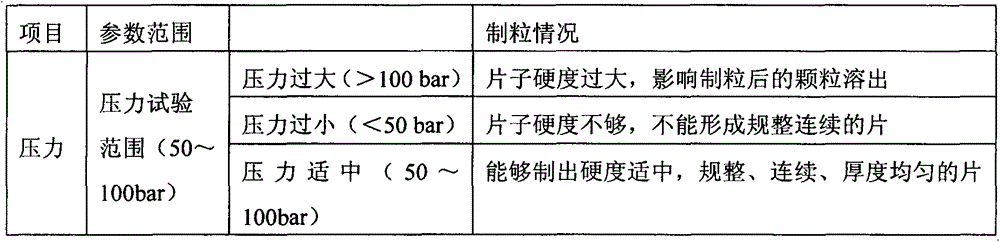
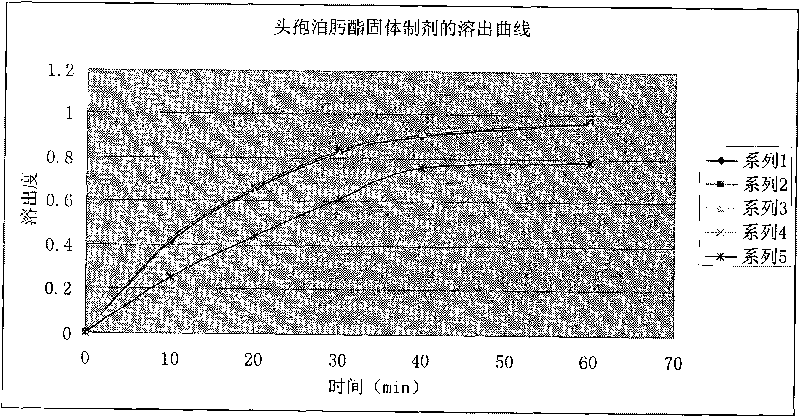
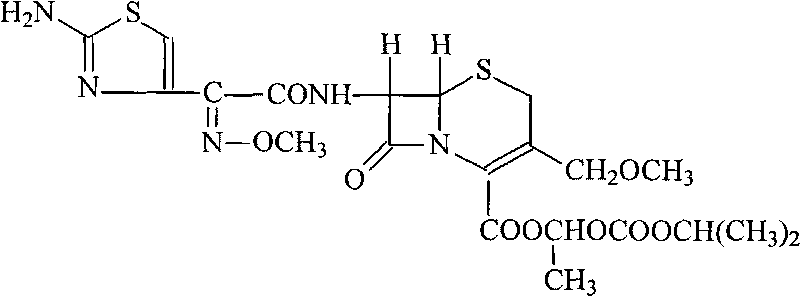
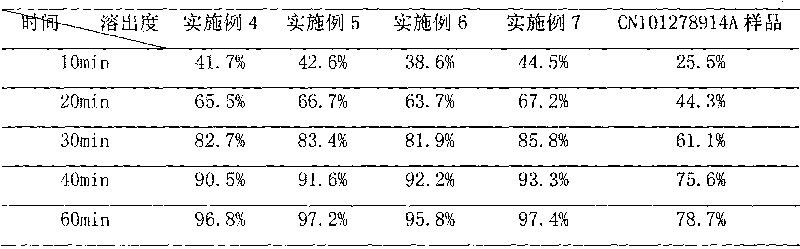
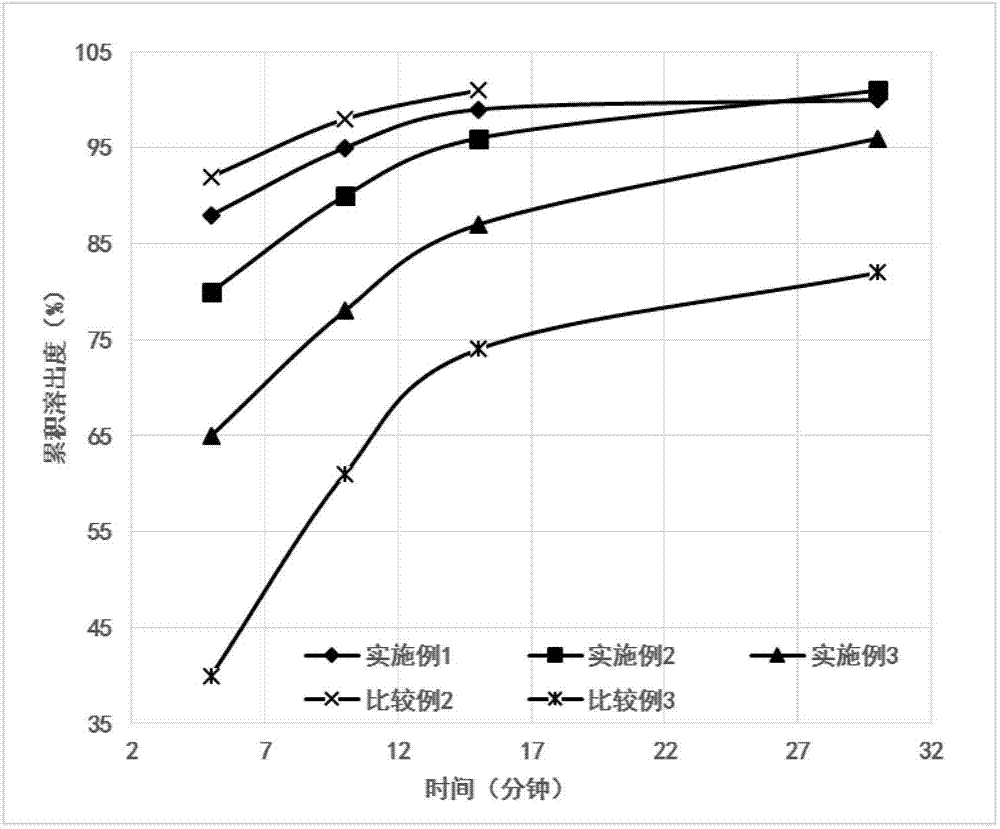
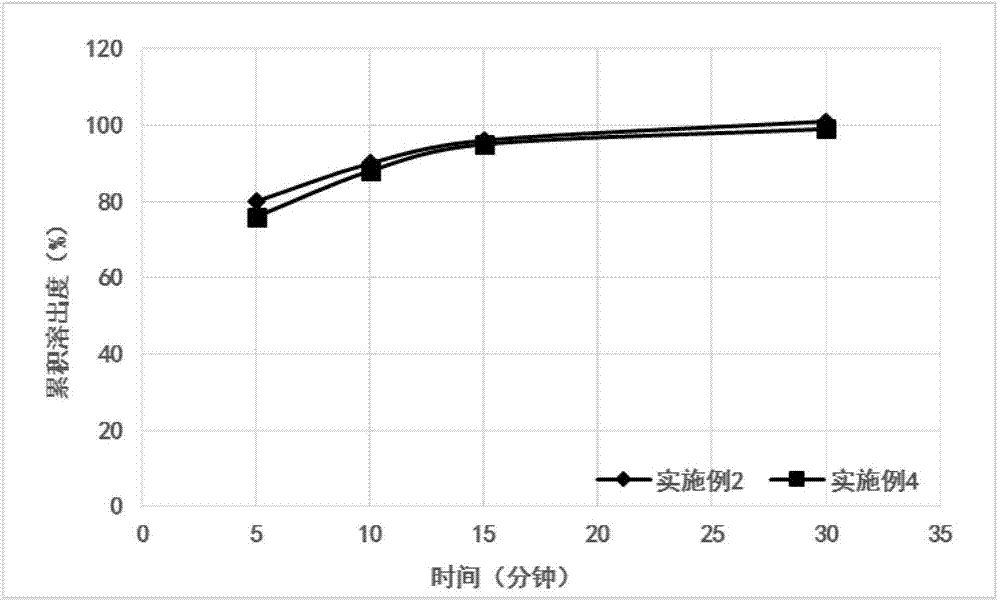
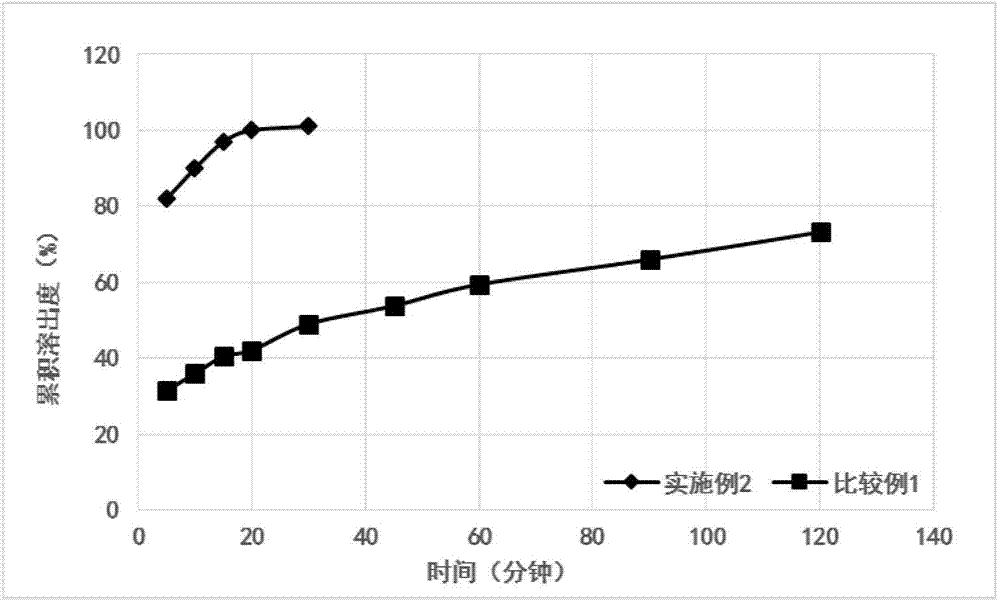
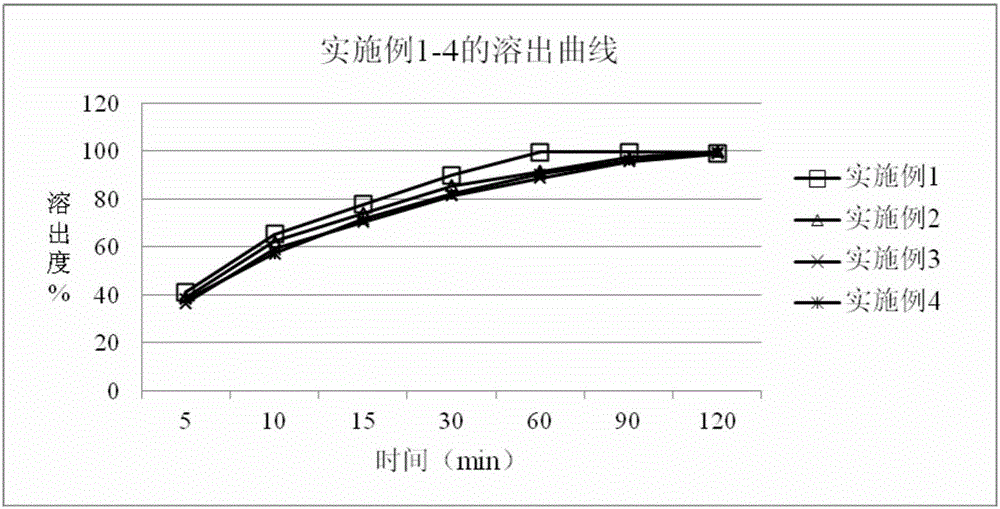
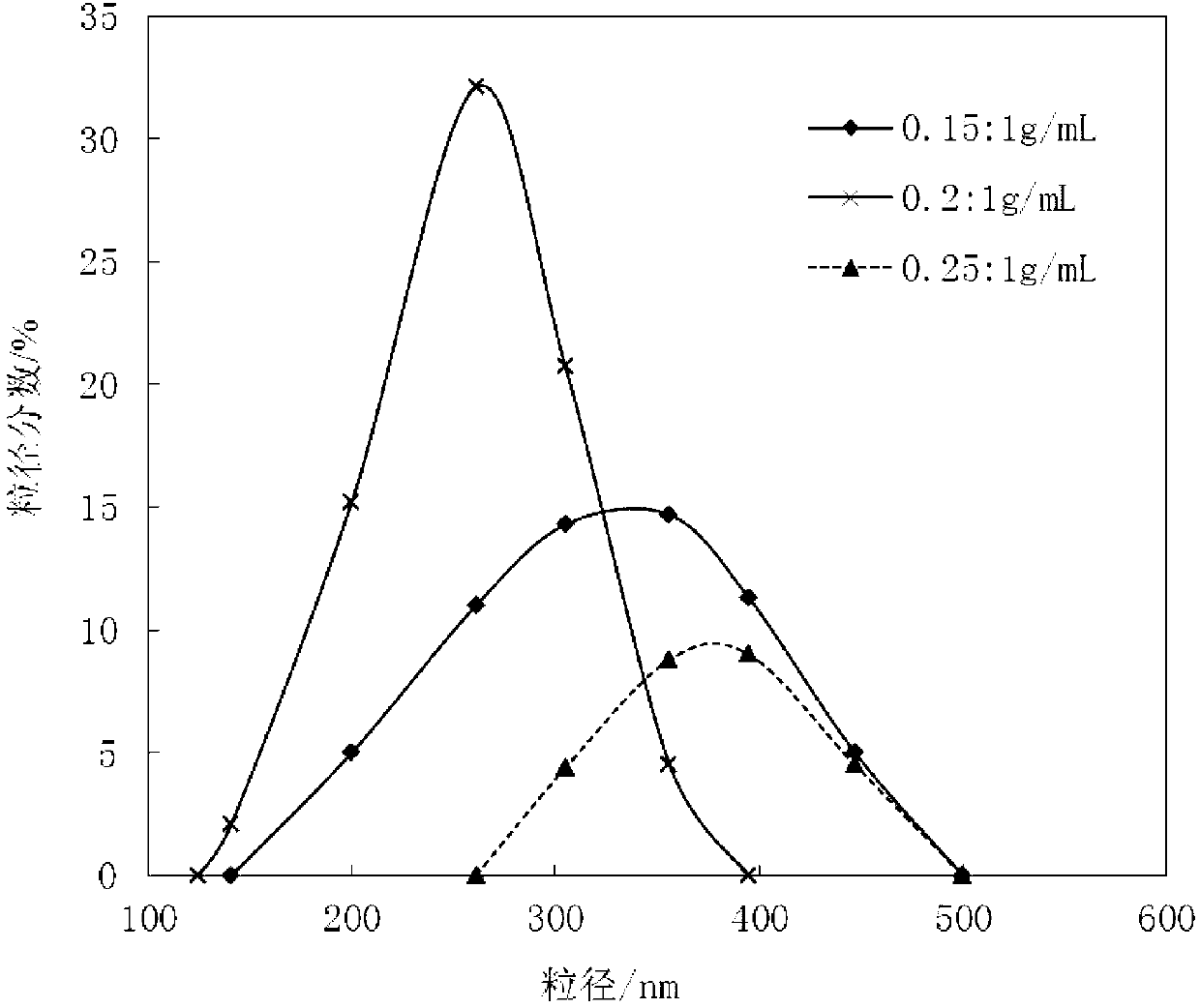
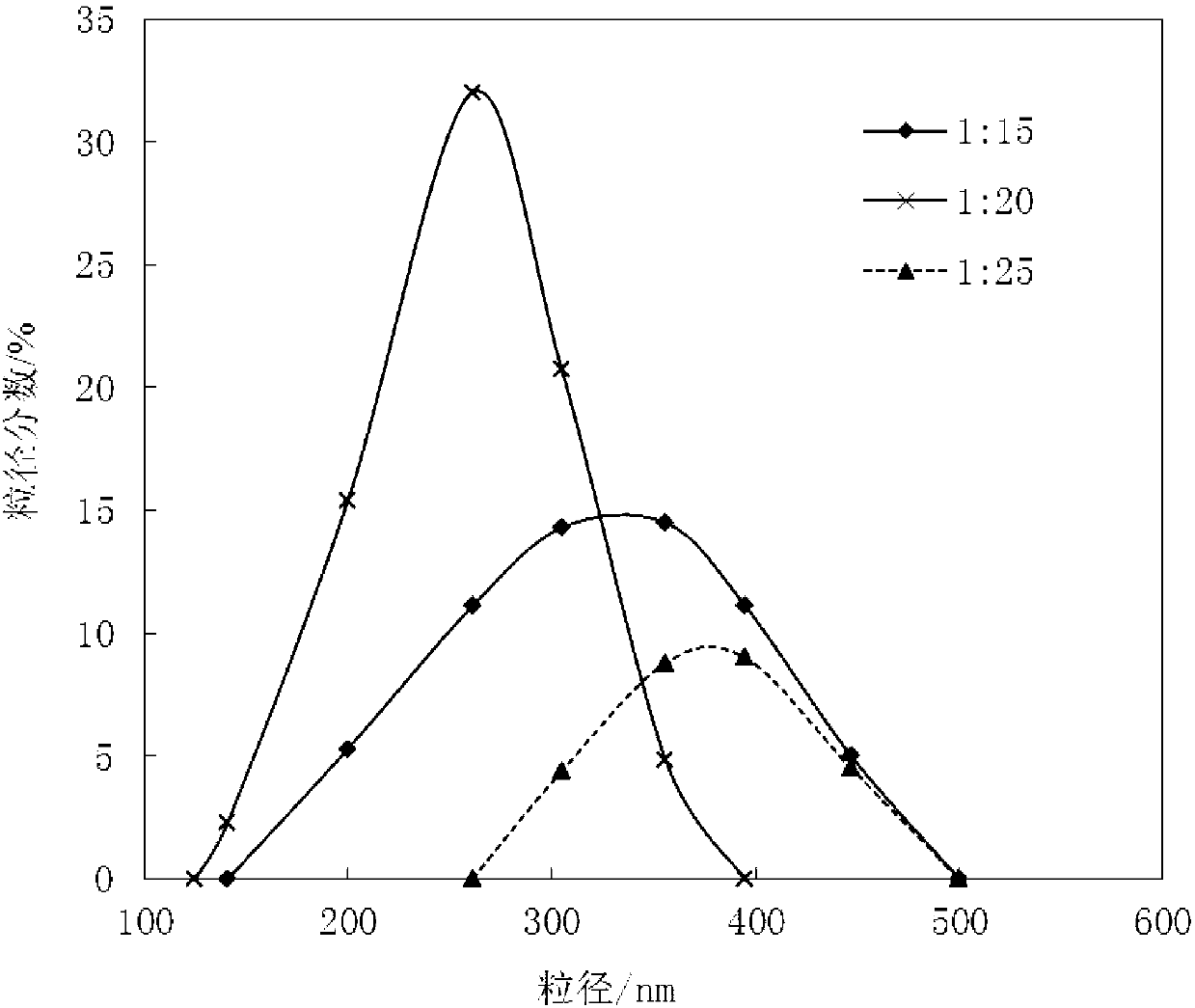
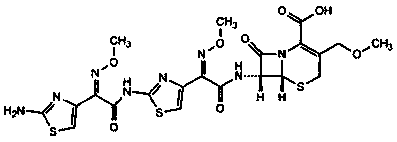
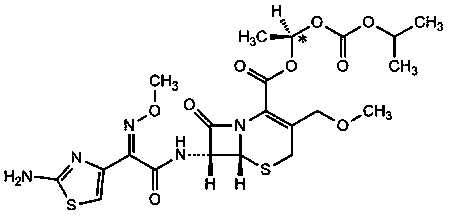
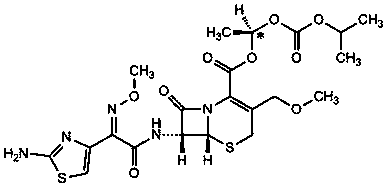
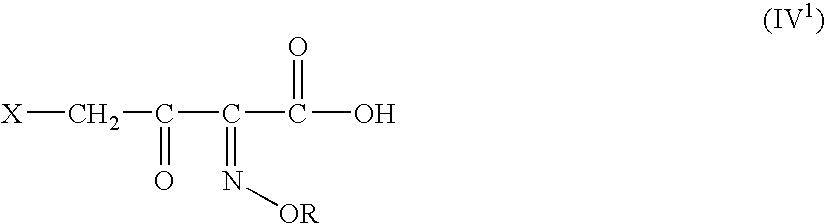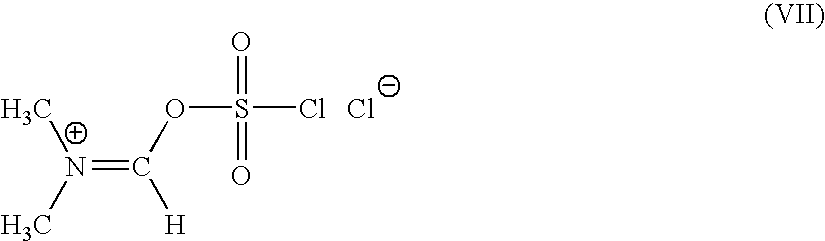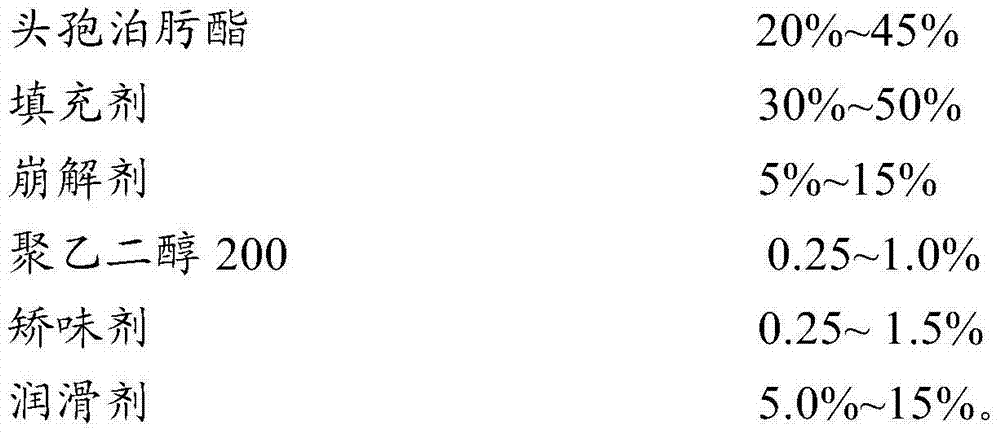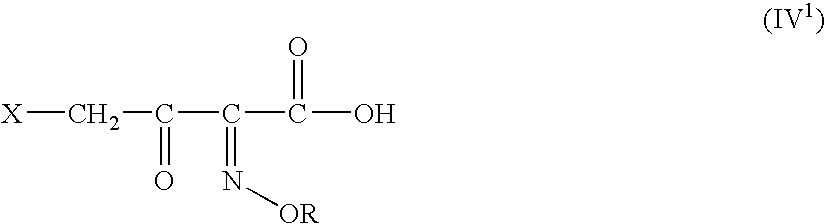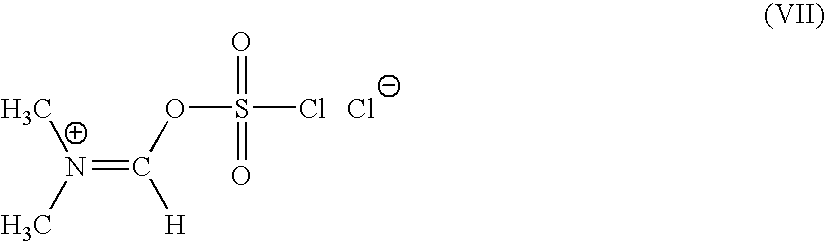Patents
