Patents
Literature
50 results about "Recombinant virus vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The recombinant virus vaccine can then multiply in infected cells and produce the antigens of a wide range of viruses. The genes of several viruses can be inserted, so the potential exists for producing polyvalent live vaccines. HBsAg, rabies, HSV and other viruses have been expressed in vaccinia.
Adenovirus vector vaccine for preventing SARS-CoV-2 infection
ActiveCN110974950AImprove securityAvoid infectionSsRNA viruses positive-senseViral antigen ingredientsVector vaccineHuman cell
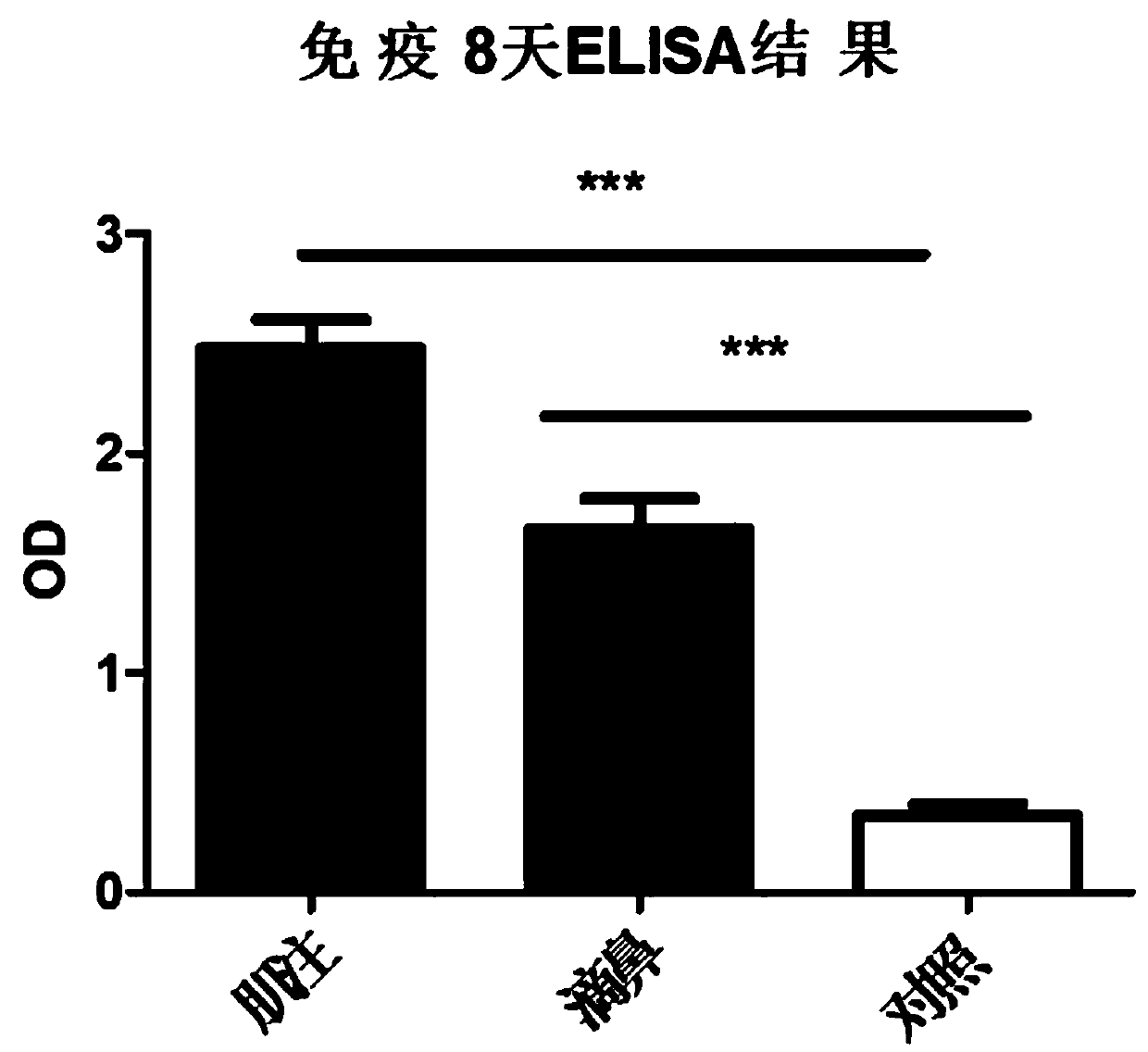
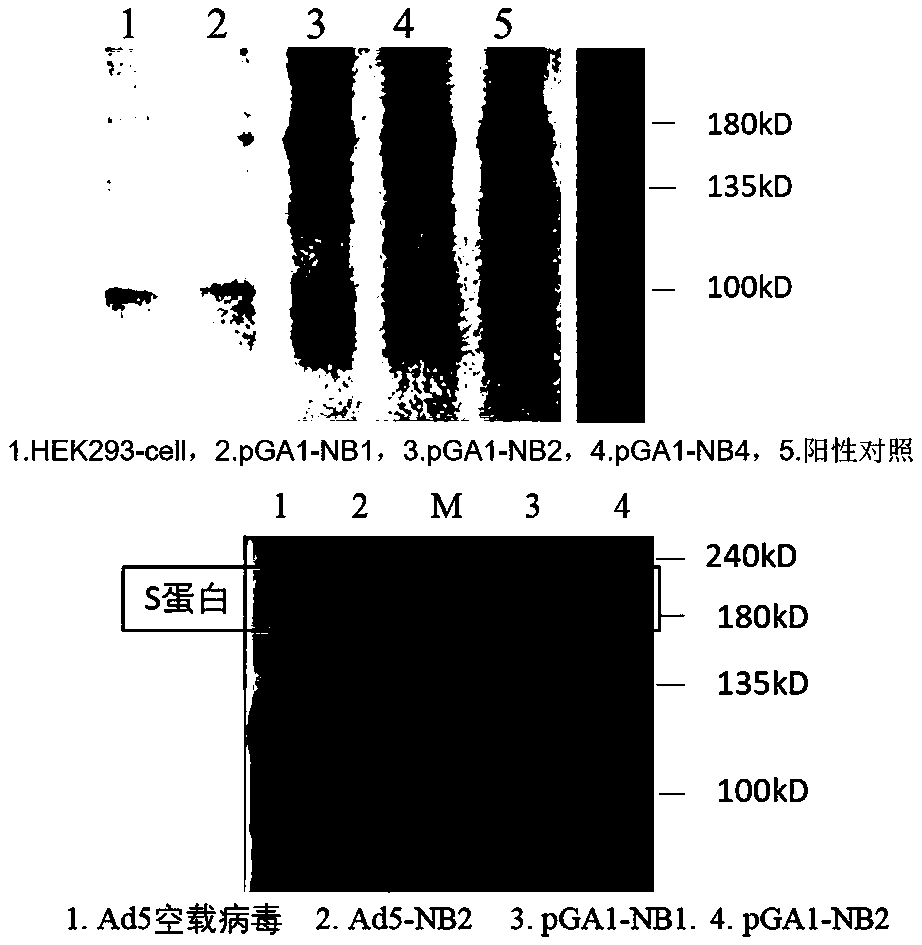
The invention discloses an adenovirus vector vaccine for preventing SARS-CoV-2 infection. The vaccine comprises a nucleic acid sequence as shown in SEQ ID NO: 1. According to a plurality of embodiments of the invention, the S protein nucleic acid sequence contained in the vaccine is easy to express in human cells, and generation of more S proteins can be induced, so the vaccine is expected to be used as a recombinant virus vaccine for preventing SARS-CoV-2 infection. According to a plurality of embodiments of the invention, the vaccine has good security.
Owner:GUANGZHOU N BIOMED LTD
Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
ActiveCN102146418AGenetic material ingredientsViral/bacteriophage medical ingredientsCurative effectRecombinant virus vaccine
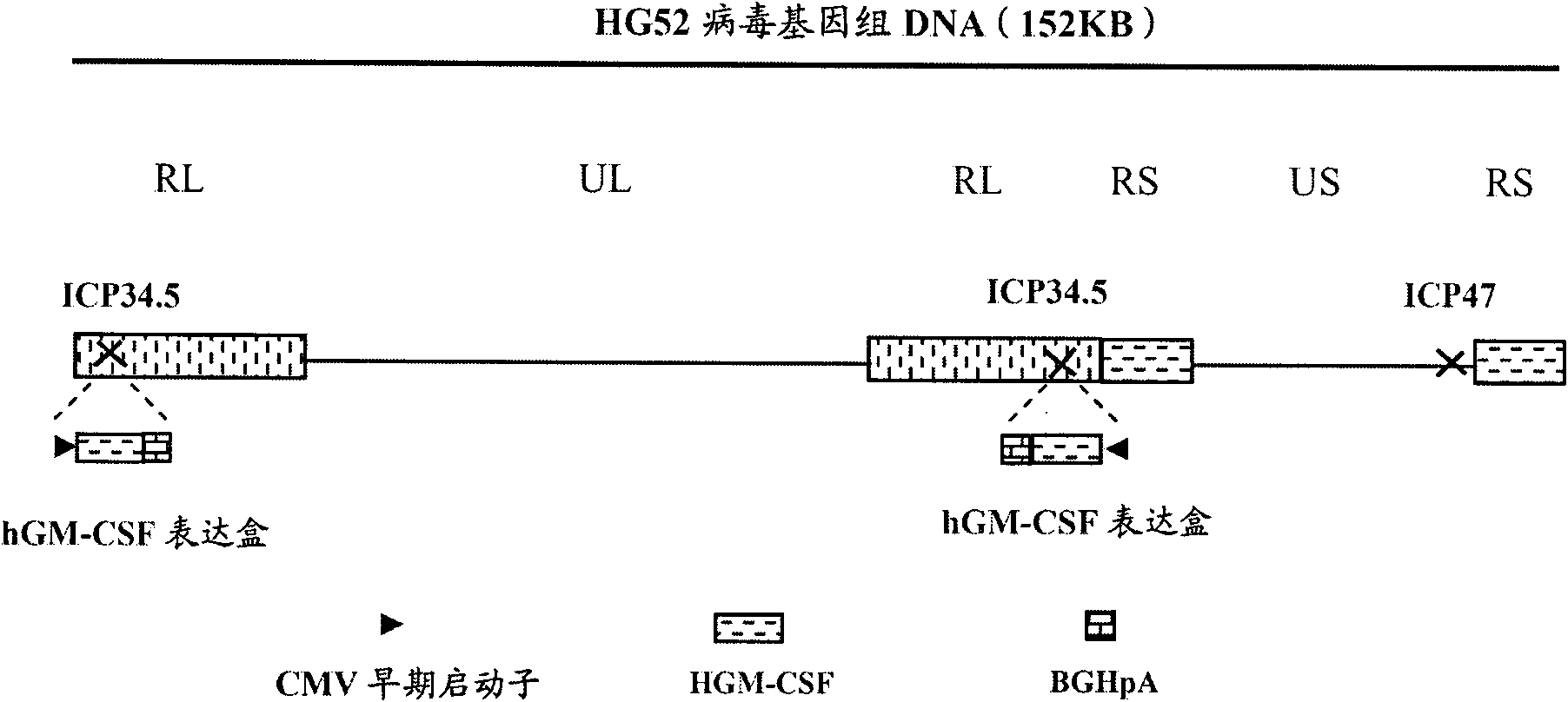
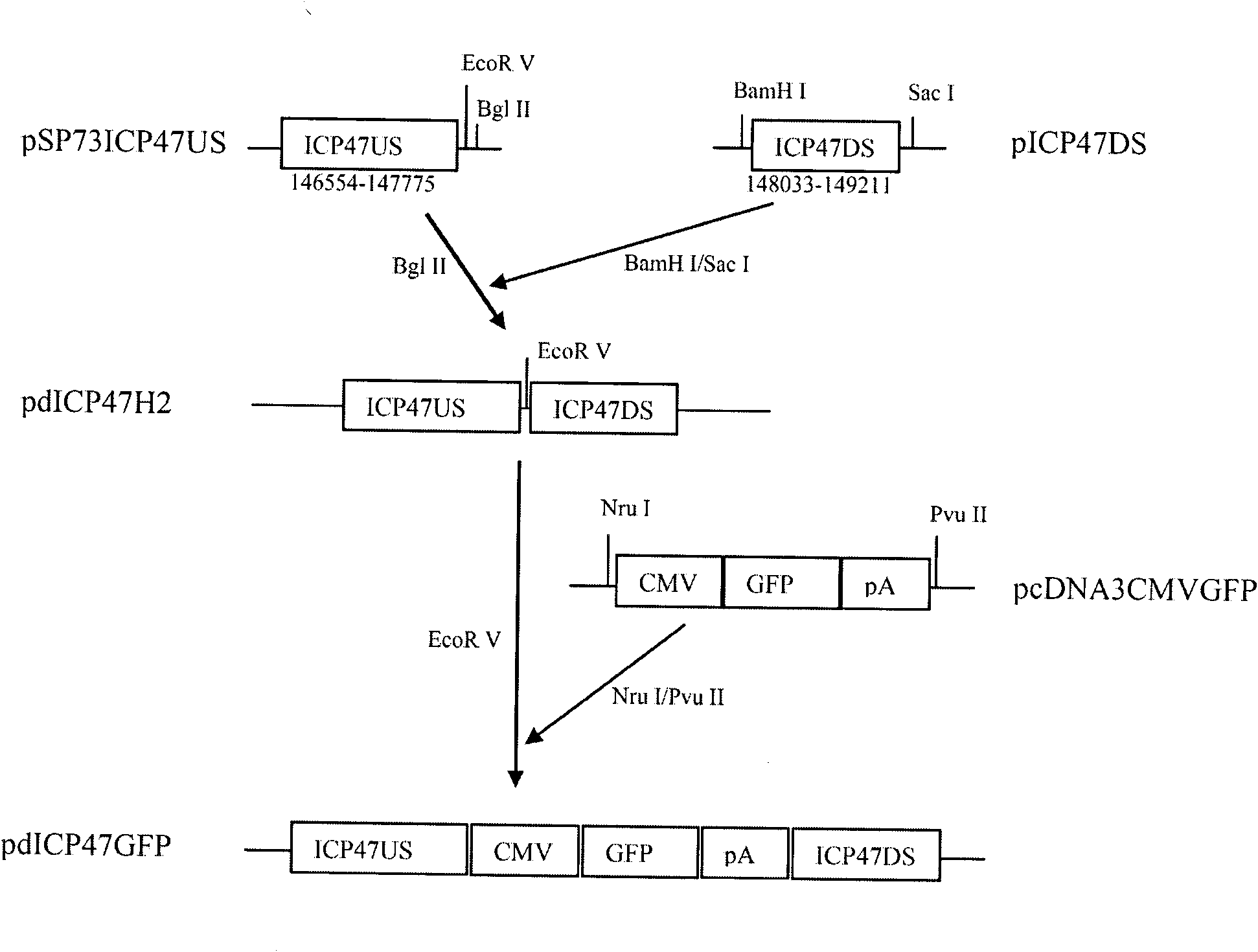
The invention provides a recombinant II type herpes simplex virus vector. An ICP34.5 gene and an ICP47 gene of a wild II type herpes simplex virus HG52 strain are removed in the virus vector, and preferably a human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression box is inserted into the position where the ICP34.5 gene is removed. The invention also provides a preparation method of the recombinant II type herpes simplex virus vector, a recombinant virus using the recombinant II type herpes simplex virus as a vector, a medicinal composition consisting of the recombinant II type herpes simplex virus vector and a pharmaceutically acceptable vector or excipient, and application of the recombinant II type herpes simplex virus vector in preparation of a gene medicament for treating tumors. As the ICP34.5 gene is removed in the recombinant II type herpes simplex virus vector provided by the invention, the oncolysis virus is safe and can selectively grow and propagate in tumor cells; the ICP47 gene is removed to promote immune response and enhance oncolysis activity; and the curative effect of the recombinant II type herpes simplex virus vector is superior to that of the conventional recombinant I type herpes simplex virus vector, and the recombinant II type herpes simplex virus vector has high safety.
