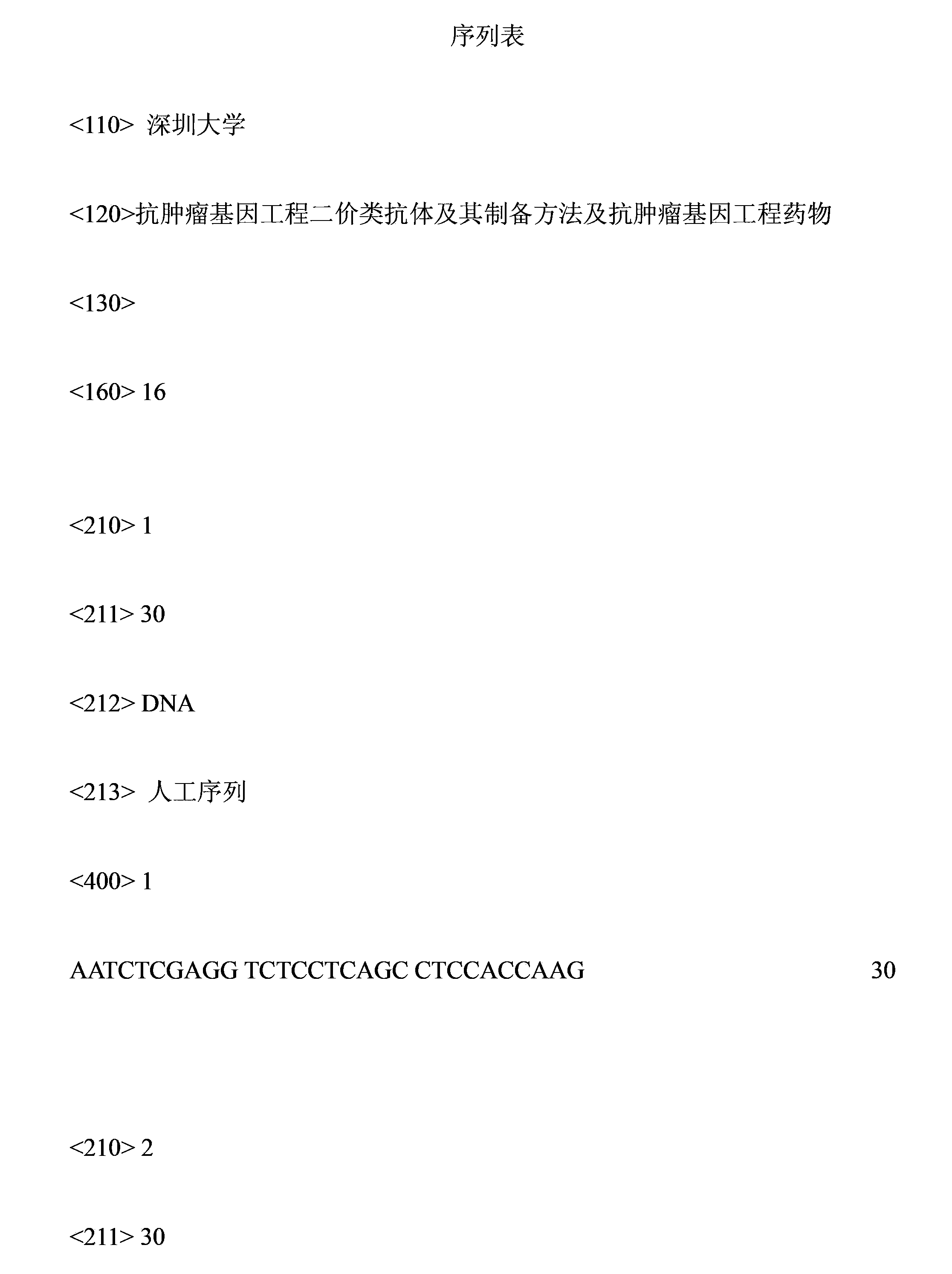
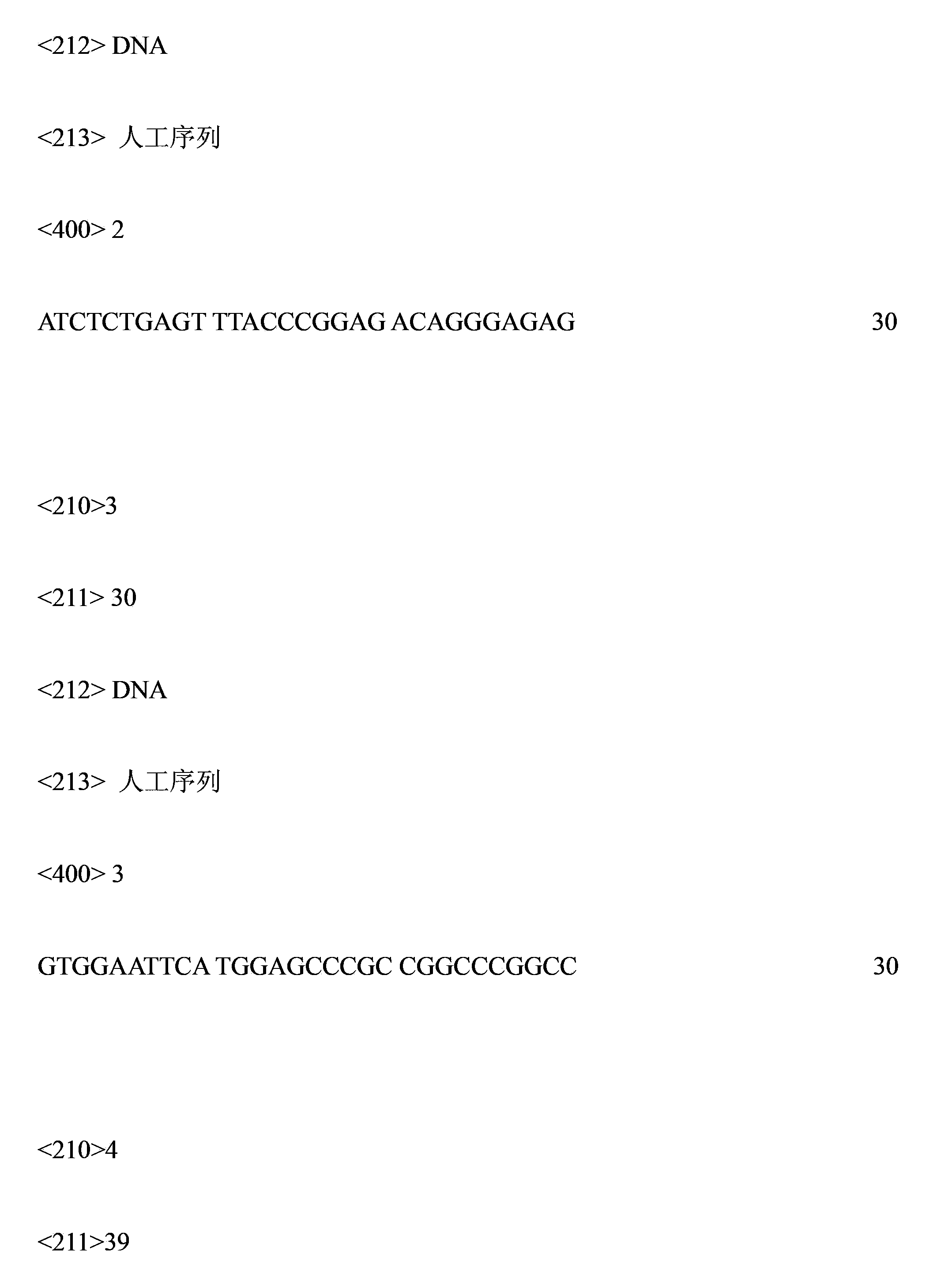
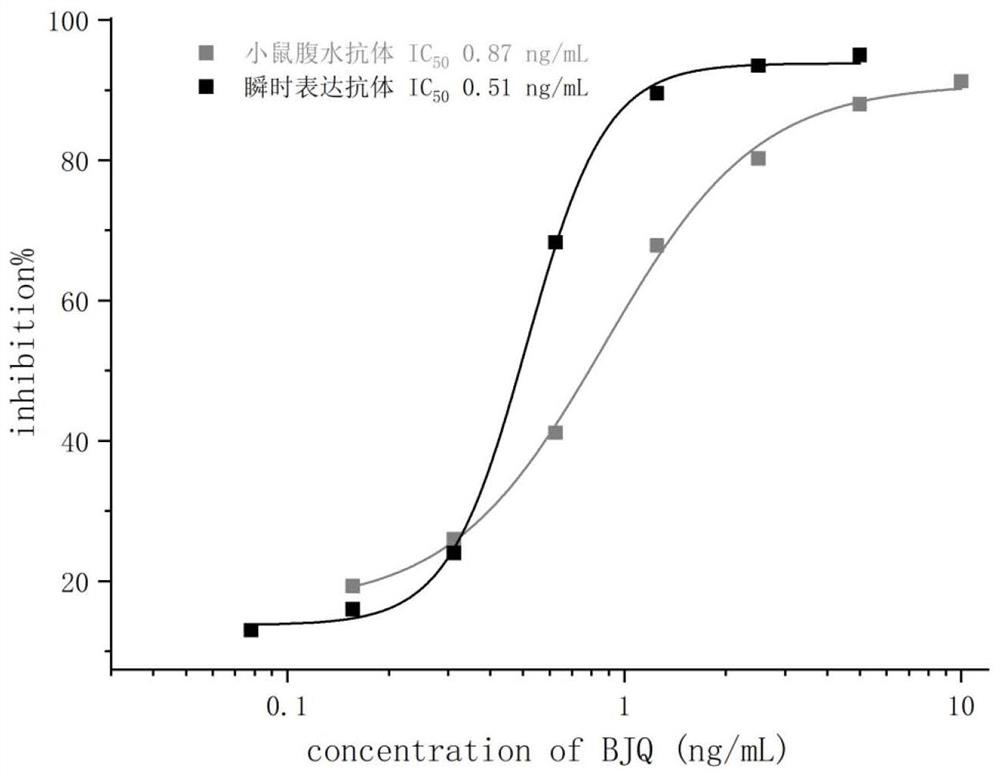
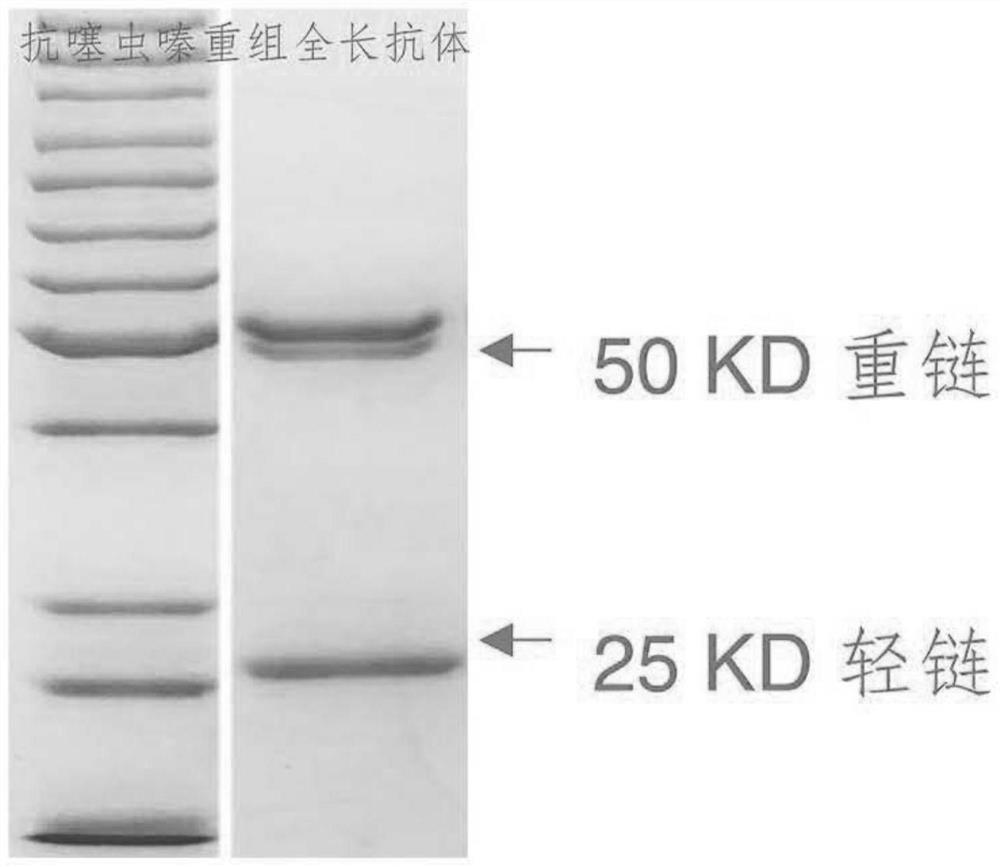
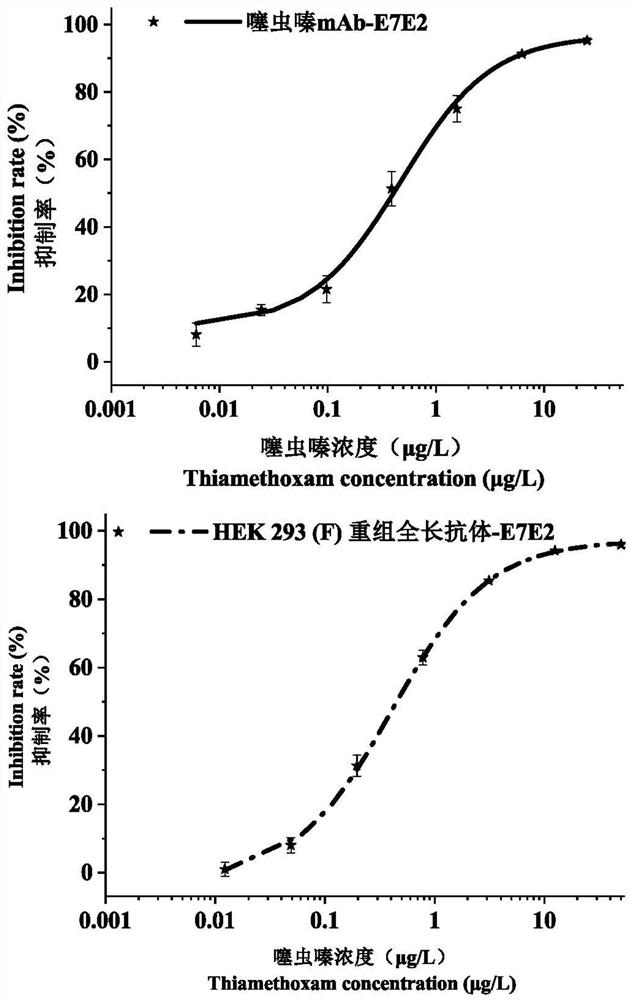
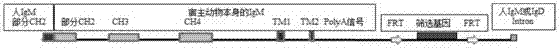Patents
Literature
36 results about "Constant region gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The T-cell receptor gamma chain is formed when 1 of 12 variable (V) genes (see 615454), which encode the N-terminal antigen recognition domain, rearranges to a joining (J) gene to create a functional V region exon that is transcribed and spliced to a constant (C) region gene segment encoding the C-terminal portion of the molecule.
Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions
InactiveUS7049426B2Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenExon
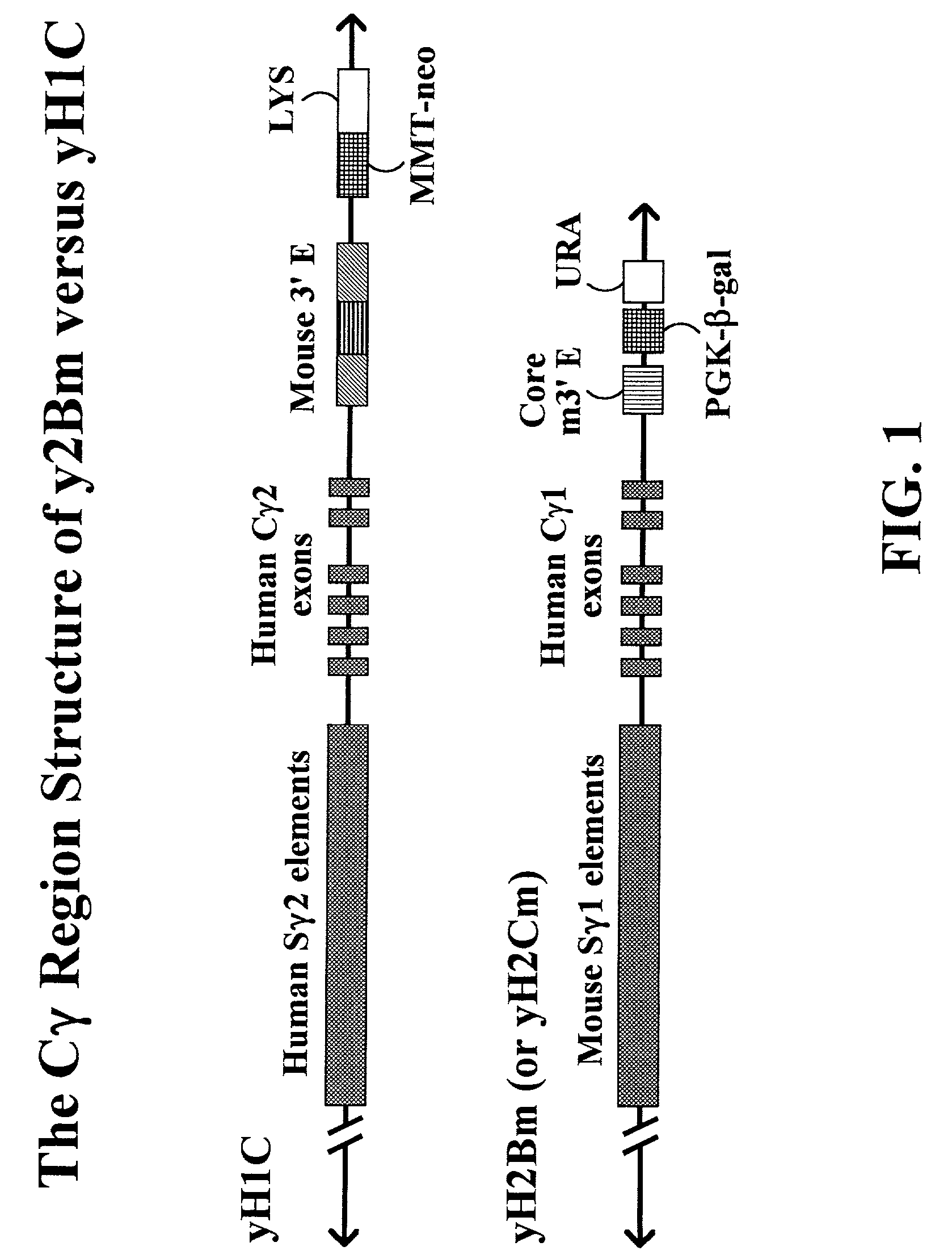
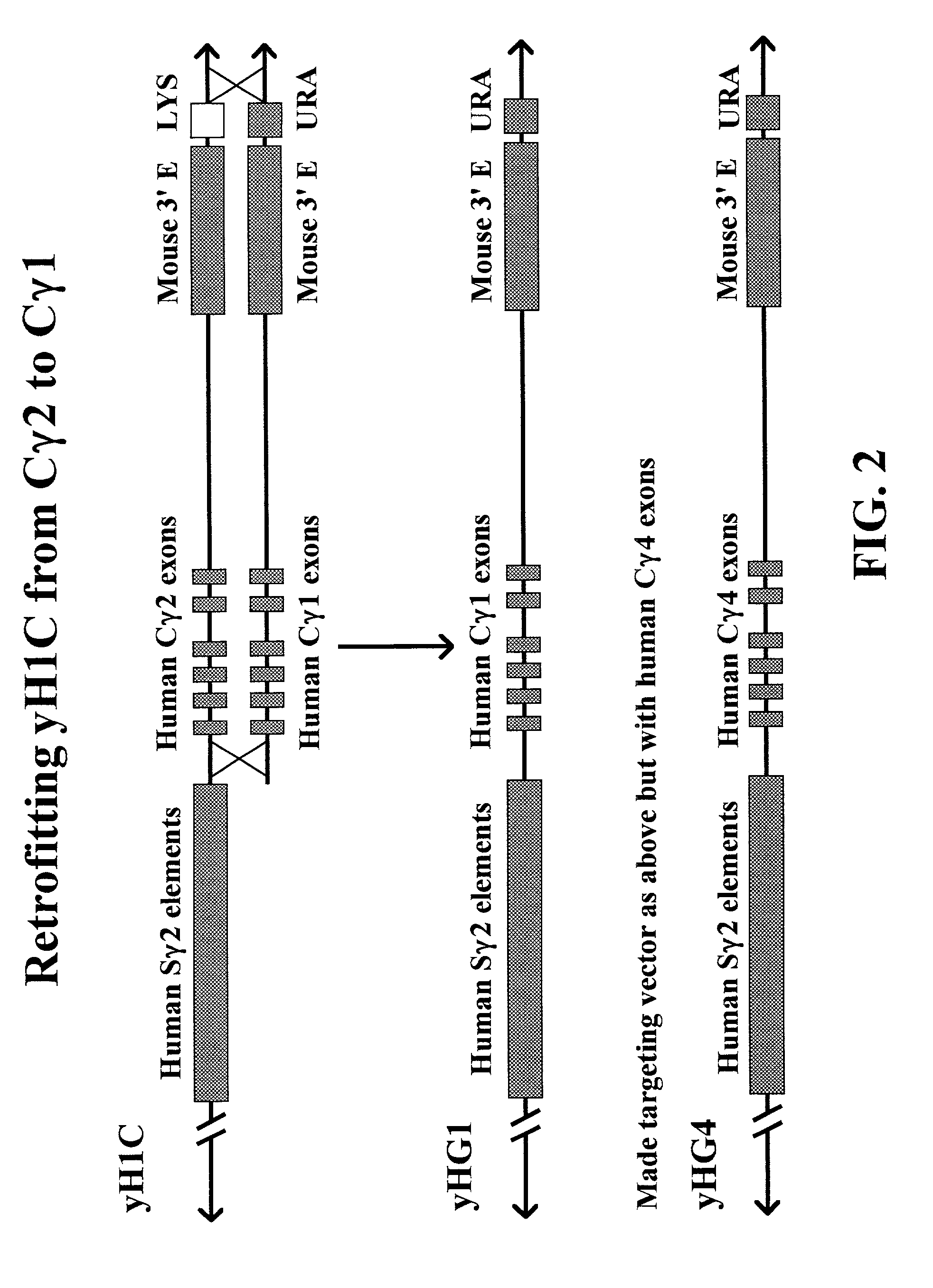
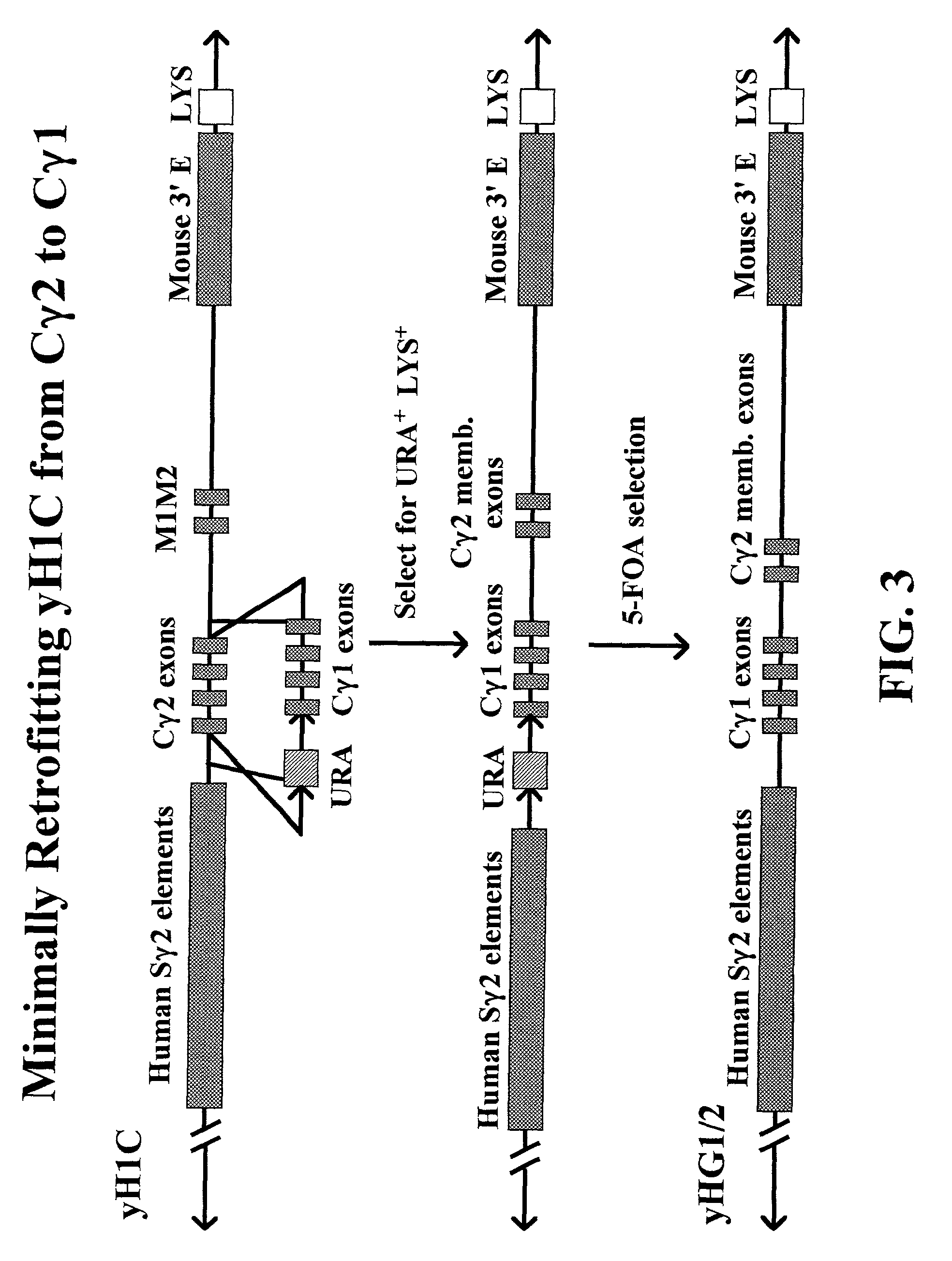
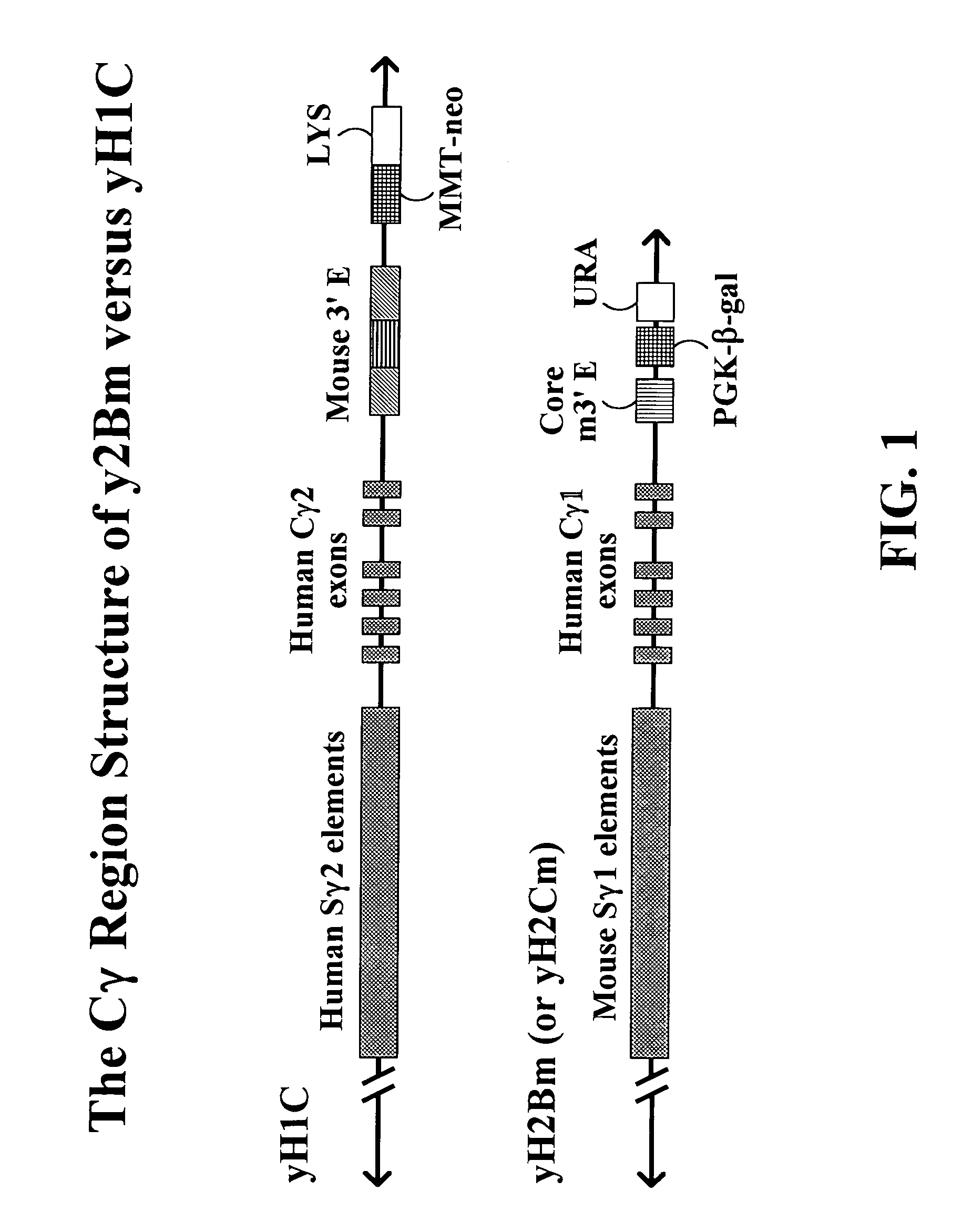
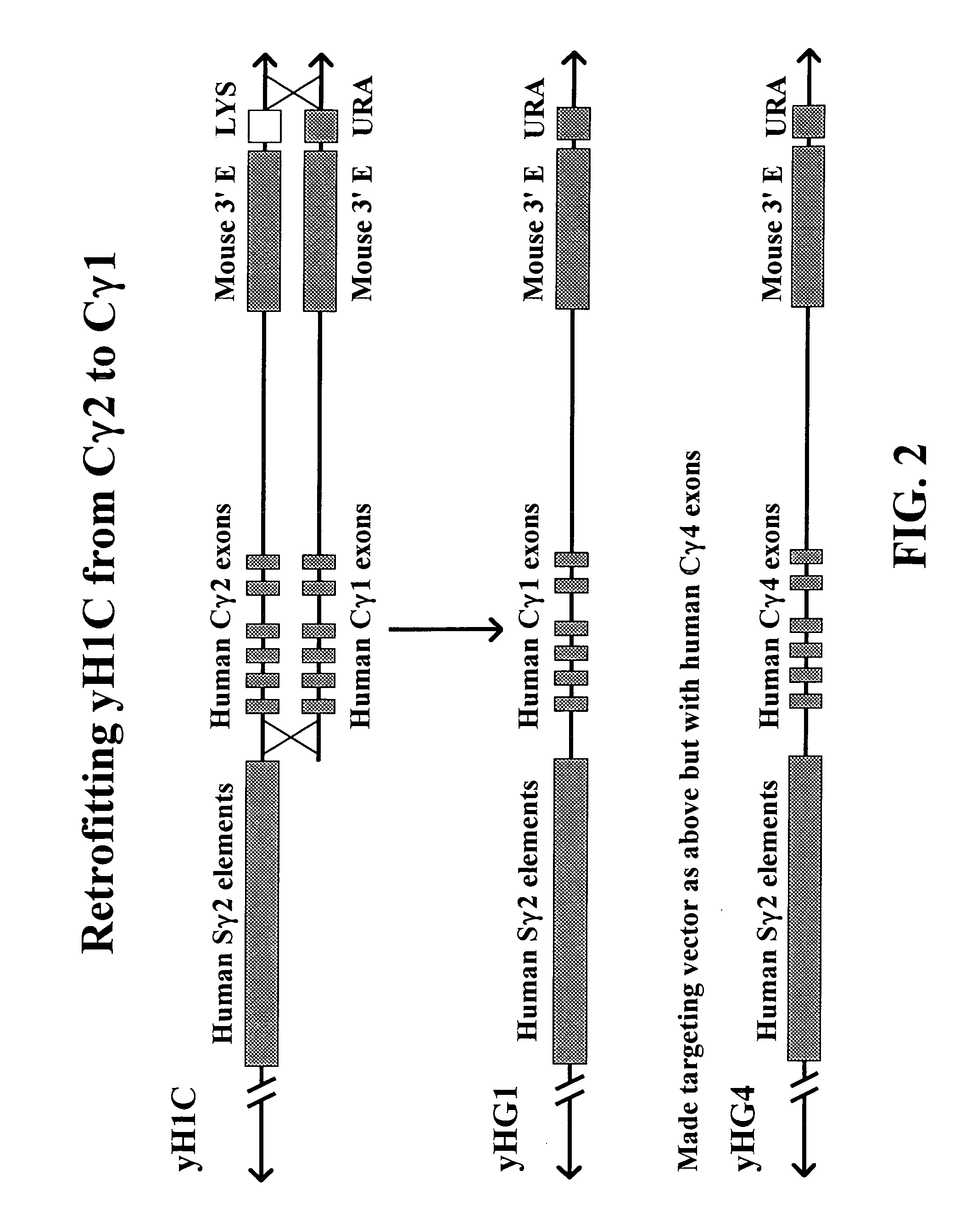
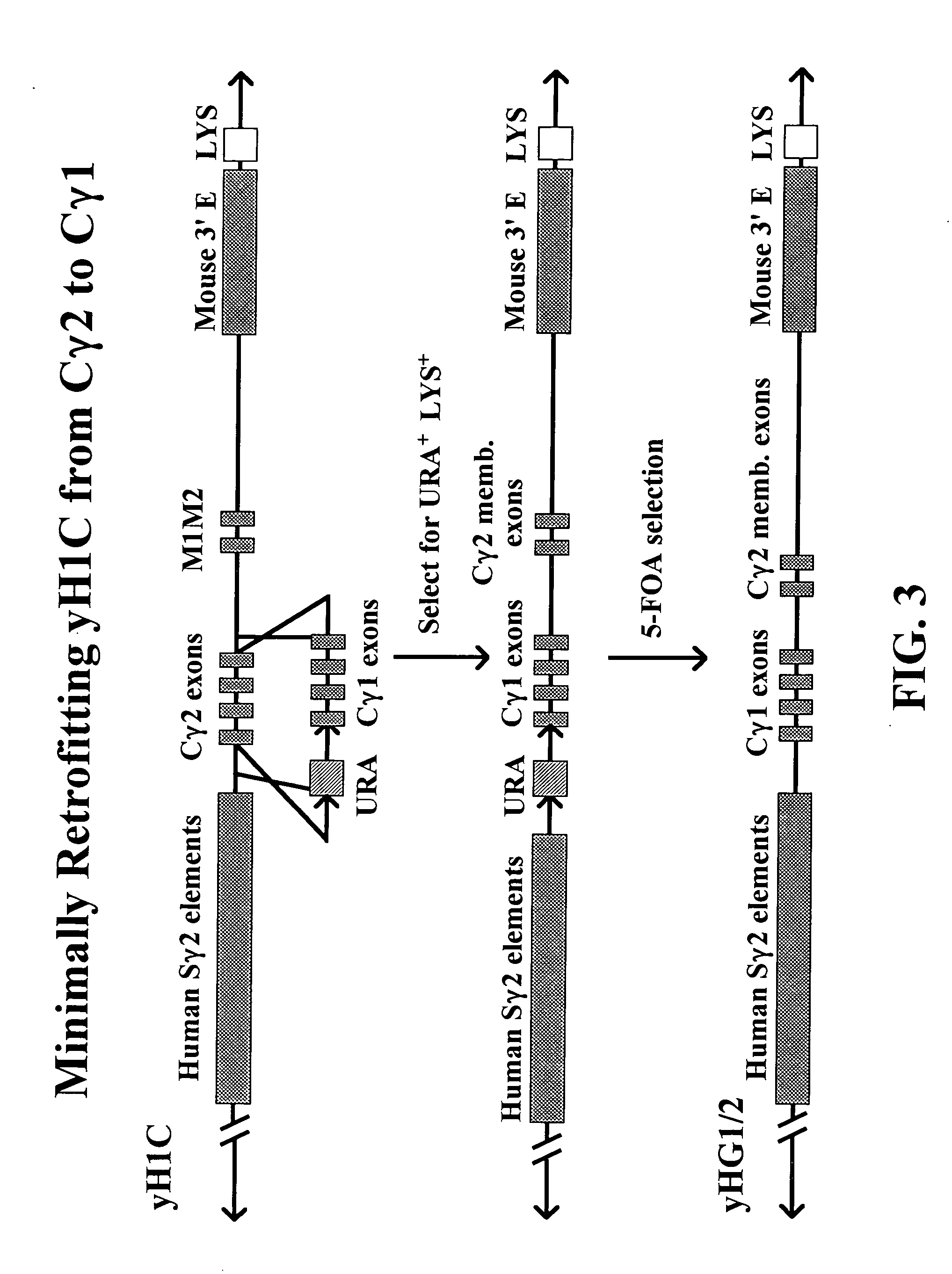
The present invention provides fully human antibodies in a transgenic animal of a desired isotype in response to immunization with any virtually any desired antigen. The human immunoglobulin heavy chain transgene in the foregoing animals comprises a human constant region gene segment comprising exons encoding the desired heavy chain isotype, operably linked to switch segments from a constant region of a different heavy chain isotype, i.e., a non-cognate switch region. Said additional constant region segment comprises a switch region and human constant region coding segment, wherein the constant region coding segment is operably linked to a switch region that it is not normally associated with, i.e., a non-cognate switch region. In the transgenes of the invention, the non-cognate switch region may be a switch region from a different species than the constant region coding segment. The switch region and membrane exons of the invention may comprise a human gamma-2 constant region and the secreted constant region exons are from a human gamma-1 or a human gamma-4 constant region.
