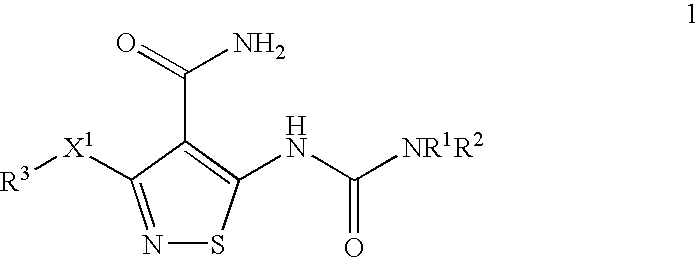
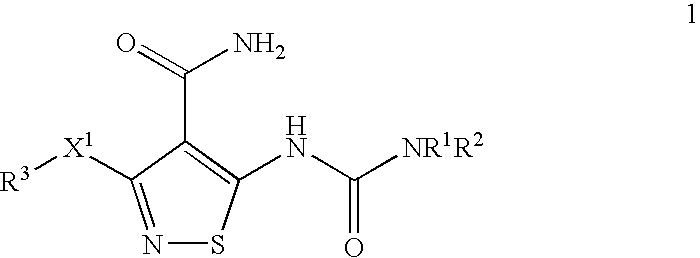
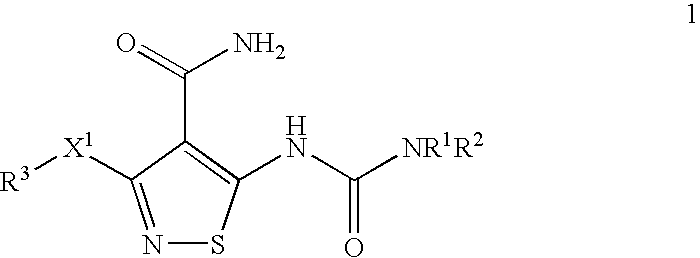
Patents
Literature
68 results about "Topotecan HCl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
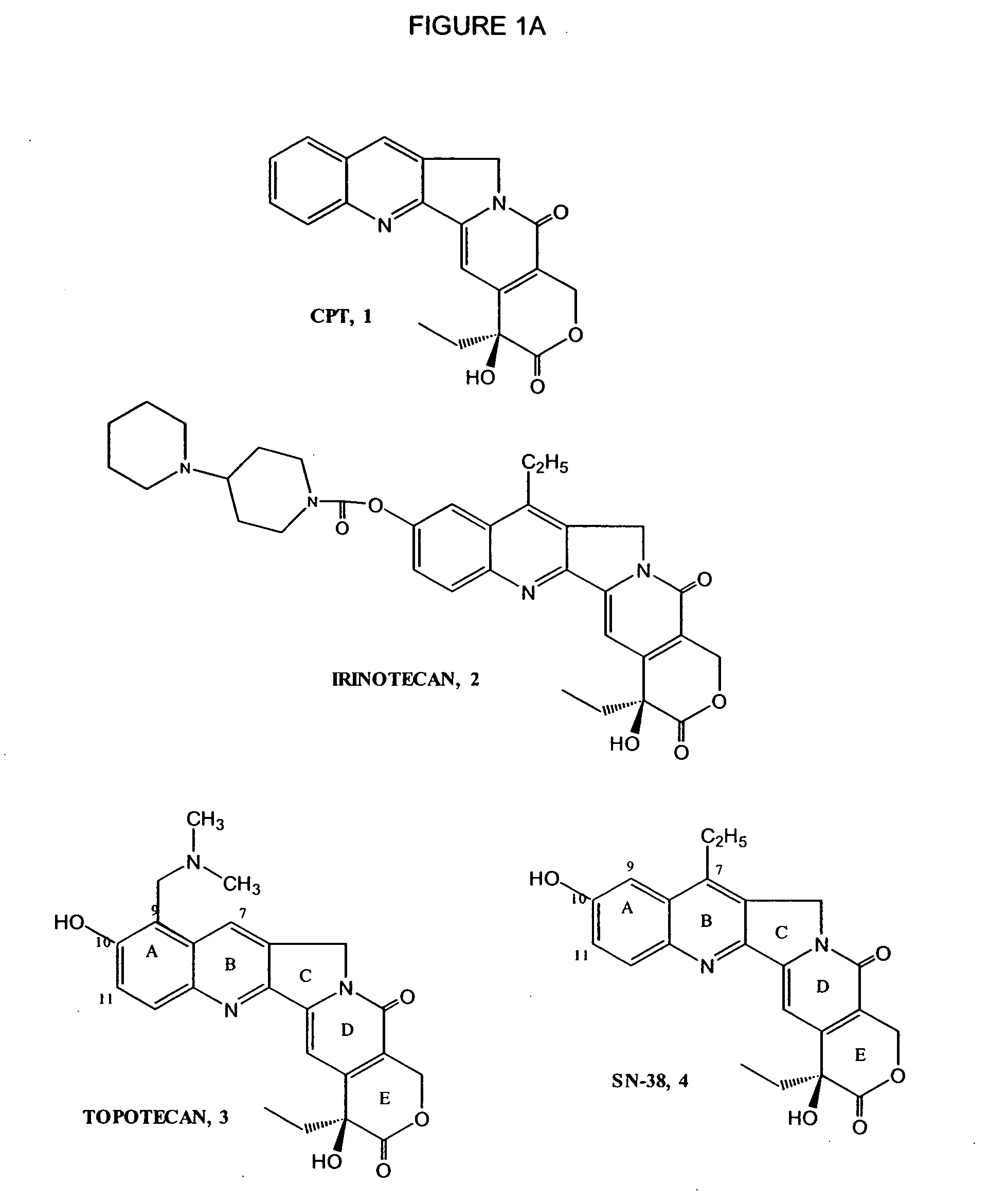
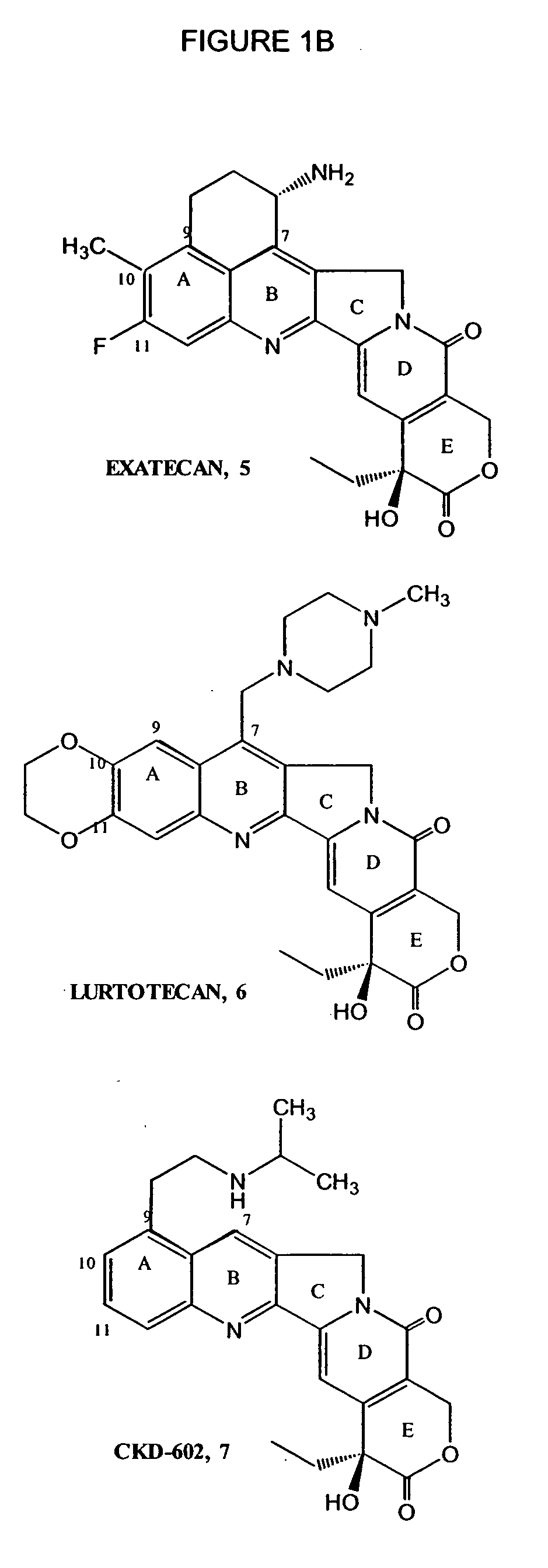
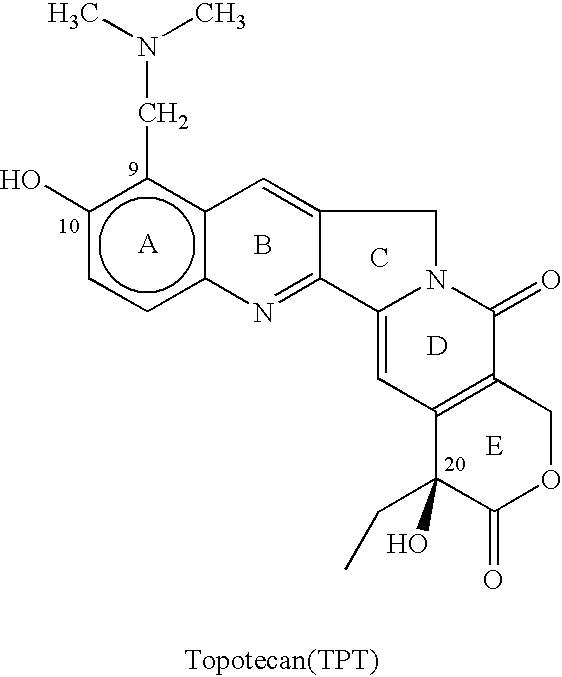
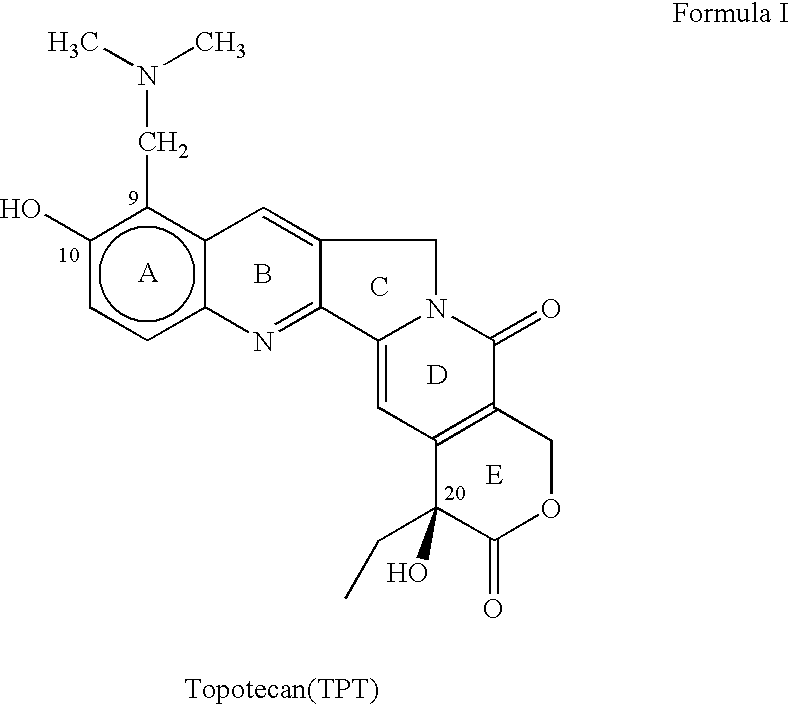
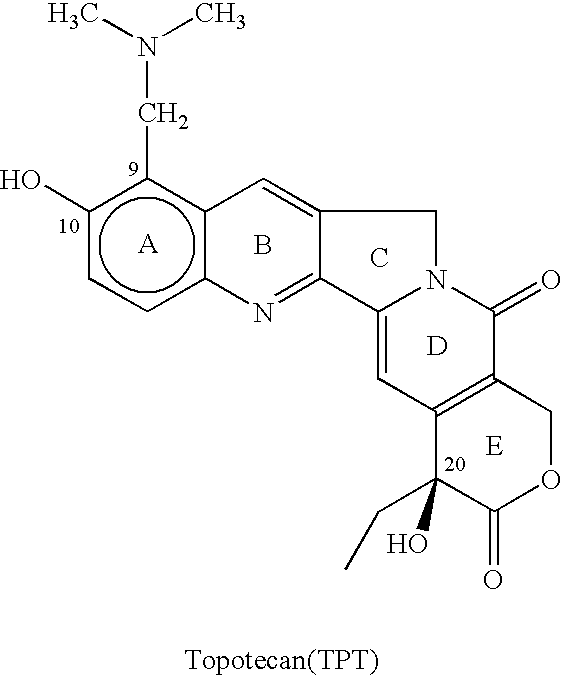
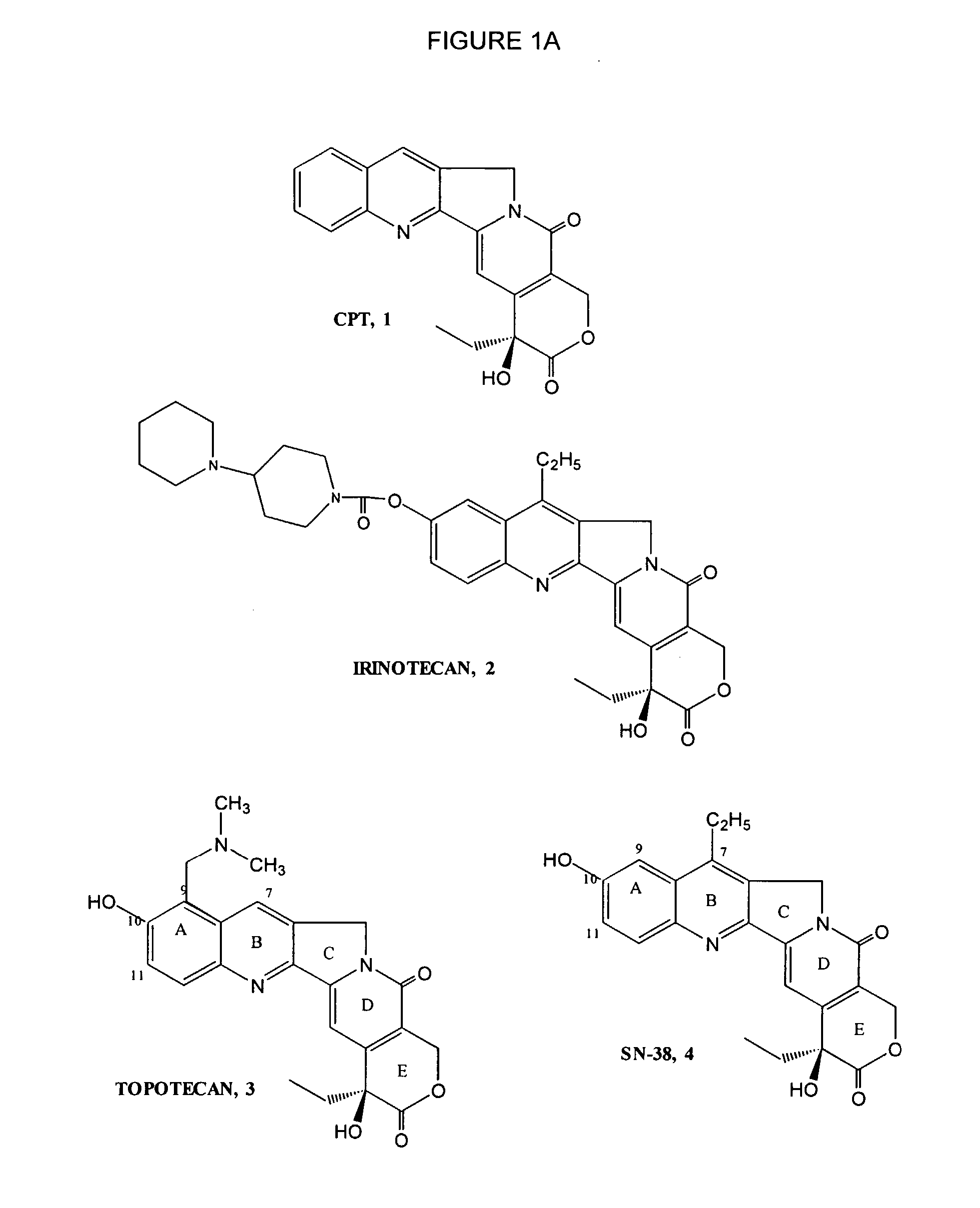
Topotecan (trade name Hycamtin) is a chemotherapeutic agent that is a topoisomerase inhibitor. It is a synthetic, water-soluble analog of the natural chemical compound camptothecin. It is used in the form of its hydrochloride salt to treat ovarian cancer, lung cancer and other cancer types.
Method of using a matrix metalloproteinase inhibitor and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia
InactiveUS6858598B1Reduce morbidityReduction in severity and frequencyHeavy metal active ingredientsBiocideDNA underwindingIrinotecan
A method of using an MMP inhibitor and optionally radiation therapy, and one or more antineoplastic agents of the topoisomerase class selected from the group consisting of irinotecan and topotecan, as a combination therapy for the treatment of neoplasia is disclosed.
Owner:GD SEARLE & CO
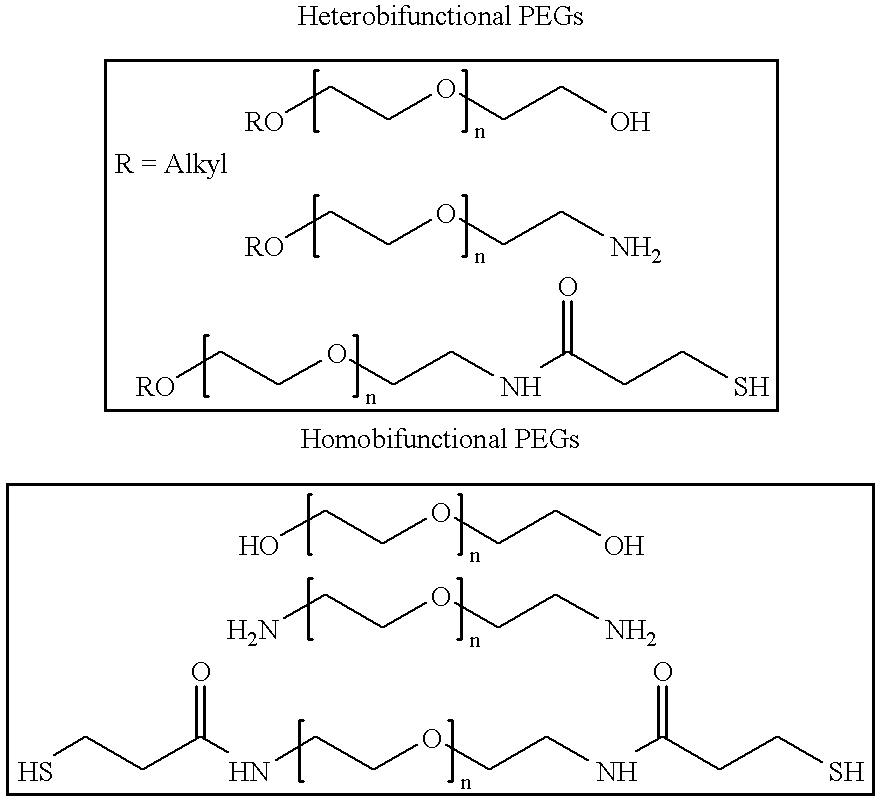
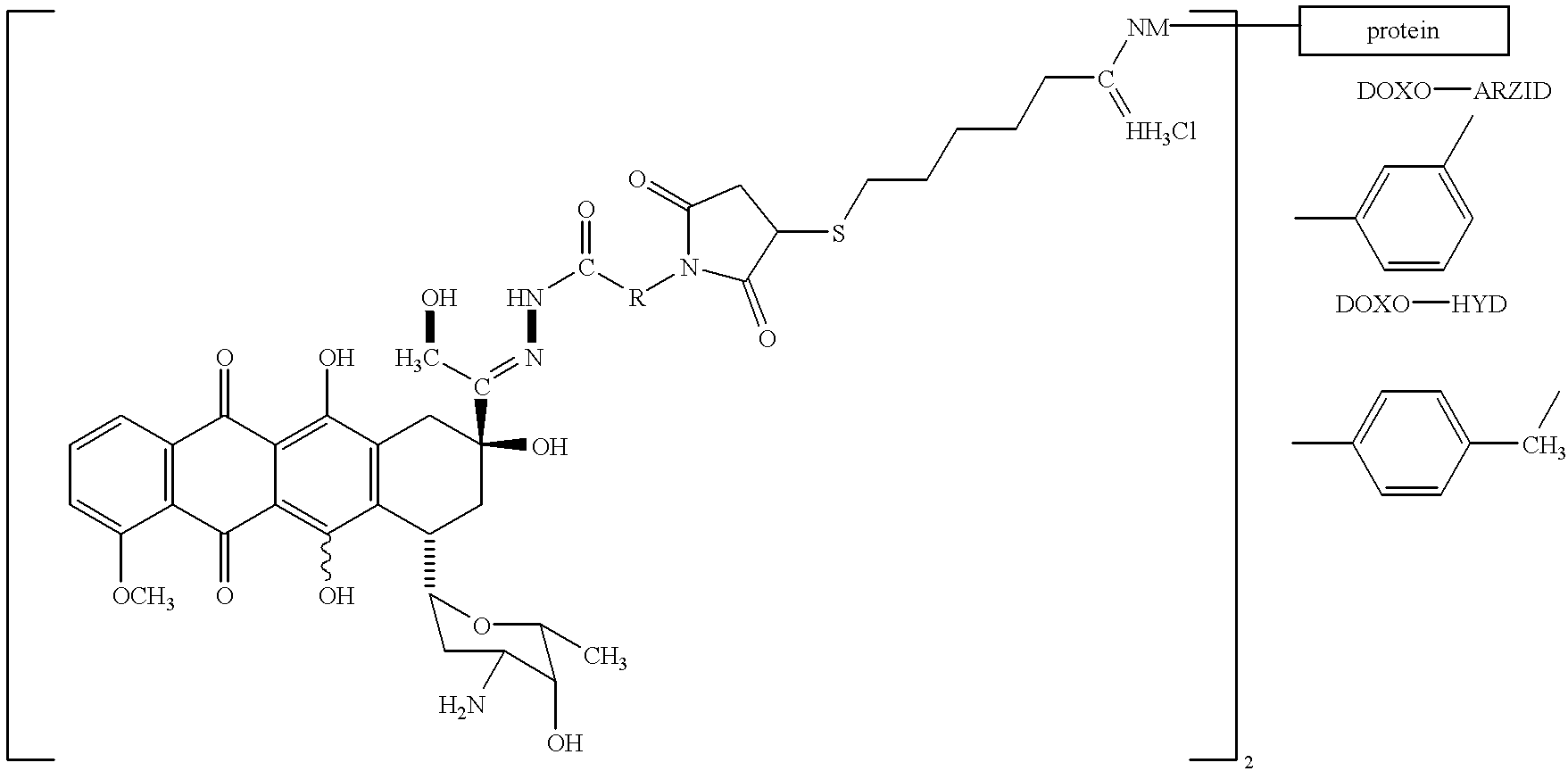
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
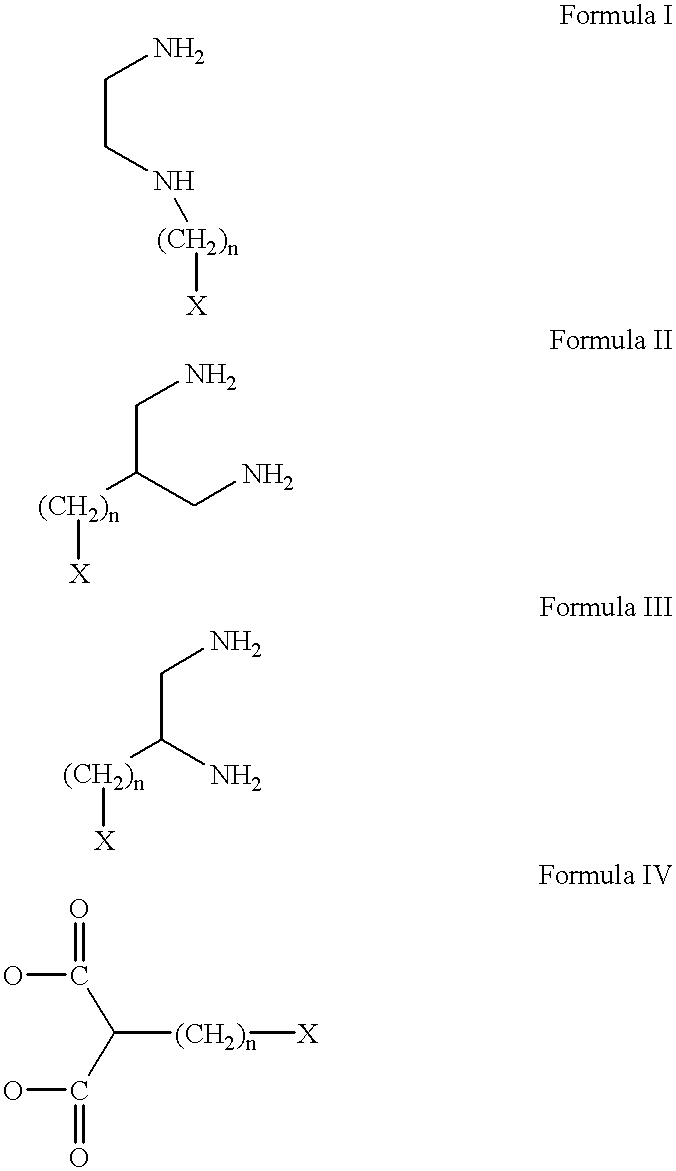
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Method of preparing and using a cold extract from the leaves of nerium oleander
A method of preparing and using a sterile non-toxic pyrogen-free cold extract from the leaves of Nerium oleander as a supplementary medication to cancer chemo-, hormon and / or radiotherapy to restore and / or ameliorate the immune system of the patient and / or to decrease side effects and increase the antitumor effects of radiotherapy and chemotherapeutics, particularly when used in combination with taxol, adriamycin, cisplatin, 5-fluoro-uracil, alimta, cyclophosphamide, mitomycin-C, navelbine, taxotere and topotecan, respectively, and its use in the manufacture of a medicament for the treatment of one or more cancers of bladder, kidney, liver, ovary, pancreas, testicle, uterus, and vagina as well as pleuramesotheliomas and Hodgkin's lymphomas.
