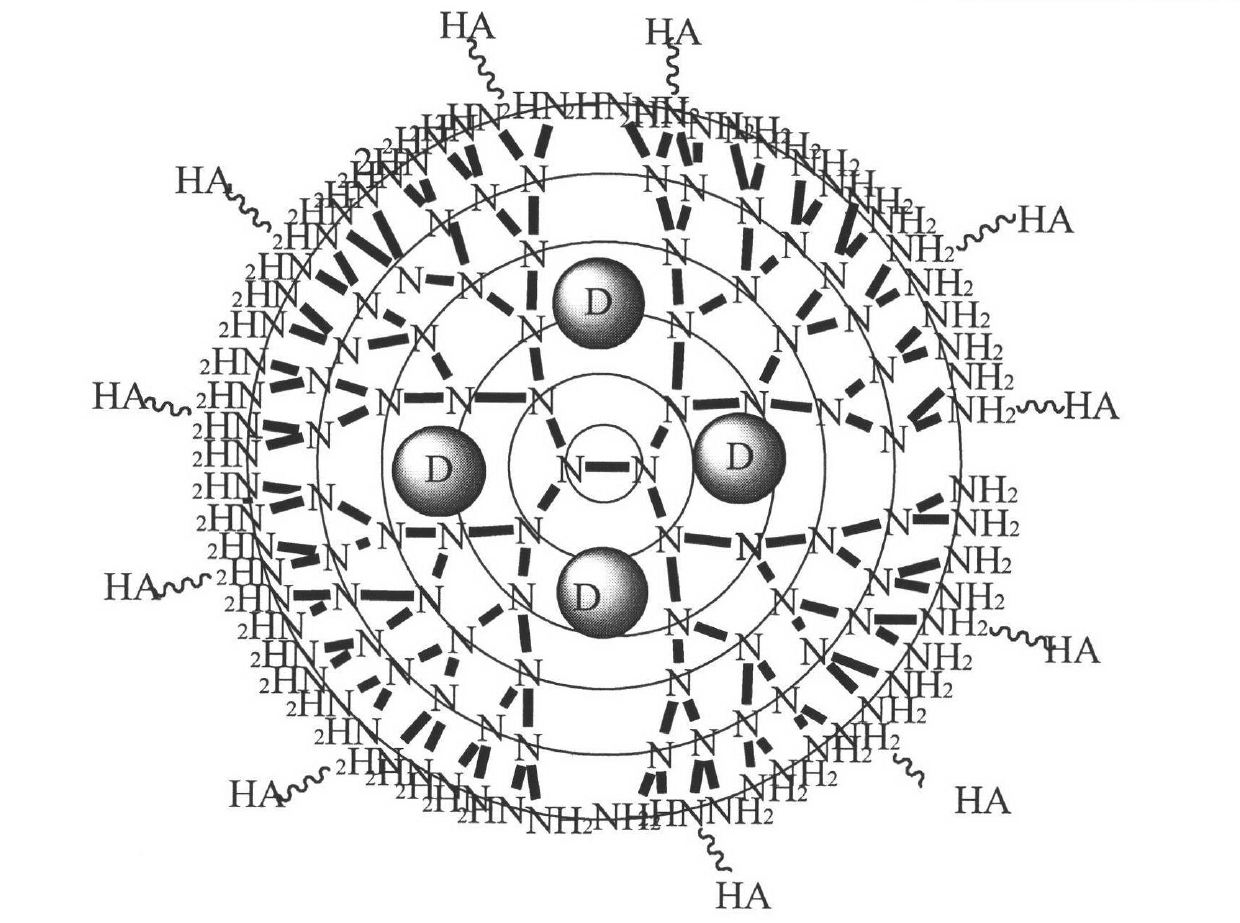
Novel tumor-targeting arboraceous polymer nano carrier of camptothecin drug
A technology of dendrimers and nanocarriers, applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve problems such as unsatisfactory positioning effect and inevitable cytotoxicity, so as to prolong blood circulation time, prevent degradation, The effect of improving targeting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
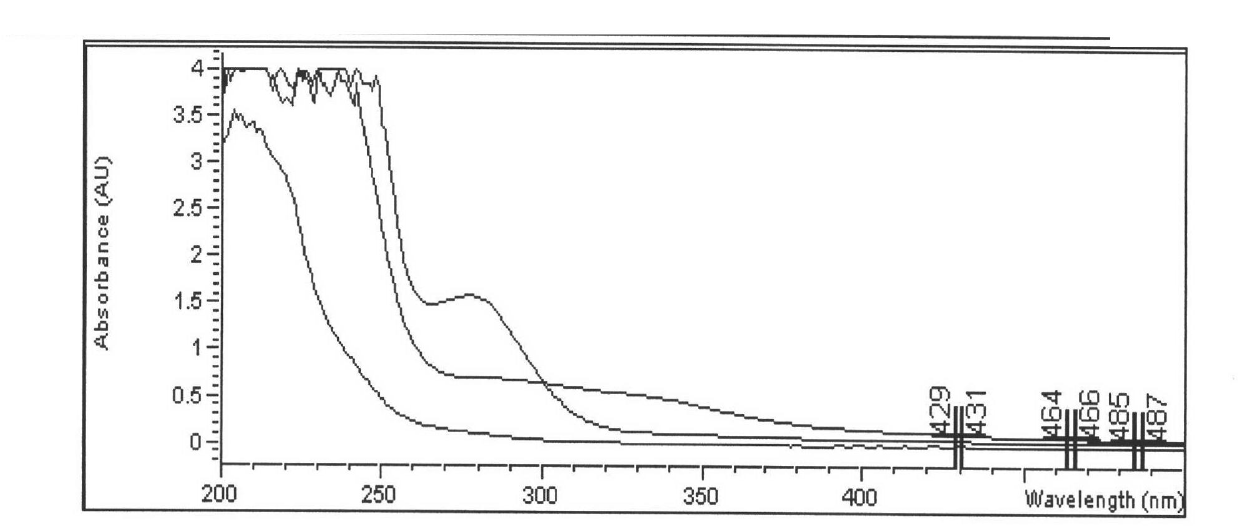
Examples
Embodiment 1
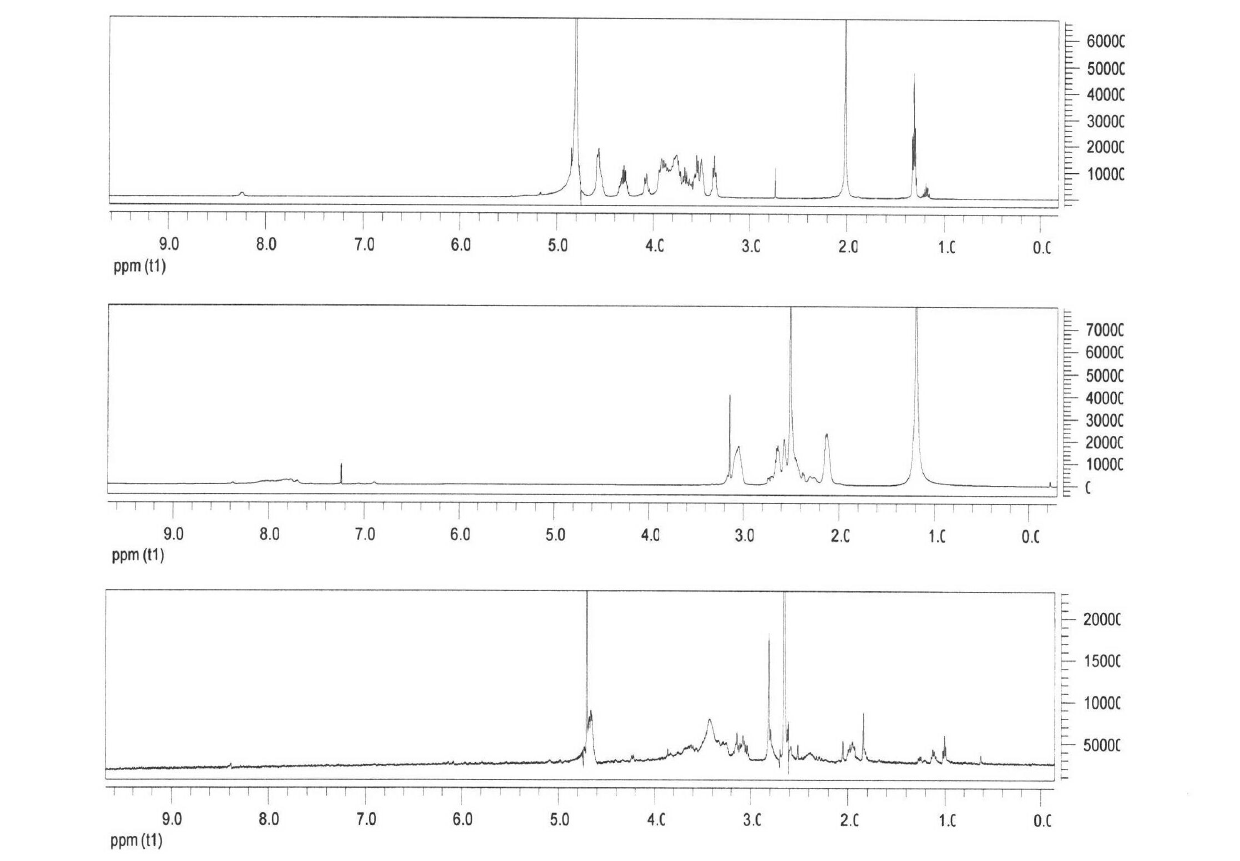
[0036] 1. Synthesis of PAMAM
[0037] A) Purification of raw materials
[0038] Methanol was dried with iodine pellets for 24h (after adding, heated to 66°C to keep small droplets dripping drop by drop, and refluxed for 24h), then distilled at atmospheric pressure, and the collected fraction was 64-66°C;