Owner:ABQENIX INC
Genetically-modified cells comprising a modified human T cell receptor alpha constant region gene
ActiveUS9889160B2Efficient insertionConvenient treatmentAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseGene Modification
Disclosed herein is a genetically-modified cell comprising in its genome a modified human T cell receptor alpha constant region gene, wherein the cell has reduced cell-surface expression of the endogenous T cell receptor. The present disclosure further relates to methods for producing such a genetically-modified cell, and to methods of using such a cell for treating a disease in a subject.
Owner:PRECISION BIOSCI
Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions
InactiveUS20060134780A1Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBeta globulins
The present invention provides fully human antibodies in a transgenic animal of a desired isotype in response to immunization with any virtually any desired antigen. The human immunoglobulin heavy chain transgene in the foregoing animals comprises a human constant region gene segment comprising exons encoding the desired heavy chain isotype, operably linked to switch segments from a constant region of a different heavy chain isotype, i.e., a non-cognate switch region. Said additional constant region segment comprises a switch region and human constant region coding segment, wherein the constant region coding segment is operably linked to a switch region that it is not normally associated with, i.e., a non-cognate switch region. In the transgenes of the invention, the non-cognate switch region may be a switch region from a different species than the constant region coding segment. The switch region and membrane exons of the invention may comprise a human gamma-2 constant region and the secreted constant region exons are from a human gamma-1 or a human gamma-4 constant region.
Owner:AMGEN FREMONT INC
Recombined chimeric antibody against human tumor necrosis factor alpha
ActiveCN101177453AExtended half-lifeFunctionalPeptide/protein ingredientsTumor necrosis factorDiseaseHuman tumor
Described herein are antibodies that bind to human tumor necrosis factor alpha (hTNFα). And list the nucleotide sequence of antibody heavy chain and light chain variable region and its derived amino acid sequence. The heavy chain and light chain variable region genes are respectively connected with the human immunoglobulin gamma 1 (hIgG1) heavy chain constant region and human kappa (k) light chain constant region genes to form a chimeric gene. Then, the vector containing the chimeric gene is introduced into the host cell line to express the antibody protein. This recombinant chimeric antibody protein can neutralize the activity of hTNFα in vitro, and is suitable for treating patients with excessive secretion of harmful hTNFα, including certain inflammations, such as rheumatoid arthritis and Crohn's disease.
Owner:PHARMAB
Preparation method and kit of cytokine-induced killing cell for inducing antibody-dependent cellular cytotoxicity
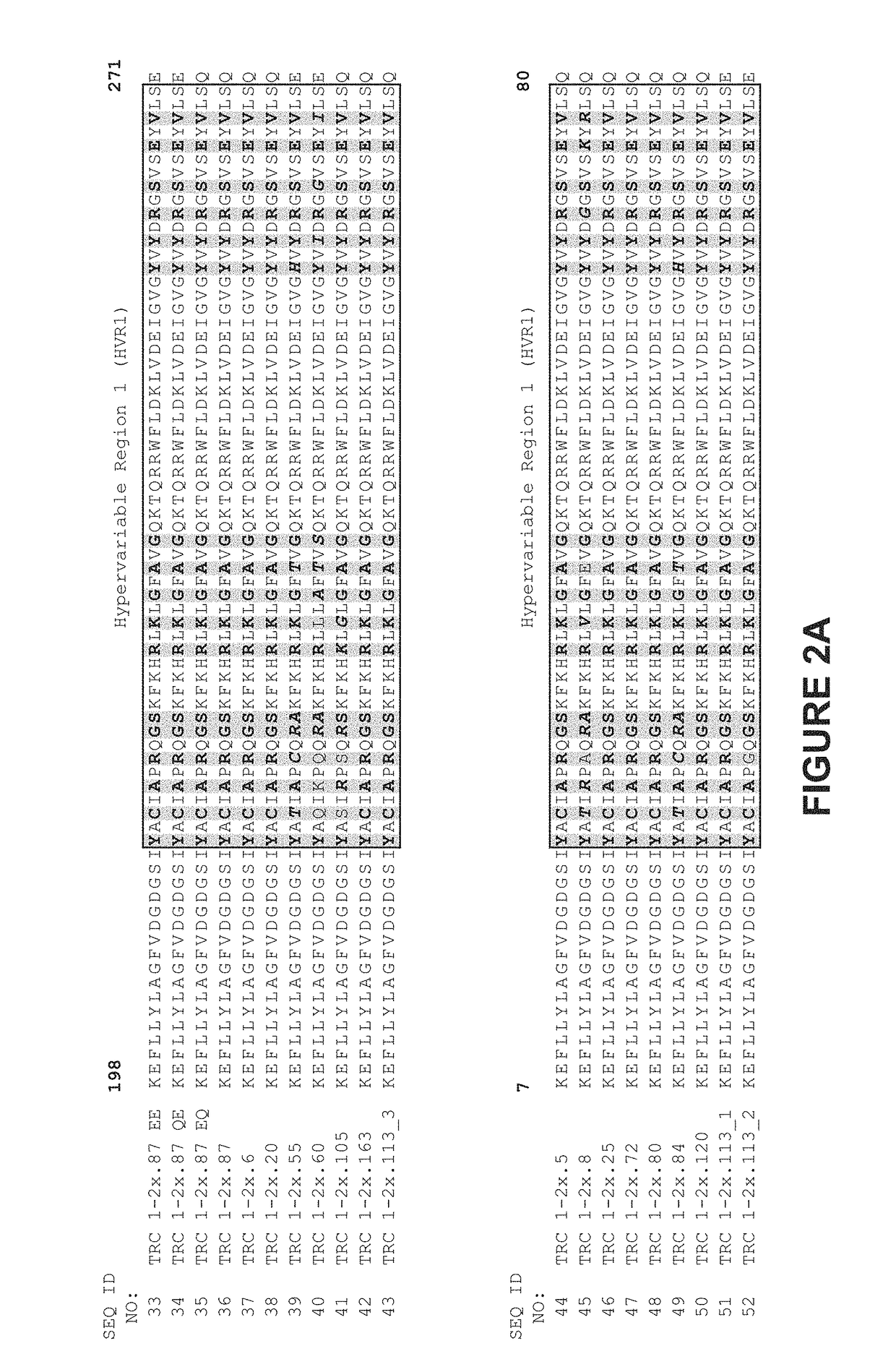
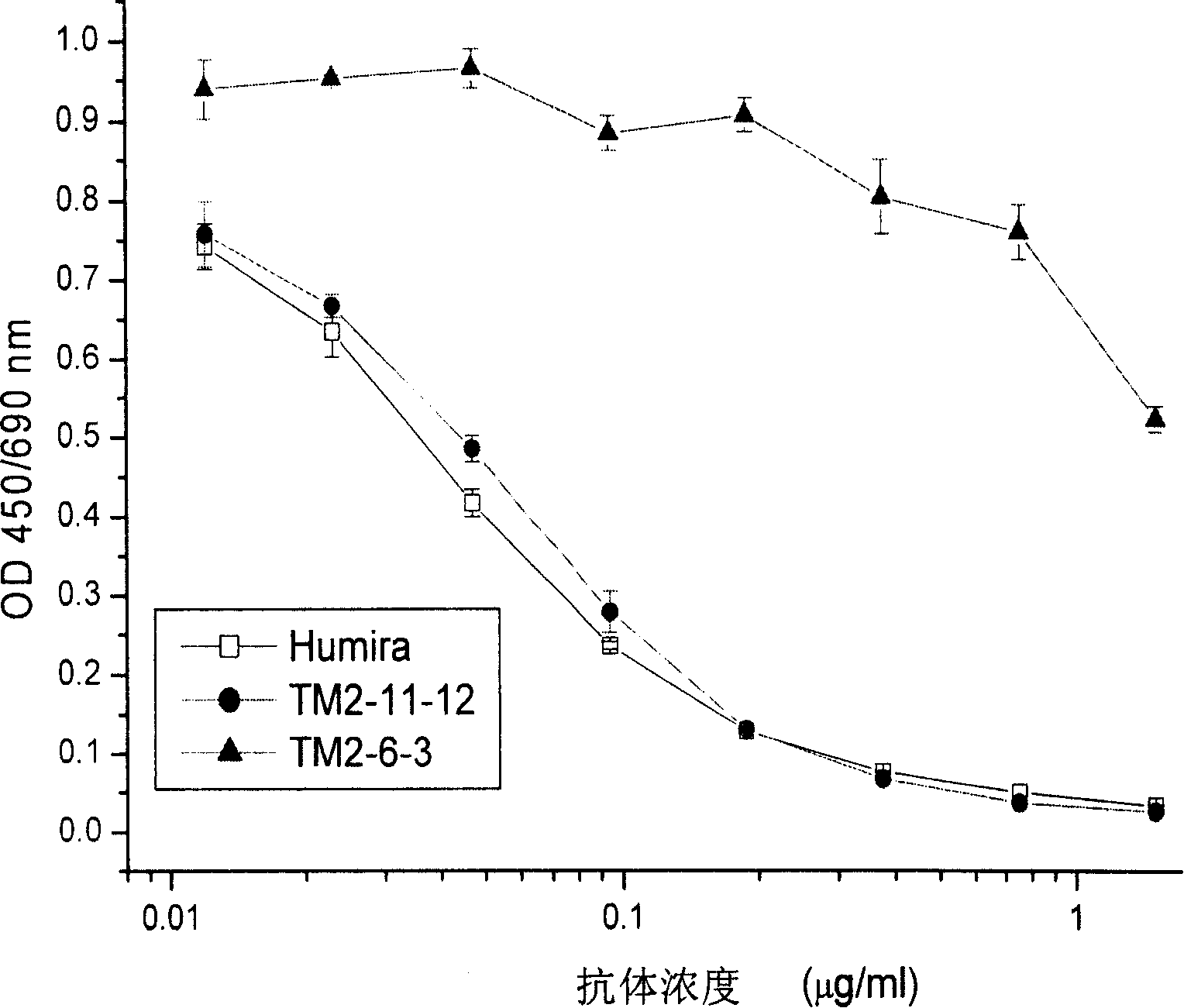
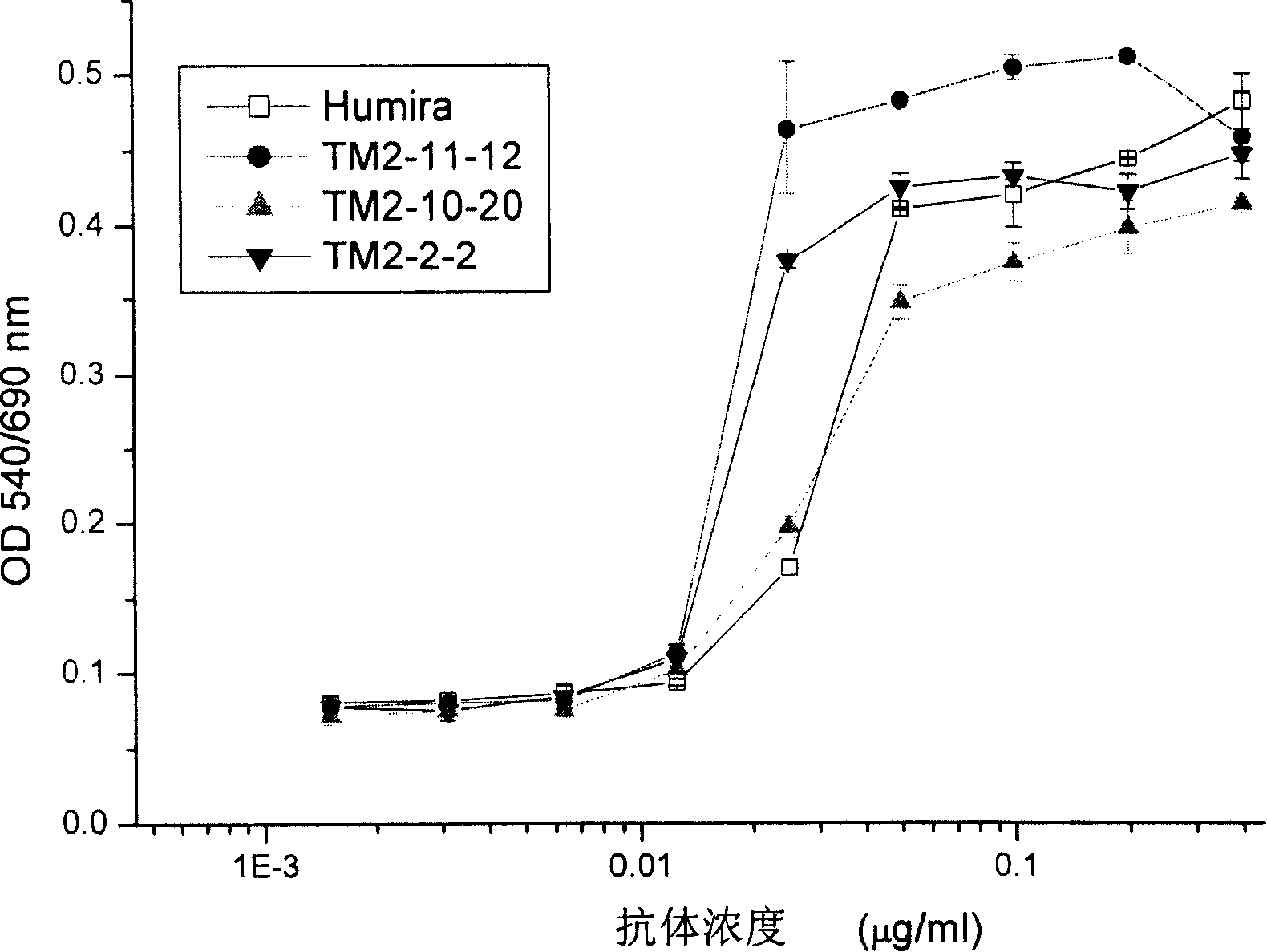
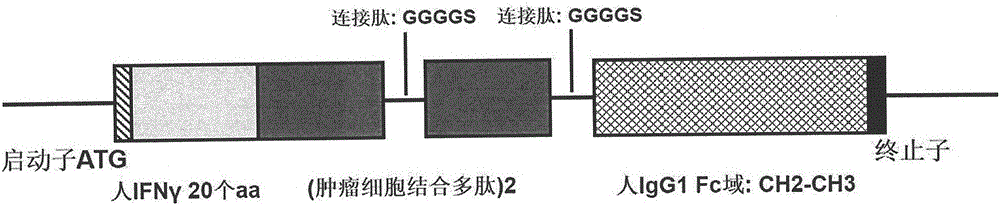
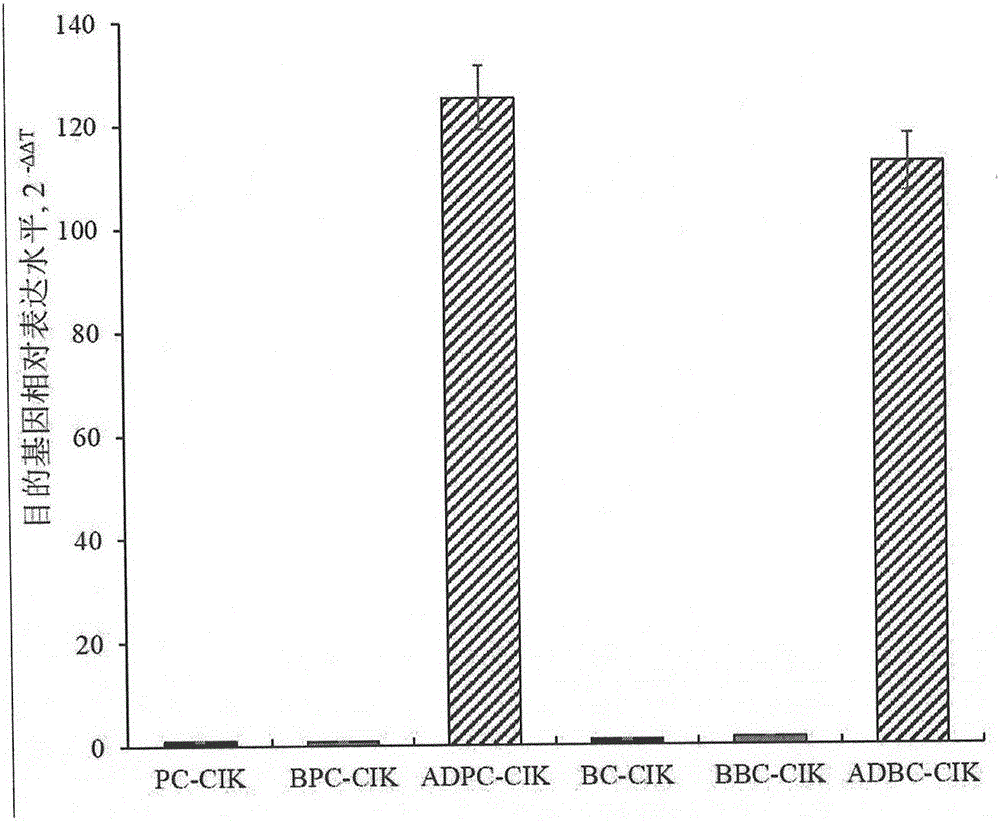
The invention provides a preparation method of a cytokine-induced killing cell for inducing antibody-dependent cellular cytotoxicity. The preparation method comprises constructing an interferon gamma signal peptide-tumor cell combined peptide, bonding 2th and 3th constant region gene segments of a crystallizable fragment domain heavy chain to the combined peptide, recombining the combined chain to a viral vector, transfecting a human cytokine-induced killing cell and carrying out high expression of a 2th and 3th constant region fusion protein of a novel polypeptide-crystallizable fragment domain heavy chain. The preparation method can cause antibody-dependent cellular cytotoxicity, improve a cytokine-induced killing cell proliferation multiple to 1000 times or more, a CD3+ / CD56+ expression rate to 40% or more and a CD16+ expression rate to 30% or more. In-vivo and in-vitro tests prove that the cytokine-induced killing cell has strong tumor killing toxicity. The invention also provides a kit for autologous cytokine-induced killing cell proliferation culture and has a high-efficiency anti-tumor function.
Owner:ZICHENG RUISHENGHUI BEIJING BIOTECH DEV CO LTD
Preparation and application of anti-human PCSK9 (pro-protein convertase subtilisin/kexin 9) antibody
The invention discloses an anti-human PCSK9 (pro-protein convertase subtilisin / kexin 9) chimeric antibody, and preparation and application thereof. The preparation method comprises the following steps: respectively amplifying mouse light chain and heavy chain variable region genes from mouse hybridoma cells, respectively carrying out chimerism with light chain and heavy chain constant region genes of human IgG, and carrying out recombination expression to obtain the human-mouse chimeric antibody. The human-mouse chimeric antibody has favorable affinity with human PCSK9, obviously inhibits the degradation activity of the PCSK9 for liver cell low-density lipoprotein receptors (LDLR), enhances the ingestion of liver cells for LDL-cholesterol (LDL-C), and lowers the cholesterol level in blood.