Literature
47 results about "Cefpodoxime Proxetil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
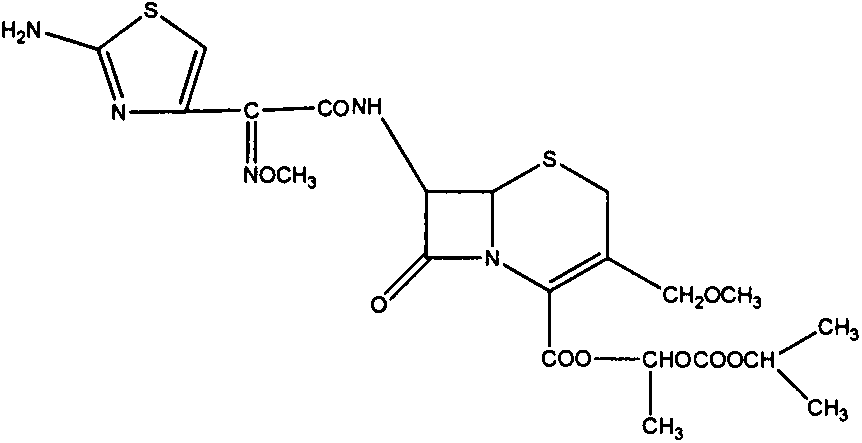
A third generation semi-synthetic cephalosporin and a beta-lactam antibiotic with bactericidal activity. Cefpodoxime's effect is dependent on its binding to penicillin-binding proteins (PBPs) located in the bacterial cytoplasmic membrane. Binding results in the inhibition of the transpeptidase enzymes, thereby preventing cross-linking of the pentaglycine bridge with the fourth residue of the pentapeptide and interrupting consequent synthesis of peptidoglycan chains. As a result, cefpodoxime inhibits bacterial septum and cell wall synthesis formation.