Owner:WUHAN BINHUI BIOTECH CO LTD
Recombinant virus for expressing swine fever virus E2 gene, and preparation method and application thereof
ActiveCN104178505AImprove expression levelHigh expressionViral antigen ingredientsAntiviralsPig farmsSwine Fever Virus
Owner:HUAZHONG AGRI UNIV
Efficient cell culture system for hepatitis C virus genotype 5A
Owner:HVIDOVRE HOSPITAL
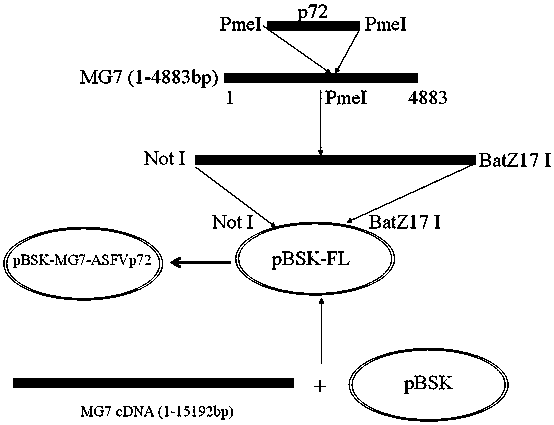
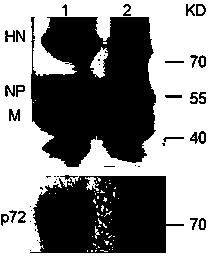
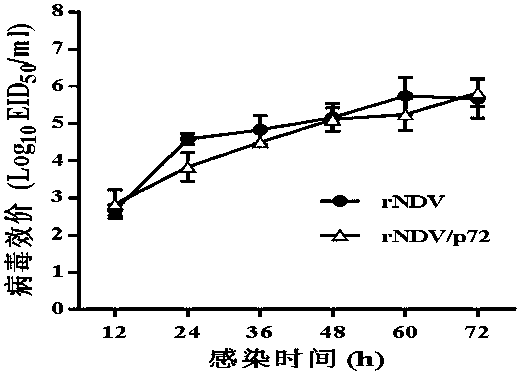
Recombined new castle disease virus vaccine strain for expressing African swine fever virus p72 proteins
ActiveCN104962581AImprove protectionEffective protectionViral antigen ingredientsMicroorganism based processesDiseaseNewcastle disease virus NDV
The invention provides a preparation method of a recombined new castle disease virus vaccine strain which expresses African swine fever virus p72 proteins and a recombined new castle disease virus vaccine strain. The method provided by the invention comprises the following steps: constructing a recombinant transcriptional plasmid which is inserted with a p72 gene of African swine fever virus (ASFV); constructing a transcriptional helper plasmid system; carrying out a contransfection for the transcriptional plasmids and the transcriptional helper plasmids into host cells BHK-21 which can be duplicated in new castle disease attenuate strains; and saving and obtaining the recombined virus stain. The vaccine strain for expressing the African swine fever virus p72 proteins provided by the invention has important preservation and strategy meaning in the aspect of animal epidemic disease prevention and control, and can be applied to the treatment and prevention of African swine fever virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation method of avian influenza virus HA gene recombinant adenovirus
InactiveCN104404005ASolve technical problems with low expression efficiencyViruses/bacteriophagesGenetic engineeringHemagglutininAvian influenza virus
The invention provides a preparation method of avian influenza virus HA gene recombinant adenovirus, which creatively comprises the following steps: carrying out a series of intermediate processes on plasmid pCAGGS, adenovirus shuttle plasmid pShuttle, adenovirus framework plasmid pAdEasy-1 and the like to obtain a gene expression plasmid and other intermediate products, and transfecting the obtained recombinant adenovirus plasmid with 293 cell; and carrying out immunohistochemical screening on the recombinant virus according to the adenovirus-infected cytopathy and specific cells. By using the CAG as the promoter to express the target gene, the method obviously enhances the expression level of the target gene. The hemagglutinin recombinant adenovirus for respectively expressing H5N1 and H9N2 subtype avian influenza viruses provides a virus model for development of the H5 / H9 subtype avian influenza virus bivalent nucleic acid vaccine, and also lays the foundation for development of the AIV (avian influenza virus) adenovirus live vector vaccine.
Owner:TIANJIN RINGPU BIO TECH
Recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes and construction method thereof
ActiveCN104195116AViral antigen ingredientsMicroorganism based processesF proteinNewcastle disease virus NDV
The invention discloses a recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes and a construction method thereof, belonging to the field of recombinant virus vaccines. The recombinant Newcastle disease virus is a goose isolate, the cleavage site amino acid sequence of F protein of the recombinant Newcastle disease virus is GRQGRL, and the P3 gene is positioned in a noncoding region between the P gene and M gene of Newcastle disease virus. The transcription plasmid pCI-NA-VP3(SEQ ID NO.1) and the transcription helper plasmid pcDNA-N, pcDNA-P and pcDNA-L(SEQ ID NO. 2-4) cotransfect a host cell licensed by application of the Newcastle disease virus to culture a transfected host cell, and the recombinant Newcastle disease virus can be saved from a cell suspension of the transfected host cell. The recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes can be used as a bivalent living-vector vaccine for preventing Newcastle disease virus and goose parvovirus.
Owner:JILIN UNIV
An adenovirus vector vaccine for the prevention of SARS-CoV-2 infection
ActiveCN110974950BAvoid infectionImprove securitySsRNA viruses positive-senseViral antigen ingredientsVector vaccineHuman cell
The invention discloses an adenovirus vector vaccine for preventing SARS-CoV-2 infection. The vaccine comprises a nucleic acid sequence as shown in SEQ ID NO: 1. According to a plurality of embodiments of the invention, the S protein nucleic acid sequence contained in the vaccine is easy to express in human cells, and generation of more S proteins can be induced, so the vaccine is expected to be used as a recombinant virus vaccine for preventing SARS-CoV-2 infection. According to a plurality of embodiments of the invention, the vaccine has good security.
