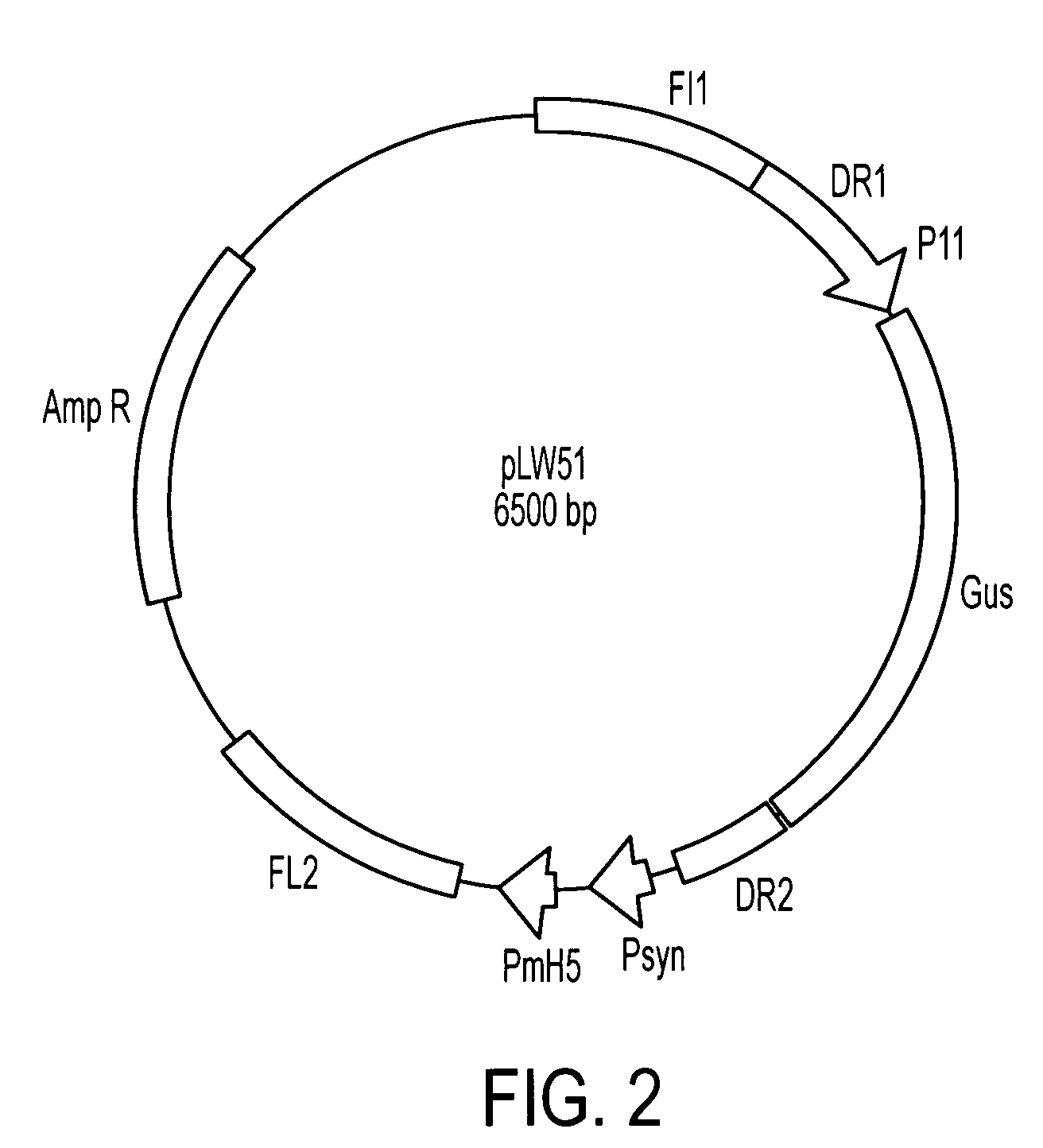
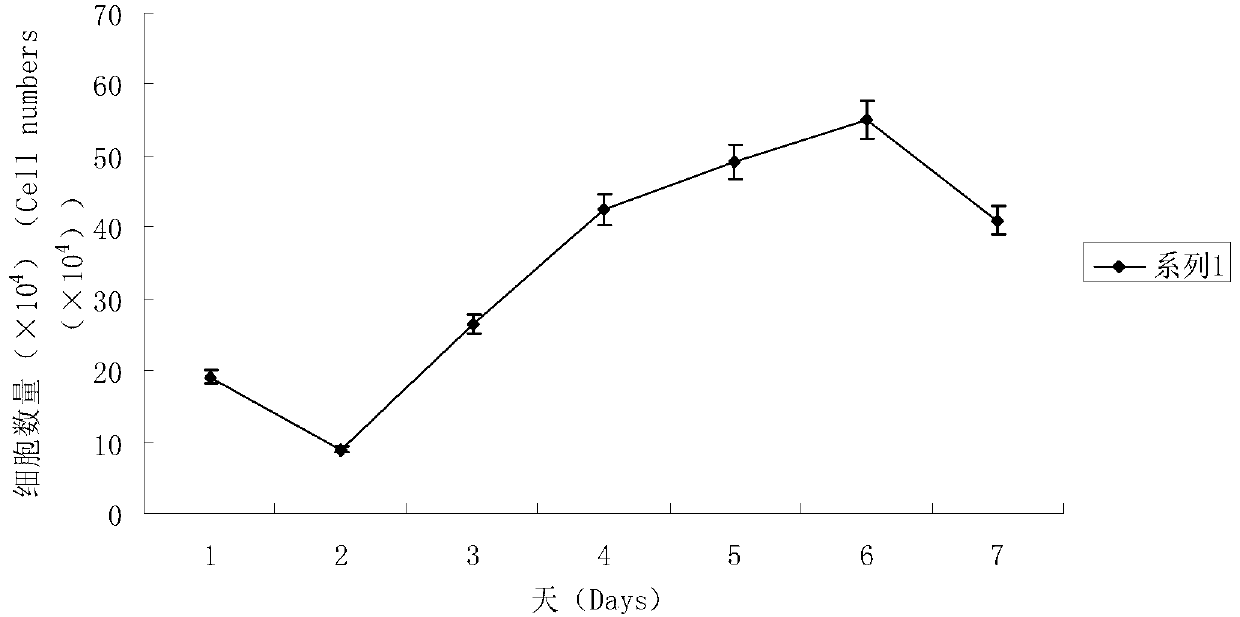
Patents
Literature
494 results about "Viral Vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any vaccine preparation composed of a live, attenuated, inactivated, killed or recombinant virus. Administration of a viral vaccine is to provide prophylaxis against viral disease or used as a vehicle to treat cancers.
Adenovirus vector vaccine for preventing SARS-CoV-2 infection
ActiveCN110974950AImprove securityAvoid infectionSsRNA viruses positive-senseViral antigen ingredientsVector vaccineHuman cell
The invention discloses an adenovirus vector vaccine for preventing SARS-CoV-2 infection. The vaccine comprises a nucleic acid sequence as shown in SEQ ID NO: 1. According to a plurality of embodiments of the invention, the S protein nucleic acid sequence contained in the vaccine is easy to express in human cells, and generation of more S proteins can be induced, so the vaccine is expected to be used as a recombinant virus vaccine for preventing SARS-CoV-2 infection. According to a plurality of embodiments of the invention, the vaccine has good security.
Owner:GUANGZHOU N BIOMED LTD
Development of a preventive vaccine for filovirus infection in primates
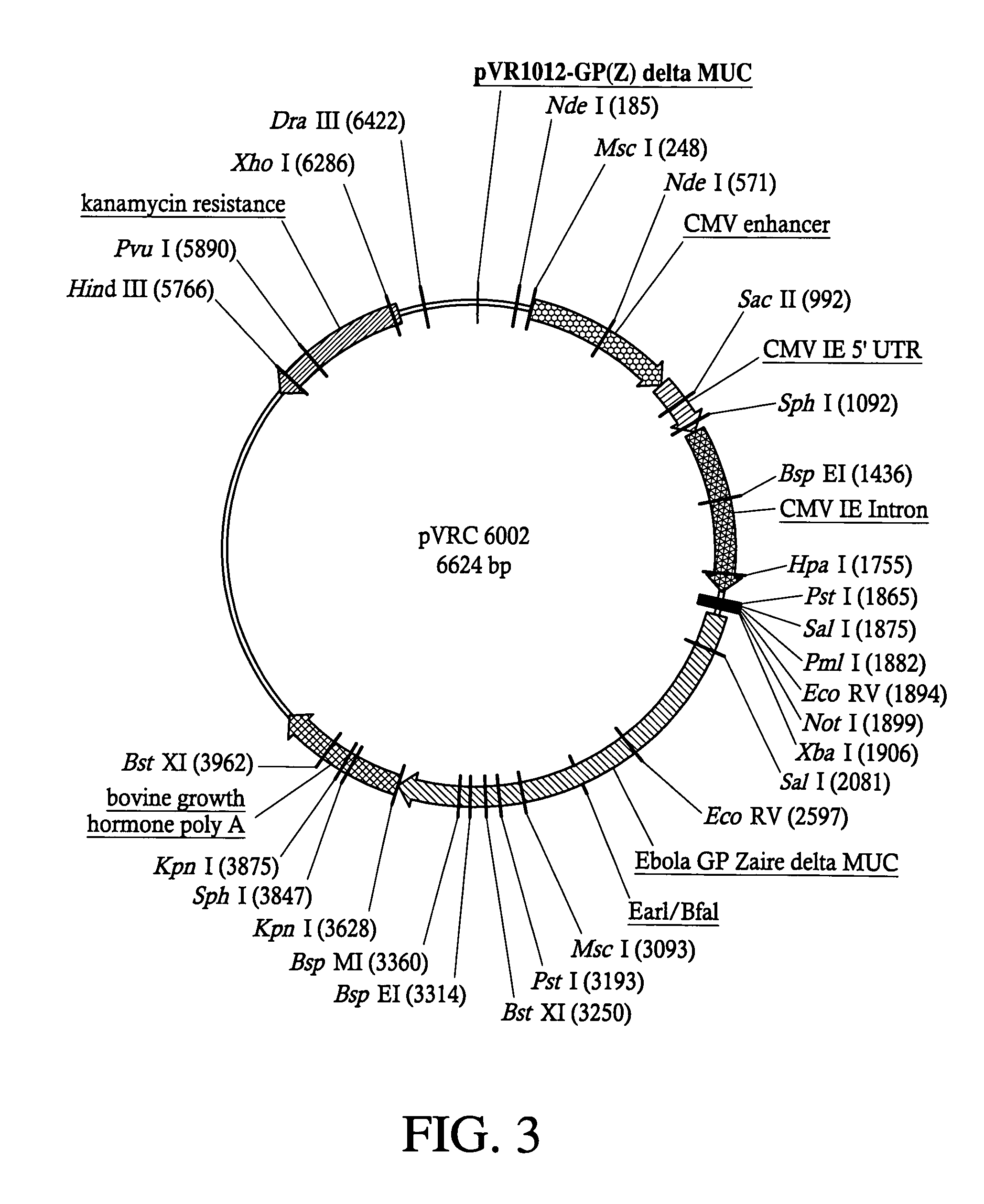
The present invention relates generally to viral vaccines and, more particularly, to filovirus vaccines and methods of eliciting an immune response against a filovirus or disease caused by infection with filovirus.