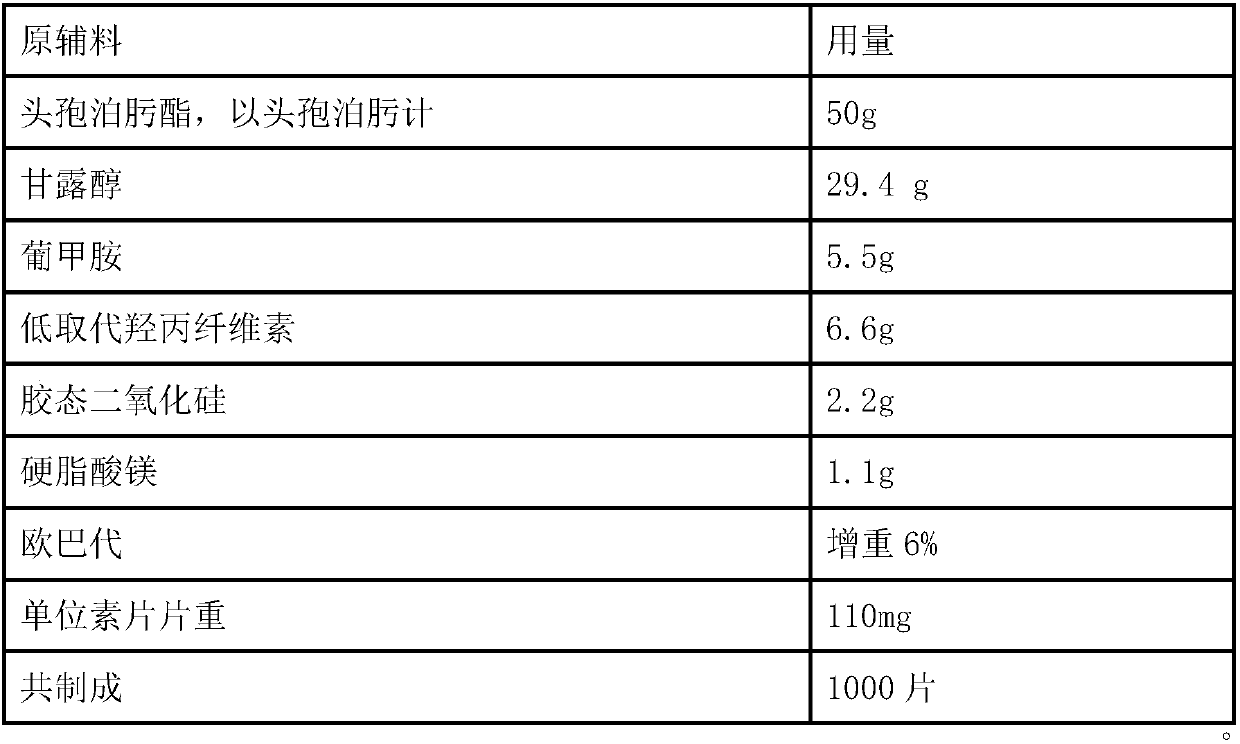
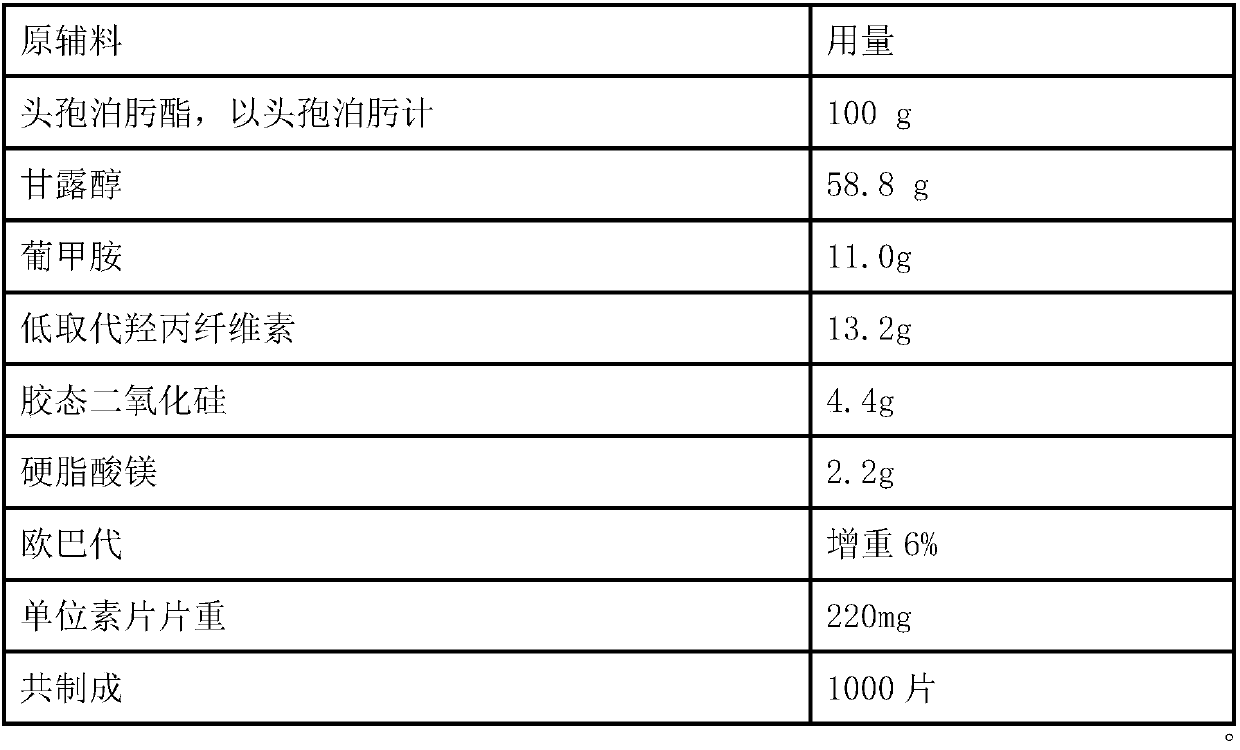
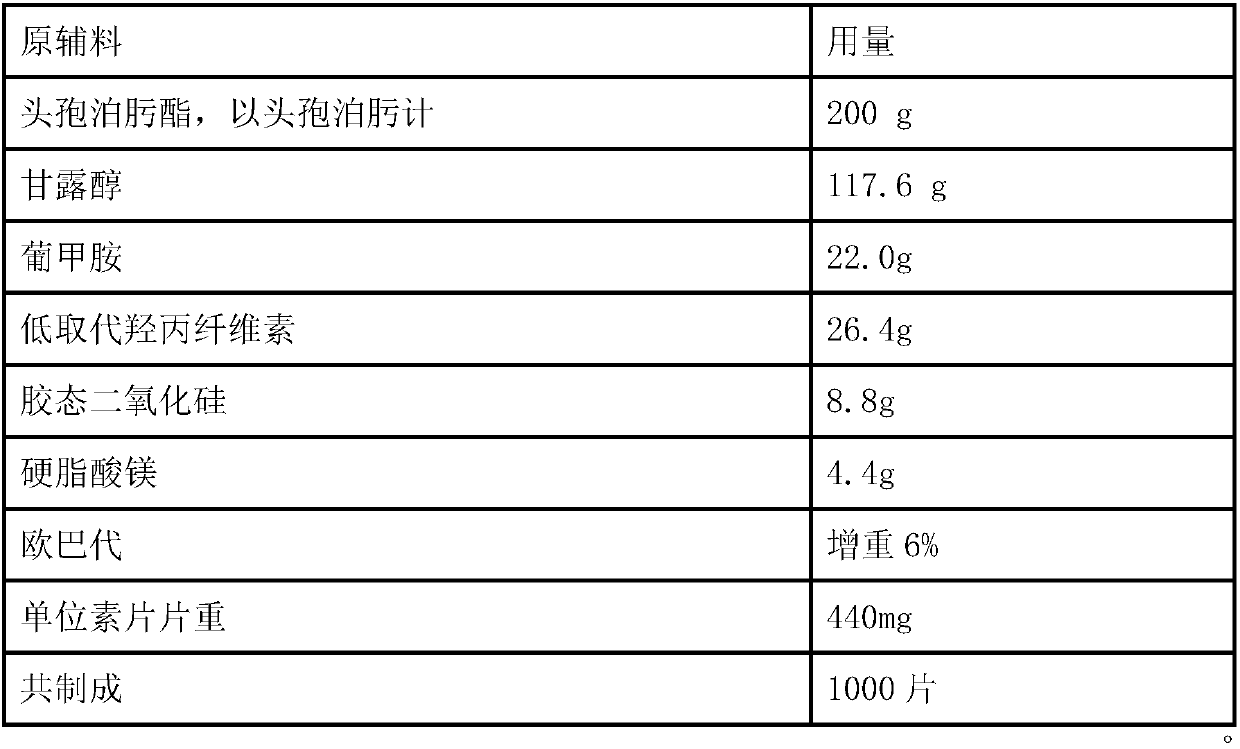
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
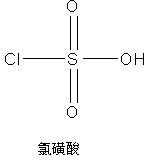
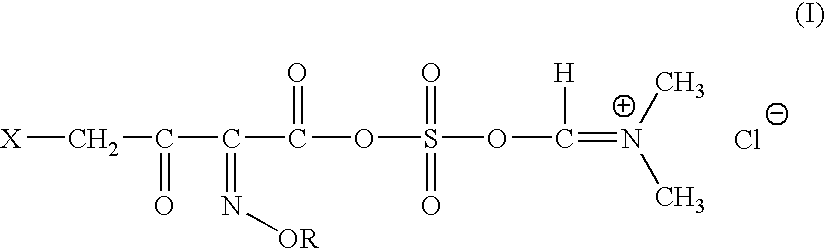
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
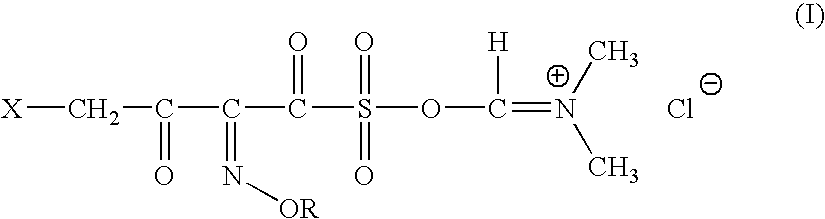
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
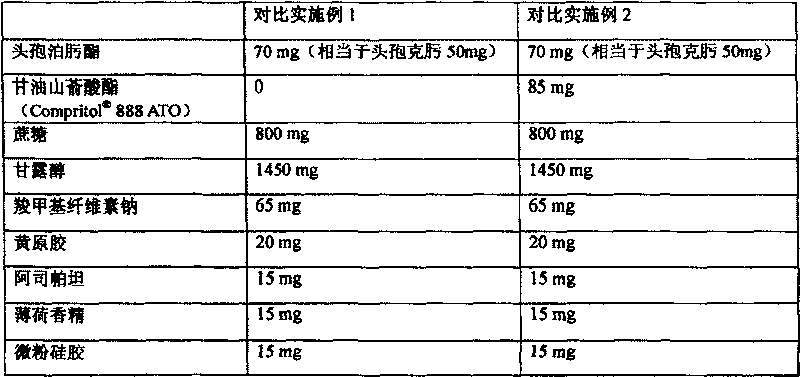
Cefpodoxime proxetil suspension composition and preparation thereof
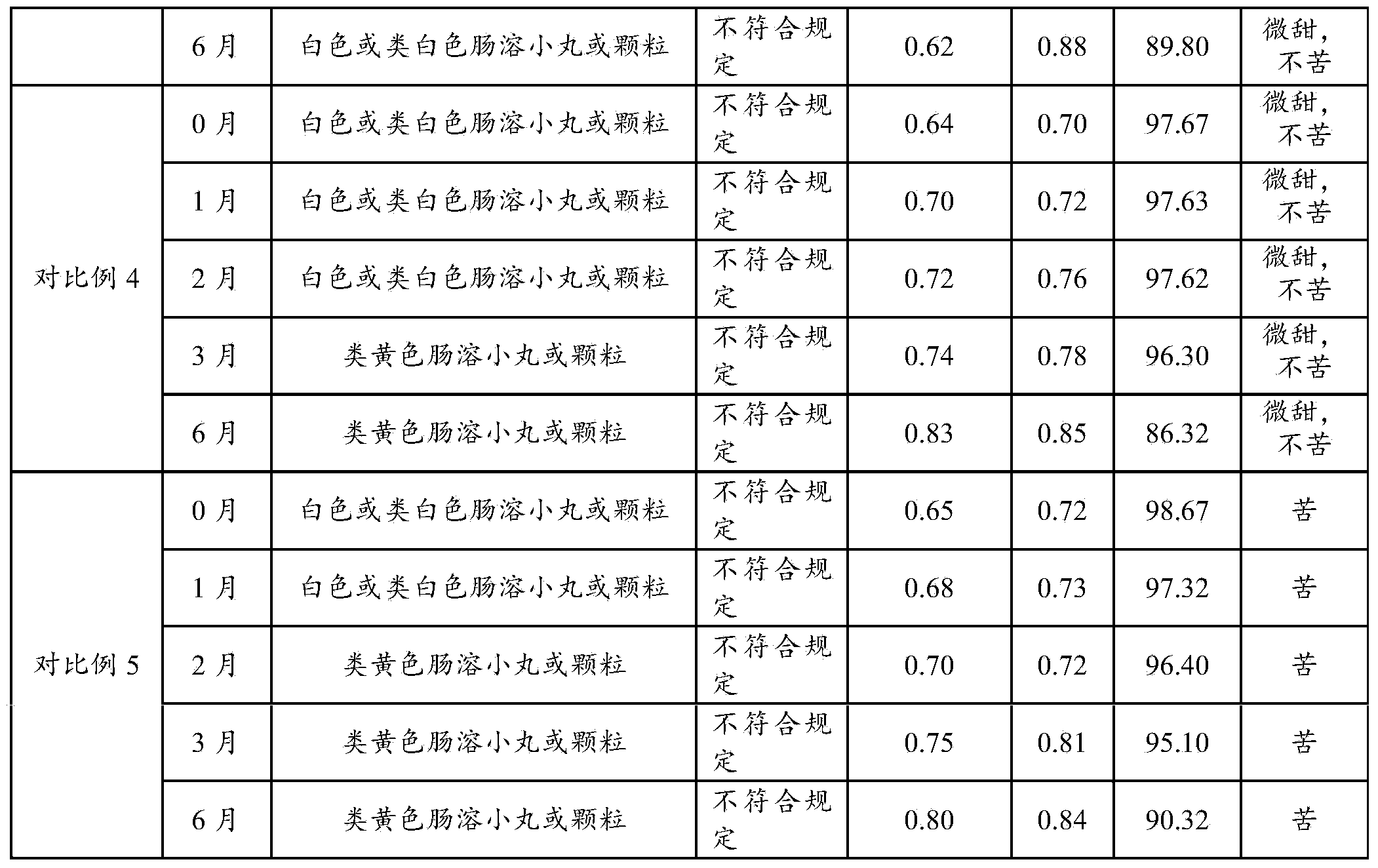
ActiveCN101278914AImprove bitternessEasy to acceptAntibacterial agentsPowder deliveryMedicineCefpodoxime Proxetil
The invention relates to a cefpodoxime proxetil dry suspension composition and a preparation method thereof. The invention provides the cefpodoxime proxetil dry suspension composition, containing 100 parts by weight of the cefpodoxime proxetil and 20 to 500 parts by weight of the glyceryl behenate. The invention also provides the preparation method of the dry suspension composition. The dry suspension composition of the invention is capable of reducing bitterness of the cefpodoxime proxetil remarkably and improving compliance of a clinical patient, thereby being suitable for a child to take a drug.
Owner:海南三叶美好制药有限公司
Cefpodoxime proxetil granules and preparation method thereof
The invention relates to cefpodoxime proxetil granules and a preparation method thereof. The granules comprise the following components in parts by weight: 40-60 parts of cefpodoxime proxetil, 700-950 parts of filler, 30-50 parts of disintegrant, 30-50 parts of binder, 12-35 parts of flavoring agent, 2-10 parts of disintegration promoting agent and 1-7 parts of other auxiliary materials. The preparation method of the granules specifically comprises the following steps of: (1) performing pretreatment on the raw materials and the auxiliary materials; (2) mixing; (3) performing granulation; and (4) performing total mixing. The cefpodoxime proxetil granules prepared by the preparation method disclosed by the invention have the advantages of capability of obviously reducing the bitterness of the raw materials, stable preparation, controllable quality, good medicament dissolution and higher safety and effectiveness in clinical medication, and is suitable for industrial mass production and the like.
