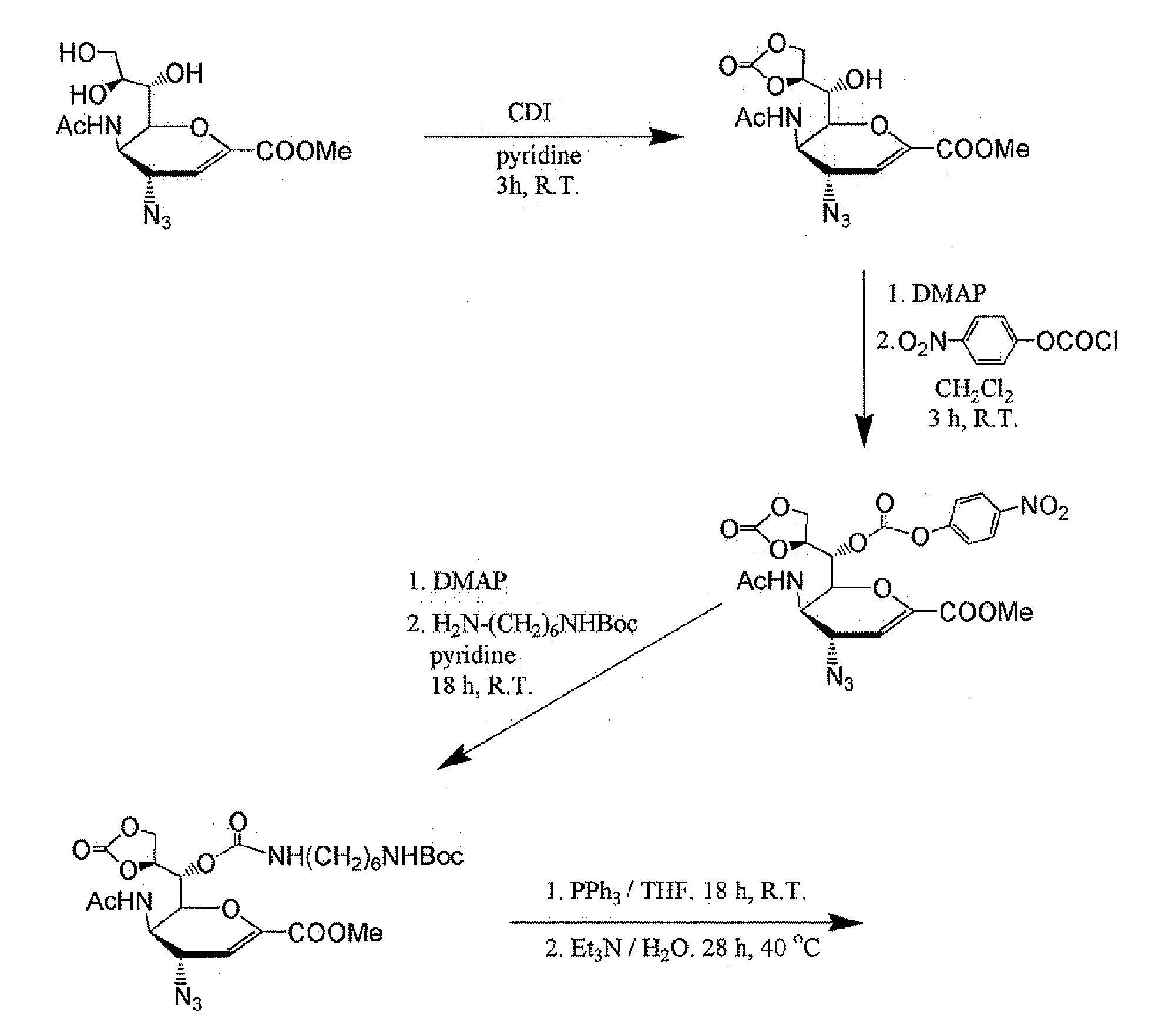
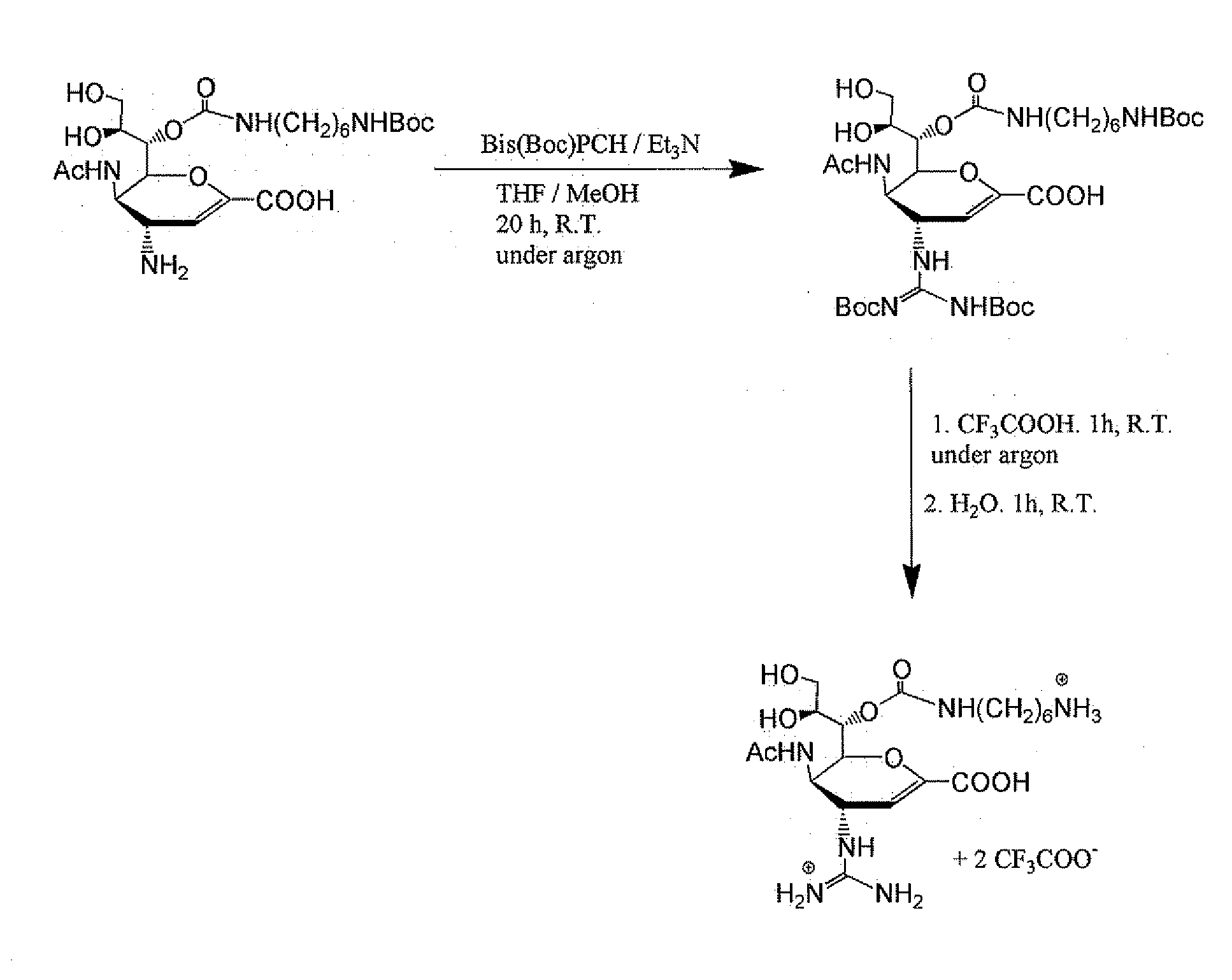
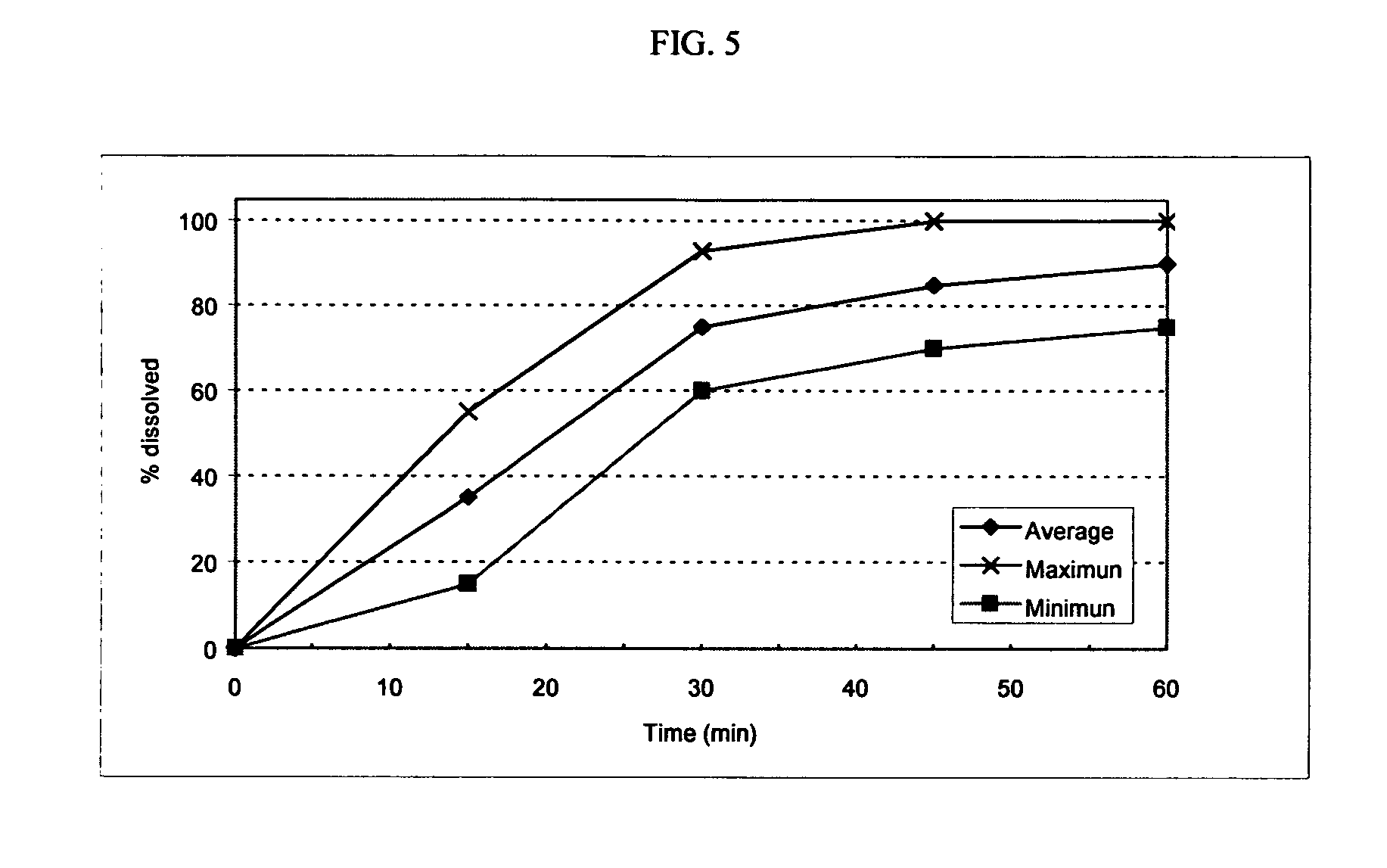
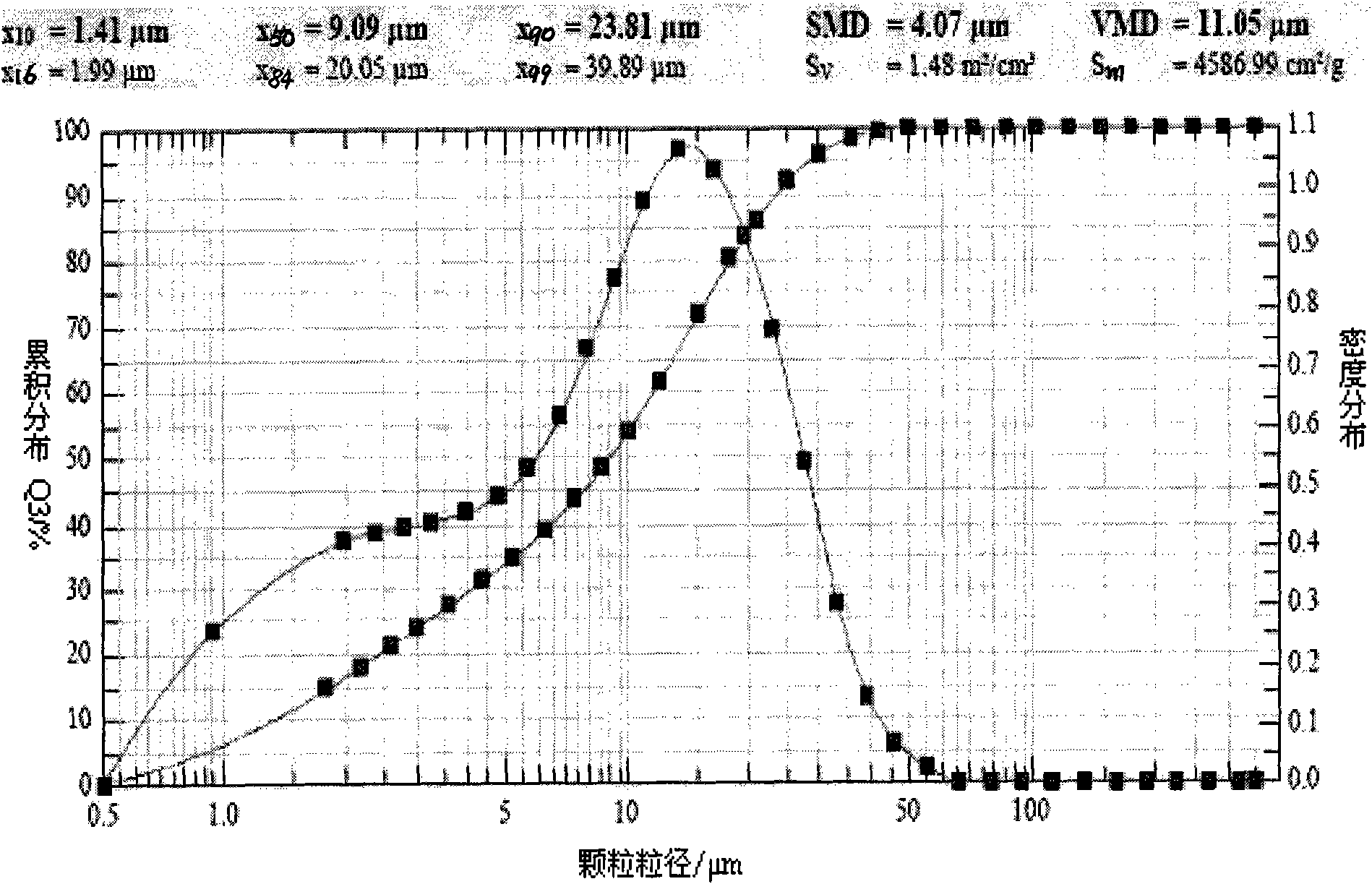
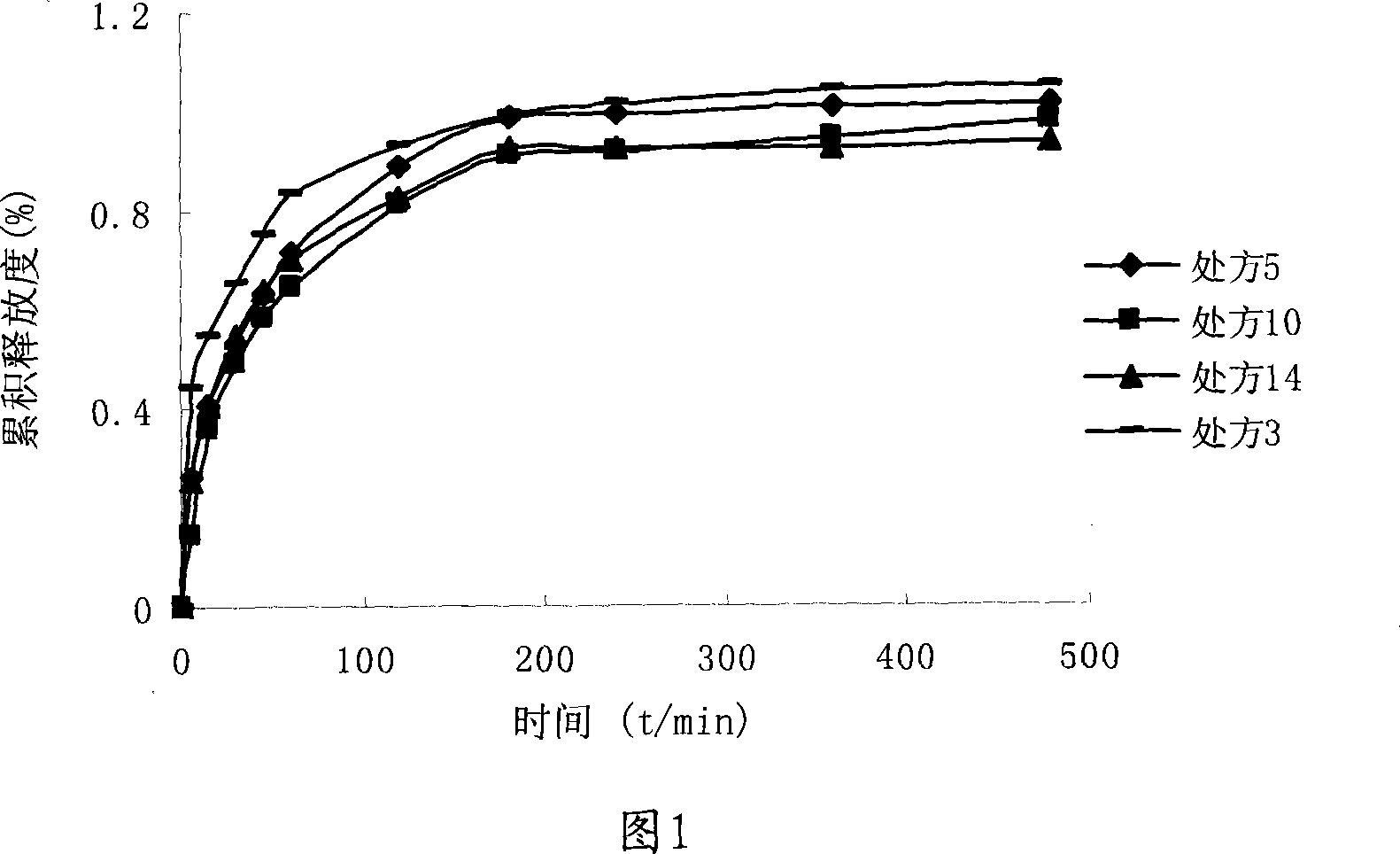
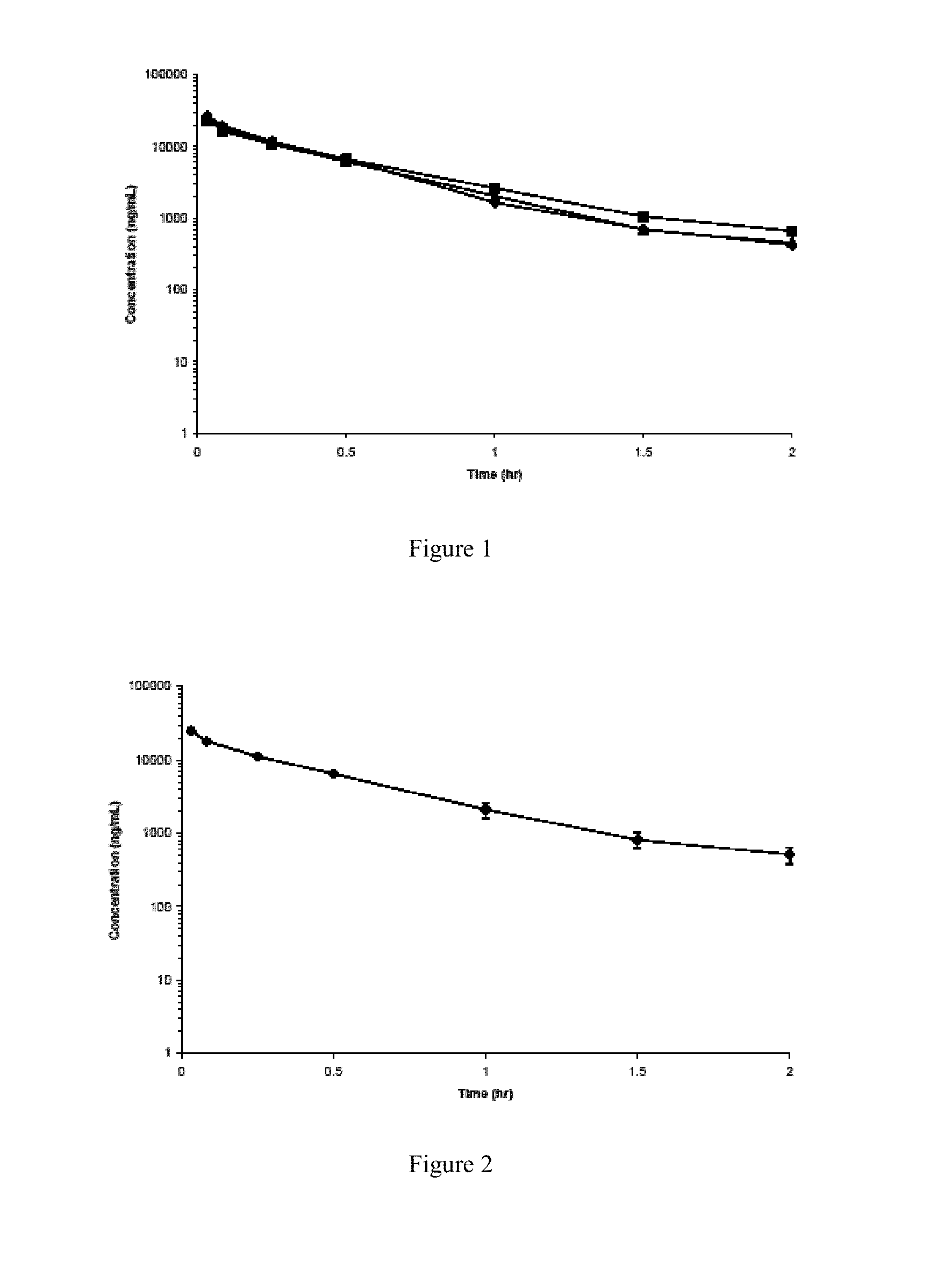
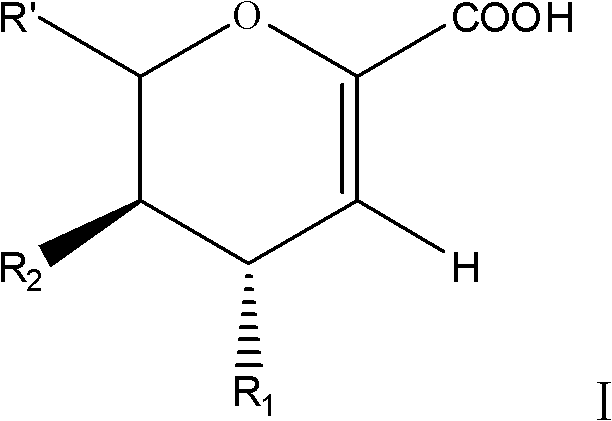
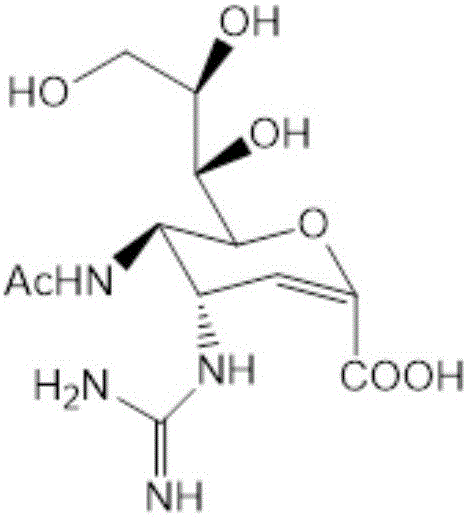
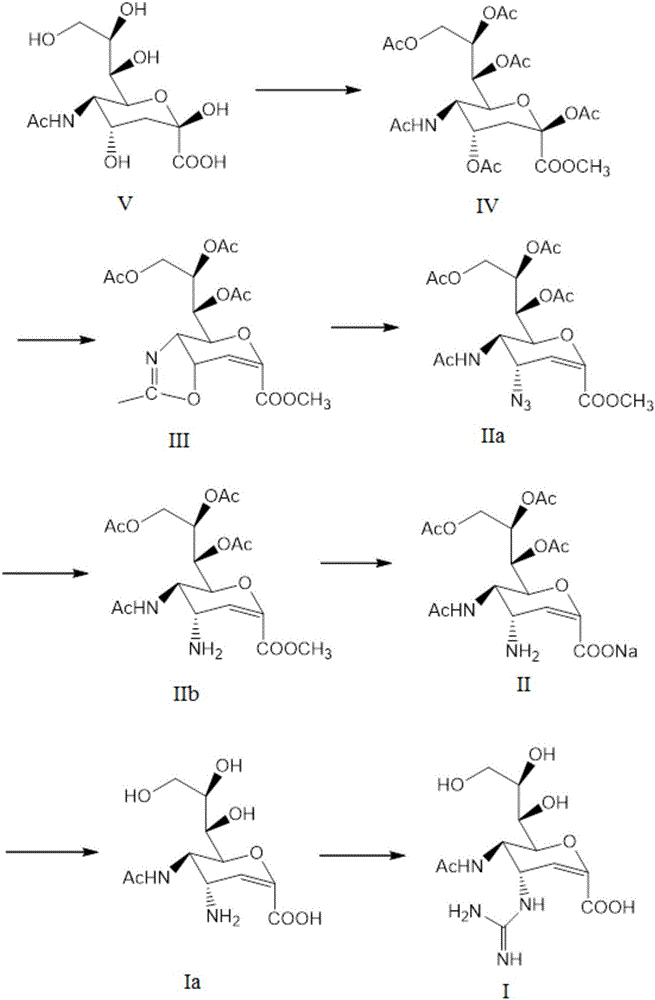
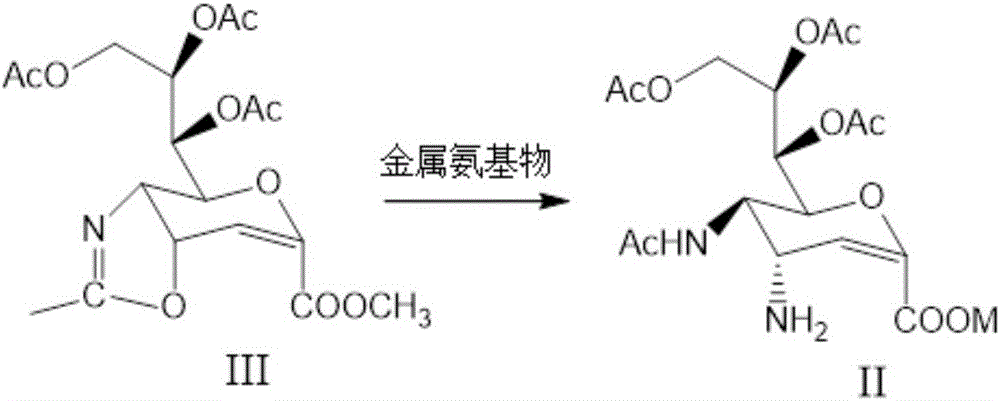
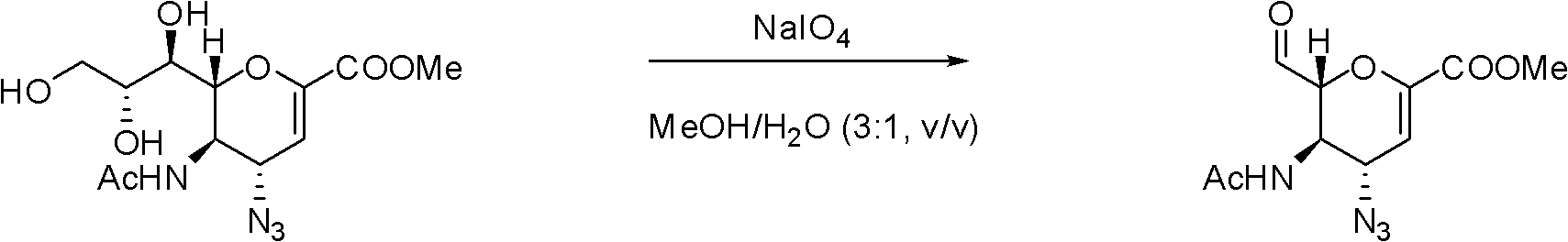Patents
Literature
58 results about "Zanamivir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
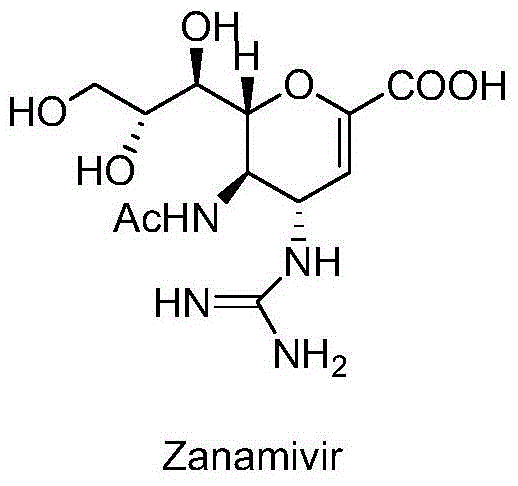
Zanamivir is used to treat symptoms caused by the flu virus (influenza) if you have had symptoms for 2 days or less.
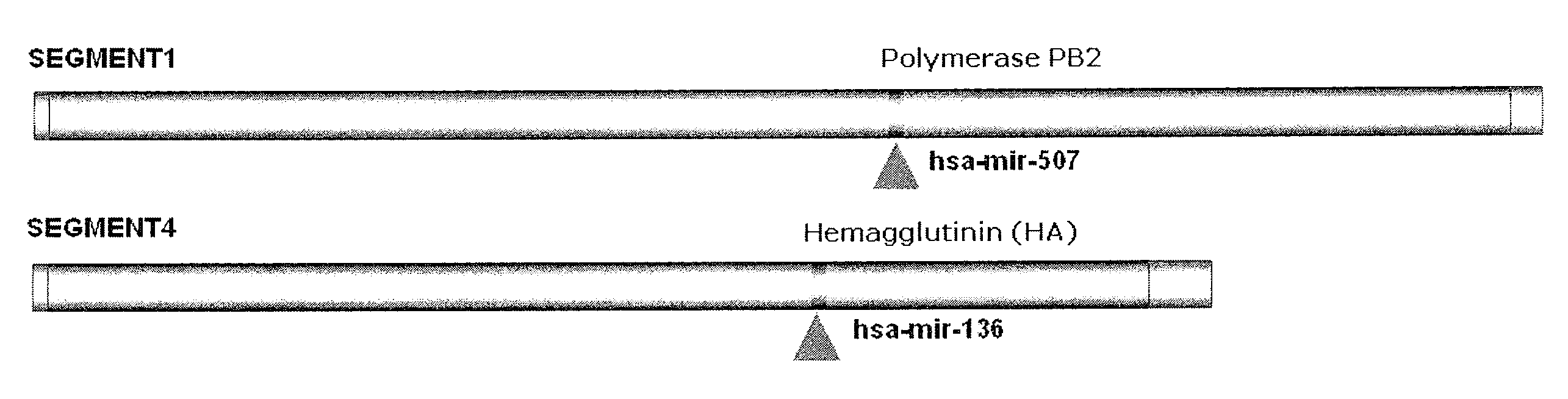
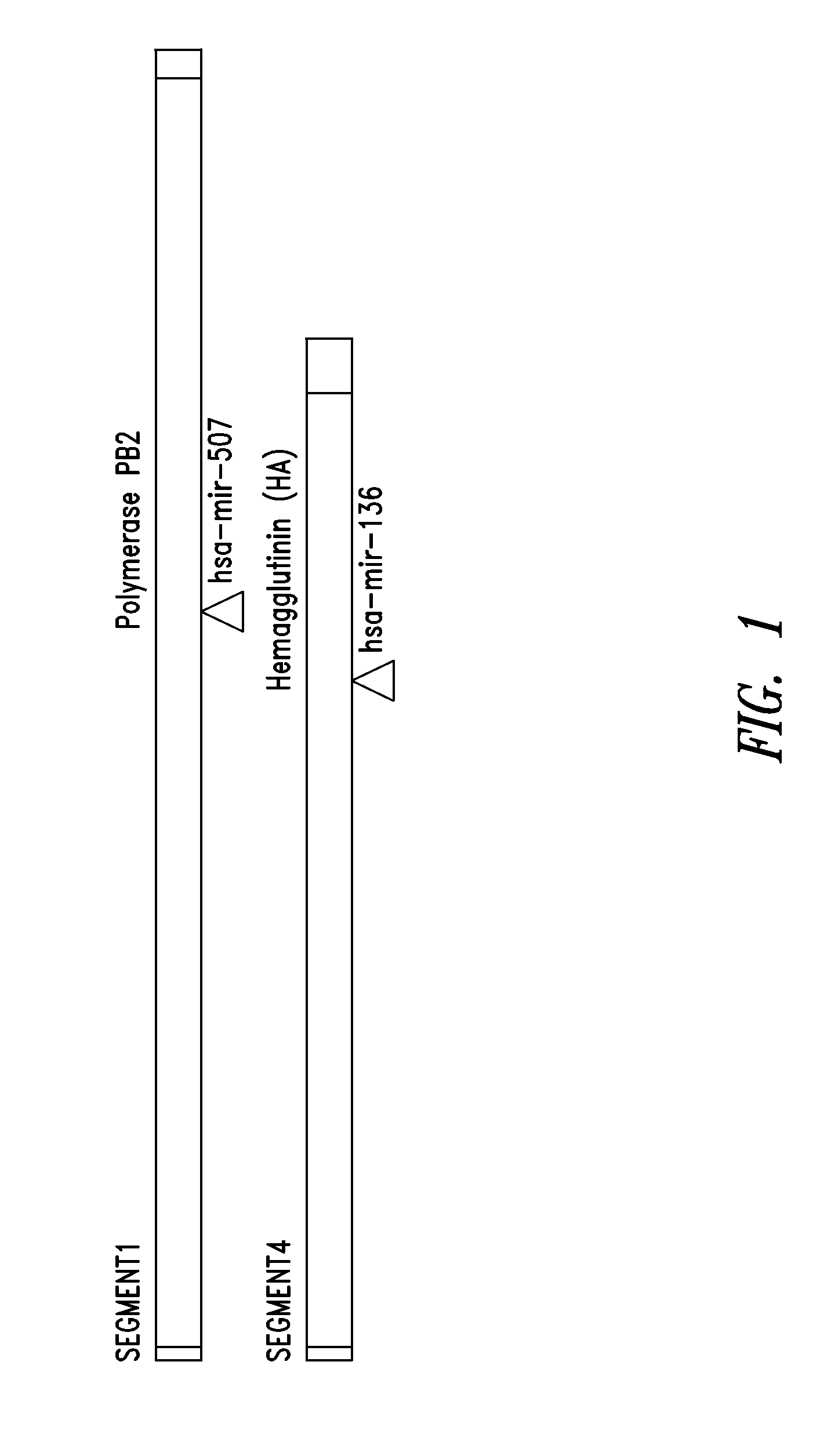
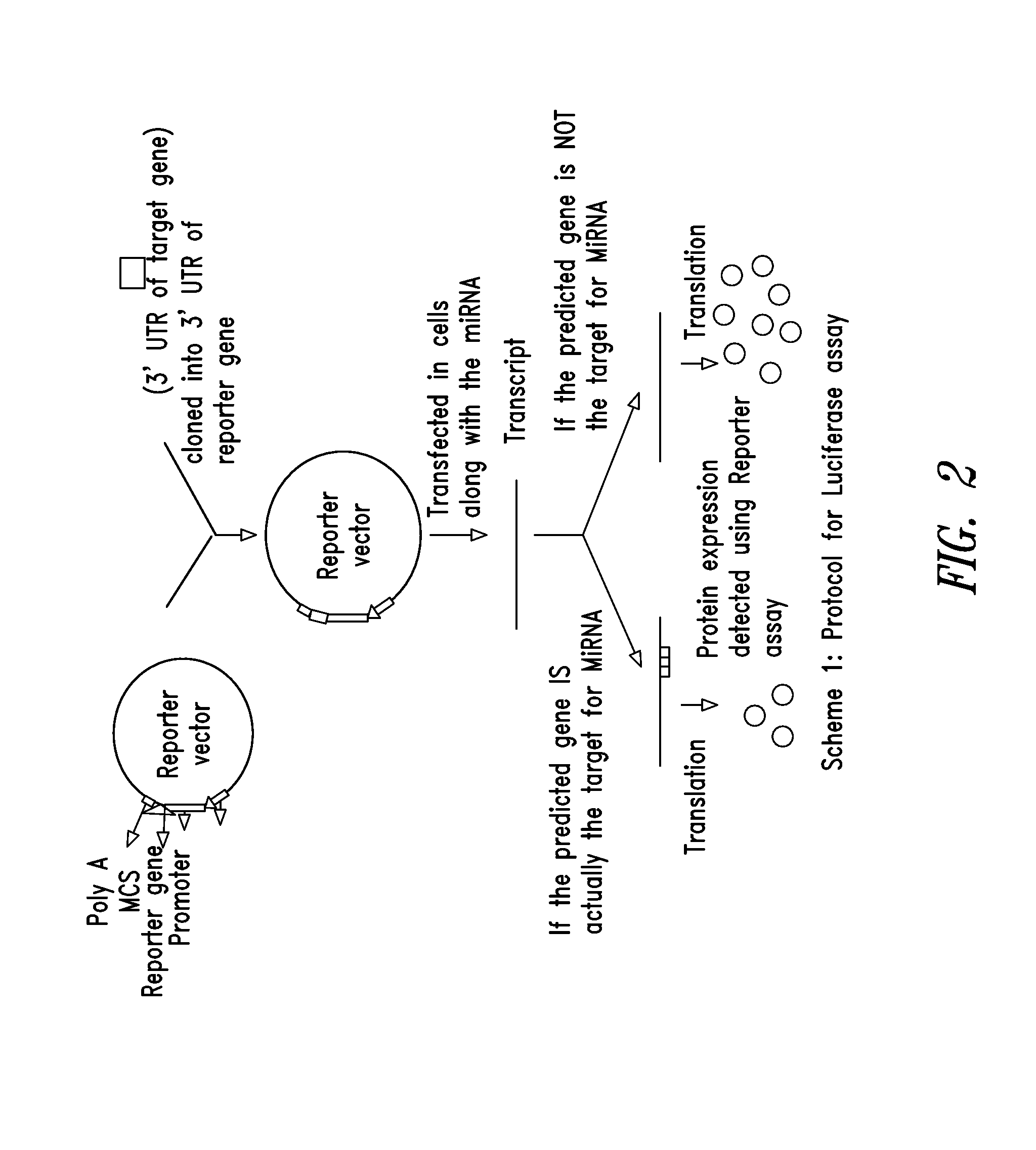
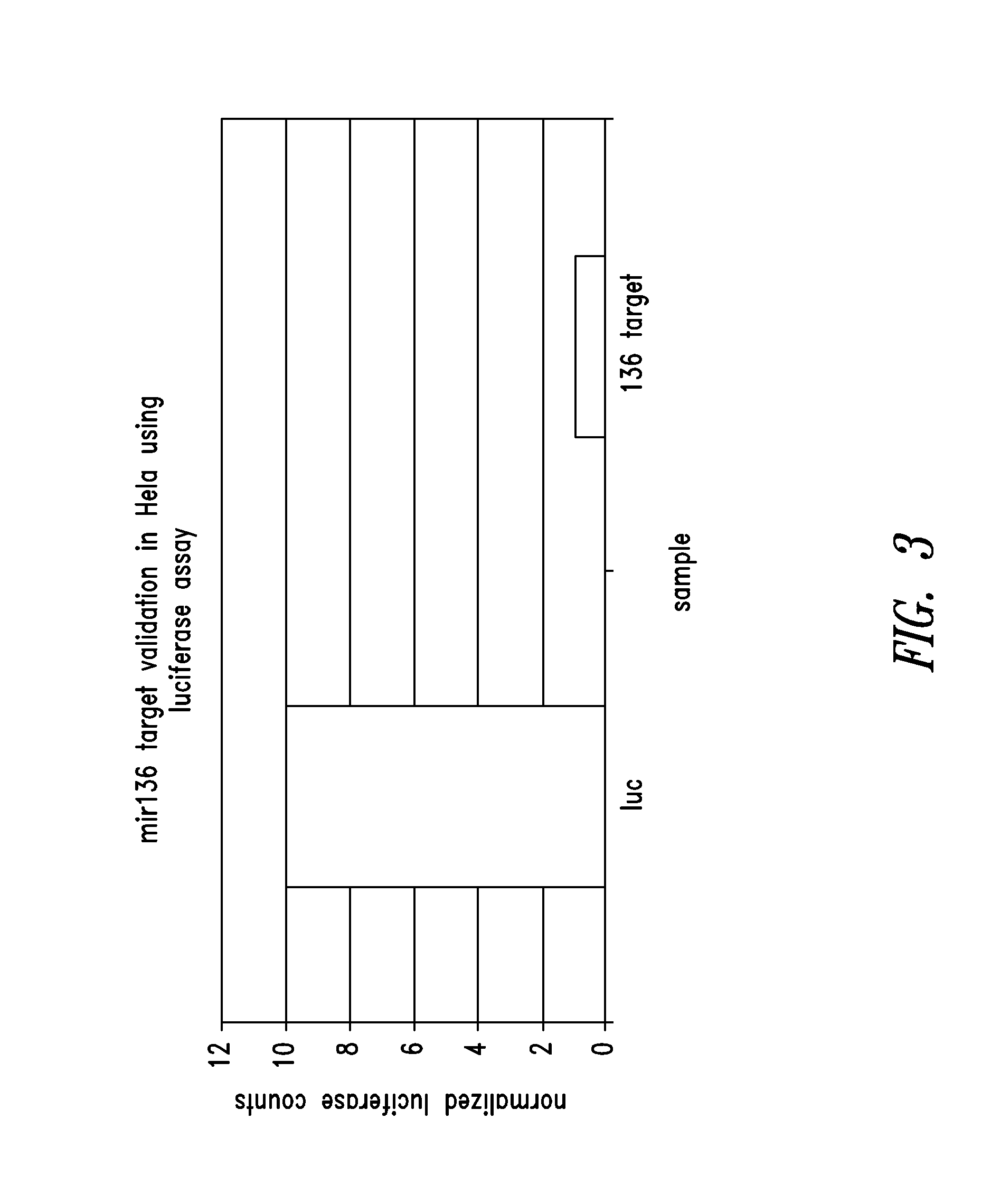
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
Bi-Functional Polymer-Attached Inhibitors of Influenza Virus
InactiveUS20090081249A1Inhibiting and preventing development of resistanceAntiviralsCarrier-bound antigen/hapten ingredientsPolyethylene glycolDextran
Antimicrobial compositions containing two or more antiviral agents coupled to a polymer and methods of making and using the compositions, are described herein. In one embodiment, two or more antiviral agents are covalently coupled to the polymer. Suitable antiviral agents include, but are not limited to, sialic acid, zanamivir, oseltamivir, amantadine, rimantadine, and combinations thereof. The polymer is preferably a water-soluble, biocompatible polymer. Suitable polymers include, but are not limited to, poly(isobutylene-alt-maleic anhydride) (PIBMA), poly(aspartic acid), poly(l-glutamic acid), polylysine, poly(acrylic acid), plyaginic acid, chitosan, carboxymethyl cellulose, carboxymethyl dextran, polyethyleneimine, and blends and copolymers thereof. In another embodiment, the compositions contain a physical mixture of polymer containing one antiviral agent and polymer containing a second antiviral agent. The compositions can be formulated for enteral or parenteral administration. Suitable oral / intranasal dosage forms include, but are not limited to, tablets, capsules, solutions, suspensions, emulsions, syrups, and lozenges. Suitable dosage forms for parenteral administration include, but are not limited to, solutions, suspensions, and emulsions. The compositions described herein are effective at treating a variety of infections, including viral infections such as influenza, while inhibiting or preventing the development of microbial resistance.
