@mSiO2-HA-RGD-MEL. According to the preparation method, gold nano-particles with an effect of photothermal therapy are effectively combined with an anti-cancer drug, namely melphalan, thereby realizing combined therapy of tumor thermotherapy and chemotherapy and increasing the therapeutic effect; and by utilizing mesoporous silicon oxide ducts for efficiently loading drugs, the drug loading capacity can be increased.
Owner:WUHAN UNIV OF TECH
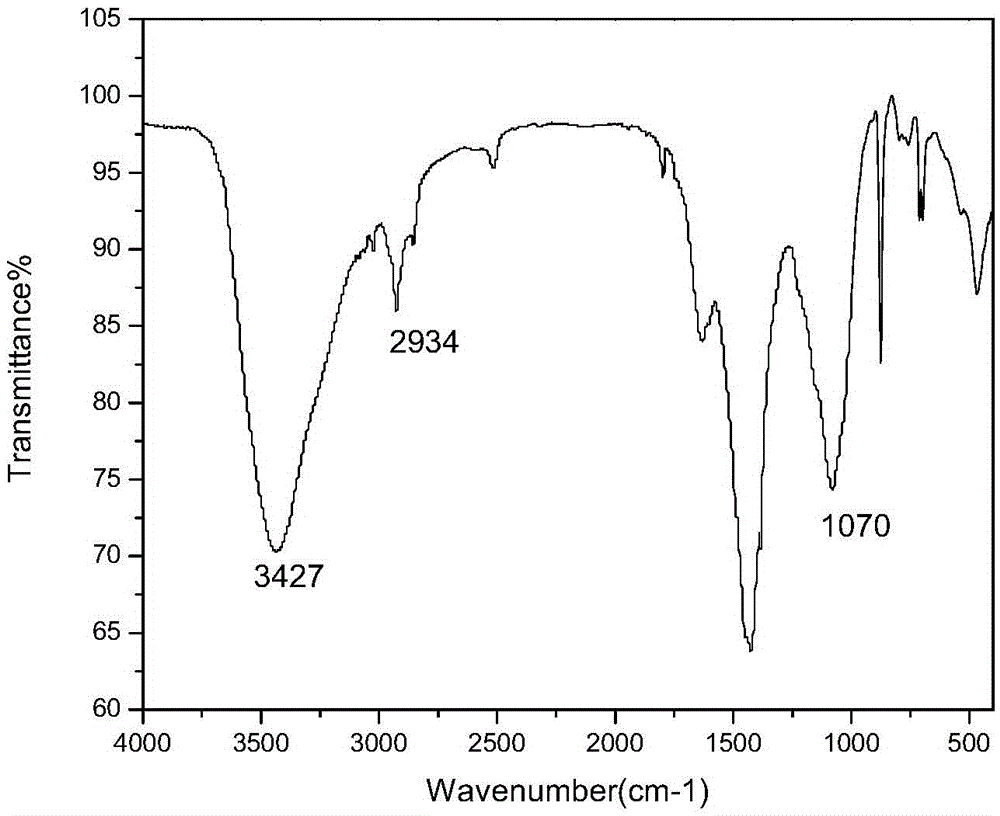
Preparation method of targeted graphene oxide binary medicine loading composite modified by HA/RGD
InactiveCN105641710ABeneficial to subsequent cellsFacilitate animal researchOrganic active ingredientsMaterial nanotechnologyFreeze-dryingFunctional modification
The invention discloses a preparation method of a targeted graphene oxide binary medicine loading composite modified by HA / RGD. The method includes the following steps of conducting functional modification on graphene oxide through ADH to obtain aminated graphene oxide GO-ADH, coupling an METH connection bridge to HA through an amido bond in a covalent mode, conducting dialyzing and freeze-drying after stirring is conducted for 4-6 hours to obtain HA-METH, adding the obtained GO-ADH to obtain GO-HA-METH, adding sulfhydrylated RGD to obtain a GO-HA-RGD composite, dropwise adding a solution containing melphalan and a solution containing adriamycin, and conducting centrifuging, washing and freeze-drying to obtain MEL / DOX-GO-HA-RGD. The preparation method is easy to operate and mild in experimental condition. The whole carrier system is high in medicine loading amount, long-acting slow release can be achieved, pH sensitivity is achieved, the release rate under the low-pH-value environment is high, and the whole carrier system is suitable for micro environments of tumor tissue and has important clinical significance.
Owner:WUHAN UNIV OF TECH
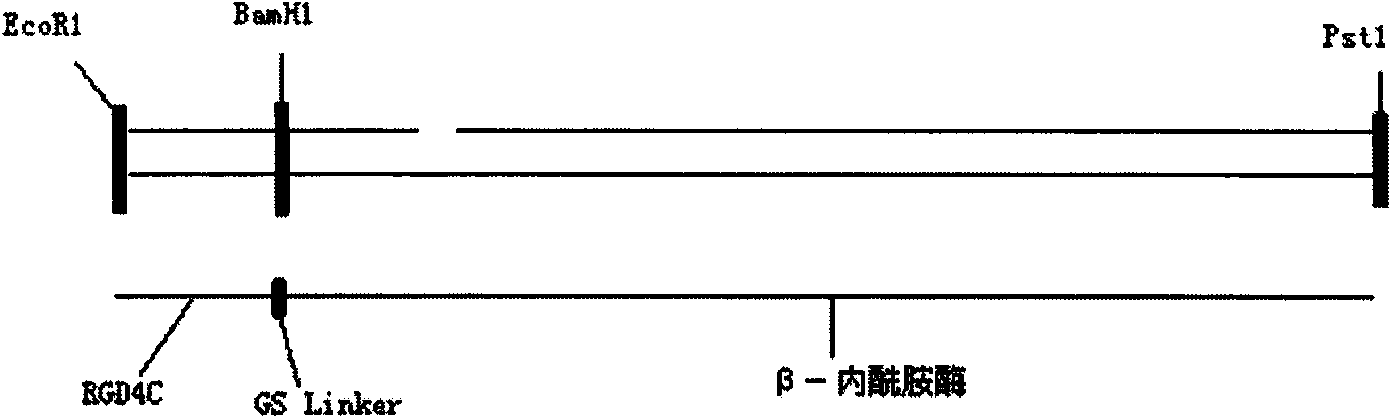
Recombinant beta-lactamase-RGD-fusion protein and application thereof in medicine
InactiveCN101824406AActiveHas cell adhesion activityPeptide/protein ingredientsMicroorganism based processesCell adhesionAmino acid
The invention discloses a beta-lactamase-RGD-fusion protein and a preparation method thereof. The amino acid sequence of the recombinant beta-lactamase-RGD-fusion protein comprises a protein sequence which is derived from beta-lactamase and a sequence which comprises short peptide RGD. The recombinant beta-lactamase-RGD-fusion protein not only has the structure and the function of the beta-lactamase, but also has cell adhesion activity, so that the fusion protein can be positioned at the place of the tumor, thereby having better target. The beta-lactamase-RGD-fusion protein can be used for the treatment of the antibody guide enzyme prodrug, can be used for treating a series of solid tumors such as breast cancer and the like jointly with the cephalosporins prodrug, and has better effect than single melphalan and low toxicity.