Owner:TIANJIN PHARMA GROUP GENCOM PHARMA
Cefpodoxime proxetil submicron emulsion solid preparation and novel application thereof
InactiveCN101708166AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsEmulsionBioavailability
The invention discloses a cefpodoxime proxetil submicron emulsion solid preparation and a novel application thereof, particularly a cefpodoxime proxetil solid preparation which is subjected to micro-emulsification and a novel application thereof. In the invention, the micro-emulsification technology is applied to process cefpodoxime proxetil raw materials so as to obtain a cefpodoxime proxetil submicron emulsion with excellent performance, the stability of the cefpodoxime proxetil is improved and the dissolution rate of the cefpodoxime proxetil preparation is obviously improved, so that the cefpodoxime proxetil submicron emulsion solid preparation has better bioavailability and can be used for preparing a medicament for treating osteomyelitis of jaws.
Owner:HAINAN MEIDA PHARMA
Cefpodoxime proxetil taste-masking granule and preparation method thereof
ActiveCN107625754ASolve the problem of inconvenient medicationImprove stabilityAntibacterial agentsOrganic active ingredientsAdjuvantAdditive ingredient
The invention specifically discloses a cefpodoxime proxetil taste-masking granule and a preparation method thereof, belonging to the field of medicinal preparations. The taste-masking granule is prepared through mixing of a coated cefpodoxime proxetil pellet and a taste-masking particle, wherein the coated cefpodoxime proxetil pellet is of a structure composed of three layers, i.e., a drug-loadedpellet, a separating layer and a smoothing layer from interior to exterior. The main objective of the invention is to overcome the problems that current commercially-available cefpodoxime proxetil products immediately produce unpleasant bitter taste when child patients take the products; the taste of the cefpodoxime proxetil products is bad, which leads to poor medication compliance of child patients; and the cefpodoxime proxetil products have to be dissolved in water before taking, which leads to inconvenience in administration. To improve the medication compliance of children, the inherent bitter taste of cefpodoxime proxetil is totally masked, and components like a sweetener applicable to children are increased, so the bitter taste of the chemical drug is eliminated and fragrant smell is increased; and thus, child patients easily accept the granule and are pleasant to the fragrant smell, so the medication compliance of children is improved. Besides improvement of the medication compliance of children, the granule provided by the invention also overcomes the problem of inconvenience in administration of children by adding special adjuvants which allow children to take the granulethrough direct swallowing without dissolving in water into a prescription.
Owner:COSCI MED TECH CO LTD
Dry suspension of cefpodoxime proxetil composition and preparation method thereof
InactiveCN102525948AAntibacterial agentsOrganic active ingredientsLower respiratory infectionSucrose
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
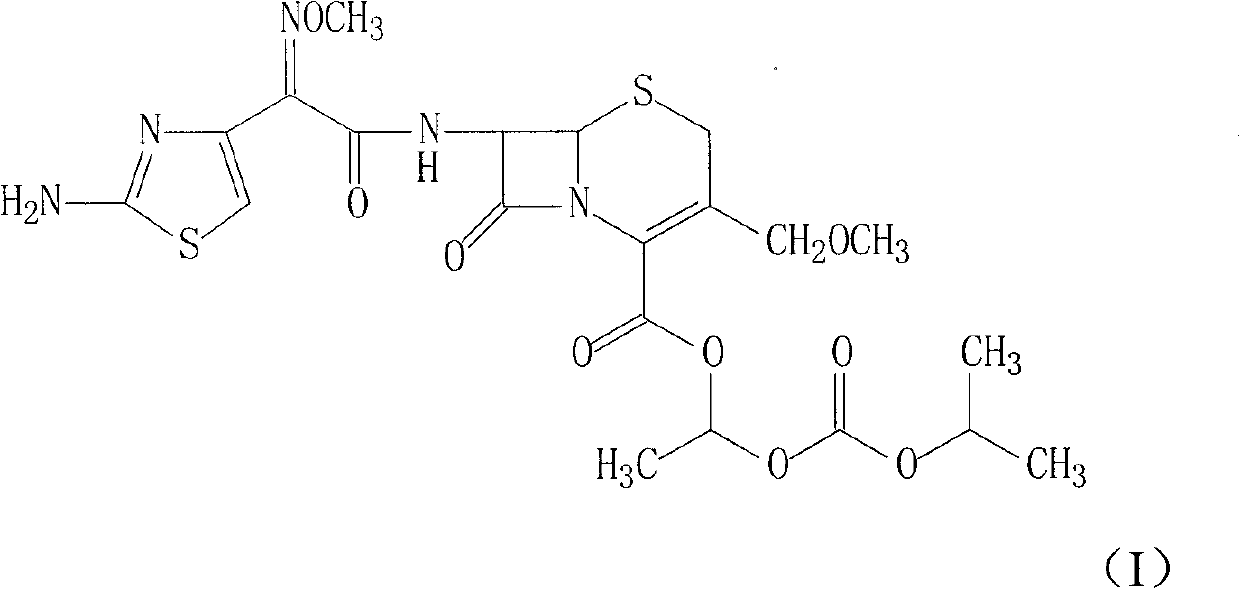
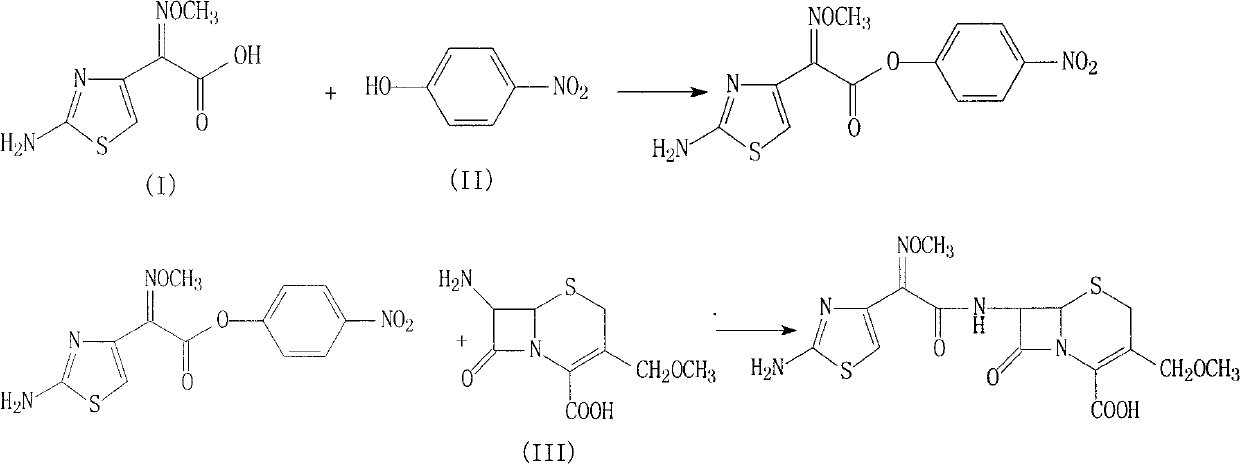
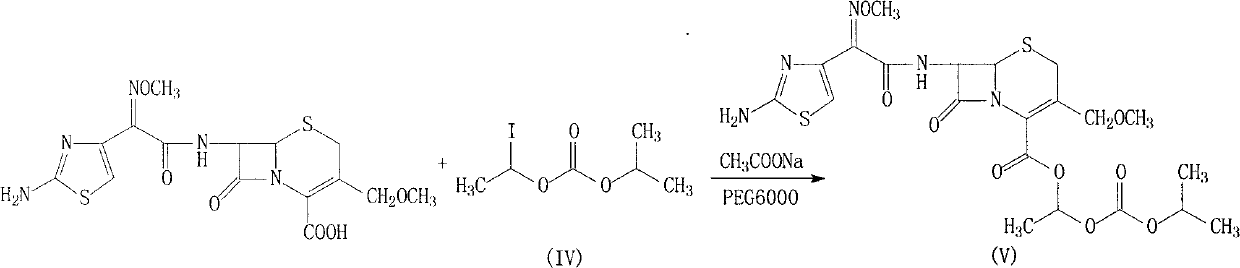
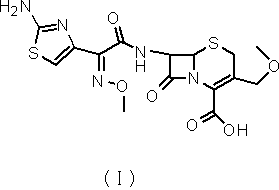
Cefpodoxime proxetil compound of new route
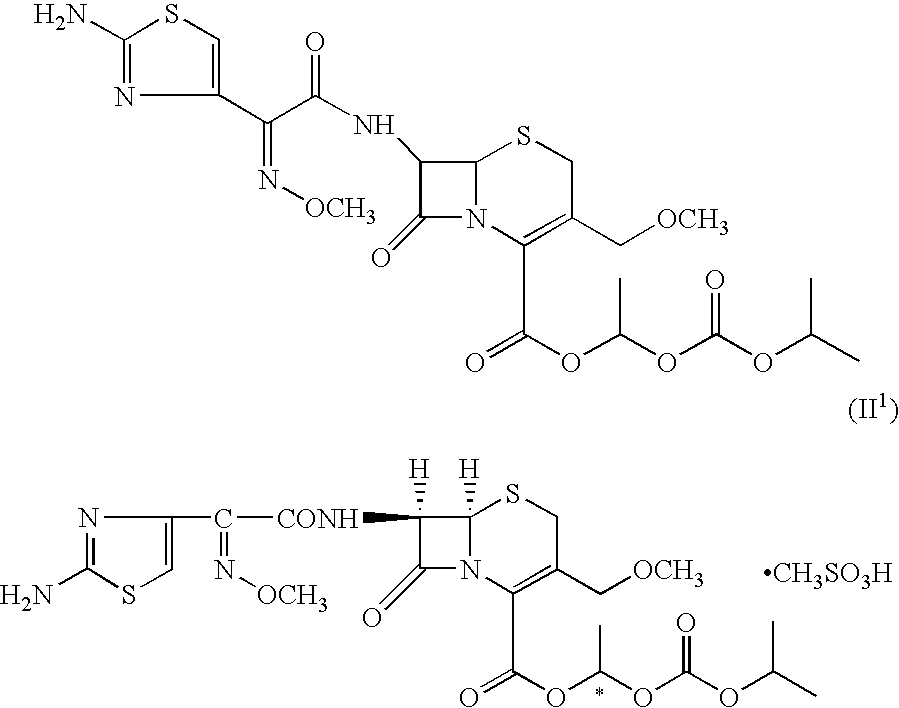
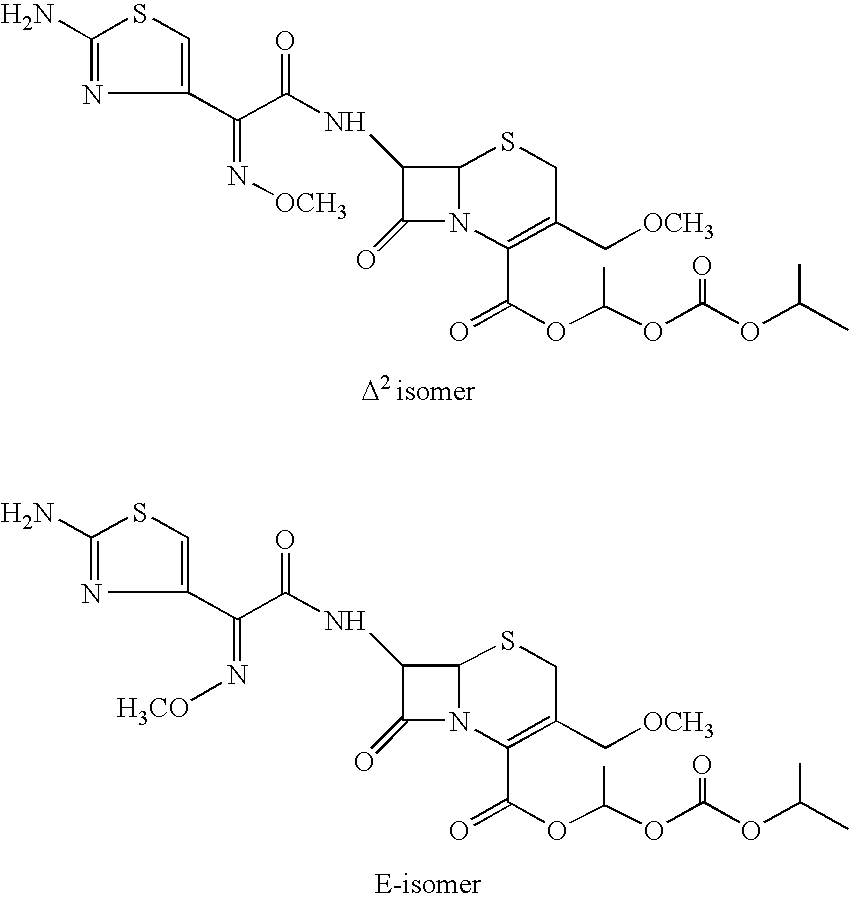
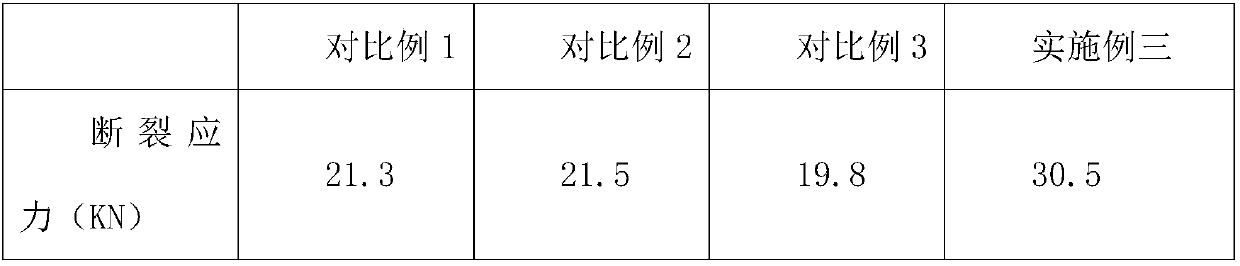
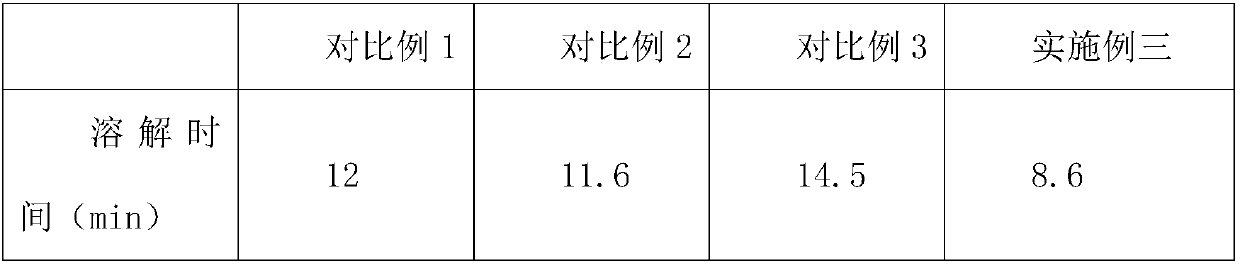
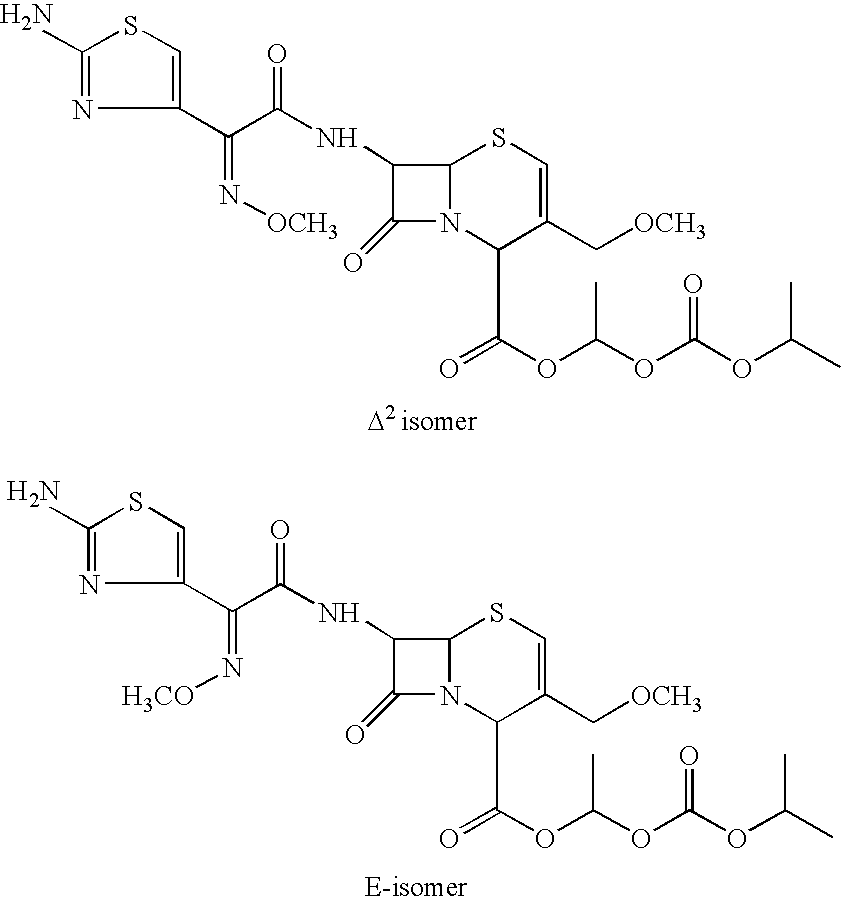
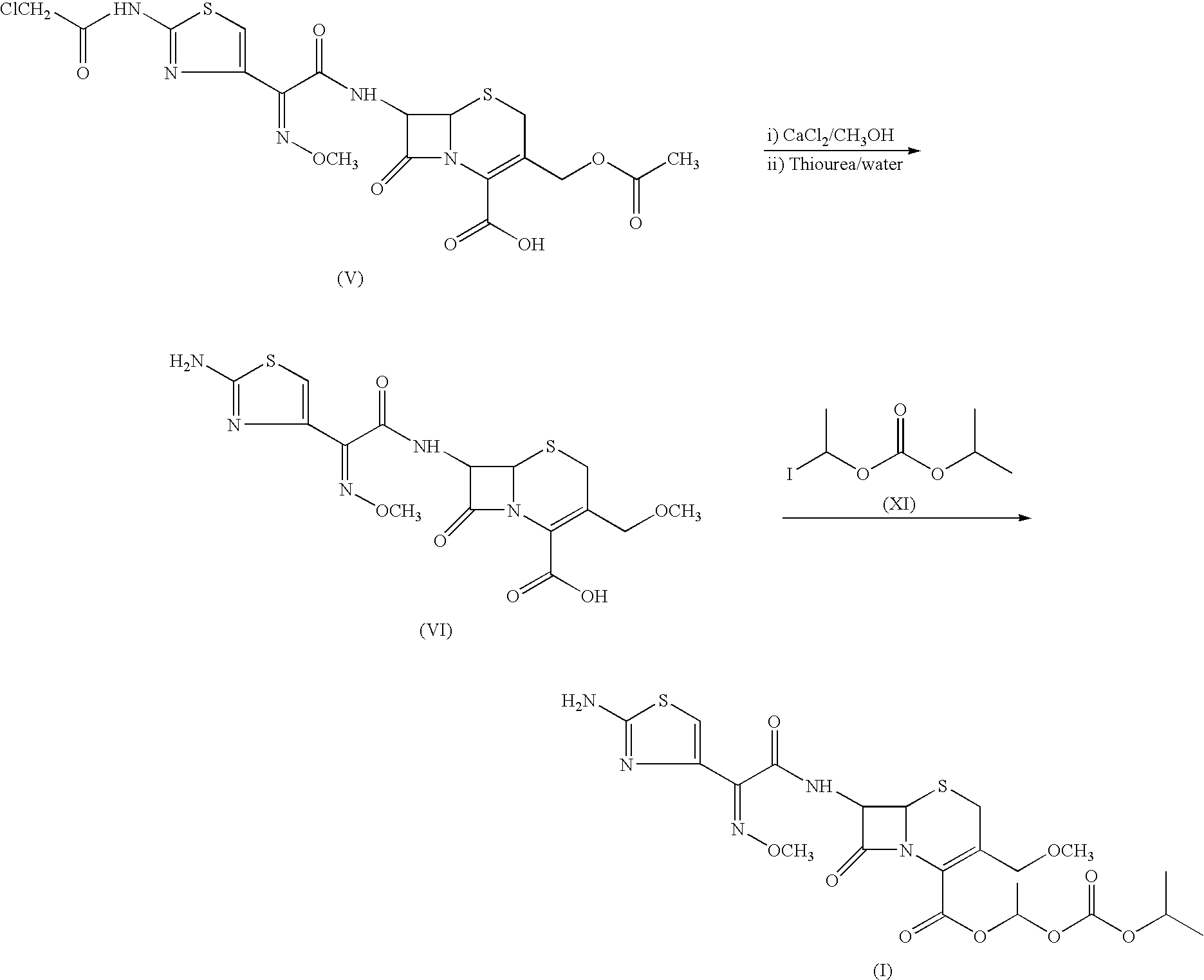
The invention relates to a cefpodoxime proxetil compound of new route, which belongs to technology field of medicine. The steps comprise: (1) (Z) -2 - (2 - aminothiazole -4 - ethyl) -2 - methoxyimino aceticacid (AMAA in short) and p-nitrophenol react, 7-AMCA is added to the mixture and is stirred for reacting, the PH value is adjusted with hydrochloric acid to obtain the cefpodoxime acid; (2) PEG6000 is used as catalyst, the cefpodoxime acid reacts with 1 - iodine ethyl isopropyl carbonate to obtain the cefpodoxime proxetil. The advantages of the invention are as follows: the method has high yield, low cost, and obtained product of high purity; the content of delta2 isomer is low, and the invention is suitable for industrial production.
Owner:HAINAN MEIDA PHARMA
Synthesis method of cefpodoxime proxetil intermediate
InactiveCN105669701AFew stepsLow costOrganic chemistryBulk chemical productionChlorosulfuric acidDesorption
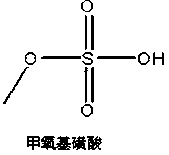
