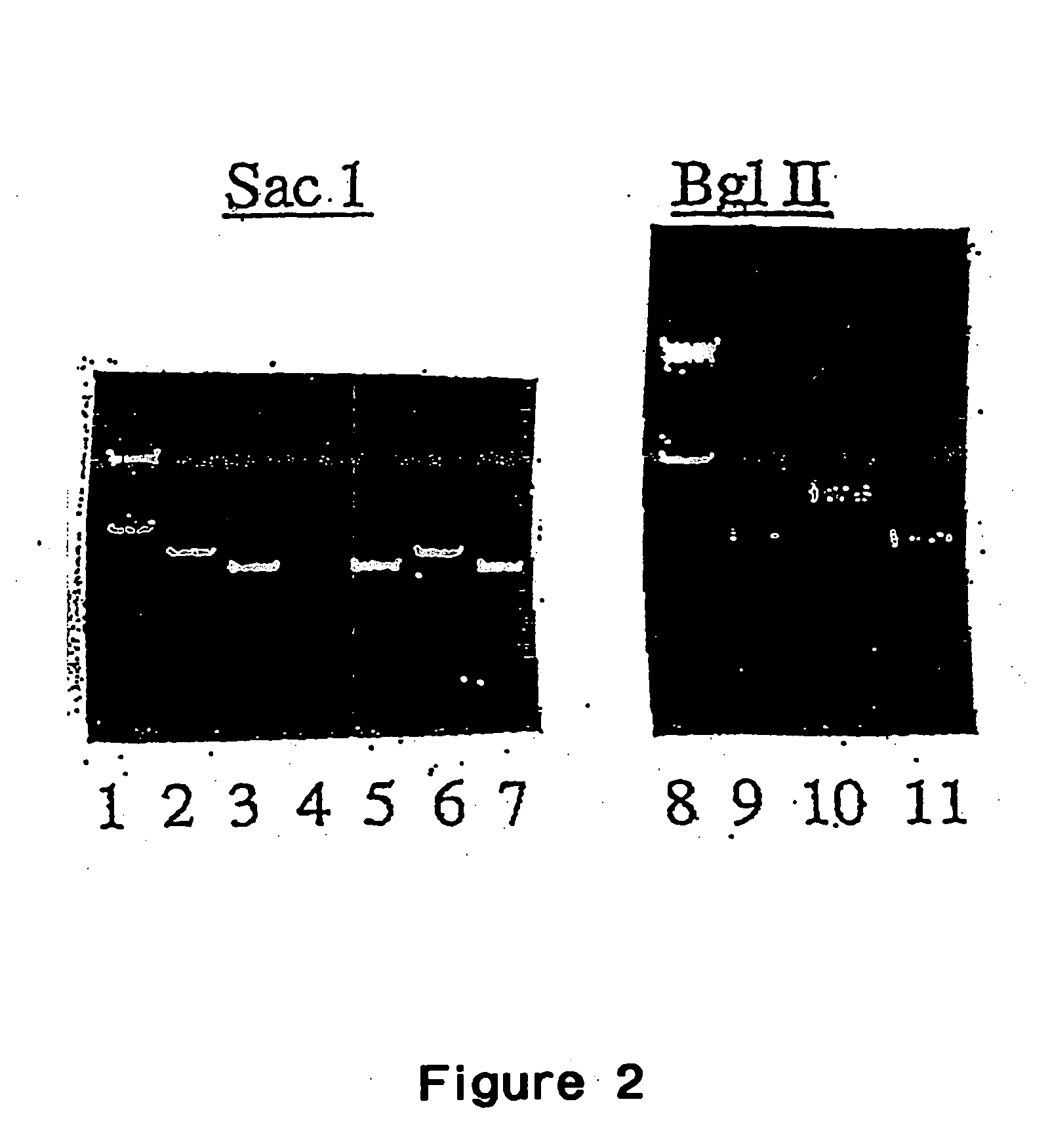
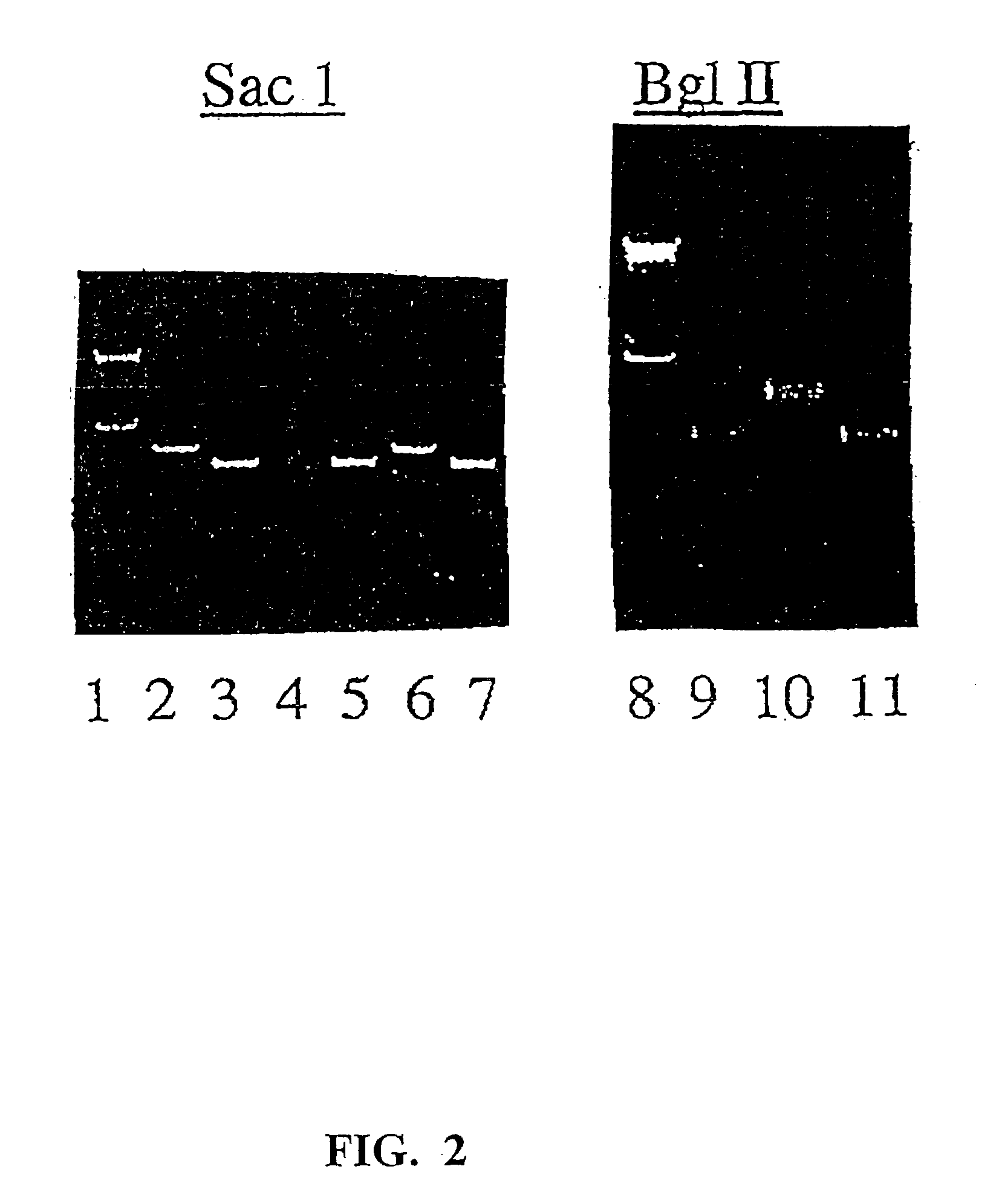
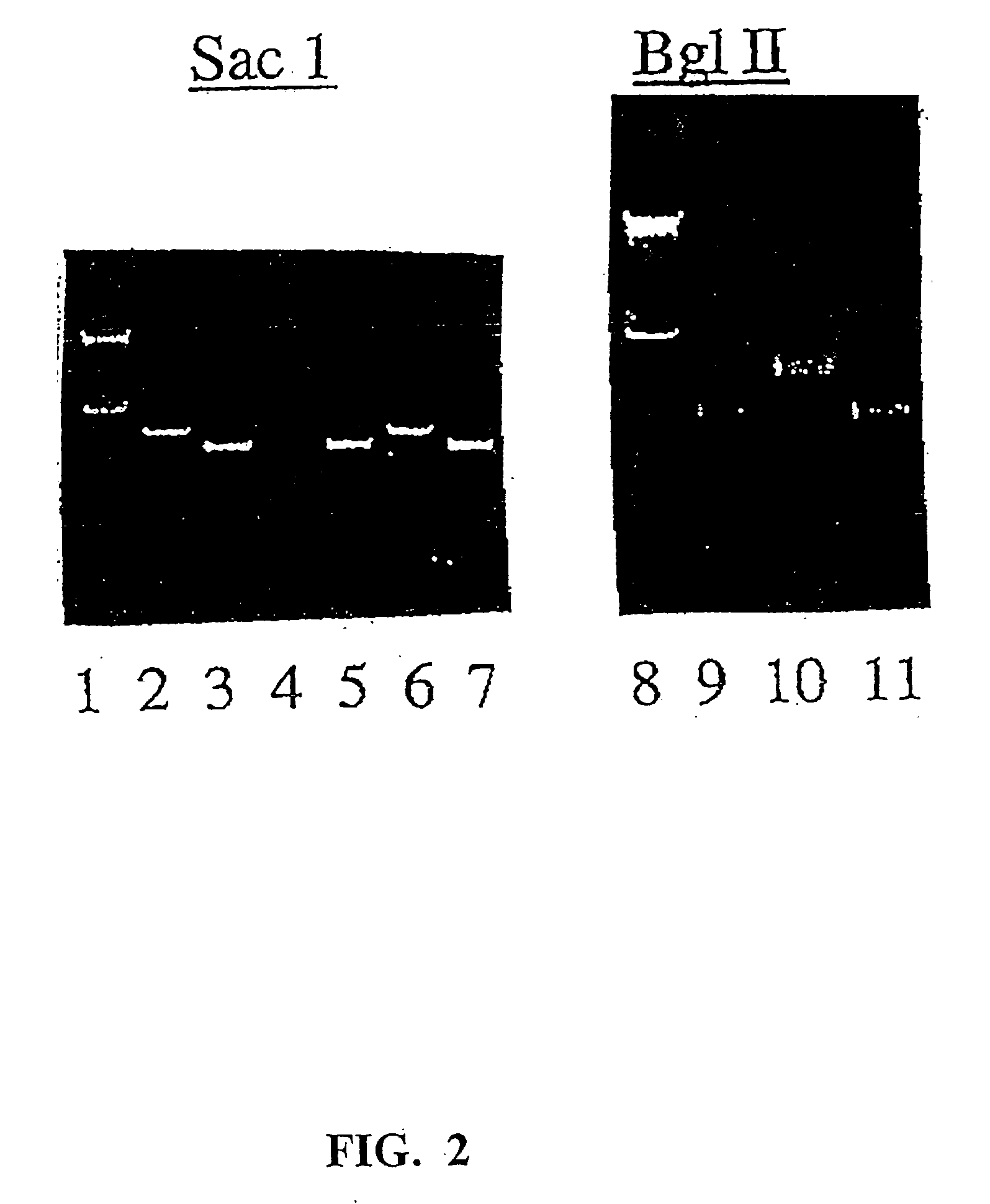
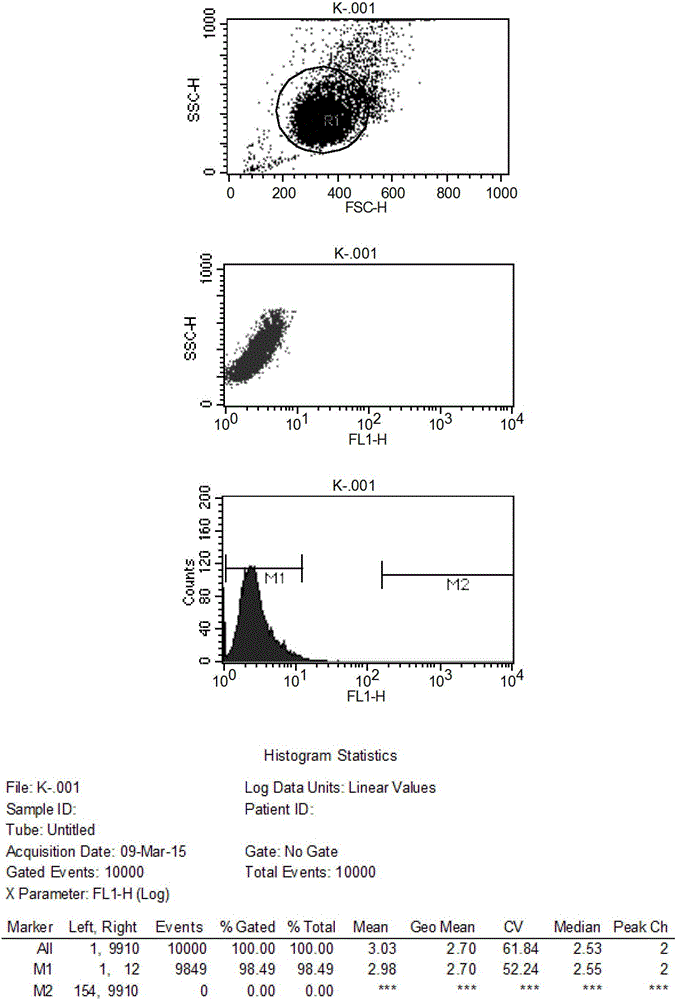
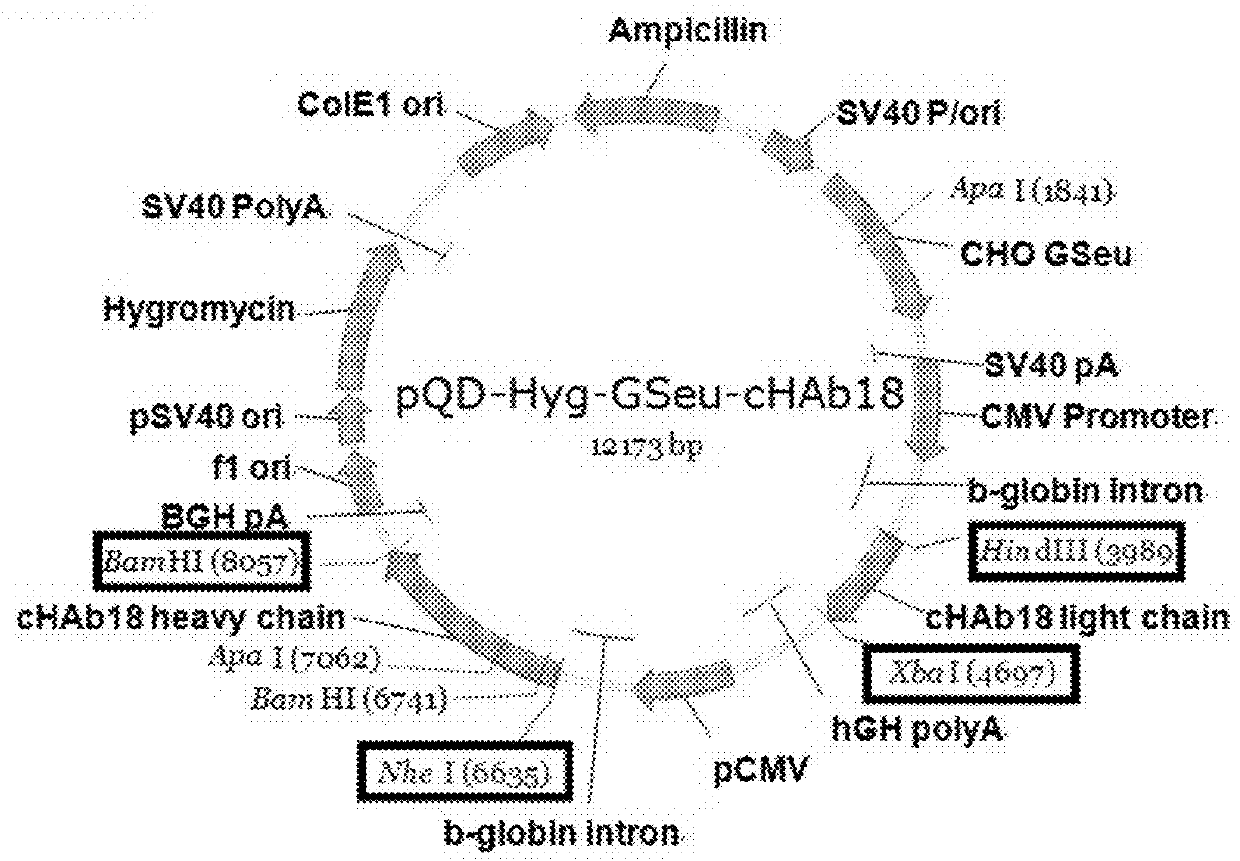
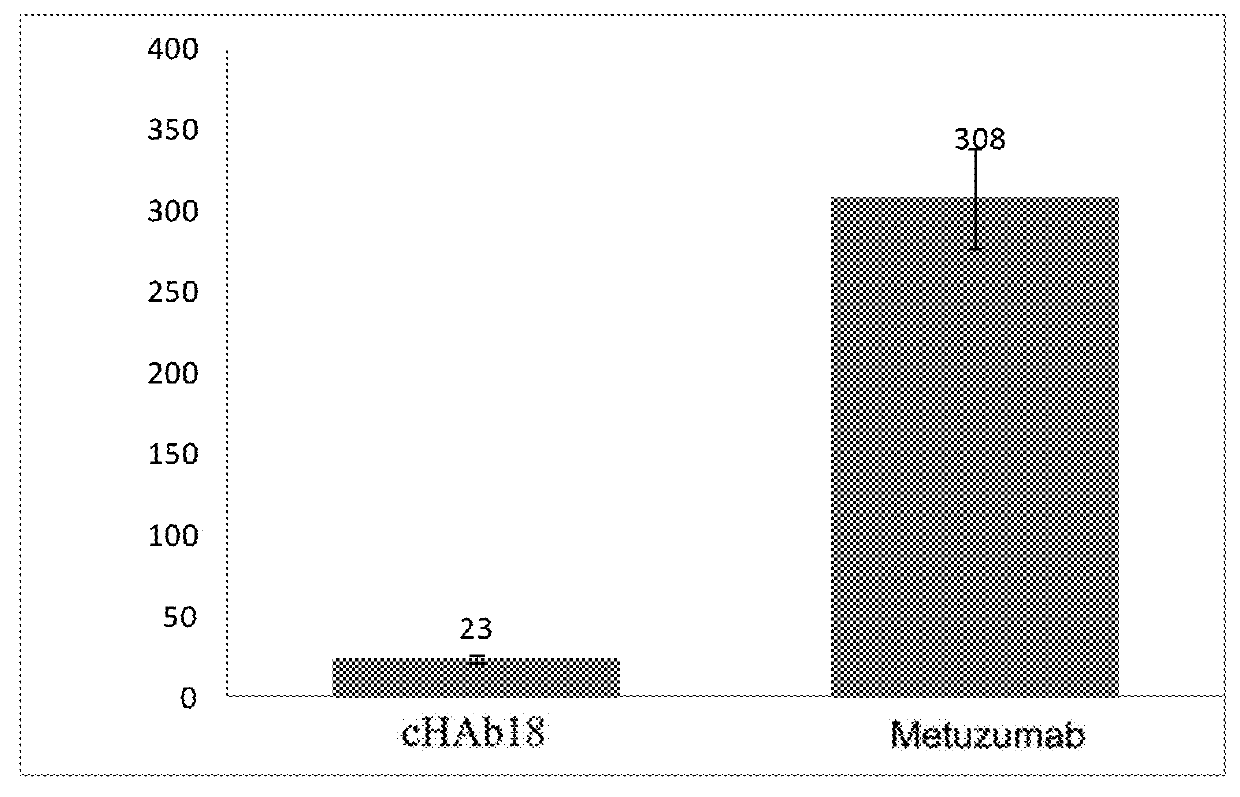
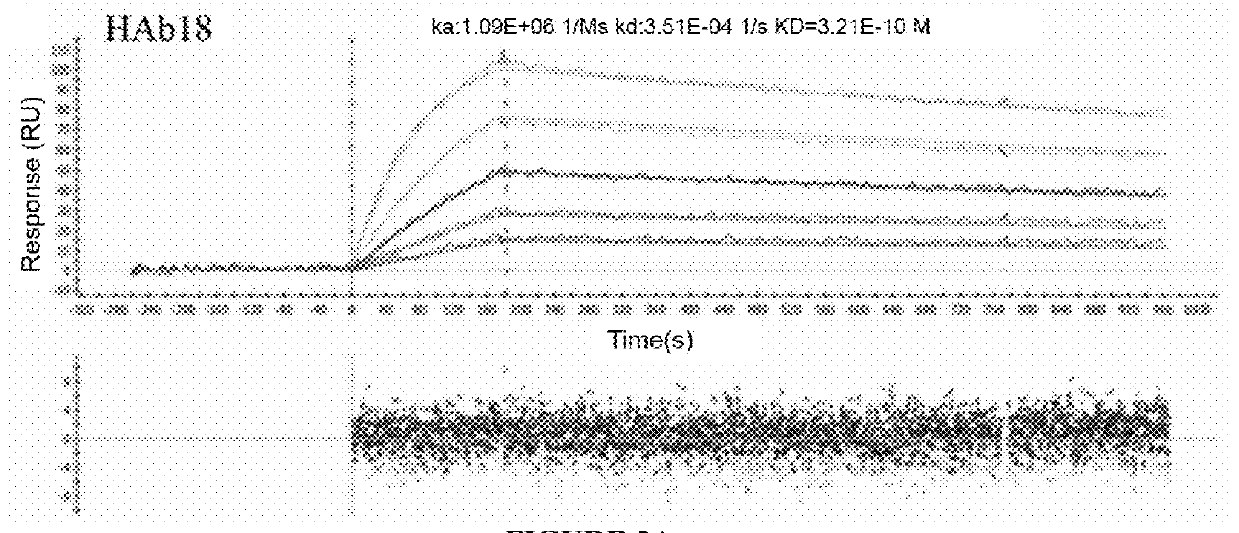
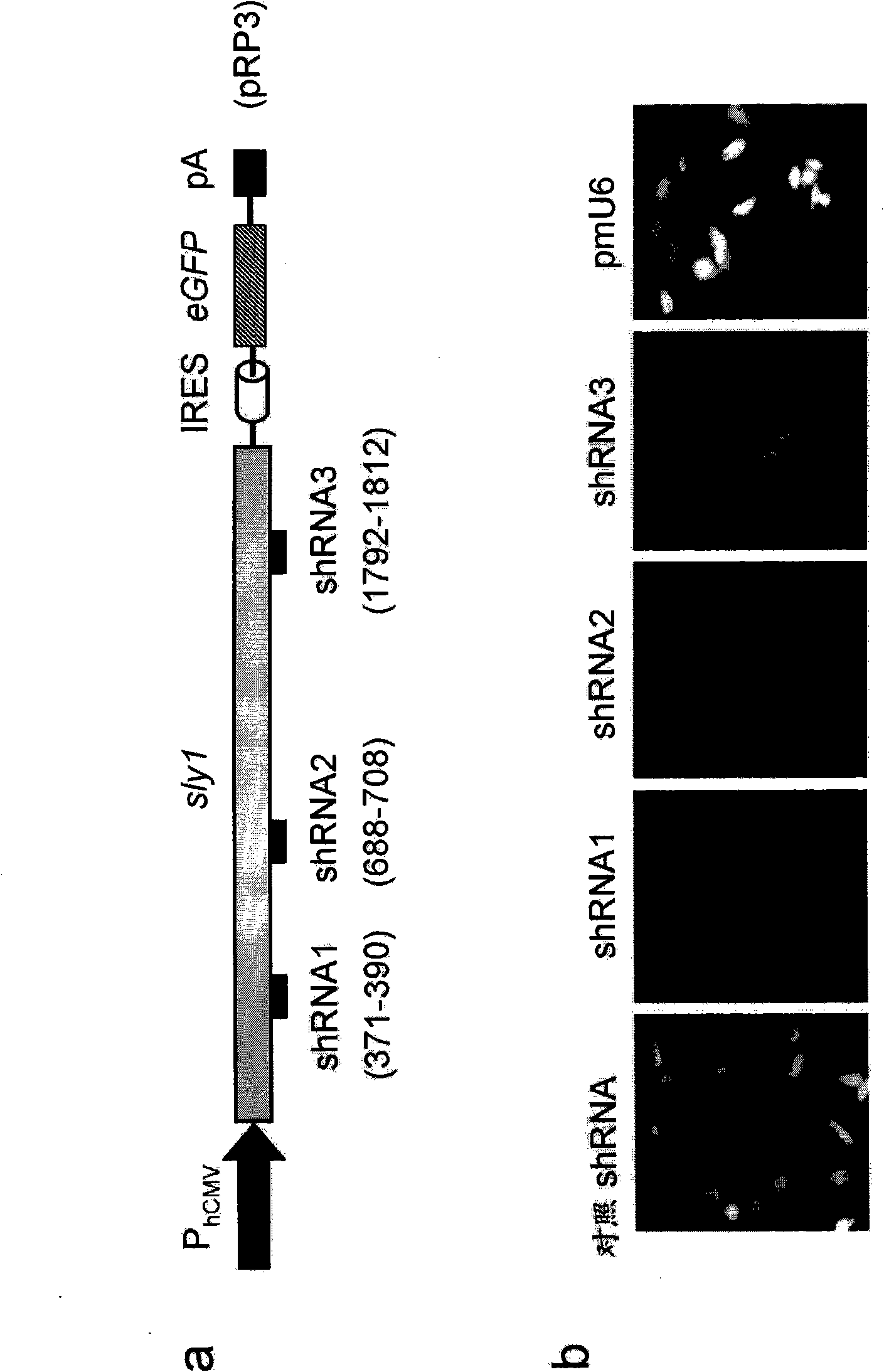
Patents
Literature
76 results about "Host cell line" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infectious cDNA clone of North American porcine reproductive and respiratory syndrome (PRRS) virus and uses thereof
InactiveUS7132106B2Effective immunoprotective responseEfficient productionAnimal cellsSsRNA viruses positive-senseA-DNARNA Sequence
The invention provides isolated polynucleotide molecules, including plasmids; viral vectors; and transfected host cells that comprise a DNA sequence encoding an infectious RNA sequence encoding a North American PRRS virus; and also North American PRRS viruses encoded thereby. The invention further provides isolated infectious RNA molecules encoding a North American PRRS virus. The invention also provides isolated polynucleotide molecules, infectious RNA molecules, viral vectors, and transfected host cells encoding genetically-modified North American PRRS viruses; and genetically-modified North American PRRS viruses encoded thereby. The invention also provides vaccines comprising such plasmids, RNA molecules, viral vectors, and North American PRRS viruses, and methods of using these vaccines in swine and in other animals. Also provided are isolated polynucleotide molecules, viral vectors, and transfected host cells that comprise a nucleotide sequence encoding a peptide of a North American PRRS virus. These viral vectors and transfected host cell lines are useful in providing peptides to compensate for mutated peptide coding sequences of DNA sequences encoding genetically-modified North American PRRS viruses so that functional virions can be generated.
Owner:ZOETIS SERVICE LLC
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
InactiveUS20030232061A1Optimization orderImprove scalabilitySsRNA viruses negative-senseVectorsNegative strandAntigen
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Method and application for screening ultralow fucose cell line