Owner:GUANGZHOU N BIOMED LTD
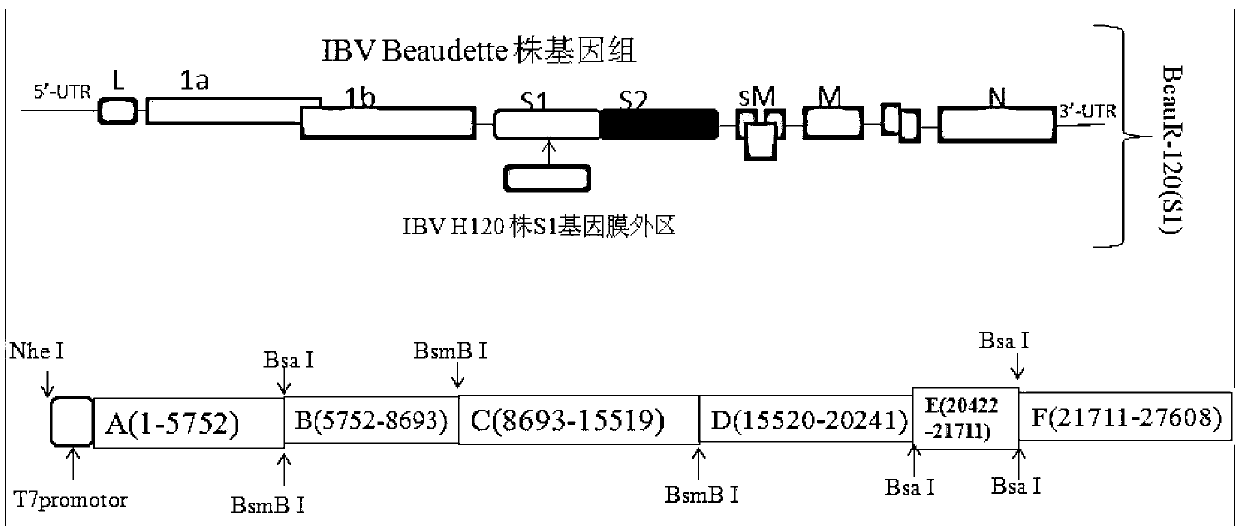
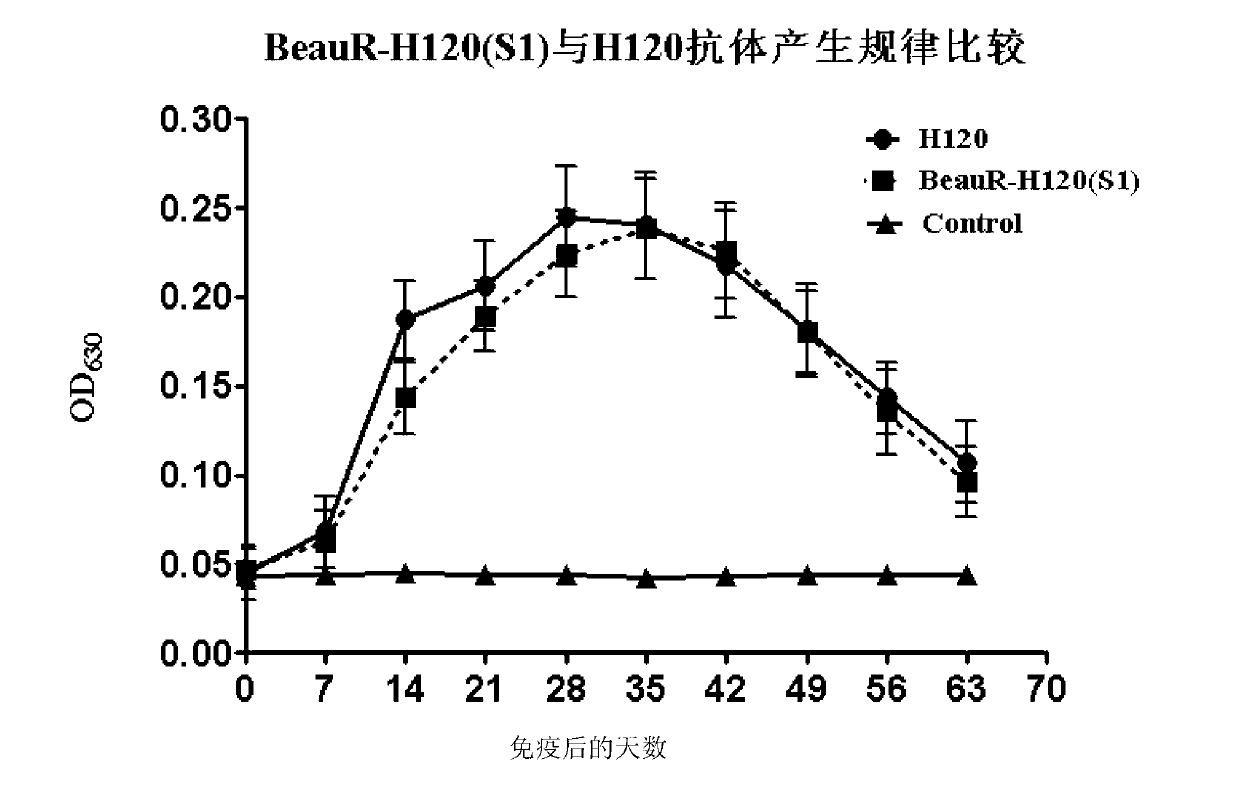
Recombinant virus of chimeric IBV H120 S1 gene ectodomain suitable for cell culture and construction method and application thereof
ActiveCN103275939AViral antigen ingredientsMicroorganism based processesCytopathic effectFreeze and thaw
The invention discloses a recombinant virus of a chimeric IBV H120 S1 gene ectodomain suitable for cell culture and a construction method and application thereof. The recombinant virus is constructed by the following steps of: replacing the S1 gene ectodomain segment of an IBV Beaudette strain with an S1 gene ectodomain segment of an IBV H120 strain to construct the recombinant virus; after successful virus rescue, passing from the Vero cell; collecting toxicity when the cell cytopathic effect area is over 90%; and repeatedly freezing and thawing for three times, and continuing passage to obtain the recombinant virus of a chimeric IBV H120 S1 gene ectodomain suitable for cell culture. The recombinant virus disclosed by the invention is used as a vaccine strain to immunize SPF chicken; after counteracting toxicity, the chicken flocks of an immunity group and a control group are continuously observed to discover that the recombinant virus disclosed by the invention can serve as a vaccine strain to provide good immunity protection to the inoculated chicken; and the recombinant virus is safe to various kinds of chicken and avoids side reaction.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
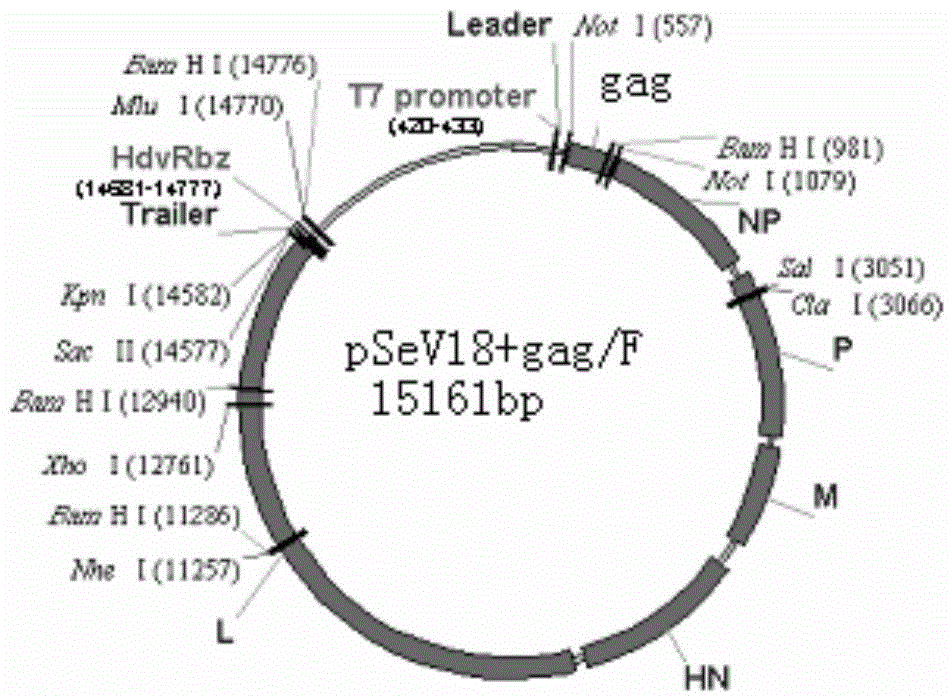
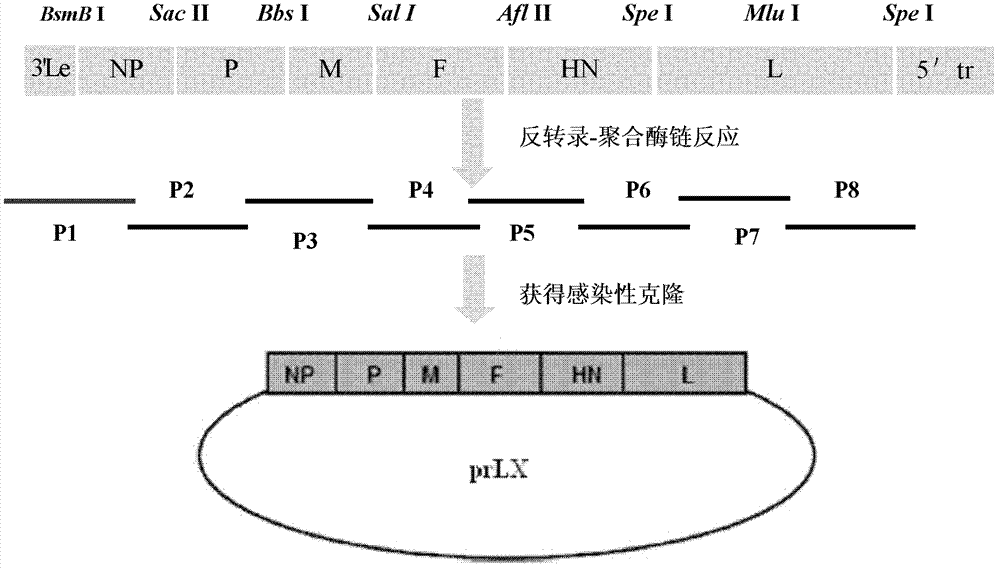
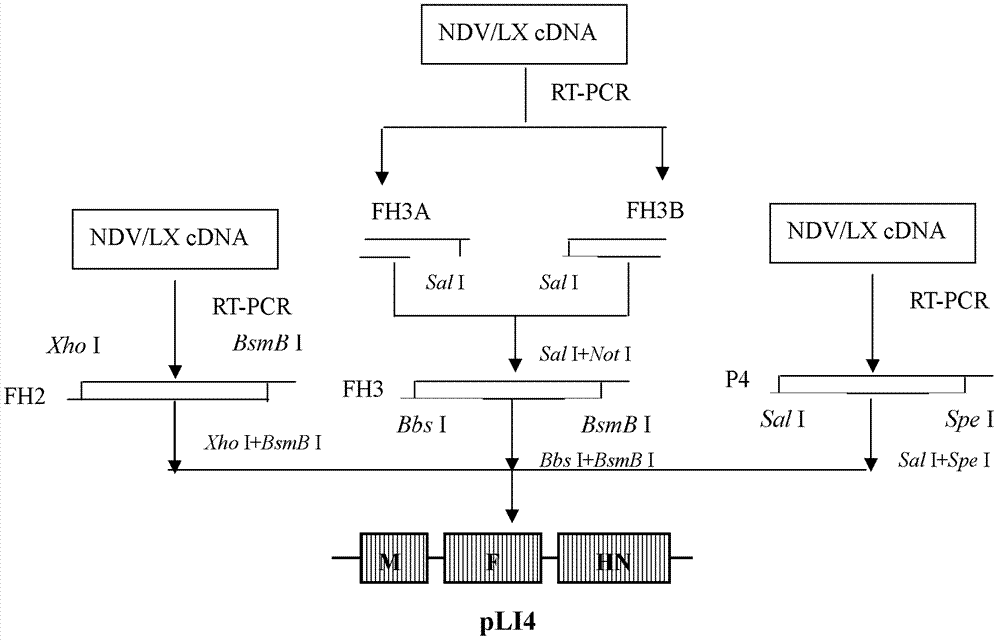
Virus recombinant vaccine A-NDV-LX/14 for newcastle disease and construction method thereof
ActiveCN102533676AHigh reproductive titerSuitable for mass productionViral antigen ingredientsMicroorganism based processesSerum igeNewcastle disease virus NDV
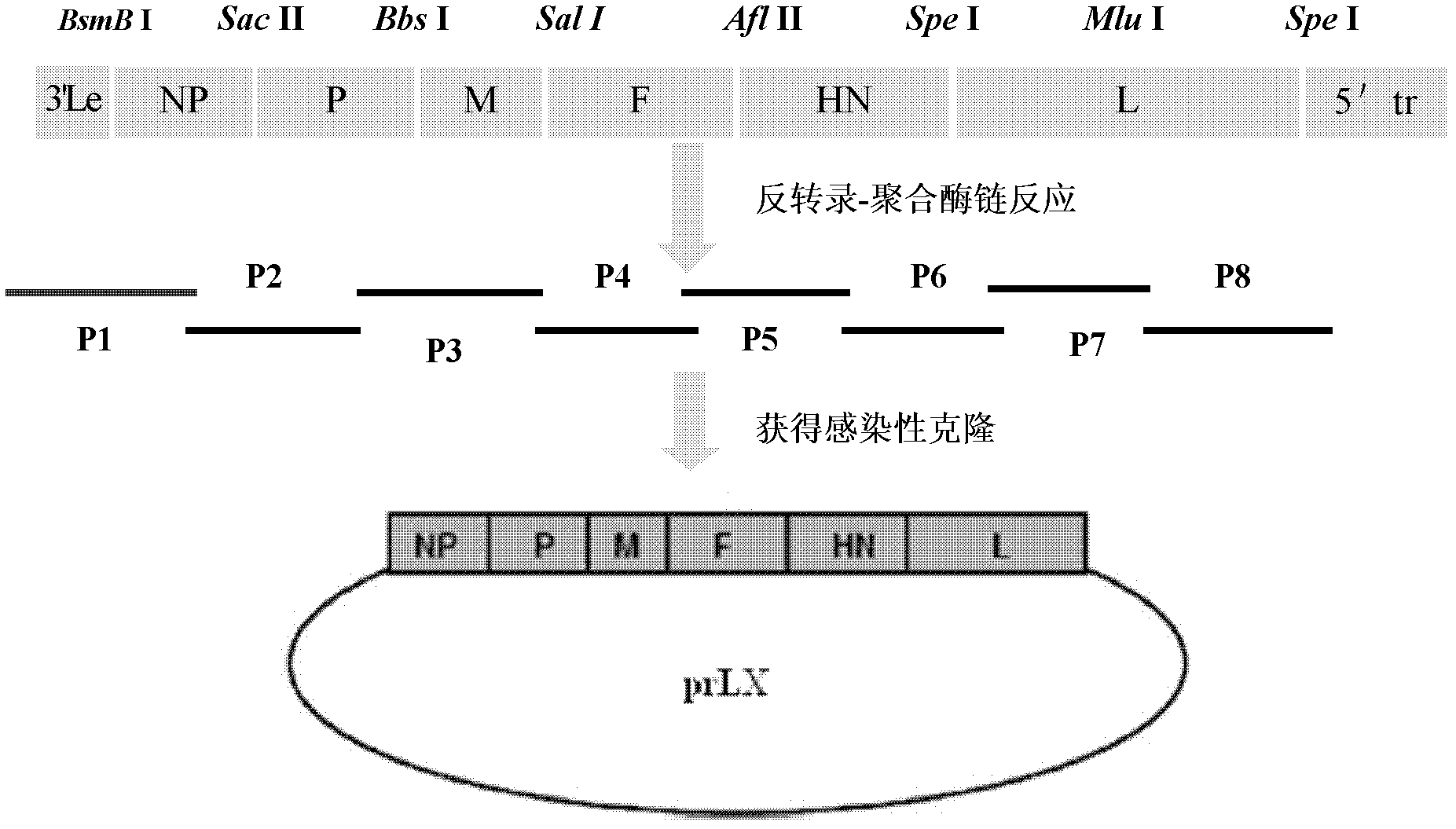
The invention relates to a virus vaccine A-NDV-LX / 14 for a newcastle disease, which is a recombinant of expressed genes VII F and HN, and the preservation number is CGMCCNO: 3844. After two weeks of the constructed virus A-NDV-LX / 14 immunity test on chicken, the average potency of the serum HI is 27. After four days of eliminating toxic material with immune group LaSota, the virus isolation ratesof SPF chicken laryngotracheal and cloacal swab samples are 10 percent and 30 percent respectively, and the virus isolation rates of chicken laryngotracheal and cloacal swab samples are 40 percent and 20 percent respectively. After four days of eliminating toxic material with immune group V4, the virus isolation rates of SPF chicken laryngotracheal and cloacal swab samples are 30 percent and 10 percent respectively, and the virus isolation rates of chicken laryngotracheal and cloacal swab samples are 30 percent and 20 percent respectively. Compared with traditional vaccine, more effective immune protection can be provided by the recombinant virus vaccine.