Owner:MASSACHUSETTS INST OF TECH
Drug delivery device containing neuraminidase inhibitor and an H1 antagonist
The present invention provides a dual release solid dosage form containing a first composition that releases a neuraminidase inhibitor, such as oseltamivir, zanamivir, or peramivir, in a controlled manner and a second composition that releases an H1 antagonist in a rapid and / or immediate manner. A wide range of H1 antagonist antihistamines, especially fexofenadine and loratadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. The device is useful for the treatment of respiratory congestion and other viral infection associated symptoms.
Owner:ACELLA HLDG LLC +1
Zanamivir inhalation solution and application thereof
InactiveCN101773491AIncrease deposition rateDoes not cause depositionOrganic active ingredientsPharmaceutical delivery mechanismPharmacyMedicine
The invention belongs to the fields of pharmacy and influenza, and discloses a zanamivir inhalation solution and the application thereof. The zanamivir inhalation solution is prepared by being added with a certain dosage of surface active agent and osmotic pressure regulator, and comprises the components by weight percent: 0.1-10wt percent of zanamivir, 0.01-0.5wt percent of surface active agent, 0.9-8wt % of osmotic pressure regulator and balance water. The inhalation solution is beneficial to forming aerosol granules with small grain diameter by ultrasonic atomization, and prevents the zanamivir liquid drops from merging and gathering after aerosol is formed, thus effectively transmitting the medicine into the lung of a patient, and ensuring the medicine to take the best effect.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD
Zanamivir nasal in situ jellies with phase variation property and preparing method thereof
InactiveCN101229122AEffective absorptionExtended stayOrganic active ingredientsAerosol deliveryNasal cavityWhole body