The invention discloses a synthesis method of a cefpodoxime proxetil intermediate, namely (6R,7R)-7-[2-(2-amino-4-thiazolyl)-(Z)-2-(methoxyimino)acetamido]-3-methoxymethyl-8-oxo-5-thio-1-azabicyclo[4.2.0]oct-2-ene-2-methanoic acid. The synthesis method includes: enabling chlorosulfonic acid and methanol to react to prepare methoxy sulfonic acid; under the action of the methoxy sulfonic acid and dimethylformamide, etherifying 7-ACA (7-aminocephalosporanic acid) and trimethyl borate prior to aftertreatment, adding into a water and methanol solution reversely to guarantee that the obtained intermediate isn't sticky and is loose, drying and grafting with AE active ester so as to obtain a target product, namely the cefpodoxime proxetil intermediate. The synthesis method of the cefpodoxime proxetil intermediate has the advantages that synthesis steps of 3-position and 7-position protection and desorption of 7-ACA can be omitted, so that low step cost, high yield and high purity are achieved, all materials are cheap and available, and industrial production and little pollution are benefited.
Owner:陕西思尔生物科技有限公司
Cefpodoxime proxetil soft capsule preparation and its preparing method
InactiveCN1586486AInhibition effectProtected from photolysisOrganic active ingredientsSenses disorderCefpodoxime ProxetilBioavailability
The present invention discloses one kind of soft cefpodoxime proxetil capsule and its preparation process. The medicine consists of medicine liquid and capsule shell, the medicine liquid contains cefpodoxime proxetil, matrix and stabilizer, and the capsule shell contains gelatin, glycerin, water, preservative and light screening agent. Compared with available cefpodoxime proxetil preparations, the present invention is even suitable for children and other people incapable of taking care of himself to take and has the advantages of high bioavailability, high stability, accurate content, etc.
Owner:北京瑞伊人科技发展有限公司 +1
Preparation method of cefpodoxime proxetil
ActiveCN106046024AEasy to separateQuality assuranceOrganic chemistryCefpodoxime ProxetilMedicinal chemistry
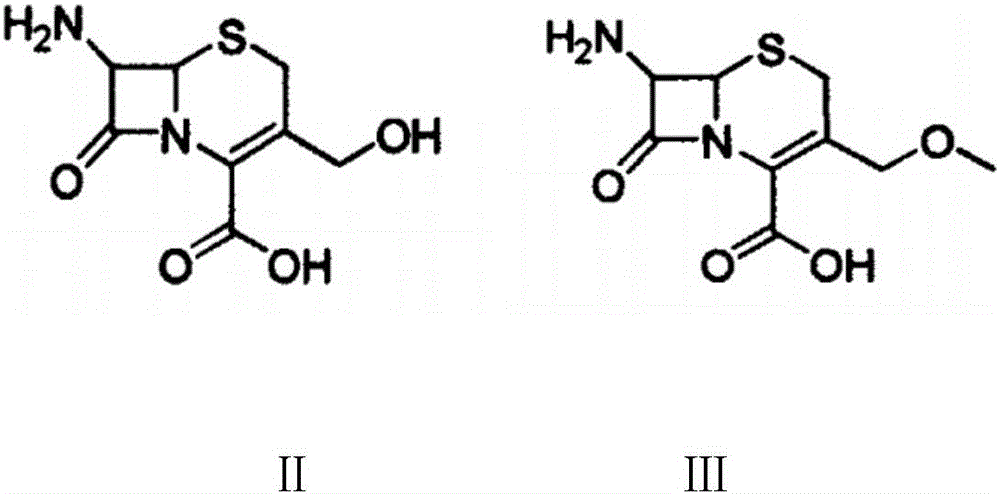
The invention discloses a preparation method of cefpodoxime proxetil. In the method, a compound of formula II is used as an initial raw material, the intermediate compound of formula III is not separated during the process, and a crude product of cefpodoxime proxetil is synthesized by one step; and after that, a simple and easy recrystallization method is adopted for purifying the crude product of cefpodoxime proxetil to obtain cefpodoxime proxetil. In the preparation method disclosed by the invention, the yield is increased without reducing the quality, the obtained product has high purity, the synthesis path is simple, and the preparation method is suitable for industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Medicament composition of cefpodoxime proxetil and cyclodextrin and preparation method thereof
InactiveCN1981765AAntibacterial agentsMacromolecular non-active ingredientsCyclodextrinCefpodoxime Proxetil
A medication compound, which include cyclodextrin and cephalosporin ester's clathrate; this invention alao related to the method to produce it.
Owner:RANBAXY LAB LTD
Method for prepairng highly purity cefpodoxime proxetil
Highly pure cefpodoxime proxetil can be prepared by a simple process comprising the step of reacting a cefpodoxime salt with 1-iodoethylisopropylcarbonate in an organic solvent in the presence of a crown ether.
Owner:HANMI PHARMA
Stable taste masked formulations of cephalosporins
A stable taste masked, pharmaceutical composition comprising a plurality of coated, non-disintegrating discrete dosage units, said units comprising of a core comprising one or more cephalosporins such as cefuroxime axetil and cefpodoxime proxetil and one or more coating layers. Cefuroxime axetil is in α-crystalline and amorphous forms, where at least 30% of the Cefuroxime axetil is in the α-crystalline form, wherein the particle size distribution of the α-crystalline form being such that 100% of the particles have a particle size below 250μ. The ratio of the crystalline fraction to the amorphous fraction ranges from 0.3:0.7 to 0.99:0.01. The particle size of cefpodoxime proxetil is such that 90% of the particles are below 15μ. The process of preparation of coated, non-disintegrating pellets comprising the steps of reducing the particle size of the one or more cephalosporins, blending with the other excipients, wet granulation, extrusion, spheronization, drying and screening to obtain pellets, said pellets being further coated with one or more layers of film coating to achieve taste masking.