Owner:成都金洛克锶生物技术有限公司
Chimeric nucleic acid molecule and application thereof to humanized antibody preparation
ActiveCN105441455AHigh affinityImprove rearrangement efficiencyHybrid immunoglobulinsFermentationHumanized antibodyImmunoglobulin M
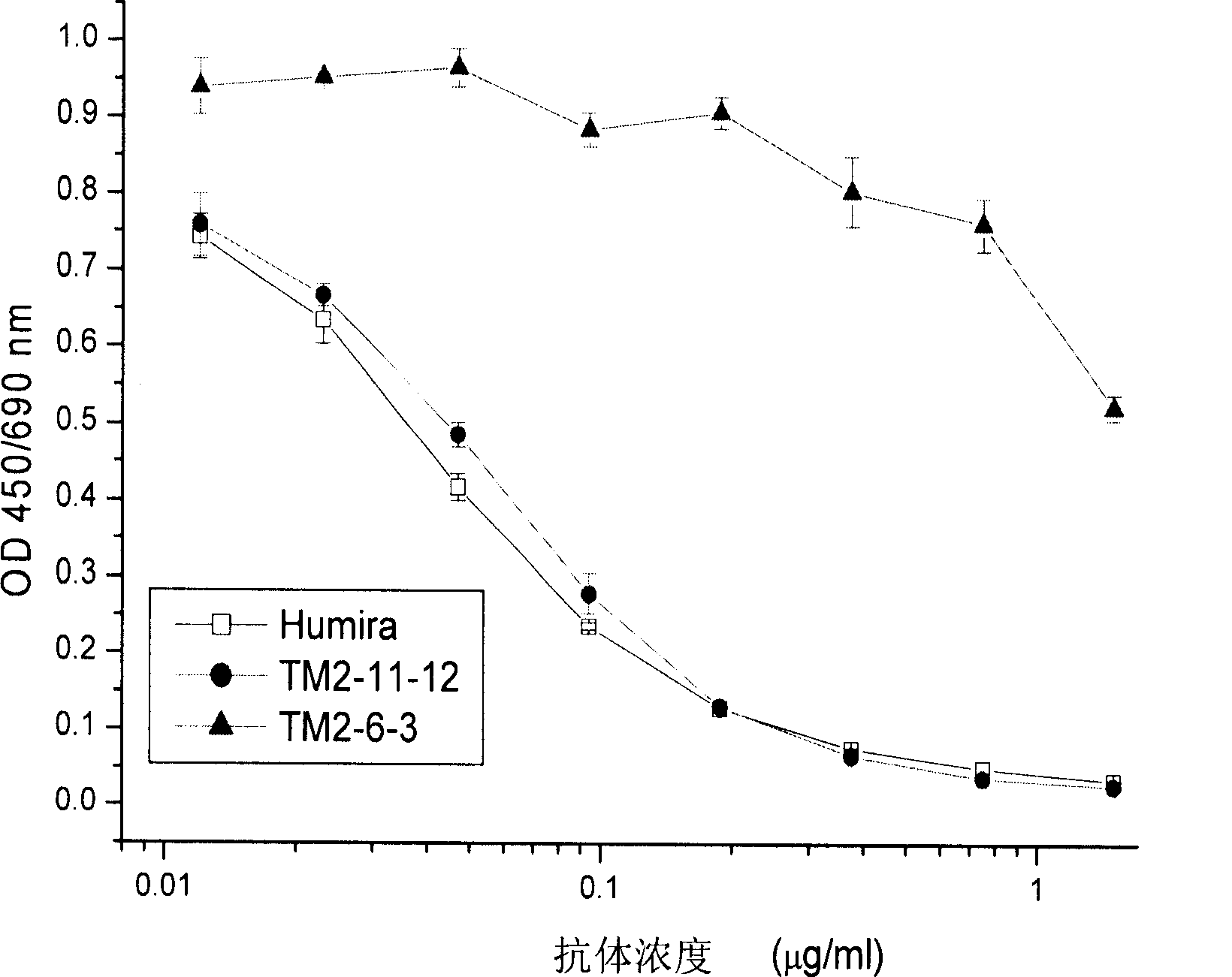
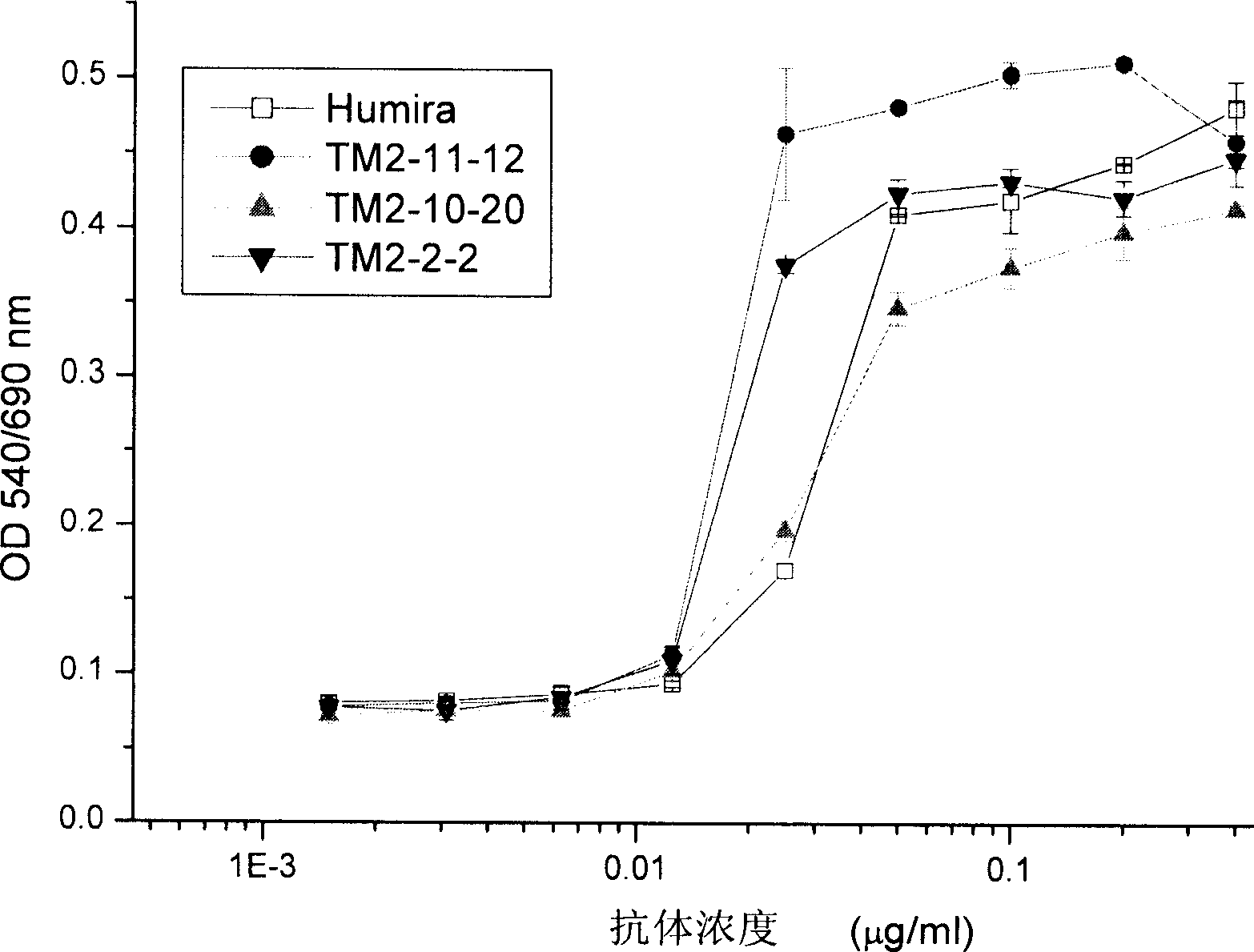
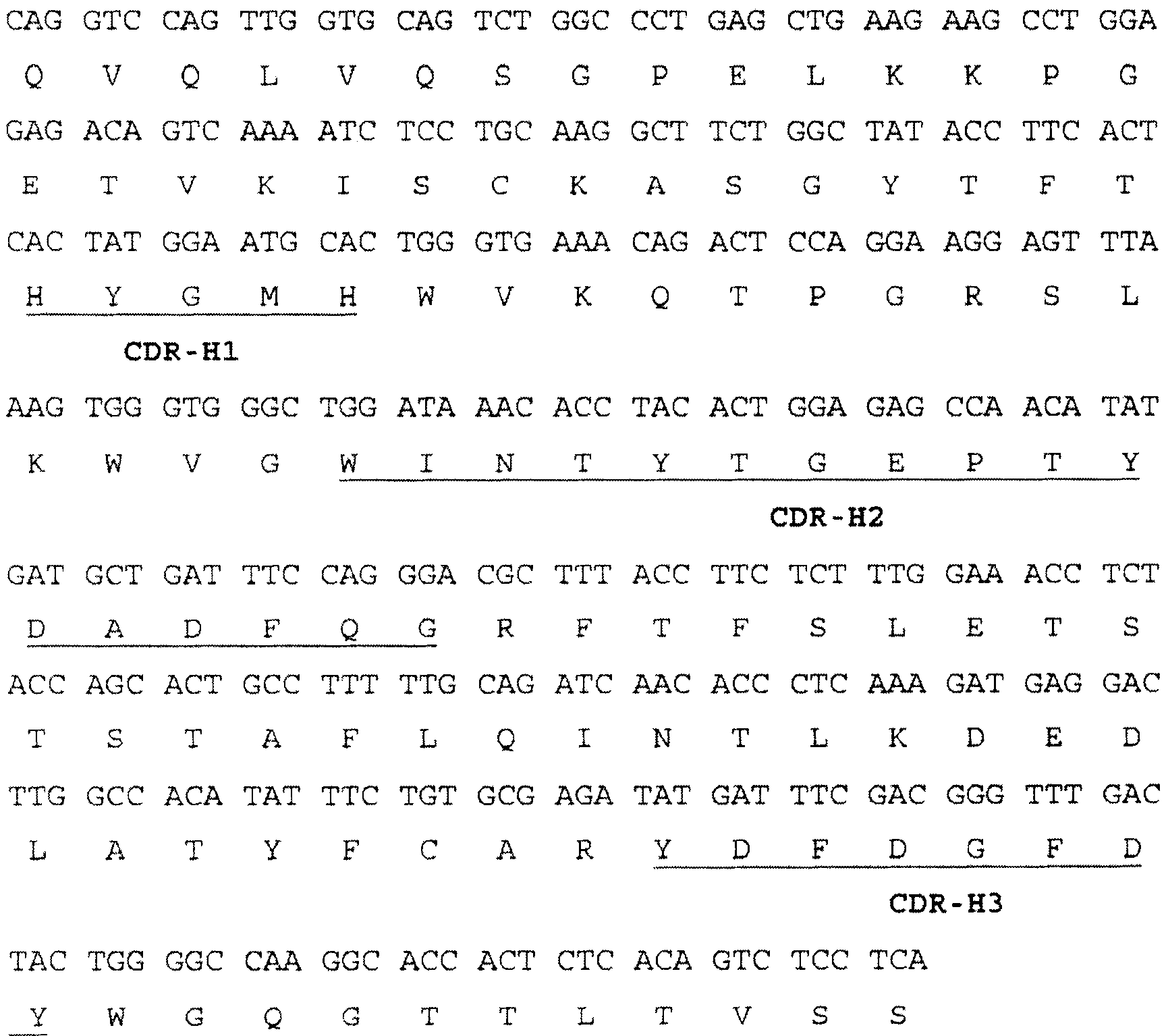
The invention provides a nucleic acid molecule. The nucleic acid molecule comprises human immunoglobulin genes or segments thereof and is characterized by also comprising gene segments in a host animal IgM (immunoglobulin M) constant region. The nucleic acid molecule can be used for efficiently preparing full humanized antibodies and has the effect of solving the problem of incompatibility of the interactions between BCRs (B cell receptors) of different species and Ig alpha and Ig beta. Meanwhile, the humanized antibodies expressed by the nucleic acid molecule are unnecessary to undergo second modification.
Owner:CHONGQING JINMAIBO BIOTEC CO LTD
Recombined chimeric antibody against human tumor necrosis factor alpha
ActiveCN101177453BExtended half-lifeFunctionalPeptide/protein ingredientsTumor necrosis factorDiseaseConstant region gene
The invention discloses an antibody combined with human tumor necrosis factor Alpha (hTNF a), juxtaposing out antibody heavy chain and nucleotide sequence of light chain variable region, and the amino acid sequence derived from the nucleotide sequence. The heavy chain and the light china are connected into chimeric genes with the genes in the human immunoglobulin gamma 1(hIgG1) heavy china constant region and in the kappa (k) light chain constant region respectively, then the carrier with the chimeric genes is introduced into the host cell line expressing antibodies protein. The restructuringchimeric antibody protein is tested outside human body and can neutralize the hTNF Alpha activity. The invention is suitable for treating disease of excessive harmful hTNF Alpha secretion, comprisinga plurality of inflammations such as rheumatoid arthritis and regional enteritis.
Owner:PHARMAB
FPV (feline parvovirus)-resistant cat-derived genetic engineering antibody
InactiveCN110283246AHigh activityPrevent agglutinationImmunoglobulins against virusesAntiviralsFeline parvovirusRed cell agglutination
The invention discloses an FPV (feline parvovirus)-resistant cat-derived genetic engineering antibody, and belongs to the technical field of antibody engineering. The antibody comprises a mouse-derived variable region and a cat-derived constant region, the nucleotide sequence of a heavy chain variable region of the antibody is represented as SEQ ID NO.1, the gene sequence of a heavy chain constant region of the antibody is represented as SEQ ID NO.5, the nucleotide sequence of a light chain variable region of the antibody is represented as SEQ ID NO.2, and the gene sequence of a light chain constant region of the antibody is represented as SEQ ID NO.6. The variable region sequence of a monoclonal antibody of a canine parvovirus is assembled with the cat-derived constant region, and the FPV-resistant cat-derived genetic engineering antibody is obtained, shows good FPV neutralization activity, has an effect of inhibiting red cell agglutination of the FPV, can be applied to the fields of cat-derived research for a canine parvovirus monoclonal antibody and the like and has great significance for promoting development of the cat-derived monoclonal antibody drugs.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Method for preparing pre-staining luminescent protein marker
InactiveCN107298719AAdd binding sitesOptical signal enhancementAntibody mimetics/scaffoldsG-proteinsBinding siteX-ray
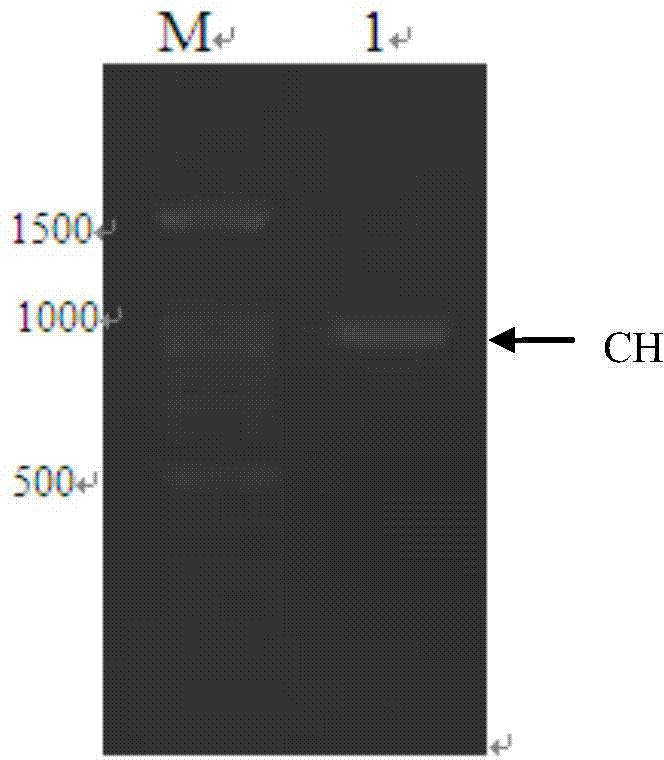
The invention provides a method for preparing a pre-staining luminescent protein marker. The method comprises the following steps: acquiring a protein A gene, a protein G gene, an MBP (Myelin Basic Protein) gene and a heavy chain constant region gene (CH gene) of a mouse and rabbit derived antibody, and cutting and combining the genes according to sizes of different proteins in a pre-staining luminescent protein marker; expressing the proteins, purifying, and mixing proteins of different molecule weights according to a certain ratio so as to obtain a luminescent protein marker; mixing the prepared luminescent protein marker with the pre-staining luminescent protein marker according to different ratios. The pre-staining luminescent protein marker prepared by using the method is capable of recognizing primary antibodies or secondary antibodies of different sources, binding sites of proteins are increased, light signals are intensified, not only are general positions of proteins indicated in the electrophoresis process and after membrane transfer, but also precise positions of the proteins can be indicated on X-ray films, no extra pre-staining marker channels are needed, no extra antibodies are needed, and the luminescent proteins can be combined with IgG or anti-rabbit and anti-mouse secondary antibodies derived from species such as human beings, rats, mice and rabbits.