Owner:UNITED STATES OF AMERICA
RNA respiratory syncytial virus vaccines
InactiveUS6060308AFaster replicationImprove efficiencySsRNA viruses negative-senseBiocideF proteinViral Vaccine
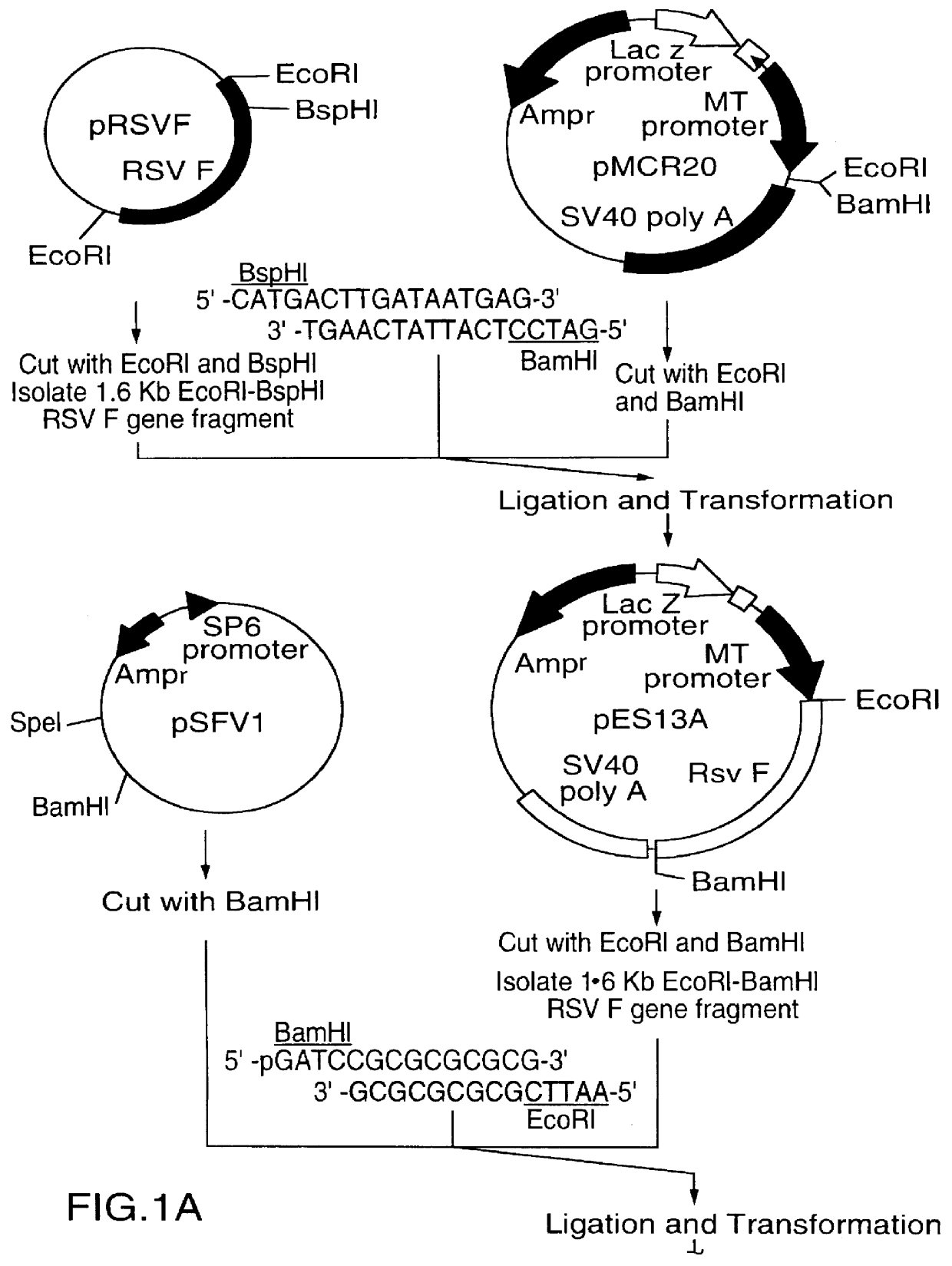
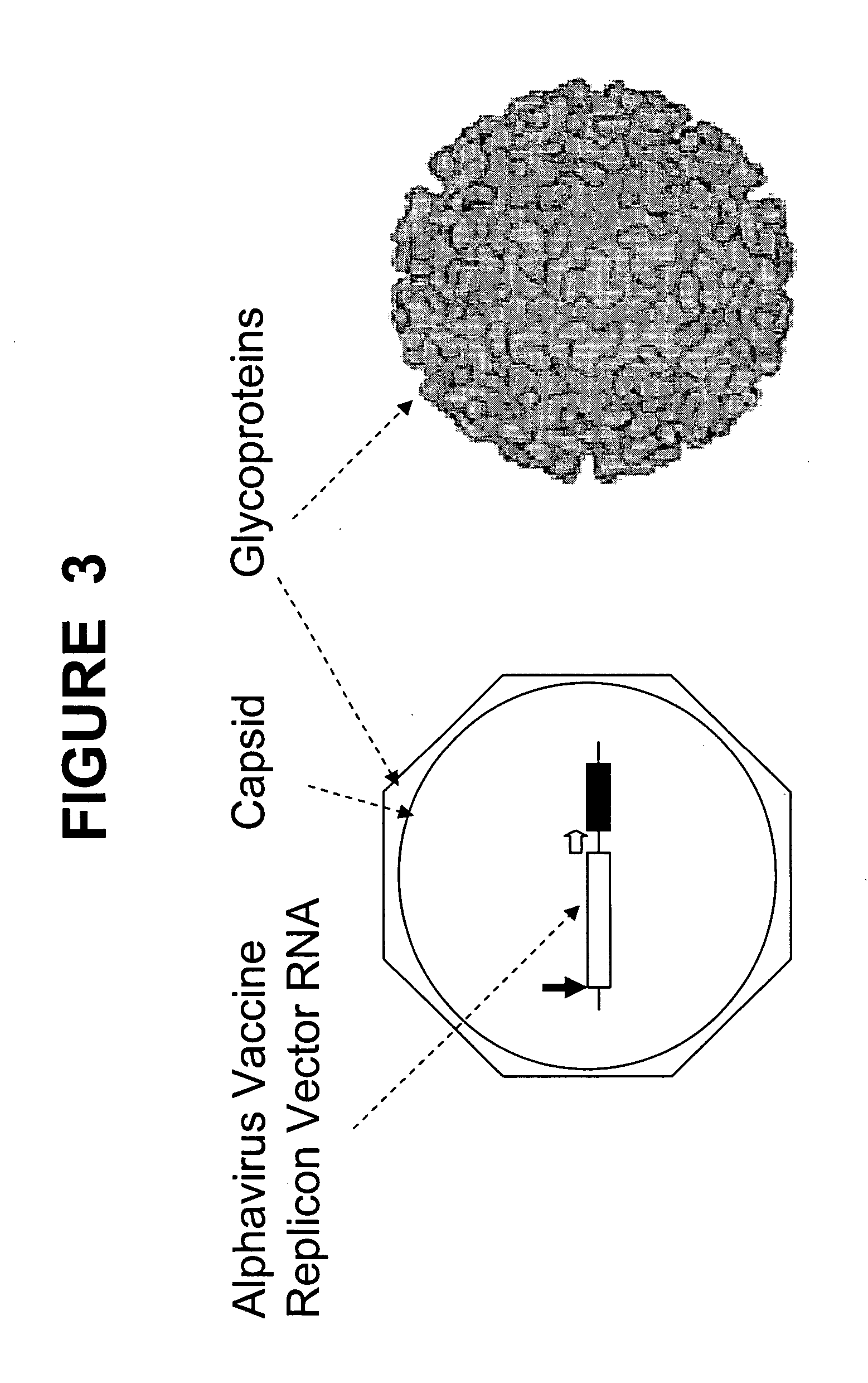
A vector comprising a first DNA sequence which is complementary to at least part of an alphavirus RNA genome and having the complement of complete alphavirus DNA genome replication regions, a second DNA sequence encoding a paramyxovirus protein, particularly a respiratory syncytial virus fusion (RSV F) protein or a RSV F protein fragment that generates antibodies that specifically react with RSV F protein, the first and second DNA sequences being under the transcriptional control of a promoter is described. Such vector may be used to produce an RNA transcript which may be used to immunize a host, including a human host, to protect the host against disease caused by paramyxovirus, particularly respiratory syncytial virus, by administration to the host.
Owner:CONNAUGHT LAB
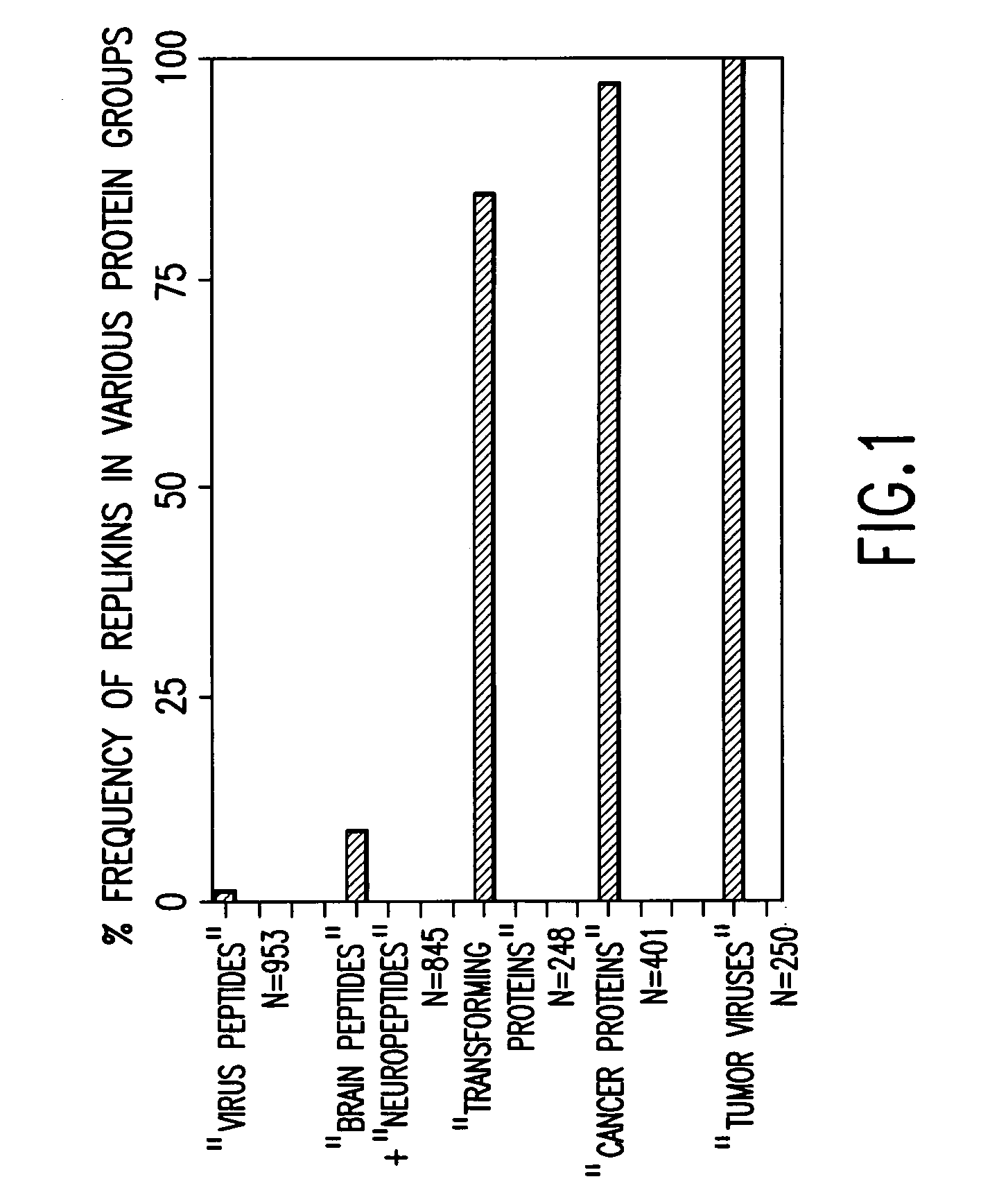
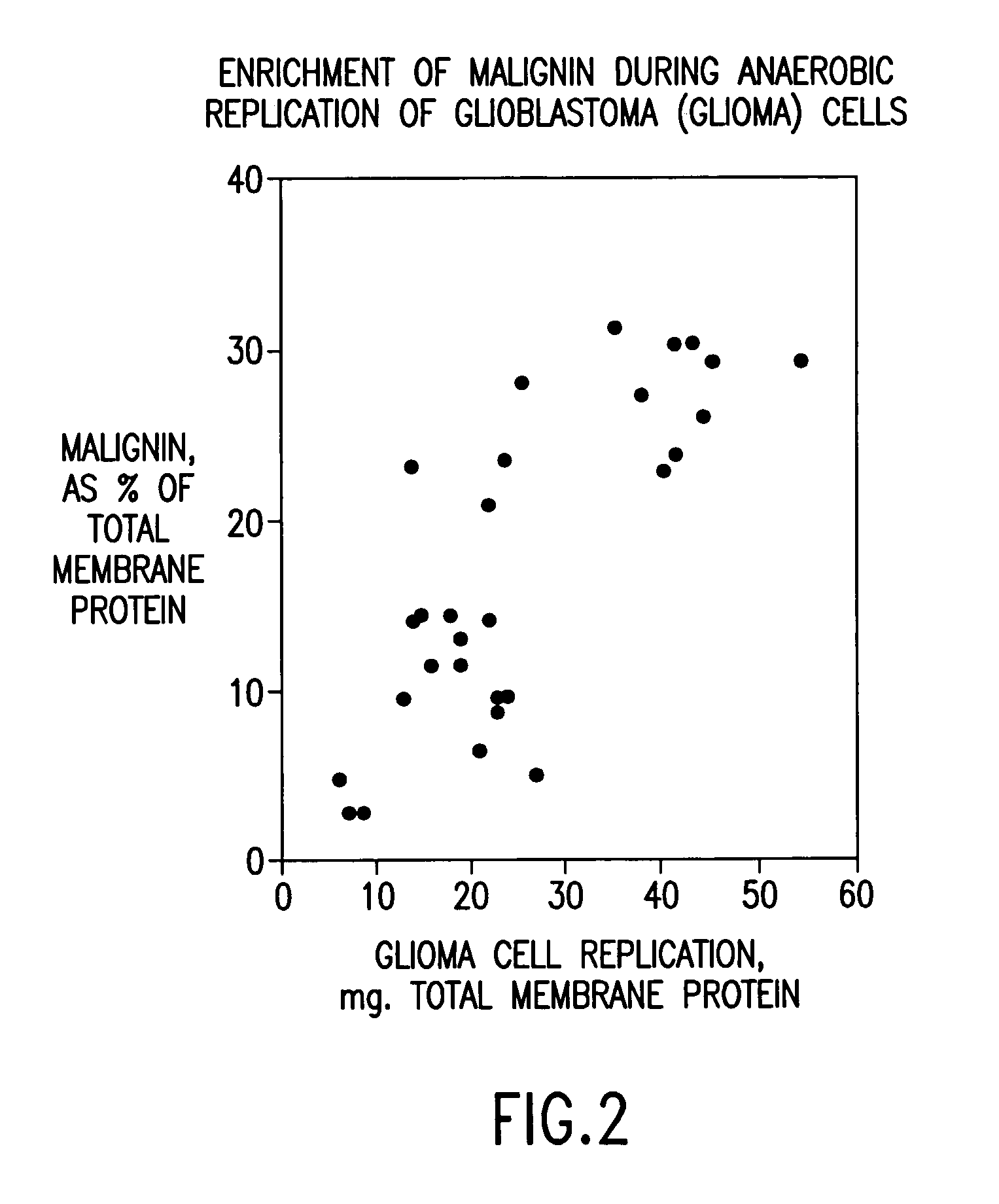
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
Feline vaccines against avian influenza
InactiveUS20080107687A1Elicit immune responseSsRNA viruses negative-senseViral antigen ingredientsEpitopeViral Vaccine
The present invention encompasses influenza vaccines, in particular avian influenza vaccines. The vaccine may be a recombinant poxvirus vaccine or an inactivated vaccine. The invention also encompasses recombinant poxvirus vectors encoding and expressing avian influenza antigens, epitopes or immunogens which can be used to protect animals, in particular felids, against avian influenza.