Owner:LUPIN LTD
Cefpodoxime proxetil rapid-release preparation and preparation method thereof
ActiveCN104771368AAvoids tendency to gel with hydrolysisAvoid introducingAntibacterial agentsPowder deliveryFluidized bed dryingAlcohol
The invention provides a cefpodoxime proxetil rapid-release solid preparation and a preparation method thereof. The cefpodoxime proxetil rapid-release solid preparation comprises cefpodoxime proxetil and a surfactant, wherein cefpodoxime proxetil accounts for 2.5-15wt% of the total preparation, and the surfactant accounts for 0.8-15.0wt% of the total preparation. The method for preparing the rapid-release solid preparation adopts wet granulation and specifically comprises the following steps: (1) dissolving cefpodoxime proxetil and the surfactant in absolute ethyl alcohol to obtain a mixture I; (2) after mixing a diluting agent with a disintegrating agent uniformly, slowly adding or spraying the mixture I and carrying out granulation and fluidized bed drying to obtain a mixture II; (3) adding a suspending aid, a flavoring agent and a lubricating agent to the mixture II and carrying out total mixing and subpackaging. The rapid-release solid preparation can be quickly dissolved out when meeting water and can not be gelated. Meanwhile, technology improvement is adopted, thus avoiding the processes of raw material grinding and micronization treatment; and special equipment, such as a dry type granulator, is unnecessary to be used, thus ensuring the product stability while simplifying the production operation.
Owner:SHIJIAZHUANG NO 4 PHARMA
Cefpodoxime proxetil flavored chewable tablet and preparation method thereof
ActiveCN105963269AImprove stabilityIsolated from direct contactAntibacterial agentsOrganic active ingredientsFluidized bedDissolution
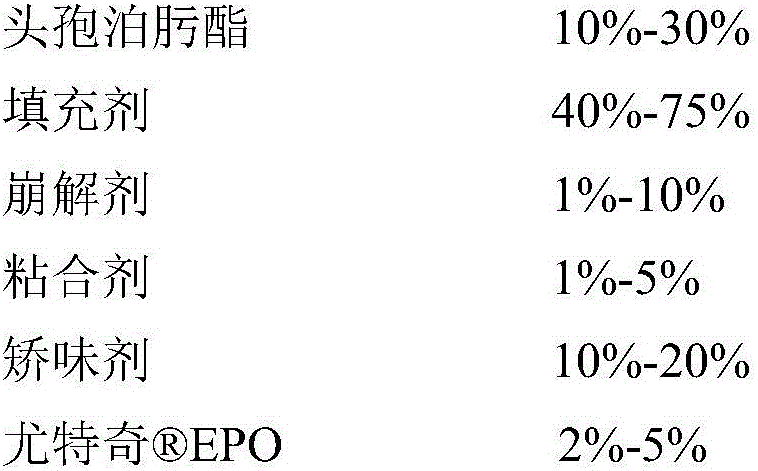
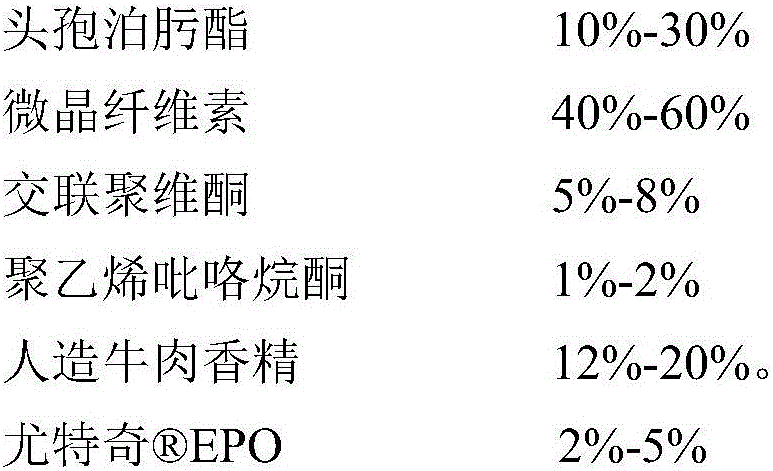
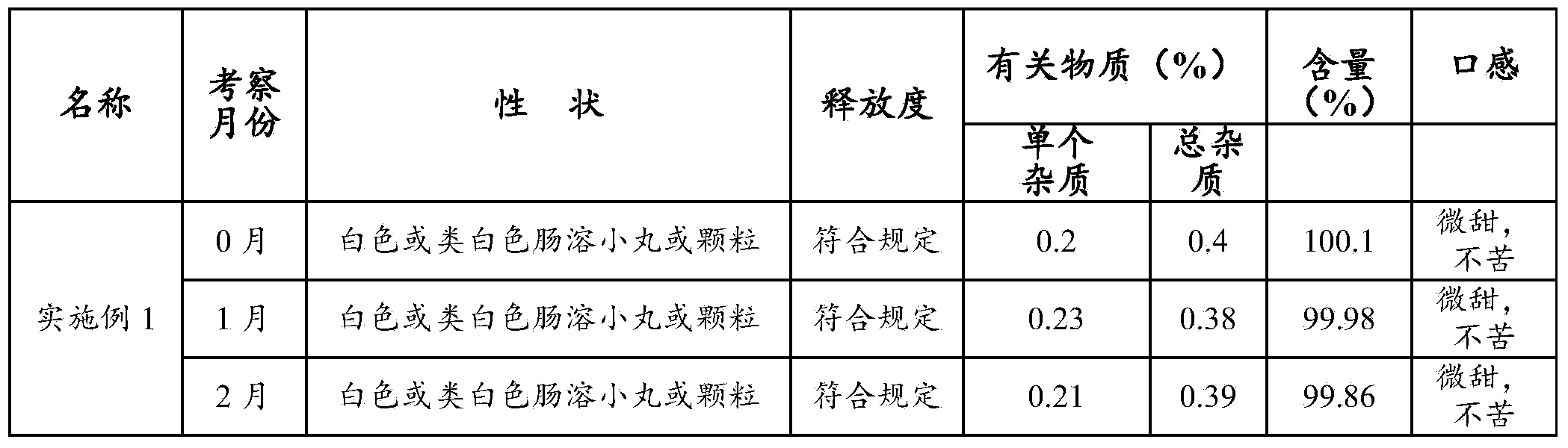
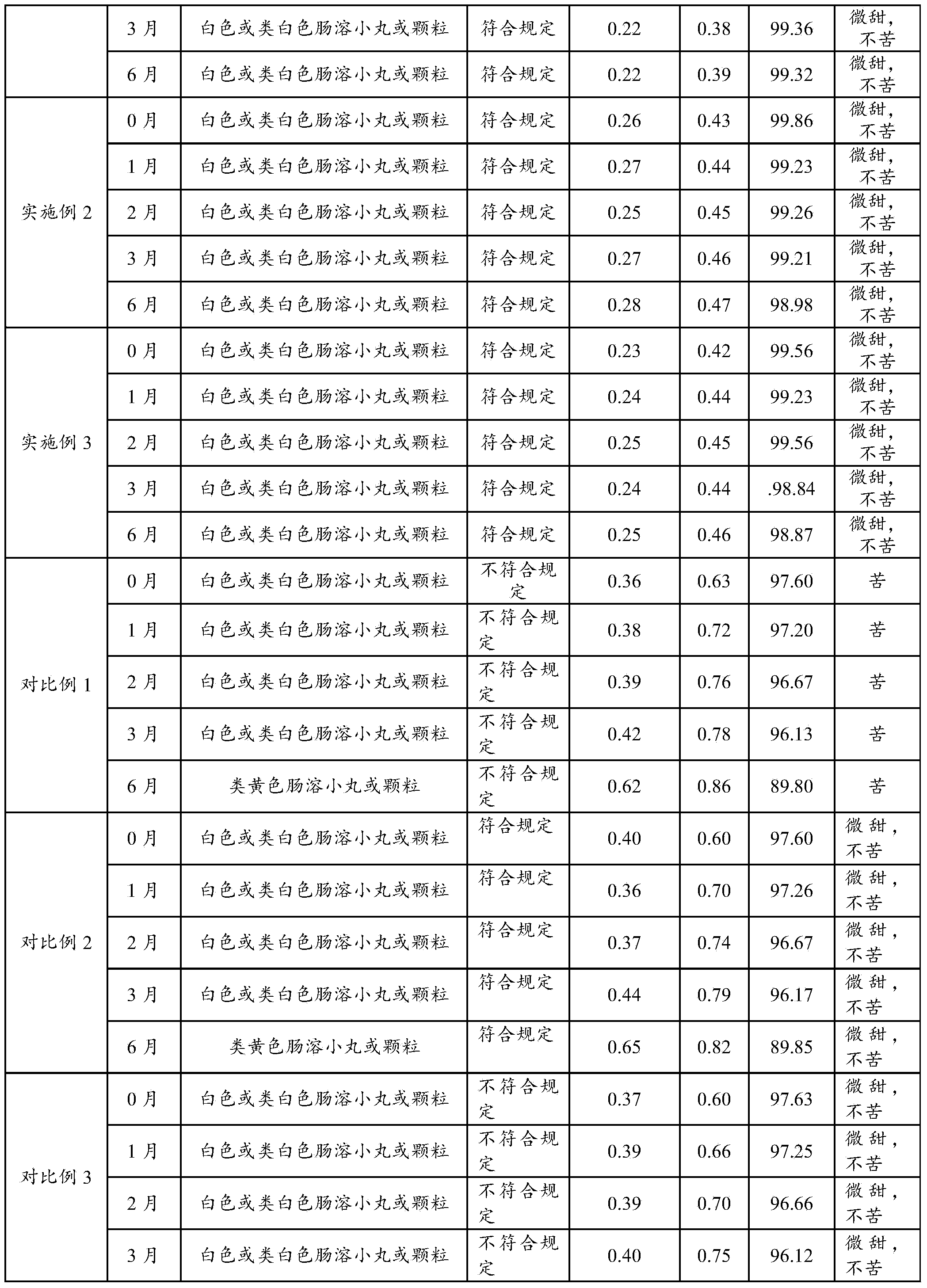
A cefpodoxime proxetil flavored chewable tablet is prepared from, by weight, 10%-30% of cefpodoxime proxetil, 40%-75% of filler, 1%-10% of a disintegrating agent, 1%-5% of an adhesive, 10%-20% of corrigent and 2%-5% of Eudragit EPO. The Eudragit EPO is used as a granule coating material to realize fluidized bed coating of granules containing the cefpodoxime proxetil, bitter taste is effectively removed for canines chewing the cefpodoxime proxetil flavored chewable tablet, direct contact between the cefpodoxime proxetil and the corrigent is isolated, and stability of the cefpodoxime proxetil is improved evidently. In addition, the cefpodoxime proxetil flavored chewable tablet is high in content uniformly and quick in disintegration and dissolution, and high stability is guaranteed while palatability is improved.
Owner:QILU ANIMAL HEALTH PROD
Cefpodoxime proxetil pellets and capsules and preparation method thereof
InactiveCN103919734AImprove liquidityGood content uniformityAntibacterial agentsOrganic active ingredientsMethyl celluloseCefpodoxime Proxetil
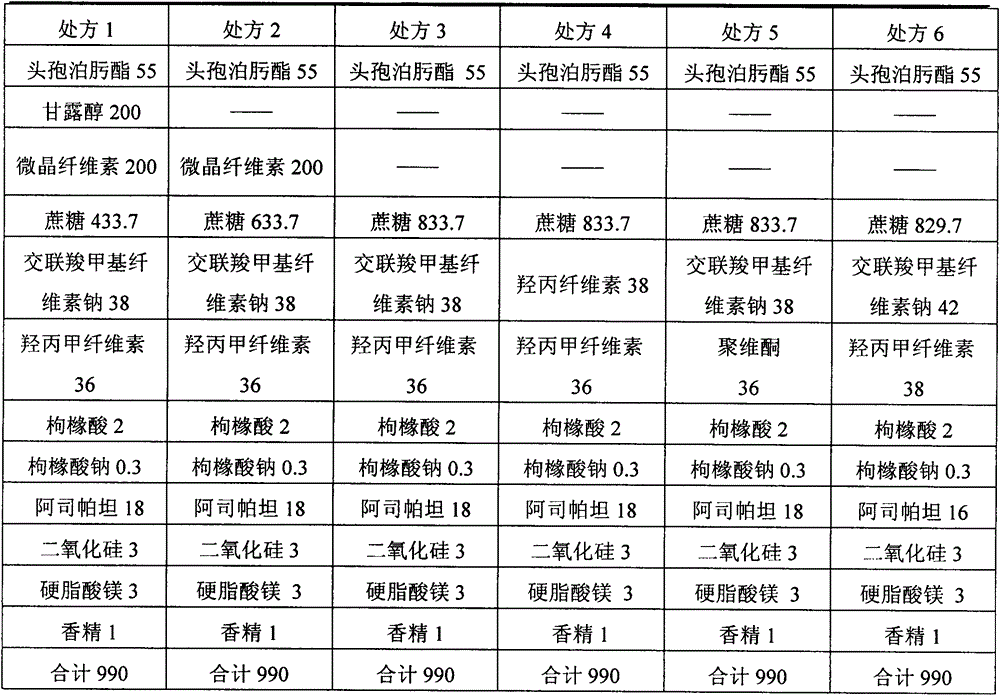
The invention discloses cefpodoxime proxetil pellets and capsules and a preparation method thereof. The pellets are composed of the following raw materials: 55 to 70 percent of cefpodoxime proxetil, 10.5 to 30 percent of hydroxypropyl methyl cellulose, 6 to 12 percent of mannitol, 1 to 5 percent of diacetyl glycerin monoester, and 1 to 3 percent of talcum powder. The method is low in cost, simple in process, and applicable for industrial production. The prepared capsules are good in quality, and are free of gravel taste after taking. The cefpodoxime proxetil has good taste, flowability, and content uniformity so as to be well preserved for a long time.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Process for the manufacture of cefpodoxime proxetil
InactiveUS20060149055A1High puritySimple preparation processOrganic chemistryOrganic solventDiastereomer
Owner:LUPIN LTD
Cefpodoxime proxetil compound and preparation method thereof
InactiveCN101550148ASolve the shortcomings of low purityQuality improvementAntibacterial agentsOrganic chemistryOrganic solventSaline solutions
The invention relates to a cefpodoxime proxetil compound and a preparation method thereof. In the method, the crude product of cefpodoxime proxetil prepared by the prior art is processed by the following steps to obtain a relatively pure cefpodoxime proxetil compound: the crude product of cefpodoxime proxetil dissolves in an organic solvent A; alkali or an organic alkalescent saline solution is added and the mixture is stirred for reaction and hydrolyzed to obtain cefpodoxime salt; the cefpodoxime salt is absorbed with added active carbon and filtered; and then 1-iodoethyl isopropyl carbonic ester is added and reaction is conducted in the presence of an organic solvent B to obtain cefpodoxime proxetil.