The invention discloses a method and application for screening an ultralow fucose cell line. The invention provides a method for constructing fucose deficit type host cells capable of expressing an antibody and an IgG-FC fusion protein, a detection method for the fucose activity of the antibody and the IgG-Fc fusion protein, and concrete application of the cell lines. The method provided by the invention is realized by highly-efficient knockout of fucose-based transferase (FUT8) gene in an engineering cell producing the antibodies and the IgG-Fc fusion protein through a TALEN (and / or CRISPR) technology; and through lens culinaris lectin (LCA) pressurizing, gene sequencing and a flow cytometry screening process, the host cells with highly-efficiently knocked-out fucose is obtained. Meanwhile, fucose deficit CHOK1 host cell lines are constructed into stable engineering cell lines capable of expressing antibody proteins; and after the antibody proteins are obtained, glycoform analysis is performed; and results show that fucose knockout efficiency reaches to 99% or above.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD +1
Infectious cDNA clone of north american porcine reproductive and respiratory syndrome (PRRS) virus and uses thereof
The invention provides isolated polynucleotide molecules, including plasmids; viral vectors; and transfected host cells that comprise a DNA sequence encoding an infectious RNA sequence encoding a North American PRRS virus; and also North American PRRS viruses encoded thereby. The invention further provides isolated infectious RNA molecules encoding a North American PRRS virus. The invention also provides isolated polynucleotide molecules, infectious RNA molecules, viral vectors, and transfected host cells encoding genetically-modified North American PRRS viruses; and genetically-modified North American PRRS viruses encoded thereby. The invention also provides vaccines comprising such plasmids, RNA molecules, viral vectors, and North American PRRS viruses, and methods of using these vaccines in swine and in other animals. Also provided are isolated polynucleotide molecules, viral vectors, and transfected host cells that comprise a nucleotide sequence encoding a peptide of a North American PRRS virus. These viral vectors and transfected host cell lines are useful in providing peptides to compensate for mutated peptide coding sequences of DNA sequences encoding genetically-modified North American PRRS viruses so that functional virions can be generated.