Owner:天津天颐科苑科技有限公司
Methods of Treating Multiple Myeloma
PendingUS20220062415A1Conducive to survivalReduce riskInorganic non-active ingredientsBoron compound active ingredientsAntiendomysial antibodiesPharmaceutical drug
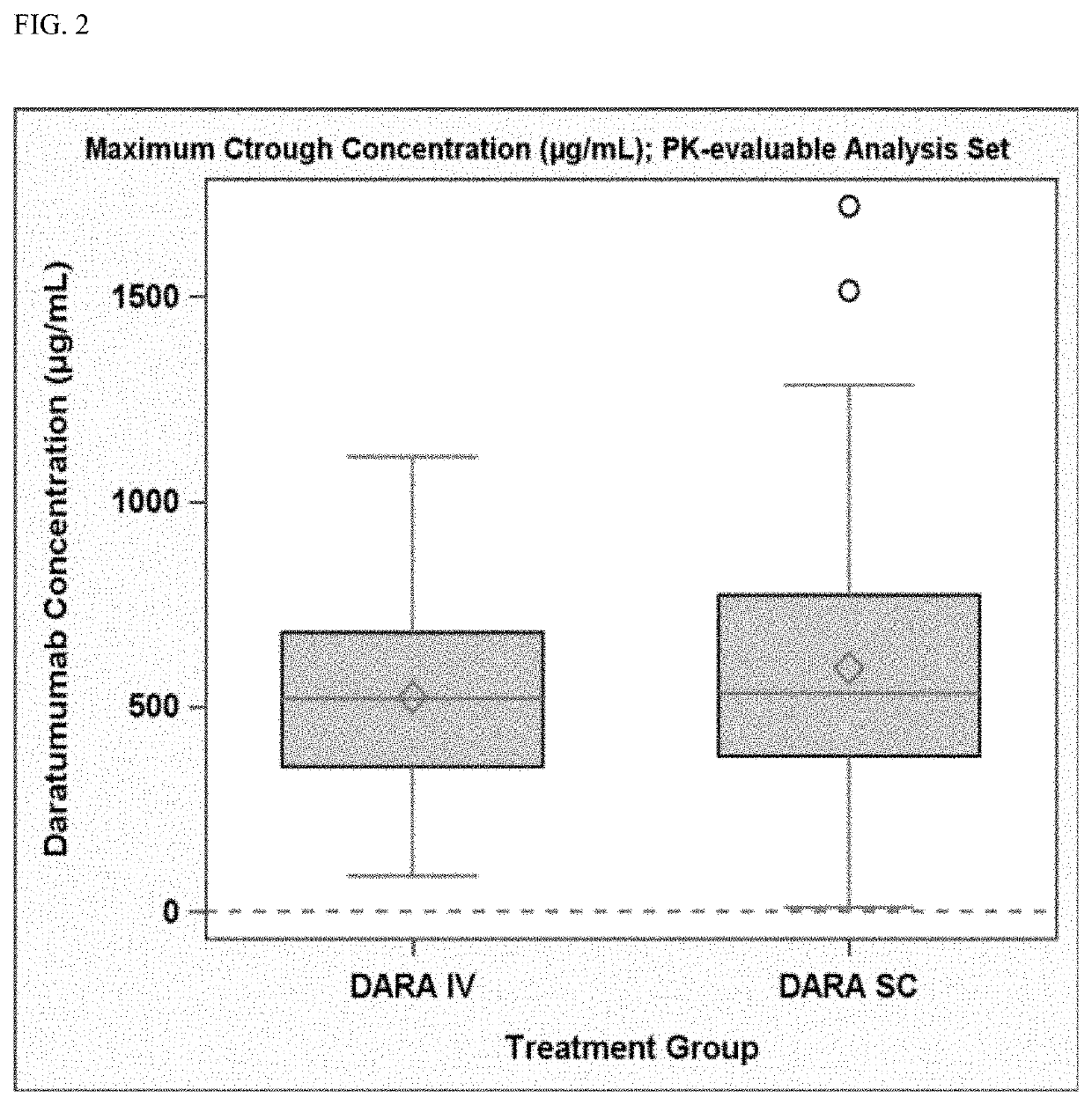
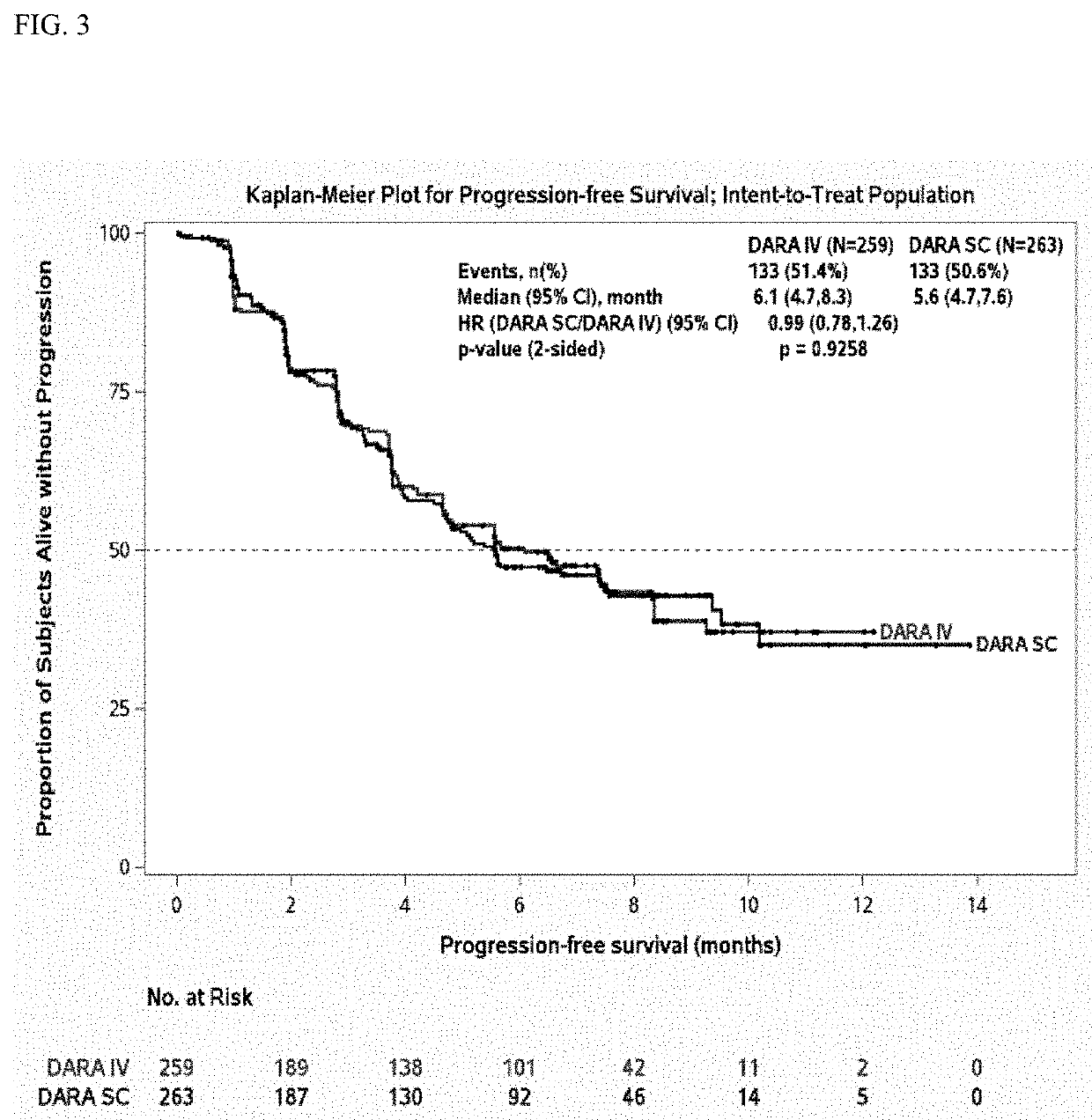
Described herein are methods of treating multiple myeloma with clinically proven safe and effective amounts of an antibody that specifically recognizes CD38 with bortezomib, melphalan, and prednisone. Also described are methods of selling or offering for sale an antibody that specifically recognizes CD38 or pharmaceutical compositions thereof with bortezomib, melphalan, and prednisone.