Owner:HAINAN MEIDA PHARMA
Cefpodoxime proxetil dispersible tablet and preparation method thereof
ActiveCN103479589AShort disintegration timeModerate hardnessAntibacterial agentsOrganic active ingredientsMedicinePolyethylene glycol
The invention discloses a cefpodoxime proxetil dispersible tablet which comprises the following raw materials in percentage by weight: 20-45 percent of cefpodoxime proxetil, 30-50 percent of filler, 5-15 percent of a disintegrating agent, 0.25-1.0 percent of a wetting agent, 0.25-1.5 percent of a flavoring agent and 5.0-15 percent of polyethylene glycol 200. The preparation method adopts a specific wetting agent, so as to improve mobility, dissolubility and uniformity in mixing of the product and facilitate the storage of the product, and after the product is stored for a period of time, no obvious changes occur. According to the invention, the cefpodoxime proxetil dispersible tablet is obtained through direct powder mixing, granulation and tabletting, and the preparation method is simple without any special equipment, and is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Cefpodoxime proxetil compound as well as preparation method and medicinal composition thereof
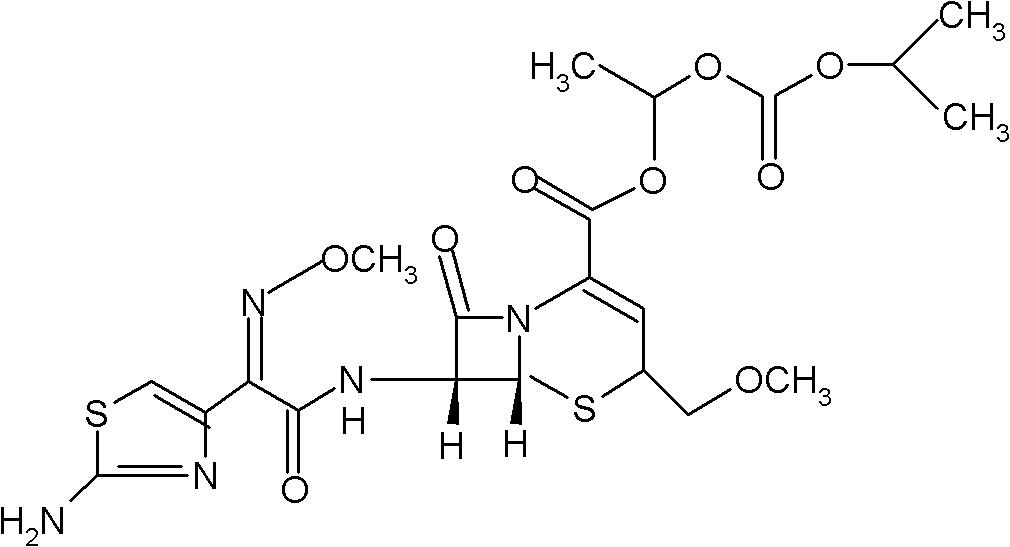
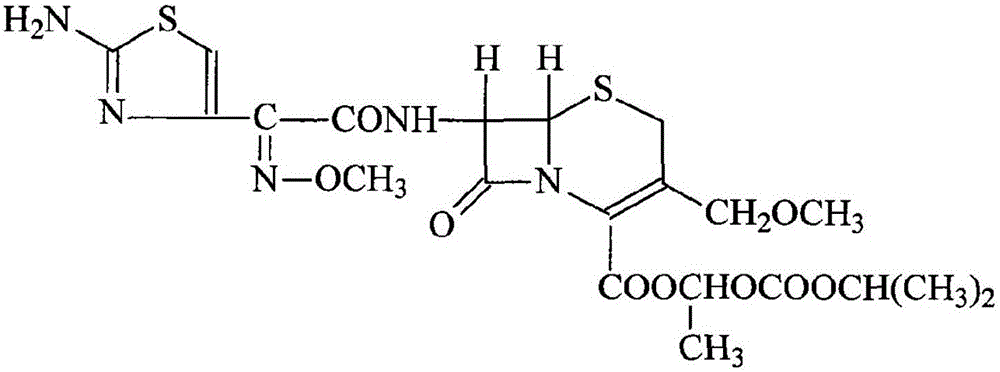
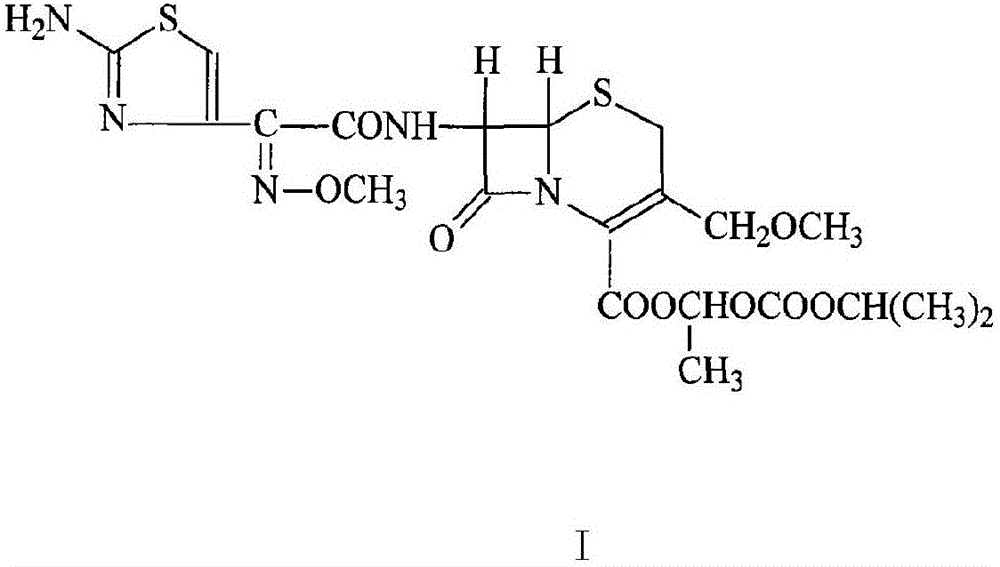
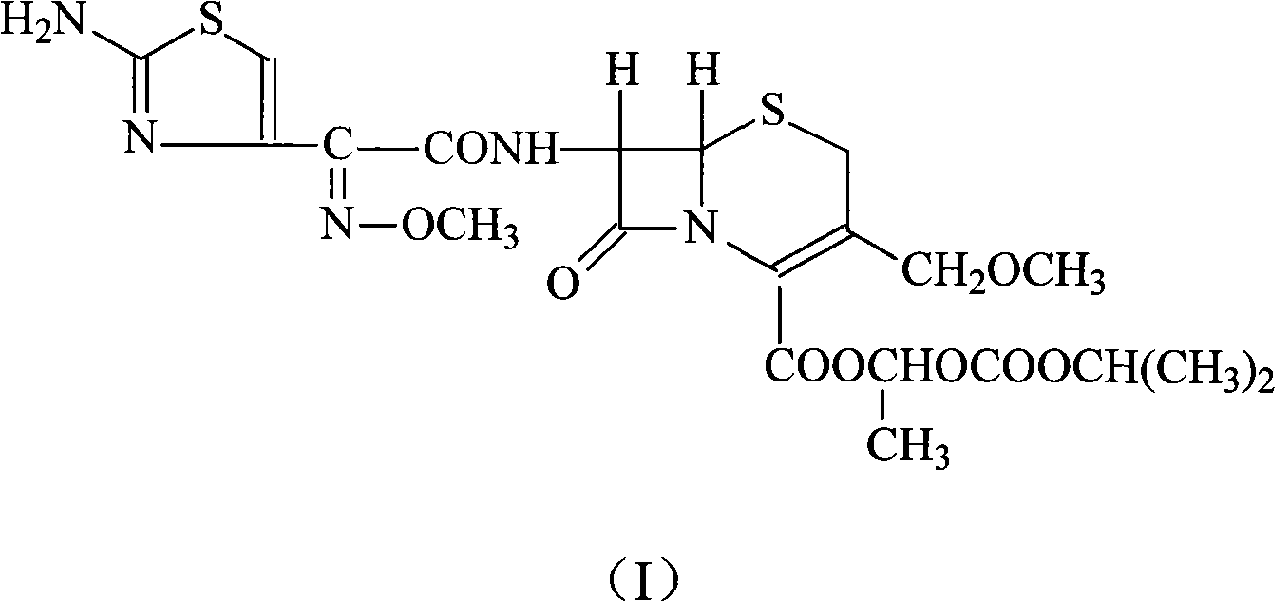
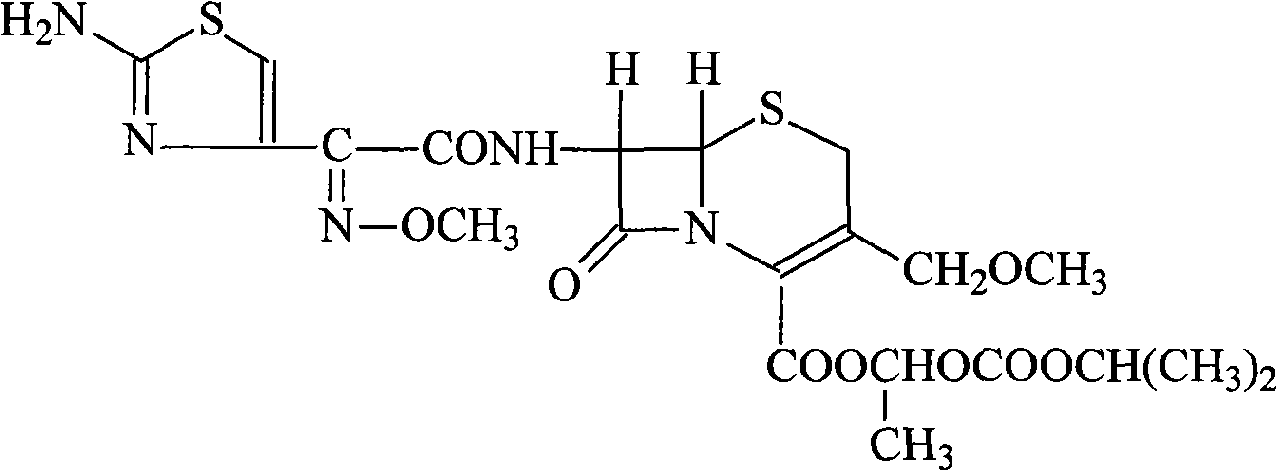
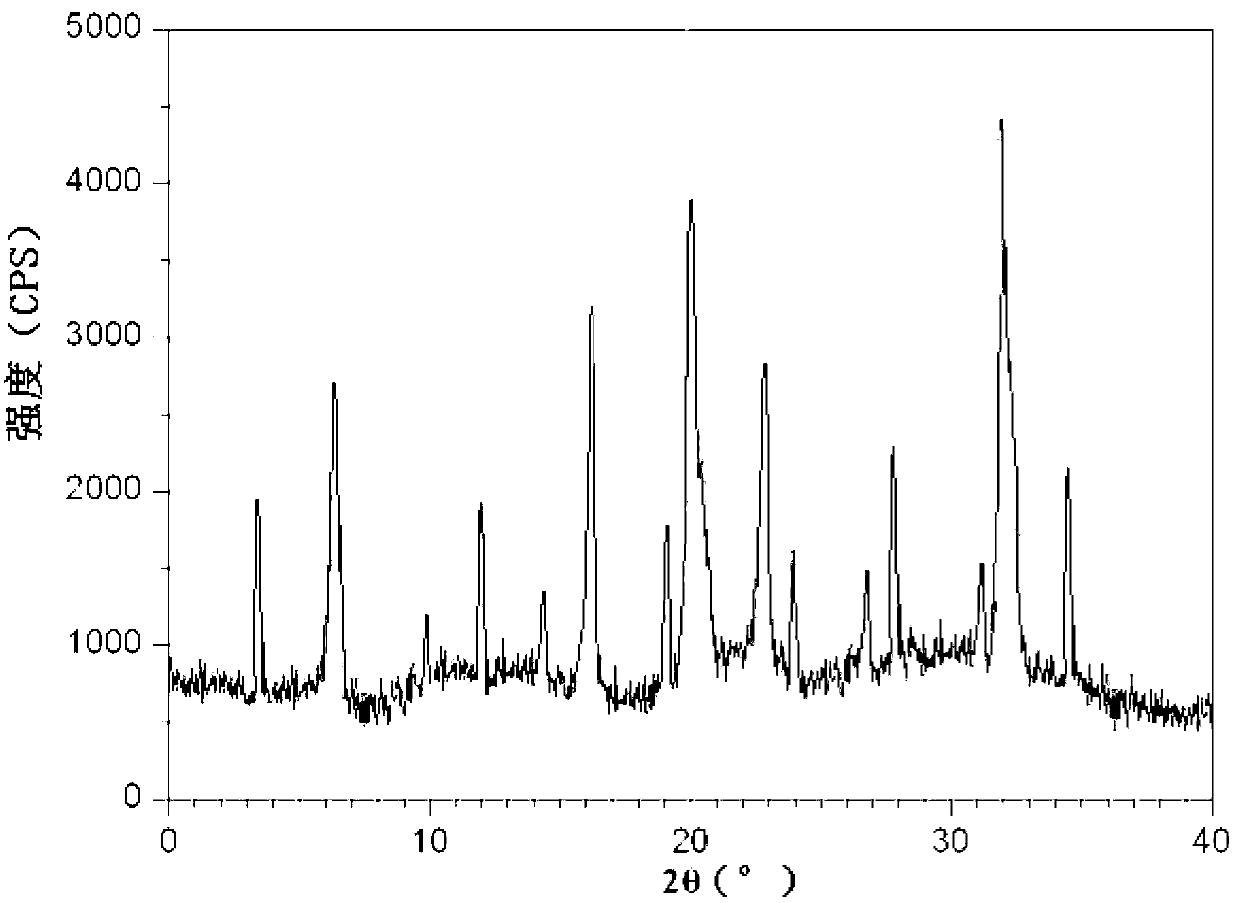
InactiveCN103275102AUniform particle size distributionGood dispersionOrganic active ingredientsOrganic chemistryStructural formulaCefpodoxime Proxetil
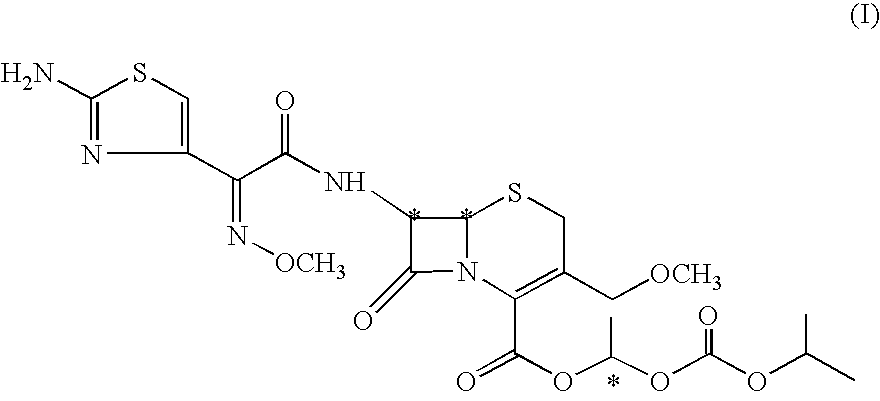
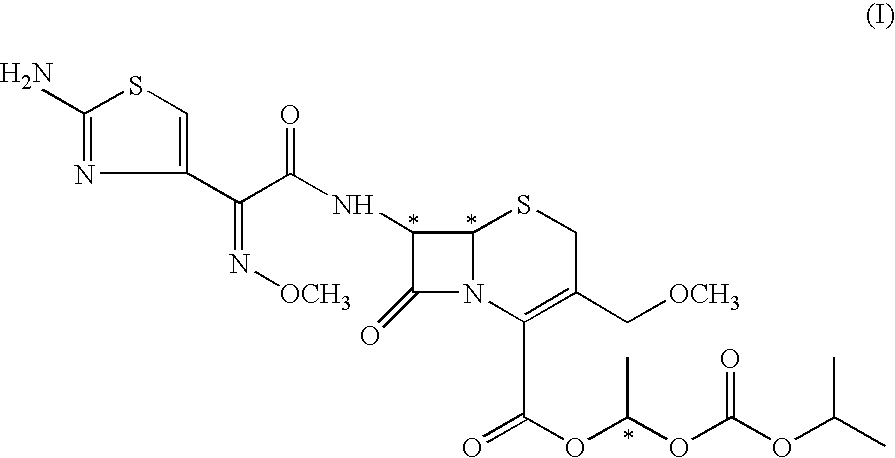
The invention belongs to the technical field of medicines, and in particular relates to a cefpodoxime proxetil compound as well as a preparation method and a medicinal composition thereof. The cefpodoxime proxetil compound has a chemical structural formula shown in a formula (I); and the cefpodoxime proxetil compound adopts an X-ray powder diffraction pattern which is obtained by Cu-Kalpha ray measurement and shown in a figure 1. The stability test shows that the humidity stability of the cefpodoxime proxetil compound is obviously superior to that of cefpodoxime proxetil in the prior art, the temperature and illumination stability of the cefpodoxime proxetil compound is also superior to that of cefpodoxime proxetil in the prior art, and the purity of the cefpodoxime proxetil compound is obviously higher than that of cefpodoxime proxetil in the prior art; and in addition, surprisingly, the cefpodoxime proxetil compound provided by the invention has good fluidity and is easy to subpackage.