The invention relates to the new dosage form of zanamivir, which is a nasal in-situ gel with phase transformation property and preparation methods, the invention is made of the original drug of zanamivir, hydrophilic gel materials which are sensitive to environments and assistant materials which can be accepted in pharmacy, after being absorbed by nasal mucosa, the invention can have systemic functions and improve the partial concentration of drugs in respiratory tracts, when room temperature is about 20 DEG C and the invention is in storing state, the invention is in the state of liquid, while after being put into the physiological conditions of nasal cavities, the invention can be quickly formed into gel on the surface of nasal mucosa, so that the detained time of drugs can be prolonged, biological availability can be improved and the compliance of patients can be perfected, and the viscosity of the invention is proper, after being formed into gel, the invention can be detained on the surface of the nasal cavity for a rather long time, which has no nasal ciliary toxicity.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
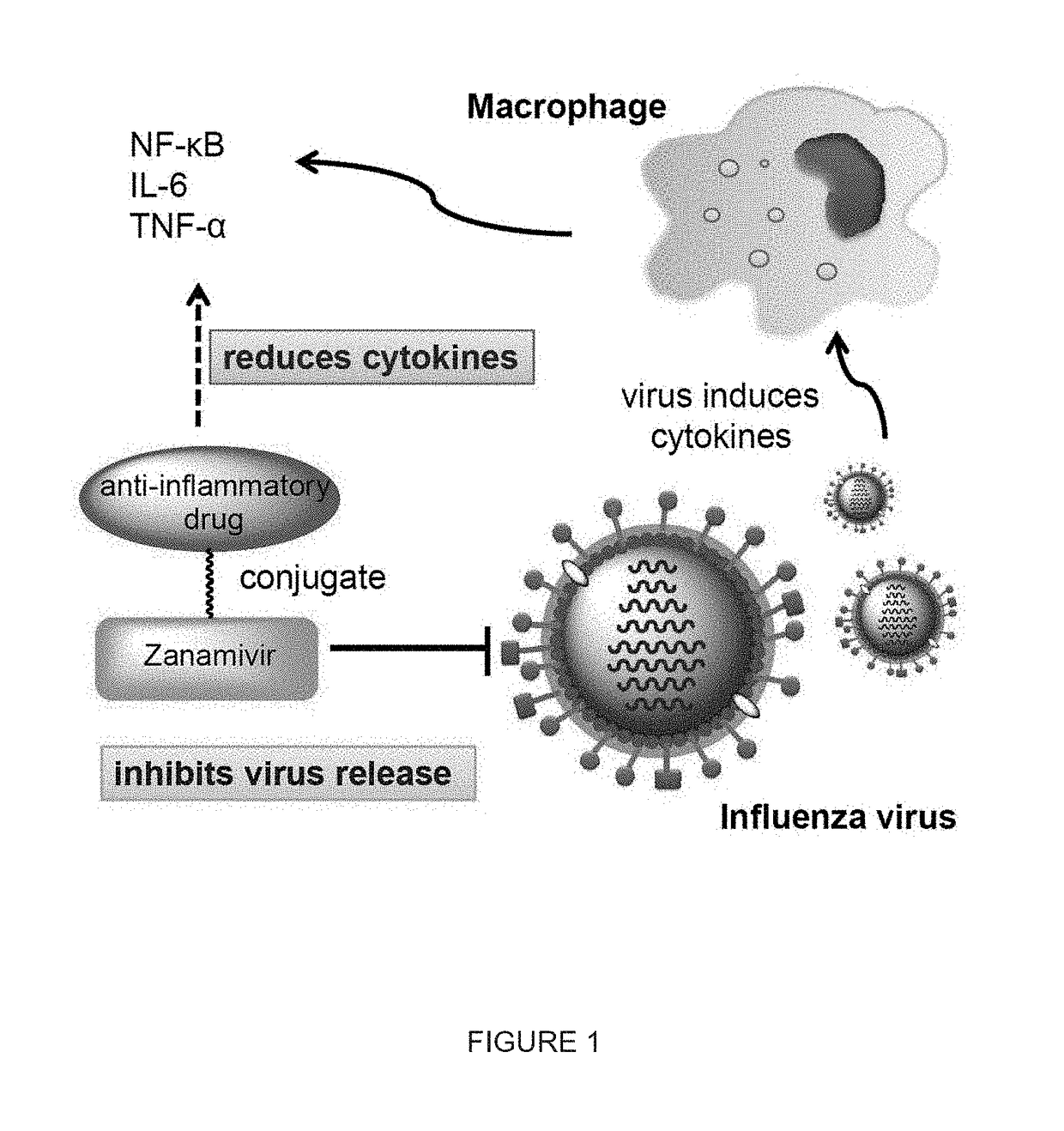
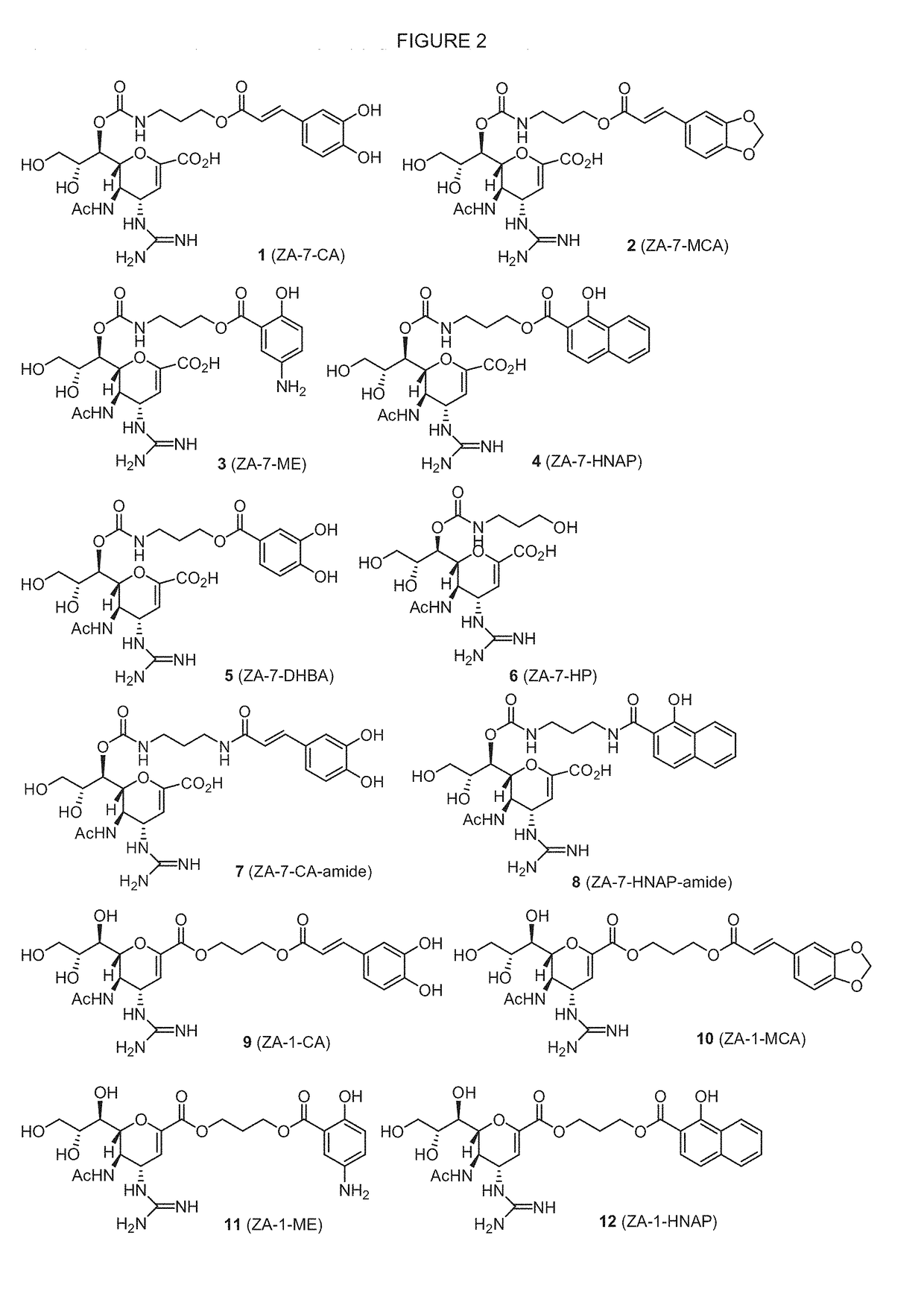
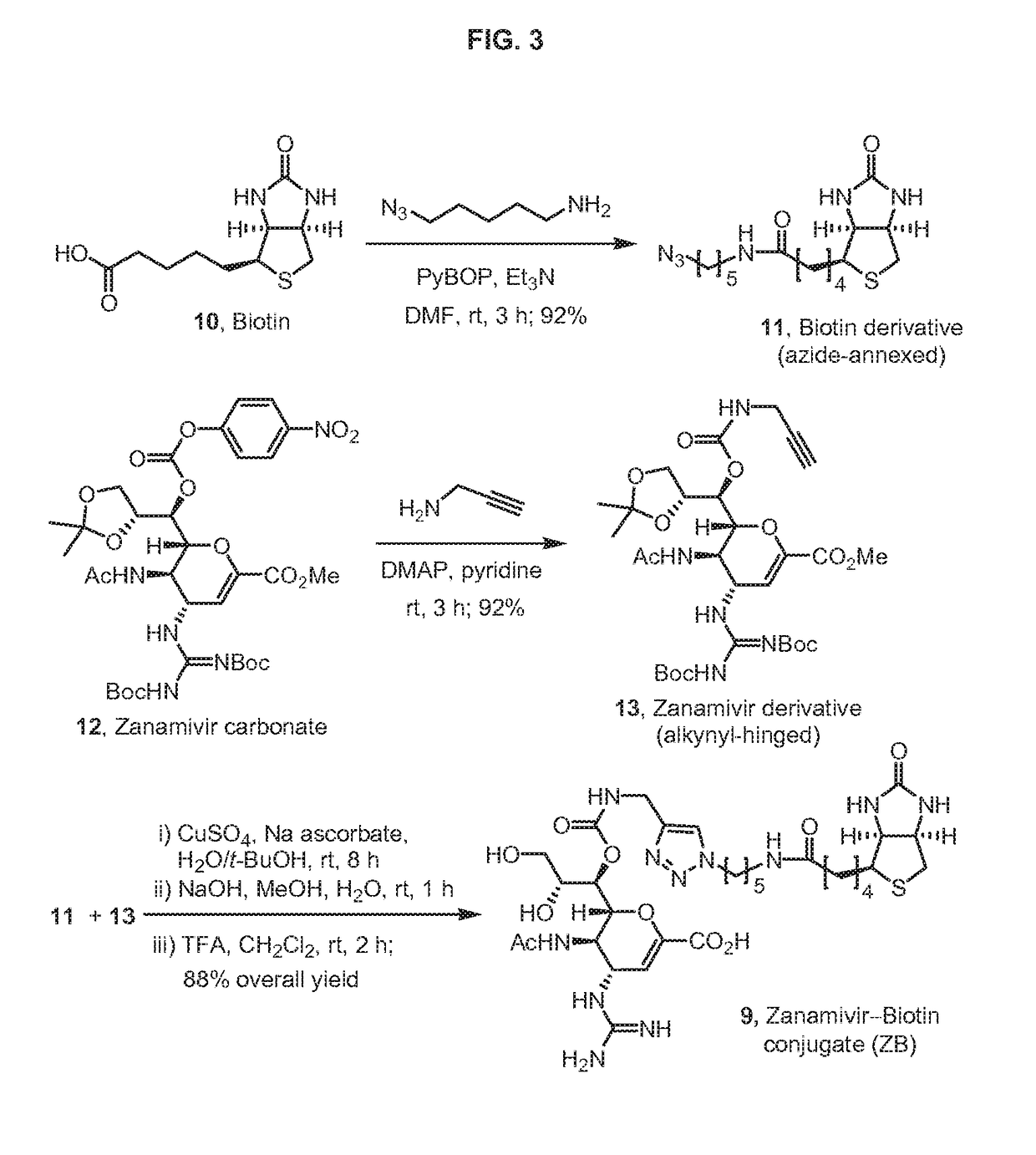
Enhanced anti-influenza agents conjugated with anti-inflammatory activity
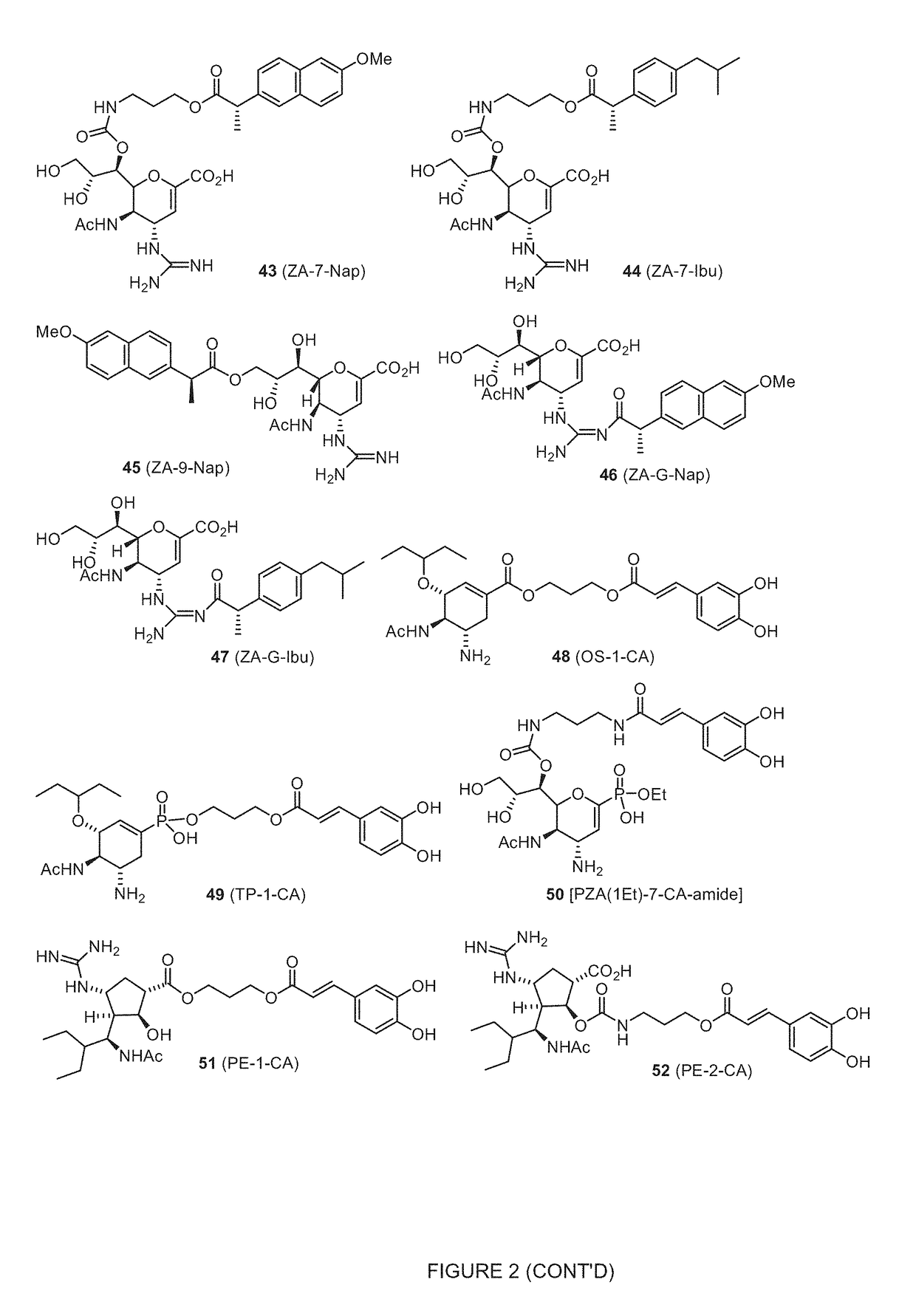
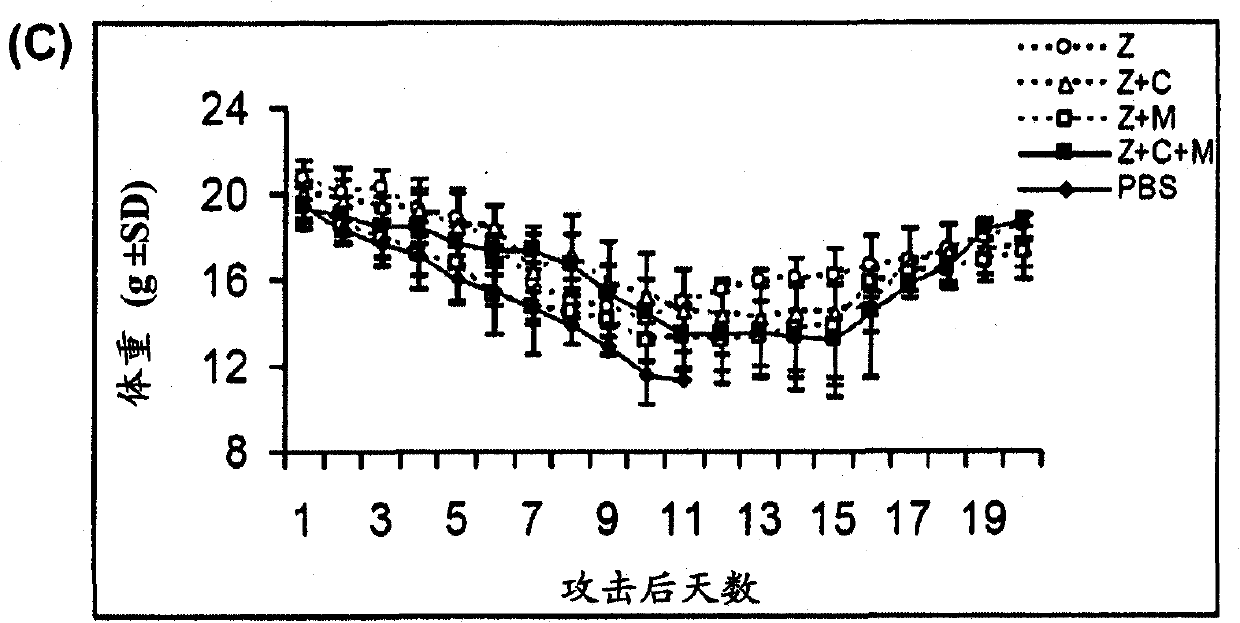
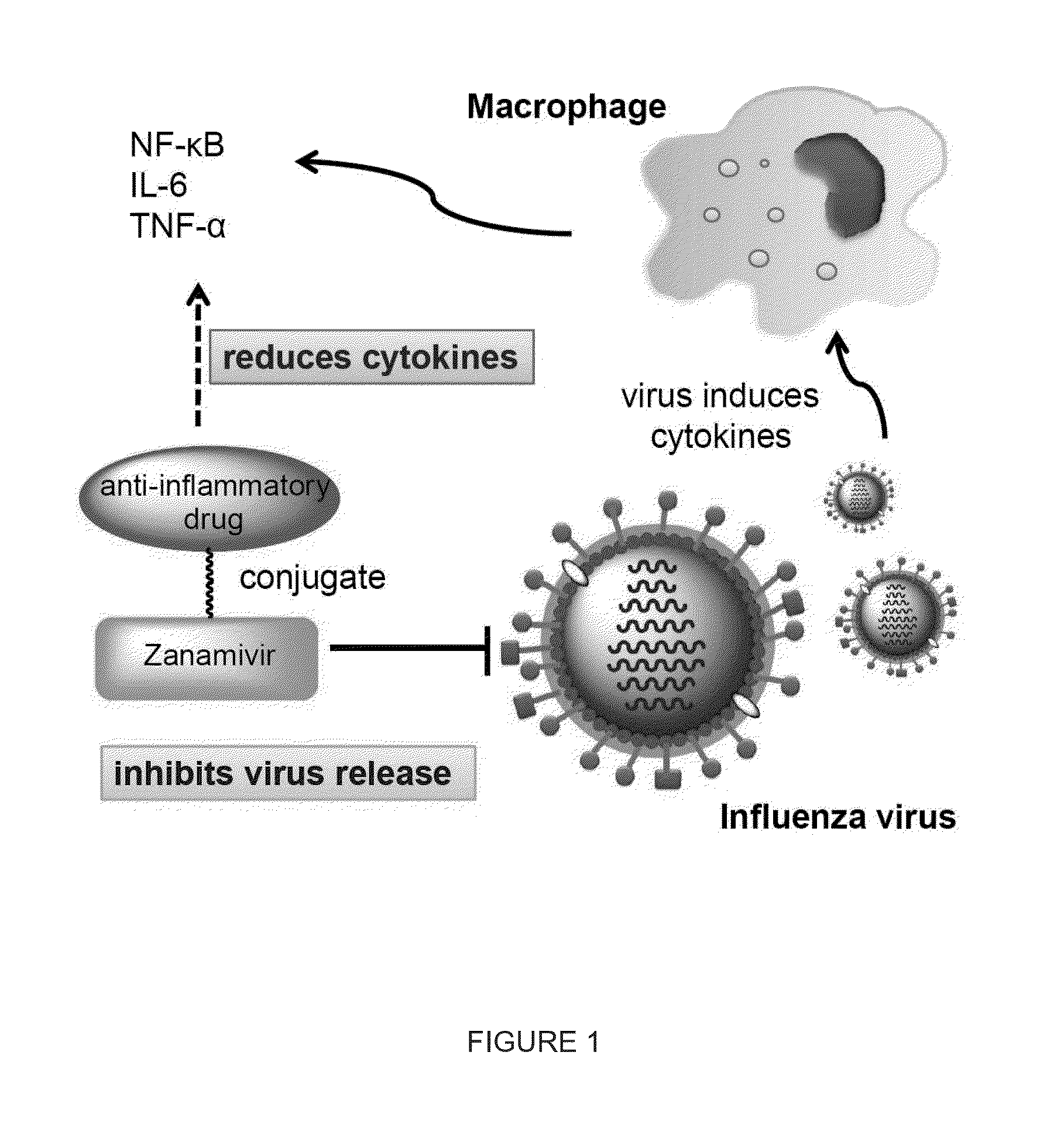
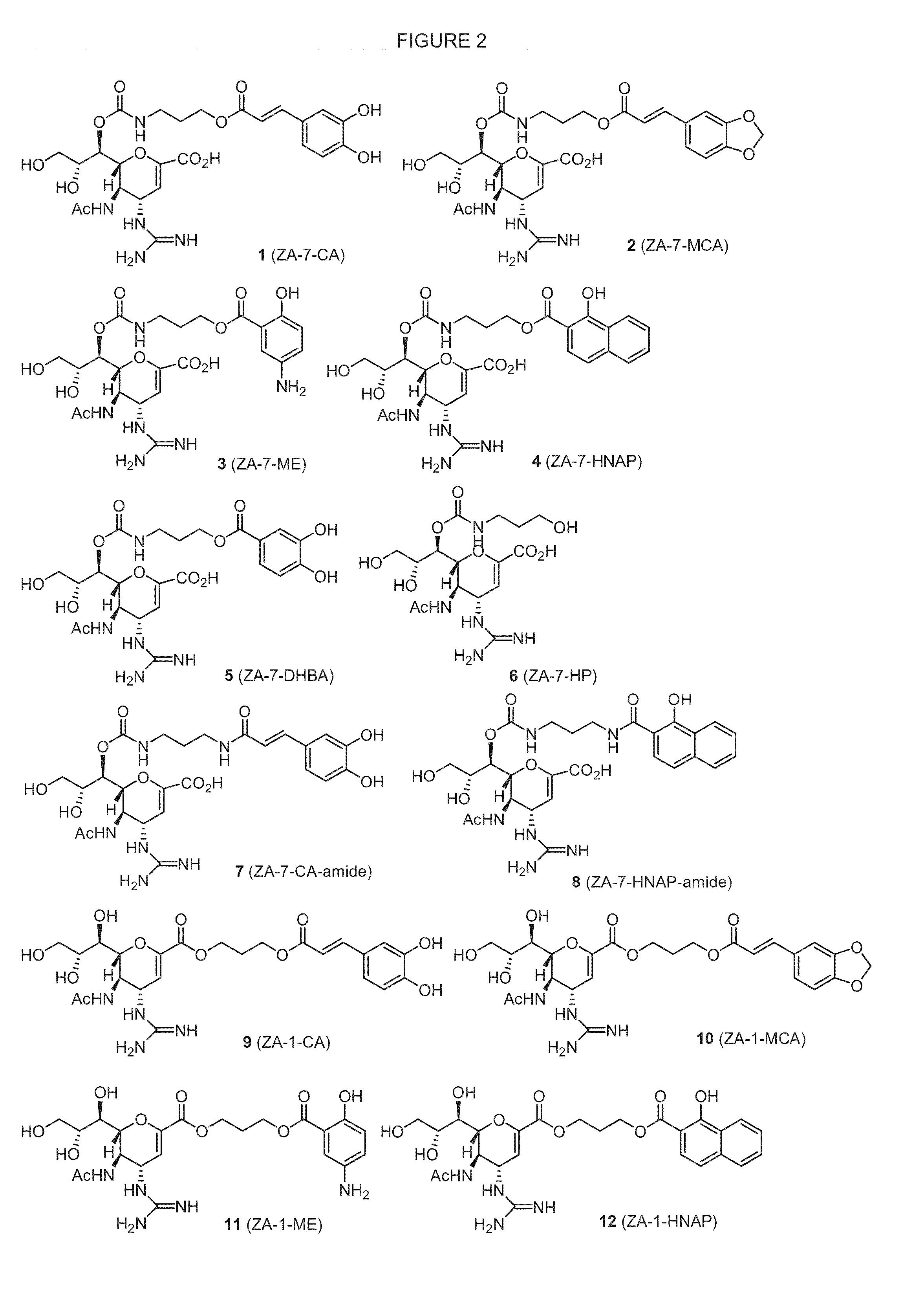
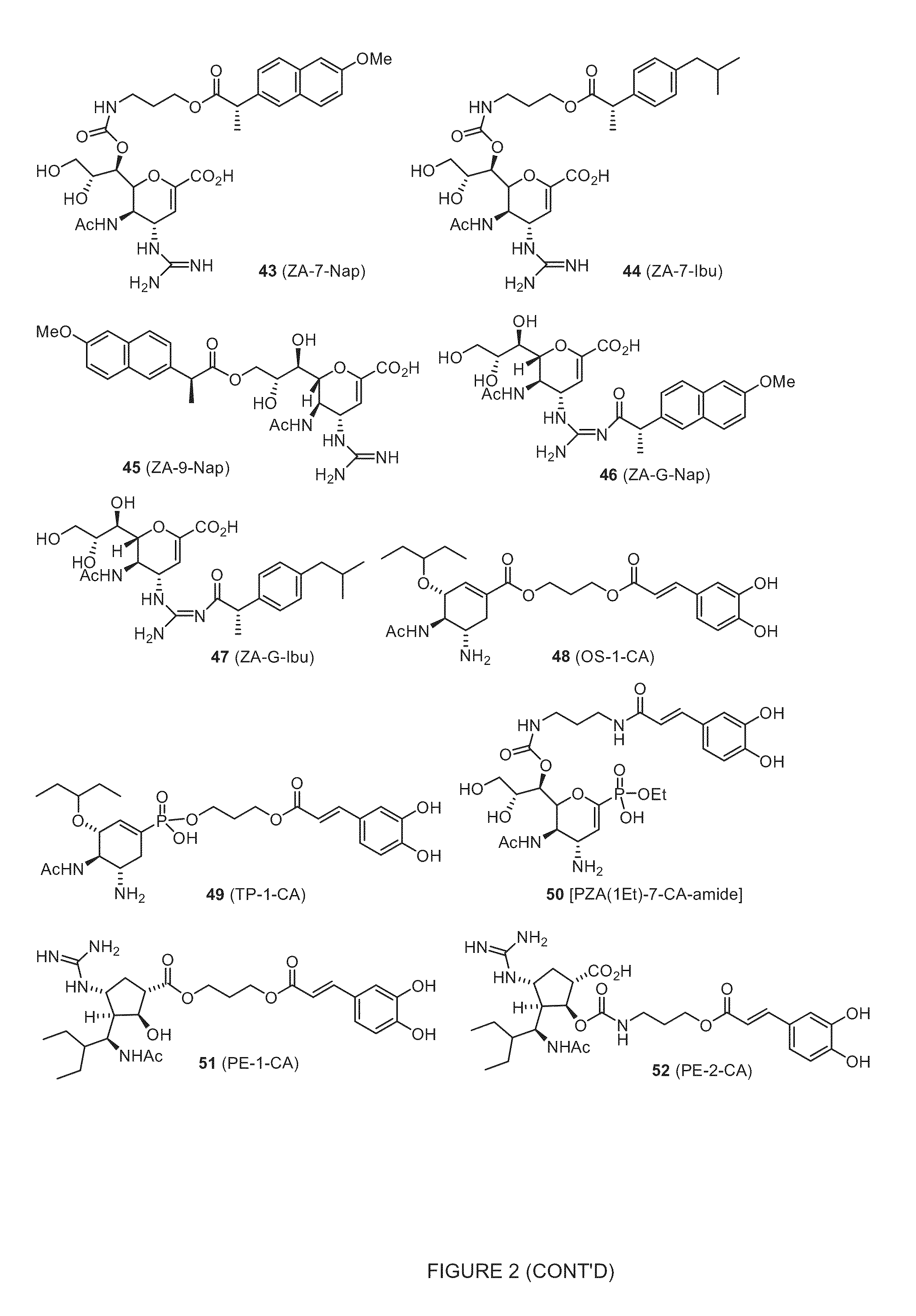
Novel dual-targeted, bifunctional anti-influenza drugs formed by conjugation with anti-inflammatory agents are disclosed. Exemplary drugs according to the invention include caffeic acid (CA)-bearing zanamivir (ZA) conjugates ZA-7-CA (1), ZA-7-CA-amide (7) and ZA-7-Nap (43) for simultaneous inhibition of influenza virus neuraminidase and suppression of proinflammatory cytokines. Synthetic methods for preparation of these enhanced anti-influenza conjugate drugs are provided. The synthetic bifunctional ZA conjugates act synergistically towards protection of mice lethally infected by H1N1 or H5N1 influenza viruses. The efficacy of ZA-7-CA, ZA-7-CA-amide and ZA-7-Nap conjugates is much greater than the combination therapy of ZA with anti-inflammatory agents.