Owner:南京赛诺博生物科技有限责任公司
Targeting-fusion DNA vaccine for caries-prevention, and its prepn. method
InactiveCN1459317ASimplify production stepsImprove immunityGenetic material ingredientsDigestive systemEscherichia coliHinge region
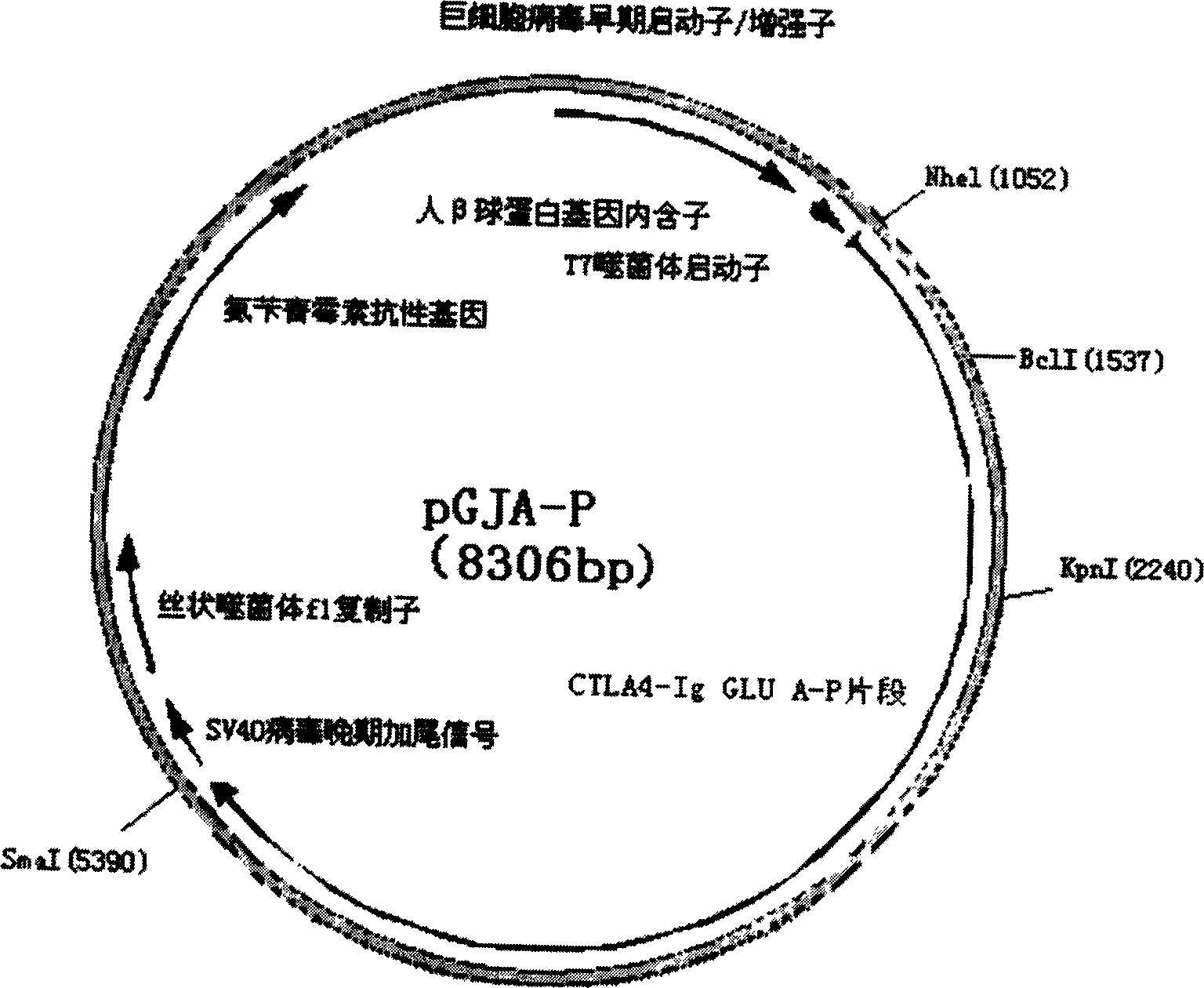
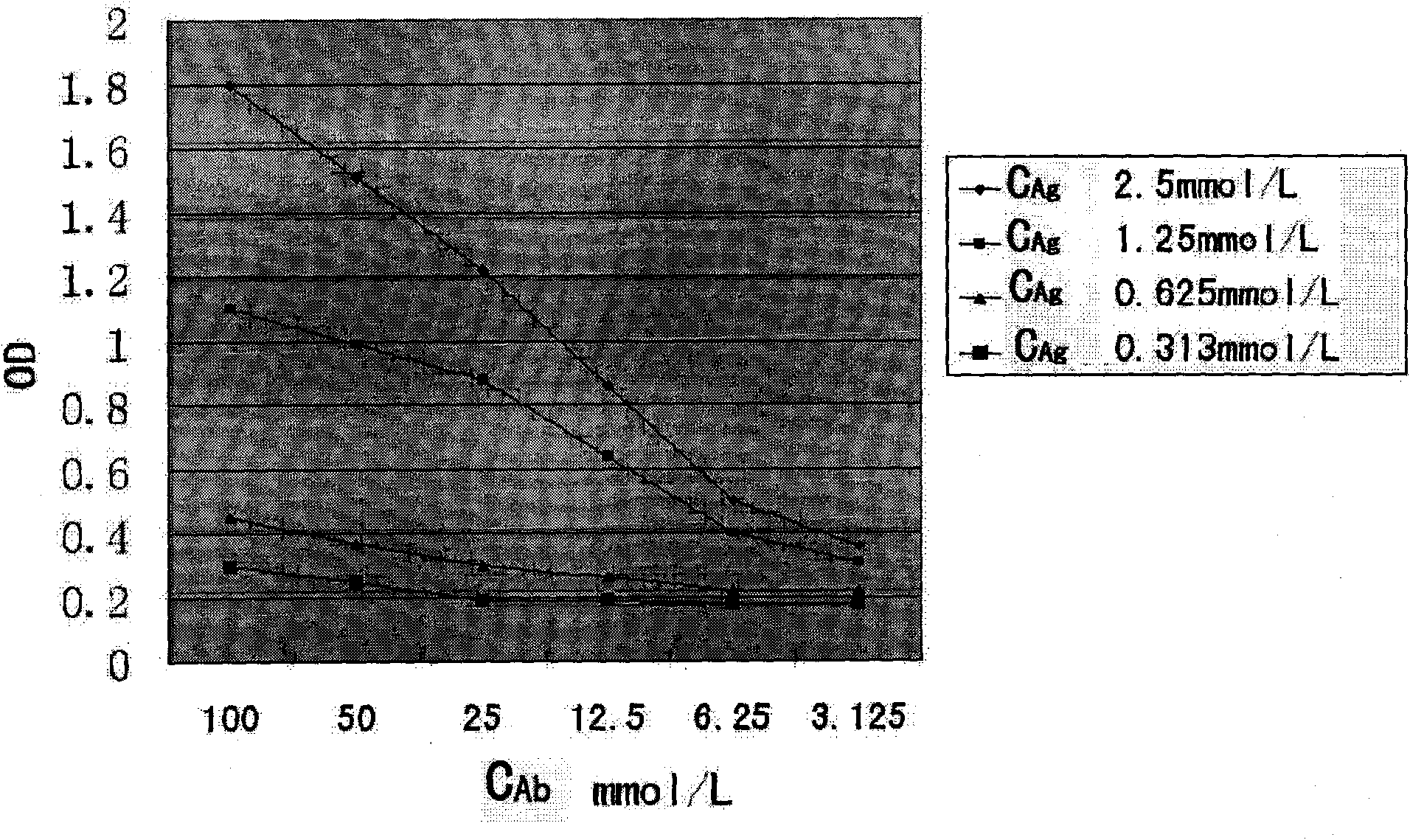
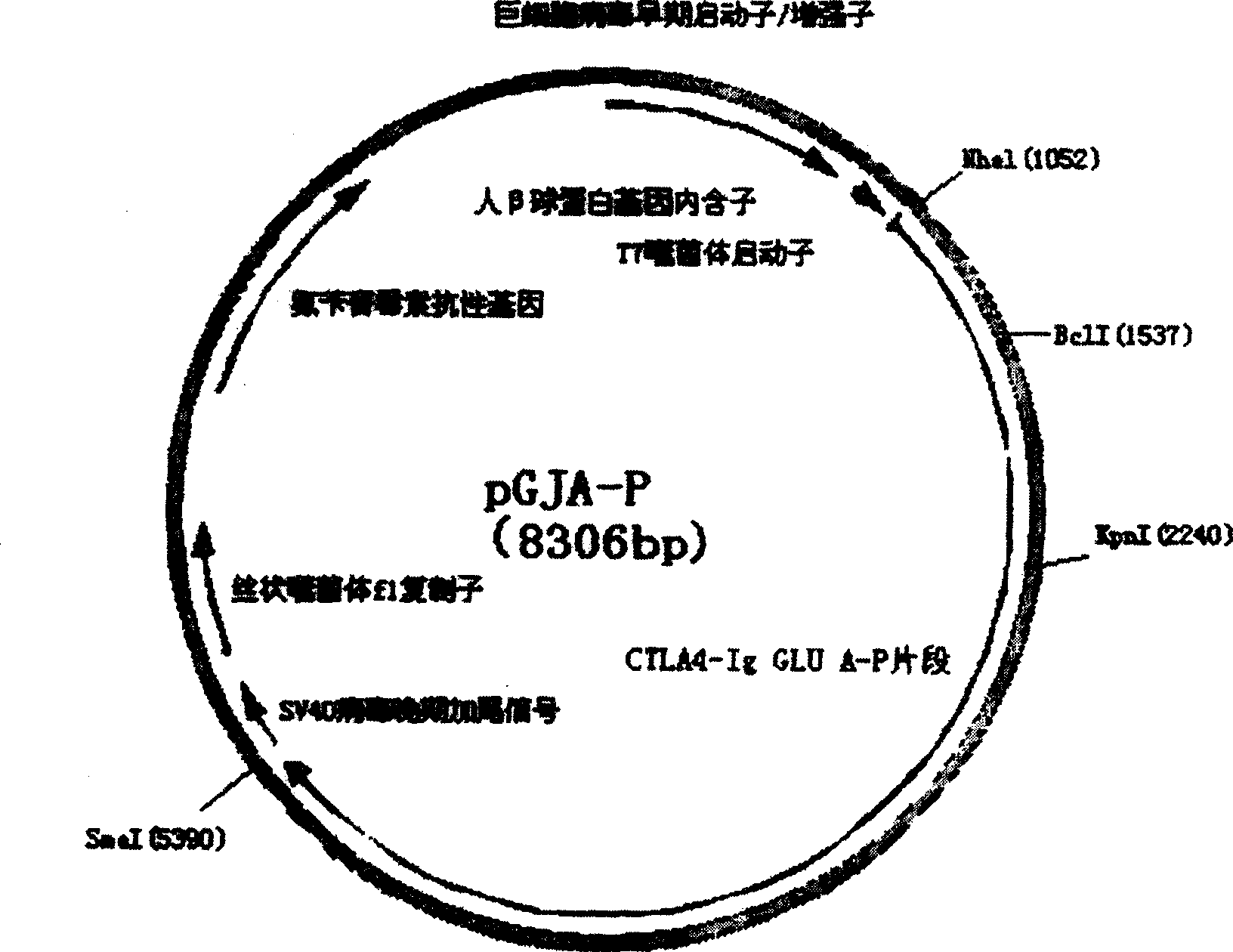
A target fused DNA vaccine pGJA-P for preventing decayed tooth is prepared by using the PCR process through amplifying the antigen 4 signal peptide of toxic T lymphocyte, the sequence of extracellular region, and the genes in Ig gamma 1 hinge region and constant region, respectively cloning them to pU cm-T carrier to configune pGTLA and pJIg, linking them to configure their recombinant plasmid, verifying the correctness of inserted fragments, and inserting the fusion gene in the front end of pGLUA-P. Its advantages are simple process, high safety, low cost, and high effect to improve immunity.
Owner:WUHAN UNIV
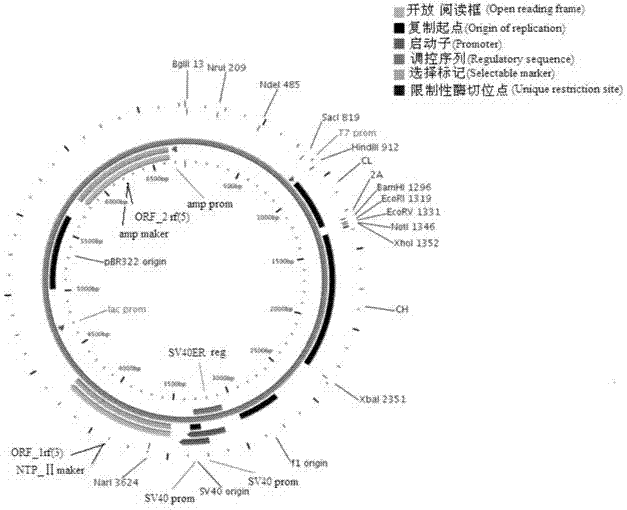
Anticancer genetic engineering bivalent antibody, preparation method thereof, and anticancer genetic engineering drug
InactiveCN103804495AAvoid infectionEffective infectionImmunoglobulins against animals/humansAntibody ingredientsWilms' tumorGenetic engineering
The invention relates to an anticancer genetic engineering bivalent antibody, a preparation method thereof, and an anticancer genetic engineering drug. The anticancer genetic engineering bivalent antibody comprises a constant region, and SIRPa and B7 connected with the constant region. The preparation method of the anticancer genetic engineering bivalent antibody comprises following steps: acquirement of constant region gene, SIRPa gene, and B7 gene; construction of an expression plasmid containing the three genes; transfection of the expression plasmid into expression cell strain for culturing; and obtaining of the bivalent antibody via separation and purification. The bivalent antibody comprises both SIRPa and B7 which are capable of realizing specific binding with CD47 locus of tumor cells and CD28 locus of lymphocyte; specific binding performance is improved greatly; double inhibition on body tumor cells is realized; the preparation method is simple and practicable; tumor cells can be influenced by the anticancer genetic engineering drug containing the anticancer genetic engineering bivalent antibody effectively; and the anticancer genetic engineering drug is capable of preventing and treating tumor diseases effectively.
Owner:SHENZHEN UNIV
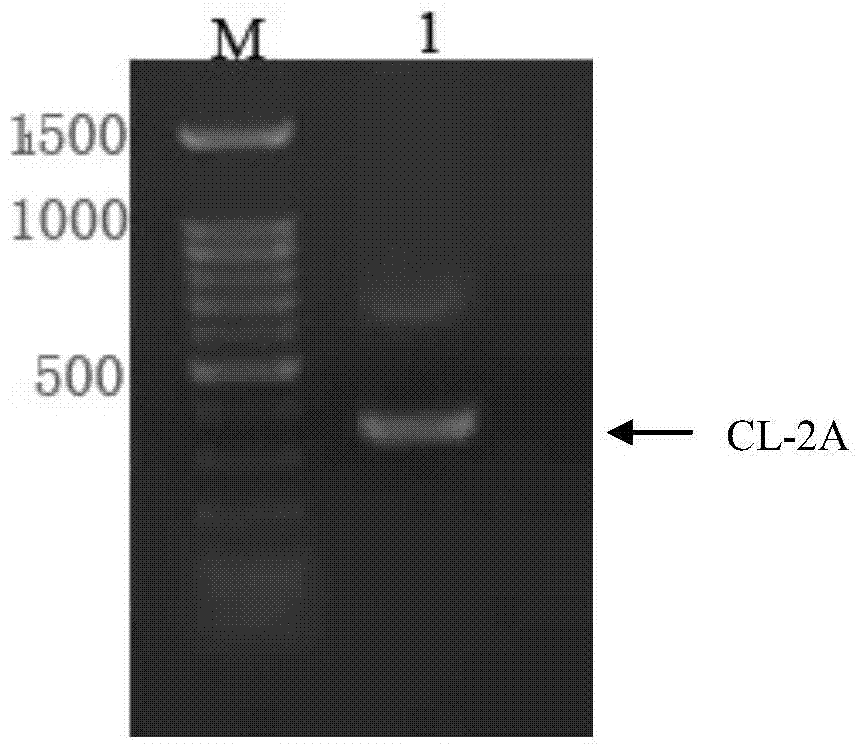
Humanized antibody expression vector and construction method thereof
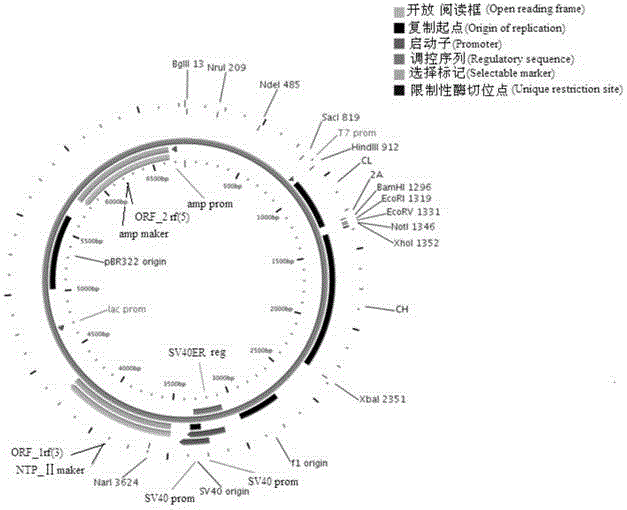
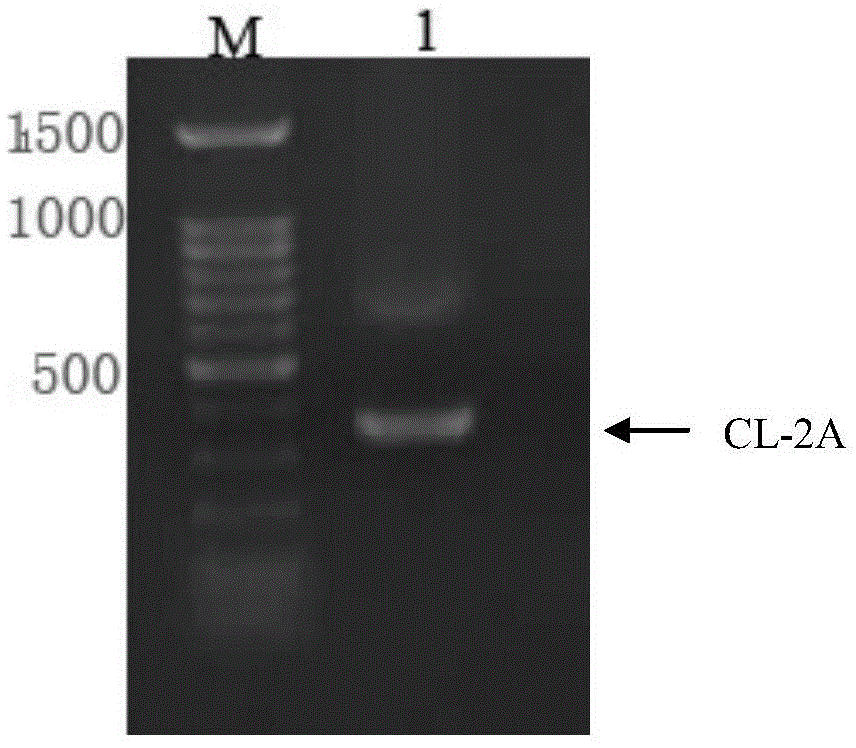
ActiveCN103898141AImprove shear efficiencyImprove balanceVector-based foreign material introductionAntibody expressionHumanized antibody
The invention discloses a humanized antibody expression vector and a construction method thereof. The construction method comprises the steps of: respectively connecting a 2A sequence-containing light chain constant region gene sequence CL-2A and a heavy chain constant region gene sequence CH with a pMD18-T vector to obtain a pMD18-T-CL-2A plasmid and a pMD18-T-CH plasmid; connecting the pMD18-T-CL-2A plasmid and a eukaryotic expression vector after being subjected to double digestion to obtain a CL-2A-containing eukaryotic expression vector; and then connecting the CL-2A-containing eukaryotic expression vector with a pMD18-T-CH plasmid to obtain a CL-2A-CH-containing eukaryotic expression vector. The construction method of the expression vector disclosed by the invention can be used for rapidly constructing a full-length complete antibody sequence only by inserting a variable region sequence of a mouse or a humanized antibody into a corresponding locus and rapidly constructing a chimeric antibody, the humanized antibody and the like, and is simple and practical.
