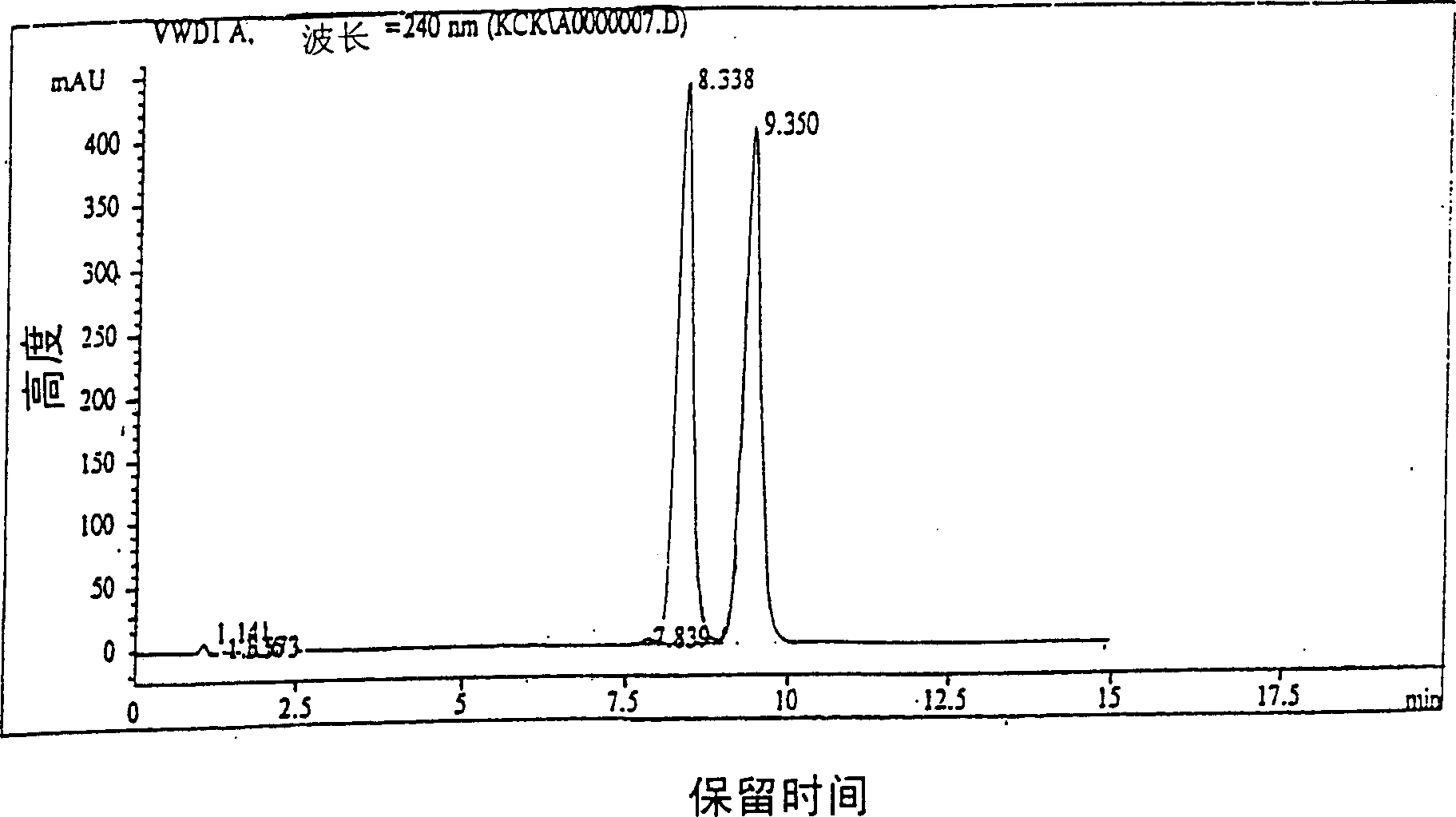
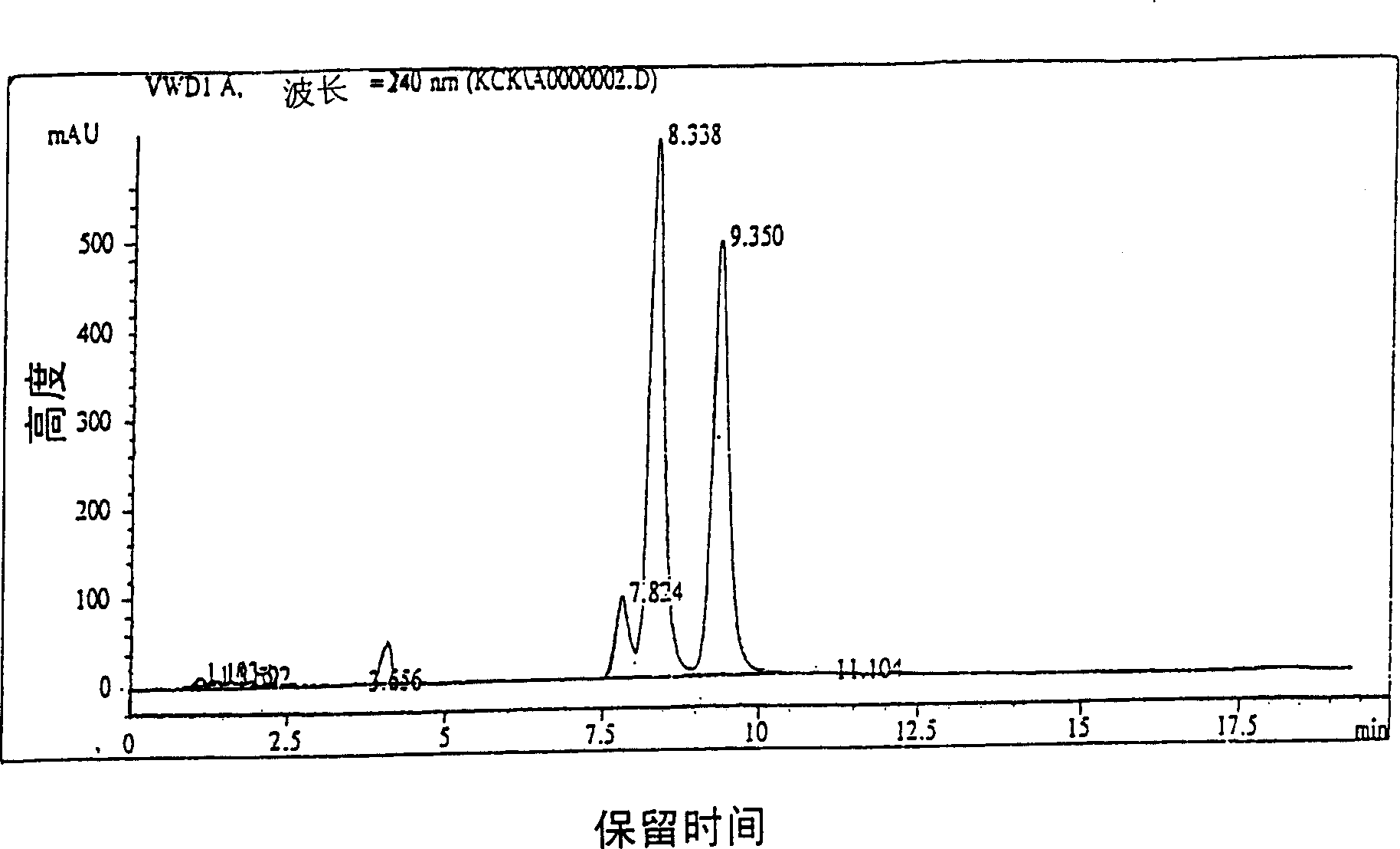
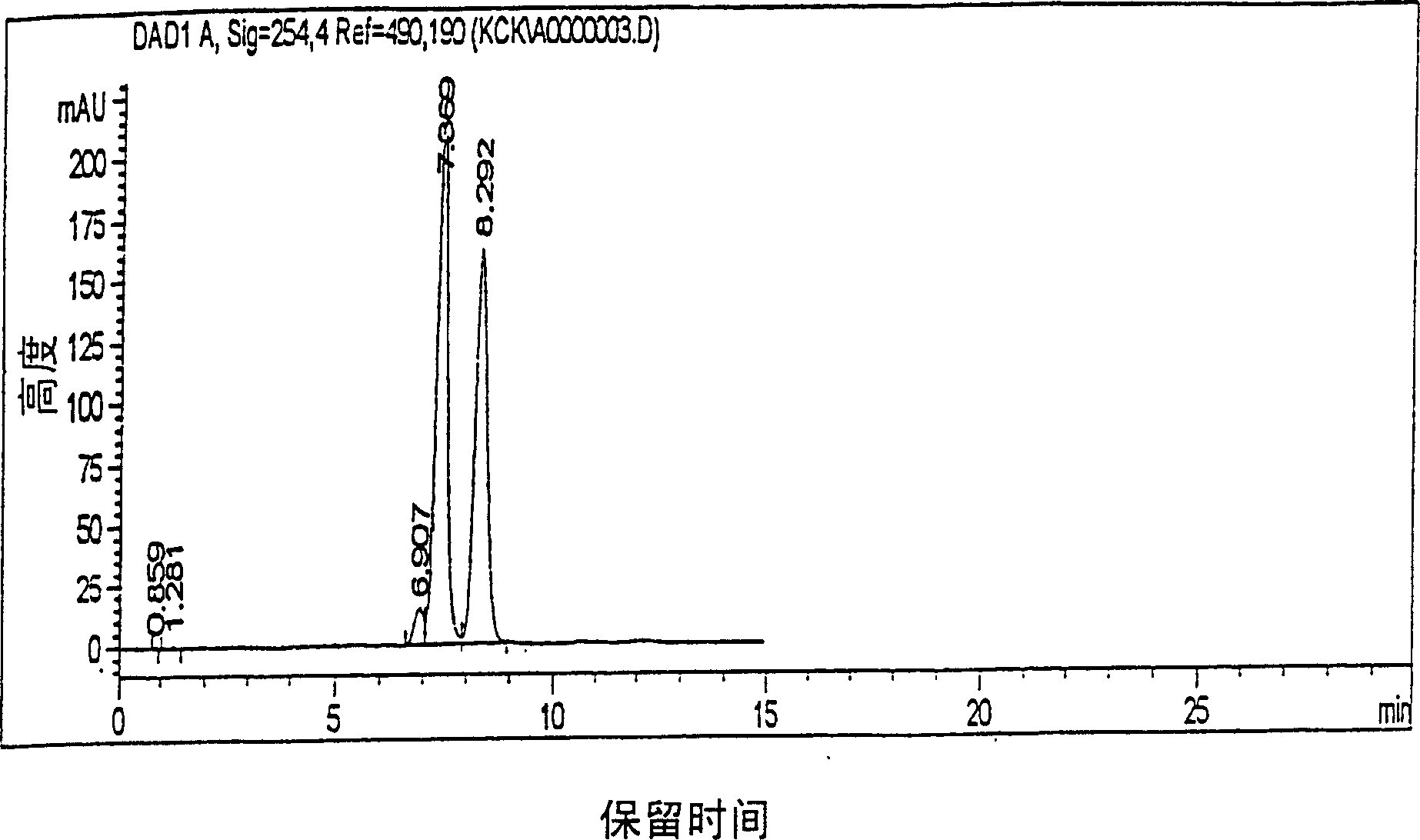
Method for preparing highly purity cefpodoxime proxetil
A technology of cefpodoxime and oxime propyl axetil, applied in the field of cefpodoxime propyl axetil, can solve problems such as low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0021] Preparation Example 1: Preparation of Cefpodoxime Sodium Salt
[0022] 2.03g sodium 2-ethylhexanoate was dissolved in 12.5ml N, in the mixture of N-dimethylacetamide and 50ml methanol, 5.0g cefpodoxime (7-[2-(2-amino-4 -thiazolyl)-(Z)-2-(methoxyimino)acetamido]-3-methoxymethyl-3-cephem-4-carbonic acid), and the mixture was stirred at room temperature for 30 minutes. Methanol was then removed under reduced pressure and 50 ml of acetone was added thereto. The solution was stirred for 30 minutes and filtered. After the filtered solid was washed