Owner:ACAD SINIC
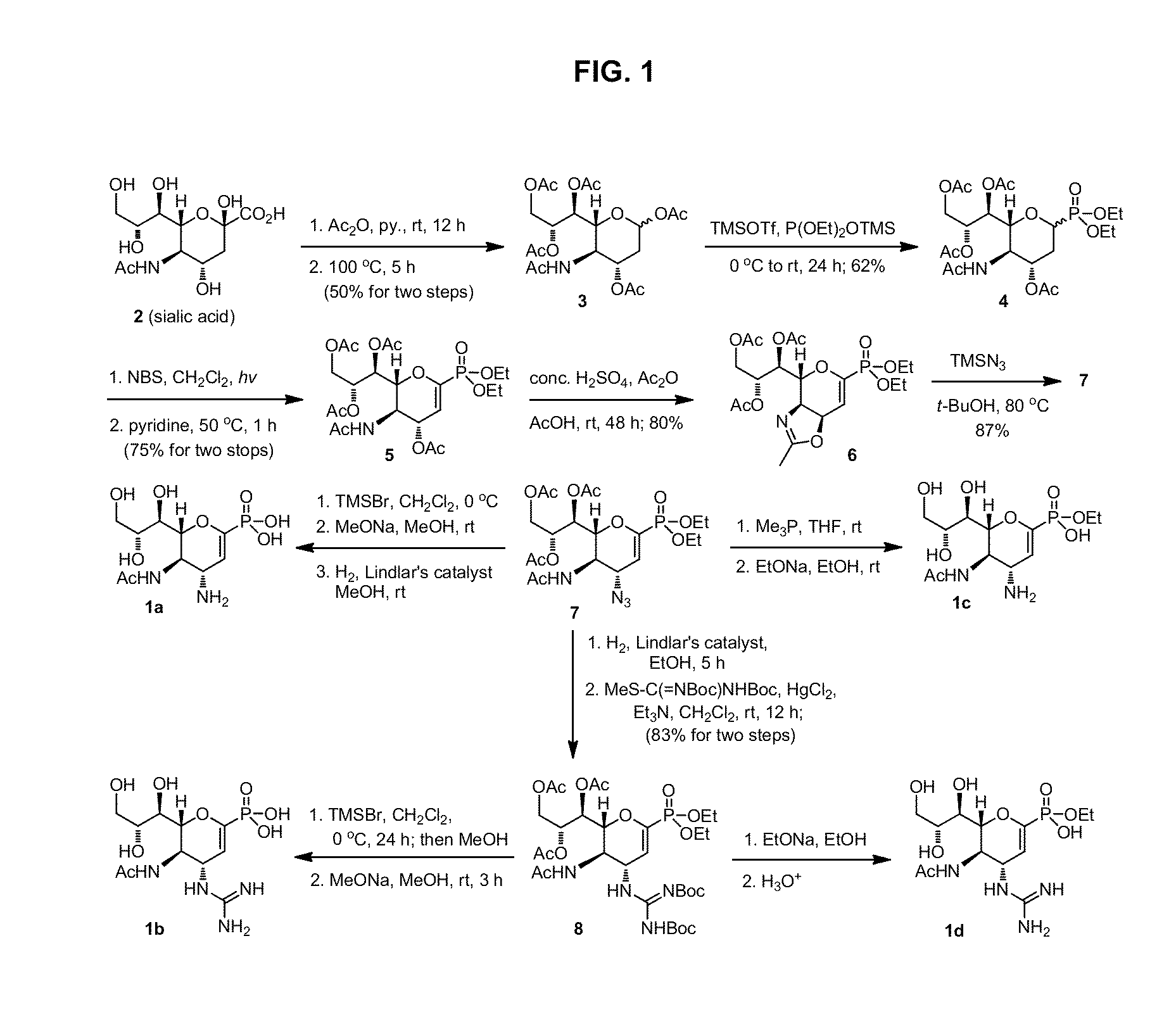
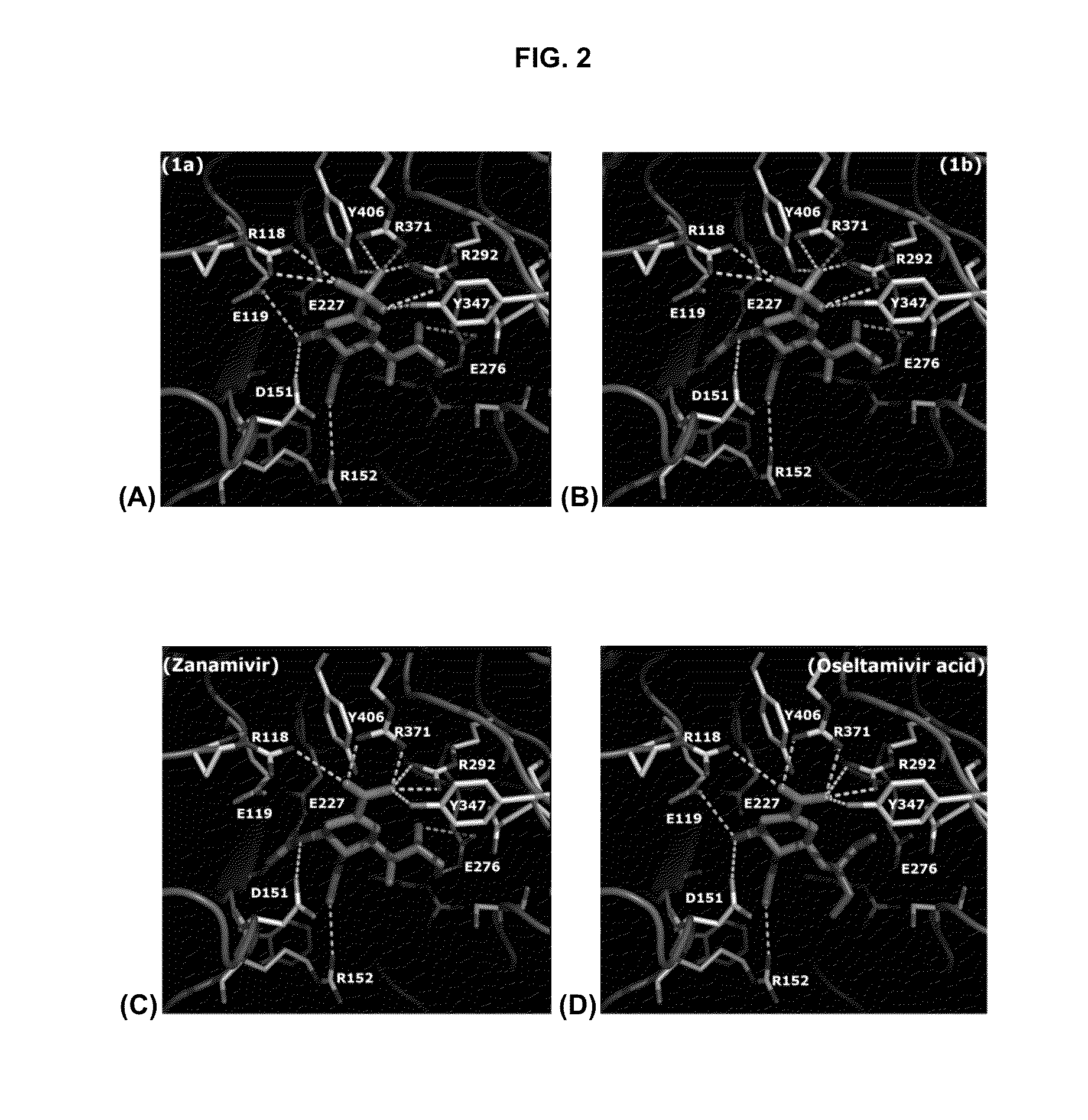
Zanamivir phosphonate congeners with Anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
ActiveUS20170102387A1Organic active ingredientsGroup 5/15 element organic compoundsWild typeDrug resistance
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINICA
Zanamivir phosphonate congeners with Anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
ActiveUS20130225532A1Reduce the binding effectBiocideOrganic active ingredientsCompetitive bindingWild type
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINIC
Combination therapy for the treatment of influenza
InactiveCN102036658AInhibit or reduce the effective amountOrganic active ingredientsAntiviralsInfected cellImmunomodulating Agent
Compositions and methods for treating one or more symptoms of influenza, preferably influenza due to infection with influenza A (H5N1) are provided. It has been discovered that administration of a combination of a neuraminidase inhibitor with two immunomodulators increases survivability in subjects 24, 48, or even 72 hours post infection compared to administration of the neuraminidase inhibitor alone. A preferred neuraminidase inhibitor includes, but is not limited to zanamivir. Preferred immunomodulators include, but are not limited to celecoxib and mesalazine. Another embodiment provides a method for treating influenza, preferably, influenza due to infection with avian influenza A (H5N1) by administering to subject infected with the influenza virus, an effective amount of a neuraminidase inhibitor to inhibit or reduce budding of the influenza virus from infected cells of the subject, and an effective amount of at least two immunomodulators effective to reduce or inhibit one or more symptoms of inflammation in the subject.
Owner:THE UNIVERSITY OF HONG KONG
Enhanced Anti-influenza agents conjugated with Anti-inflammatory activity
Novel dual-targeted, bifunctional anti-influenza drugs formed by conjugation with anti-inflammatory agents are disclosed. Exemplary drugs according to the invention include caffeic acid (CA)-bearing zanamivir (ZA) conjugates ZA-7-CA (1), ZA-7-CA-amide (7) and ZA-7-Nap (43) for simultaneous inhibition of influenza virus neuraminidase and suppression of proinflammatory cytokines. Synthetic methods for preparation of these enhanced anti-influenza conjugate drugs are provided. The synthetic bifunctional ZA conjugates act synergistically towards protection of mice lethally infected by H1N1 or H5N1 influenza viruses. The efficacy of ZA-7-CA, ZA-7-CA-amide and ZA-7-Nap conjugates is much greater than the combination therapy of ZA with anti-inflammatory agents.
Owner:ACAD SINIC
Zanamivir capsule type inhalation aerosol powder and preparation method thereof
The invention relates to a zanamivir capsule type inhalation aerosol powder and a preparation method thereof. The capsule type inhalation aerosol powder comprises a capsule shell and a capsule content, wherein the capsule content is composed of 5 parts by weight of zanamivir super micropowder, 10-18 parts by weight of inhalation crystallization lactose and 2-10 parts by weight of inhalation grinding lactose 40M, and average particle size of the zanamivir super micropowder is less than 5mu m and particle size of an excipient is within the range of 40-100mu m. The zanamivir capsule type inhalation aerosol powder provided by the invention has a simple preparation process, good mobility, low hygroscopicity, high content uniformity and high deposit rate on an effective part.
Owner:SHANDONG NEWTIME PHARMA
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
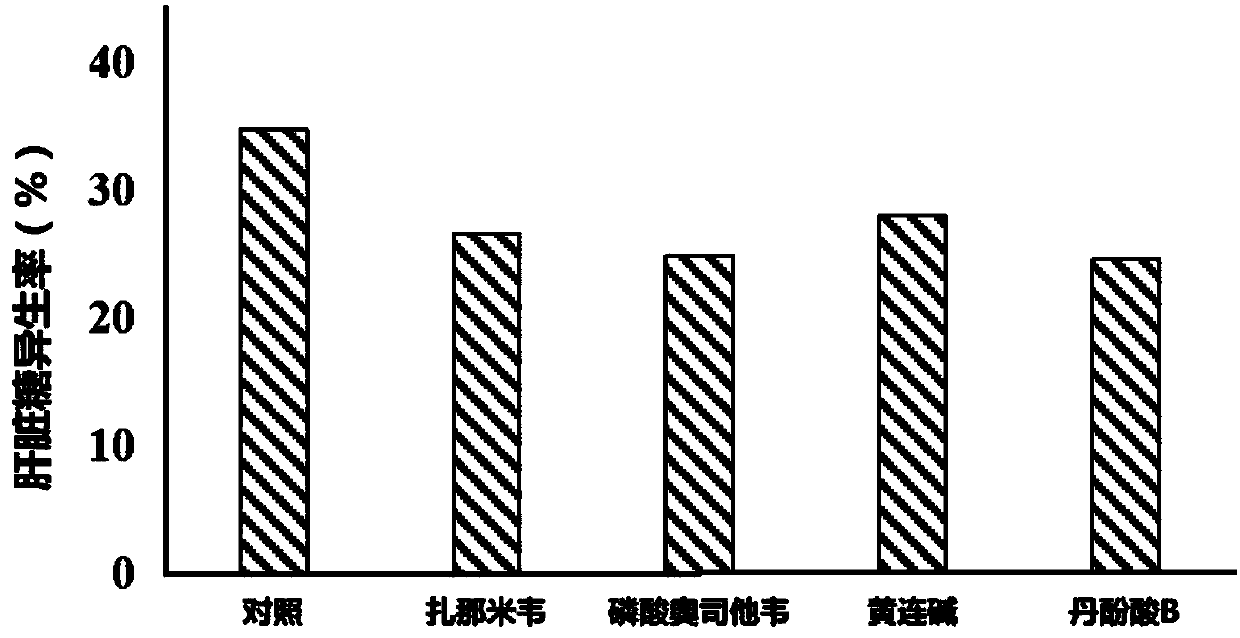
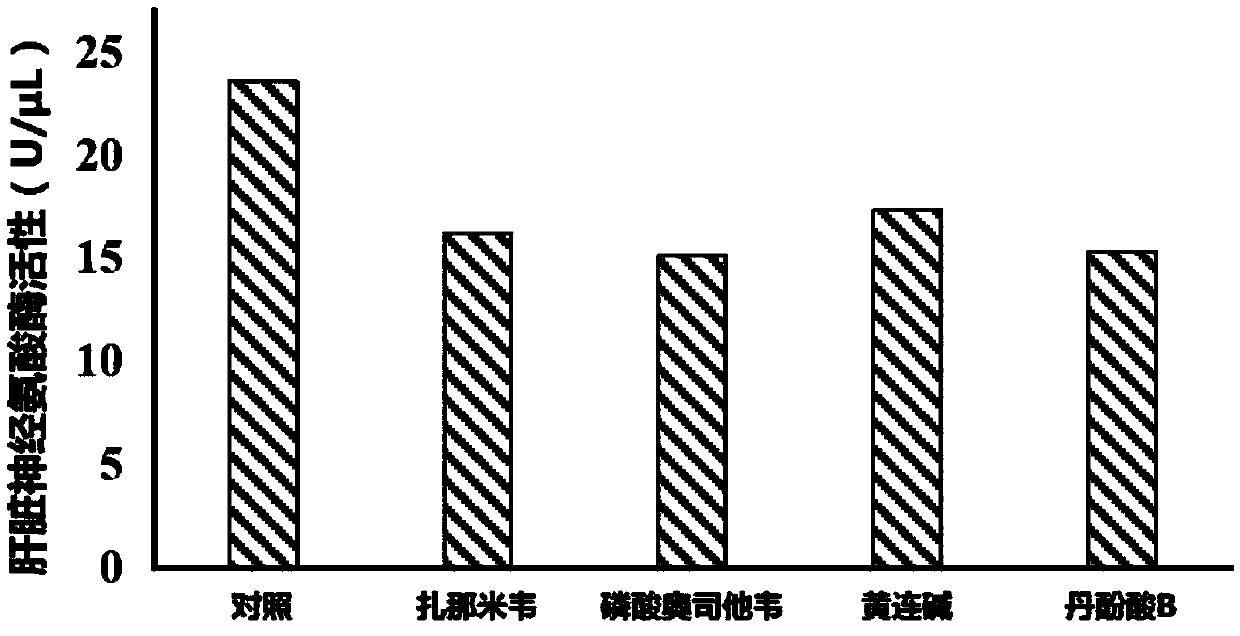
Application of neuraminidase and neuraminidase inhibitors to preparation of medicines for inhibiting hepatic gluconeogenesis
ActiveCN107812182AInhibitory activityOrganic active ingredientsPeptide/protein ingredientsZanamivirOseltamivir Phosphate
The invention discloses application of neuraminidase and neuraminidase inhibitors to preparation of medicines for inhibiting hepatic gluconeogenesis. Zanamivir and oseltamivir phosphate are effectiveinhibitors of the neuraminidase; the inhibition effect of coptisine on neuraminidase is also disclosed in the previous patent application of the applicant. The applicant also discovers that salvanic acid B is capable of inhibiting the activity of the neuraminidase in vitro and is used as an inhibitor of the neuraminidase. In-vivo test proves that the several neuraminidase inhibitors are capable ofeffectively inhibiting the activity of the neuraminidase in the liver and then inhibiting hepatic gluconeogenesis. Therefore, the neuraminidase and the neuraminidase inhibitors can be used for preparing the medicines for inhibiting hepatic gluconeogenesis and can also be used for treating diabetes, obesity and non-alcoholic fatty liver disease.
Owner:CHINA PHARM UNIV
Zanamivir solid lipid nanosphere oral preparation and preparation method thereof
InactiveCN102028655AHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsDrugs solutionOil phase
The invention provides a zanamivir solid lipid nanosphere oral preparation and a preparation method thereof. In the method, medicinal solution with different concentrations is used as inner water phases, dichloromethane in which glycerin monostearate / granulesten mixture is melted serves as an oil phase, and poloxamer 188 solution is used as an outer water phase so as to prepare the solid lipid nanosphere suspension or a free-dried preparation by a water-in-oil-in-water (W / O / W) composite emulsion solvent volatilization method. Solid lipid nanospheres prepared by the method have round shapes, the particle diameter of between 150 and 500nm, the surface potential of between -45 and -55mV, the entrapment rate of over 30 percent and release rate of more than 80 percent. The method is reliable and is easy and convenient to operate, the prepared solid lipid nanospheres can promote oral absorption of medicaments, and improve bioavailability, and the compliance of patients.
Owner:SUZHOU UNIV
Neuraminidase inhibitor zanamivir derivative and preparation method thereof
InactiveCN107266404AEasy to manufactureShorten the timeOrganic active ingredientsOrganic chemistryDrug biological activityStereochemistry
The invention discloses a neuraminidase inhibitor zanamivir derivative and a preparation method thereof. Different from the previous synthetic route of zanamivir derivatives, the present invention uses zanamivir as the starting material for the first time to prepare derivatives; the one-step reaction obtains the product, which greatly simplifies the synthesis steps; the prepared compounds are all New compound molecules with high predicted biological activity.
Owner:SHANGHAI INST OF TECH
Laninamivir octanoate preparation method
ActiveCN103435582ARaw materials are easy to getEasy to control intermediateOrganic chemistryChlorideReagent
The invention relates to a laninamivir octanoate preparation method, wherein the method is performed in the same reactor or a plurality of reactors in a step-by-step manner. The reaction process sequentially comprises: 1) mixing zanamivir and methanol while adding an acid cation resin, and carrying out a reaction to obtain zanamivir methyl ester; 2) carrying out a reaction of the zanamivir methyl ester and dimethyl carbonate under an alkaline condition to produce a compound represented by a formula (17); 3) carrying out a reaction of the compound represented by the formula (17) and methyl iodide under an alkaline condition to obtain a compound represented by a formula (18); 4) carrying out a reaction of the compound represented by the formula (18) and an alkali, and adopting a cation resin to adjust the pH value to the neutral state to obtain a compound represented by a formula (19); and 5) carrying out a reaction of the compound represented by a formula (19) and octanoyl chloride to obtain the target product laninamivir octanoate. The present invention provides the laninamivir octanoate preparation method, which has characteristics of simple route, low cost, low energy consumption, high product purity, and avoidance of use of hypertoxic reagents and explosive reagents.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Zanamivir phosphonate congeners with anti-influenza activity and determining oseltamivir susceptibility of influenza viruses
Methods and compositions for detection of drug resistant pathogens and treatment against infections thereof are provided. Methods for detection of oseltamivir-resistant influenza viruses by competitive binding assays utilizing non-oseltamivir influenza virus neuraminidase inhibitors and oseltamivir carboxylate are provided. Influenza virus neuraminidase inhibitors coupled to sensors and useful for employment in the methods of the invention are disclosed. Novel phosphonate compounds active as neuraminidase inhibitors against wild-type and oseltamivir-resistant influenza strains of H1N1, H5N1 and H3N2 viruses are disclosed. An enantioselective synthetic route to preparation of these phosphonate compounds via sialic acid is provided.
Owner:ACAD SINIC
Application of neuraminidase inhibitor to preparation of drug for treating myocarditis
ActiveCN108125942ARemission of lesionEffective therapeutic effectOrganic active ingredientsCardiovascular disorderSerum igeCardiac muscle
The invention discloses application of a neuraminidase inhibitor to preparation of a drug for treating myocarditis. The invention discovers that oseltamivir phosphate or zanamivir as the neuraminidaseinhibitor has an effective treatment effect on a myocarditis model mouse. After the myocarditis model mouse is treated by the oseltamivir phosphate or the zanamivir, the serum cTnI concentration is significantly reduced, so that the degree of myocardial injury is improved; and myocardial case scores are significantly reduced, so that the degree of cardiomyopathy is relieved. Therefore, the oseltamivir phosphate or the zanamivir as the neuraminidase inhibitor can be used for preparing the drug for treating the myocarditis.