Owner:ZOETIS SERVICE LLC
Recombinant negative strand virus rna expression systems and vaccines
ActiveUS20050221489A1Improving immunogenicityAttenuated phenotypeSsRNA viruses negative-senseAntibacterial agentsHeterologousNegative strand
The present invention relates to recombinant RNA virus templates derived from and applicable to negative strand naturally non-segmented viruses, including the families Bornaviridae, Filoviridae, and Paramyxoviridae, and methods for generating such recombinant RNA virus templates, wherein the templates are RNA generated from two or more recombinant RNA molecules. The invention relates to the use of segmented recombinant RNA virus templates for naturally non-segmented RNA viruses to express heterologous gene products in appropriate host cell systems and / or to construct recombinant viruses taken from that family and that express, package, and / or present the heterologous gene product. The invention includes the expression products and recombinant and chimeric viruses thus prepared and vaccine and therapeutic formulations comprising the recombinant RNA viruses.
Owner:MT SINAI SCHOOL OF MEDICINE
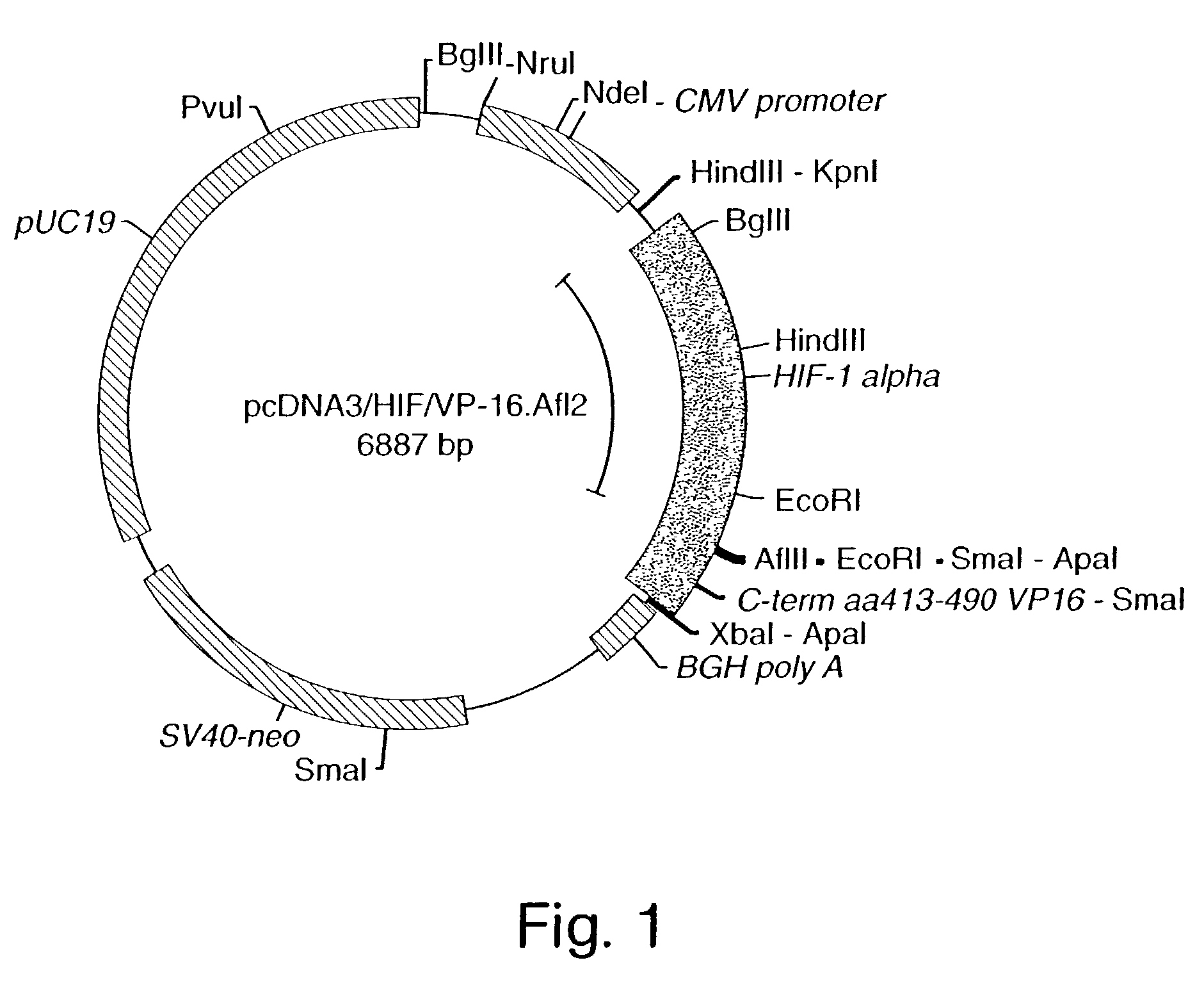
Compositions and methods for inducing gene expression
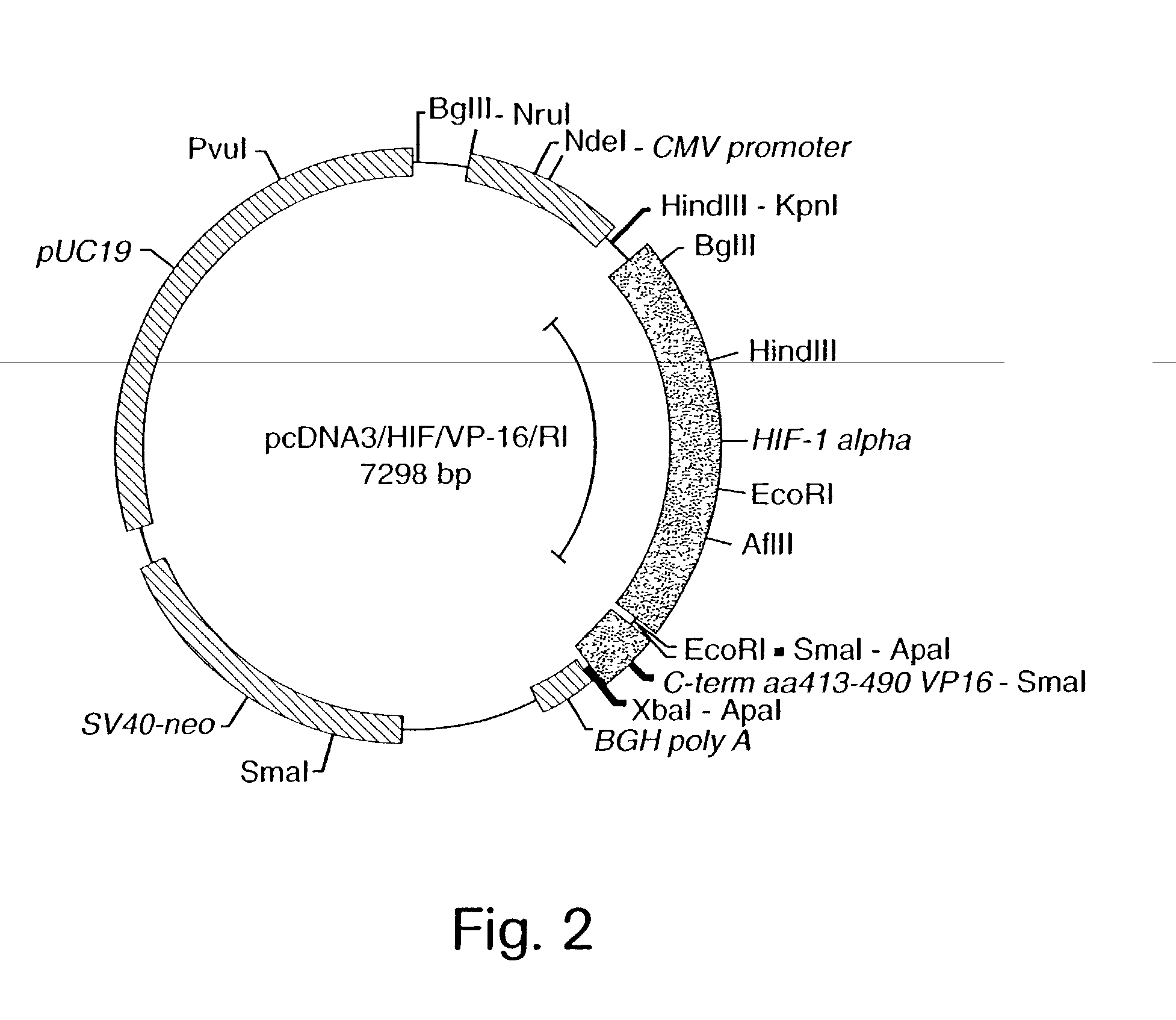
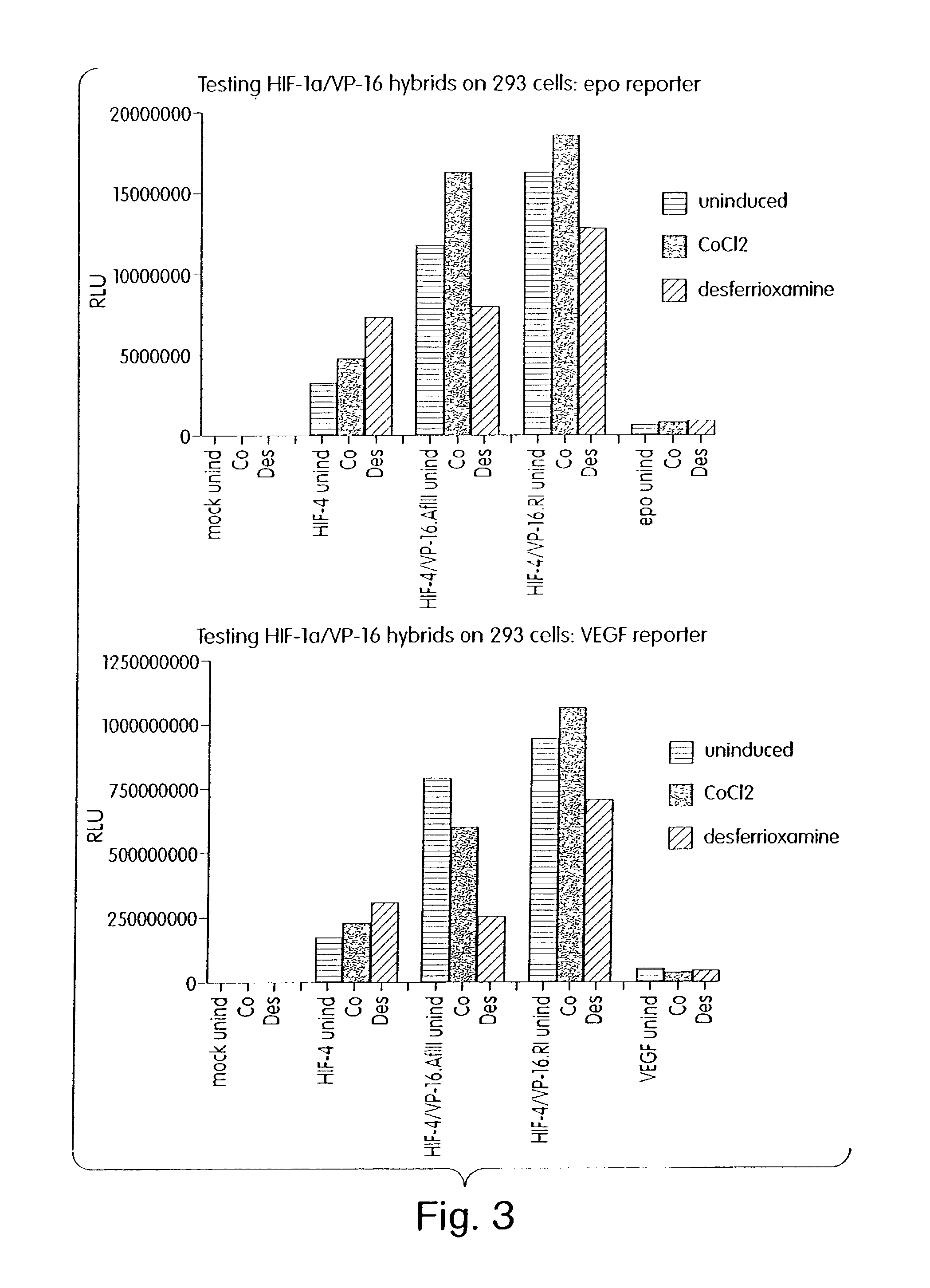
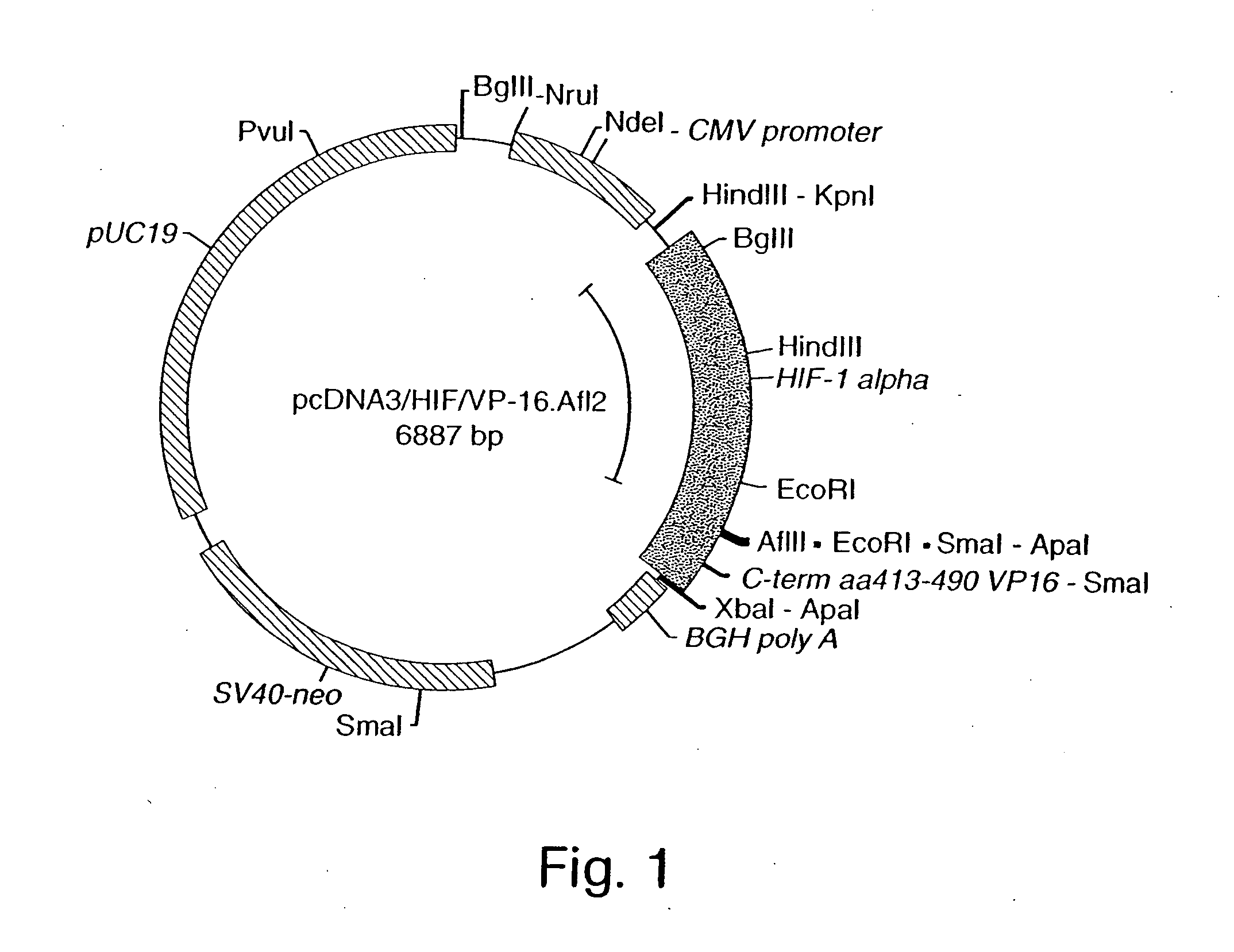
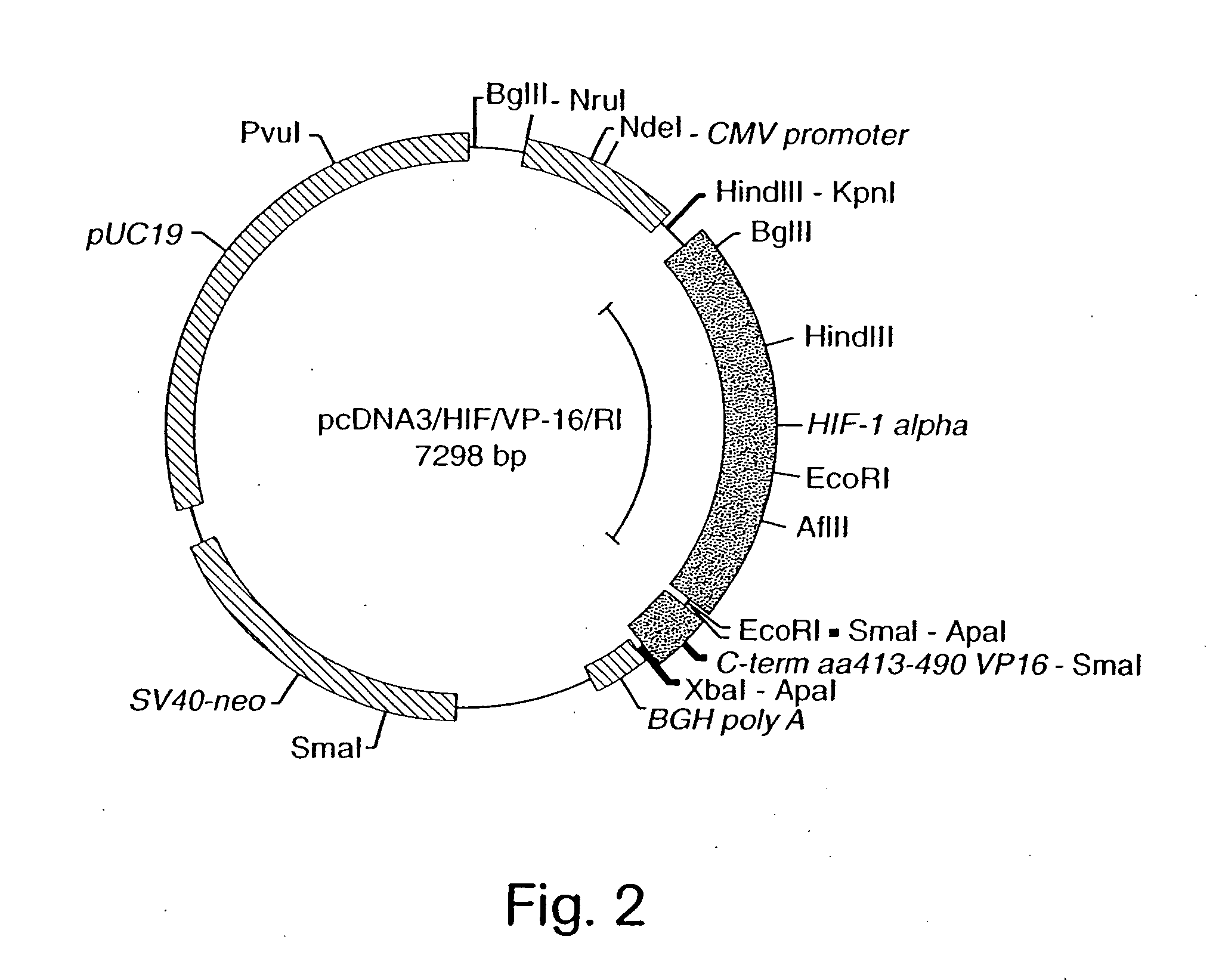
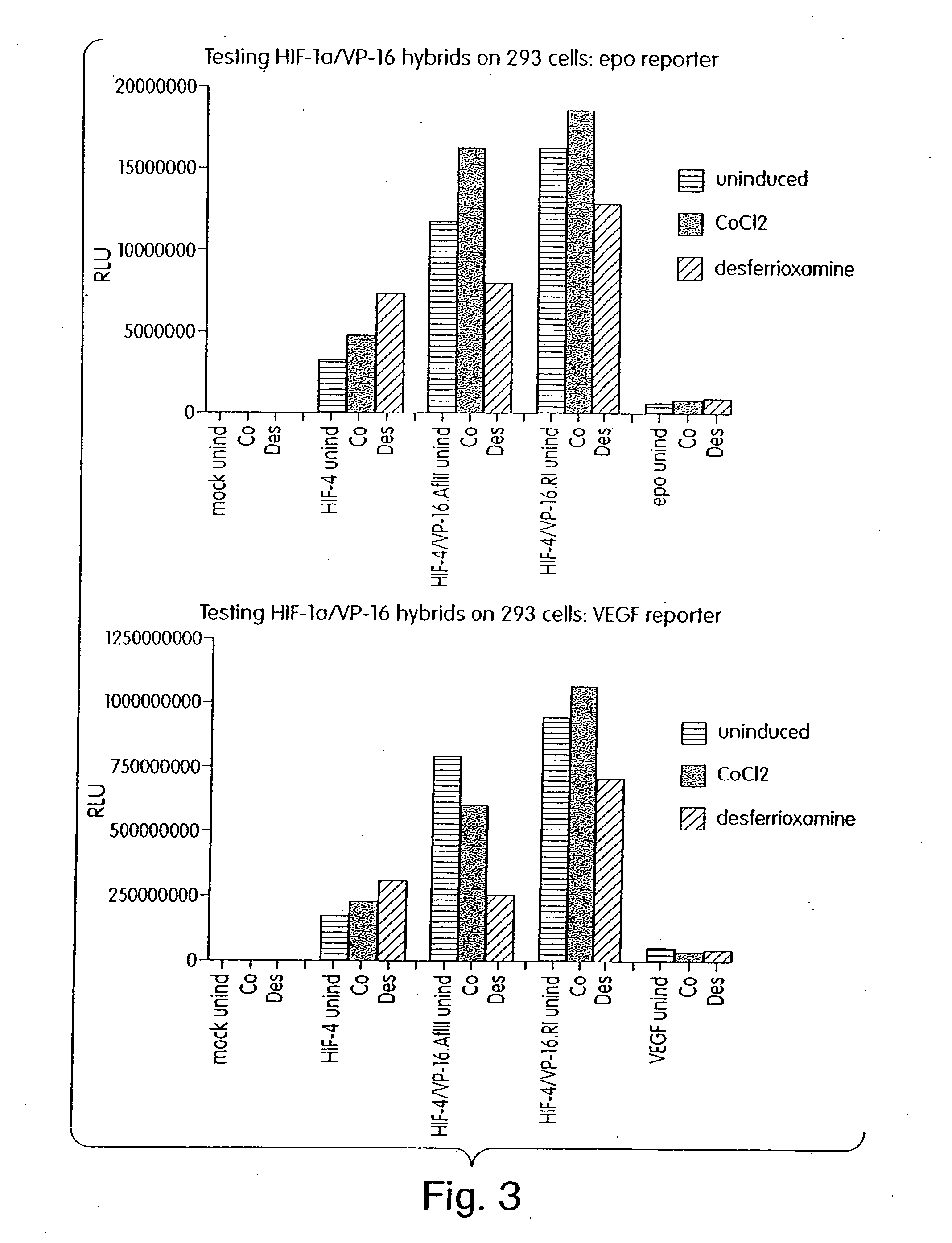
InactiveUS7053062B2High expressionIncreased formationVirusesFusion with DNA-binding domainA-DNABiological activation
The present invention provides recombinant nucleic acid molecules encoding a chimeric transactivator protein including a DNA binding domain of a DNA binding protein and a protein domain capable of transcriptional activation. The present invention also provides recombinant viral and non-viral vectors that are able to infect and / or transfect and sustain expression of a biologically active chimeric transactivator proteins in mammalian cells. Also provided are host cell lines and non-human transgenic animals capable of expressing biologically active chimeric transactivator proteins. In another aspect, compositions and methods for treating or preventing ischemic damage associated with hypoxia-related disorders are provided.