Owner:RASHAN JUAY JAMIL
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Anticancer composition
InactiveCN1961864AOrganic active ingredientsPharmaceutical delivery mechanismAdjuvantTreatment effect
Disclosed is an anti-cancer composition slow release injection which comprises slow release microspheres and dissolvent, the slow release microspheres comprise slow release auxiliary materials, Epothilone derivatives, glyoxaline piperazidine, Topo enzyme inhibitor combination selected from SN-38, CPT-11, HCPT, Topotecan, Irinotecan, Etoposide, Teniposide, Amrubicin, Valtaxin, XK469, AD312, ICRF-187 or ICRF-193, the dissolvent being specific dissolvent containing suspension adjuvant, the slow release auxiliary materials are selected from polylactic acid and its copolymer, polyethylene / polylactic acid, glycollic acid copolymer, aliphatic acid and sebacylic acid copolymer, the viscosity of the suspension adjuvant is 80-3000cp (at 20-30 deg C), and is selected from carboxymethylcellulose, The slow release microspheres can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a release period of about 40 days. The slow release injection and slow release implanting agent can be used independently for effectively suppressing tumor accretion, or used in combination with non-operative methods such as chemotherapy and / or radiotheraphy with the function of improving their treatment effects.
Owner:JINAN SHUAIHUA PHARMA TECH
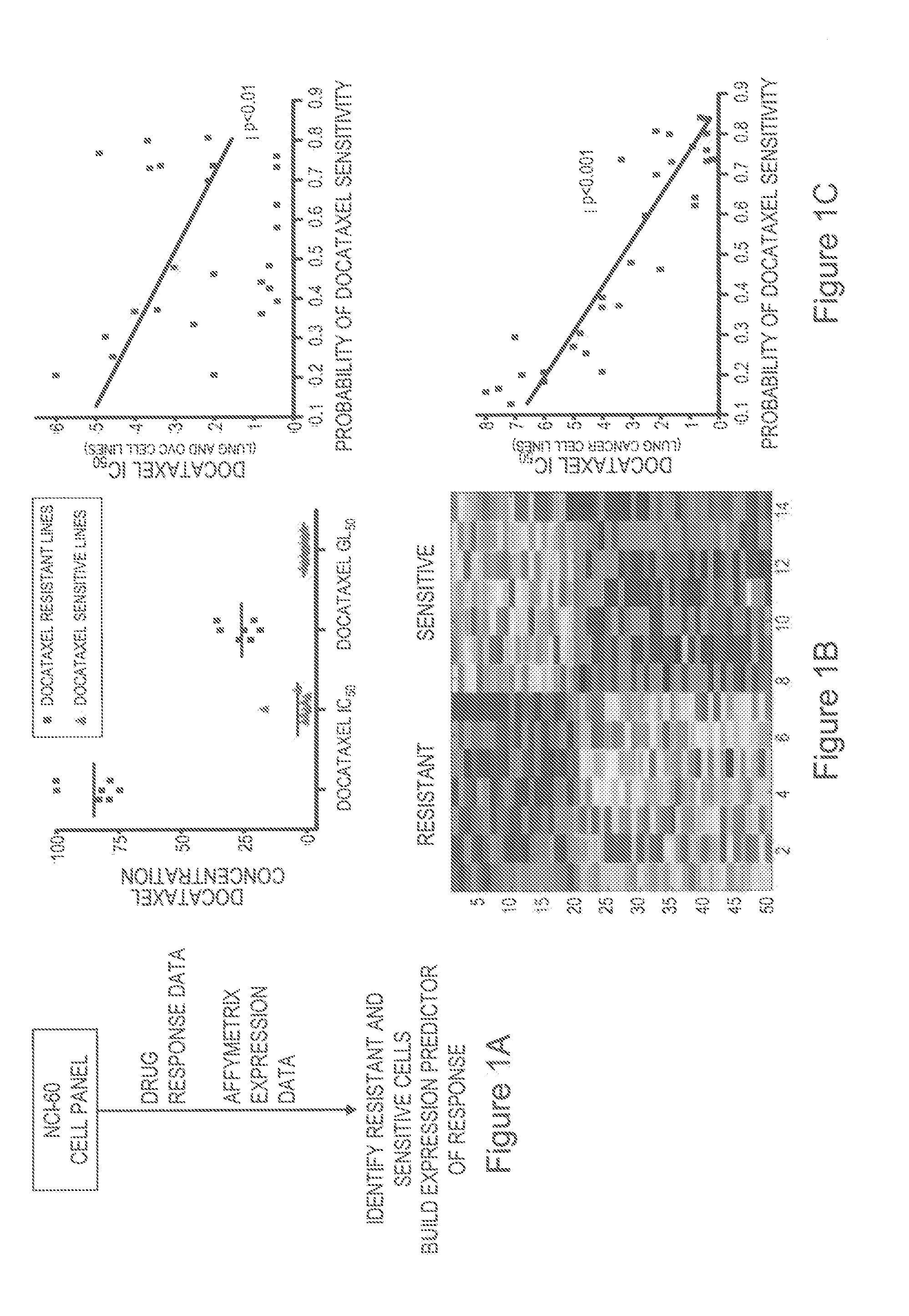
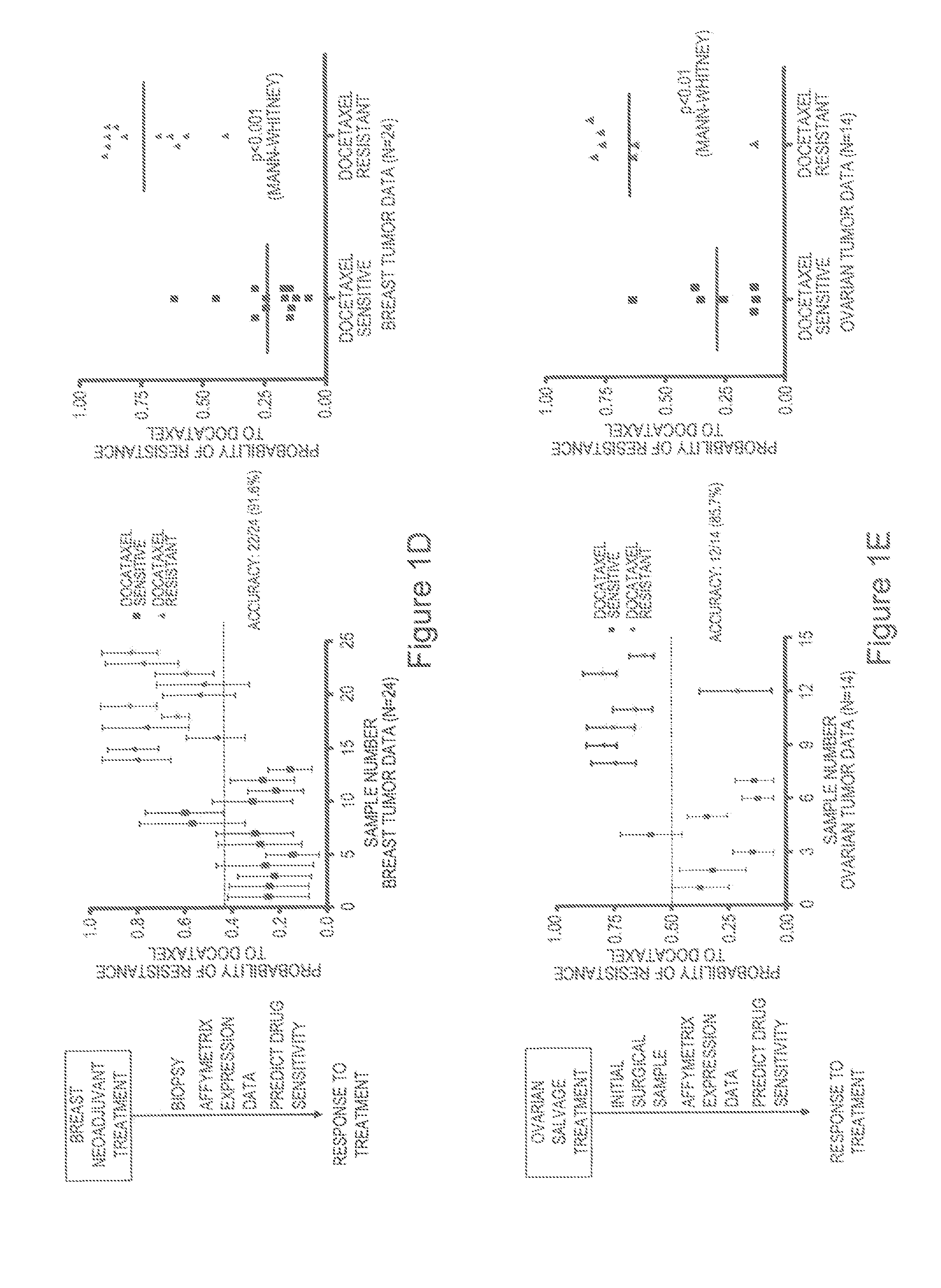
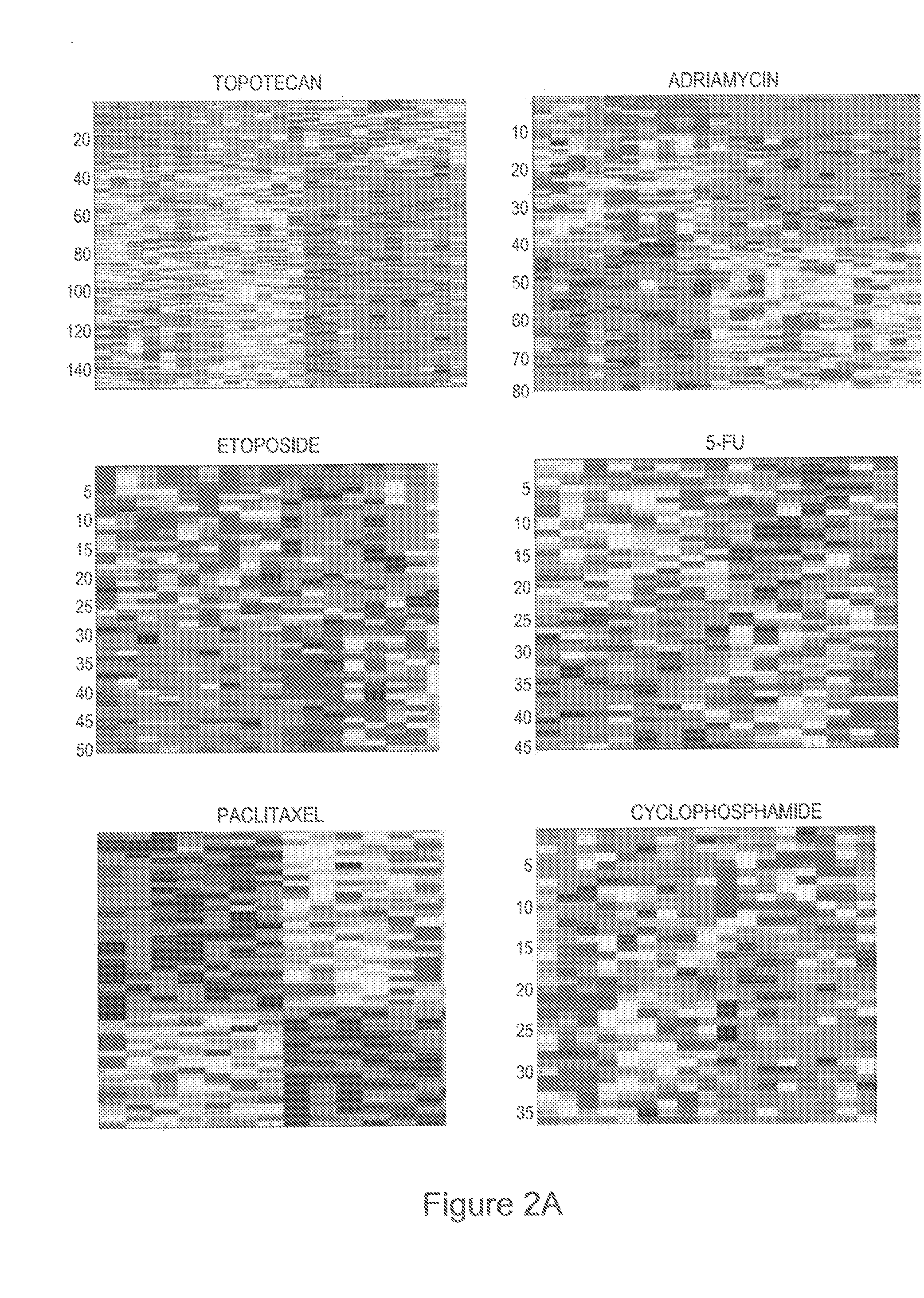
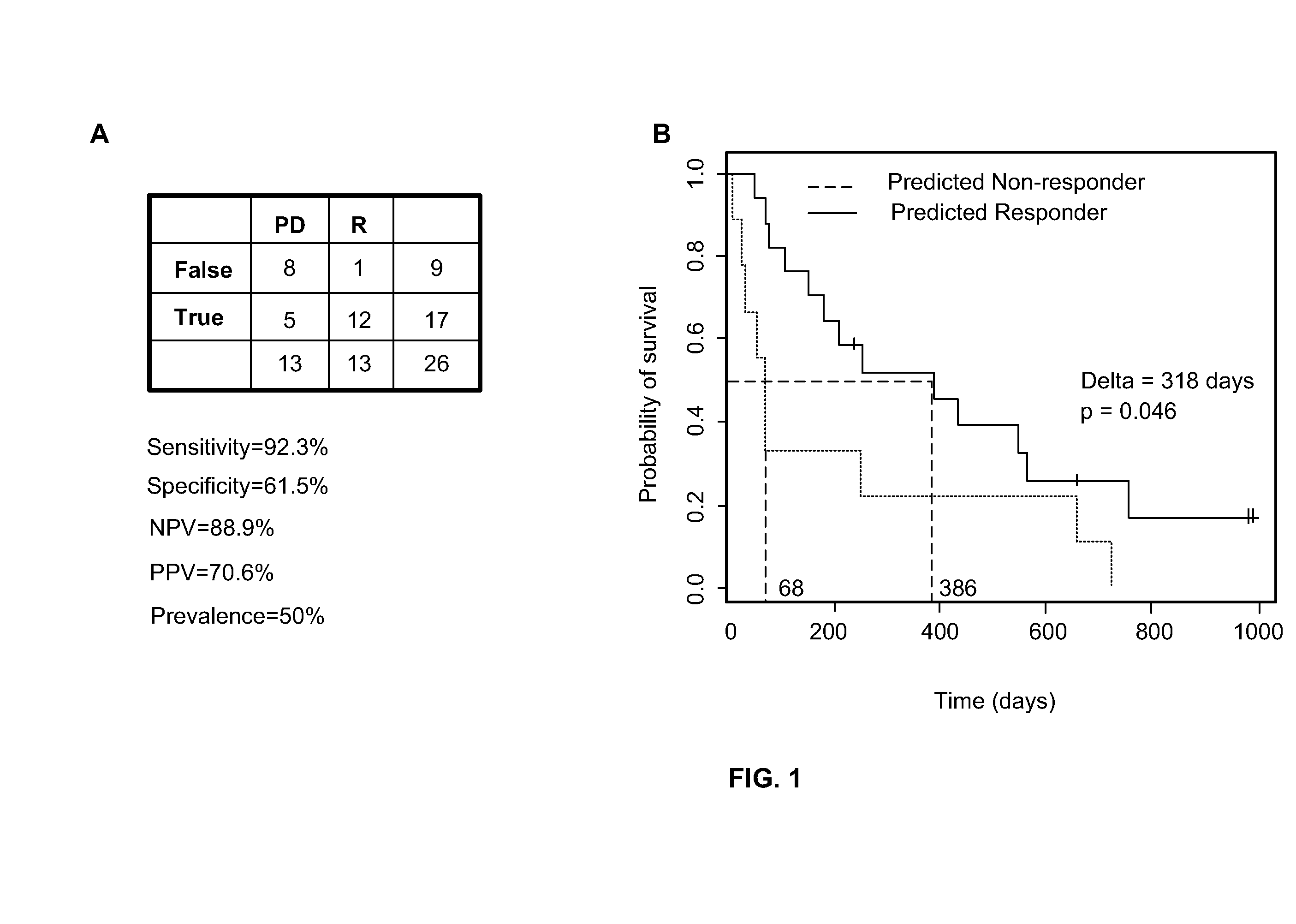
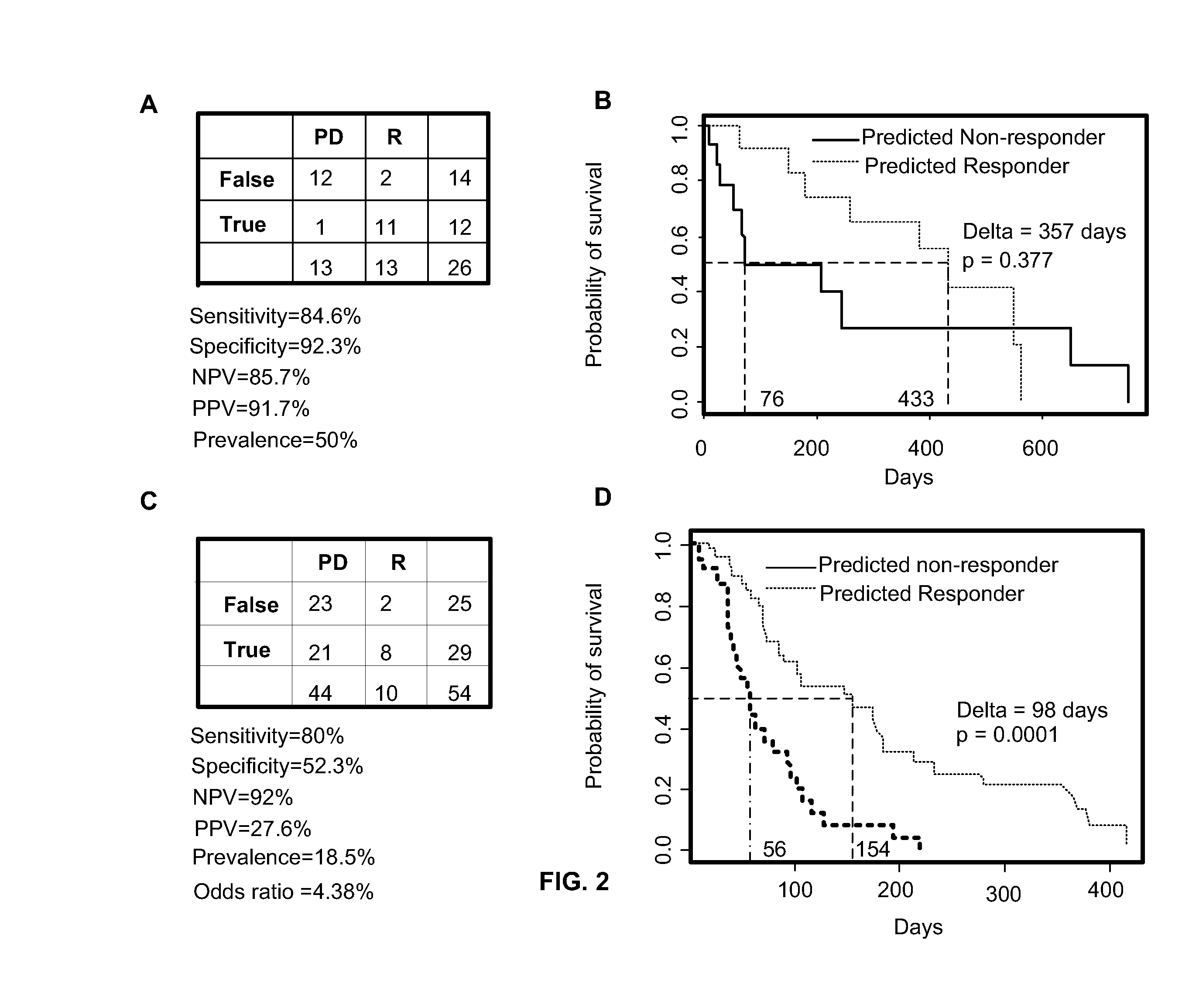
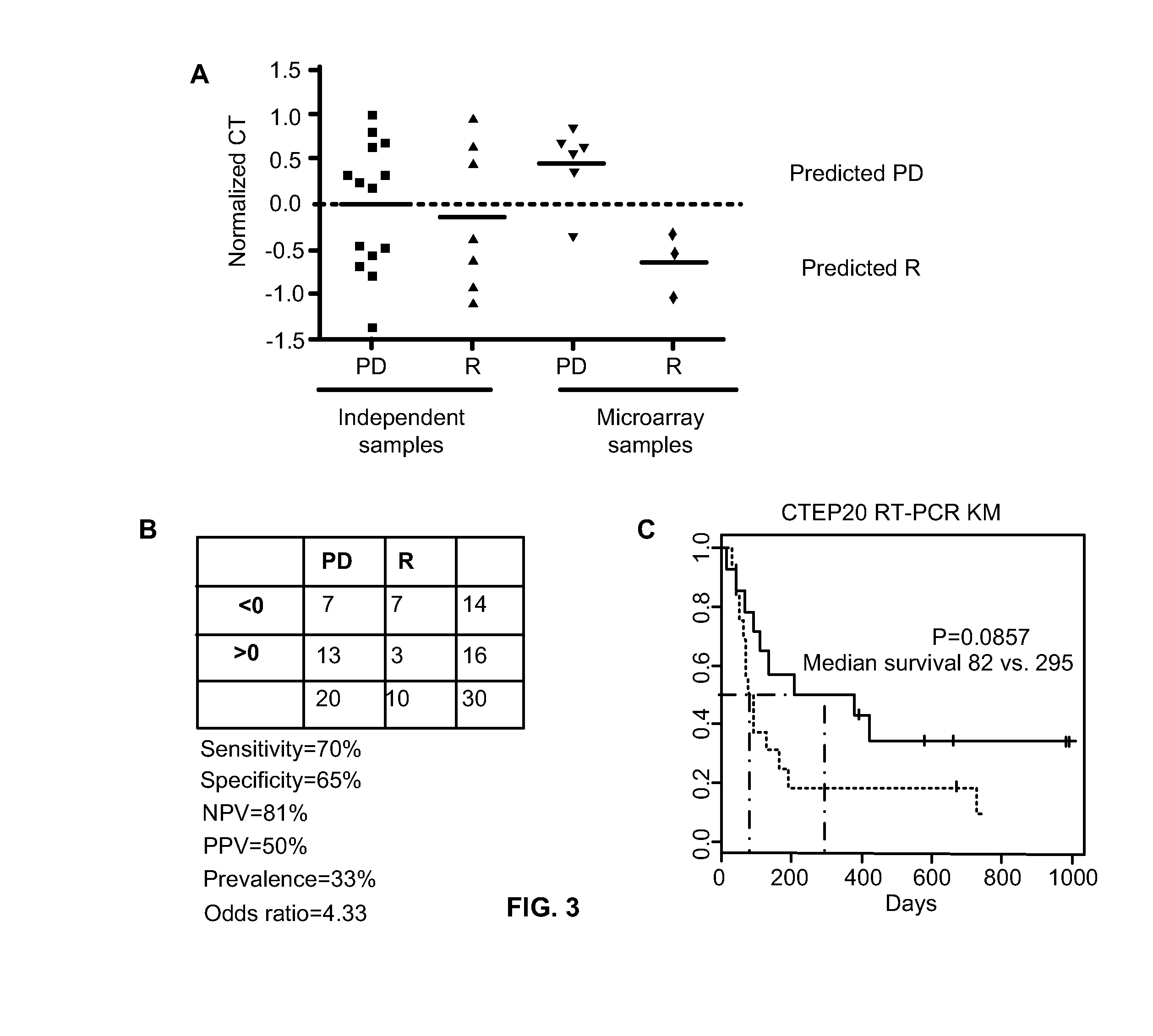
Predicting responsiveness to cancer therapeutics
Owner:UNIV OF SOUTH FLORIDA +1
Novel tumor-targeting arboraceous polymer nano carrier of camptothecin drug
InactiveCN102429870AFinely adjustable structure sizeControllable numberOrganic active ingredientsPowder deliveryTumor targetSafe delivery
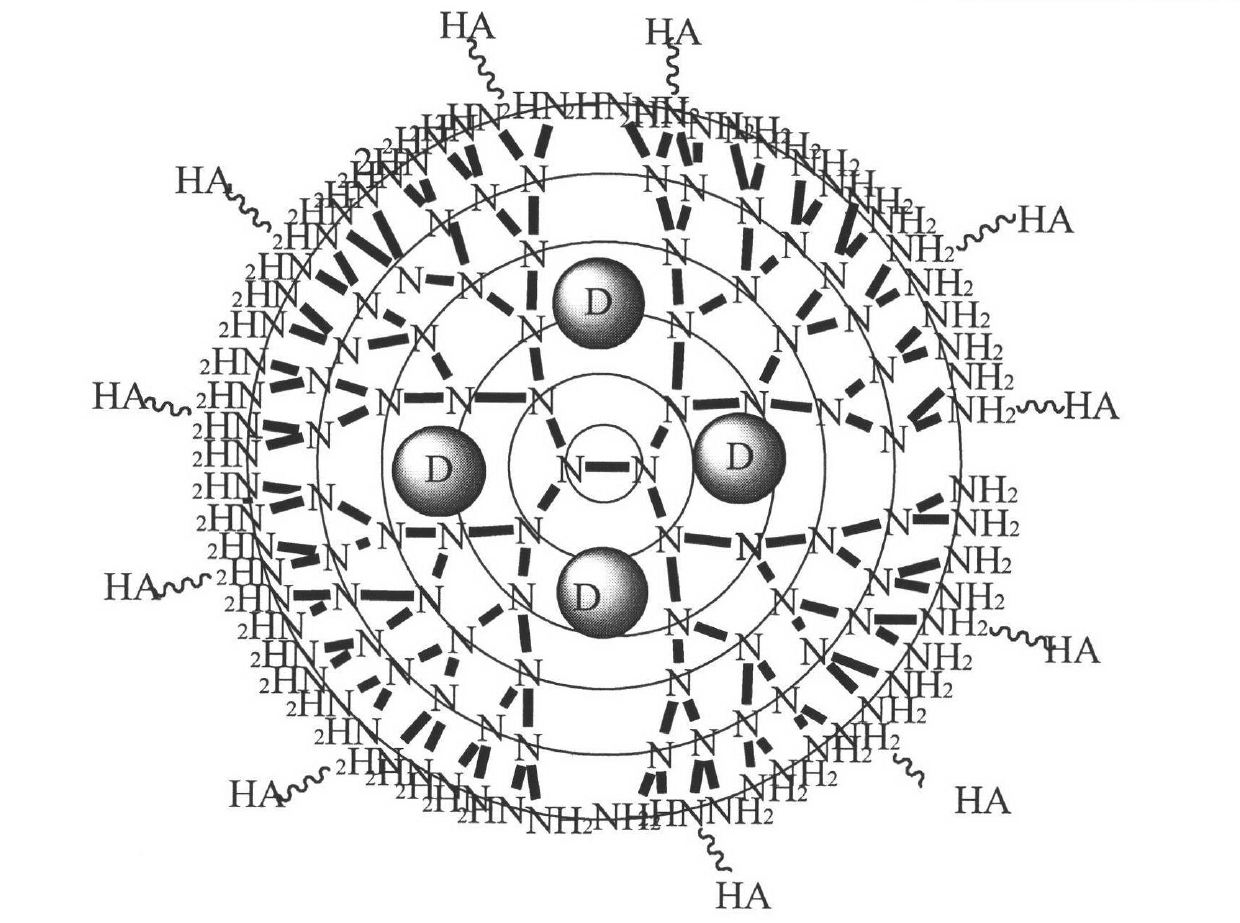
The invention belongs to the field of pharmaceutical preparations, and relates to preparation of a novel target nano carrier and application of the novel target nano carrier in a camptothecin drug targeting delivery system. The system can be represented as PAMAM-HA / drug, wherein PAMAM is polyamide amine-amine arboraceous macromolecules of different generations, HA is hyaluronic acid of different molecular weights, and the drug is one or more of camptothecin anticancer drugs such as camptothecin, 10- hydroxycamptothecine, topotecan and the like. The carrier is used for wrapping the camptothecin drugs, and has the actions of prolonging the drug blood circulation time, improving the drug bioavailability, reducing the non-tumor part concentration of the drug, reducing the drug cytotoxicity, and simultaneously protecting the lactonic rings of the camptothecin drugs from damage, thereby improving the tumor treatment efficiency from multiple aspects. The nano carrier provided by the invention has important significance in promoting the safe delivery of the camptothecin drugs and the effective treatment of tumor.
