Patents
Literature
458 results about "Alphabaculovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alphabaculovirus is a genus of viruses in the family Baculoviridae. Its natural hosts include a wide range of invertebrates, among them winged insects, Lepidopterans, Hymenopterans, Dipterans, and decapods. There are currently 47 species in the genus, including the type species Autographa californica multiple nucleopolyhedrovirus.
Therapy of cancer by insect cells containing recombinant baculovirus encoding genes
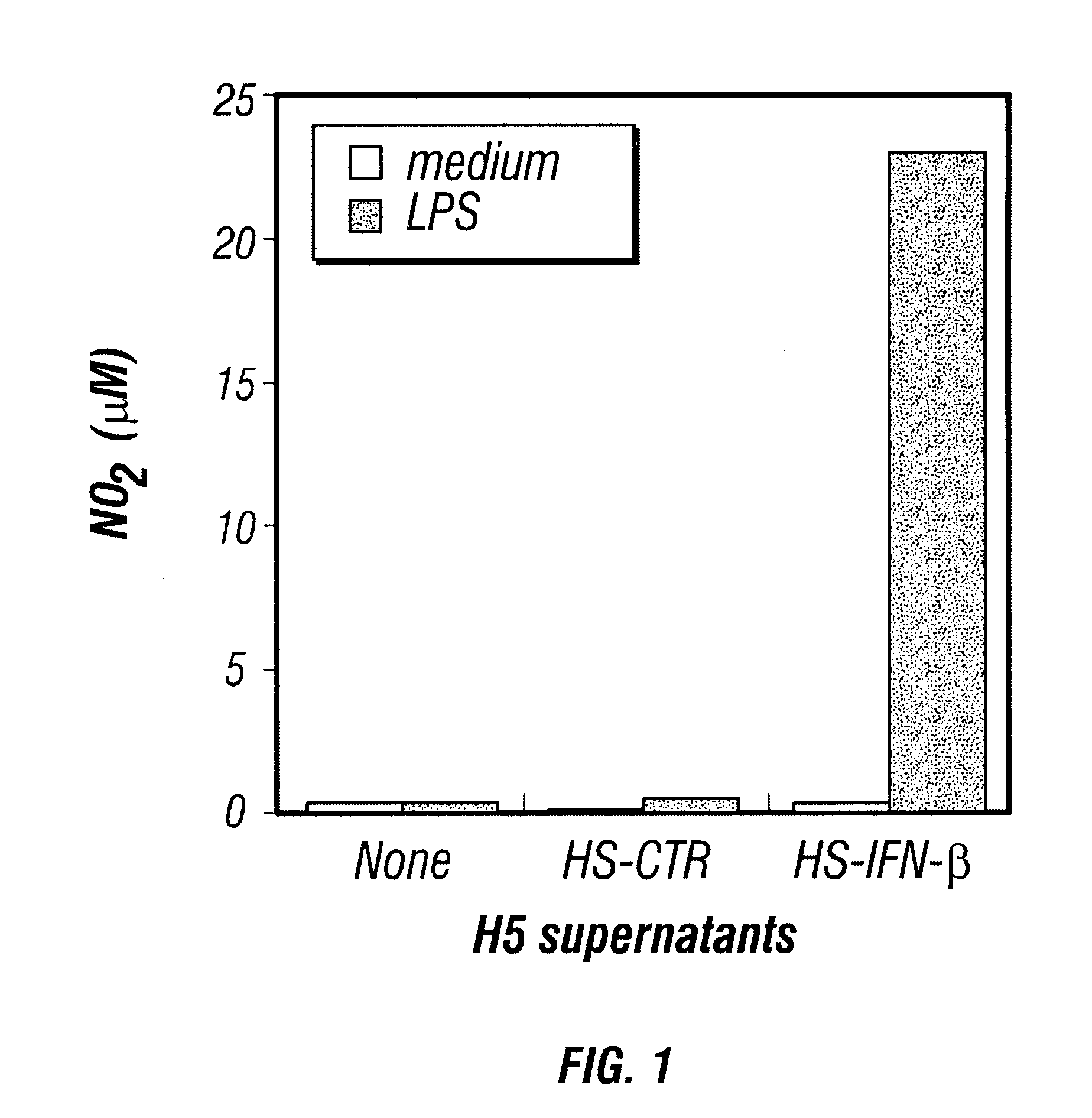
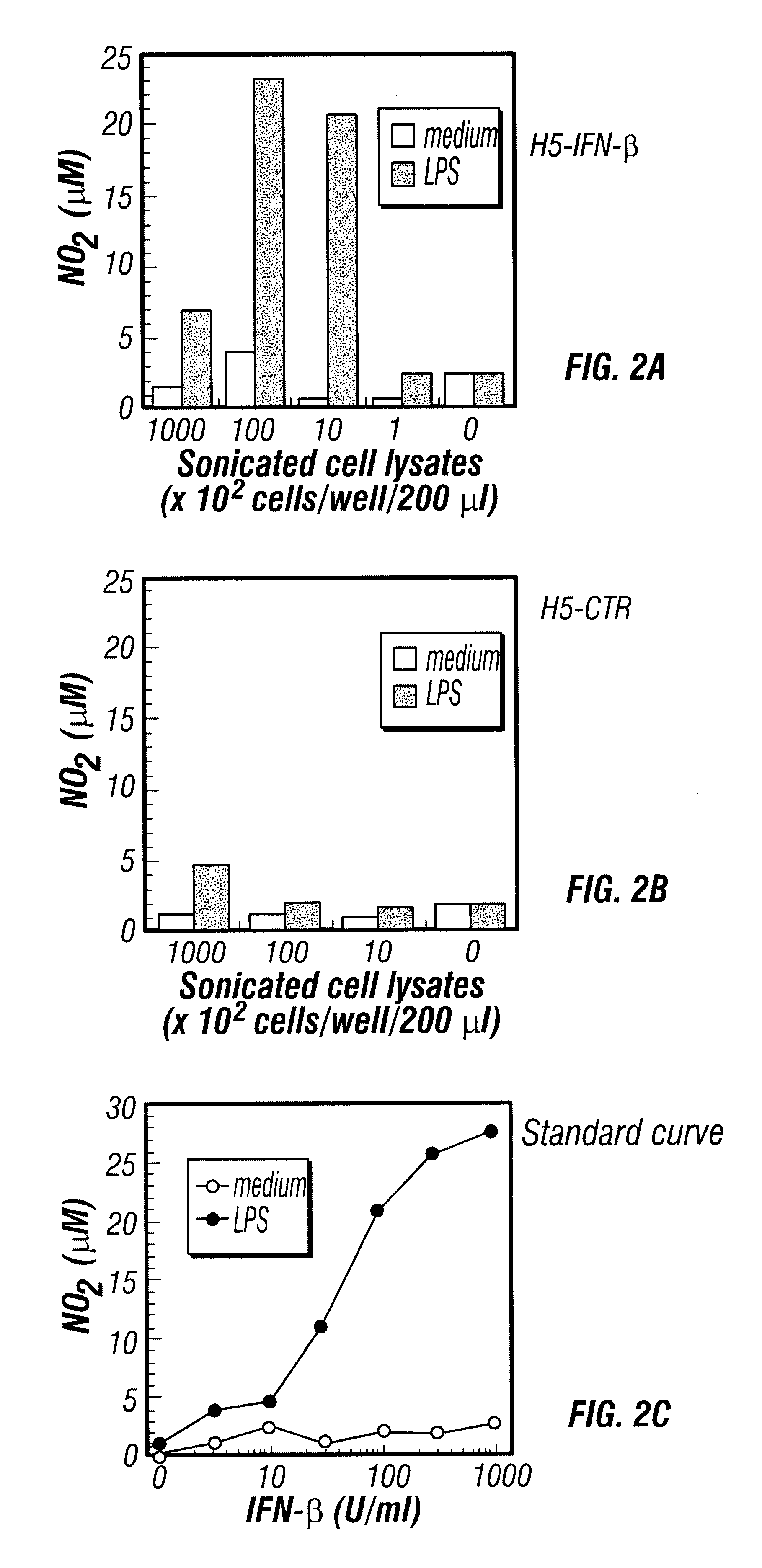
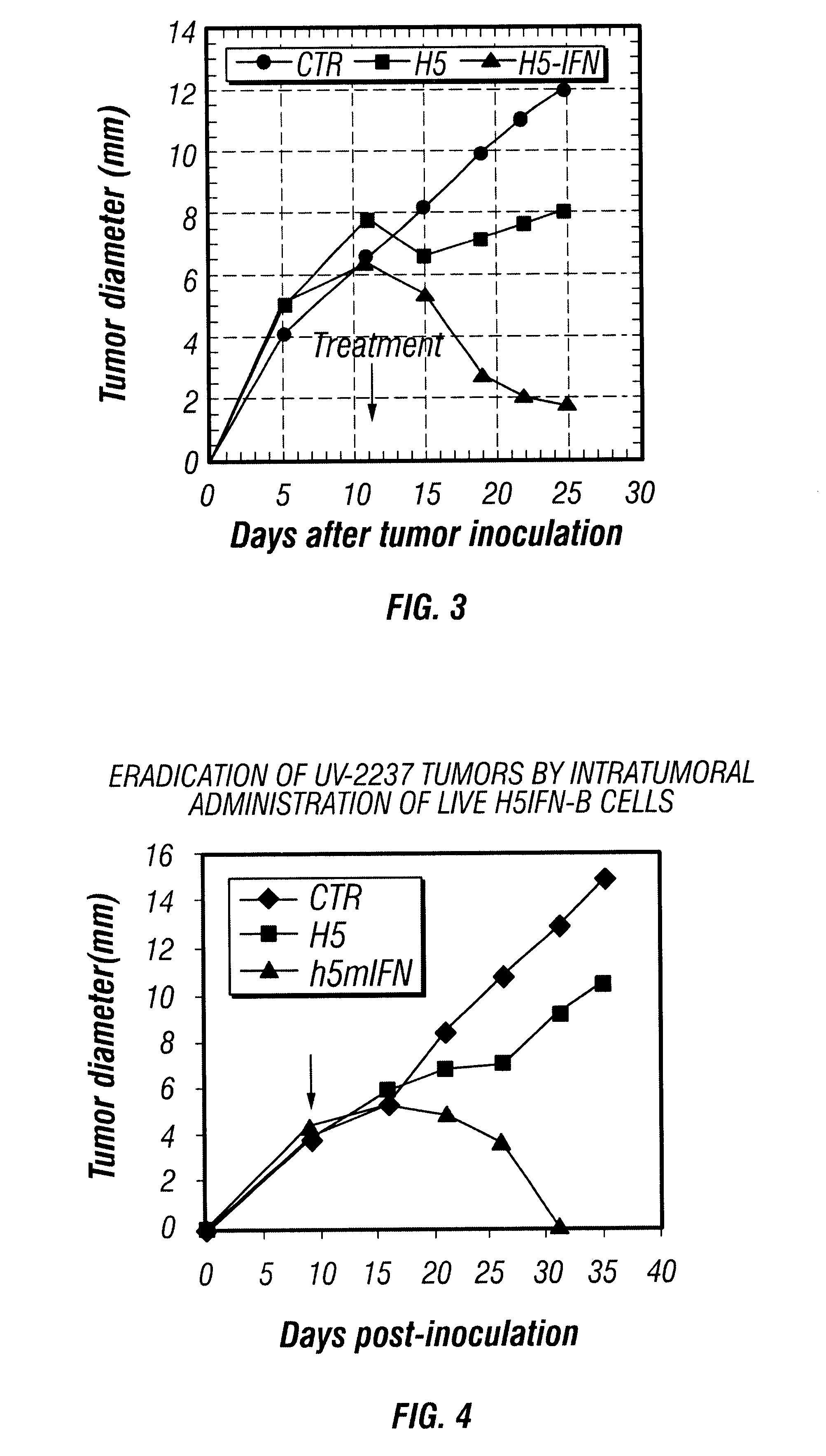
Provided are compositions and methods of use for insect cells comprising baculovirus encoding non-surface expressed proteins and peptides. The claimed invention particularly relates to compositions comprising insect cells containing baculovirus that express cytokines. Such compositions may be administered by, for example, direct intratumoral injection into tumors in mammals, resulting in tumor reduction or recission. Another aspect of the claimed invention concerns methods of promoting resistance to the reoccurence of tumors in mammals who have undergone such tumor recission. In a specific aspect of the claimed invention, the mammals are human subjects presenting with various forms of cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
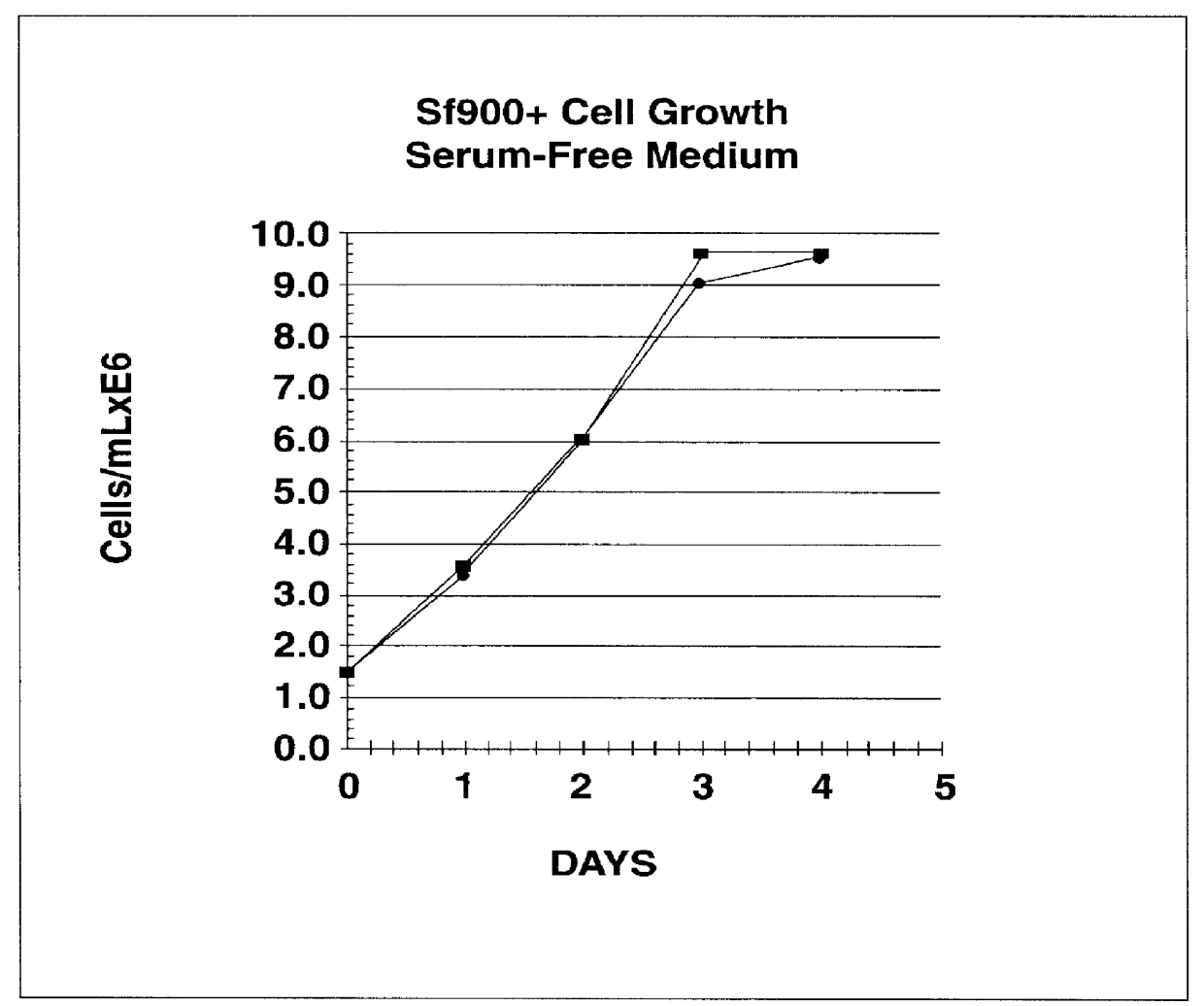
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
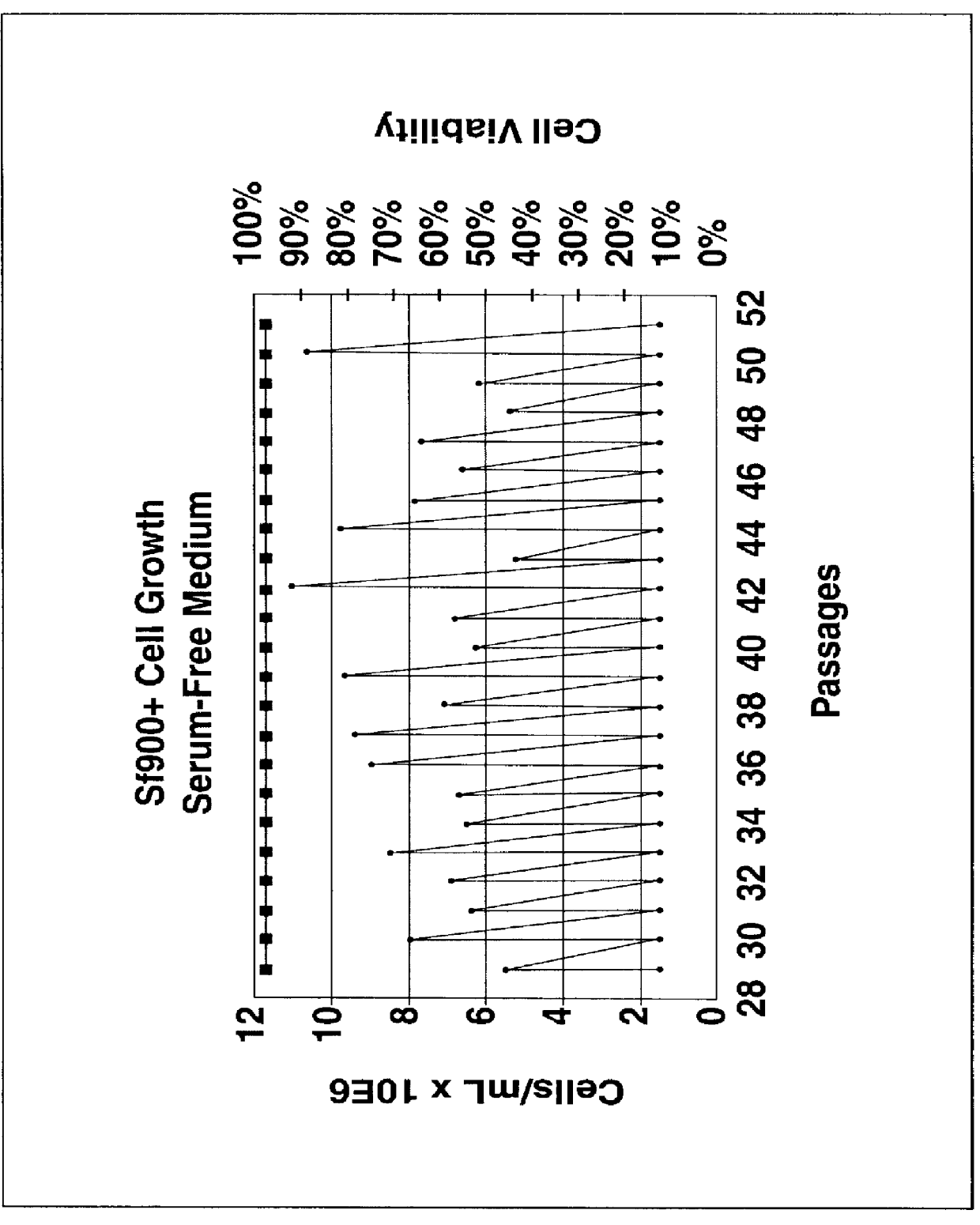
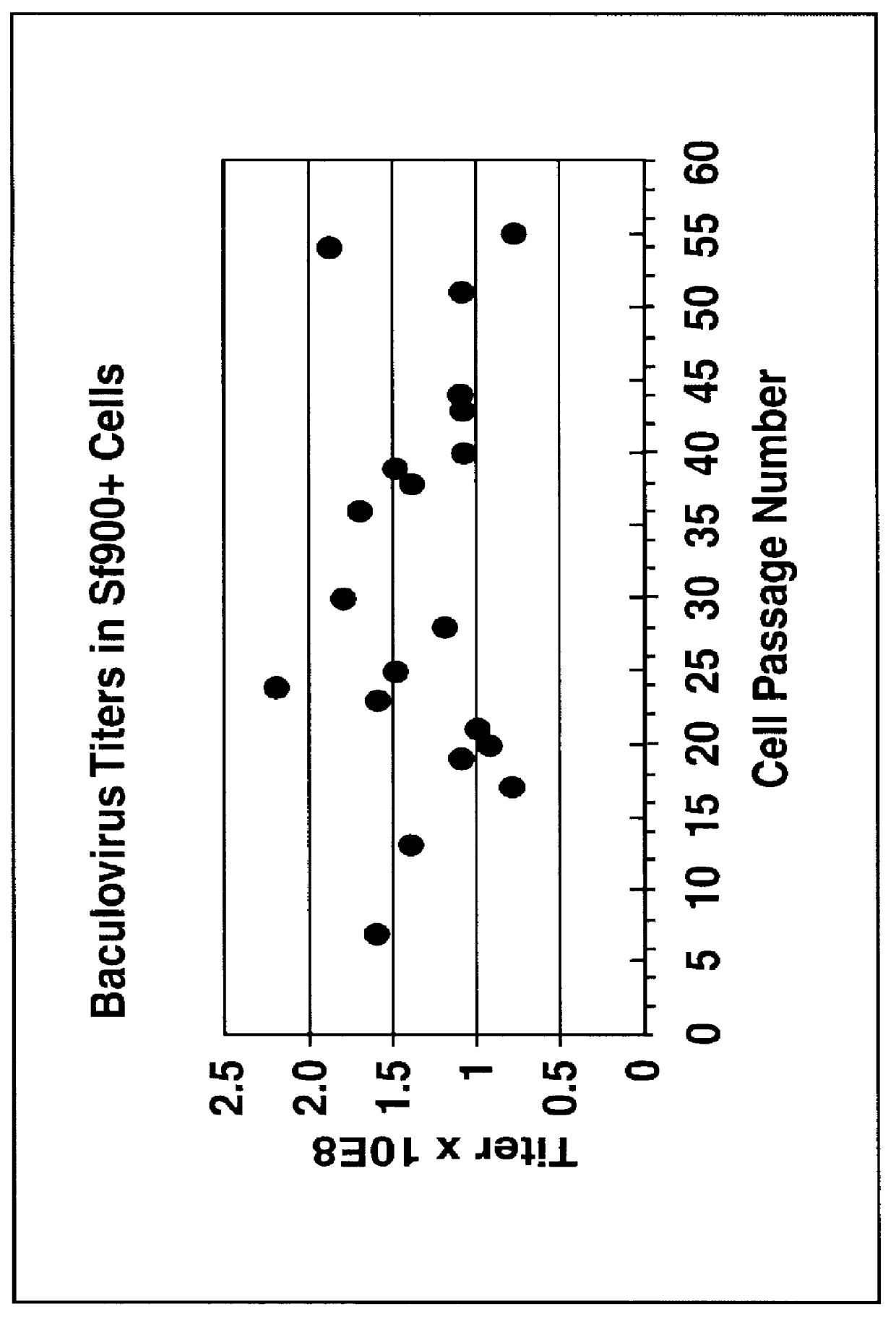
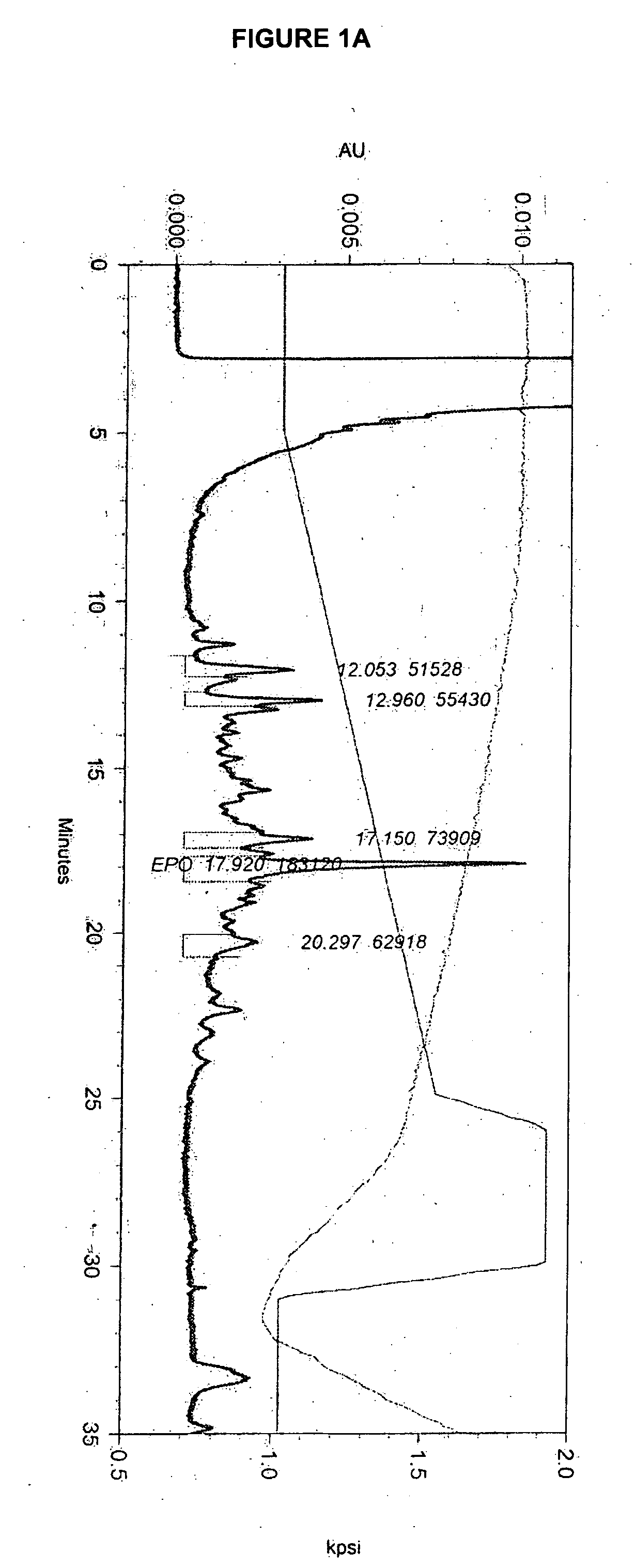