Owner:MERIAL LTD
Norovirus Vaccine Formulations
ActiveUS20080299152A1Enhance antigen uptakeExtended retention timeBiocideSsRNA viruses positive-senseAntigenAdjuvant
The present invention relates to antigenic and vaccine compositions comprising Norovirus antigens and adjuvants, in particular, mixtures of monovalent VLPs and mixtures of multivalent VLPs, and to a process for the production of both monovalent and multivalent VLPs, the VLPs comprising capsid proteins from one or more Norovirus genogroups.
Owner:TAKEDA VACCINES INC
West nile virus vaccine
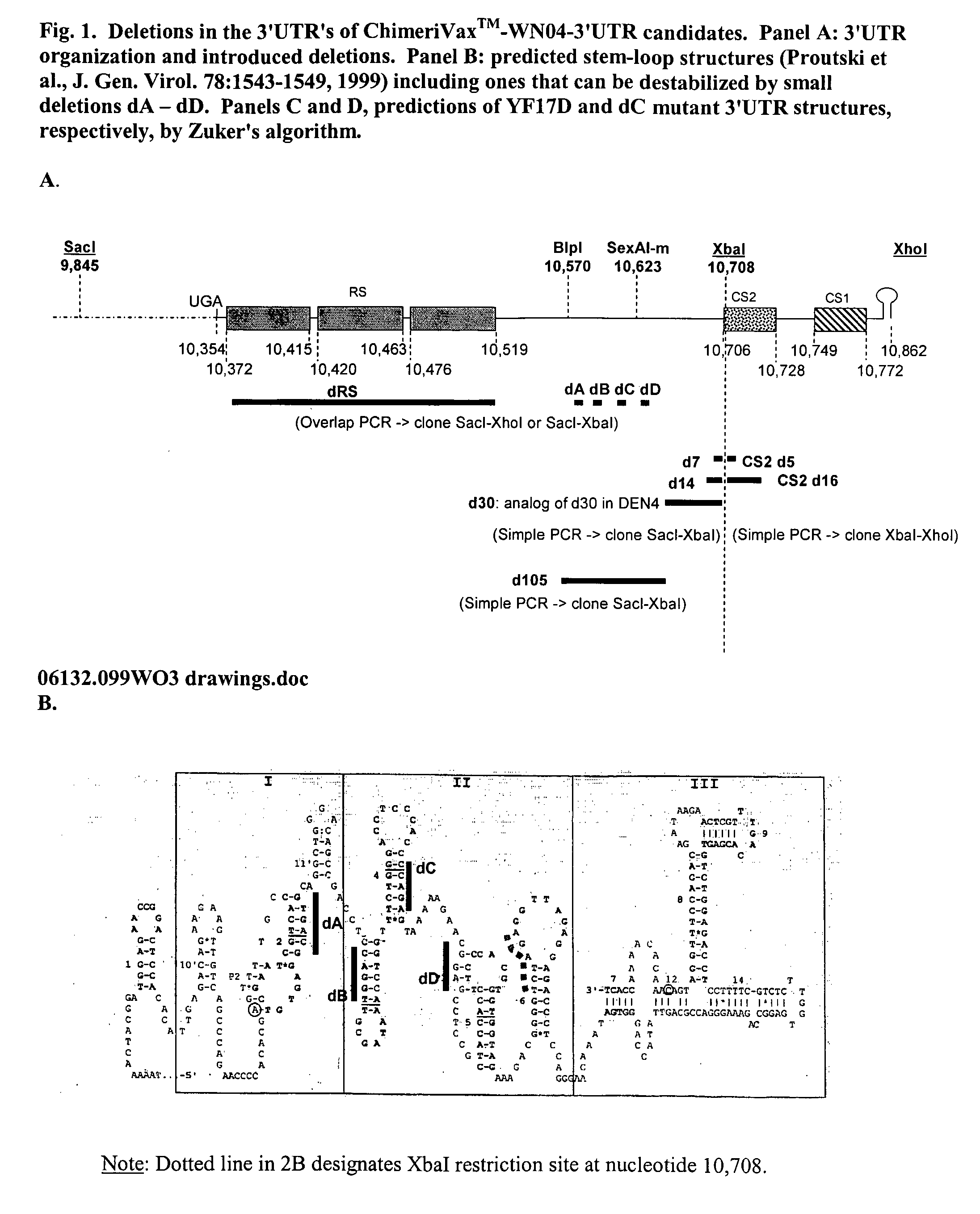
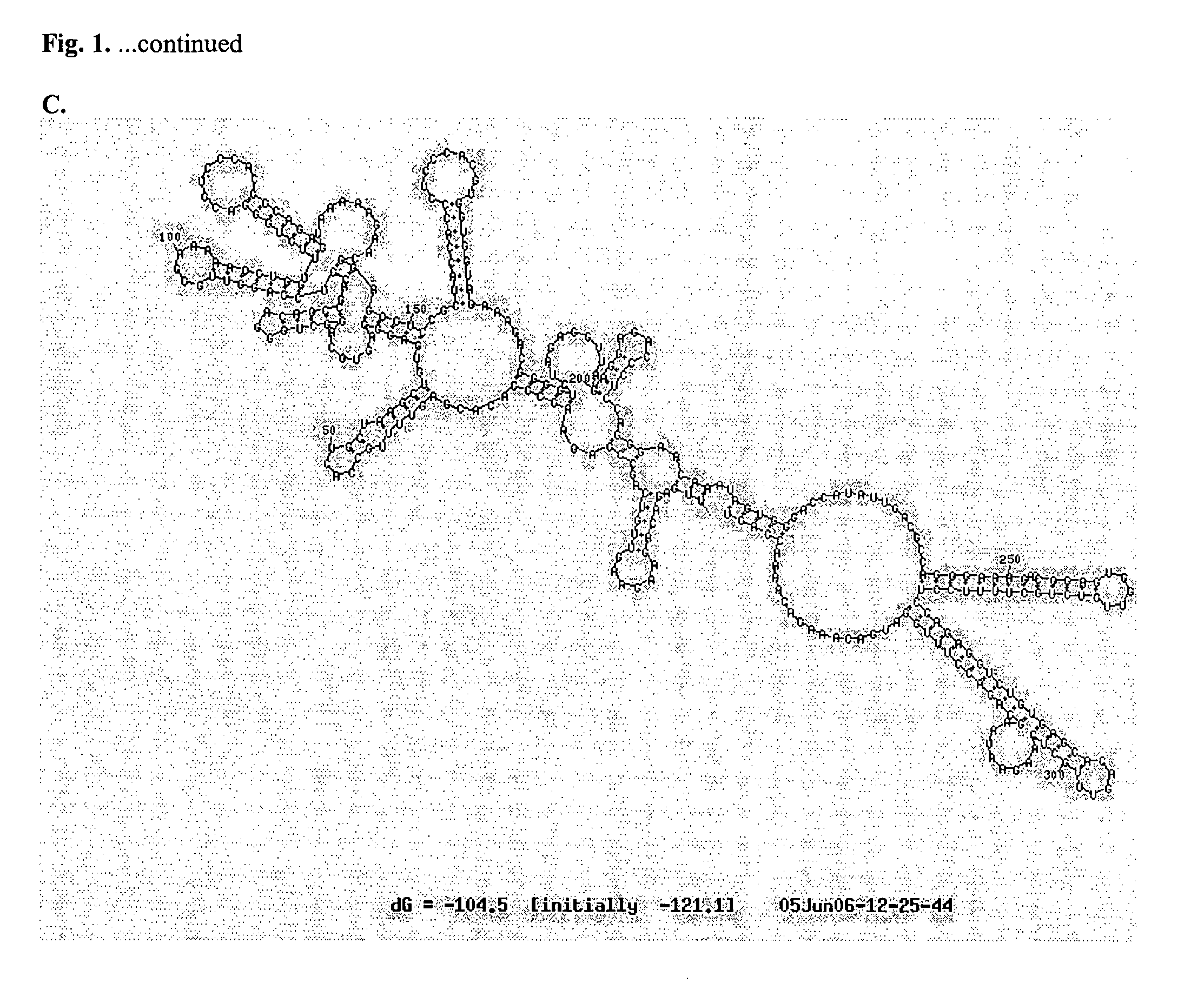
InactiveUS20050053624A1Decreased neurovirulenceEffective and safe approachSsRNA viruses positive-senseSugar derivativesWest Nile Virus InfectionViral Vaccine
The invention provides chimeric flavivirus vaccines against West Nile virus and methods of using these vaccines to prevent or treat West Nile virus infection.
Owner:SANOFI PASTEUR BIOLOGICS CO
Nucleic acid respiratory syncytial virus vaccines
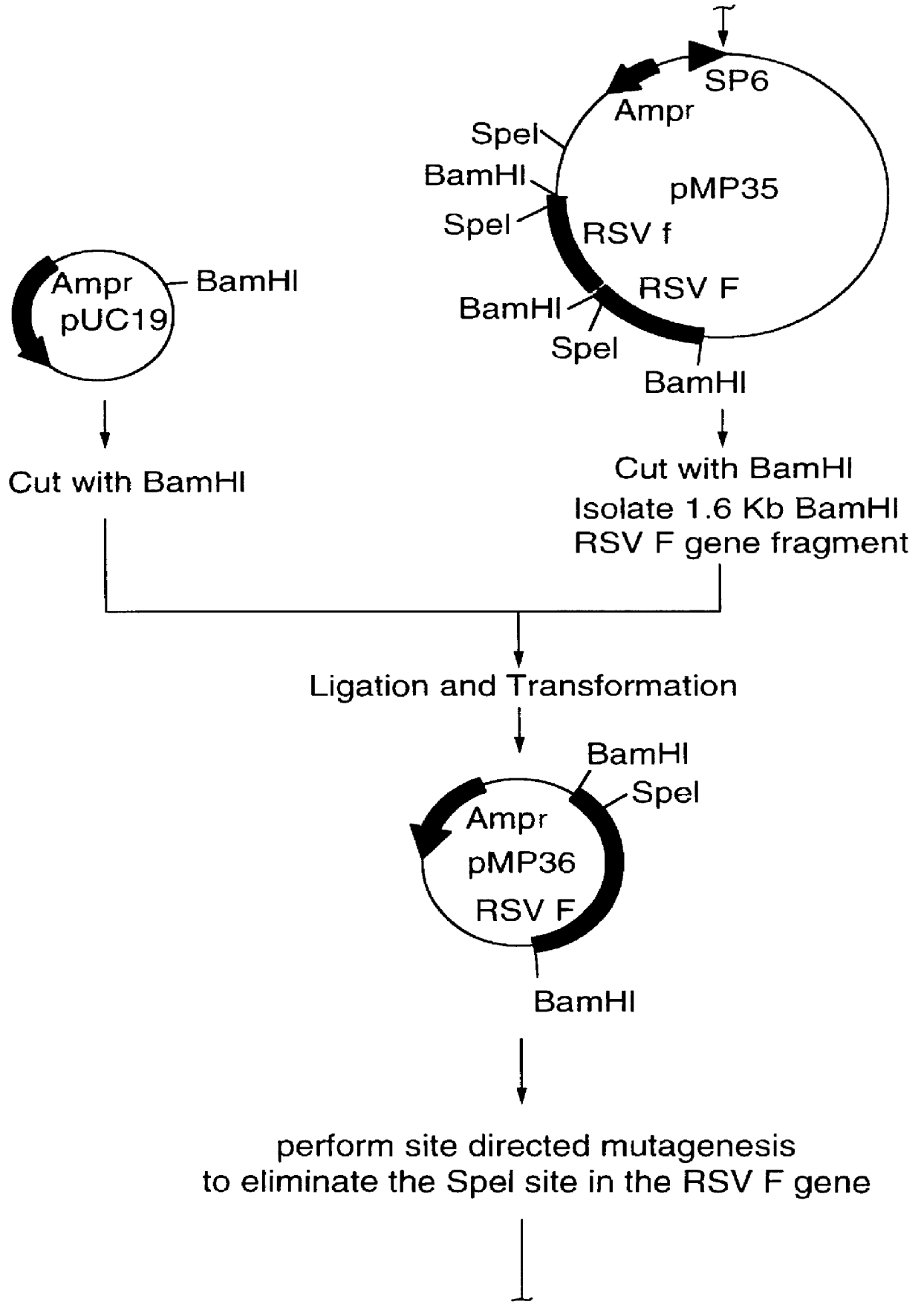
InactiveUS6083925AGood immune protectionImprove expression levelSsRNA viruses negative-senseGenetic material ingredientsHeterologousF protein
Non-replicating vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described for in vivo immunization. The nucleotide sequence encloding the RSV F protein may lack a sequence encoding the homologous signal peptide but possessing a heterologous signal peptide enhancing RSV F protein expression. Such non-replicating vectors, including plasmids, also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such non-replicating vectors may be used to immunize a host against disease caused by infection with RSV, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purpose. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:CONNAUGHT LAB
Vaccines expressed in plants
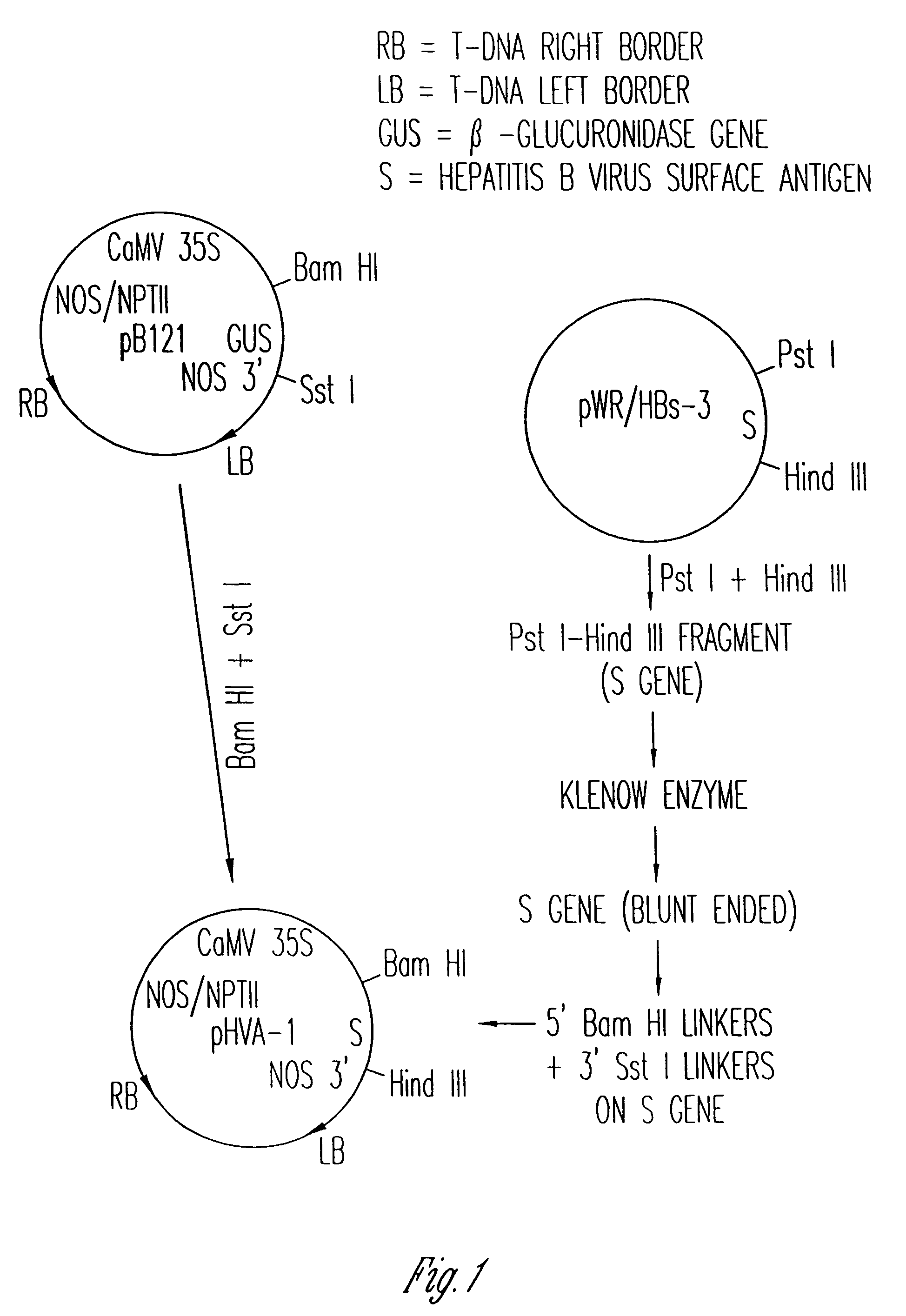
The anti-viral vaccine of the present invention is produced in transgenic plants and then administered through standard vaccine introduction method or through the consumption of the edible portion of those plants. A DNA sequence encoding for the expression of a surface antigen of a viral pathogen is isolated and ligated to a promoter which can regulate the production of the surface antigen in a transgenic plant. This gene is then transferred to plant cells using a procedure that results in its integration into the plant genome, such as through the use of an Agrobacterium tumenfaciens plasmid vector system. Preferably, the foreign gene is expressed in an portion of the plant that is edible by humans or animals. In a preferred procedure, the vaccine is administered through the consumption of the edible plant as food, preferably in the form of a fruit or vegetable juice which can be taken orally.