Owner:GENZYME CORP
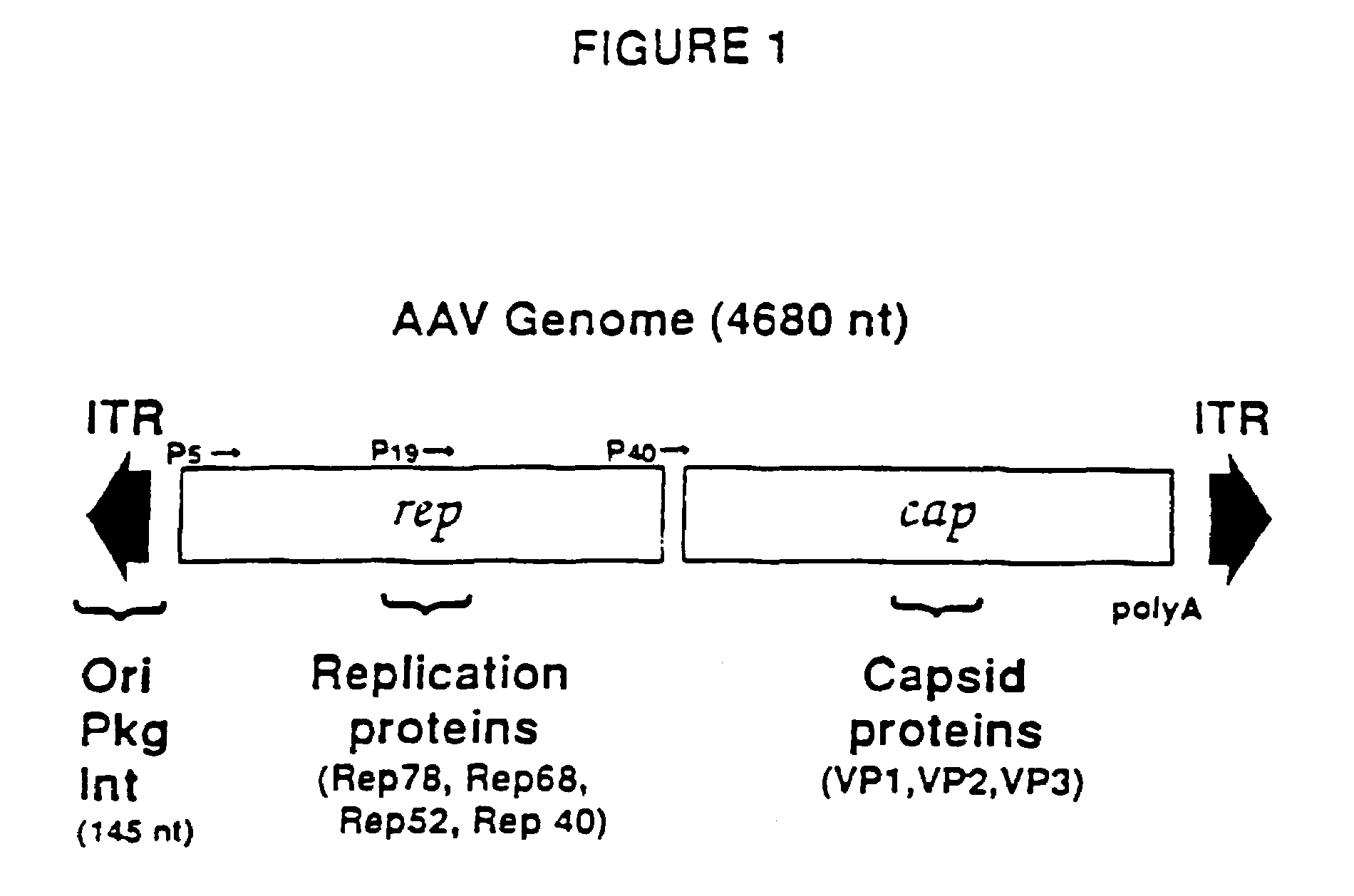
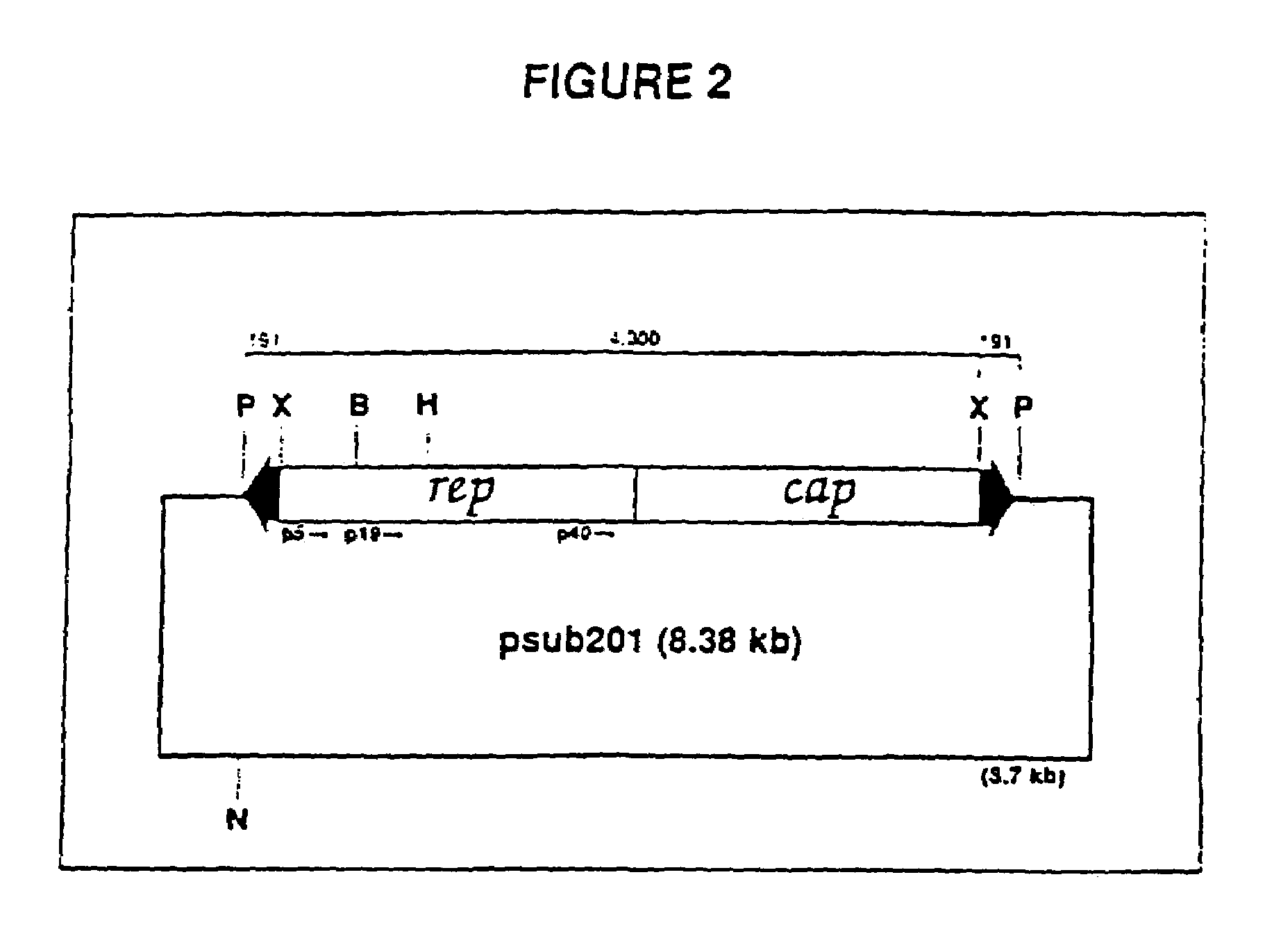
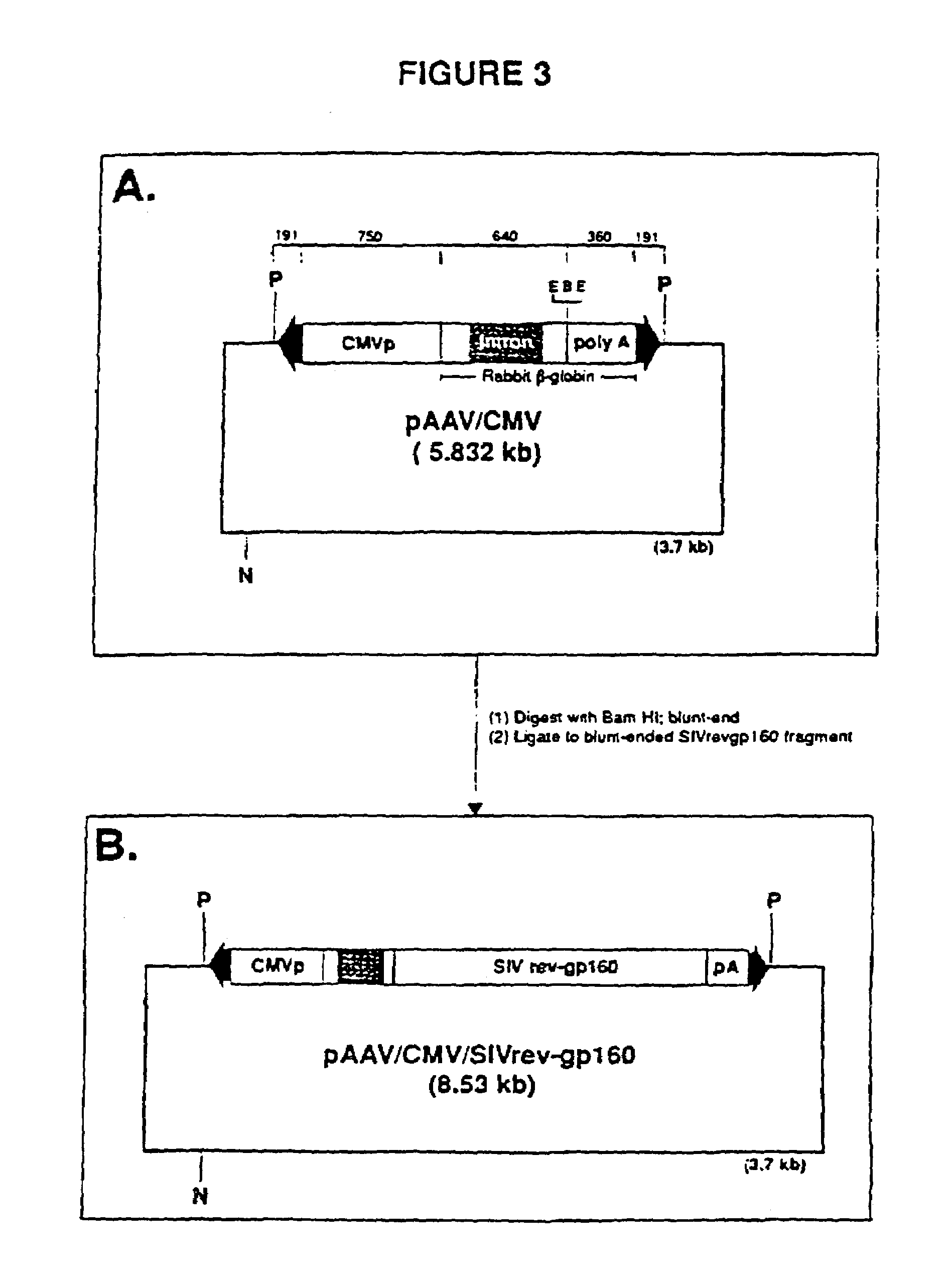
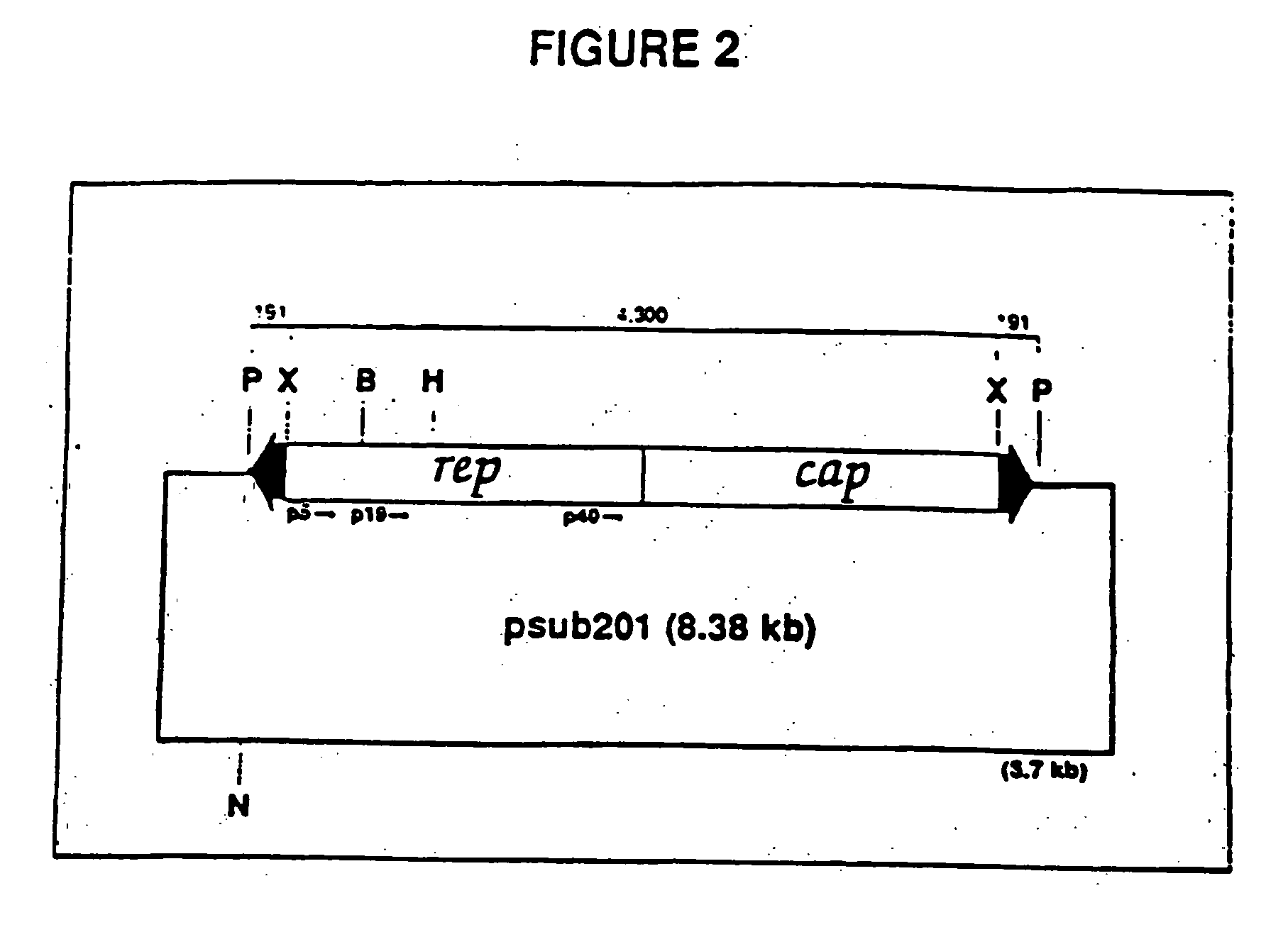
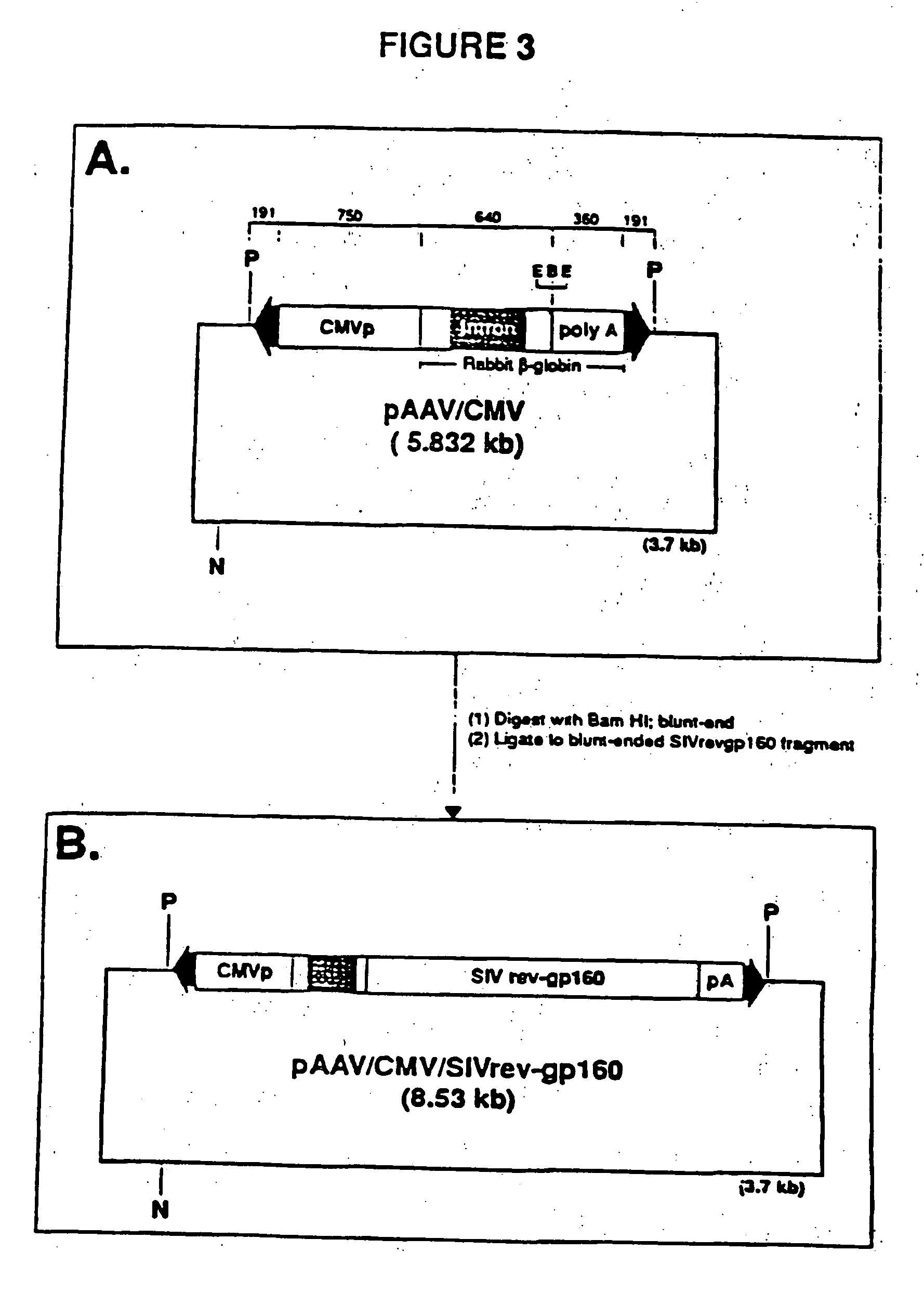
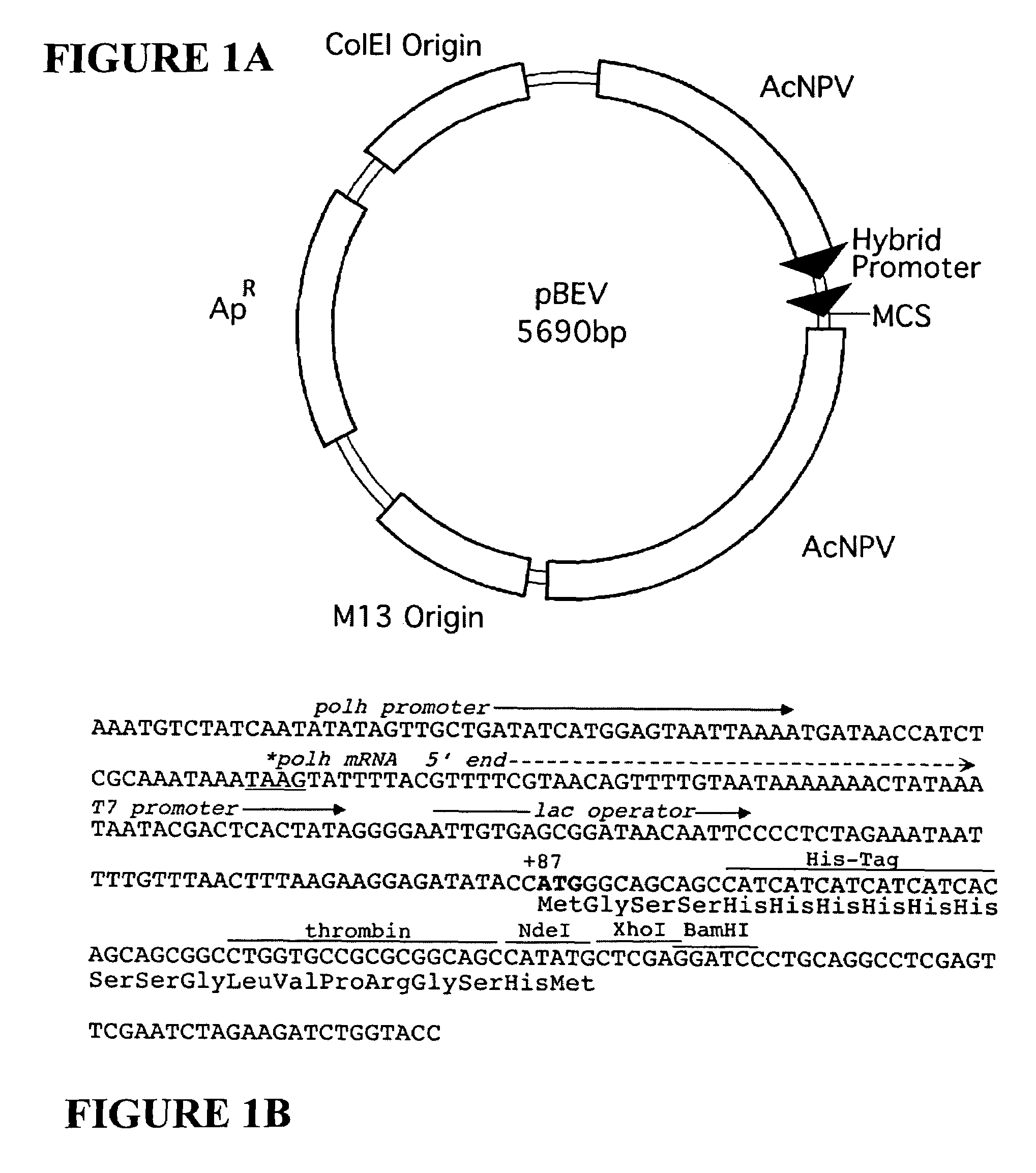
Adeno-associated virus materials and methods
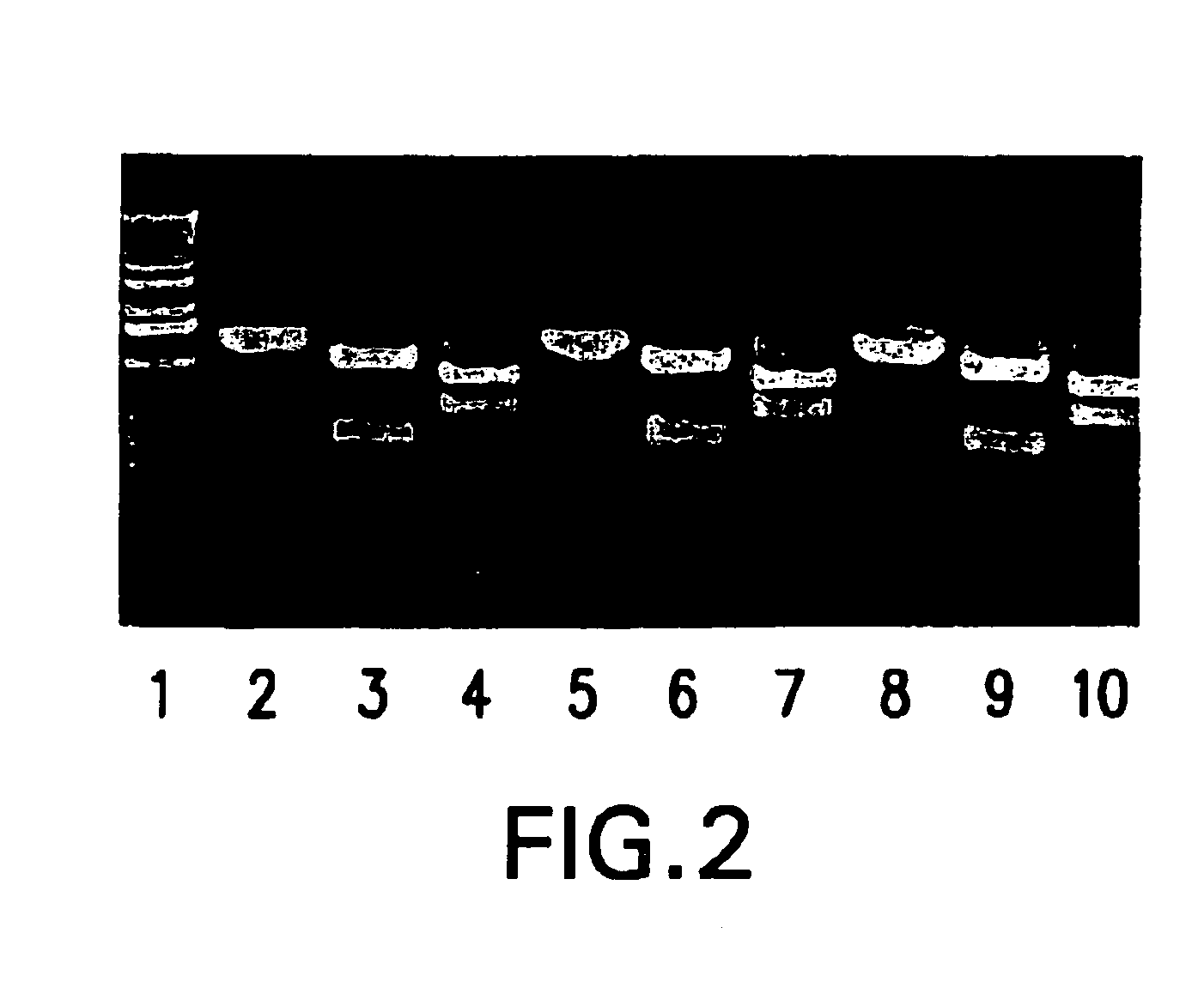
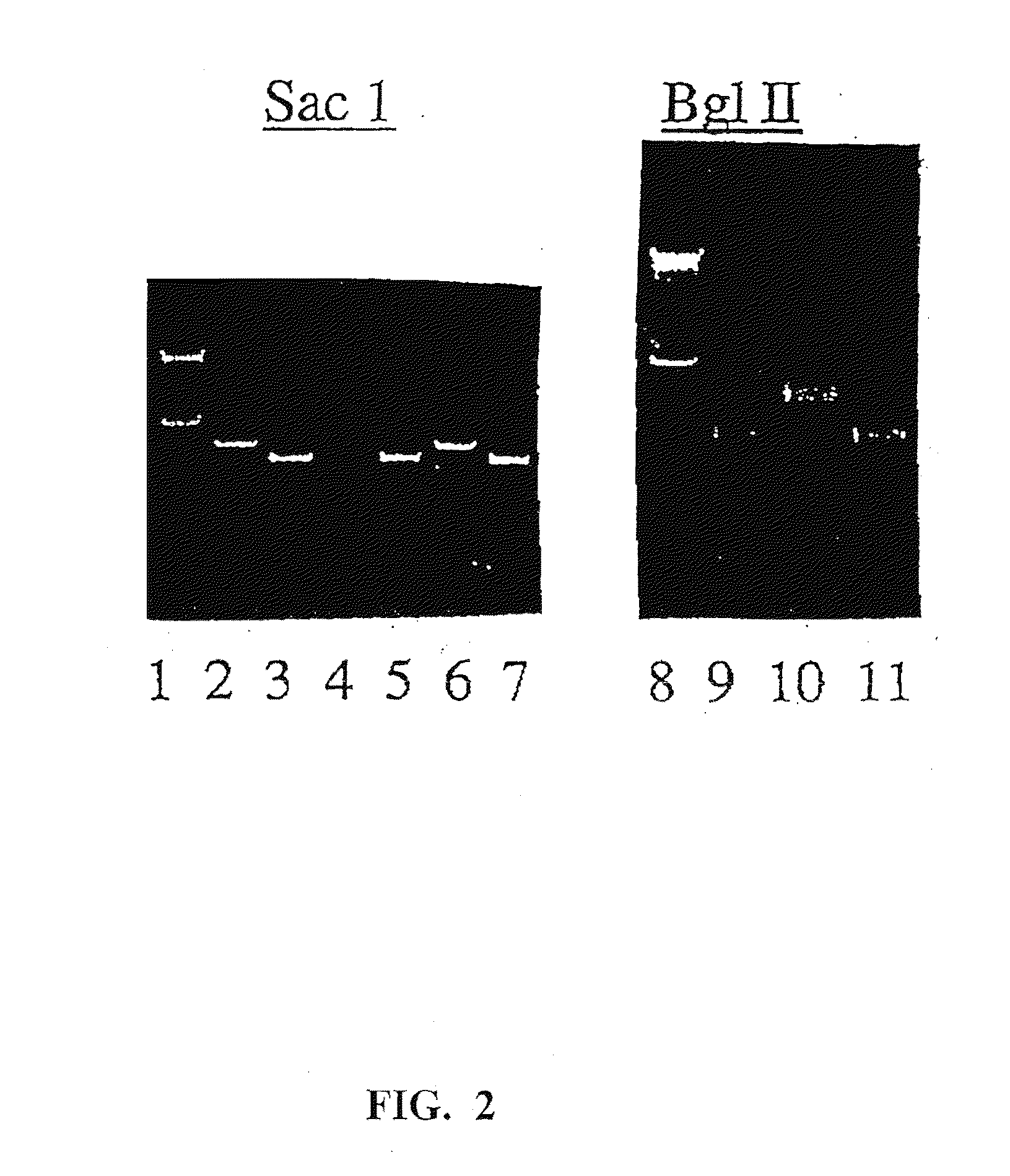
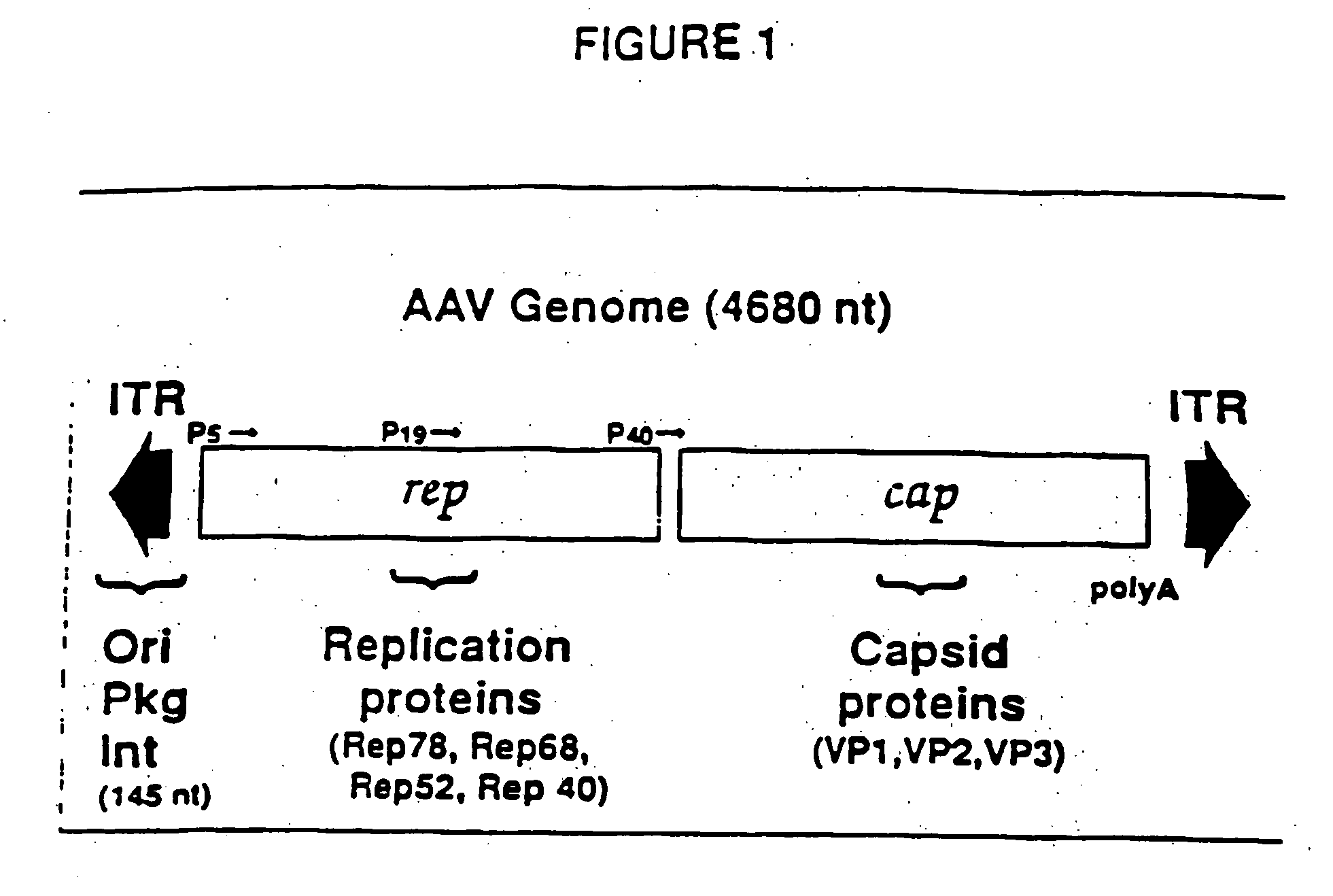
The present invention provides adeno-associated virus (AAV) materials and methods which are useful for DNA delivery to cells. More particularly, the invention provides recombinant AAV (rAAV) genomes, methods for packaging rAAV genomes, stable host cell lines producing rAAV and methods for delivering genes of interest to cells utilizing the rAAV. Particularly disclosed are rAAV useful in generating immunity to human immunodeficiency virus-1 and in therapeutic gene delivery for treatment of neurological disorders.
Owner:CHILDRENS HOSPITAL
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
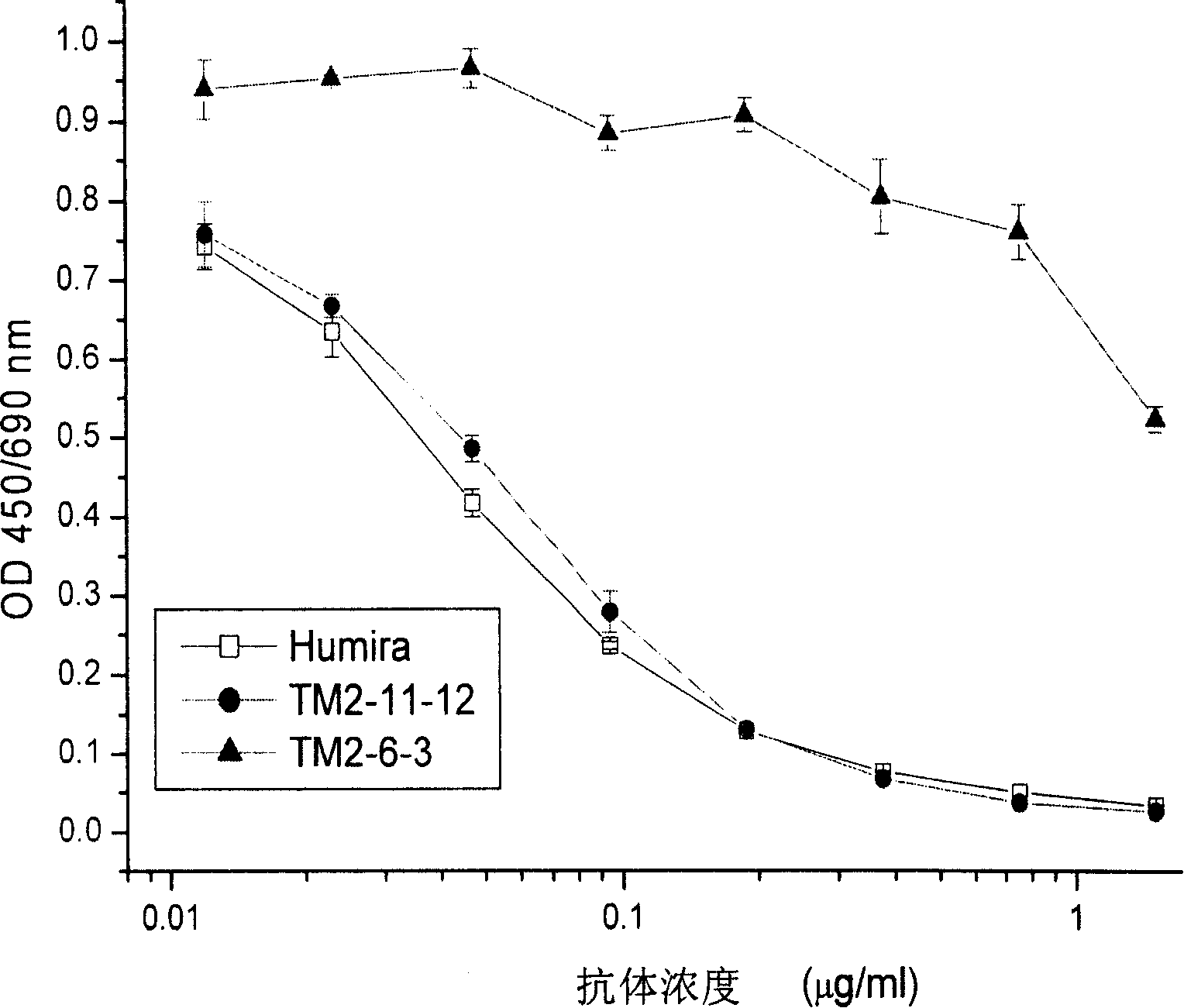