Owner:YANGZHOU UNIV
Construction of recombinant virus vaccines by direct transposon-mediated insertion of foreign immunologic determinants into vector virus proteins
InactiveCN101528254APromote exchangeIncrease antigen qualityViral antigen ingredientsAgainst vector-borne diseasesProtein targetVirus Protein
The invention provides viral vectors, such as chimeric flavivirus vectors, including foreign peptides inserted into the target proteins of the vectors, methods of making and using these vectors, and compositions including the vectors.
Owner:SANOFI PASTEUR BIOLOGICS CO
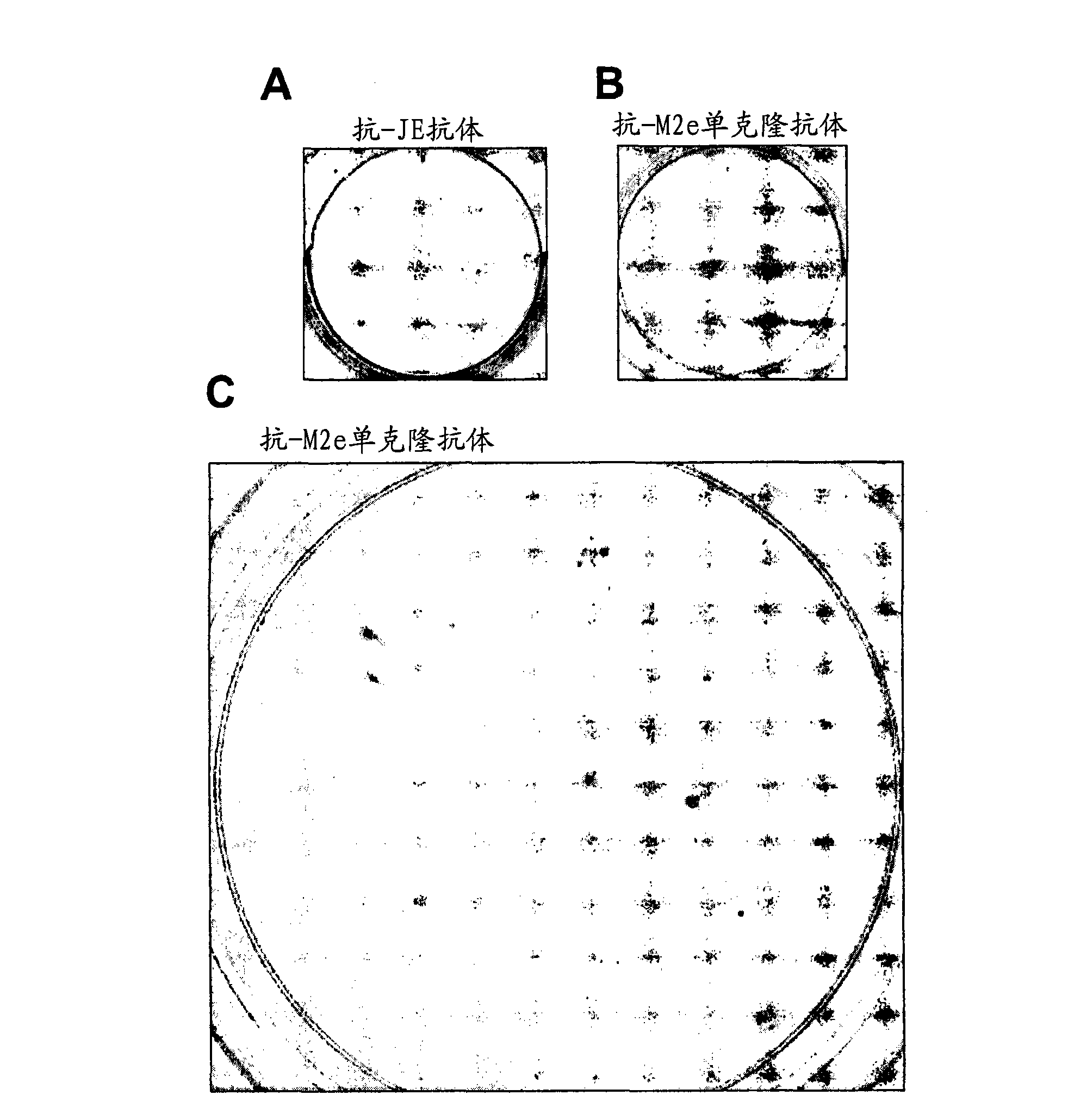
Infectious laryngotracheitis recombinant virus strain for expressing Newcastle disease virus F protein and establishment and application of strain
ActiveCN108611328AGood immune effectFast immune protectionSsRNA viruses negative-senseViral antigen ingredientsMicroorganism preservationMicroorganism
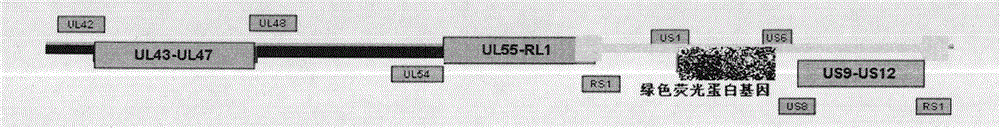
The invention discloses an infectious laryngotracheitis recombinant virus strain for expressing a Newcastle disease virus F protein and establishment and the application of the strain and relates to the field of recombinant virus vaccines. Aiming at problems of recombinant virus establishment and the application of an exogenous immunogenic gene with a nonobligatory gene US9 locus of an ILTV (Infectious Laryngotracheitis Virus), the invention provides an infectious laryngotracheitis recombinant virus strain which is named as rILTV US9-NDV-F, the microorganism preservation number of the strain is CGMCC No.14738, and the virus strain is an infectious laryngotracheitis recombinant virus which is established by replacing a US9 gene of the infectious laryngotracheitis recombinant virus by the Newcastle disease virus F protein, establishing an acquirement deficiency US9 gene and by inserting an NDV-F (Newcastle Disease Virus-F Protein) expression box into a corresponding position of the gene,and is used for recombining the Newcastle disease virus F protein. The recombinant infectious laryngotracheitis recombinant virus strain disclosed by the invention is used for preparing vaccines forpreventing infectious laryngotracheitis and other chicken infectious diseases.
Owner:HARBIN WEIKE BIOTECH DEV
Recombinant protein vaccine, recombinant expression vector containing genes for coding recombinant protein vaccine and application of recombinant protein vaccine
ActiveCN104707135AInhibition of activationActivation blockBacteriaMicroorganism based processesDiseaseTreatment effect
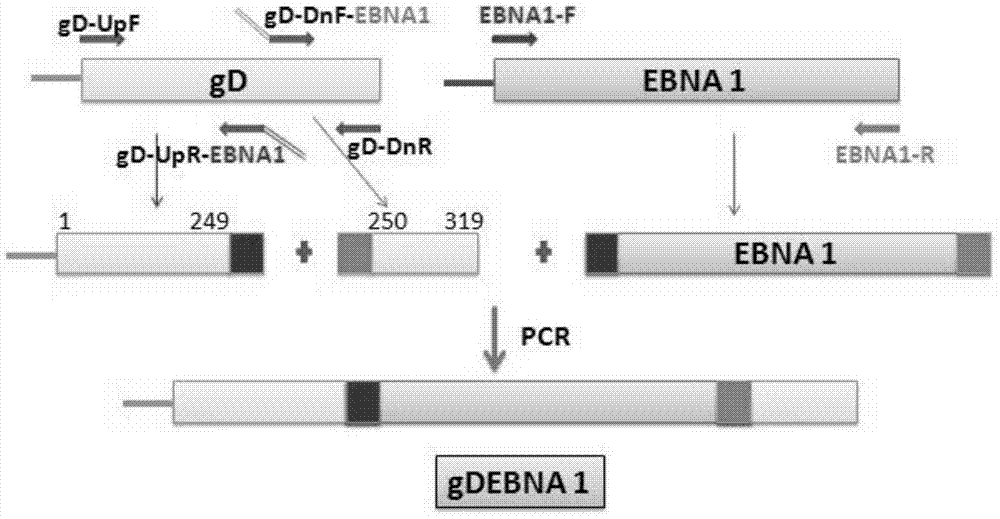
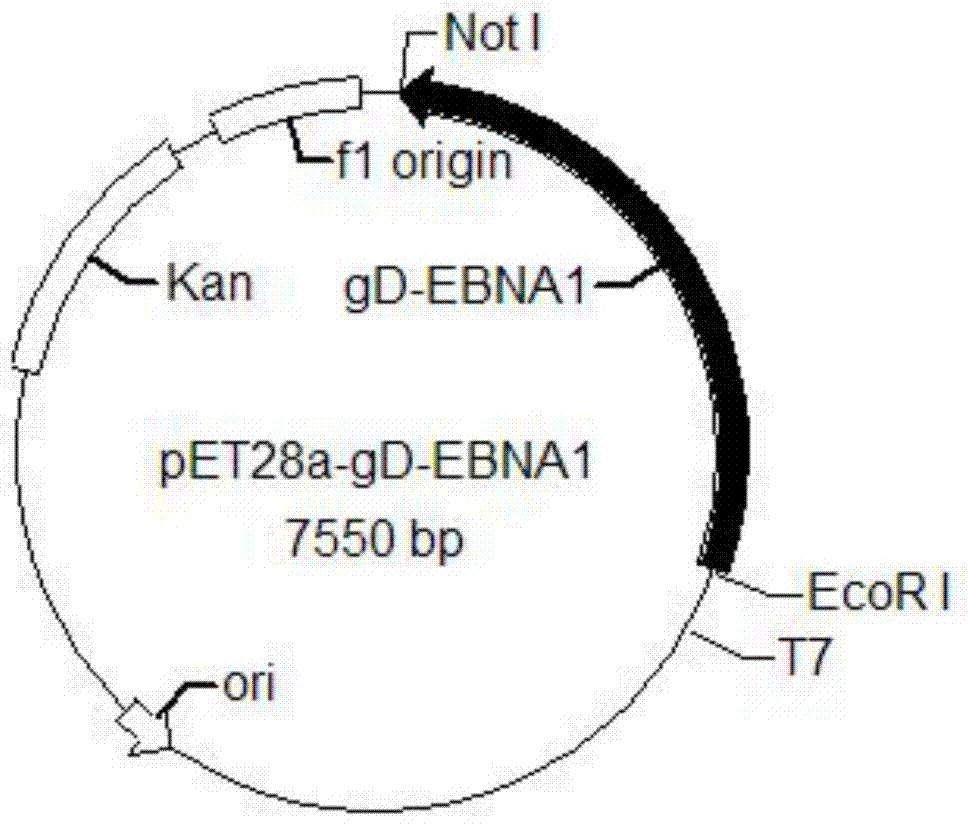
The invention provides a recombinant protein vaccine. The recombinant protein vaccine contains an epitope of EB virus antigen 1 (EBNA1) and an epitope of human herpes virus glycoprotein; fusion proteins can effectively activate a specific cell toxic T lymphocyte (CTL) reaction, directly restrain the expression EBNA1, and give play to treatment effects on EB virus-associated tumors; an immunosuppression path can be directly blocked through herpes virus glycoprotein, and the activity of regulatory T cells is improved. In this way, by means of the recombinant protein vaccine, EB virus infections and diseases related to the EB virus infections and tumors related to the EB virus infections can be prevented or treated in a multi-way manner more comprehensively. The invention further provides a recombinant expression vector containing genes for coding the recombinant protein vaccine and application of the recombinant protein vaccine.