Owner:PRODI GENE INC
Momlv-based pseudovirion packaging cell line
The present invention discloses Moloney murine leukemia virus (MoMLV)-based viral packaging cell line for the production of anti-viral vaccines. The invention also includes methods of making, administering and formulating pseudovirions and replicon deficient viral particles of the invention and methods of inducing immunity.
Owner:BIOPROTECTION SYST
Hemorrhagic feline calicivirus, calicivirus vaccine and method for preventing calicivirus infection or disease
The present invention relates to a novel, isolated and purified hemorrhagic feline calicivirus FCV-DD1. The invention further embraces monovalent and multivalent vaccines containing the new FCV-DD1 strain. In addition, the invention encompasses methods of protecting felines against infection or preventing disease caused by feline calicivirus alone or in addition to other pathogens that comprises administering to the felines an immunologically effective amount of the monovalent and multivalent vaccines described herein. Also, the invention concerns methods for diagnosing or detecting the hemorrhagic feline calicivirus in a susceptible host, asymptomatic carrier and the like by detecting the presence of feline calicivirus FCV-DD1 or antibodies raised or produced against feline calicivirus FCV-DD1 antigen.
Owner:ELANCO US INC
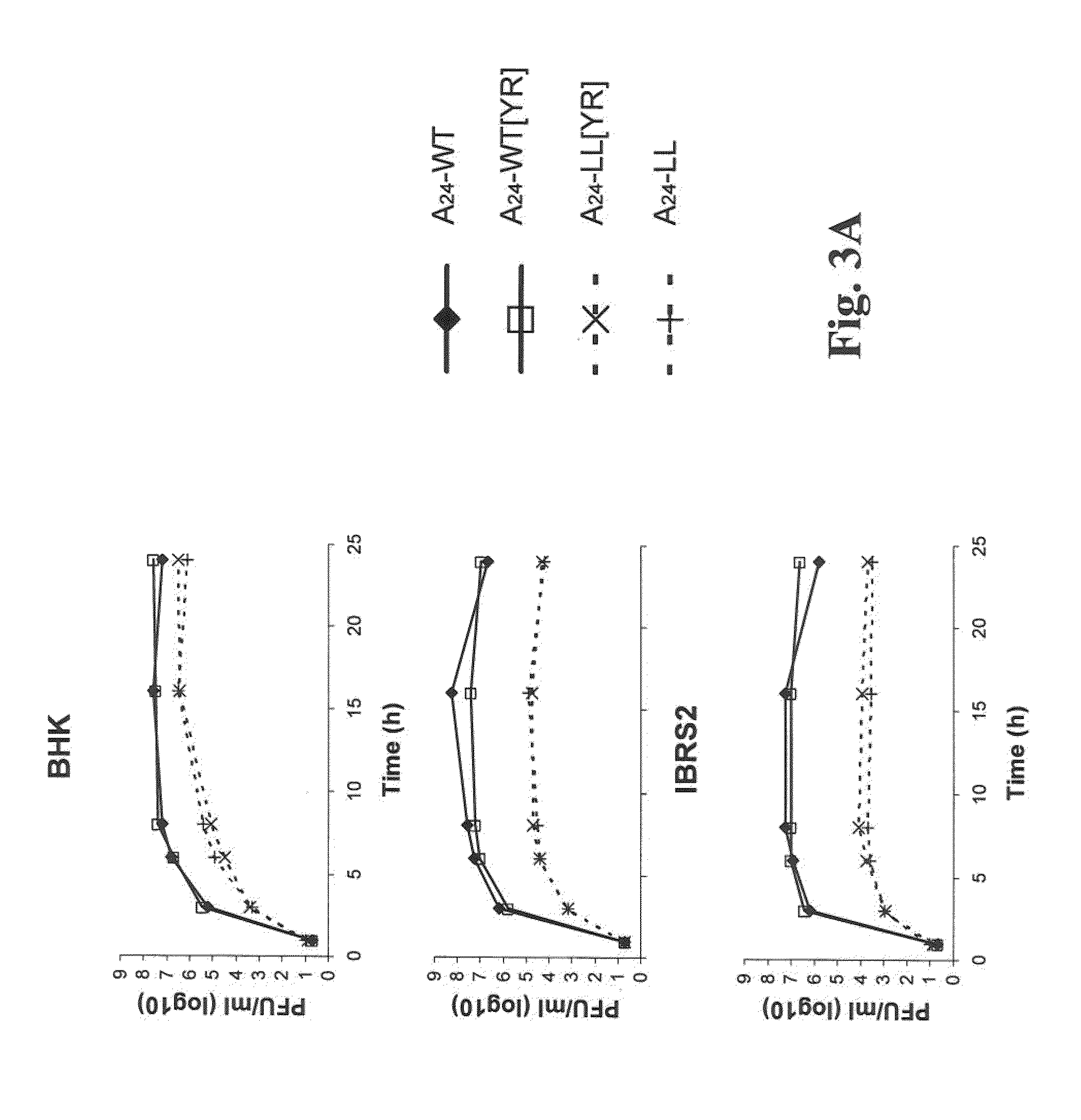
Development of a Marker Foot and Mouth Disease Virus Vaccine Candidate That is Attenuated in the Natural Host
ActiveUS20120315295A1Easy to replaceQuick changeSsRNA viruses positive-senseSugar derivativesSerotypeViral Vaccine
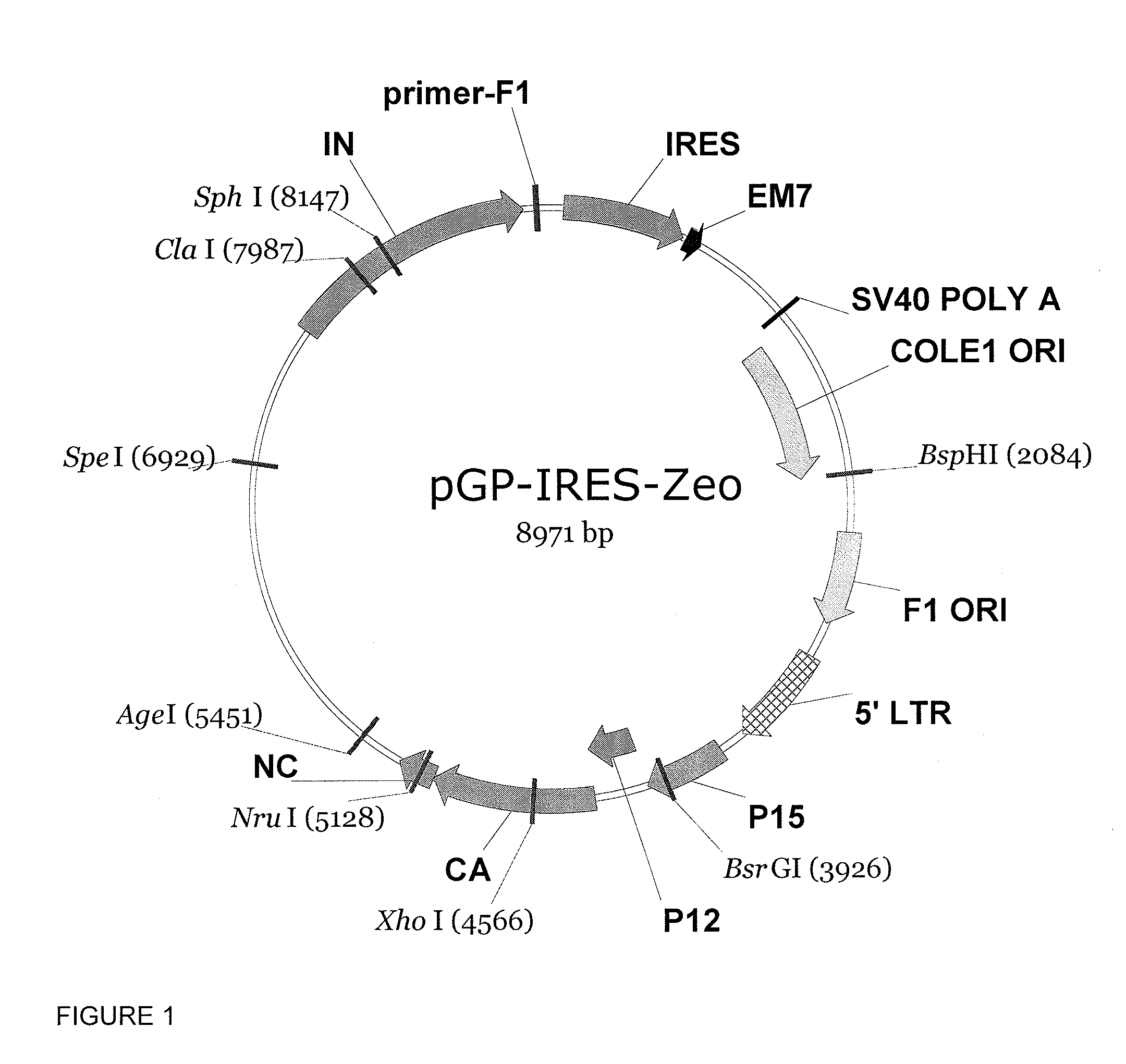
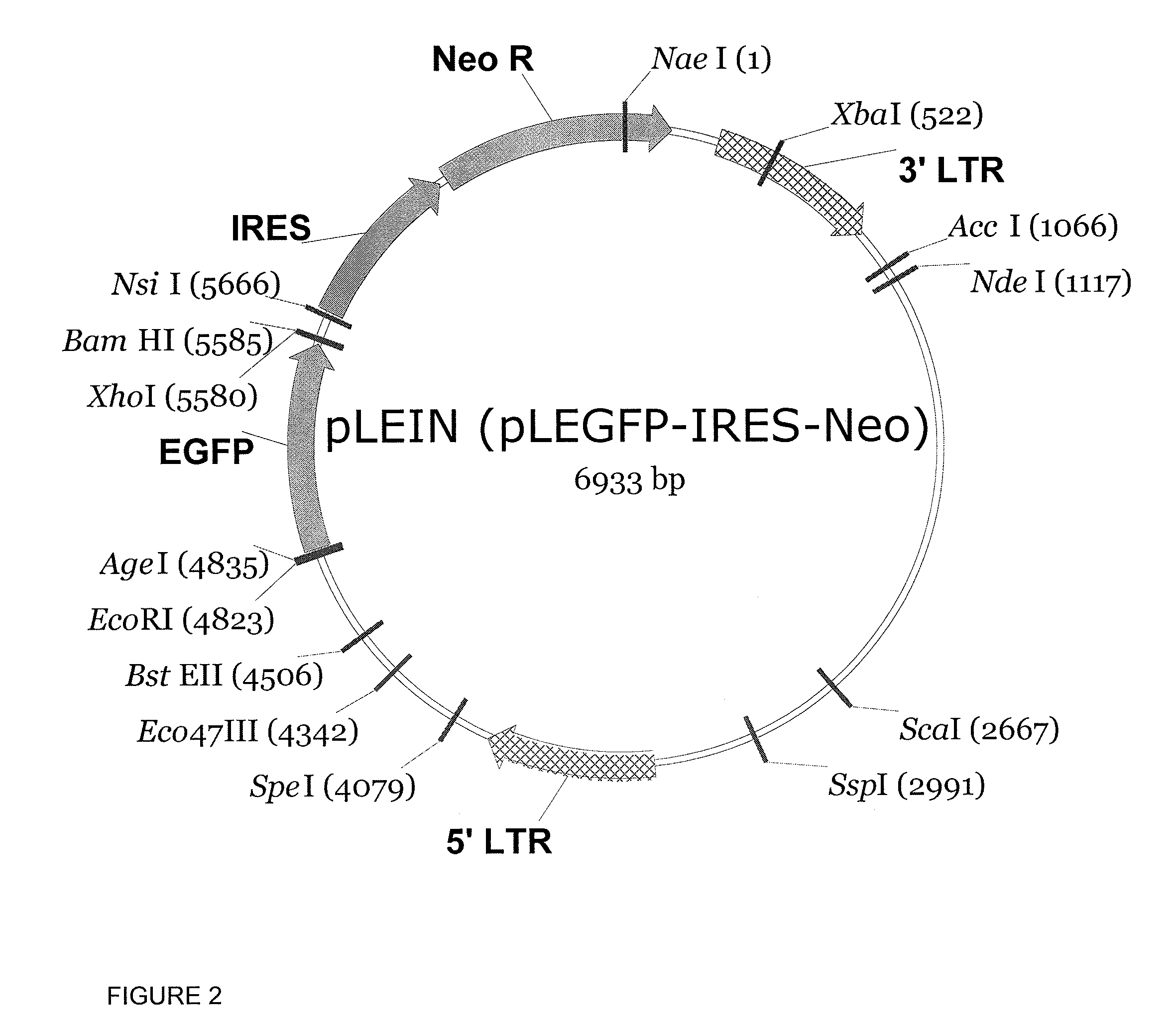
We have generated novel molecularly marked FMDV A24LL3DYR and A24LL3BPVKV3DYR vaccine candidates. The mutant viruses contain a deletion of the leader coding region (LL) rendering the virus attenuated in vivo and negative antigenic markers introduced in one or both of the viral non-structural 3Dpol and 3B proteins. The vaccine platform includes unique restriction endonuclease sites for easy swapping of capsid proteins for different FMDV subtypes and serotypes. The mutant viruses produced no signs of FMD and no shedding of virulent virus in cattle. No clinical signs of disease or fever were observed and no transmission to in-contact animals was detected in pigs inoculated with live A24LL3DYR. Cattle immunized with chemically inactivated vaccine candidates showed an efficacy comparable to a polyvalent commercial FMDV vaccine. These vaccine candidates used in conjunction with a cELISA provide a suitable target for DIVA companion tests.