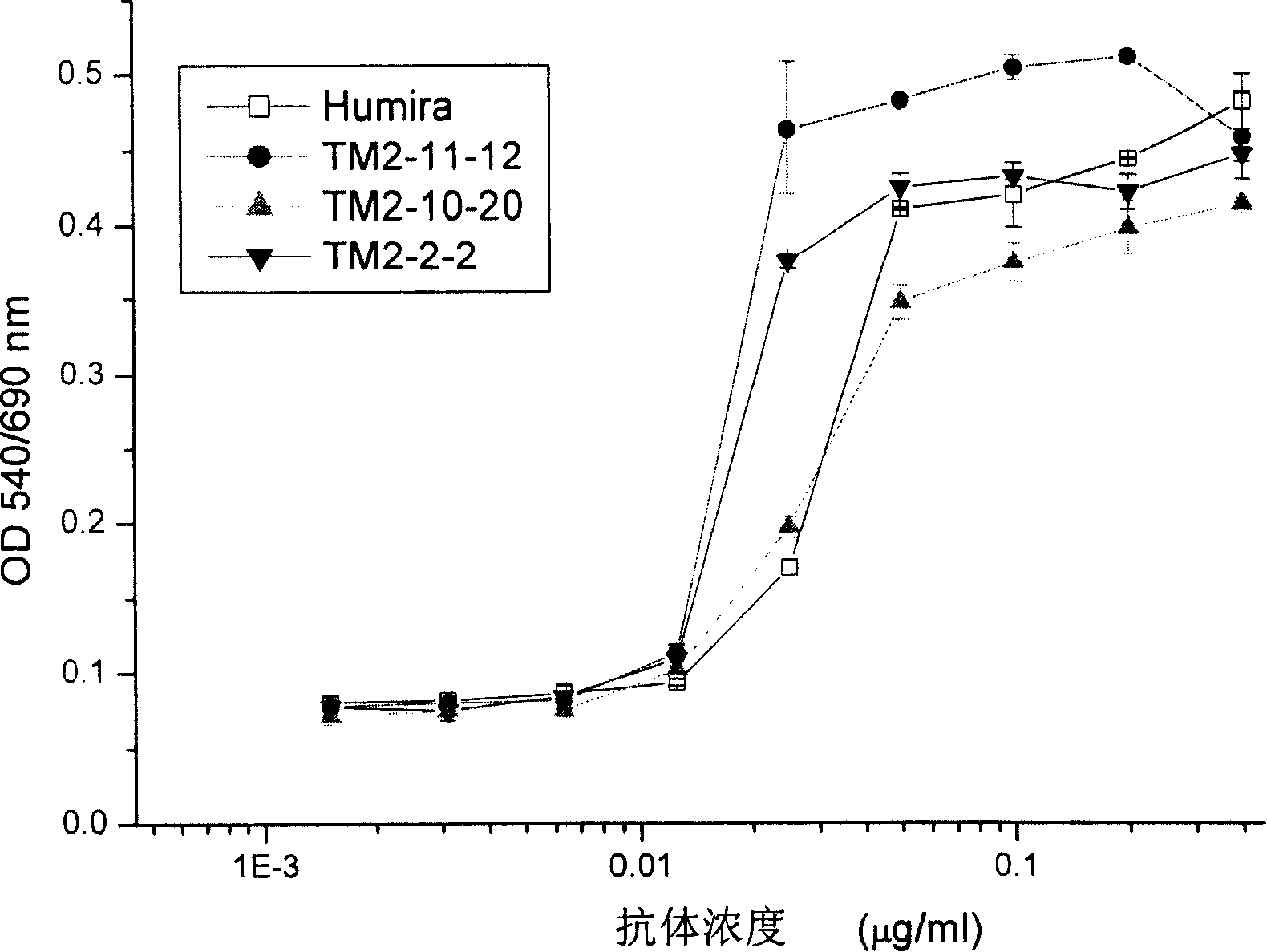
Recombined chimeric antibody against human tumor necrosis factor alpha
ActiveCN101177453AExtended half-lifeFunctionalPeptide/protein ingredientsTumor necrosis factorDiseaseHuman tumor
Described herein are antibodies that bind to human tumor necrosis factor alpha (hTNFα). And list the nucleotide sequence of antibody heavy chain and light chain variable region and its derived amino acid sequence. The heavy chain and light chain variable region genes are respectively connected with the human immunoglobulin gamma 1 (hIgG1) heavy chain constant region and human kappa (k) light chain constant region genes to form a chimeric gene. Then, the vector containing the chimeric gene is introduced into the host cell line to express the antibody protein. This recombinant chimeric antibody protein can neutralize the activity of hTNFα in vitro, and is suitable for treating patients with excessive secretion of harmful hTNFα, including certain inflammations, such as rheumatoid arthritis and Crohn's disease.