Owner:CHINA PHARM UNIV +1
Process for preparing topotecan
Owner:DR REDDYS LAB LTD +1
Liposomal Composition for Convection-Enhanced Delivery to the Central Nervous Centre
ActiveUS20110274625A1Improved tissue distributionConvection-enhanced delivery (CED)BiocideNervous disorderPhospholipidDrug release
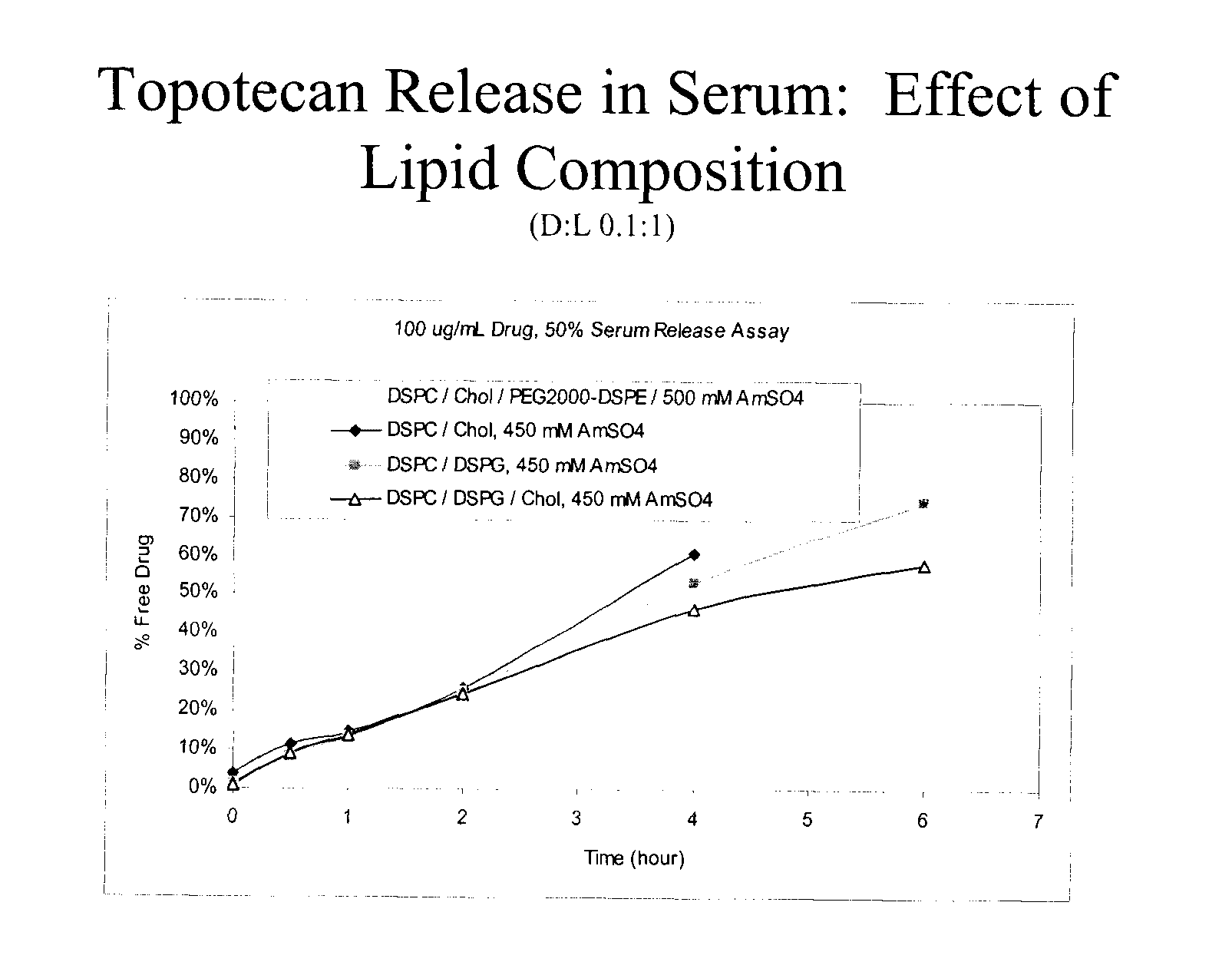
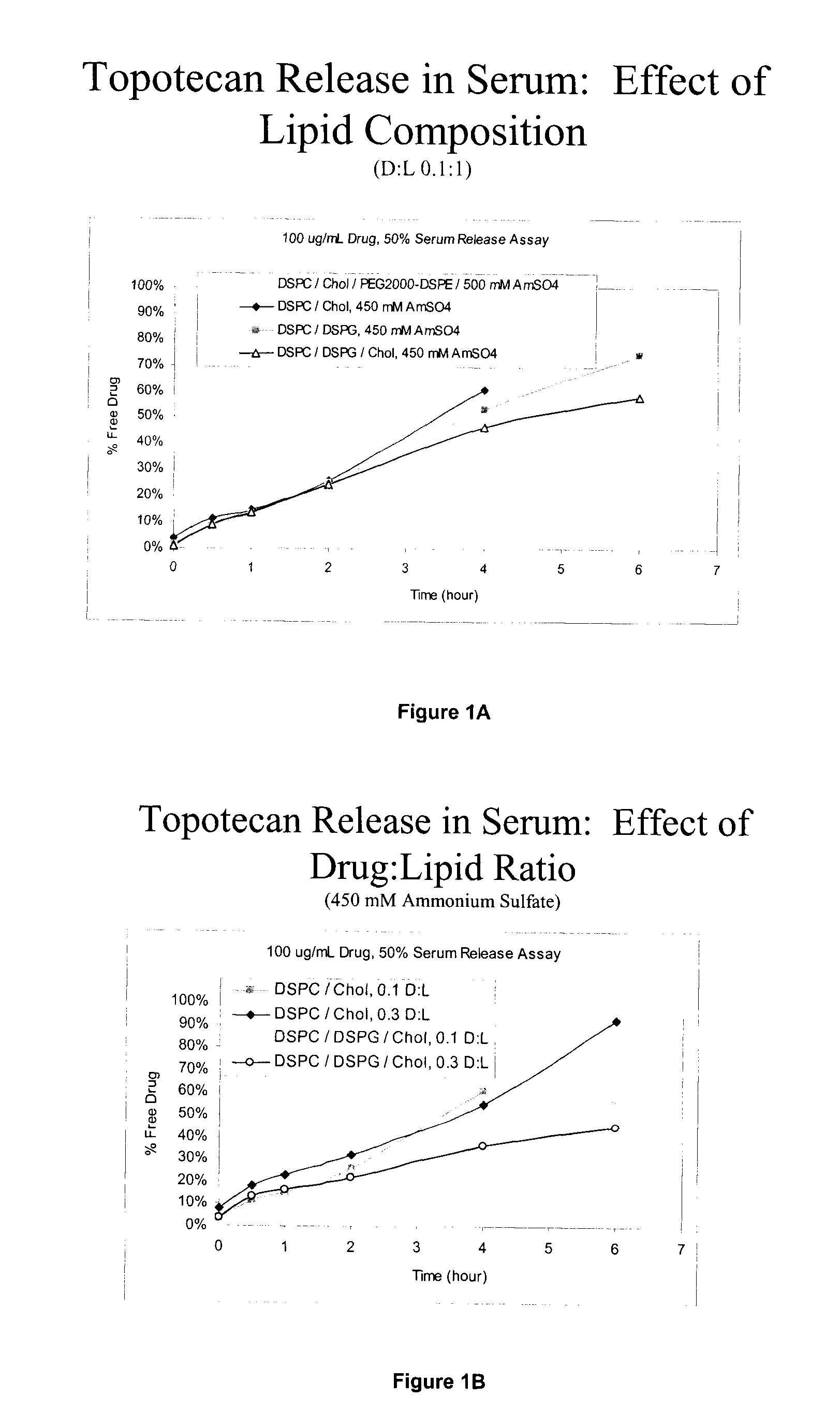
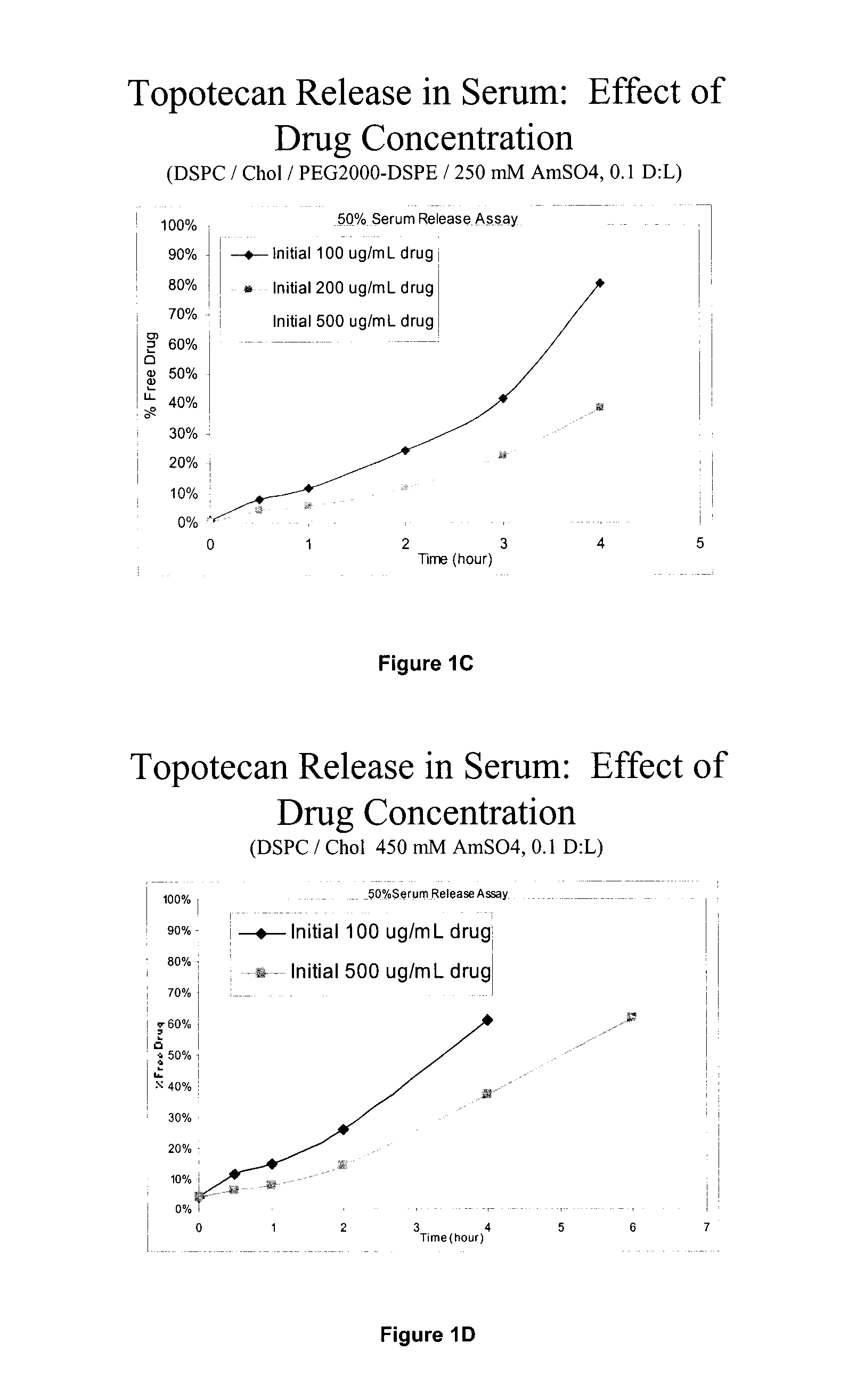
Convection-enhanced delivery (CED) is used as a method to deliver a direct infusion of therapeutic agents to the central nervous centre thus circumventing the blood-blood barrier. A non-PEGylated liposomal composition comprising at least one saturated neutral phospholipid and at least one saturated anionic phospholipid and a therapeutic or diagnostic agent encapsulated therein is used to overcome toxicity associated with high peak drug concentration delivered locally CED as well as to increase tissue distribution volume for an improved sustained drug release. In one embodiment, the liposome composition comprises a molar ratio of DSPC:DSPG:CHOL of 7:2:1 and the therapeutic or diagnostic agent is selected from topotecan, conotoxin, gadodiamide or rhodamine, and is used in the treatment of epilepsy
Owner:MEDGENESIS THERAPEUTIX INC
Combination therapy for hyperproliferative disease
InactiveUS20070197517A1Ease of detectabilityEasy to prepareBiocideHeavy metal active ingredientsCarboplatinDisease
This invention relates a method of treating hyperproliferative diseases. More particularly, the present invention relates to a method of treating hyperproliferative diseases, such as cancer, comprising the step of administering to a mammal in need of such treatment, either simultaneously or sequentially, (i) a therapeutically effective amount of a taxane derivative, a platinium coordination complex selected from the group consisting of carboplatin, tetraplatin, and topotecan, a nucleoside analog selected from the group consisting of gemcitabine hydrochloride and 5-FU, an anthracycline, a topoisomerase selected from the group consisting of etoposide, teniposide, amsacrine, topotecan, and Camptosar®, an aromatase inhibitor; and (ii) a therapeutically effective amount of an isothiazole derivative. The combinations of the present invention may optionally include an anti-hypertensive agent. This invention also relates to pharmaceutical compositions useful in the treatment of hyperproliferative diseases in mammals, containing such combinations. The present invention also relates to kits having a first compartment with a compound of formula 1 and a second compartment containing a taxane derivative, a platinum coordination complex, a nucleoside analog, an anthracycline, a topoisomerase inhibitor, or an aromatase inhibitor and a third compartment containing an anti-hypertensive agent.