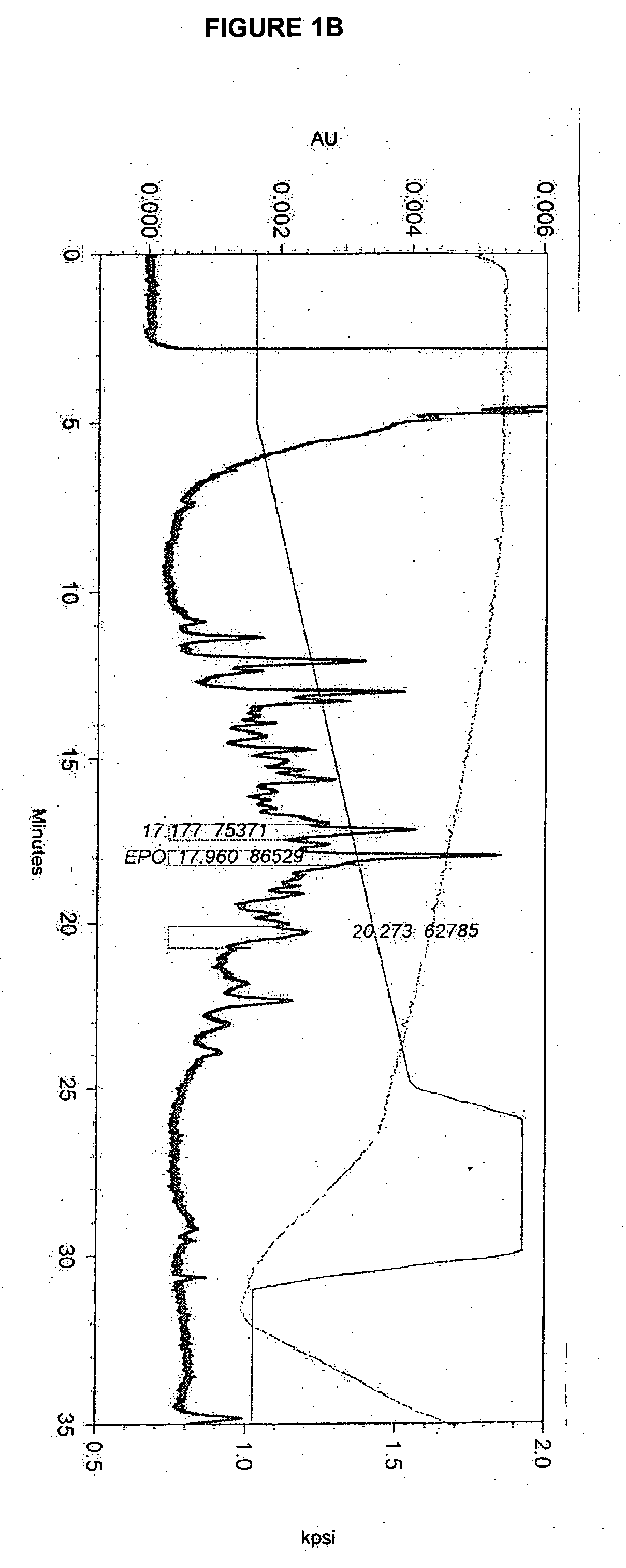
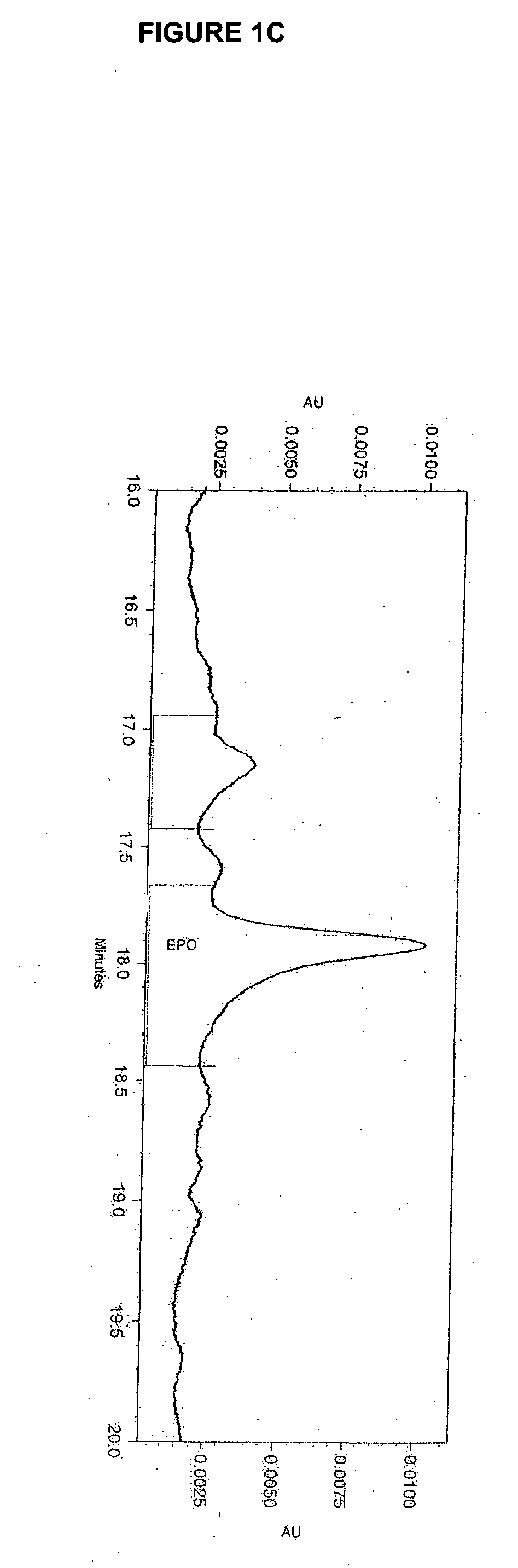
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Functional influenza virus-like particles (VLPs)
ActiveUS20050009008A1SsRNA viruses negative-senseVirus peptidesMultiple copyVirus Structural Proteins
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Manufacturing process for the production of peptides grown in insect cell lines
InactiveUS20060246544A1Improve purityHigh concentrationHydrolasesPeptide/protein ingredientsPeptideGlycosyltransferase
The present invention provides a manufacturing method for the production of peptides that are grown in insect cell lines. The peptides are grown in insect cell cultures that are infected with baculovirus particles in a culture supplemented with a lipid mixture. The peptides are then isolated from the insect cell culture using a method that employs a tangential flow filtration cascade. The isolated peptides are glycopeptides having an insect specific glycosylation pattern. The glycopeptides may then be conjugated to a modifying group via linkage through a glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase.
Owner:NOVO NORDISK AS
Composition and vaccine for treating lung cancer
InactiveUS20160168227A1High in proteinEffectively stimulating the (adaptive) immune systemOrganic active ingredientsTumor rejection antigen precursorsAntigenDisease