Owner:PHARMAB
Recombinant parainfluenza virus expression systems and vaccines
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
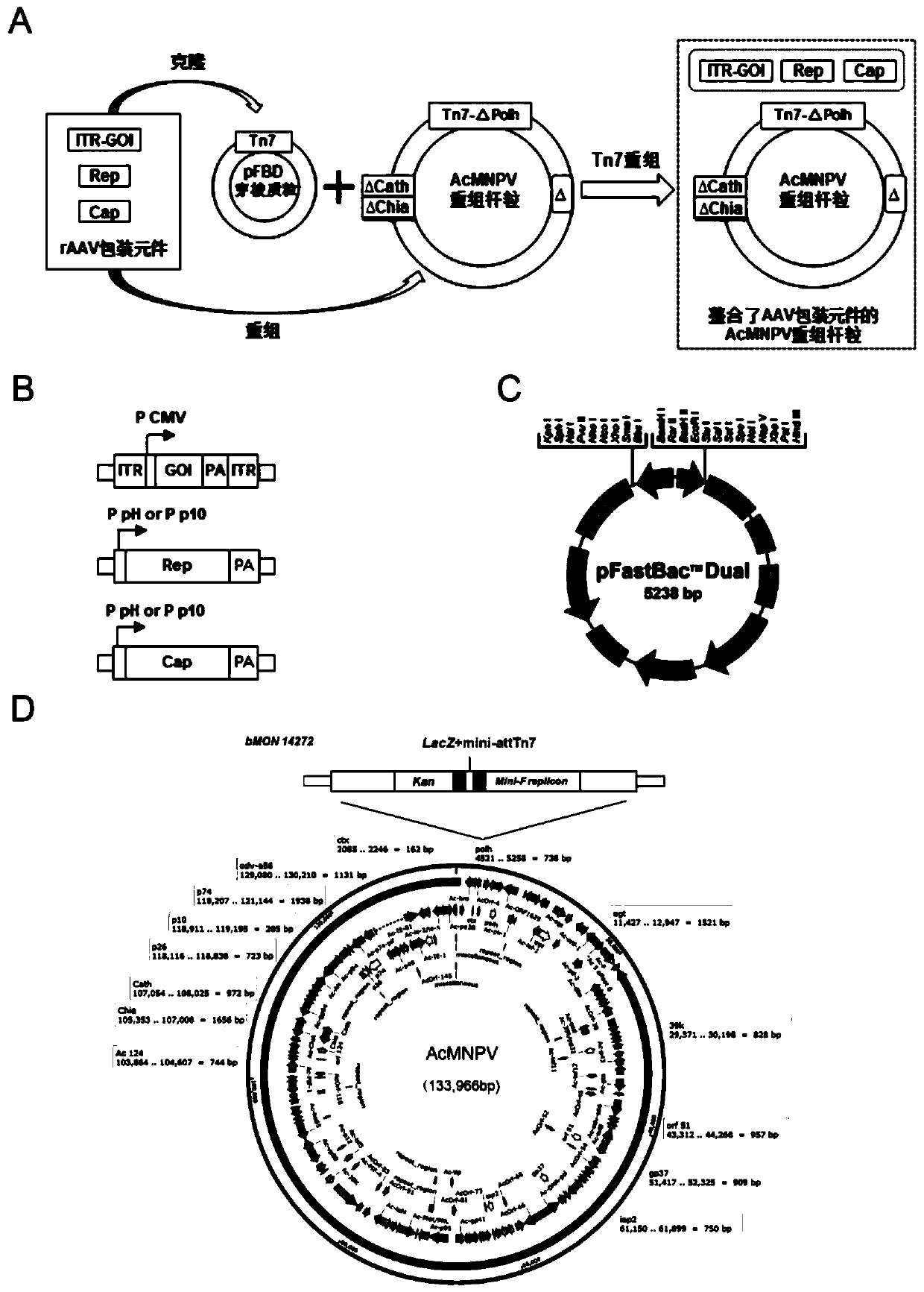
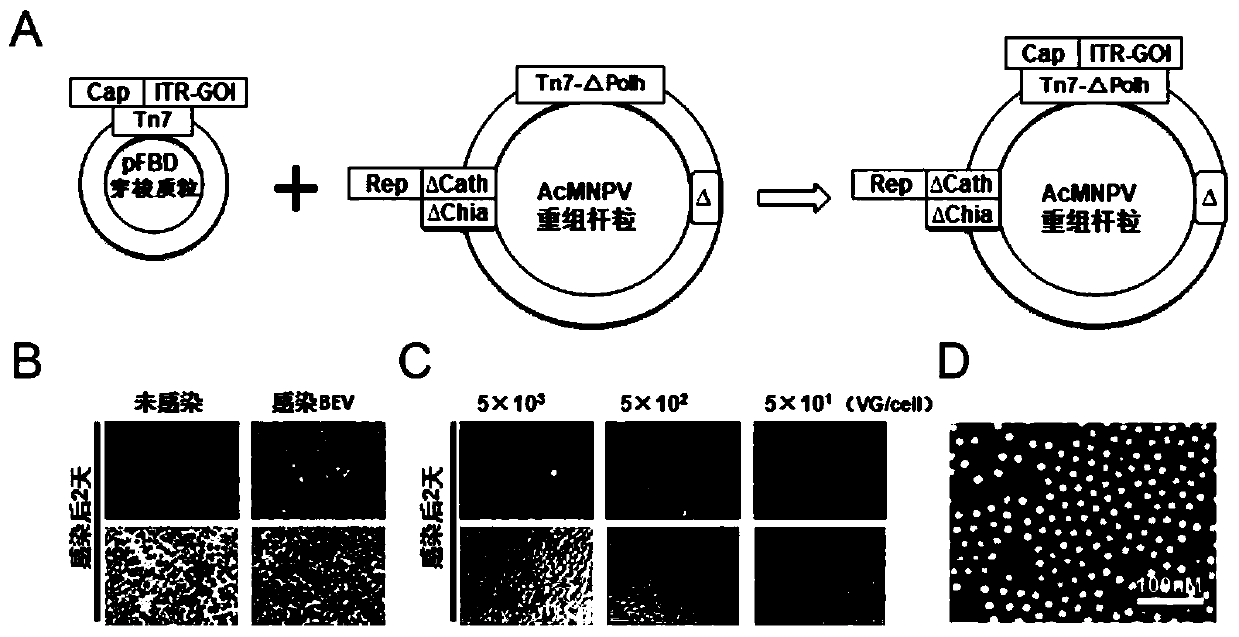
Preparation method and system of recombinant adeno-associated virus and recombinant bacmid
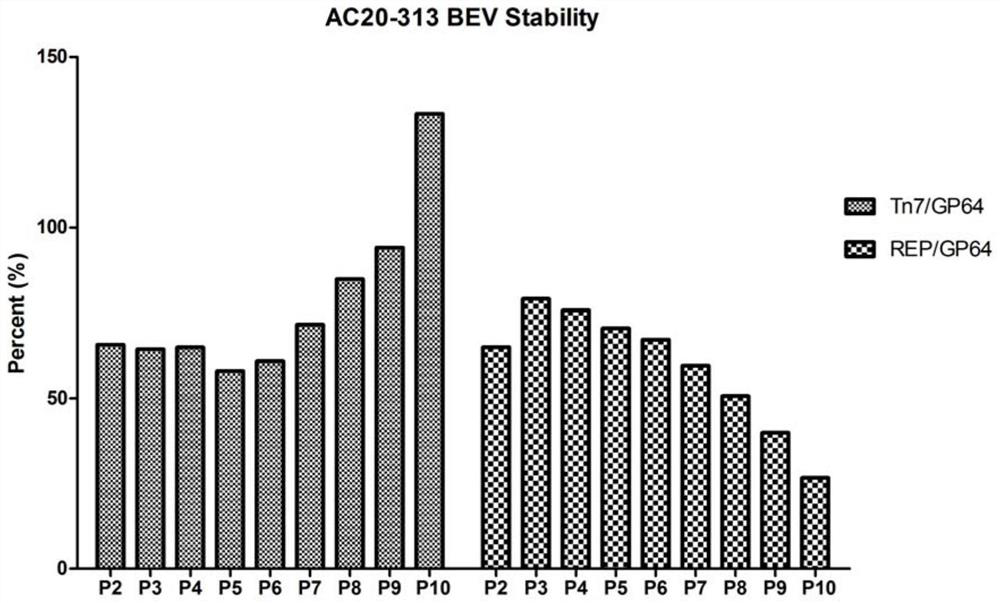
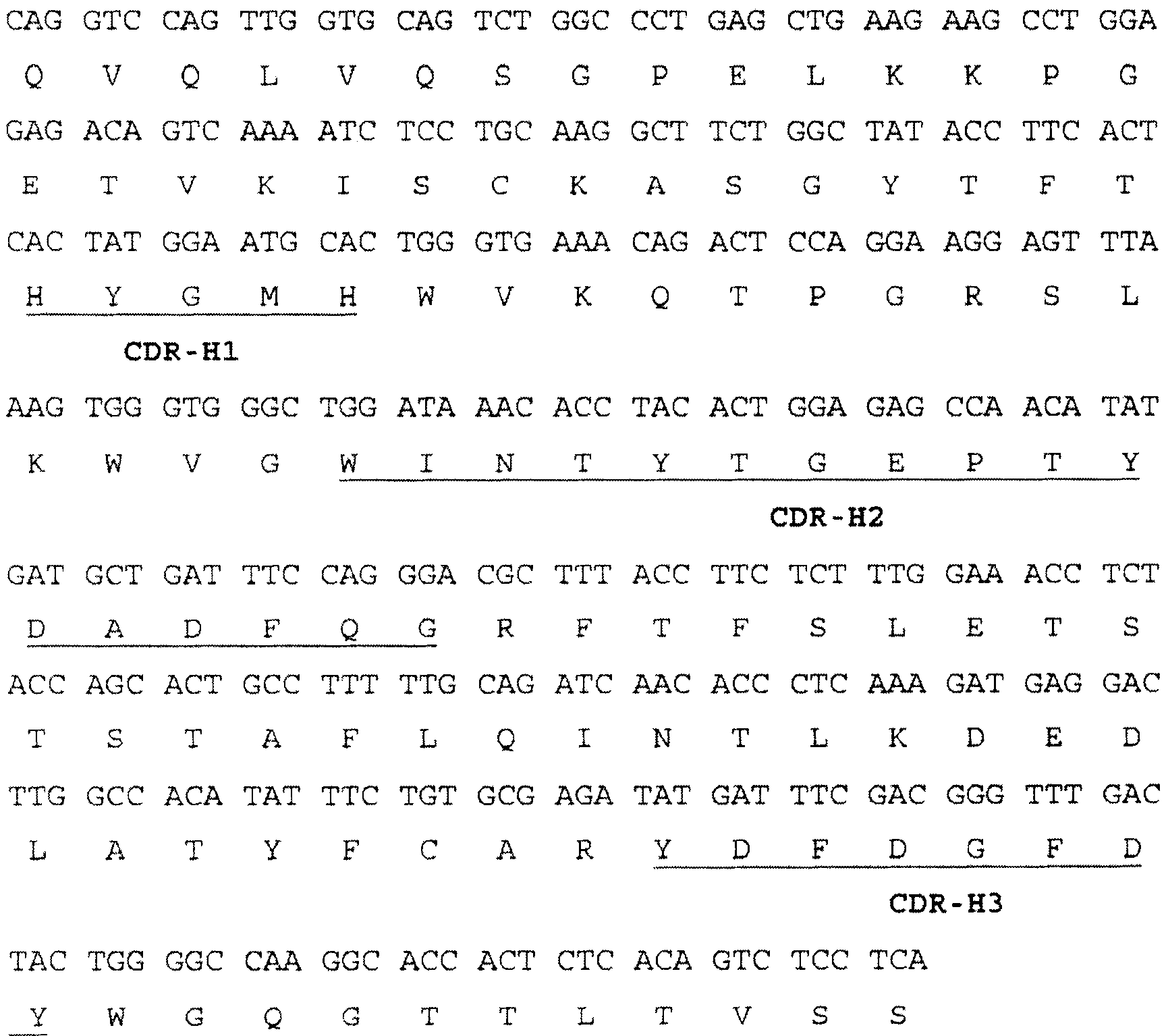
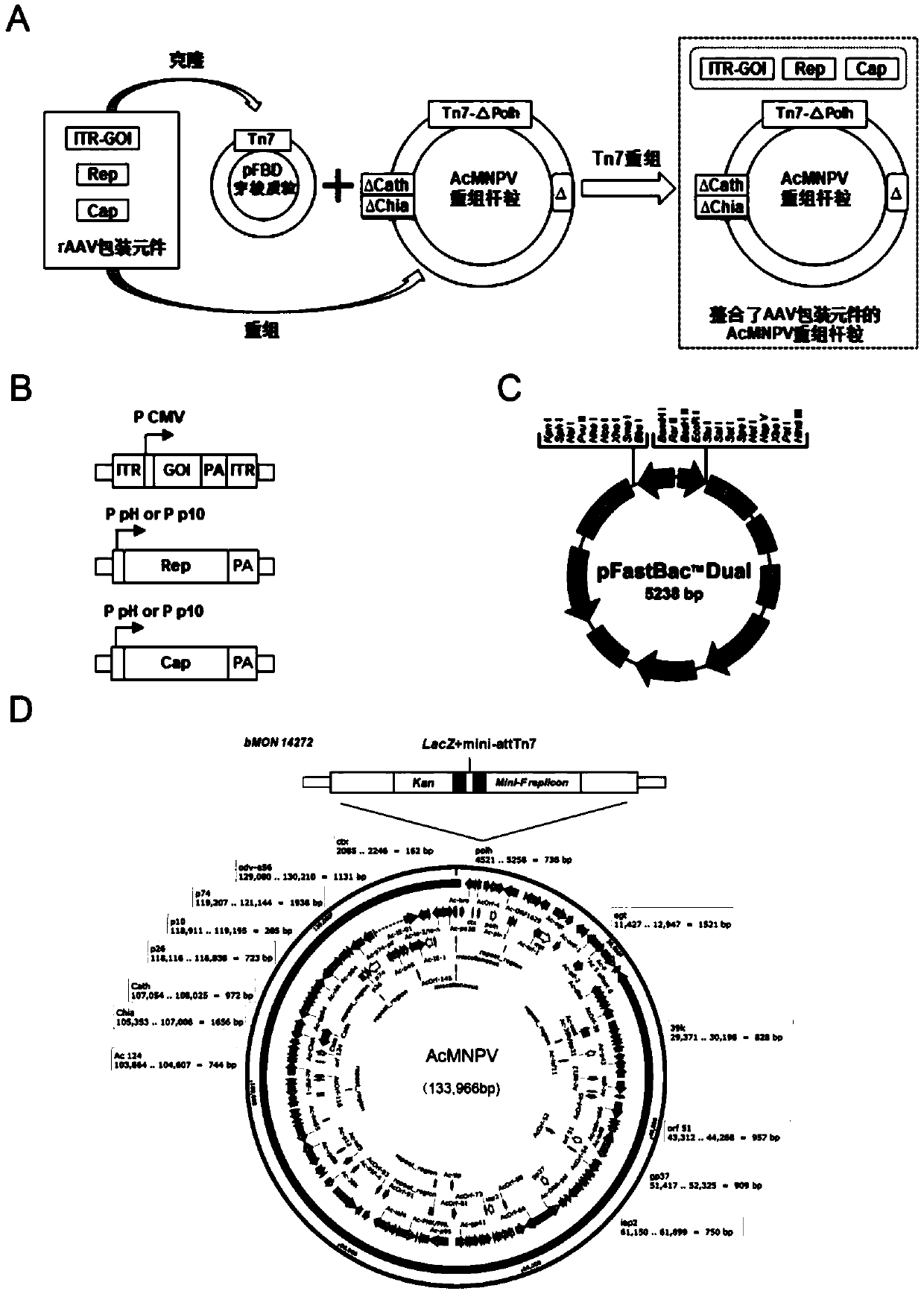
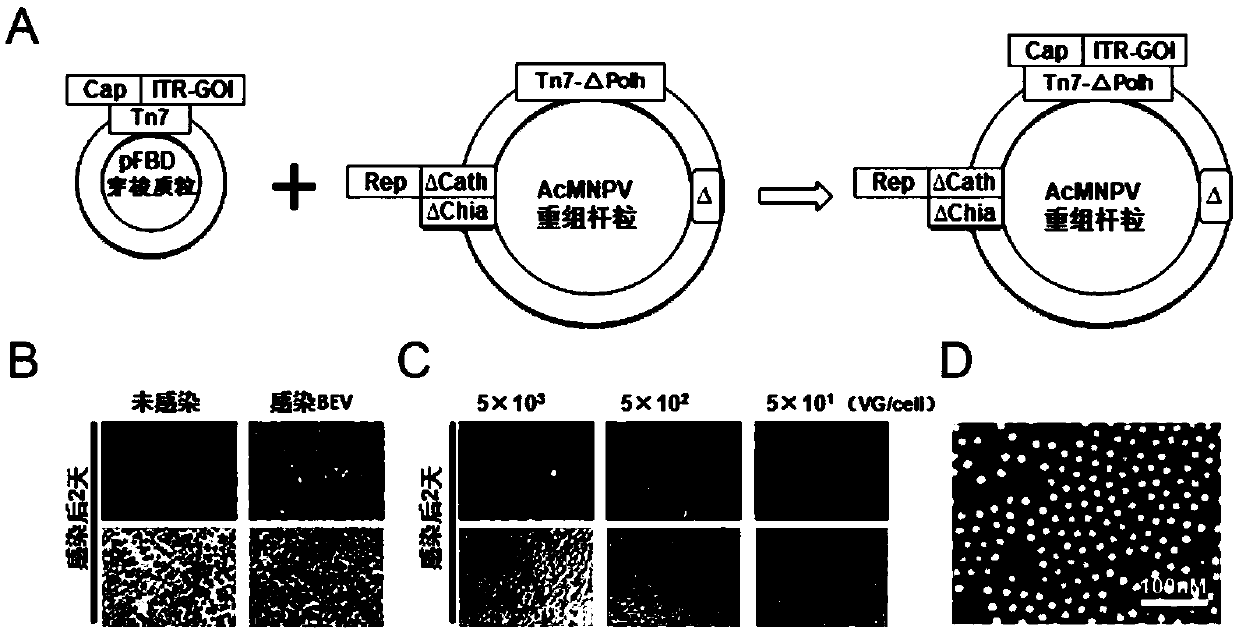
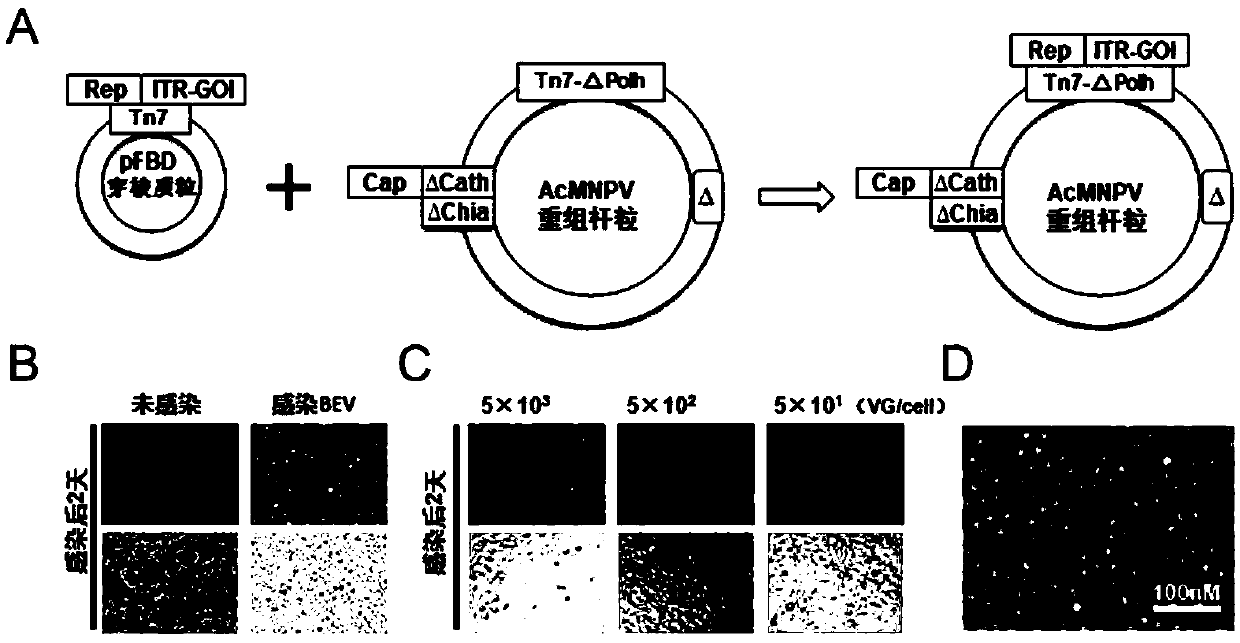
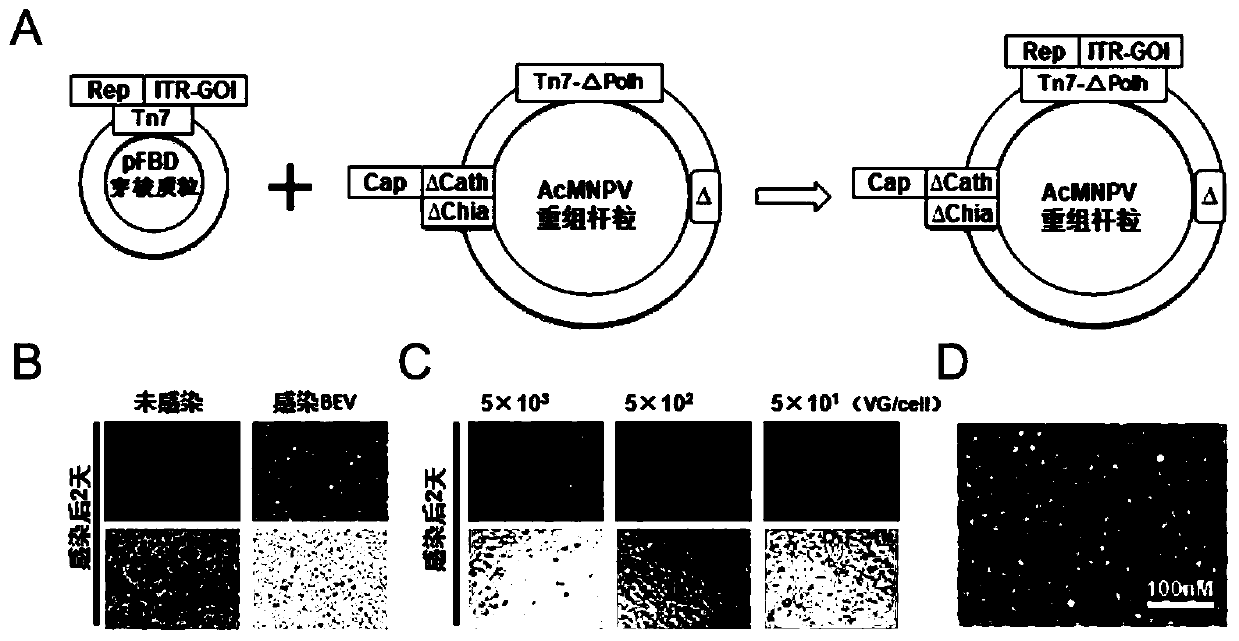
ActiveCN112553257ASolve the technical problem of poor passaging stabilityAddressing massive scale-upVirus peptidesMicroorganism based processesHost cell lineVirus
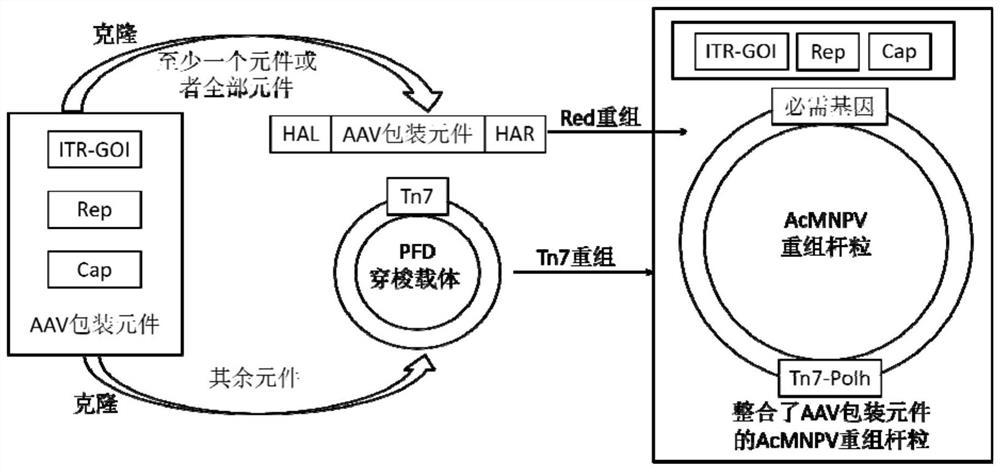
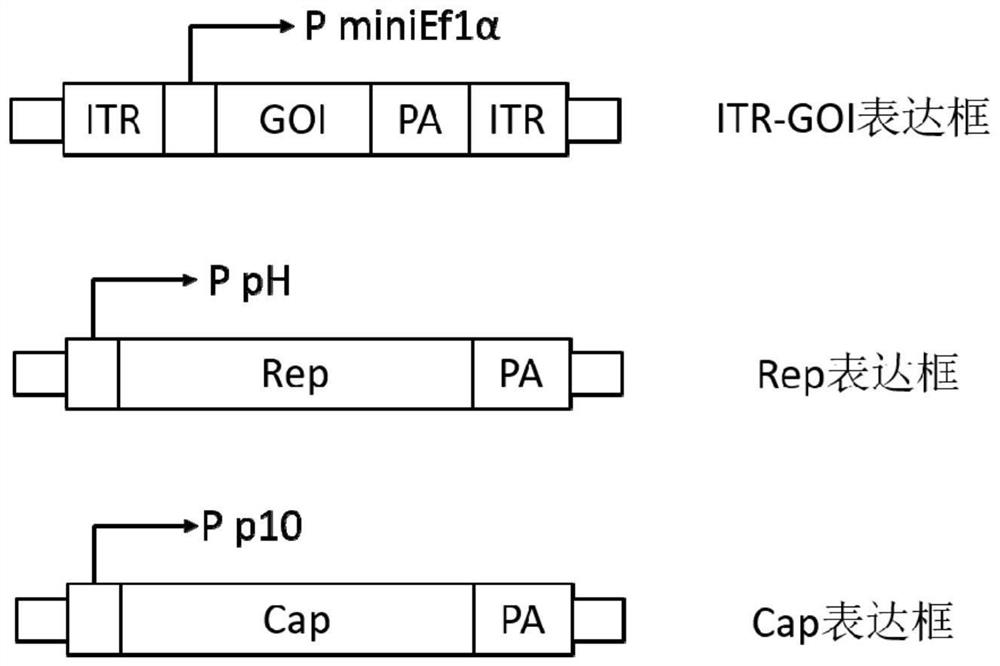
The invention belongs to the field of gene therapy, and particularly relates to a preparation method and system of recombinant adeno-associated virus and recombinant bacmids. Firstly, constructing recombinant bacmids of a recombinant baculovirus genome containing necessary functional elements for producing the recombinant adeno-associated virus; wherein at least one necessary functional element isinserted into the N terminal or the C terminal of the necessary locus of the recombinant baculovirus genome; and then transfecting the obtained recombinant bacmids containing the recombinant baculovirus genome for producing the recombinant adeno-associated virus into a host cell line for culturing to prepare the recombinant adeno-associated virus. Compared with a recombinant baculovirus obtainedby preparing recombinant bacmids through traditional Tn7 recombination, the recombinant baculovirus obtained by inserting core elements containing Cap, Rep and ITR into the two sides of an essential gene of the baculovirus has the advantages that the production level of continuous passage rAAV in cells is more stable, and the rAAV yield is higher.