Owner:PFIZER INC
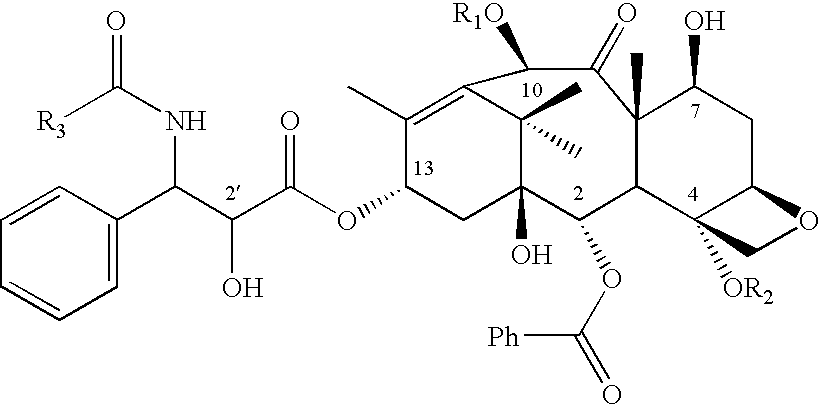
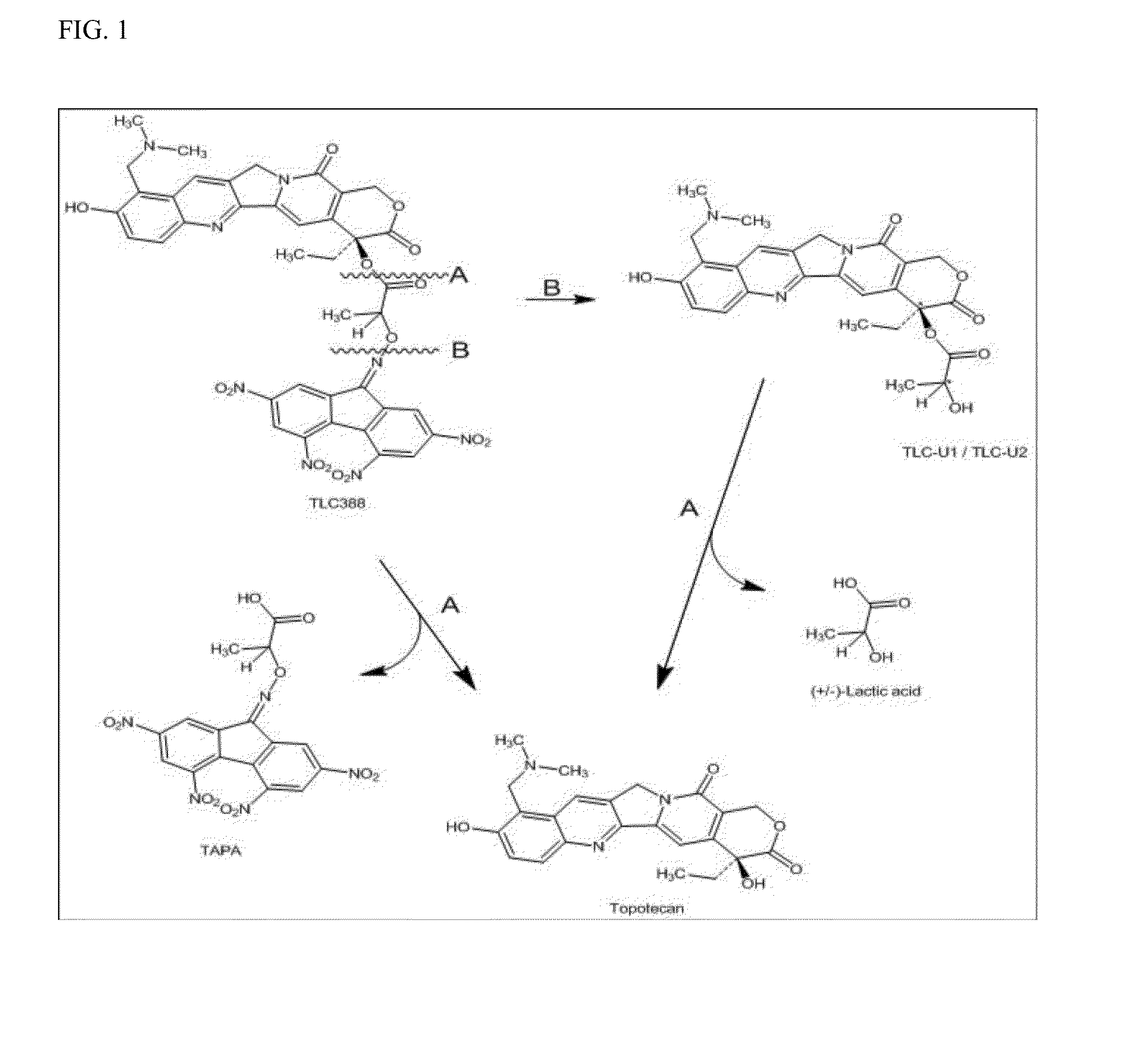
Process to prepare camptothecin derivatives and novel intermediate and compounds thereof
New processes are disclosed for the preparation of camptothecin derivatives, such as, irinotecan and topotecan, as well as new intermediates and compounds related thereof.
Owner:INNOVATIONAL HLDG LLC
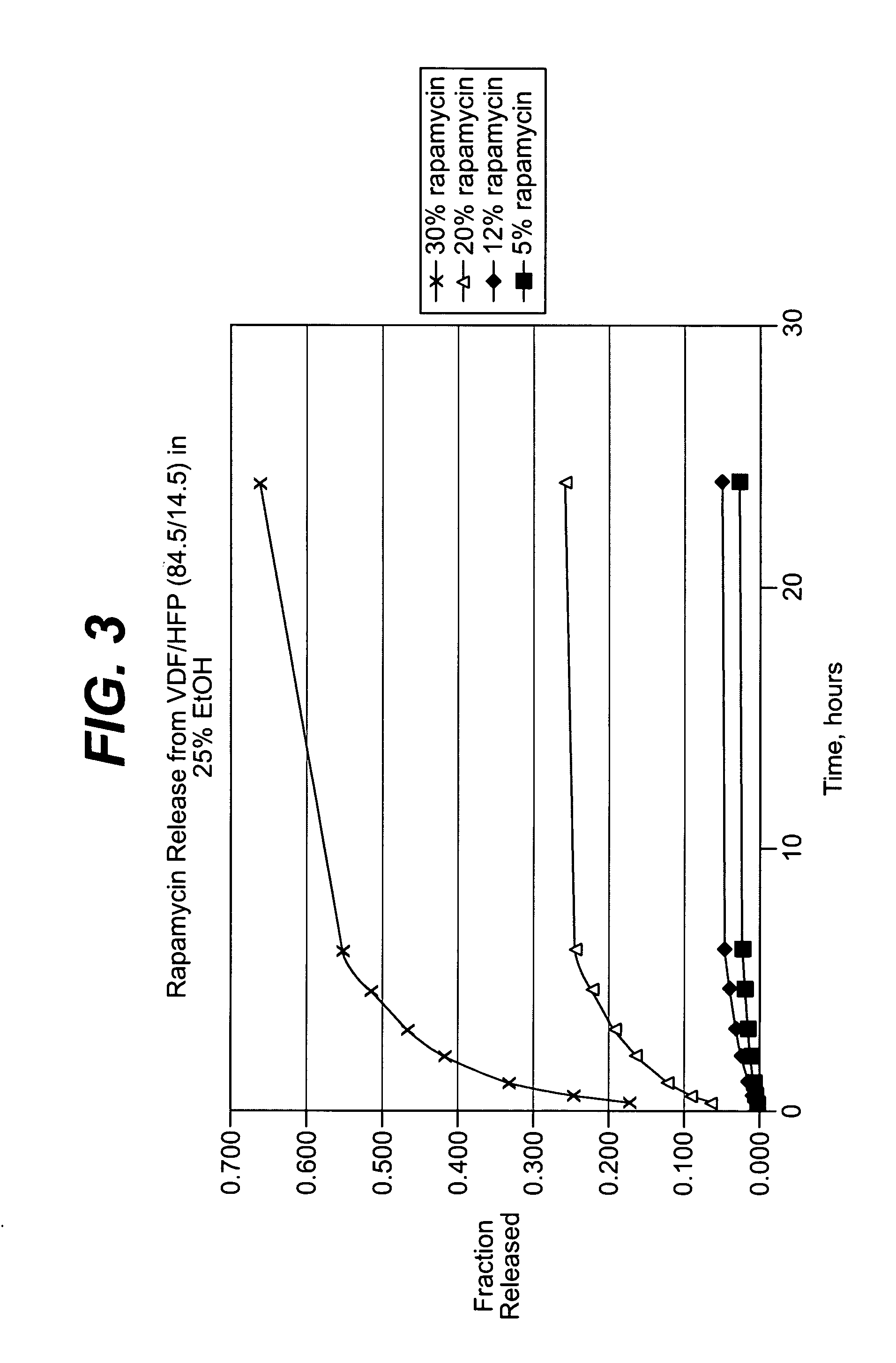
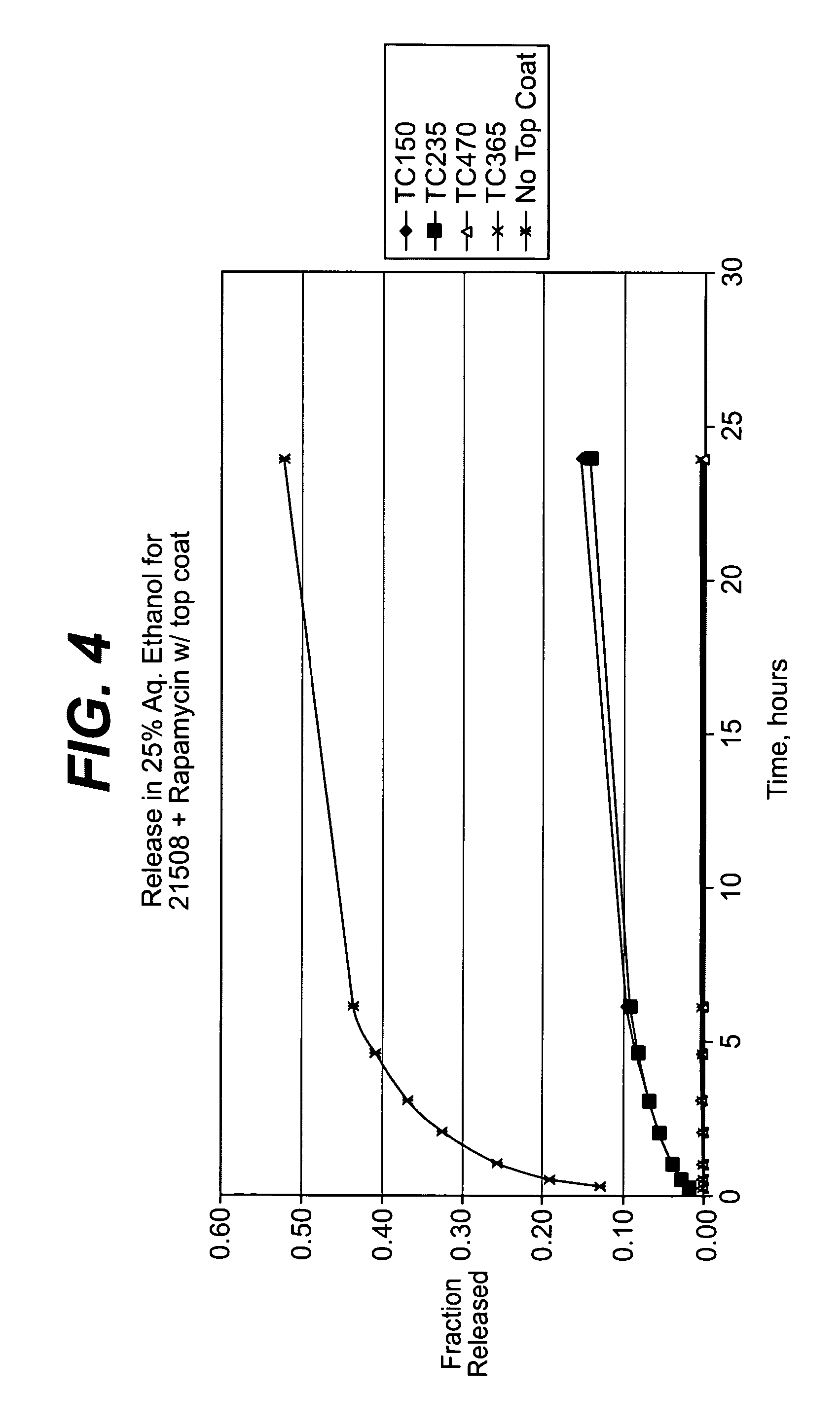
Local vascular delivery of topotecan in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050202059A1Minimize potential risk of damageReduce frictionStentsSurgeryThrombusPercent Diameter Stenosis
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
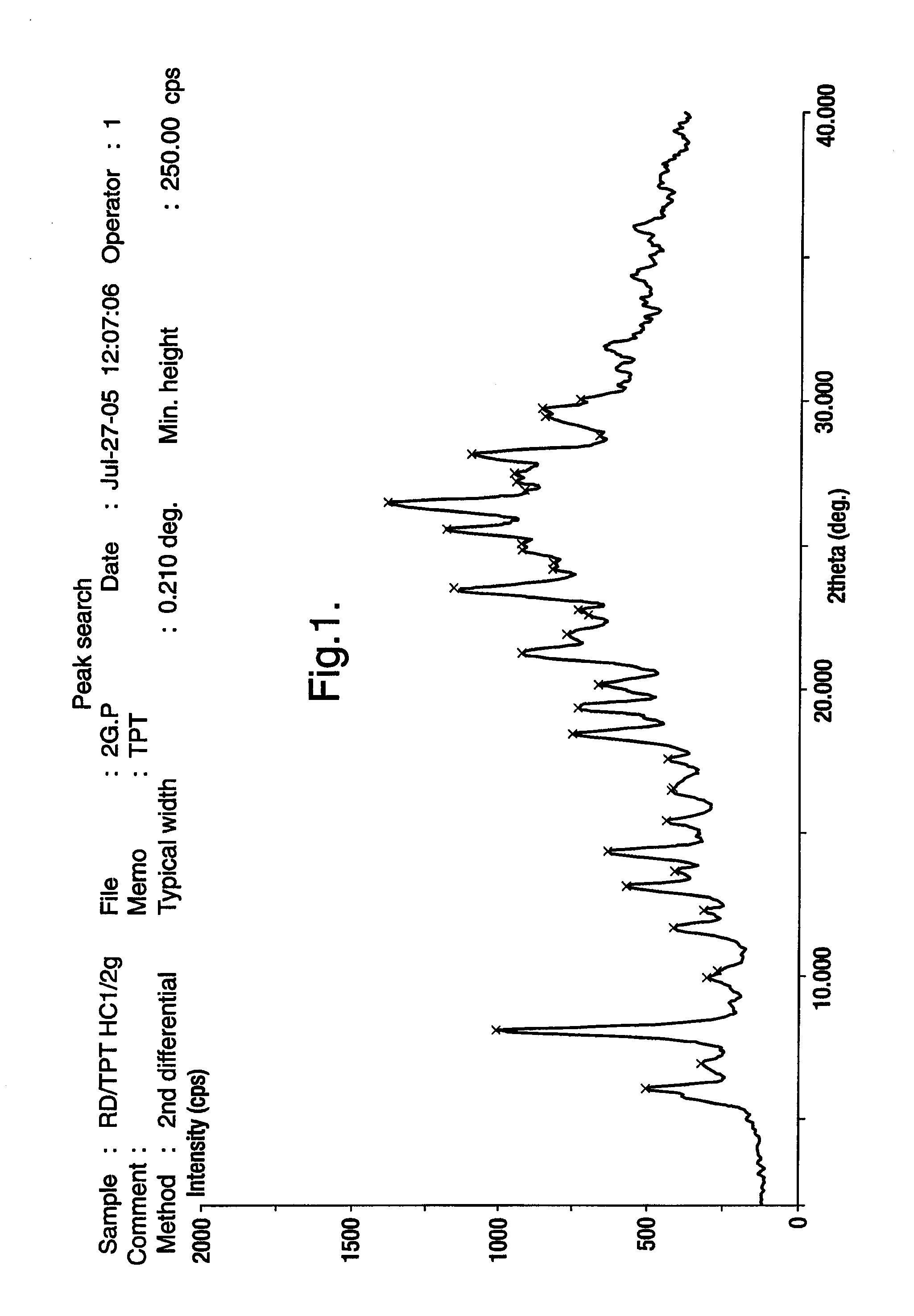