with 50 ml of acetone and then with 30 ml of isopropyl ether, it was vacuum-dried at room temperature to obtain 4.98 g of light yellow cefpodoxime sodium salt (yield 95%).
[0023] 1 H-NMR (δ, D 2 O): 3.15(s, 3H, C-OCH 3 ), 3.37 (ABq, 2H, C-2), 3.85 (s, 3H, =N-OCH 3 ), 4.07 (d, 2H, -CH 2 -OCH 3 ), 5.09 (d, 1H, C-6), 5.66 (d, 1H, C-7), 6.88 (s, 1H, aminothiazole ring-H).
preparation example 2
[0024] Preparation Example 2: Preparation of Cefpodoxime Sodium Salt
[0025] 1.01 g of sodium acetate was dissolved in a mixture of 1 ml of water and 5 ml of methanol, to which 5.0 g of cefpodoxime was added, followed by 15 ml of N,N-dimethylacetamide. The mixture was stirred at room temperature for 30 minutes, and then 100 ml of acetone was added thereto. The solution was stirred for 20 minutes and filtered. After the filtered solid was washed with 50 ml of acetone and then with 30 ml of isopropyl ether, it was vacuum-dried at room temperature to obtain 5.04 g of pale yellow cefpodoxime sodium salt (96% yield).
[0026] income 1 The H-NMR data are the same as those in Preparation Example 1.
preparation example 3
[0027] Preparation Example 3: Preparation of Cefpodoxime Potassium Salt
[0028] 0.7 g of potassium acetate was dissolved in 5 ml of methanol, and 3.0 g of cefpodoxime was added thereto, followed by 9 ml of N,N-dimethylacetamide. The mixture was stirred at room temperature for 30 minutes, and then 60 ml of acetone was added thereto. The solution was stirred for 20 minutes and filtered. After the filtered solid was washed with 50 ml of acetone and then with 30 ml of isopropyl ether, it was vacuum-dried at room temperature to obtain 3.11 g of pale yellow cefpodoxime potassium salt (96% yield).
[0029] income 1 The H-NMR data are the same as those in Preparation Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com