Owner:SHENZHEN INST OF ADVANCED TECH
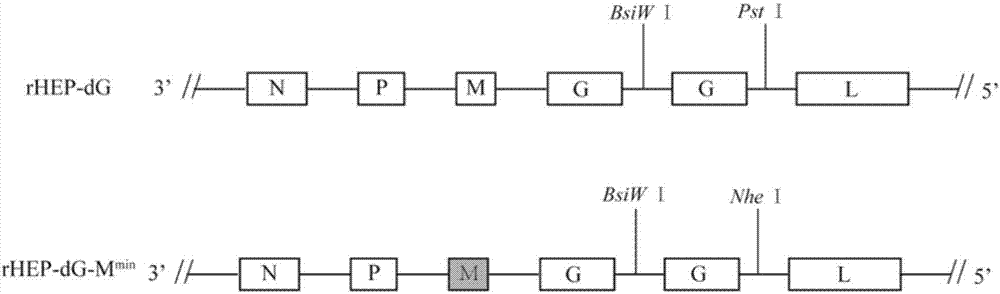
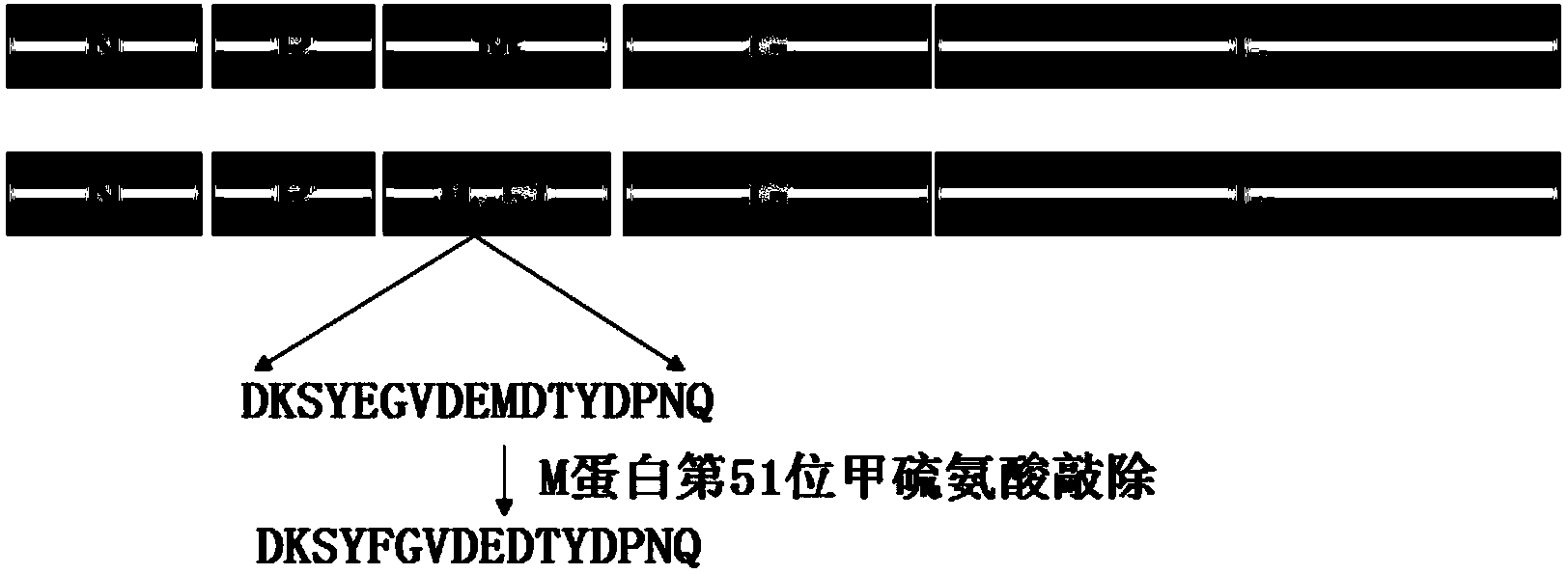
Recombinant rabies virus carrying deoptimized M gene and two G genes
ActiveCN107201371AHigh viral titerLow costSsRNA viruses negative-senseViral antigen ingredientsRabies virus strainRecombinant virus vaccine
The invention discloses a recombinant rabies virus carrying a deoptimized M gene and two G genes. Firstly, a reading frame part of the M gene is deoptimized, wherein the sequence after deoptimization is as shown in SEQ ID NO.2; secondly, a rabies virus HEP-Flury strain is taken as a skeleton, an M gene in the HEP-Flury is replaced by the deoptimized M gene, and an additional rabies virus G gene is inserted to obtain recombinant rabies virus pHEP-dG-Mmin plasmid carrying the deoptimized M gene and two G genes; finally, rescuing and screening are conducted to obtain a recombinant rabies virus strain rHEP-dG-Mmin. The recombinant virus has higher virus titer, and the cost of canine vaccine can be reduced. Under the condition of multiplicity of infection, G protein with higher level can be expressed, virus replication and transcription of each structural gene are increased, and the recombinant virus has the potential of serving as a rabies vaccine candidate strain.
Owner:SOUTH CHINA AGRI UNIV
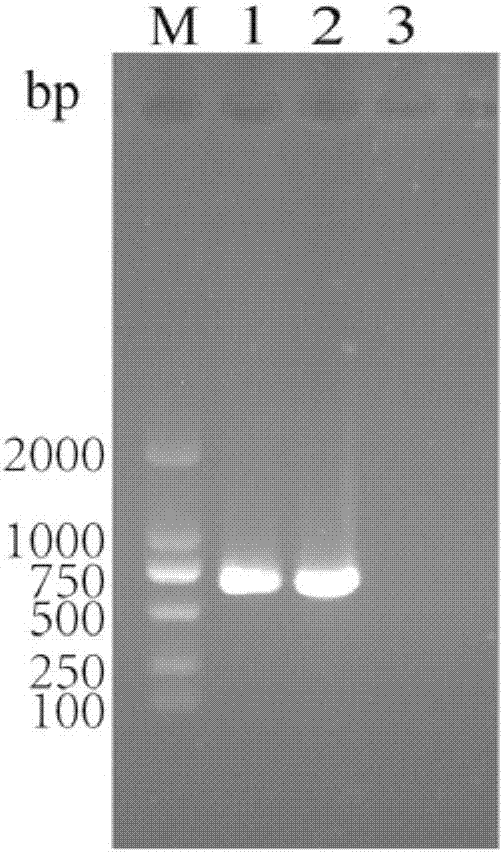
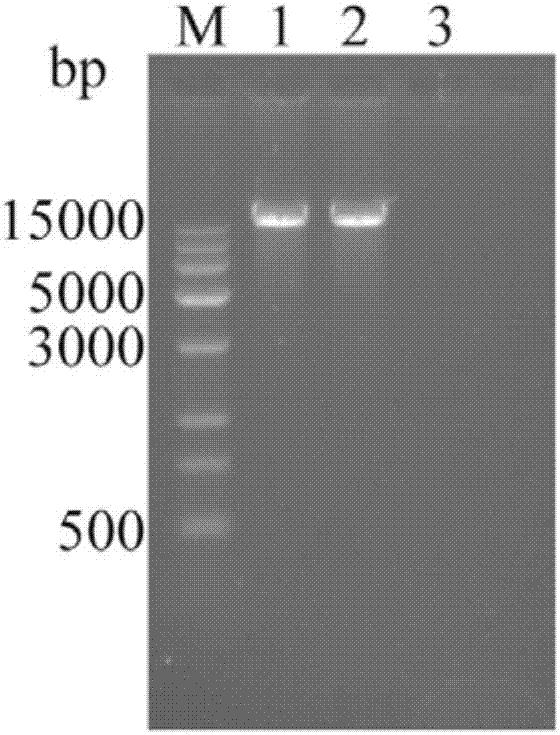
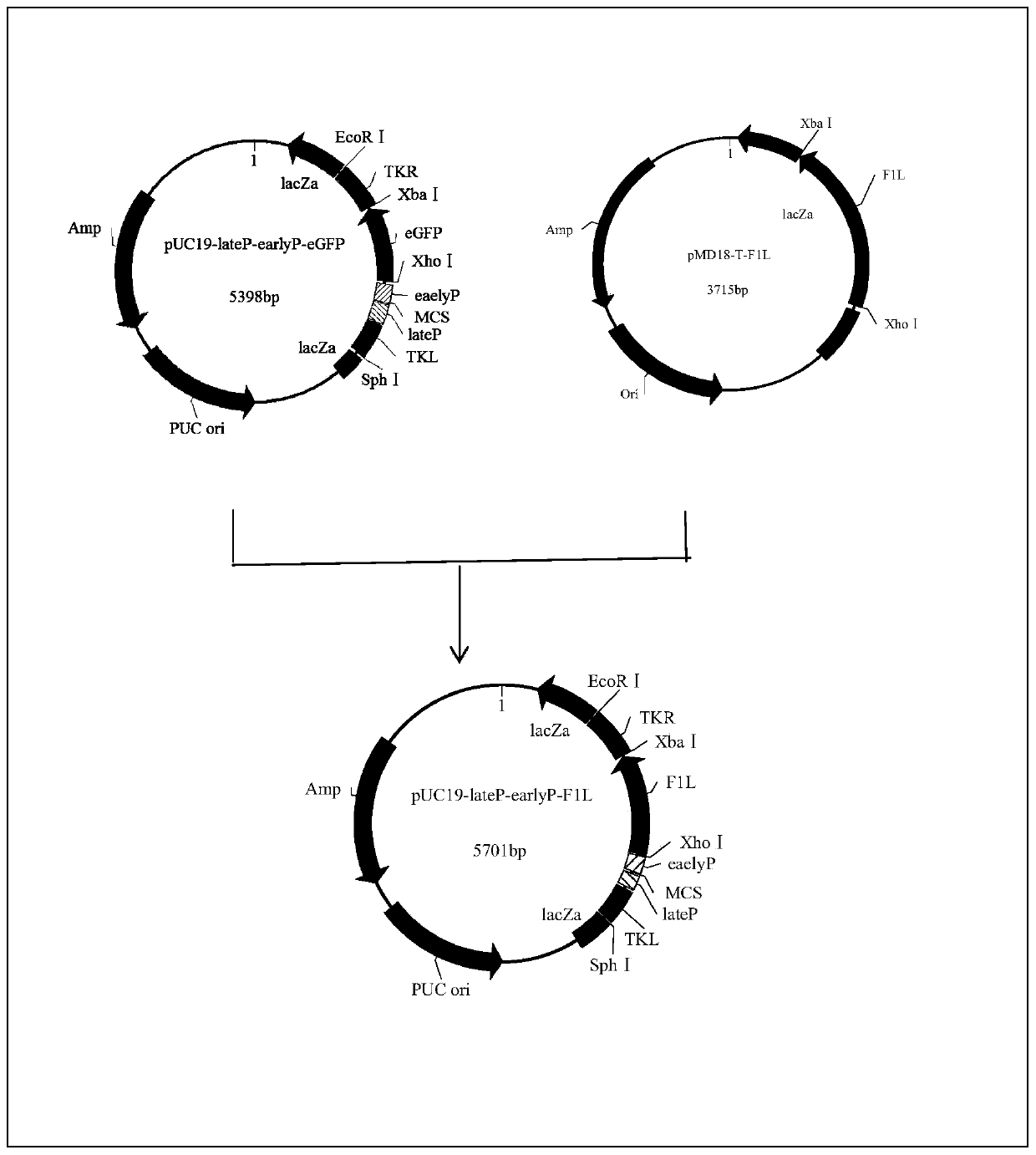
Selection marker-free recombinant goat-pox virus for expression of Orf virus F1L protein and construction method thereof
InactiveCN110724674AHigh expressionContinuous and efficient expressionViral antigen ingredientsMicroorganism based processesEngineeringRecombinant virus vaccine
Through homologous recombination and by innovative utilization of plaque and reverse plaque screening technologies, the selection marker-free recombinant virus, especially the rGTPV-F1L recombinant goat-pox virus is finally obtained. The method is simple and effective, is easy to operate, provides the selection marker-free recombinant virus for the development of a recombinant virus vaccine, provides new ideas and measures for the researches on a novel ORF vaccine, and has broad application prospects.