Owner:US SEC AGRI
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
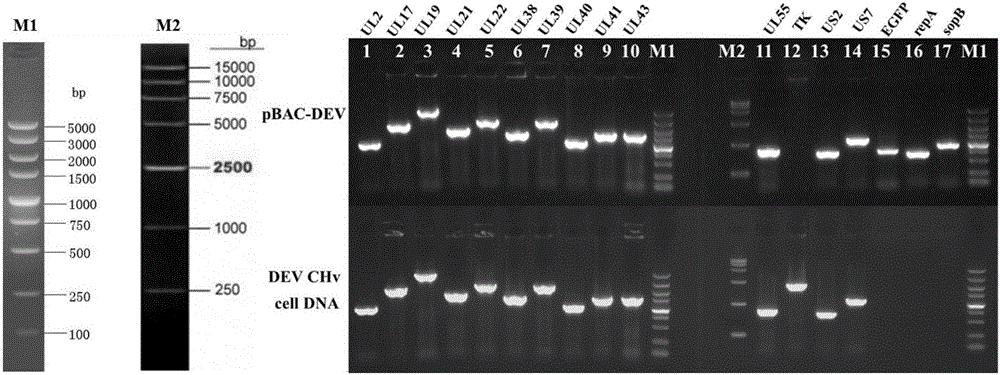
Establishing method of bacterial artificial chromosome recombinant duck plague virus rescue system platform and application
InactiveCN105802922ALower titerDoes not affect the replication cycleVirus peptidesNucleic acid vectorBacteroidesRecombinant vaccines
The invention discloses an establishing method of a bacterial artificial chromosome recombinant duck plague virus rescue system platform and application of the platform. A bacterial artificial chromosome recombinant duck plague virus is obtained by inserting a recombinant duck plague virus transfer vector pUC18 / EGFP-TKAB-BAC11 in a TK domain, wherein the recombinant duck plague virus transfer vector contains a TK gene left-right homologous arm, a reporter gene EGFP and a bacterial artificial chromosome core function component. By means of the platform, the in-vitro biologics characteristics of a UL55 gene-deleted strain established through an inside-bacterium two-step RED recombination method and a back mutation strain and parent strain of the UL55 gene-deleted strain are quite close; the functions are not related to positioning of a UL26.5 gene in a cell. The method is beneficial to development of pathogenic mechanism and gene function research of DPV CHv and is beneficial to the duck plague virus prevention and the research and application of recombinant duck plague virus vaccines of other poultry infectious diseases based on the platform; in addition, due to the fact that the recombinant virus carries a TK deletion mark and an EGFP gene, a mark vaccine can be developed to clinically distinguish a wild virus and a recombinant vaccine virus.
Owner:SICHUAN AGRI UNIV
Vaccines Against Japanese Encephalitis Virus and West Nile Virus
ActiveUS20070269458A1Decrease viscerotropism/viremiaHigh genetic stabilitySsRNA viruses positive-senseSugar derivativesViral VaccineWest Nile virus RNA
The invention provides attenuated Flavivirus vaccines, such as vaccines against Japanese encephalitis virus and West Nile virus, as well as methods of making and using these vaccines.
Owner:SANOFI PASTEUR BIOLOGICS CO
Method for purifying recombinant human serum albumin protein and application thereof
The present invention relates to a method for purifying recombinant human serum albumin (rHSA) protein. The method comprises the following steps: fermented liquid containing rHSA is processed by a ceramic membrane, supernatant liquid is orderly purified by high salt cation exchange chromatography, hydrophobic layer exchange chromatography and weak anion exchange chromatography, and purified rHSA is obtained. The present invention is characterized in that solution processed by high salt cation exchange chromatography is processed by borate and then filtered by hollow fibers. The rHSA obtained can be used for producing vaccines for humans against viruses with a cell culture method, particularly rabies vaccines.
Owner:NCPC NEW DRUG RES & DEV
Recombinant multivalent viral vaccine
InactiveUS7087234B1Elicit immune responseReadily apparentSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininViral Vaccine