Owner:SHENZHEN ZHONGKE AMSHENN MEDICINE CO LTD
Anti-p185<erbB2> human-mouse chimeric antibody lentivirus expression vector and construction method thereof
InactiveCN102618582ALarge capacityNo toxicityFermentationVector-based foreign material introductionEnzyme digestionChimeric antibody
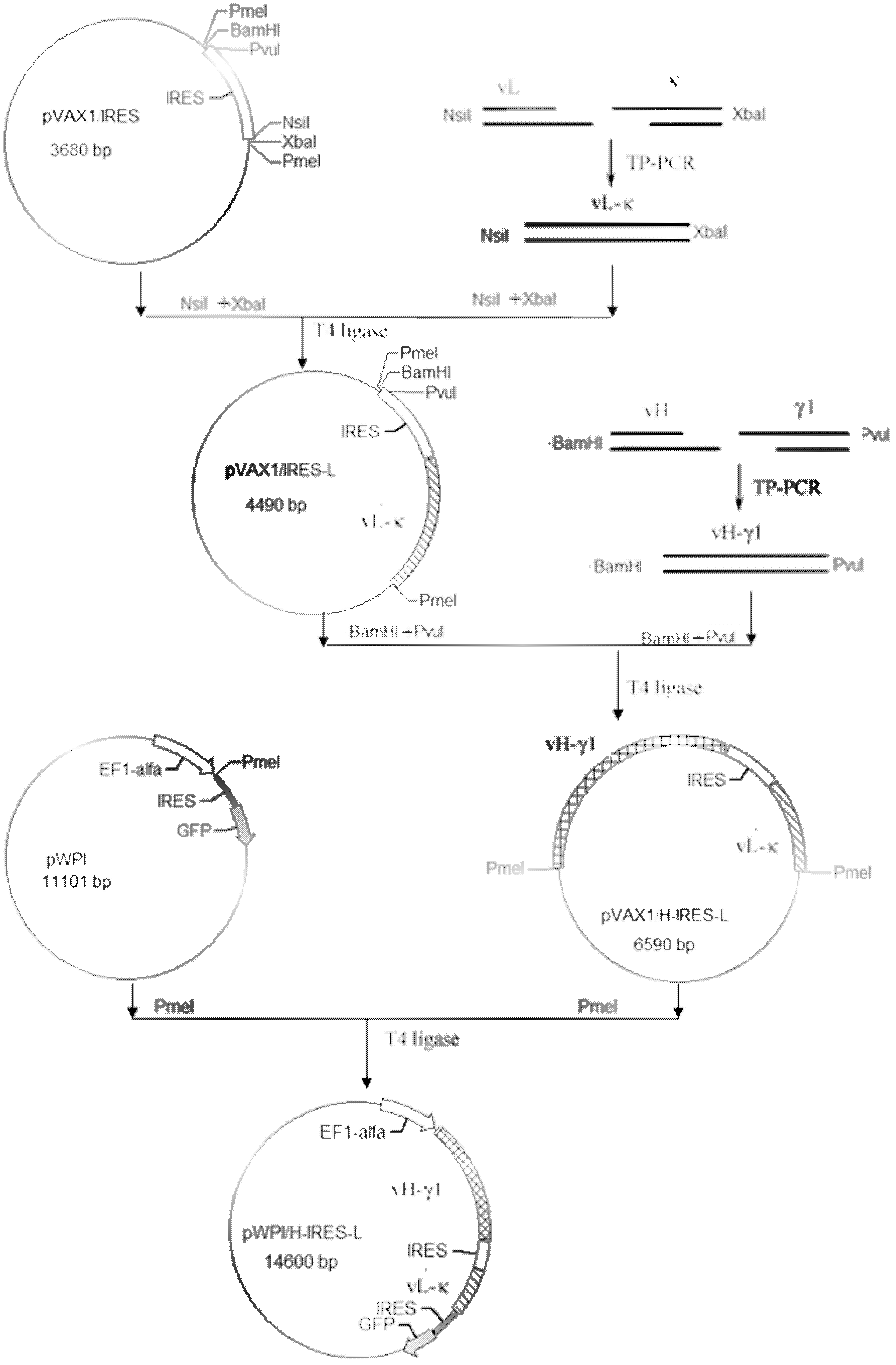
The invention discloses an anti-p185<erbB2> human-mouse chimeric antibody lentivirus expression vector and a construction method thereof. Antihuman-p185<erbB2> mouse-derived monoclonal antibody variable region genes (vH and vL) and human-IgG1 constant region genes (gamma 1 and kappa) are obtained by amplification by PCR (polymerase chain reaction) process. The vH is spliced with the gamma 1 and the vL is spliced with the kappa by three-primer PCR process so as to obtain a chimeric heavy chain (H) gene and a chimeric light chain (L) gene. The chimeric heavy chain (H) gene and the chimeric light chain (L) gene are inserted onto an IRES (internal ribosome entry site) element of plasmid pVAX1 / IRES downstream and upstream respectively. H-IRES-L is cut off from the pVAX1 / H-IRES-L using endonucleases and is inserted into the lentivirus expression vector pWPI so as to construct a lentivirus expression pWPI / H-IRES-L. Corresponding enzyme digestion and sequencing appraisal show that the expression is consistent to that of the expected design. After transfection of cells 293T, the chimeric heavy chain (H) gene and the chimeric light chain (L) gene are co-expressed, and the chimeric antibody can be combined with p185<erbB2> molecular specificity. By the vector and the construction method, basis is laid for anti-p185<erbB2> engineering antibodies in the future.
Owner:广西壮族自治区肿瘤防治研究所
Anti-human immunodeficiency virus (HIV) gene engineering recombinant virus and preparation method thereof, and anti-HIV gene engineering medicament
InactiveCN102690792AEffective infectionAvoid infectionViral/bacteriophage medical ingredientsAntiviralsHIV receptorHuman immunodeficiency
The invention relates to an anti-HIV gene engineering recombinant virus and a preparation method thereof, and an anti-HIV gene engineering medicament. The anti-HIV gene engineering recombinant virus comprises a constant region of a human antibody, and a cluster of differentiation 4 (CD4) and a chemokine receptor 5 (CCR5) which are connected with the constant region. The preparation method for the anti-HIV gene engineering recombinant virus comprises the steps as follows: obtaining a heavy chain constant region gene of the human antibody, a light chain constant region gene of the human antibody, a CD4 gene and a CCR5 gene; constructing a virus vector shuttle plasmid comprising the four genes; co-transforming the shuttle plasmid and a virus auxiliary plasmid to generate a recombinant virus plasmid; and transferring the recombinant virus plasmid to a cell strain for culturing and purifying the virus. The recombinant virus has both the CD4 and CCR5 capable of specifically binding with the CD4 site and CCR5 site of an HIV virus, an obviously enforced specific binding effect, and the function of doubly preventing the HIV virus from infecting a host cell. The preparation method is simple and practical. The anti-HIV gene engineering medicament with the recombinant virus can effectively act on the HIV virus, and can further effectively prevent and treat the infection of the HIV virus.
Owner:SHENZHEN UNIV
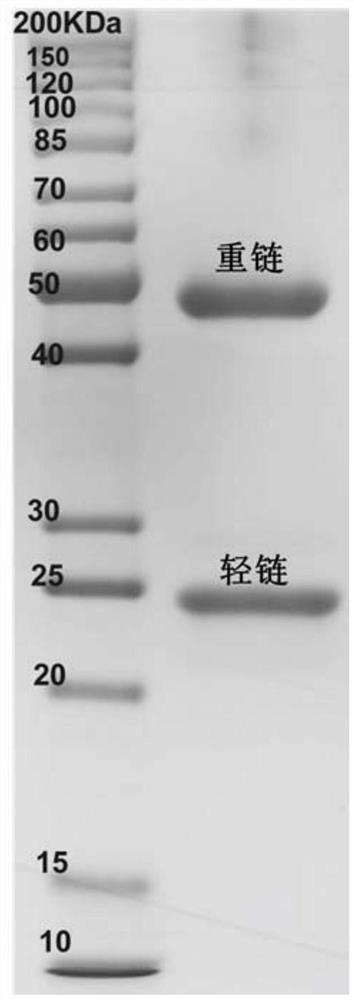
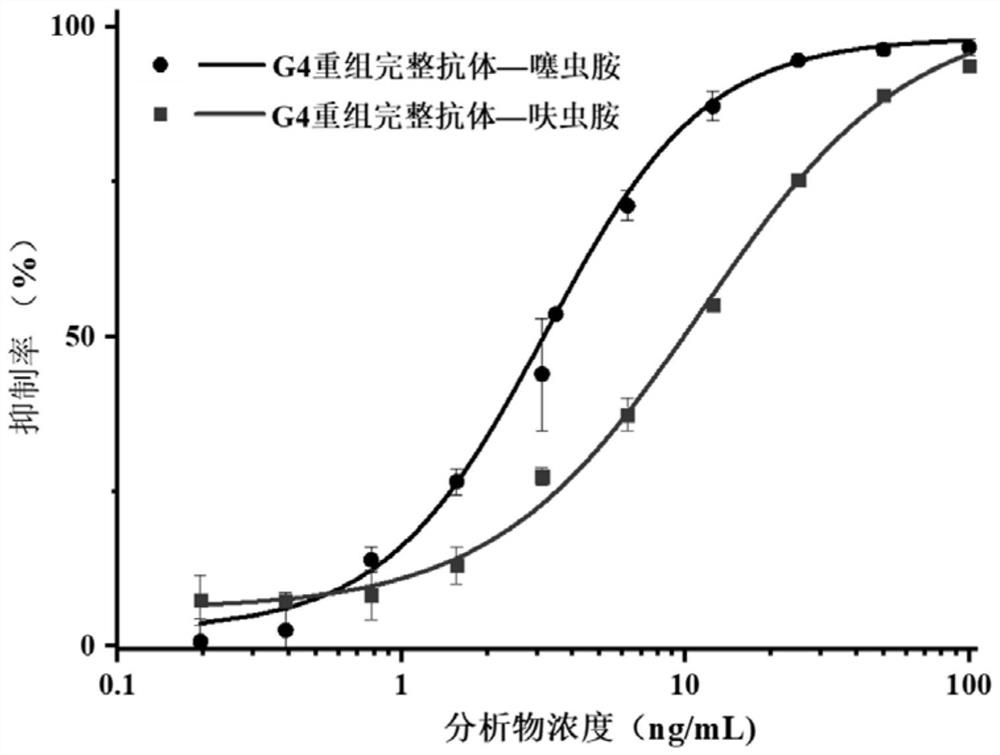
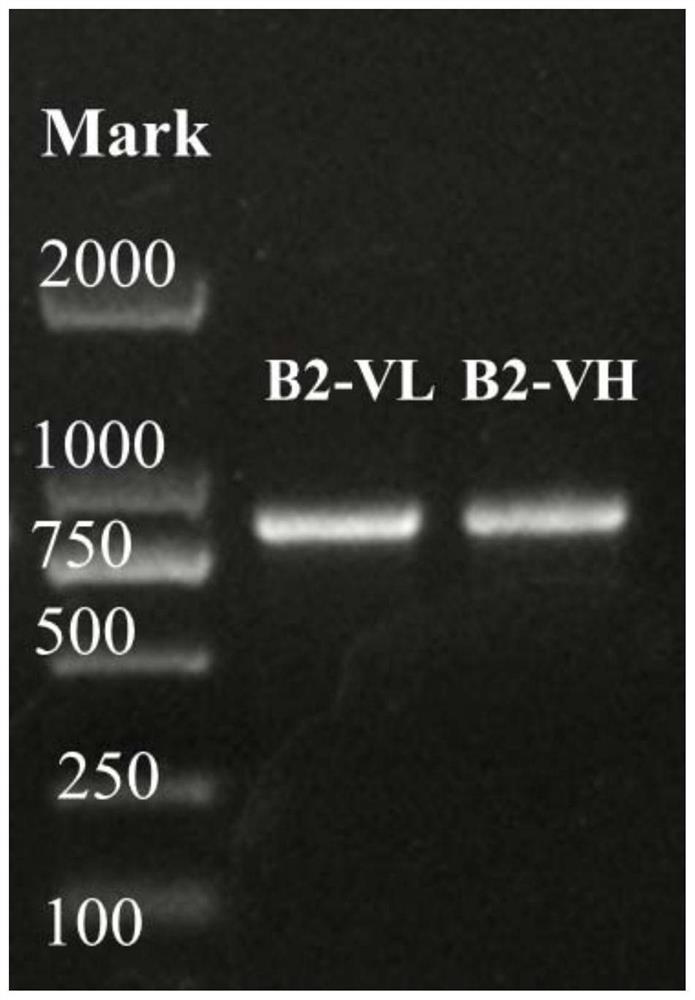
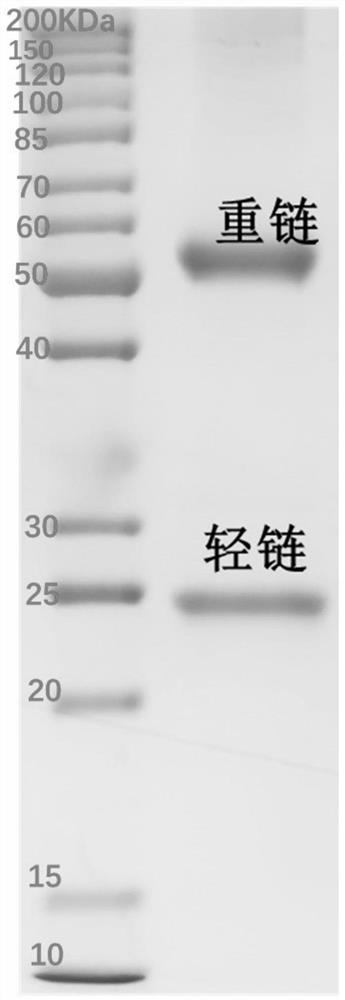
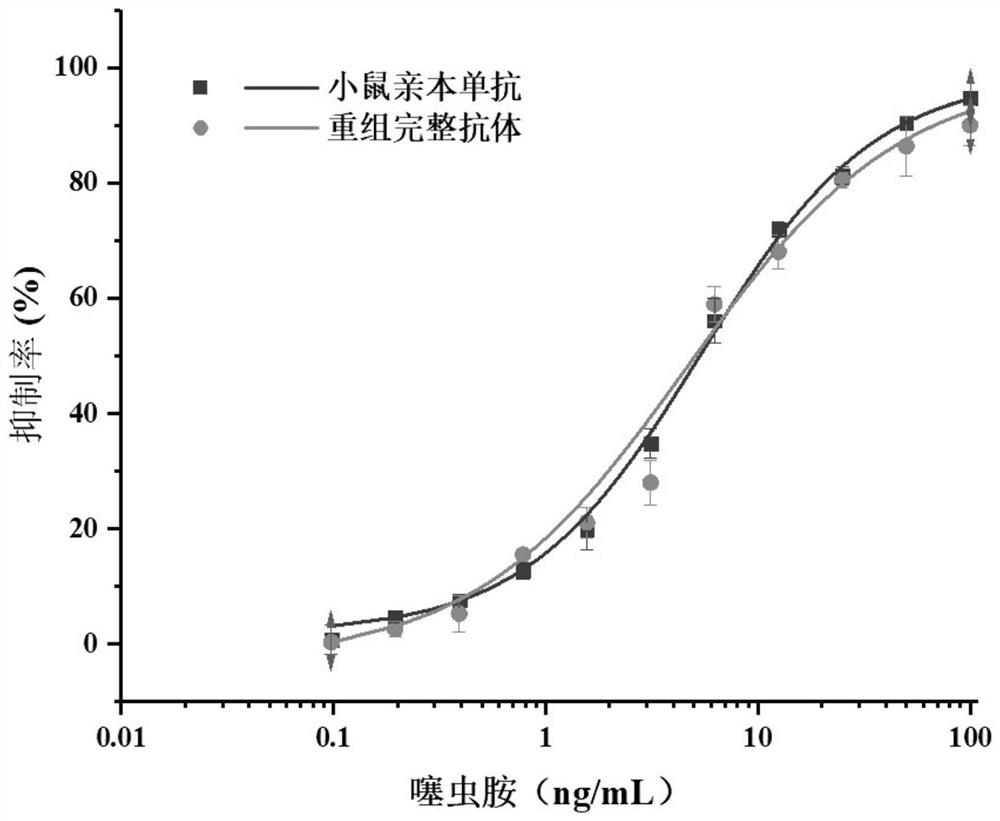
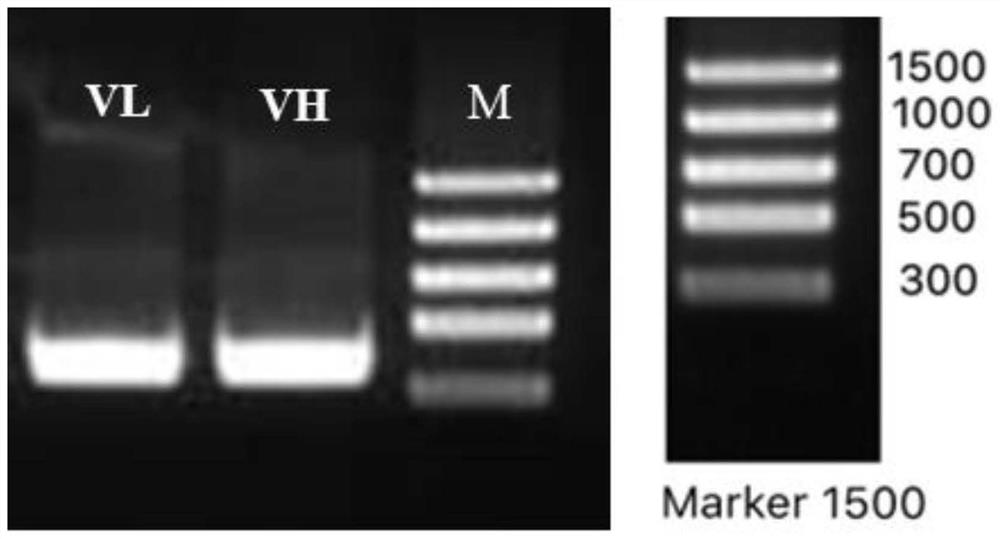
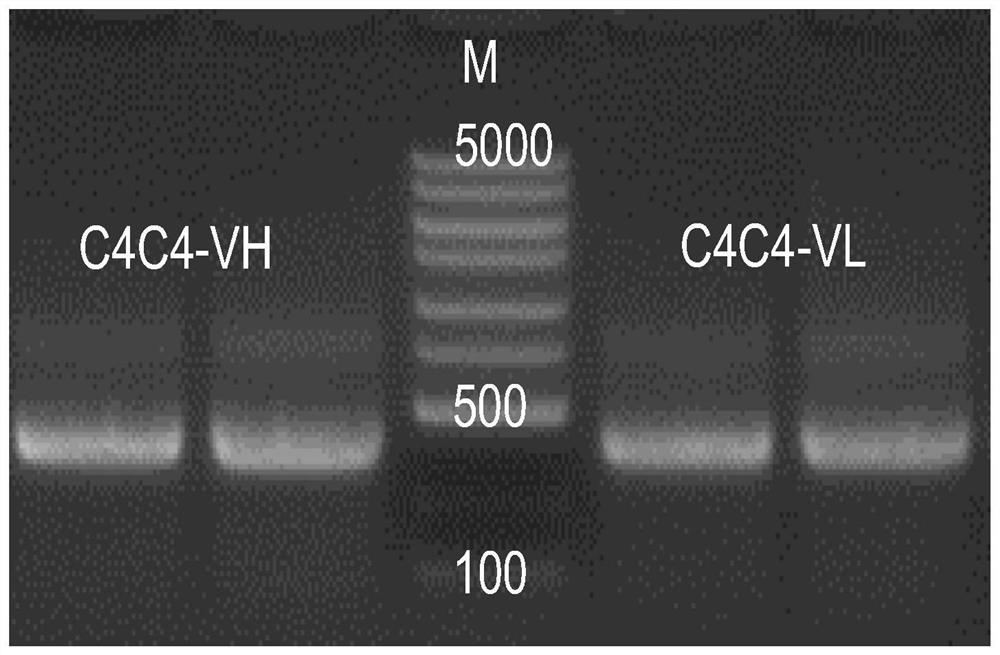
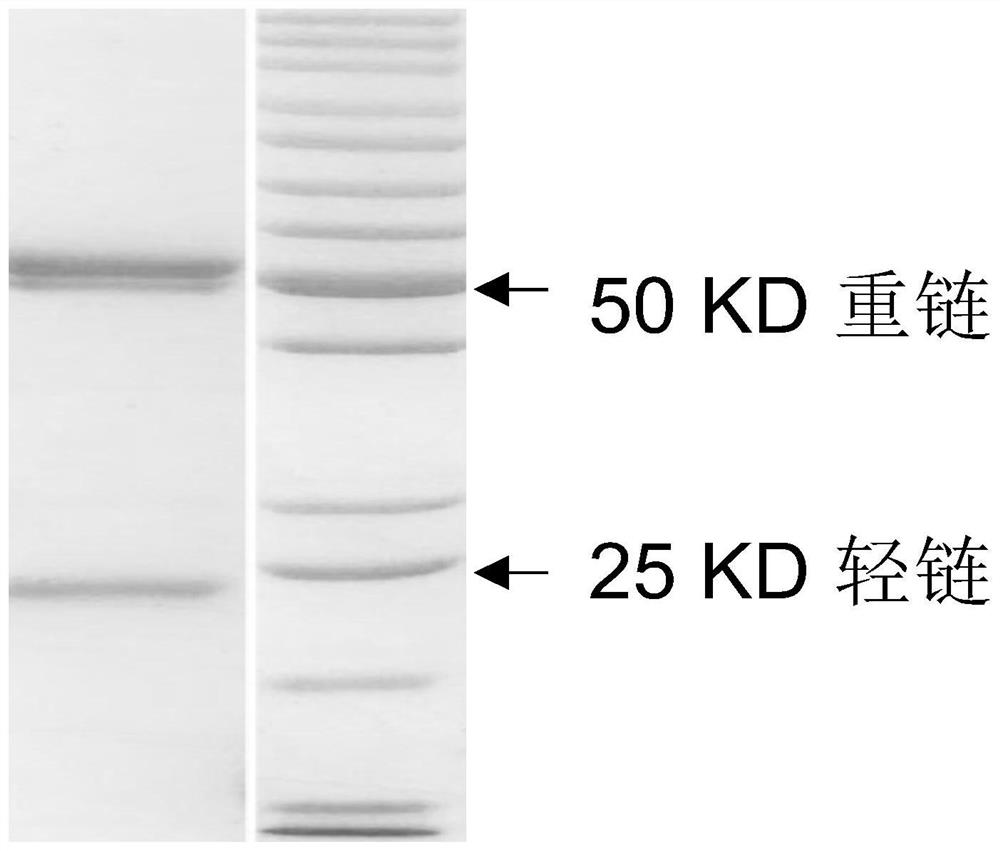
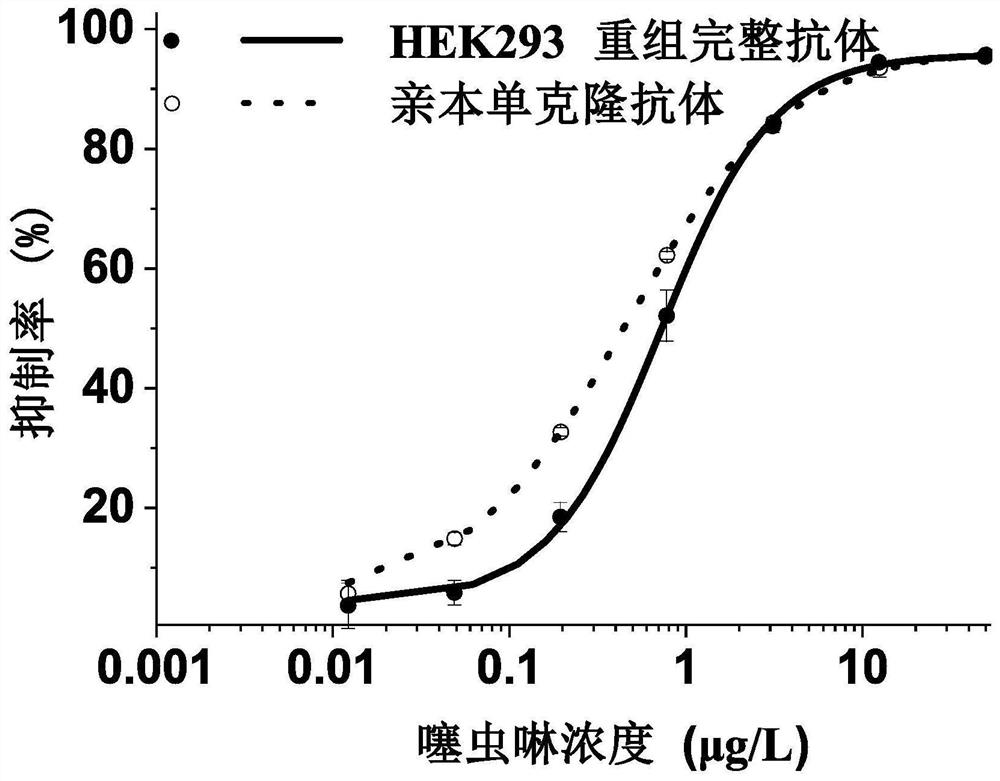
Variable region sequence of broad-spectrum antibody resisting clothianidin and dinotefuran and preparation of recombinant complete antibody of variable region sequence
ActiveCN112062852AHigh purityAvoid mutationImmunoglobulinsFermentationImmune profilingConstant region gene
The invention discloses a variable region sequence of a broad-spectrum antibody resisting clothianidin and dinotefuran. The amino acid sequence of a heavy chain variable region coding gene is shown asSEQ ID NO: 2. The invention also discloses a broad-spectrum recombinant complete antibody resisting clothianidin and dinotefuran. The broad-spectrum recombinant complete antibody resisting clothianidin and dinotefuran comprises a heavy chain constant region, a heavy chain variable region, a light chain constant region and a light chain variable region, and the amino acid sequence of the heavy chain variable region coding gene is shown as SEQ ID NO: 2. The sequence genes obtained by the invention are respectively connected to an expression vector containing a heavy chain constant region gene and a light chain constant region gene, and a recombinant complete antibody is obtained by adopting mammalian cell expression of a double-plasmid system, so that the recognition activity of a murine parent antibody is ensured, the broad-spectrum antibody resisting clothianidin and dinotefuran can be stably produced on a large scale, and a reliable core reagent is provided for various immunoassay methods for synchronously detecting the clothianidin and the dinotefuran.
Owner:ZHEJIANG UNIV
Bifunctional antibody, construction method thereof, and bifunctional antibody gene engineering drug
InactiveCN104342452AStrong targetingHybrid peptidesVector-based foreign material introductionProtein targetConstant region gene
The invention provides a bifunctional antibody and a construction method thereof. The bifunctional antibody comprises an antibody heavy chain constant area, an antibody light chain constant area, a first target protein, which is connected to the antibody heavy chain constant area, and a second target protein, which is connected to the antibody light chain constant area. The construction method of the bifunctional antibody comprises the following steps: individually obtaining the antibody heavy chain constant area gene, antibody light chain constant area gene, the first target gene, and the second target gene; adopting expression carriers to construct recombinant carrier shuttle plasmids of the genes mentioned above; transfecting the recombinant carrier shuttle plasmids to an expression bacterium strain to carry out culture so as to obtain the bifunctional antibody; carrying out centrifugation, and collecting the supernate or purifying the cells so as to obtain the bifunctional antibody. The construction method assembles antibody molecules in a gene level, thus the pertinence is strong, and the method is easy to achieve. The obtain bifunctional antibody comprises two antigen combining sites, so the bifunctional antibody can combine two different antigens at the same time.
Owner:SHENZHEN UNIV
Variable region sequence of specific anti-clothianidin antibody, and preparation and application of recombinant complete antibody thereof
ActiveCN112194725AStable production in large quantitiesGuaranteed recognition activityHybrid immunoglobulinsBiological material analysisImmune profilingAntiendomysial antibodies
The invention discloses a variable region sequence of a specific anti-clothianidin antibody. The amino acid sequence of a heavy chain variable region coding gene is shown as SEQ ID NO:2. The inventionalso discloses an anti-clothianidin recombinant complete antibody, which comprises a heavy chain constant region, a heavy chain variable region, a light chain constant region and a light chain variable region; and the amino acid sequence of the heavy chain variable region coding gene is shown as SEQ ID NO:2. The invention also discloses an antibody expression plasmid. The invention further discloses an immune test strip for rapidly detecting clothianidin residues. The sequence gene obtained by the invention is respectively connected to an expression vector containing a heavy chain constant region gene and a light chain constant region gene; the recombinant complete antibody is obtained by adopting mammalian cell expression of a double-plasmid system, so that the recognition activity of amouse parent antibody is ensured; the anti-clothianidin antibody can be stably produced in batches; and a reliable core reagent is provided for various immunoassay methods for clothianidin residue detection.
Owner:ZHEJIANG UNIV
Variable region sequence of specific anti-chlorothalonil antibody and recombinant full-length IgG antibody thereof
ActiveCN113563472AHigh sensitivityHigh selectivityImmunoglobulinsVector-based foreign material introductionImmune profilingAntiendomysial antibodies