Owner:JINFAN BIOMEDICAL TECH (WUHAN) CO LTD
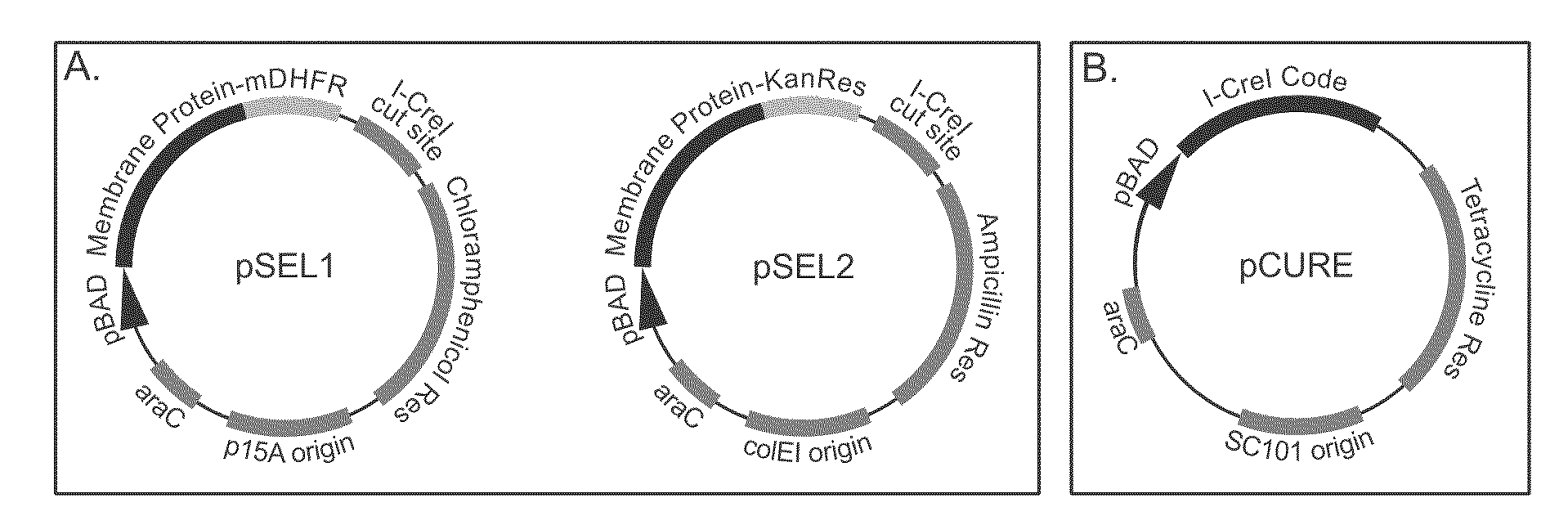
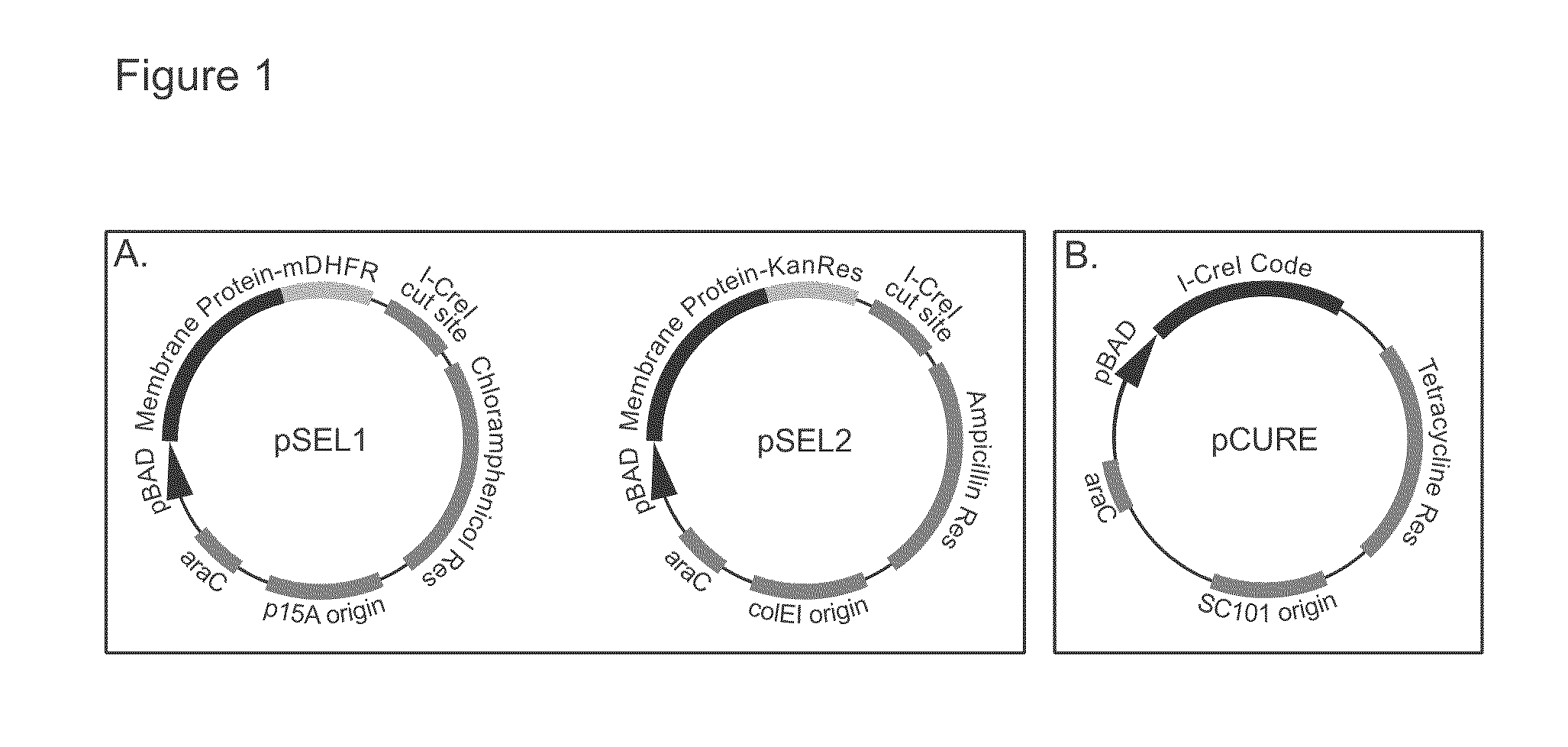
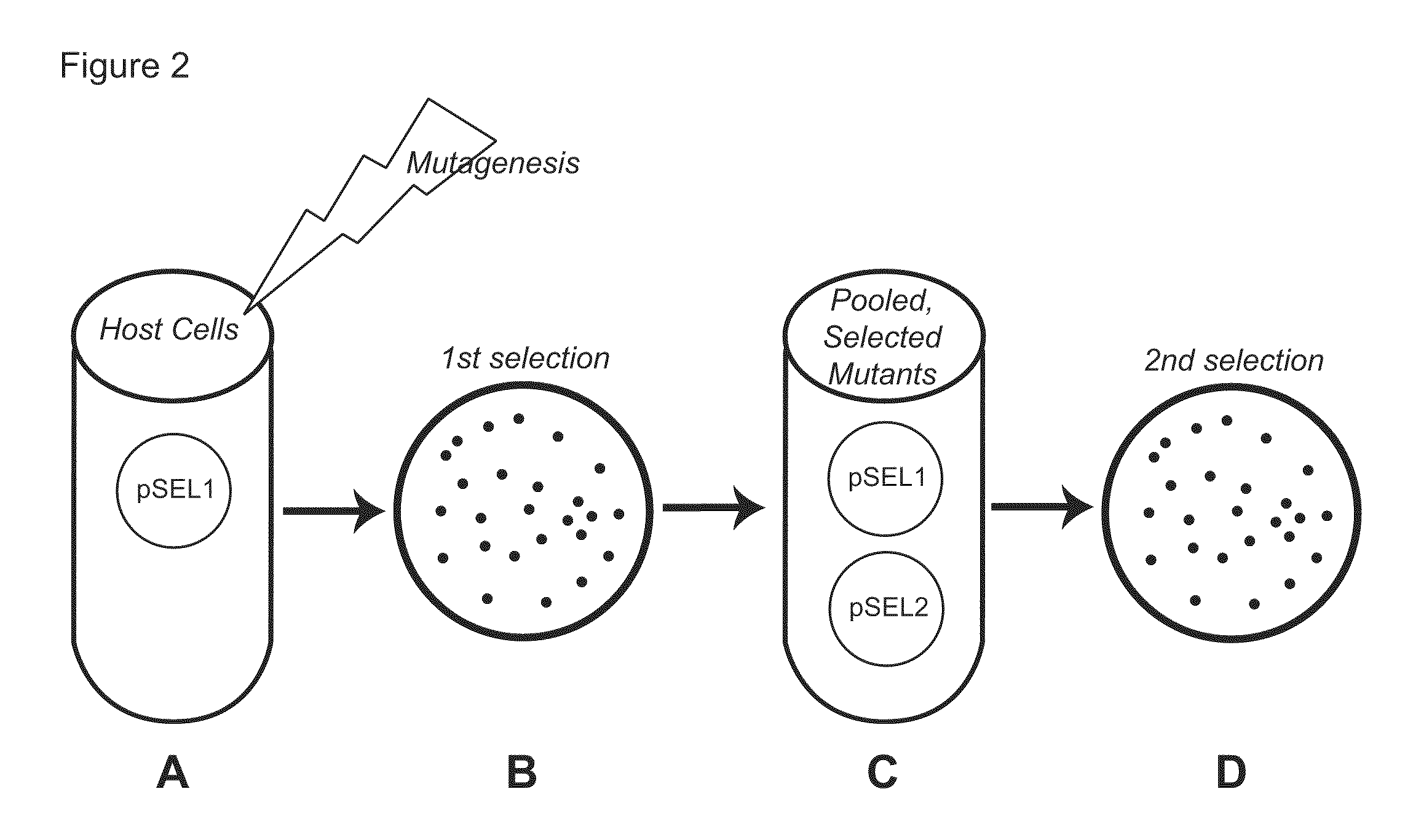
Genetic selection system for improving recombinant protein expression
InactiveUS20100099169A1Improve abilitiesImprove propertiesBacteriaMutant preparationProtein targetHost cell line
A method for selecting host cells with an improved ability to recombinantly overexpress a target protein; the host cells thus generated and their use. The invention also provides a curing method to remove plasmids from host cell lines.
Owner:RGT UNIV OF CALIFORNIA
Recombinant parainfluenza virus expression system and vaccines
InactiveUS20030095987A1Suitable for useSsRNA viruses negative-sensePharmaceutical delivery mechanismAntigenNegative strand
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Recombined chimeric antibody against human tumor necrosis factor alpha
ActiveCN101177453BExtended half-lifeFunctionalPeptide/protein ingredientsTumor necrosis factorDiseaseConstant region gene
The invention discloses an antibody combined with human tumor necrosis factor Alpha (hTNF a), juxtaposing out antibody heavy chain and nucleotide sequence of light chain variable region, and the amino acid sequence derived from the nucleotide sequence. The heavy chain and the light china are connected into chimeric genes with the genes in the human immunoglobulin gamma 1(hIgG1) heavy china constant region and in the kappa (k) light chain constant region respectively, then the carrier with the chimeric genes is introduced into the host cell line expressing antibodies protein. The restructuringchimeric antibody protein is tested outside human body and can neutralize the hTNF Alpha activity. The invention is suitable for treating disease of excessive harmful hTNF Alpha secretion, comprisinga plurality of inflammations such as rheumatoid arthritis and regional enteritis.
Owner:PHARMAB
Preparation method and system of recombinant adeno-associated virus (rAAV), and recombinant bacmid
ActiveCN109609552AImprove passaging stabilityImprove compatibilityVirus peptidesFermentationHeterologousShuttle plasmid
The invention discloses a preparation method and system of recombinant adeno-associated virus (rAAV), and recombinant bacmid. The method comprises: (1) separately preparing shuttle plasmids and a corresponding recombinant bacmid containing baculovirus genome; (2) integrating a rAAV core expression element which has a heterologous functional gene fragment with other expression cassettes which produce functional protein components necessary for rAAV so as to obtain a recombinant bacmid containing recombinant baculovirus genome producing the rAAV; and (3) transfecting the obtained recombinant bacmid into a host cell line for culturing. The system comprises the shuttle plasmids and the corresponding recombinant bacmid containing the baculovirus genome. The recombinant bacmid comprises at leastone expression cassette that produces functional protein components necessary for rAAV. The system has flexibility, compatibility, and higher passage stability.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Compositions and methods for inducing gene expression
InactiveUS20060127361A1Increased formationHigh expressionOrganic active ingredientsBiocideA-DNABiological activation
The present invention provides recombinant nucleic acid molecules encoding a chimeric transactivator protein including a DNA binding domain of a DNA binding protein and a protein domain capable of transcriptional activation. The present invention also provides recombinant viral and non-viral vectors that are able to infect and / or transfect and sustain expression of a biologically active chimeric transactivator proteins in mammalian cells. Also provided are host cell lines and non-human transgenic animals capable of expressing biologically active chimeric transactivator proteins. In another aspect, compositions and methods for treating or preventing ischemic damage associated with hypoxia-related disorders are provided.