The present invention relates to multivalent recombinant raccoon poxviruses, containing more than one exogenous gene inserted into either the thymidine kinase gene, the hemagglutinin gene, or a combination thereof. Disclosed is the use of the multivalent recombinant raccoon poxviruses as vaccines to immunize felines against subsequent challenge by feline pathogens. Also disclosed is a method of making a multivalent recombinant raccoon poxvirus by a recombination process involving the construction of an insertion vector into which the exogenous genes are inserted, and flanking the inserted genes are sequences which can recombine into the raccoon poxvirus thymidine kinase gene, or the hemagglutinin gene, or a combination thereof; introducing both the insertion vector containing the exogenous genes, and raccoon poxvirus into susceptible host cells; and selecting the recombinant raccoon poxvirus from the resultant plaques.
Owner:CORNELL RES FOUNDATION INC +1
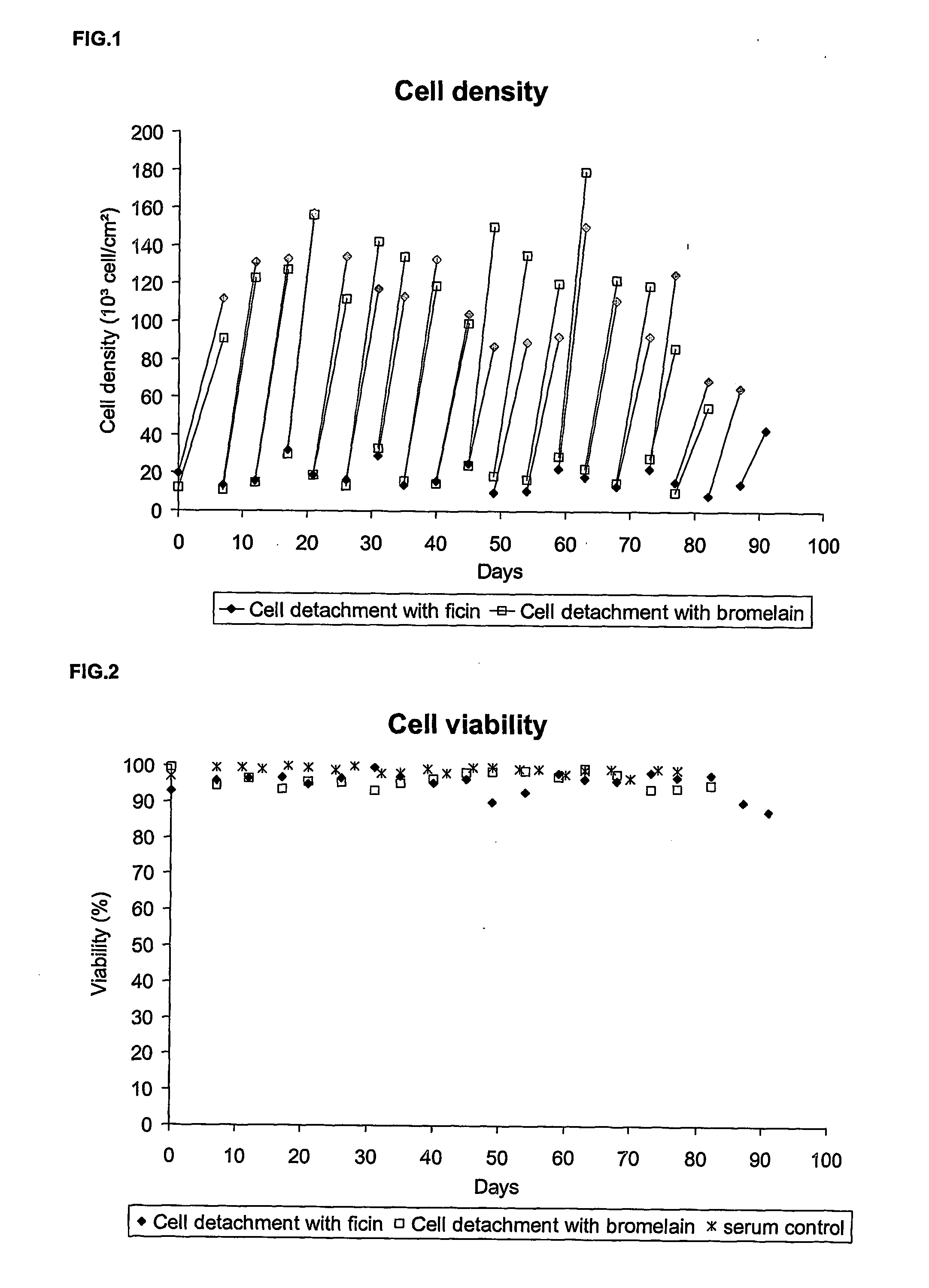
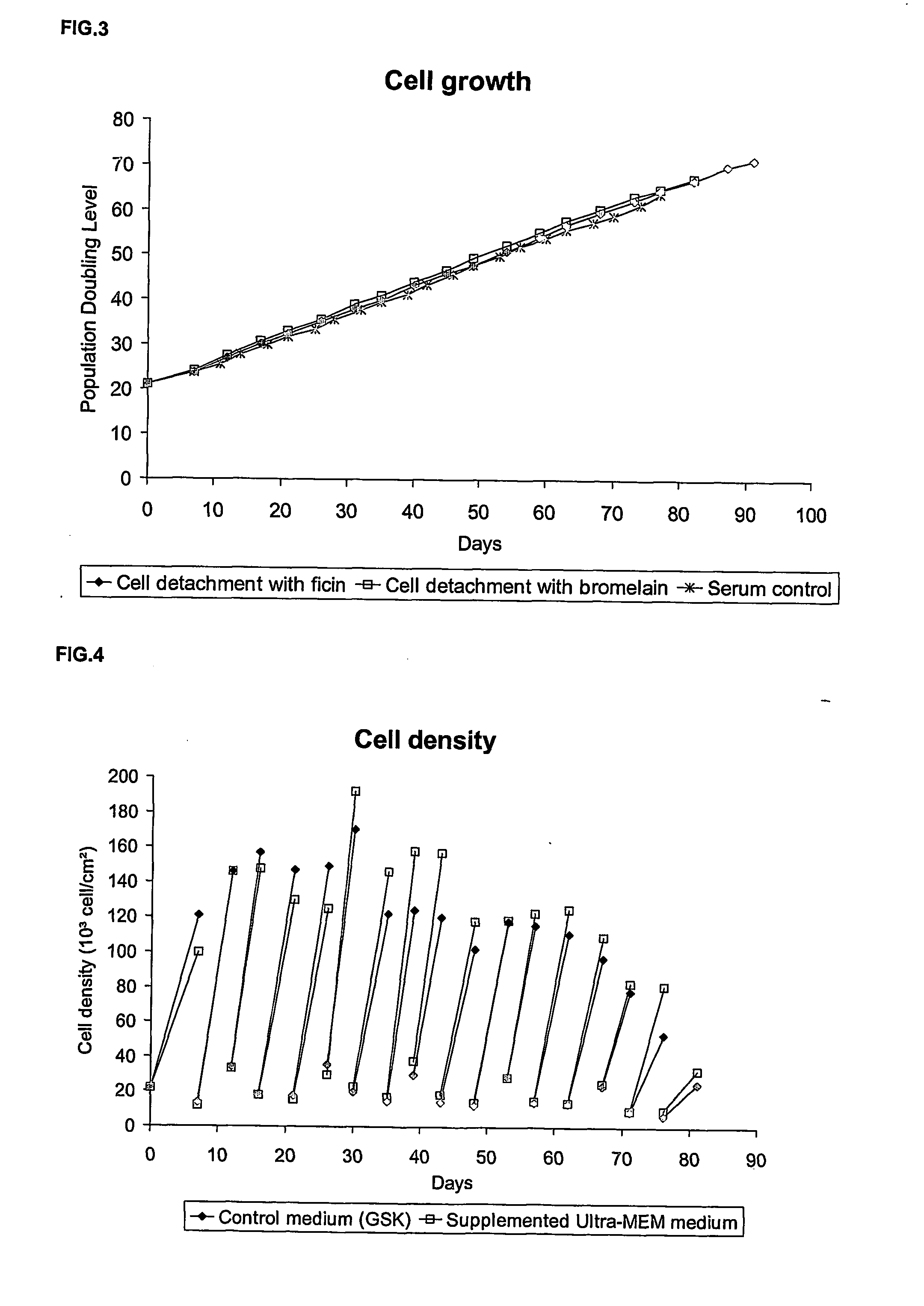
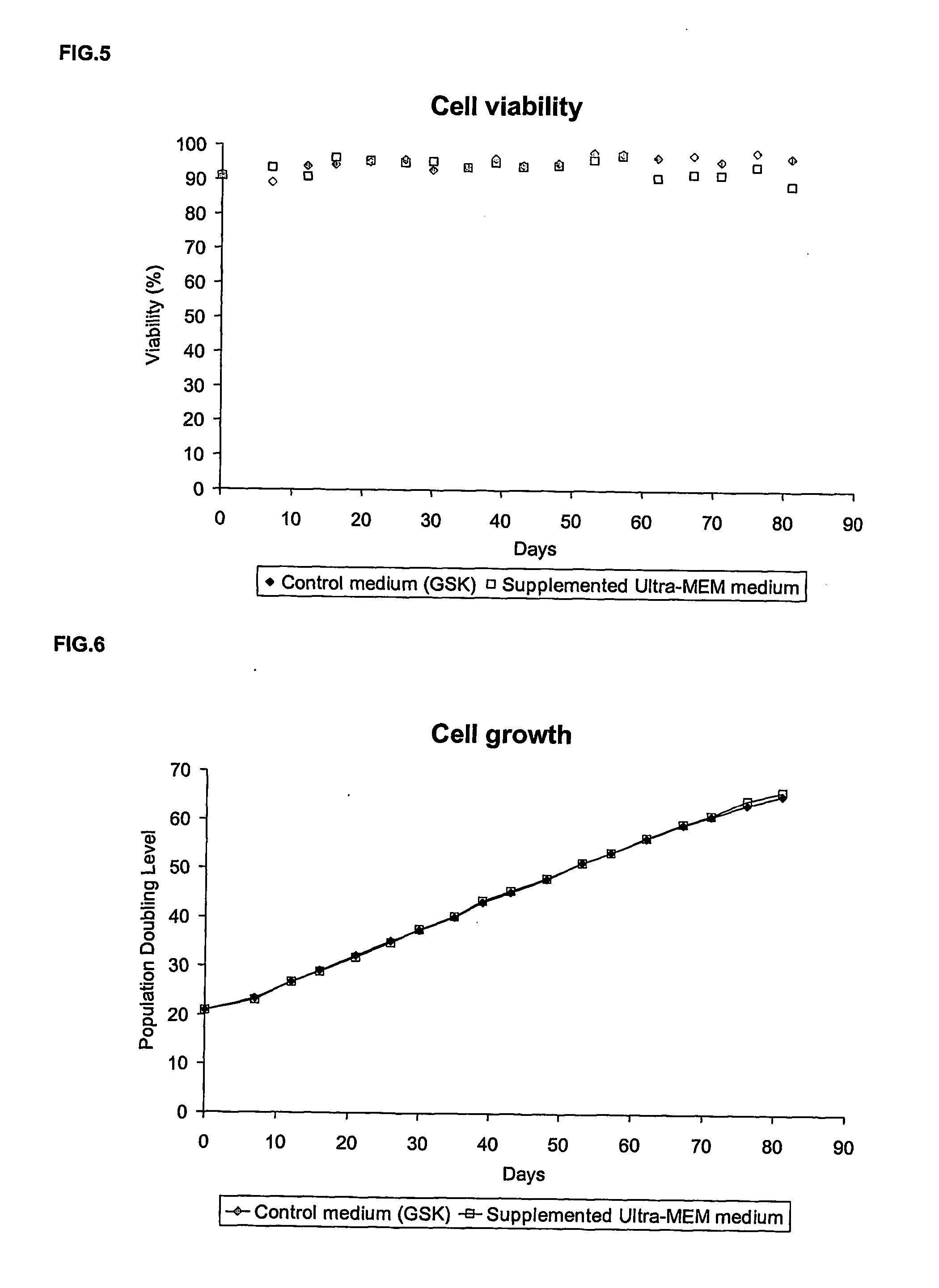
Animal-free cell culture method
InactiveUS20060183224A1Easy to replaceSsRNA viruses positive-senseCell culture mediaBiotechnologyCell culture media
The present invention relates to a process for animal, preferably human, diploid anchorage-dependent cell culture, in the absence of exogenous components of primary animal origin, and to a cell culture medium substantially free of exogenous components of primary animal origin suitable for carrying out said process. In particular the invention concerns a cell culture medium which comprises at least one, more preferably several, exogenous animal-free growth factors. The present invention also relates to a process for cultivating animal, preferably human diploid anchorage-dependent cells in a medium according to the invention, involving the use of a trypsin substitute of non-animal origin for passaging cells. The invention further relates to a process for producing viruses, viral vaccines and the like.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
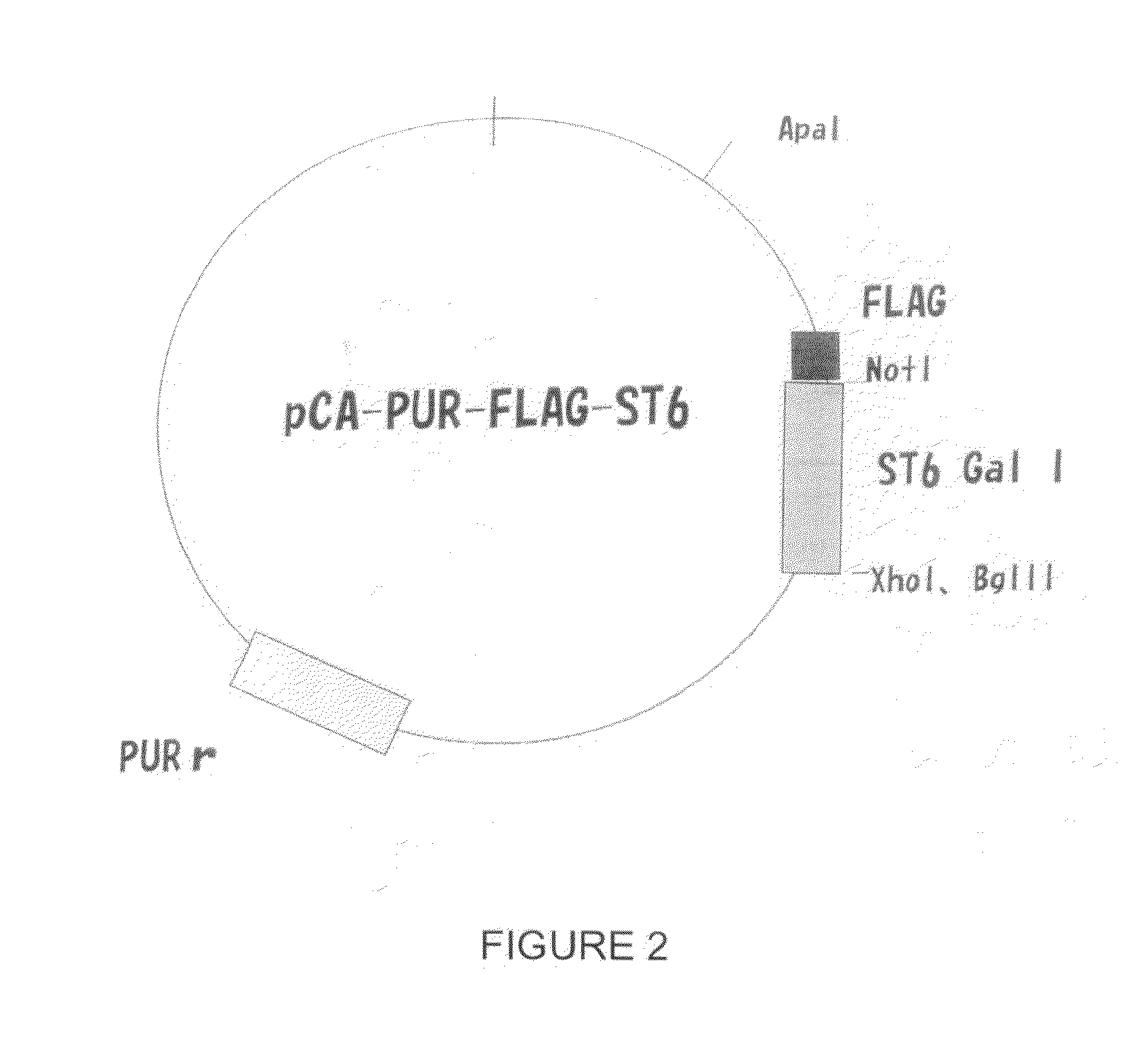
Cell-based systems for producing influenza vaccines
ActiveUS20100021499A1Minimize and prevent virus infectionAvoid componentsSsRNA viruses negative-senseAnimal cellsHemagglutininBinding site
The present invention relates to a cell-based method for producing influenza virus vaccines by enriching the population of surface-bound α2,6-sialic acid receptors on a cell surface, such as on a Chinese Hamster Ovary (CHO) cell surface. The host cell therefore presents numerous binding sites to which an influenza virus can bind via its hemagglutinin spike protein and infect the host cell. In contrast to wild-type CHO cells, the surface of the mutated CHO cells of the present invention contains an enriched population of α2,6-sialic acid receptors which makes the inventive CHO cells highly susceptible to viral infection, and therefore safe, effective, and highly efficient cells for rapidly producing influenza vaccines.
Owner:FLUGEN
Porcine reproductive and respiratory syndrome virus (PRRSV) recombinant poxvirus vaccine
What is described is a recombinant vector, such as a virus; for instance, a poxvirus, such as avipox virus, containing foreign DNA from porcine reproductive and respiratory syndrome virus. What are also described are immunological compositions containing the recombinant poxvirus for inducing an immunological response in a host animal to which the immunological composition is administered. Also described are methods of treating or preventing disease caused by porcine reproductive and respiratory syndrome virus by administering the immunological compositions of the invention to an animal in need of treatment or susceptible to infection by porcine reproductive and respiratory syndrome virus.