Owner:CHONGQING ACAD OF ANIMAL SCI
Matrix protein mutated recombinant vesicular stomatitis virus serving as porcine vaccine vector
InactiveCN103768592AExperimental results directlyThe experimental results are accurateAntiviralsAntibody medical ingredientsArginineVariant virus
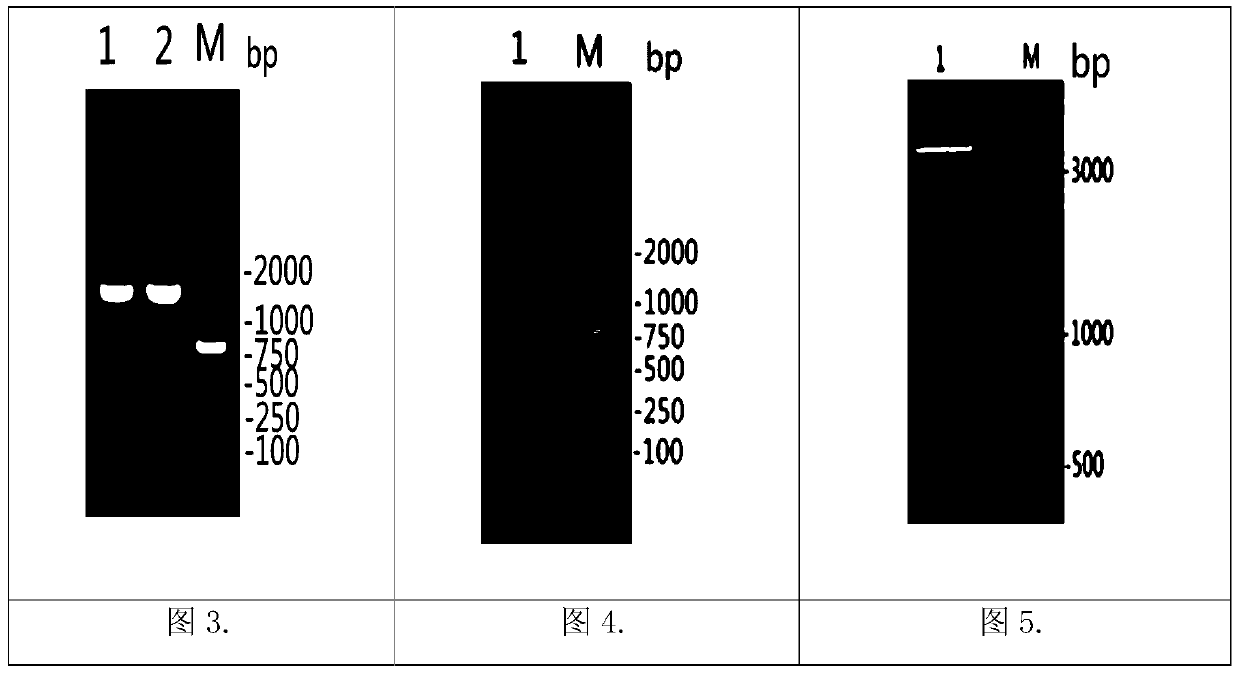
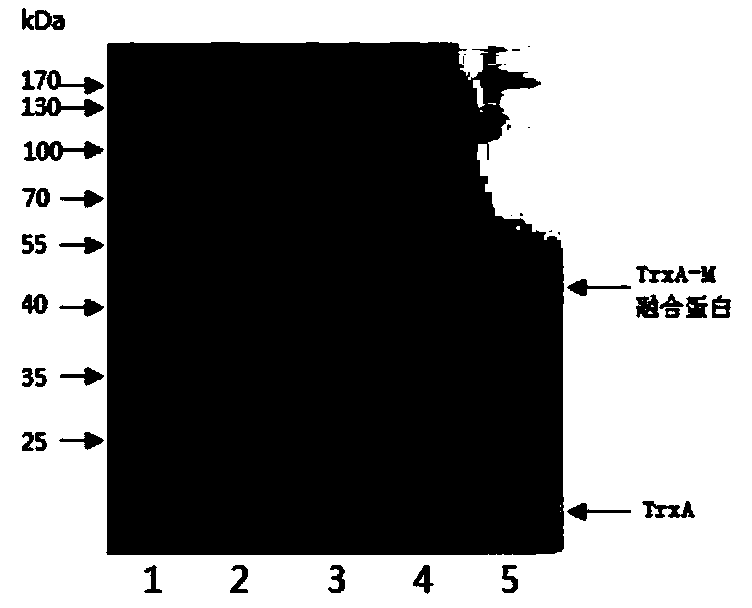
The invention discloses a matrix protein mutated recombinant vesicular stomatitis virus serving as a porcine vaccine vector. The vaccine vector is a recombinant virus VSV (vesicular stomatitis virus) delta M51. The invention also provides a kit for detecting the specific M antibody in porcine serum. The using method of the kit comprises the following steps: establishing an ELISA method by utilizing high-purity M protein prepared by recombinant expression to detect the level of the specific M antibody in porcine serum which is inoculated with VSV delta M51 virus. The invention firstly provides the variant virus strain (VSV delta M51) of which the 51st arginine of the matrix protein of an VSV critical virulence factor serving as a candidate VSV virus strain with application values, provides a systematic evaluation to the pathogenicity of VSV delta M51 on pigs for the first time at home and abroad, and provides basis to VSV delta M51 serving as the vaccine vector.
Owner:SHANGHAI JIAO TONG UNIV
Application of recombinant virus vaccine in galliformes poultry by adopting duck virus enteritis virus vaccine strain as vector
ActiveCN103667351AViral antigen ingredientsGenetic material ingredientsAvian influenza virusInfluenza virus A hemagglutinin
The invention provides an application of living virus vector duck virus enteritis virus (DEV) attenuated vaccine vector in galliformes poultry. The DEV attenuated vaccine vector can be used for expressing relevant genes of the galliformes poultry pathogeny and can carry exogenous genes to be expressed in the galliformes poultry, and the protection for the pathogeny can be well induced in an immune animal body. Through the application of the recombinant duck virus enteritis virus vaccine strain (preservation serial number is CCTCC V201125, named as rDEVus78Ha-Re6) for expressing the avian influenza virus haemagglutinin (HA) genes and the recombinant duck virus enteritis virus vaccine strain (preservation serial number is CCTCC V201220, and named as rDEV F) for expressing newcastle disease virus gene F in chicken, the characteristics of the DEV attenuated vaccine vector can be proved.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Anti-human immunodeficiency virus (HIV) gene engineering recombinant virus and preparation method thereof, and anti-HIV gene engineering medicament
InactiveCN102690792AEffective infectionAvoid infectionViral/bacteriophage medical ingredientsAntiviralsHIV receptorHuman immunodeficiency
The invention relates to an anti-HIV gene engineering recombinant virus and a preparation method thereof, and an anti-HIV gene engineering medicament. The anti-HIV gene engineering recombinant virus comprises a constant region of a human antibody, and a cluster of differentiation 4 (CD4) and a chemokine receptor 5 (CCR5) which are connected with the constant region. The preparation method for the anti-HIV gene engineering recombinant virus comprises the steps as follows: obtaining a heavy chain constant region gene of the human antibody, a light chain constant region gene of the human antibody, a CD4 gene and a CCR5 gene; constructing a virus vector shuttle plasmid comprising the four genes; co-transforming the shuttle plasmid and a virus auxiliary plasmid to generate a recombinant virus plasmid; and transferring the recombinant virus plasmid to a cell strain for culturing and purifying the virus. The recombinant virus has both the CD4 and CCR5 capable of specifically binding with the CD4 site and CCR5 site of an HIV virus, an obviously enforced specific binding effect, and the function of doubly preventing the HIV virus from infecting a host cell. The preparation method is simple and practical. The anti-HIV gene engineering medicament with the recombinant virus can effectively act on the HIV virus, and can further effectively prevent and treat the infection of the HIV virus.
Owner:SHENZHEN UNIV
Recombinant turkey herpesvirus candidate vaccine strain for expressing gene VII-type Newcastle disease virus fusion protein and preparation method thereof
InactiveCN110331135ASsRNA viruses negative-senseViral antigen ingredientsNewcastle disease virus NDVTurkey Herpesvirus
The invention belongs to the technical field of genetic engineering vaccines, and particularly relates to a recombinant turkey herpesvirus candidate vaccine strain for expressing gene VII-type Newcastle disease virus fusion protein and a preparation method thereof. The recombinant turkey herpesvirus candidate vaccine strain for expressing the gene VII-type Newcastle disease virus fusion protein isnamed as rHVT-NDV-VII-F, and has a preservation number of CCTCC NO: V201904. The recombinant virus can express a gene VII-type NDV F protein, is completely matched with the genotype of a currently domestically popular NDV wild strain, and can be used as a gene engineering vaccine candidate strain for preventing NDV infection.
Owner:YANGZHOU UNIV
Swine influenza virus H1N1 subtype hemagglutinin (HA)-1 protein recombinant suipoxvirus and preparation method thereof
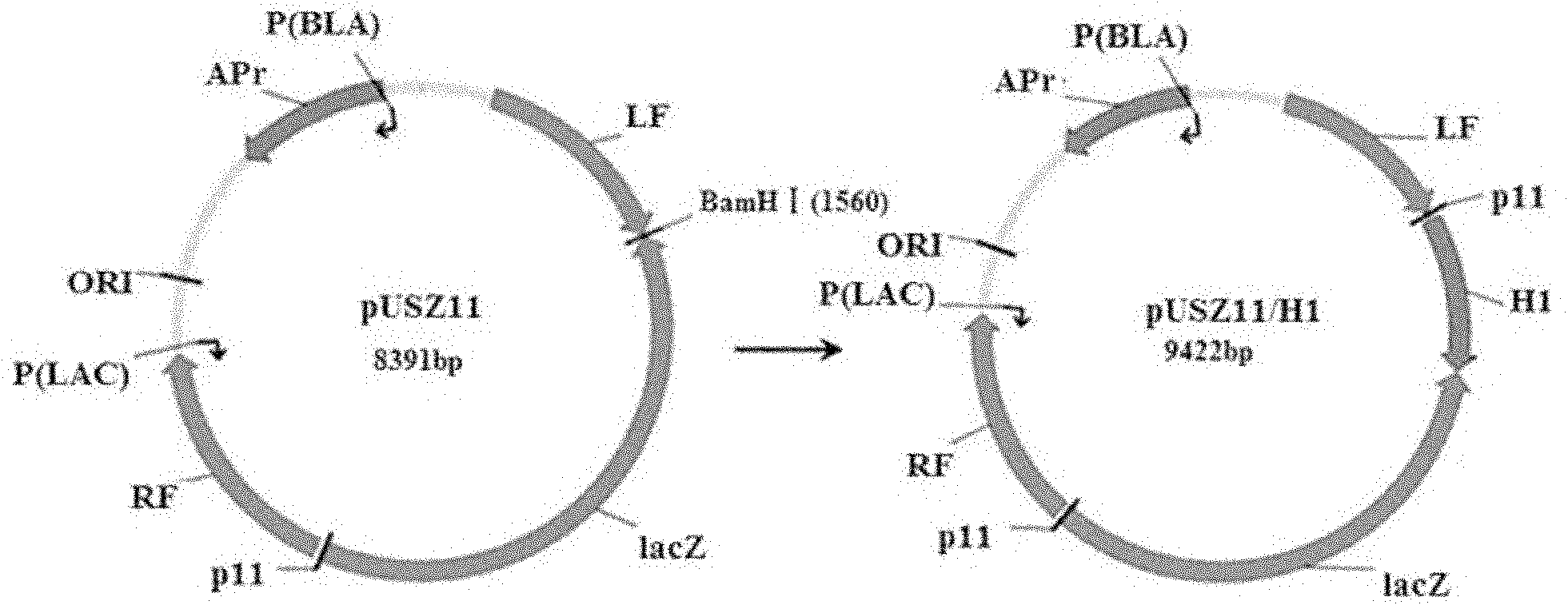
InactiveCN102154365AObvious blue spotEasy to filterMicroorganism based processesAntiviralsHemagglutininRecombinant virus vaccine
The invention belongs to the technical field of biology and discloses swine influenza virus H1N1 subtype HA-1 protein recombinant suipoxvirus and a preparation method thereof. An HA1 gene is designed and synthesized according to the gene sequence of HA1 of an A / swine / Shanghai / 1 / 2005(H1N 1) strain of the swine influenza virus, and the gene is cloned in a suipoxvirus vector pUSZ11 to obtain a transfer vector pUSZ11 / H1. The recombinant virus is a positive clone obtained by infecting and transfecting PK15 cells with the suipoxvirus and the transfer vector pUSZ11 / H1 and performing homologous recombination. The recombinant suipoxvirus can express HA1 protein stably, and after infection with the virus, the cytopathy becomes regular, the toxic effect of the breeding virus is stabilized at 107TCID50 / mL, the breeding virus can effectively stimulate the immune protect reaction of organisms and can be used in the preparation of medicines for preventing and / or treating infection with swine influenza virus H1N1 subtype.