The invention discloses a variable region sequence of a specific anti-chlorothalonil antibody. The amino acid sequence of a heavy chain variable region coding gene is shown in SEQ ID NO: 2. The invention also discloses an anti-chlorothalonil recombinant full-length IgG antibody. The invention also discloses a recombinant antibody expression plasmid. The heavy chain variable region and light chain variable region sequences of the anti-chlorothalonil recombinant full-length IgG antibody are derived from a monoclonal cell strain which secretes a high-affinity and high-specificity anti-chlorothalonil antibody and is obtained by immunizing mice for multiple times, performing cell fusion and screening. The sequence genes are respectively connected to expression vectors containing a mouse IgG1 heavy chain constant region gene and a mouse kappa light chain constant region gene, the recombinant full-length IgG antibody is obtained by expression of mammalian cells of a double-plasmid system, the recognition activity of the recombinant full-length IgG antibody is very close to that of a mouse-derived parent antibody, large-scale standardized production of the anti-chlorothalonil antibody can be realized, and reliable core raw materials are provided for various immunoassay methods for rapid detection of chlorothalonil residues.
Owner:ZHEJIANG UNIV +1
Variable region sequence of specific anti-thiacloprid antibody and anti-thiacloprid recombinant overall-length antibody
ActiveCN112111011AHigh sensitivityHigh selectivityImmunoglobulinsFermentationImmune profilingAntiendomysial antibodies
The invention discloses a variable region sequence of a specific anti-thiacloprid antibody. The amino acid sequence of a heavy chain variable region encoding gene is disclosed by SEQ ID NO: 2. The invention also discloses an anti-thiacloprid recombinant overall-length antibody, which comprises a heavy chain constant region, a heavy chain variable region, a light chain constant region and a light chain variable region, wherein the amino acid sequence of the heavy chain variable region encoding gene is disclosed by SEQ ID NO: 2. The invention also discloses an antibody expression plasmid. A sequence gene obtained by the invention is independently connected to expression carriers containing a heavy chain constant region gene and a light chain constant region gene, mammal cell of a double-plasmid system is adopted to obtain the recombinant overall-length antibody, the identification activity of a murine parent antibody is guaranteed, the anti-thiacloprid antibody can be stably produced ona large scale, and a reliable core reagent is provided for various immunoassay methods for detecting thiacloprid residues.
Owner:ZHEJIANG UNIV
Single chain antibody of human anti-placenta growth factor
InactiveCN101643508AHigh affinityImmunoglobulins against growth factorsPeptide preparation methodsAntigenCDNA library
The invention relates to a single chain antibody of human anti-placenta growth factor, which is encoded by a heavy-chain gene with an SEQ NO.1 sequence and light-chain gene with an SEQ NO.2 sequence.The invention also provides a preparation method thereof, comprising the following steps in sequence: amplifying human total RNA heavy-chain variable region, light-chain variable region and constant region gene of the whole set by a PCR technique, splicing and constructing an overall-length single-chain antibody cDNA library; expressing the cDNA library by an externally-coupled transcription / translation system to obtain an antibody-ribosome-mRNA compound; performing affiliation selection and clution on the compound by a magnetic bead coated by specific antigen; amplifying selected destinationantibody gene by the PCR technique; and expressing the amplified antibody gene using the externally-coupled transcription / translation system repeatedly to obtain the single-chain antibody. The antibody is adapted to prepare medicament for treating ovarian cancer.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Targeting-fusion DNA vaccine for caries-prevention, and its prepn. method
InactiveCN100462104CSimplify production stepsImprove immunityGenetic material ingredientsDigestive systemEscherichia coliHinge region
A target fused DNA vaccine pGJA-P for preventing decayed tooth is prepared by using the PCR process through amplifying the antigen 4 signal peptide of toxic T lymphocyte, the sequence of extracellular region, and the genes in Ig gamma 1 hinge region and constant region, respectively cloning them to pU cm-T carrier to configune pGTLA and pJIg, linking them to configure their recombinant plasmid, verifying the correctness of inserted fragments, and inserting the fusion gene in the front end of pGLUA-P. Its advantages are simple process, high safety, low cost, and high effect to improve immunity.
Owner:WUHAN UNIV
Anti-HIV genetic engineering divalent antibody and preparation method thereof, and anti-HIV genetic engineering medicine
InactiveCN103183735AImprove specific binding abilitySimple processImmunoglobulins against virusesAntiviralsEffective actionEukaryotic plasmids
The invention relates to an anti-HIV genetic engineering divalent antibody and a preparation method thereof, and an anti-HIV genetic engineering medicine. The anti-HIV genetic engineering divalent antibody comprises a human antibody constant region and CD4 and CCR5 connected with the human antibody constant region. The preparation method of the anti-HIV genetic engineering antibody comprises the steps that: human antibody constant region gene, CD4 gene, and CCR5 gene are obtained; expression plasmids comprising the three genes are constructed; the expression plasmids are transfected into cell lines and are cultured; and the divalent antibody is obtained after separation and purification. The divalent antibody provided by the invention has both CD4 and CCR5 which can be specifically bound with CD4 and CCR5 loci on HIV virus, specific binding effect is substantially improved, and HIV-infected host cell dual inhibition can be realized. The preparation method provided by the invention is simple and feasible. The anti-HIV genetic engineering medicine with the divalent antibody can effectively act upon HIV virus, and assists in effectively preventing and treating HIV virus infections.
Owner:SHENZHEN UNIV
Preparation method of allergen-specific IgE antibody composite quality control product and allergen-specific IgE antibody composite quality control product
PendingCN113004403AHigh purityImprove stabilityImmunoglobulins against animals/humansImmunoglobulins against plantsConstant region geneSpecific antibody
The invention discloses a preparation method of an allergen-specific IgE antibody composite quality control product and the allergen-specific IgE antibody composite quality control product. The preparation method specifically comprises the following steps: preparing an allergen-specific IgE antibody; obtaining a target gene, and obtaining a constant region gene sequence and a variable region gene sequence of human IgE; the method comprises the following steps: designing a primer containing an XhoI restriction enzyme cutting site according to a heavy chain constant region gene sequence of human IgE, designing a primer containing an Hind III restriction enzyme cutting site according to a light chain constant region gene sequence of human IgE, and constructing an expression empty vector containing a light chain signal peptide, a heavy chain signal peptide, a human IgE heavy chain and a light chain C region gene fragment; constructing recombinant expression vectors of different allergens; transfecting the cells; cloning, recognizing and screening; the quality control product has the advantages of high titer, higher purity, favorable dilution linearity and favorable stability, and has higher use value in the aspects of quality control, conventional quality control, performance verification and product development of large-scale production.
Owner:湖南携光生物技术有限公司
A variable region sequence of a specific anti-thiacloprid antibody and its recombinant complete antibody
ActiveCN112111010BHigh sensitivityHigh selectivityImmunoglobulinsFermentationImmune profilingAntiendomysial antibodies
The invention discloses a variable region sequence of a specific anti-thiacloprid antibody. The amino acid sequence of the gene encoding the heavy chain variable region is shown in SEQ ID NO:2. The present invention also discloses an anti-thiacloprid recombinant complete antibody, including heavy chain constant region, heavy chain variable region, light chain constant region and light chain variable region, the amino acid sequence of the gene encoding the heavy chain variable region is as follows: Shown in SEQ ID NO:2. The invention also discloses an antibody expression plasmid. The sequence genes obtained in the present invention are respectively connected to the expression vectors containing the heavy chain constant region gene and the light chain constant region gene, and the recombinant complete antibody is obtained by using the mammalian cell expression of the double plasmid system, which ensures the recognition of the mouse parent antibody activity, and enables the stable production of anti-thiacloprid antibodies in large quantities, and provides reliable core reagents for various immunoassay methods for the detection of thiacloprid residues.