The present invention relates to a composition comprising at least one mRNA encoding a combination of antigens capable of eliciting an (adaptive) immune response in a mammal, wherein the antigens are selected from the group consisting of 5T4 (Trophoblast glycoprotein, TPBG), Survivin (Baculoviral TAP repeat-containing protein 5; BIRC5), NY-ESO-1 (New York esophageal squamous cell carcinoma 1, CTAG1B), MAGE-C1 (Melanoma antigen family C1), MAGE-C2 (Melanoma antigen family C2), and MUC1 (Mucin 1). The invention furthermore relates to a vaccine comprising at least one mRNA encoding such a combination of antigens, and to the use of said composition (for the preparation of a vaccine) and / or of the vaccine for eliciting an (adaptive) immune response for the treatment of lung cancer, preferably of non-small cell lung cancer (NSCLC), and diseases or disorders related thereto. Finally, the invention relates to kits, particularly to kits of parts, containing the composition and / or the vaccine.
Owner:CUREVAC AG
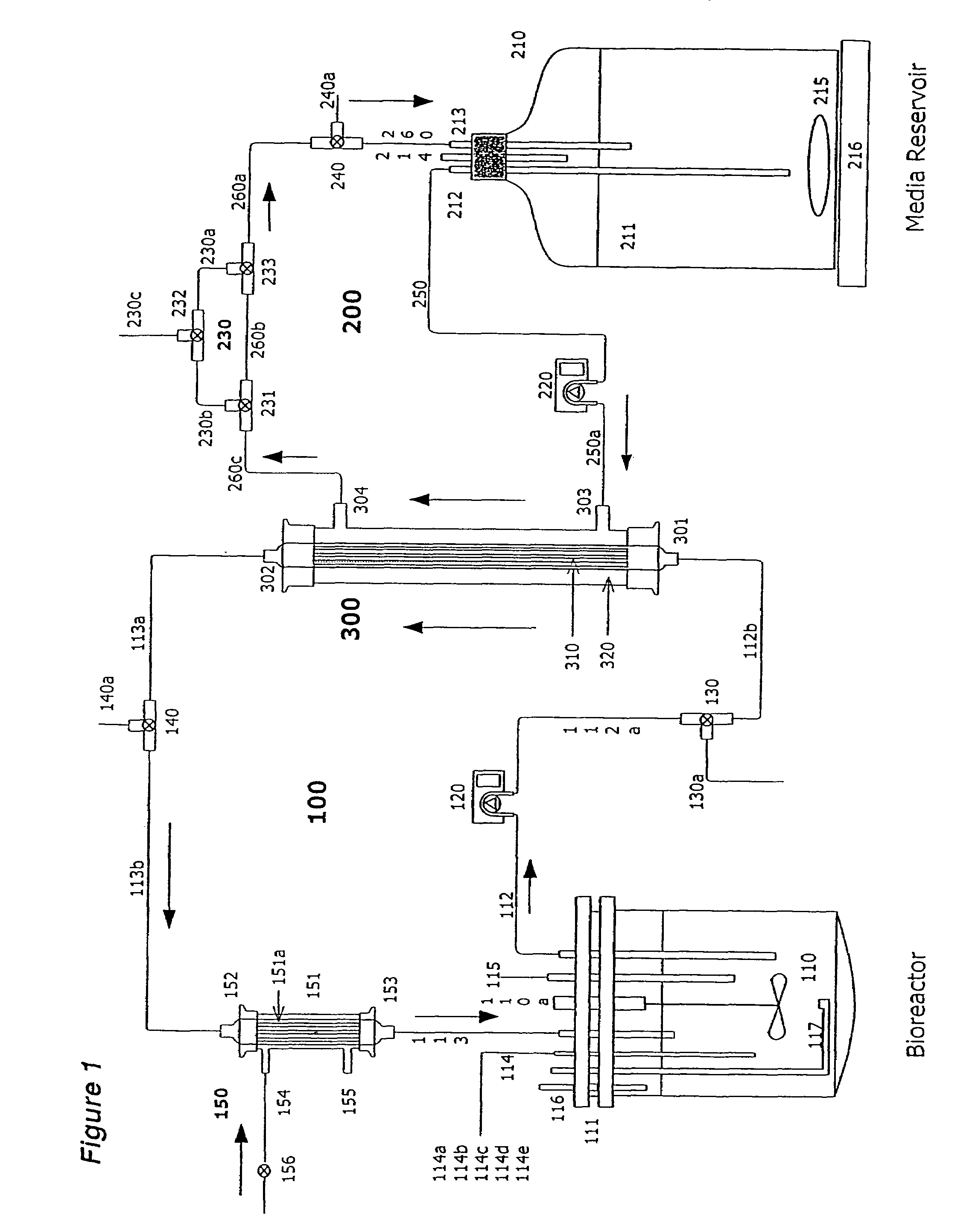
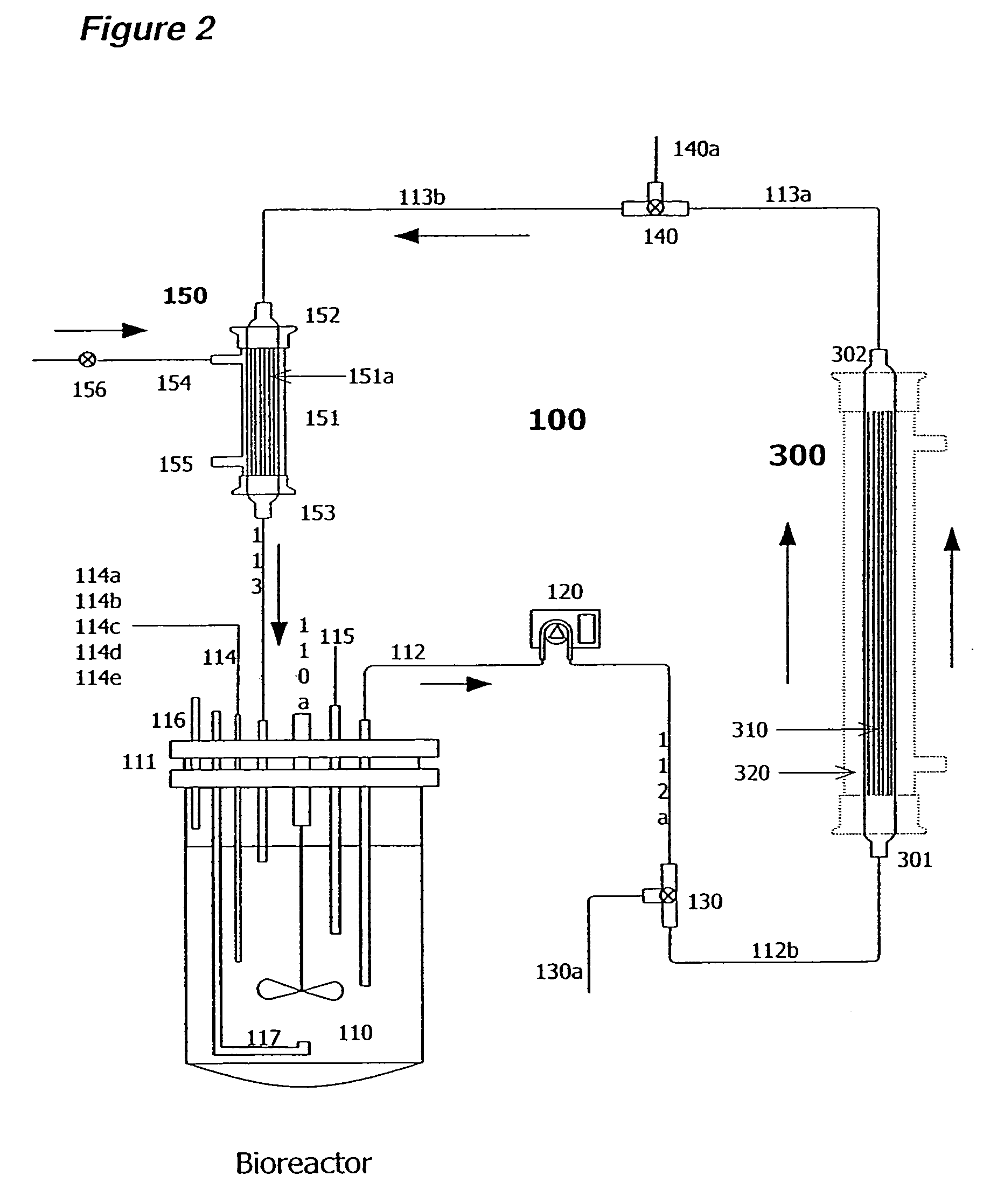
Apparatus and methods for producing and using high-density cells and products therefrom
InactiveUS20060019385A1High densityQuick exchangeAnimal cellsBioreactor/fermenter combinationsHigh cellHigh density
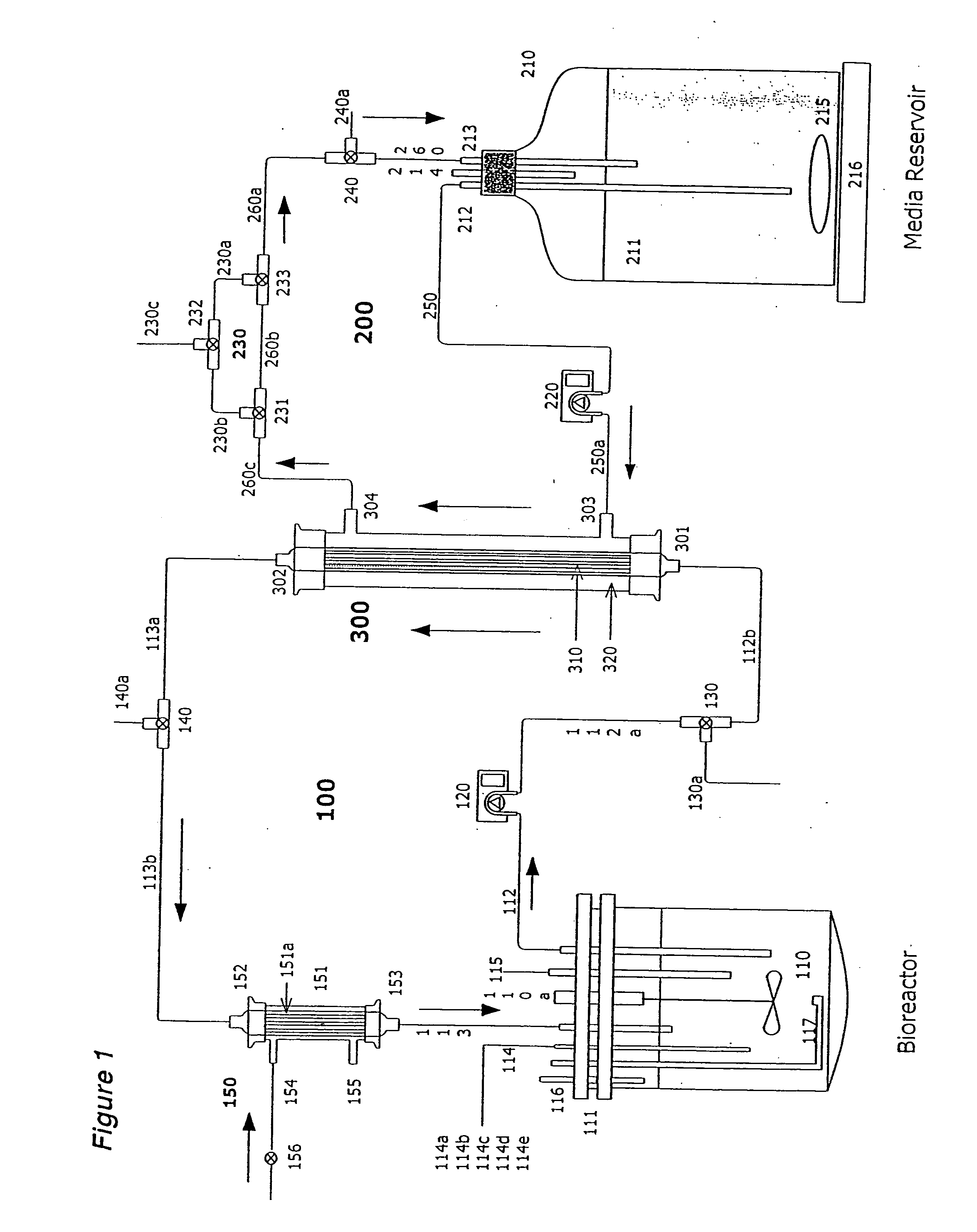
Disclosed and claimed is apparatus and methods for the growth of cells to high density, products therefrom and uses thereof. Also disclosed and claimed is the use of this method for the growth to high-density insect cells, such as the Spodoptera frugiperda Sf900+ cell line (ATCC: CRL 12579). Further disclosed is the infection of Sf900+ cells at high cell density with wild type and recombinant baculoviruses to produce baculovirus and DNA or gene or expression products.