Owner:MERIAL SAS
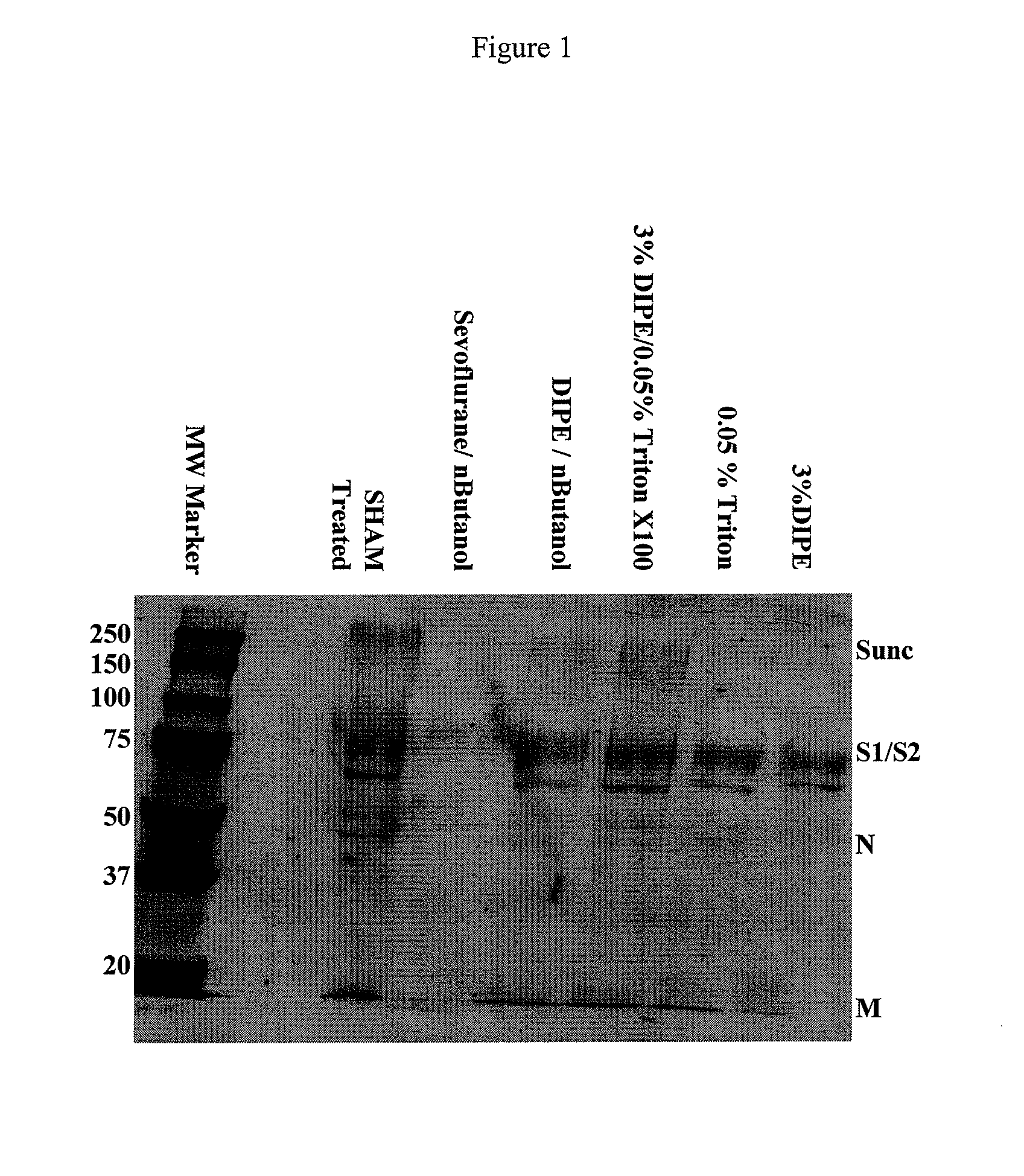
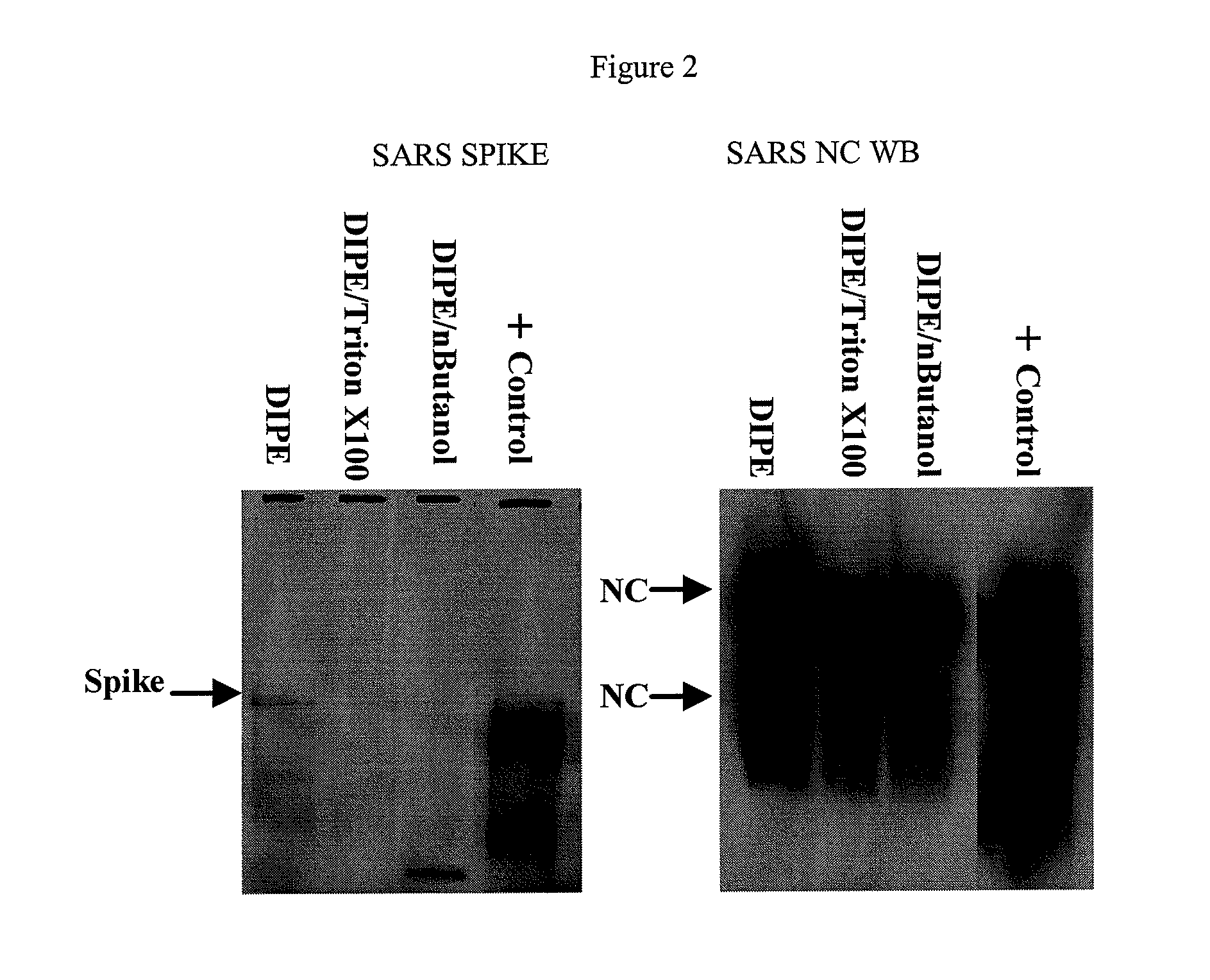
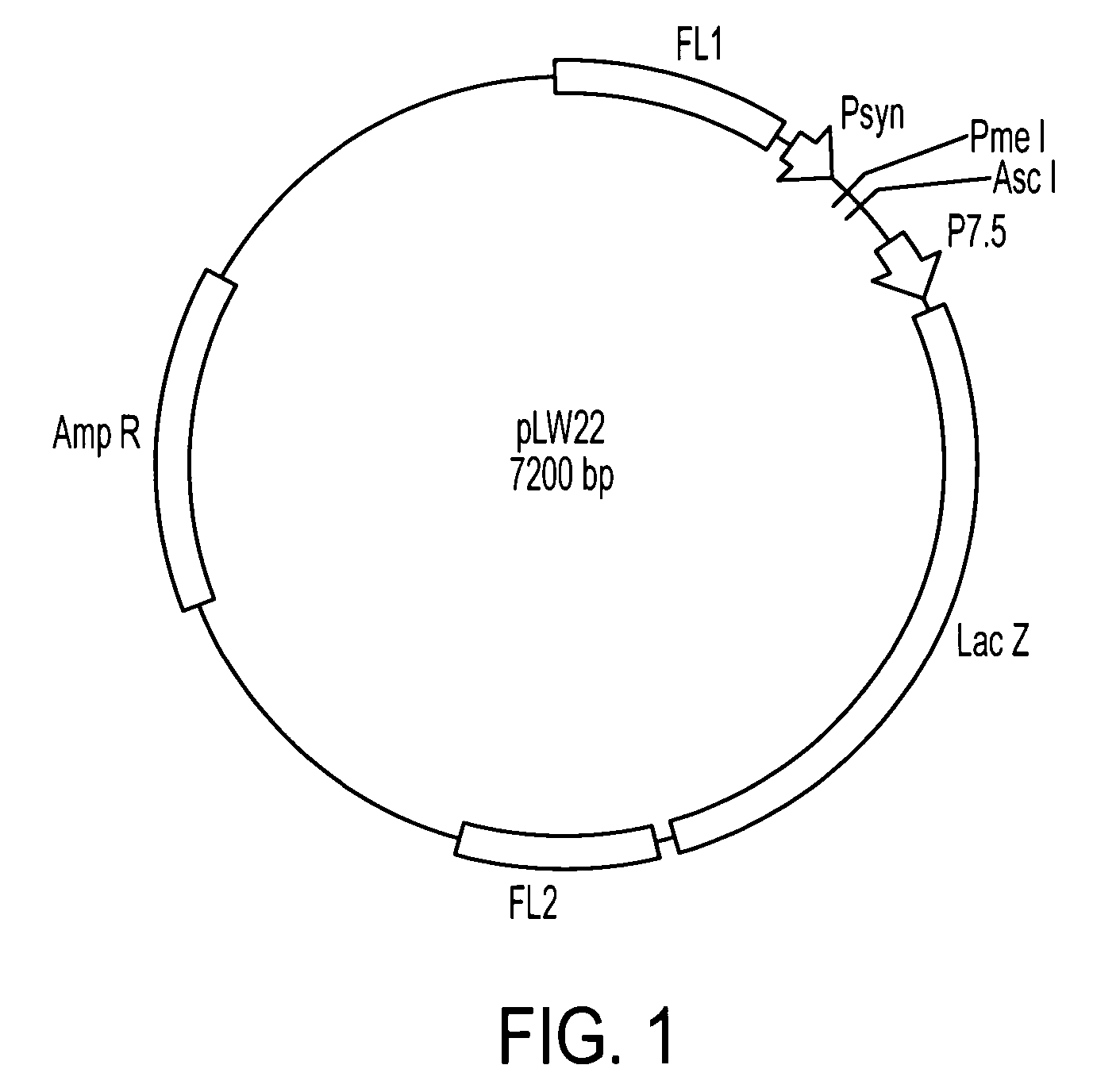
SARS Vaccine Compositions and Methods of Making and Using Them
InactiveUS20090017069A1The process is simple and effectiveThe method is simple and efficientSsRNA viruses positive-senseViral antigen ingredientsLipid formationViral Vaccine
Owner:ELI LILLY & CO +1
Nipah virus vaccines
ActiveUS20070031455A1Easy to storeSsRNA viruses negative-senseSugar derivativesViral glycoproteinViral Vaccine
The present invention relates to recombinant anti-Nipah virus vaccines and the administration of such vaccines to animals, advantageously pigs. Advantageously, the anti-Nipah virus vaccine may comprise a recombinant avipox virus containing a Nipah virus glycoprotein gene. The invention encompasses methods of vaccinating animals, advantageously pigs, by administration of anti-Nipah virus vaccines that may comprise a recombinant avipox virus that may contain a Nipah virus glycoprotein gene.
Owner:MERIAL INC
Vaccine for hand-foot-and-mouth disease viruses
ActiveCN101897963AEnsure safetyGood immune effectMicroorganism based processesAntiviralsAdjuvantInfectious Disorder
The invention relates to preparation of a vaccine for hand-foot-and-mouth disease viruses and an application method thereof, belonging to a novel vaccine for preventing infectious diseases. The vaccine of the invention mainly comprises the components of high-purified inactivated human enteropathogenic virus 71 (EV 71) and an aluminum adjuvant. The vaccine, which is prepared according to the method of the invention, has excellent immunogenicity, and after immunity, organisms can selectively generate a high titer serum neutralization antibody, thereby preventing infectious diseases caused by the human EV 71.
Owner:BEIJING LUZHU BIOTECH +1
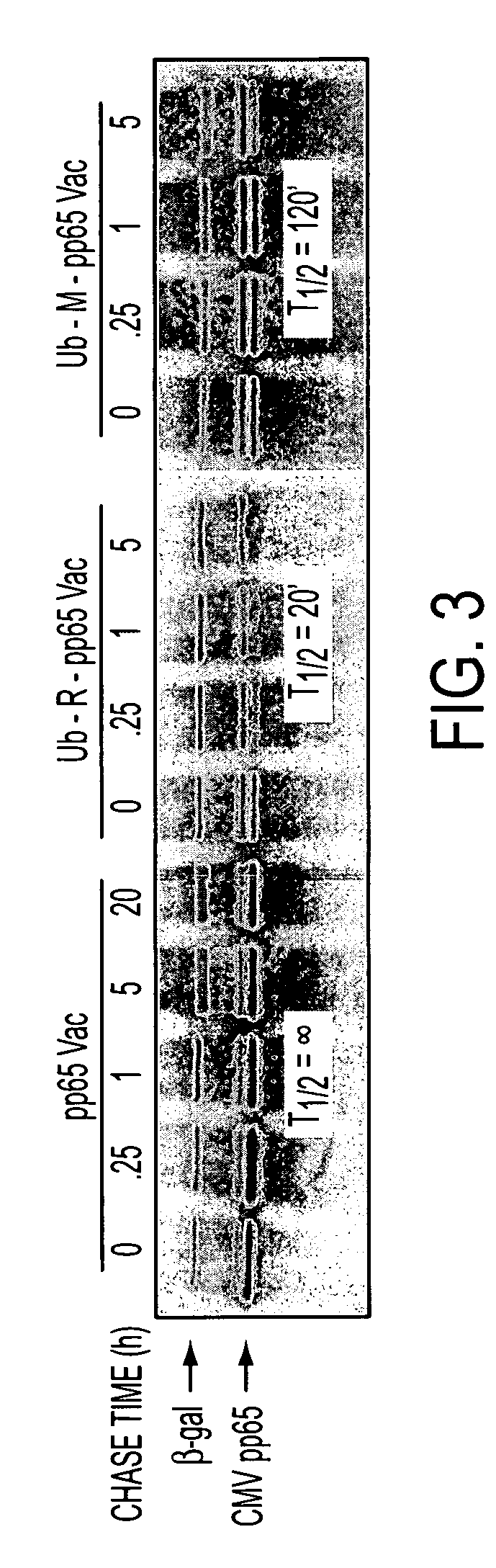
Human cytomegalovirus antigens expressed in MVA and methods of use
ActiveUS7163685B2Improve immunitySugar derivativesViral antigen ingredientsModified vaccinia AnkaraVaccine virus
DNA and protein constructs useful in producing vaccines against human cytomegalovirus contain optionally N-end modified and N-terminal ubiquitinated human cytomegalovirus antigenic proteins, including pp65, pp150, IE1, gB and antigenic fragments thereof. Vaccine viruses, in particular poxviruses such as vaccinia and Modified Vaccinia Ankara, that express the constructs may be used as vaccines to augment the immune response to human cytomegalovirus, both prophylatically and in patients already carrying human cytomegalovirus, as well as to create and expand cytomegalovirus-reactive T cells for transfer of adoptive immunity.