Owner:CHINA PHARM UNIV
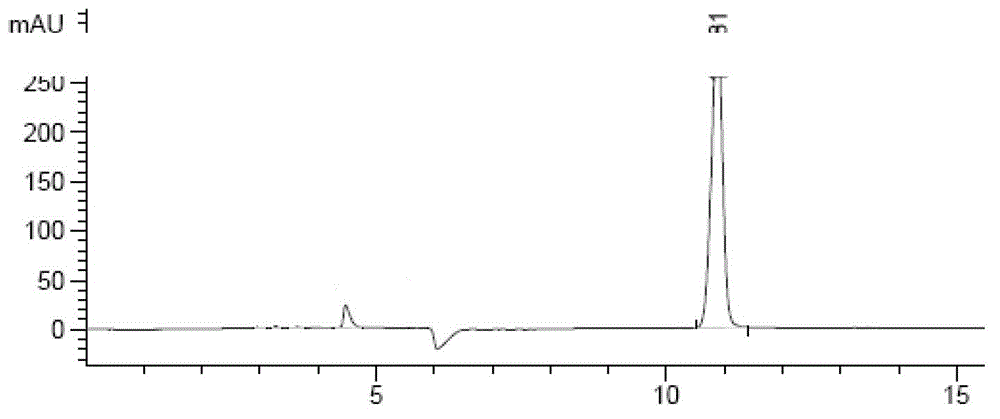
HPLC determination method for detecting impurities in zanamivir and zanamivir-containing preparation
ActiveCN104007185ASolution to short lifeStrong retentionComponent separationOrganic solventLength wave
The invention discloses an HPLC determination method for detecting impurities in zanamivir and a zanamivir-containing preparation. Hydrophilic interaction liquid chromatography (HILIC) is selected to separate zanamivir and its relevant substances, and the determination method for detecting impurities in zanamivir and the zanamivir-containing relevant preparation is concretely characterized in that a chromatographic column is an HILIC column, a mobile phase selects a polar organic solvent-buffer salt solution, a polar organic solvent-weak acid solution or an aqueous solution of a polar organic solvent, and the detection wavelength is 200-240nm. The determination method enables process impurities and degraded products of zanamivir to be rapidly and accurately detected, and has the characteristics of simple operation, high sensitivity, good repeatability and reliable result.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD +1
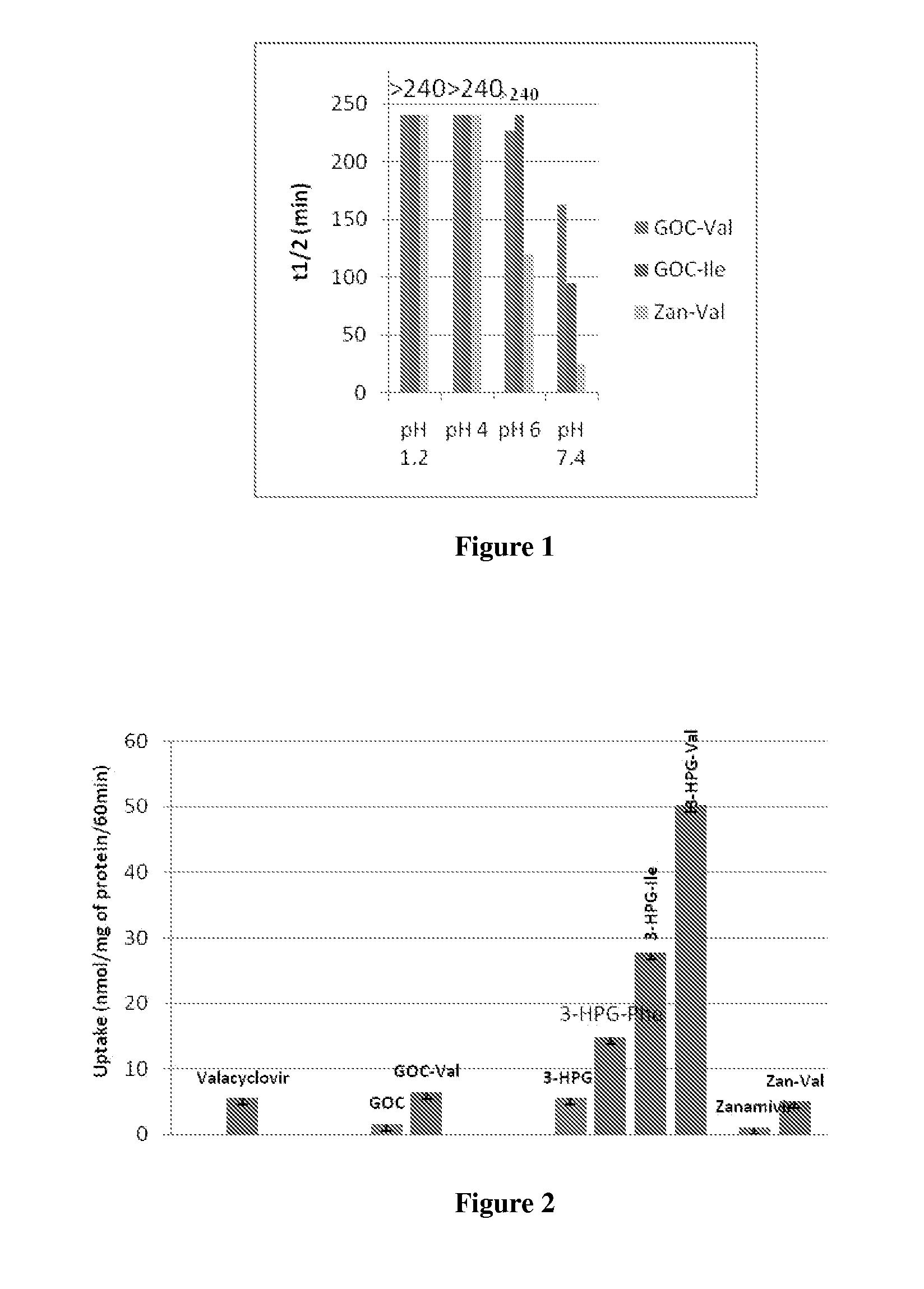
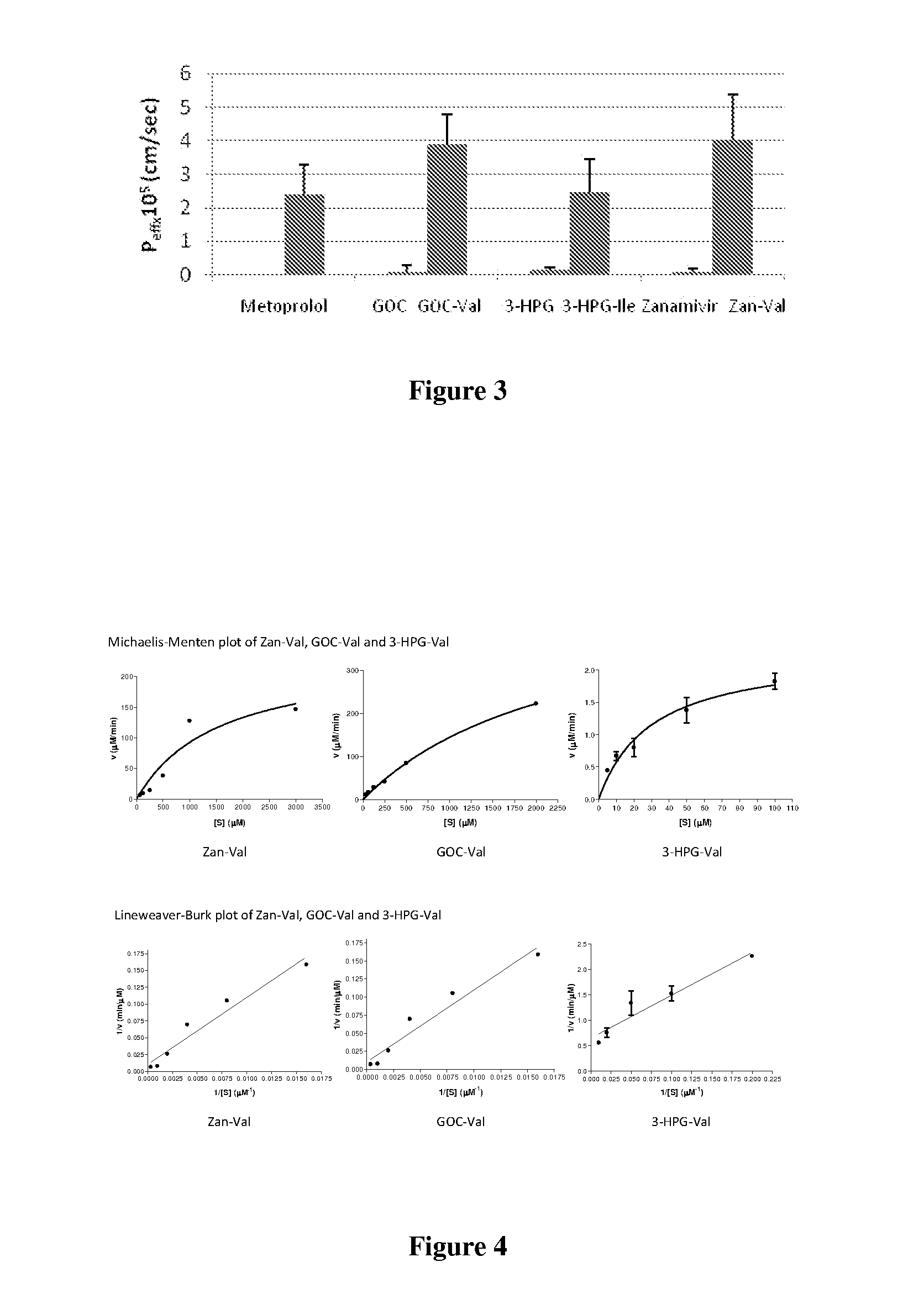
Formulations for enhanced bioavailability of zanamivir
In accordance with the present invention, there are provided compositions comprising zanamivir and at least one permeability enhancer. The compositions can increase the amount of zanamivir capable of being transported across a cell membrane (such as a Caco-2 cell membrane), and can increase this amount by at least 150% relative to the amount capable of being transported across the cell membrane in the absence of the permeability enhancer. Also provided are oral dosage forms of the compositions, which comprise a therapeutically effective amount of zanamivir and a permeability-enhancing amount of a permeability enhancer. The oral dosage forms can further comprise an enteric- or pH-sensitive coating or layer surrounding the composition. Also provided in accordance with the present invention are methods for treating or preventing influenza infection.
Owner:ALA WAI PHARMA
Method for preparing zanamivir intermediate serving as compound resisting highly pathogenic avian influenza
InactiveCN101704851AHigh purityChange physical formSugar derivativesSugar derivatives preparationSodium acetateDistillation
The invention relates to a method for preparing zanamivir intermediate serving as compound resisting highly pathogenic avian influenza (5-acetylamino-3,5-didehydro-D-glycerol-D-galactose-2-enol methyl palmitate). The method comprises: obtaining hydrogen chloride solution of methanol through the reaction of acyl chloride and methanol, wherein the weight ratio of the acyl chloride to the methanol is 1:100-1,000; adding sialic acid to the hydrogen chloride solution of methanol, stirring, heating, performing reaction at the temperature between 20 and 60 DEG C for 2 to 8 hours, adding 0.1 equivalent (mol) of sodium acetate, adjusting pH between 6 and 7, continuing to stir for 10 minutes and stopping reaction, wherein the weight ratio of the sialic acid to the hydrogen chloride solution of methanol is 1:2-20, and reaction temperature is 20 to 60 DEG C; performing concentration under reduced pressure, performing distillation under reduced pressure till drying the obtained product and using water and ethyl acetate for recrystallization, wherein the weight ratio of the water to the ethyl acetate is 1:10-100; and filtering, drying under vacuum and obtaining the zanamivir intermediate serving as compound resisting highly pathogenic avian influenza.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD
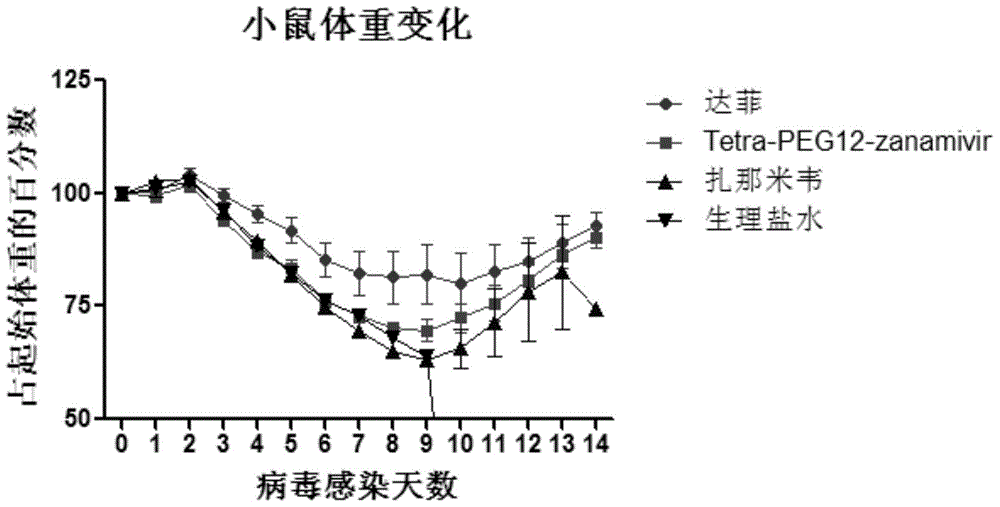
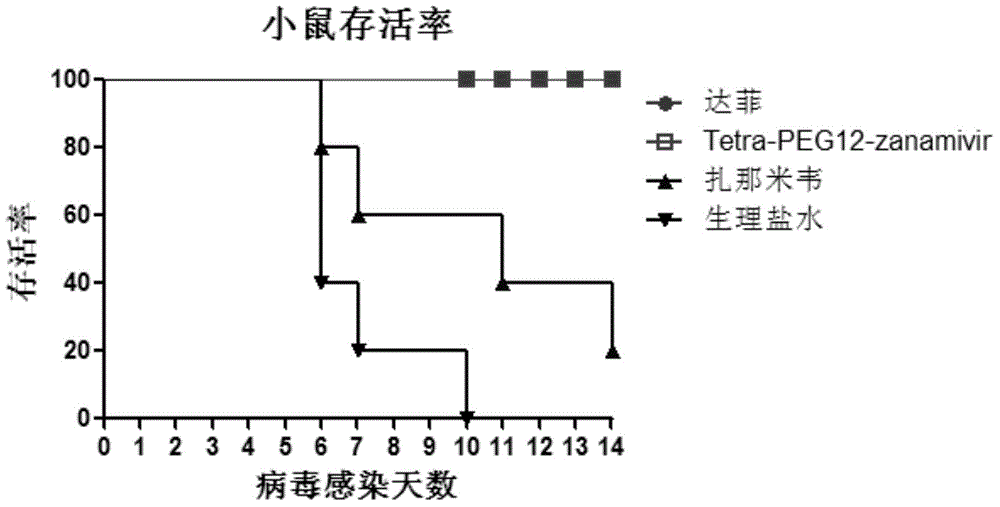
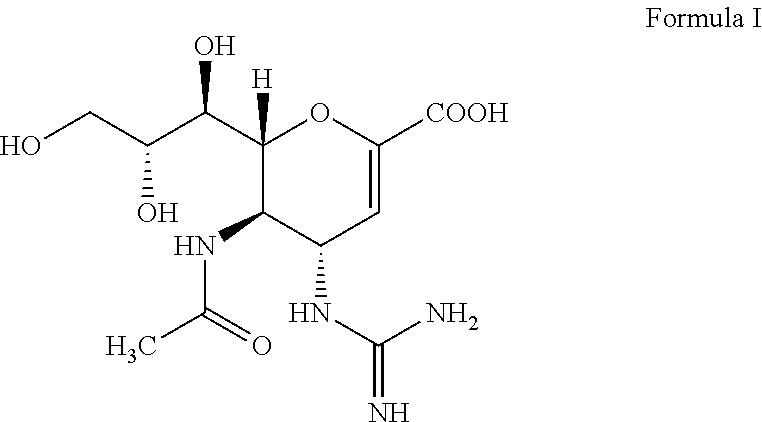
Tetravalent zanamivir and its preparation method and application
ActiveCN103819665BEfficient inhibitory effectHigh anti-influenza virus activityOrganic chemistryAntiviralsNeuraminidaseVirus influenza
The invention discloses tetravalent zanamivir, its preparation method and its application. A structural formula of tetravalent zanamivir is shown as a formula I, wherein m in the formula is a number between 3 and 12. The invention also provides the application of tetravalent zanamivir in the formula I in preparation of medicines for resisting influenza, and the application concretely appears as 1) or 2): 1) the anti-influenza medicines can inhibit enzymatic activity of neuraminidase; and 2)the anti-influenza medicines can inhibit the growth of the cells infected by influenza. The provided tetravalent zanamivir can used for treating various viral influenza. Compared with the current zanamivir, tetravalent zanamivir has anti-influenza virus activity with higher efficiency, and has inhibition effect with high efficiency to the drug-resistant strains of influenza virus; and the provided tetravalent zanamivir can be prepared to oral medicines.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Sialic acid analog and application thereof to preparation of anti-influenza-virus medicaments
InactiveCN102127041AInhibitory activityOrganic active ingredientsOrganic chemistryAnti virusAntiviral drug
The invention provides a sialic acid analog with obvious activity of inhibiting influenza virus and application of the sialic acid analog to preparation of anti-influenza-virus medicaments. Zanamivir serves as a lead compound; and a C6 position side chain in the molecular structure is modified, so that a novel zanamivir analog of which the C6 position is provided with an amino side chain and a C6position side chain is provided with hydrophilic hydroxyl is obtained. A vitro anti-virus activity research is performed on the compound. The result shows that the compound in vitro has obvious activity of inhibiting the influenza virus. Therefore, the sialic acid analog is expected to be applied to preparation of the anti-influenza-virus medicaments.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Preparation method of zanamivir intermediate and preparation method of zanamivir
ActiveCN106632186AFew synthetic stepsSimple process operationOrganic chemistryBulk chemical productionChemistrySialic acid
The invention relates to a preparation method of a zanamivir intermediate, namely an acetylation protected amino compound, and a preparation method of zanamivir. The preparation method of the zanamivir, provided by the invention, comprises the following steps: performing cyclization by taking sialic acid as a raw material and through group protection to produce a cyclization substance, treating the cyclization substance by a metal amino substance at one step to obtain the acetylation protected amino compound, and removing a hydroxyl protective agent acetic acid ester and removing guanidyl from imipyrozole reaction to obtain the zanamivir, wherein the process of treating the cyclization substance by the metal amino substance at one step to obtain the acetylation protected amino compound is great substantial breakthrough in preparation of the zanamivir, the synthesis steps are greatly shortened and the process operation is simplified. Ring opening is conducted by the cheap metal amino substance instead of expensive trimethylsilyl azid, so that the cost is reduced and the safety of the production process is guaranteed. According to thepreparation method of the zanamivir, provided by the invention, the total mass yield can reach 50 percent or above and is greatly increased as compared with that in the prior art.
Owner:湖北浩信药业有限公司
Formulations for enhanced bioavailability of zanamivir
In accordance with the present invention, there are provided compositions comprising zanamivir and at least one permeability enhancer. The compositions can increase the amount of zanamivir capable of being transported across a cell membrane (such as a Caco-2 cell membrane), and can increase this amount by at least 150% relative to the amount capable of being transported across the cell membrane in the absence of the permeability enhancer. Also provided are oral dosage forms of the compositions, which comprise a therapeutically effective amount of zanamivir and a permeability-enhancing amount of a permeability enhancer. The oral dosage forms can further comprise an enteric- or pH-sensitive coating or layer surrounding the composition. Also provided in accordance with the present invention are methods for treating or preventing influenza infection.