Owner:JANSSEN BIOTECH INC
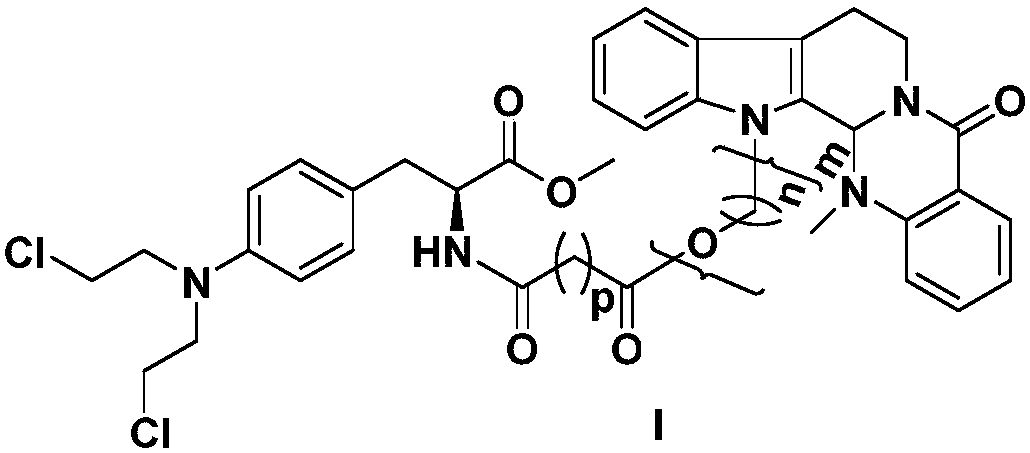
Chlormethine fructus evodiamine derivatives, and preparation method and application thereof
ActiveCN107602557AImprove pharmacological activityHigh activityOrganic active ingredientsOrganic chemistryDNANatural medicine
The invention relates to the fields of natural medicines and medicinal chemistry, and provides chlormethine fructus evodiamine derivatives, and a preparation method and application thereof. In particular, the invention relates to a preparation method for introducing DNA alkylating agent melphalan derivatives at N-13 site of evodiamine, and application to preparation of anti-tumor medicines. The structure of the evodiamine splicing melphalan derivatives and pharmaceutically acceptable salt is shown in a general formula I shown in the description, wherein n, m and p are described in the claims and the description.
Owner:SHENYANG PHARMA UNIVERSITY
Receptor-mediated quantum dot tracing targeted drug delivery system, preparation method thereof and application
InactiveCN102743762AIncrease drug concentrationAchieving passive targetingOrganic active ingredientsGenetic material ingredientsPolyethylene glycolMelphalan
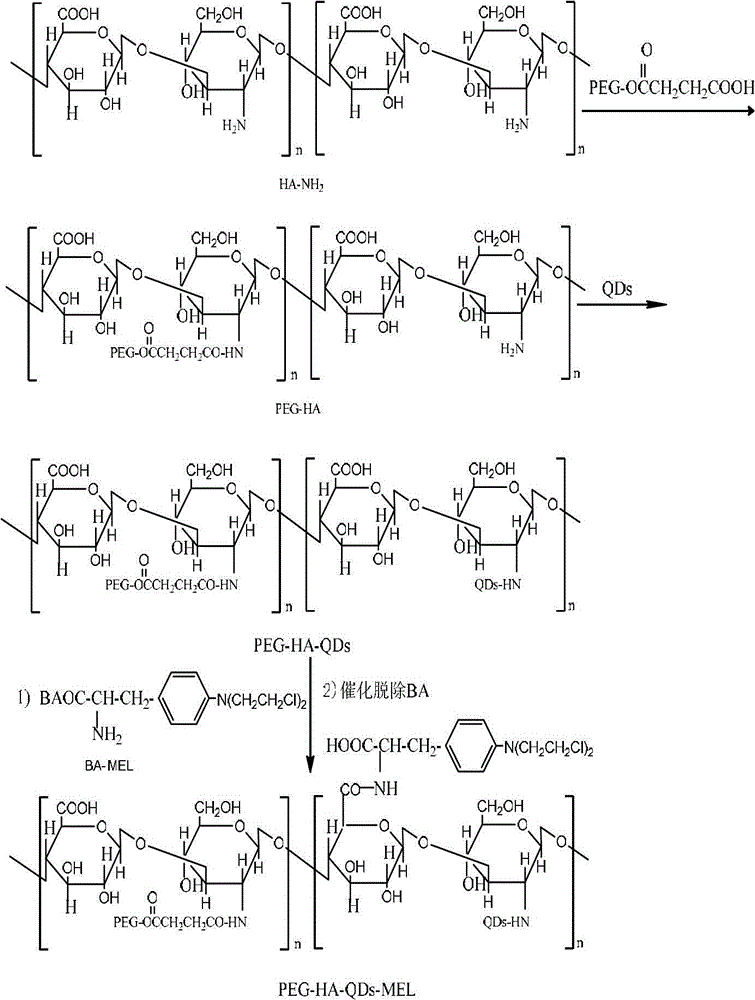
Disclosed are a receptor-mediated quantum dot tracing targeted drug delivery system, a preparation method thereof and application. The targeted drug delivery system comprises polyethylene glycol, hyaluronic acid, quantum dots and melphalan complex which are abbreviated as PEG-HA-QDs-MEL. The PEG is connected with HA amide in a dewatered and condensed manner, the water-soluble CdTe / CdS quantum dots are connected into the HA, and finally, the PEG, the HA and the QDs are prepared with the MEL complex to obtain the targeted drug delivery system. The component content of the PEG-HA-QDs-MEL includes, by weight, 30-100 parts of the MEL, 600-1500 parts of the PEG, 200-500 parts of the HA, 5-50 parts of the CdTe / CdS quantum dots, 20-50 parts of DCC (dicyclohexylcarbodiimide), 1-10 parts of DMAP (dimethylamino-pyridine), 100-500 parts of succinic anhydride, 20-50 parts of NHS, 20-50 parts of EDC (carbodiimide) and 10-100 parts of sodium bisulfite. The targeted drug delivery system is applied to preparing antitumor drugs.