Owner:CITY OF HOPE
Construction method of mandarin fish brain cell system
ActiveCN103275925AGood growthGood repeatabilityMicroorganism based processesVertebrate cellsCulture cellFishery
The invention discloses a construction method of a mandarin fish brain cell system. Aiming at the characteristic of mandarin fish brain cells, the primary cell culture is carried out by separating the brain cells through a serum-contained collagenase digestion method, so that the mandarin fish brain cell system is constructed successfully. Thus, a great quantity of mandarin fish brain source cells can be provided; and the good growth status of sub-culture cells can be maintained. The construction method is strong in repeatability and brings convenience for researching fish virus infection paths and fish virus infection mechanisms, developing viral vaccines and the like.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Membrane virus host range mutations and their uses as vaccine substrates
InactiveUS7128915B2Lower levelSsRNA viruses positive-senseSugar derivativesViral VaccineTransmembrane domain
The present invention is directed to genetically engineered, membrane-enveloped viruses with deletion mutations in the protein transmembrane domains. Also provided are viral vaccines based on the engineered viruses, methods of producing and using such vaccines.
Owner:RES DEVMENT FOUND
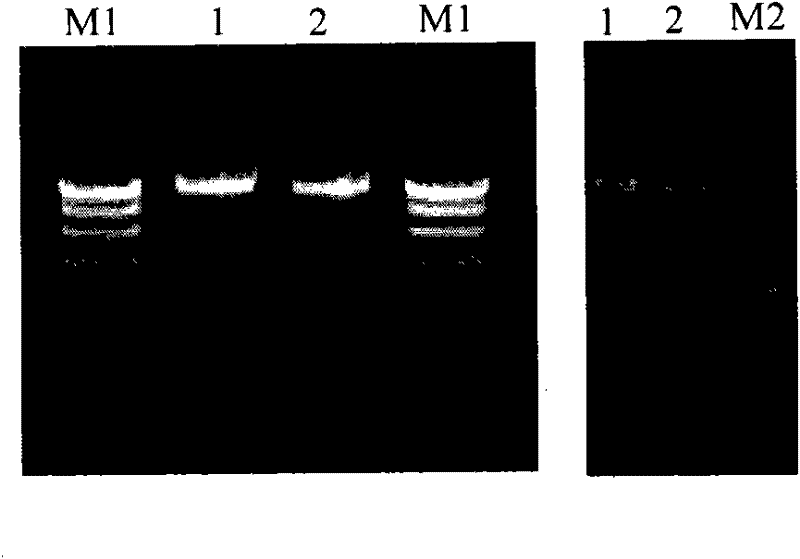
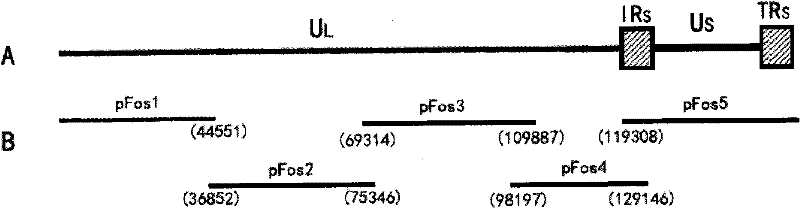
Duck viral enteritis virus vaccine strain infectious cloning system and its construction method and application
The invention relates to a duck viral enteritis virus infective cloned system, which is characterized in that the system comprises multiple cosmids, each cosmid possesses clone of a gene fragment of duck viral enteritis viral vaccine strain, the gene fragment of the duck viral enteritis viral vaccine strain contains a mutual overlapped area, and splices to cover the whole gene group of the duck viral enteritis viral vaccine strain. The invention also relates to a construction method and an application of the duck viral enteritis virus infective cloned system.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
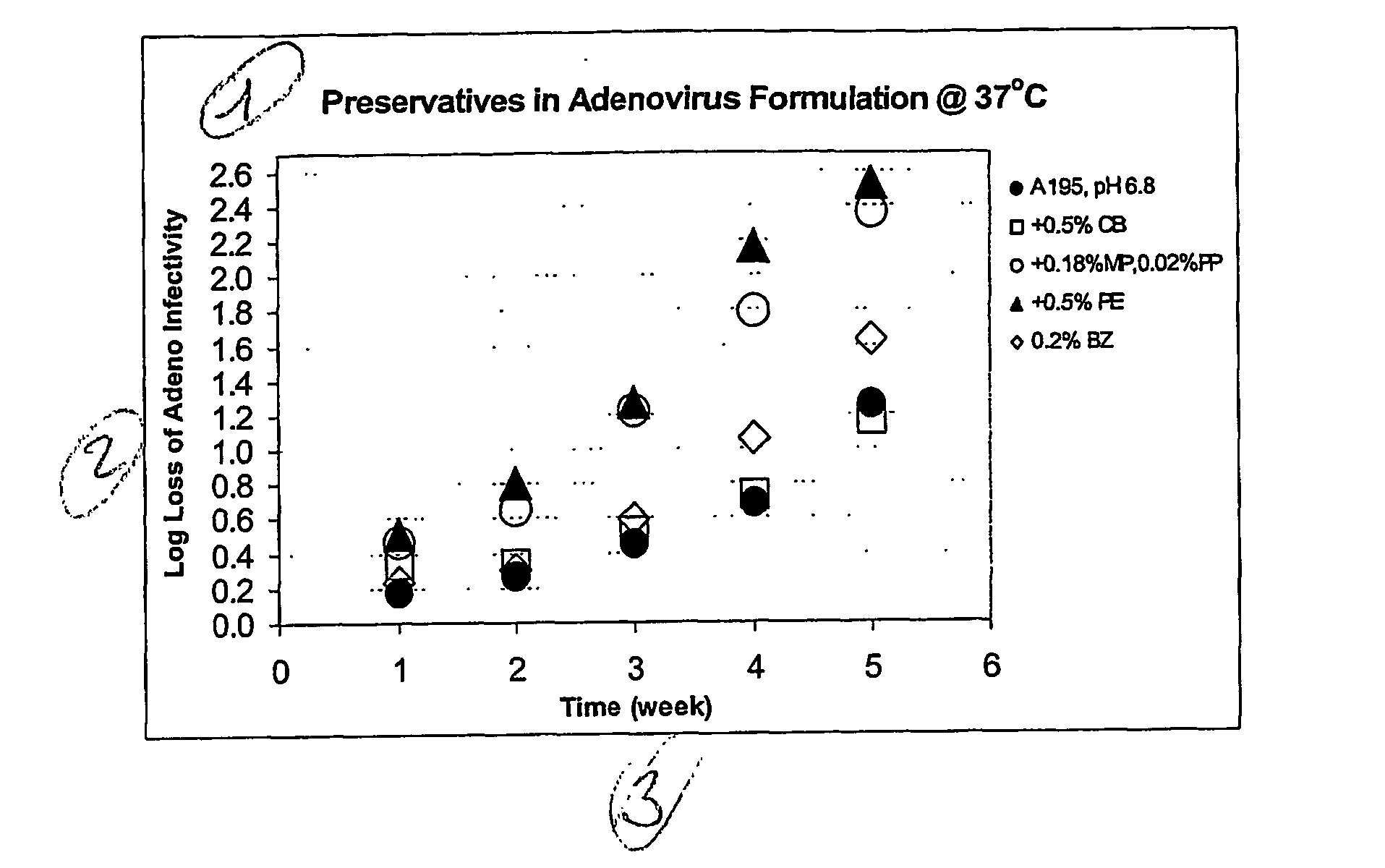
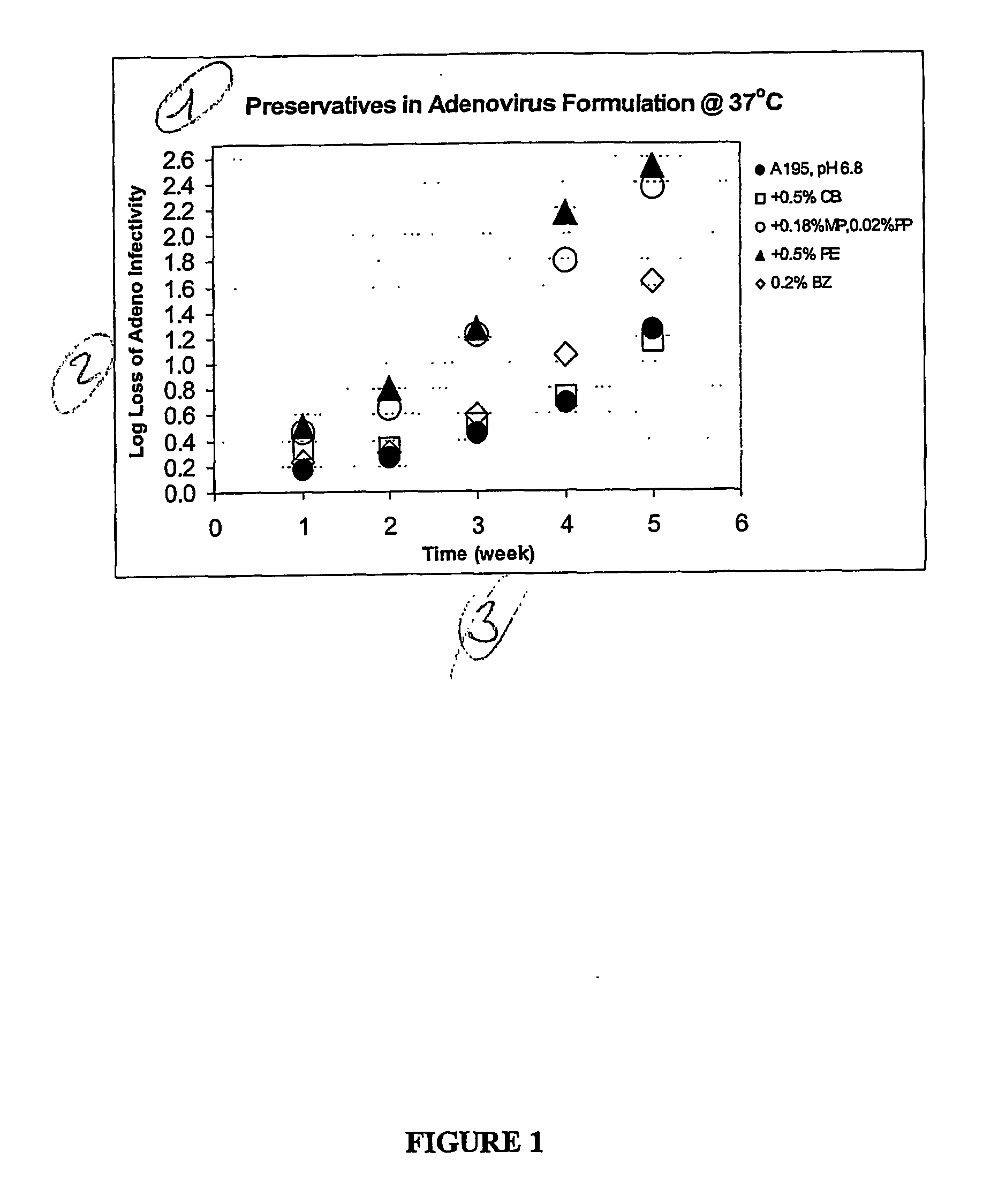
Preservative-containing virus formulations
InactiveUS20070148765A1Negligible lossHigh antibacterial activityMicrobiological testing/measurementPharmaceutical delivery mechanismViral VaccineGene
The preservation of live viral vaccines is disclosed. These liquid formulations comprise a live virus and a preservative, namely chlorobutanol. The preserved, live virus formulations of the present invention are (1) suitable for a vaccine or gene therapy product with a multi-dose image; (2) compatible with parenteral administration; and (3) are stable for extended periods of time with negligible loss of activity.
Owner:MERCK SHARP & DOHME CORP
Immunological Compositions Effective for Lessening the Severity or Incidence of PRRSV Signs and Methods of Use Thereof
InactiveUS20100003278A1Reduce severityReduce percentageSsRNA viruses positive-senseViral antigen ingredientsAdjuvantHaemophilus
The present application describes improved an immunogenic compositions of virus vaccines wherein the virus vaccines comprise adjuvants selected from the group consisting of MCP-1, Haemophilus sonmus fractions, carbomer and combinations thereof. Methods and compositions using such improved compositions are described.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com