Owner:ZHEJIANG UNIV
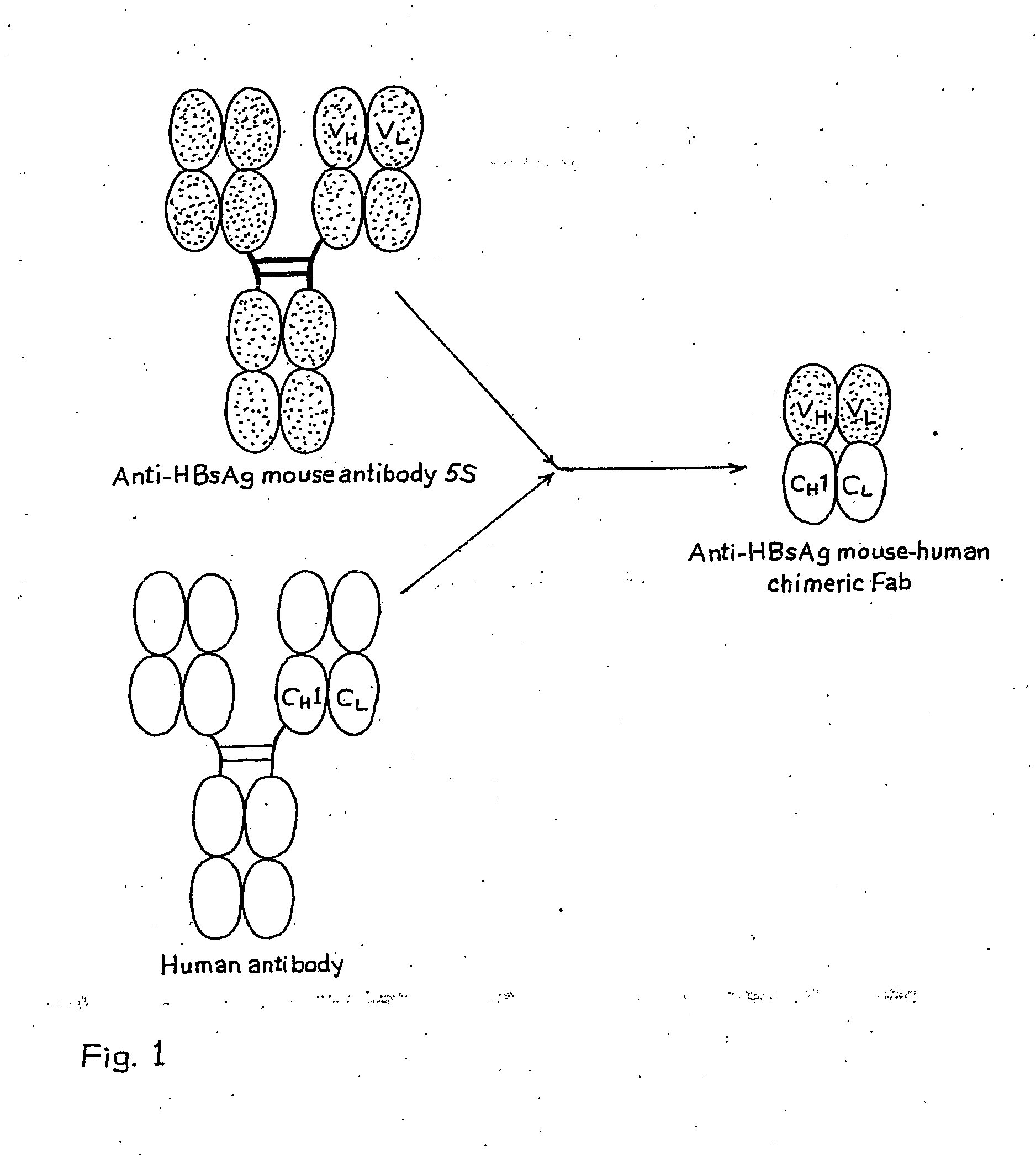
Recombinant Mouse-Human Chimeric Fab Against Hepatitis B Surface Antigen
InactiveUS20100145025A1Safer and cheapHybrid immunoglobulinsVirus peptidesHepatitis B Surface AntigensConstant region gene
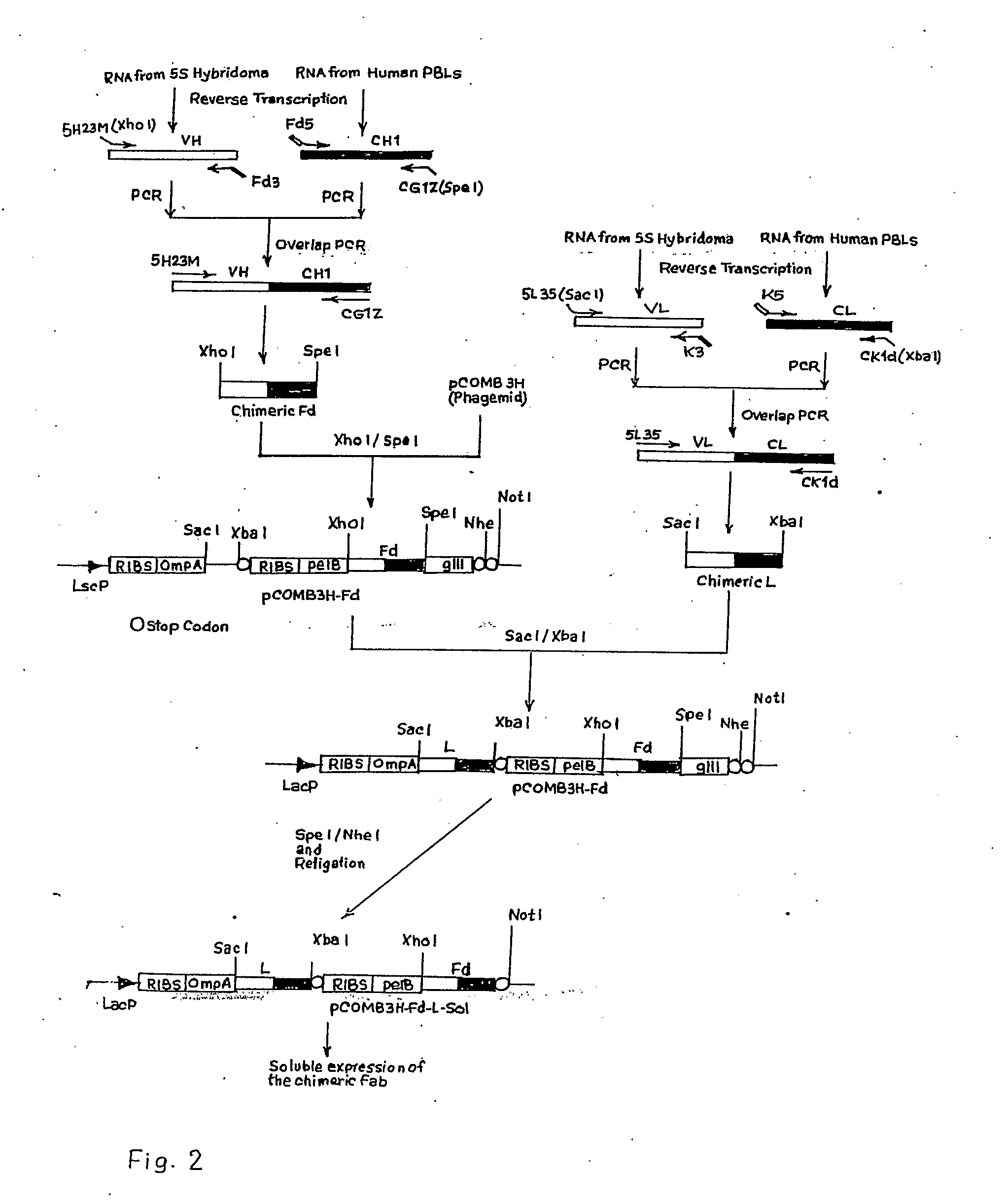
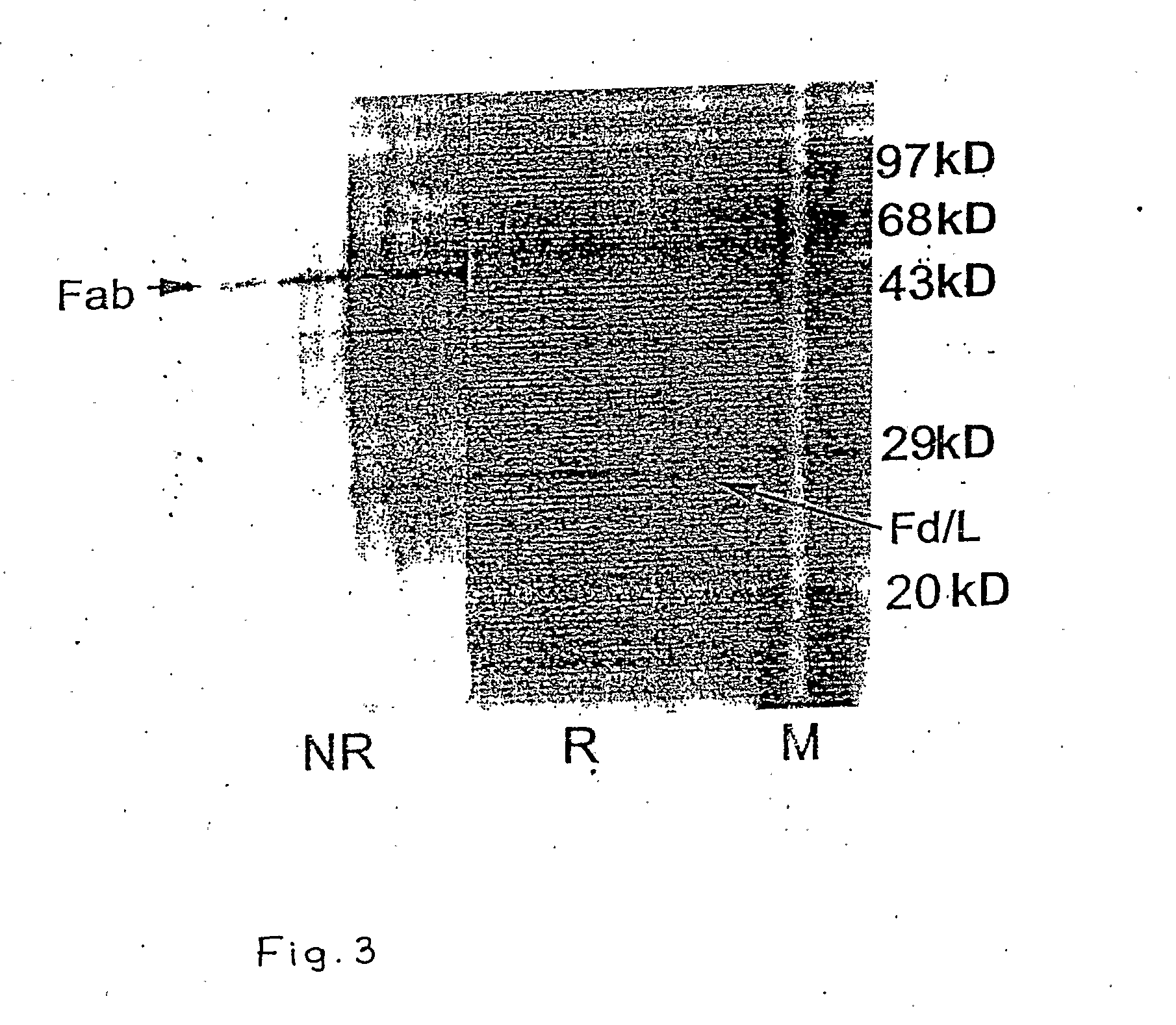
The invention relates to a recombinant chimeric Fab antibody comprising a recombinant Fd and a recombinant chimeric light chain which binds to hepatitis B surface antigen with high affinity, generated by fusing variable region genes (VH and VL) of an anti-HBsAg mouse antibody 5S and constant region genes of human, CH1 region of human IgGI and the CL region of human kappa chain, wherein mouse VL and human CL are linked by overlap PCR to generate chimeric light chain and mouse VH and CH1 are linked by overlap PCR to generate chimeric Fd.
Owner:DEPT OF BIOTECHNOLOGY MINIST OF SCI & TECH GOVERNMENT OF INDIA +2
Humanized antibody expression vector and construction method thereof
The invention discloses a humanized antibody expression vector and a construction method thereof. The construction method comprises the steps of: respectively connecting a 2A sequence-containing light chain constant region gene sequence CL-2A and a heavy chain constant region gene sequence CH with a pMD18-T vector to obtain a pMD18-T-CL-2A plasmid and a pMD18-T-CH plasmid; connecting the pMD18-T-CL-2A plasmid and a eukaryotic expression vector after being subjected to double digestion to obtain a CL-2A-containing eukaryotic expression vector; and then connecting the CL-2A-containing eukaryotic expression vector with a pMD18-T-CH plasmid to obtain a CL-2A-CH-containing eukaryotic expression vector. The construction method of the expression vector disclosed by the invention can be used for rapidly constructing a full-length complete antibody sequence only by inserting a variable region sequence of a mouse or a humanized antibody into a corresponding locus and rapidly constructing a chimeric antibody, the humanized antibody and the like, and is simple and practical.
Owner:SHENZHEN ZHONGKE AMSHENN MEDICINE CO LTD
Preparation and application of anti-human pcsk9 antibody
The invention discloses an anti-human PCSK9 (pro-protein convertase subtilisin / kexin 9) chimeric antibody, and preparation and application thereof. The preparation method comprises the following steps: respectively amplifying mouse light chain and heavy chain variable region genes from mouse hybridoma cells, respectively carrying out chimerism with light chain and heavy chain constant region genes of human IgG, and carrying out recombination expression to obtain the human-mouse chimeric antibody. The human-mouse chimeric antibody has favorable affinity with human PCSK9, obviously inhibits the degradation activity of the PCSK9 for liver cell low-density lipoprotein receptors (LDLR), enhances the ingestion of liver cells for LDL-cholesterol (LDL-C), and lowers the cholesterol level in blood.
Owner:成都金洛克锶生物技术有限公司
Expression vector containing detection label IgG (immunoglobulin g), construction method and application thereof
InactiveCN103898136AReduce problemsSimple detection operationBiological testingVector-based foreign material introductionProtein targetComplementary deoxyribonucleic acid
The invention discloses an expression vector containing detection label IgG (immunoglobulin g), a construction method, and an application thereof in expression detection of target protein. The detection label IgG is the constant region gene of at least one species of IgG, and the expression detection of the target protein is realized through direct reaction of the constant region gene of at least one species of IgG and at least one species of secondary antibody. The construction method for the expression vector containing detection label IgG comprises the steps of designing an upstream primer and a downstream primer of the constant region gene of at least one species of IgG; taking at least one species of cDNA (complementary deoxyribonucleic acid) as a template, carrying out PCR (polymerase chain reaction) amplification through the upstream primer and the downstream primer so as to obtain the constant region gene of at least one species of IgG; and connecting the constant region gene of at least one species of IgG with the expression vector so as to construct the expression vector containing detection label IgG. When being used for detection of target protein, the expression vector can lower the probability of false negative detection result, and improve the accuracy and sensitivity of the detection.
Owner:SHENZHEN UNIV
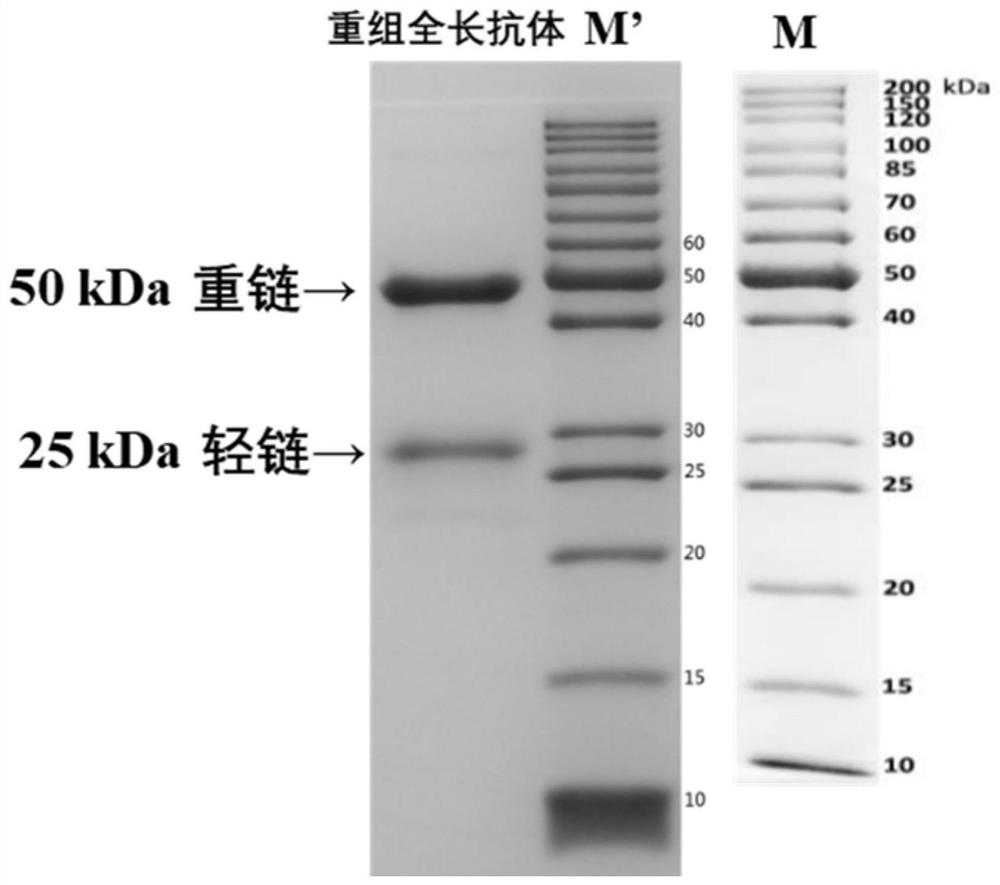
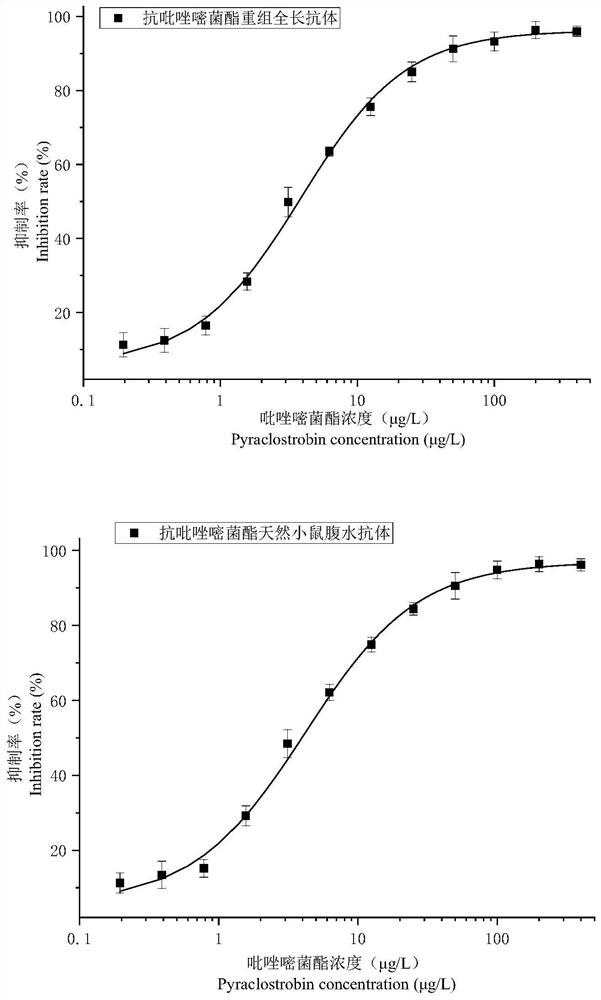
A variable region sequence of a specific anti-pyraclostrobin antibody and its recombinant full-length antibody
ActiveCN112111009BGuaranteed recognition activityStable productionNucleic acid vectorImmunoglobulinsImmune profilingAntiendomysial antibodies
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com