Owner:GENZYME CORP
Improved cell lines having reduced expression of NOCR and use thereof
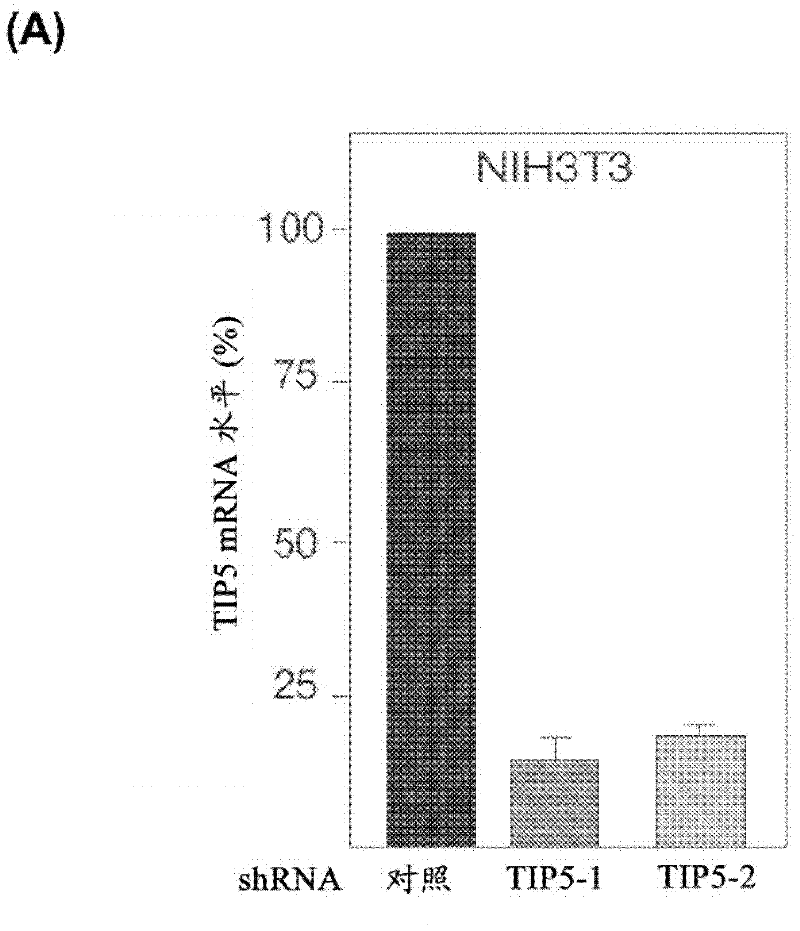
InactiveCN102414320AIncrease productionImprove developmentFermentationVector-based foreign material introductionControl cellRibosomal RNA
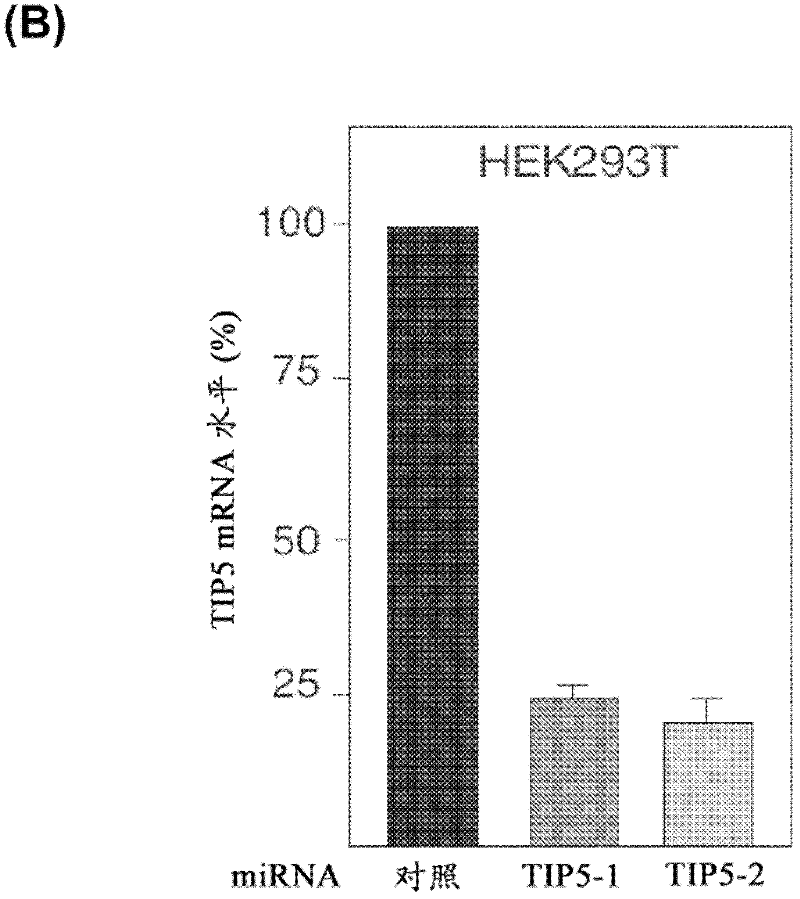
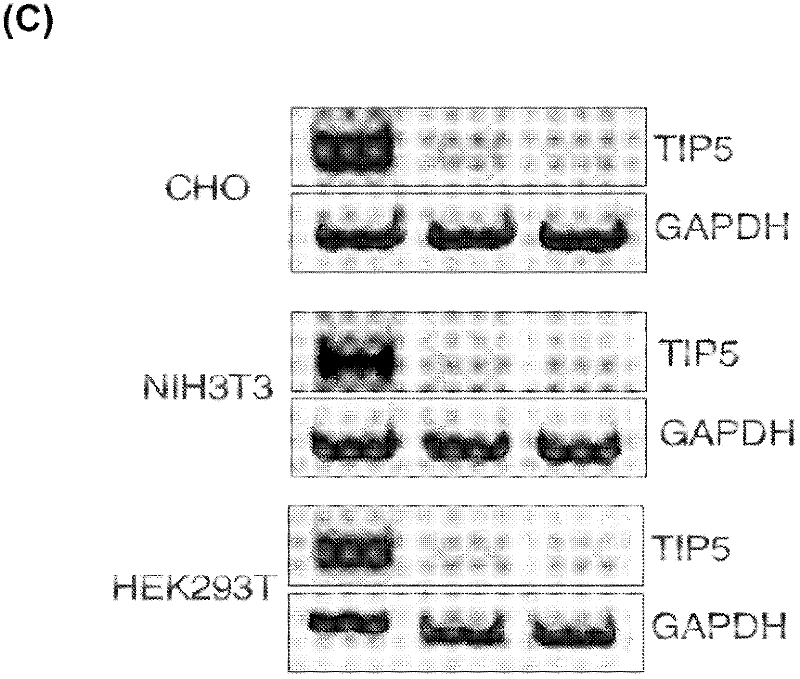
The invention concerns the field of cell culture technology. It concerns production host cell lines with increased expression of ribosomal RNA (rRNA) achieved through reducing expression of NoCR proteins, especially of TIP-5. Those cell lines have improved secretion and growth characteristics in comparison to control cell lines. The invention further concerns a method of producing proteins using the cells generated by the described method.
Owner:BOEHRINGER INGELHEIM INT GMBH
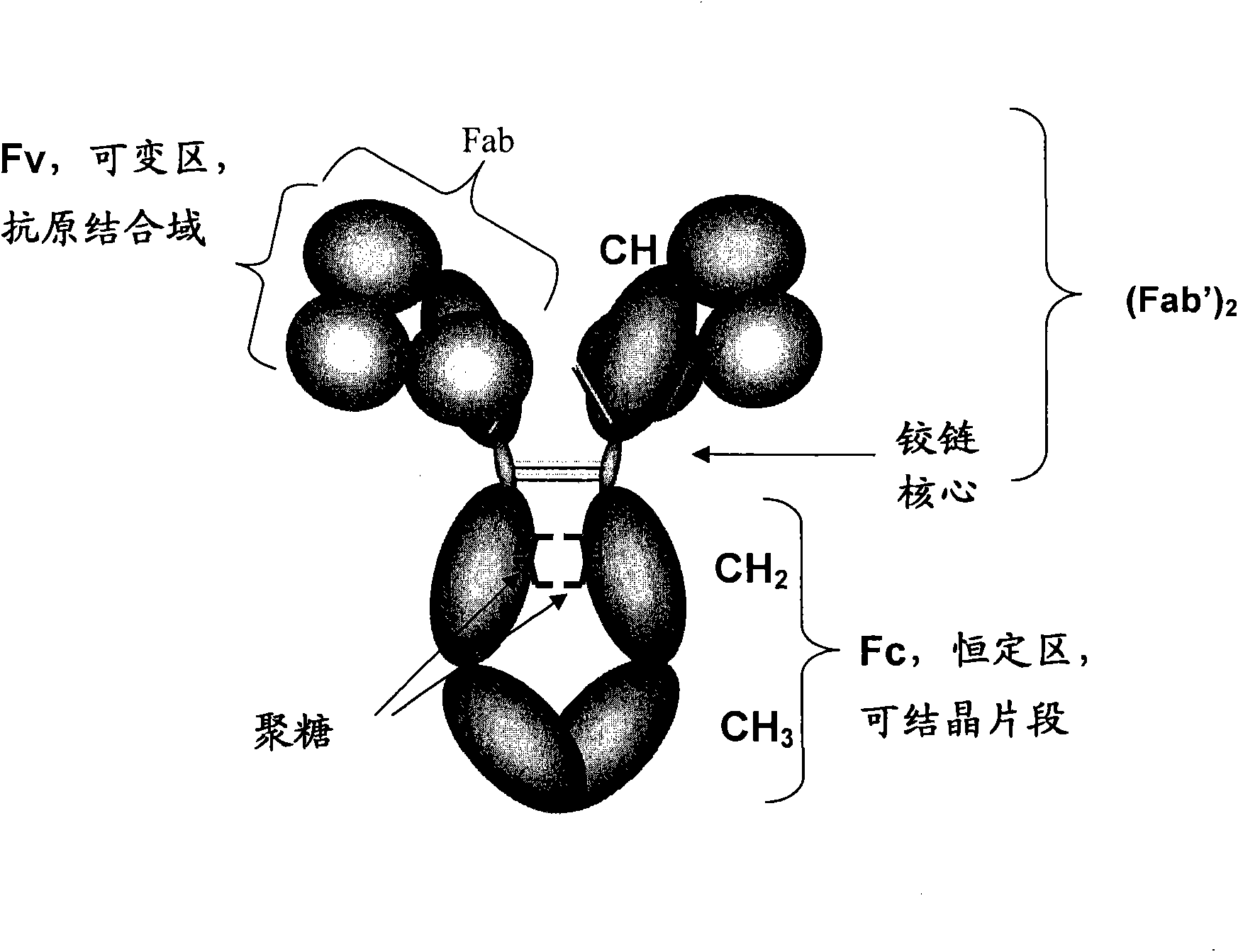
Host cell lines for production of antibody constant region with enhanced effector function
InactiveCN101627111AReduce or prevent growthHigh activityAntibody ingredientsTissue cultureSerum free mediaChemical composition
Host cell lines for biopharmaceutical production of antibodies, antibody fragments or antibody-derived fusion proteins are selected as having the capability of inducing improved cellular effector functions, e.g., Fc-medicated effector functions. The host cells are derived from the rat myeloma cell line YB2 / 0 and are adapted to growth in chemically-defined medium.
Owner:CENTOCOR
Recombinant parainfluenza virus expression systems and vaccines
InactiveUS20040208895A1SsRNA viruses negative-senseMicroorganism based processesHeterologousNegative strand
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE VACCINES
Adeno-associated virus materials and methods
The present invention provides adeno-associated virus (AAV) materials and methods which are useful for DNA delivery to cells. More particularly, the invention provides recombinant AAV (rAAV) genomes, methods for packaging rAAV genomes, stable host cell lines producing rAAV and methods for delivering genes of interest to cells utilizing the rAAV. Particularly disclosed are rAAV useful in generating immunity to human immunodeficiency virus-1 and in therapeutic gene delivery for treatment of neurological disorders.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Cross-barrier targeted drug delivery system for lesions, drug-carrier system and host cell system
InactiveCN109517843AEfficient deliverySecure and Directed DeliveryOther foreign material introduction processesMacromolecular non-active ingredientsVirus-like particleDrug carrier
The invention relates to a cross-barrier targeted drug delivery system for lesions, a drug-carrier system and a host cell system. The drug delivery system comprises cross-barrier virus-like particlesand lesion-targeting virus-like particles, wherein the cross-barrier virus-like particles carry cross-barrier signals, and the lesion-targeting virus-like particles carry lesion-targeting signals. Thecross-barrier virus-like particles and the lesion-targeting virus-like particles are capable of performing self-assembly to form heteropoly virus-like particles. The drug delivery system has the dualfunctions of cross-barrier and targeted transportation to the lesions, and meanwhile, the virus-like particles have the advantages of safety and high transportation efficiency.
Owner:BEIJING DIRECTION BIOTECH CO LTD
Mixtures of antibodies
ActiveCN109475627APharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsHost cell lineChemistry
Described herein are antibodies and mixtures of antibodies optionally produced by a host cell line, nucleic acids encoding the antibodies and mixtures of antibodies, host cells containing such nucleicacids, and methods of treatment using the antibodies, mixtures of antibodies, or nucleic acids encoding the antibodies or mixtures of antibodies. Also described are methods of producing mixtures of antibodies in host cells.
Owner:QILU PUGET SOUND BIOTHERAPEUTICS CORP
Human-mouse chimeric anti-CD147 antibody with non-fucosylated glycosylation
The present disclosure relates to a nucleotide sequence comprising the sequence of SEQ ID NO: 5, and / or sequence of SEQ ID NO: 6, vector and host cell line comprising the nucleotide. The present disclosure also relates to an antibody that binds to extracellular region of human CD147, wherein the antibody comprises a heavy chain variable region having the amino acid sequence of SEQ ID NO: 2, and / or a light chain variable region having the amino acid sequence of SEQ ID NO: 1, and the antibody contains a glycoform lacking both fucose residues and xylose residues, pharmaceutical composition comprising the antibody or fragment thereof, the method of producing the antibody or fragment thereof, and use of the pharmaceutical composition in treatment of CD147 expression-related diseases.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
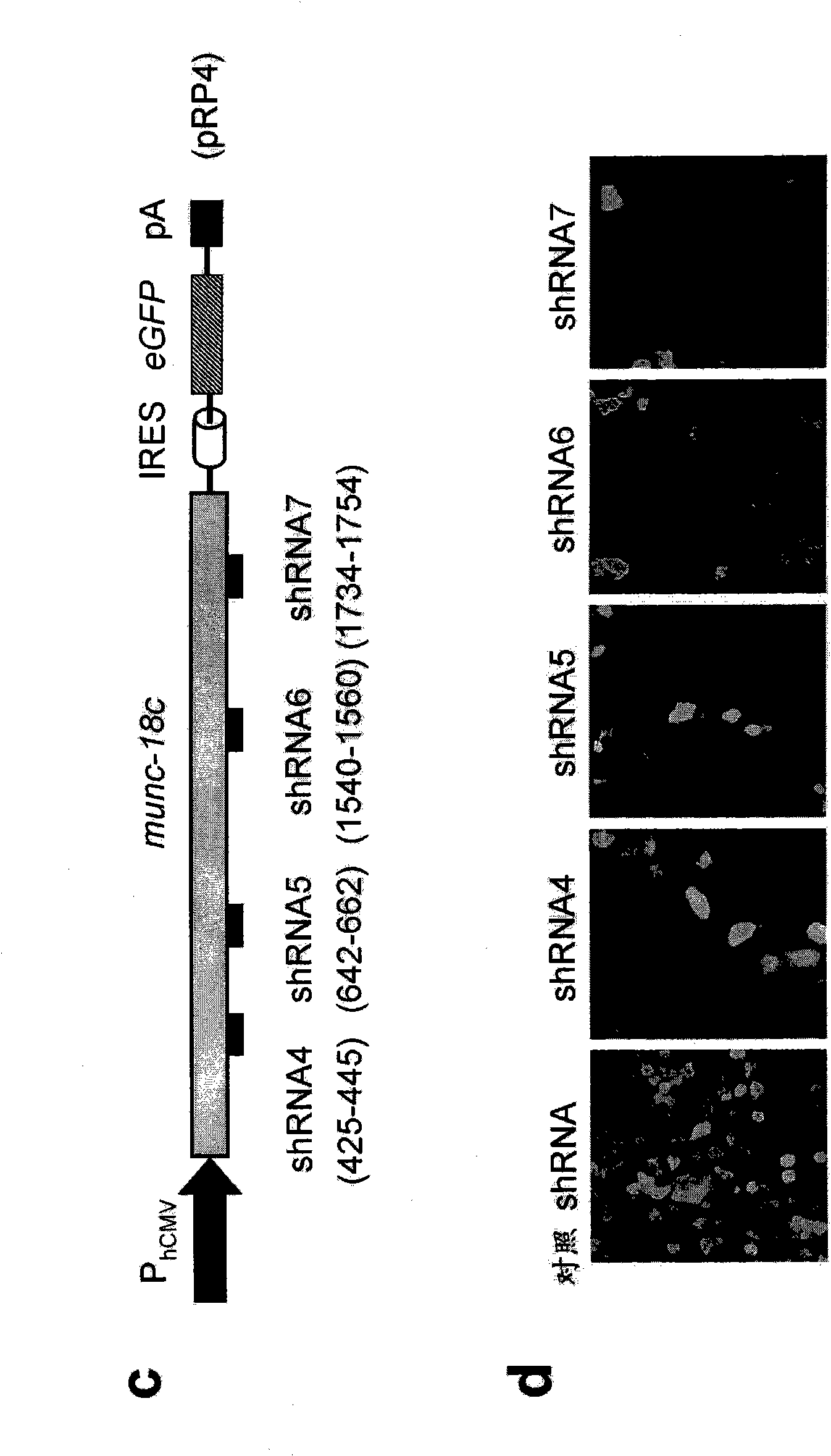
SM-protein based secretion engineering
The present invention concerns the field of cell culture technology. It describes a novel method for enhancing the secretory transport of proteins in eukaryotic cells by heterologous expression of Munc18c, Sly1 or other members of the SM protein family. This method is particularly useful for the generation of optimized host cell systems with enhanced production capacity for the expression and manufacture of recombinant protein products.
Owner:BOEHRINGER INGELHEIM PHARM KG
Dual expression vector system and screening methods
Owner:VERTEX PHARMA INC
Preparation method, system and recombinant bacmid of recombinant adeno-associated virus
ActiveCN109609552BImprove passaging stabilityImprove compatibilityVirus peptidesFermentationHeterologousShuttle plasmid
The invention discloses a preparation method, system and recombinant bacmid of recombinant adeno-associated virus (rAAV). The method comprises: (1) separately preparing shuttle plasmids and their corresponding recombinant bacmids containing the baculovirus genome; (2) combining rAAV core expression elements with heterologous functional gene fragments with other functions necessary to produce rAAV The expression frame of the protein component is integrated to obtain a recombinant bacmid comprising the recombinant baculovirus genome producing the rAAV; (3) transfected into a host cell line for culture. The system includes a shuttle plasmid and its corresponding recombinant bacmid containing the baculovirus genome. The recombinant bacmid contains at least one expression box of functional protein components required for rAAV production. The invention has flexibility, compatibility and higher generation stability.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Medium being able to optimize virus replication
InactiveCN105985446AImprove expression efficiencyIncrease Titer TiterViral antigen ingredientsMicroorganism based processesCytotoxicityHerpes simplex virus DNA
The invention relates to a medium being able to optimize virus replication. The medium contains a fusion protein E with the concentration of 100-1000nM, and a cell line is a host cell line over-expressing the fusion protein E gene. The fusion protein E can improve the TCID50 tilter of various viruses by 1-3 lg units and improve the HA tilter by 2-4 lg2 units, the various viruses comprise coronavirus, paramyxovirus, orthomyxovirus, reovirus and herpes virus, the fusion protein E has no cytotoxicity in a prescribed dosage, breaks a boundary between DNA viruses and RNA viruses, and also breaks the boundary between viruses with envelopes and viruses without envelopes. The fusion protein E obtained through a prokaryotic expression system has a high expression efficiency, and can be easily purified, the one-step purification efficiency can reach 85% or above, and the final concentration of the protein can reach 3mg / ml. The fusion protein E can be directly added to the virus medium in order to improve the tilter of various virus vaccines, so the medium is simple and fast. The medium has great influences and meanings in the production, learning and research of the virus subject.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com