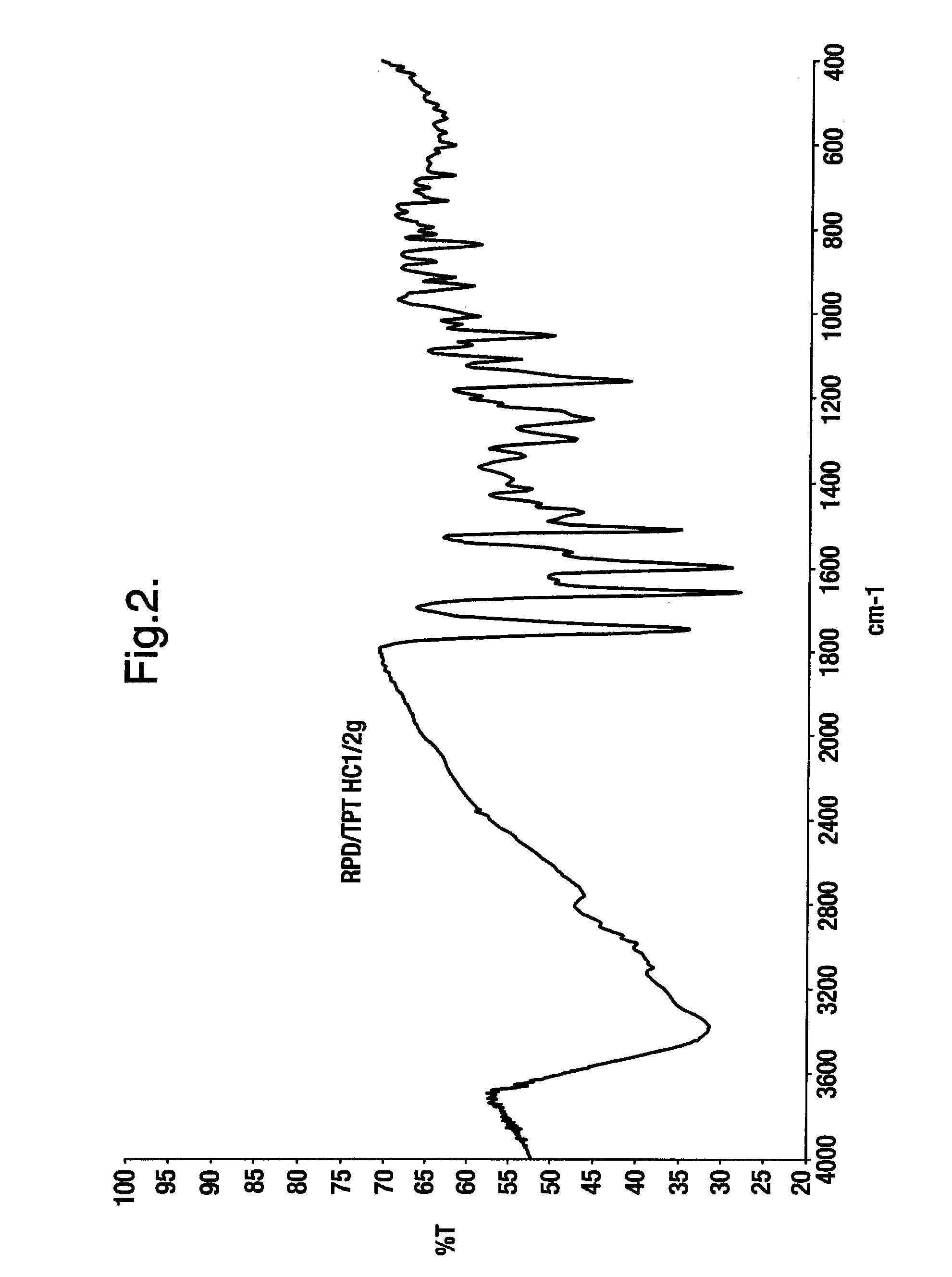
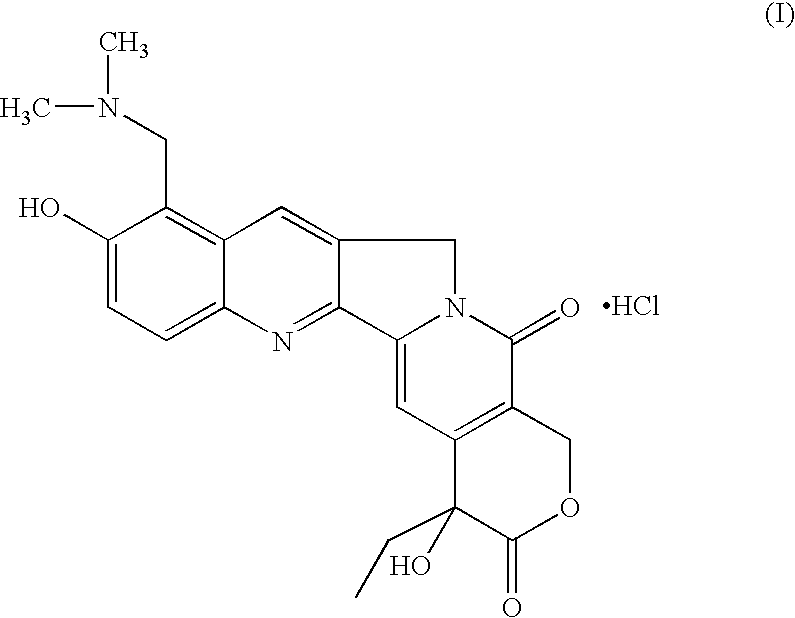
Novel crystalline polymorphic form of a camptothecin analogue
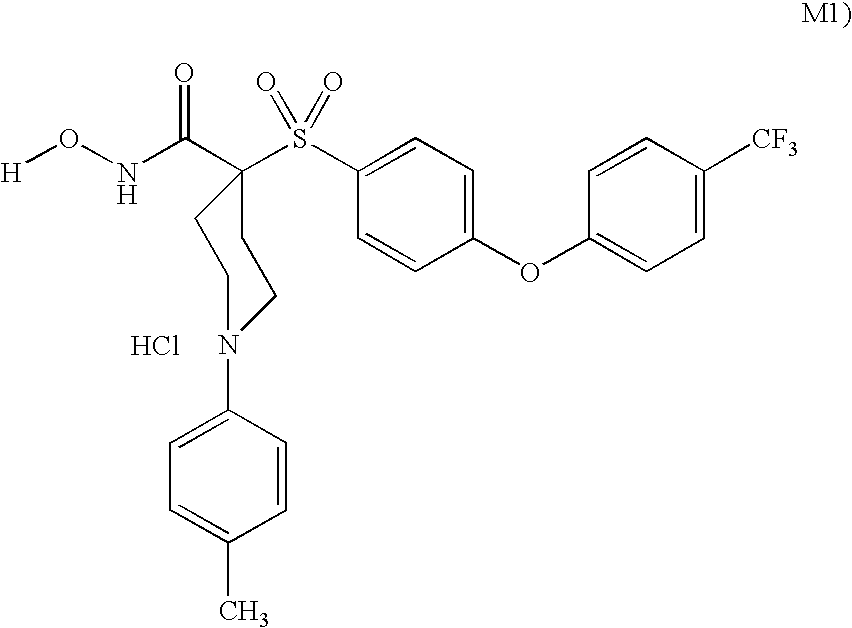
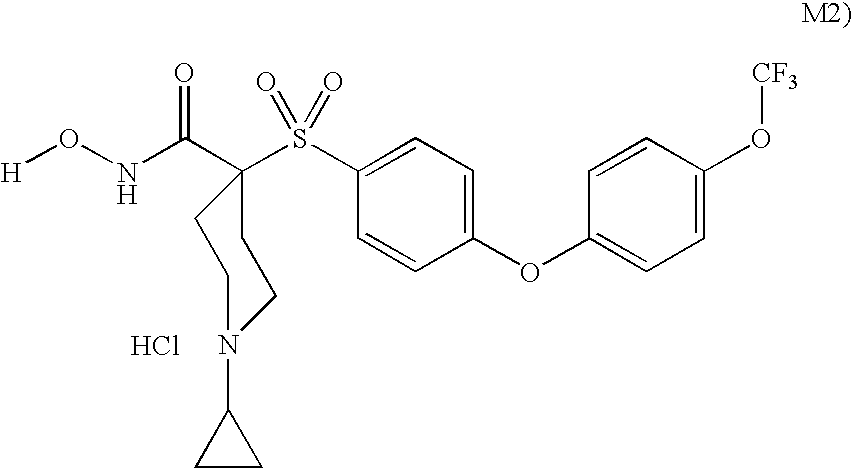
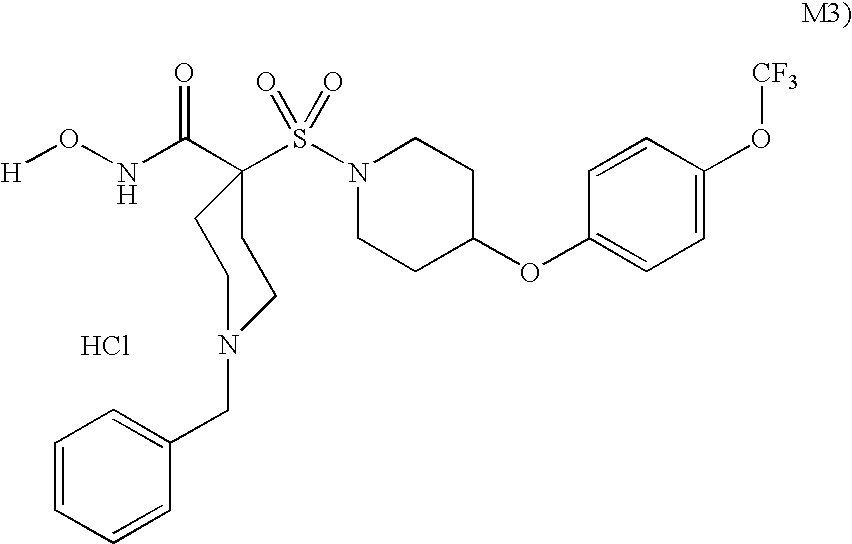
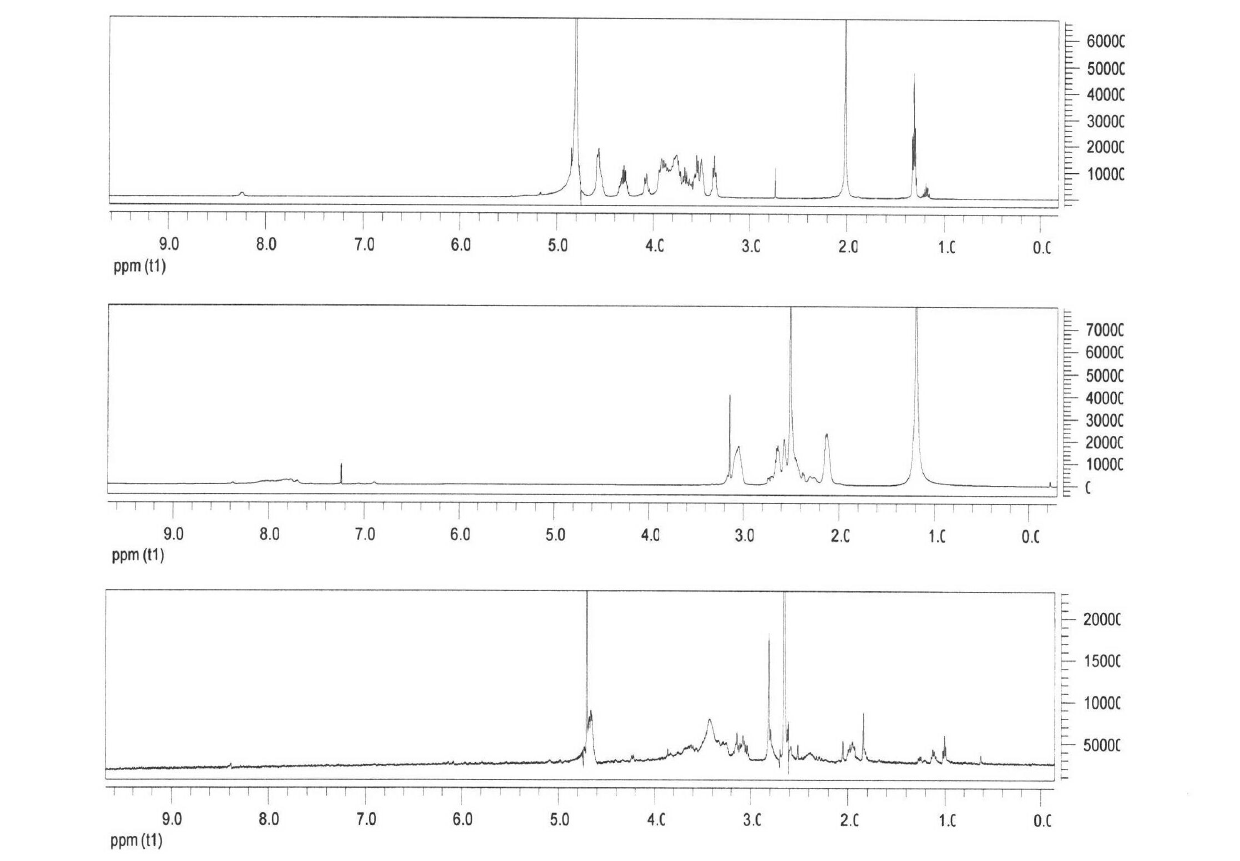
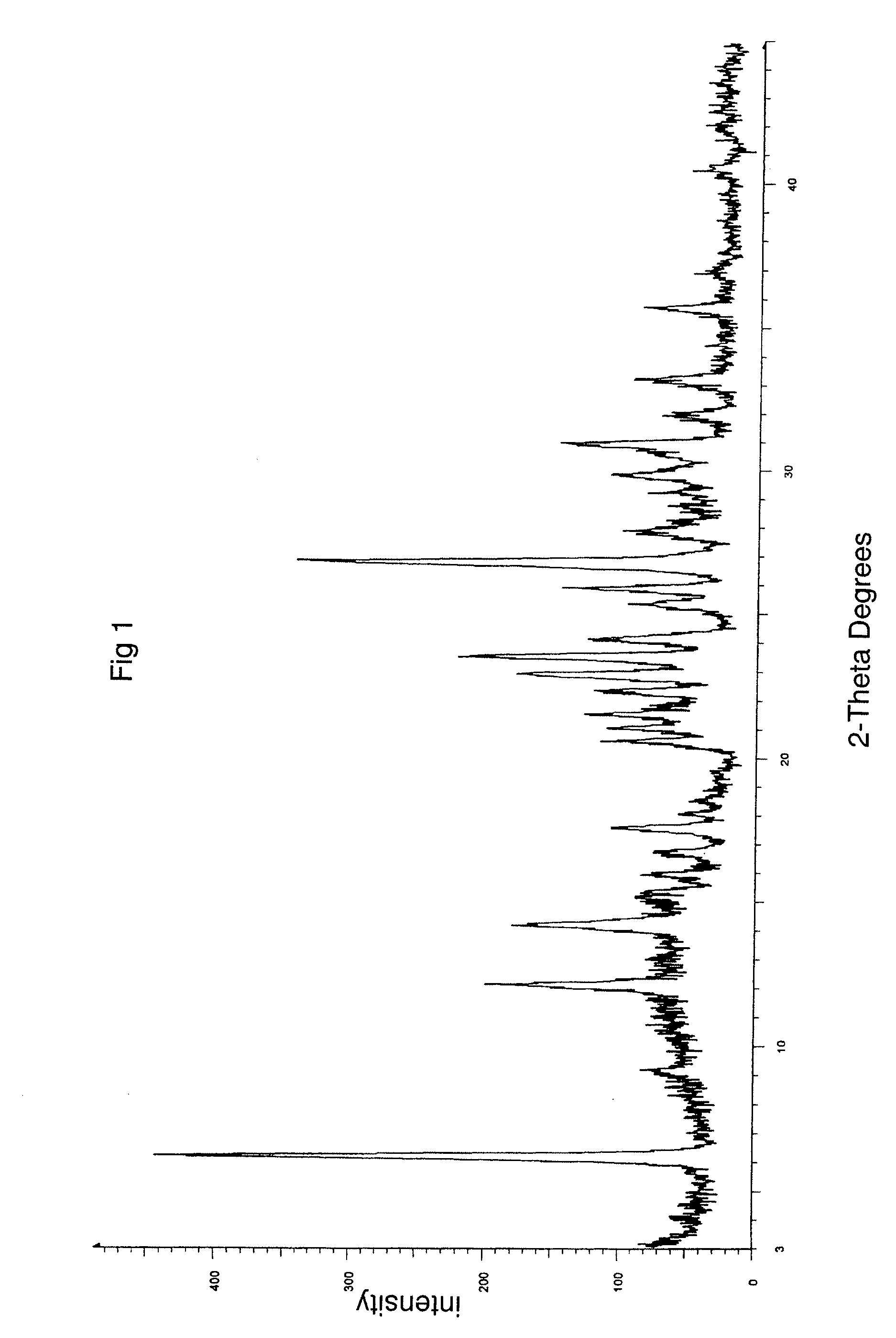
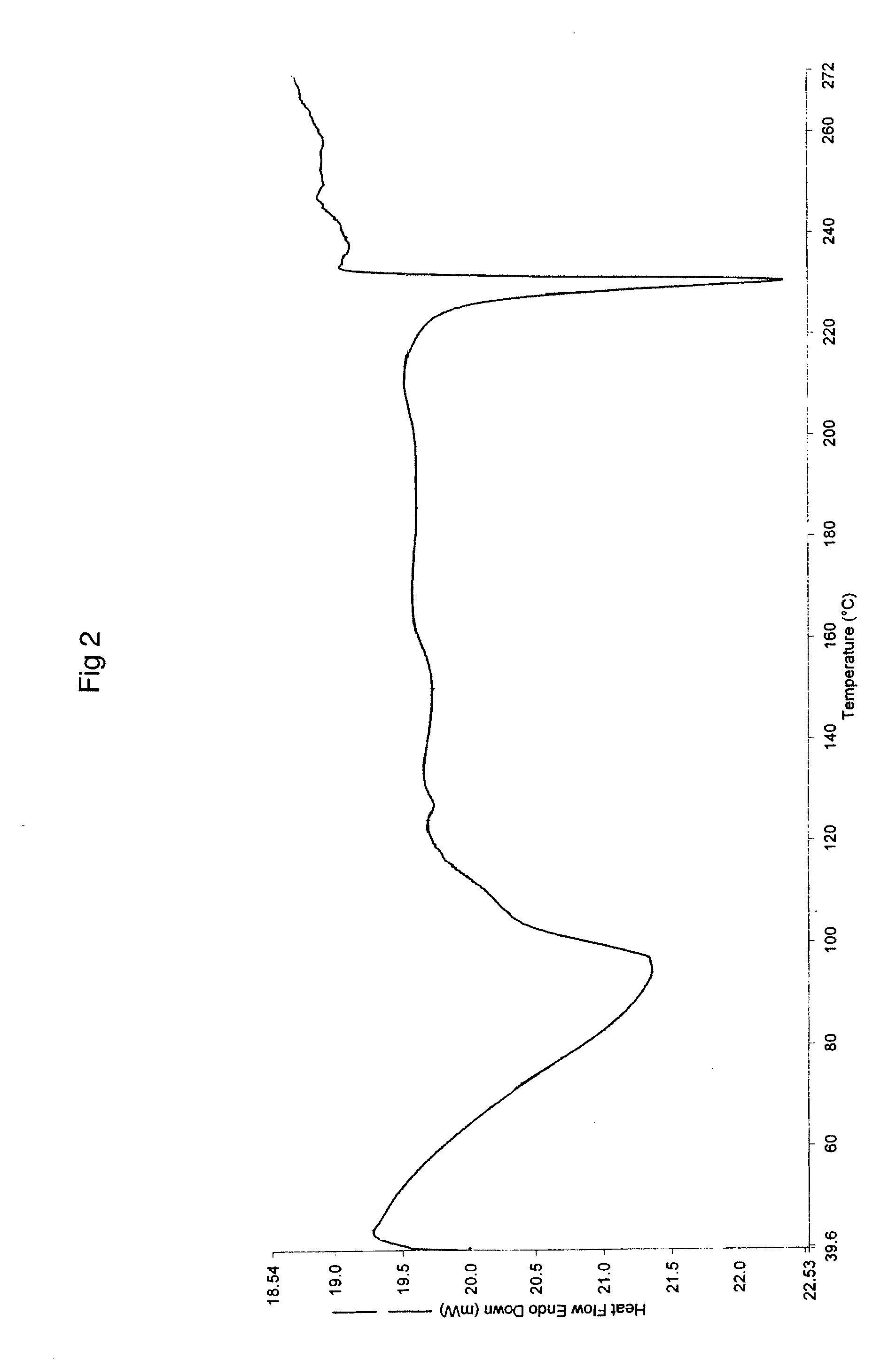
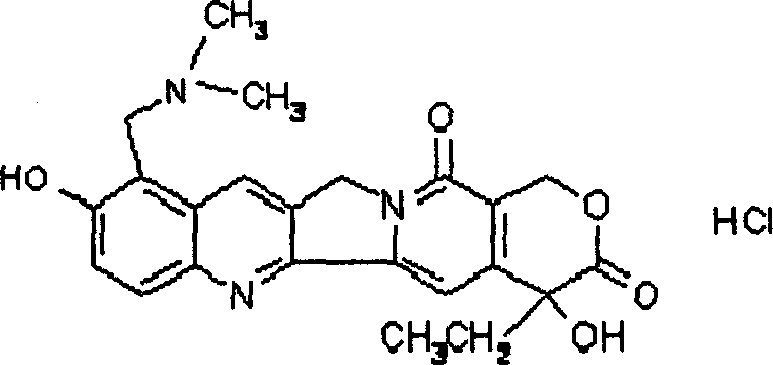
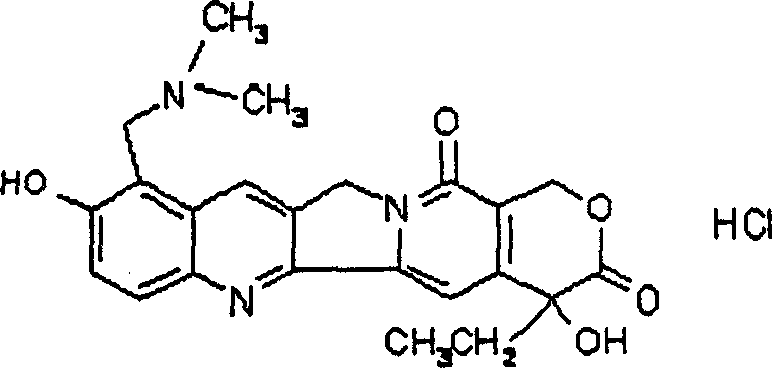
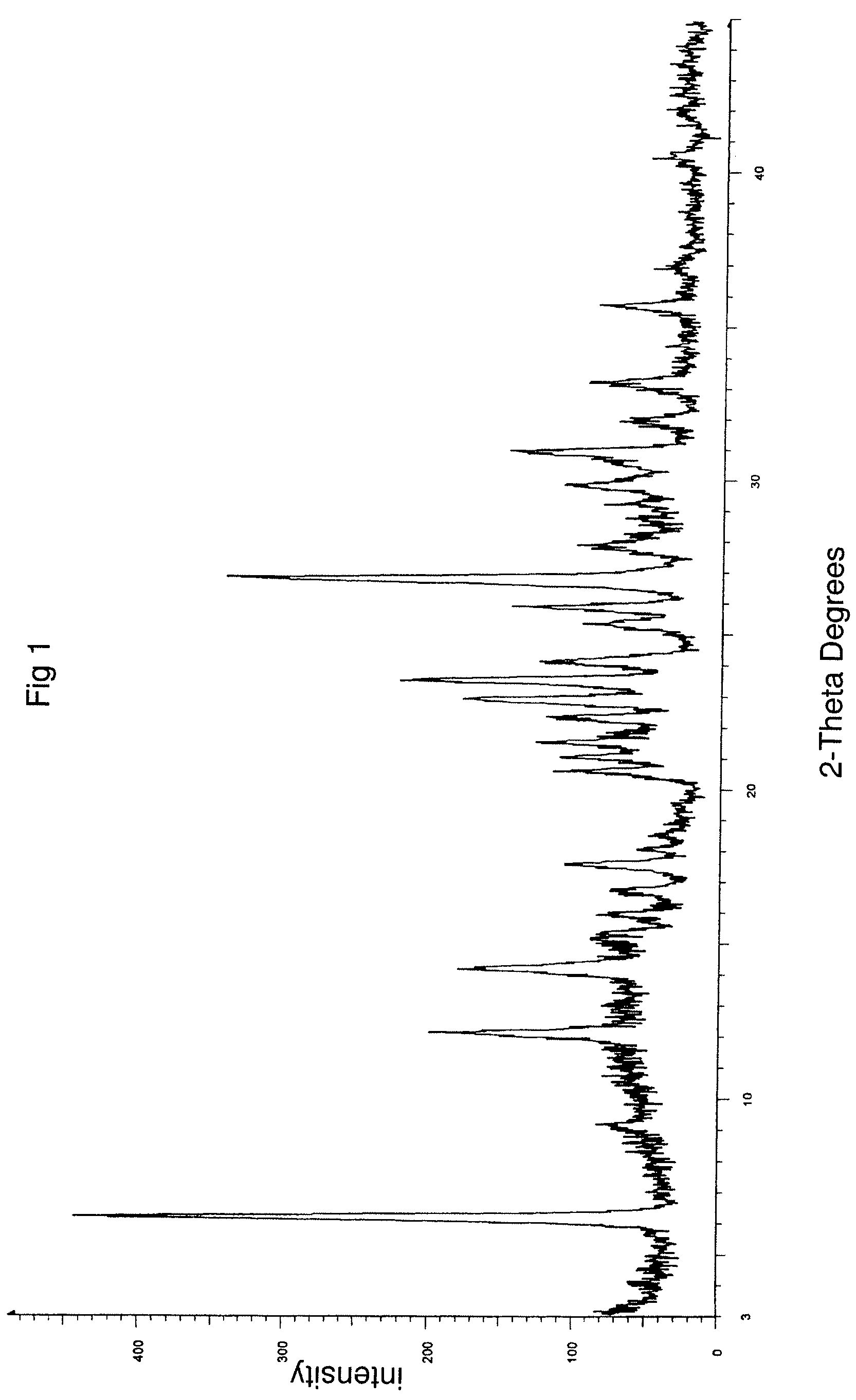
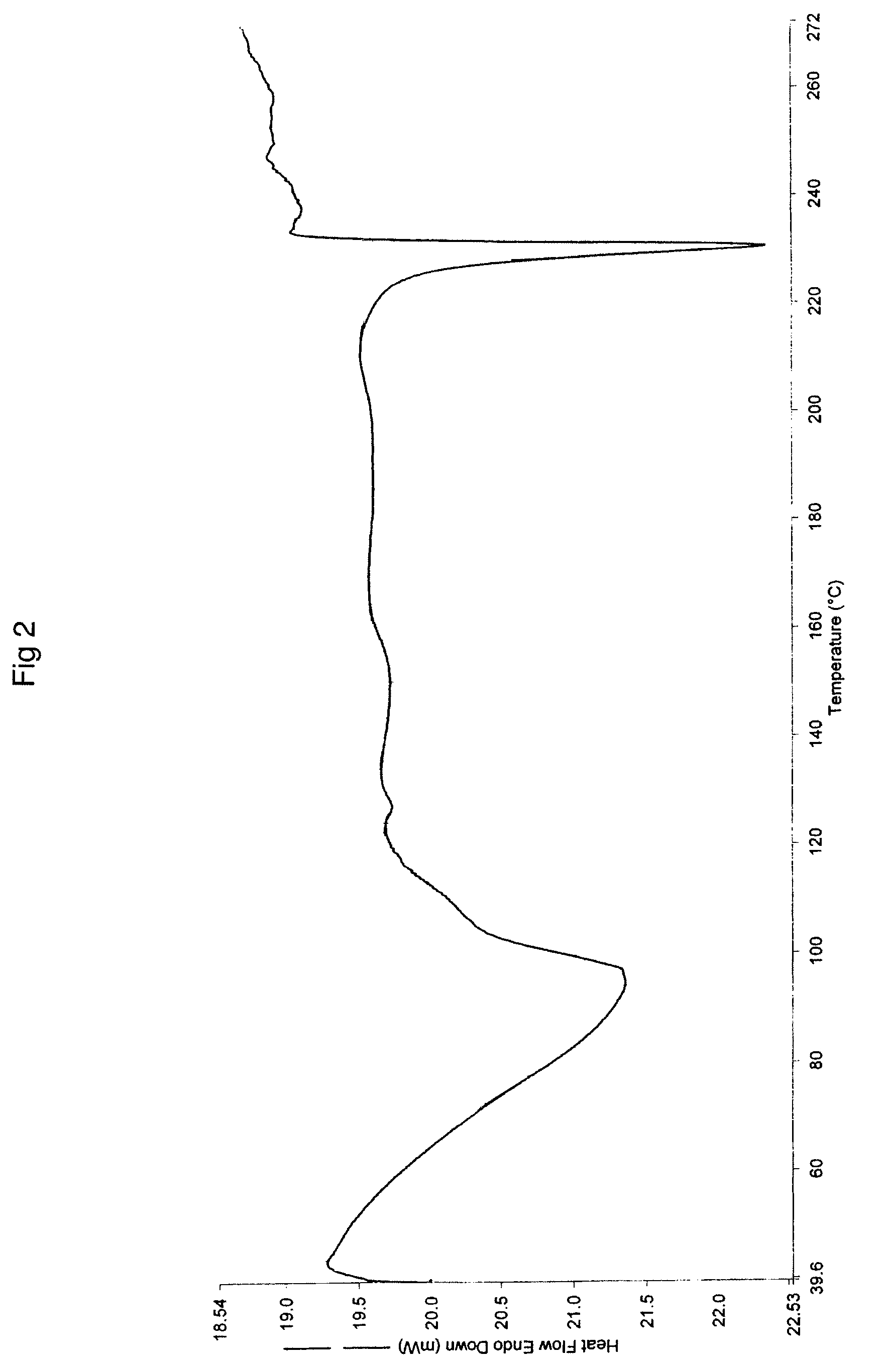
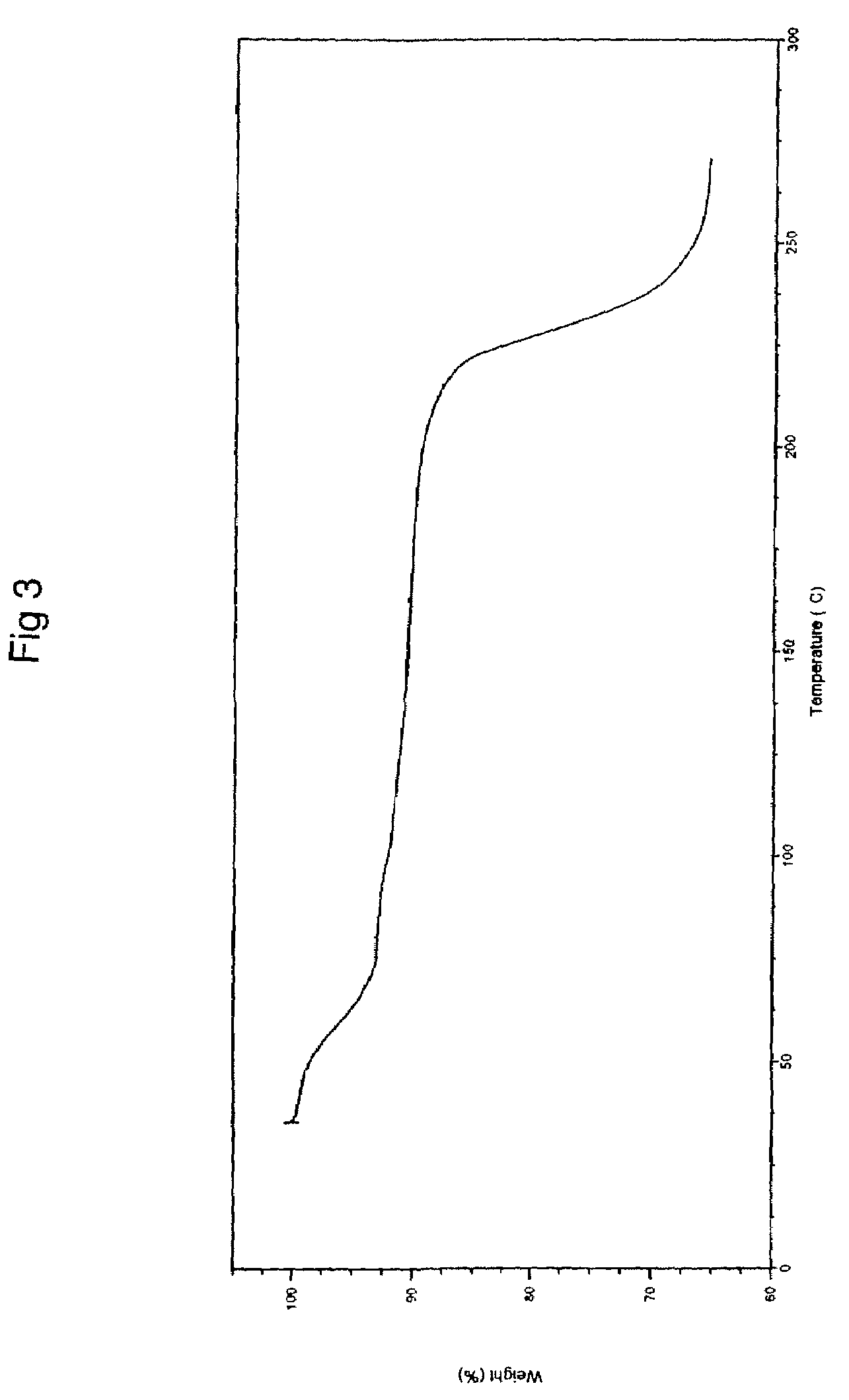
The invention relates to a novel crystalline form of topotecan hydrochloride, and methods of making the same. The characteristic XRPD pattern and FT-IT patterns are shown in FIGS. 1 and 2.