Owner:NANJING AGRICULTURAL UNIVERSITY
Lentivirus vaccine based on the recombinant viral vaccine against yellow fever
The present invention relates to an attenuated, recombinant viral vaccine against yellow fever which expresses heterologous sequences of a lentivirus and is used as an immunisation agent to induce an immune response to lentivirus.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ +1
Preparation method of viral vaccine expressing plasmodium ovale AMA1 protein
ActiveCN109022456AStrong immune responseEffective immune protectionSsRNA viruses negative-sensePeptidesImmune cycleStructural protein
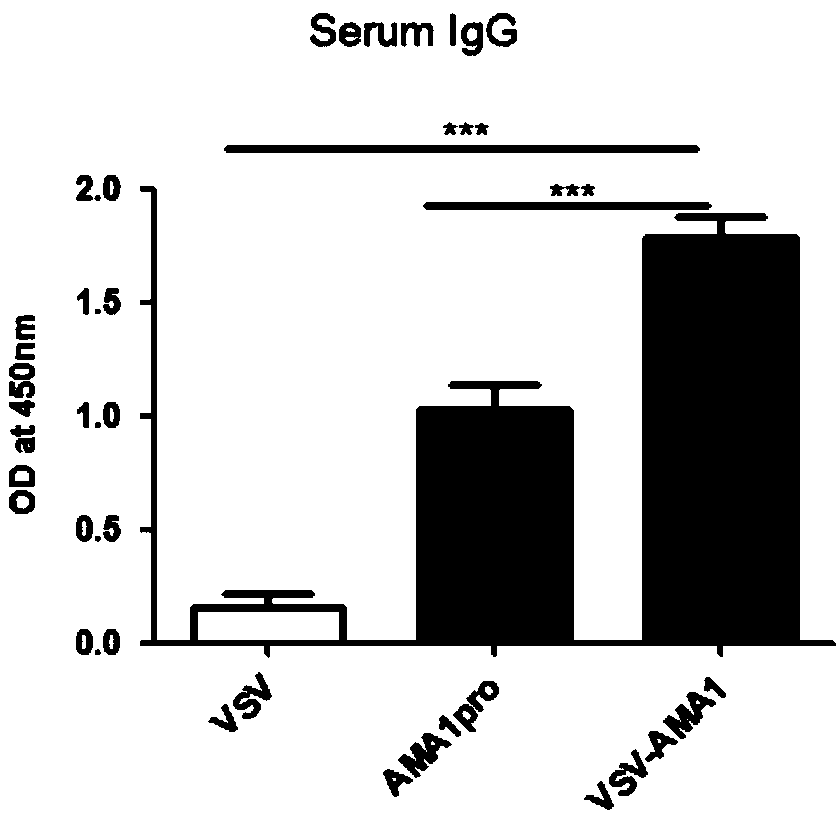
Belonging to the technical field of virology and genetic engineering, the invention discloses a preparation method of a viral vaccine expressing plasmodium ovale AMA1 protein. According to the invention, a gene expressing plasmodium ovale apical membrane protein-1 is inserted into a vesicular stomatitis virus VSV genome vector to construct the recombinant virus vector skeleton plasmid XN2-AMA1, apoxvirus containing T7RNA polymerase is employed to infect the baby hamster kidney cell (BHK-21), then the plasmid XN2-AMA1 and the structural protein plasmids pN, pP, pL are utilized for cotransfection of BHK-21 cells to realize rescue of recombinant virus in cells, virus-like particles expressing AMA1 are packaged, and the recombinant virus rVSV-AMA1, i.e. the vaccine can be formed. The vaccineprovided by the invention has the advantages of simple operation, high production titer and short immune cycle, can induce strong humoral and cellular immune response, and has the great potential of clinical application.
Owner:JIANGNAN UNIV
Recombinant expression vector and construction method thereof, recombinant virus strain and application thereof, recombinant protein and subunit vaccine
InactiveCN105039405AHigh expression yieldImproving immunogenicityViral antigen ingredientsVirus peptidesDiseaseRecombinant virus vaccine
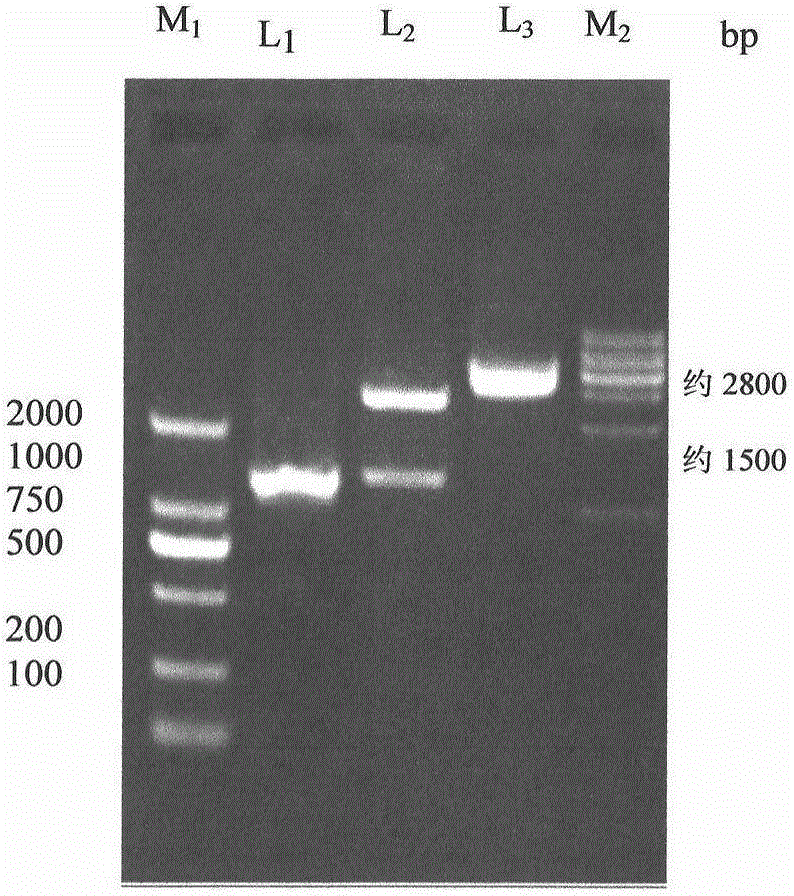
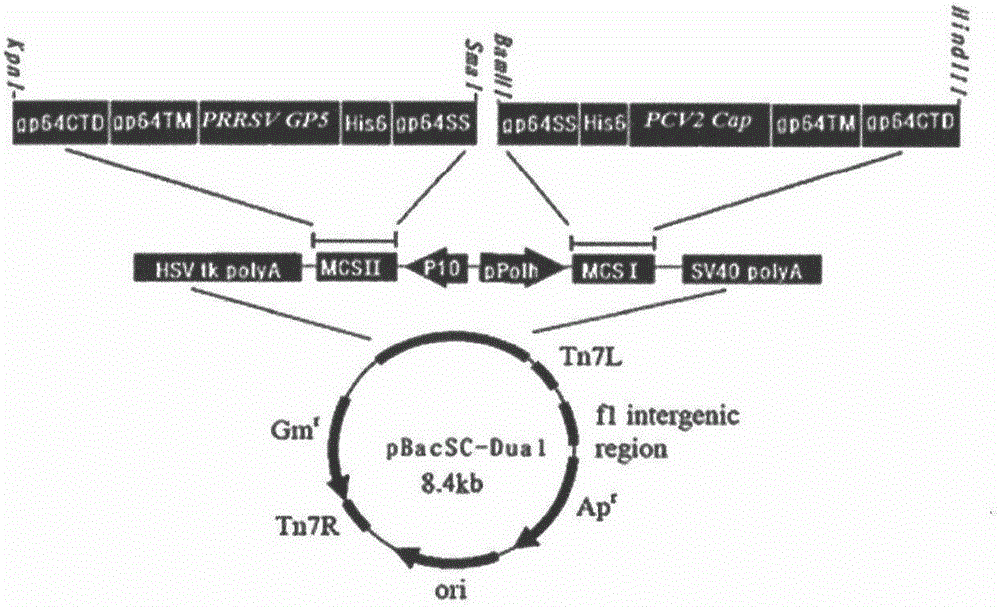
The invention relates to the technical fields of animal virology and genetic engineering and especially relates to a recombinant expression vector and a construction method thereof, a recombinant virus strain and an application thereof, a recombinant protein and a subunit vaccine. According to the invention, PCR amplification is successfully carried out for amplifying PRRSV-GP5 gene and PCV2-Cap gene and constructing a high-effective recombinant expression plasmid BacSC-Dual-GP5-Cap. In addition, the recombinant protein expressed by the recombinant virus strain is very high in product expression yield. The BacSC-Dual-GP5-Cap subunit vaccine product in the invention is safe to animal. An immune efficacy test result of the vaccine proves that PRRSV and PCV2 researched through a genetic engineering method have a strong immunogenicity. The subunit vaccine has a wide application prospect in the field of prevention and treatment of porcine reproductive and respiratory syndrome and porcine circovirus disease.
Owner:JILIN HEYUAN BIOENG LIMITED
Preparation method of recombinant baculovirus vaccine for preventing and treating avian influenza H5N1
The invention relates to a preparation method of a recombinant baculovirus vaccine for preventing and treating avian influenza H5N1, in particular to an H5N1 virus vaccine strain for expressing avian influenza virus hemagglutinin HA gene and a preparation method thereof. The preparation method concretely comprises the following steps of 1) building pS-ITRs-HA plasmids; 2) converting the pS-ITRs-HA plasmids into E .coli DH10 Bac; 3) transfecting Sf9 insect cells by recombinant baculovirus shuttle vectors; 4) amplifying the recombinant baculovirus. When the recombinant H5N1 virus vaccine strain obtained through preparation is used for performing immunodetection on blood serum of immune chicken; the level of IgG antibodies in the blood serum of the immune chicken can reach 0.966 to the highest degree; the concentrations of IL-2, IL-4 and IFN-gamma can respectively reach 89 .67ng / L, 80 .21ng / L and 64 .24ng / L to the highest degree. The results show that the recombinant baculovirus vaccine can effectively irritate the chicken bodies to simultaneously generate the body fluid immunity and the cell immunity; the dual immunity protection effect is provided for the chicken group; the invention lays the theoretical foundation for the research and development of the avian influenza virus based on the baculovirus.
