Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
a technology of pathogenic microorganisms and compositions, applied in the directions of antibody medical ingredients, spray delivery, aerosol delivery, etc., can solve the problems of human contact with infected animals or animal products, animal anthrax infection still represents a significant problem, and domestic, agricultural and wild animals are affected by fatal diseases, etc., to reduce the morbidity, mortality, and/or infectivity , the effect of minimizing microbial resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
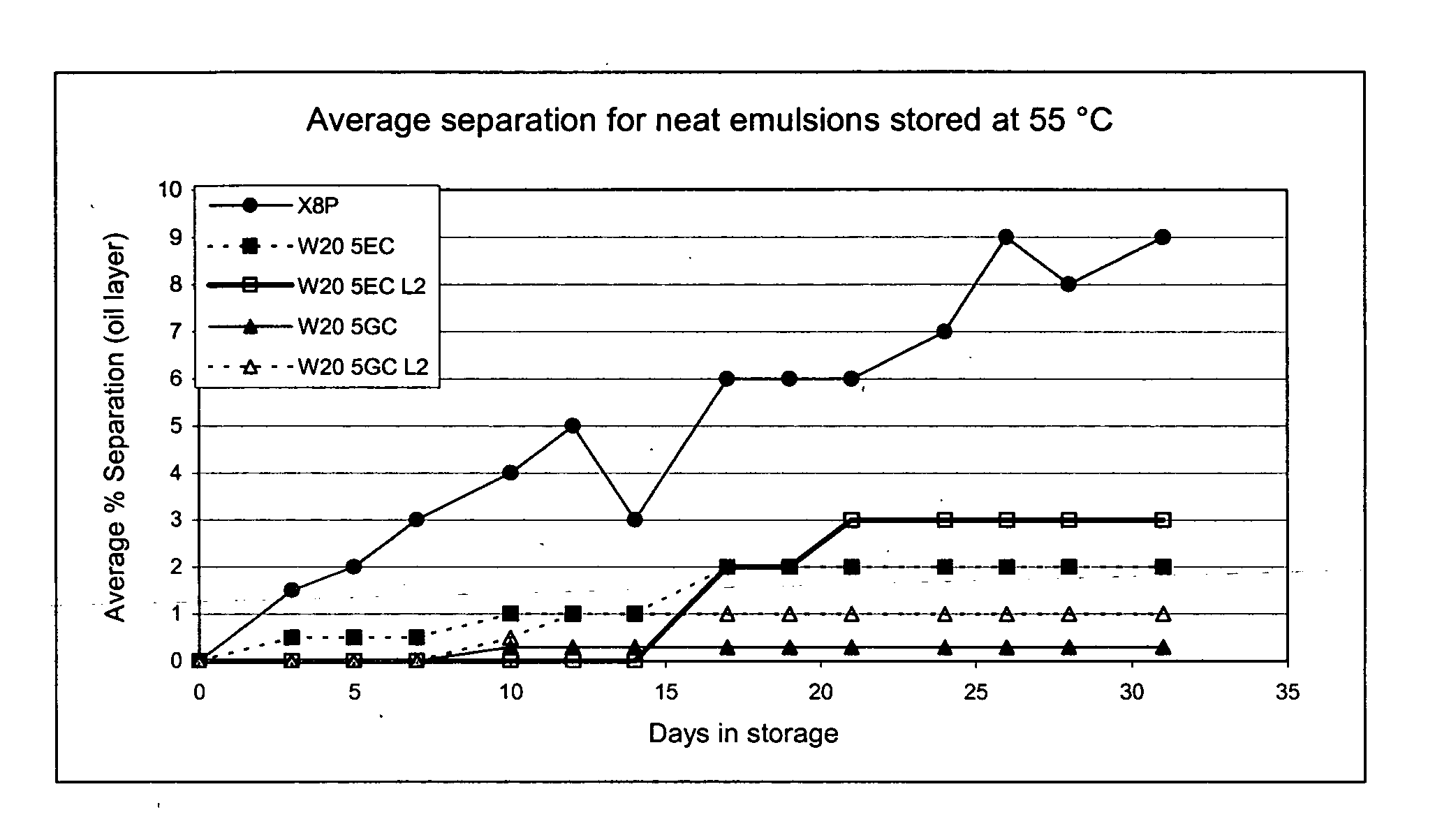
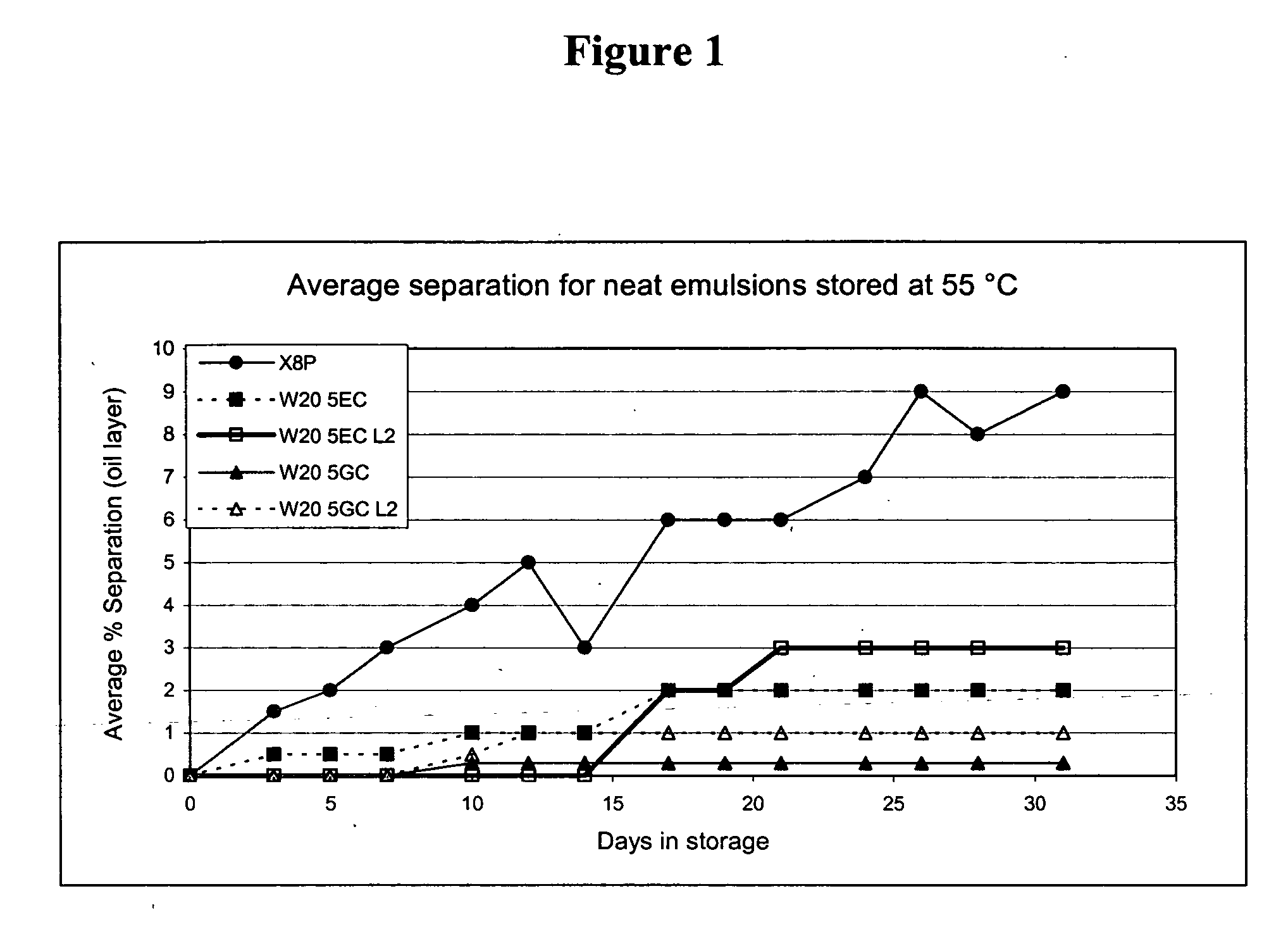
Comparison of Standard Emulsions and Small pParticle Size Nanoemulsions
[0111] The nanoemulsions are described by the components of the nanoemulsion according to Table 1. Unless otherwise noted, the oil is soybean oil. In the formulations, the detergent is listed first, followed by the volume percentage of the detergent (e.g., W205 refers to 5 vol % of Tween 20). In the formulations, the designation L2 refers to a small particle size nanoemulsion produced by a microfluidizer, while the absence of the L2 designation refers to a standard nanoemulsion (i.e., average particle sizes of 250 nm to about 1 micrometer). The designation L3 refers to nanoemulsions produced using an Avesting high pressure homogenizer.
TABLE 1ComponentSymbolTween 20W20EthanolECetylpyridinium chlorideCEDTAEDTriton X-100XTributyl phosphatePGlycerolGBenzalkonium chlorideBA
[0112] A first nanoemulsion is produced from a mixture containing 548 milliliters of water, 2.24 grams of EDTA, 25 grams of cetylpyridiunium chl...
example 2
Method of Making a Small Particle Size Nanoemulsion
