Preparation method and system of recombinant adeno-associated virus and recombinant bacmid
A technology of recombinant baculovirus and virus, which is applied in the field of preparation of recombinant adeno-associated virus, can solve the problems of poor passaging stability of BEV and easy loss of foreign genes, etc., achieve good system compatibility, improve passaging stability, and improve overall stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
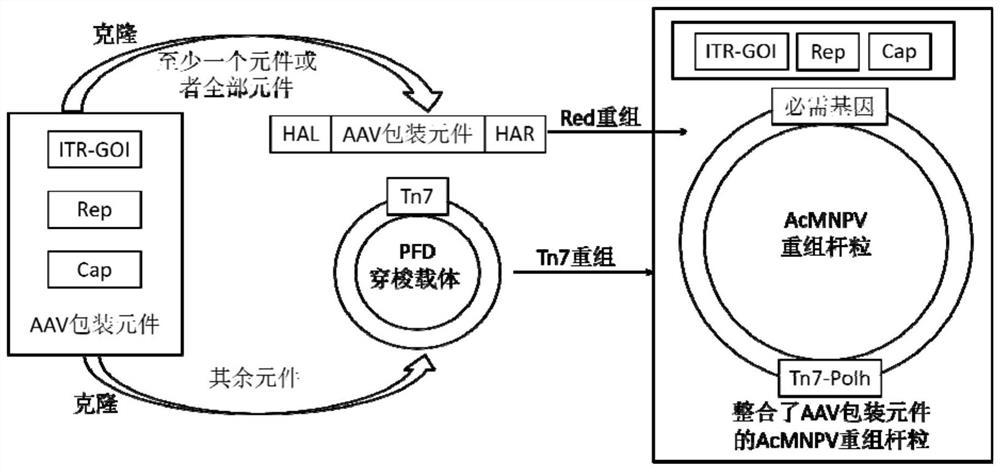
[0060] A method for preparing recombinant adeno-associated virus provided by the invention, such as figure 1 shown, including the following steps:
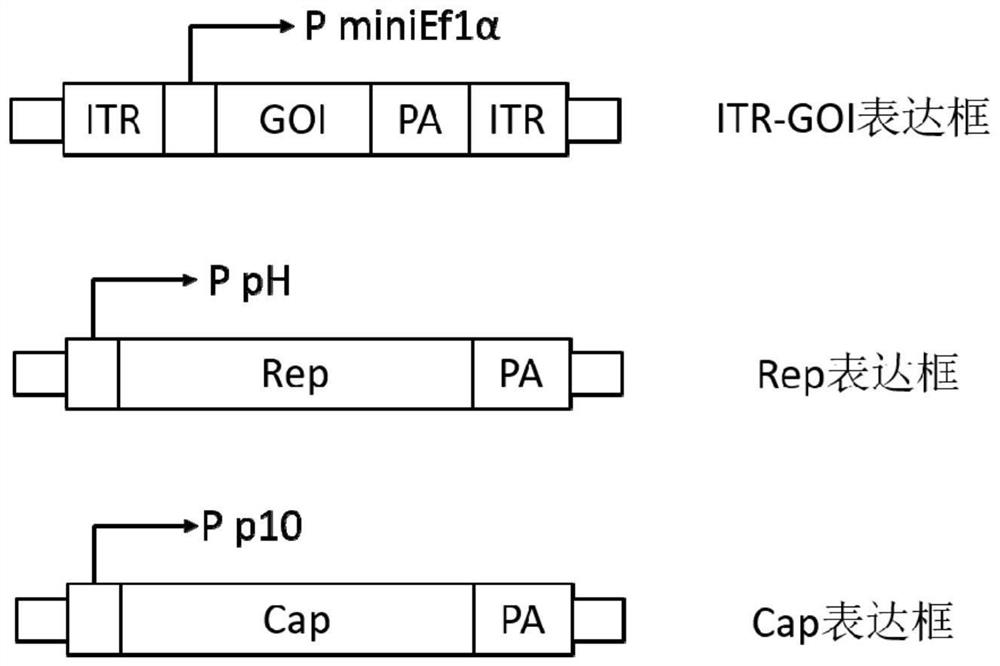
[0061] (1) Constructing a recombinant bacmid comprising a recombinant baculovirus genome for producing a recombinant adeno-associated virus; the recombinant baculovirus genome contains essential functional elements for producing the recombinant adeno-associated virus, and the essential functional elements include the Cap gene , Rep gene and core expression element ITR-GOI with exogenous target gene;
[0062] (2) Transfecting the recombinant bacmid comprising the recombinant baculovirus genome for producing recombinant adeno-associated virus obtained in step (1) into a host cell line for culture;
[0063] Wherein, at least one of the essential functional elements is inserted into the N-terminal or C-terminal of the essential gene locus of the recombinant baculovirus genome. The N-terminal or C-terminal of the essential gene locus...