Owner:CIPLA LTD
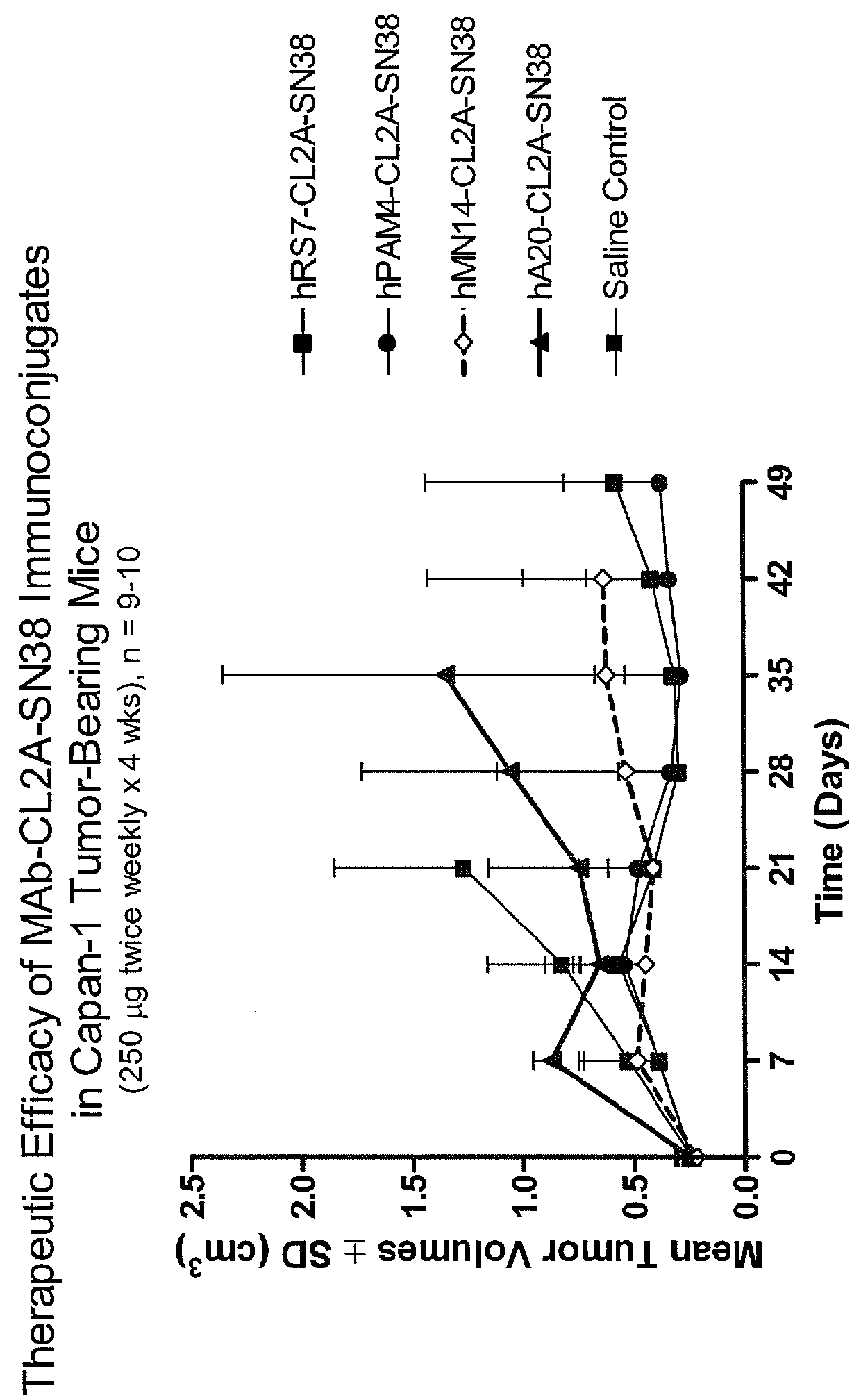
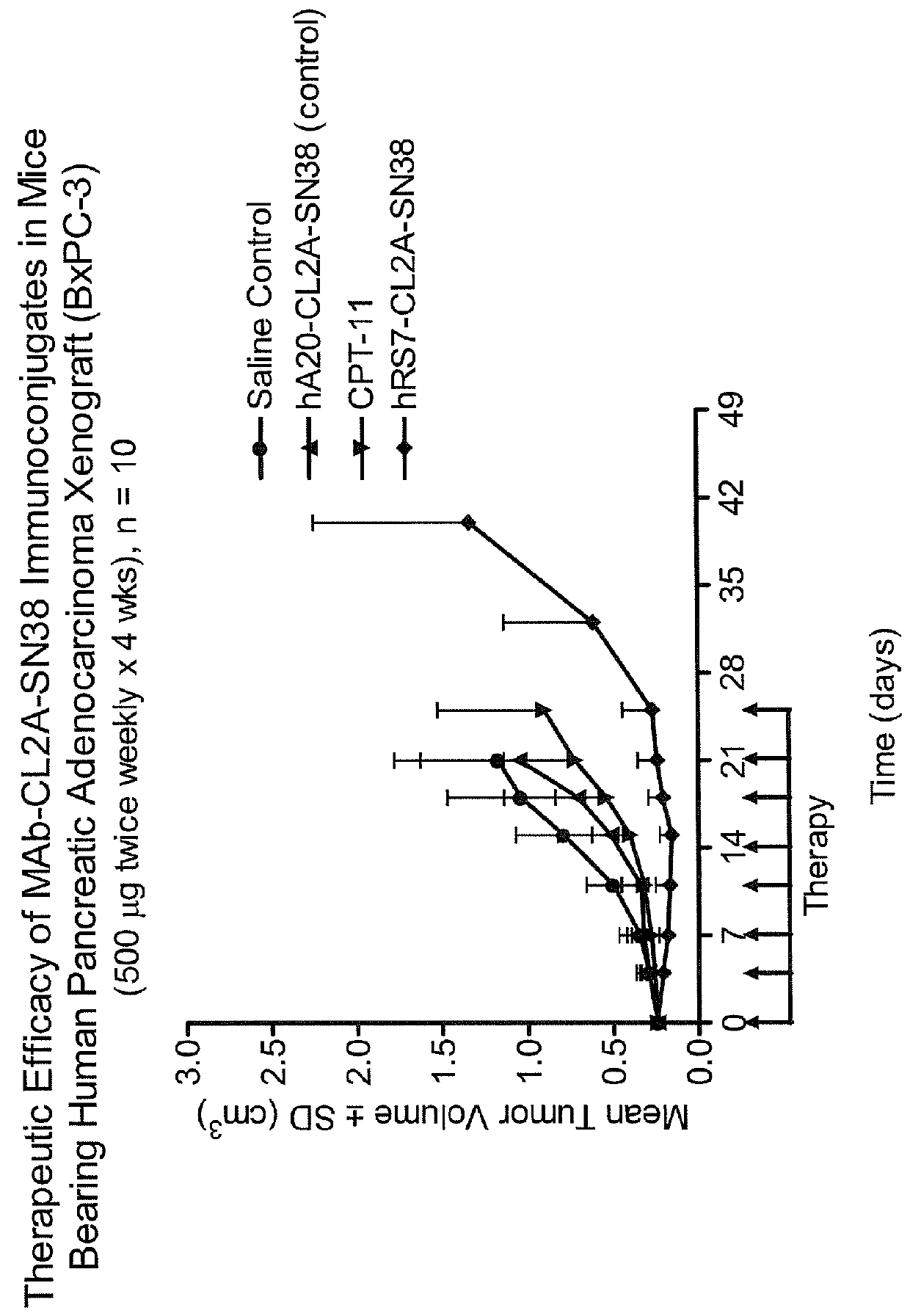
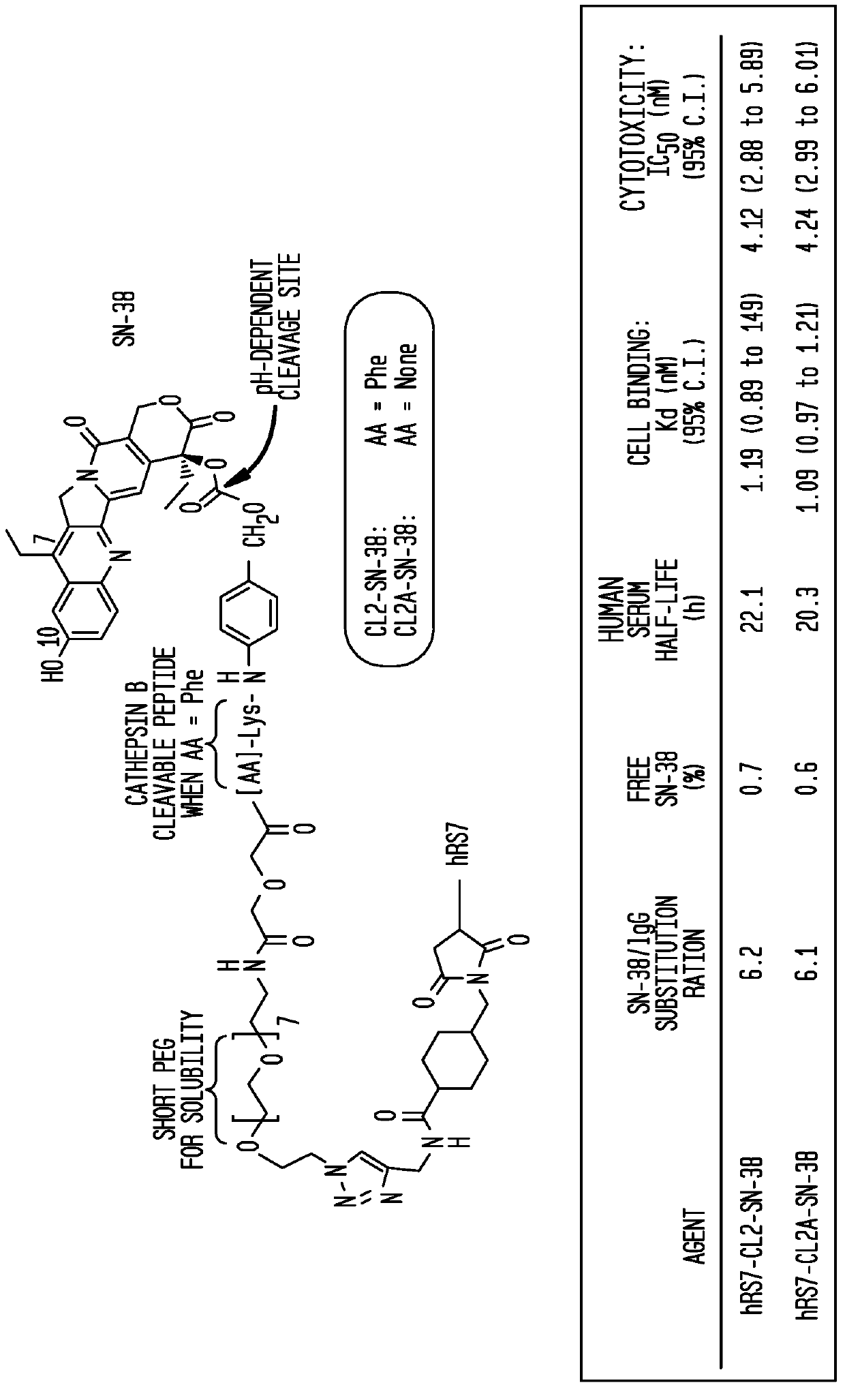
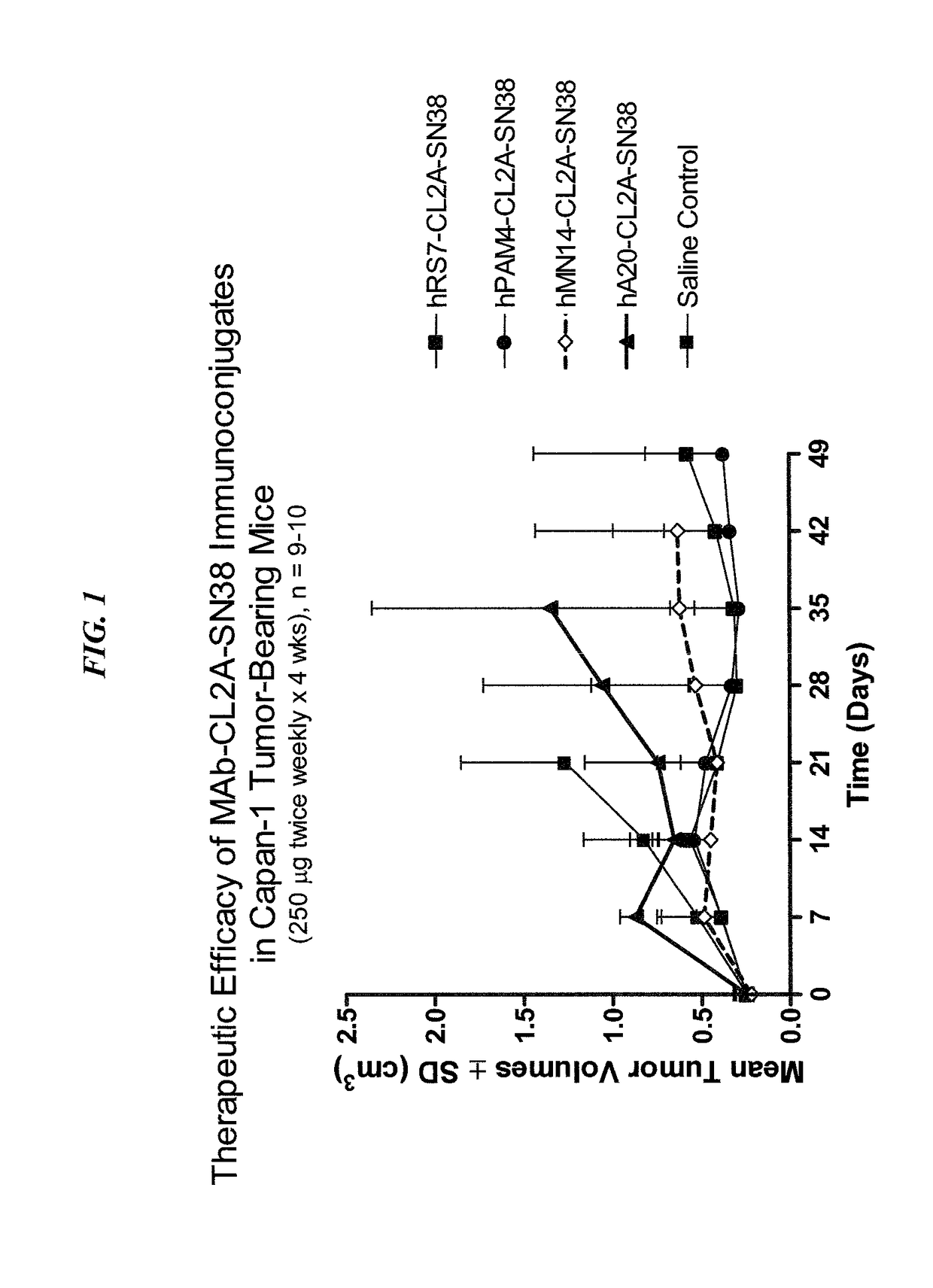
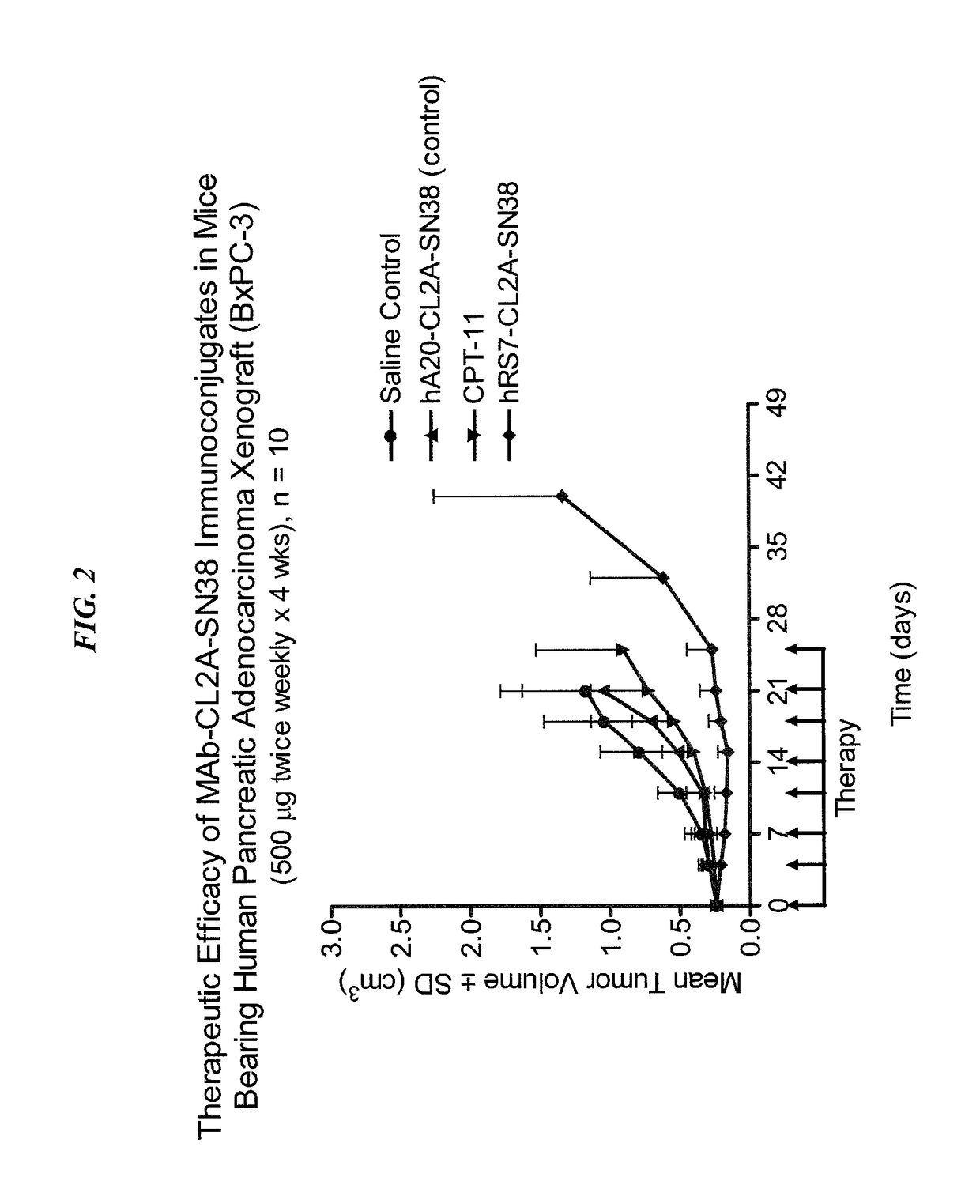
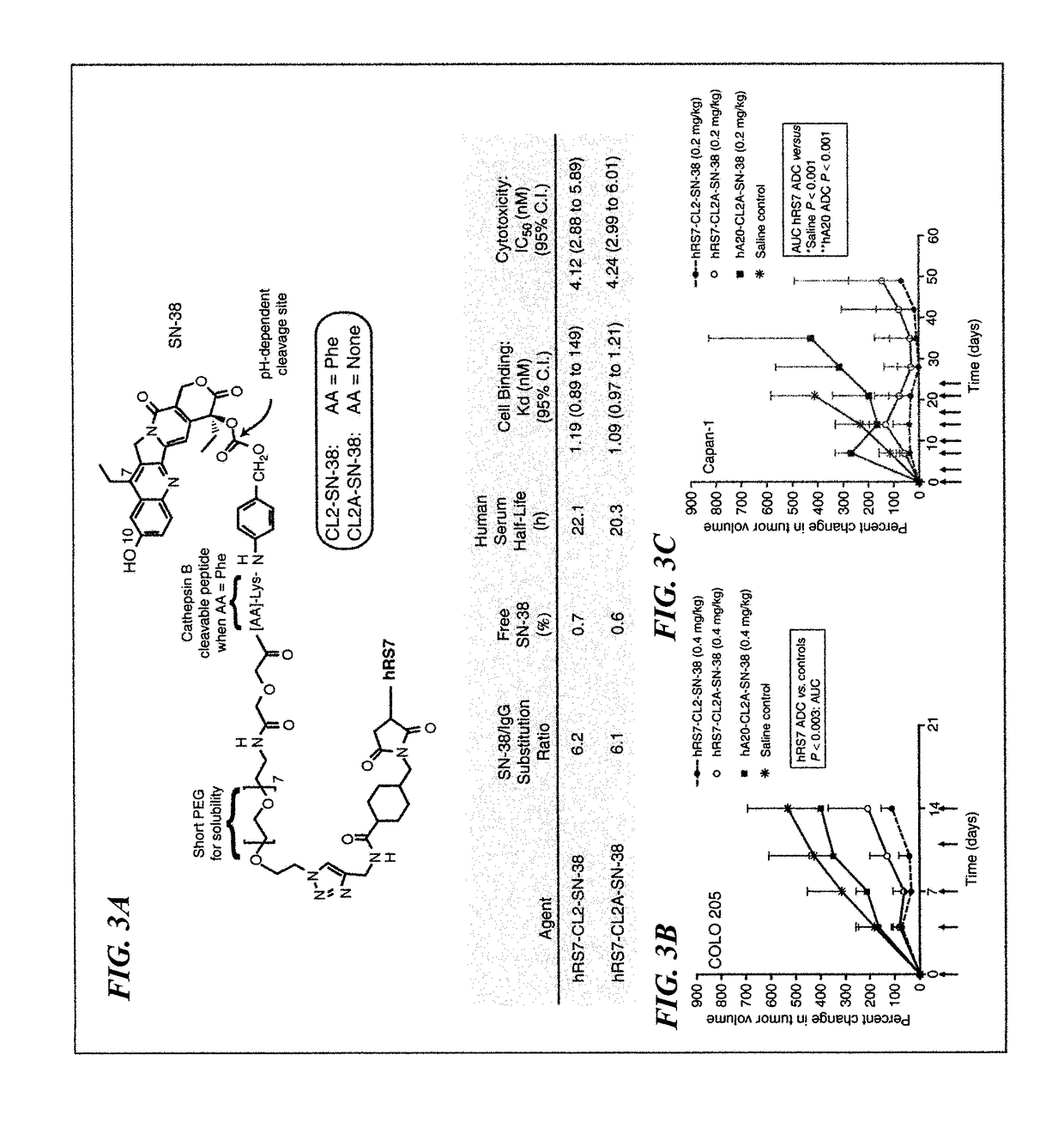
Treatment of high trop-2 expressing triple negative breast cancer (TNBC) with sacituzumab govitecan (immu-132) overcomes homologous recombination repair (HRR) rescue mediated by rad51
ActiveUS20180271992A1Good effectLow toxicityHeavy metal active ingredientsOrganic active ingredientsRAD51Lymphatic Spread
The present invention relates to treatment of Trop-2 positive cancers with the combination of anti-Trop-2 ADC and a Rad51 inhibitor. Preferably the drug conjugated to the antibody is SN-38, and the ADC is sacituzumab govitecan. The ADC may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg. When administered at specified dosages and schedules, the combination of ADC and Rad51 inhibitor can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the combination is effective to treat cancers that are refractory to or relapsed from irinotecan or topotecan.