[0115] A standard nanoemulsion (i.e., particles sizes of 250 nm to 5 micrometers) is formed as follows. A mixture of 22 vol % distilled water, 1 wt / vol % cetylpyridinium chloride, 5 vol % Tween 20, 64 vol % soybean oil, and 8 vol % ethanol based on the total volume of the mixture is formed. The nanoemulsion is formed by mixing for 5 minutes at 10,000±500 revolutions per minute with a Silverson L4RT mixer with a standard mixing assembly and a fine emulsion screen. The standard nanoemulsion is denoted as W205EC.
[0116] A small particle size nanoemulsion is formed by passing the W205EC nanoemulsion 4 times through a Microfluidics M-110EH microfluidizer processor using an H210Z (200 μm) chamber downstream of an H230Z (400 μm) chamber. The small particle size nanoemulsion is denoted as W205EC L2.
[0117] After formation, the W205EC and W205EC L2 emulsions are diluted with water for further testing. Particle sizes are determined by Partic...
example 3
Effect of Microfluidizer Chamber Size on the Size of Small Particle Size Nanoemulsion Particles
[0119] A W205G BA2 nanoemulsion is passed through different combinations of microfluidizer chambers as shown in Table 4. The W205G BA2 L2 small particle size nanoemulsion is made with 1 pass with a Silverson L4RT mixer and 4 passes through a microfluidizer. Combinations of chamber having 75, 200, 400 micrometer microchannels are used to determine the relationship between the size of the microchannels and the size of the particles produced.
TABLE 4FirstSecond chamber,ParticleSamplechamber, μmμmsize, nm175100174210075165375200185420075180575400211640075199
[0120] As shown in Table 4, the chamber size utilized in the microfluidizer, when varied between 75 and 400 μm, does not significantly affect the particle size of the emulsions. In all cases, the particle size is less than or equal to about 250 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com