Owner:PROTEIN SCI
Baculoviral vectors comprising repeated coding sequences with differential codon biases
ActiveUS8697417B2Improve stabilityReduced expression levelAnimal cellsSugar derivativesViral vectorParvovirus
The present invention relates to production of proteins in insect cells whereby repeated coding sequences are used in baculoviral vectors. In particular the invention relates to the production of parvoviral vectors that may be used in gene therapy and to improvements in expression of the viral rep proteins that increase the productivity of parvoviral vectors.
Owner:UNIQURE IP BV
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Subunit coronavirus vaccine for dimerization-based receptor binding domains
ActiveCN106928326AOvercoming the disadvantage of insufficient immunogenicityIncrease neutralizing antibody productionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationMiddle East respiratory syndrome coronavirus
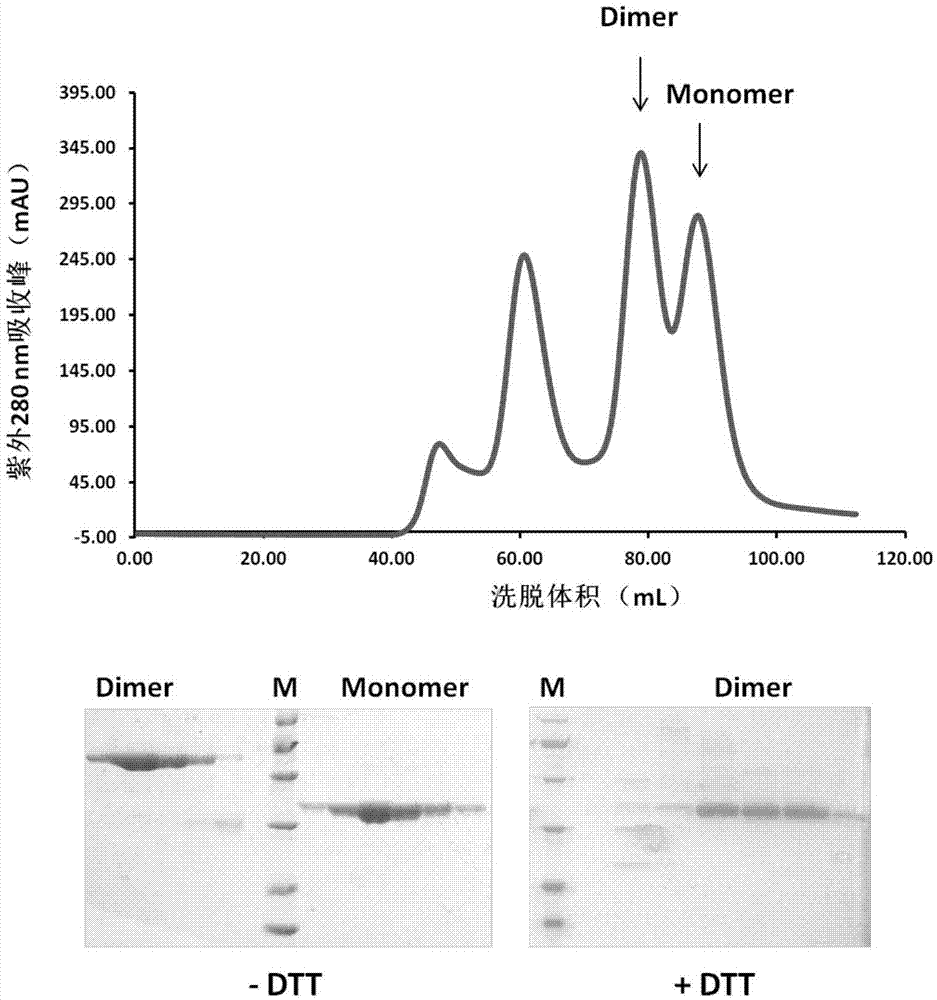
The invention discloses a subunit coronavirus vaccine for dimerization-based receptor binding domains and belongs to the technical field of medicine. A baculovirus expresses RBD (receptor binding domain (E367-Y606) of MERS-CoV (middle east respiratory syndrome coronavirus) protein and RBD (R294-F515) of SARS-CoV (severe acute respiratory syndrome coronavirus) in insect cells, the RBDs may form a dimer through cysteine residue at 603 of S (spike) protein or form a dimer through cysteine residue at 512 of the S protein, and purified RBD protein dimer and monomer are used respectively to immunize Bald / c mice. The dimerized RBDs have the advantages that the defect that RBD monomers have poor immunogenicity is overcome and the generation of neutralizing antibodies in against MERS-CoV is increased greatly.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD
Recombinant baculovirus strain of porcine circovirus type 2 Cap protein expression, construction method and application thereof
InactiveCN101358182AImprove immune activityViral antigen ingredientsAntiviralsMicroorganism preservationImmunocompetence
The present invention discloses a recombinant baculovirus strain rBac / PCV2Cap (microorganism preservation number: CGMCC NO.2083) efficiently expressing Porcine circovirus type 2 Cap protein and applications thereof. The recombinant baculovirus strain rBac / PCV2Cap constructed by the present invention can efficiently express recombinant PCV2-Cap protein in insect cells, and the expressed recombinant Cap protein, which has good immunocompetence and antigenicity, can serve as a subunit vaccine used to prevent the related plague caused by Porcine circovirus type 2 infection as well as a detecting and diagnostic antigen for Porcine circovirus type 2 serum antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
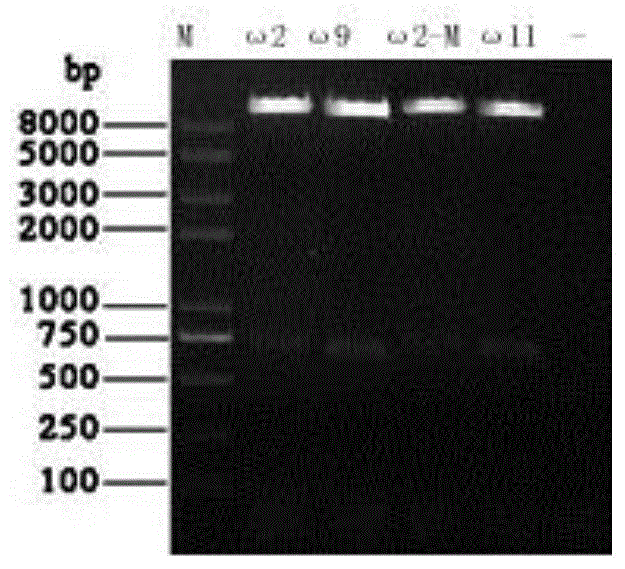
Cat omega interferon mutant as well as preparation method and application thereof
ActiveCN104788554AStrong antiviral activityPeptide/protein ingredientsAntiviralsDiseaseTransfer vector
The invention discloses a cat omega interferon mutant as well as a preparation method and an application thereof, and belongs to the field of preparation and application of cat omega interferons. A cat omega 2 or omega 11 interferon mutant is obtained by comparing gene sequences and amino acid sequences of 13 subtypes of cat omega interferon and performing amino acid site-directed mutation on cat omega 2 or omega 11 interferon. The invention further discloses the method for preparing the cat omega interferon mutant. The method comprises the following steps: cloning a gene for coding the cat omega 2 or omega 11 interferon mutant into a baculovirus transfer vector, recombining with a baculovirus for infecting an insect host, expressing an exogenous gene, and obtaining a cat omega interferon protein expression product. The method is simple in process, the cat omega interferon capable of being safe to use can be efficiently and stably obtained, and the antiviral activity of the cat omega interferon mutant is remarkably improved. The cat omega 2 or omega 11 interferon mutant can be used for preparing drugs or reagents for preventing or treating cat viral diseases.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
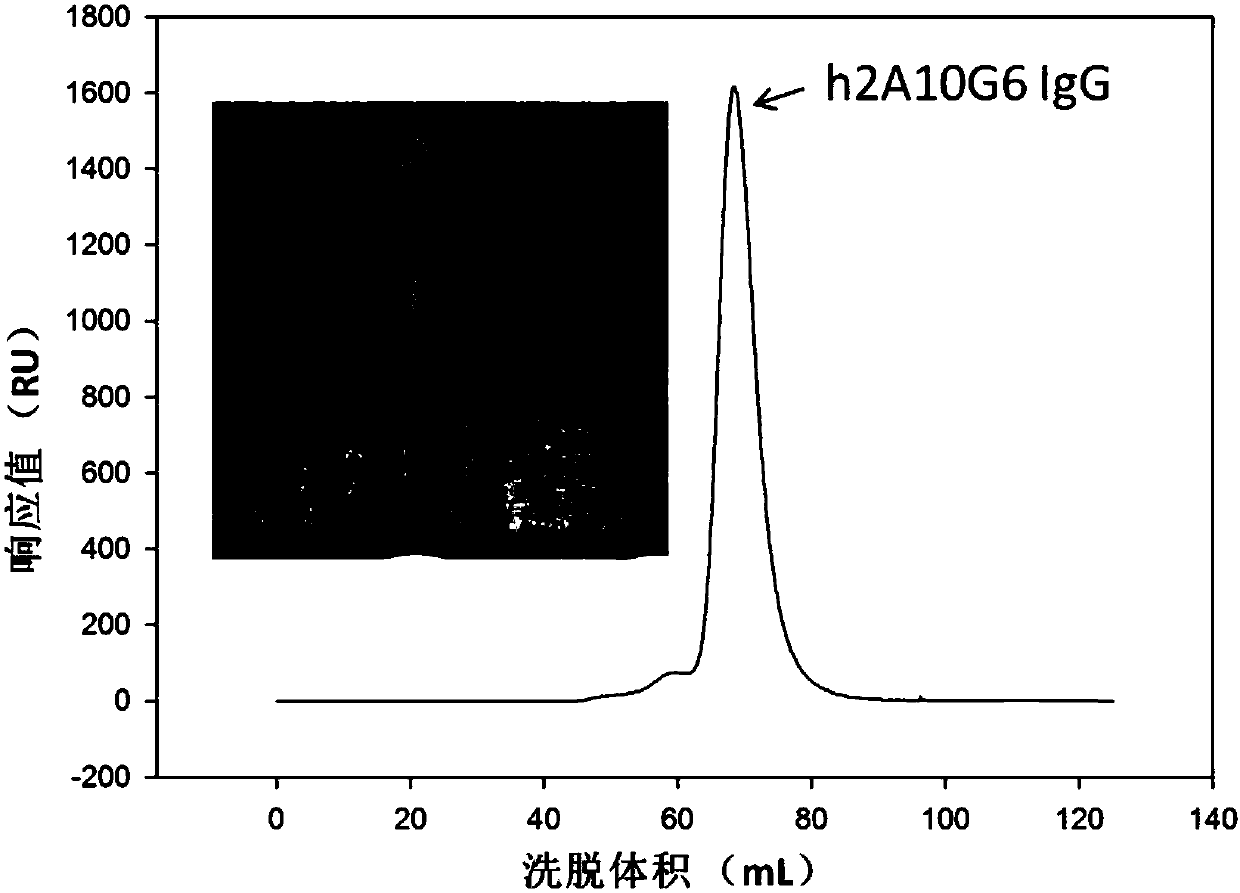
Humanized monoclonal antibody and application thereof
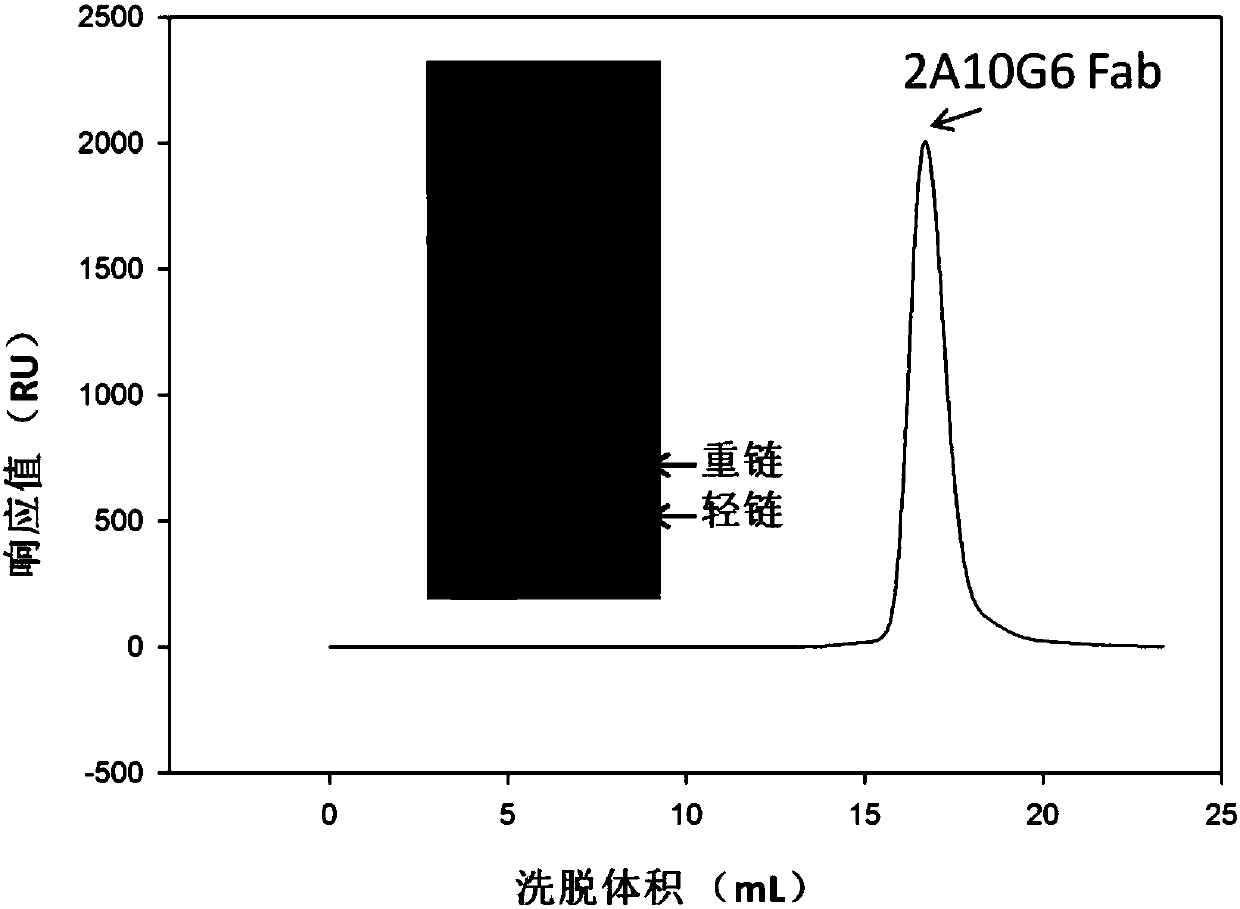
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Method for preparing rabies virus antigen
ActiveCN101307317AReduce consumptionImprove securityAntiviralsDepsipeptidesAntigenBaculovirus expression