Embodiment 1
[0103] rAAV was prepared using DH10Bac-Cap-Rep(Ac135)-Tn7(ITR-GOI).
[0104] This embodiment includes a homologous recombination vector and a shuttle vector: the homologous recombination vector targets the essential gene Ac135 gene, contains the expression cassettes of functional protein components necessary for the production of rAAV, the Chol expression cassette, and the ETL-EGFP expression cassette, wherein Chol The expression cassette is used as a screening marker for Red homologous recombination positive clones, and the ETL-EGFP expression cassette is used for TCID of BEV 50 Titer determination; the shuttle vector contains the core expression element ITR-GOI expression cassette of rAAV.
[0105] The method for preparing rAAV using the rAAV preparation system provided in this embodiment includes the following steps:
[0106] 1.1 Construction of a homologous recombination vector comprising the essential functional elements Cap and Rep gene expression cassettes described in...
Embodiment 2
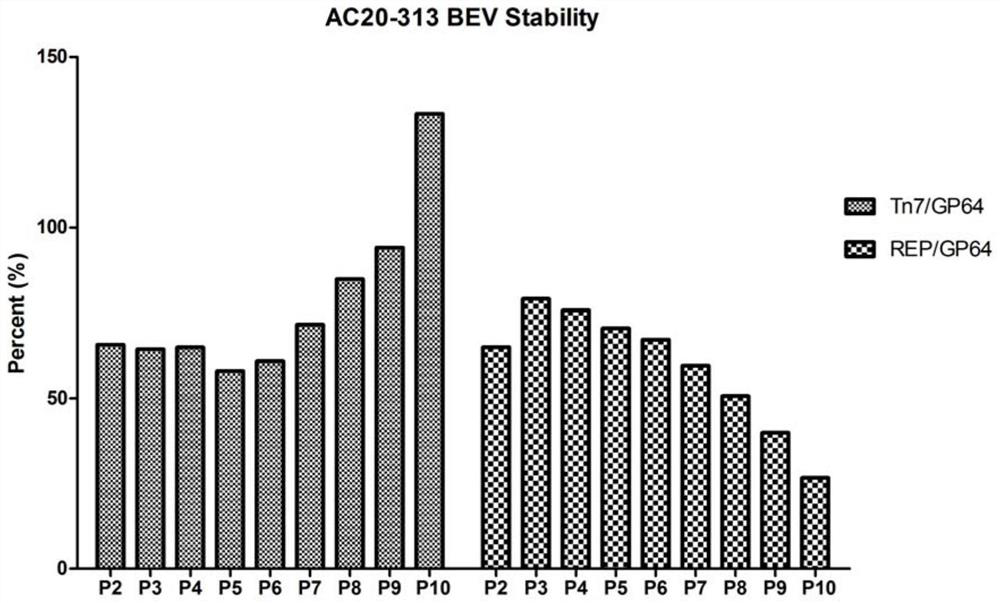
[0128] In view of Example 1, we targeted the rAAV capsid protein gene expression cassette (Cap) and the Rep gene expression cassette (Rep) to the essential gene Ac135 gene, and obtained BEV stability and rAAV packaging rate better than placing Rep / Cap in Recombinant baculovirus bacmid at the PH locus of the non-essential gene. Therefore, in the following examples, Rep / Cap is placed next to the essential gene GP64, and the core expression element ITR-GOI expression cassette of rAAV is placed at the C-terminus of various essential genes of baculovirus to evaluate the stability of BEV Sex and packaging rate of rAAV.
[0129] rAAV was prepared using DH10Bac-ΔCC-Cap-Rep(GP64)-ITR-GOI(Ac135).
[0130] This example contains two homologous recombination vectors: one is a homologous recombination vector targeting the essential gene GP64 containing the Cap and Rep expression cassettes, the Chol expression cassette and the ETL-EGFP expression cassette containing the functional protein c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com