Owner:WUHAN UNIV OF TECH
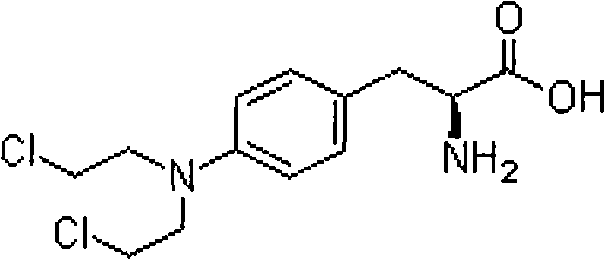
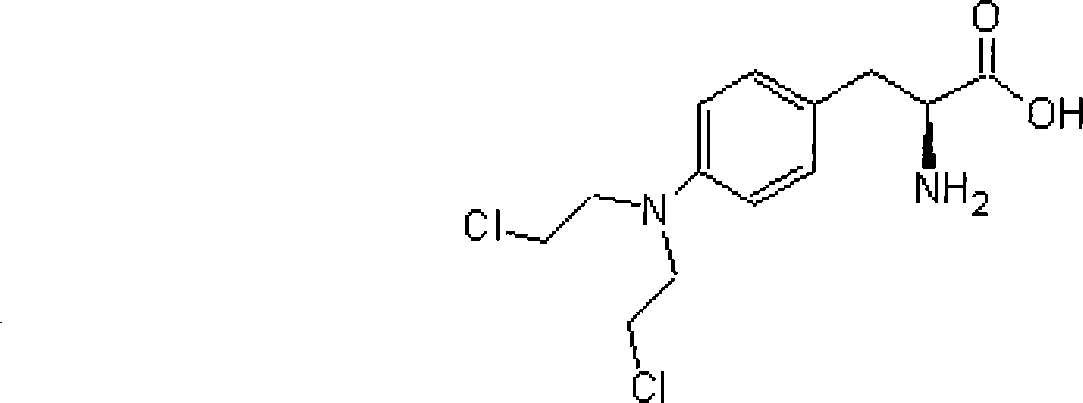
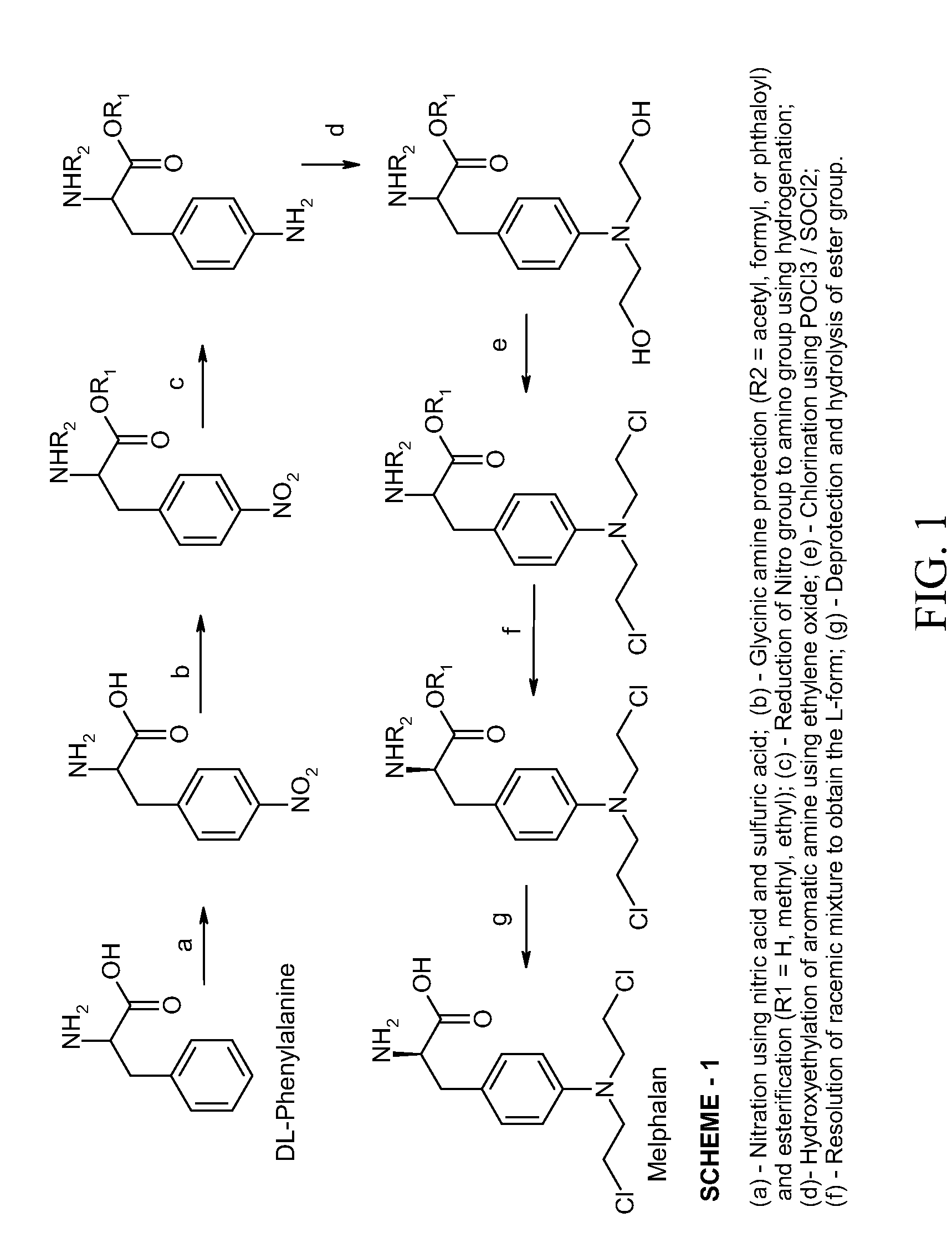
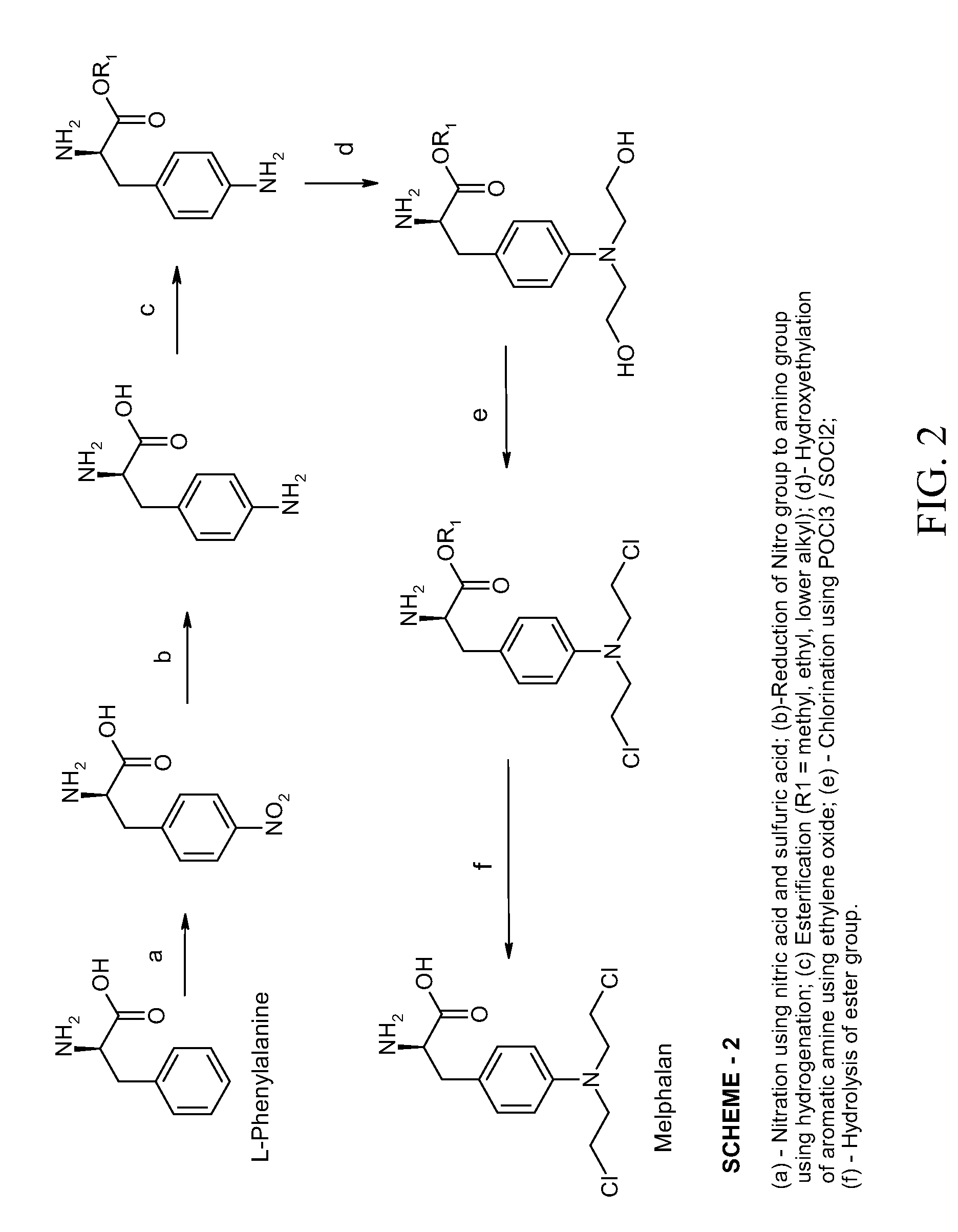
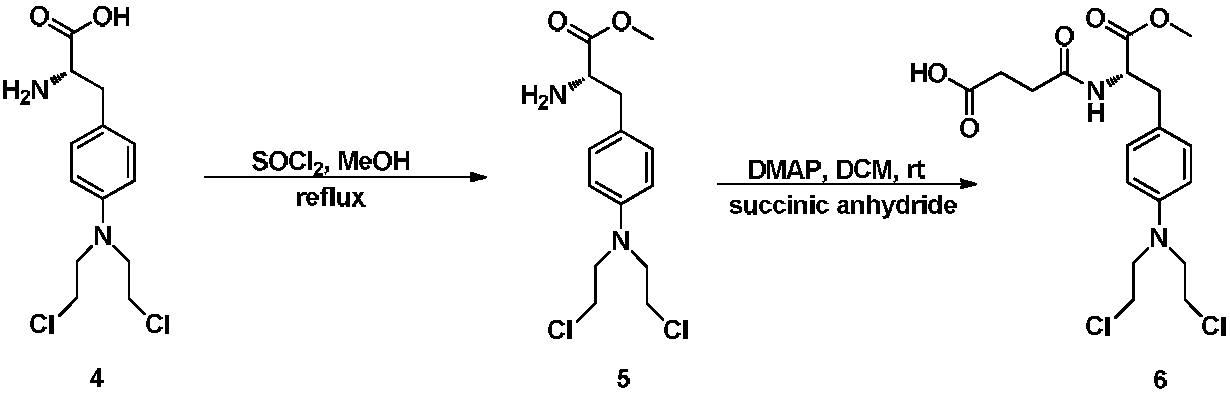
Process Of Making Optically Pure Melphalan
ActiveUS20090240074A1Organic compound preparationAmino-carboxyl compound preparationRegioselectivityPhenylalanine
This invention provides a process of making 4-(bis-(2-hydroxyethyl)amino)-L-phenylalanine of the formulaby hydroxyethylation, in a regioselective manner, of the aromatic amino group rather than the glycinic amino group.
Owner:NAVINTA
Injectable Melphalan Compositions Comprising a Cyclodextrin Derivative and Methods of Making and Using the Same
ActiveUS20140221488A1Minimize toxicology and side-effect profileImprove bioavailabilityBiocidePeptide/protein ingredientsCyclodextrin derivativeMelphalan
The present invention is directed to pharmaceutical compositions comprising melphalan and a cyclodextrin derivative, and methods of making and using the same.
Owner:CYDEX PHARMA INC
Methods for the Effective Treatment of Metastatic Cancer
InactiveUS20150265641A1Avoid survivalReduced survivalBiocideDipeptide ingredientsCancer cellOxidative stress
The present invention relates to methods for the treatment of metastatic cancer. Methods of treating metastatic cancer by administration of a set of drugs overcome multiple mechanisms of melphalan resistance and hypersensitize cancer cells to melphalan are described. The methods involve the administration of drug(s) that induce oxidative stress in cancer cells, in conjunction with melphalan on a defined schedule.
Owner:GENERAL ONCOLOGY INC
Temperature controlled sustained-release injection comprising alkyl agent and method for preparing the same
InactiveCN101273962APharmaceutical delivery mechanismPharmaceutical non-active ingredientsWater insolubleWhole body
The invention relates to a temperature-controlled sustained-release injection containing an alkylating agent and a preparation method thereof, the temperature-controlled sustained-release injection comprises effective anti-cancer amount of the alkylating agent, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained angiogenesis inhibitor to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months. The sustained-release gel injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug and being used for the treatment of the tumors in different stages. The alkylating agent is selected from cyclophosphamide, melphalan, leukeran, 4H-cyclophosphamide peroxide, norcantharidin, mannosulfan, treosulfan, ritrosulfan, ethoglucid, pipobroman, piposulfan, pumitepa, uredepa, azatepa and so on.