The invention provides a method for making rabies virus antigen. The method comprises the following steps that: the antigen gene of rabies virus or the combined expression combination of the antigen gene is respectively cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the transfer expression carrier and baculovirus undergo cotransfection so as to carry out homologous recombination or transposition, thereby obtaining recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding rabies antigen; and the expressed antigen is ingathered and purified so as to obtain rabies virus antigen. The method adopts a baculovirus expression system to make safe and efficient rabies virus antigen in a domestic silkworm bioreactor; moreover, due to having extremely high safety, the made antigen can be directly used to make injection vaccine and oral vaccine used for animal immunization. The method can substantially reduce the production cost of rabies virus antigen, and has the advantages of safety, high efficiency, less energy consumption and low cost, etc.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Apparatus and methods for producing and using high-density cells and products therefrom
InactiveUS7598075B2Quick exchangeImprove performanceBioreactor/fermenter combinationsBiological substance pretreatmentsHigh cellHigh density
Disclosed and claimed is apparatus and methods for the growth of cells to high density, products therefrom and uses thereof. Also disclosed and claimed is the use of this method for the growth to high-density insect cells, such as the Spodoptera frugiperda Sf900+ cell line (ATCC: CRL 12579). Further disclosed is the infection of Sf900+ cells at high cell density with wild type and recombinant baculoviruses to produce baculovirus and DNA or gene or expression products.
Owner:PROTEIN SCI
Preparation method and application of classical swine fever virus recombinant subunit vaccine
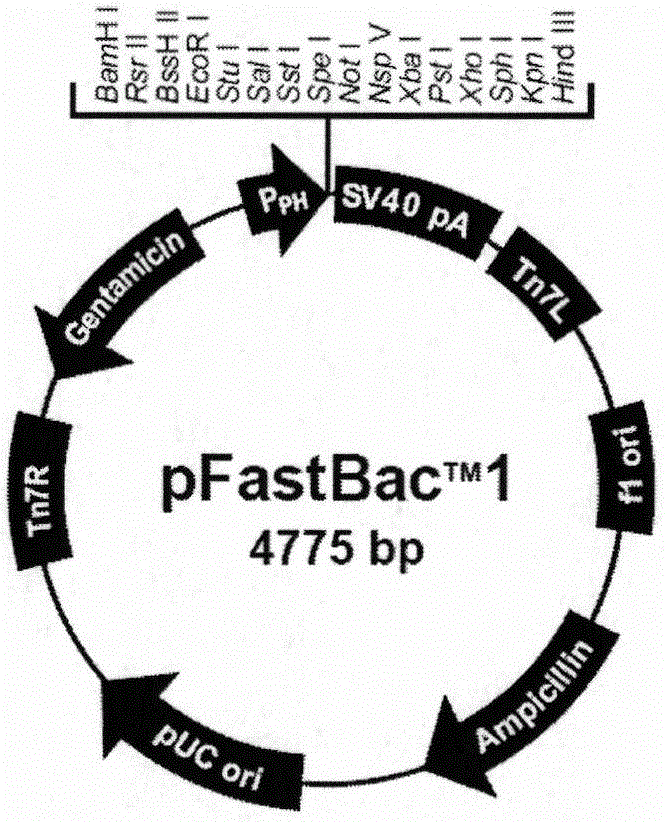
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
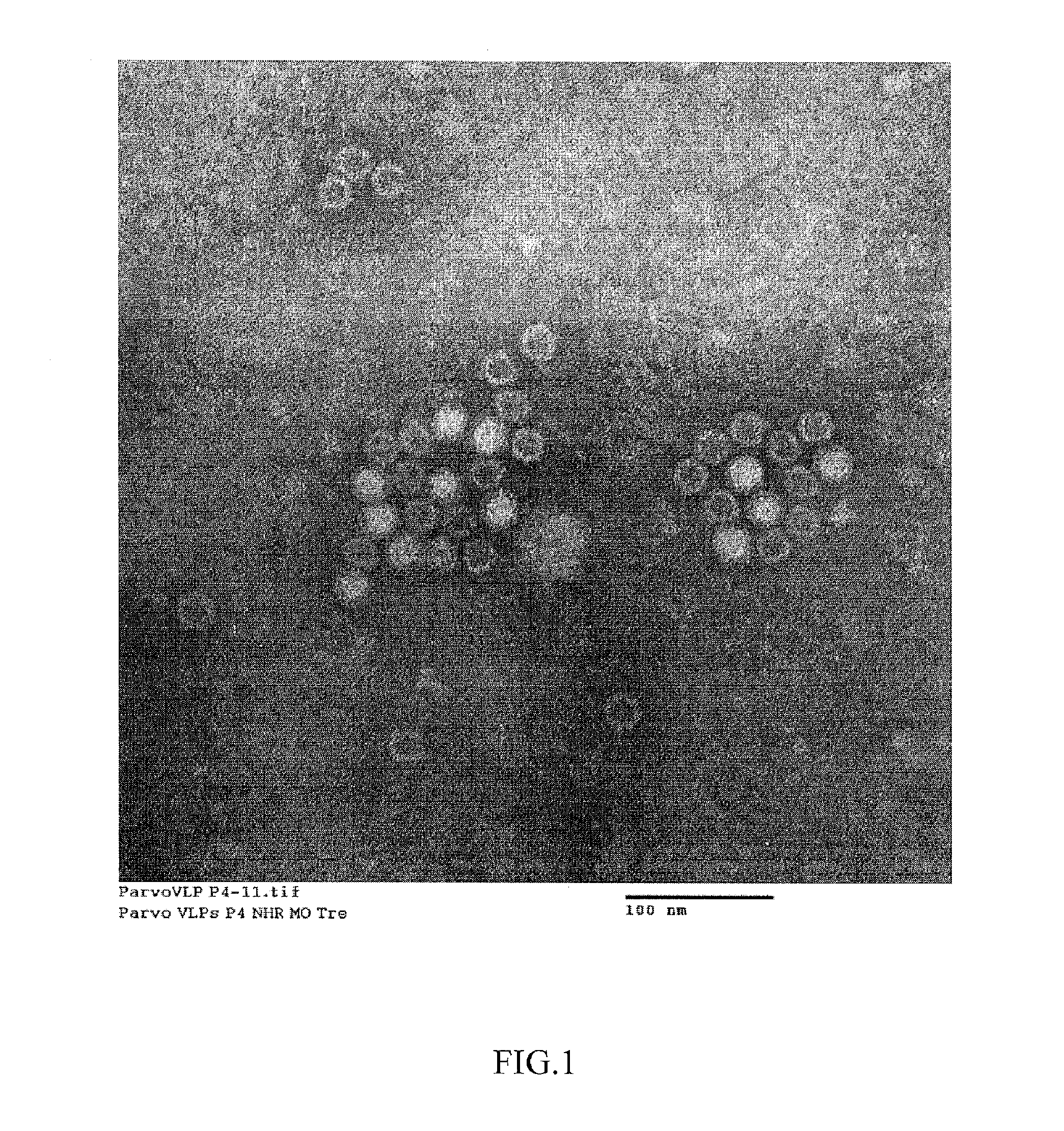
Method for producing porcine parvovirus antigen and its product
ActiveCN102382845AImprove immune activityReduce manufacturing costGenetic material ingredientsAntiviralsAntigenBaculovirus expression
The invention discloses a method for producing a porcine parvovirus antigen and its product. The method comprises the following steps: porcine parvovirus capsid protein VP2 gene or optimized VP2 gene is cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the constructed transfer expression carrier and baculovirus DNA are carried out cotransfection to obtain recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding porcine parvovirus capsid protein; and the expressed antigen is ingathered and purified so as to obtain the porcine parvovirus antigen. The method adopts a baculovirus expression system to make safe and efficient porcine parvovirus antigen capsid particles in a domestic silkworm bioreactor; the prepared purified antigen by the method has high safety, and can be directly produced to vaccines for animal immunity. The method for producing porcine parvovirus antigen has the advantages of high expression efficiency, high immunization activity of the expressed antigen, low production cost, large scale production realization and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Method for preparing foot-and-mouth disease antigen
ActiveCN101121938APromote safe productionReduce consumptionSsRNA viruses positive-senseVirus peptidesAntigenTransfer vector
The invention provides a method for expressing foot-and-mouth disease antigens in insects using recombinant baculoviruses, which includes: cloning different gene combinations of foot-and-mouth disease into baculovirus delivery vectors to construct transfer vectors; using the constructed transfer vectors to transfer Infect the baculovirus and perform DNA recombination to obtain the recombinant baculovirus; infect the insect host with the recombinant baculovirus; culture the infected insect host to express the foot-and-mouth disease antigen; collect and purify the expressed foot-and-mouth disease antigen. The method of the present invention uses a baculovirus expression system to safely and efficiently produce foot-and-mouth disease antigens in a silkworm bioreactor. The prepared antigens are extremely safe and can directly produce vaccines to immunize animals. The method of preparing foot-and-mouth disease antigen of the present invention does not require investment in building a factory, has no three wastes, consumes very little energy such as electricity and water resources, and its production cost is also significantly lower than the traditional method of preparing foot-and-mouth disease antigen. It is safe, efficient, has low energy consumption and low cost. Low and many advantages.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
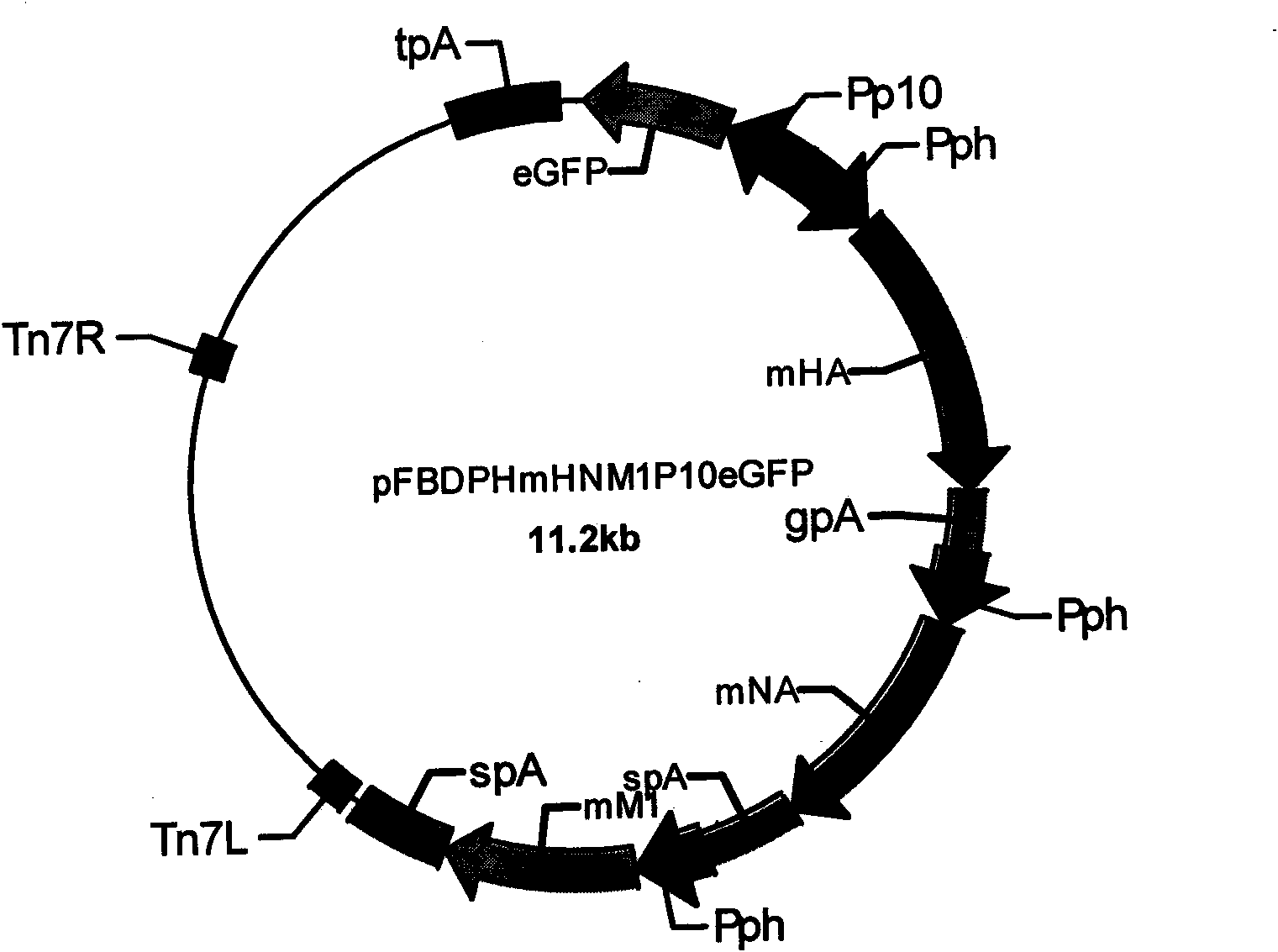
Recombinant baculovirus expressing manually modified and synthesized influenza A H1N1 virus HA-NA-M1 gene
InactiveCN101624580AImprove expression levelImprove screening efficiencyGenetic material ingredientsVirus peptidesInfluenza A (H1N1) virusSapovirus