Owner:IMMUNOMEDICS INC
Therapy of small-cell lung cancer (SCLC) with a topoisomerase-i inhibiting antibody-drug conjugate (ADC) targeting trop-2
ActiveUS20180185351A1Good effectLow toxicityOrganic active ingredientsHeavy metal active ingredientsCarboplatinLymphatic Spread
The present invention relates to treatment of SCLC with therapeutic ADCs comprising a drug attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. Preferably, the drug is SN-38. More preferably, the antibody is an hRS7 antibody and the ADC is sacituzumab govitecan. The ADC may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg, mostly preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the ADC is effective to treat cancers that are refractory to or relapsed from irinotecan or topotecan. Preferably, the ADC is administered as a combination therapy with one or more other anti-cancer treatments, such as carboplatin or cisplatinum.
Owner:IMMUNOMEDICS INC
Method of determining acute myeloid leukemia response to treatment with farnesyltransferase inhibitors
ActiveUS20130130999A1Faster assayQuick forecastBiocideMicrobiological testing/measurementMyeloid leukemiaAptX
The disclosed method rapidly identifies with desired accuracy AML patients, including elderly AML patients, likely to respond to treatment with a combination of a farnesyltransferase inhibitor and one or more of etoposide, teniposide, tamoxifen, sorafenib, paclitaxel, temozolomide, topotecan, trastuzumab and cisplatinum. In an embodiment, the improvements include the use of whole blood rather than the customary bone marrow sample, thus making the assay more accurate, rapid, less intrusive, less expensive as well as less painful. The method includes evaluation of a two-gene expression ratio (RASGRP1:APTX), which with a corresponding threshold, provides sufficient accuracy for predicting the response to the combination treatment. In the preferred embodiment the combination treatment combines tipifarnib (R115777, ZARNESTRA®) with etoposide. Further, the elderly AML patients identified as being likely responsive to the combination treatment with tipinifarb and etoposide have a complete recovery rate comparable to the best therapy available for younger patients.
Owner:JANSSEN PHARMA NV
Imaging of drug accumulation as a guide to antitumor therapy
InactiveUS7175830B2Effective judgmentAvoid the needX-ray constrast preparationsRadioactive preparation carriersDocetaxel-PNPDocetaxel
The use of radio-labeled antitumor drugs in the treatment of solid tumors by the method of administering a radio-labeled anticancer drug to a patient and imaging at least a part of the patient using Positron Emission Tomography imaging is described. The method can be used to monitor delivery of antitumor drugs to tumors, to predict the effectiveness of therapy with a particular antitumor drug or combination of antitumor drugs, to assess the effectiveness of modulators of cellular accumulation, to individualize therapy and to evaluate the effectiveness of antitumor drugs with respect to particular cancers. Particularly preferred drugs are labeled taxanes, e.g., 11C-paclitaxel and 11C-docetaxel, labeled anthracyclines, e.g., 11C-doxorubicin and 11C-epirubicin, and other radiolabeled drugs, e.g. 11C-topotecan, 11C-SN-38, and 11C-imatinib. The invention further describes antitumor drugs labeled with the radioactive label 11C and methods of preparing radio-labeled drugs.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US REPRESENTED BY THE SEC
Thermal sensitive liposome preparation containing camptothecin antineoplastic agents
InactiveCN101744767AOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention relates to a thermal sensitive liposome preparation containing camptothecin antineoplastic agents, which contains the camptothecin antineoplastic agents, common lipoid, a phase-change regulator and an optional long-circulating material, wherein the phase-change regulator accounts for 6-100% of the weight of liposome, the long-circulating material accounts for 0-40% of the weight of the liposome, and the weight ratio of the amptothecin antineoplastic agents to the liposome is 1:0.5-1:100. Since the liposome preparation is characterized by thermal sensitivity, when the tissues of a tumor are heated partially, the liposome is targeted to the position of the tumor and releases a lot of medicine at the position of the tumor, thereby improving the curative effect and reducing the overall toxic and side effect. The camptothecin antineoplastic agents of the inveition can include water-solubility derivatives, such as irinotecan and topotecan, and water-insolubility derivatives, such as hydroxycamptothecine, SN-38, 9-NC, and the like.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
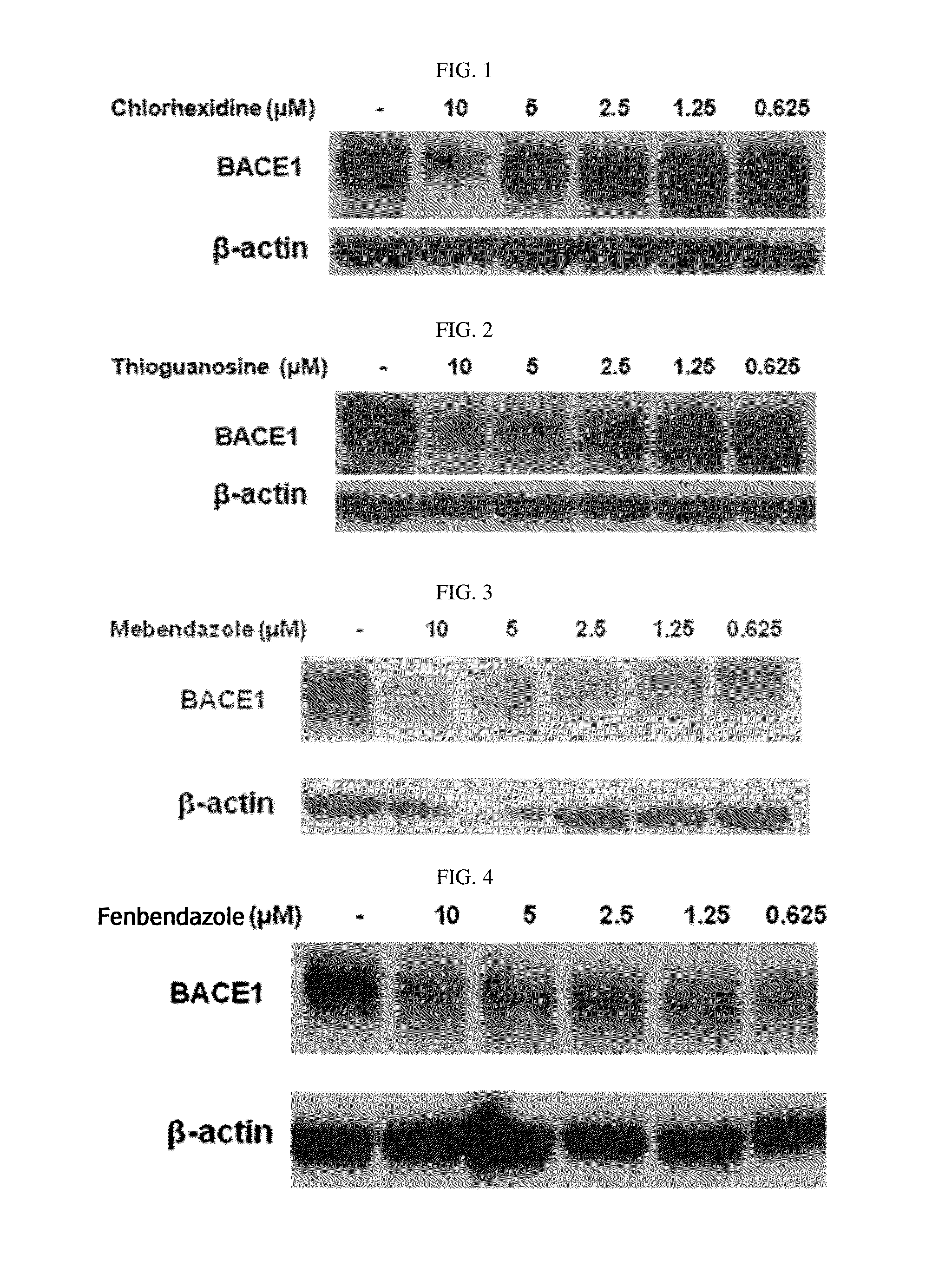
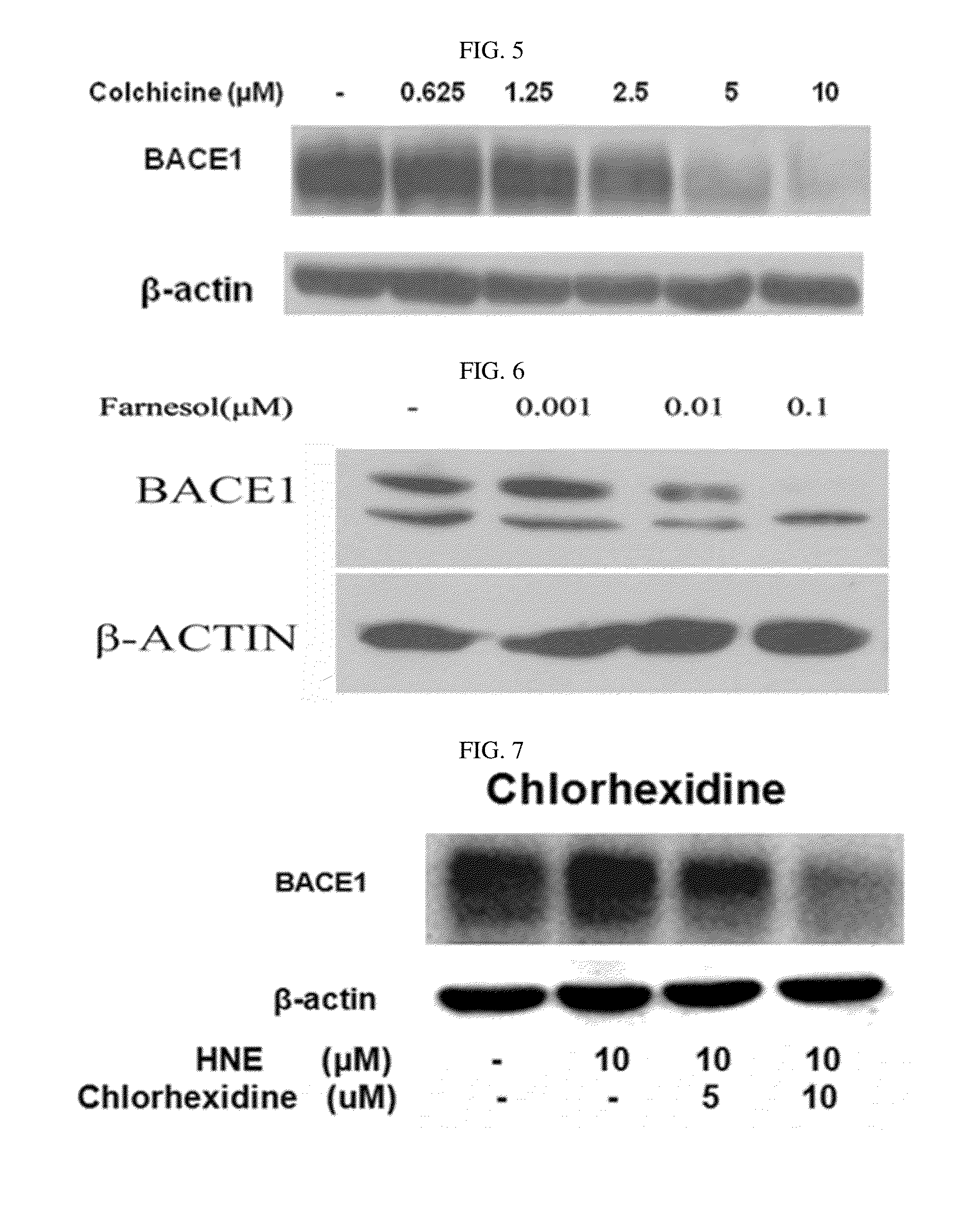
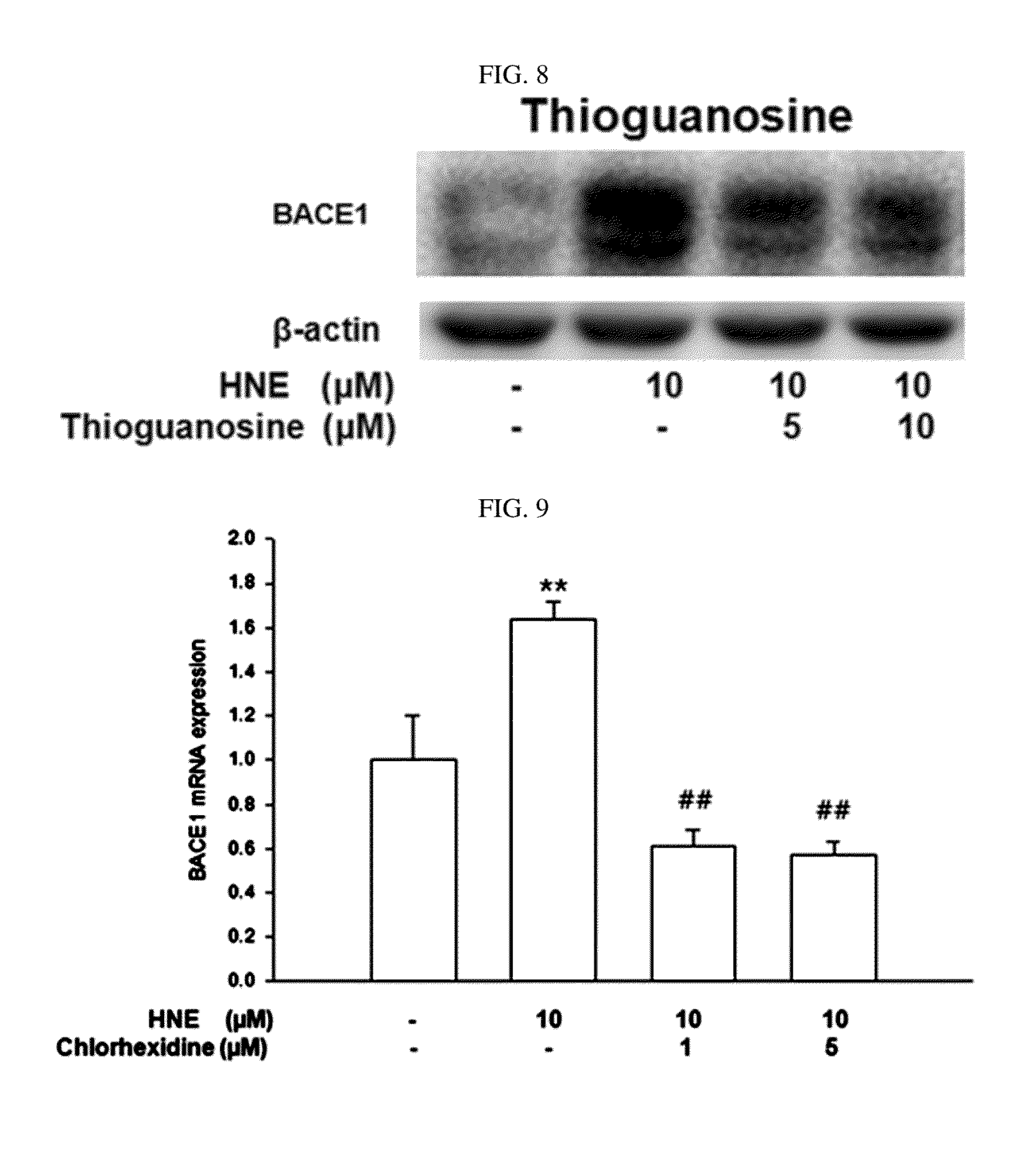
Composition for preventing or treating degenerative brain diseases including compound downregulating expression of BACE1 proteins
ActiveUS20150018297A1Reduce expressionSuppress generationBiocideSugar derivativesEfavirenzRaloxifene Hydrochloride
The present disclosure relates to a pharmaceutical composition, a health functional food composition, and a method for preventing or treating a brain disease or diabetes. The pharmaceutical composition, health functional food composition, and method includes at least one active ingredient that is chlorhexidine, thioguanosine, mebendazole, fenbendazole, colchicine, farnesol, trimethobenzamide hydrochloride, disulfuram, azathioprine, mebeverine hydrochloride, zaprinast, tosufloxacin hydrochloride, efavirenz, thiostrepton, probenecid, entacapone, harmine hydrochloride, flunisolide, thimerosal, hexestrol, sulfaquinoxaline sodium salt, monensin sodium salt, raloxifene hydrochloride, 2-chloropyrazine, or topotecan.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
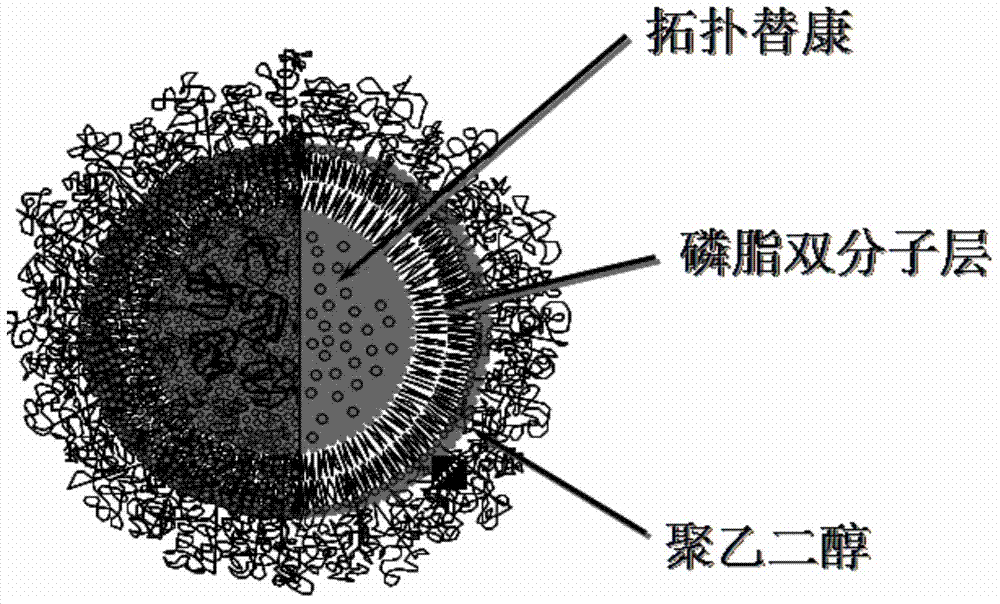
Topotecan hydrochloride targeted liposome preparation and preparation method thereof
InactiveCN102764234ASolve the problem that the mass effect reduces the drug loadingHigh drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetingPolyethylene glycol
The invention provides a topotecan hydrochloride targeted liposome preparation and a preparation method of the topotecan hydrochloride targeted liposome preparation. According to the topotecan hydrochloride targeted liposome preparation, antitumor drug topotecan hydrochloride is encapsulated into a targeted long-circulating liposome modified by hydrophilic polymer polyethylene glycol and targeted ligand RGD cyclopeptide, and the lipsome is capable of prolonging the circulating time of the medicine in the body, thereby the tumor targeting is achieved, the curative effect is improved and the toxic and side effects are reduced.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
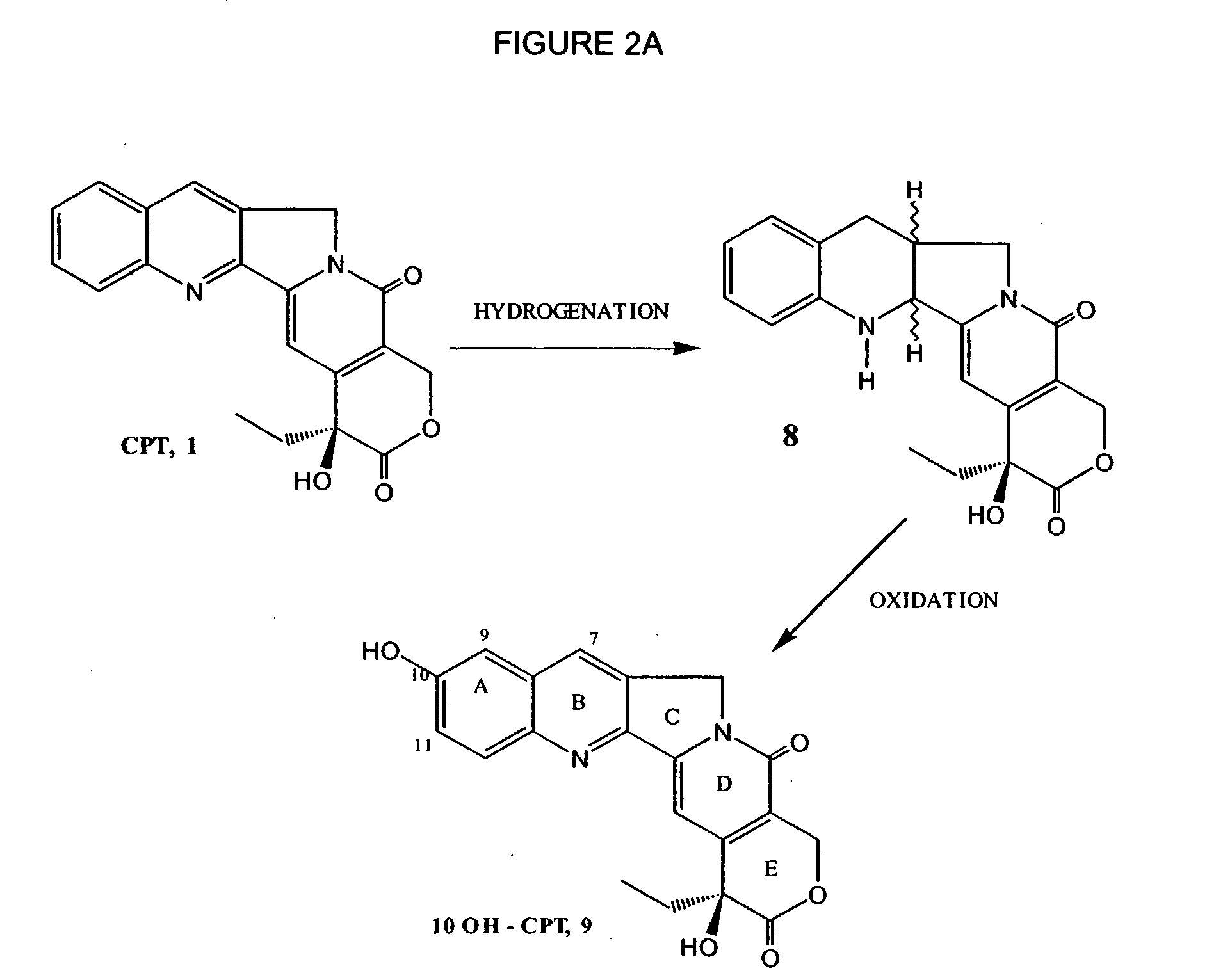
Process for preparing Topotecan from 10-hydroxy-4-(S) camptothecin
The present invention relates to the use of dihalomethanes as reagents for the preparation of Topotecan{4-(S)-10(dimethylamino)-methyl-4-ethyl 4,9 dihydroxyl-H-pyrano[3'4':6,7]indolizino-[1,2-b]quinoline-3,14(4H,12H)dione} from 10-hydroxycamptothecin. The invention discloses the rationale use of dichloromethane under solid-liquid phase transfer catalysis, which can behave both as solvent and a reactant when it serves as a source for C-1 unit for amino-alkylation of 10-hydroxy-4-(S)camptothecin.