Owner:ALA WAI PHARMA
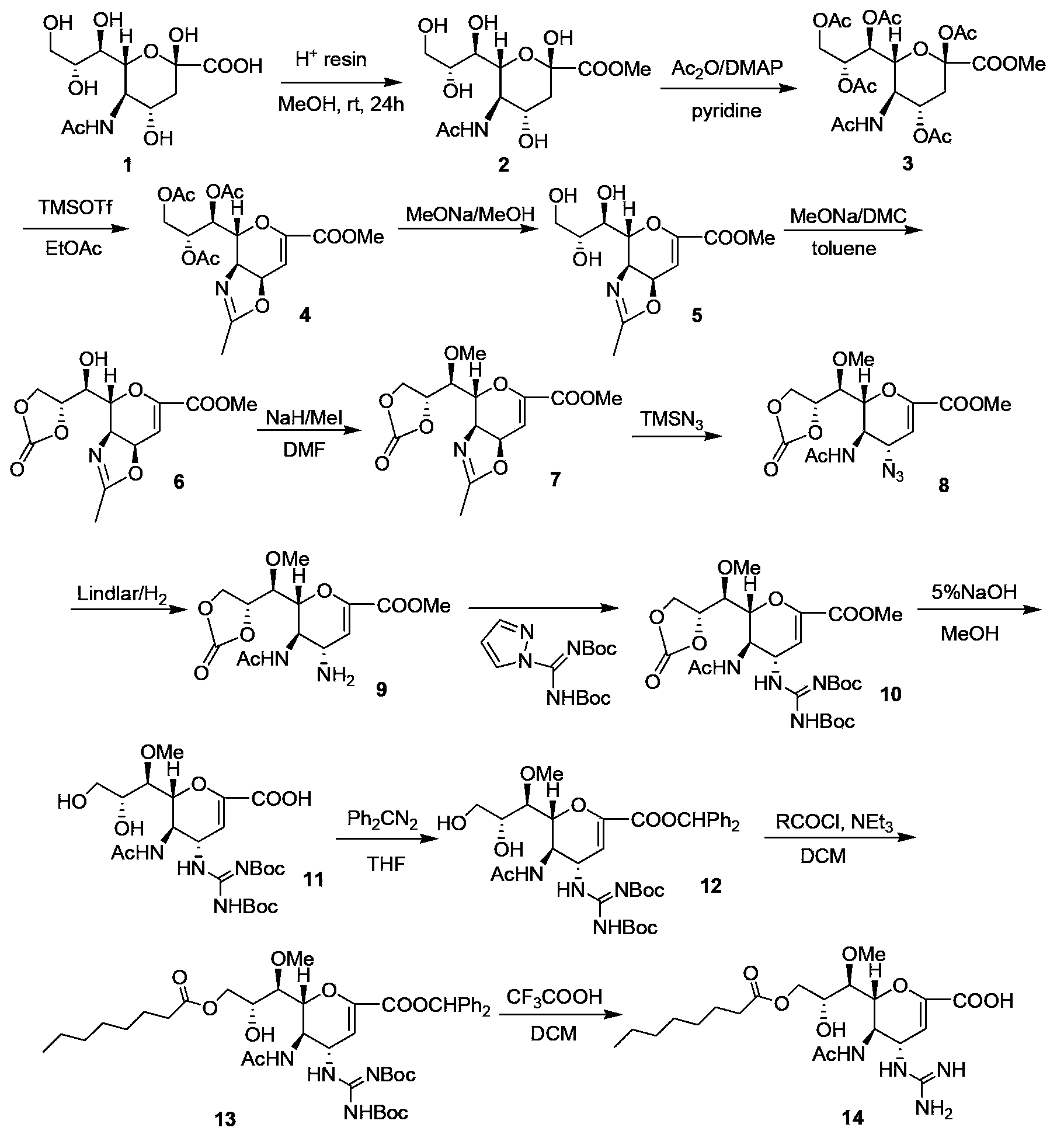
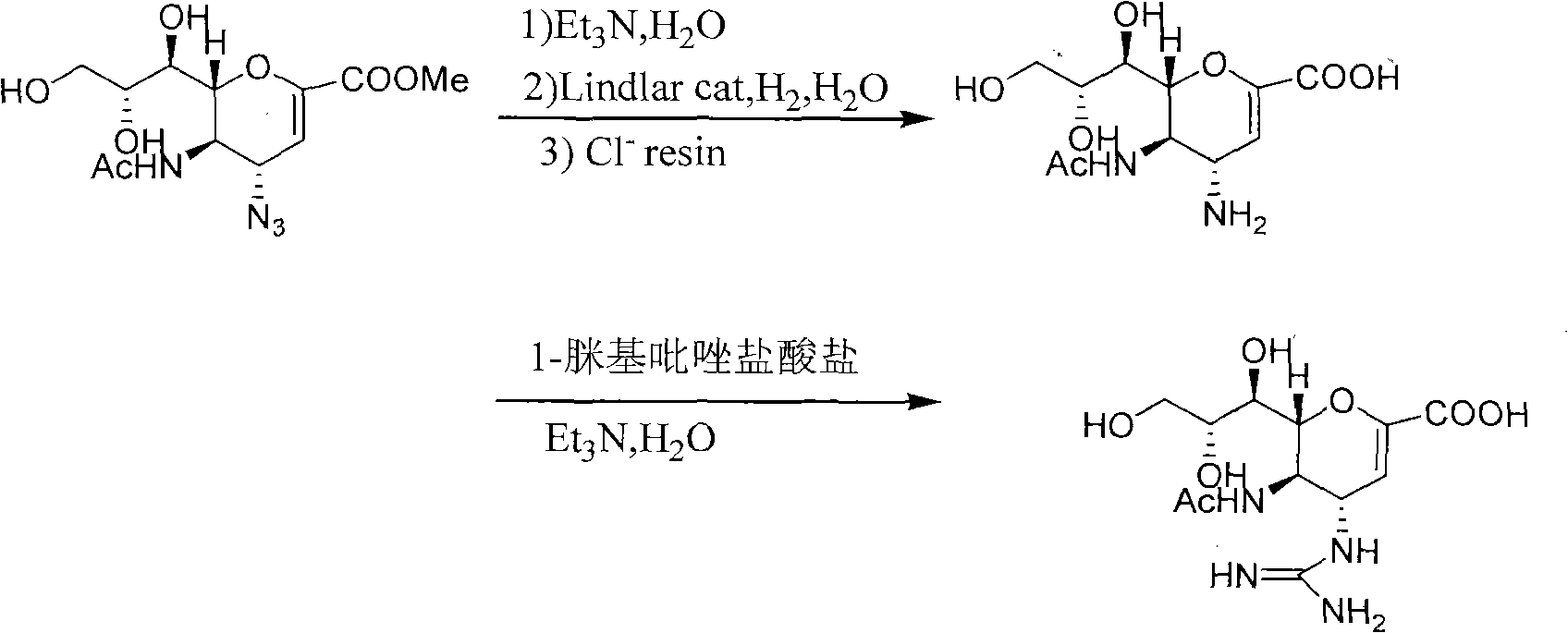
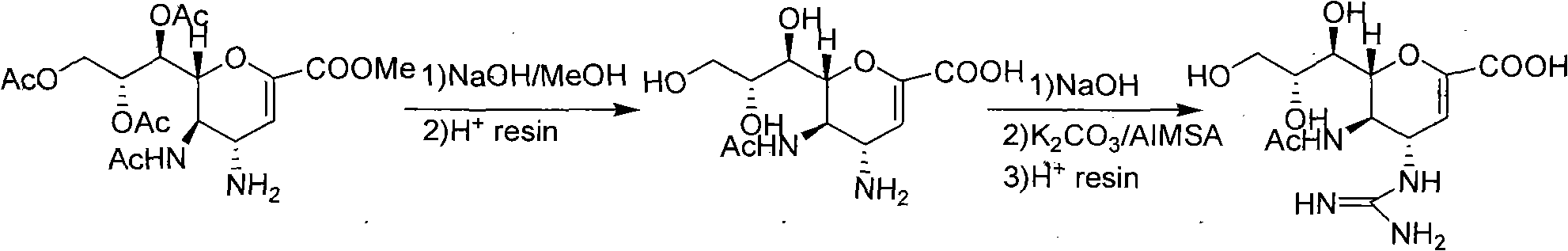
Preparation method of zanamivir
The invention relates to a preparation method of an antiviral drug zanamivir, which comprises the following steps: amino in an intermediate neuraminic acid methyl ester derivative is conversed to guanidine, then ester group is subjected to hydrolyzation, so that zanamivir is prepared. Compared with current synthesis technology of zanamivir, the method of the invention has the advantages that the usage of resin can be avoided, the cost is reduced, no separation or purification is required after each step of the reaction, the next step reaction can be directly carried out, the loss of the intermediate during the purifying process can be avoided, the yield is high, the operation is convenient, and the preparation method of the zanamivir is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Anti-influenza virus compound as well as preparation method and application thereof
ActiveCN111303235AStrong inhibitory activityGood water solubilityOrganic active ingredientsAntiviralsChemical compoundAqueous solubility
The invention discloses an anti-influenza virus compound as well as a preparation method and application thereof, and belongs to the technical field of medicinal chemistry. The anti-influenza virus compound has a structural general formula shown in the specification, wherein m is any natural number selected from 2-11, n is any natural number selected from 3-12, X is selected from OCOO or O, and the expression of Y is described in the specification. The anti-influenza virus compound provided by the invention has obvious inhibition activity on various influenza viruses, especially on zanamivir-resistant influenza viruses (H3N2, E119V), the water solubility of the original medicine is remarkably improved, the lipid-water distribution coefficient is tens of times higher than that of the original medicine, and the compound can be prepared into an oral preparation and has a great breakthrough in medicine dosage forms.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
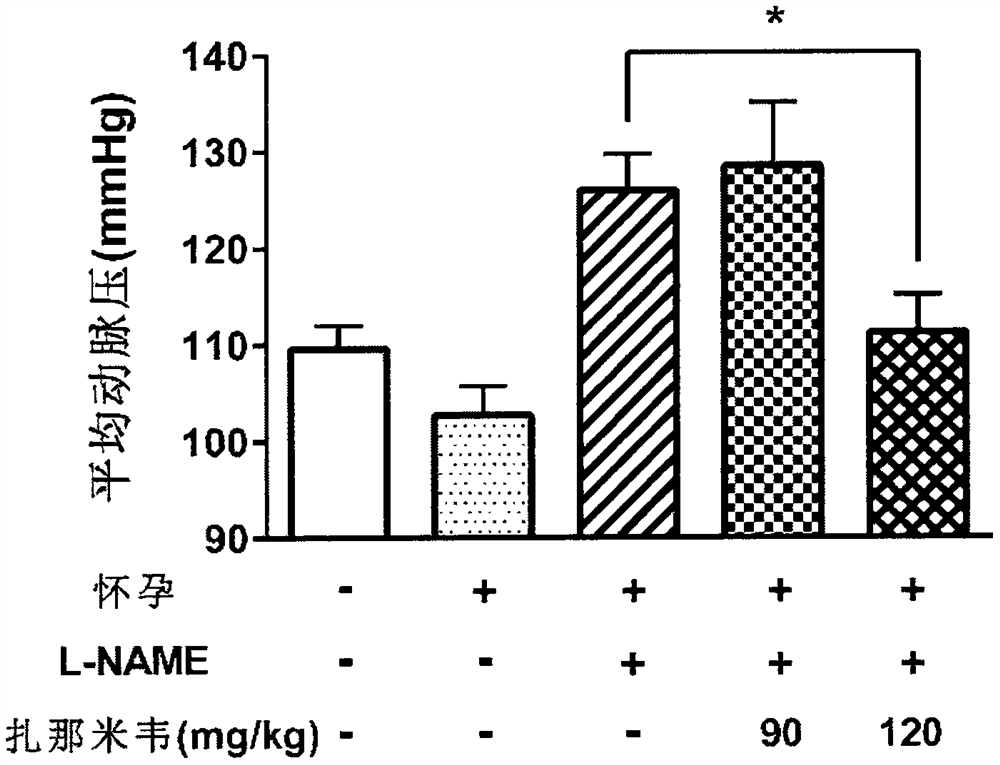
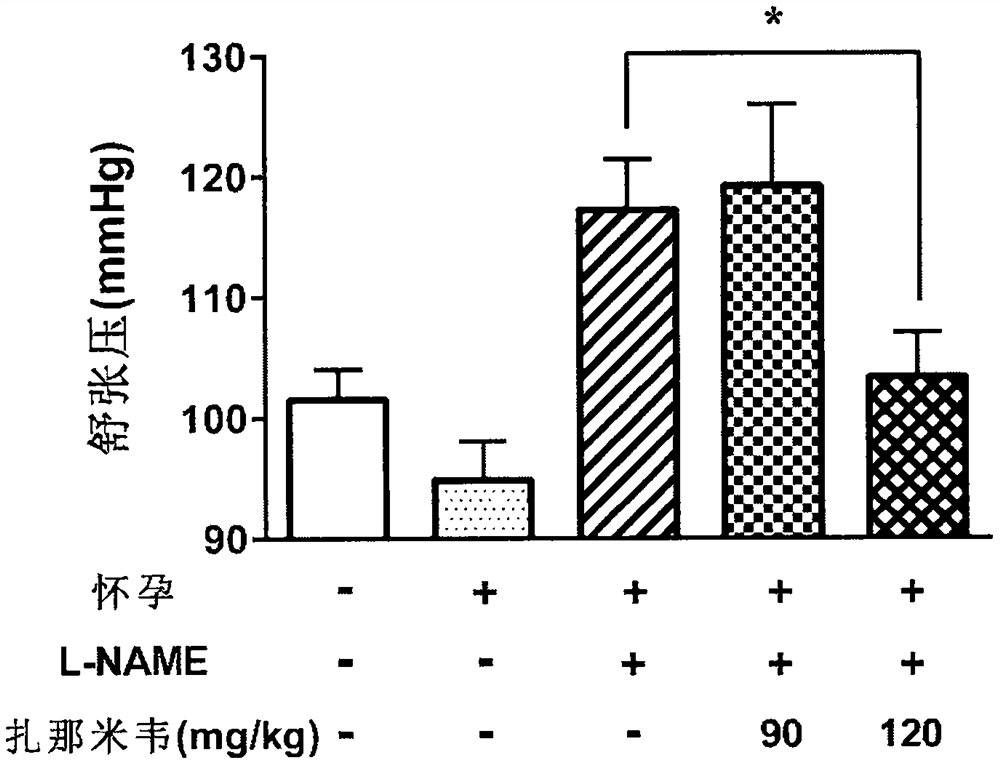
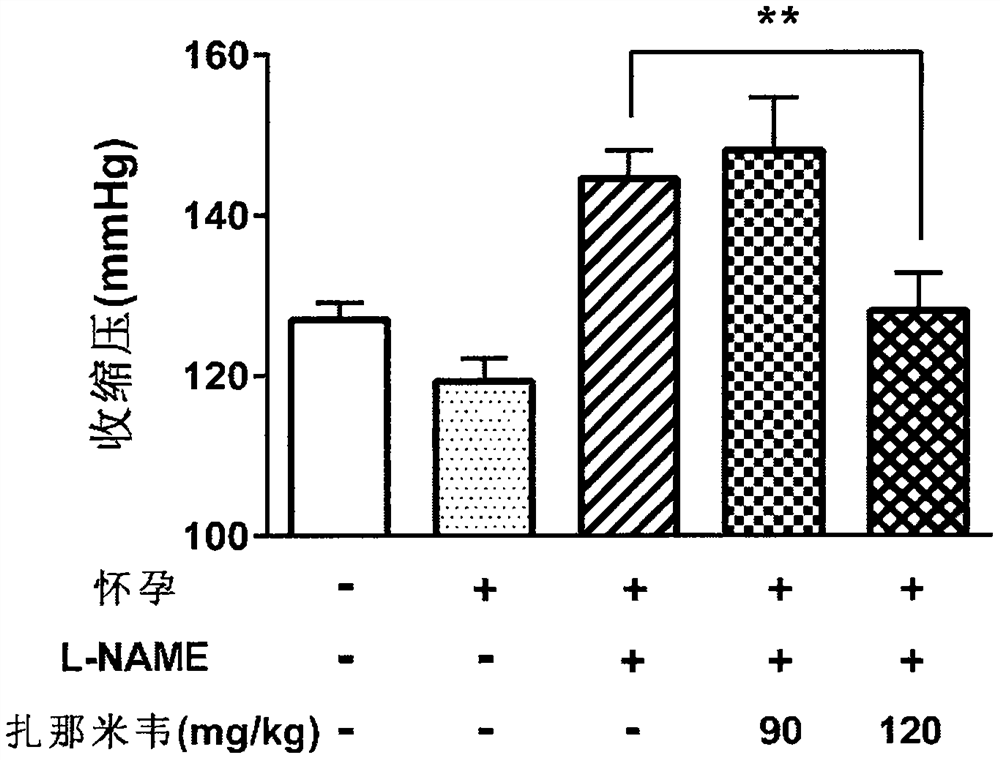
Application of zanamivir in preparation of medicine for treating or preventing preeclampsia
PendingCN114533721AImprove high blood pressureGood treatment effectOrganic active ingredientsSenses disorderPregnanetriolonePharmacology
The invention provides application of zanamivir in preparation of a medicine for treating or preventing preeclampsia, a corresponding medicine and a treatment method. Zanamivir can effectively improve the symptoms of hypertension, proteinuria, creatinine, neuraminidase and sialic acid rise of preeclampsia and increase the NO level; the pregnancy outcome is improved. The combination of zanamivir and hydroxyprogesterone hexanoate shows a better effect.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
Prodrugs of Neuraminidase Inhibitors
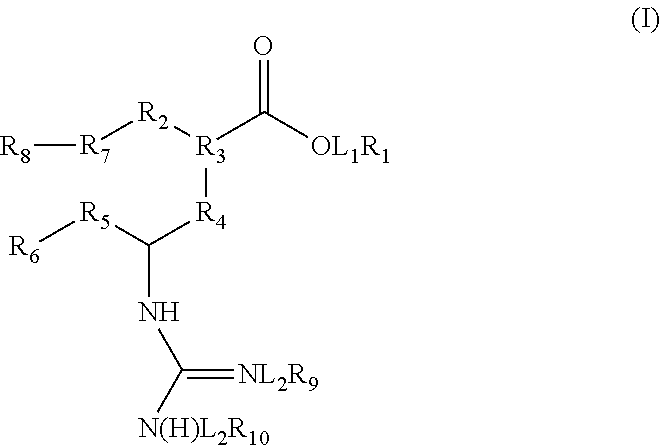
InactiveUS20120058937A9Improve oral bioavailabilityInhibition is effectiveGroup 4/14 element organic compoundsBiocideDipeptidePrimary alcohol
A new class of neuramidase inhibitor prodrugs is provided characterized by a prodrug moiety of a carboxyl group modified to form a carbonyl ethoxy amino acid, a carbonyl ethoxy dipeptide or a carbonyl ethoxy tripeptide, a guanidine group modified to form a carbonyl ethoxy amino acid, a carbonyl ethoxy dipeptide, a carbonyl ethoxy tripeptide; a primary alcohol modified to form an esterified single amino acid, dipeptide or tripeptide of zanavimir of the unaltered therapeutic agent. Exemplary therapeutic agents so modified to form prodrugs include zanavimir, oseltamivir and peramivir. The prodrug has increased oral bioavailability relative to the unaltered neuraminidase inhibitor and is effective in the inhibition of viral infections involving neuraminidase in the viral reproductive cycle.