Owner:SHANDONG LANJIN PHARMA +1
Method for inactivating gonadotrophs
InactiveUS20050277582A1Easy to transportImprove solubilityPeptide/protein ingredientsDepsipeptidesGeloninPseudomonas aeruginosa exotoxin A
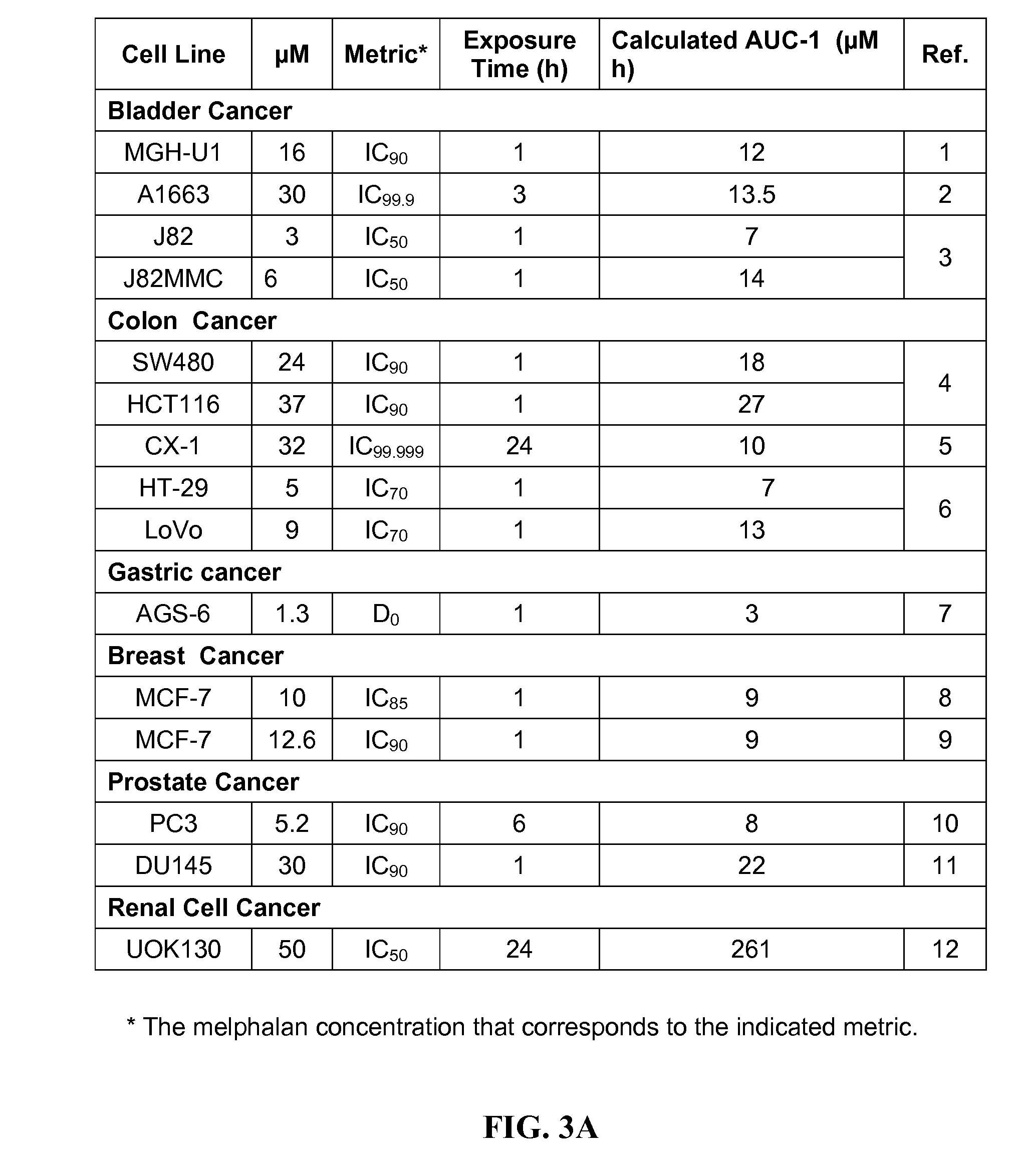
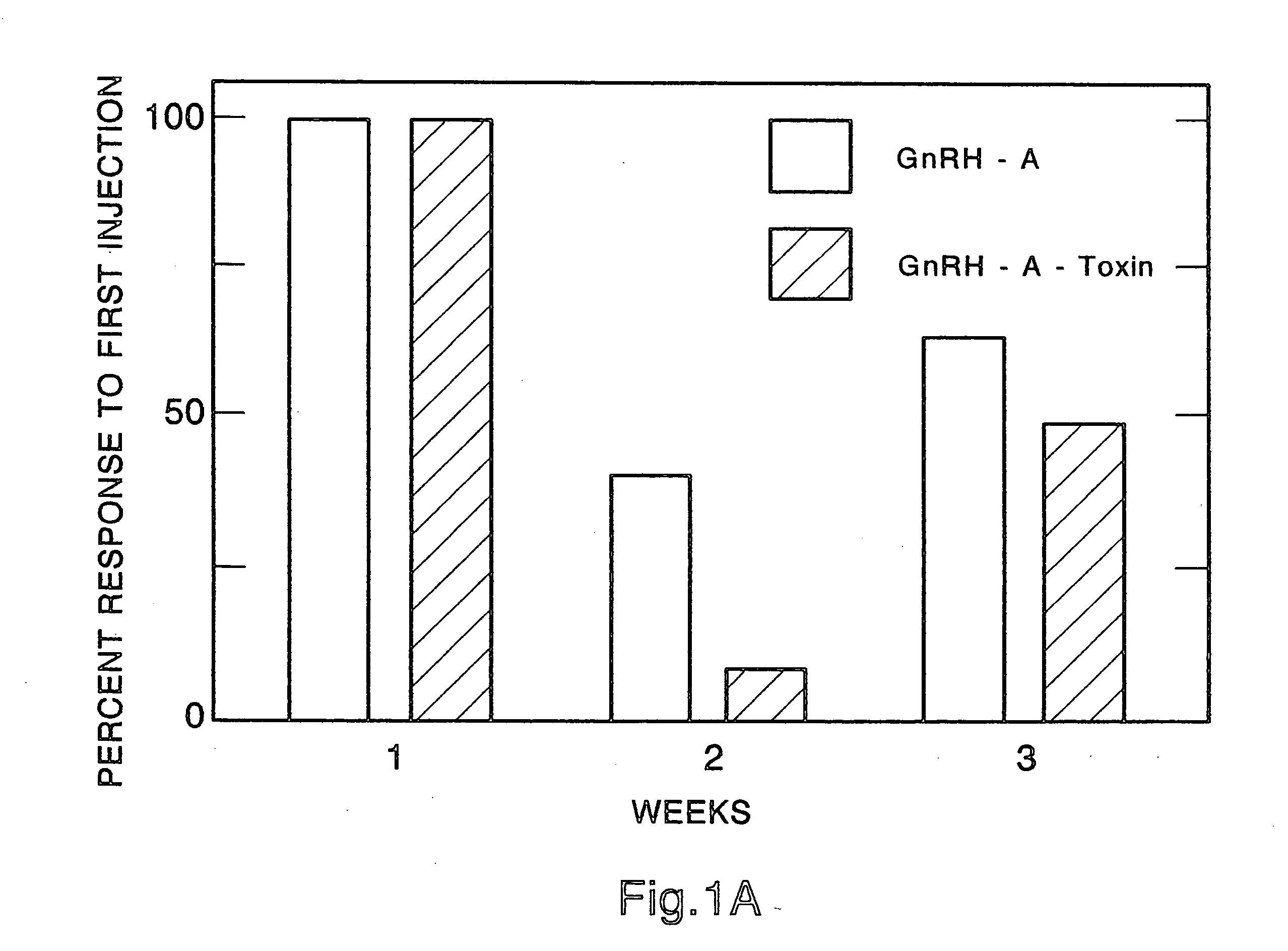
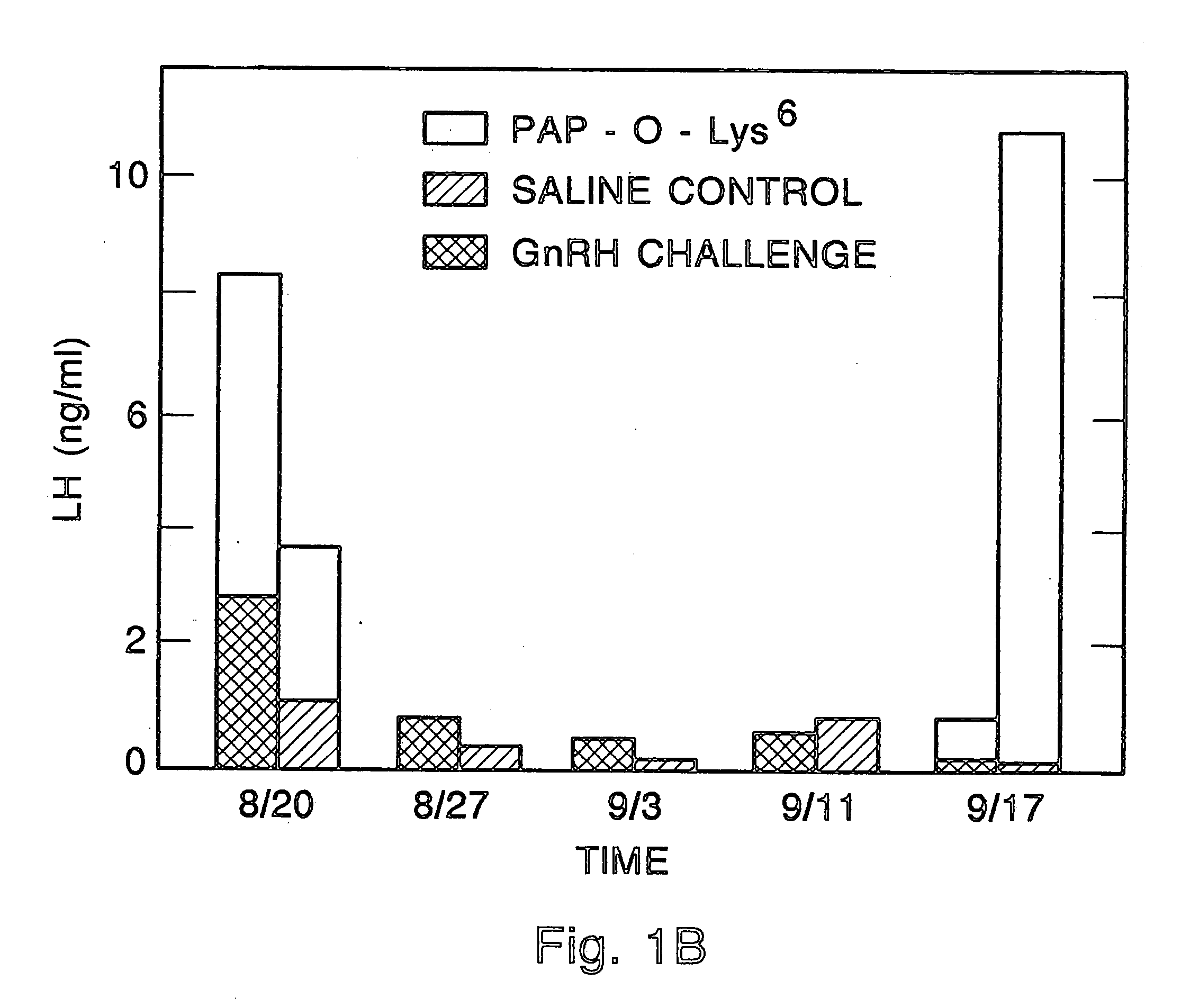
Certain toxic compounds (T) such as, for example, compounds based upon diphtheria toxin, ricin toxin, pseudomonas exotoxin, .alpha.-amanitin, pokeweed antiviral protein (PAP), ribosome inhibiting proteins, especially the ribosome inhibiting proteins of barley, wheat, corn, rye, gelonin and abrin, as well as certain cytotoxic chemicals such as, for example, melphalan and daunomycin can be conjugated to certain analogs of gonadotropin-releasing hormone to form a class of compounds which, when injected into an animal, destroy the gonadotrophs of the animal's anterior pituitary gland. Hence such compounds may be used to sterilize such animals and / or to treat certain sex hormone related diseases.
Owner:NETT TORRANCE +3
Enmein diterpene-melphalan derivative as well as preparation method and application thereof
ActiveCN108101925AHigh activityImprove pharmacological activityOrganic active ingredientsOrganic chemistryA-DNADiterpene