The invention relates to the field of virology, in particular to a recombinant baculovirus which is manually modified and synthesized and contains a main immunogenic gene HA-NA-M1 of an influenza A H1N1 virus. The strain QP-Ac-HNM1 belongs to the baculovirus (Baculovirus) and is preserved in the China Center for Type Culture Collection (CCTCC) with the preserving number of CCTCC-V200912. The recombinant virus is capable of synchronously expressing the HA and NA of the influenza A H1N1 virus and M1 proteins to form virus particles which can be used for developing vaccines so as to prevent human beings and swine from being infected with the influenza A H1N1 virus.
Owner:HUAZHONG AGRI UNIV
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombination baculovirus for expressing Africa swine fever CD2V protein in SF9 cell
InactiveCN110157737AHigh protein expressionEasy to purifyViral antigen ingredientsVirus peptidesSf9Amino acid
The invention provides a recombination baculovirus for expressing Africa swine fever CD2V protein in an SF9 cell. The CD2V protein can be recombined and expressed in the SF9 cell; the amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; the sequence of the nucleotide fragment is shown in SEQ ID NO:3. The recombination baculovirus is used for preparing the Africa swine fever CD2V protein in an insect cell. The insect cell Sf9 is used for recombining and expressing the Africa swine fever CD2V protein, the protein expression amount is high, purification is easy, the recombination baculovirus is used for preparing and identifying a diagnosis product, and a solid foundation is laid for producing Africa swine fever subunit vaccines and diagnosis reagents.
Owner:YEBIO BIOENG OF QINGDAO
Canine parvovirus-like particles and preparation method and application thereof
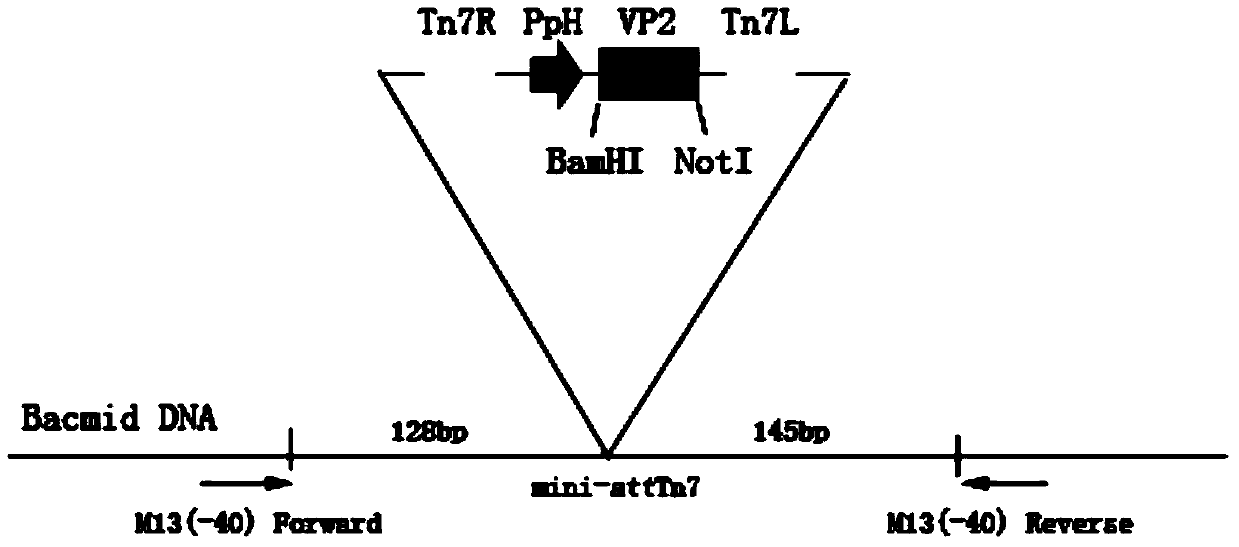
ActiveCN103387996AIncrease productionReduce manufacturing costInactivation/attenuationAntiviralsAdjuvantCanine parvovirus
The invention provides canine parvovirus-like particles. The VP2 gene of canine parvovirus is optimized according to the preferred codons of insect cells, the optimized VP2 gene is connected with an insect expression vector, and the canine parvovirus-like particles are produced by use of a rhabdovirus / insect cell expression system. The canine parvovirus-like particles are combined with a preservative and an adjuvant to prepare a canine parvovirus enteritis vaccine. By adopting the canine parvovirus-like particles provided by the invention, the prepared canine parvovirus enteritis vaccine has the advantages of high output, low production cost, high immunogenicity, good safety, convenience in large-scale production and the like, and has good immunization and prevention effects on canine parvovirus enteritis.
Owner:CHANGCHUN SR BIOLOGICAL TECH
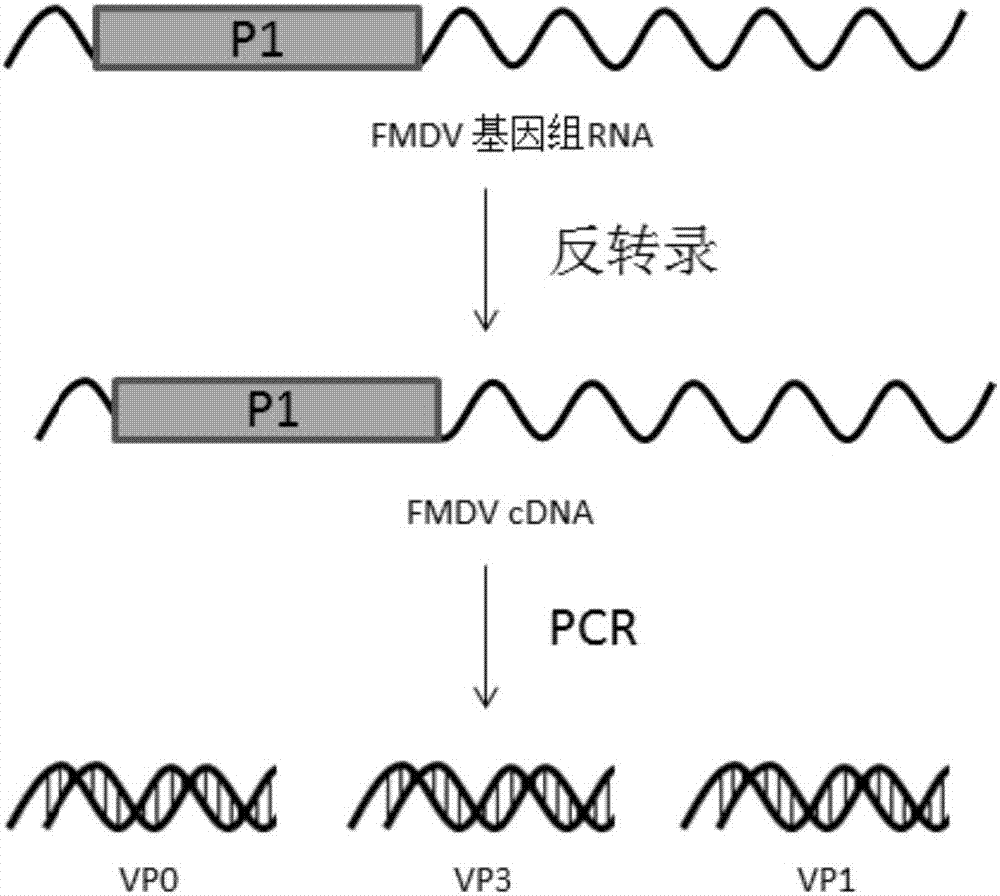
Foot and mouth disease virus recombinant virus sample particle as well as preparation method and application thereof
The invention discloses a foot and mouth disease virus recombinant virus sample particle as well as a preparation method and application thereof. The recombinant virus sample particle is commonly assembled and expressed by components in a DNA molecule composition. The DNA molecule composition contains an O-type foot and mouth disease VP0 gene, an O-type foot and mouth disease VP1 gene and an O-type foot and mouth disease VP3 gene and further contains a green fluorescent protein gene. By virtue of the property that FMDV VLPs is self-assembled through VP0, VP1 and VP3, a construction method of a baculovirus recombinant vector is improved, a green fluorescent protein label is added into the vector, and FMDV VLPs is successfully prepared by virtue of a pFBDM Bac-to-Bac system, so that a theoretical foundation is laid for the further development of safe and efficient O-type FMDV genetic engineering vaccines.
Owner:CHINA ANIMAL HUSBANDRY IND
Vectors expressing SARS immunogens, compositions containing such vectors or expression products thereof, methods and assays for making and using
SARS (severe acute respiratory syndrome virus, a coronavirus) immunogens, antigens, or epitopes, nucleic acid molecules encoding such immunogens, antigens, or epitopes; vectors containing such nucleic acid molecules, e.g., viral vectors such as baculovirus vectors, DNA vectors, such as DNA plasmid vectors, e.g., DNA plasmids that express a nucleic acid molecule in a mammalian cell, uses for such immunogens, antigens or epitopes and vectors, e.g., as an active component immunogenic, immunological or vaccine compositions, or to generate antibodies, such as monoclonal antibodies, and methods for making, and using such immunogens, antigens or epitopes, vectors, antibodies, including in methods for eliciting an immunological or immunogenic or vaccine response, as well as in assays or diagnostic kits or methods, are discussed, as well as a seamless fusion of sequences in a plasmid or vector, e.g., a sequence encoding a leader sequence and a sequence encoding a protein, epitope or immunogen or antigen.