Owner:SINEVIR THERAPEUTICS
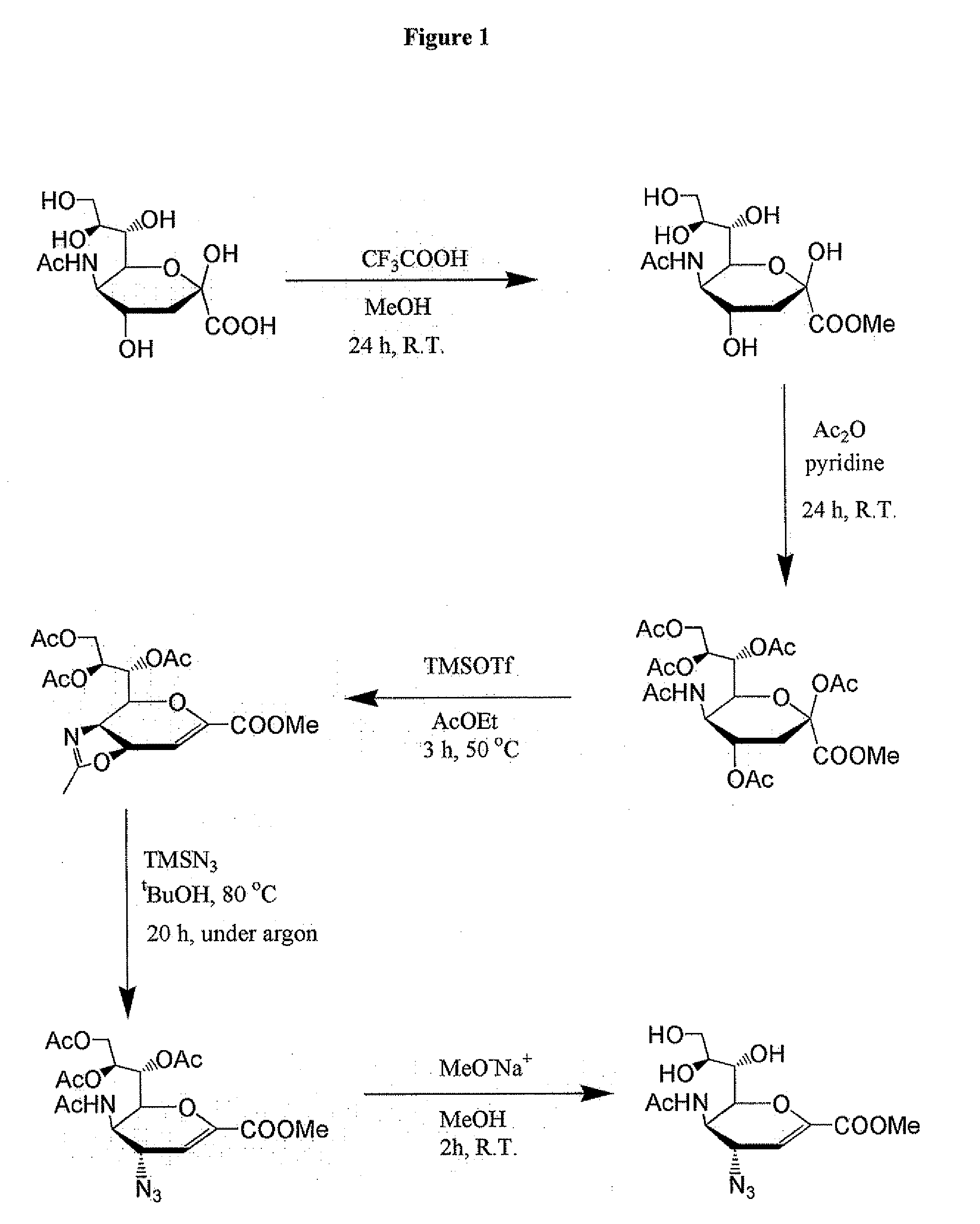
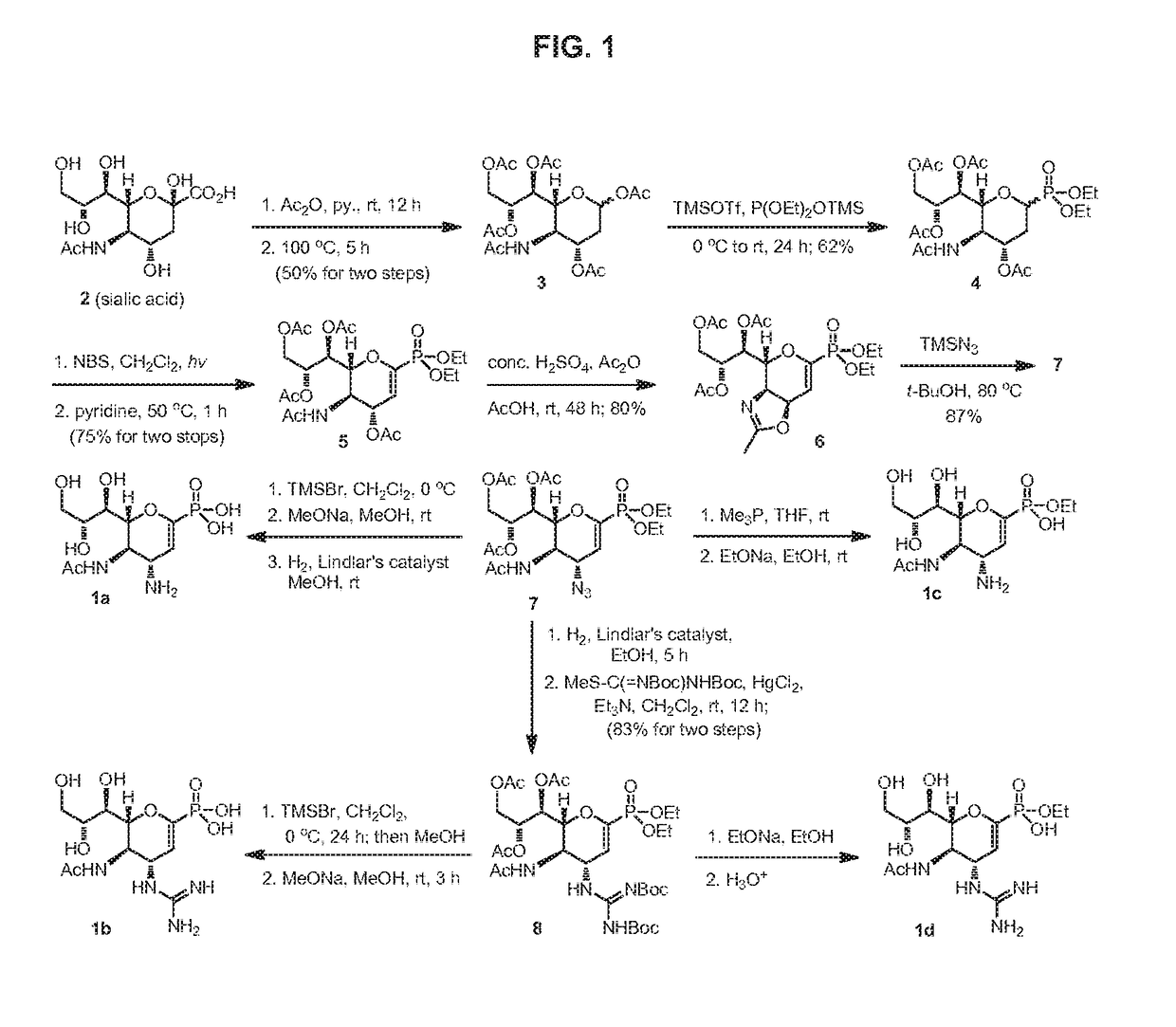
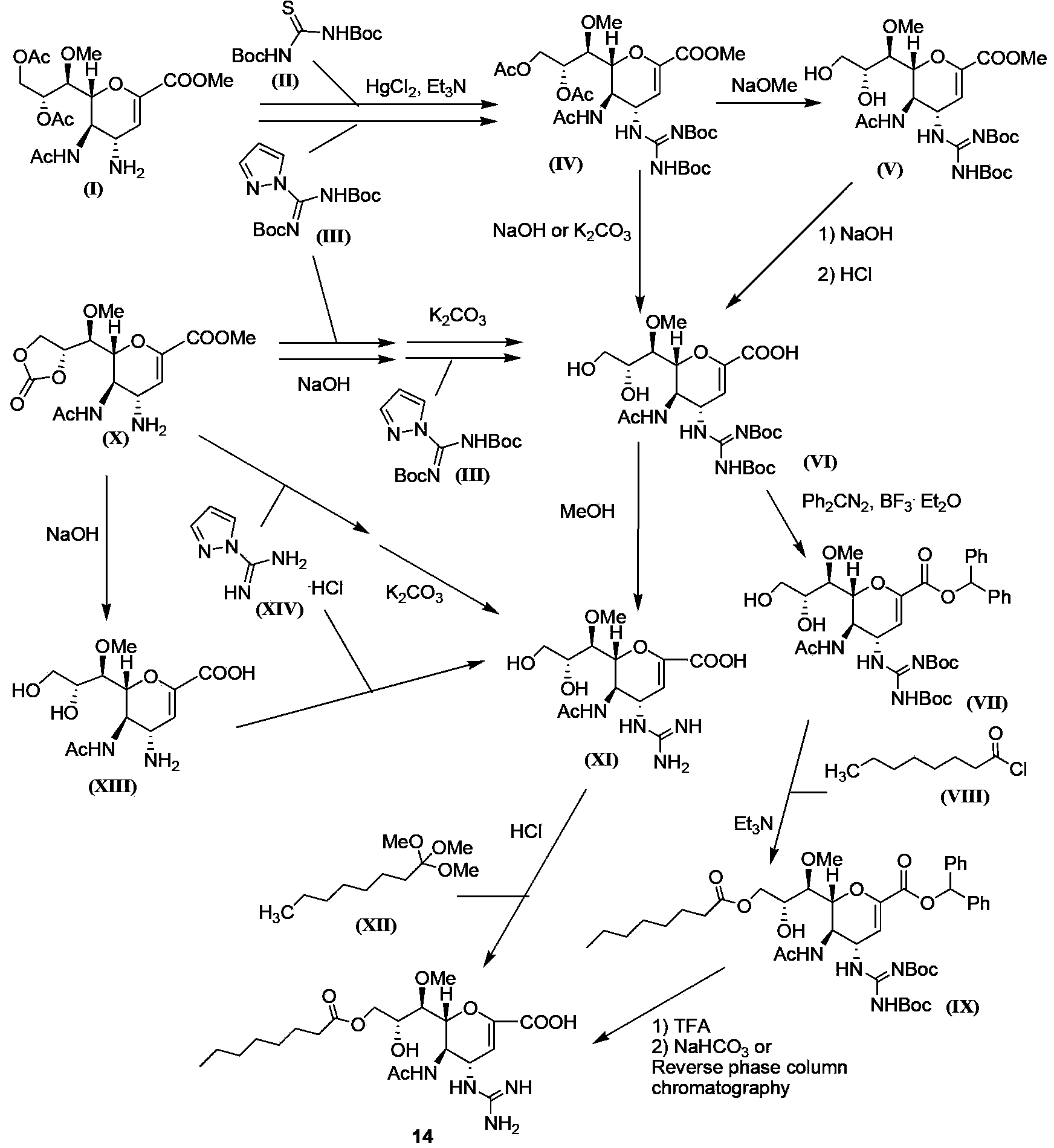
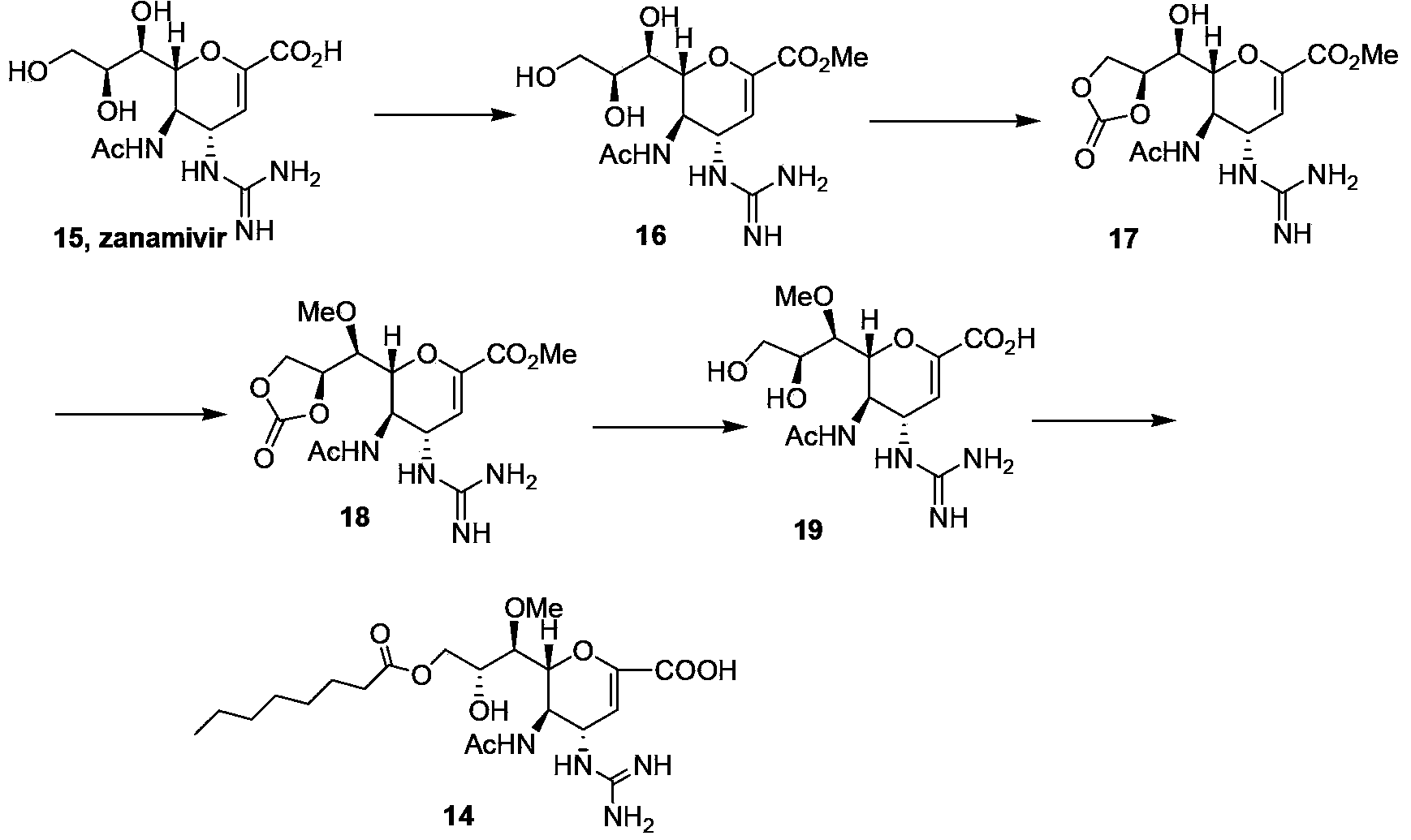
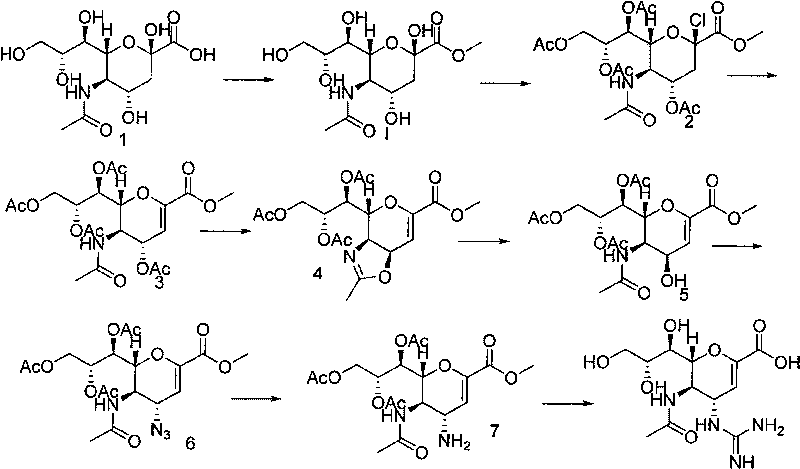
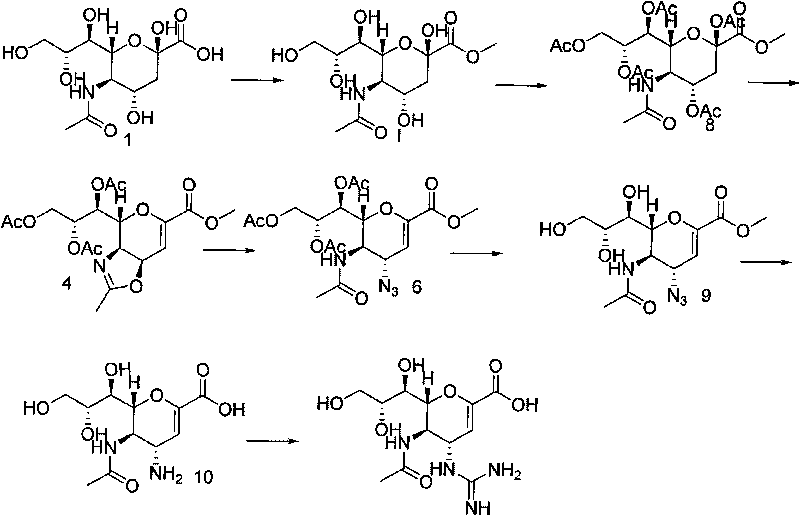
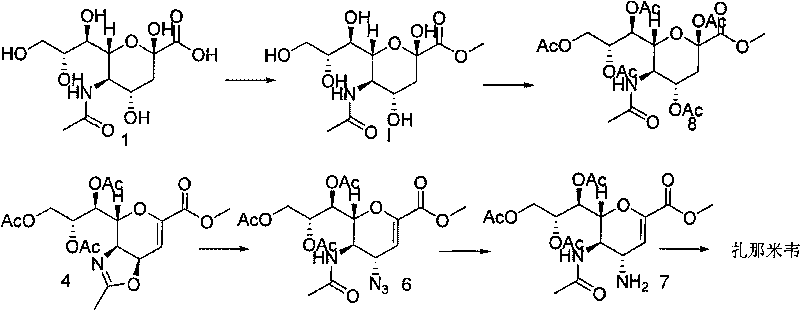
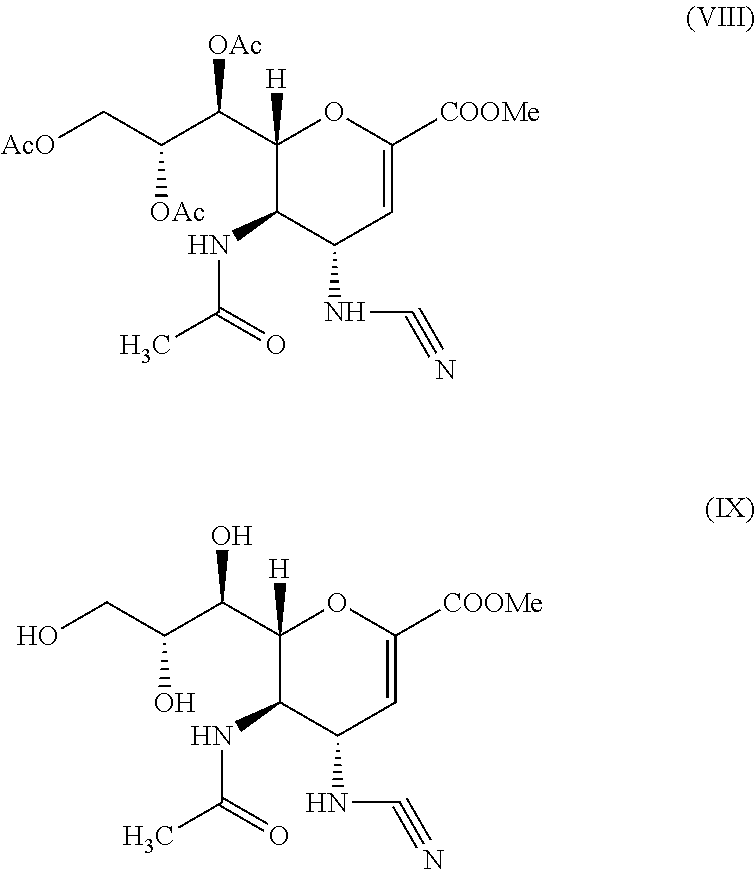
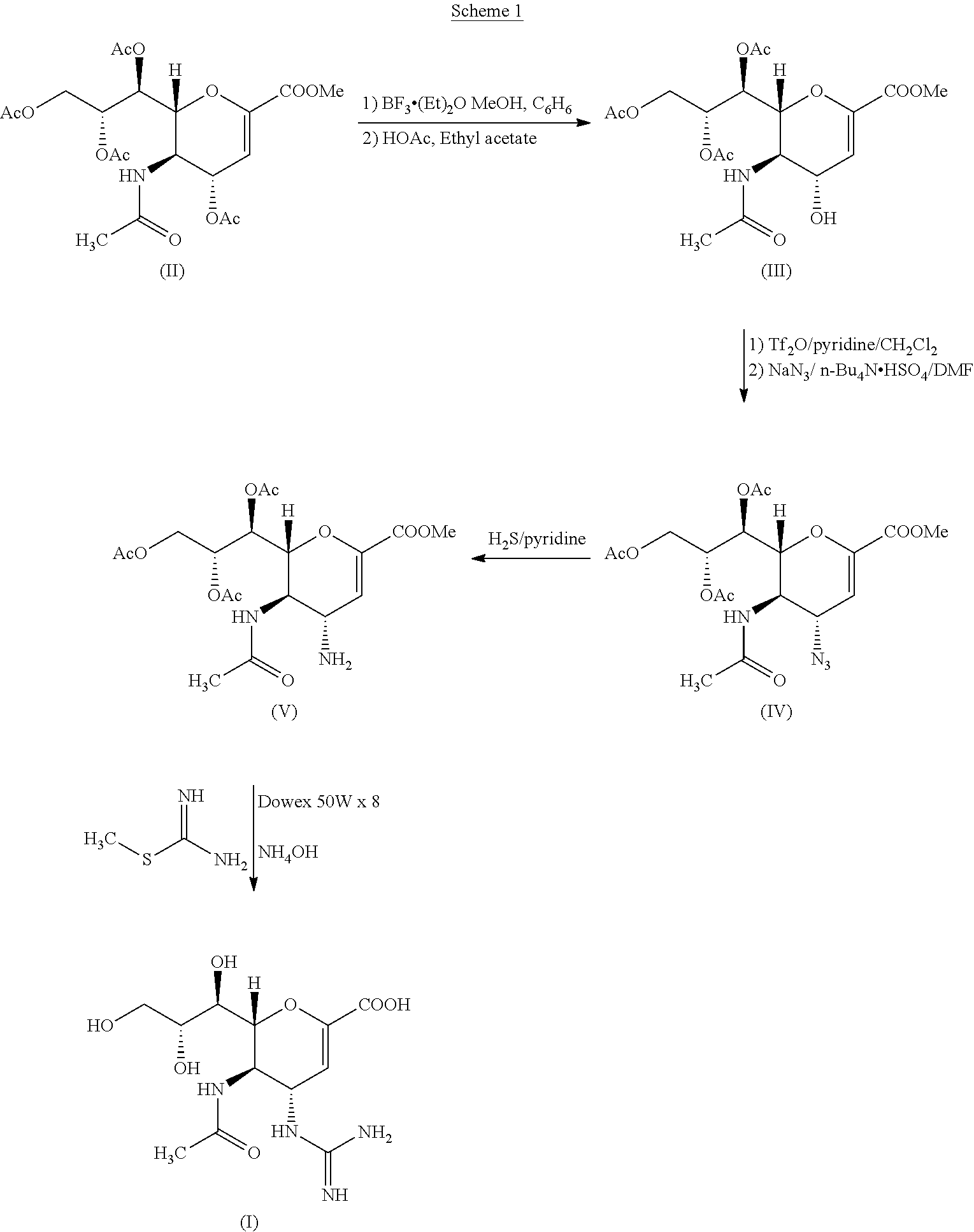
Process for Preparing Zanamivir and Intermediates for Use in the Process
The present invention provides a process for preparing methyl 5-acetamido-4-amino-6-(1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylate (V), which process comprises reducing methyl 5-acetamido-4-azido-6-(1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylate (IV) in the presence of a reducing agent selected from the group consisting of lithium aluminium hydride, sodium borohydride, zinc / ammonium chloride, zinc-ferric chloride and ferric chloride / sodium iodide. The present invention also provides compounds of formula (VIII) and (IX) which may be used in the synthesis of zanamivir. The present invention also provides processes for preparing compounds (VIII) and (IX) and processes involving their use, including in the synthesis of zanamivir.
Owner:CIPLA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com