Owner:HEILONGJIANG UNIV
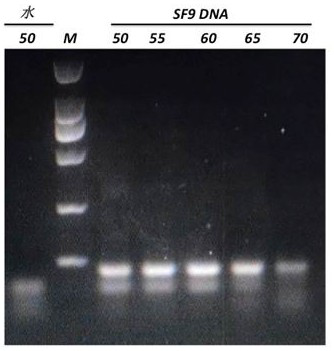
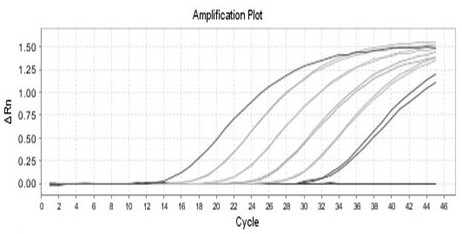
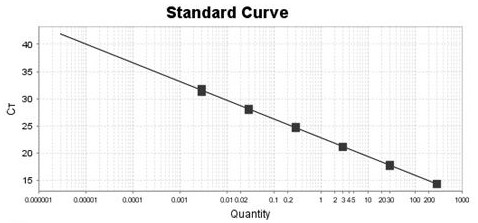
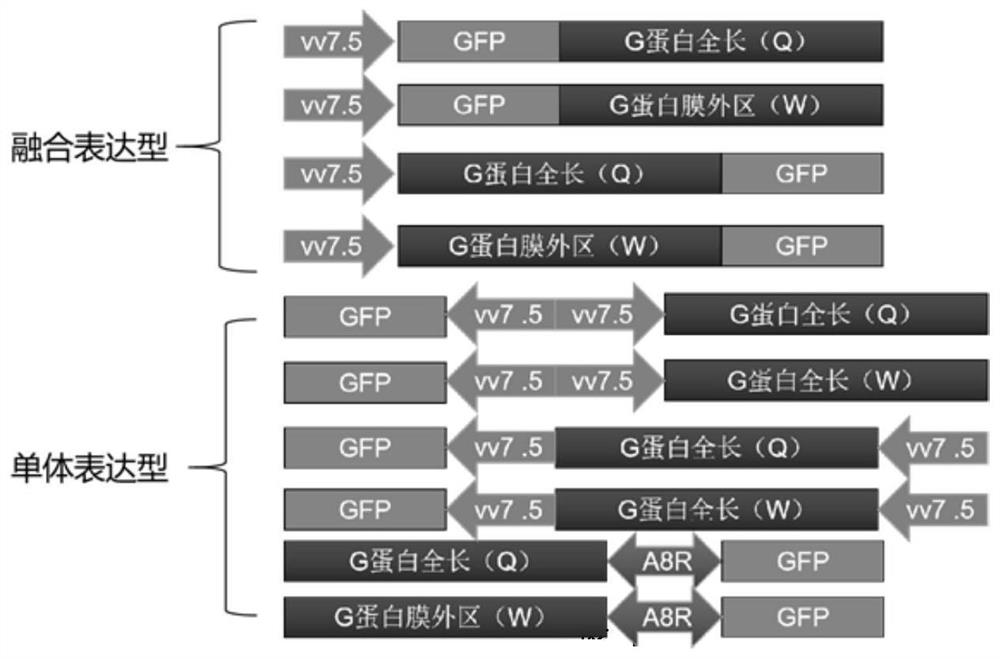
Primer, kit and detection method used for detecting insect cell DNA residues
InactiveCN112094928ALow limit of quantitationHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationRecombinant virus vaccinePcr method
The invention discloses a primer, a kit and a detection method for detecting insect cell DNA residues. Through a method of using a real-time fluorescent quantitative PCR technology for qualitatively and quantitatively detecting the insect cell DNA residues and a PCR kit prepared according to the method, a quantitative limit of as low as 3fg / ul for detecting the insect cell DNA residues is realized; and in other words, the method has good sensitivity and specificity. The real-time fluorescent quantitative PCR method can be used for detecting recombinant protein or recombinant virus vaccines orgene therapy vectors produced by insect cells which are taken as expression host cells, such as recombinant adeno-associated viruses and the like, and can provide reliable quality detection data for research, development and safe production of the gene therapy vectors.
Owner:天津科佰迪生物医药科技有限公司
Construction method of rabies virus G protein-goatpox virus recombinant vaccine
ActiveCN111944769AThe immune effect is stable and significantThe build process is greatly simplifiedSsRNA viruses negative-senseVirus peptidesImmune effectsRecombinant vaccines
The invention discloses a construction method of a rabies virus G protein-goatpox virus recombinant vaccine. The construction method comprises the following steps of: 1, design of a recombinant virusvaccine construction model; 2, amplification of each gene and fusion amplification of each combined gene; 3, construction of various combined recombinant transfer vectors; 4, recombination of rabies virus G protein and goatpox virus and virus purification; 5, rabies virus G protein expression detection; and step 6, detection of the immune effect of a rabies virus G protein-goatpox recombinant virus vaccine strain. The vaccine constructed by the method can effectively prevent rabies of goat, has the characteristics of high production efficiency, simplicity, convenience and practicability, and is suitable for popularization and application.
Owner:TARIM UNIV
Recombinant vector vaccine expressing HIV antigen
InactiveCN105441482AStrong immune responseGenetic material ingredientsAntiviralsVector vaccineRecombinant virus vaccine
The invention provides a recombinant sendai vector vaccine expressing HIVGag, construction method and application thereof. The vaccine is constructed based on an F gene defective sendai virus vector. The polynucleotide expressing HIVGag is properly modified and reconstructed so as to be able to realize high expression in eukaryotic cells without depending on regulatory protein Rev. The test result shows that the recombinant vector vaccine can express HIVGag correctly and can effectively induce in-vivo and in-vitro cellular immunity.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Virus recombinant vaccine A-NDV-LX/14 for newcastle disease and construction method thereof
ActiveCN102533676BImprove separation rateSolid Immune Protection EfficiencyViral antigen ingredientsMicroorganism based processesSerum igeNewcastle disease virus NDV
The invention relates to a virus vaccine A-NDV-LX / 14 for a newcastle disease, which is a recombinant of expressed genes VII F and HN, and the preservation number is CGMCCNO: 3844. After two weeks of the constructed virus A-NDV-LX / 14 immunity test on chicken, the average potency of the serum HI is 27. After four days of eliminating toxic material with immune group LaSota, the virus isolation ratesof SPF chicken laryngotracheal and cloacal swab samples are 10 percent and 30 percent respectively, and the virus isolation rates of chicken laryngotracheal and cloacal swab samples are 40 percent and 20 percent respectively. After four days of eliminating toxic material with immune group V4, the virus isolation rates of SPF chicken laryngotracheal and cloacal swab samples are 30 percent and 10 percent respectively, and the virus isolation rates of chicken laryngotracheal and cloacal swab samples are 30 percent and 20 percent respectively. Compared with traditional vaccine, more effective immune protection can be provided by the recombinant virus vaccine.
Owner:YANGZHOU UNIV
A recombinant virus vaccine strain expressing African swine fever virus p72 protein
ActiveCN104962581BImprove protectionEffective protectionViral antigen ingredientsMicroorganism based processesNewcastle disease virus NDVAnimals diseases
The invention provides a preparation method of a recombinant Newcastle disease virus vaccine strain expressing African swine fever virus p72 protein and the recombinant Newcastle disease virus vaccine strain. The method of the present invention includes: constructing a recombinant transcription plasmid in which the p72 gene of African swine fever virus (ASFV) is inserted, constructing a transcription auxiliary plasmid system, and co-transfecting the above transcription plasmid and the transcription auxiliary plasmid into the attenuated Newcastle disease strain to be able to replicate The host cell BHK‑21 was rescued to obtain the recombinant virus strain. The vaccine strain expressing the African swine fever virus p72 protein prepared by the invention has important reserve and strategic significance in the prevention and control of the animal disease, and can be used in the treatment and prevention of the African swine fever virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
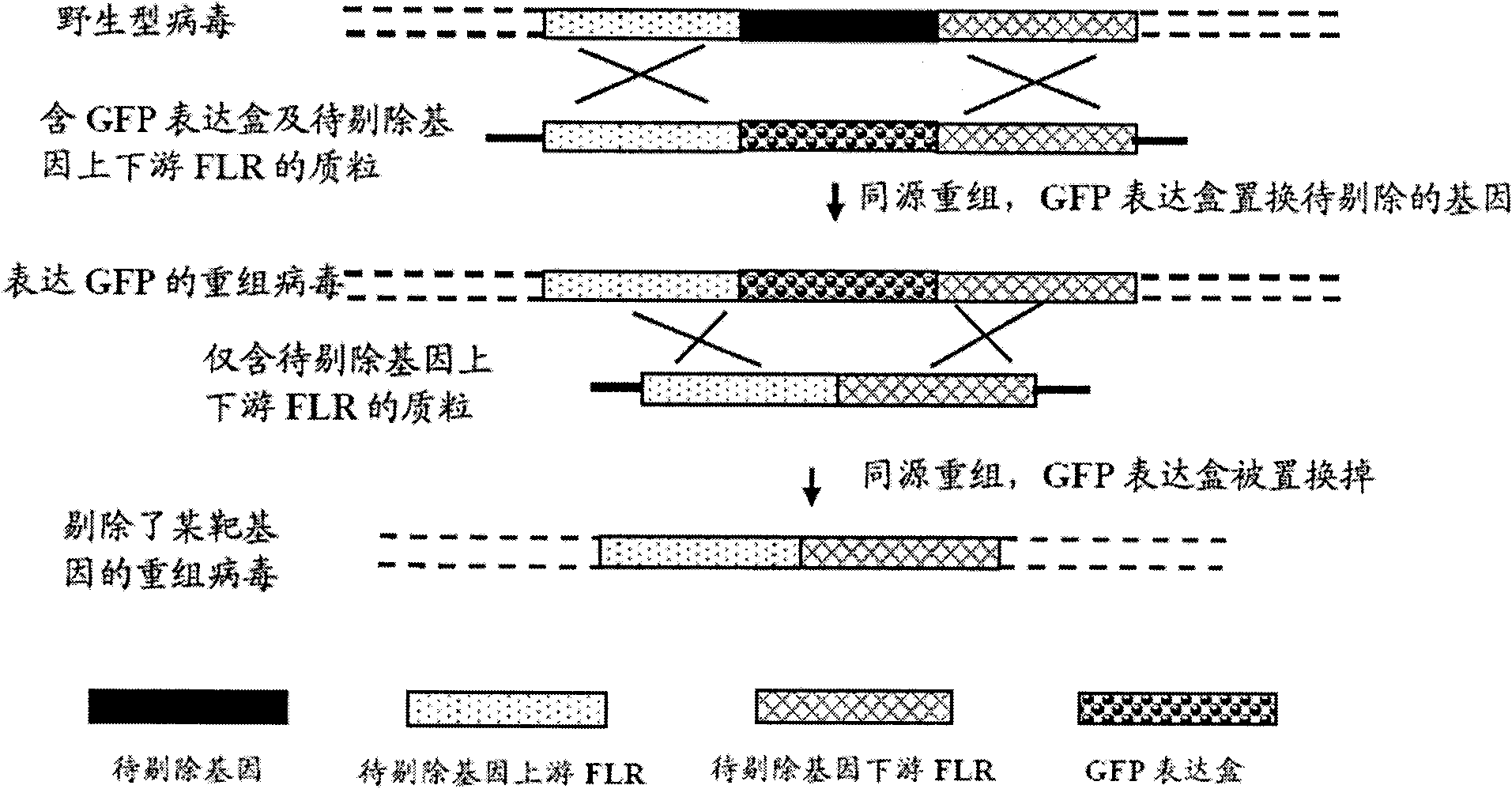
Herpes simplex virus type Ⅱ gene recombinant attenuated live vaccine and preparation method thereof
InactiveCN102657862BRelapse controlControl spreadMicroorganism based processesAntiviralsShuttle vectorFluorescence
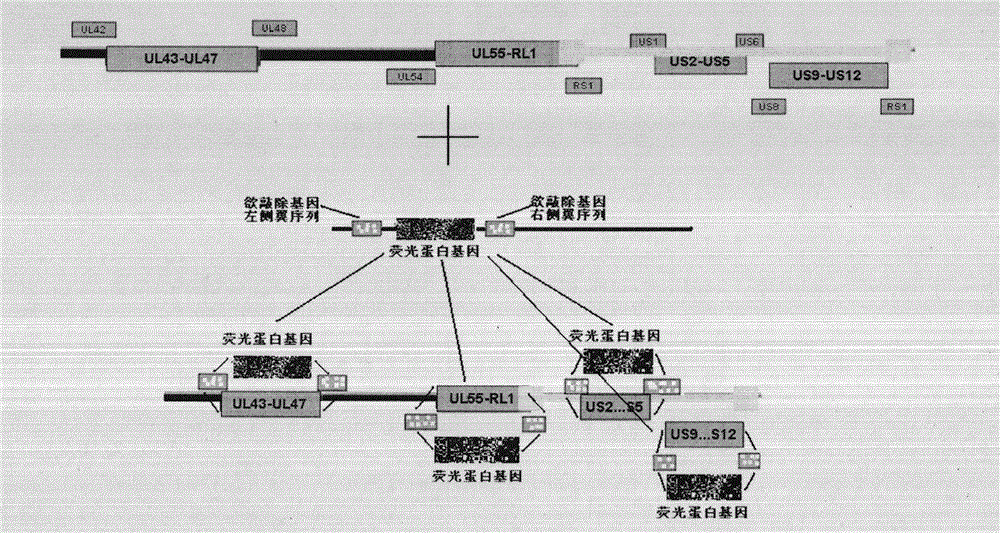
The invention discloses a herpes simplex virus type 2 (HSV-2) genetic recombinant attenuated live vaccine and a preparation method thereof. US2, US3, US4 and US5 genes in a genome are knocked out. The method comprises the following steps of: separating and identifying vesicle liquid of a patient who suffers from oral herpes and genital herpes and has obvious vesicles to obtain wild type HSV-1 and HSV-2; performing amplifying culture, and extracting a virus complete genome; transfecting Vero cells by using a shuttle vector containing a fluorescent expressed gene and 700bp to 2,000bp homologous flanking sequences on the left and right sides of an HSV gene which is prepared to be knocked out; and picking recombinant virus out under a fluorescent microscope by using one or more fluorescent protein genes as markers, and performing plaque purification to obtain the needed recombinant attenuated live vaccine. The vaccine effectively induces mucosal and humoral immune response of organisms, so that the morbidity of people is reduced; and cellular immune response is excited, so that the infected virus can be cleared, and the relapse and spreading of herpes are controlled.
Owner:郑州金森生物科技工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com