[0039] Methyl acrylate was dried with anhydrous sodium sulfate for 24 hours (after adding, heated to 78 degrees to keep small droplets dripping drop by drop, and refluxed for 24 hours), then distilled at atmospheric pressure, and the collected fractions were 77-79 degrees Celsius;
[0040] Ethylenediamine was dried with potassium hydroxide for 24h and then distilled under reduced pressure (pressure at 1000±50Pa), collecting fractions at 45-47°C.
[0041] B) Synthesis of PAMAM G0.5
[0042] Weigh 2.0068g of ethylenediamine (about 0.033mol) in a 250mL three-necked flask, add 22.0103g of methanol under conditions of ice-water bath, electromagnetic stirring, and nitrogen prote...
Embodiment 2
[0056] 1. Synthesis of PAMAM
[0057] A) Purification of raw materials
[0058] Methanol was dried with iodine pellets for 24 hours (after adding, heated to 66°C to keep small droplets dripping drop by drop, and refluxed for 24 hours), then distilled at atmospheric pressure, and the collected fraction was 64-66°C;
[0059] Methyl acrylate was dried with anhydrous sodium sulfate for 24 hours (after adding, heated to 78°C to keep small droplets dripping drop by drop, and refluxed for 24 hours), then distilled at atmospheric pressure, and the collected fraction was 77-79°C;
[0060] Ethylenediamine was dried with potassium hydroxide for 24h and then distilled under reduced pressure (pressure at 1000±50Pa), collecting fractions at 45-47°C.
[0061] B) Synthesis of PAMAM G0.5
[0062] Weigh 2.0068g of ethylenediamine (about 0.033mol) in a 250mL three-necked flask, add 22.0103g of methanol under conditions of ice-water bath, electromagnetic stirring, and nitrogen protection, mix w...
Embodiment 3-4
[0076] Except replacing PAMAM G4.0 with PAMAM G3.0 and PAMAM G5.0 respectively, others are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com