Owner:UNKNOWN
Preparation method and system of recombinant adeno-associated virus and recombinant bacmid
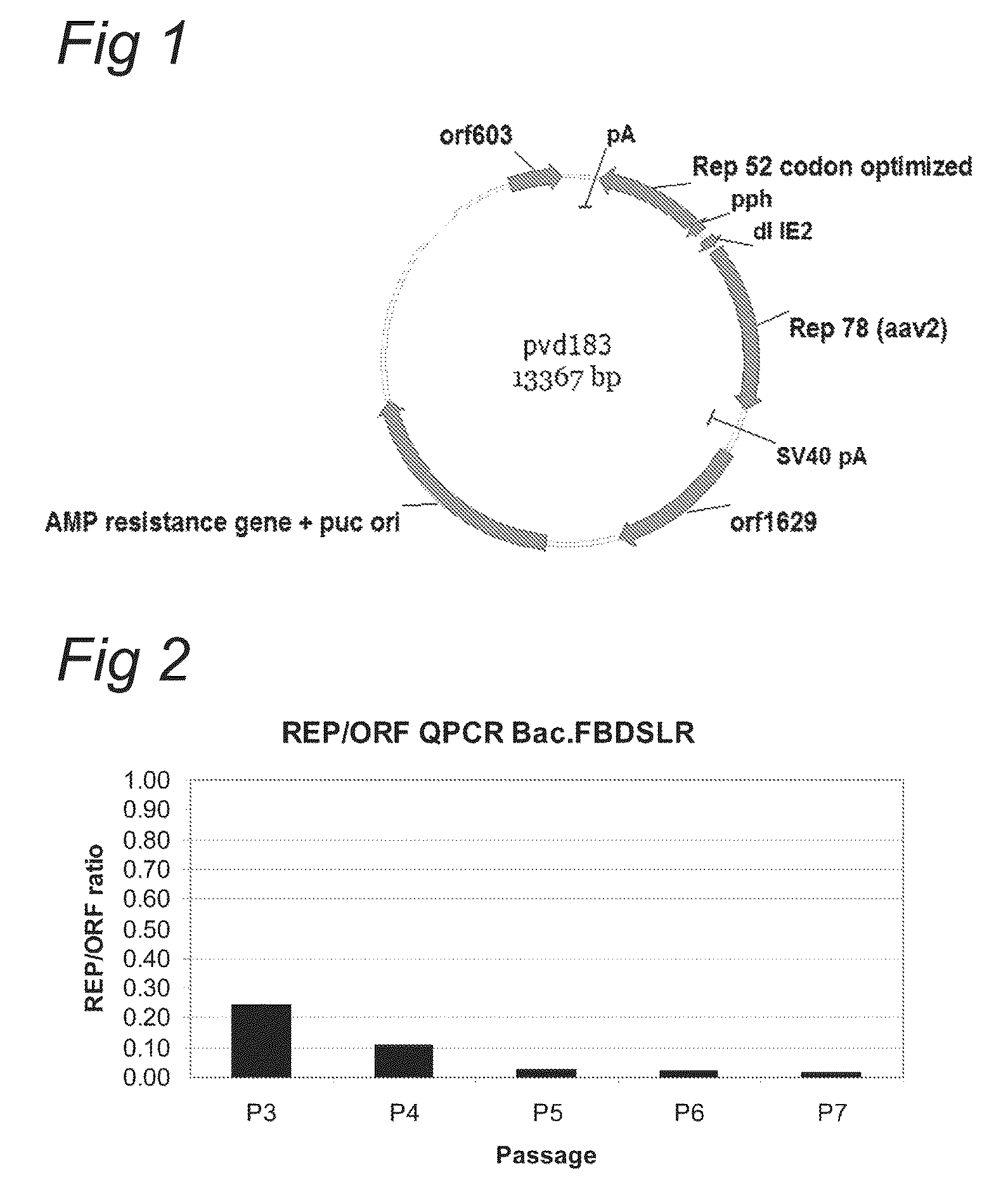
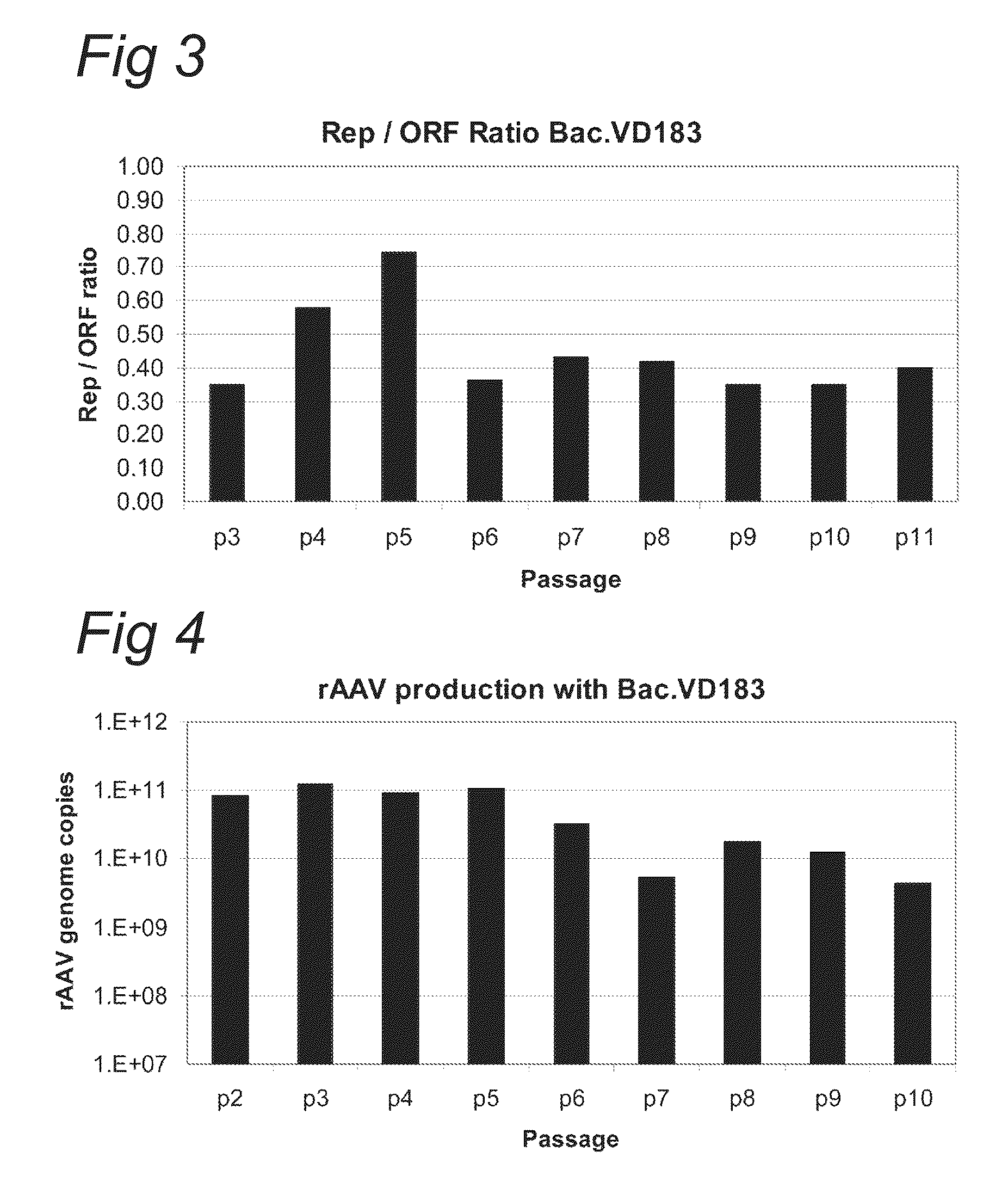
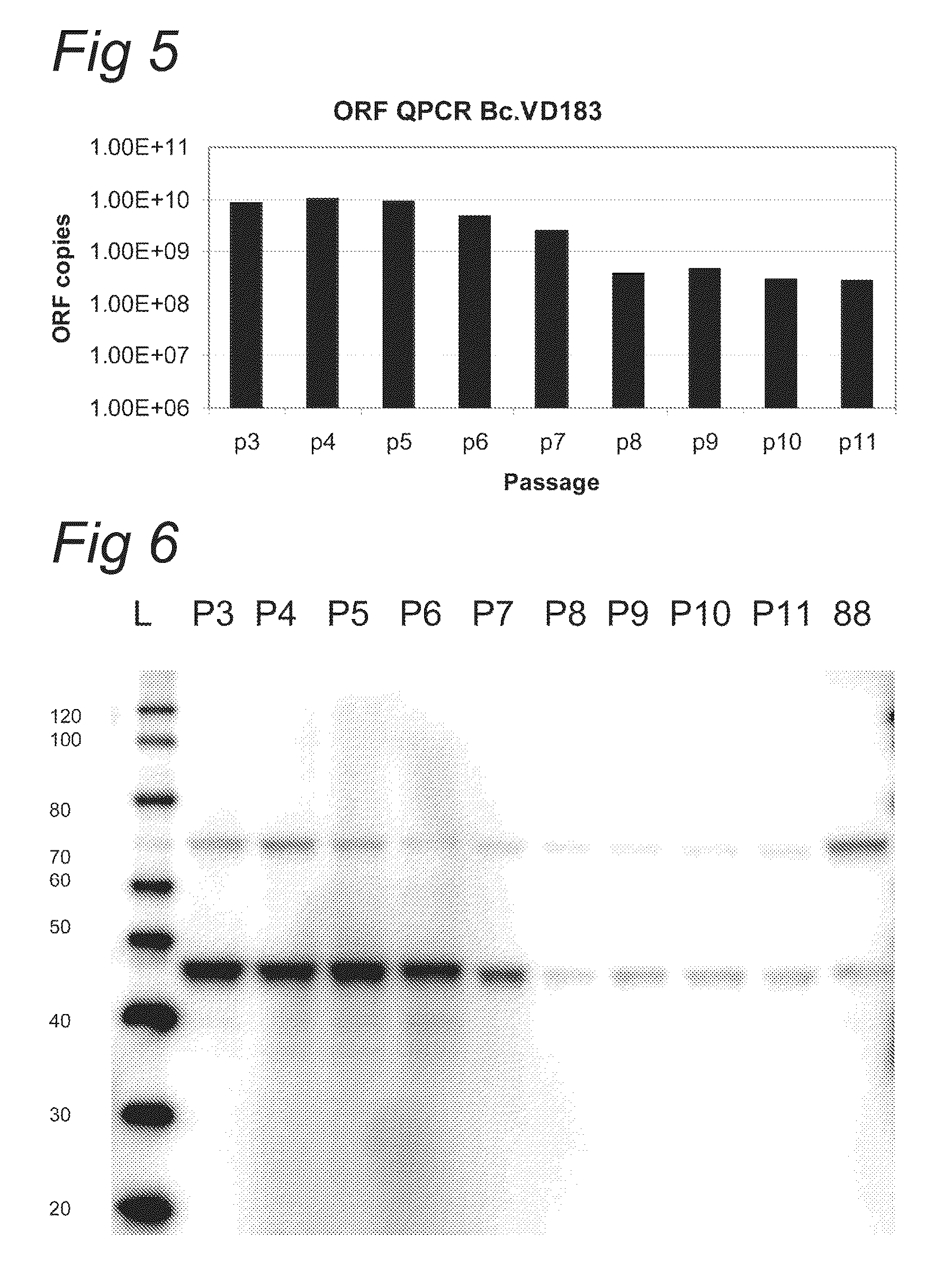
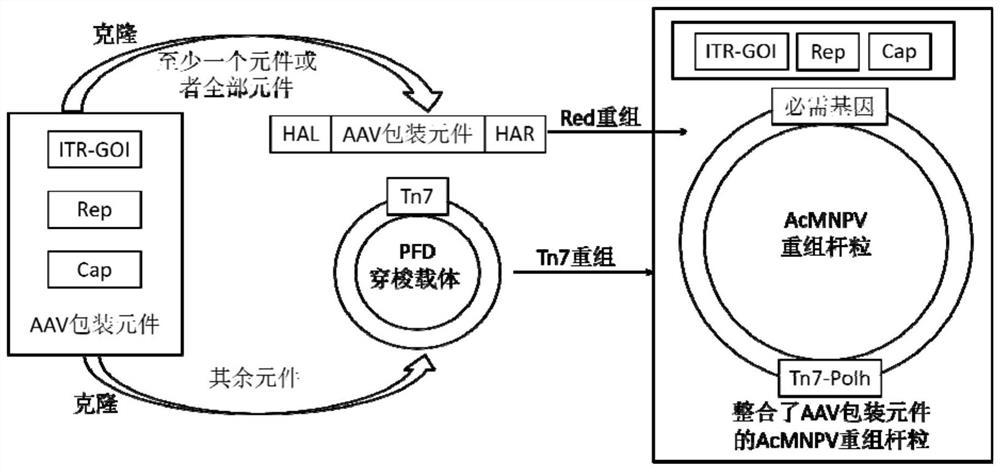
ActiveCN112553257ASolve the technical problem of poor passaging stabilityAddressing massive scale-upVirus peptidesMicroorganism based processesHost cell lineVirus
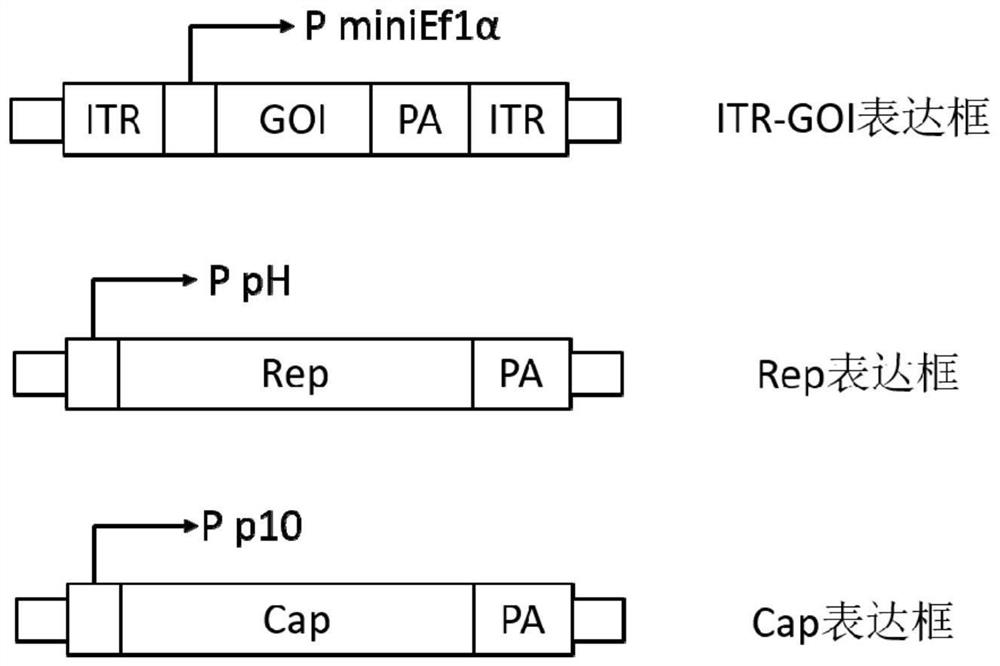
The invention belongs to the field of gene therapy, and particularly relates to a preparation method and system of recombinant adeno-associated virus and recombinant bacmids. Firstly, constructing recombinant bacmids of a recombinant baculovirus genome containing necessary functional elements for producing the recombinant adeno-associated virus; wherein at least one necessary functional element isinserted into the N terminal or the C terminal of the necessary locus of the recombinant baculovirus genome; and then transfecting the obtained recombinant bacmids containing the recombinant baculovirus genome for producing the recombinant adeno-associated virus into a host cell line for culturing to prepare the recombinant adeno-associated virus. Compared with a recombinant baculovirus obtainedby preparing recombinant bacmids through traditional Tn7 recombination, the recombinant baculovirus obtained by inserting core elements containing Cap, Rep and ITR into the two sides of an essential gene of the baculovirus has the advantages that the production level of continuous passage rAAV in cells is more stable, and the rAAV yield is higher.