Owner:四川省惠达药业有限公司
Cefpodoxime proxetil impurity, preparation method thereof and application of cefpodoxime proxetil impurity
InactiveCN108530468AQuality improvementValid data supportOrganic chemistryComponent separationQuality controlCefpodoxime Proxetil
The invention relates to a cefpodoxime proxetil impurity, a preparation method thereof and an application of the cefpodoxime proxetil impurity, discovers a novel impurity of cefpodoxime proxetil in the preparation process, and discloses a preparation method and an application of the impurity. A pair of mono-impurities of the cefpodoxime proxetil are analyzed, forming processes of the impurities are derived, a synthetic method is designed, an impurity monomer is acquired and can be used for quality research and process research of raw medicines and preparations of cefpodoxime proxetil, researchof impurity comparison products and the like, effective data support is provided for improvement of the quality of the cefpodoxime proxetil, and effective guarantee is provided for safe clinical useof the cefpodoxime proxetil. Quality research of the medicines is important, and research of the impurities is particularly important along with popularization of consistency evaluation of generic medicines. Research of the impurities has great significance for product quality control and medication safety of people.
Owner:SHANDONG RUIYING PIONEER PHARMA
Medicinal composition containing cefpodoxime proxetil cyclodextrin inclusion compound and preparation thereof
InactiveCN101264087AGood water solubilityImprove solubilityAntibacterial agentsOrganic active ingredientsSolubilityHemolysis
The invention provides a medicine composition comprising the cefpodoxime proxetil cyclodextrin inclusion compound. The basic composition comprises the cefpodoxime proxetil and the pharmacy-acceptable cyclodextrin; the cyclodextrin is chosen from one or a plurality of Beta -cyclodextrin, sulfobutyl- Beta -cyclodextrin, hydroxypropyl- Beta -cyclodextrin or hydroxypropyl-sulfobutyl- Beta -cyclodextrin. The invention has the advantages of increasing the solubility and stability of the medicine, meanwhile lower hemolysis irritability and higher activity. The invention also provides the preparation method of the medicine composition.
Owner:NANJING NORMAL UNIVERSITY +1
Cefpodoxime proxetil tablet and production process thereof
InactiveCN108815130AHigh purityAvoid wastingAntibacterial agentsOrganic active ingredientsOrganic solventRegistered trademark
The invention discloses a cefpodoxime proxetil tablet. The cefpodoxime proxetil tablet comprises the following raw materials by weight: 30 to 50 parts of crude cefpodoxime proxetil, 10 to 20 parts ofan organic solvent A, 5 to 15 parts of an organic weakly-alkaline salt solution, 10 to 20 parts of active carbon, 5 to 10 parts of 1-iodoethyl isopropyl carbonate, 6 to 12 parts of an organic solventB, 2 to 8 parts of a filling agent, 2 to 6 parts of a disintegrating agent, 6 to 12 parts of an aqueous binder solution, 3 to 7 parts of Eudragit with a registered trademark of EPO, 1 to 7 parts of acorrigent, 2 to 9 parts of sodium alginate and 1 to 5 parts of isomaltitol. The cefpodoxime proxetil tablet provided by the invention is directly prepared by using the crude cefpodoxime proxetil, so the waste of the cefpodoxime proxetil tablet caused by crude cefpodoxime proxetil is avoided, and a plurality of unnecessary preparation steps can be reduced at the same time; meanwhile, the quality ofa clinical preparation is improved for better solidification of pills of master drug components, so medication safety is guaranteed; and the method provided by the invention has the advantages of simple process, convenient operation, low cost and applicability to large-scale production.
Owner:邓倩
Process for the preparation of cefpodoxime procetil
InactiveUS20060293296A1High puritySimple and cost-effectiveOrganic active ingredientsOrganic chemistryCefpodoxime ProxetilPhotochemistry
A process for the preparation of cefpodoxime proxetil of high purity conforming to pharmacopoeial specifications.
Owner:LUPIN LTD
Medicine composition containing cefpodoxime proxetil
InactiveCN108261404AImprove stabilityImprove dissolution rateAntibacterial agentsOrganic active ingredientsDissolutionHydrolysis
The invention discloses a medicine composition containing cefpodoxime proxetil. Meglumine is added into the composition to be used as a gelatin inhibitor; the gelation phenomenon of the solid preparation in an aqueous solution can be effectively inhibited; the dissolution-out performance of a solid preparation is effectively improved; the bioavailability is further improved. A flavoring agent of sodium glutamate is added in the composition to achieve the cooperated effect with meglumine, the problem of bitter taste of the particle preparation is solved. Through a dry process granulation process, the introduction of adverse factors such as moisture and high temperature is avoided; a preparation recipe is matched; the problems of hydrolysis or oxidization degradation of ester bonds, amido bonds, lactam bonds and primary amine groups in raw medicine molecules is solved. Experiments prove that the effect is good.
Owner:天津双硕医药科技有限公司
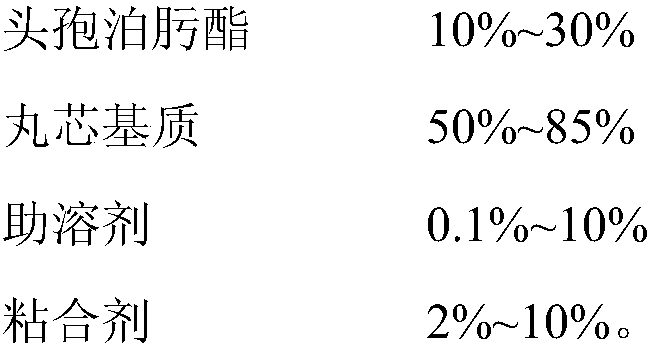
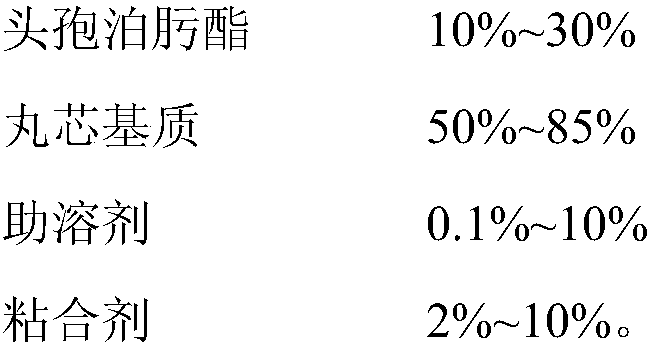
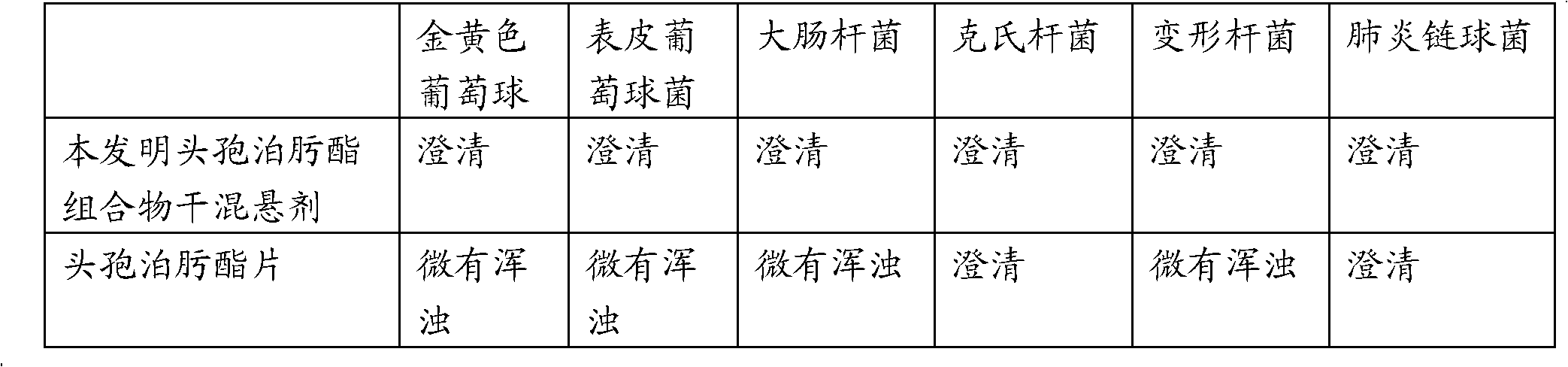
Cefpodoxime proxetil composition dry suspension and preparation method thereof
ActiveCN103230367BHigh dissolution rateImprove antibacterial propertiesAntibacterial agentsPowder deliveryMANNITOL/SORBITOLSucrose
The invention relates to the technical field of medicines and in particular relates to a cefpodoxime proxetil composition dry suspension and a preparation method thereof. The cefpodoxime proxetil composition dry suspension comprises the following materials: cefpodoxime proxetil, sucrose, mannitol and starch slurry. Through the combined action of sucrose, mannitol and starch slurry, the cefpodoxime proxetil composition dry suspension, which is high in dissolution rate, stronger in antibacterial action and good in stability, is obtained on the condition that the process of adding suspending aid is omitted. Moreover, the prepared cefpodoxime proxetil composition dry suspension can be used for preventing medicines from packing and further can be used for strengthening the antibacterial action of the cefpodoxime proxetil.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Method for preparing highly purity cefpodoxime proxetil
Owner:HANMI PHARMA
Cefpodoxime proxetil suspension composition and preparation thereof
ActiveCN101278914BImprove bitternessEasy to acceptAntibacterial agentsOrganic active ingredientsCefpodoxime ProxetilChemistry
The invention relates to a cefpodoxime proxetil dry suspension composition and a preparation method thereof. The invention provides the cefpodoxime proxetil dry suspension composition, containing 100parts by weight of the cefpodoxime proxetil and 20 to 500 parts by weight of the glyceryl behenate. The invention also provides the preparation method of the dry suspension composition. The dry suspension composition of the invention is capable of reducing bitterness of the cefpodoxime proxetil remarkably and improving compliance of a clinical patient, thereby being suitable for a child to take adrug.
Owner:海南三叶美好制药有限公司
Intermediates for synthesis of cephalosporins and process for preparation of such intermediates
Owner:LUPIN LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com