The invention relates to the fields of natural drugs and medicinal chemistry, relates to an enmein diterpene-melphalan derivative as well as a preparation method and application thereof and specifically relates to a preparation method of a melphalan derivative with a DNA alkylating agent induced on a 14-OH or 6, 14-OH site in a mother nucleus structure of enmein diterpene and application of the melphalan derivative to an antitumor drug. The structures of enmein diterpene-melphalan derivative and pharmaceutically acceptable salt thereof are shown as the general formula I or the general formulaII, wherein n is described as the claims and the specification.
Owner:SHENYANG PHARMA UNIVERSITY
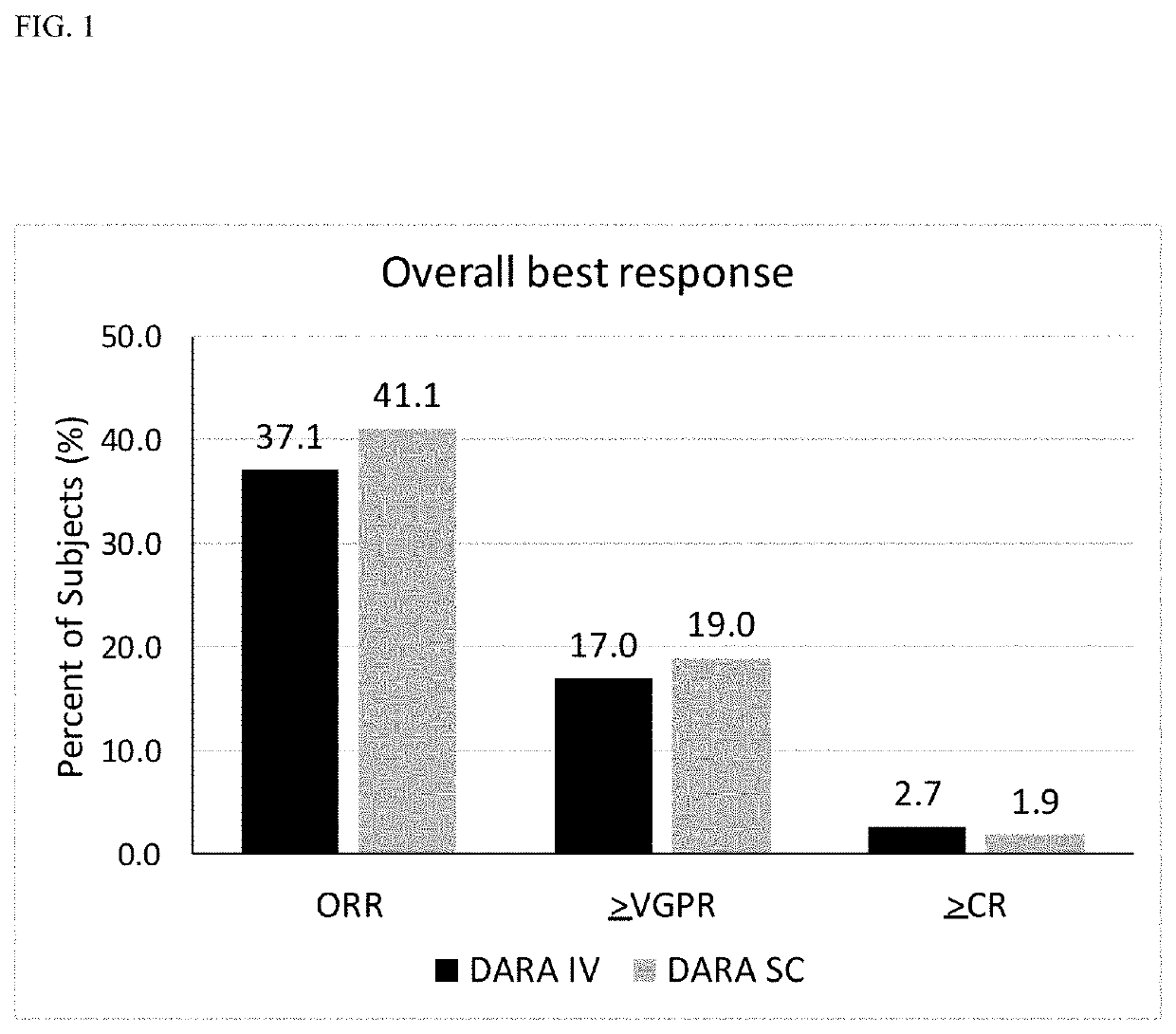
Clinically Proven Subcutaneous Pharmaceutical Compositions Comprising Anti-CD38 Antibodies and Their Uses in Combination with Bortezomib, Mephalan and Prednisone
InactiveUS20200330593A1Peptide/protein ingredientsPharmaceutical delivery mechanismAntiendomysial antibodiesPharmaceutical drug
The present invention relates to clinically proven subcutaneous pharmaceutical compositions comprising anti-CD38 antibodies and methods of their uses in combination with bortezomib, melphalan and prednisone.