Owner:JINFAN BIOMEDICAL TECH (WUHAN) CO LTD
Virus-like particle recombinant protein of virus variation strain VP2 gene of infectious bursal disease
InactiveCN101624421ANon-infectiousImproving immunogenicityViral antigen ingredientsVirus peptidesVp2 geneVirus-like particle
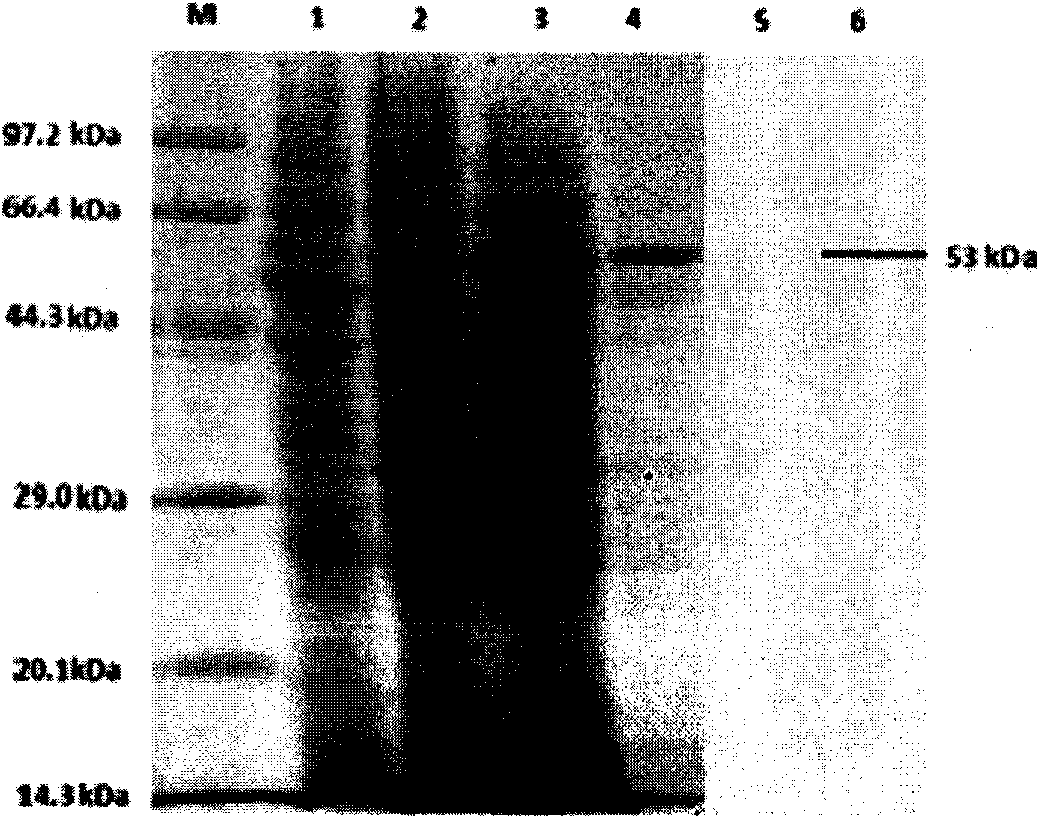
The invention relates to virus-like particle recombinant protein of a virus variation strain VP2 gene of an infectious bursal disease, belonging to the field of biologic pharmacy. The IBDV variation strain (AH1) VP2 gene is cloned, converted and transfected to obtain a recombinant baculovirus vBac-VP2; an infected Sf9 insect cell has specific fluorescence, and the antigen valence of the infected Sf9 insect cell is above 1.6*10<3>; the molecular weight of a recombinant VP2 protein is 53kDa, and the recombinant VP2 protein is in the state of virus-like particles; an indirect ELISA detection method established for an envelope antigen by the purified recombinant VP2 protein has good specificity and sensibility; an immune chicken can resist IBDV virulent attack, and the protection ratio achieves 100 percent. The novel virus-like particle recombinant VP2 protein prepared by the IBDV variation strain VP2 gene has high pertinence on the immune prevention of a current prevalent IBDV virulent strain and good practical value and popularization prospects.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Anti-chicken infectious bursal disease recombinant protein subunit vaccine
ActiveCN103360498AEnhance immune responseImprove protectionViral antigen ingredientsAntiviralsFlagellinVaccine Immunogenicity
The invention provides an anti-chicken infectious bursal disease (IBD) recombinant protein subunit vaccine. The vaccine is a fusion protein having high immunogenicity of Salmonella typhimurium flagellin and an infectious bursal disease virus (VP2). The above flagellin + VP2 fusion protein is obtained through the expression of a recombinant baculovirus containing a flagellin + VP2 gene by utilizing a Bac-to-Bac baculovirus expression system. The recombinant baculovirus obtained through the system has a short period, and the expressed flagellin + VP2 fusion protein has high immune protection force.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
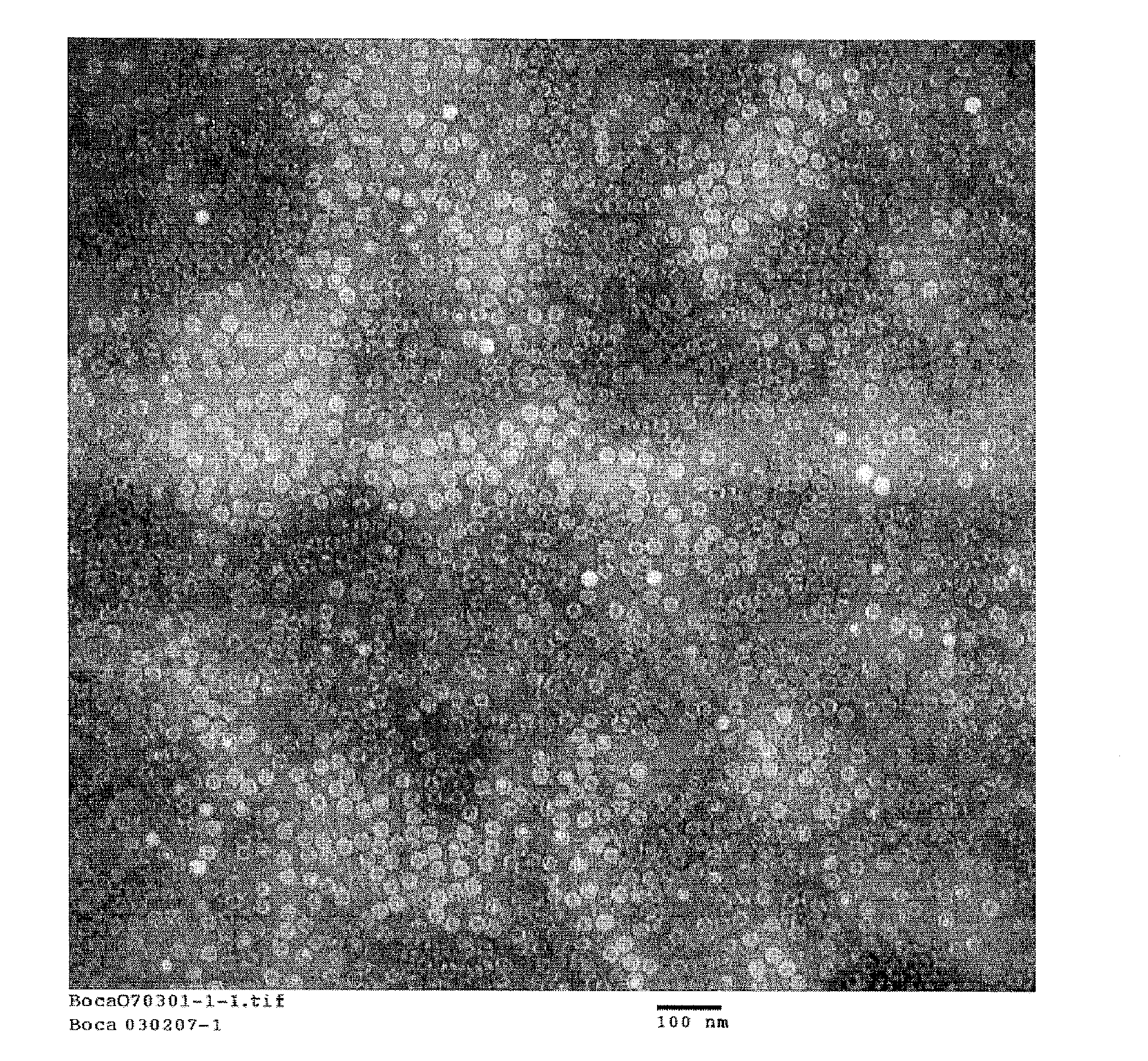
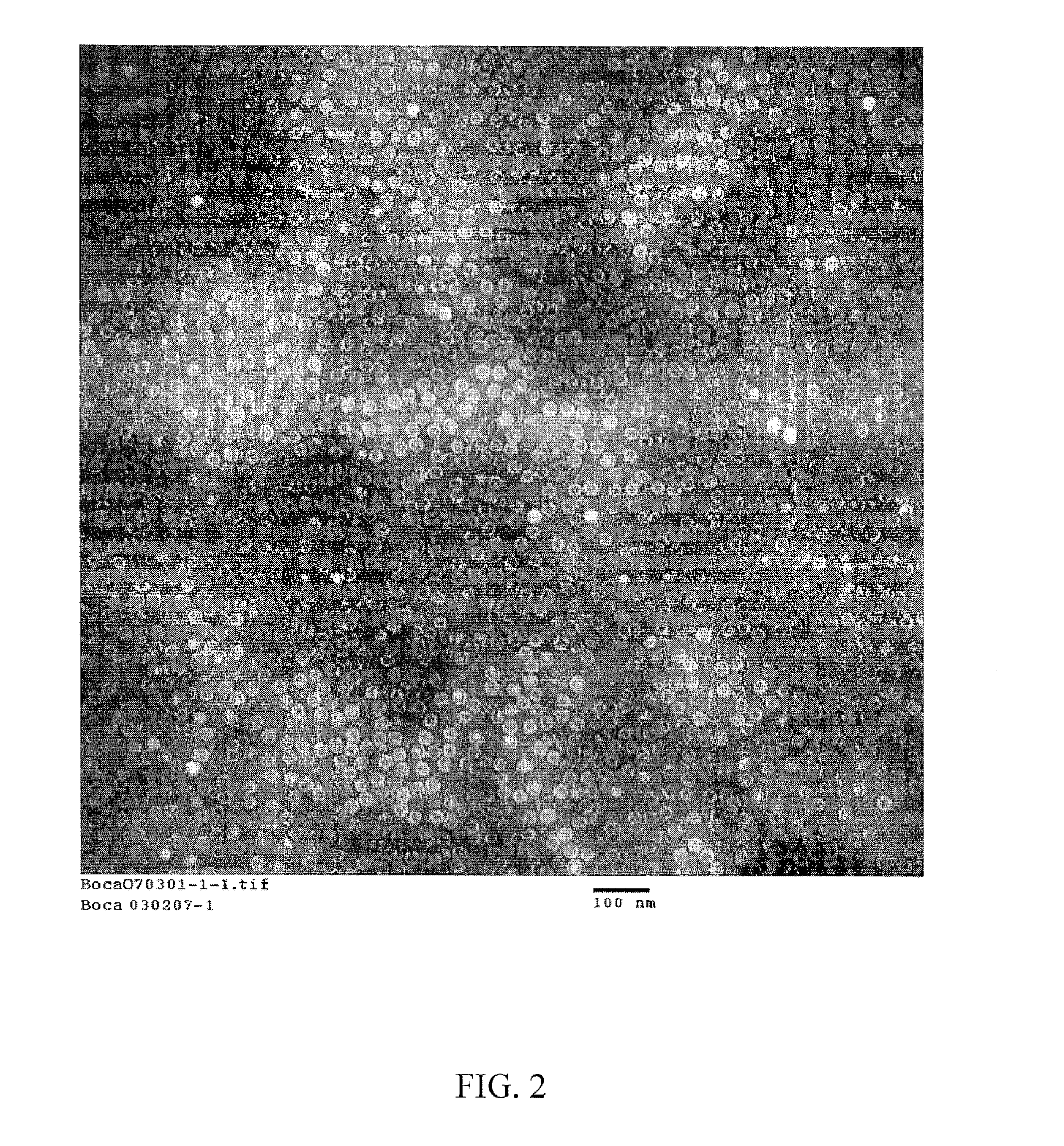
Compositions and processes relating to human bocavirus
Non-replicating, antigenic, human bocavirus virus-like particles (HBoV VLPs) are provided by the present invention along with assays using the HBoV VLPs to detect anti-HBoV antibodies in a biological sample. Pharmaceutical compositions including HBoV VLPs and / or anti-HBoV antibodies are described herein along with novel antibodies generated using HBoV VLPs as an antigen. A recombinant baculovirus is provided including a DNA sequence encoding an expressible human bocavirus VP2 with or without a DNA sequence encoding an expressible human bocavirus VP1 polypeptide, and / or a non-HBoV peptide or protein, and culturing the cells to form the VP1 and / or VP2 proteins that self assemble to form the HBoV VLPs which are then amenable to isolation.
Owner:ERDMAN DEAN D +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com