Owner:COUNCIL OF SCI & IND RES
Method for treating abnormal cell growth
InactiveUS20050267140A1Ease of detectabilityEasy to prepareBiocideAnimal repellantsNitrocamptothecinSN-38
Therapeutic pharmaceutical compositions and methods of treatment of abnormal cell growth comprising a pyrimidine derivative or a pharmaceutically acceptable salt, solvate or prodrug thereof in combination with an oral camptothecin, an oral camptothecin derivative, an indolopyrrocarbazole derivative or a pharmaceutically acceptable salt, solvate or prodrug thereof for the treatment of cancer are described. In one embodiment of the present invention the oral camptothecin derivative is selected from the group consisting of 10-hydroxycamptothecin, 9-aminocamptothecin, 9-nitrocamptothecin, irinotecan, irinotecan salt, SN-38, CPT-11, and topotecan and the indolopyrrocarbazole derivative is edotecarin. In one embodiment the pyrimidine derivative is selected from the group consisting gemcitabine, MTA and capecitabine. In one preferred embodiment, the pyrimidine derivative is capecitabine and the camptothecin derivative is CPT-11.
Owner:PFIZER INC
Topotecan liposome and preparation method therefor
InactiveCN101015526AAnti-tumor effect is effectiveImprove anti-tumor effectPowder deliveryOrganic active ingredientsFreeze thawingSucrose
This invention relates to a topotecan liposome and its preparation method. The preparation can be used as therapeutic drug of multiple tumours such as non-small cell lung cancer, liver cancer, stomach cancer, hematopathy, and lymphomata. The topotecan liposome is characterised in containing topotecan and at least one biologic receivable phospholipid at a weight ratio of 1:0.5. The phospholipid is selected from one of natural phospholipid, hydrogenated soybean phosphatide, and synthesized phospholipid. The liposome can also contain cholesterol and supported reagent. The supported reagent is selected from one of glucose, mannit, sucrose, sorbitol, dextran, lactose, and trehalose. The weight ratio of phospholipid and supported agent is 1:0.01-500. The preparation method comprise film dispersion, injection, ultrasonic dispersion, lyophilization, freeze thawing, forceful discharging, preparing with a viscolizer, and preparing through microjet under high pressure.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Dose regime for camptothecin derivatives
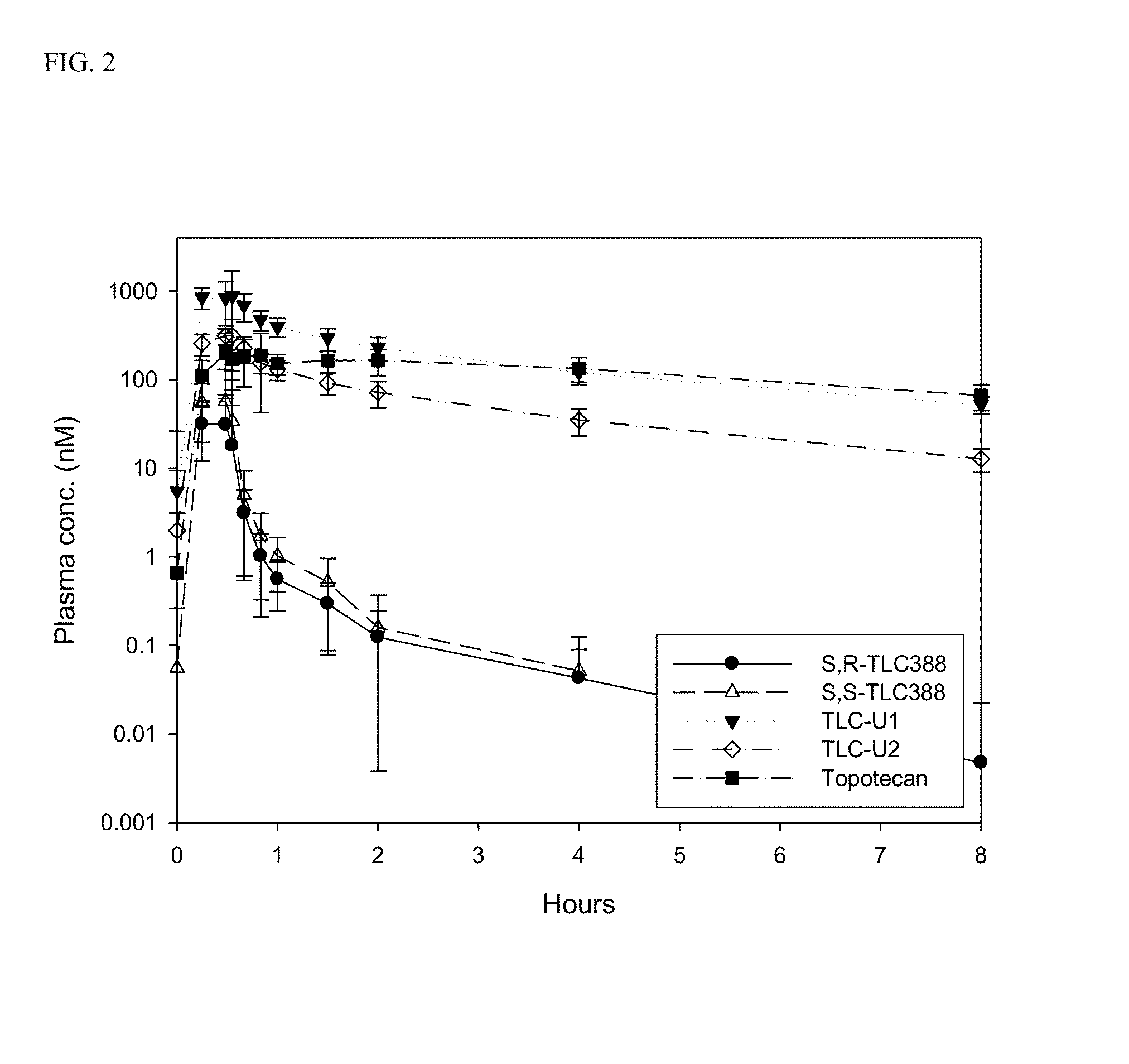
The present invention is directed to a method of inhibiting cancer cell growth, comprising administering a pharmaceutical composition to a subject in need thereof. The pharmaceutical composition comprises at least one camptothecin derivative or a pharmaceutically acceptable salt thereof; and at least one PEG phospholipid, and provides a sustained release of topotecan as an active ingredient.
Owner:TLC BIOPHARMACEUTICALS INC +1
Process to Prepare Camptothecin Derivatives and Novel Intermediate and Compounds Thereof
InactiveUS20080051580A1Organic chemistryAntineoplastic agentsCompound (substance)Camptothecin derivative
New processes are disclosed for the preparation of camptothecin derivatives, such as, irinotecan and topotecan, as well as new intermediates and compositions thereof.
Owner:CHATHAM BIOTEC LTD
Compounds and compositions for the treatment of cancer
New uses for phenylketone carboxylate compounds and substituted aromatic compounds of Formula I, Formula I.1, Formula I.2, Formula IA, Formula IB, Formula IC and Formula II and their pharmaceutical acceptable salts for the treatment of cancer. The use of a combination of two of these compounds is described and the use of the combination of one of these compounds with an anticancer agent such as decarbazine, doxorubicin, daunorubicin, cyclophosphamide, busulfex, busulfan, vinblastine, vincristine, bleomycin, etoposide, topotecan, irinotecan, taxotere, taxol, 5-fluorouracil, methotrexate, gemcitabine, cisplatin, carboplatin and chlorambucil.
Owner:PROMETIC PHARMA SMT LTD
Process for preparing topotecan
Owner:DR REDDYS LAB LTD +1
Topotecan hydrochloride lipidosome nano preparation and preparing method thereof
InactiveCN104771361AParticle size has no effectEffect of particle sizeOrganic active ingredientsPharmaceutical product form changeChemistryLiposome
The invention provides a preparing method of a topotecan hydrochloride lipidosome nano preparation and the topotecan hydrochloride lipidosome nano preparation prepared by the same, wherein the method comprises the following steps: a), dissolving phospholipid, cholesterol and a modification material in ethanol to obtain an organic phase, injecting the organic phase into an aqueous solution containing a gradient regulator, stirring, and homogenizing to form a blank liposome; b) with adopting of a tangential flow filtration method, utilizing deionized water to replace the ionic gradient regulator in the aqueous phase outside the blank liposome, and thus forming an ion concentration gradient of the aqueous phases inside and outside the blank liposome; c) taking topotecan hydrochloride, mixing with the blank liposome formed in the step b), incubating under a temperature higher than a phospholipid phase transition temperature, and thus obtaining the topotecan hydrochloride liposome nano preparation. The method is simple to operate, has less time-consuming, has the quality easy to control, and is suitable for industrialized mass production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
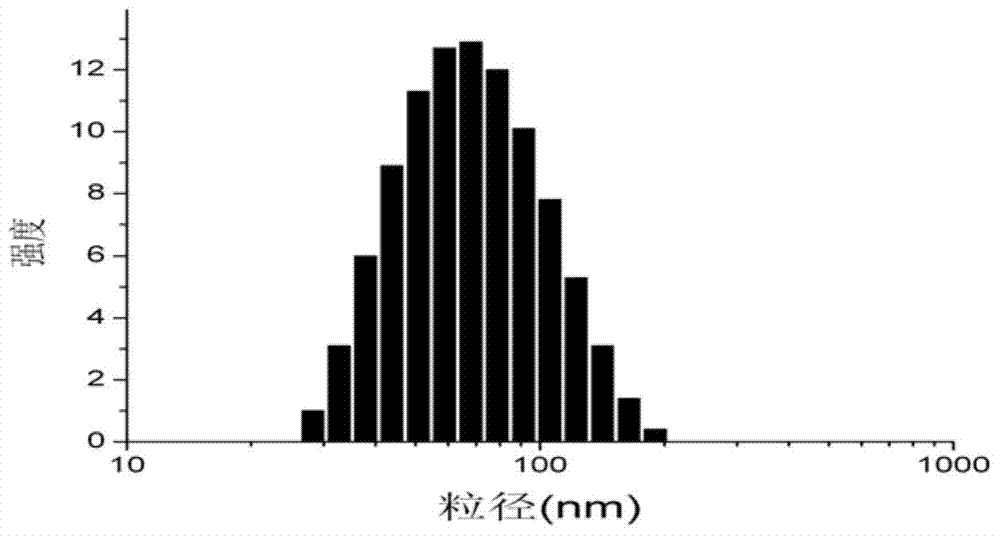
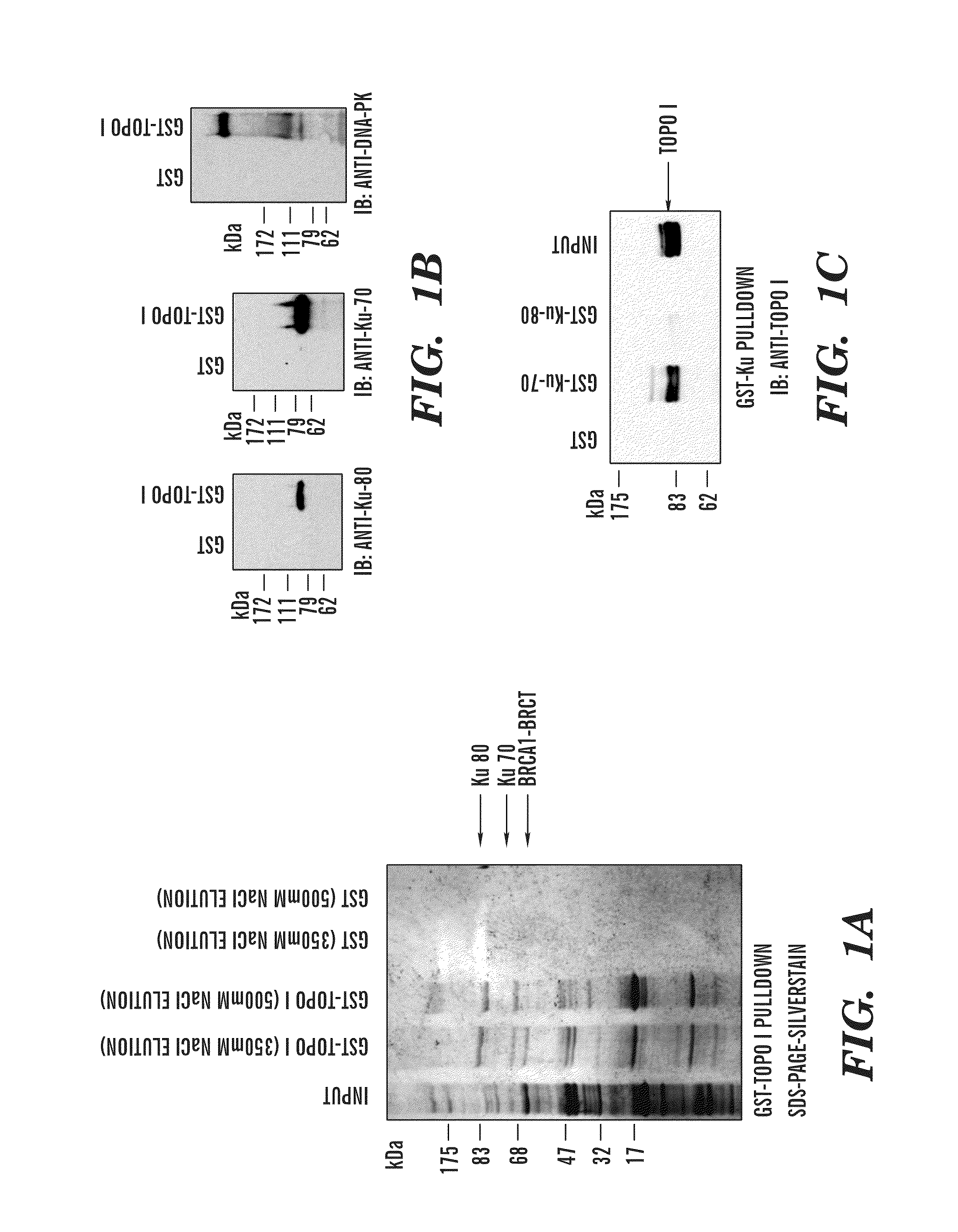
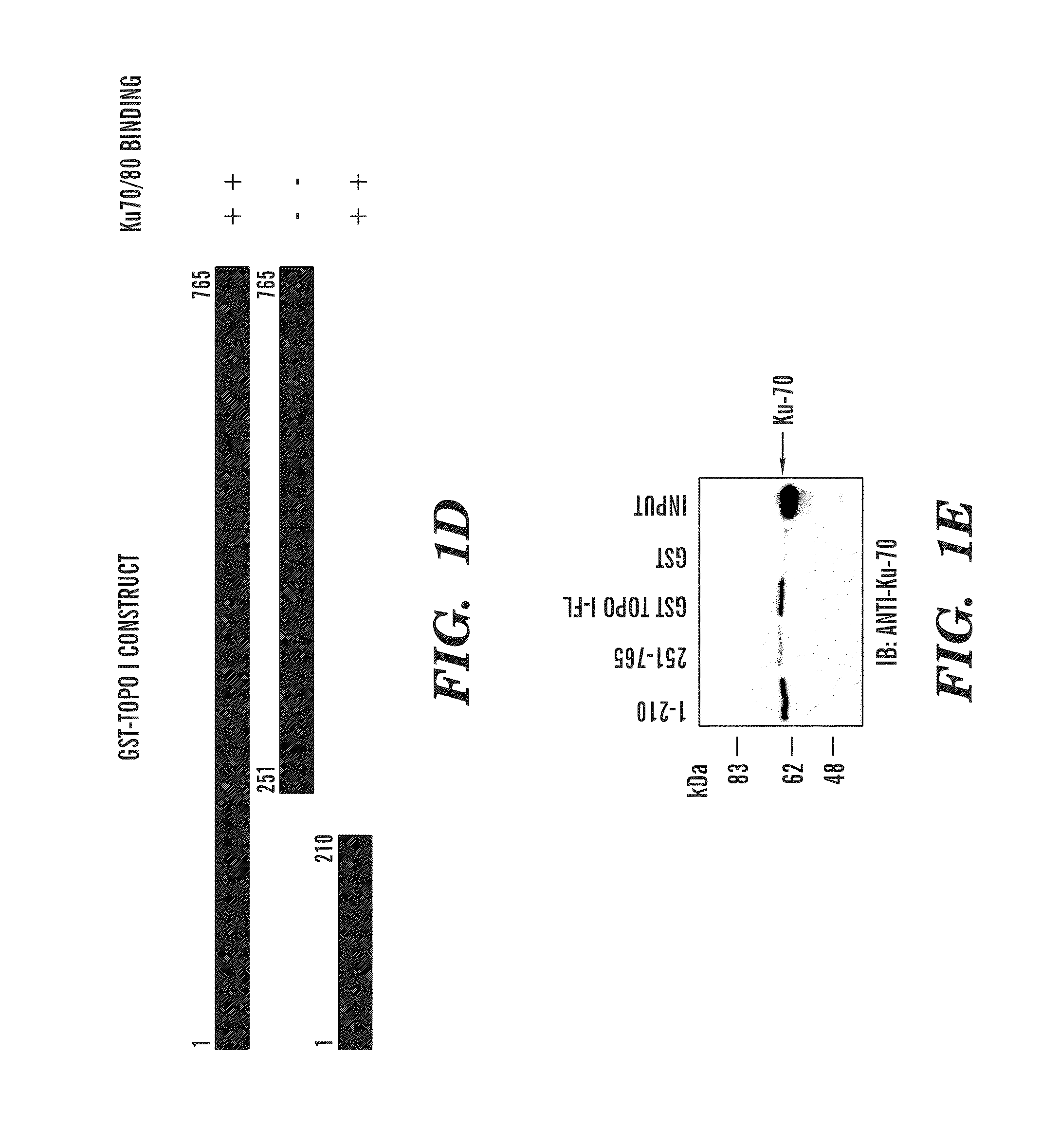
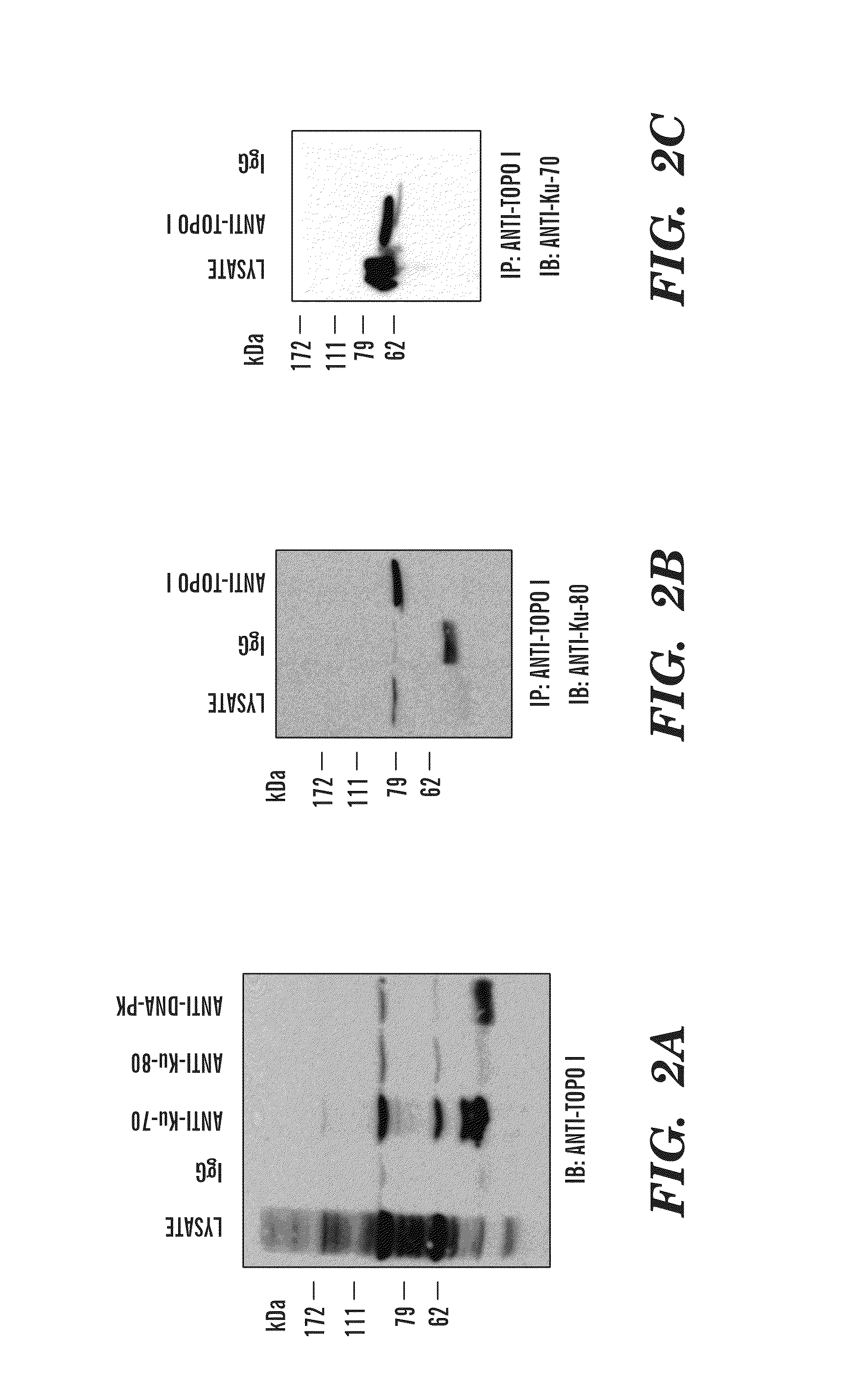
Predictive marker for topoisomerase I inhibitors
ActiveUS8993309B2Organic active ingredientsBioreactor/fermenter combinationsCancer preventionPredictive marker
The present invention generally relates to the fields of cancer therapy and cancer prevention. More particularly, the present invention generally relates to a diagnostic marker for predicting the efficacy of topoisomerase I (topo I) inhibitors in the treatment of cancers. More specifically, the present invention relates to methods, machines, computer systems, computable readable media and kits which can be used to identify and determine the effectiveness of topoisomerase I (topo I) inhibitors in the treatment of cancers, and in some embodiments, the level of sensitivity or resistance of a tumor cell to a topoisomerase I inhibitor, such as camptothecin (CPT), or CTP analogues such as topotecan and irinotecan and derivatives thereof. More specifically, the present invention related to methods, machines, computer systems, computable readable media and kits which can be used to determine the presence of phosphorylation of topoisomerase I polypeptide, in some embodiments phosphorylation at residue serine 10 (S10) of a topoisomerase I polypeptide, wherein the presence of phosphorylation, in particular the phosphorylation at serine 10 of a topoI polypeptide indicates a cancer is likely to be unresponsive to a topo I inhibitor, whereas the absence of phosphorylation, in particular, the absence of phosphorylation at residue serine 10 (S10) identifies a cancer is likely to be responsive to a topo I inhibitor. Other aspect of the present invention relate to phospho-serine 10 topoisomerase I antibodies and other protein binding moieties, and uses thereof.
Owner:BOSTON MEDICAL CENTER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com