Owner:JANSSEN BIOTECH INC
Histone deacetylase (HDAC) inhibitors (PXD101) for the treatment of cancer alone or in combination with chemotherapeutic agent
The present invention relates generally to methods for treating cancer. In one respect, the present invention relates to a method of treating a hematological cancer (e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof a therapeutically effective amount of a histone deacetylase inhibitor, for example, a histone deacetylase (HDAC) inhibitor as described herein, for example, PXD-101. In another respect, the present invention relates to a method of treating cancer (e.g., solid tumor cancer, e.g., rectal cancer, colon cancer, ovarian cancer; hematological cancer, e.g., multiple myeloma, leukemia, lymphoma) comprising administering to a patient in need thereof, a first amount of a histone deacetylase (HDAC) inhibitor, for example, a histone deacetylase inhibitor as described herein, for example, PXD-101, and a second amount of an other chemotherapeutic agent, for example, an other chemotherapeutic agent selected from: an antibody against VEGF, AVASTIN® (bevacizumab), an antibody against CD20, rituximab, bortezomib, thalidomide, dexamethasone, vincristine, doxorubicin, and melphalan, wherein the first and second amounts together comprise a therapeutically effective amount.
Owner:TOPOTARGET UK LTD
Combination For The Effective Treatment Of Metastatic Cancer In Patients
ActiveUS20180338935A1Increase chanceLong-term and durable CRsHydroxy compound active ingredientsAmide active ingredientsHemolysisCancer cell
The present invention relates to methods and sets of drugs for the effective treatment of metastatic cancer and the administration of a set of drugs that overcome mechanisms of resistance to DNA-damaging agents, thereby sensitizing cancer cells to said DNA-damaging agents. The methods involve the administration of a set of drugs comprising melphalan, BCNU, hydroxocobalamin, and ascorbic acid. In a preferred embodiment, ethanol is also added to the set of drugs and bone marrow toxicity is reversed with an infusion of bone marrow stem cells. The methods also involve the depletion of GSH in tumors and the selective delivery of drugs to solid tumors. The methods also involve preventing the loss of catalase function and preventing oxidant-induced hemolysis and / or methemoglobin formation in subjects treated with oxidant drugs or agents that generate hydrogen peroxide, wherein said methods comprise the systemic administration of ethanol.
Owner:GENERAL ONCOLOGY INC
Antineoplastic conjugates of transferin, albumin and polyethylene glycol
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R*H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Medicine composition for treating tumors and application thereof
ActiveCN103933044AImprove tumor treatment effectLow toxicityOrganic active ingredientsAntineoplastic agentsTumor therapyPharmaceutical medicine
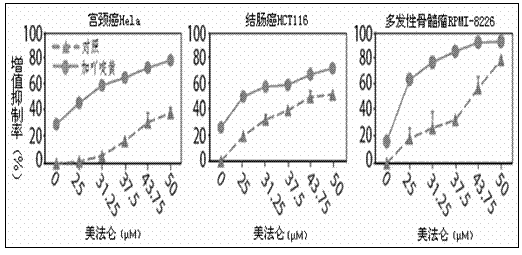
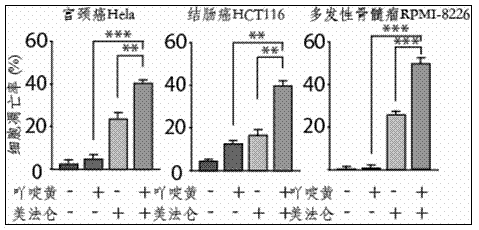
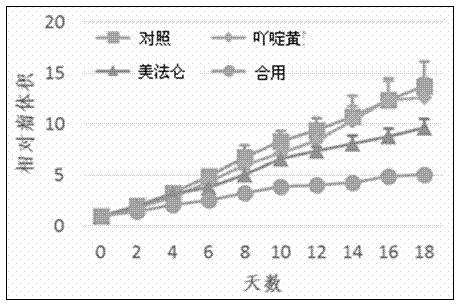
The invention provides a medicine composition for treating tumors and an application thereof. The medicine composition is composed of acriflavine or pharmaceutically acceptable salts thereof, melphalan or pharmaceutically acceptable salts thereof, and a medicinal carrier, wherein the molar ratio of the acriflavine to the melphalan is 1:100 to 1:10, and the tumors comprise blood tumors and solid tumors. The forms of the medicine comprise single preparations prepared from acriflavine or melphalan respectively, or a compound preparation prepared from acriflavine and melphalan. The medicine composition provided by the invention has the effects of improving the curative effect on tumors, the toxicity of the medicine composition can be reduced, and the growth speeds of the tumors can be slowed down.
Owner:ZHEJIANG UNIV
Anticancer sustained-release preparation loaded with anti-cancer medicine and synergist thereof
InactiveCN101380308AGood treatment effectLow toxicitySolution deliveryEmulsion deliveryGoserelinSuspending Agents
An anticarcinogenic slow release formulation carrying an anticancer drug and a synergist is a slow release injection or a slow release implant, and the slow release injection is made from slow release microspheres and a dissolvant. The slow release microspheres comprise anticancer active components and a slow adjuvant, and the dissolvant is a special dissolvant containing a suspending agent which is selected from sodium carboxymethyl cellulose and the like, and the viscosity of the suspending agent is 80cp-3,000cp (at room temperature). The anticancer active components are alkylating agents such as melphalan, ifosfamide and the like, purine analogues such as O6-BG and the like, and / or hormones anticancer drugs selected from triptorelin, goserelin, leuprorelin and a composition selected from epothilone (A-F) and derivatives thereof; the slow release adjuvant is chosen from one of or the copolymer or the mixture of polylactic acid and a copolymer thereof, polifeprosan and the copolymer or the mixture of polylactic acid and sebacic acid copolymer; the slow release implant and the slow release injection are injected or put in tumors or around the tumors, which is beneficial to diffusing the drug in the solid tumors, maintaining high concentration, reducing drug tolerance, being capable of mutual synergy and enhancing curative effects of chemotherapy and / or radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com